Abstract
Glia constitute roughly half of the cells of the central nervous system (CNS), but were long-considered static bystanders to its formation and function. Here we provide an overview of how the diverse and dynamic functions of glial cells orchestrate essentially all aspects of nervous system formation and function. Radial glia, astrocytes, oligodendrocyte progenitor cells, oligodendrocytes and microglia each influence nervous system development, from neuronal birth, migration, axon specification and growth, through to circuit assembly and synaptogenesis. As neural circuits mature, distinct glia fulfil key roles in synaptic communication, plasticity, homeostasis, and networklevel activity, through dynamic monitoring and alteration of CNS structure and function. Continued elucidation of glial cell biology, and the dynamic interactions of neurons and glia, will enrich our understanding of nervous system formation, health and function.
Introduction
Cursed perhaps by the incorrect assertion of Virchow in 1846 that the brain contains a connective structure called “nervenkitt”, the mundane notion of glia as glue (deriving from Greek) emerged, and stuck (1). This is despite the fact that soon after Virchow’s “discovery” of glia, the famous neuroanatomists of the late 19th and early 20th century had not only identified the major glia of the CNS, but speculated with keen foresight on their potential diverse functions (1). Here we provide an overview of insights that are now addressing the question posed by Ramon y Cajal in 1909, “What is the function of glia?” (1). We focus on the function of glia of the vertebrate CNS: radial glia, astrocytes, oligodendrocyte progenitor cells (OPCs, also called NG2 cells), oligodendrocytes, and microglia (Figure 1). For example, we now know that radial glia are CNS progenitors that generate the majority of our neurons and glia, either directly, or via intermediate progenitors (Figure 1). Astrocytes, starshaped cells with thousands of processes that interact with likely all cell types of the CNS, exhibit a range of functions that help drive nervous system development and sculpt its activity (Figure 2). OPCs are the most proliferative cells in the CNS, generate myelinating oligodendrocytes throughout life, and likely serve additional yet to be identified roles in circuit formation and function (Figure 3). Mature oligodendrocytes generate myelin sheaths that speed up nerve impulse conduction and provide metabolic support to axons (Figure 3), and it now appears that dynamic regulation of myelination may regulate the precise timing of information propagation and communication across functional circuits. Finally, microglia are the guests of the CNS, best known as the brain’s resident macrophages, but with increasingly evident roles during multiple stages of nervous system development and activity (Figure 4).
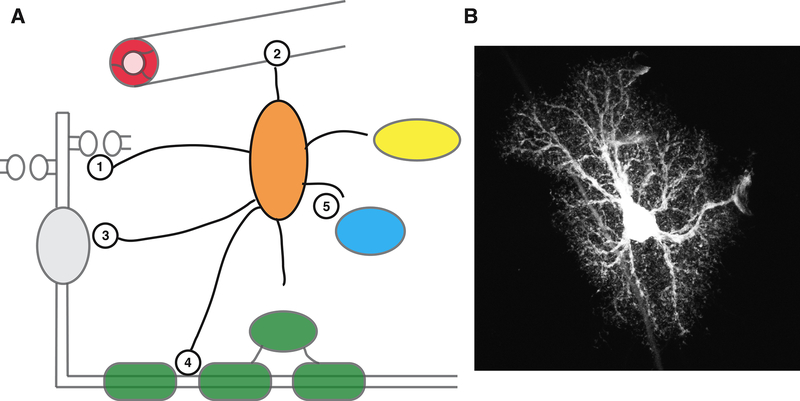
Figure 1. Origin and overview of CNS glial cells.
A: Radial Glial Cell in an E14 mouse cortex, visualised following in utero electroporation of GFP (green), and co-stained for apical centrosomes (pericentrin, red) and cell nuclei (DAPI, blue). Image courtesy of Sven Falk and Magdalena Goetz, Helmholtz Centre, Munich.
B: Radial glial cells are the principal neuroepithelial progenitor cells of the central nervous system, and generate the majority of CNS neurons and glia, either directly (e.g. neurons), or indirectly through intermediate progenitors (e.g. OPCs). Microglia (yellow) enter the CNS during embryonic development.
C: Neurons and glia interact in a myriad of ways (indicated by circles, explained in text and subsequent figures).
Figure 2. Astrocytes.
A: Astrocytes have a characteristic star-like morphology and send out multiple branches that terminate in thousands of fine processes that interact with synapses, blood vessels, and other cells. Astrocytes regulate synapse formation, elimination and function (1), have endfeet that ensheath CNS vasculature (2), contributing to metabolic support and homeostatic function, and reciprocal interactions with neurons regulating circuit function (3). Astrocytes also make contact with nodes of Ranvier (4), the function of which is unclear, and interact bidirectionally with OPCs, oligodendrocytes and microglia (5), the relevance of which is emerging.
B: Astrocyte image courtesy of The Cell Image Library, image CIL 48001, https://doi.org/doi:10.7295/W9CIL48001.
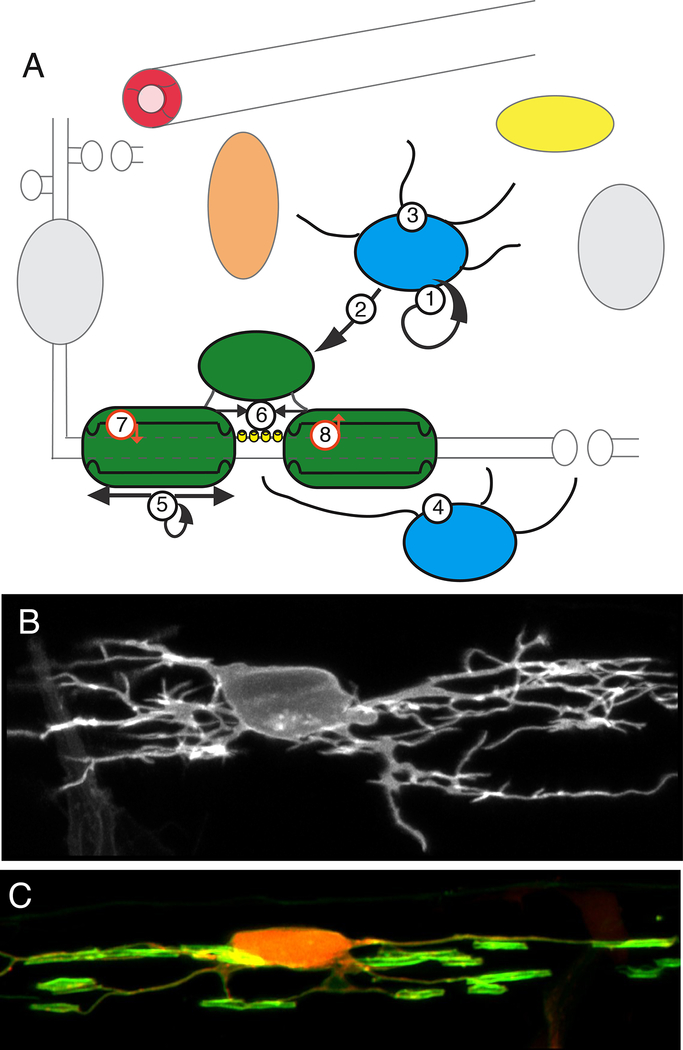
Figure 3. Oligodendrocyte progenitor cells and oligodendrocytes.
A: OPCs (blue) are the most proliferative cells of the CNS (1) and generate mature myelinating oligodendrocytes throughout life (2). OPCs interact with many other cells of the CNS (3), particularly in disease. OPCs extend processes that contact nodes of Ranvier, receive synapses from axons, and regulate synaptic function (4). Whether there are distinct subtypes of OPCs or simply different functional states remains unclear.
Oligodendrocytes (dark green) produce lipid rich myelin sheaths that wrap around axons and regulate action potential conduction velocity. Dynamic regulation of myelination in response to neuronal signals (5) may be a fundamental mechanism by which neural circuit function is fine-tuned. Myelinating oligodendrocytes organise axonal domains (6), including nodes of Ranvier, where the voltage-gated Na2+ channels (yellow) that mediate action potential propagation are localised, provide metabolic support to axons (7) and facilitate ion homeostasis essential to normal action potential conduction, e.g. the uptake by myelin of K+ ions (8).
B: OPC expressing membrane tethered fluorescent protein imaged in a living transgenic zebrafish larvae at 3 days postfertilisation.
C: Oligodendrocyte expressing membrane tethered GFP and cytoplasmic RFP imaged in a living transgenic zebrafish larvae at 4 days post-fertilisation. Images courtesy of Dr. Marion Baraban, Lyons lab.
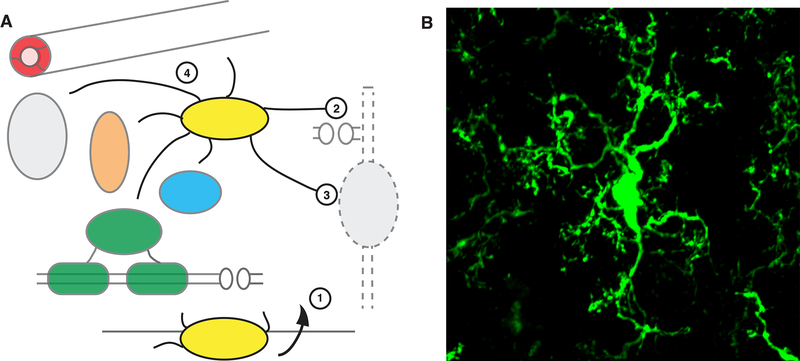
Figure 4. Microglia.
A: Microglia are the resident immune cells of the brain, entering during early development from the periphery (1). In addition to immune surveillance roles (not shown), microglia interact with multiple cell types of the CNS and regulate numerous developmental and functional processes, including synaptic pruning (2), clearing apoptotic neurons (3) and interacting with multiple CNS cell types, in health and disease (4). B: Microglia expressing GFP in mouse cortex (Cx3cr1-GFP), courtesy of Youtong Huang and Greg Lemke, Salk Institute.
One of the most quoted “facts” about glia is that they outnumber neurons by 10:1 in the human brain, but it is now clear that we have a roughly equal proportion of neurons and glia (2). The relative proportions vary by region (e.g. grey matter vs white matter), developmental stage, and species, but as a very general rule human brains are composed of 20% astrocytes (2), 3–10% OPCs (3), 25% oligodendrocytes (2), and 5–15% microglia (4). We have known of neuronal diversity for over a hundred years, and it is now emerging that glia also exhibit significant functional diversity, (57), likely to be driven by both intrinsic programmes based on developmental origins, and extrinsic interactions with their environment. In this piece, we take a chronological perspective that aims to chart how glia first drive neurogenesis and subsequently regulate neuronal migration, axon growth, synapse formation, and ultimately, circuit function. We focus on the role of glia and their reciprocal interactions with neurons in regulating healthy nervous system formation and function, and refer interested readers to other excellent sources on the development of glia (3, 8–10), and the myriad roles of glia in disease (4, 11–13).
Glia in CNS formation
Glia are the principle regulators of cell number in the CNS.
During embryonic development, neuroepithelial progenitors take on characteristics of astrocytes, including the expression of factors such as glutamate transporters, glycogen granules, and intermediate filaments. These neuroeiptheial cell derived “radial glia” go on to generate neurons during embryonic stages of mammalian development (14), and then switch to generating mature glia, which they do either directly by generating proliferating astrocytes, or through generation of intermediate progenitors, such as OPCs (3). Towards the end of gliogenesis, many radial glia differentiate directly into mature astrocytes, whereas in anamniotic vertebrates (e.g. fish and amphibians) mature radial glia retain a radial morphology, but may subserve the functional roles of astrocytes (15). Subtypes of radial glia with distinct neurogenic and gliogenic potentials have been described, and radial glia-derived progenitors persist through adulthood and give rise to adult neural stem cells (16). In addition to cells derived from the neuroepithelium, microglia, derived from erythromyeloidprogenitors enter the vertebrate CNS early in development at the onset of neurogenesis, and subsequently proliferate and migrate to colonise the entire brain and spinal cord (10). Microglia regulate neuronal survival, phagocytose excess neurons undergoing apoptosis during both early development and in neurogenic regions of the adult brain (17), and have multiple roles in refining CNS formation and function (18). Although glia regulate neuron number (14), neurons can, in turn, regulate glial cell number. For example, the programmed cell death of specific neurons influences initial microglial cell entry to the CNS (18), and neuronal activity can regulate OPC proliferation and oligodendrocyte generation (19). As will become clear, many aspects of nervous system formation and function are underpinned by dynamic bidirectional interactions of neurons and glia.
Glia influence neuronal migration, axon specification and growth
Following their birth, neurons in the CNS migrate, either radially through the neuroepithelium, e.g. establishing specific cortical layers, or tangentially, as exemplified by interneuron migration from the ganglionic eminences to the cortex. The role of glia in regulating tangential migration has not been intensively studied, although microglia have been implicated (10). In contrast, it is well known that radial glia support radial migration of neurons, with the underlying mechanisms extensively investigated (14). For example, disruption of radial glial cell polarity dysregulates neuronal migration to such an extent that a duplicated cortex can form underneath a normal one (14). Radial glia also exhibit polarised mRNA transport and localised protein translation along their processes (20), suggesting that they may locally regulate additional aspects of neuronal development. Indeed, axon specification occurs on the opposite side of the neuron to that of radial glial contact, at least in vitro (21). In addition, microglia (10), astrocytes and OPCs (22) have been shown to subsequently help guide axons to their destinations, and analogous roles for glia in axon specification (23), and guidance have also been identified in C. elegans (24).
Glia can coordinate circuitwide neuronal differentiation
Our understanding of the mechanisms that coordinate neuronal differentiation across circuits is rudimentary, but it is now clear that glial cells can coordinate the timing of axondriven target neuron differentiation. In the Drosophila visual system, photoreceptor neurons (PRNs) first secrete a signal to their associated wrapping glia (analogous to myelinating glia), which then secrete cues that induce target neuron differentiation. This indirect signalling via glia introduces a delay that controls the timely differentiation of target neurons, because the wrapping glia arrive at specific target locations with a delay relative to axons (25). The mechanisms that might coordinate differentiation of neurons distributed across local and long-range circuits in vertebrates are unclear. However, given that invertebrate models have served as mechanistic pathfinders for many aspects of vertebrate neural development (26, 27), the prospective role of glia in regulating circuit-level neuron differentiation warrants further investigation.
Glia regulate synapse formation and pruning
After neurogenesis, neuronal migration, and axon guidance are complete, prospective partner neurons begin to make synaptic connections. Synaptogenesis involves a series of formation, strengthening and remodelling steps, with glia playing distinct roles at each stage. The timing of initial synaptogenesis coincides with the generation of astrocytes, putting them in the right place at the right time (28). First, astrocytes contact immature neurons, making them competent to form synapses (28). Then neurons become receptive to astrocyte-secreted signals that instruct formation of immature synapses, including Thrombospondins that induce silent synapses, and Glypicans, which induce functionally active synapses (28). Synapses are then matured by additional glial signals, including Sparcl1 from astrocytes, which induces synaptic strengthening and stabilization (18). As a balance to the pro-synaptogenic signals, astrocytes also produce signals (e.g. Sparc) that negatively regulate synapse formation and function (18). In addition to astrocytes, microglia have been implicated in synapse formation (18). The various roles of distinct glia in regulating synapse formation, and the extent to which they do so throughout the nervous system and over time in vivo remains to be fully determined.
Towards the end of the synaptogenic period, weak and inappropriate synapses are eliminated by astrocytes and microglia, ultimately leaving neurons with their adult connectivity. Astrocytes phagocytose synapses via Megf10 and Mertk, whereas microglia prune synapses tagged with components of the classical complement pathway (18), or through the Trem2 pathway (29). IL33 signalling from astrocytes to microglia has also been shown to regulate synapse elimination (30), highlighting the complexity of cell-cell interactions in the CNS.
A role for glia in regulating synapse development appears broadly conserved across evolution. For example, human astrocytes transplanted into mice are capable of regulating multiple aspects of the structure and function of host synapses (5). Invertebrate glia also regulate synapses, with glia in C. elegans determining the precise location of specific synapses and promoting synaptogenesis (27), and Drosophila glia also regulating synapse maturation (26). The distinct roles of glia in synapse formation and remodelling is likely to be in part regulated by their regional identity (8) and complex interactions with their environment. For example, neurons can regulate astrocyte maturation and diversity in vivo (28), and neural activity can regulate hundreds of astrocyte genes in vitro (31). Therefore, distinct neurons may be able to influence and employ specialised glial functions to sculpt circuit-specific architecture. Determining how interactions between neurons and glia regulate different synaptic classes in different brain regions in vivo will be an important area of exploration.
Glia in regulating CNS function
Once neuronal circuits are assembled, glia regulate numerous aspects of nervous system function.
Glia adjust synaptic communication and plasticity
Glia regulate many aspects of synaptic function. Astrocytes regulate neurotransmitter uptake, e.g. via glutamate transporters (32), which is dynamically regulated, with glutamate transporters on astrocyte processes being upregulated by high activity and downregulated during prolonged periods of low activity, providing a way to modulate the concentration of transmitter available at synapses (32). Furthermore, increased synaptic activity can also lead to a greater ensheathment of synapses by astrocyte processes, decreasing the chance of neurotransmitter diffusing and activating a neighbouring synapse (33). Astrocytes express receptors for multiple neurotransmitters and neuromodulators. Many of these are GPCRs coupled to intracellular signalling cascades that converge on intracellular calcium ion (Ca2+) signalling (33). Astrocytes can in turn release gliotransmitters, thus signalling back to neurons and other glial cells (34). Whether gliotransmitter release primarily regulates the function of individual synapses or groups of synapses (given that an individual astrocyte can contact thousands of synapses) is likely to depend on the synapse type and gliotransmitter in question. Gliotransmitters include ATP, which, when converted to adenosine, acts on presynaptic adenosine receptors to alter neurotransmitter release probability and short term plasticity. In addition, D-serine is a co-agonist for the NMDA glutamate receptor and is released from astrocytes in a calcium-dependent manner, through which it can also regulate synaptic plasticity (32–34). Astrocyte activation has even been shown to enhance both neuronal plasticity and memory in vivo (35).
In addition to astrocytes, OPCs and microglia have also been shown to regulate synaptic function. For example, OPCs have been implicated in controlling postsynaptic NMDA and AMPA receptor mediated currents and plasticity through cleavage of the transmembrane proteoglycan NG2, and NG2 mutant mice exhibit deficits in sensorimotor function (19). Dysregulation of microglial signalling also affects synaptic and circuit function. For example, celltype specific knockout of purinergic receptors leads to disruption of ocular dominance plasticity in the visual cortex (4), and TNFα, a cytokine secreted by microglia that regulates homeostatic synaptic scaling, influences ocular dominance plasticity (36). In addition, depletion of microglia from the juvenile mouse brain inhibits motor learning, with microglial-BDNF proposed to mediate the effect. However, the elimination of microglia at adult ages does not affect behaviour (4, 18), further indicating that stage and region specific neuron-glial interactions regulate neural circuit function.
Glial regulation of ion homeostasis affects circuit function
Glia can affect neuronal excitability by regulating ion homeostasis, for example by clearing potassium (K+) ions from the extracellular space, which facilitates neuronal membrane repolarisation after an action potential and thus continued firing (37). The glial cell responsible for K+ uptake can vary by brain region, and includes astrocytes, OPCs and oligodendrocytes (37). Along myelinated axons most K+ channels are localised under the myelin sheath, suggesting that K+ clearance might occur at the interface of the axon and the myelin sheath, rather than at the node of Ranvier where astrocytes and OPCs make contact. Indeed, knockout of the inward rectifying potassium channel Kir4.1 from mature myelinating oligodendrocytes leads to poor K+ clearance and consequent conduction defects (38), revealing a function for mature oligodendrocytes in facilitating circuit function.
Glial regulation of extracellular K+ can influence diverse aspects of neural circuit function. For example, in the rat lateral habenula (LHb), Kir4.1 is expressed at low levels in astrocytes, but is upregulated in models of depression. Regulation of astrocyte Kir4.1 can alter both the bursting activity of LHb neurons and associated depression-like symptoms (39). In contrast, in the spinal cord, the astrocytes surrounding large fast motor neurons express a very high level of Kir4.1, which is necessary to maintain the high firing rate of these specific neurons (40). In the cortex specialised satellite oligodendrocytes tightly associate with the cell bodies of cortical neurons, and regulate extracellular K+ concentration and neuronal activity through formation of a glial syncytium with neighbouring astrocytes and oligodendrocytes (19). It remains unknown to what extent region specific glial phenotypes (e.g. high or low Kir4.1 expression) are driven by intrinsically distinct developmental programmes or by dynamic interactions with the extrinsic environment.
Neuro-glial-vascular coupling provides metabolic support
Interactions between the vasculature and cells of the CNS are increasingly implicated in brain health. CNS blood vessels have specialized barrier properties, known as the blood brain barrier (BBB), which limit movement of substances, including nutrients, between the blood and the brain (41). However, astrocytes are ideally positioned to actively supply neurons with nutrients, as they extend processes that contact both blood vessels and neurons (42). Astrocytes are not just relays, but also process precursor molecules for delivery to neurons. For example, astrocytes are enriched in lipid synthesis pathways and provide lipids to neurons, including cholesterol, essential for maintaining presynaptic function (28). Astrocytes are also enriched in glycolytic pathways and make glycogen, which can be converted into lactate and transferred to neighbouring neurons by monocarboxylate transporters (43). Although there has been debate about the specific role of lactate as an energy source for neurons, mice lacking monocarboxylate transporters do show impairments in long term memory formation (43).
Along myelinated axons, myelin sheathes create a barrier between the axon and the extracellular environment, limiting direct metabolic support routes to the axon to the short unmyelinated gaps between myelin sheaths at nodes of Ranvier. Instead, myelinating oligodendrocytes generate glycolytic products including lactate, which can be transported to the axon via monocarboxylate transporters at the myelin-axon interface (44). It has been proposed that high-frequency axonal firing activates NMDA receptors on oligodendrocytes, triggering increased glucose uptake, and the subsequent transport of lactate to the axon to support the energy requirements associated with maintaining high levels of activity (44). Visualising this metabolic support system in real-time and in response to neural activity, and investigating how astrocytes and oligodendrocytes collaborate on metabolic support, are important areas of ongoing investigation.
Glia regulate network level function
Astrocytes dynamically alter brain states
There is now compelling evidence of glial involvement in dynamic regulation of circuitlevel function. For example, networks of astrocytes in the mouse visual cortex can be activated by the neuromodulator norepinephrine in line with dynamic changes in the arousal state of the animal. This increased astrocyte activity can in turn enhance the ability of astrocytes to detect altered neuronal activity (5). The regulation of circuit function via astrocytes has also been demonstrated in Drosophila, where Octopaminergic neurons signal through astrocytes to control downstream neurons and behaviour (28). Furthermore, dynamic regulation of astrocyte population level Ca2+ activity has been shown to alter ensemble-level oscillatory states in mice (5). Over a century ago Ramon y Cajal proposed that astrocytes may regulate sleep state, a suggestion now supported by extensive evidence (5).
Myelination dynamically alters circuit function
Myelination of axons by oligodendrocytes has long been known to increase conduction velocity. In addition to whether an axon is myelinated or not, changes to the number, distribution, length and thickness of myelin sheaths all in principle affect conduction velocity and thus timing of information propagation, prompting speculation that dynamic regulation of myelination in response to neural activity may fine-tune the timing of communication between neurons in a circuit. Indeed, neural activity can regulate many aspects of oligodendrocyte lineage progression in developing, juvenile and adult animals (19). OPCs receive bona fide synapses from neuronal axons, which may mediate the effects of neural activity on their proliferation, and/ or differentiation (19). Mature myelinating oligodendrocytes also express a range of neurotransmitter receptors, ion channels and transporters, whose diverse functions remain to be fully elucidated, but which likely mediate the effects of neural activity on myelination itself. The fact that only some neuronal subtypes regulate myelination via activity (19), suggests that activity-regulated myelination may be circuit-specific.
Direct evidence that activity-regulated myelination in turn affects circuit function comes from several studies. Social isolation of mice, for a distinct postnatal period or a prolonged period in adulthood, disrupts myelination in the prefrontal cortex (19): in addition, the behavioural traits associated with social isolation can be phenocopied by loss of oligodendrocyte ErbB receptor signalling during the corresponding postnatal period, and reversed by chemically promoting myelination in adulthood (19). These results indicate that the changes in neural activity associated with isolation lead to behavioural deficits via changes in myelination. In addition, learning new motor tasks promotes new oligodendrocyte generation in adult mice, and the prevention of such oligodendrogenesis impairs learning (19). Nonetheless, it remains to be determined how dynamic regulation of myelination affects the timing of conduction, synaptic communication, or the function of neuronal populations within intact circuits in vivo (19). Evidence that neurons regulate myelinating glia, and that myelinating glia in turn regulate circuit function further exemplifies how reciprocal interactions between neurons and glia play fundamental roles in circuit function.
Conclusions
Here we have highlighted integral roles of glial cells in many aspects of CNS formation and function, and we refer interested readers to our more extensive supplementary reading list for further information. Given the emerging evidence of diverse subtypes/ dynamic states of glia, we expect that further new functions will be revealed, and their mechanistic underpinnings elucidated, in years to come. This will require interrogation using a variety of models and approaches, bridging of scales from molecule to system, and investigating mechanisms from development through the entire life-course. The study of glia reminds us that nervous system development does not end at birth, nor indeed adolescence, and that the dynamic behaviour of glial cells and ongoing neuron-glial interactions actively sculpt and remodel the CNS throughout life. We expect that further insight into how the brain forms, functions and ages in a healthy manner will derive from integrating our understanding of how neurons interact with glia, how different glial cells interact with each other, and how the brain interacts with the rest of the body.
Supplementary Material
Acknowledgments
We dedicate this piece to Ben A. Barres, a pioneer in glial cell biology, who challenged neuroscientists to think of glia not as glue, but as active participants in nervous system development, function and disease. We thank members of the Allen and Lyons laboratories for helpful feedback on the manuscript. We apologise to colleagues for omitting original references, due to space constraints, and refer interested readers to our supplementary reading list.
Funding: Work in the Allen lab is supported by NIH-NINDS NS105742 and NS089791, and the Hearst, Pew, Dana, CART and LouLou Foundations; and in the Lyons lab by a Wellcome Trust Senior Research Fellowship (102836/Z/13/Z), and MRC Project Grant (MR/P006272/1) and a Strategic Research Agreement with Biogen.
Footnotes
Competing interests: No competing interests.
Data and materials availability: Not applicable.
Supplementary Materials
Supplementary Reading list
This manuscript has been accepted for publication in Science. This version has not undergone final editing. Please refer to the complete version of record at http://www.sciencemag.org/. The manuscript may not be reproduced or used in any manner that does not fall within the fair use provisions of the Copyright Act without the prior, written permission of AAAS.
References
- 1.Somjen GG, Nervenkitt: notes on the history of the concept of neuroglia. Glia. 1, 2–9 (1988). [DOI] [PubMed] [Google Scholar]
- 2.von Bartheld CS, Bahney J, Herculano S Houzel, The search for true numbers of neurons and glial cells in the human brain: A review of 150 years of cell counting. J. Comp. Neurol 524, 3865–3895 (2016). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Dimou L, Götz M, Glial Cells as Progenitors and Stem Cells: New Roles in the Healthy and Diseased Brain. Physiological reviews. 94, 709–737 (2014). [DOI] [PubMed] [Google Scholar]
- 4.Salter MW, Stevens B, Microglia emerge as central players in brain disease. Nat Med. 23, 1018–1027 (2017). [DOI] [PubMed] [Google Scholar]
- 5.Poskanzer KE, Molofsky AV, Dynamism of an Astrocyte In Vivo: Perspectives on Identity and Function. Annu. Rev. Physiol 80, 143–157 (2018). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Tomassy GS, Dershowitz LB, Arlotta P, Diversity Matters: A Revised Guide to Myelination. Trends in Cell Biology. 26, 135–147 (2016). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Wolf SA, Boddeke HWGM, Kettenmann H, Microglia in Physiology and Disease. Annu. Rev. Physiol. 79, 619–643 (2017). [DOI] [PubMed] [Google Scholar]
- 8.Bayraktar OA, Fuentealba LC, Alvarez-Buylla A Rowitch DH, Astrocyte development and heterogeneity. Cold Spring Harbor Perspectives in Biology. 7, a020362 (2015). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Bergles DE, Richardson WD, Oligodendrocyte Development and Plasticity. Cold Spring Harbor Perspectives in Biology. 8, a020453 (2015). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Ginhoux F, Prinz M, Origin of microglia: current concepts and past controversies. Cold Spring Harbor Perspectives in Biology. 7, a020537–16 (2015). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Dossi E, Vasile F, Rouach N, Human astrocytes in the diseased brain. Brain Res. Bull 136, 139–156 (2018). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Tognatta R, Miller RH, Contribution of the oligodendrocyte lineage to CNS repair and neurodegenerative pathologies. Neuropharmacology. 110, 539–547 (2016). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Nave K-A, Ehrenreich H, Myelination and oligodendrocyte functions in psychiatric diseases. JAMA Psychiatry. 71, 582–584 (2014). [DOI] [PubMed] [Google Scholar]
- 14.Taverna E, Götz M, Huttner WB, The Cell Biology of Neurogenesis: Toward an Understanding of the Development and Evolution of the Neocortex. Annu. Rev. Cell Dev. Biol 30, 465–502 (2014). [DOI] [PubMed] [Google Scholar]
- 15.Lyons DA, Talbot WS, Glial cell development and function in zebrafish. Cold Spring Harbor Perspectives in Biology. 7, a020586 (2015). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Falk S, Götz M, Glial control of neurogenesis. Current Opinion in Neurobiology. 47, 188–195 (2017). [DOI] [PubMed] [Google Scholar]
- 17.Kierdorf K, Prinz M, Microglia in steady state. J. Clin. Invest 127, 3201–3209 (2017). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Reemst K, Noctor SC, Lucassen PJ, Hol EM, The Indispensable Roles of Microglia and Astrocytes during Brain Development. Front. Hum. Neurosci 10, 15983–28 (2016). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Almeida RG, Lyons DA, On Myelinated Axon Plasticity and Neuronal Circuit Formation and Function. J. Neurosci 37, 10023–10034 (2017). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Pilaz L-J, Lennox AL, Rouanet JP, Silver DL, Dynamic mRNA Transport and Local Translation in Radial Glial Progenitors of the Developing Brain. Curr. Biol 26, 3383–3392 (2016). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Xu C et al. , Radial Glial Cell-Neuron Interaction Directs Axon Formation at the Opposite Side of the Neuron from the Contact Site. Journal of Neuroscience. 35, 14517–14532 (2015). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Minocha S et al. , Nkx2.1-derived astrocytes and neurons together with Slit2 are indispensable for anterior commissure formation. Nature Communications. 6, 6887 (2015). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Meng L, Zhang A, Jin Y, Yan D, Regulation of neuronal axon specification by glia-neuron gap junctions in C. elegans. Elife. 5, e19510 (2016). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Rapti G, Li C, Shan A, Lu Y, Shaham S, Glia initiate brain assembly through noncanonical Chimaerin-Furin axon guidance in C. elegans. Nat Neurosci. 20, 1350–1360 (2017). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Fernandes VM, Chen Z, Rossi AM, Zipfel J, Desplan C, Glia relay differentiation cues to coordinate neuronal development in Drosophila. Science. 357, 886–891 (2017). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Freeman MR, Drosophila Central Nervous System Glia. Cold Spring Harbor Perspectives in Biology. 7, a020552 (2015). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Shaham S, Glial Development and Function in the Nervous System of Caenorhabditis elegans. Cold Spring Harbor Perspectives in Biology. 7, a020578–15 (2015). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Allen NJ, Eroglu C, Cell Biology of Astrocyte-Synapse Interactions. Neuron. 96, 697–708 (2017). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Filipello F et al. , The Microglial Innate Immune Receptor TREM2 Is Required for Synapse Elimination and Normal Brain Connectivity. Immunity. 48, 979–991.e8 (2018). [DOI] [PubMed] [Google Scholar]
- 30.Vainchtein ID et al. , Astrocyte-derived interleukin-33 promotes microglial synapse engulfment and neural circuit development. Science. 359, 1269–1273 (2018). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Hasel P et al. , Neurons and neuronal activity control gene expression in astrocytes to regulate their development and metabolism. Nature Communications. 8, 15132 (2017). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Allen NJ, Astrocyte regulation of synaptic behavior. Annu. Rev. Cell Dev. Biol 30, 439–463 (2014). [DOI] [PubMed] [Google Scholar]
- 33.Khakh BS, McCarthy KD, Astrocyte calcium signaling: from observations to functions and the challenges therein. Cold Spring Harbor Perspectives in Biology. 7, a020404 (2015). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Araque A et al. , Gliotransmitters travel in time and space. Neuron. 81, 728–739 (2014). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Adamsky A et al. , Astrocytic Activation Generates De Novo Neuronal Potentiation and Memory Enhancement. Cell. 174, 59–71.e14 (2018). [DOI] [PubMed] [Google Scholar]
- 36.Kaneko M, Stellwagen D, Malenka RC, Stryker MP, Tumor necrosis factoralpha mediates one component of competitive, experience-dependent plasticity in developing visual cortex. Neuron. 58, 673–680 (2008). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Larsen BR et al. , Contributions of the Na⁺/K⁺-ATPase, NKCC1, and Kir4.1 to hippocampal K⁺ clearance and volume responses. Glia. 62, 608–622 (2014). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Larson VA et al. , Oligodendrocytes control potassium accumulation in white matter and seizure susceptibility. Elife. 7, e34829 (2018). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Cui Y et al. , Astroglial Kir4.1 in the lateral habenula drives neuronal bursts in depression. Nature. 554, 323–327 (2018). [DOI] [PubMed] [Google Scholar]
- 40.Kelley KW et al. , Kir4.1-Dependent Astrocyte-Fast Motor Neuron Interactions Are Required for Peak Strength. Neuron. 98, 306–319.e7 (2018). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Daneman R, Prat A, The blood-brain barrier. Cold Spring Harbor Perspectives in Biology. 7, a020412 (2015). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Nortley R, Attwell D, Control of brain energy supply by astrocytes. Current Opinion in Neurobiology. 47, 80–85 (2017). [DOI] [PubMed] [Google Scholar]
- 43.Magistretti PJ, Allaman I, Lactate in the brain: from metabolic endproduct to signalling molecule. Nat Rev Neurosci. 19, 235–249 (2018). [DOI] [PubMed] [Google Scholar]
- 44.Saab AS, Nave K-A, Myelin dynamics: protecting and shaping neuronal functions. Current Opinion in Neurobiology. 47, 104–112 (2017). [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.