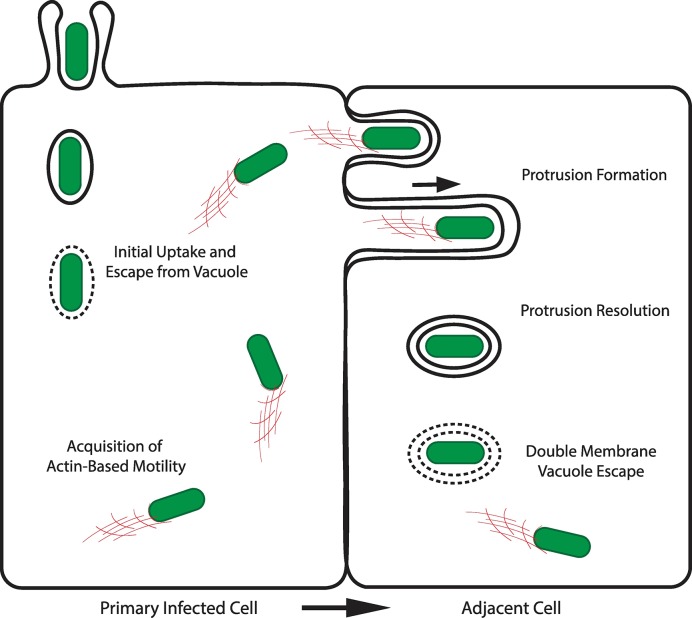
A subset of intracellular pathogens, including Listeria monocytogenes, Shigella flexneri, Rickettsia spp., and Burkholderia spp. disseminate within nonphagocytic cells, such as epithelial and endothelial cells, through a process referred to as cell-to-cell spread [1]. These pathogens utilize the host cell actin cytoskeleton to move in the cytosol of infected cells and project into adjacent cells through formation of membrane protrusions. The formed protrusions resolve into vacuoles from which the pathogen escapes, thereby gaining access to the cytosol of adjacent cells (Fig 1). Here, we present the general principles and summarize the underlying mechanisms supporting this bacterial dissemination process.
Fig 1. Intracellular bacterial spread from cell to cell.
Steps supporting the intracellular dissemination of Listeria monocytogenes [46]. Important variations in this process during Shigella flexneri, Rickettsia spp., and Burkholderia spp. dissemination are discussed in this review.
Step 1: Gaining access to the actin assembly machinery
Pathogenic bacteria gain access to the host cell actin assembly machinery through bacterial engulfment in membrane-bound compartments termed primary vacuoles from which the bacteria escape through secretion of bacterial factors that challenge the integrity of the vacuole membrane [2]. This invasion and vacuole escape process is a critical first step that grants the pathogens access to the cytosolic actin assembly machinery (Fig 1).
L. monocytogenes primary vacuole escape in human epithelial cells is facilitated by the production of the pore-forming toxin Listeriolysin O (LLO) [3] and phospholipases C (PlcA and PlcB) [4].
S. flexneri vacuole escape depends on the type-three secretion system (T3SS). Similar to L. monocytogenes LLO, the T3SS translocases, primarily IpaC, contribute to vacuole escape by forming pores in the vacuole membrane [5,6]. The host factor Rab11 has also been shown to contribute to S. flexneri vacuole escape [7]. The recruitment of Rab11 relies on the S. flexneri T3SS effector protein IpgD [7], suggesting that, in addition to the translocases, T3SS effector proteins are also involved in primary vacuole escape. Rickettsia spp. produce phospholipases that are implicated in vacuole escape; however, this mechanism is still unclear [8–11]. Burkholderia spp. escape vacuoles through the activity of the T3SS [12].
Step 2: Acquisition of actin-based motility
Once in the cytosol, intracellular bacteria spread from cell to cell by first acquiring actin-based motility (ABM). In uninfected cells, actin polymerization relies on actin nucleators and their cognate regulators [13]. In infected cells, bacteria display ABM by hijacking host cell actin nucleators or by expressing bacterial actin nucleators [14]. These factors are localized at the bacterial pole on the bacterial surface, resulting in polar actin polymerization that propels the bacteria throughout the cytosol (Fig 1). Pathogens have evolved bacterial factors that mimic the activity of all types of host cell actin nucleators and cognate regulators known to date.
In S. flexneri and L. monocytogenes, the actin-related protein ARP2/3 complex—a critical host cell actin nucleator—is recruited to the bacterial pole. S. flexneri secretes a bacterial autotransporter protein IcsA (also known as VirG), whose activity recruits the ARP2/3 nucleation-promoting factor Wiskott−Aldrich Syndrome protein (N-WASP), and consequently ARP2/3, to the bacterial pole [15,16].
By contrast, L. monocytogenes expresses a bacterial factor, ActA [17], that recruits ARP2/3 at the bacterial pole [18] through structural and regulatory mimicry of N-WASP [19–21] and activates the ARP2/3 complex directly.
Rickettsia spp. encode RickA, an N-WASP mimic that recruits ARP2/3 to the bacterial surface and induces ABM [22,23]. In addition, R. rickettsii produce Sca2, which is required for cell-to-cell spread and resembles actin nucleators of the formin family [24]. While RickA leads to ARP2/3-mediated nucleation of branched actin filaments, Sca2 catalyzes the processive nucleation at the barbed end, producing networks of long, bundled actin filaments [25]. RickA is implicated in ABM early after invasion, whereas Sca2 is proposed to be the primary nucleator later in infection [26].
Similarly to Listeria and Rickettsia, B. thailandensis BimA acts as a nucleator that activates Arp2/3. However, orthologs of BimA from pathogenic counterparts B. pseudomallei and B. mallei mimic actin nucleators of the host Ena/VASP family [27]. Similar to VASP, BimA oligomerizes and binds multiple filaments to increase their elongation rate by outcompeting capping proteins [27].
Step 3: Membrane protrusion formation
Cytosolic ABM allows intracellular pathogens to reach the plasma membrane at sites of cell−cell contacts, where they form membrane protrusions that project into adjacent cells (Fig 1). This process differs from ABM in the cytosol because (i) it requires countering tension at the plasma membrane and (ii) it occurs in a membrane-bound compartment that, as opposed to the cytosol, displays finite amounts of actin network components [28].
Step 3a: Reducing tension at cell−cell contacts
L. monocytogenes releases Tuba/N-WASP-mediated tension at cell−cell contacts by secreting Internalin C (InlC), which binds Tuba, thereby displacing N-WASP [29]. The R. parkeri effector protein Sca4 has been proposed to release tension by interfering with vinculin−α-catenin interactions, potentially creating unequal actomyosin tension at cell junctions and promoting bacterial spread. This mechanism has been shown to contribute to protrusion resolution [30]. How S. flexneri and Burkholderia spp. overcome membrane tension is unknown.
Step 3b: Protrusion elongation
In addition to the ARP2/3-dependent actin assembly machinery required for L. monocytogenes cytosolic ABM, membrane protrusion formation relies on the AIP1/CFL1-dependent disassembly machinery [28]. The disassembly of the distal actin network in membrane protrusions fuels the continuous actin assembly at the bacterial pole, a process termed local actin network recycling. Local recycling in a membrane-bound compartment is critical for efficient protrusion elongation [28]. Efficient membrane protrusion formation also requires host ERM family proteins [31] and formins [32], whose functions in protrusion are unknown.
It is presumed that, similar to cytosolic ABM, IcsA and N-WASP/Arp2-3 are responsible for actin polymerization in S. flexneri protrusions. In addition to ARP2/3, the host formins mDia1/2 localize to protrusions and are required for their proper formation [33]. Myosin-X also localizes to protrusions and was proposed to facilitate protrusion formation by bridging actin filaments and the plasma membrane [34].
Although Rickettsia spp. ABM relies on the actin cytoskeleton, it was recently observed that R. parkeri protrusions uniquely lack actin tails, suggesting that the R. parkeri protrusion formation may not rely on the forces generated by actin assembly [30]. This potentially actin-independent mechanism of protrusion formation remains to be elucidated.
Actin-containing membrane protrusions are formed during B. pseudomallei and B. thailandensis infection [12,35], but their exact contribution to the dissemination process remains unclear. It has been suggested that cell−cell fusion may support Burkholderia dissemination [12,35]. However, the potential contribution of membrane protrusions in the fusion process remains to be determined.
Step 4: Resolution of protrusions into vacuoles in adjacent cells
During bacterial spread from cell to cell, membrane protrusions resolve into double membrane vacuoles (DMVs), whose inner and outer membranes are contributed by the primary infected cell and the adjacent cell, respectively (Fig 1). The formation of DMVs is a multistep process that requires the disassembly of the actin network (when involved) and the scission of the inner and outer membranes. Although the mechanisms supporting the scission of protrusion membranes remain poorly understood, the bacterial and cellular factors supporting the remodeling of the actin network in protrusions have been recently uncovered.
In L. monocytogenes, the host AIP1/CFL1-dependent disassembly machinery is critical not only for the formation but also for the resolution of protrusions [28]. It was proposed that local actin network recycling in protrusions allows for the generation of membrane tension through efficient actin polymerization at the bacterial pole, as well as exhaustion of the actin network in the distal part of protrusions, where membrane scission occurs [28]. The bacterial metallo-protease Mpl has been suggested to facilitate the resolution process through maturation of the bacterial nucleation-promoting factor ActA, although the exact role of ActA processing remains unknown [36]. An additional mechanism of protrusion resolution involving LLO, phosphatidylserine, and receptor T-cell immunoglobulin and mucin-domain containing protein 4 (TIM-4) has been described in macrophages [37]; however, the implication of this mechanism in L. monocytogenes spread in epithelial cells is unclear.
In S. flexneri, protrusions are resolved in a two-step process. The collapse of the protrusion neck, presumably due to the disassembly of the actin cytoskeleton network, results in the formation of intermediate structures termed vacuole-like protrusions (VLPs) [38]. Formation of VLPs requires several cellular signaling events, including tyrosine kinase and phosphoinositide signaling [38,39]. The subsequent severing of the VLP membrane tether leads to vacuole formation. On the bacterial side, tyrosine kinase and phosphoinositide signaling-dependent resolution of protrusions requires the integrity of the bacterial T3SS [40], but the T3SS effector proteins potentially involved have yet to be elucidated.
As mentioned above, the Rickettsia effector protein Sca4 contributes to Rickettsia spp. protrusion resolution [41]. Although Burkholderia spp. have been observed in protrusions, they have not been observed in DMVs [12]; therefore, the mechanism of protrusion resolution is unknown.
Step 5: DMV escape
In order to resume ABM in the cytosol of adjacent cells, spreading bacterial pathogens must escape from the DMVs formed as a result of protrusion resolution. In contrast with primary vacuole escape, DMV escape is a complex process that requires the destabilization of two membranes (Fig 1).
L. monocytogenes accomplishes DMV escape by using pore-forming toxins and enzymes that challenge the integrity of the vacuole membranes [4]. Similar to primary vacuole escape, LLO and Plcs play seemingly complementary roles in DMV escape in human epithelial cells [42].
Because S. flexneri mutants lacking functional T3SS are trapped in DMVs, it was proposed that, similar to primary vacuole escape, the T3SS translocases may mediate DMV escape through pore formation [43]. In addition, the T3SS effector protein IcsB has recently been shown to be specifically required for effective DMV escape [44]. IcsB is an 18-carbon fatty acyltransferase that modifies several membrane-associated host proteins [45], although its exact function in DMV escape remains to be determined.
The mechanism supporting Rickettsia spp. DMV escape is poorly understood, and whether Burkholderia spp. forms DMVs altogether is unknown.
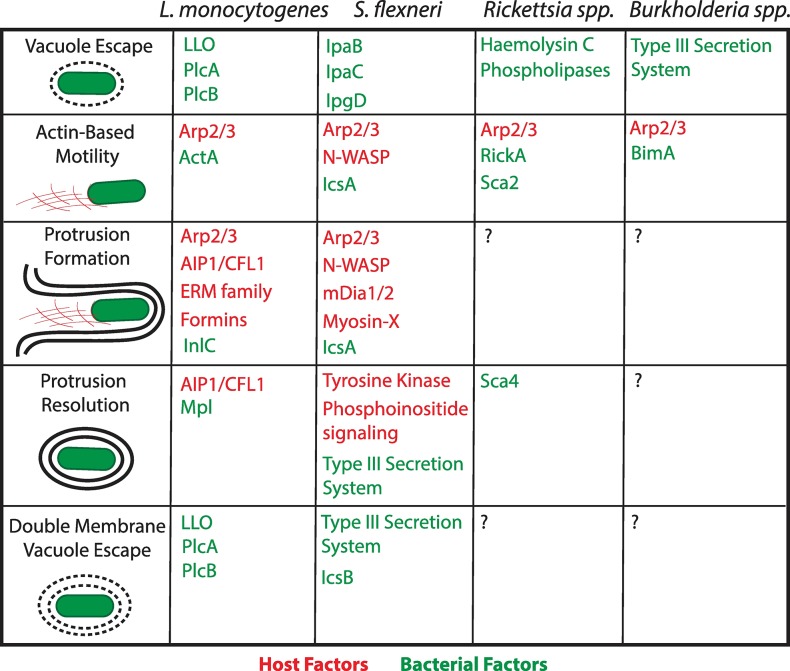
In conclusion and as shown in Fig 2, the host/pathogen interface supporting the first steps of bacterial dissemination, including cytosolic ABM, have been extensively investigated for L. monocytogenes and S. flexneri and are now fairly well understood for Rickettsia spp. and Burkholderia spp. By contrast, further investigation will be required to uncover the mechanisms supporting the formation and resolution of protrusions resolution and DMV escape for most pathogens that spread from cell to cell.
Fig 2. Host and bacterial factors that facilitate cell-to-cell spread.
For each step of spread, the key factors involved are shown for L. monocytogenes, S. flexneri, Rickettsia spp., and Burkholderia spp. Host factors are indicated in red and bacterial factors are indicated in green. ERM, Ezrin, Radixin, Moesin Family Proteins; LLO, Listeriolysin O; Plc, phospholipase.
Funding Statement
The work was supported by NIH grant AI073904. The funders had no role in study design, data collection and analysis, decision to publish, or preparation of the manuscript.
References
- 1.Kuehl CJ, Dragoi A-M, Talman A, Agaisse H. Bacterial spread from cell to cell: beyond actin-based motility. Trends Microbiol. 2015;23: 558–566. 10.1016/j.tim.2015.04.010 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Cossart P, Sansonetti PJ. Bacterial invasion: the paradigms of enteroinvasive pathogens. Science. 2004;304: 242–248. 10.1126/science.1090124 [DOI] [PubMed] [Google Scholar]
- 3.Gaillard JL, Berche P, Mounier J, Richard S, Sansonetti P. In vitro model of penetration and intracellular growth of Listeria monocytogenes in the human enterocyte-like cell line Caco-2. Infect Immun. 1987;55: 2822–2829. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Marquis H, Doshi V, Portnoy DA. The broad-range phospholipase C and a metalloprotease mediate listeriolysin O-independent escape of Listeria monocytogenes from a primary vacuole in human epithelial cells. Infect Immun. 1995;63: 4531–4534. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.High N, Mounier J, Prévost MC, Sansonetti PJ. IpaB of Shigella flexneri causes entry into epithelial cells and escape from the phagocytic vacuole. EMBO J. 1992;11: 1991–1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Du J, Reeves AZ, Klein JA, Twedt DJ, Knodler LA, Lesser CF. The type III secretion system apparatus determines the intracellular niche of bacterial pathogens. Proc Natl Acad Sci U S A. 2016;113: 4794–4799. 10.1073/pnas.1520699113 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Mellouk N, Weiner A, Aulner N, Schmitt C, Elbaum M, Shorte SL, et al. Shigella subverts the host recycling compartment to rupture its vacuole. Cell Host Microbe. 2014;16: 517–530. 10.1016/j.chom.2014.09.005 [DOI] [PubMed] [Google Scholar]
- 8.Silverman DJ, Santucci LA, Meyers N, Sekeyova Z. Penetration of host cells by Rickettsia rickettsii appears to be mediated by a phospholipase of rickettsial origin. Infect Immun. 1992;60: 2733–2740. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Whitworth T, Popov VL, Yu X-J, Walker DH, Bouyer DH. Expression of the Rickettsia prowazekii pld or tlyC Gene in Salmonella enterica Serovar Typhimurium Mediates Phagosomal Escape. Infect Immun. 2005;73: 6668–6673. 10.1128/IAI.73.10.6668-6673.2005 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Rahman MS, Gillespie JJ, Kaur SJ, Sears KT, Ceraul SM, Beier-Sexton M, et al. Rickettsia typhi Possesses Phospholipase A(2) Enzymes that Are Involved in Infection of Host Cells. Isberg RR, editor. PLoS Pathog. 2013;9: e1003399 10.1371/journal.ppat.1003399 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Driskell LO, Yu XJ, Zhang L, Liu Y, Popov VL, Walker DH, et al. Directed mutagenesis of the Rickettsia prowazekii pld gene encoding phospholipase D. Infect Immun. 2009;77: 3244–3248. 10.1128/IAI.00395-09 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.French CT, Toesca IJ, Wu T-H, Teslaa T, Beaty SM, Wong W, et al. Dissection of the Burkholderia intracellular life cycle using a photothermal nanoblade. Proc Natl Acad Sci U S A. 2011;108: 12095–12100. 10.1073/pnas.1107183108 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Rottner K, Faix J, Bogdan S, Linder S, Kerkhoff E. Actin assembly mechanisms at a glance. J Cell Sci. 2017;130: 3427–3435. 10.1242/jcs.206433 [DOI] [PubMed] [Google Scholar]
- 14.Choe JE, Welch MD, Welch M. Actin-based motility of bacterial pathogens: mechanistic diversity and its impact on virulence. 2016; 1–10. 10.1093/femspd/ftw099 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Bernardini ML, Mounier J, D’Hauteville H, Coquis-Rondon M, Sansonetti PJ. Identification of icsA, a plasmid locus of Shigella flexneri that governs bacterial intra- and intercellular spread through interaction with F-actin. Proc Natl Acad Sci U S A. 1989;86: 3867–3871. 10.1073/pnas.86.10.3867 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Makino S, Sasakawa C, Kamata K, Kurata T, Yoshikawa M. A genetic determinant required for continuous reinfection of adjacent cells on large plasmid in S. flexneri 2a. Cell. 1986;46: 551–555. [DOI] [PubMed] [Google Scholar]
- 17.Kocks C, Gouin E, Tabouret M, Berche P, Ohayon H, Cossart P. L. monocytogenes-induced actin assembly requires the actA gene product, a surface protein. Cell. 1992;68: 521–531. [DOI] [PubMed] [Google Scholar]
- 18.Welch MD, Rosenblatt J, Skoble J, Portnoy DA, Mitchison TJ. Interaction of Human Arp2/3 Complex and the Listeria monocytogenes ActA Protein in Actin Filament Nucleation. Science (80-). 1998;281: 105–108. [DOI] [PubMed] [Google Scholar]
- 19.Boujemaa-Paterski R, Gouin E, Hansen G, Samarin S, Le Clainche C, Didry D, et al. Listeria Protein ActA Mimics WASP Family Proteins: It Activates Filament Barbed End Branching by Arp2/3 Complex. Biochemistry. 2001;40: 11390–11404. 10.1021/bi010486b [DOI] [PubMed] [Google Scholar]
- 20.Skoble J, Portnoy DA, Welch MD. Three Regions within Acta Promote Arp2/3 Complex-Mediated Actin Nucleation and Listeria monocytogenes Motility. J Cell Biol. 2000;150: 527–538. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Chong R, Swiss R, Briones G, Stone KL, Gulcicek EE, Agaisse H. Regulatory mimicry in Listeria monocytogenes actin-based motility. Cell Host Microbe. 2009;6: 268–278. 10.1016/j.chom.2009.08.006 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Jeng RL, Goley ED, D'Alessio JA, Chaga OY, Svitkina TM, Borisy GG, Heinzen RA, Welch MD. A Rickettsia WASP-like protein activates the Arp2/3 complex and mediates actin-based motility. Cell Microbiol. 2004;6: 761–769. 10.1111/j.1462-5822.2004.00402.x [DOI] [PubMed] [Google Scholar]
- 23.Gouin E, Egile C, Dehoux P, Villiers V, Adams J, Gertler F, et al. The RickA protein of Rickettsia conorii activates the Arp2/3 complex. Nature. 2004;427: 457–461. 10.1038/nature02318 [DOI] [PubMed] [Google Scholar]
- 24.Kleba B, Clark TR, Lutter EI, Ellison DW, Hackstadt T. Disruption of the Rickettsia rickettsii Sca2 autotransporter inhibits actin-based motility. Infect Immun. 2010;78: 2240–2247. 10.1128/IAI.00100-10 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Haglund CM, Choe JE, Skau CT, Kovar DR, Welch MD. Rickettsia Sca2 is a bacterial formin-like mediator of actin-based motility. Nat Cell Biol. 2010;12: 1057–1063. 10.1038/ncb2109 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Reed SCO, Lamason RL, Risca VI, Abernathy E, Welch MD. Rickettsia actin-based motility occurs in distinct phases mediated by different actin nucleators. Curr Biol. 2014;24: 98–103. 10.1016/j.cub.2013.11.025 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Benanti EL, Nguyen CM, Welch MD. Virulent Burkholderia species mimic host actin polymerases to drive actin-based motility. Cell. 2015;161: 348–360. 10.1016/j.cell.2015.02.044 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Talman AM, Chong R, Chia J, Svitkina T, Agaisse H. Actin network disassembly powers dissemination of Listeria monocytogenes. J Cell Sci. 2014;127: 240–249. 10.1242/jcs.140038 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Rajabian T, Gavicherla B, Heisig M, Müller-Altrock S, Goebel W, Gray-Owen SD, et al. The bacterial virulence factor InlC perturbs apical cell junctions and promotes cell-cell spread of Listeria. Nat Cell Biol. 2009;11: 1212–1218. 10.1038/ncb1964 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Lamason RL, Bastounis E, Kafai NM, Serrano R, del Álamo JC, Theriot JA, et al. Rickettsia Sca4 Reduces Vinculin-Mediated Intercellular Tension to Promote Spread. Cell. 2016;167: 670–683.e10. 10.1016/j.cell.2016.09.023 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Pust S, Morrison H, Wehland J, Sechi AS, Herrlich P. Listeria monocytogenes exploits ERM protein functions to efficiently spread from cell to cell. EMBO J. 2005;24: 1287–1300. 10.1038/sj.emboj.7600595 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Fattouh R, Kwon H, Czuczman MA, Copeland JW, Pelletier L, Quinlan ME, et al. The Diaphanous-Related Formins Promote Protrusion Formation and Cell-to-Cell Spread of Listeria monocytogenes. J Infect Dis. 2015;211: 1185–1195. 10.1093/infdis/jiu546 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Heindl JE, Saran I, Yi C, Lesser CF, Goldberg MB. Requirement for Formin-Induced Actin Polymerization during Spread of Shigella flexneri. Infect Immun. 2010;78: 193–203. 10.1128/IAI.00252-09 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Bishai EA, Sidhu GS, Li W, Dhillon J, Bohil AB, Cheney RE, et al. Myosin-X facilitates Shigella-induced membrane protrusions and cell-to-cell spread. Cell Microbiol. 2013;15: 353–367. 10.1111/cmi.12051 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Kespichayawattana W, Rattanachetkul S, Wanun T, Utaisincharoen P, Sirisinha S. Burkholderia pseudomallei Induces Cell Fusion and Actin-Associated Membrane Protrusion: a Possible Mechanism for Cell-to-Cell Spreading. Infect Immun. 2000;68: 5377–5384. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Alvarez DE, Agaisse H. The Metalloprotease Mpl Supports Listeria monocytogenes Dissemination through Resolution of Membrane Protrusions into Vacuoles. Infect Immun. 2016;84: 1806–1814. 10.1128/IAI.00130-16 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Czuczman MA, Fattouh R, van Rijn JM, Canadien V, Osborne S, Muise AM, et al. Listeria monocytogenes exploits efferocytosis to promote cell-to-cell spread. Nature. 2014;509: 230–234. 10.1038/nature13168 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Dragoi AM, Agaisse H. The class II phosphatidylinositol 3-phosphate kinase PIK3C2A promotes Shigella flexneri dissemination through formation of vacuole-like protrusions. Infect Immun. 2015;83: 1695–1704. 10.1128/IAI.03138-14 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Dragoi AM, Agaisse H. The serine/threonine kinase STK11 promotes Shigella flexneri dissemination through establishment of cell-cell contacts competent for tyrosine kinase signaling. Infect Immun. 2014;82: 4447–4457. 10.1128/IAI.02078-14 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Kuehl CJ, Dragoi AM, Agaisse H. The shigella flexneri type 3 secretion system is required for tyrosine kinase-dependent protrusion resolution, and vacuole escape during bacterial dissemination. PLOS ONE. 2014;9 10.1371/journal.pone.0112738 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Lamason RL, Bastounis E, Kafai NM, Serrano R, del Álamo JC, Theriot JA, et al. Rickettsia Sca4 reduces vinculin-mediated intercellular tension to promote spread. Cell. 2016;167: 670–683.e10. 10.1016/j.cell.2016.09.023 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Grundling A, Gonzalez MD, Higgins DE. Requirement of the Listeria monocytogenes broad-range phospholipase PC-PLC during infection of human epithelial cells. J Bacteriol. 2003;185: 6295–6307. 10.1128/JB.185.21.6295-6307.2003 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Raymond S, Sandlin RC, Maurelli AT. A system for identifying post-invasion functions of invasion genes: requirements for the Mxi–Spa type III secretion pathway of Shigella flexneri in intercellular dissemination. 2002;34: 675–689. 10.1046/j.1365-2958.1999.01627.x [DOI] [PubMed] [Google Scholar]
- 44.Weddle E, Agaisse H. Spatial, temporal and functional assessment of LC3-dependent autophagy in Shigella flexneri dissemination. Infect Immun. 2018; 10.1128/IAI.00134-18 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Liu W, Zhou Y, Peng T, Zhou P, Ding X, Li Z, et al. Nε-fatty acylation of multiple membrane-associated proteins by Shigella IcsB effector to modulate host function. Nat Microbiol. 2018; 10.1038/s41564-018-0215-6 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Tilney LG, Portnoy DA. Actin filaments and the growth, movement, and spread of the intracellular bacterial parasite, Listeria monocytogenes. J Cell Biol. 1989;109: 1597 LP–1608. [DOI] [PMC free article] [PubMed] [Google Scholar]