Leoimyosarcoma is aggressive and responds poorly to chemotherapy. Novel treatment options are now available, but the challenge is the selection and optimal use of these new therapies. This review examines current guidelines to build consensus for optimal treatment guidelines for advanced or recurrent uterine leiomyosarcoma.
Keywords: Uterine cancer, Leiomyosarcoma, Soft tissue sarcoma, Uterine sarcoma, L‐sarcoma chemotherapy
Abstract
The treatment of metastatic and recurrent uterine leoimyosarcoma (uLMS) has evolved rapidly in the past several years. Leoimyosarcoma is extremely aggressive and responds poorly to traditional chemotherapeutics. Recent regulatory approval of novel treatment options has significantly expanded the therapeutic armamentarium, and the addition of these therapies has challenged clinicians to select and optimally sequence these new compounds. Additionally, the potential role of immunotherapy is being assessed in current uLMS clinical trials. Given the increasing number of agents available both in the U.S. and globally, a treatment template that addresses optimal sequencing based upon expert consensus would be useful. Current guidelines, although listing various options, lack granularity by line of therapy. Most patients with leiomyosarcoma, even in early stage, are treated with surgery followed by adjuvant chemotherapy despite uLMS being relatively chemoresistant. Adjuvant chemotherapy often includes the combination of gemcitabine and docetaxel with or without doxorubicin in first‐line systemic therapy, but these cytotoxic agents only provide patients with advanced disease a 5‐year survival <30%. This review will focus on examination of current guidelines and consensus building for optimal sequencing of systemic therapies for advanced or recurrent uLMS. Critical ongoing studies investigating novel approaches including immunotherapeutics and genetic alterations also will be discussed.
Implications for Practice.
Recent regulatory approval of novel treatment options has significantly expanded the therapeutic armamentarium, and the addition of these therapies has challenged clinicians to select and optimally sequence these compounds. This review will focus on examination of current guidelines and consensus building for optimal sequencing of systemic therapies for advanced or recurrent uterine leoimyosarcoma.
Introduction
Approximately 5,058 cases of uterine sarcomas are expected to be diagnosed in the U.S. in 2018 with a mortality rate as high as 29% [1], [2]. For leiomyosarcoma (LMS), high recurrence rates are observed in all stages despite surgery and adjuvant treatment [3]. The 5‐year survival rate for stage I disease is 76%, whereas stages II–IV are associated with 60%, 45%, and 29% survival, respectively [4]. Most patients will not live more than 12 months after recurrence [5], [6]. In advanced‐stage patients (stage III/IV), current guidelines support the role of chemotherapy in patients following definitive surgery (adjuvant) and in patients who have unresectable disease. However, the optimal regimen, order, and dose of systemic chemotherapy remains unclear. Here, we review the rationale behind the regimens and types of chemotherapy that are being used for metastatic and recurrent uterine LMS (uLMS).
Background
Uterine sarcomas are a heterogeneous group of tumors, including carcinosarcomas, leiomyosarcomas, endometrial stromal sarcomas, adenosarcomas with sarcomatous overgrowth, and high‐grade undifferentiated sarcomas (Table 1) [7]. The natural history, prognosis, and treatment of uterine sarcomas vary by histology and grade. There are limited data regarding the rarest subtypes. When carcinosarcoma is excluded, LMS is the most common type of uterine sarcoma [8], [9]. uLMS is composed of malignant smooth muscle with significant cellularity, nuclear atypia, necrosis, high mitotic rate, invasion, and metastases. LMS is diagnosed based on the Stanford criteria, requiring the presence of at least two of the following characteristics: (a) high mitotic rate >10 figures per 10 high‐power fields, (b) moderate to severe cellular atypia, and (c) areas of coagulative tumor cell necrosis [10]. Leiomyosarcomas typically express smooth muscle markers, including smooth muscle actin and h‐caldesmon. There has been significant variation in the literature as to the percentage of estrogen and/or progesterone receptor positivity, varying from 7% to 71% [11]. uLMS harbors multiple complex chromosomal abnormalities with no single specific translocation. Abnormalities of the p53, MDM2, and DCC genes have been described [12]. Given the complexity of the histology of uLMS, it is important that all cases are reviewed by a gynecological pathologist with expertise in this area.
Table 1. Types of uterine sarcomas.
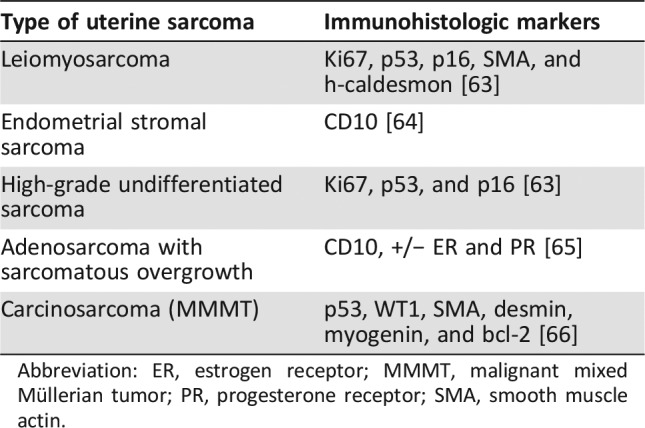
Abbreviation: ER, estrogen receptor; MMMT, malignant mixed Müllerian tumor; PR, progesterone receptor; SMA, smooth muscle actin.
Median age at diagnosis is 56 years. Most experts believe uLMS arises de novo and only rarely transitions from benign leiomyoma, occuring approximately 5%–10% of the time. The incidence of occult LMS is between 0.2% and 0.7% [13]. It typically presents with abnormal bleeding like most uterine cancers; however, it has been linked to some unusual presentations, including the Leser‐Trélat sign, which is the rapid onset of seberroheic keratoses that can be a sign of underlying malignancy [14].
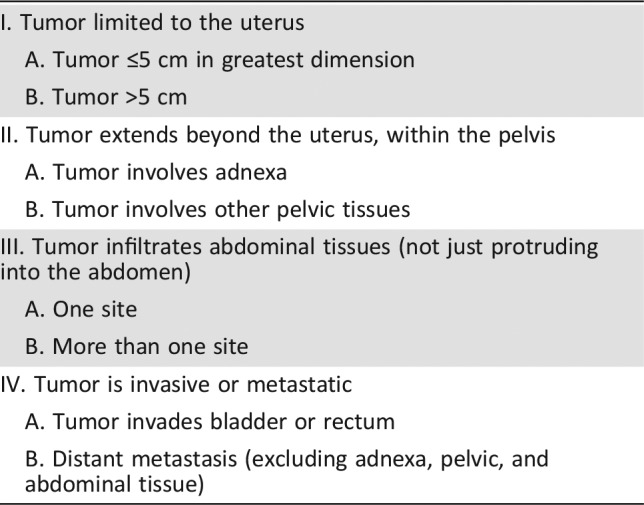
Prior to 2009, all uterine sarcomas were staged according to the criteria for uterine carcinomas. In 2009, the International Federation of Gynecology and Obstetrics created a distinct surgical staging system for uterine sarcomas excluding uterine carcinosarcoma (Table 2).
Table 2. International Federation of Gynecology and Obstetrics staging.
The initial treatment of LMS involves removal via hysterectomy; however, bilateral salpingo‐oophorectomy remains controversial. These tumors may express estrogen receptors, and thus eliminating estradiol production may limit recurrence. However, this remains hypothetical as retrospective data analysis has revealed no outcome differences between hysterectomy with bilateral salpingo‐oophorectomy versus hysterectomy alone and must be weighed against the advantages of ovarian preservation [4], [15]. A review of cases by Pritts et al. did not conclusively reveal any outcome differences related to morcellation verses en bloc removal [16]. However, an expert opinion by Chalas, as well as a consensus review by Hensley et al., states that morcellation should be avoided in any suspected or diagnosed malignancy, including LMS [15], [17].
In stage I uLMS, Littell et al. found that observation with imaging after hysterectomy was equivalent to administration of adjuvant gemcitabine and docetaxel, which has been recommended for ongoing management in these women [18], [19]. The rate of lymphatic involvement is relatively low, ranging between 3.5% and 11% [3]. Therefore, if final pathology for presumed benign leiomyoma returns as LMS, it is not generally recommended to perform a second procedure to procure lymph nodes. In addition, administration of adjuvant gemcitabine and docetaxel is equivalent to observation in these patients. So, for stage I uLMS it is reasonable to defer therapy to use if a patient recurs. The most common metastatic sites are lung, liver, and bone. Even if the cancer has not metastasized, the risk of recurrence within 2–5 years is 40%–70% [3]. Once metastatic disease is diagnosed, either initially or at recurrence, median survival is 1 year [5], [6]. Given the devastating nature of this disease, establishing a treatment consensus for advanced and recurrent uLMS is vitally important.
Single Agents in Metastatic uLMS
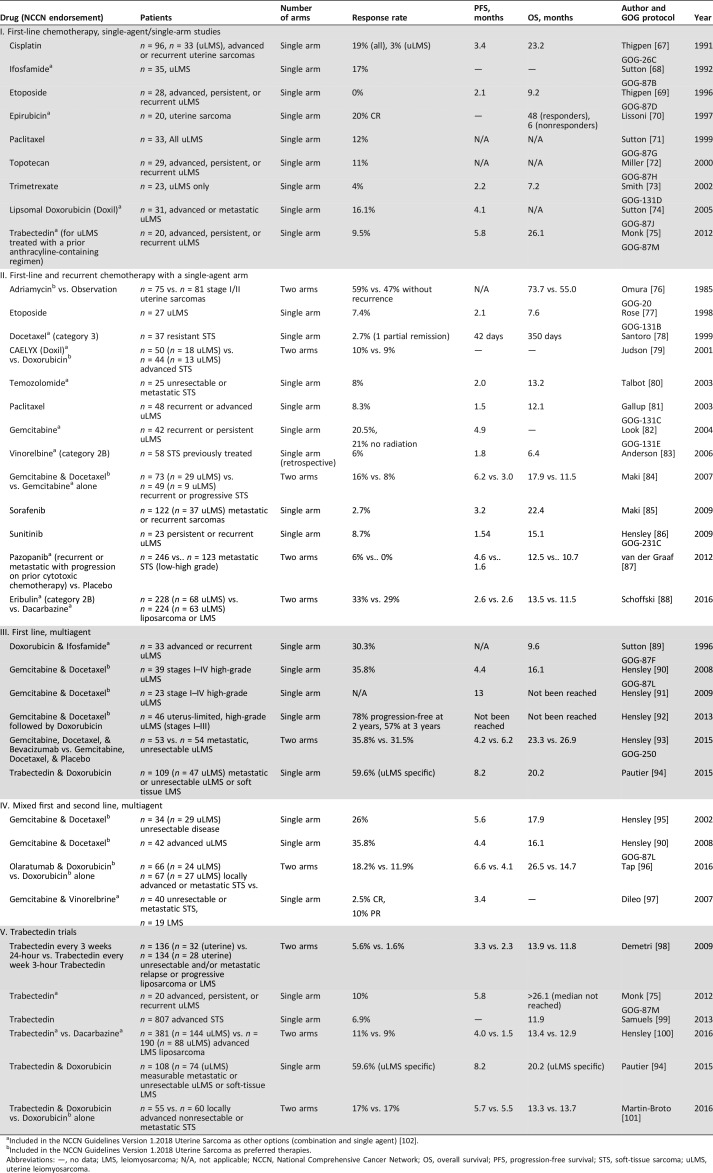
Doxorubicin (adriamycin) is an active single agent for uLMS and is less toxic than combination regimens. Three GOG trials (GOG 20, 21, 42) conducted in the 1980s assessed single‐agent adriamycin for the treatment of uLMS. In GOG 20, 56% of patients did not recur, but there was no survival difference observed for adjuvant doxorubicin versus observation, with or without radiation [20]. GOG 21 and 42 compared single‐agent doxorubicin to doxorubicin with dimethyl tiazenoimidazole carboxamide (GOG 21) or cyclophosphamide (GOG 42), which showed no outcome difference for single‐agent versus combination treatments [21], [22]. Judson et al. compared the efficacy of doxorubicin to pegylated liposomal doxorubicin (Doxil; Janssen Pharmaceutica, Beerse, Belgium) in 94 patients (35% with LMS) with advanced soft‐tissue sarcoma (STS), and demonstrated similar activity in both with an improved toxicity profile in Doxil patients [23]. Gynecologic oncologists favor a dose of 60 mg/m2 of doxorubicin for systemic treatment of LMS. However, medical oncoligists recommend that the threshold dose for optimal activity appears be ≥60 mg/m2 per 3‐week cycle for metastatic STS, with most settling on a standard dose of 75 mg/m2 per cycle [24]. This is an important distinction because there is not a consensus in regard to dosages between the two specialties. Cisplatin, ifosfamide, etoposide, paclitaxel, topotecan, trimetrexate, doxorubicin, temozolomide, and trabectedin have all shown modest to minimal response rates in advanced LMS in the upfront setting: 3%, 17%, 0%, 12%, 11%, 4%, 16%, 8%, and 9.5%, respectively (Table 3).
Table 3. Chemotherapy agents and trials.
Included in the NCCN Guidelines Version 1.2018 Uterine Sarcoma as other options (combination and single agent) [102].
Included in the NCCN Guidelines Version 1.2018 Uterine Sarcoma as preferred therapies.
Abbreviations: —, no data; LMS, leiomyosarcoma; N/A, not applicable; NCCN, National Comprehensive Cancer Network; OS, overall survival; PFS, progression‐free survival; STS, soft‐tissue sarcoma; uLMS, uterine leiomyosarcoma.
Other therapies included in the National Comprehensive Cancer Network (NCCN) list of recommended single agents include the following: (category 2A: dacarbazine, epirubicin, gemcitabine, pazopanib, temoxolomide), (category 2B: vinorelbine, eribulin), and (category 3: docetaxel; Table 3). Gemcitabine as a single agent has had response rates as high as 21% when a 1,000 mg/m2 dose was administered over 30 minutes on days 1, 8, and 15, with cycles repeated every 28 days. Eribulin, an antimicrotular antineoplastic agent, is listed in the NCCN guidelines as an option for treatment of uterine sarcomas; however, it is not U.S. Food and Drug Administration (FDA) approved for this indication. It had equal efficacy when compared with dacarbazine, an alkylating antineoplastic agent, in a phase III trial that included patients with both LMS and liposarcoma (LPS). The median overall survival (OS) was 13.5 versus 11.5 months (hazard ratio [HR] 0.79), although the study was not powered for OS. When a subgroup analysis was performed in just the uLMS patients, there was no difference in OS (12.7 vs. 13) with an HR of 0.93 [25]. Although eribulin is not approved for uLMS in the U.S., it is approved for use in advanced LPS in the U.S., and for LMS and LPS in other countries.
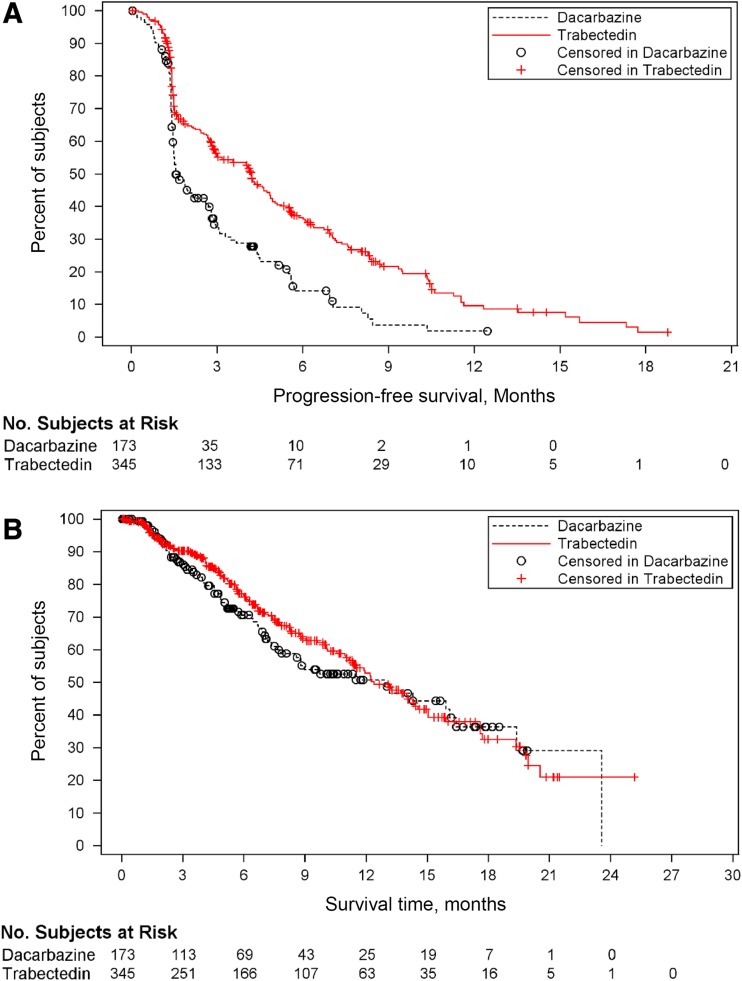
Trabectedin interacts with DNA repair mechanisms as a minor groove binder and major groove binder. It modulates transcriptional regulation by displacing transcription factor and fusion proteins, specifically the FUS‐CHOP fusion gene, the hallmark of myxoid liposarcoma. Additionally, it inhibits transcription by blocking RNA polymerase II and affects the tumor microenvironment by inhibiting CCL2, a proinflammatory mediator [26]. Trabectedin (ET‐743) gained approval in Europe in 2007, and in 2015 it was approved in the U.S. for use in advanced LMS and LPS after failure of anthracycline therapy [27], [28]. The dose and schedule was determined by a phase II randomized trial of two different schedules in advanced or metastatic LPS or LMS after failure of prior anthracyclines and ifosfamide, which included 260 patients (177 LMS patients). The median time to progression favored the every‐3‐week 24‐hour schedule. Its FDA approval was based on SAR 3007, which was a phase III randomized trial comparing trabectedin with dacarbazine in metastatic LPS or LMS after an anthracycline‐ and ifosphamide‐containing regimen or an anthracycline‐containing regimen and one additional chemotherapy regimen. The median progression‐free survival (PFS) favored trabectedin over dacarbazine: 4.2 versus 1.5 months (HR 0.55; p < .001) [29], [30]. In patients with uLMS specifically, trabectedin significantly improved PFS compared with treatment with dacarbazine (median PFS: 4.01 months vs. 1.54 months [HR 0.57; p = .0012]; Fig. 1) [31]. Clinical trials with trabectedin are listed separately in Table 3.
Figure 1.
Kaplan‐Meier curves of uLMS patients in Trabectedin vs. Dacarbazine trial. (A): Progression‐free survival comparison. (B): Survival time comparison.
Targeted Therapy
The oral multikinase angiogenesis inhibitor, pazopanib, is FDA approved for advanced STS other than LPS or gastrointestinal stromal tumors that fail anthracycline therapy. It was initially explored in advanced STS in the EORTC Study 62043, which was a phase II study that included 41 patients with LMS. The 12‐week progression‐free rate (primary endpoint) was 44% in this population [32]. It was later studied in a multicenter, international, double‐blind, placebo‐controlled phase III PALETTE study (EORTC study 62072) in STS patients, including 165 LMS patients, who had received at least two lines of prior chemotherapy. This trial showed a 6% response rate (RR) with a 4.6‐month PFS and a 12.5‐month OS, compared with the 1.6‐month PFS (HR 0.31) and 10.7‐month OS (HR 0.86) in the placebo arm [33]. The LMS cohort specifically had a PFS of 4.6 months versus 1.9 months (HR 0.37).
Additionally, Benson et al. published a retrospective analysis of 44 patients with uterine sarcoma treated with pazopanib; 39 patients had uLMS. Despite over 85% receiving prior treatment in the uterine sarcoma group, pazopanib showed signs of activity with similar outcomes to patients with nonuterine STS. Median PFS was 3.0 months (95% confidence interval [CI] 2.5–4.7) in uterine versus 4.5 (95% CI 3.7–5.1) in nonuterine STS. Median OS was 17.5 months (95% CI 11.1–19.6), which was longer than the nonuterine population OS of 11.1 months (95% CI 10.2–12.0; p = .352) [34].
Sorafenib and sunitinib, tyrosine kinase inhitors, showed minimal response rates in advanced and/or recurrent LMS (2.7% and 8.7%, respectively) and thus are not included in the NCCN recommended guidelines (Table 3) [35].
Combination Agents in Metastatic uLMS
Combination chemotherapy yields better response rates than single agents in patients who can tolerate more aggressive regimens. Given that doxorubicin and ifosfamide are the most active single agents in STS, the combination of the two was evaluated by the GOG in a phase II study and showed a response rate of 29%; however, this response rate was similar to that observed in STS with doxorubicin alone (~25% RR) [36].
In 2002, Hensley et al. published the first trial using gemcitabine and docetaxel, the most commonly used combination regimen for treatment of uLMS. It was a single‐institution phase II trial in patients with unresectable LMS who had received 0–2 prior chemotherapy regimens both with and without prior radiation [37]. This trial evaluated two different treatment doses depending on if the patient received prior radiation (675 gem/75 docetaxel) or did not receive prior radiation (900 gem/100 docetaxel). The two agents were given every 3 weeks for six cycles. The overall response rate in this trial was 53% (3 with complete response [CR], 15 with partial response [PR]) with a median PFS of 5.6 months and a median OS of 17.9 months. This was the first trial to demonstrate that combination gemcitabine and docetaxel was a tolerable and active treatment for unresectable disease, both with and without prior treatment. In 2007, Maki et al. published a study that compared gemcitabine with combination gemcitabine + docetaxel. This trial was done in all STS tumors and showed a response rate of 8% versus 16%, PFS of 3 versus 6.2 months, and an OS of 11.5 versus 17.9 months, supporting the use of the combination of gemcitabine with docetaxel over gemcitabine alone [38]. Interestingly, although the PFS and OS was calculated for all STS tumors, the authors reported treatment outcomes for the LMS tumors only. In LMS patients, 1/9 (11%) responded to gemcitabine alone, whereas 15/29 (52%) responded to the combination.
GOG 87L investigated the same regimen as Hensely in 2002, but this phase II trial was limited to patients with advanced, unresectable disease who had received no prior chemotherapy. Fifty percent of all patients (19/38) received six or more cycles. The response rate was 36% (15/42; CR 2/39, 4.8%; PR 13/39, 31%); 26.2% had stable disease (11/32); and 62% had some clinical benefit. Sixty percent of patients at 12 weeks had not progressed, and 41% of patients had not progressed at 24 weeks. Those who had either a complete or partial response had a response duration of 6 months, and the PFS was 4.4 months (Table 3) [39]. The same fixed‐dose rate gemcitabine plus docetaxel achieved a high response rate in advanced, recurrent uLMS as a second‐line treatment in GOG 131G. This was a phase II trial that demonstrated 50% of patients had stable disease and 27% showed a response to treatment (CR 6.3%, PR 20.8%) [39]. Given the activity of this regimen in advanced disease, it was later studied in patients with stage I–IV uLMS that had been completely resected, showing a median PFS of 13 months [40].
In 2013, Hensley et al. published SARC 005, a phase II trial of four cycles of fixed‐dose‐rate gemcitabine plus docetaxel followed by four cycles of doxorubicin in patients with disease limited to the uterus after complete resection. The median time to recurrence was 27.4 months (range 3–40 months). Seventy‐eight percent (95% CI 67%–91%) were progression‐free at 2 years, and 57% (95% CI 44%–74%) were progression‐free at 3 years [41]. Despite a reported improvement in response with addition of doxorubicin to gemcitabine and docetaxel, not all providers are using this regimen as their standard. Many providers at large academic centers continue to use gemcitabine and docetaxel without doxorubicin and later use doxorubicin when patients progress. Two years after SARC 005, Hensley et al. published GOG 250, which evaluated the role of bevacizumab in the management of metastatic LMS. The study was closed early for futility; there was no difference in PFS, OS, or objective response rates. The median PFS was 6.2 months in the control arm versus 4.2 months with bevacizumab (HR 1.12; p = .58). The mean duration of response was 8.6 months for gemcitabine and docetaxel plus placebo versus 8.8 months for gemcitabine and docetaxel plus bevacizumab. The response rate was 32% in the gemcitabine and docetaxel arm versus 36% in the gemcitabine and docetaxel plus bevacizumab arm [42].
Olaratumab, an anti‐PDGFa monocolonal antibody, was FDA approved in 2016 for STS, but is not included in the most recent version of the Uterine 2017 NCCN guidelines. Approval was based on data comparing olaratumab with doxorubicin with doxorubicin alone in recent open‐label phase Ib and phase II trials in advanced STS [43]. The median PFS was 6.6 versus 4.1 months (HR 0.672). The median OS was 26.5 versus 14.7 months (HR 0.46). The response rate was 18.8% with combination versus 12.3% with doxorubicin alone. The phase II trial was analyzed separately and showed a PFS of 8.2 versus 4.4 months and a response rate of 18% versus 8% with the same OS. The statistically significant difference in OS was consistent across stratifications, including LMS versus non‐LMS subgroups [44].
The combination of trabectedin and doxorubicin was evaluated by the French Sarcoma Group as first‐line treatment in advanced STS, and in uLMS patients the median PFS was 8.2 months [45]. The Spanish Group performed a phase II trial comparing the combination of trabectedin and doxorubicin with doxorubicin alone as first‐line treatment for advanced STS, but the combination did not demonstrate superiority over doxorubicin alone [46].
Neoadjuvant Chemotherapy in Soft Tissue Sarcomas
The use of neoadjuvant chemotherapy has been studied in a phase Ib/II trial for STS, which compared gemcitabine and docetaxel with gemcitabine and docetaxel with pazopanib [47]. This trial only accrued five patients (two LMS). Three patients discontinued because of toxicity, and the study closed. To our knowledge, there are no recommendations regarding neoadjuvant chemotherapy in uLMS.
Completed Clinical Trials with Unpublished Results
The Gynecologic Oncology Group phase III trial (GOG 277) was designed to compare gemcitabine and docetaxel plus doxorubicin with observation with and without an aromatase inhibitor in early‐stage uLMS; however, it was closed early because of poor accrual. Therefore, observation after resection, according to the NCCN guidelines, can be considered in early‐stage disease. Investigational agents that are being evaluated in STS include evofosfamide, a hypoxia‐activated prodrug. Trials with evofosfamide alone or in combination with doxorubicin have been completed in STS, but the results are not yet available [48].
Ongoing Clinical Trials
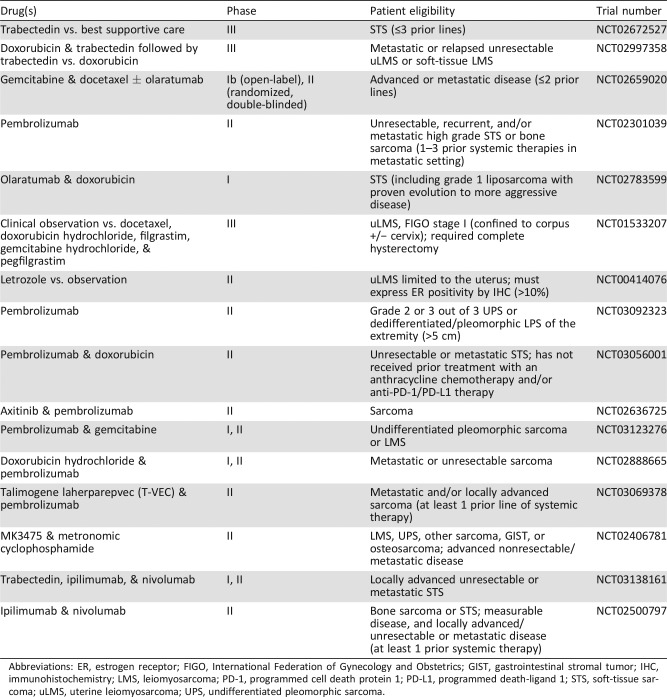
There are multiple ongoing trials that include uLMS (Table 4). Table 4 is not an all‐inclusive list, but it summarizes some of the ongoing research in the field. Immunotherapeutic agents, such as pembrolizumab (anti‐programmed cell death protein 1 [PD‐1]), nivolumab (anti‐PD‐1), and ipilimumab (anti‐CTLA4), are being studied in STS. George et al. recently published a study in which 12 women received nivolumab alone. The median PFS was only 1.8 months (95% CI 0.8–not defined), and no objective responses were observed. Nivolumab as a single agent failed to demonstrate antitumor activity among uLMS patients without any biomarker selection. However, immunohistochemistry (IHC) expression of archival tumor material and analysis of changes in circulating immunophentypes are ongoing, and the study is being amended to evaluate treatment of metastatic disease [49].
Table 4. Ongoing clinical trials.
Abbreviations: ER, estrogen receptor; FIGO, International Federation of Gynecology and Obstetrics; GIST, gastrointestinal stromal tumor; IHC, immunohistochemistry; LMS, leiomyosarcoma; PD‐1, programmed cell death protein 1; PD‐L1, programmed death‐ligand 1; STS, soft‐tissue sarcoma; uLMS, uterine leiomyosarcoma; UPS, undifferentiated pleomorphic sarcoma.
Pembrolizumab, an anti‐PD‐1 agent, is also being evaluated alone (SARC028) and in combination with other agents: olaratumab, doxorubicin, axitinib, gemcitabine, tamlimogene laherparepvec, and cyclophosphamide. Tawbi et al. recently studied 80 patients, including 10 LMS patients, receiving pembrolizumab. No objective responses were observed in the STS cohort. PFS rates at 8 weeks were 50% in LMS. Longer clinical follow‐up data are being evaluated to determine the therapeutic impact of single‐agent pembrolizumab in STS and bone sarcoma and will be published along with immune monitoring in peripheral blood and tumor tissues [50]. Additionally, a phase II trial investigating nivolumab with and without ipilumumab was initiated in uLMS patients specifically, but has been suspended [51]. Nivolumab and ipilumumab are continuing to be evaluated together and in combination with trabectedin in STS. Whether the efficacy of immunotherapy in LMS is correlated with specific biomarkers such as PI3K pathway activation, loss of PTEN (both associated with anti‐programmed death‐ligand 1 [PD‐L1] activity in melanoma), percentage of PD‐L1 expression by IHC, amount of tumor‐infiltrating lymphocytes, neoantigen load, or tumor mutational burden is currently being investigated [52], [53], [54], [55].
Molecular Genetics and Potential Future Targets
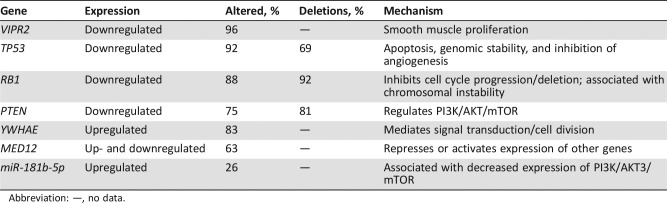
As previously alluded to, specific targeting of tumors is likely the future of treatment. A recent study attempted to identify potential targets in uLMS by sequencing 84 samples across multiple centers. Although the authors identified reduced expression of established tumor suppressor genes such as TP53, RB1, and PTEN, they also identified VIPR2, a negative regulator of smooth muscle proliferation. VIPR2 was affected in 96% of samples and reduced in uLMS in comparison with normal myometrium. In addition, its deletion was a negative prognostic indicator in uLMS [53]. By identifying new potential oncogenes or tumor suppressor genes in individual tumors, we can potentially use established therapies or novel therapies to upregulate or downregulate specific genes. For example, imipramine, which is an FDA‐approved antidepressant that upregulates VIPR2 expresssion, was proposed as an established therapy that could act by upregulating this tumor suppressor gene identified in uLMS [56]. The comprehensive and integrated genomic characterization of adult STS name many of the same tumor suppressor genes as above, but also make mention of miR‐181b‐5p, which is upregulated in LMS and associated with recurrence‐free survival [57]. Interestingly, miR‐181‐b was thought to increase vascular smooth muscle proliferation and migration via the PI3K pathway [58]; however, this study found an association between increased expression of miR‐181b‐5p and low expression of PI3K pathway and targets mammalian target of rapamycin (mTOR) and AKT3 that are associated with phosphatase and tensin homolog (PTEN). Table 5 offers a summary of the major genes mutated in uLMS, how often they are affected, and whether they are up‐ or downregulated.
Table 5. Mutated genes in uLMS [103], [104] .
Abbreviation: —, no data.
How Should Systemic Chemotherapy Be Sequenced?
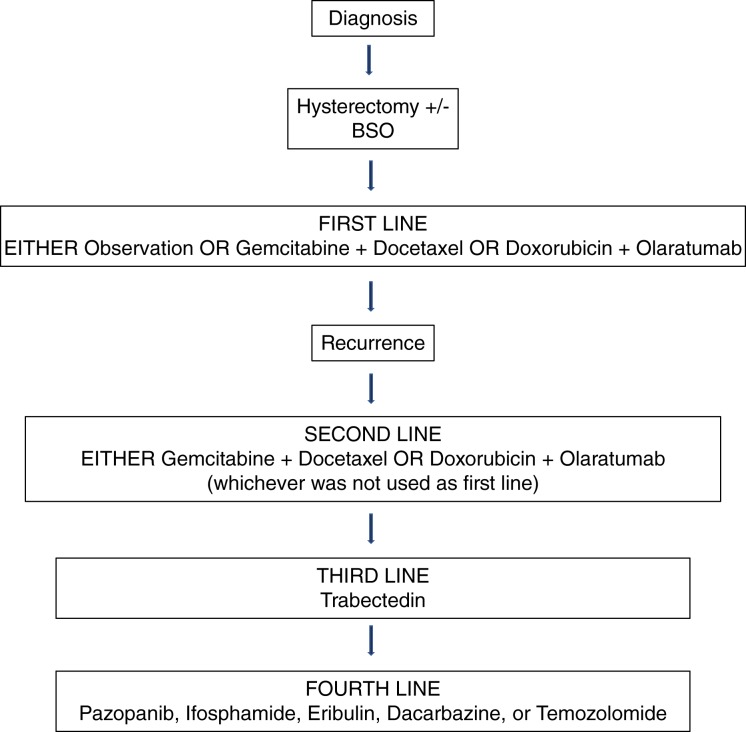
Based on the data presented in this review, it is our opinion that frontline chemotherapy for uLMS should include either gemcitabine or doxorubicin. These agents should be administered alone if the patient cannot tolerate combination treatment, but ideally in combination with docetaxel (gemcitabine with docetaxel) or olaratumab (doxorubicin with olaratumab). According to all the clinical trials for uLMS, the most effective regimen in disease limited to the uterus has been the SAR005 regimen: gemcitabine (900 mg/m2 day 1 and 8) plus docetaxel (75 mg/m2 on day 8) every 21 days for four cycles, followed by doxorubicin (60 mg/m2) every 21 days for four cycles. It is important to note that this regimen is not described in the NCCN guidelines and has not been studied in advanced disease.
Once patients with uLMS have recurred, patients should be encouraged to enroll in a clinical trial. If no trial is available and patients have not received prior doxorubicin, doxorubicin should serve as second‐line systemic therapy. If doxorubicin was used previously without the use of gemcitabine and docetaxel, then gemcitabine and docetaxel should be used. If the patient already received gemcitabine and docetaxel, then doxorubicin could be administered with olaratumab. If the patient received gemcitabine and docetaxel followed by doxorubicin initially, trabectedin 1.5 mg/m2 over a 24‐hour infusion every 3 weeks would be an appropriate next line of therapy.
If not previously used, the third‐line therapy for all uLMS patients should be trabectedin, as the phase II trial using this agent in advanced or recurrent disease had a 5.8‐month PFS. If the patient already received trabectedin, another option for third‐line or fourth‐line therapy includes pazopanib. Other options could include ifosphamide, eribulin, or dacarbazine. Eribulin, although not FDA approved for uLMS, should be considered because it had comparable efficacy and fewer side effects than dacarbazine. See Figure 2 for suggested treatment algorithm.
Figure 2.
Suggested algorithm for chemotherapy treatment in uLMS.
Conclusion
uLMS is a devastating disease manifested by poor survival outcomes and short overall survival intervals once advanced or recurrent disease is diagnosed. Survival for stage I disease is poor in comparison with other uterine cancers [59], [60]. The cause is multifaceted—both tumor aggression and a lack of efficacious therapy in advanced or recurrent disease contribute to poor outcomes. Poor response to traditional chemotherapies has caused difficulty in establishing a consensus standard regimen among gynecological oncologists.
Starting in the 1980s, trials were commissioned to identify chemotherapeutics to treat sarcomas. Initially, single‐agent doxorubicin was studied in three early GOG trials and no survival benefit was observed. Since that time, many agents have shown modest benefit; however, the NCCN has only supported ifosfamide, doxorubicin, and trabectedin as single‐agent options for uLMS. Multiple trials have shown combination therapy to be efficacious in treating both previously resected and unresectable disease—most notably Hensley et al. and Maki et al. showed gemcitabine and docetaxel were both tolerable and active agents against STS [37], [38]. Both GOG87L and GOG131G confirmed that this regimen was active in patients with advanced, unresectable uLMS without prior chemotherapy and in patients with advanced, recurrent uLMS as a second‐line treatement. SARC005 demonstrated improved response by adding four cycles of doxorubicin after the fixed‐dose gemcitabine and docetaxel regimen, although the addition of bevacizumab showed no appreciable benefit [41].
Targeted therapy, specifically olaratumab, has shown promise in combination with doxorubicin versus doxorubicin alone. In addition, there are multiple ongoing trials that point to immunotherapy as a possible option to improve survival in uLMS. Tailoring immunotherapies to specific tumors in combination with traditional chemotherapies could provide great strides in an effort to more adequately treat uLMS. As targeted therapy and immunotherapy continue to evolve, it will be essential for providers to become more aware of the genetic and inflammatory profile of tumors. Ongoing clinical trials will hopefully offer new options to be used in combination with established agents. We continue to advocate for enrollment in these trials if available.
Additionally, researchers continue to explore other areas of evaluation and treatment of sarcomas, including surgical technique, staging methods, and response criteria [61]. This includes a proposal by Choi to embrace a new imaging‐based response criteria specifically for sarcomas to better assess response [62].
The recommendations put forth in this review article are an attempt to establish the most current and effective treatment of recurrent and advanced uLMS. By forming a consensus for the efficacious treatment of this disease, a standard regimen will be established, which will be perpetually built upon and consequently result in increased survival. We realize that the ever‐changing landscape of research in this arena will require consummate appraisal, and are excited by the multiple ongoing sarcoma trials. We look forward to further treatment development in the field, but until that time, the sequence of systemic chemotherapy proposed above has shown the most benefit in past trials.
Author Contributions
Conception/design: Rebecca C. Arend, Thomas J. Herzog
Collection and/or assembly of data: Rebecca C. Arend, Michael D. Toboni, Allison M. Montgomery
Data analysis and interpretation: Rebecca C. Arend, Michael D. Toboni, Allison M. Montgomery, Robert A. Burger, Alexander B. Olawaiye, Bradley J. Monk, Thomas J. Herzog
Manuscript writing: Rebecca C. Arend, Michael D. Toboni, Allison M. Montgomery, Robert A. Burger, Alexander B. Olawaiye, Bradley J. Monk, Thomas J. Herzog
Final approval of manuscript: Rebecca C. Arend, Robert A. Burger, Alexander B. Olawaiye, Bradley J. Monk, Thomas J. Herzog
Disclosures
Rebecca C. Arend: Clovis Oncology, AstraZeneca, VBL Therapeutics, Janseen, Tesaro, Puma (C/A); Robert A. Burger: Amgen, AstraZeneca, Clovis Oncology, Gradalis, Janssen, Merck, Morphotek, Tesaro, VBL Therapeutics (C/A); Bradley J. Monk: AstraZeneca, Tesaro, Inc. (C/A), Tesaro, Inc. (RF), AstraZeneca (H). The other authors indicated no financial relationships; Thomas J. Herzog: AstraZeneca, Caris, Clovis Oncology, Johnson & Johnson, Tesaro (SAB).
(C/A) Consulting/advisory relationship; (RF) Research funding; (E) Employment; (ET) Expert testimony; (H) Honoraria received; (OI) Ownership interests; (IP) Intellectual property rights/inventor/patent holder; (SAB) Scientific advisory board
References
- 1.ACS . Cancer Facts and Figures 2017. Available at https://www.cancer.org/research/cancer‐facts‐statistics/all‐cancer‐facts‐figures/cancer‐facts‐figures‐2017.html. Accessed February 1, 2018. [Google Scholar]
- 2.Nordal RR, Thoresen SO. Uterine sarcomas in Norway 1956‐1992: Incidence, survival and mortality. Eur J Cancer 1997;33:907–911. [DOI] [PubMed] [Google Scholar]
- 3.Giuntoli RL 2nd, Metzinger DS, DiMarco CS et al. Retrospective review of 208 patients with leiomyosarcoma of the uterus: Prognostic indicators, surgical management, and adjuvant therapy. Gynecol Oncol 2003;89:460–469. [DOI] [PubMed] [Google Scholar]
- 4.Kapp DS, Shin JY, Chan JK. Prognostic factors and survival in 1396 patients with uterine leiomyosarcomas: Emphasis on impact of lymphadenectomy and oophorectomy. Cancer 2008;112:820–830. [DOI] [PubMed] [Google Scholar]
- 5.Van Glabbeke M, van Oosterom AT, Oosterhuis JW et al. Prognostic factors for the outcome of chemotherapy in advanced soft tissue sarcoma: An analysis of 2,185 patients treated with anthracycline‐containing first‐line regimens—A European Organization for Research and Treatment of Cancer Soft Tissue and Bone Sarcoma Group Study. J Clin Oncol 1999;17:150–157. [DOI] [PubMed] [Google Scholar]
- 6.Italiano A, Mathoulin‐Pelissier S, Cesne AL et al. Trends in survival for patients with metastatic soft‐tissue sarcoma. Cancer 2011;117:1049–1054. [DOI] [PubMed] [Google Scholar]
- 7.D'Angelo E, Prat J. Uterine sarcomas: a review. Gynecol Oncol 2010;116:131–139. [DOI] [PubMed] [Google Scholar]
- 8.George S, Serrano C, Hensley ML et al. Soft tissue and uterine leiomyosarcoma. J Clin Oncol 2018;36:144–150. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Shah SH, Jagannathan JP, Krajewski K et al. Uterine sarcomas: Then and now. AJR Am J Roentgenol 2012;199:213–223. [DOI] [PubMed] [Google Scholar]
- 10.Bell SW, Kempson RL, Hendrickson MR. Problematic uterine smooth muscle neoplasms. A clinicopathologic study of 213 cases. Am J Surg Pathol 1994;18:535–558. [PubMed] [Google Scholar]
- 11.George S, Feng Y, Manola J et al. Phase 2 trial of aromatase inhibition with letrozole in patients with uterine leiomyosarcomas expressing estrogen and/or progesterone receptors. Cancer 2014;120:738–743. [DOI] [PubMed] [Google Scholar]
- 12.Patterson H, Gill S, Fisher C et al. Abnormalities of the p53 MDM2 and DCC genes in human leiomyosarcomas. Br J Cancer 1994;69:1052–1058. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Leibsohn S, d'Ablaing G, Mishell DR Jr et al. Leiomyosarcoma in a series of hysterectomies performed for presumed uterine leiomyomas. Am J Obstet Gynecol 1990;162:968–974; discussion 974–966. [DOI] [PubMed] [Google Scholar]
- 14.Abakka S, Elhalouat H, Khoummane N et al. Uterine leiomyosarcoma and Leser‐Trelat sign. Lancet 2013;381:88. [DOI] [PubMed] [Google Scholar]
- 15.Hensley ML, Barrette BA, Baumann K et al. Gynecologic Cancer InterGroup (GCIG) consensus review: Uterine and ovarian leiomyosarcomas. Int J Gynecol Cancer 2014;24:S61–S66. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Pritts EA, Parker WH, Brown J et al. Outcome of occult uterine leiomyosarcoma after surgery for presumed uterine fibroids: A systematic review. J Minim Invasive Gynecol 2015;22:26–33. [DOI] [PubMed] [Google Scholar]
- 17.Chalas E. Morcellation in gynecologic oncology. Curr Opin Obstet Gynecol 2018;30:96–98. [DOI] [PubMed] [Google Scholar]
- 18.Hensley ML. Difficult choices in stage I uterine leiomyosarcoma: It's okay to “stand there”. Gynecol Oncol 2017;147:1–2. [DOI] [PubMed] [Google Scholar]
- 19.Littell RD, Tucker LY, Raine‐Bennett T et al. Adjuvant gemcitabine‐docetaxel chemotherapy for stage I uterine leiomyosarcoma: Trends and survival outcomes. Gynecol Oncol 2017;147:11–17. [DOI] [PubMed] [Google Scholar]
- 20.Omura GA, Blessing JA, Major F et al. A randomized clinical trial of adjuvant adriamycin in uterine sarcomas: A gynecologic oncology group study. J Clin Oncol 1985;3:1240–1245. [DOI] [PubMed] [Google Scholar]
- 21.Omura GA, Major FJ, Blessing JA et al. A randomized study of adriamycin with and without dimethyl triazenoimidazole carboxamide in advanced uterine sarcomas. Cancer 1983;52:626–632. [DOI] [PubMed] [Google Scholar]
- 22.Muss HB, Bundy B, DiSaia PJ et al. Treatment of recurrent or advanced uterine sarcoma. A randomized trial of doxorubicin versus doxorubicin and cyclophosphamide (a phase III trial of the gynecologic oncology group). Cancer 1985;55:1648–1653. [DOI] [PubMed] [Google Scholar]
- 23.Judson I, Radford JA, Harris M et al. Randomised phase II trial of pegylated liposomal doxorubicin (DOXIL/CAELYX) versus doxorubicin in the treatment of advanced or metastatic soft tissue sarcoma: A study by the EORTC Soft Tissue and Bone Sarcoma Group. Eur J Cancer 2001;37:870–877. [DOI] [PubMed] [Google Scholar]
- 24.Borden EC, Amato DA, Rosenbaum C et al. Randomized comparison of three adriamycin regimens for metastatic soft tissue sarcomas. J Clin Oncol 1987;5:840–850. [DOI] [PubMed] [Google Scholar]
- 25.Schoffski P, Chawla S, Maki RG et al. Eribulin versus dacarbazine in previously treated patients with advanced liposarcoma or leiomyosarcoma: A randomised, open‐label, multicentre, phase 3 trial. Lancet 2016;387:1629–1637. [DOI] [PubMed] [Google Scholar]
- 26.D'Incalci M, Galmarini CM. A review of trabectedin (ET‐743): A unique mechanism of action. Mol Cancer Ther 2010;9:2157–2163. [DOI] [PubMed] [Google Scholar]
- 27.Barone A, Chi DC, Theoret MR et al. FDA Approval Summary: Trabectedin for unresectable or metastatic liposarcoma or leiomyosarcoma following an anthracycline‐containing regimen. Clin Cancer Res 2017;23. [DOI] [PubMed] [Google Scholar]
- 28.Thway K, Noujaim J, Jones RL et al. Advances in the pathology and molecular biology of sarcomas and the impact on treatment. Clin Oncol (R Coll Radiol) 2017;29:471–480. [DOI] [PubMed] [Google Scholar]
- 29.Demetri GD, von Mehren M, Jones RL et al. Efficacy and safety of trabectedin or dacarbazine for metastatic liposarcoma or leiomyosarcoma after failure of conventional chemotherapy: Results of a phase III randomized multicenter clinical trial. J Clin Oncol 2016;34:786–793. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Hensley ML, Patel SR, von Mehren M et al. Efficacy and safety of trabectedin or dacarbazine in patients with advanced uterine leiomyosarcoma after failure of anthracycline‐based chemotherapy: Subgroup analysis of a phase 3, randomized clinical trial. Gynecol Oncol 2017;146:531–537. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Hensley ML, Patel SR, von Mehren M et al. Efficacy and safety of trabectedin or dacarbazine for the treatment of patients with uterine leiomyosarcoma after prior chemotherapy: A subgroup analysis of the randomized phase 3 SAR‐3007 study. Gynecol Oncol 2017;146:531–537. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Sleijfer S, Ray‐Coquard I, Papai Z et al. Pazopanib, a multikinase angiogenesis inhibitor, in patients with relapsed or refractory advanced soft tissue sarcoma: A phase II study from the European Organisation for Research and Treatment of Cancer‐Soft Tissue and Bone Sarcoma Group (EORTC study 62043). J Clin Oncol 2009;27:3126–3132. [DOI] [PubMed] [Google Scholar]
- 33.van der Graaf WT, Blay JY, Chawla SP et al. Pazopanib for metastatic soft‐tissue sarcoma (PALETTE): A randomised, double‐blind, placebo‐controlled phase 3 trial. Lancet 2012;379:1879–1886. [DOI] [PubMed] [Google Scholar]
- 34.Benson C, Ray‐Coquard I, Sleijfer S et al. Outcome of uterine sarcoma patients treated with pazopanib: A retrospective analysis based on two European Organisation for Research and Treatment of Cancer (EORTC) Soft Tissue and Bone Sarcoma Group (STBSG) clinical trials 62043 and 62072. Gynecol Oncol 2016;142:89–94. [DOI] [PubMed] [Google Scholar]
- 35.Mahmood ST, Agresta S, Vigil CE et al. Phase II study of sunitinib malate, a multitargeted tyrosine kinase inhibitor in patients with relapsed or refractory soft tissue sarcomas. Focus on three prevalent histologies: Leiomyosarcoma, liposarcoma and malignant fibrous histiocytoma. Int J Cancer 2011;129:1963–1969. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Sutton G, Blessing JA, Malfetano JH. Ifosfamide and doxorubicin in the treatment of advanced leiomyosarcomas of the uterus: A gynecologic oncology group study. Gynecol Oncol 1996;62:226–229. [DOI] [PubMed] [Google Scholar]
- 37.Hensley ML, Maki R, Venkatraman E et al. Gemcitabine and docetaxel in patients with unresectable leiomyosarcoma: Results of a phase II trial. J Clin Oncol 2002;20:2824–2831. [DOI] [PubMed] [Google Scholar]
- 38.Maki RG, Wathen JK, Patel SR et al. Randomized phase II study of gemcitabine and docetaxel compared with gemcitabine alone in patients with metastatic soft tissue sarcomas: Results of sarcoma alliance for research through collaboration study 002 [corrected]. J Clin Oncol 2007;25:2755–2763. [DOI] [PubMed] [Google Scholar]
- 39.Hensley ML, Blessing JA, Mannel R et al. Fixed‐dose rate gemcitabine plus docetaxel as first‐line therapy for metastatic uterine leiomyosarcoma: A gynecologic oncology group phase II trial. Gynecol Oncol 2008;109:329–334. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Hensley ML, Ishill N, Soslow R et al. Adjuvant gemcitabine plus docetaxel for completely resected stages I‐IV high grade uterine leiomyosarcoma: Results of a prospective study. Gynecol Oncol 2009;112:563–567. [DOI] [PubMed] [Google Scholar]
- 41.Hensley ML, Wathen JK, Maki RG et al. Adjuvant therapy for high‐grade, uterus‐limited leiomyosarcoma: Results of a phase 2 trial (SARC 005). Cancer 2013;119:1555–1561. [DOI] [PubMed] [Google Scholar]
- 42.Hensley ML, Miller A, O'Malley DM et al. Randomized phase III trial of gemcitabine plus docetaxel plus bevacizumab or placebo as first‐line treatment for metastatic uterine leiomyosarcoma: An NRG oncology/gynecologic oncology group study. J Clin Oncol 2015;33:1180–1185. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Klug LR, Heinrich MC. PDGFRA antibody for soft tissue sarcoma. Cell 2017;168:555. [DOI] [PubMed] [Google Scholar]
- 44.Tap WD, Jones RL, Van Tine BA et al. Olaratumab and doxorubicin versus doxorubicin alone for treatment of soft‐tissue sarcoma: An open‐label phase 1b and randomised phase 2 trial. Lancet 2016;388:488–497. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Pautier P, Floquet A, Chevreau C et al. Trabectedin in combination with doxorubicin for first‐line treatment of advanced uterine or soft‐tissue leiomyosarcoma (LMS‐02): A non‐randomised, multicentre, phase 2 trial. Lancet Oncol 2015;16:457–464. [DOI] [PubMed] [Google Scholar]
- 46.Martin‐Broto J, Pousa AL, de Las Penas R et al. Randomized phase II study of trabectedin and doxorubicin compared with doxorubicin alone as first‐line treatment in patients with advanced soft tissue sarcomas: A Spanish Group for Research on Sarcoma Study. J Clin Oncol 2016;34:2294–2302. [DOI] [PubMed] [Google Scholar]
- 47.Munhoz RR, D'Angelo SP, Gounder MM et al. A phase Ib/II study of gemcitabine and docetaxel in combination with pazopanib for the neoadjuvant treatment of soft tissue sarcomas. The Oncologist 2015;20:1245–1246. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.von Gottberg A, de Gouveia L, Tempia S et al. Effects of vaccination on invasive pneumococcal disease in South Africa. N Engl J Med 2014;371:1889–1899. [DOI] [PubMed] [Google Scholar]
- 49.George S, Barysauskas CM, Solomon S et al. Phase 2 study of nivolumab in metastatic leiomyosarcoma of the uterus. J Clin Oncol 2016;34:11007–11007. [Google Scholar]
- 50.Tawbi HA, Burgess MA, Crowley J et al. Safety and efficacy of PD‐1 blockade using pembrolizumab in patients with advanced soft tissue (STS) and bone sarcomas (BS): Results of SARC028—A multicenter phase II study. J Clin Oncol 2016;34(suppl 15):11006a.
- 51.Groome MJ, Page N, Cortese MM et al. Effectiveness of monovalent human rotavirus vaccine against admission to hospital for acute rotavirus diarrhoea in South African children: A case‐control study. Lancet Infect Dis 2014;14:1096–1104. [DOI] [PubMed] [Google Scholar]
- 52.Cuppens T, Annibali D, Coosemans A et al. Potential targets’ analysis reveals dual PI3K/mTOR pathway inhibition as a promising therapeutic strategy for uterine leiomyosarcomas—An ENITEC group initiative. Clin Cancer Res 2017;23:1274–1285. [DOI] [PubMed] [Google Scholar]
- 53.Cuppens T, Moisse M, Depreeuw J et al. Integrated genome analysis of uterine leiomyosarcoma to identify novel driver genes and targetable pathways. Int J Cancer 2017. [DOI] [PubMed] [Google Scholar]
- 54.Pollack SM, He Q, Yearley JH et al. T‐cell infiltration and clonality correlate with programmed cell death protein 1 and programmed death‐ligand 1 expression in patients with soft tissue sarcomas. Cancer 2017;123:3291–3304. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.George S, Miao D, Demetri GD et al. Loss of PTEN Is associated with resistance to anti‐PD‐1 checkpoint blockade therapy in metastatic uterine leiomyosarcoma. Immunity 2017;46:197–204. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Reichenstein M, Rehavi M, Pinhasov A. Involvement of pituitary adenylate cyclase activating polypeptide (PACAP) and its receptors in the mechanism of antidepressant action. J Mol Neurosci 2008;36:330–338. [DOI] [PubMed] [Google Scholar]
- 57.Cancer Genome Atlas Research Network . Comprehensive and integrated genomic characterization of adult soft tissue sarcomas. Cell 2017;171:950–965 e928. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Li TJ, Chen YL, Gua CJ et al. MicroRNA 181b promotes vascular smooth muscle cells proliferation through activation of PI3K and MAPK pathways. Int J Clin Exp Pathol 2015;8:10375–10384. [PMC free article] [PubMed] [Google Scholar]
- 59.Abeler VM, Royne O, Thoresen S et al. Uterine sarcomas in Norway. A histopathological and prognostic survey of a total population from 1970 to 2000 including 419 patients. Histopathology 2009;54:355–364. [DOI] [PubMed] [Google Scholar]
- 60.Dinh TA, Oliva EA, Fuller AF Jr et al. The treatment of uterine leiomyosarcoma. Results from a 10‐year experience (1990‐1999) at the Massachusetts General Hospital. Gynecol Oncol 2004;92:648–652. [DOI] [PubMed] [Google Scholar]
- 61.Jeys L, Morris G, Evans S et al. Surgical innovation in sarcoma surgery. Clin Oncol (R Coll Radiol) 2017;29:489–499. [DOI] [PubMed] [Google Scholar]
- 62.Choi H. Role of imaging in response assessment and individualised treatment for sarcomas. Clin Oncol (R Coll Radiol) 2017;29:481–488. [DOI] [PubMed] [Google Scholar]
- 63.D'Angelo E, Prat J. Uterine sarcomas: A review. Gynecol Oncol 2010;116:131–139. [DOI] [PubMed] [Google Scholar]
- 64.Chu PG, Arber DA, Weiss LM, et al. Utility of CD10 in distinguishing between endometrial stromal sarcoma and uterine smooth muscle tumors: An immunohistochemical comparison of 34 cases. Mod Pathol 2001;14:465–471. [DOI] [PubMed] [Google Scholar]
- 65.Pinto A, Howitt B. Uterine adenosarcoma. Arch Pathol Lab Med 2016;140:286–290. [DOI] [PubMed] [Google Scholar]
- 66.Kaspar HG, Crum CP. The utility of immunohistochemistry in the differential diagnosis of gynecologic disorders. Arch Pathol Lab Med 2015;139:39–54. [DOI] [PubMed] [Google Scholar]
- 67.Thigpen JT, Blessing JA, Beecham J, et al. Phase II trial of cisplatin as first‐line chemotherapy in patients with advanced or recurrent uterine sarcomas: A gynecologic oncology group study. J Clin Oncol 1991;9:1962–1966. [DOI] [PubMed] [Google Scholar]
- 68.Sutton GP, Blessing JA, Barrett RJ, et al. Phase II trial of ifosfamide and mesna in leiomyosarcoma of the uterus: A gynecologic oncology group study. Am J Obstet Gynecol 1992;166:556–559. [DOI] [PubMed] [Google Scholar]
- 69.Thigpen T, Blessing JA, Yordan E, et al. Phase II trial of etoposide in leiomyosarcoma of the uterus: A gynecologic oncology group study. Gynecol Oncol 1996;63:120–122. [DOI] [PubMed] [Google Scholar]
- 70.Lissoni A, Cormio G, Colombo N, et al. High‐dose epirubicin in patients with advanced or recurrent uterine sarcoma. Int J Gynecol Cancer 1997;7:241–244. [Google Scholar]
- 71.Sutton G, Blessing JA, Ball H. Phase II trial of paclitaxel in leiomyosarcoma of the uterus: A gynecologic oncology group study. Gynecol Oncol 1999;74:346–349. [DOI] [PubMed] [Google Scholar]
- 72.Miller DS, Blessing JA, Kilgore LC, et al. Phase II trial of topotecan in patients with advanced, persistent, or recurrent uterine leiomyosarcomas: A gynecologic oncology group study. Am J Clin Oncol 2000;23:355–357. [DOI] [PubMed] [Google Scholar]
- 73.Smith HO, Blessing JA, Vaccarello L. Trimetrexate in the treatment of recurrent or advanced leiomyosarcoma of the uterus: A phase II study of the gynecologic oncology group. Gynecol Oncol 2002;84:140–144. [DOI] [PubMed] [Google Scholar]
- 74.Sutton G, Blessing J, Hanjani P, et al. Phase II evaluation of liposomal doxorubicin (Doxil) in recurrent or advanced leiomyosarcoma of the uterus: A gynecologic oncology group study. Gynecol Oncol 2005;96:749–752. [DOI] [PubMed] [Google Scholar]
- 75.Monk BJ, Blessing JA, Street DG, et al. A phase II evaluation of trabectedin in the treatment of advanced, persistent, or recurrent uterine leiomyosarcoma: A gynecologic oncology group study. Gynecol Oncol 2012;124:48–52. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76.Omura GA, Blessing JA, Major F, et al. A randomized clinical trial of adjuvant adriamycin in uterine sarcomas: A gynecologic oncology group study. J Clin Oncol 1985;3:1240–1245. [DOI] [PubMed] [Google Scholar]
- 77.Rose PG, Blessing JA, Soper JT, et al. Prolonged oral etoposide in recurrent or advanced leiomyosarcoma of the uterus: A gynecologic oncology group study. Gynecol Oncol 1998;70:267–271. [DOI] [PubMed] [Google Scholar]
- 78.Santoro A, Romanini A, Rosso A, et al. Lack of activity of docetaxel in soft tissue sarcomas: Results of a phase II study of the Italian Group on Rare Tumors. Sarcoma 1999;3:177–181. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79.Judson I, Radford JA, Harris M, et al. Randomised phase II trial of pegylated liposomal doxorubicin (DOXIL/CAELYX) versus doxorubicin in the treatment of advanced or metastatic soft tissue sarcoma: A study by the EORTC Soft Tissue and Bone Sarcoma Group. Eur J Cancer 2001;37:870–877. [DOI] [PubMed] [Google Scholar]
- 80.Talbot SM, Keohan ML, Hesdorffer M, et al. A phase II trial of temozolomide in patients with unresectable or metastatic soft tissue sarcoma. Cancer 2003;98:1942–1946. [DOI] [PubMed] [Google Scholar]
- 81.Gallup DG, Blessing JA, Andersen W, et al. Evaluation of paclitaxel in previously treated leiomyosarcoma of the uterus: A gynecologic oncology group study. Gynecol Oncol 2003;89:48–51. [DOI] [PubMed] [Google Scholar]
- 82.Look KY, Sandler A, Blessing JA, et al. Phase II trial of gemcitabine as second‐line chemotherapy of uterine leiomyosarcoma: A gynecologic oncology group (GOG) Study. Gynecol Oncol 2004;92:644–647. [DOI] [PubMed] [Google Scholar]
- 83.Anderson SE, Keohan ML, D'Adamo DR, et al. A retrospective analysis of vinorelbine chemotherapy for patients with previously treated soft‐tissue sarcomas. Sarcoma 2006;2006:15947. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 84.Maki RG, Wathen JK, Patel SR, et al. Randomized phase II study of gemcitabine and docetaxel compared with gemcitabine alone in patients with metastatic soft tissue sarcomas: Results of sarcoma alliance for research through collaboration study 002 [corrected]. J Clin Oncol 2007;25:2755–2763. [DOI] [PubMed] [Google Scholar]
- 85.Maki RG, D'Adamo DR, Keohan ML, et al. Phase II study of sorafenib in patients with metastatic or recurrent sarcomas. J Clin Oncol 2009;27:3133–3140. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 86.Hensley ML, Sill MW, Scribner DR Jr., et al. Sunitinib malate in the treatment of recurrent or persistent uterine leiomyosarcoma: A gynecologic oncology group phase II study. Gynecol Oncol 2009;115:460–465. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 87.van der Graaf WT, Blay JY, Chawla SP, et al. Pazopanib for metastatic soft‐tissue sarcoma (PALETTE): A randomised, double‐blind, placebo‐controlled phase 3 trial. Lancet 2012;379:1879–1886. [DOI] [PubMed] [Google Scholar]
- 88.Schoffski P, Chawla S, Maki RG, et al. Eribulin versus dacarbazine in previously treated patients with advanced liposarcoma or leiomyosarcoma: A randomised, open‐label, multicentre, phase 3 trial. Lancet 2016;387:1629–1637. [DOI] [PubMed] [Google Scholar]
- 89.Sutton G, Blessing JA, Malfetano JH. Ifosfamide and doxorubicin in the treatment of advanced leiomyosarcomas of the uterus: A gynecologic oncology group study. Gynecol Oncol 1996;62:226–229. [DOI] [PubMed] [Google Scholar]
- 90.Hensley ML, Blessing JA, Mannel R, et al. Fixed‐dose rate gemcitabine plus docetaxel as first‐line therapy for metastatic uterine leiomyosarcoma: A gynecologic oncology group phase II trial. Gynecol Oncol 2008;109:329–334. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 91.Hensley ML, Ishill N, Soslow R, et al. Adjuvant gemcitabine plus docetaxel for completely resected stages I‐IV high grade uterine leiomyosarcoma: Results of a prospective study. Gynecol Oncol 2009;112:563–567. [DOI] [PubMed] [Google Scholar]
- 92.Hensley ML, Wathen JK, Maki RG, et al. Adjuvant therapy for high‐grade, uterus‐limited leiomyosarcoma: Results of a phase 2 trial (SARC005). Cancer 2013;119:1555–1561. [DOI] [PubMed] [Google Scholar]
- 93.Hensley ML, Miller A, O'Malley DM, et al. Randomized phase III trial of gemcitabine plus docetaxel plus bevacizumab or placebo as first‐line treatment for metastatic uterine leiomyosarcoma: An NRG oncology/gynecologic oncology group study. J Clin Oncol 2015;33:1180–1185. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 94.Pautier P, Floquet A, Chevreau C, et al. Trabectedin in combination with doxorubicin for first‐line treatment of advanced uterine or soft‐tissue leiomyosarcoma (LMS‐02): A non‐randomised, multicentre, phase 2 trial. Lancet Oncol 2015;16:457–464. [DOI] [PubMed] [Google Scholar]
- 95.Hensley ML, Maki R, Venkatraman E, et al. Gemcitabine and docetaxel in patients with unresectable leiomyosarcoma: Results of a phase II trial. J Clin Oncol 2002;20:2824–2831. [DOI] [PubMed] [Google Scholar]
- 96.Tap WD, Jones RL, Van Tine BA, et al. Olaratumab and doxorubicin versus doxorubicin alone for treatment of soft‐tissue sarcoma: An open‐label phase 1b and randomised phase 2 trial. Lancet 2016;388:488–497. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 97.Dileo P, Morgan JA, Zahrieh D, et al. Gemcitabine and vinorelbine combination chemotherapy for patients with advanced soft tissue sarcomas: Results of a phase II trial. Cancer 2007;109:1863–1869. [DOI] [PubMed] [Google Scholar]
- 98.Demetri GD, Chawla SP, von Mehren M, et al. Efficacy and safety of trabectedin in patients with advanced or metastatic liposarcoma or leiomyosarcoma after failure of prior anthracyclines and ifosfamide: Results of a randomized phase II study of two different schedules. J Clin Oncol 2009;27:4188–4196. [DOI] [PubMed] [Google Scholar]
- 99.Samuels BL, Chawla S, Patel S, et al. Clinical outcomes and safety with trabectedin therapy in patients with advanced soft tissue sarcomas following failure of prior chemotherapy: Results of a worldwide expanded access program study. Ann Oncol 2013;24:1703–1709. [DOI] [PubMed] [Google Scholar]
- 100.Hensley ML, Patel SR, von Mehren M, et al. Efficacy and safety of trabectedin or dacarbazine for the treatment of patients with uterine leiomyosarcoma after prior chemotherapy: A subgroup analysis of the randomized phase 3 SAR‐3007 study. Gynecol Oncol 2017;146:531–537. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 101.Martin‐Broto J, Pousa AL, de Las Penas R, et al. Randomized phase II study of trabectedin and doxorubicin compared with doxorubicin alone as first‐line treatment in patients with advanced soft tissue sarcomas: A Spanish Group for Research on Sarcoma Study. J Clin Oncol 2016;34:2294–2302. [DOI] [PubMed] [Google Scholar]
- 102.National Comprehensive Cancer Network. NCCN Clinical Practice Guidelines in Oncology (NCCN Guidelines). Available at https://www.nccn.org/professionals/physician_gls/default.aspx. Accessed May 21, 2018.
- 103.Cuppens T, Moisse M, Depreeuw J, et al. Integrated genome analysis of uterine leiomyosarcoma to identify novel driver genes and targetable pathways. Int J Cancer 2018;142:1230–1243. [DOI] [PubMed] [Google Scholar]
- 104.Cancer Genome Atlas Research Network . Comprehensive and integrated genomic characterization of adult soft tissue sarcomas. Cell 2017;171:950–965 e928. [DOI] [PMC free article] [PubMed] [Google Scholar]