Abstract
Physical exercise leads to beneficial effects in numerous tissues and organ systems and offers protection against obesity and type 2 diabetes. Recent studies have investigated the role of exercise on brown adipose tissue (BAT) and white adipose tissue (WAT), and have indicated marked adaptations to each tissue with exercise. Studies investigating the effects of exercise on BAT have produced conflicting results, with some showing an increase in the thermogenic activity of BAT and some demonstrating a decrease in the thermogenic activity of BAT. Human studies have observed a down-regulation of BAT activity (measured by a reduction in glucose uptake) in response to exercise. In WAT, exercise decreases adipocyte size, alters gene expression, and increases mitochondrial activity. Transplantation of exercise-trained subcutaneous WAT (scWAT) improves whole-body metabolic health. In rodents, exercise also results in a beiging of scWAT. Thus, exercise-induced changes to adipose tissue may be part of the mechanism by which exercise improves metabolic health.
Keywords: Exercise, BAT, Beige adipose tissue, Metabolism
1. Introduction
Physical activity causes numerous adaptations in the body resulting in beneficial effects on health. Exercise improves cardiovascular health [1] and can prevent or delay the onset of type 2 diabetes, obesity, and metabolic disease [2]. Physical exercise also improves metabolic health by enhancing insulin sensitivity, increasing glucose tolerance, and reducing circulating lipid concentrations [3–6], primarily through adaptations to skeletal muscle. The effects of exercise-induced adaptations to skeletal muscle, as well as the cardiovascular system, have been well-established [1,3–7]. More recently, studies have begun to examine exercise-induced adaptations on other tissues, including adipose tissue [8–10].
2. BAT, WAT, and beige adipocytes
There are three distinct types of adipose tissue in rodents and humans: brown adipose tissue (BAT), white adipose tissue (WAT), and beige adipocytes, which are found interspersed within WAT. Each adipose tissue depot or type of adipocyte has distinct morphological and physiological functions and, important with respect to this review, a specific response to exercise. Brown adipose tissue (BAT) is a thermogenic tissue that produces energy as heat. It is defined by a multilocular appearance, the presence of numerous mitochondria, and increased expression of uncoupling protein 1 (UCP1) [11]. Modulation of BAT metabolism influences whole body energy balance, and increased BAT activity counteracts obesity and diabetes [11–16].
Beige adipocytes are found interspersed within the WAT, particularly the scWAT, and have a multilocular morphology similar to brown adipocytes. Beige adipocytes express the distinct cell surface markers Tmem26 and Cd137 [22]. In rodents, beige cells are induced in response to various stimuli including cold exposure, β-adrenergic stimulation and exercise [22–25]. Upon stimulation, beige adipocytes have an increased capacity for fuel oxidation and thermogenesis [26]. Additionally, although beige adipocytes appear similar to brown adipocytes in many senses, they are derived from different lineages: brown adipocytes originate from a Myf5+ lineage while beige adipocytes are derived from a Myf5-lineage [22,26]. The different morphological and functional characteristics of each adipose tissue depot (brown, white, and beige) contribute to their distinctive response to exercise.
3. Potential mechanisms of BAT activation during exercise
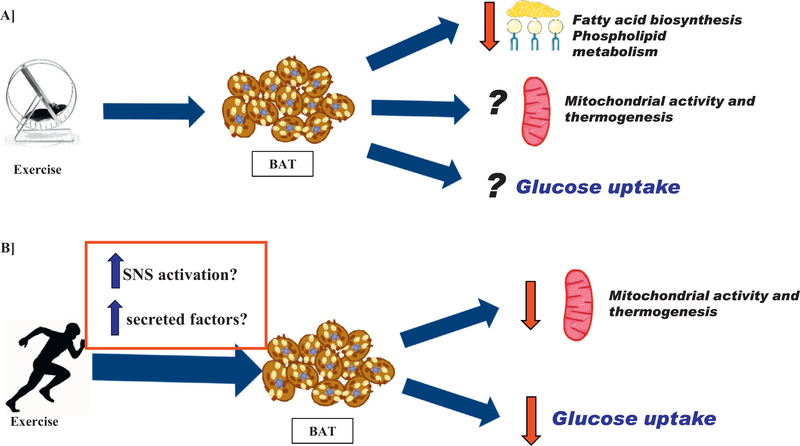
The effects of exercise to influence BAT activity are not yet well-defined (Fig. 1). BAT is a thermogenic tissue involved in heat production and energy expenditure [11]. Exercise is also a thermogenic activity, resulting in an increase in both muscle and core body temperature [27]. Since both an increase in BAT activity and exercise can raise core body temperature, it is not intuitive that exercise would increase the thermogenic role of BAT. In fact, it has been proposed that BAT is “hypoactive” during exercise [28]. While this argues against a role for BAT to be activated by exercise, BAT is mainly regulated by the sympathetic nervous system (SNS). SNS innervation is required in BAT to quickly release lipids for a fast energy supply, and rapidly activate thermogenesis [29]. Exercise also stimulates the SNS and corresponding catecholamine release. The duration and intensity of exercise are the predominant factors that stimulate the SNS and alter the catecholamine response to exercise [30]. Thus it is possible that exercise could stimulate BAT via increased SNS activation and in turn increase UCP1 expression and mitochondrial biogenesis [31].
Fig. 1.
Effects of exercise on A) mouse BAT and B) human BAT.
There are also mechanisms of BAT activation that are independent of the SNS, specifically a group of secreted factors that are increased during exercise. These factors include cardiac natriuretic peptides [32,33], interleukin-6 (IL-6) [34], and fibroblast growth factor 21 (FGF21) [35–37]. Cardiac natriuretic peptides are increased in response to acute exercise and then induce UCP1 expression, mitochondrial biogenesis, lipolysis, and uncoupled respiration in human adipocytes [32,33]. Exercise also promotes the release of IL-6 [34], and previous work in our laboratory has shown that an increase in circulating IL-6 can improve the metabolic activity of BAT [16]. FGF21 is increased in humans and rats with exercise [35–39], and an increase in circulating FGF21 levels are associated with increased BAT activity in male human subjects [40]. While it is possible that an exercise-induced increase in these secreted factors could influence BAT activity during exercise, to this point no investigation has made a direct link between these secreted factors and BAT activity during exercise.
4. Effects of exercise on BAT
To determine the effects of exercise on BAT, studies have investigated BAT mitochondrial activity and gene expression, BAT glucose uptake, the lipidome of BAT, and thermogenesis in BAT after acute and chronic exercise, all with varying results. Some rodent studies have identified increased mitochondrial activity and thermogenesis in rodent BAT with exercise [8,25,41], while others have shown decreased mitochondrial gene expression and thermogenesis [42,43]. In humans, studies have shown that exercise decreases glucose uptake in BAT [43,44].
4.1. Effects of exercise on the thermogenic activity of BAT
Several studies have investigated the effects of various modalities of exercise (swimming, forced treadmill exercise, and voluntary wheel running) on the thermogenic activity of BAT in rodetns [41,45–50]. Six to eight weeks of swim training in rodents increased UCP1 protein in BAT [47], enhanced blood flow to BAT [46], and upregulated type 2 deiodinase (dio2) enzymatic activity and mitochondrial respiration in BAT [41]. Moderate intensity treadmill running of rodents for 6–8 wks also increased BAT activity, measured by an increase in cytochrome oxidase activity and oxygen consumption rates [48], as well as an upregulation of BAT specific gene markers including Ucp1, Dio2, Prdm16, and Pgc1α [48–50]. These data suggest that exercise training in rodents can increase mitochondrial biogenesis and activity in BAT.
In contrast, other studies have indicated that exercise decreased the thermogenic effect of BAT [42,45,51]. Six to eight wks of moderate intensity treadmill exercise in rats decreased UCP1 expression in BAT and total BAT mass [42,45]. One of these studies determined a decrease in PGC1α protein expression and a “whitening” of the BAT, or increase in lipid droplets, and a decrease in fatty acid oxidation in BAT [42]. However, the second study determined that exercise increased PGC1α protein expression, decreased the brown adipocyte size, and increased sympathetic tone and vascularization of the BAT [45]. The reason for the discrepancies in the data is unclear, but both studies indicated that moderate intensity exercise decreased the thermogenic activity of BAT. A third study showed that 6 wks of moderate intensity treadmill exercise resulted in no change in BAT mitochondrial gene expression or thermogenic activity [52].
The reasons for the discrepancies in the thermogenic response of BAT to exercise have not been thoroughly investigated. With regard to swimming, it is possible that UCP1 is increased as a response to the environment activating a thermogenic response instead of an exercise adaptation. The difference in UCP1 in BAT in moderate intensity treadmill exercise compared to voluntary wheel running could be related to the amount of time spent running. Treadmill running is typically limited to an hour per day while mice given open access to wheel cages may run for a longer period of time, particularly during the dark cycle. This continuous running could prompt a continuous supply of substrate to fuel BAT [53], which could be a potential reason for the increase in UCP1 with voluntary wheel running [54,55]. It is also possible that exercise is not affecting UCP1 but a difference in protein appears because exercise decreases BAT mass [56].
4.2. Effects of exercise on glucose uptake in BAT
When activated by cold, both the thermogenic activity of BAT and the ability of BAT to take up glucose increases [11–16,57,58]. Previous studies in mice have demonstrated that increasing the amount of BAT by transplantation improved whole-body metabolic health and increased insulin-stimulated glucose uptake [16,58]. Measuring glucose uptake by PET/CT scan with [18F] Fluorodeoxyglucose (FDG) is the most common and well-established measurement for BAT activity, and studies investigating the effects of exercise on BAT activity in human subjects have used this measurement as their primary outcome.
Two recent studies have investigated the effects of exercise-training on BAT using [18F]FDG-PET-CT as the measurement for BAT activity. In the first study, age and BMI matched male subjects who were either sedentary or endurance athletes underwent 2 h of mild cold exposure to determine BAT activity [43]. Cold-stimulated BAT activity was significantly decreased in endurance-trained subjects compared to their sedentary controls, suggesting that endurance training decreases the metabolic activity of BAT in humans [43]. A second study examined the effects of short-term exercise training, both high-intensity interval training and moderate-intensity continuous training in healthy, sedentary, male subjects [44]. Basal BAT activity was measured and subjects were subdivided into those that had high or low basal BAT activity. Exercise training decreased insulin-stimulated glucose uptake in BAT in subjects who had highly active BAT prior to the exercise intervention. Interestingly, there was no change in insulin-stimulated glucose uptake in BAT in subjects who had low BAT activity prior to the exercise intervention [44]. It is important to note that these studies have been performed only in male subjects. Female subjects have higher BAT activity than males [12], and it is possible that exercise could have a different response on BAT in female subjects.
Together these data suggest that endurance exercise, high intensity exercise, and moderate intensity exercise decrease the ability of BAT to take up glucose in human subjects. This is not to say that there are no exercise-induced adaptations to BAT, and while glucose uptake via PET/CT is an important measurement, it might not be the best mechanism to assess the effects of exercise on BAT. To this point, exercise-induced adaptations to the endocrine activity of BAT have not been investigated. Additionally, incorporating new techniques that use MRI or fMRI to measure activity, volume, and blood flow in BAT in human exercise studies will allow greater insight into exercise-induced adaptations to BAT [44,59–61].
4.3. Effects of exercise on the lipid profile of BAT in mice
Recent work in our laboratory has investigated the effects of exercise on the BAT lipidome in mice [62]. Three weeks of voluntary wheel cage running significantly decreased the abundance of TAGs, phosphatidylcholines (PC) and cholesterol esters (CE), and increased specific molecular species of PC and phosphatidylethanolamines (PE) in BAT. Exercise decreased expression of genes involved in phospholipid metabolism (Agpat3, Gpd1, Lgpat1, Ptdss2 and Pld1) and fatty acid biosynthesis (Acaca, Scd1, Agpat3, Dgkd and Mlxipl). The decrease in genes involved in fatty acid biosynthesis corresponded to a decrease in overall TAGs in BAT. These data indicate that exercise causes significant adaptations to the BAT lipidome, although the physiological effects of these changes on insulin sensitivity and glucose tolerance are still topics of investigation.
5. Exercise-induced adaptations to WAT
Exercise-training results in several well-established adaptations to white adipose tissue (WAT). While some adaptations, such as decreased adipocyte size, increased mitochondrial activity [6,10,63,64], altered secretion of adipokines [65–69], and striking changes in gene expression [6] occur in both visceral (vWAT) and subcutaneous (scWAT) WAT, there are also several exercise-induced adaptations to WAT that are specific to either the vWAT or scWAT depot. For the purpose of this review, we will focus on exercise-induced adaptations specifically to the scWAT (Fig. 2).
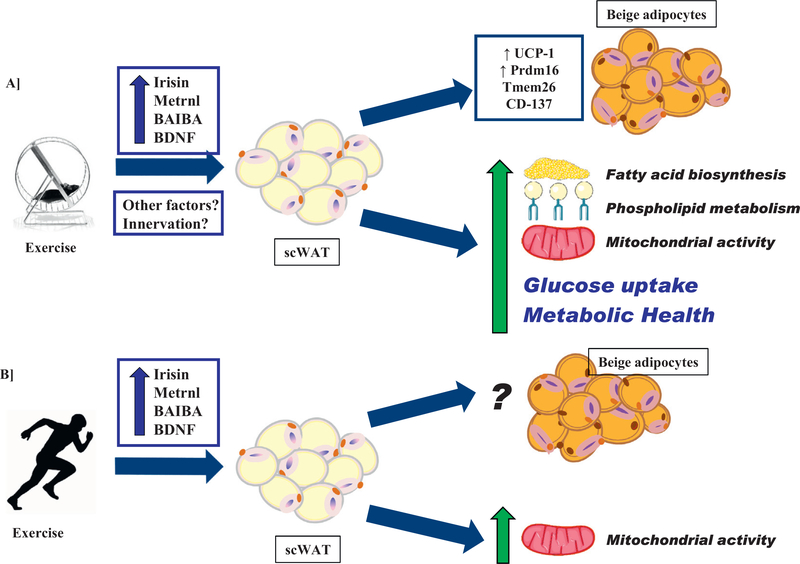
Fig. 2.
Effects of exercise on A) mouse scWAT and B) human scWAT.
6. Exercise alters the gene expression profile of scWAT
Recently, we investigated the effects of 11 days of exercise training on the gene expression profile of scWAT [6]. The purpose of this experiment was to determine the degree of plasticity of scWAT in response to exercise. We compared the scWAT of 10 wk. old male, C57BL/6 mice that were either sedentary or exercise-trained using voluntary wheel running paradigm and determined that 11 days of exercise significantly increased the expression of 1844 genes and decreased the expression of 1156 genes. Gene set enrichment analysis (P < 0.05 and Q < 0.25) showed that exercise training increased gene pathways involved in glucose metabolism, fatty acid oxidation, oxidative stress and signaling, mitochondrial biogenesis, membrane transport, cell stress, and apoptosis. Genes involved in beiging, Wnt signaling, and Pgc1α-related pathways were also significantly increased with exercise in scWAT. Importantly, the number of genes that were upregulated by exercise in scWAT was signficantly greater than the number of genes increased in skeletal muscle with exercise [70–72]. This degree of plasticity indicates that exercise has a marked effect on scWAT.
7. Exercise increases mitochondrial activity and expression ofmitochondrial genes in scWAT
Numerous studies have determined that mitochondrial activity is increased in both rodent and human scWAT with exercise [6,42,64,73–75]. In rodents, work in our laboratory determined an increase in mitochondrial activity by measuring basal oxygen consumption rate and mitochondrial gene expression in scWAT after 11 days of voluntary wheel cage running [6]. Other studies have investigated the effects of 4–8 wks of swim training on mitochondrial gene expression and observed an increase in expression of the mitochondrial marker Pgc1α in scWAT [64,75]. Eight wks of treadmill training in rats also increased in Pgc1α in scWAT [42]. Together these studies demonstrate that exercise training has marked effects on mitochondrial gene expression and activity in scWAT. The changes in mitochondrial gene expression in scWAT occurred in response to various durations (11 days to 9 wks) and modalities (swimming, treadmill running, voluntary wheel cage running) of exercise.
In humans, studies have also determined an increase in mitochondrial gene expression in scWAT in response to both mild and intense aerobic exercise. A 6 month intervention of mild exercise (3 h of aerobic exercise per week) in healthy, previously sedentary men, increased genes involved in oxidative phosphorylation in scWAT [73]. An intensive 4 wk exercise intervention (1 h, 3 times per week) in sedentary men and women who had either normal glucose tolerance, impaired glucose tolerance, or were diabetic (type 2), also significantly increased PGC1α in scWAT [74]. This is of particular importance because these data indicate that exercise can increase mitochondrial gene expression in both normoglycemic and type 2 diabetic subjects, providing more evidence for exercise as a therapeutic tool to improve metabolic health.
8. Exercise and the ‘beiging’ of white adipose tissue
One adaptation that has been the focus of several recent investigations is the increased ‘beiging’ of scWAT in rodents that occurs with exercise. Rodent studies investigating various modalities (voluntary wheel running, forced treadmill running, and swimming) and durations (11–63 days) of exercise have each identified a significant increase in beiging of scWAT, measured by upregulation of brown and beige adipocyte markers (Ucp1, Prdm16, Cidea, Elovl3, Pgc1α, Pparγ, Cox8b, Dio2, and otopetrin), increased UCP1 immunofluorescence, and the increased presence of multilocular cells [6,63,75–77].
While all modalities and intensities of exercise increased the beiging of scWAT, the type and duration of exercise training do influence the extent of beiging. Multiple studies determined that voluntary wheel running consistently increased Ucp1 expression in scWAT. Interestingly, exercise durations of < 3 wks had the most significant fold change in Ucp1 expression in exercise-trained compared to sedentary mice. Voluntary wheel running exercise paradigms ranging from 11 to 21 days in male C57BL/6 mice resulted in a ~25–30 fold increase in Ucp1 in scWAT [6,76]. Ucp1 was increased in studies that used voluntary wheel running over a longer period of time but not to the same extent; 28 days of voluntary wheel cage running resulted in an ~8 fold increase in Ucp1 in scWAT [77], and 63 days of voluntary wheel cage running increased Ucp1 ~5 fold in scWAT [77]. Moderate intensity treadmill running for 5–8 wks resulted in a ~2 fold increase in Ucp1 gene expression [78] and a ~6–8 fold increase in UCP1 protein in scWAT [42,78]. Swimming also increased Ucp1 expression in scWAT; in one study Ucp1 expression was increased ~60 fold after 21 days of swimming [76], and another study determined a ~2 fold increase in Ucp1 in scWAT after 30 days of swimming. These studies indicate that it isn’t the type or intensity of exercise that influences the change in Ucp1 expression, but the duration; exercise training paradigms < 3 wks in duration resulted in the greatest increases in Ucp1 in scWAT. The reason for this is not clear; it is possible that exercise-induced lipolysis is stimulated 3 wks post exercise, or that innervation to the scWAT is increased at this time point, decreasing adipocyte size and increasing the number of multilocular droplets. Full longitudinal studies examining the extent of beiging and how that corresponds to glucose metabolism and mitochondrial gene expression over time have not been performed and are necessary to fully determine the optimal duration and intensity of exercise required to maximize the beneficial effects of exercise on scWAT.
8.1. Mechanisms of exercise-induced beiging of scWAT
The mechanism behind the exercise-induced beiging of scWAT is an important topic of investigation. Non-exercise stimuli that induce beiging, including cold exposure, environmental factors, or pharmaceutical agents, typically do so through increased heat loss and compensatory adrenergic stimulation. Increased heat loss results in a greater thermogenic demand, leading to increased sympathetic tone and UCP1 expression to increase heat production [79]. While this is a well-established mechanism with regard to cold-induced beiging of scWAT, exercise likely does not work through this mechanism because exercise increases heat production [27].
There are multiple hypotheses that have been brought forth to explain the mechanisms responsible for the exercise-induced increase in beiging of rodent scWAT. Exercise decreases adipocyte size and lipid content in scWAT, which reduces body insulation and could result in the need for increased heat production [10,25,79,80]. It has also been hypothesized that an exercise-induced increase in sympathatic innervation causes the beiging of scWAT [79,81] or that exercise-induced adaptations to other peripheral tissues, in particular skeletal muscle, results in the beiging of scWAT. Studies have shown that beiging occurs in response to increased secretion of the hypothalamic brain-derived neurotrophic factor (BDNF) during exercise [77] as well as myokines including irisin [76], meteorin-like 1 (Metrnl) [82], myostatin [83], lactate [84], and β-aminoisobutyric acid (BAIBA) [85], which are released from skeletal muscle during exercise. Each of these hypotheses is intriguing and plausible, and further investigation is needed to fully elucidate the mechanisms responsible for the exercise-induced beiging of scWAT.
8.2. Transplantation of an exercise-induced ‘beige’ tissue improves metabolic health
Regardless of the mechanism responsible, it is clear that exercise induces a beiging of scWAT in rodents. Since beiging results in more metabolically active cells [24], previous work in our laboratory investigated if transplantation of this more metabolically active, exercise-trained scWAT would improve whole-body metabolism [6]. To address this hypothesis, sedentary recipient mice were transplanted with scWAT from exercise-trained mice (mice that had undergone 11 days of exercise-training by voluntary wheel running). Control mice were transplanted with scWAT from sedentary mice or were sham operated. There was no effect of transplantation of exercise-trained scWAT on body weight, food intake, energy expenditure, or activity. At 9 days post-transplantation there was a significant improvement in glucose tolerance in mice transplanted with exercise-trained scWAT compared with both sham-operated mice and mice transplanted with sedentary scWAT. Mice transplanted with exercise-trained scWAT also had a decrease in fasting blood glucose, insulin, and cholesterol concentrations. This improvement in glucose tolerance was transient; there was no effect of transplanting exercise-trained vs. sedentary scWAT on glucose tolerance 4 wks after transplantation.
A previous study examined the effects of transplanting scWAT or vWAT from sedentary donor mice into the subcutaneous or visceral cavity of recipient mice to distinguish if the differences in metabolic function of scWAT and vWAT were due to anatomic location or intrinsic differences of these two adipose tissue depots [86]. This study showed that mice transplanted with vWAT into either the visceral or subcutaneous cavity had no improvements in glucose metabolism or metabolic health. Mice transplanted with scWAT into the visceral cavity, however, had decreased body weight and fat mass, increased insulin sensitivity, and decreased circulating glucose and insulin concentrations 12 wks post-transplantation compared to sham-operated mice. Mice transplanted with scWAT into the subcutaneous cavity also had decreased body weight and increased insulin sensitivity compared to sham-operated mice, but not to the same extent as the mice transplanted with scWAT into the visceral cavity [86]. These findings indicated that both the type of adipose tissue and the location of the adipose tissue transplantation are significant in determining the effect on glucose metabolism in recipient mice.
We then investigated if the beneficial effects of transplanting scWAT from exercise-trained mice were specific to the type adipose tissue transplanted (scWAT) or the location of the adipose tissue transplant (into the visceral cavity). To address this, we transplanted sedentary and exercise-trained vWAT (from the perigonadal WAT depot) into recipient mice and measured glucose tolerance 9 days post transplantation. There was no difference in glucose tolerance among sham-operated mice, mice that received sedentary vWAT, or mice that received exercise-trained vWAT [6].
To determine if the effects of transplanting scWAT from exercise-trained mice were specific to the location of the adipose tissue transplantation, scWAT from sedentary and exercise-trained mice was transplanted into the subcutaneous cavity (directly atop the inguinal adipose tissue). Nine days post-transplantation, mice transplanted with exercise-trained scWAT into the subcutaneous cavity had a significant improvement in glucose tolerance compared with sham-operated mice, but not improved to the same extent as mice receiving exercise-trained scWAT into the visceral cavity [6]. These studies, taken together, indicate that the type of adipose tissue transplanted, the location of the transplant, and whether the transplanted tissue came from a sedentary or exercise-trained mouse are all important factors in determining the effect of the transplant on the metabolic health of the recipient mice. Transplantation of scWAT into the visceral cavity resulted in the strongest improvements in the recipient mice.
8.3. Transplantation of exercise-trained scWAT increases glucose uptake inskeletal muscle, BAT
To determine which tissue was responsible for the increase in glucose uptake in the mice that were transplanted with exercise-trained scWAT we measured insulin-stimulated glucose uptake in vivo and performed pyruvate tolerance tests to determine the contribution of the liver. Our results showed that glucose uptake was increased in the oxidative skeletal muscles and BAT in mice transplanted with exercise-trained scWAT, while pyruvate tolerance was improved in mice receiving scWAT from both sedentary and exercise-trained donors. Since transplantation of scWAT improved liver function, but only transplantation of scWAT from exercise-trained mice improved glucose tolerance, the enhanced glucose tolerance is a result of improved peripheral insulin sensitivity, particularly in the skeletal muscle and BAT, and not of altered liver glucose metabolism. It is likely that the effects on peripheral tissues are mediated through an endocrine-related mechanism [6]. These endocrine effects are likely mediated by the release of adipokines that are unique to exercise-trained scWAT. While the specific adipokine(s) secreted from trained scWAT that could influence skeletal muscle and BAT glucose uptake have not been identified, microarray analysis revealed several putative secreted proteins that were significantly increased in scWAT from exercise-trained mice [6].
These exciting data demonstrate that transplantation of exercise-trained scWAT dramatically improves glucose metabolism in the recipient mice in the short-term. It is not clear, however, whether the beneficial effects of transplanting exercise-trained scWAT is a result of transplanting a ‘beige’ tissue, or transplanting scWAT that has undergone exercise-training and has an altered adipokine profile. Future investigations will parse apart these two components and determine if there are beneficial effects of exercise-training independent of beiging that improve whole-body metabolic health.
8.4. Lipidomic profile of an exercise-induced ‘beige’ tissue
Several recent studies have investigated the effects of lipids on metabolic health and determined that lipids, released from adipose tissue, can act as signaling molecules and influence glucose and fatty acid metabolism [87–89]. Recent work in our laboratory investigated the effects of exercise on the structural lipid profile of scWAT [62]. Three wks of exercise resulted in a significant decrease in the overall abundance of phosphatidylserines (PS), lysophosphatidylglycerols (LPG), lysophosphatidylinositols (LPI), and triacylglycerols (TAG). There were also numerous decreases in specific molecular species of phosphatidic acid (PA), phosphatidylethanolamines (PE), and PS in scWAT. Genes involved in phospholipid metabolism (Agpat3, Gpd1, Pla2g12a, Gpam, Ipla2g) were also significantly up-regulated in response to exercise. The overall abundance of TAGs was decreased, while the genes regulating fatty acid biosynthesis and elongation (Evol3, Evol4, Acaca, Gpam, Agpat3 and Pparα) were upregulated in scWAT with exercise. Together these data suggest molecular species specific remodeling of phospholipids and TAGs in scWAT in response to exercise. The decrease in overall TAGs in scWAT, combined with the upregulation of genes that code for fatty acid biosynthesis and elongation suggest that scWAT could be working to create a fuel source for the working muscle during exercise [62]. To this point, it is not clear if these adaptations are specific to exercise or the result of an increased beiging in scWAT. Investigations examining the effects of cold-exposure on the lipidome of scWAT will allow insight into this question. Future studies are also necessary to determine the physiological consequences of the exercise-induced changes to the lipidome of scWAT and if they contribute to insulin sensitivity or other metabolic changes seen with exercise.
8.5. Exercise-induced beiging of human scWAT
The exercise-induced beiging of rodent scWAT has been very well-established, but the exercise-induced beiging of human scWAT is less clear [43,90]. Several studies have investigated the expression of myokines known to cause beiging of rodent scWAT and have identified several that are increased with exercise in humans, including irisin [76,91–93], Metrnl [82], and BAIBA [85]. However, in each of these studies, beiging of human scWAT (i.e., presence of multilocular droplets or UCP1 expression) has either not been increased or has not been measured [76,91–93].
One study examined the effects of a 12 wk. combined strength and endurance exercise intervention on scWAT of normoglycemic and pre-diabetic subjects and saw no change in UCP1, PRDM16, TBX1, TMEM26, or CD137 expression [90]. A study comparing scWAT from sedentary and endurance exercise-trained BMI-matched subjects observed no difference in UCP1 expression among groups [43]. While it is clear that there are numerous exercise-induced adaptations to human scWAT [10], further investigation is necessary to establish if exercise results in the beiging of human scWAT.
8.6. Physiological consequences of the exercise-induced ‘beiging’ of scWAT
Since it is unclear if exercise induces a beiging of human scWAT, the question arises: What is the physiological consequence of the exerciseinduced beiging of scWAT? The beiging of scWAT during exercise is counterintuitive; exercising skeletal muscles generate heat [27] so why would exercise induce a thermogenic, heat-producing beige adipocyte? There are several potential hypotheses put forth to explain why an increase in beiging during exercise could be beneficial. First, in most animal studies, rodents are housed below thermoneutral temperatures (> 30 °C), which could induce a thermal stress and the resulting increase in UCP1 may be a mechanism to compensate for changes in insulation and total fat mass after exercise [54,79]. Other studies have indicated that UCP1 may decrease the effects of lipid-induced reactive oxygen species (ROS) [94,95]; because exercise can increase ROS [96], it is possible that the increase in UCP1 maybe be an adaptive mechanism to reduce the detrimental effects of ROS [54].
Our study, discussed above, hypothesized that increasing a ‘beige’ exercise-trained scWAT depot by transplantation would improve glucose tolerance into recipient mice and our data demonstrated that transplanting an exercise-trained ‘beige’ scWAT depot improved whole-body glucose tolerance and glucose uptake into the skeletal muscle and BAT depots. In contrast to this, a recent study indicated that scWAT could be dispensable for the metabolic health benefits of mice after exercise. Mice that had scWAT surgically removed still exhibited wholebody metabolic benefits of exercise including improved glucose tolerance and increased skeletal muscle mitochondrial gene expression [54]. These data are intriguing in a mouse model, but it is unclear how to interpret these data in a clinical setting since a human subject would not likely have a similar complete loss of scWAT mass.
Another potential explanation is that exercise itself may result in the loss of adipose tissue mass, thus decreasing insulation and requiring heat production, resulting in the beiging of scWAT [25,79]. This hypothesis is of potential evolutionary significance; foraging animals were likely required to travel great distances to search for food and needed plasticity in their adipose tissue to maintain body temperature and thus the beiging of white adipocytes would be evolutionarily advantageous [97]. Together each of these explanations is a plausible physiological reason for why exercise increases the beiging of scWAT in a rodent model, but they are not a likely explanation for an exercise-induced beiging in humans. It is important to note, as discussed above, that more investigation is necessary to fully delineate the effects of exercise in human subjects particularly since there is limited information on how exercise influences various adipose tissue depots in humans. Future work is needed to understand how exercise alters adipose tissue, if an exercise-stimulated increase in beiging is required to determine the metabolic benefits of exercise on scWAT, and to determine how exercise-induced adaptations to adipose tissue effect whole-body metabolic health.
9. Summary
Exercise results in profound adaptations to multiple tissues, including adipose tissue. Exercise decreases mitochondrial gene expression and glucose uptake in BAT in rodents and humans and alters the lipidomic profile of BAT in rodents. In WAT, exercise-induced adaptations include increased mitochondrial biogenesis and gene expression, alterations in the lipidome of scWAT, and an increased beiging of scWAT in rodents. Still, there are a number of unanswered questions. Two essential questions are whether exercise induces the beiging of scWAT in humans, and determining why scWAT beiges in response to exercise. Another important question is if measuring glucose uptake in BAT is the best determinant for BAT activity in response to exercise. More investigation is needed to fully elucidate exercise-induced metabolic adaptations to adipose tissue, and to use this information to identify novel therapeutic targets for obesity, type 2 diabetes, and other metabolic diseases.
Supplementary Material
Acknowledgements
This work was supported by National Institutes of Health Grant K01-DK-105109 and R01-HL-138738 (to K.I.S.).
Footnotes
The authors have no conflict of interest.
This article is part of a Special Issue entitled Brown and Beige Fat: From Molecules to Physiology Guest Editor: Paul Cohen
Transparency document
The Transparency document associated this article can be found, in online version.
References
- [1].Myers J, Exercise and cardiovascular health, Circulation 107 (1) (2003) e2–e5. [DOI] [PubMed] [Google Scholar]
- [2].Crandall JP, et al. , The prevention of type 2 diabetes, Nat. Clin. Pract. Endocrinol. Metab 4 (7) (2008) 382–393. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [3].Bonadonna RC, et al. , Transmembrane glucose transport in skeletal muscle of patients with non-insulin-dependent diabetes, J. Clin. Invest 92 (1) (1993) 486–494. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [4].Joyner MJ, Green DJ, Exercise protects the cardiovascular system: effects beyond traditional risk factors, J. Physiol. 587 (Pt 23) (2009) 5551–5558. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [5].Goodyear LJ, Kahn BB, Exercise, glucose transport, and insulin sensitivity, Annu. Rev. Med 49 (1998) 235–261. [DOI] [PubMed] [Google Scholar]
- [6].Stanford KI, et al. , A novel role for subcutaneous adipose tissue in exercise-induced improvements in glucose homeostasis, Diabetes 64 (6) (2015) 2002–2014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [7].Holloszy JO, Coyle EF, Adaptations of skeletal muscle to endurance exercise and their metabolic consequences, J. Appl. Physiol. Respir. Environ. Exerc. Physiol 56 (4) (1984) 831–838. [DOI] [PubMed] [Google Scholar]
- [8].Yoshioka K, et al. , Effects of exercise training on brown adipose tissue thermogenesis in ovariectomized obese rats, Endocrinol. Jpn 36 (3) (1989) 403–408. [DOI] [PubMed] [Google Scholar]
- [9].Xu X, et al. , Exercise ameliorates high-fat diet-induced metabolic and vascular dysfunction, and increases adipocyte progenitor cell population in brown adipose tissue, Am. J. Phys. Regul. Integr. Comp. Phys 300 (5) (2011) R1115–25. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [10].Stanford KI, Middelbeek RJ, Goodyear LJ, Exercise effects on white adipose tissue: beiging and metabolic adaptations, Diabetes 64 (7) (2015) 2361–2368. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [11].Rothwell NJ, Stock MJ, Effects of age on diet-induced thermogenesis and brown adipose tissue metabolism in the rat, Int. J. Obes 7 (6) (1983) 583–589. [PubMed] [Google Scholar]
- [12].van Marken Lichtenbelt WD, et al. , Cold-activated brown adipose tissue in healthy men, N. Engl. J. Med 360 (15) (2009) 1500–1508. [DOI] [PubMed] [Google Scholar]
- [13].Lowell BB, et al. , Development of obesity in transgenic mice after genetic ablation of brown adipose tissue, Nature 366 (6457) (1993) 740–742. [DOI] [PubMed] [Google Scholar]
- [14].Bartelt A, et al. , Brown adipose tissue activity controls triglyceride clearance, Nat. Med 17 (2) (2011) 200–205. [DOI] [PubMed] [Google Scholar]
- [15].Guerra C, et al. , Emergence of brown adipocytes in white fat in mice is under genetic control. Effects on body weight and adiposity, J. Clin. Invest 102 (2) (1998) 412–420. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [16].Stanford KI, et al. , Brown adipose tissue regulates glucose homeostasis and insulin sensitivity, J. Clin. Invest. 123 (1) (2013) 215–223. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [17].Tran TT, Kahn CR, Transplantation of adipose tissue and stem cells: role in metabolism and disease, Nat. Rev. Endocrinol 6 (4) (2010) 195–213. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [18].Wang Y, et al. , Comparison of abdominal adiposity and overall obesity in predicting risk of type 2 diabetes among men, Am. J. Clin. Nutr 81 (3) (2005) 555–563. [DOI] [PubMed] [Google Scholar]
- [19].Carey VJ, et al. , Body fat distribution and risk of non-insulin-dependent diabetes mellitus in women. The nurses’ health study, Am. J. Epidemiol 145 (7) (1997) 614–619. [DOI] [PubMed] [Google Scholar]
- [20].Misra A, et al. , Relationship of anterior and posterior subcutaneous abdominal fat to insulin sensitivity in nondiabetic men, Obes. Res 5 (2) (1997) 93–99. [DOI] [PubMed] [Google Scholar]
- [21].Snijder MB, et al. , Associations of hip and thigh circumferences independent of waist circumference with the incidence of type 2 diabetes: the Hoorn study, Am. J. Clin. Nutr 77 (5) (2003) 1192–1197. [DOI] [PubMed] [Google Scholar]
- [22].Wu J, et al. , Beige adipocytes are a distinct type of thermogenic fat cell in mouse and human, Cell 150 (2) (2012) 366–376. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [23].Petrovic N, et al. , Chronic peroxisome proliferator-activated receptor gamma (PPARgamma) activation of epididymally derived white adipocyte cultures reveals a population of thermogenically competent, UCP1-containing adipocytes molecularly distinct from classic brown adipocytes, J. Biol. Chem 285 (10) (2010) 7153–7164. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [24].Ishibashi J, Seale P, Medicine. Beige can be slimming, Science 328 (5982) (2010) 1113–1114. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [25].Stanford KI, Goodyear LJ, Exercise regulation of adipose tissue, Adipocytes 5 (2) (2016) 153–162. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [26].Seale P, et al. , PRDM16 controls a brown fat/skeletal muscle switch, Nature 454 (7207) (2008) 961–967. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [27].Saugen E, Vøllestad NK, Nonlinear relationship between heat production and force during voluntary contractions in humans, J. Appl. Physiol. 79 (6) (1995) 2043–2049. [DOI] [PubMed] [Google Scholar]
- [28].Cannon B, Nedergaard JAN, Brown adipose tissue: function and physiological significance, Physiol. Rev 84 (1) (2004). [DOI] [PubMed] [Google Scholar]
- [29].van Marken Lichtenbelt W, Brown adipose tissue and the regulation of nonshivering thermogenesis, Curr. Opin. Clin. Nutr. Metab. Care 15 (6) (2012) 547–552. [DOI] [PubMed] [Google Scholar]
- [30].Zouhal H, et al. , Catecholamines and the effects of exercise, training and gender, Sports Med 38 (5) (2008) 401–423. [DOI] [PubMed] [Google Scholar]
- [31].Sanchez-Delgado G, et al. , Role of exercise in the activation of brown adipose tissue, Ann. Nutr. Metab 67 (1) (2015) 21–32. [DOI] [PubMed] [Google Scholar]
- [32].Hansen D, et al. , Effect of acute endurance and resistance exercise on endocrine hormones directly related to lipolysis and skeletal muscle protein synthesis in adult individuals with obesity, Sports Med 42 (5) (2012) 415–431. [DOI] [PubMed] [Google Scholar]
- [33].Koppo K, et al. , Lipid mobilization in subcutaneous adipose tissue during exercise in lean and obese humans. Roles of insulin and natriuretic peptides, Am. J. Physiol. Endocrinol. Metab 299 (2) (2010) E258–65. [DOI] [PubMed] [Google Scholar]
- [34].Pedersen BK, Fischer CP, Physiological roles of muscle-derived interleukin-6 in response to exercise, Curr. Opin. Clin. Nutr. Metab. Care 10 (3) (2007) 265–271. [DOI] [PubMed] [Google Scholar]
- [35].Kim KH, Lee MS, FGF21 as a stress hormone: the roles of FGF21 in stress adaptation and the treatment of metabolic diseases, Diabetes Metab. J 38 (4) (2014) 245–251. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [36].Kim KH, et al. , Acute exercise induces FGF21 expression in mice and in healthy humans, PLoS One 8 (5) (2013) e63517. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [37].Catoire M, et al. , Identification of human exercise-induced myokines using secretome analysis, Physiol. Genomics 46 (7) (2014) 256–267. [DOI] [PubMed] [Google Scholar]
- [38].Fletcher JA, et al. , Modulating fibroblast growth factor 21 in hyperphagic OLETF rats with daily exercise and caloric restriction, Appl. Physiol. Nutr. Metab 37 (6) (2012) 1054–1062. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [39].Loyd C, et al. , Fibroblast growth factor 21 is required for beneficial effects of exercise during chronic high-fat feeding, J. Appl. Physiol. 121 (3) (2016) 687–698. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [40].Hanssen MJ, et al. , Serum FGF21 levels are associated with brown adipose tissue activity in humans, Sci. Rep 5 (2015) 10275. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [41].Ignacio DL, et al. , Blunted response of pituitary type 1 and brown adipose tissue type 2 deiodinases to swimming training in ovariectomized rats, Horm. Metab. Res. 44 (11) (2012) 797–803. [DOI] [PubMed] [Google Scholar]
- [42].Wu MV, et al. , Thermogenic capacity is antagonistically regulated in classical brown and white subcutaneous fat depots by high fat diet and endurance training in rats: impact on whole-body energy expenditure, J. Biol. Chem. 289 (49) (2014) 34129–34140. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [43].Vosselman MJ, et al. , Low brown adipose tissue activity in endurance-trained compared with lean sedentary men, Int. J. Obes. (Lond) 39 (12) (2015) 1696–1702. [DOI] [PubMed] [Google Scholar]
- [44].Motiani P, et al. , Decreased insulin-stimulated brown adipose tissue glucose uptake after short-term exercise training in healthy middle-aged men, Diabetes Obes. Metab. 19 (10) (2017) 1379–1388. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [45].De Matteis R, et al. , Exercise as a new physiological stimulus for brown adipose issue activity, Nutr. Metab. Cardiovasc. Dis 23 (6) (2013) 582–590. [DOI] [PubMed] [Google Scholar]
- [46].Hirata K, Blood flow to brown adipose tissue and norepinephrine-induced calorigenesis in physically trained rats, Jpn. J. Physiol. 32 (2) (1982) 279–291. [DOI] [PubMed] [Google Scholar]
- [47].Oh-ishi S, et al. , Swimming training improves brown-adipose-tissue activity in young and old mice, Mech. Ageing Dev. 89 (2) (1996) 67–78. [DOI] [PubMed] [Google Scholar]
- [48].Yoshioka K, et al. , Effects of exercise training on brown adipose tissue thermogenesis in ovariectomized obese rats, Endocrinol. Jpn. 36 (3) (1989) 403–408. [DOI] [PubMed] [Google Scholar]
- [49].Xu X, et al. , Altered adipocyte progenitor population and adipose-related gene profile in adipose tissue by long-term high-fat diet in mice, Life Sci. 90 (25–26) (2012) 1001–1009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [50].Xu X, et al. , Exercise ameliorates high-fat diet-induced metabolic and vascular dysfunction, and increases adipocyte progenitor cell population in brown adipose tissue, Am. J. Phys. Regul. Integr. Comp. Phys. 300 (5) (2011) R1115–R1125. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [51].Richards MR, et al. , Fatty acid transport protein 1 and long-chain acyl coenzyme A synthetase 1 interact in adipocytes, J. Lipid Res. 47 (3) (2006) 665–672. [DOI] [PubMed] [Google Scholar]
- [52].Richard D, Arnold J, Leblanc J, Energy balance in exercise-trained rats acclimated at two environmental temperatures, J. Appl. Physiol. 60 (3) (1986) 1054–1059. [DOI] [PubMed] [Google Scholar]
- [53].Townsend KL, Tseng YH, Brown fat fuel utilization and thermogenesis, Trends Endocrinol. Metab. 25 (4) (2014) 168–177. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [54].Peppler WT, et al. , Subcutaneous inguinal white adipose tissue is responsive to, but dispensable for, the metabolic health benefits of exercise, Am. J. Physiol. Endocrinol. Metab 314 (1) (2018) E66–e77. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [55].Peppler WT, et al. , Habitual physical activity protects against lipopolysaccharideinduced inflammation in mouse adipose tissue, Adipocytes 6 (1) (2017) 1–11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [56].Kalinovich AV, et al. , UCP1 in adipose tissues: two steps to full browning, Biochimie 134 (2017) 127–137. [DOI] [PubMed] [Google Scholar]
- [57].Dulloo AG, Miller DS, Energy balance following sympathetic denervation of brown adipose tissue, Can. J. Physiol. Pharmacol. 62 (2) (1984) 235–240. [DOI] [PubMed] [Google Scholar]
- [58].Gunawardana SC, Piston DW, Reversal of type 1 diabetes in mice by brown adipose tissue transplant, Diabetes 61 (3) (2012) 674–682. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [59].Hu HH, Gilsanz V, Developments in the imaging of brown adipose tissue and its associations with muscle, puberty, and health in children, Front. Endocrinol 2 (2011) 33. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [60].Chen YI, et al. , Anatomical and functional assessment of brown adipose tissue by magnetic resonance imaging, Obesity (Silver Spring) 20 (7) (2012) 1519–1526. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [61].Chen YC, et al. , Measurement of human brown adipose tissue volume and activity using anatomic MR imaging and functional MR imaging, J. Nucl. Med. 54 (9) (2013) 1584–1587. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [62].May FJ, et al. , Lipidomic adaptations in white and brown adipose tissue in response to exercise demonstrate molecular species-specific remodeling, Cell Rep. 18 (6) (2017) 1558–1572. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [63].Trevellin E, et al. , Exercise training induces mitochondrial biogenesis and glucose uptake in subcutaneous adipose tissue through eNOS-dependent mechanisms, Diabetes 63 (8) (2014) 2800–2811. [DOI] [PubMed] [Google Scholar]
- [64].Stallknecht B, et al. , Increased activities of mitochondrial enzymes in white adipose tissue in trained rats, Am. J. Phys. 261 (3 Pt 1) (1991) (p. E410–4). [DOI] [PubMed] [Google Scholar]
- [65].Golbidi S, Laher I, Exercise induced adipokine changes and the metabolic syndrome, J. Diabetes Res. 2014 (2014) (pp. 726861–726861). [DOI] [PMC free article] [PubMed] [Google Scholar]
- [66].Zachwieja JJ, et al. , Voluntary wheel running decreases adipose tissue mass and expression of leptin mRNA in Osborne-Mendel rats, Diabetes 46 (7) (1997) 1159–1166. [DOI] [PubMed] [Google Scholar]
- [67].Bradley RL, et al. , Voluntary exercise improves insulin sensitivity and adipose tissue inflammation in diet-induced obese mice, Am. J. Physiol. Endocrinol. Metab 295 (3) (2008). [DOI] [PMC free article] [PubMed] [Google Scholar]
- [68].Kraemer RR, et al. , Serum leptin concentrations in response to acute exercise in postmenopausal women with and without hormone replacement therapy, Proceedings of the Society for Experimental Biology and Medicine. Society for Experimental Biology and Medicine (New York, N.Y.), 221(3) 1999, pp. 171–177. [DOI] [PubMed] [Google Scholar]
- [69].Kanaley JA, et al. , Resting leptin responses to acute and chronic resistance training in type 2 diabetic men and women, Int. J. Obes 25 (10) (2001) 1474–1480. [DOI] [PubMed] [Google Scholar]
- [70].Mahoney DJ, et al. , Analysis of global mRNA expression in human skeletal muscle during recovery from endurance exercise, FASEB J. 19 (11) (2005) 1498–1500. [DOI] [PubMed] [Google Scholar]
- [71].Fu L, et al. , Effects of high-fat diet and regular aerobic exercise on global gene expression in skeletal muscle of C57BL/6 mice, Metabolism 61 (2) (2012) 146–152. [DOI] [PubMed] [Google Scholar]
- [72].Keller P, et al. , A transcriptional map of the impact of endurance exercise training on skeletal muscle phenotype, J. Appl. Physiol. 110 (1) (2011) 46–59. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [73].Ronn T, et al. , A six months exercise intervention influences the genome-wide DNA methylation pattern in human adipose tissue, PLoS Genet. 9 (6) (2013) e1003572. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [74].Ruschke K, et al. , Gene expression of PPARgamma and PGC-1alpha in human omental and subcutaneous adipose tissues is related to insulin resistance markers and mediates beneficial effects of physical training, Eur. J. Endocrinol. 162 (3) (2010) 515–523. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [75].Sutherland LN, et al. , Exercise and adrenaline increase PGC-1{alpha} mRNA expression in rat adipose tissue, J. Physiol. 587 (Pt 7) (2009) 1607–1617. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [76].Bostrom P, et al. , A PGC1-alpha-dependent myokine that drives brown-fat-like development of white fat and thermogenesis, Nature 481 (7382) (2012) 463–468. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [77].Cao L, et al. , White to brown fat phenotypic switch induced by genetic and environmental activation of a hypothalamic-adipocyte axis, Cell Metab. 14 (3) (2011) 324–338. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [78].Knudsen JG, et al. , Role of IL-6 in exercise training- and cold-induced UCP1 expression in subcutaneous white adipose tissue, PLoS One 9 (1) (2014) e84910. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [79].Nedergaard J, Cannon B, The browning of white adipose tissue: some burning issues, Cell Metab. 20 (3) (2014) 396–407. [DOI] [PubMed] [Google Scholar]
- [80].Hirata M, et al. , Genetic defect in phospholipase Cdelta1 protects mice from obesity by regulating thermogenesis and adipogenesis, Diabetes 60 (7) (2011) 1926–1937. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [81].Ranallo RF, Rhodes EC, Lipid metabolism during exercise, Sports Med. 26 (1) (1998) 29–42. [DOI] [PubMed] [Google Scholar]
- [82].Rao RR, et al. , Meteorin-like is a hormone that regulates immune-adipose interactions to increase beige fat thermogenesis, Cell 157 (6) (2014) 1279–1291. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [83].Feldman BJ, et al. , Myostatin modulates adipogenesis to generate adipocytes with favorable metabolic effects, Proc. Natl. Acad. Sci. U. S. A 103 (42) (2006) 15675–15680. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [84].Carriere A, et al. , Browning of white adipose cells by intermediate metabolites: an adaptive mechanism to alleviate redox pressure, Diabetes 63 (10) (2014) 3253–3265. [DOI] [PubMed] [Google Scholar]
- [85].Roberts LD, et al. , beta-Aminoisobutyric acid induces browning of white fat and hepatic beta-oxidation and is inversely correlated with cardiometabolic risk factors, Cell Metab. 19 (1) (2014) 96–108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [86].Tran TT, et al. , Beneficial effects of subcutaneous fat transplantation on metabolism, Cell Metab. 7 (5) (2008) 410–420. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [87].Cao H, et al. , Identification of a lipokine, a lipid hormone linking adipose tissue to systemic metabolism, Cell 134 (6) (2008) 933–944. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [88].Yore MM, et al. , Discovery of a class of endogenous mammalian lipids with antidiabetic and anti-inflammatory effects, Cell 159 (2) (2014) 318–332. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [89].Lynes MD, et al. , The cold-induced lipokine 12,13-diHOME promotes fatty acid transport into brown adipose tissue, Nat. Med. 23 (5) (2017) 631–637. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [90].Norheim F, et al. , The effects of acute and chronic exercise on PGC-1α, irisin and browning of subcutaneous adipose tissue in humans, FEBS J. 281 (3) (2014) 739–749. [DOI] [PubMed] [Google Scholar]
- [91].Daskalopoulou SS, et al. , Plasma irisin levels progressively increase in response to increasing exercise workloads in young, healthy, active subjects, Eur. J. Endocrinol 171 (3) (2014) 343–352. [DOI] [PubMed] [Google Scholar]
- [92].Jedrychowski MP, et al. , Detection and quantitation of circulating human irisin by tandem mass spectrometry, Cell Metab. 22 (4) (2015) 734–740. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [93].Kim H, et al. , Effect of aerobic training and resistance training on circulating irisin level and their association with change of body composition in overweight/obese adults: a pilot study, Physiol. Res 65 (2) (2016) 271–279. [DOI] [PubMed] [Google Scholar]
- [94].Kazak L, et al. , UCP1 deficiency causes brown fat respiratory chain depletion and sensitizes mitochondria to calcium overload-induced dysfunction, Proc. Natl. Acad. Sci. U. S. A 114 (30) (2017) 7981–7986. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [95].Shabalina IG, et al. , ROS production in brown adipose tissue mitochondria: the question of UCP1-dependence, Biochim. Biophys. Acta 1837 (12) (2014) 2017–2030. [DOI] [PubMed] [Google Scholar]
- [96].Powers SK, Jackson MJ, Exercise-induced oxidative stress: cellular mechanisms and impact on muscle force production, Physiol. Rev 88 (4) (2008) 1243–1276. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [97].Lehnig AC, Stanford KI, Exercise induced adaptations to white and brown adipose tissue, J. Exp. Biol 221 (Pt Suppl 1) (2018). [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.