Medullary thyroid cancer (MTC) is a rare neuroendocrine tumor that derives from calcitonin‐secreting thyroid C cells and accounts for fewer than 5% of all thyroid cancers. Recent advances in the understanding of genetic defects and altered molecular pathways have led to progress in treatment. This article details the underlying molecular mechanism of sporadic MTC and provides novel candidate prognostic biomarkers or potential therapeutic targets for sporadic MTC.
Keywords: FN1, RPS6KA3, Sporadic medullary thyroid cancer, Quantitative proteomics, Tandem mass tag
Abstract
Background.
Sporadic medullary thyroid cancer (MTC) is a rare neuroendocrine tumor. Currently, although the diagnosis of sporadic MTC is relatively simple, the need to discover novel candidate prognostic biomarkers for sporadic MTC and investigate the underlying mechanism involved in this rare disease is urgent.
Materials and Methods.
We employed tandem mass tag‐based liquid chromatography‐mass spectrometry to identify and analyze differentially expressed proteins (DEPs) in sporadic MTC. Western blotting was used to validate the DEPs. Immunohistochemistry was performed to investigate FN1 and RPS6KA3 in an independent set of sporadic MTC tissues. Immunohistochemical data were analyzed by different statistical methods.
Results.
Three hundred eighty‐eight DEPs were identified in mass spectrometry, mainly involved in the extracellular matrix, cytoskeletal remodeling, or oxidoreductase activity. Among them, THBS1, MMP9, FN1, RPS6KA3, SYT1, and carcinoembryonic antigen were successfully validated by Western blot. In addition, FN1 and RPS6KA3, enriched in extracellular matrix (ECM) remodeling and the mitogen‐activated protein kinase (MAPK) signaling pathway, respectively, were investigated in an independent set of sporadic MTC tissues. Receiver‐operator characteristic curve analysis showed that FN1 and RPS6KA3 can be used for discriminating sporadic MTC tumorous tissues from paired normal thyroid tissues, and the clinical biomarker calcitonin was positively correlated with FN1 and RPS6KA3 in tumorous tissues. Furthermore, the immunohistochemical scores of FN1 in tumorous tissue showed an inverse relationship with tumor classification, lymph node classification, and American Joint Committee on Cancer stage. Through univariate and multivariate analysis for progression‐free survival, we also found that low FN1 expression in tumorous tissues was an independent worse prognostic factor for progression‐free survival.
Conclusion.
We identified that the pathophysiology of sporadic MTC involve numerous pathways, including the synaptic vesicle pathway, the MAPK signaling pathway, and the ECM remodeling pathway. Furthermore, our study also identified FN1 as novel prognostic biomarkers related to the pathophysiologic changes in sporadic MTC.
Implications for Practice.
Proteomic dissection and prognostic biomarkers are scarce in sporadic medullary thyroid cancer (MTC). This article reports the use of proteomics technology to comprehensively investigate the molecular mechanisms of sporadic MTC, which resulted in the identification of FN1 as a novel candidate prognostic biomarker.
Introduction
Medullary thyroid cancer (MTC) is a rare neuroendocrine tumor that derives from calcitonin‐secreting thyroid C cells and accounts for less than 5% of all thyroid cancers [1]. Approximately 75% of MTC patients present in sporadic form, whereas the remaining 25% are hereditary, with MTC occurring as a dominant component of multiple endocrine neoplasia type 2 (MEN2) syndromes, including MEN2A and MEN2B [2]. Until now, the diagnosis of sporadic MTC has been based on elevated serum calcitonin levels and ultrasonography and fine‐needle aspiration cytology of suspicious thyroid nodules [2], [3]. MTC confined to the thyroid gland has a favorable prognosis, with overall 10‐year survival rates of 70%–80%. However, in patients with advanced MTC at the time of initial treatment or at recurrence, survival is only 26% at 5 years and 10% at 10 years [4]. Focal interventions such as surgery, radiofrequency ablation, and cryoablation have poor results in advanced MTC [5]; therefore, prognostic biomarkers are needed that can help in early identification of advanced sporadic MTC. Although age at diagnosis and stage of disease at the time of initial treatment are currently the main prognostic indicators in sporadic MTC [1], [6], wide variation in tumor behavior strongly supports the need for identification of novel prognostic biomarkers and investigation of the underlying mechanism.
Recent advances in our understanding of genetic defects and altered molecular pathways have led to enormous progress in the treatment of sporadic MTC. The main targets of the drugs used to treat patients with sporadic MTC are the rearranged during transfection (RET) kinases, which are mutated in roughly 50% of sporadic tumors [7]. Furthermore, clinicopathologic evidence demonstrates a strong correlation between RET mutations and phenotype in the clinical aggressiveness of sporadic MTC [2]. RET mutations are gain‐of‐function mutations that trigger downstream signaling pathways and effectors independent of the binding of correspondent ligands and coreceptors [8]. These downstream signaling cascades include the PI3K/AKT/mTOR, RAS/MAPK/STAT or MAPK/NK‐кB, and JNK/STAT3 pathways, which play key roles in regulating cell motility, proliferation, differentiation, apoptosis, and survival [9]. Other downstream effectors of RET mutations include SRC and phospholipase Cγ (PLCγ), which may contribute to advanced MTC [10]. Furthermore, Ameur et al. suggested that similar oncogenic pathways could be activated independently of RET mutations in sporadic MTC cases [11]. In addition to RET mutations, RAS mutations and RET or ALK fusions are known to be important drivers of sporadic MTC [12], [13], [14]. Several studies using gene array technology have been conducted to achieve transcriptional profiling related to different RET mutations in MTC [11], [15]. However, mRNA profiling may not elucidate differences in protein expression and post‐transcriptional regulation that may control the biological processes involved in tumorigenesis [16]. Therefore, the pathophysiologic changes and the specific proteins involved in these pathways in the development of this rare neuroendocrine disease require further investigation.
Quantitative proteomics techniques, such as tandem mass tag (TMT)‐based mass spectrometry (MS), are powerful methods that can be used to obtain accurate and comprehensive protein profiles of small samples [17]. Therefore, in this study, we employed TMT‐6plex‐based liquid chromatography (LC)‐MS/MS to identify and analyze the differentially expressed proteins (DEPs) in sporadic MTC tissues compared with paired normal thyroid tissues. To the best of our knowledge, this is the first comprehensive investigation of the molecular mechanisms of sporadic MTC using proteomics technology. The DEPs identified in the present study will provide both a better understanding of the underlying molecular mechanism of sporadic MTC and novel candidate prognostic biomarkers or potential therapeutic targets for sporadic MTC.
Materials and Methods
Patient Selection and Sample Collection
Because of the rarity of MTC, only three fresh sporadic medullary thyroid tumorous tissues and their paired normal thyroid tissues away from the tumor were collected from the Department of Head and Neck Surgery, Peking University Cancer Hospital & Institute (People's Republic of China) from January 2016 to October 2016. These tissues were obtained from each patient by a single surgeon during thyroidectomy. The tissues were immediately snap‐frozen in liquid nitrogen and then stored at −80°C prior to the following assays and RET mutational screening according to the previous study [11]. All tissues were evaluated by surgical pathologists to confirm the diagnosis of sporadic MTC, which have no familial history and the presence of MEN2 syndromes. The clinical and genetic features of these patients are shown in supplemental online Table 1. All tissues used in this study were obtained with informed consent of the patients, and this study was approved by the Ethics Committee of Peking University Cancer Hospital & Institute.
Protein Extraction and TMT Labeling
TMT labeling was performed as described previously with slight modifications [18]. Briefly, six samples from three sporadic MTC patients were pooled as Group A (mixed tumorous tissues) and Group B (mixed paired normal thyroid tissues). Subsequently, TMT isobaric label reagents (0.8 mg TMT dissolved in 40 μL 99.9% acetonitrile) were used to label each group of peptides according to the manufacturer's instructions: Group A and Group B were labeled with TMT‐127 and TMT‐131, respectively. Equal amounts of the labeled peptide digests from each group were then combined for subsequent high‐performance liquid chromatography (HPLC) and LC‐MS/MS analysis.
HPLC Analysis
For details, see supplemental online Appendix 1.
LC‐MS/MS analysis
The labeled fractions were analyzed using the Lumos mass spectrometer as described previously [19]. For details, see supplemental online Appendix 1.
Protein Identification
The MS proteomic data were deposited in the ProteomeXchange Consortium via the PRIDE partner repository with the dataset identifier PXD005898 [20]. For details, see supplemental online Appendix 1.
Bioinformatic Analysis
For analysis of the generated proteomics data, 388 DEPs were uploaded into the ClueGO (version 2.1.7) and CluePedia (version 1.17) software plugins folder of Cytoscape (version 3.1.1) for Gene Ontology (GO) enrichment analysis. The kappa core level was set at ≥0.4, and the Bonferroni method was used to correct the p value for multiple testing. The WebGestalt online toolkit (http://webgestalt.org) was used for Wikipathways and disease enrichment analysis; p < .01 and p < .001, respectively, were considered to indicate statistical significance. Protein‐protein interaction (PPI) analysis was conducted using the String website (http://string-db.org), with a confidence value of 0.6. The networks were downloaded as test files and visualized using Cytoscape software.
Western Blot Analysis
The same set of tissues used in the proteomic analysis was then further evaluated by Western blotting. For details, see supplemental online Appendix 1.
Immunohistochemistry and Evaluation of Staining
An independent set of 47 paraffin‐embedded archival sporadic MTC specimens, collected between January 2008 and December 2015, was obtained from the Department of Pathology, Peking University Cancer Hospital & Institute, People's Republic of China. Immunohistochemical staining was performed as previously described [21]. For details, see supplemental online Appendix 1.
Statistical Analysis of Immunohistochemical Data
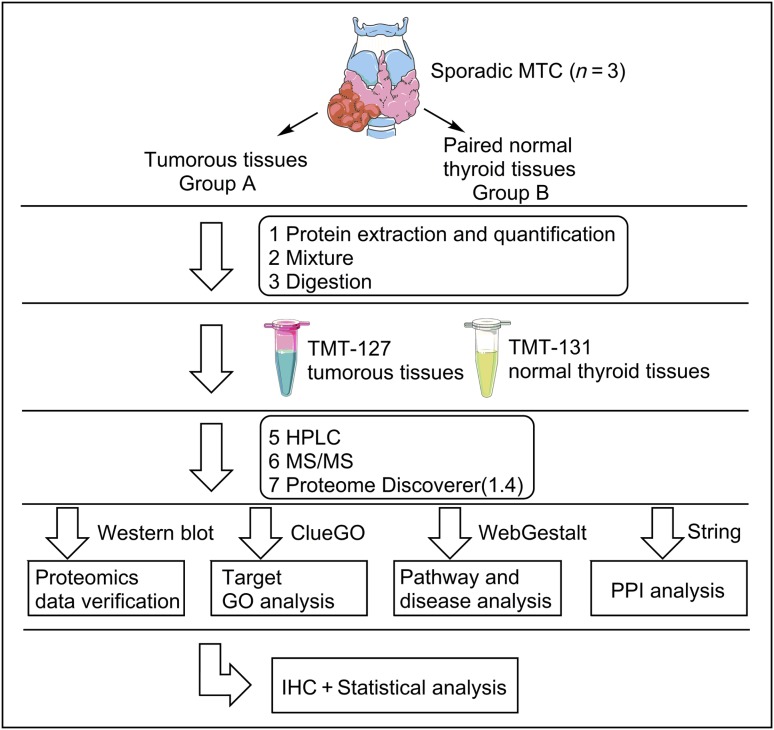
SPSS for Windows version 19.0 (IBM Corp., Armonk, NY) was used for analyses of protein expression levels in individual samples determined by immunohistochemistry (IHC) analysis. Student's t test was used for comparison of quantitative data between the two groups. Relationships between the expression level of candidate prognostic biomarkers and different clinicopathological characteristics were analyzed using the nonparametric Mann‐Whitney U test. Receiver‐operator characteristic (ROC) curves were constructed by plotting sensitivity versus (1 − specificity), and the areas under curves (AUC) were analyzed by the Hanley‐McNeil method. The optimal cutoff points for each biomarker were determined by the Youden's index, which is calculated as sensitivity + (specificity − 1). Correlations between candidate biomarkers and calcitonin (CALCA) were assessed using Pearson correlation analysis and linear regression analysis. Cumulative survival was calculated by the Kaplan‐Meier method. The Cox proportional hazards model was used for multivariate analysis to identify independent predictors of progression‐free survival (PFS). Two‐tailed p < .05 indicated statistical significance. Figure 1 shows the study flow diagram.
Figure 1.
Schematic representation of the experimental workflow to quantitatively identify the total protein profiles of sporadic MTC tumorous tissues and paired normal thyroid tissues using TMT‐based LC‐MS/MS.
Abbreviations: GO, gene ontology; HPLC, high‐performance liquid chromatography; IHC, immunohistochemistry; MS/MS, tandem mass spectrometry; MTC, medullary thyroid cancer; PPI, protein‐protein interaction; TMT, tandem mass tag.
Results
Global Protein Profiles of Sporadic MTC Tumorous Tissues and Their Paired Normal Thyroid Tissues
To comprehensively investigate the molecular mechanisms of sporadic MTC, we obtained the global protein profiles of sporadic MTC tissues and their paired normal thyroid tissues by TMT‐based LC‐MS/MS. In total, 5,865 proteins were identified, of which 3,328 with scores ≥10 were extracted; these proteins contained two or more unique peptides. The UniProt KB IDs of these 3,328 proteins were converted into Entrez Gene IDs, and four proteins [FBLL1 (A6NHQ2), APOA4 (P06727), ACAN (P16112), and CAPS (Q13938)] with no‐paired matching gene IDs were excluded. Subsequently, we compared the expression level of these 3,324 proteins in tumorous tissues and their paired normal thyroid tissues. Using cutoff ratios for DEPs set as ≥1.5‐fold and ≤0.6‐fold, a total of 388 DEPs (257 upregulated and 131 downregulated) were selected for further bioinformatics analysis (supplemental online Table 2).
GO Enrichment Analysis and PPI Analysis Based on DEPs‐Related Disease Enrichment
We used ClueGO and CluePedia software to analyze the GO enrichment of the selected 388 DEPs as described previously [22]. The results of the GO analysis showed that the DEPs in tumorous tissues were involved mainly in either extracellular matrix or cytoskeletal remodeling, or oxidoreductase activity, which are the pathological hallmarks of cancer. Furthermore, these categories are also associated with transport vesicle, SNARE complex, and hormone activity, which may reflect the active features of the neuroendocrine neoplasm markers from sporadic MTC patients (supplemental online Fig. 1). To further investigate the in‐depth pathophysiology of sporadic MTC, we used the WebGestalt online toolkit to perform disease enrichment analysis of the uploaded Entrez Gene IDs of the DEPs. In total, 234 enriched diseases were identified in the disease database using the following parameters: statistical method, hypergeometric; multiple test adjust, Benjamini‐Hochberg (BH); significance level, p < .001; minimum genes for a category, 5 (supplemental online Table 3) [23]. Among the proteins identified, DEPs enriched in 22 diseases, including live carcinoma, lung neoplasms, bone neoplasms, and neuroblastoma, may be closely related to the initiation and progression of sporadic MTC, as well as distant metastases of sporadic MTC (supplemental online Table 4).
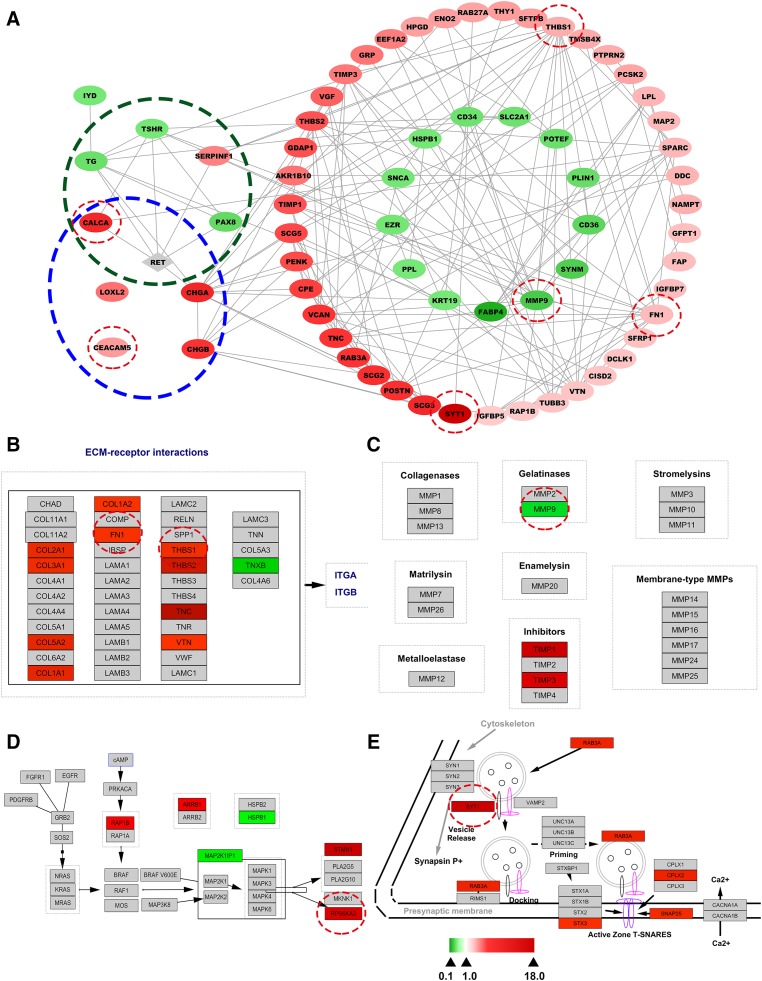
To clarify the PPI network among the DEPs enriched in these 22 diseases, we used the String website to create a comprehensive interaction network of the extracted DEPs (supplemental online Table 5) plus the RET protein (not identified in our proteomics data). Most connected proteins were found to be associated with cell adhesion/migration as well as the remodeling of the extracellular matrix and cytoskeleton, which was consistent with the GO and pathway analyses (Fig. 2A). Additionally, neuroendocrine specific markers of MTC, including CALCA, carcinoembryonic antigen (CEACAM5), and chromogranin‐A (CHGA), were all upregulated in tumorous tissues. LOXL2 and CHGB were also found to be overexpressed in tumorous tissues. All these DEPs were in accordance with those identified in previous studies [15], [24], [25].
Figure 2.
Bioinformatic analysis of differentially expressed proteins (DEPs) identified in this study. (A): Protein‐protein interaction network constructed with selected disease‐enriched proteins in sporadic medullary thyroid cancer (MTC) tumorous tissues compared with paired normal thyroid tissues using Cytoscape software. No connecting DEPs were excluded. The gray node, RET, represents a protein not identified in the present study. Blue dotted ovals represent known biomarkers of MTC. Dark green dotted ovals represent RET‐associated DEPs. (B–E): Visualization of Wikipathways enrichment analyses of DEPs. Gray nodes represent proteins not identified in the present study. (B): Visualization of the focal adhesion pathway. (C): Visualization of matrix metalloproteinase pathways. (D): Visualization of the mitogen‐activated protein kinase signaling pathway. (E): Visualization of the synaptic vesicle pathway. All DEPs are represented by colored nodes labeled with the gene name. Upregulated and downregulated DEPs are shown in red and green, respectively, whereas the fold‐changes of DEPs are reflected by color intensity. Five shared DEPs and clinical biomarkers (CALCA and CEACAM5) subsequently investigated by Western blot or immunohistochemistry (see Fig. 3) are highlighted with red circles.
Wikipathways Enrichment Analysis of DEPs
To further investigate the biological pathways involved in the development of sporadic MTC, we performed Wikipathways enrichment analysis of directly uploaded Entrez Gene IDs of the DEPs. Thirty Wikipathways were identified using the WebGestalt online toolkit (parameters: statistical method, hypergeometric; multiple test adjusts, BH; significance level, p < .01; minimum genes for a category, 2; supplemental online Table 6). Of particular note among the DEP‐related enriched pathways were a sub‐cluster of pathways (focal adhesion pathway, inflammatory response pathway, regulation of actin cytoskeleton pathway, matrix metalloproteinases pathway, and MAPK signaling pathway) associated with both cell migration/adhesion and extracellular matrix and cytoskeleton remodeling. These significantly enriched pathways were consistent with the GO analysis of the same DEPs. Among 11 proteins enriched in the focal adhesion pathway, 10 (COL2A1, COL3A1, COL5A2, COL1A1, COL1A2, FN1, THBS1, THBS2, TNC, and VTN) were obviously upregulated in tumorous tissues, whereas only one, TNXB, was downregulated (Fig. 2B). In the matrix metalloproteinases pathway, TIMP1 and TIMP3 were upregulated in tumorous tissues, and MMP9, possibly inhibited by TIMP1 and TIMP3, was downregulated (Fig. 2C). In the MAPK signaling pathway, six proteins were matched in the Wikipathways database. Of these proteins, RAP1B, ARRB1, STMN1, and RPSKA3 were upregulated, and MAP2K1IP1 and HSPB1 were downregulated (Fig. 2D). Furthermore, in accordance with the GO analysis of sporadic MTC patients, three pathways (calcium regulation in the cardiac cell, the synaptic vesicle pathway, and the TSH signaling pathway) were involved in the calcium regulation and hormone secretion categories. Of particular note, five DEPs (SYT1, RAB3A, STX3, SNAP25, and CPLX2) were upregulated in the synaptic vesicle pathway, suggesting that vesicle activity is greatly promoted in the sporadic MTC patients (Fig. 2E). These findings indicated the validity of our bioinformatics results.
Verification of DEPs Identified by TMT‐Based LC‐MS/MS by Western Blot Analysis
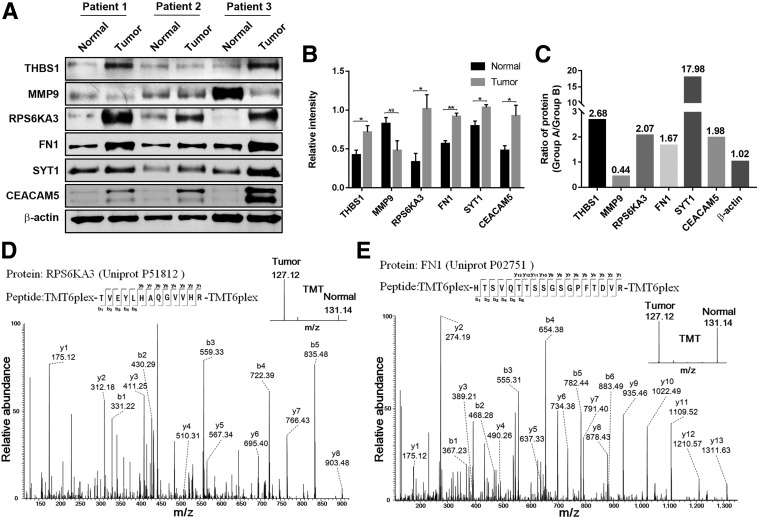
To validate the proteomics data, we performed Western blot analysis of six DEPs, THBS1, MMP9, RPS6KA3, FN1, SYT1, and CEACAM5, with β‐actin as an internal control. According to the proteomics analysis, the fold changes in these proteins were 2.68, 0.44, 2.07, 1.67, 17.98, 1.98, and 1.02, respectively (Fig. 3C). In the Western blot analysis, THBS1, RPS6KA3, FN1, SYT1, and CEACAM5 were confirmed to be upregulated in each tumorous tissue compared with the normal control, whereas MMP9 was downregulated (Fig. 3A, 3B). Thus, the patterns of DEPs identified in the Western blot analysis were consistent with the TMT‐based proteomics data. The criteria for selecting FN1 and RPS6KA3 for further investigation were as follows: (a) DEPs identified in sporadic MTC that are involved in cancer‐related signaling pathways; (b) DEPs as candidate prognostic biomarkers for sporadic MTC have not been reported by PubMed or Google research. Figure 3D, 3E shows the MS/MS spectra of FN1 and RPS6KA3 proteins, respectively.
Figure 3.
Validation of TMT‐based proteomics data. (A): Six differentially expressed proteins (DEPs) identified in proteomics data were validated by Western blot analysis. Compared with paired normal tissues (normal), the upregulation of THBS1, RPS6KA3, FN1, SYT1, and CEACAM5 and downregulation of MMP9 in tumorous tissues (tumor) were in conformity with the TMT‐based proteomics results. (B): The relative intensity of these six DEPs (normalized by corresponding β‐actin expression) in tumor and paired normal tissues was analyzed by Student's t test (*, p < .05; **, p < .005; NS, not statistically significant). (C): The fold‐changes of selected DEPs identified in TMT‐based proteomics results. (D, E): The sequence TVEYLHAQGVVHR allows the identification of RPS6KA3, and the TMT reporter signals provide relative quantitation of RPS6KA3 between tumorous and normal tissues. The sequence HTSVQTTSSGSGPFTDVR allows the identification of FN1, and the TMT reporter signals provide relative quantitation of FN1 between tumorous and normal tissues.
Abbreviation: TMT, tandem mass tag.
Verification of Candidate Biomarkers by Immunohistochemistry
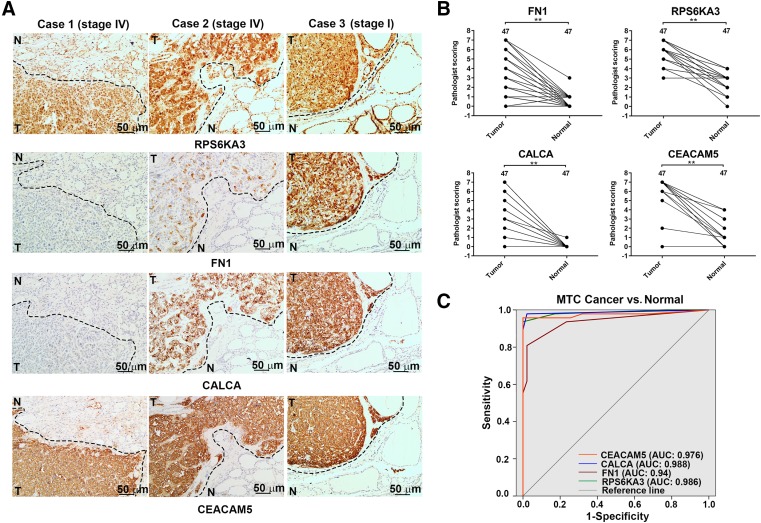
Immunohistochemistry was performed to verify the expression levels of the candidate prognostic biomarkers—FN1 and RPS6KA3—in an independent set of sporadic MTC specimens. The IHC scores for these two candidate prognostic biomarkers were significantly higher in tumorous tissues than paired normal thyroid tissues (p < .005; Fig. 4B). Furthermore, tumorous cells showed strong cytoplasmic and nuclear staining for RPS6KA3, whereas normal cells showed weak cytoplasmic and moderate nuclear staining. Additionally, two stage IV (American Joint Committee on Cancer [AJCC]) sporadic MTC patients had low or moderate FN1 and CALCA staining with strong CEACAM5 staining in the same section of tumor cells, but all stained intensely in the primary tumor cells of stage I sporadic MTC patient (Fig. 4A). These results indicate that FN1 staining in tumor cells inversely correlates with CEACAM5 staining in some aggressive sporadic MTC patients, which is consistent with the relationship between CALCA and CEACAM5 found in a previous study [26].
Figure 4.
Immunohistochemical images and diagnostic value of FN1, RPS6KA3, CALCA, and CEACAM5 proteins in tissues. (A): Consecutive staining reactions for FN1, RPS6KA3, CEACAM5, and CALCA were performed in the same tissue sections and compared, and representative images of immunostaining of these four differentially expressed proteins are shown for samples from three patients, including two stage IV and one stage I (American Joint Committee on Cancer) sporadic medullary thyroid cancer (MTC) patients. Compared with paired normal tissues, FN1, RPS6KA3, CALCA, and CEACAM5 proteins were upregulated in tumorous tissues (scale bars, 50 μm). The three tumorous cell samples show moderate and intense cytoplasmic and nuclear staining for RPS6KA3, whereas they respectively show low, moderate, and intense cytoplasmic and extracellular matrix staining for FN1. Furthermore, the three tumorous cell samples respectively show low, moderate, and intense cytoplasmic staining for CALCA, whereas they all show intense intracytoplasmic and membrane‐associated staining for CEACAM5. (B): Histopathological scoring of FN1, RPS6KA3, CALCA, and CEACAM5 in 47 paraffin‐embedded sporadic MTC tumorous tissues and paired normal thyroid tissues. **, p < .005 (Student's t test). (C): Receiver‐operator characteristic analysis of CEACAM5 (orange line), CALCA (blue line), FN1 (red line), and RPS6KAS (green line) for discriminating sporadic MTC tumorous tissue from normal tissue.
Abbreviation: TMT, tandem mass tag.
Statistical Analysis of Immunohistochemical Data
Linear regression and Pearson correlation analysis was used to assess the association between FN1 and RPS6KA3 and CALCA and CEACAM5 in tumor cells. As shown in supplemental online Table 7, a significant positive linear relationship was observed between the IHC scores of FN1 and CALCA (r = .512; p = .001) as well as between the IHC scores of RPS6KA3 and CALCA (r = .335; p = .021), with a weakly positive linear relationship between the IHC scores of FN1 and CEACAM5 (r = .124; p = .084). These results suggest that FN1 and RPS6KA3 may have clinical value for the prognostic diagnosis of sporadic MTC.
To further investigate the value of RPS6KAS and FN1 for sporadic MTC, we used ROC curve analysis to determine the sensitivity and specificity of these DEPs (Fig. 4C), with CALCA and CEACAM5 as the gold standard. The AUCs of FN1, RPS6KA3, CALCA, and CEACAM5 for discriminating the sporadic MTC tumorous tissues from the paired normal thyroid tissues were 0.94, 0.986, 0.988, and 0.976, respectively. Supplemental online Table 8 shows the optimal Youden's index and the corresponding specificity and sensitivity. These results confirm that FN1 and RPS6KA3 can be used for discriminating MTC tumorous tissue from normal tissue.
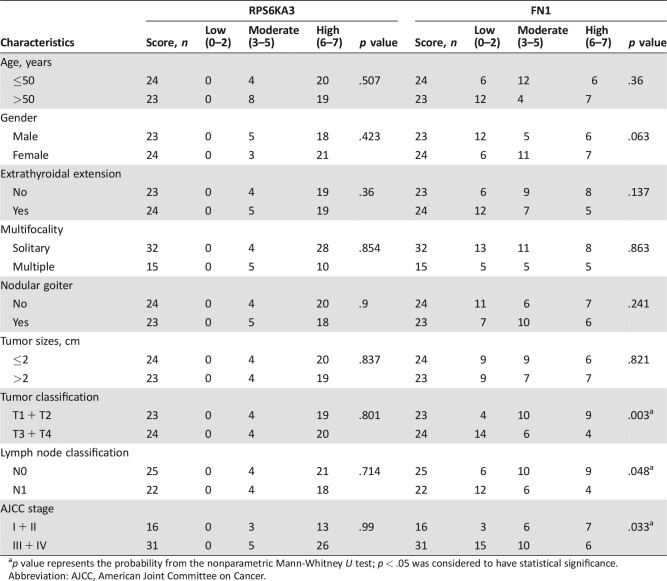
Additionally, we used the nonparametric Mann‐Whitney U test to examine how upregulation of RPS6KA3 and FN1 proteins in tumorous tissues was associated with the clinicopathological characteristics of sporadic MTC patients (Table 1). The IHC scores of FN1 were inversely correlated with sporadic MTC patients with higher tumor classification (p = .003). Furthermore, the expression levels of FN1 were significantly lower in MTC patients with advanced AJCC stage than in early‐stage disease (p = .033). Sporadic MTC patients with lymph node metastasis showed significantly lower IHC scores for FN1 than patients without metastasis (p = .048). However, the IHC score of RPS6KA3 was not significantly associated with clinicopathologic characteristics of sporadic MTC patients, probably due to the high immunohistochemical staining of RPS6KA3 in 80% of the sporadic MTC tumorous tissues.
Table 1. Correlations of FN1 and RPS6KA3 protein expression with clinicopathological characteristics.
p value represents the probability from the nonparametric Mann‐Whitney U test; p < .05 was considered to have statistical significance.
Abbreviation: AJCC, American Joint Committee on Cancer.
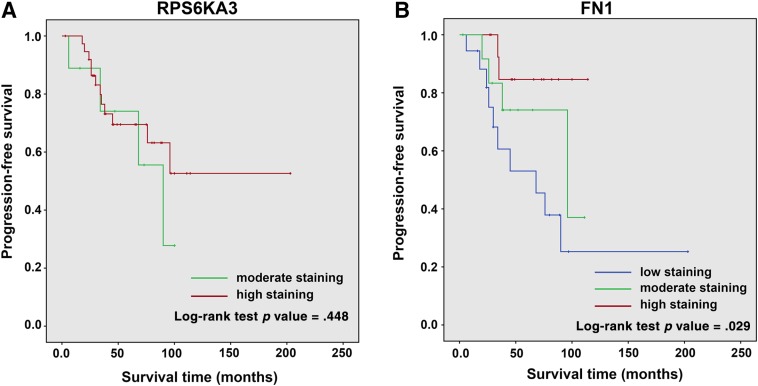
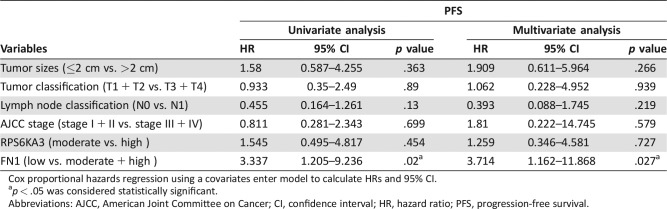
The prognostic significance of RPS6KA3 and FN1 in sporadic MTC were assessed. Kaplan‐Meier analysis indicated that patients with low FN1 expression in tumorous tissue have worse PFS than patients with moderate or high expression (p = .029); however, RPS6KA3 had no significant association with PFS in MTC patients (p = .448; Fig. 5A, 5B). Thus, elevated FN1 expression was adversely associated with sporadic MTC progression. On univariate and multivariate analysis for PFS using the Cox proportional hazards model, we also found that low FN1 expression in tumorous tissues was an independent worse prognostic factor for PFS (p = .02 and p = .027, respectively), whereas tumor size, tumor classification, lymph node classification, AJCC stage, and RPS6KA3 expression were not associated with PFS (Table 2). Because only three sporadic MTC patients died before the final follow‐up, we did not attempt to analyze the prognostic significance of the different variables for overall survival.
Figure 5.
Cumulative progression‐free survival (PFS) curves of sporadic medullary thyroid cancer patients; the patients were separated into different groups according to the immunohistochemistry scores for RPS6KA3 and FN1. (A): Kaplan‐Meier method (log‐rank analysis) showed no prognostic significance of PFS in RPS6KA. (B): Kaplan‐Meier method (log‐rank analysis) showed that there was an inverse association between elevated FN1 expressions in tumorous tissues and progression.
Table 2. Univariate and multivariate analyses of variables associated with PFS.
Cox proportional hazards regression using a covariates enter model to calculate HRs and 95% CI.
p < .05 was considered statistically significant.
Abbreviations: AJCC, American Joint Committee on Cancer; CI, confidence interval; HR, hazard ratio; PFS, progression‐free survival.
Discussion
In the present study, we used TMT‐based proteomics technology to identify a total of 5,865 proteins in sporadic MTC tissues, of which 388 were differently expressed in tumorous tissues compared with paired normal thyroid tissues. These DEPs may play pivotal roles in the pathophysiology of sporadic MTC. Of these DEPs, COL1A1, COL1A2, and LOXL2, which were upregulated in tumorous tissues, have been previously demonstrated to be closely related to the RETM918T mutation in MTC [11], [15]. This may be due to a sporadic RETM918T‐mutated MTC tissue included in this study. In addition, MTC is a neuroendocrine tumor deriving from parafollicular C cells, whereas other types of thyroid cancers originate from follicular cells [27]. In accordance with previous reports, TSHR, TG, and PAX8, which are expressed mainly in follicular cells, were shown to be downregulated in sporadic MTC tumorous tissues in our study [28]. Collectively, these results suggest the reliability of our proteomics data. Therefore, we subsequently performed bioinformatics analyses of the 388 DEPs to investigate the mechanisms underlying the pathophysiology of sporadic MTC. This analysis of the DEPs enriched in the sporadic MTC tumorous tissues indicated the involvement of a variety of pathways, including the synaptic vesicle pathway, the MAPK signaling pathway, and ECM remodeling pathways (including focal adhesion, regulation of the actin cytoskeleton, and matrix metalloproteinases pathway).
In the present study, SYT1 was greatly upregulated in sporadic MTC tumorous tissues. Through the construction of disease‐enriched PPI networks, we also found that SYT1 interacts directly with CHGA and CHGB, which have been used as candidate biomarkers of MTC in the clinic [29], [30]. Furthermore, Sudhof reported that SYT1 plays a significant role in both fast synaptic exocytosis and endocrine exocrine exocytosis, which are important in the production of neurotransmitter markers in MTC, including neuron‐specific enolase and CALCA [31], [32]. In pathway‐enrichment analysis, five DEPs were shown to be greatly upregulated in the synaptic vesicle pathway category. These included SYT1 and CPLX2 (fold change = 3.2), which is the most important cofactor for SYT1 in this pathway [32]. Collectively, these observations indicate that the synaptic vesicle pathway may be associated with the pathological process of sporadic MTC, and SYT1 may be the most important involved DEPs.
The MAPK signaling pathway plays pivotal roles in the regulation of cancer cell proliferation, migration, and survival, as well as in human thyroid tumorigenesis [33]. In sporadic MTC, it has been demonstrated that wild‐type RET or RET mutations activate several different signaling pathways, including the MAPK signaling pathway [34]. Our results revealed enrichment of six DEPs in the MAPK signaling pathway. Notably, Cho et al. discovered that RPS6KA3, which is activated by its upstream kinases, ERK1 and ERK2, plays a central role in human skin cancer development [35]. It was also reported that RPS6KA3 is activated by different growth factors that are closely related to cell proliferation, anchorage‐independent cell transformation, and cancer development [36]. In the present study, although RPS6KAS was not associated with clinicopathologic characteristics of sporadic MTC, it was significantly upregulated in MTC tumorous tissues and could be used to distinguish sporadic MTC tumorous tissues from the paired normal thyroid tissues; the AUC of RPS6KAS was similar to that of the clinical biomarker CALCA and CEACAM5. Although RPS6KA3 cannot serve as a predictor for PFS in sporadic MTC, these observations suggest that RPS6KA3 involved in the MAPK signaling pathway may play important roles in the pathological process of sporadic MTC and therefore could be combined with CALCA and CEACAM5 to improve accuracy of diagnosis of sporadic MTC.
During neoplastic progression, the composition and organization of the ECM may undergo radical changes that affect cancer cell functions such as proliferation, survival, and migration [37]. We also identified several DEPs enriched in sporadic MTC tumorous tissues associated with the ECM remodeling pathway, including overexpression of COL2A1, COL3A1, COL5A2, COL1A1, COL1A2, POSTN, VACN, TNC, THBS1, THBS2, LOXL2, FN1, and VTN, as well as downregulation of CD36, CD34, S100A9, and MMP9. These enriched DEPs have been reported previously in association with the acquisition and maintenance of several cancer hallmarks [37], [38], [39], [40], [41]. Notably, the increase in matrix stiffness attributed to LOXL2 upregulation and increased collagen cross‐linking could be enhanced by the PI3K/Akt and MAPK signaling pathway [42]. Interestingly, our proteomics data showed that MMP9 was downregulated in MTC tumorous tissues compared with paired normal thyroid tissues. Although most studies indicate that MMP9 not only regulates components of the ECM but also promotes cancer progression, a few cases showing a positive association between MMP9 and good prognosis have also been reported [40]. For instance, Takeha and colleagues discovered that stromal expression of MMP9 is negatively associated with liver metastases in colorectal cancer [43]. However, the specific functions and molecular mechanisms of MMP9 downregulation in sporadic MTC tumorous tissue remain to be determined. In contrast, TIMP1 and TIMP3, which are both MMP9 inhibitors, were found to be upregulated in sporadic MTC tumorous tissues and have been previously associated with poor prognosis in a variety of human tumors, including melanoma, as well as colon, gastric, lung, and breast carcinomas [40]. Therefore, it can be speculated that the balance between TIMPs and MMP9 plays a vital role in sporadic MTC progression. Of particular note, Prasad et al. have demonstrated that FN1 was upregulated in PTC but not in non‐neoplastic thyroid [44]. Furthermore, Jain et al. demonstrated that compared with MEN2A MTCs, several genes associated with ECM remodeling are specifically enriched in MEN2B MTC, including FN1 [45]. In the present study, we used immunohistochemical staining and found that FN1 expression was significantly upregulated in sporadic MTC tumorous tissues compared with normal tissues. FN1 had an AUC of 0.94 for discriminating sporadic MTC tumorous tissue from normal tissue. We also observed a statistically significant positive linear relationship between FN1 expression and CALCA, with a weakly positive linear relationship with CEACAM5, whereas FN1 staining in tumor cells inversely correlated with CEACAM5 staining in some aggressive sporadic MTC patients. Furthermore, the IHC scores of FN1 showed an inverse relationship with several clinicopathological characteristics, including tumor classification, lymph node classification, and AJCC stage. In addition, multivariate analysis showed that low FN1 expression in tumorous tissues was an independent predictor of poor prognosis in sporadic MTC. Consistent with these observations, previous studies have demonstrated poor expression of CALCA in the primary tumor and in metastases in aggressive MTC, and these sections manifested positive and even intense CEACAM5 staining [26]. Patients with such tumors also have poorer prognosis than those with CALCA‐rich tumors [46]. These studies suggested that MTC tumorous cells, which have a relatively low degree of differentiation and maturation, are unable to perform some mature cellular functions, such as secretion of CALCA. Therefore, we suspect that the clinical value of FN1 may be similar to that of CALCA in MTC patients. Furthermore, on the basis of these data, although we have obtained no metastasis samples, it may be expected that the intensity of FN1 staining in metastatic lesions will be weak or absent in aggressive sporadic MTC patients; this should be investigated in the future. Taken together, these findings suggest that all these DEPs enriched in the ECM remodeling pathways play vital roles in the malignancy of sporadic MTC, and most importantly, the low levels of FN1 in tumorous tissues may serve as a novel worse prognostic biomarker for sporadic MTC.
Conclusion
In summary, our quantitative proteomics analysis of sporadic MTC has confirmed changes in the expression of several known biomarkers and identified FN1 as novel prognostic biomarkers related to the pathophysiologic changes in sporadic MTC. Our results also indicate that the molecular mechanisms underlying the pathophysiology of MTC involve numerous pathways, such as the synaptic vesicle pathway, the MAPK signaling pathway, and the ECM remodeling pathway. Thus, the findings of this study highlight new therapeutic possibilities for the improved management of sporadic MTC, which need to be further explored in vivo and in vitro.
See http://www.TheOncologist.com for supplemental material available online.
Acknowledgments
This work was supported by the CAMS Innovation Fund for Medical Sciences (CIFMS, 2016‐I2M‐1–003), the National Science Foundation of China (No. 81572625), and the Beijing Municipal Administration of Hospital's Youth Program (No. QML20161103).
Contributor Information
Tianxiao Wang, Email: tianxiao_w2000@hotmail.com.
Wei Ge, Email: wei.ge@chem.ox.ac.uk.
Author Contributions
Conception/design: Tianxiao Wang, Wei Ge
Collection and/or assembly of data: Shaohua Zhan, Tianxiao Wang
Data analysis and interpretation: Shaohua Zhan
Manuscript writing: Shaohua Zhan, Jinming Li, Tianxiao Wang, Wei Ge
Final approval of manuscript: Shaohua Zhan, Jinming Li, Tianxiao Wang, Wei Ge
Disclosures
The authors indicated no financial relationships.
References
- 1. Wells SA Jr, Asa SL, Dralle H et al. Revised American Thyroid Association guidelines for the management of medullary thyroid carcinoma. Thyroid 2015;25:567–610. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2. Fahiminiya S, de Kock L, Foulkes WD. Biologic and clinical perspectives on thyroid cancer. N Engl J Med 2016;375:2306–2307. [DOI] [PubMed] [Google Scholar]
- 3. Kloos RT, Eng C, Evans DB et al. Medullary thyroid cancer: Management guidelines of the american thyroid association. Thyroid 2009;19:565–612. [DOI] [PubMed] [Google Scholar]
- 4. Bergholm U, Bergström R, Ekbom A. Long term follow‐up of patients with medullary carcinoma of the thyroid. Cancer 1997;79:132–138. [DOI] [PubMed] [Google Scholar]
- 5. Hadoux J, Pacini F, Tuttle RM et al. Management of advanced medullary thyroid cancer. Lancet Diabetes Endocrinol 2016;4:64–71. [DOI] [PubMed] [Google Scholar]
- 6. Roman S, Lin R, Sosa JA. Prognosis of medullary thyroid carcinoma: Demographic, clinical, and pathologic predictors of survival in 1252 cases. Cancer 2006;107:2134–2142. [DOI] [PubMed] [Google Scholar]
- 7. Elisei R, Cosci B, Romei C et al. Prognostic significance of somatic RET oncogene mutations in sporadic medullary thyroid cancer: A 10‐year follow‐up study. J Clin Endocrinol Metab 2008;93:682–687. [DOI] [PubMed] [Google Scholar]
- 8. Hu MI, Ying AK, Jimenez C. Update on medullary thyroid cancer. Endocrinol Metab Clin North Am 2014;43:423–442. [DOI] [PubMed] [Google Scholar]
- 9. Ernani V, Kumar M, Chen AY et al. Systemic treatment and management approaches for medullary thyroid cancer. Cancer Treat Rev 2016;50:89–98. [DOI] [PubMed] [Google Scholar]
- 10. Cerrato A, De Falco V, Santoro M. Molecular genetics of medullary thyroid carcinoma: The quest for novel therapeutic targets. J Mol Endocrinol 2009;43:143–155. [DOI] [PubMed] [Google Scholar]
- 11. Ameur N, Lacroix L, Roucan S et al. Aggressive inherited and sporadic medullary thyroid carcinomas display similar oncogenic pathways. Endocr Relat Cancer 2009;16:1261–1272. [DOI] [PubMed] [Google Scholar]
- 12. Grubbs EG, Ng PK, Bui J et al. RET fusion as a novel driver of medullary thyroid carcinoma. J Clin Endocrinol Metab 2014;100:788–793. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Ji JH, Oh YL, Hong M et al. Identification of driving ALK fusion genes and genomic landscape of medullary thyroid cancer. PLoS Genet 2015;11:e1005467. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Moura MM, Cavaco BM, Leite V. RAS proto‐oncogene in medullary thyroid carcinoma. Endocr Relat Cancer 2015;22:R235–R252. [DOI] [PubMed] [Google Scholar]
- 15. Maliszewska A, Leandro‐Garcia LJ, Castelblanco E et al. Differential gene expression of medullary thyroid carcinoma reveals specific markers associated with genetic conditions. Am J Pathol 2013;182:350–362. [DOI] [PubMed] [Google Scholar]
- 16. Lian Z, Wang L, Yamaga S et al. Genomic and proteomic analysis of the myeloid differentiation program. Blood 2001;98:513–524. [DOI] [PubMed] [Google Scholar]
- 17. Eagle GL, Zhuang J, Jenkins RE et al. Total proteome analysis identifies migration defects as a major pathogenetic factor in immunoglobulin heavy chain variable region (IGHV)‐unmutated chronic lymphocytic leukemia. Mol Cell Proteomics 2015;14:933–945. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Xu L, Gao YP, Chen YY et al. Quantitative proteomics reveals that distant recurrence‐associated protein R‐RAS and transgelin predict post‐surgical survival in patients with stage III colorectal cancer. Oncotarget 2016;7:43868–43893. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Wang S, Lu Y, Sun X et al. Identification of common and differential mechanisms of glomerulus and tubule senescence in 24‐month‐old rats by quantitative LC‐MS/MS. Proteomics 2016;16:2706–2717. [DOI] [PubMed] [Google Scholar]
- 20. Vizcaíno JA, Deutsch EW, Wang R et al. Proteomexchange provides globally coordinated proteomics data submission and dissemination. Nat Biotechnol 2014;32:223–226. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. Zeng GQ, Zhang PF, Deng X et al. Identification of candidate biomarkers for early detection of human lung squamous cell cancer by quantitative proteomics. Mol Cell Proteomics 2012;11:M111.013946. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. Bindea G, Mlecnik B, Hackl H et al. ClueGO: A cytoscape plug‐in to decipher functionally grouped gene ontology and pathway annotation networks. Bioinformatics 2009;25:1091–1093. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23. Wang J, Duncan D, Shi Z et al. WEB‐based GEne SeT AnaLysis Toolkit (WebGestalt): Update 2013. Nucleic Acids Res 2013;41:W77–W83. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24. Erovic BM, Kim D, Cassol C et al. Prognostic and predictive markers in medullary thyroid carcinoma. Endocr Pathol 2012;23:232–242. [DOI] [PubMed] [Google Scholar]
- 25. Erickson LA, Vrana JA, Theis J et al. Analysis of amyloid in medullary thyroid carcinoma by mass spectrometry‐based proteomic analysis. Endocr Pathol 2015;26:291–295. [DOI] [PubMed] [Google Scholar]
- 26. Mendelsohn G, Wells SA, Baylin SB. Relationship of tissue carcinoembryonic antigen and calcitonin to tumor virulence in medullary thyroid carcinoma. An immunohistochemical study in early, localized, and virulent disseminated stages of disease. Cancer 1984;54:657–662. [DOI] [PubMed] [Google Scholar]
- 27. Elisei R, Pinchera A. Advances in the follow‐up of differentiated or medullary thyroid cancer. Nat Rev Endocrinol 2012;8:466–475. [DOI] [PubMed] [Google Scholar]
- 28. Puglisi F, Cesselli D, Damante G et al. Expression of Pax‐8, p53 and bcl‐2 in human benign and malignant thyroid diseases. Anticancer Res 2000;20:311–316. [PubMed] [Google Scholar]
- 29. Blind E, Schmidt‐Gayk H, Sinn HP et al. Chromogranin A as tumor marker in medullary thyroid carcinoma. Thyroid 1992;2:5–10. [DOI] [PubMed] [Google Scholar]
- 30. Taupenot L, Harper KL, O'Connor DT. The chromogranin‐secretogranin family. N Engl J Med 2003;348:1134–1149. [DOI] [PubMed] [Google Scholar]
- 31. Sudhof TC. Synaptotagmins: Why so many? J Biol Chem 2002;277:7629–7632. [DOI] [PubMed] [Google Scholar]
- 32. Südhof TC. A molecular machine for neurotransmitter release: Synaptotagmin and beyond. Nat Med 2013;19:1227–1231. [DOI] [PubMed] [Google Scholar]
- 33. Xing M. Molecular pathogenesis and mechanisms of thyroid cancer. Nat Rev Cancer 2013;13:184–199. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34. de Groot JW, Links TP, Plukker JT et al. RET as a diagnostic and therapeutic target in sporadic and hereditary endocrine tumors. Endocr Rev 2006;27:535–560. [DOI] [PubMed] [Google Scholar]
- 35. Cho YY, Lee MH, Lee CJ et al. RSK2 as a key regulator in human skin cancer. Carcinogenesis 2012;33:2529–2537. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36. Yoo SM, Cho SJ, Cho YY. Molecular targeting of ERKs/RSK2 signaling axis in cancer prevention. J Cancer Prev 2015;20:165–171. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37. Hynes RO. The extracellular matrix: Not just pretty fibrils. Science 2009;326:1216–1219. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38. Lu P, Weaver VM, Werb Z. The extracellular matrix: A dynamic niche in cancer progression. J Cell Biol 2012;196:395–406. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39. Pickup MW, Mouw JK, Weaver VM. The extracellular matrix modulates the hallmarks of cancer. EMBO Rep 2014;15:1243–1253. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40. Egeblad M, Werb Z. New functions for the matrix metalloproteinases in cancer progression. Nat Rev Cancer 2002;2:161–174. [DOI] [PubMed] [Google Scholar]
- 41. Liu Y, Cao X. Characteristics and significance of the pre‐metastatic niche. Cancer Cell 2016;30:668–681. [DOI] [PubMed] [Google Scholar]
- 42. Levental KR, Yu H, Kass L et al. Matrix crosslinking forces tumor progression by enhancing integrin signaling. Cell 2009;139:891–906. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43. Takeha S, Fujiyama Y, Bamba T et al. Stromal expression of MMP‐9 and urokinase receptor is inversely associated with liver metastasis and with infiltrating growth in human colorectal cancer: A novel approach from immune/inflammatory aspect. Jpn J Cancer Res 1997;88:72–81. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44. Prasad ML, Pellegata NS, Huang Y et al. Galectin‐3, fibronectin‐1, CITED‐1, HBME1 and cytokeratin‐19 immunohistochemistry is useful for the differential diagnosis of thyroid tumors. Mod Pathol 2005;18:48–57. [DOI] [PubMed] [Google Scholar]
- 45. Jain S, Watson MA, DeBenedetti MK et al. Expression profiles provide insights into early malignant potential and skeletal abnormalities in multiple endocrine neoplasia type 2b syndrome tumors. Cancer Res 2004;64:3907–3913. [DOI] [PubMed] [Google Scholar]
- 46. Saad MF, Ordonez NG, Guido JJ et al. The prognostic value of calcitonin immunostaining in medullary carcinoma of the thyroid. J Clin Endocrinol Metab 1984;59:850–856. [DOI] [PubMed] [Google Scholar]