Abstract
Calcium (Ca2+) is an important ion in physiology and is found both outside and inside cells. The intracellular concentration of Ca2+ is tightly regulated as it is an intracellular signal molecule and can affect a variety of cellular processes. In immune cells Ca2+ has been shown to regulate e.g. gene transcription, cytokine secretion, proliferation and migration. Ca2+ can enter the cytoplasm either from intracellular stores or from outside the cells when Ca2+ permeable ion channels in the plasma membrane open. The Ca2+ release-activated (CRAC) channel is the most prominent Ca2+ ion channel in the plasma membrane. It is formed by ORAI1-3 and the channel is opened by the endoplasmic reticulum Ca2+ sensor proteins stromal interaction molecules (STIM) 1 and 2. Another group of Ca2+ channels in the plasma membrane are the voltage-gated Ca2+ (CaV) channels. We examined if a change in immunological tolerance is accompanied by altered ORAI, STIM and CaV gene expression in peripheral blood mononuclear cells (PBMCs) in pregnant women and in type 1 diabetic individuals. Our results show that in pregnancy and type 1 diabetes ORAI1-3 are up-regulated whereas STIM1 and 2 are down-regulated in pregnancy but only STIM2 in type 1 diabetes. Expression of L-, P/Q-, R- and T-type voltage-gated Ca2+ channels was detected in the PBMCs where the CaV2.3 gene was up-regulated in pregnancy and type 1 diabetes whereas the CaV 2.1 and CaV3.2 genes were up-regulated only in pregnancy and the CaV1.3 gene in type 1 diabetes. The results are consistent with that expression of ORAI, STIM and CaV genes correlate with a shift in immunological status of the individual in health, as during pregnancy, and in the autoimmune disease type 1 diabetes. Whether the changes are in general protective or in type 1 diabetes include some pathogenic components remains to be clarified.
Introduction
The immune system is inherently flexible. Both in pregnancy and with the onset of an autoimmune disease, an immunological shift takes place. In pregnancy, the immunological tolerance is expanded whereas in an autoimmune disease, it is decreased. How this plasticity comes about is still not clear despite intensive research [1–4]. The calcium (Ca2+) ion is an intracellular messenger in cells where it regulates multitude of mechanisms that in immune cells includes; activation, differentiation, proliferation, secretion and migration [5–11]. If calcium is involved in the change in immunological tolerance as observed during pregnancy and autoimmune diseases, then it is possible that expression of plasma membrane ion channels that regulate entry of the calcium ions into the cells may be altered when the immunological shift takes place resulting in altered expression in pregnancy and autoimmune diseases i.e. type 1 diabetes.
Ca2+ ions are present in both extra- and intracellular fluids in mammals. The intracellular concentration is tightly regulated. Increase of Ca2+ ion concentration in the cytoplasm of immune cells most often is associated with either release of Ca2+ from intracellular stores, like the endoplasmic reticulum (ER), or entry through ion channels in the plasma membrane. Store-operated Ca2+ entry (SOCE) through Ca2+ release-activated Ca2+ (CRAC) channels are present in many tissues including neurons, cardiac myocytes, skeletal muscle cells, pregnant human myometrium, vascular smooth muscle cells, pancreatic islet β cells, endothelial cells and most immune cells [6, 12–20] and are thought to be responsible for the majority of Ca2+ influx in at least the immune cells [6, 8]. Other Ca2+ permeable channels may be present in the immune cells like the voltage-gated Ca2+ channels (Cav), glutamate-gated NMDA receptors, TRP channels and P2X receptors but generally less is known about the role of these channels in the immune system [8, 21–24]. The CRAC and some Cav channels are regulated by stromal interaction molecules (STIM1 and 2) which are located in the ER membrane [6, 8, 25–29].
The CRAC channels are tetramers of ORAI proteins that form the channel pore in the plasma membrane. The channels can be formed from homo- or heteromeric ORAI proteins (ORAI 1, 2 or 3) that differ in their kinetic properties [6, 9]. The store operated Ca2+ entry is initiated by STIM that are sensors for Ca2+ in the ER and when the ER Ca2+ concentration drops significantly, they cluster and at the ER-plasma membrane junction they bind to and open the CRAC channels. There are 10 members in the voltage-gated Ca2+ channel family [30]. In excitable cells the channels are opened by depolarization of the membrane potential but in immune cells additional mechanism involving STIM appears to participate in regulating the channels activation mechanism [25–27]. STIM 1 and STIM 2 are structurally similar molecules but STIM 2 has lower affinity for Ca2+ [31, 32].
We examined if the ORAI, Cav and STIM mRNAs expression in peripheral blood mononuclear cells (PBMCs) was correlated to altered immunity state in humans. We examined mRNAs isolated from PBMCs from healthy individuals, healthy pregnant women and individuals with type 1 diabetes. The results show that in pregnancy and in type 1 diabetes, the mRNA levels of genes encoding the CRAC channels and the Ca2+ sensing proteins were significantly altered. Cav channels were normally expressed at lower levels but significant changes were observed for specific L, R and T-type Ca2+ channels.
Material and methods
Study design
The studies were approved by the Regional Ethics Review Board in Uppsala. All individuals participating in the study were given oral and written information regarding the study and provided a written consent before entering the study. There were two groups in the study (A) healthy pregnant women and their age and body mass index (BMI) matched healthy control individuals including both men and non-pregnant women (S1 Table) and (B) type 1 diabetes individuals and their age, sex and BMI matched healthy control individuals (S1 Table). Both control groups were medical students, hospital employees and individuals recruited through poster advertising. Inclusion criteria for both control groups were self-reported physical health, no ongoing infection and no daily medication. None of the healthy controls had a first degree relative diagnosed with type 1 diabetes. Additional exclusion criteria for healthy control women were pregnancy, breast feeding and use of hormonal contraception. Pregnant participant in this study were recruited from women participating in a study at the Department for Women’s and Children’s Health, Uppsala University. They visited the lab between gestational weeks 36 and 41. Individuals with type 1 diabetes were recruited at the Department of Endocrinology and Diabetology and routine blood samples were analyzed at the department of Clinical Chemistry and Pharmacology, Uppsala University Hospital. Venous blood samples were collected into EDTA tubes for later isolation of PBMCs, see description below.
PBMCs preparation
Blood samples were subjected to density gradient centrifugation to isolate PBMCs. In brief, samples were diluted in 1:1 ratio in MACS buffer (Miltenyi Biotec, Madrid, Spain) and layered on Ficoll-paque plus (Sigma-Aldrich, Hamburg, Germany). These diluted samples were centrifuged at 400g for 30 minutes at room temperature. The lymphocyte layer (PBMCs) was carefully withdrawn and washed twice in MACS buffer. PBMCs were saved in RNAlater (Sigma) at -80°C for later mRNA extraction.
Total RNA isolation and real-time quantitative reverse transcription PCR
PBMC samples were processed for total RNA extraction using Gen Elute total RNA Miniprep (Sigma-Aldrich) or RNA/DNA/Protein Purification Plus Kit (Norgen Biotek, Ontario, Canada) and the concentration of total RNA was measured by Nanodrop (Nanodrop Technologies, Thermo Scientific, Inc., Wilmington, DE, USA). Further, 1.0–1.5 μg RNA was treated with 0.6 U DNAse I (Roche, Basel, Switzerland) for 30 minutes at 37°C, with 8 mM EDTA for 10 minutes at 75°C and then converted to cDNA using Superscript III or IV reverse transcriptase (Invitrogen, Stockholm, Sweden) in a 20 μl reaction. Reverse transcriptase negative control was performed in order to exclude genomic DNA contamination. Real-time quantitative PCR (RT-qPCR) was performed in 10 μl volume containing 4 μl cDNA (8–15 ng), 1×PCR reaction buffer, 3 mM MgCl2, 0.3 mM dNTP, 0.8 U JumpStart Taq DNA polymerase (Sigma-Aldrich), 1×ROX reference dye, 5×SYBR Green I (Invitrogen) and 0.4 μM each of forward and reverse primers. The gene-specific primer pairs (S2 Table) were designed using NCBI Primer-Blast, synthesized by Sigma Aldrich and further validated on human prefrontal cortex cDNA by the identification of the single peak in the melt curve and the single band of amplicon size on agarose gel. Amplification was performed in 384-well optical plates (Corning, 3757, Sigma-Aldrich) using the ABI PRISM 7900HT Sequence Detection System (Applied Biosystems, Stockholm, Sweden) with an initial denaturation of 5 min at 95°C, followed by 45 cycles of 95°C for 15 s, 60°C for 30s, and 72°C for 30 s, further followed by melt curve to ensure single product amplification. Cycle threshold (Ct) values were analyzed using SDS 2.4 and RQ Manager 1.2 softwares provided with the instrument. Since the expression of reference gene may differ between different cell types, it is very important to use validated and stable reference genes for normalization of qPCR data. We used importin 8 (IPO8) and TATA-binding protein (TBP) for normalization [33, 34]. The expression of each target gene relative to a normalization factor (geometric mean of two reference genes—IPO8 and TBP) was calculated with Data Assist v2.0 using the 2−ΔCt method as previously described [35].
Western blot analysis
Protein extraction from PBMC samples was performed using RNA/DNA/Protein Purification Plus Kit (Norgen Biotek, Ontario, Canada). Proteins were measured using the RC DCTM protein assay kit (Bio-Rad, USA) in Multiskan MS plate reader (Labsystems, Vantaa, Finland) and the concentration was calculated by plotting standard curve. Protein samples (20–60 μg) subjected to SDS-PAGE using 8% polyacrylamide gels and transferred to Amersham Hybond PVDF membranes by either wet or semi-dry transfer systems. The membranes were blocked with 10% FBS in Tris buffered saline containing 0.1% Tween (TBS-T) for 1 h and incubated overnight at 4°C with primary antibodies against STIM2 (1:200, Cell Signaling Technology, Cat No. 4917), ORAI1 (1:500, Alomone labs, Cat No. ACC-060), ORAI2 (1:500, Alomone labs, Cat No. ACC-061), CaV1.3 (1:500, Alomone labs, Cat No. ACC-005), CaV2.3 (1:500, Alomone labs, Cat No. ACC-006) and GAPDH (1:3000; Merck Millipore, Cat No. ABS16). After intensive washing with TBS-T, the membranes were further incubated with horseradish peroxidase-conjugated secondary antibody (1:3000; Cell Signaling Technology, Cat No. 7074) for 2 h. Further, membranes were washed intensively and developed for detection of immunoreactive protein bands using ECL Prime Western Blotting System (GE Healthcare, RPN2232). Bands were visualized in ChemiDocMP imaging system (Bio-Rad).
Statistical analysis
Statistical analysis and data mining were done by using Statistica 12 (StatSoft Scandinavia, Uppsala, Sweden) and GraphPad Prism 7 (La Jolla, CA, USA). The statistical tests were performed after omitting outliers identified by Tukey test. The whiskers and the outliers are plotted by the Tukey method which uses +/- 1.5 inter-quartile distance i.e. interquartile range (IQR), the difference between the 25th and 75th percentiles. All data points were included when calculating IQR. The differences between groups were assessed by nonparametric Kruskal–Wallis ANOVA on ranks with Dunn’s post hoc test or by one-way ANOVA with Bonferroni post hoc test depending on the normality of the data. Normality of the data was determined by Shapiro-Wilk normality distribution test (S3 Table). A general stepwise linear regression model was used to identify covariates (e.g., age, gender and BMI). Variables with a significant association with groups were included in the final statistical model as covariates. The significance level was set to p < 0.05. We further correlated expression level of all CRAC and VDCC channel subunits with demographic characteristics of type 1 diabetic donors such as age at onset of T1D and duration of T1D. The correlation was accessed using non-parametric Spearman rank correlation.
Results
The demographic characteristics of individuals in this study were shown in S1 Table. As expected, the blood glucose, C-peptide and HbA1c levels in type 1 diabetic individuals were significantly different when compared with healthy controls (S1 Table). There was no significant difference in age, BMI (controls A vs. pregnant women, and nondiabetic controls B vs. type 1 diabetic) and gender (nondiabetic controls B vs. type 1 diabetic). The mRNAs expression of three ORAI (1–3), STIM1 and 2 and ten voltage-gated Ca2+ channel-forming α1 subunits (CaV1.1–1.4, CaV2.1–2.3, CaV3.1–3.3) in PBMCs was quantified by RT-qPCR in samples from controls (A) and (B), pregnant women and type 1 diabetic individuals. The primers covered all transcripts known today for the particular gene (S2 Table) and ORAI 1 mRNA expression was also tested by an additional pair of primers to verify the biological results obtained.
The expression of ORAIs and STIM mRNAs in PBMCs is altered in pregnancy
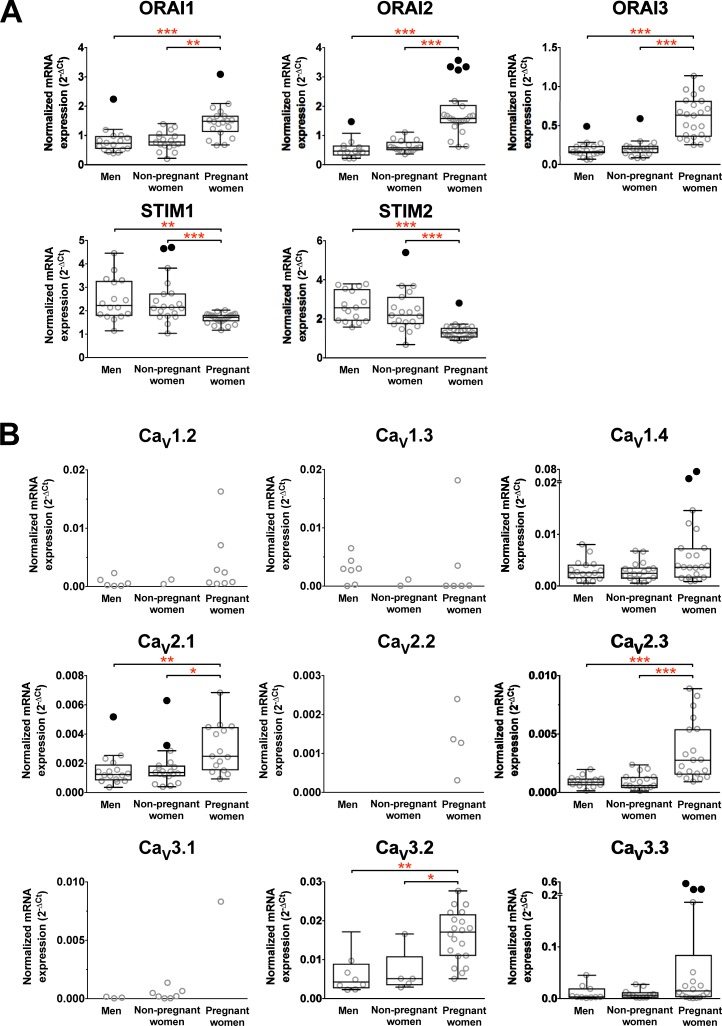
Comparison of PBMCs gene expression from controls and pregnant women is shown in Fig 1A and the number of individuals expressing the target mRNAs in Table 1. ORAI1, ORAI2, ORAI3, STIM1 and STIM2 were expressed in the majority of the PBMCs samples from both controls and pregnant women. Interestingly, the average expression level of ORAI1, ORAI2 and ORAI3 were significantly up-regulated in pregnant women whereas, in contrast, both STIM1 and STIM2 were down-regulated.
Fig 1. Altered mRNA expression of specific calcium release activated calcium (CRACs) channel and voltage-gated calcium channel (Cav) subunits in PBMCs from non-pregnant controls and pregnant women.
Data from each group is presented as scatter dot plot (°) or box and whiskers plot with median and whiskers plotted by Tukey method to determine outliers (• - above or below the whiskers). CaV1.1 subunit mRNA was not detected in any sample. Statistical analysis was performed by excluding outliers depending on normality distribution of the data and only the subunits with statistically significant differences are mentioned below. One-Way ANOVA with Bonferroni post-hoc test: ORAI1, df = 48, p = 0.003; Kruskal–Wallis ANOVA on ranks with Dunn’s post hoc test: ORAI2, H(1, 46) = 28.5, p < 0.001; ORAI3, H(1, 54) = 37.2, p < 0.001; STIM1, H(1, 58) = 18.3, p < 0.001; STIM2, H(1, 58) = 32.5, p < 0.001; CaV2.1, H(1, 48) = 13.1, p < 0.001; CaV2.3, H(1, 49) = 22.1, p < 0.001; CaV3.2, H(1, 31) = 17.7, p < 0.001. ** p < 0.01, *** p < 0.001.
Table 1. Number of individuals expressing CRAC or Ca2+V channel mRNAs in PBMCs.
| Controls (A) (n = 35) | Pregnant Women (n = 24) | Controls (B) Nondiabetic individuals (n = 21) | Type 1 diabetic individuals (n = 33) | |
|---|---|---|---|---|
| CRAC | ||||
| ORAI1 | 33 | 19 | 21 | 33 |
| ORAI2 | 31 | 22 | 21 | 33 |
| ORAI3 | 33 | 23 | 21 | 33 |
| STIM1 | 35 | 24 | 21 | 33 |
| STIM2 | 35 | 24 | 20 | 32 |
| VGCCs | ||||
| CaV1.1 | 0 | 0 | 0 | 8 |
| CaV1.2 | 8 | 8 | 9 | 17 |
| CaV1.3 | 9 | 6 | 13 | 18 |
| CaV1.4 | 33 | 23 | 20 | 28 |
| CaV2.1 | 25 | 15 | 11 | 18 |
| CaV2.2 | 0 | 4 | 5 | 2 |
| CaV2.3 | 30 | 19 | 17 | 25 |
| CaV3.1 | 10 | 1 | 2 | 1 |
| CaV3.2 | 13 | 20 | 18 | 23 |
| CaV3.3 | 21 | 18 | 13 | 21 |
The expression of CaV 2 and 3 channel subtypes may be altered in pregnancy
PBMCs from control and pregnant women expressed 8 and 9 Cav subtypes, respectively, of the 10 known genes encoding the α1 pore-forming CaV (Fig 1B). The number of individuals expressing each subtype is given in Table 1. CaV1.4, CaV2.1, CaV2.3 and CaV3.3 were expressed in more than 50% of samples and CaV3.2 was more prominently expressed by pregnant women than controls. The frequency of expression for the remaining subtypes was lower. The CaV1.1 was not detected in any of the samples. The average mRNA expression level of CaV2.1, CaV2.3 and CaV3.2 was significantly increased in pregnant women. The expression profile of the CaV channel-forming α1 subunit in PBMCs is consistent with that specific subtypes of voltage-gated Ca2+ channels respond to altered immunological status of the pregnant woman.
The expression of ORAIs and STIM2 mRNAs in PBMCs is altered in type 1 diabetes
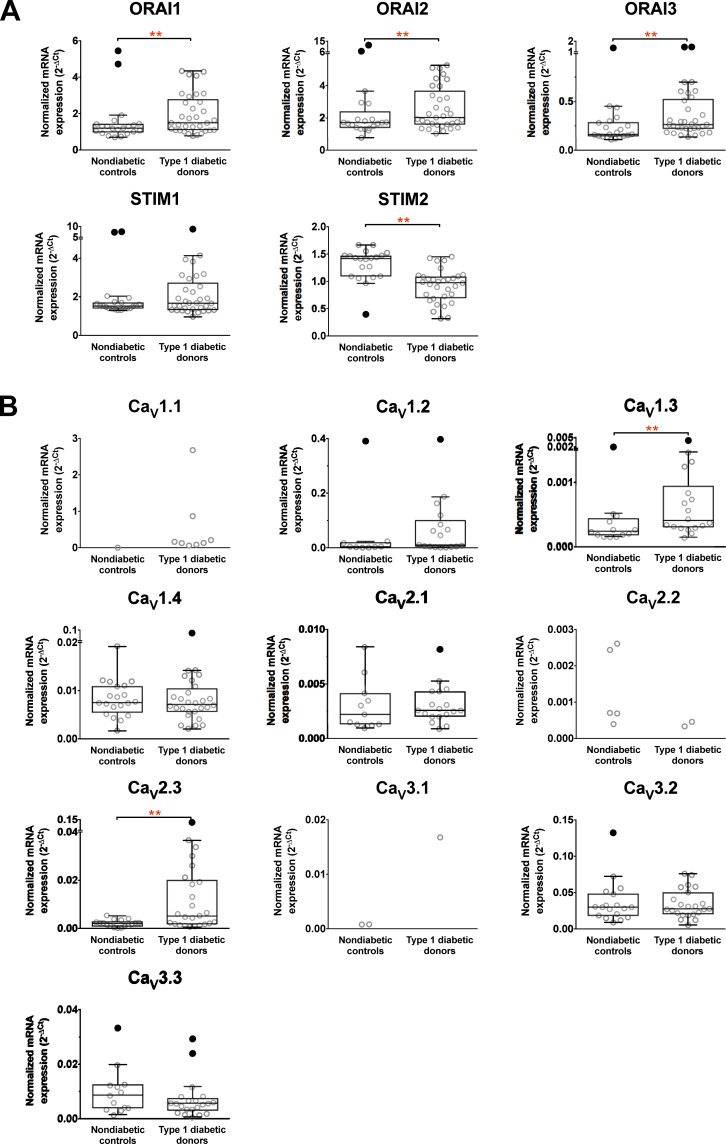
Comparison of PBMCs genes from nondiabetic and type 1 diabetic individuals is shown in Fig 2A and the number of individuals expressing the specific genes in Table 1. ORAI1, ORAI2, ORAI3, STIM1 and STIM2 were expressed in the majority of the samples from both nondiabetic and type 1 diabetic individuals. Similar to the expression in PBMCs from pregnant women, the average expression level of ORAI1, ORAI2 and ORAI3 were significantly up-regulated by type 1 diabetes. In contrast, the STIM2 mRNAs was down-regulated but the level of STIM1 gene expression was unaltered when samples from nondiabetic and type 1 diabetic individuals were compared.
Fig 2. Altered mRNA expression of specific calcium release activated calcium (CRACs) channel and voltage-gated calcium channel (Cav) subunits in PBMCs from non-diabetic controls and T1D patients.
Data from each group is presented as scatter dot plot (°) or box and whiskers plot with median and whiskers plotted by Tukey method to determine outliers (• - above or below the whiskers). Statistical analysis was performed by excluding outliers depending on normality distribution of the data and only the subunits with statistically significant differences are mentioned below. One-Way ANOVA with Bonferroni post-hoc test: STIM2, df = 45, p = 0.002; Kruskal–Wallis ANOVA on ranks with Dunn’s post hoc test: ORAI1, H(1, 47) = 9.1, p < 0.003; ORAI2, H(1, 47) = 7.9, p < 0.005; ORAI3, H(1, 48) = 8.2, p < 0.004; CaV1.3, H(1, 29) = 6.9, p < 0.008; CaV2.3, H(1, 41) = 7.4, p < 0.006. ** p < 0.01.
The expression of Cav 1 and 2 channel subtypes may be altered by type 1 diabetes
PBMCs from nondiabetic and type 1 diabetic individuals expressed 9 and 10 CaV subtypes, respectively, of the 10 known α1 pore-forming CaV genes (Fig 2B). The number of individuals expressing each subtype is given in Table 1. CaV1.3, CaV1.3, CaV2.1, CaV2.3, CaV3.2 and CaV3.3 were expressed in more than 50% of samples, CaV1.2 was more prominently expressed in individuals with type 1 diabetes but for the remaining subtypes frequency of expression was lower. The average gene expression level of CaV1.3 and CaV2.3 was significantly increased in type 1 diabetes. The expression profile of the CaV channel-forming α1 subunit in PBMCs is consistent with that specific subtypes of voltage-gated Ca2+ channels respond to altered immunological status of individuals with type 1 diabetes.
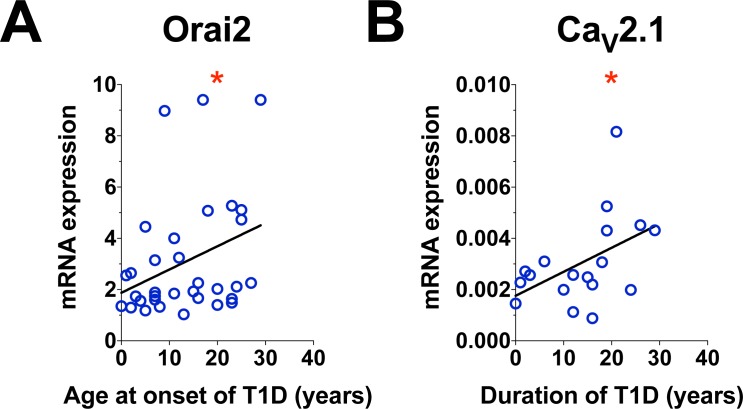
Type 1 diabetes duration and age of onset influences expression of specific channels
We examined if disease duration or age at onset affected the expression of the ORAIs, STIM1 and 2 and the Cav1.2 channels. Significant correlation was found for ORAI2 with age of onset of type 1 diabetes (Fig 3A, p <0.05) and CaV1.2 (Fig 3B, p <0.05) with duration of type 1 diabetes.
Fig 3. Correlation between the mRNA expression in PBMCs and demographic characteristics of type 1 diabetic donors.
A. Correlation between ORAI2 and age at onset of type 1 diabetes. B. Correlation between CaV2.1 and duration of Type 1 diabetes. The correlation was accessed using non-parametric Spearman rank test. * indicates p value < 0.05.
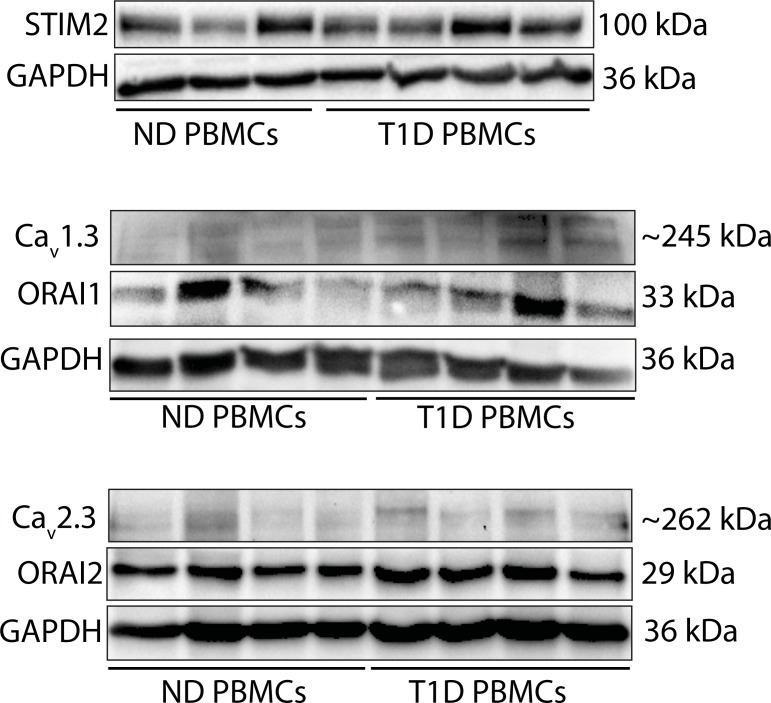
STIM2, Cav1.3, Cav2.3 and ORAI1 and 2 proteins are expressed in PBMCs from controls and type 1 diabetic individuals
Western blot analysis showed that STIM2, Cav1.3, Cav2.3 and ORAI1 and 2 proteins were detected in PBMCs samples from nondiabetic (ND) individuals (controls B) and type 1 diabetic (T1D) individuals (Fig 4). Due to the limited protein sample size, we did not quantify the protein expression levels in these samples.
Fig 4. Western blot analysis.
Western blot analysis showing the expression of Ca2+ sensor protein stromal interaction molecules (STIM) 2, voltage-gated Ca2+ (CaV) channel subunits Cav1.3 and Cav2.3, and Ca2+ release-activated Ca2+ (CRAC) channel proteins ORAI1 and 2 in PBMCs from ND (n = 3 or 4) and T1D (n = 4) individuals. GAPDH (Glyceraldehyde 3-phosphate dehydrogenase) served as loading controls.
Discussion
Here we examined if we could detect any differences in expression of the plasma membrane Ca2+ channels in immune cells in pregnancy and in an autoimmune disease, type 1 diabetes. In both cases an immunological shift takes place but the outcome in terms of health differs. In one case, pregnancy, the effects are beneficial whereas in the other, type 1 diabetes, the effects are detrimental and contribute to establishing the disease. Ca2+ is an important intracellular messenger and a wide variety of cellular functions depends on the dynamics of the intracellular Ca2+ concentration. Recent evidence has suggested associations of altered SOCE with altered diabetes complications in diabetic nephropathy, diabetic vasculopathy and diabetic cardiomyopathy [14] whereas in pregnancy, SOCE expressed in the pregnant preterm and term human myometrium is not altered by the onset of labor [18].
The most prominent Ca2+ channels in the plasma membrane of immune cells are the CRAC channels that are opened by the ER Ca2+ sensor proteins STIM1 and 2 [6]. In this study we showed that both in pregnancy and in type 1 diabetes the mRNAs encoding proteins forming the pore of the CRAC channels, the ORAI1-3, were up-regulated whereas the low-affinity Ca2+ sensor gene, STIM2, was down-regulated. In pregnancy, even the high Ca2+-affinity STIM1 was down-regulated. Interestingly, differential expression has been observed for the ORAI 1–3 and STIM 1 during T cells activation where the mRNAs were up-regulated [36] and in Sjögren’s syndrome, an autoimmune disease, where the STIM1 and 2 proteins were reduced [37]. The opposite effects on expression of the pore-forming and the gating partner of the store operated Ca2+entry in our study is intriguing. Since the stoichiometry of ORAI:STIM protein ratio determines the Ca2+ current size and inactivation properties, the altered expression level of the genes is bound to affect the cellular responses [11, 38]. Indeed, Ca2+ entry in PBMCs from patients with Sjögren’s syndrome was reduced as compared with cells from healthy controls [37]. Furthermore, in neutrophils, a loss of STIM2 has been associated with lower levels of cytokine production whereas STIM1 was required and enough for classical short-term neutrophil responses [32]. Together the results suggest that the cellular Ca2+ dynamics and thus intracellular signaling are, at least in part, regulated by the expression levels of the plasma membrane CRAC channels and the STIM proteins.
The contribution of the CaV channels to the Ca2+ influx into the cells and its effects on immunity is less well defined than that of the CRAC channels [7, 8, 21, 22]. The expression profile of the Cav pore-forming α1 subunit in the PBMCs in this study was consistent with expression of L-, P/Q-, R- and T-type voltage-gated Ca2+ channels [30] in the cells and included channels with high-to-low threshold for voltage activation. The Cav2.3 gene (R-type) was up-regulated in pregnancy and in type 1 diabetes whereas the CaV 2.1 (P/Q-type) and CaV3.2 (T-type) genes were up-regulated only in pregnancy and CaV1.3 (L-type) gene in type 1 diabetes. Voltage-gated Ca2+ channels are generally opened by a depolarizing voltage change in excitable cells like neurons and some endocrine cells but how these channels operate in immune cells has been somewhat of an enigma [6]. Importantly, however, selective opening of depolarizing channels is possible in immune cells expressing ligand-gated channels, e.g glutamate receptors or GABAA receptors [23, 24, 39, 40] and, in addition, the STIM proteins have been shown to modulate both L- and T-type CaV channels [25–27]. A reciprocal relationship has been proposed between CaV and ORAI regulated by the potency of TCR signaling [26].
Temporal and spatial organization of Ca2+ entry can be created by organization of specific Ca2+ microdomains in the cells and, thereby, enabling selective activation of specific intracellular signaling cascades. These domains may be created by differential location of ion channels and intracellular proteins organized into distinct macromolecular complexes for mediation of biological effects channeled through specific signaling pathway [41–44]. How a change in the expression of CRAC, STIM or CaV genes then leads to altered intracellular Ca2+ signaling and immune function remains to be elucidated. Our results indicate that the expression levels of these genes are associated with the immune status in health, during pregnancy, and in the autoimmune disease type 1 diabetes. Further studies are required on specific immune cell populations i.e. CD4+, CD8+, Treg, dendritic cells or macrophages, were channel function, intracellular Ca2+ concentration dynamics and concomitant activation of signaling cascades is coupled to specific function.
In conclusion, these studies will contribute to the understanding of how cellular Ca2+ fluctuations are related to the immune shift observed in pregnancy and type 1 diabetes. Whether the changes are in general protective or in type 1 diabetes include some pathogenic components remains to be clarified.
Supporting information
(DOCX)
(DOCX)
(DOCX)
Acknowledgments
We thank to Karin Nygren for technical assistance.
Data Availability
All relevant data are within the manuscript and its Supporting Information files.
Funding Statement
This study was funded by the Swedish Research Council, Swedish Children Diabetes Foundation (grant 2015-2017), Swedish Diabetes Foundation (grant 2016-2017), Stiftelsen Familjen Ernfors Fond, and EXODIAB. The funders had no role in study design, data collection and analysis, decision to publish, or preparation of the manuscript.
References
- 1.Kinder JM, Jiang TT, Way SS. Offspring's Tolerance of Mother Goes Viral. Immunity. 2016;44(5):1085–7. Epub 2016/05/19. 10.1016/j.immuni.2016.04.021 . [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Bonney EA. Alternative theories: Pregnancy and immune tolerance. J Reprod Immunol. 2017;123:65–71. Epub 2017/09/25. 10.1016/j.jri.2017.09.005 . [DOI] [PubMed] [Google Scholar]
- 3.Pugliese A. Autoreactive T cells in type 1 diabetes. J Clin Invest. 2017;127(8):2881–91. Epub 2017/08/02. 10.1172/JCI94549 ; PubMed Central PMCID: PMCPMC5531393. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Dawson NAJ, Vent-Schmidt J, Levings MK. Engineered Tolerance: Tailoring Development, Function, and Antigen-Specificity of Regulatory T Cells. Front Immunol. 2017;8:1460 Epub 2017/11/23. 10.3389/fimmu.2017.01460 ; PubMed Central PMCID: PMCPMC5675854. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Hartzell CA, Jankowska KI, Burkhardt JK, Lewis RS. Calcium influx through CRAC channels controls actin organization and dynamics at the immune synapse. Elife. 2016;5 Epub 2016/07/22. 10.7554/eLife.14850 ; PubMed Central PMCID: PMC4956410. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Feske S, Wulff H, Skolnik EY. Ion channels in innate and adaptive immunity. Annual review of immunology. 2015;33:291–353. 10.1146/annurev-immunol-032414-112212 . [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Omilusik KD, Nohara LL, Stanwood S, Jefferies WA. Weft, warp, and weave: the intricate tapestry of calcium channels regulating T lymphocyte function. Frontiers in immunology. 2013;4:164 10.3389/fimmu.2013.00164 ; PubMed Central PMCID: PMC3690356. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Davenport B, Li Y, Heizer JW, Schmitz C, Perraud AL. Signature Channels of Excitability no More: L-Type Channels in Immune Cells. Front Immunol. 2015;6:375 Epub 2015/08/11. 10.3389/fimmu.2015.00375 ; PubMed Central PMCID: PMC4512153. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Vaeth M, Yang J, Yamashita M, Zee I, Eckstein M, Knosp C, et al. ORAI2 modulates store-operated calcium entry and T cell-mediated immunity. Nat Commun. 2017;8:14714 Epub 2017/03/16. ncomms14714 [pii] 10.1038/ncomms14714 ; PubMed Central PMCID: PMC5355949. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Demaurex N, Nunes P. The role of STIM and ORAI proteins in phagocytic immune cells. Am J Physiol Cell Physiol. 2016;310(7):C496–508. Epub 2016/01/15. ajpcell.00360.2015 [pii] 10.1152/ajpcell.00360.2015 ; PubMed Central PMCID: PMC4824159. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Niemeyer BA. Changing calcium: CRAC channel (STIM and Orai) expression, splicing, and posttranslational modifiers. Am J Physiol Cell Physiol. 2016;310(9):C701–9. Epub 2016/02/26. ajpcell.00034.2016 [pii] 10.1152/ajpcell.00034.2016 . [DOI] [PubMed] [Google Scholar]
- 12.Parekh AB, Putney JW Jr. Store-operated calcium channels. Physiol Rev. 2005;85(2):757–810. Epub 2005/03/25. 10.1152/physrev.00057.2003 . [DOI] [PubMed] [Google Scholar]
- 13.Targos B, Baranska J, Pomorski P. Store-operated calcium entry in physiology and pathology of mammalian cells. Acta Biochim Pol. 2005;52(2):397–409. Epub 2005/06/04. . [DOI] [PubMed] [Google Scholar]
- 14.Chaudhari S, Ma R. Store-operated calcium entry and diabetic complications. Exp Biol Med (Maywood). 2016;241(4):343–52. Epub 2015/10/16. 10.1177/1535370215609693 ; PubMed Central PMCID: PMCPMC4935416. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Gruszczynska-Biegala J, Pomorski P, Wisniewska MB, Kuznicki J. Differential roles for STIM1 and STIM2 in store-operated calcium entry in rat neurons. PLoS One. 2011;6(4):e19285 Epub 2011/05/05. 10.1371/journal.pone.0019285 ; PubMed Central PMCID: PMCPMC3082561. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Uehara A, Yasukochi M, Imanaga I, Nishi M, Takeshima H. Store-operated Ca2+ entry uncoupled with ryanodine receptor and junctional membrane complex in heart muscle cells. Cell Calcium. 2002;31(2):89–96. Epub 2002/04/24. 10.1054/ceca.2001.0257 . [DOI] [PubMed] [Google Scholar]
- 17.Pan Z, Brotto M, Ma J. Store-operated Ca2+ entry in muscle physiology and diseases. BMB Rep. 2014;47(2):69–79. Epub 2014/01/15. 10.5483/BMBRep.2014.47.2.015 ; PubMed Central PMCID: PMCPMC3967412. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Chin-Smith EC, Slater DM, Johnson MR, Tribe RM. STIM and Orai isoform expression in pregnant human myometrium: a potential role in calcium signaling during pregnancy. Front Physiol. 2014;5:169 Epub 2014/05/17. 10.3389/fphys.2014.00169 ; PubMed Central PMCID: PMCPMC4018559. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Leung FP, Yung LM, Yao X, Laher I, Huang Y. Store-operated calcium entry in vascular smooth muscle. Br J Pharmacol. 2008;153(5):846–57. Epub 2007/09/19. 10.1038/sj.bjp.0707455 ; PubMed Central PMCID: PMCPMC2267267. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Tian G, Tepikin AV, Tengholm A, Gylfe E. cAMP induces stromal interaction molecule 1 (STIM1) puncta but neither Orai1 protein clustering nor store-operated Ca2+ entry (SOCE) in islet cells. J Biol Chem. 2012;287(13):9862–72. Epub 2012/02/03. 10.1074/jbc.M111.292854 ; PubMed Central PMCID: PMCPMC3323006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Feske S. Ca(2+) influx in T cells: how many ca(2+) channels? Front Immunol. 2013;4:99 Epub 2013/05/01. 10.3389/fimmu.2013.00099 ; PubMed Central PMCID: PMC3633966. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Wang H, Zhang X, Xue L, Xing J, Jouvin MH, Putney JW, et al. Low-Voltage-Activated CaV3.1 Calcium Channels Shape T Helper Cell Cytokine Profiles. Immunity. 2016;44(4):782–94. Epub 2016/04/03. 10.1016/j.immuni.2016.01.015 . [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Levite M. Neurotransmitters activate T-cells and elicit crucial functions via neurotransmitter receptors. Curr Opin Pharmacol. 2008;8(4):460–71. Epub 2008/06/27. S1471-4892(08)00065-9 [pii] 10.1016/j.coph.2008.05.001 . [DOI] [PubMed] [Google Scholar]
- 24.Bhandage AK, Jin Z, Hellgren C, Korol SV, Nowak K, Williamsson L, et al. AMPA, NMDA and kainate glutamate receptor subunits are expressed in human peripheral blood mononuclear cells (PBMCs) where the expression of GluK4 is altered by pregnancy and GluN2D by depression in pregnant women. J Neuroimmunol. 2017;305:51–8. Epub 2017/03/13. 10.1016/j.jneuroim.2017.01.013 . [DOI] [PubMed] [Google Scholar]
- 25.Park CY, Shcheglovitov A, Dolmetsch R. The CRAC channel activator STIM1 binds and inhibits L-type voltage-gated calcium channels. Science. 2010;330(6000):101–5. 10.1126/science.1191027 . [DOI] [PubMed] [Google Scholar]
- 26.Wang Y, Deng X, Mancarella S, Hendron E, Eguchi S, Soboloff J, et al. The calcium store sensor, STIM1, reciprocally controls Orai and CaV1.2 channels. Science. 2010;330(6000):105–9. 10.1126/science.1191086 ; PubMed Central PMCID: PMCPMC3601900. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Nguyen N, Biet M, Simard E, Beliveau E, Francoeur N, Guillemette G, et al. STIM1 participates in the contractile rhythmicity of HL-1 cells by moderating T-type Ca(2+) channel activity. Biochim Biophys Acta. 2013;1833(6):1294–303. 10.1016/j.bbamcr.2013.02.027 . [DOI] [PubMed] [Google Scholar]
- 28.Clemens RA, Chong J, Grimes D, Hu Y, Lowell CA. STIM1 and STIM2 cooperatively regulate mouse neutrophil store-operated calcium entry and cytokine production. Blood. 2017;130(13):1565–77. Epub 2017/07/21. 10.1182/blood-2016-11-751230 ; PubMed Central PMCID: PMCPMC5620414. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Zhang S, Miao Y, Zheng X, Gong Y, Zhang J, Zou F, et al. STIM1 and STIM2 differently regulate endogenous Ca(2+) entry and promote TGF-beta-induced EMT in breast cancer cells. Biochem Biophys Res Commun. 2017;488(1):74–80. Epub 2017/05/10. 10.1016/j.bbrc.2017.05.009 . [DOI] [PubMed] [Google Scholar]
- 30.Catterall WA, Perez-Reyes E, Snutch TP, Striessnig J. International Union of Pharmacology. XLVIII. Nomenclature and structure-function relationships of voltage-gated calcium channels. Pharmacol Rev. 2005;57(4):411–25. Epub 2005/12/31. 57/4/411 [pii] 10.1124/pr.57.4.5 . [DOI] [PubMed] [Google Scholar]
- 31.Brandman O, Liou J, Park WS, Meyer T. STIM2 is a feedback regulator that stabilizes basal cytosolic and endoplasmic reticulum Ca2+ levels. Cell. 2007;131(7):1327–39. Epub 2007/12/28. S0092-8674(07)01543-7 [pii] 10.1016/j.cell.2007.11.039 ; PubMed Central PMCID: PMC2680164. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Clemens RA, Chong J, Grimes D, Hu Y, Lowell CA. STIM1 and STIM2 cooperatively regulate mouse neutrophil store operated calcium entry and cytokine production. Blood. 2017. Epub 2017/07/21. blood-2016-11-751230 [pii] 10.1182/blood-2016-11-751230 . [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Kreth S, Heyn J, Grau S, Kretzschmar HA, Egensperger R, Kreth FW. Identification of valid endogenous control genes for determining gene expression in human glioma. Neuro-oncology. 2010;12(6):570–9. 10.1093/neuonc/nop072 ; PubMed Central PMCID: PMC2940642. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Ledderose C, Heyn J, Limbeck E, Kreth S. Selection of reliable reference genes for quantitative real-time PCR in human T cells and neutrophils. BMC research notes. 2011;4:427 10.1186/1756-0500-4-427 ; PubMed Central PMCID: PMC3229292. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Schmittgen TD, Livak KJ. Analyzing real-time PCR data by the comparative C(T) method. Nature protocols. 2008;3(6):1101–8. . [DOI] [PubMed] [Google Scholar]
- 36.Lioudyno MI, Kozak JA, Penna A, Safrina O, Zhang SL, Sen D, et al. Orai1 and STIM1 move to the immunological synapse and are up-regulated during T cell activation. Proc Natl Acad Sci U S A. 2008;105(6):2011–6. Epub 2008/02/06. 10.1073/pnas.0706122105 ; PubMed Central PMCID: PMCPMC2538873. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Cheng KT, Alevizos I, Liu X, Swaim WD, Yin H, Feske S, et al. STIM1 and STIM2 protein deficiency in T lymphocytes underlies development of the exocrine gland autoimmune disease, Sjogren's syndrome. Proc Natl Acad Sci U S A. 2012;109(36):14544–9. Epub 2012/08/21. 10.1073/pnas.1207354109 ; PubMed Central PMCID: PMCPMC3437853. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Hoover PJ, Lewis RS. Stoichiometric requirements for trapping and gating of Ca2+ release-activated Ca2+ (CRAC) channels by stromal interaction molecule 1 (STIM1). Proc Natl Acad Sci U S A. 2011;108(32):13299–304. Epub 2011/07/27. 1101664108 [pii] 10.1073/pnas.1101664108 ; PubMed Central PMCID: PMC3156176. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Bjurstom H, Wang J, Ericsson I, Bengtsson M, Liu Y, Kumar-Mendu S, et al. GABA, a natural immunomodulator of T lymphocytes. J Neuroimmunol. 2008;205(1–2):44–50. 10.1016/j.jneuroim.2008.08.017 . [DOI] [PubMed] [Google Scholar]
- 40.Bhandage AK, Hellgren C, Jin Z, Olafsson EB, Sundstrom-Poromaa I, Birnir B. Expression of GABA receptors subunits in peripheral blood mononuclear cells is gender dependent, altered in pregnancy and modified by mental health. Acta Physiol (Oxf). 2015;213(3):575–85. Epub 2014/12/23. 10.1111/apha.12440 . [DOI] [PubMed] [Google Scholar]
- 41.Wehbi VL, Tasken K. Molecular Mechanisms for cAMP-Mediated Immunoregulation in T cells—Role of Anchored Protein Kinase A Signaling Units. Front Immunol. 2016;7:222 Epub 2016/07/05. 10.3389/fimmu.2016.00222 ; PubMed Central PMCID: PMCPMC4896925. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Zhang J, Carver CM, Choveau FS, Shapiro MS. Clustering and Functional Coupling of Diverse Ion Channels and Signaling Proteins Revealed by Super-resolution STORM Microscopy in Neurons. Neuron. 2016;92(2):461–78. Epub 2016/10/21. 10.1016/j.neuron.2016.09.014 ; PubMed Central PMCID: PMCPMC5553284. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Kar P, Parekh AB. Distinct spatial Ca2+ signatures selectively activate different NFAT transcription factor isoforms. Mol Cell. 2015;58(2):232–43. Epub 2015/03/31. 10.1016/j.molcel.2015.02.027 ; PubMed Central PMCID: PMCPMC4405353. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Wolf IMA, Guse AH. Ca(2+) Microdomains in T-Lymphocytes. Front Oncol. 2017;7:73 Epub 2017/05/18. 10.3389/fonc.2017.00073 ; PubMed Central PMCID: PMCPMC5411426. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
(DOCX)
(DOCX)
(DOCX)
Data Availability Statement
All relevant data are within the manuscript and its Supporting Information files.