Abstract
Lentil, black soybean and black turtle bean are commonly consumed legumes of different genera, containing high phenolic contents, which are effective antioxidants and angiotensin-I converting enzyme (ACE) inhibitors. However, these legumes’ phenolic compositions and ACE inhibition ability have not been compared. Crude water extract (CE) was semi-purified (SPE) and fractionated using column chromatography. Results showed that purification and fractionation could substantially increase phenolic contents and antioxidant capacities. Heating and variety had great effect on phenolic substances, antioxidant potential and mass yield of extracts and fractions. Only crude extracts showed potent ACE inhibitory activity. Black turtle bean’s ACE inhibition potential was largely reduced by cooking. The order from low to high in terms of ACE inhibitory activity was black turtle bean < lentil < black soybean. Identification and quantification of individual phenolic compounds by UV spectroscopy and LC-MSn analysis confirmed 18, 22, and 14 compounds, respectively, for the three legumes.
Keywords: Legume, Phenolics, Purification, Fractionation, Identification, ACE inhibition, Antioxidant activity
1. Introduction
Clinical trials have demonstrated that legume consumption is inversely related to the incidence of cardiovascular diseases (Afshin, Micha, Khatibzadeh, & Mozaffarian, 2014; Belski et al., 2011; Hermsdorff, Zulet, Abete, & Martínez, 2010). These health benefits are partially attributed to the attenuation of oxidative stress by antioxidant components, which exert an array of cellular activities (Wang, Melnyk, Tsao, & Marcone, 2011). Oxidative stress has been established as the major factor in the development of a wide range of cardiovascular diseases including hypertension (Siti, Kamisah, & Kamsiah, 2015). Dietary antioxidants are able to attenuate the oxidative stress and counteract the onset and progression of cardiovascular diseases. With human and rat cardiomyocytes, we have proven that crude lentil phenolic extract is able to attenuate angiotensin II-induced cardiomyocyte hypertrophy and to reduce intracellular reactive oxygen species (ROS) levels (Yao, Sun, & Chang, 2010). In animal studies, we have demonstrated that administration of crude lentil phenolic extract could substantially reduce aorta ROS level and increase total phenolic content (TPC) and oxygen radical absorbance capacity (ORAC) in artery serum. Meanwhile, significant alleviation of angiotensin II-induced hypertension, peripheral vascular remodeling and perivascular fibrosis have also been observed (Xuan et al., 2013; Yao, Sun, & Chang, 2012). Hypertension is a predominant factor in the development of various cardiovascular diseases including atherosclerosis, heart attack and coronary disease. With spontaneously hypertensive rats (SHRs), other researchers also have proven that phenolic extracts from legume could reduce blood pressure and suppress inflammatory responses, such as intracellular ROS level, overexpression of proinflammatory enzymes including iROS, COX-1, generation of O2−, as well as NADPH oxidase (Mukai & Sato, 2009, 2011).
Lentil (Lens culinaris), black soybean (Glycine max), and black turtle bean (Phaseolus vulgaris) are dry legumes, and belong to three different scientific genera, which are widely cultivated in the world, and preferred by different groups of consumers in different parts of the world. Numerous studies have proven that lentil, black soybean and black turtle bean have high concentration of phenolics and potent antioxidant capacity (Tan, Chang, & Zhang, 2016; Wang et al., 2016; Xu, Yuan, & Chang, 2007; Zhang, Chang, & Liu, 2015). However, a direct comparison of the compositions and relative health-promotion potential of these phenolic-rich legume varieties, particularly when they are cooked, is not available in the literature. Cooking (thermal treatment) is essential for human consumption since raw legumes contain antinutritional factors that will cause illness without heating. In addition, dry legumes are not texturally palatable unless they are soaked and cooked to softness.
In addition to the suppression of oxidative stress, one commonly used therapeutic approach to treat hypertension is the inhibition of angiotensin-I converting enzyme (ACE), which mediates the formation of angiotensin II, a vasoconstrictor and ROS initiator. Various plant extracts and pure phenolics possess ACE inhibitory activity and the ACE inhibition varies greatly according to their chemical structures (Afonso, Passos, Coimbra, Silva, & Soares-da-Silva, 2013; Al Shukor et al., 2013; Guerrero et al., 2012; Ojeda et al., 2010). However, the ACE inhibition capability of cooked legume extract has received little study (Xuan et al., 2013). In addition, phenolic composition and antioxidant activity of legumes are largely affected by processing conditions (Haileslassie, Henry, & Tyler, 2016; Xu & Chang, 2009; Zhang & Chang, 2016). It is logical to assume that the processing-induced change in phenolic composition might affect ACE inhibitory activity, but information regarding the effect of thermal processing on ACE inhibition is not available. Our previous animal study (Xuan et al., 2013) with rats revealed that phenolic extracts of cooked lentil showed lower effectiveness than raw extracts in the attenuation of angiotensin II-induced blood pressure elevation, peripheral vascular remodeling and perivascular fibrosis.
High phenolic content and compositions in these three legume varieties have been reported in the literature. However, results are inconsistent or even contradictory due to differences in extraction methods and equipment employed for analysis. Therefore, the objectives of this study were (1) to investigate and compare the effects of thermal treatments, purification, and fractionation on phenolic substances, antioxidant activity, and ACE inhibition of the three legume varieties, and (2) to identify phenolic compounds using UV spectroscopy and LC-MSn analysis.
2. Material and methods
2.1. Legumes
Lentil (Lens culinaris var. Meritt) and black turtle bean (Phaseolus vulgaris var. Turtle) of 2012 harvest were kindly provided by Geogre F. Brocke & Sons, INC (Kendrick, Idaho, USA), and by Goya Foods (Jersey City, NJ, USA), respectively. Black soybean (Glycine max) with black seed coat and green cotyledon imported from China was obtained from a local market (Asian Food Market, Starkville, MS, USA). All samples were stored in −20 °C until use.
2.2. Chemicals
Eighteen phenolic acids and aldehydes (gallic acid, protocatechuic acid, 2,3,4-trihydroxybenzoic acid, protocatechuic aldehyde, p-hydroxybenzoic acid, genistic acid, chlorogenic acid, vanillic acid, caffeic acid, vanillin, syringic acid, syringaldehyde, p-coumaric acid, ferulic acid, m-coumaric acid, sinapic acid, o-coumaric acid, trans-cinnamic acid), sixteen flavonoids (catechin, catechin gallate, gallocatechin gallate, epicatechin, epigallocatechin, epicatechin gallate, epigallocatechin gallate, kaempferol, kaempferol-3-o-glucoside, kaempferol-3-o-rutinoside, quercetin, quercetin-3-o-glucoside, myricetin, luteolin, rutin, apigenin), four procyanidins (A2, B1, B2, C1), polydatin and resveratrol, were purchased from Sigma-Aldrich Chemical Co (St. Louis, MO, USA). HPLC-grade and other analytical grade solvents were purchased from VWR International (West Chester, PA, USA).
2.3. Preparation of cooked legumes
For each legume variety, one selected thermal treatment was applied to make a comparison with the raw material. The cooking conditions were selected based on our preliminary study and reports in the literature (Xu & Chang, 2008a,b; Xu & Chang, 2009). The processing conditions, which produced palatable bean texture and gave rise to highest phenolic contents and antioxidant activities, were chosen to prepare the cooked samples. Lentil was soaked for 2 h and steamed for 10 min at 100 °C. Black turtle bean was soaked for 2 h and steamed for 30 min at 100 °C. Black soybean was soaked for 4 h and steamed for 50 min at 100 °C.
2.4. Preparation of crude phenolic extracts
Phenolics were extracted as described in our previous study (Xu & Chang, 2007). The phenolic contents and antioxidant capacities were measured from the aqueous organic extract and expressed on the basis of seed. The organic solvent in the extract was removed by vacuum rotary evaporator (38 °C) and the concentrate was freeze-dried. The resultant extract powder was referred to as crude extract (CE).
2.5. Semi-purification of crude phenolic extracts with XAD-7 resin packed column
Semi-purification of crude phenolic extracts was conducted according to our previous study (Tan, Chang, & Zhang, 2017; Zou, Chang, Gu, & Qian, 2011) with slight modification. Crude phenolic extract was suspended in water and mixed vigorously. The suspension was centrifuged at 20,000g for 15 min and the supernatant was vacuum-filtered through two layers of Whatman No. 3 filter paper. The sediment was washed and filtered two more times and the supernatants were combined. The final crude extract to water ratio was 1:10 (w/v). Thirty milliliters of clear filtered solution were loaded on an Amberlite® XAD-7 resin packed column (40 × 2.6 cm, i.d.; bed volume (BV) = 180 mL). The column was first eluted with water at 4 mL/min for 1.5 h (2 BV) to remove sugars, organic acids and proteins. Then the column was eluted with 80% methanol at 4 mL/min for 2.25 h (3 BV). The eluate from 80% methanol was collected. After the organic solvent was removed by vacuum rotary evaporator at 38 °C, the concentrate was lyophilized and the resultant powder was designated as semi-purified extract (SPE).
2.6. Fractionation of semi-purified phenolic extracts
The semi-purified extracts were further fractionated according to our reported protocol (Zou et al., 2011) with some modifications. Two hundred milligrams of semi-purified extract were dissolved in 2 mL of distilled water. The solution was centrifuged at 14,000g for 10 min and the supernatant was loaded onto a Sephadex LH-20 resin packed column (40 × 2.6 cm, i.d.; bed volume (BV) = 180 mL). The column was eluted sequentially by distilled water, 50% ethanol, and 50% acetone. The flow rate of eluents was set at 0.4 mL/min and the fractions were collected based on the peaks monitored by a UV detector set at 280 nm. For lentil and black soybean, four fractions were collected: F1 and F2 (water eluted), F3 (50% ethanol eluted), and F4 (50% acetone eluted). For black turtle bean, three fractions were collected: F1 (water eluted), F2 (50% ethanol eluted) and F3 (50% acetone eluted). After removing the solvent from all fractions by vacuum rotary evaporator at 38 °C, the concentrate was lyophilized.
2.7. Determination of total phenolic content (TPC)
Total phenolic content was determined with Folin-Ciocalteu assay (Singleton & Rossi, 1965) with slight modification (Xu & Chang, 2007). The absorbance was measured at 765 nm by a 96-well plate reader (Flex Station 3.0, Molecular Devices, CA, USA). TPC was expressed as milligrams of gallic acid equivalents per gram of dry sample (mg of GAE/g).
2.8. Determination of total flavonoid content (TFC)
Total flavonoid content was determined according to Jia, Tang, and Wu (1999). The absorbance was measured at 510 nm by a 96-well plate reader (Flex Station 3.0, Molecular Devices). TFC was expressed as milligrams of (+)-catechin equivalents per gram of dry sample (mg of CAE/g).
2.9. Determination of condensed tannin content (CTC)
Condensed tannin was determined according to Broadhurst and Jones (1978) with slight modification by our laboratory (Xu & Chang, 2007). The absorption was measured at 500 nm against methanol as the blank by a 96-well plate reader (Flex Station 3.0, Molecular Devices). CTC was expressed as milligrams of (+)-catechin equivalents per gram of dry sample (mg of CAE/g).
2.10. Determination of DPPH free radical scavenging activity
DPPH free radical scavenging activity assay was conducted according to the method of Chen and Ho (1995) with slight modification by our laboratory (Xu & Chang, 2007). The absorbance was measured at 517 nm by a 96-well plate reader (Flex Station 3.0, Molecular Devices). The DPPH scavenging activity was expressed as micromoles of Trolox equivalents per gram of dry sample (μmol of TE/g).
2.11. Determination of oxygen radical absorbing capacity (ORAC)
ORAC assay was conducted according to the method reported by Prior et al. (2003) with some modifications. The test was done by a plate reader (Flex Station 3, Molecular Devices). Twenty microliters of a series of concentrations of Trolox standard solutions in 75 mM phosphate buffer (pH 7.0), blank (water), and properly diluted samples were added into the wells of a black 96-well Costar plate. After incubation in the chamber at 37 °C for 20 min, two hundred microliters of 94 μM sodium fluorescein in phosphate buffer and twenty microliters of 0.16 M 2,2′-azobis (2-methylpropionamidine) dihydrochloride (AAPH) in phosphate buffer were added into the wells. Before addition, the fluorescein solution was kept at 37 °C in water bath and AAPH was dissolved in buffer immediately before adding to the plate. The plate was immediately put back into the chamber of plate reader and was read. The fluorescence filters were set at 485 nm for excitation and 520 nm for emission. Kinetic reading was recorded for 121 cycles with 30 s each. The calculation was done by the SoftMax Pro Software. The result was expressed as micromoles of Trolox equivalents per gram of dry sample (μmol of TE/g).
2.12. ACE inhibitory activity analysis
ACE inhibitory activity was determined according to Wu and Ding (2002) with slight modification. A portion of 60 μL of ACE (10 mU/mL) in 100 mM borate buffer (pH 8.3, 300 mM NaCl) was mixed with 30 μL of sample or water (for control) and the mixture was let to stand for 10 min at room temperature. A portion of 60 μL of 2.5 mM hippuryl-histidyl-leucine (HHL) in the borate buffer was added and the reaction mixture was kept at 37 °C for 30 min. The reaction was stopped by boiling at 85 °C for 10 min. The reaction mixture was filtered through a 0.20 μm nylon syringe filter into HPLC vials for HPLC analysis. Hippuric acid (HA) was separated and quantified by Agilent 1260 Infinity HPLC system (Agilent Technologies, Santa Clara, CA, USA). Agilent Eclipse XDB-C18 column (4.6 × 150 mm; particle size 5 μm; Agilent Technologies, Rising Sun, MD, USA) was used and the wavelength of diode array detector (DAD) was set at 228 nm. Mobile phases included solvent A (0.1% trifluoroacetic acid in water) and solvent B (0.1% trifluoroacetic acid in acetonitrile). The elution gradient was as follow: 0–10 min, 5–60% B; 10–12 min, 60% B; 12–13 min, 60–5% B. The percentage of ACE inhibition was calculated according to the following equation:
where HAsample denotes the content of HA when inhibitor was present; HAcontrol denotes the content of HA when inhibitor was replaced by water. The IC50 value was defined as the concentration to inhibit 50% ACE activity and was calculated by GraphPad Prism software based on five concentrations.
2.13. HPLC analysis of phenolic acids from fractions
HPLC analysis of phenolic acids was performed according to Robbins and Bean (2004) with slight modification by our lab (Xu & Chang, 2009). In current study, an Agilent 1260 Infinity HPLC system (Agilent Technologies) equipped with a DAD was used. Extracts and fractions were dissolved in water and filtered through 0.20 μm nylon syringe filter before loading. Wavelengths of 270 and 325 nm, which are representative of absorbance peaks of hydroxybenzoic and hydroxycinnamic acids, respectively, were chosen. An Agilent ZORBAX SB-C18 column (4.6 × 250 mm; particle size 5 μm; Agilent Technologies, Rising Sun, MD, USA) was used. Peak width was set at 0.02 for integrator to distinguish peaks from base line noise. Identification was accomplished by comparing retention time and spectrum with phenolic acid standards. Contents of individual phenolic acids were expressed as micrograms per gram of dry sample (μg/g).
2.14. HPLC analysis of flavonoids and condensed tannins
HPLC analyses of flavonoids and condensed tannins were conducted according to Murphy, Song, Buseman, and Barua (1997) with slight modification by our lab (Xu & Chang, 2009). In current study, an Agilent 1260 Infinity HPLC system (Agilent Technologies) equipped with a DAD was used. Extracts and fractions were dissolved in water and filtered through 0.20 μm nylon syringe filter before loading. Wavelengths of 279 and 262 nm which are representative of absorbance peaks of flavan-3-ol monomers/oligomers and flavonols were selected. An Agilent ZORBAX SB-C18 column (4.6 × 250 mm; particle size 5 μm; Agilent Technologies) was used. Peak width was set at 0.02 for integrator to distinguish peaks from base line noise. Identification was conducted by comparing retention time and spectrum with flavonoid and condensed tannin standards. Contents of detected compounds were expressed as micrograms per gram of dry sample (μg/g).
2.15. Identification of phenolic compounds with LC-MSn analysis
Small molecules extracts were subjected to nano LC-MSn analysis using Ultimate 3000 HPLC system and LTQ Orbitrap Velos mass spectrometer (both by ThermoFisher Scientific, Waltham, MA, USA). A 45-min linear acetonitrile gradient from 2 to 55% was employed at the flow rate of 0.3 μL min−1, using Acclaim C18 column (75 μm × 15 cm, particle size 2 μm; ThermoFisher Scientific). Mass spectra were measured in positive mode at highest Orbitrap resolution of 100,000. The replicate spectral data were analyzed by the peak alignment and feature (combination of ions retention time, RT, and mass to charge ratio, m/z) detection software SIEVE 2.1 (ThermoFisher Scientific). Derived monoisotopic Mr. values were scanned against a custom database comprised of monoisotopic masses of known phenolic compounds using the “small molecule identification” module of the SIEVE software. Matches were filtered by delta mass < 10 ppm. These tentative identifications (based on accurate mass only) were further followed by LC-MSn analysis (measuring mass spectra of fragmented precursors to obtain molecule structural information). Two approaches were utilized: (a) the mass trees of commercially available standards were measured via direct injection method using LTQ Orbitrap Velos mass spectrometer. The method selected the non-fragmented molecule, subjected it to collision induced decay (CID) and measured the fragment spectra (MS2). Three most abundant peaks in MS2 spectra were selected for another fragmentation and spectra were measured (MS3). In analogy, spectra up to MS5 (in some cases) were measured, creating structure information rich mass trees for given standard. Mass trees were uploaded to Mass Frontier 7.0 (HighChem, Bratislava, SK) software and compiled into the custom database. The LC-MSn raw files of particular samples were then manually examined using Mass Frontier software. Mass trees respective to monoisotopic masses of tentatively identified compounds were compared to custom mass tree database of known standards. “Identity search” option was selected, and the HighChem high resolution algorithm (with default parameters) of Mass Frontier was used for high confidence matching. Particular MSn spectra were also exported from Mass Frontier and manually searched against the mzCloud on-line database (mzcloud.org; 5893 compounds as of Nov 03, 2016), using the HighChem high resolution algorithm, and “From Source Accuracy” (derived from raw spectral files). (b) The LC-MSn raw files were also analyzed using the Compound Discoverer 2.0 (Thermo Scientific) software, which allows for automated generation of MSn spectra, their export to, and matching against the mzCloud database. The “Unknown detection in single file” workflow was used, comprising six processing nodes: Input files, Select spectra, Detect Unknown Compounds, Group Unknown Compounds, Predict Compositions, and Search mzCloud. The most critical parameter of Mass Tolerance was set to 5 ppm. Only compounds matched by at least one of the (a) or (b) approaches were considered as positively identified.
2.16. Statistical analysis
Data were subject to analysis of variance (ANOVA) with SAS 9.4 package. Significant differences among variables were determined by Duncan’s multiple range test (α = 0.05). Data were expressed as means ± SD (n = 3).
3. Results and discussion
3.1. Effects of processing, purification, and fractionation on phenolic contents, antioxidant capacities, and mass yields of three legume varieties
In this study, only water soluble phenolics in extracts and fractions were characterized. However, the water soluble phenolics in freeze-dried crude extracts made up the majority of the extracted phenolic compounds by aqueous organic solvent. For example, as shown in Table 1, in the case of raw lentil, 13.81 g of crude extract was obtained from 100 g of raw material. Water soluble TPC from the 13.81 g of crude lentil extract was 534 mg GAE, which was about 94% of TPC extracted from 100 g of raw lentil (570 mg GAE). In this study, differences in phenolic content from other studies in the literature existed. This is reasonable with consideration of different legume materials used. As shown in Table 1, for all legume varieties, either raw or cooked, crude phenolic extracts contained significantly higher phenolic contents and antioxidant activities compared with legume seeds. For example, with raw lentil, TPC, TFC, CTC, DPPH and ORAC values in crude extract were 6.8, 4.4, 7.7, 28.5 and 6.7 times those values in seeds; for cooked lentil, these values were 7.8, 3.9, 2.9, 7.4 and 6.8 times those in seeds. After removal of proteins, sugars and other water-soluble components through XAD-7 packed column, the phenolics were further concentrated in semi-purified extracts. For raw lentil, TPC, TFC, CTC, DPPH and ORAC values were further increased by 11.7, 12.0, 10.5, 8.3 and 9.9 times, respectively; for cooked lentil, these parameters were improved by 14.0, 21.9, 19.5, 16.3 and 18.3 times, respectively. The uneven increase of the above parameters substantiated the disparity of the reaction mechanisms, on which the assays were based. Therefore, different assays were required to elucidate phenolic composition and antioxidant potentials from different angles. The highly concentrated phenolics make crude extracts and semi-purified extracts a better choice to be incorporated into functional and nutraceutical foods. Even though the mechanism is not clear, it is known that only about 5% of phenolic intake in the diet can be absorbed and the rest will pass to the large intestine for microflora fermentation (Clifford, 2004). Given the low phenolic absorption in the human body, it should be health-beneficial to ingest food supplements with higher level of phenolics because health effects of phenolics are dependent on intake and their bioavailability (Bohn, 2014). After fractionation, water-eluted fractions (F1 and F2 for lentil and black soybean; F1 for black bean) showed the lowest phenolic content and antioxidant activity for all legume varieties. That should be due to high content of sugars and proteins, which were firstly eluted with water. In fact, high level of protein (5–10%) still remained in the semi-purified extracts (Table 2). However, in most cases, 50% acetone-eluted fractions, which were predominantly composed of proanthocyanidins exhibited highest antioxidant potential. Except the fraction from cooked black soybean, all other 50% ethanol-eluted fractions showed high CTC values ranging from 250.0 to 470.8 mg CAE/g. In our current fractionation system with Sephadex-LH 20, condensed tannins should be mainly present in the fraction eluted by 50% acetone. These high CTC values must come mainly from flavanol monomers which can also react with vanillin due to the lack of specificity of vanillin/HCl assay (Schofield, Mbugua, & Pell, 2001). The assumption was substantiated by our following assay presented in Table 4, which shows that in the 50% ethanol-eluted fraction from raw black soybean, the epicatechin content was extremely high: 97,343 μg/g.
Table 1.
Mass yields, phenolic contents, and antioxidant capacities of extracts and fractions from three legume varieties.
| Fractions | TPC mg GAE/g | TFC (mg CAE/g) | CTC (mg CAE/g) | DPPH (μmol TE/g) | ORAC (μmol TE/g) | Mass Yield (g/100 g) | TPC Recovery (mg GAE/100 g) | % TPC Recovery | ||
|---|---|---|---|---|---|---|---|---|---|---|
| Lentil | Raw | Seed | 5.7 ± 0.1gA1 | 3.1 ± 0.0fA2 | 5.8 ± 0.3eA1 | 12.5 ± 0.3fA2 | 114.1 ± 6.2fA1 | |||
| CE | 38.7 ± 0.0fA1 | 14.1 ± 0.1dA1 | 44.7 ± 0.1dA1 | 356.8 ± 8.9dA1 | 761.2 ± 12.4eA1 | 13.81 | 534.4 | 100.00 | ||
| SPE | 491.3 ± 3.1bA1 | 184.0 ± 0.6aA2 | 513.8 ± 10.1bA2 | 3308.8 ± 51.6bA2 | 8268.8 ± 159.1cA1 | 1.00 | 491.3 | 91.93 | ||
| F1 | 55.8 ± 0.7eA | 5.4 ± 0.2eA | 6.7 ± 0.2eA | 64.8 ± 1.4eB | 962.0 ± 36.1eA | 0.18 | 10.0 | 1.88 | ||
| F2 | 114.1 ± 2.8dB | 13.8 ± 0.1dA | 9.8 ± 0.3eB | 92.7 ± 2.9eB | 3785.5 ± 79.5dB | 0.18 | 20.5 | 3.84 | ||
| F3 | 382.4 ± 2.5cB5 | 46.1 ± 0.7cB6 | 361.7 ± 5.9cB4 | 3027.8 ± 7.3cB4 | 8759.8 ± 307.4bB3 | 0.12 | 45.9 | 8.59 | ||
| F4 | 597.8 ± 5.1aB3 | 66.2 ± 0.7bB6 | 674.8 ± 24.0aB2 | 4802.9 ± 12.7aB2 | 10526.9 ± 263.2aB2 | 0.30 | 179.3 | 33.56 | ||
| Cooked | Seed | 2.6 ± 0.0gB3 | 1.4 ± 0.1fB5 | 5.1 ± 0.3deB2 | 12.4 ± 0.0fA2 | 51.0 ± 1.8gB4 | ||||
| CE | 20.2 ± 0.1fB4 | 5.5 ± 0.4dB5 | 14.8 ± 0.1dB4 | 91.6 ± 0.9eB5 | 349.3 ± 1.4fB5 | 11.12 | 224.6 | 100.00 | ||
| SPE | 303.1 ± 0.7cB4 | 125.7 ± 1.0cB3 | 303.8 ± 0.7cB3 | 1586.3 ± 13.0cB4 | 6738.9 ± 133.5cB2 | 0.55 | 166.7 | 74.22 | ||
| F1 | 46.3 ± 0.5eB | 2.6 ± 0.0eB | 3.9 ± 0.1eB | 97.2 ± 1.9eA | 957.0 ± 35.2eA | 0.08 | 3.7 | 1.65 | ||
| F2 | 136.6 ± 1.6dA | 2.5 ± 0.0efB | 11.6 ± 0.1deA | 158.3 ± 4.4dA | 5134.6 ± 178.6dA | 0.13 | 17.8 | 7.91 | ||
| F3 | 513.2 ± 1.4bA1 | 313.5 ± 1.1aA1 | 470.8 ± 11.5bA1 | 3379.9 ± 40.4bA2 | 14891.7 ± 262.0aA1 | 0.04 | 20.5 | 9.14 | ||
| F4 | 676.6 ± 10.6aA1 | 242.8 ± 0.7bA2 | 796.7 ± 9.5aA1 | 6192.3 ± 40.4aA1 | 12473.7 ± 260.8bA1 | 0.08 | 54.1 | 24.10 | ||
| Black soy-bean | Raw | Seed | 3.2 ± 0.1gA3 | 2.2 ± 0.0gA3 | 3.9 ± 0.0fB4 | 12.3 ± 0.5gA2 | 89.7 ± 2.0gA2 | |||
| CE | 24.6 ± 0.5fA2 | 10.3 ± 0.0fA3 | 14.2 ± 0.2dA5 | 139.5 ± 1.7fA4 | 463.4 ± 3.4fA3 | 19.45 | 478.5 | 100.00 | ||
| SPE | 268.1 ± 7.5cA5 | 108.8 ± 0.4cA5 | 126.5 ± 1.9cA4 | 948.6 ± 8.5cA5 | 4779.9 ± 98.2cA3 | 1.38 | 370.0 | 77.32 | ||
| F1 | 129.6 ± 0.7dA | 19.7 ± 0.1eA | 8.4 ± 0.3eA | 232.9 ± 0.0eA | 2839.1 ± 27.3eA | 0.27 | 35.0 | 7.31 | ||
| F2 | 114.3 ± 0.7eA | 20.9 ± 0.3dA | 0.0 ± 0.0fA | 283.2 ± 4.1dA | 3938.8 ± 63.9dA | 0.02 | 2.3 | 0.48 | ||
| F3 | 410.7 ± 2.2bA3 | 205.8 ± 1.0bA2 | 250.0 ± 2.5bA5 | 1899.3 ± 13.1bA5 | 9703.5 ± 78.2bA2 | 0.11 | 45.2 | 9.44 | ||
| F4 | 636.4 ± 0.0aA2 | 429.4 ± 0.0aA1 | 455.4 ± 5.6aA5 | 2785.1 ± 18.9aA5 | 10693.6 ± 70.4aA2 | 0.07 | 44.5 | 9.31 | ||
| Cooked | Seed | 1.9 ± 0.1fB4 | 1.0 ± 0.0fB6 | 4.5 ± 0.3dA3 | 7.0 ± 0.2gB3 | 62.5 ± 3.4eB3 | ||||
| CE | 8.2 ± 0.0eB5 | 1.61 ± 0.0fB6 | 0.8 ± 0.0fB6 | 18.0 ± 0.1fB6 | 181.3 ± 3.2eB6 | 17.93 | 147.0 | 100.00 | ||
| SPE | 122.4 ± 2.2bB6 | 27.9 ± 0.6cB6 | 18.3 ± 0.1bB5 | 219.7 ± 0.8cB6 | 3099.5 ± 13.6bB5 | 0.70 | 85.7 | 58.29 | ||
| F1 | 84.5 ± 0.6cB | 13.8 ± 0.1dB | 3.4 ± 0.2eB | 116.2 ± 1.1dB | 1953.1 ± 51.9dB | 0.27 | 22.8 | 15.52 | ||
| F2 | 53.9 ± 0.1dB | 10.9 ± 0.3eB | 0.0 ± 0.0gA | 102.4 ± 2.9eB | 2311.3 ± 123.7cB | 0.04 | 2.2 | 1.47 | ||
| F3 | 161.5 ± 2.7aB6 | 59.4 ± 0.6bB5 | 16.9 ± 0.0cB6 | 476.6 ± 8.2bB6 | 5913.3 ± 246.8aB6 | 0.13 | 21.0 | 14.28 | ||
| F4 | 160.2 ± 1.7aB6 | 89.4 ± 0.7aB5 | 22.5 ± 0.0aB6 | 899.8 ± 9.6aB6 | 2337.0 ± 66.0cB4 | 0.01 | 1.6 | 1.09 | ||
| Black turtle bean | Raw | Seed | 5.9 ± 0.1fA1 | 3.2 ± 0.2fA1 | 5.2 ± 0.4fA2 | 12.1 ± 0.3fB2 | 67.9 ± 1.7fA3 | |||
| CE | 39.0 ± 0.1eA1 | 12.1 ± 0.1eA2 | 40.9 ± 0.4dA2 | 290.9 ± 1.1dA2 | 506.4 ± 6.1eA2 | 14.08 | 549.1 | 100.00 | ||
| SPE | 479.1 ± 3.8aA2 | 196.8 ± 0.6bA1 | 566.8 ± 6.6bA1 | 3836.1 ± 26.6bA1 | 6824.8 ± 30.0bA2 | 0.97 | 464.7 | 84.63 | ||
| F1 | 87.3 ± 0.6dB | 24.5 ± 0.1dB | 19.4 ± 1.0eA | 228.6 ± 2.2eB | 2155.0 ± 19.3dB | 0.23 | 20.1 | 3.66 | ||
| F2 | 396.7 ± 1.8cB4 | 160.0 ± 0.0cB4 | 405.5 ± 1.4cA2 | 3245.3 ± 15.2cB3 | 7355.2 ± 146.6aA4 | 0.14 | 55.5 | 10.11 | ||
| F3 | 461.9 ± 0.4bB5 | 222.5 ± 1.8aA3 | 603.6 ± 3.8aA3 | 4259.8 ± 20.1aB4 | 6279.9 ± 12.9cB3 | 0.24 | 110.9 | 20.19 | ||
| Cooked | Seed | 3.4 ± 0.1fB2 | 2.0 ± 0.1fB4 | 3.9 ± 0.0eB4 | 18.4 ± 1.1fA1 | 42.0 ± 1.1fB5 | ||||
| CE | 21.3 ± 0.1eB3 | 6.3 ± 0.0eB4 | 18.6 ± 0.1dB3 | 147.2 ± 1.1eB3 | 362.0 ± 3.6eB4 | 13.26 | 282.4 | 100.00 | ||
| SPE | 325.8 ± 3.1cB3 | 118.1 ± 0.3cB4 | 303.8 ± 6.4cB3 | 2043.6 ± 30.9cB3 | 4575.8 ± 61.3cB4 | 0.75 | 244.4 | 86.53 | ||
| F1 | 119.8 ± 1.2dA | 30.1 ± 0.1dA | 18.4 ± 0.4dA | 284.7 ± 1.6dA | 3108.3 ± 22.5dA | 0.18 | 21.6 | 7.64 | ||
| F2 | 455.7 ± 3.3bA2 | 169.8 ± 2.4bA3 | 377.7 ± 6.5bB3 | 3420.5 ± 7.3bA1 | 6864.2 ± 34.5aB5 | 0.08 | 36.5 | 12.91 | ||
| F3 | 515.3 ± 3.4aA4 | 215.6 ± 1.1aB4 | 530.6 ± 4.7aB4 | 4343.8 ± 7.3aA3 | 6566.1 ± 94.0bA3 | 0.07 | 36.1 | 12.77 |
Values are expressed as mean ± SD (n = 3); values followed by different lowercase letters are significantly different (p < 0.05) among different extracts and fractions within the same variety and processing condition (raw or cooked); values followed by different capital letters are significantly different (p < 0.05) between processing conditions within the same type of extract or fraction and variety; values followed by different numbers are significantly different (p < 0.05) across variety and processing condition but within the same type of extract or fraction. Seed: determined from the aqueous organic extract and expressed on seed weight; mass yield: the yield in grams per 100 g of bean on dry basis; TPC recovery: total phenolic content of extracts or fractions derived from 100 g of legume material; CE: crude extract; SPE: semi-purified extract; F1: fraction one; F2: fraction two; F3: fraction three; F4: fraction four. TPC: total phenolic content; TFC: total flavonoid content; CTC: condensed tannin content; DPPH: DPPH free radical scavenging activity; ORAC: oxygen radical absorption capacity.
Table 2.
Protein contents of crude extracts and semi-purified extracts (%).
| Crude extracts | Semi-purified extracts | |
|---|---|---|
| Raw lentil | 8.07 ± 0.05 | 8.55 ± 0.21 |
| Cooked lentil | 8.13 ± 0.11 | 8.76 ± 0.33 |
| Raw black soybean | 4.81 ± 0.10 | 4.76 ± 0.06 |
| Cooked black soybean | 5.11 ± 0.16 | 4.98 ± 0.13 |
| Raw black turtle | 9.78 ± 0.22 | 9.59 ± 0.20 |
| Cooked black turtle | 9.89 ± 0.19 | 9.59 ± 0.30 |
Values are expressed as means ± SD (n = 2).
Table 4.
Flavonoid contents in fractions and legume seeds (μg/g).
| Catechin | Epicatechin | Quercetin-3 glucoside | Kaempferol-3 glucoside | Kaempferol-3 rutinoside | Myricetin | ||
|---|---|---|---|---|---|---|---|
| Lentil | FRLS | ND | 3537 ± 34bD | ND | ND | ND | ND |
| RLS | ND | 4.2 ± 0.04aB | ND | ND | ND | ND | |
| FCLS | ND | 9014 ± 518aB | ND | ND | ND | ND | |
| CLS | ND | 3.86 ± 0.22bC | ND | ND | ND | ND | |
| Black soybean | FRLS | ND | 97,343 ± 1130A | 1897 ± 28C | ND | ND | ND |
| RLS | ND | 103.36 ± 1.2A | 2.01 ± 0.03C | ND | ND | ND | |
| FCLS | ND | ND | ND | ND | ND | ND | |
| CLS | ND | ND | ND | ND | ND | ND | |
| Black turtle ean | FRLS | ND | 2493 ± 49bE | 4106 ± 100aA | 2550 ± 27aA | 1509 ± 64bB | 1642 ± 66aA |
| RLS | ND | 3.50 ± 0.07aD | 5.76 ± 0.14aA | 3.58 ± 0.04aA | 2.12 ± 0.09aA | 2.30 ± 0.09aA | |
| FCLS | 23,961 ± 538 | 4094 ± 122aC | 3426 ± 93bB | 2302 ± 86bB | 2236 ± 134aA | 1357 ± 15bB | |
| CLS | 19.60 ± 0.44 | 3.35 ± 0.1bD | 2.80 ± 0.08bB | 1.88 ± 0.07bB | 1.83 ± 0.11bB | 1.11 ± 0.01bB |
Values are expressed as mean ± SD (n = 3); values followed by lowercase letters are significantly (p < 0.05) different between processing conditions (raw and cooked) within the same legume variety and sample type (legume seed or fraction). Values followed by different capital letters are significantly (p < 0.05) different across variety and processing condition within the same sample type. Flavonoid content of each type of legume seed (raw and cooked) was estimated based on the flavonoid content and the mass yield of the fraction derived from the legume seed. ND: not detected; FRLS: fraction from raw legume seed; RLS: raw legume seed; FCLS: fraction from cooked legume seed; CLS: cooked legume seed.
As shown in Table 1, cooking not only affected phenolic contents and antioxidant capacities, but also mass yields (grams of extracts or fractions from 100 g of legume material). The heating mediated-changes were variety dependent. Cooking reduced mass yields in crude and semi-purified extracts and most fractions for the three legume varieties. On the contrary, Siddhuraju and Becker (2007) found the crude extract yields of cowpea after dry heating and soaking plus autoclaving were increased. This study is the first in reporting cooking effect on the yields of crude extracts. The different mass yield changes induced by thermal treatments were likely due to the different legume varieties and the processing (cooking) methods employed.
If we order the phenolic contents and antioxidant capacities within the same type of extract or fraction but across varieties and processing conditions (raw and cooked) as indicated by the significant labels in Table 1, the orders changed greatly, which suggested the disparity of legume composition. In addition, as shown in Table 1, legume variety factor contributed substantially to the differences in the mass yields of extracts and fractions. Karamac, Kosinska, Rybarczyk, and Amarowicz (2007) also observed the great variation of extract yields among legume varieties including red and green lentils. However, the extraction yields of these two lentils were only 40% of the lentil yields observed in our current study, which suggested the substantial effect of extraction protocols. In comparison with the other two legume varieties, cooked black soybean showed extremely lower mass yield and CTC (0.01 g/100 g and 22.5 mg CAE/g, respectively) in F4. This might be due to heat-induced complexation between condensed tannins and protein (Awika, Dykes, Gu, Rooney, & Prior, 2003). It is commonly known that soybean contained much higher protein than the other two legumes. The low level of condensed tannins makes the extracts from cooked black soybean a better choice for consumption in view of the low protein digestibility that may be induced by condensed tannins (Gilani, Xiao, & Cockell, 2012).
For both raw and cooked black soybean, F2 possessed lower (p < 0.05) total phenolic content than F1. This phenomenon can be explained by the non-specificity of Folin-Ciocalteu assay. In the Sephadex LH-20 elution, sugars and proteins were eluted first and these compounds could react with Folin-Ciocalteu reagent to increase TPC values (Singleton, Orthofer, & Lamuela-Raventos, 1999). Our results showed that the semi-purified black soybean fractions still contained about 5% nitrogen materials, which presumably are protein/peptides as determined by Kjeldhal (Table 2).
In order to investigate the stepwise phenolic loss during semi-purification and fractionation, total phenolic content (TPC) of crude extracts, semi-purified extracts and fractions derived from 100 g of legume materials were presented in Table 1. In addition, the total phenolic content from crude extracts was expressed as 100% and the percentage recoveries of following semi-purified extracts and fractions were also calculated (Table 1). The phenolic loss, on the one hand, was due to the freeze-drying step, which made some extract insoluble as evidenced by the residue observed after centrifugation. On the other hand, the degradation of phenolics during the lengthy extraction and fractionation process could occur. As shown in Table 1, percentages of TPC recovery was not only affected by thermal treatment, but was also by variety. After fractionation, for raw lentil, cooked lentil and raw black turtle bean, F4 predominantly represented 33.56, 24.10 and 20.19% TPC recovery, respectively. This high recovery is consistent with the strong correlation (r = 0.997, p < 0.05) between CTC and TPC in seed, crude extract and semi-purified extracts, because F4 was mainly composed of condensed tannins.
3.2. ACE inhibitory activity of crude extracts from three legumes varieties
High ACE inhibition was only found in crude extracts. This observed phenomenon was contrary to our initial hypothesis that the more concentrated or purified extracts might be more potent in the ACE inhibition. Afonso et al. (2013) found that oligomeric procyanidin-rich extracts from grape seeds showed much higher ACE inhibitory activity than pure flavanols. Ojeda et al. (2010) demonstrated that water extract from Hibiscus sabdariffa had only less than a half of the IC50 of the anthocyanin-rich fraction indicating the crude water extract was more potent against ACE. Our recent study (Tan et al., 2017) demonstrated that phenolic extracts and fractions from black soybean and black turtle bean had higher inhibitory activity against α-amylase and lipase than most of pure phenolic compounds tested. Based on our previous and current findings, it could be postulated that there must be some synergism among phenolic compounds in the inhibition against ACE. In order to verify this speculation, protocatechuic acid, which was reported to possess high ACE inhibitory activity by Al Shukor et al. (2013) was tested with the same protocol as used for extracts and fractions. The IC50 of protocatechuic acid was over 1000 μg/g, which was much higher than crude extracts. Another explanation was the presence of peptides in the crude extracts. In order to confirm this, the protein contents of the crude and semi-purified phenolic extracts were measured by the Kjeldahl method. All extracts were confirmed to contain certain level of proteins (Table 2). Actually, these nitrogen-containing substances were most likely peptides or other reactive compounds that were soluble in the organic solvent, because large proteins were not likely soluble in organic solvent. ACE inhibition by peptides present in various legumes, including lentil and soybean have been found (Garcia-Mora et al., 2015; Mamilla & Mishra, 2017). The possible peptides involved in the ACE inhibition will be further explored in our future research. Our current study is the first to report the decreased ACE inhibition after purification and fractionation from crude phenolic extracts and further investigation is needed to elucidate the underlying mechanism.
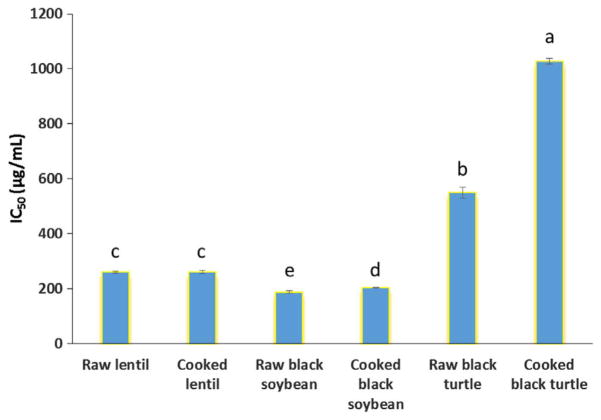
As shown in Fig. 1, the crude extracts from black soybean had the highest ACE inhibitory activity (lowest IC50 values), followed by lentil, and then by black turtle bean. For lentil, cooking had no effect on the ACE inhibitory activity, which might partly explain our previous animal study that cooked and raw lentil crude extracts had no significant differences in the attenuation of angiotensin II-induced blood pressure elevation, peripheral vascular remodeling and perivascular fibrosis (Xuan et al., 2013). For black soybean and black turtle bean, cooking significantly (p < 0.05) decreased ACE inhibitory activities of crude extracts. In particular, for black turtle bean, the IC50 value was almost doubled after cooking, indicating a 50% reduction in potency. IC50 values of protein hydrolysates after simulated in vitro gastrointestinal digestion from lentil, soybean and black bean have been reported as 430, 380 and 150 μg/mL, respectively (Jakubczyk & Baraniak, 2013; Lo, Farnworth, & Li-Chan, 2006; Rui, Boye, Barbana, Simpson, & Prasher, 2012). However, if the enzyme activity and enzyme/sample ratios are considered, crude phenolic extracts from our study exhibited more potent ACE inhibition in comparison with their respective protein hydrolysate counterparts. When we correlated the IC50 values presented in Fig. 1 with the corresponding TPC values in Table 1, we found that these two parameters were not significantly correlated, suggesting ACE inhibitory activity might be more related to the composition of the extract than the sum (total content) of the phenolics.
Fig. 1.
IC50 values of ACE inhibitory activity of crude extracts. Different letters above bars mean significant difference (p < 0.05).
3.3. Identification and quantification of phenolic acids through purification and fractionation
After purification and fractionation, some phenolic acids, which could not be identified due to co-elution or low levels, were identified and accurately quantified with improved purity. These phenolic acids were found in 50% ethanol-eluted fractions (F3 for lentil and black soybean; F2 for black turtle bean). As shown in Table 3, for lentil, five phenolic acids were identified and quantified and their contents were all significantly (p < 0.05) increased by cooking. Gallic acid and protocatechuic aldehyde were only detected after cooking, suggesting these two phenolic acids only existed in conjugated or bound forms and can be released from complexation with other compounds during thermal treatment. Our conclusion was consistent with the observations of Duenas, Hernandez, and Estrella (2007), who found gallic acid only when lentil was subjected to exogenous enzymes. This study, with another approach, demonstrated that gallic acid did not exist in free form in lentil. Three phenolic acids were identified and quantified in black soybean. The fraction from raw black soybean contained significantly (p < 0.05) higher protocatechuic acid than that from cooked black soybean, but when the protocatechuic acid contents were expressed on the basis of seed weight, opposite trends appeared due to the mass yield differences. p-Coumaric acid and syringic acid were detected only after cooking, implying the heat-induced liberation. For black turtle bean, raw seed and cooked seed showed equal contents of gallic acid. However, raw seed had higher contents of ferulic acid and sinapic acid than cooked seed, indicating heat-induced degradation.
Table 3.
Phenolic acid contents in fractions and legume seeds (μg/g).
| Gallic acid | Protocatechuic acid | Protocatechuic aldehyde | p-hydroxybenzoic acid | p-Coumaric acid | Syringic acid | Ferulic acid | Sinapic acid | ||
|---|---|---|---|---|---|---|---|---|---|
| Lentil | FRLS | ND | 166 ± 25bD | ND | 449 ± 25bB | 1064 ± 15bC | ND | ND | ND |
| RLS | ND | 0.20 ± 0.03bD | ND | 0.54 ± 0.03bB | 1.29 ± 0.02bC | ND | ND | ND | |
| FCLS | 988 ± 47C | 1483 ± 71aC | 4.9 ± 0.1 | 2865 ± 116aA | 3582 ± 258aA | ND | ND | ND | |
| CLS | 0.42 ± 0.02B | 0.63 ± 0.03aC | 0.002 ± 0.00 | 1.23 ± 0.05aA | 1.53 ± 0.11aB | ND | ND | ND | |
| Black soybean | FRLS | ND | 10,149 ± 161aA | ND | ND | ND | ND | ND | ND |
| RLS | ND | 10.78 ± 0.17bB | ND | ND | ND | ND | ND | ND | |
| FCLS | ND | 8600 ± 88bB | ND | ND | 1998 ± 38B | 829 ± 1 | ND | ND | |
| CLS | ND | 11.2 ± 0.11aA | ND | ND | 2.60 ± 0.05A | 1.08 ± 0.00 | ND | ND | |
| Black turtle bean | FRLS | 3307 ± 86bB | ND | ND | ND | ND | 3394 ± 71bB | 840 ± 10bB | |
| RLS | 4.64 ± 0.12aA | ND | ND | ND | ND | ND | 4.76 ± 0.10aA | 1.18 ± 0.01aA | |
| FCLS | 5641 ± 322aA | ND | ND | ND | ND | ND | 3897 ± 49aA | 1140 ± 25aA | |
| CLS | 4.53 ± 0.26aA | ND | ND | ND | ND | ND | 3.19 ± 0.04bB | 0.92 ± 0.02bB |
Values are expressed as mean ± SD (n = 3); values followed by lowercase letters are significantly (p < 0.05) different between processing conditions (raw and cooked) within the same legume variety and sample type (legume seed or fraction). Values followed by different capital letters are significantly (p < 0.05) different across variety and processing condition within the same sample type. Phenolic acid content of each type of legume seed (raw and cooked) was estimated based on the phenolic acid content and the mass yield of the fraction derived from the legume seed. ND: not detected; FRLS: fraction from raw legume seed; RLS: raw legume seed; FCLS: fraction from cooked legume seed; CLS: cooked legume seed.
3.4. Identification and quantification of flavonoids through purification and fractionation
In the three legume varieties, flavonoids were detected in the fractions eluted by 50% ethanol (F3 for lentil and black soybean; F2 for black turtle bean). As shown in Table 4, in lentil, only epicatechin was detected. The epicatechin content from the cooked lentil fraction was 2.8 times that from the raw lentil fraction. However, when expressed on the basis of lentil seed with consideration of mass yield, the epicatechin content of cooked lentil was lower than that of raw lentil. Two flavonoids were identified and quantified from black soybean. Epicatechin was only detected in raw black soybean and the content was up to 97,343 μg/g. The absence of epicatechin in cooked black soybean might be due to the heating-induced oxidative condensation or decomposition (Asami, Hong, Barrett, & Mitchell, 2003). As with epicatechin, quercetin-3-glucoside also showed thermal susceptibility. Correa et al. (2010) identified quercetin-o-hexoside in black soybean with LC/ESI/MS, but the sugar moiety attached was not specified. In black turtle bean, six flavonoids were identified and quantified. Catechin was only found in cooked sample, suggesting the heating-induced depolymerization of condensed tannins. For all flavonoids, thermal processing had significant (p < 0.05) effect on their contents, both in fractions and seeds. The different change patterns of these compounds upon thermal process reflected their different thermal stability and forms of existence. Among the three legumes, myricetin only existed in black turtle bean. Our previous study (Tan et al., 2017) had shown myricetin was the most potent phenolic compound against α-amylase, α-glucosidase and lipase. Therefore, the fractions, either from raw or from cooked black turtle bean, might be a promising therapeutic approach to diabetes through the inhibition against the above enzymes.
3.5. Identification and quantification of condensed tannins through purification and fractionation
In this study, four procyanidin standards were used. Because procyanidin A2 was not found in all varieties, it was not included in Table 5. Condensed tannin predominantly existed in 50% acetone-eluted fractions (F4 for lentil and black soybean and F3 for black turtle bean). The spectrum analysis showed that most of the peaks in these fractions exhibited similar pattern: absorbance peaks at about 232 and 279 nm, which are representative of condensed tannins. However, the three legume varieties showed different chromatographic profiles not only in the number of peaks, but also in the retention time of peaks. This chromatographic disparity implied the compositional differences of condensed tannins. For the same legume variety, the fractions from raw and cooked materials also exhibited great discrepancy, suggesting the effect of thermal process. For lentil, only procyanidin C1 was identified and quantified. As shown in Table 5, procyanidin C1 content from cooked lentil fraction was more than two times that from raw lentil fraction. However, after correction for mass yield, the procyanidin content from cooked lentil seed was significantly (p < 0.05) lower than that from raw lentil seed. Bartolome, Estrella and Hernandez (1997) also found procyanidin C1 in lentil, but the content reported was only about one tenth of the content of our current study. In black soybean, procyanidin B2 and procyanidin C1 were detected and quantified. However, they existed only in raw black soybean. The disappearance of these two condensed tannins after thermal process might be due to the interaction of condensed tannins with macronutrients, especially protein during the long processing duration (50 min 100 °C steaming) (Awika et al., 2003; Le Bourvellec & Renard, 2012). For the same type of condensed tannin C1, as described above, in lentil, even though cooking significantly reduced its content, it did not vanish. The differences might be partly attributed to the differences of composition and heating conditions of the two varieties. The contents of protein, which had the high affinity for tannins, of lentil and black soybean were 22.63 and 37.59%, respectively (data not shown). The much longer exposure to heating (50 min steaming) of black soybean could enhance the interaction between tannins and macronutrients either non-covalently or covalently (Le Bourvellec & Renard, 2012). In black turtle bean, only procyanidin B1 was identified after cooking, suggesting this compound existed only in bound form, which was released by heating or was formed from higher MW condensed tannins by depolymerization.
Table 5.
Condensed tannin contents in fractions legume seeds (μg/g).
| Procyanidin B1 | Procyanidin B2 | Procyanidin C1 | ||
|---|---|---|---|---|
| Lentil | FRLS | ND | ND | 731 ± 38bC |
| RLS | ND | ND | 2.17 ± 0.11aB | |
| FCLS | ND | ND | 1792 ± 129aB | |
| CLS | ND | ND | 1.39 ± 0.10bC | |
| Black soybean | FRLS | ND | 1186 ± 48 | 8287 ± 376A |
| RLS | ND | 0.86 ± 0.03 | 5.99 ± 0.27A | |
| FCLS | ND | ND | ND | |
| CLS | ND | ND | ND | |
| Black turtle bean | FRLS | ND | ND | ND |
| RLS | ND | ND | ND | |
| FCLS | 2454 ± 139 | ND | ND | |
| CLS | 1.72 ± 0.10 | ND | ND |
Values are expressed as mean ± SD (n = 3); values followed by lowercase letters are significantly (p < 0.05) different between processing conditions (raw and cooked) within the same legume variety and sample type (legume seed or fraction). Values followed by different capital letters are significantly (p < 0.05) different across variety and processing condition within the same sample type. Condensed tannin content of each type of legume seed (raw and cooked) was estimated based on the condensed tannin content and the mass yield of the fraction derived from the legume seed. ND: not detected; FRLS: fraction from raw legume seed; RLS: raw legume seed; FCLS: fraction from cooked legume seed; CLS: cooked legume seed.
3.6. Identification of individual phenolic compounds
Some phenolic compounds have been identified from these three legume varieties by other researchers (Bartolome et al., 1997; Correa et al., 2010; Duenas et al., 2007; Hart, Tako, Kochian, & Glahn, 2015; Zhang et al., 2015). However, these reports were inconsistent or even contradictory due to the limitation of methods or equipment employed. In this study, we compared LC retention time and UV spectrum with available external standards after purification and fractionation; and we also used mass spectrometry analysis measuring both accurate monoisotopic masses of intact compounds (MS), and spectra of fragmented compounds (MSn or MS trees) for confident identification. In lentil, black soybean and black turtle bean, 18, 22, and 14 compounds were identified, respectively (Table 6). Even though the overall antioxidant and ACE inhibitory capacities of phenolic extracts result from the collective actions of numerous phenolics related to their specific chemical structure and content, the identified phenolic compounds in Table 6 can still give some insight. For example, the much weaker ACE inhibition of black turtle bean might be partially due to the lack of p-coumaric acid, caffeic acid and procyanidin C1, which are reported to be potent ACE inhibitors (Actis-Goretta, Ottaviani, Keen, & Fraga, 2003; Al Shukor et al., 2013). Some of these compounds have not been reported in the literature and can be used as reference database for later studies. It should be noted that in our current study, only three isoflavone forms were identified, which was different from our previous study (Zhang et al., 2015). This was likely due to different extraction procedures employed for this study, since our current study aimed to extract flavonoids (not isoflavonoids) in legumes for comparison.
Table 6.
Summary of compounds identified in three legume varieties via LC-MSn analysis.
| Compounds | Monoisotopic mass | Lentil | Black soybean | Black turtle bean | |
|---|---|---|---|---|---|
| Phenolic acids | Gallic acid | 170.0215 | x | ||
| Protocatechuic acid | 154.0266 | x | x | ||
| P-Hydroxybenzoic acid | 138.0316 | x | x | ||
| p-Coumaric acid | 164.0473 | x | x | ||
| 2-Hydroxycinnamic acid | 164.0473 | # | # | ||
| 3-Hydroxycinnamic acid | 164.0473 | # | # | ||
| Vanillic acid | 168.0422 | x | x | ||
| Caffeic acid | 180.0422 | x | x | ||
| Ferulic acid | 194.0579 | x | x | x | |
| Syringaldehyde | 182.0579 | x | x | ||
| Trans-cinnamic acid | 148.0524 | x | |||
| Sinapic acid | 224.0684 | x | x | x | |
| Flavonoids | Kaempferol | 286.0477 | x | * | x |
| Epicatechin | 290.0790 | x | x | x | |
| Cyanidin | 287.0550 | * | * | ||
| Kaempferol 3- | 432.1056 | * | * | ||
| rhamnoside | |||||
| Kaempferol-3-O-rutinoside | 594.1585 | x | x | x | |
| Luteolin 7-glucoside | 448.1006 | * | * | * | |
| Kaempferol-3-O-glucoside | 448.1005 | x | x | x | |
| Kaempferol 3-(6 -malonylglucoside) | 534.1010 | x | x | ||
| Quercetin-3-O-glucopyranoside | 464.0950 | x | x | ||
| Myricetin | 464.0950 | x | |||
| Apigenin | 270.0528 | x | x | ||
| Pelargonidin | 270.0528 | * | |||
| Genistein | 270.0528 | x | |||
| Glycitein | 284.0685 | x | |||
| Genistin | 432.1057 | x | |||
| Condensed tannins | Procyanidin B1 | 578.1425 | x | ||
| Procyanidin B2 | 578.1425 | x | |||
| Procyanidin C1 | 866.2058 | x | x | ||
| Other compounds | Riboflavin | 376.1383 | x | ||
| Indole-3-acrylic acid | 187.0633 | x | |||
| Carvone | 150.1045 | x | |||
| Indole-3-acrylic acid | 187.0633 | x | |||
| N,N′-Dicyclohexylurea | 224.1886 | x | x | ||
| Adenosine | 267.0968 | x | |||
| 3-Nitro-2-6-dipiperidinopyridine | 290.1743 | x | |||
| Dioctyl phthalate | 390.2770 | x |
X indicates confident identification via matching mass spectra of analytes to spectra of standards in the in-house, or on-line database (see text for details).
Indicates compounds that are indistinguishable via mass spectra matching.
Indicates tentative identification based only on matching of monoisotopic masses.
4. Conclusion
Purification and fractionation of phenolic extracts could significantly increase phenolic contents and antioxidant capacity. High ACE inhibitory activity was only observed in the crude extracts. Three legume varieties exhibited great variations in phenolic composition during purification and fractionation. Black soybean showed highest ACE inhibition, followed by lentil, and black turtle bean. Except lentil, the ACE inhibitory activity of crude extracts from the other two legumes decreased upon heating. With our novel analytical methods, different groups of compounds were identified and accurately quantified in these three legumes. The findings on antioxidant and ACE inhibitory properties of phenolic extracts and fractions contributed to the scientific basis for future development of legume nutritional supplements for controlling hypertension.
Acknowledgments
The metabolomics-mass spectrometry analysis was performed at the Institute for Genomics, Biocomputing and Biotechnology, Mississippi State University, with partial support from Mississippi Agriculture and Forestry Experimental Station. T.P. was funded by INBRE-NIH award 4P20GM103476-15. USDA-ARS SCA No. 58-402-2729 Mississippi Center for Food Safety and Post-Harvest Technology and Mississippi Agricultural and Forestry Experiment Station contributed funding for this CRIS project No. MIS 501170.
References
- Actis-Goretta L, Ottaviani JI, Keen CL, Fraga CG. Inhibition of angiotensin converting enzyme (ACE) activity by flavan-3-ols and procyanidins. FEBS letters. 2013;555:597–600. doi: 10.1016/s0014-5793(03)01355-3. [DOI] [PubMed] [Google Scholar]
- Afonso J, Passos CP, Coimbra MA, Silva CM, Soares-da-Silva P. Inhibitory effect of phenolic compounds from grape seeds (Vitis vinifera L.) on the activity of angiotensin I converting enzyme. LWT – Food Science and Technology. 2013;54(1):265–270. [Google Scholar]
- Afshin A, Micha R, Khatibzadeh S, Mozaffarian D. Consumption of nuts and legumes and risk of incident ischemic heart disease, stroke, and diabetes: A systematic review and meta-analysis. The American Journal of Clinical Nutrition. 2014;100:278–288. doi: 10.3945/ajcn.113.076901. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Al Shukor N, Van Camp J, Gonzales GB, Staljanssens D, Struijs K, Zotti MJ, … Smagghe G. Angiotensin-converting enzyme inhibitory effects by plant phenolic compounds: A study of structure activity relationships. Journal of Agricultural and Food Chemistry. 2013;61(48):11832–11839. doi: 10.1021/jf404641v. [DOI] [PubMed] [Google Scholar]
- Asami DK, Hong YJ, Barrett DM, Mitchell AE. Comparison of the total phenolic and ascorbic acid content of freeze-dried and air-dried marionberry, strawberry, and corn grown using conventional, organic, and sustainable agricultural practices. Journal of Agricultural and Food Chemistry. 2003;51(5):1237–1241. doi: 10.1021/jf020635c. [DOI] [PubMed] [Google Scholar]
- Awika JM, Dykes L, Gu L, Rooney LW, Prior RL. Processing of sorghum (Sorghum bicolor) and sorghum products alters procyanidin oligomer and polymer distribution and content. Journal of Agricultural and Food Chemistry. 2003;51(18):5516–5521. doi: 10.1021/jf0343128. [DOI] [PubMed] [Google Scholar]
- Bartolomé B, Estrella I, Hernández T. Changes in phenolic compounds in lentils (Lens culinaris) during germination and fermentation. Zeitschrift für Lebensmitteluntersuchung und -Forschung A. 1997;205(4):290–294. [Google Scholar]
- Belski R, Mori TA, Puddey IB, Sipsas S, Woodman RJ, Ackland TR, … Hodgson JM. Effects of lupin-enriched foods on body composition and cardiovascular disease risk factors: A 12-month randomized controlled weight loss trial. International Journal of Obesity. 2011;35(6):810–819. doi: 10.1038/ijo.2010.213. [DOI] [PubMed] [Google Scholar]
- Bohn T. Dietary factors affecting polyphenol bioavailability. Nutrition Reviews. 2014;72(7):429–452. doi: 10.1111/nure.12114. [DOI] [PubMed] [Google Scholar]
- Broadhurst RB, Jones WT. Analysis of condensed tannins using acidified vanillin. Journal of Agricultural and Food Chemistry. 1978;29:788–794. [Google Scholar]
- Chen CW, Ho CT. Antioxidant properties of polyphenols extracted from green and black teas. Journal of Food Lipids. 1995;2:35–46. [Google Scholar]
- Clifford MN. Diet-derived phenols in plasma and tissues and their implications for health. Planta Medica. 2004;70(12):1103–1114. doi: 10.1055/s-2004-835835. [DOI] [PubMed] [Google Scholar]
- Correa CR, Li L, Aldini G, Carini M, Oliver Chen CY, EllipsisYeum KJ. Composition and stability of phytochemicals in five varieties of black soybeans (Glycine max) Food Chemistry. 2010;123(4):1176–1184. [Google Scholar]
- Duenas M, Hernandez T, Estrella I. Changes in the content of bioactive polyphenolic compounds of lentils by the action of exogenous enzymes. Effect on their antioxidant activity. Food Chemistry. 2007;101:90–97. [Google Scholar]
- Garcia-Mora P, Frias J, Peñas E, Zieliński H, Giménez-Bastida JA, Wiczkowski W, … Martínez-Villaluenga C. Simultaneous release of peptides and phenolics with antioxidant, ACE-inhibitory and anti-inflammatory activities from pinto bean (Phaseolus vulgaris L. var. pinto) proteins by subtilisins. Journal of Functional Foods. 2015;18(Part A):319–332. [Google Scholar]
- Gilani GS, Xiao CW, Cockell KA. Impact of antinutritional factors in food proteins on the digestibility of protein and the bioavailability of amino acids and on protein quality. British Journal of Nutrition. 2012;108(S2):S315–S332. doi: 10.1017/S0007114512002371. [DOI] [PubMed] [Google Scholar]
- Guerrero L, Castillo J, Quinones M, Garcia-Vallve S, Arola L, Pujadas G, Muguerza B. Inhibition of angiotensin I converting enzyme activity by flavonoids structure-activity relationship studies. PLoS ONE. 2012;7:e49493. doi: 10.1371/journal.pone.0049493. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Haileslassie HA, Henry CJ, Tyler RT. Impact of household food processing strategies on antinutrient (phytate, tannin and polyphenol) contents of chickpeas (Cicer arietinum L.) and beans (Phaseolus vulgaris L.): A review. International Journal of Food Science and Technology. 2016;51:1947–1957. [Google Scholar]
- Hart JJ, Tako E, Kochian LV, Glahn RP. Identification of black bean (Phaseolus vulgaris L.) polyphenols that inhibit and promote iron update by Caco-2 cells. Journal of Agricultural and Food Chemistry. 2015;63:5950–5956. doi: 10.1021/acs.jafc.5b00531. [DOI] [PubMed] [Google Scholar]
- Hermsdorff HHM, Zulet MÁ, Abete I, Martínez JA. A legume-based hypocaloric diet reduces proinflammatory status and improves metabolic features in overweight/obese subjects. European Journal of Nutrition. 2010;50(1):61–69. doi: 10.1007/s00394-010-0115-x. [DOI] [PubMed] [Google Scholar]
- Jakubczyk A, Baraniak B. Activities and sequences of the angiotensin I-converting enzyme (ACE) inhibitory peptides obtained from the digested lentil (Lens culinaris) globulins. International Journal of Food Science and Technology. 2013;48:2363–2369. [Google Scholar]
- Jia Z, Tang M, Wu J. The determination of flavonoid content in mulberry and their scavenging effects on superoxide radicals. Food Chemistry. 1999;64:555–559. [Google Scholar]
- Karamac M, Kosinska A, Rybarczyk A, Amarowicz R. Extraction and chromatographic separation of tannin fractions from tannin-rich plant material. Polish Journal of Food and Nutrition Science. 2007;57:471–474. [Google Scholar]
- Le Bourvellec C, Renard CMGC. Interactions between polyphenols and macromolecules: Quantification methods and mechanisms. Critical Review in Food Science and Nutrition. 2012;52:213–248. doi: 10.1080/10408398.2010.499808. [DOI] [PubMed] [Google Scholar]
- Lo WMY, Farnworth ER, Li-Chan ECY. Angiotensin I-converting enzyme inhibitory activity of soy protein digests in a dynamic model system simulating the upper gastrointestinal tract. Journal of Food Science. 2006;71(3):231–237. [Google Scholar]
- Mamilla RK, Mishra VK. Effect of germination on antioxidant and ACE inhibitory activities of legumes. LWT – Food Science and Technology. 2017;75:51–58. [Google Scholar]
- Mukai Y, Sato S. Polyphenol-containing azuki bean (Vigna angularis) extract attenuates blood pressure elevation and modulates nitric oxide synthase and caveolin-1 expressions in rats with hypertension. Nutrition, Metabolism and Cardiovascular Diseases. 2009;19(7):491–497. doi: 10.1016/j.numecd.2008.09.007. [DOI] [PubMed] [Google Scholar]
- Mukai Y, Sato S. Polyphenol-containing azuki bean (Vigna angularis) seed coats attenuate vascular oxidative stress and inflammation in spontaneously hypertensive rats. The Journal of Nutritional Biochemistry. 2011;22(1):16–21. doi: 10.1016/j.jnutbio.2009.11.004. [DOI] [PubMed] [Google Scholar]
- Murphy PA, Song T, Buseman G, Barua K. Isoflavones in soy-based infant formulas. Journal of Agricultural and Food Chemistry. 1997;45:4635–4638. [Google Scholar]
- Ojeda D, Jimenez-Ferrer E, Zamilpa A, Herrera-Arellano A, Tortoriello J, Alvarez L. Inhibition of angiotensin converting enzyme (ACE) activity by anthocyanins delphinidin- and cynidin-3-o-sambubiosides from Hibiscus sabdariffa. Journal of Ethnopharmacology. 2010;127:7–10. doi: 10.1016/j.jep.2009.09.059. [DOI] [PubMed] [Google Scholar]
- Prior RL, Hoang H, Gu L, Wu X, Bacchiocca M, Howard L, … Jacob R. Assays for hydrophilic and lipophilic antioxidant capacity (oxygen radical absorbance capacity (ORACFL)) of plasma and other biological and food samples. Journal of Agricultural and Food Chemistry. 2003;51(11):3273–3279. doi: 10.1021/jf0262256. [DOI] [PubMed] [Google Scholar]
- Robbins RJ, Bean SR. Development of a quantitative high-performance liquid chromatograph-photodiode array detection measurement system for phenolic acids. Journal of Chromatography A. 2004;1038:97–105. doi: 10.1016/j.chroma.2004.03.009. [DOI] [PubMed] [Google Scholar]
- Rui X, Boye JI, Barbana C, Simpson BK, Prasher SO. Electrophoretic profiles and angiotensin I-converting enzyme inhibitory activities of nine varieties of Phaseolus vulgaris protein hydrolysates. Journal of Nutrition and Food Sciences. 2012;2(8):1–6. [Google Scholar]
- Schofield P, Mbugua DM, Pell AN. Analysis of condensed tannins: A review. Animal Feed Science and Technology. 2001;91(1–2):21–40. [Google Scholar]
- Siddhuraju P, Becker K. The antioxidant and free radical scavenging activities of processed cowpea (Vigna unguiculata (L.) Walp.) seed extracts. Food Chemistry. 2007;101(1):10–19. [Google Scholar]
- Singleton VL, Orthofer R, Lamuela-Raventos RM. Analysis of total phenols and other oxidation substrates and antioxidants by means of Folin-Ciocalteu Reagent. Methods in Enzymology. 1999;299:152–178. [Google Scholar]
- Singleton VL, Rossi JA. Colorimetry of total phenolics with phosphomolybdic-phosphotungstic acid reagents. American Journal of Enology and Viticulture. 1965;16:144–158. [Google Scholar]
- Siti HN, Kamisah Y, Kamsiah J. The role of oxidative stress, antioxidants and vascular inflammation in cardiovascular disease (a review) Vascular Pharmacology. 2015;71:40–56. doi: 10.1016/j.vph.2015.03.005. [DOI] [PubMed] [Google Scholar]
- Tan YY, Chang SKC, Zhang Y. Innovative soaking and grinding methods and cooking affect the retention of isoflavones, antioxidant and antiproliferative properties in soymilk prepared from black soybean. Journal of Food Science. 2016;81(4):H1016–H1023. doi: 10.1111/1750-3841.13266. [DOI] [PubMed] [Google Scholar]
- Tan Y, Chang SKC, Zhang Y. Comparison of α-amylase, α-glucosidase and lipase inhibitory activity of the phenolic substances in two black legumes of different genera. Food Chemistry. 2017;214:259–268. doi: 10.1016/j.foodchem.2016.06.100. [DOI] [PubMed] [Google Scholar]
- Wang S, Melnyk JP, Tsao R, Marcone MF. How natural dietary anti-oxidants in fruits, vegetables and legumes promote vascular health. Food Research International. 2011;44(1):14–22. [Google Scholar]
- Wang YK, Zhang X, Chen GL, Yu J, Yang LQ, Gao YQ. Antioxidant property and their free, soluble conjugate and insoluble-bound phenolic contents in selected beans. Journal of Functional Foods. 2016;24:359–372. [Google Scholar]
- Wu JP, Ding XL. Characterization of inhibition and stability of soy-protein derived angiotensin I-converting enzyme inhibitory peptides. Food Research International. 2002;35:367–375. [Google Scholar]
- Xu BJ, Chang SKC. A comparative study on phenolic profiles and antioxidant activities of legumes as affected by extraction solvents. Journal of Food Science. 2007;72(2):S159–S166. doi: 10.1111/j.1750-3841.2006.00260.x. [DOI] [PubMed] [Google Scholar]
- Xu BJ, Chang SKC. Total phenolics, phenolic acids, isoflavones, and anthocyanins and antioxidant properties of yellow and black soybeans as affected by thermal processing. Journal of Agricultural and Food Chemistry. 2008a;56:7165–7175. doi: 10.1021/jf8012234. [DOI] [PubMed] [Google Scholar]
- Xu BJ, Chang SKC. Effect of soaking, boiling, and steaming on total phenolic content and antioxidant activities of cool season food legumes. Food Chemistry. 2008b;110:1–13. doi: 10.1016/j.foodchem.2008.01.045. [DOI] [PubMed] [Google Scholar]
- Xu B, Chang SKC. Total Phenolic, Phenolic Acid, Anthocyanin, Flavan-3-ol, and Flavonol Profiles and Antioxidant Properties of Pinto and Black Beans (Phaseolus vulgaris L.) as Affected by Thermal Processing. Journal of Agricultural and Food Chemistry. 2009;57(11):4754–4764. doi: 10.1021/jf900695s. [DOI] [PubMed] [Google Scholar]
- Xu BJ, Yuan SH, Chang SKC. Comparative analysis of phenolic composition, antioxidant capacity, and color of cool season legumes and other selected food legumes. Journal of Food Science. 2007;72(2):S167–S177. doi: 10.1111/j.1750-3841.2006.00261.x. [DOI] [PubMed] [Google Scholar]
- Xuan CL, Yao FR, Guo LR, Liu Q, Chang SKC, Liu KX, Sun CW. Comparison of extracts from cooked and raw lentil in antagonizing angiotensin II-induced hypertension and cardiac hypertrophy. European Review for Medical and Pharmacological Sciences. 2013;17:2644–2653. [PubMed] [Google Scholar]
- Yao FR, Sun CW, Chang SKC. Morton lentil extract attenuated angiotensin II-induced cardiomyocyte hypertrophy via inhibition of intracellular reactive oxygen species levels in vitro. Journal of Agricultural and Food Chemistry. 2010;58(19):10382–10388. doi: 10.1021/jf101648m. [DOI] [PubMed] [Google Scholar]
- Yao FR, Sun CW, Chang SKC. Lentil polyphenos extract prevents angiotensin II-induced hypertension, vascular remodelling and perivascular fibrosis. Food Function. 2012;3:127–133. doi: 10.1039/c1fo10142k. [DOI] [PubMed] [Google Scholar]
- Zhang Y, Chang SKC. Isoflavone profile and kinetic changes during ultra-high temperature processing of soymilk. Journal of Food Science. 2016;81(3):C593–C599. doi: 10.1111/1750-3841.13236. [DOI] [PubMed] [Google Scholar]
- Zhang Y, Chang SKC, Liu Z. Isoflavone profile in soymilk as affected by soybean variety, grinding, and heat-processing methods. Journal of Food Science. 2015;80(5):C983–C988. doi: 10.1111/1750-3841.12839. [DOI] [PubMed] [Google Scholar]
- Zhang B, Deng Z, Ramdath DD, Tang Y, Chen PX, Liu R, … Tsao R. Phenolic profiles of 20 Canadian lentil cultivars and their contribution to antioxidant activity and inhibitory effects on α-glucosidase and pancreatic lipase. Food Chemistry. 2015;172:862–872. doi: 10.1016/j.foodchem.2014.09.144. [DOI] [PubMed] [Google Scholar]
- Zou Y, Chang SKC, Gu Y, Qian SY. Antioxidant activity and phenolic compositions of lentil (Lens culinaris var. Morton) extract and its fractions. Journal of Agricultural and Food Chemistry. 2011;59(6):2268–2276. doi: 10.1021/jf104640k. [DOI] [PMC free article] [PubMed] [Google Scholar]