Abstract
Phloretin (Phl) is a dihydrochalcone flavonoid with significant cytoprotective properties; e.g., free radical trapping, electrophile scavenging. Based on this, it has been suggested that Phl might be useful in the treatment of pathogenic processes and prevention of drug toxicities. Therefore, we determined the ability of Phl to provide route- and dose-dependent hepatoprotection in a mouse model of acetaminophen (APAP) overdose. Intraperitoneal (i.p.) administration of Phl produced a bimodal effect; i.e., the highest dose (2.40 mmol/kg) did not prevent APAP-induced lethality, whereas lower doses (0.2 – 0.4 mmol/kg) afforded modest hepatoprotection. When given alone, the highest i.p. Phl dose was lethal within 24 hrs, whereas the lower doses were not toxic. Oral Phl (0.40 – 2.40 mmol/kg) did not prevent APAP-induced hepatotoxicity. The highest oral dose given alone (2.4 mmol/kg) produced 64% lethality, whereas lower doses were not lethal. This toxicity profile was reflected in a study using APAP-exposed isolated mouse hepatocytes, which showed that the Phl pharmacophores, 1,3,5-trihydroxyacetophenone (PG) and 2’,4’,6’-trihydroxyacetophenone (THA) where protective. Corroborative cell free studies showed that polyphenol protectants prevented glutathione loss mediated by the APAP metabolite, N-acetyl-p-benzoquinone imine (NAPQI). Thus, in spite of possesing cytoprotective attributes, Phl was generally toxic in our APAP models. These and earlier findings suggest that Phl is not a candidate for drug design. In contrast, we have found that the enol-forming pharmacophores, THA and PG, are potential platforms for pharmacotherapeutic development.
Keywords: phytopolyphenol, hepatoprotection, acetaminophen overdose, druginduced toxicity, enol-based cytoprotectants
1.0. Introduction
Phloretin (Phl) is a dihydrochalcone flavonoid that is found in apple skins. This phytopolyphenol has been shown to have significant antioxidant properties and might, therefore, be useful in treating pathogenic conditions that have oxidative stress as a common mechanism (de Oliveira, 2016; Jiang et al., 2010). Indeed, some evidence suggests that Phl is a pharmacotherapeutic approach to cardiovascular disease (Stangl et al., 2005), cancer (Ma et al., 2016), ischemia-reperfusion injury (Liu et al, 2015) and neurodegeneration (Ghumatkar et al., 2018). In this regard, we have previously shown that Phl prevented electrophile (e.g., acrolein)-induced nerve terminal toxicity (LoPachin et al., 2011). We also found that the Phl pharmacophores, 2’,4’,6’-trihydroxyacetophenone (THA) and 1,3,5-trihydroxyacetophenone (phloroglucinol), could prevent hepatotoxicity as indicated by prevention of cell lethality and mitochondrial dysfunction in an isolated mouse hepatocyte model of acetaminophen (APAP) overdose (Geohagen et al., 2016). Results indicated that these derivatives were hepatoprotective and that the corresponding levels of protection were significantly greater than that provided by either 2-acetylcyclopentanone (2-ACP), a 1,3-dicarbonyl enol derivative of curcumin, or N-acetylcysteine (NAC; Mucomyst™), the thiol-based acetaminophen (APAP) antidote (Geohagen et al., 2016; Zhang et al., 2013).
The apparent broad protective abilities of Phl are consistent with certain chemical attributes of this polyphenol. Specifically, Phl is an aromatic flavonoid that can act as an antioxidant and trap toxic free oxygen/nitrogen radicals (Calliste et al., 2001). In addition, Phl has several enolizable sites (e.g., C-3 enol on the A ring) that can ionize to form enolates with modest nucleophilicity (LoPachin et al., 2011). Therefore, Phl cytoprotection could also involve scavenging electrophiles such N-acetyl-p-benzoquinone imine (NAPQI) and unsaturated carbonyl species (e.g., acrolein) that mediate APAP hepatotoxicity (Geohagen et al., 2016; LoPachin et al., 2011; Zhu et al., 2009, 2012; reviewed in LoPachin et al., 2016; Zhu et al., 2011). These chemical properties suggest that Phl could treat pathogenic conditions and drug toxicities that involve electrophiles. Electrophilic species cause toxicity by forming covalent adducts with biological nucleophiles, which subsequently produces mitochondrial damage, glutathione (GSH) depletion, protein inactivation and secondary oxidative stress (Hinson et al., 2010; LoPachin et al., 2016). Therefore, to further our understanding of enolatebased cytoprotection, the route- and dose-dependent effects of Phl were evaluated in a mouse model of acute APAP overdose (Zhang et al., 2013). During the course of these studies, we found that Phl produced dose-dependent toxicity. To evaluate this bimodal effect, corroborative studies were conducted in freshly isolated mouse hepatocytes. Results were compared to findings from previous pharmacological studies involving enol- and thiol-based cytoprotectants (Geohagen et al., 2016; LoPachin et al., 2011; Zhang et al., 2013). Research has provided evidence that electrophile-based GSH depletion was a significant index of cytotoxicity (reviewed in LoPachin et al., 2016). Therefore, as a measure of enolate scavenging kinetics, we conducted cell-free in chemico studies to quantify polyphenol prevention of NAPQI-induced GSH loss.
2.0. Materials and Methods
2.1. Reagents.
All chemicals, reagents and phytopolyphenols were of the highest grade commercially available and were purchased from Sigma-Aldrich (St. Louis, MO).
2.2. Animals and Treatments.
All aspects of animal use in this study were in accordance with the National Institutes of Health Guide for Care and Use of Laboratory Animals and were approved by the Montefiore Medical Center Animal Care Committee. Three month old male C57BL/6N mice (mean weight 27gm) were purchased from Charles River Laboratory (Wilmington, MA). Mice were housed individually in polycarbonate boxes, and filtered drinking water and Purina Rodent Laboratory Chow (Purina Mills, Inc., St. Louis, MO) were available ad libitum. The animal room was maintained at approximately 22° C and 50% humidity with a 12 hr light/dark cycle. Prior to each experiment, mice were fasted overnight and treatments began at 0800 the following morning. Food was returned 1 hr post-treatment. APAP and all experimental compounds were administered in phosphate buffered polyethylene glycol (PEG). Preliminary studies demonstrated that this vehicle did not affect the experimental outcome (Zhang et al., 2013). As a general protocol, groups of mice (n=15) were pretreated by i.p. (10 ml/kg) injection or oral (4 ml/kg) administration of putative hepatoprotectant followed 20 minutes later by oral (4 ml/kg) APAP (500 mg/kg). Initial research indicated that the onset and development of enol hepatoprotection was similar regardless of APAP administration time; i.e., 20 mins before, 20 mins after or simultaneously (Zhang et al., 2013). A separate group of animals were given vehicle by i.p. injection or oral administration followed twenty minutes later by oral APAP. Control mice received an i.p./oral sequence of vehicle injections. Separate groups (n=15) of animals were administered a cytoprotectant by either the oral or i.p. route. An assessment of general toxicity was conducted at two day intervals over a 7day post-intoxication period. Specifically, animals were weighed and assessed by a blinded observer for changes in grooming, nest building, open field behavior, recumbancy and gait (Zhang et al., 2013). Kaplan-Meier survival curves were used to illustrate the cumulative percent daily lethality of mice in different experimental groups and were generated in Prism 6.0 (Graphpad software). In previous studies, we (e.g., see Geohagen et al., 2016; Zhang et al., 2013) fully characterized the molecular pathogenesis of APAP-induced hepatotoxicity and enol-based hepatoprotection using temporal measurements of histopathology, liver-specific enzymes, GSH/GSSG ratio, mitochondrial function and unsaturated aldehyde production.
2.3. Hepatocyte Isolation Procedures and Incubations
Hepatocytes were prepared from anesthetized mice (isoflurane inhalation) using the collagenase perfusion method of Geohagen et al. (2016). Briefly, to separate dead hepatocytes, isolated cells were centrifuged (140g × 8 mins) in a Percoll gradient and then washed in media (140g × 3 mins) to remove Percoll. The isolation procedure yielded ~30–40 million cells with 80–90% viability as determined by trypan blue exclusion. Hepatocytes (100,000 cells/ml) were incubated in covered 35mm plastic dishes containing supplemented RPMI-1640 media at 37°C in a humidified atmosphere of 95% O2/5% CO2. The concentration-dependent cytoprotection (0.01–3.0mM) of individual test compounds was determined in isolated hepatocytes exposed to APAP (e.g., 1 mM × 4 hrs incubation). Control conditions included: vehicle alone (0.1% DMSO in media), vehicle plus APAP (e.g., 1 mM × 4 hrs incubation) and cytoprotectants alone (3.0 mM × 4 hrs). As sensitive indices of hepatocyte injury (Geohagen et al., 2016; Zhang et al., 2013), we measured the respective activities of lactate dehydrogenase (LDH) and the liver specific enzyme, alanine aminotransferase (ALT) in hepatocyte medium. The concentration-response data were fitted by nonlinear regression analyses (Geohagen et al., 2016). In all studies, hepatocyte viability and other toxic measures (see ahead) were determined in at least n = 4–6 independent experiments.
2.4. In Chemico Studies – Effects of Cytoprotectants on Electrophile-Induced GSH Loss.
Graded concentrations of NAPQI (2–128μM) were pre-incubated (15 mins) in phosphate buffered saline (PBS; pH 7.4, 25° C) with selected cytoprotectants (50 μM) from different chemical classes (thiol and phytopolyphenols) or vehicle (PEG). Following pre-incubation, GSH (30 μM) was added and remaining sulfhydryl content was measured after 15 mins by the DTNB method of LoPachin et al. (2009a). For each hepatoprotectant, respective sulfhydryl data were fitted by nonlinear regression analyses (r2 for all curves ≥ 0.90) and electrophile concentrations that produced 50% thiol loss (IC50’s) and their 95% confidence intervals were calculated by the Cheng-Prusoff equation (LoPachin et al., 2007a).
2.5. Calculations of Hard and Soft, Acids and Bases (HSAB) Parameters
The Lowest Unoccupied Molecular Orbital (LUMO) energy (ELUMO) and Highest Occupied Molecular Orbital (HOMO) energy (EHOMO), were determined using Spartan 14 (version 1.1.8) software (Wavefunction Inc., Irvine CA). For each structure, ground state equilibrium geometries were calculated with Density Functional B3LYP 6–31G* in water starting from 6–31G* geometries. Global (whole molecule) hardness (η) was calculated as η = (ELUMO-EHOMO)/2 and softness (σ) was calculated as the inverse of hardness (i.e., σ= 1/η). The electrophilicity index (ω) was calculated as ω = μ2/2η where μ is chemical potential of the electrophile (μ = (ELUMO+EHOMO)/2). An index of nucleophilicity (ω−, see LoPachin et al., 2012 for more detailed discussion) was calculated as ω− = ηA(μA - μB)2/2(ηA+ηB)2, where A = reacting nucleophile and B = NAPQI (μ = −5.235 ev, η = 2.005 ev).
2.6. Statistical Analyses
All statistical analyses were conducted using Prism 6.0 (GraphPad software; San Diego, CA) with significance set at the 0.05 level of probability. In studies evaluating the relative abilities of potential hepatoprotectants to modify APAP lethality in experimental groups of mice, the Mantel-Cox log-rank test was used to compare survival rates among the experimental groups.
3.0. Results
3.1. Hepatoprotection in a Mouse Model of APAP Overdose.
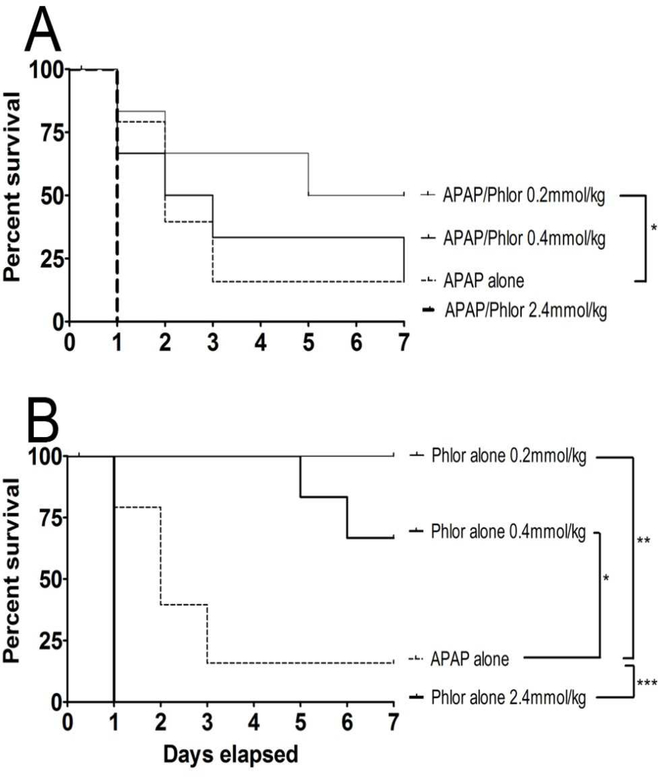
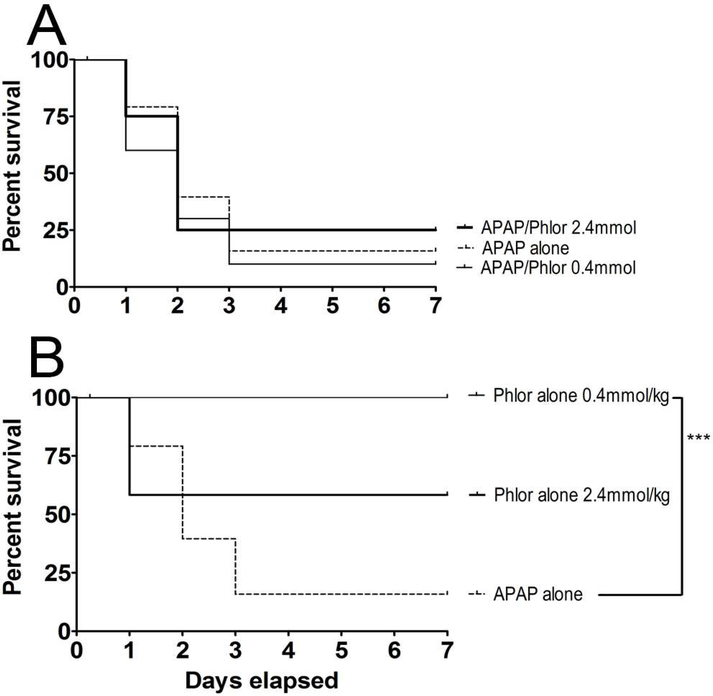
In this study, hepatoprotectants were administered by either the oral or i.p. route 20 mins before oral APAP (Zhang et al., 2013). Results showed that i.p. Phl was modestly protective at the lowest dose-rate tested (0.20 mmol/kg; Fig. 1A). Results showed that i.p. Phl was modestly protective at the lowest dose-rate tested (0.20 mmol/kg; Fig. 1A); i.e surviving APAP-treated animals exhibited normal weight gain, nesting and ambulation. However, an intermediate dose of Phl (0.4 mmol/kg) did not provide significant protection, whereas the highest Phl dose (2.40 mmol/kg, i.p.) combined with oral APAP caused 100% lethality within the first 24 hrs (Fig. 1A). In a corroborative study of toxicity, we found that Phl administered alone at the highest dose-rate (2.4 mmol/kg) also produced total lethality within 24 hrs (Fig. 1B). Mice administered Phl at the 0.40 mmol/kg dose-rate exhibited 38% delayed lethality at the end of the 7 day observation period. Phl administered orally at either 0.4 or 2.40 mmol/kg did not prevent lethality in the APAP mouse model (Fig. 2A). When administered alone (Fig. 2B), the larger Phl exposure was associated with early-onset lethality (45% within 24 hours), whereas the smaller dose did not cause lethality over the 7 day observation period.
Figure 1.
(A) Dose-dependent hepatoprotective effects of Phl (0.20 – 2.40 mmol/kg) administered by intraperitoneal injection 20 minutes prior to oral APAP (500 mg/kg) overdose. (B) Dose-dependent toxicity (lethality) of Phl (0.20 – 2.40 mmol/kg) administered alone by intraperitoneal injection. Kaplan-Meier survival curves illustrate the cumulative percent daily lethality in the APAP alone and APAP/Phl groups (n= 15 mice/group). Joining lines indicate statistically significant differences in treatment groups at *p<0.05, *p<0.01 and *** p<0.001 levels of significance.
Figure 2.
(A) Dose-dependent hepatoprotective effects of oral Phl (0.40 – 2.40 mmol/kg) administered 20 minutes prior to oral APAP (500 mg/kg) overdose. (B) Dosedependent toxicity (lethality) of oral Phl (0.40 – 2.40 mmol/kg). Kaplan-Meier survival curves illustrate the cumulative percent daily lethality in the APAP alone, APAP/Phl groups (n= 15 mice/group). Joining line indicates statistically significant differences in treatment groups at *** p<0.001 level of significance.
3.2. Cytoprotection in APAP-Exposed Isolated Mouse Hepatocytes.
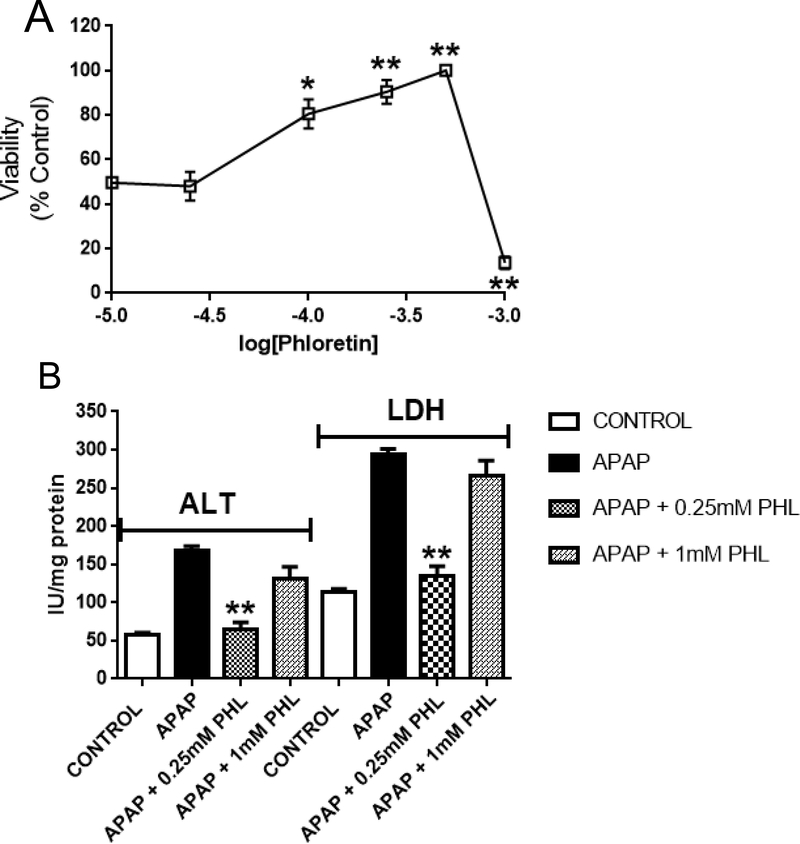
Results from animal studies suggest that Phl had bimodal actions; i.e., when given by i.p. administration, this phytopolyphenol is protective at lower dose-rates and toxic at higher rates (Fig. 1A). To examine this bimodality further, we characterized the hepatoprotective properties of Phl and related pharmacophores in an isolated mouse hepatocyte system. Figure 3A shows that Phl provided hepatic cell protection (viability) from APAP (1.0mM × 4 hrs) over a relatively broad dose-range (100–750 mM). However, the combination of APAP and the highest Phl dose tested (1.0 mM) produced nearly 100% hepatocyte lethality. Previously, we showed that the development of irreversible APAP-induced liver toxicity was closely correlated to increases in the activities of ALT and LDH (Geohagen et al., 2016; Zhang et al., 2013). In the present study, changes in these enzyme indices corroborated the bimodal effect of Phl on hepatocyte injury. Specifically, exposure of hepatocytes to APAP alone caused significant increases in LDH and ALT (Fig. 3B). Addition of Phl at the lower concentration (0.25 mM) was protective as indicated by normalization of corresponding liver injury markers (Fig. 3B). In contrast, the highest Phl concentration (1.0 mM) did not prevent the APAP-induced changes in ALT and LDH. Parallel control studies indicated that Phl alone was cytotoxic at 1.0 mM (59 ± 11% loss of viability), whereas the lower concentration range (100–750 μM) did not alter hepatocyte viability (data not shown).
Figure 3.
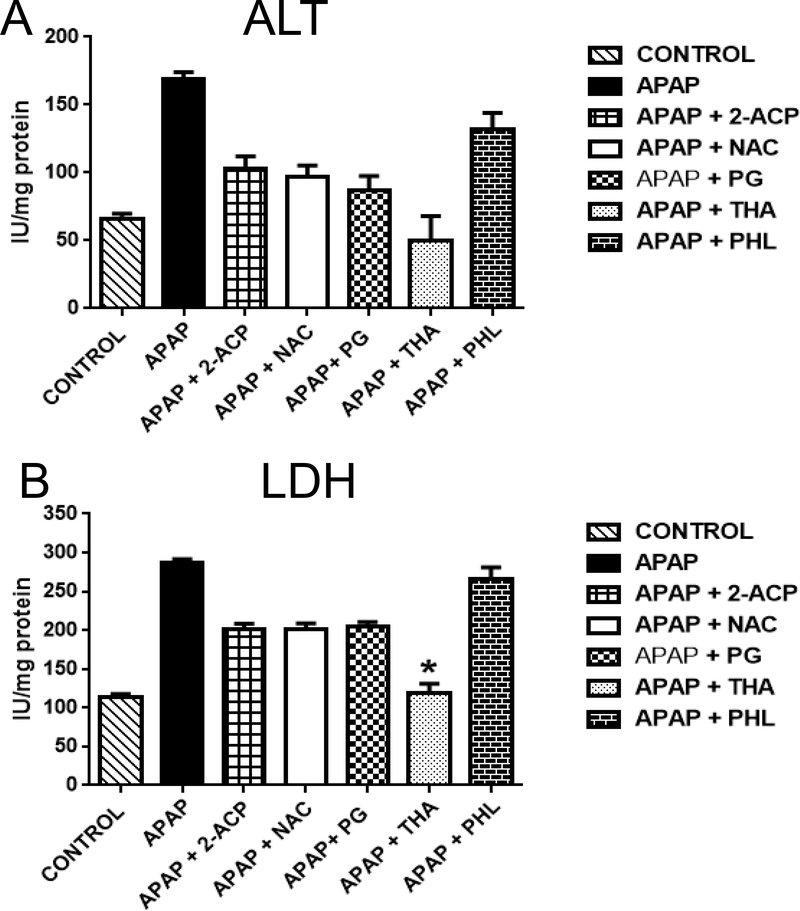
(A) Effects of graded Phl concentrations (0.1 – 1.0 mM) on the viability of APAP (1.0 mM × 4 hrs)-exposed freshly isolated mouse hepatocytes. Data are expressed as mean percent of control ± SEM cell viability (n = 6–8). Exposure of hepatocytes to APAP alone (1.0 mM × 4 hrs) caused a decrease in mean cell viability of 48 ± 8% (see ordinate of Fig. 3A). Exposure of hepatocytes to phloretin concentrations ≤ 0.75 mM did not alter viability, whereas the 1.0 mM concentration caused a 59 ± 11% loss of viability (data not shown). (B) Concentration-dependent effects of phloretin (0.25 and 1.0 mM) on alanine aminotransferase (ALT) and lactate dehydrogenase (LDH) measured in incubation medium. Data are expressed as mean IU/mg protein ± SEM cell viability (n = 4–6). Statistically significant differences in treatment groups at *p<0.05 and **p<0.01 levels of significance.
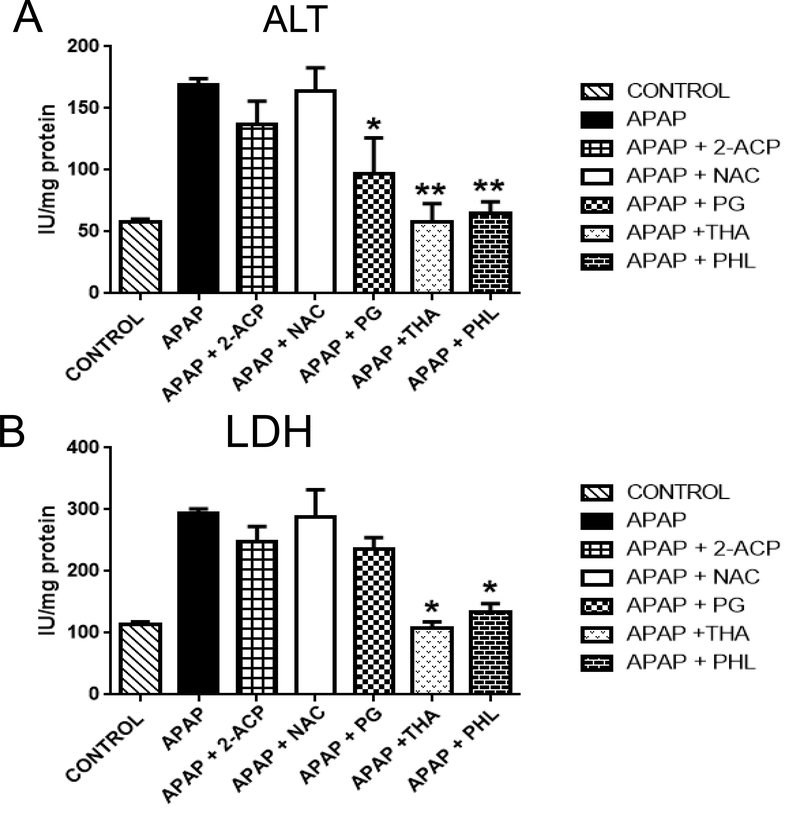
For comparative purposes, we determined the protective abilities of several compounds from different chemical classes; e.g., thiols (NAC). 1,3-dicarbonyl enols (2ACP and polyphenols (THA, PG). Thus, the lower concentration of both 2-ACP and NAC (0.25 mM) did not prevent APAP (1.0 mM × 4 hrs)-induced ALT (Fig. 4A) and LDH (Fig. 4B) increases in media. In contrast, the Phl pharmacophores, THA and PG (0.25 mM), were protective as indicated by the normalization of the hepatocyte-injury biomarker, ALT. At the higher concentration (1.0 mM), 2-ACP, NAC, PG and THA significantly reduced the APAP-induced rise in both ALT (Fig. 5A) and LDH (Fig. 5B).
Figure 4.
Effects of 0.25 mM protectant concentrations on APAP (1.0 mM × 4 hrs)induced changes in medium (A) alanine aminotransferase (ALT) and (B) lactate dehydrogenase (LDH) activities. Data are expressed as mean IU/mg protein ± SEM cell viability (n = 3–6). Statistically significant differences in treatment groups at *p<0.05 and **p<0.01 levels of significance. Abbreviations: NAC – N-acetylcysteine; 2-ACP – 2- acetylcyclopentanone; THA - 2’,4’,6’-trihydroxyacetophenone; PHL – phloretin; PG - 1,3,5-trihydroxyacetophenone.
Figure 5.
Effects of 1.0 mM protectant concentrations on APAP (1.0 mM × 4 hrs)induced changes in medium (A) alanine aminotransferase (ALT) and (B) lactate dehydrogenase (LDH) activities. Data are expressed as mean IU/mg protein ± SEM cell viability (n = 3–6). Statistically significant differences in treatment groups at *p<0.05 and **p<0.01 levels of significance. Abbreviations: NAC – N-acetylcysteine; 2-ACP – 2- acetylcyclopentanone; THA - 2’,4’,6’-trihydroxyacetophenone; Phl – phloretin; PG - 1,3,5-trihydroxyacetophenone.
3.3. HSAB description of Cytoprotectants.
Our research suggested that the cytoprotection afforded by carbon (enolate)based nucleophiles involves the covalent scavenging of electrophiles (e.g., NAPQI, acrolein) that mediate many types of drug toxicity. However, these electrophilenucleophile reactions do not occur indiscriminately and instead exhibit a significant degree of selectivity as defined by the HSAB theory of Pearson. Based on relative electron mobility (polarizability), electrophiles and nucleophiles are classified as being either soft (polarizable) or hard (non-polarizable) and, in accordance with HSAB principles, electrophiles will react preferentially with nucleophilic targets of comparable softness or hardness. The “hard” or “soft” nature of a chemical can be calculated based on inherent electronic characteristics; i.e., respective frontier molecular orbital energies. These values can be used to quantify corresponding electrophilicity (ω) or nucleophilicity (ω−; reviewed in LoPachin et al., 2012; LoPachin and Gavin, 2015).
Table 2 shows that the quinones, NAPQI and p-benzoquinone (pBQ), are exceptionally soft, highly electrophilic chemicals (larger σ and ω values), whereas the α,β -unsaturated carbonyl derivatives, N-ethyl maleimide (NEM), acrolein and acrylamide exhibit significantly lower ranges of softness and electrophilicity (see also LoPachin et al., 2007a,b, 2009a,b). It is evident from these data that relative electrophilicity is directly related to the respective IC50 values for loss of GSH sulfhydryl groups. As a soft electrophile, NAPQI will rapidly form covalent adducts with soft nucleophiles and to identify potential targets, the corresponding relative nucleophilicities (ω−) can be calculated. The data of Table 3 show that the listed thiolate- and enolateforming compounds are relatively soft but differ significantly with respect to nucleophilicities and pKa values.
Table 2. Softness, Electrophilicity and IC50 Values for Quinone and α β -Unsaturated Carbonyl Derivatives.
Softness (σ) and electrophilicity (ω) for the selected compounds were calculated as described in the Materials and Methods section. The respective IC50 values represent in vitro electrophile concentrations that produce 50% thiol loss and reflect the relative electrophilic potency of each chemical (Geohagen et al., 2016). Abbreviations: NAPQI - N-acetyl-p-benzoquinone imine; pBQ, benzoquinone; NEM – N-ethylmaleimide.
| Electrophile | σ (×103 ev−1) | ω(ev) | IC50 (μM) |
|---|---|---|---|
| NAPQI | 500 | 6.83 | 8.4 |
| pBQ | 524 | 7.78 | 11.6 |
| NEM | 410 | 5.10 | 17.2 |
| Acrolein | 379 | 3.57 | 52.5 |
| Acrylamide | 346 | 2.62 | 436,515 |
Table 3. Nucleophilicity, Softness and pKa Values of Thiolate and Enolate Anions .
HSAB and ionization parameters for thiolate and enolate nucleophilicities. Softness (σ) and nucleophilicity (ω− with NAPQI as the reacting electrophile) for the selected chemicals were calculated as described in Materials and Methods. Abbreviations: NAC – N-acetylcysteine; GSH – glutathione; 2-ACP – 2-acetylcyclopentanone; THA - 2’,4’,6’-trihydroxyacetophenone; PG - 1,3,5-trihydroxyacetophenone.
| Anion | σ (×10−3 ev−1) | ω− (×10−3 ev) | pKa |
|---|---|---|---|
| NAC | 367 | 667 | 9.5 |
| GSH | 427 | 548 | 8.6 |
| 2-ACP | 418 | 485 | 7.8 |
| THA | 485 | 325 | 7.7 |
| PG | 366 | 540 | 8.5 |
| Curcumin | 604 | 307 | 7.8 |
| Phloretin | 494 | 221 | 7.3 |
If the nucleophiles in Table 3 are cytoprotective through electrophile scavenging, then they should be capable of slowing the adduct reaction between NAPQI and a competing nucleophilic target such as GSH. Thus, the anionic thiolate forms of NAC and GSH are more nucleophilic (larger ω− values) than the enolate state of 2-ACP. However, due to the lower acidity (higher pKa) of the sulfhydryl group, at physiological pH (7.4) the nucleophilic enolate of 2-ACP will be present in significantly higher concentrations than either thiolate. THA, a Phl pharmacophore, has a similar enolate pKa (7.7) and is a relatively soft but less reactive nucleophile (Table 3). Curcumin and Phl are relatively weak nucleophiles (ω− values of 0.307 and 0.221ev respectively) but stronger acids (lower pKa values; i.e., 7.3 and 7.8, respectively). Accordingly, a large proportion of these polyphenols will exist in the nucleophilic enolate state at cellular pH.
3.4. Nucleophiles Scavenge NAPQI.
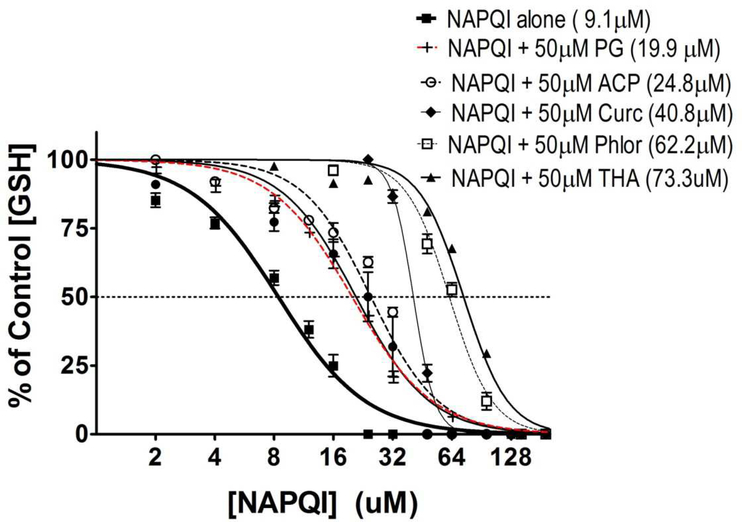
To examine these concepts experimentally, we determined the relative abilities of equimolar (50 μM) nucleophile concentrations to prevent NAPQI-induced GSH thiol loss in an in chemico model (Fig. 6). Results indicate that NAPQI alone produced a concentration-dependent depletion of GSH-derived sulfhydryl groups (IC50 = 9.1μM), whereas the test nucleophiles significantly increased this IC50 in a manner that reflected dependency on both the respective pKa values and nucleophilic reactivity (ω−) of the enolate or sulfhydryl group (see Discussion). Thus, Phl and curcumin provided the most thiol-protection among the phytopolyphenol compounds tested; e.g., in the presence of Phl (50 μM) the IC50 of NAPQI increased from 9.1 to 62.2 μM. The thiolnucleophile NAC did not cause a significant shift in IC50, whereas 2-ACP was minimally effective (Fig. 6).
Figure 6.
Cell free in chemico evaluation of the relative abilities of selected cytoprotectants to inhibit NAPQI-induced loss of sulfhydryl groups on GSH. Graded concentrations of NAPQI were incubated with putative hepatoprotectants. Data are expressed as mean percent control ± SEM (n = 3 to 4 separate experiments) and corresponding IC50 values are provided in parentheses. Abbreviations: NAC – N-acetylcysteine; 2-ACP – 2-acetylcyclopentanone; PG - 1,3,5-trihydroxyacetophenone; THA - 2’,4’,6’-trihydroxyacetophenone; Phl – phloretin and Curc - curcumin.
4.0. Discussion
4.1. The Dose-Dependent Effects of Phl.
Substantial evidence (Introduction) suggests that Phl is a potential therapeutic approach to numerous pathogenic and injury processes. Therefore, as an index of general cytoprotective potential and pharmacotherapeutic development, we determined the relative ability of Phl to prevent hepatotoxicity in a well described mouse model of APAP overdose. Results indicated that although low level Phl doses provided modest hepatoprotection, this effect was complicated by inherent toxicity. These in vivo findings were recapitulated in our in vitro study of Phl protection in APAP-exposed isolated hepatocytes. Also notable in this hepatocyte study, the Phl pharmacophores, THA and PG, were highly protective and significantly more potent than either 2-ACP or NAC. Corroborative in chemico studies showed that the nucleophiles tested in this study increased the NAPQI IC50 (9.1 μM) for GSH loss in accordance with the respective pKa values and nucleophilic reactivities (ω−) of the enolate or sulfhydryl group.
4.2. Mechanisms of Phl Cytoprotection and Toxicology.
We have proposed a chemical mechanism of polyphenol cytoprotection where corresponding enol moieties ionize to form nucleophilic enolates (Table 1) that play an important protective role (Geohagen et al., 2016; Jiao et al., 2006; LoPachin et al., 2011; Zhu et al., 2012). From a physicochemical perspective, Phl should be an effective hepatoprotectant in APAP poisoning. Although structural rigidity restricts metal chelation, Phl can trap free radicals and has multiple enolizable sites (LoPachin et al., 2011). Despite the modest nucleophilicity of this compound (e.g., the ω− value of the C3 enolate on the A ring shown in Table 3 is 221 × 10−3ev), the corresponding pKa value is approximately 7.3. Therefore, at physiological pH, Phl is more than 50% ionized; i.e., more than half of the molecules exist as the nucleophilic enolate (Table 3). We and others have shown that Phl can readily scavenge acrolein, NAPQI and other electrophiles that are likely involved in APAP hepatotoxicity (LoPachin et al., 2011; Zhu et al., 2009, 2012).
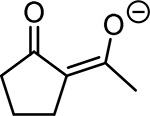
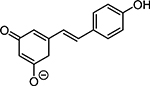
Table 1. Enolates, Enol and Diketo Tautomers of 2-ACP and hytopolyphenols.
Structures of at least one enol containing tautomer and one diketo tautomer for three plant derived polyphenols and 2-ACP (2-acetylcyclopentanone). In addition, a diketo containing resonance form of the enolate anion from each compound is shown. It should be noted that tautomers exist in temperature and pH dependent equilibrium with each other and their enolates and that the diketo form is generally not the predominant tautomer for 1,3-dicarbonyl compounds. Each of the enolates has additional resonance forms (not shown) that contain anionic oxygen atoms.
| Enol Tautomers | Diketo Tautomers | Enolates | |
|---|---|---|---|
| A. 2-ACP | 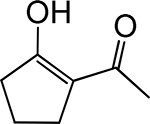 |
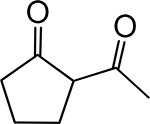 |
 |
| B. Curcumin | 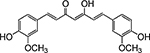 |
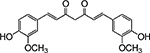 |
 |
| C. Phloretin | 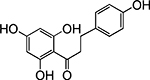 |
 |
 |
| D. Resveratrol |  |
 |
 |
The magnitude of Phl hepatoprotection was significantly less than anticipated, which might be a consequence of conflicting toxicity. Additionally, our previous cell culture studies showed that Phl was a significant toxicant (LC50 = 362 μM) that potentiated, rather than prevented, H2O2 toxicity (LoPachin et al., 2011). These data are consistent with the previously noted cytotoxicity of Phl and other hydroxychalcones, which research suggests is due to the pro-oxidant activities of these polyphenols and possibly to direct mitochondrial toxicity (Galati and O’Brien, 2004; Halliwell et al., 2008; Lambert et al., 2007).
4.3. Cytoprotective Mechanisms of the Respective Phl and Curcumin Pharmacophores.
Our studies of APAP- exposed isolated hepatocytes suggested that NAC and the curcumin pharmacophore, 2-ACP, were significantly weaker protectants than the Phl derivatives, THA and PG. Mechanistic studies of 2-ACP indicated that hepatoprotection involved ionization of the dicarbonyl moiety to an enolate (Geohagen et al., 2016; LoPachin et al., 2016; Zhang et al., 2013). Whereas the ability of curcumin and other phytopolyphenols to function as aromatic antioxidants and trap free radicals has been the focus of much mechanistic research, determination of structure-activity relationships revealed that the central 1,3-dicarbonyl was critically involved in the well-documented protective effects of curcumin (Begum et al., 2008; Weber et al., 2006; reviewed in LoPachin et al., 2016). We showed that this nucleophile could scavenge NAPQI and could also detoxify (via adduction) lipid-derived unsaturated aldehyde electrophiles (e.g., acrolein, 4-hydroxy-2-nonenal) that mediate the oxidative stress-induced hepatocyte injury process. Although free radical trapping was not involved, our earlier studies provided direct evidence that metal chelation was an important cytoprotective property of 2-ACP (reviewed in LoPachin et al., 2016). In this case, the 2-ACP enolate is a bidentate chelator of metal ions such as iron [Fe(III)] and copper [Cu(II)] that participate in the radical generating Fenton reaction (Eames, 2009; Jiao et al., 2006). Regarding the cytoprotective mechanisms of THA and PG, in addition to possessing the 2-ACP mechanisms, these pharmacophores also exhibit an ability to trap free oxygen and nitrogen radicals (reviewed in LoPachin et al., 2016). The multifunctional demeanor of these aromatic enolate-forming compounds suggests an ability to block toxic cascades (e.g, oxidative stress) at several stages. Accordingly, our data indicate that THA and PG were significantly more protective than 2-ACP and NAC (Fig. 4A).
4.4. Conclusion.
In this study, although Phl provided low dose hepatoprotection, higher doses were either ineffective or associated with lethality. With respect to cytoprotection, we have identified several chemical attributes that appear to constitute a multi-functional molecular mechanism of enolate-based cytoprotection; i.e., scavenging electrophiles, metal ion chelation and free radical trapping. It is possible that these chemical properties act in concert with cell-level effects (e.g., activation of sirtuin 1) to prevent toxicant-induced cell injury; e.g., NAPQI-induced hepatotoxicity. However, it should be noted that Phl and other phytopolyphenols (e.g., curcumin) are significant toxicants at higher doses (e.g. see LoPachin et al, 2011). In fact, the development of phytopolyphenols as cytoprotectants has been hampered by their toxicity, poor bioavailability and chemical instability (Halliwell, 2008; Lambert et al., 2007). In contrast, the 1,3-dicarbonyl enols (e.g., 2-ACP) and polyphenols (e.g., THA) are stable, non-toxic and relatively water soluble compounds (Ballantyne and Cawley, 2001; LoPachin et al., 2011; Zhang et al., 2013). Our present findings and recent studies demonstrating the ability of enolate-forming compounds to inhibit liver ischemia/reperfusion injury (Kosharskyy et al., 2015) suggest that 2-ACP, THA, PG and other enolate-forming derivatives might be viable platforms for development of new pharmacotherapeutic approaches to diseases, injury states or drug-induced toxicities that involve electrophile-mediated cellular damage.
Supplementary Material
Highlights.
Phloretin (Phl) is a phytopolyphenol with significant antioxidant properties.
Phl might be useful in treating oxidative stress induced pathogenic conditions.
In experimental acetaminophen (APAP) toxicity, Phl was cytoprotective at low doses.
Phl was, however, toxic at higher doses in this model and when given alone.
Phl derivatives (e.g., THA) were protective and are candidates for drug development.
5.0 Acknowledgments
The authors would like to thank the MMC Chemistry laboratory for their analyses of liver enzymes.
Abbreviations:
- APAP
acetaminophen
- NAPQI
N-acetyl-p-benzoquinonimine
- PBS
phosphate buffered saline
- 2-ACP
2-acetylcyclopentanone
- NAC
N-acetylcysteine
- GSH
glutathione
- DMSO
Dimethyl sulfoxide
- THA
2’,4’,6’-trihydroxyacetophenone
- PG
1,3,5-trihydroxyacetophenone
- PEG
polyethylene glycol
- LDH
lactate dehydrogenase
- ALT
alinine aminotransferase
- pBQ
para-benzoquinone
- MDA
malondialdehyde
- HSAB
Hard and Soft, Acids and Bases
- ELUMO
Lowest Unoccupied Molecular Orbital energy
- EHOMO
Highest Occupied Molecular Orbital energy
Footnotes
7.0 Conflicts of interest.
The authors declare no conflicts of interest.
Footnotes The research described in this manuscript was supported by an NIH grant from the National Institute of Environmental Health Sciences (RO1 ES003830–30).
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
8.0 References
- Ballantyne B and Cawley TJ, 2001. Toxicology update: 2,4-Pentanedione. J. Appl. Toxicol 21, 165–171. [DOI] [PubMed] [Google Scholar]
- Begum AN, Jones MR, Lim GP 2008. Curcumin structure-function, bioavailability and efficacy in models of neuroinflammation and Alzheimer’s disease. J. Pharmacol. Exp. Ther 326, 196–208. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Calliste C LeBail JC, Trounilas P, Pouget C, Habrioux G, Chulia AJ and Duroux JL 2001. Chalcones: structural requirements for antioxidant estrogenic and antiproliferative activities. Anticancer Res. 21: 3949–3956. [PubMed] [Google Scholar]
- Eames J 2009. Acid-base properties of enols and enolates In The Chemistry of Metal Enolates (Zablicky J ed) Chapt 8, pp 411–460. John Wiley & Sons, West Sussex, England. [Google Scholar]
- Galati G and O’Brien PJ 2004. Potential toxicity of flavonoids and other dietary phenolics: significance for their chemopreventive and anticancer properties. Free Rad. Biol. Med 37, 287–303. [DOI] [PubMed] [Google Scholar]
- Geohagen B, Vydyanathan A, Kosharskyy B, Shaparin N, Zhang L, Gavin T and LoPachin RM. 2016 Enolate-Forming Phloretin Pharmacophores: Hepatoprotection in an Experimental Model of Drug-Induced Toxicity J. Pharmacol. Exp. Ther 357: 476–486, 2016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ghumatkar P, Peshattiwar V, Patil S, Muke S, Whitfield D, Howlett D, Grancis P, Sathaye S 2018. The effect of phloretin on synaptic proteins and adult hippocampal neurogenesis in Aβ (1–42)-injected male Wistar rats. J Pharm Pharmacol. DOI:1111/jphp.12925. [DOI] [PubMed] [Google Scholar]
- Halliwell B 2008. Are polyphenols antioxidants or pro-oxidants? What do we learn from cell culture and in vivo studies? Arch. Biochem. Biophys 476, 107–112. [DOI] [PubMed] [Google Scholar]
- Hinson JA, Roberts DW and James LP 2010. Mechanisms of acetaminophen-induced liver necrosis. Handbook Exp. Pharmacol 196, 369–405. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jiao Y, Wilkinson J, Pietsch EC, Buss JL, Wang W, Planalp R, Torti FM and Torti SV 2006. Iron chelation in the biological activity of curcumin. Free Rad. Biol. Med 40, 1152–1160. [DOI] [PubMed] [Google Scholar]
- Jiang F, Chang CW and Dusting GJ 2010. Cytoprotection by natural and synthetic polyphenols in the heart: novel mechanisms and perspectives. Curr. Pharm. Des 16, 4103–4112. [DOI] [PubMed] [Google Scholar]
- Kosharskyy B, Vydyanathan Zhang L, Shaparin N, Geohagen BC, Bivin W, Qiang L, Gavin T and LoPachin RM 2015. 2-Acetylcyclopentanone, an enolate-forming 1,3-dicarbonyl compound, is cytorprotective in warm ischemia-reperfusion injury of rat liver. J. Pharmacol. Exp. Ther 353, 150–158. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lambert JD, Sang S and Yang CS 2007. Possible controversy over dietary polyphenols: benefits vs risks. Chem. Res. Toxicol 20, 583–585. [DOI] [PubMed] [Google Scholar]
- Liu Y, Zhang L and Lian J. (2015) Activation of the Nrf2 defense pathway contributes to neuroprotective effects of phloretin on oxidative stress injury after cerebral ischemia/reperfusion in rats. J Neurolog Sci 351, 88–92. [DOI] [PubMed] [Google Scholar]
- LoPachin RM, Lehning EJ, Ross JF, Reid M, Das S and Mansukhani S 2007a. Structure-toxicity analysis of Type-2 alkenes: in vitro neurotoxicity. Tox. Sci 95: 136–146. [DOI] [PubMed] [Google Scholar]
- LoPachin RM, Barber DS, Geohagen BC, Gavin T, Das S and He D 2007b. Neurotoxic mechanisms of electrophilic type-2 alkenes: soft-soft interactions described by quantum mechanical parameters. Tox Sci 98, 561–570. [DOI] [PubMed] [Google Scholar]
- LoPachin RM, Gavin T, Petersen DR and Barber DS. 2009a. Molecular mechanisms of 4-hydroxy-2-nonenal and acrolein toxicity: nucleophilic and adduct formation. Chem. Res. Toxicol 22, 1499–1508. [DOI] [PMC free article] [PubMed] [Google Scholar]
- LoPachin RM, Gavin T and Geohagen BC 2009b. Synaptosomal toxicity and nucleophilic targets of 4-hydroxy-2-nonenal. Tox, Sci, 107, 171–181. [DOI] [PMC free article] [PubMed] [Google Scholar]
- LoPachin RM, Gavin T, Geohagen BC, Zhang L, Casper D, Lekrhaj R and Barber DS, 2011. β-Dicarbonyl enolates: a new class of neuroprotectants J Neurochem 116: 132–143. [DOI] [PMC free article] [PubMed] [Google Scholar]
- LoPachin RM, Gavin T. DeCaprio APand Barber DS, 2012. Application of the hard and soft acids and bases (HSAB) theory to toxicant-target interactions. Chem. Res. Toxicol 25, 239–251. [DOI] [PMC free article] [PubMed] [Google Scholar]
- LoPachin RM and Gavin T 2015. Reactions Of Electrophiles With Nucleophilic Thiolate Sites: Relevance to Pathophysiological Mechanisms and Remediation. Free Rad Res 50: 195–205. [DOI] [PMC free article] [PubMed] [Google Scholar]
- LoPachin RM, Geohagen BC and Gavin T. 2016. Enolate-forming compounds as a novel approach to cytoprotection. Chem. Res. Toxicol 29, 2096–2107. [DOI] [PubMed] [Google Scholar]
- Ma L, Wang R, Nan Y, Li W, Wang Q and Jin F (2016) Phloretin exhibits an anticancer effect and enhances the anticander ability of cisplatin on non-small cell lung cancer cell lines by regulationg expression of apoptotic pathways and matrix metalloproteinases. Int J Oncol 48, 843–853. [DOI] [PubMed] [Google Scholar]
- de Oliveira MR 2016. Phloretin-induced cytoprotective effects on mammalian cells: a mechanistic view and future directions. Biofactors 42, 13–40. [DOI] [PubMed] [Google Scholar]
- Stangl V, Lorenz M, Ludwig A, Grimbo N, Guether C, Sanad W, Ziemer S, Martus P, Baumann G and Stangle K. (2005) The flavonoid phloretin suppresses stimulated expression of endothelial adhesion molecules and reduces activation of human platelets. J Nutr. 135, 172–178. [DOI] [PubMed] [Google Scholar]
- Weber WM, Hunsaker LA, Gonzales AM, Heynekamp JJ, Orlando RA, Deck LM and Vander Jagt DL 2006. TPA-induced up-regulation of activator protein-1 can be inhibited or enhanced by analogs of the natural product curcumin. Biochem. Pharmacol 72, 928–940. [DOI] [PubMed] [Google Scholar]
- Zhang L, Gavin T, Geohagen BC, Liu Q, Downey KJ and LoPachin RM, 2013. Protective properties of 2-acetylcyclopentanone in a mouse model of acetaminophen hepatotoxicity. J. Pharmacol. Exp. Ther 346, 259–269. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhu Q, Zheng AP, Cheng KW, Wu JJ, Zhang S, Tang YS, Sze KH, Chen J, Chen F and Wang M. 2009. Natural polyphenols as direct trapping agents of lipid peroxidation – derived acrolein and 4-hydroxy-trans-nonenal. Chem Res Toxicol 22, 1721–1729. [DOI] [PubMed] [Google Scholar]
- Zhu Q, Sun Z, Jiang Y, Chen F and Wang M, 2011. Acrolein scavengers: reactivity, mechanism and impact on health. Mol. Nutr. Food Res 55, 1–16. [DOI] [PubMed] [Google Scholar]
- Zhu Q, Zhang NQ, Lau CF, Chao J, Sun Z Chang RC-C, Chen F and Wang M, 2012. In vitro attenuation of acrolein-induced toxicity by phloretin, a phenolic compound from apple. Food Chem 135, 1762–1768. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.