Abstract
Exposure therapy for Social Anxiety Disorder (SAD) utilizes fear extinction, a memory process enhanced by sleep. We investigated whether naps following exposure sessions might improve symptoms and biomarkers in response to social stress in adults undergoing 5-week exposure-based group SAD therapy. Thirty-two participants aged 18-39 (18 females) with SAD were randomized. Before and after treatment, participants completed the Liebowitz Social Anxiety Scale (LSAS) and underwent a Trier Social Stress Test with psychophysiological monitoring (mpTSST) that included skin conductance (SCL), electromyographic (EMG) and electrocardiographic recording, and an auditory startle procedure while anticipating the social stressor. At sessions 3 and 4, exposure was followed by either a 120-min polysomnographically monitored sleep opportunity (Nap, N=17) or wakefulness (Wake, N=15). Primary hypotheses about SAD symptom change (LSAS) and EMG blink-startle response failed to differ with naps, despite significant symptom improvement (LSAS) with therapy. Some secondary biomarkers, however, provided preliminary support for enhanced extinction learning with naps, with trend-level Time (pre-, post-treatment) × Arm interactions and significant reduction from pre- to post treatment in the Nap arm alone for mpTSST SCL and salivary cortisol rise. Because of the small sample size and limited sleep duration, additional well-powered studies with more robust sleep interventions are indicated.
Keywords: sleep, social anxiety disorder, exposure therapy, extinction, psychophysiology, cortisol
1. Introduction
Social Anxiety Disorder (SAD) (APA, 2013) can occur with a range of severities varying from a circumscribed fear of public speaking to a debilitating generalized condition that severely limits social interactions (Morrison and Heimberg, 2013). The National Comorbidity Survey Replication reported the lifetime prevalence of SAD at 12.1%, higher than any other anxiety disorder except specific phobia (Kessler et al., 2005). SAD can lead to significant disability in education, employment and relationships (Stein and Kean, 2000; Stein et al., 2000), and is a major risk factor for the development of depression and alcohol abuse (Beesdo et al., 2007).
Exposure therapy is an effective, evidence based treatment for SAD (Jorstad-Stein and Heimberg, 2009; Ponniah and Hollon, 2008). However, because exposure therapy often does not lead to full remission and relapse is common (Hofmann and Smits, 2008), methods to enhance its efficacy have received strong research interest. Fear extinction, i.e., learning that something once perceived as dangerous no longer need be feared, is the neurobehavioral basis for the efficacy of exposure therapy (Craske et al., 2008). Extinction does not erase fear; rather, it forms an inhibitory memory that opposes fear (Milad and Quirk, 2012) and, if strengthened, can enhance the efficacy of exposure therapy. The current study investigated whether sleep might serve as a non-pharmacological way to enhance consolidation of therapeutic extinction learning as measured by reduction in social anxiety symptoms as well as stress related biomarker responses to a standardized social stressor, the Trier Social Stress Test (TSST) (Kirschbaum et al., 1993), employed as an experimental test of the robustness of fear extinction learning.
Sleep promotes consolidation in multiple memory systems (Rasch and Born, 2013). Sleep may facilitate key neural processes required for memory consolidation (Abel et al., 2013), and sleep disturbance can disrupt these same processes (da Costa Souza and Ribeiro, 2015; Krause et al., 2017). Thus, sleep might also strengthen and generalize extinction learning and memory (Pace-Schott et al., 2015a, b).
Preliminary findings from our laboratory and others support this possibility. For example, among spider fearful women, those allowed a night’s sleep following simulated (video) exposure therapy, unlike those who remained awake across a day, retained the lowered negative emotion ratings, skin conductance response (SCR) and heart-rate acceleration that they achieved during their exposure session when they returned 12 hr. later (Pace-Schott et al., 2012a). Additionally, those who slept unlike those who remained awake, did not show sensitization of subjective ratings, corrugator supercilii EMG or SCR when shown a novel spider during their second session. Control groups exposed and re-tested in the morning and evening of this study ruled out circadian effects. Similar findings for self-report measures were reported in persons with spider phobia who either napped or remained awake after a single session of virtual reality exposure treatment (Kleim et al., 2013). In a de-novo fear conditioning and extinction paradigm, we have shown that sleep promotes generalization of SCR indices of fear extinction (Pace-Schott et al., 2009). Whereas most studies on sleep and extinction utilize SCR (e.g., Menz et al., 2016; Spoormaker et al., 2012), other studies employ fear-potentiated startle indexed by the orbicularis oculii blink startle response (Marshall et al., 2014; Straus et al., 2017). In both cases, REM sleep has been implicated in memory for extinction (reviewed in Pace-Schott et al. 2015a,b). In a nap study, sleep-dependent inter-session habituation was seen for SCR and corrugator EMG when a 2-h nap opportunity, versus 2h of wakefulness, was interposed between habituation to aversive photographs and re-challenge with these same images (Pace-Schott et al., 2011). Finally, both corrugator EMG in this same study, as well as heart rate deceleration in another study on habituation to acoustic startle (Pace-Schott et al., 2014) showed wake-related sensitization of responses that were not seen when participants slept.
The current pilot study of a small (N=32) sample of adults with SAD investigated whether sleep could be used to strengthen extinction memories formed during a brief 5-week exposure-based group therapy for SAD (Hofmann, 2004). We investigated whether naps following social exposure sessions might serve to enhance consolidation of therapeutic extinction memory and lead to improved clinical outcomes. Psychophysiological measures were used to assess responses to an experimental social stressor, the Trier Social Stress Test (TSST), presented before and after the exposure therapy. We specifically hypothesized that, from pre- to post-treatment, participants who napped following exposure would: (1) show greater reduction in social anxiety symptom reporting on the Liebowitz Social Anxiety Scale (LSAS) (Liebowitz, 1987); and (2) show greater reduction in the EMG blink-startle response to an acoustic-startle stimulus during anticipation of the social-stress challenge.
2. Methods
2.1. Participants
Participants were recruited using advertisements in electronic bulletin boards and social media. Thirty-two participants aged 18-39 years (mean 26 years, SD 6.26, 18 females) completed an evidence-based standardized 5-week exposure-based, group therapy for SAD (Hofmann, 2004; Hofmann et al., 2006; Smits et al., 2014). Each participant underwent telephone screenings followed by psychiatric and sleep-disorders screening interviews. Inclusion criteria included an LSAS score of at least 60, and all met criteria for DSM-IV-TR SAD. Exclusion Criteria included potentially confounding medical, sleep, neurological, substance use or severe psychiatric illnesses (see Supplementary Methods for details). In accordance with the dimensional approach of the NIMH Research Domain Criteria initiative (Morris and Cuthbert, 2012), milder current anxiety and mood comorbidities were permitted (see Supplementary Methods). All study procedures accorded with the Declaration of Helsinki and were approved by the Partners Healthcare Institutional Review Board. All participants provided written informed consent, received group treatment free of charge and were paid a participant fee.
2.2. Procedures
Figure 1 provides a CONSORT diagram (Thabane et al., 2016) of study participants and Figure 2 displays the study protocol. Each eligible participant was assigned to one of nine, 5-week/5-session therapy groups, each with 3 to 4 participants, run successively by 2 experienced Ph.D. clinical psychologists certified in the protocol. All subjects underwent the mpTSST as an assessment of psychophysiological reactivity to a social performance challenge (detailed below). Each 2 hour pre-treatment mpTSST occurred approximately 1 week before therapy and the identical procedure was repeated approximately 1 week after the last (fifth) therapy session (post-treatment mpTSST). Subjects participated in the 2-h mpTSST, one at a time, from approximately 1330-1530 or 1530-1730. Three days prior to the 2 mpTSSTs and 5 therapy sessions, participants were asked to follow a basic sleep hygiene regimen (see Supplementary Methods for details and compliance data). Throughout the entire protocol participants wore the Actiwatch 2 (Phillips Respironics, Bend, OR) and completed daily sleep diaries (see Supplementary Methods).
Figure 1.
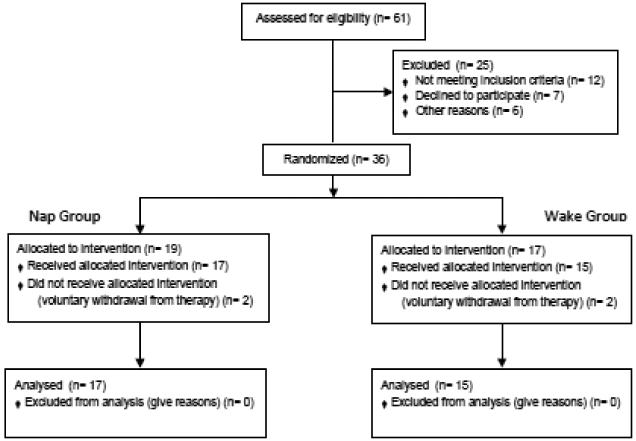
CONSORT diagram of subject recruitment, randomization and analysis.
Figure 2.
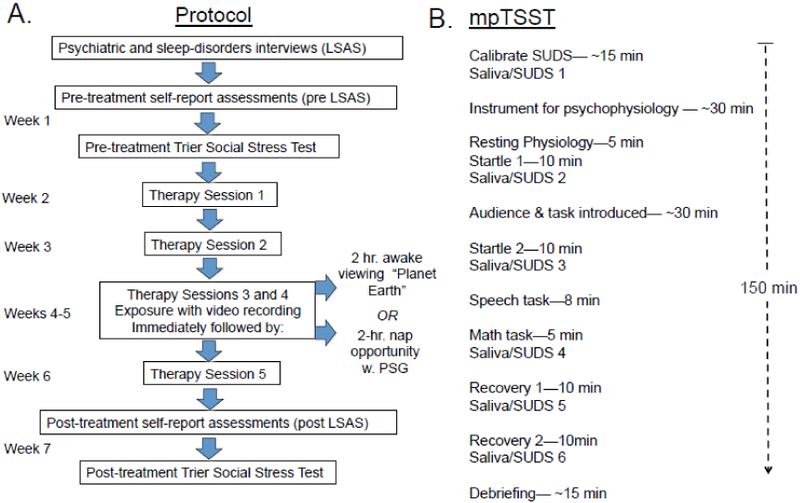
Experimental procedures showing: A. Treatment schedule, mpTSST and intervention; B. mpTSST procedure. mpTSST - Trier Social Stress Test modified for psychophysiology; SUDS - Subjective units of distress.
Before the third therapy session, participants were randomized in blind fashion to either the Nap (N=17, 9 females) or Wake (N=15, 9 females) condition. The third and fourth therapy sessions ended with an exposure exercise after which, at approximately 1430, participants were escorted to the Massachusetts General Hospital Sleep Laboratory. When initially providing consent, participants were made aware that they would either nap or remain awake following the third and fourth therapy sessions and all participants were instrumented to undergo polysomnography (PSG). However, the Wake arm received sham instrumentation. Participants were assigned to the same condition at both sleep-lab visits; however, they were not told whether they would nap or remain awake until after being instrumented. The 2 psychotherapists and the independent rater remained blind to each subject’s assigned arm. Nap-arm subjects were given a 2-h sleep opportunity with PSG recording while those in the Wake arm spent an equal duration watching 2 episodes of Planet Earth. (See Supplementary Methods for rationale of nap/wake timing parameters.) At 1600, lights were turned off (Nap arm) and videos began (Wake arm). All were monitored via video with 15-min, in-person check-ins for Wake-arm subjects. At 1800, electrodes were removed and all were instructed to go to bed at their usual hour. Approximately 1 week following the fifth therapy session, each participant completed their post-treatment mpTSST followed by a comprehensive debriefing.
2.3. Exposure-based therapy for individuals with SAD (Hofmann, 2004)
Treatment was a manualized and validated (Hofmann et al 2006, Smits et al 2013) behavior-based treatment for social anxiety consisting of five 90-min sessions carried out weekly by the same 2-therapist team for all participants. Treatment utilized public speaking exposures during which extinction learning could take place. During all but the first therapy session, participants each gave a videotaped speech to the group. Session 2 speeches focused on the model of social anxiety. From therapy session 3 onward, participants' speech topics were personalized and designed to increase in level of challenge such that the therapy session-5 speech was typically the most difficult. Only sessions 3 and 4 were followed by naps as they were the 2 sessions that focused exclusively on exposure. Subjects in all but the first group provided standard 100-point Subjective Units of Distress (SUDS) ratings (Heimberg, 1991) before and after their speech (N=28). An additional 5 (3 Nap, 2 Wake) did not provide SUDS ratings at therapy session 5 leaving 12 Nap and 11 Wake participants whose SUDS ratings were able to be compared across therapy sessions.
2.4. Trier Social Stress Test modified for psychophysiological measurements (mpTSST)
The TSST is an extensively used and widely validated paradigm for eliciting the in vivo effects of both normal and pathological social anxiety (Allen et al., 2014; Frisch et al., 2015). The standard TSST was modified to assess physiological reactivity during a period of anticipatory anxiety prior to the social stressor as well as during the stressor (mpTSST). During the anticipatory anxiety phase, we examined as primary the blink-startle orbicularis oculi electromyographic (EMG) response to a loud acoustic stimulus— a procedure analogous to that used in fear-potentiated startle paradigms (Grillon and Baas, 2003; Norrholm et al., 2011). Additional secondary physiological measures were obtained during the mpTSST including: (1) Elevation of salivary cortisol by the mpTSST. Cortisol reactivity is the standard assay of HPA axis response to social stress in the TSST (Allen et al., 2014; 2017; Frisch et al., 2015; Kirschbaum et al. 1993; Kirschbaum and Hellhammer, 1994). The time points of saliva samples roughly followed Kirschbaum et al. 1993 (viz., baseline, beginning and end of anticipation and then 0, 10 and 20 min following end of stressor) as illustrated in Figure 2B. Cortisol reactivity has been reported elevated in SAD versus controls in some paradigms (Zorn et al., 2017). (2) Skin conductance level (SCL) and response (SCR): The TSST is known to elevate indices of sympathetic activation such as SCR (Allen et al., 2014) and, in some studies, this has been shown to be further elevated in SAD (Rozenman et al., 2017). Thus, SCR to the auditory startle was also measured as was the elevation of SCL brought about by the mpTSST stressor. (3) Cardiac measures using electrocardiography (ECG): Heart-rate acceleration (HRA) to the acoustic startle, heart rate (HR) during the mpTSST stressor, and heart-rate variability during quiescent periods were measured as additional indices of sympathetic activation.
Procedures and timing for the mpTSST were adapted from Kirschbaum et al. (1993) (see Supplementary Methods). Throughout the mpTSST, participants provided 6 SUDS ratings immediately followed by saliva samples for cortisol measurements using the Salimetrics oral swab (Salimetrics, LLC, State College, PA). The mpTSST procedure was divided into the following phases: (1) Orientation/instrumentation phase: Participants were trained in and provided their first SUDS ratings and saliva sample, and were instrumented for SCL, facial EMG and ECG. (2) Resting physiology phase: Participants were monitored as they sat quietly for 5 min in a comfortable chair. (3) Startle 1 phase: The first of 2 startle procedures (Buhlmann et al., 2007; Pace-Schott et al., 2014), added to the mpTSST to assess physiological reactivity during periods of anticipatory anxiety, was performed. Across the next 10 min, the participant received, binaurally through headphones, 10 randomly spaced (but fixed across subjects) 500-ms, 100-102 dB, 1000-Hz, sudden-onset pure tones to elicit the startle reflex. After Startle 1, participants provided their second SUDS and saliva. (4) Instruction phase: An investigator read the standard TSST instructions (Kirschbaum et al., 1993). Two “evaluators” (one male, one female) then entered the room, reiterated instructions for the speech, showed the participant a video camera and tape recorder and stated that they would record the speech for later evaluation. Each participant had a different pair of evaluators for their pre- vs. post-treatment mpTSST (additional detail in Supplementary Methods). (5) Startle 2 phase: Each participant was given 10 min to prepare their speech while wearing headphones and hearing loud tones identical to those presented during Startle 1 but with different inter-stimulus intervals (again fixed across subjects). During the first 5 min (Early Startle 2 subphase), participants prepared their speech “in their head.” They were then given 5 minutes (Late Startle 2 subphase) to write down their ideas, but were not allowed to use their notes during the speech. Five startle stimuli were delivered during each interval. At the end of the second 5-min period, participants provided their third SUDS and saliva sample. (6) Performance phase: After evaluators returned and started the video camera and tape recorder, the participant delivered their speech for 8 min (Speech subphase) and next performed serial subtractions out loud for 5 min (Math subphase), then provided their fourth SUDS and saliva sample. (7) Recovery phase: Participants were then left alone for 10 min while completing a series of evaluations (Early Recovery subphase), after which they provided their fifth SUDS and saliva sample. They then sat quietly for an additional 10 minutes (Late Recovery subphase), after which they provided their sixth SUDS and saliva sample. (8) Debriefing phase: Following both mpTSSTs the participant was evaluated by clinical staff to ensure that any distress engendered by the mpTSST had dissipated.
2.5. Stimuli and physiological recording
Throughout the mpTSST, SCL, HR and facial EMG were continuously monitored using the Biopac MP150 system (Biopac Systems, Inc., Goleta, CA) with AcqKnowledge 4.1.5 data-acquisition software. Startle stimuli were timed and triggered using Superlab 4.5 (Cedrus Corp., San Pedro, CA) which simultaneously transmitted event marks allowing precise synchronization of each stimulus onset with the ongoing physiological recording. Previously published techniques were used for electrode placement (Pace-Schott et al., 2012b) and processing responses to loud tones including SCR, orbicularis oculi blink EMG and HRA (Pace-Schott et al., 2014). Details of data reduction are provided in Supplementary Methods.
2.6. Polysomnographic recordings
Subjects underwent polysomnography using the Somte-PSG (Compumedics USA, Inc., Charlotte, NC) ambulatory sleep monitor (with sham for Wake-arm participants). A standard PSG montage was employed (see Supplementary Methods) and a trained technician scored naps using standard criteria (Iber & al. 2007, Rechtschaffen & Kales 1968).
2.7. Salivary cortisol
Saliva samples were frozen and stored at −20 C. Unbound cortisol was assayed by Salimetrics, LLC using enzyme immunoassay. Cortisol levels from the fourth to sixth samples were subtracted from the first baseline level to calculate changes during the mpTSST. Cortisol rise in the second and third samples were not analyzed due to the known delay in salivary peak levels following an experimental stressor (Lopez-Duran et al., 2014). Change scores were square-root transformed to reduce heteroscedasticity. Participants with missing values were excluded from analyses.
2.8. Outcome and predictor variables
Primary and secondary outcome measures were posted on Clinicaltrials.gov (NCT02325128) before collection of data began. The primary clinical outcome measure was the LSAS (total, fear and avoidance scores). Secondary clinical outcome variables included a battery of additional social anxiety questionnaires (detailed in Supplementary Methods and Table S1).
The primary psychophysiological (mpTSST) outcome variable was the mean of the first 5 orbicularis EMG responses to the loud tones during the Early Startle 2 subphase. Physiological responses to these 5 stimuli occurred after 10 habituation stimuli during Startle 1 and before participants began taking notes during Late Startle 2 and were, thus, putatively least influenced by habituation or distraction. Mean EMG responses were also examined at Startle 1 (last 5) and Late Startle 2 (detailed rationale and methods in Supplementary Methods). Secondary mpTSST psychophysiological outcome variables included: (1) mean SCR and HRA during Early Startle 2; (2) mean SCL and heart rate (HR) during Performance (Speech, Math) and Recovery (Late recovery) (Resting-physiology phase SCL and HR were also compared to rule out baseline differences); and (3) mean mpTSST-induced cortisol rise (Orientation phase cortisol levels were also compared to rule out baseline differences). The secondary mpTSST self-report variables were mean SUDS ratings and 4 additional assessments described in Supplementary Methods.
Potential covariates influencing the effect of the Nap intervention on outcome measures included: (1) retrospective and mean longitudinal home sleep measures (diary and actigraph), (2) sleep-architectural characteristics of naps and (3) within-session extinction at therapy sessions 3 and 4. These are described and analyzed in Supplementary Materials.
2.9. Sample size consideration
The sample size required for this study was initially estimated from a large effect size in a previous study of spider-fearful subjects (Pace-Schott et al., 2012a) in which between-session extinction of SCR responses to a 10ms, 80dB white noise stimulus was compared between daytime wakefulness and nocturnal sleep groups. The mean difference in stimulus-evoked SCR between the initial presentations of a spider video (to which subjects then underwent simulated exposure treatment in the first session) and their stimulus-evoked responses during a video of a new spider during the second session (that followed sleep vs. wakefulness) served as an index of the generalization of extinction learning to novel phobic stimuli. Superior generalization was seen in the sleep group with an effect size of d= −1.07. With 15 participants per arm, this effect size would allow detection of a group difference with 90% power and a 2-sided alpha of 0.05.
2.10. Statistical analyses
Comparisons of self-report assessments between Nap and Wake arms were carried out using ANOVA with 1 between-subjects factor (“Arm”: Nap, Wake) and 1 within-subject factor (“Time”: pre-, post-treatment). Such ANOVAs were also used to compare psychophysiological responses to the startle stimuli during the mpTSST. Additionally, for the mpTSST data, a second within-subject variable was added for cortisol rise (3 levels of “Order”: fourth to sixth sample), SCL (3 levels of “Subphase”) and SUDS ratings (“Order”: first to sixth). Similarly, ANOVA analyses of SUDS ratings during therapy sessions included a within-subject factor for the 2 therapy sessions that occurred after at least one nap-wake intervention (Sessions 4 and 5) and, within sessions, the Phase of exposure (Pre- and Post-speech). The Greenhouse-Geisser correction was applied to all analyses containing within-subject comparisons. Post-hoc sub-group analyses were used to examine potential interactive effects identified in the main models. Significance levels were set at α=0.05 and reported effect sizes of ANOVA model effects are partial η2 (pη2) with small, medium and large effect sizes of 0.0099, 0.0588, and 0.1379 respectively (Richardson, 2011). Analyses of primary outcome variables were repeated comparing the Wake arm to the 13 Nap participants who achieved slow wave sleep (SWS) and to the 7 Nap participants who achieved rapid eye movement sleep (REM) in at least one nap. (See Supplementary Methods for analyses of covariates.)
3. Results
3.1. Baseline Characteristics and Nap Success
The Nap and Wake arms did not differ in baseline characteristics including sleep and LSAS severity (Table 1). All Nap-arm participants (except one in nap 1) achieved sleep during both naps. Nap-1 sleep durations averaged 68.9 min (median 79.5, 11/17 > 50 min), Nap 2 averaged 68.8 min (median 80.5, 14/17 > 50), summed sleep across both naps averaged 137.7 (median 149.5, 16/17 > 50). Table 2 shows mean sleep stage composition for both naps. Within the Nap arm, 14 individuals achieved slow wave sleep (SWS) and 7 achieved REM sleep in at least 1 nap.
Table 1.
Comparison of Nap and Wake arms’ demographic, pre-treatment retrospective sleep and clinical outcome variables using un-paired t-tests.
| Nap | SD | Wake | SD | df | t | p | d | |
|---|---|---|---|---|---|---|---|---|
| N (females) | 17 (9) | 15 (9) | ||||||
| Age | 27.24 | 6.79 | 24.60 | 5.49 | 1,30 | 1.20 | 0.24 | 0.43 |
| Appx. years of education a | 16 | 2.06 | 16.13 | 2.47 | 1,30 | 0.66 | 0.87 | −0.05 |
| PSQI-PRE | 5.35 | 3.72 | 4.73 | 2.43 | 1,30 | 0.55 | 0.59 | 0.20 |
| MEQ-PRE | 46.82 | 10.38 | 47.47 | 11.70 | 1,30 | 0.17 | 0.87 | −0.06 |
| Self-report h mean TST | 7.1 | 0.92 | 7.4 | 1.08 | 1,30 | 0.97 | 0.34 | −0.30 |
| Self-report naps/week b,c | 0.87 | 2.13 | 1.21 | 2.52 | 1,27 | 0.40 | 0.69 | −0.15 |
| Clinical outcome | ||||||||
| total LSAS-PRE | 84.24 | 21.87 | 84.87 | 16.14 | 1,30 | 0.09 | 0.93 | −0.03 |
| total LSAS-POST b | 62.82 | 23.68 | 55.29 | 19.27 | 1,29 | 0.96 | 0.35 | 0.35 |
| LSAS Fear-PRE | 43.06 | 12.31 | 43.27 | 9.04 | 1,30 | 0.05 | 0.96 | −0.02 |
| LSAS Fear-POST b | 33.29 | 12.93 | 30.21 | 12.03 | 1,29 | 0.68 | 0.50 | 0.25 |
| LSAS Avoidance-PRE | 41.18 | 11.35 | 41.60 | 10.06 | 1,30 | 0.11 | 0.91 | −0.04 |
| LSAS Avoidance-POST b | 29.53 | 13.03 | 25.07 | 10.10 | 1,29 | 1.05 | 0.30 | 0.38 |
PSQI – Pittsburgh Sleep Quality Index; MEQ – Morningness-Eveningness Questionnaire; TST – total sleep time
Derived from Structured Clinical Interview for DSM-IV-TR, where high school grad = 12, partial college = 14, college graduate = 16, post college = 19
N Wake=14
N Nap=15; t – t-value of a two-sample t-testd – Cohen’s d.
Table 2.
Sleep architecture of the two naps of Nap arm showing minutes in each sleep stage.
| Nap 1 | Nap 2 | |||||
|---|---|---|---|---|---|---|
| Mean | SD | N | Mean | SD | N | |
| All Participants | ||||||
| TST (min) | 68.91 | 37.67 | 17 | 68.82 | 39.76 | 17 |
| N1 (min) | 8.59 | 8.64 | 17 | 8.15 | 6.67 | 17 |
| N2 (min) | 40.82 | 21.13 | 17 | 39.56 | 21.57 | 17 |
| N3 (min) | 13.79 | 14.72 | 17 | 17.18 | 16.79 | 17 |
| REM (min) | 5.00 | 8.65 | 17 | 3.94 | 7.04 | 17 |
| Those who achieved sleep states* | ||||||
| TST (min) | 73.22 | 34.32 | 16 | 68.82 | 39.76 | 17 |
| Sleep onset latency (min) | 20.28 | 22.39 | 16 | 16.41 | 22.87 | 17 |
| N1 (min) | 9.13 | 8.62 | 16 | 8.15 | 6.67 | 17 |
| N2 (min) | 43.38 | 18.92 | 16 | 39.56 | 21.57 | 17 |
| N3 (min) | 19.54 | 13.88 | 12 | 24.33 | 14.84 | 12 |
| REM (min) | 17.00 | 6.63 | 5 | 11.17 | 7.87 | 6 |
| REM latency (min) | 68.40 | 11.67 | 5 | 79.67 | 11.31 | 6 |
Mean, SD and N for only those individuals who achieved each sleep stage. Neither total sleep time nor sleep onset latency differed between naps (p=0.99 and p=0.56 respectively).
3.2. Clinical outcome variables
Participants’ social anxiety disorder symptoms improved significantly across treatment on LSAS total [F(1,29)=85.93, p<0.0001, pη2=0.748], as well as fear [F(1,29)=47.15, p<0.0001, pη2=0.619] and avoidance [F(1,29)=69.70, p<0.0001, pη2=0.706] subscales. However, this primary clinical outcome did not differ by Nap vs. Wake arm, with non-significant Arm × Time interactions for LSAS Total [F(1,29)=2.04, p=0.16, pη2=0.066], fear [F(1,29)=0.88, p=0.36, pη2=0.029] and avoidance [F(1,29)=1.97, p=0.17, pη2=0.064] scales. All secondary self-report measures showed a similar pattern of significant improvement across treatment but no difference between arms (Table S1).
3.3. Psychophysiological responses to startle procedures
For the orbicularis blink startle EMG primary outcome measure (at Early Startle 2), there was no Arm × Time interaction [F(1,28)=0.17, p=0.69, pη2=0.006]. Similarly, there was no such interaction for the secondary HRA measure [F(1,28)=1.23, p=0.28, pη2=0.042]. However, for the secondary SCR measure, the Arm × Time interaction was non-significant but with a moderate effect size [F(1,28)=2.81, p=0.105, pη2=0.091]. SCR showed a significant decrease from pre- to post-treatment mpTSST in the Nap arm [F(1,15)=7.13, p=0.018, pη2=0.322] but not the Wake arm [F(1,13)=0.21, p=0.65, pη2=0.015]. For comparison of Nap and Wake arms at different times during the startle procedures, see Supplementary Results and Table S2.
3.4. Skin conductance level during performance and recovery
The Arm × Time interaction for SCL approached but did not quite reach significance [F(1,25)=4.21, p=0.051, pη2=0.144]. This interaction remained for Speech alone [F(1,27)=4.65, p=0.04, pη2=0.147] and at a trend level for Math alone [F(1,27)=3.32, p=0.08, pη2=0.109] but not Late Recovery alone [F(1,27)=2.32, p=0.14, pη2=0.082]. Within the Nap Arm, there was a significant decrease in SCL from pre- to post-treatment [Time main effect: F(1,14)=6.18, p=0.026 pη2=0.306] driven by a significant pre- to post-treatment decrease during Speech [F(1,15)=6.12, p=0.026 pη2=0.290] and Math [F(1,15)=5.78, p=0.03 pη2=0.278] but not Late Recovery [F(1,15)=2.83, p=0.12 pη2=0.168] subphases (Figure 3). In contrast, SCL did not change across treatment in the Wake Arm [Time main effect: F(1,11)=0.07, p=0.49, pη2=0.044] (Figure 3). Indicating the absence of baseline differences in SCL, there were no Arm or Time main effects or interactions during the Resting physiology phase (all p’s>0.26).
Figure 3.
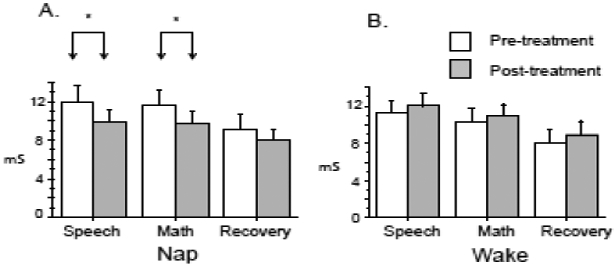
Skin conductance level during Performance (Speech, Math) and Recovery phases. Nap arm, N=15, Wake Arm N= 12, Error bars represent standard error of the mean. mS – microSiemens, * p < 0.05.
3.5. mpTSST salivary cortisol outcome variables
Significant elevation above baseline did not occur until the fourth sample collected at the end of the Presentation phase (Figure 4A). Among the fourth to sixth cortisol-rise values, there was only a trend level Arm × Time interaction [F(1,26)=2.93, p=0.099, pη2=0.101]. However, within the Nap Arm, there was a significant Time main effect [F(1,14)=5.40, p=0.036, pη2=0.278, Pre->Post-treatment], whereas this effect was absent in the Wake Arm [F(1,12)=0.05, p=0.83, pη2=0.004]. Indicating the absence of baseline differences in salivary cortisol, Orientation-phase levels did not differ between the Nap and Wake arms at the pre- [F(1,28)=0.73, p=0.40, pη2=0.024] or post-treatment [F(1,28)=0.99, p=0.33, pη2=0.035] mpTSST.
Figure 4.
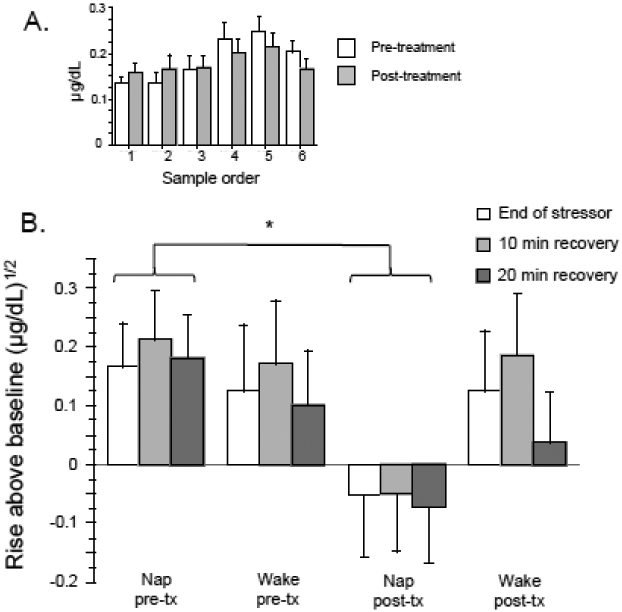
A. Delayed rise of salivary cortisol until fourth sample (conclusion of the Performance phase). B. Salivary cortisol at the end of mpTSST stressor (Performance phase), after 10 min of Recovery, and after 20 min of Recovery. Nap arm, N=15, Wake Arm N= 13. Error bars represent standard error of the mean. * p < 0.05, paired t-test.
3.6. SUDS ratings during exposure therapy sessions and mpTSST
SUDS ratings during therapy exposures showed a highly significant main effect of Phase across the second to fifth therapy sessions [F(1, 21)=41.76, p<0.0001, pη2=0.665; Pre- > Post-speech] as well as during the fourth and fifth therapy session [F(1, 21)=28.41, p<0.0001, pη2=0.575]. These latter two sessions represented treatment sessions following one and both nap interventions respectively. Ratings during the fourth and fifth therapy session showed a significant Arm × Session × Phase interaction [F(1,21)=7.40, p=0.013, pη2=0.260]. This interaction resulted from, during therapy session 5, a significant decrease from Pre- to Post-speech SUDS in the Nap arm [F(1,11)=23.29, p=0.0005, pη2=0.679] which remained only a trend in the Wake arm [F(1,10)=4.44, p=0.06, pη2=0.307]. Among all six mTSST SUDS ratings, there was a main effect of Order [F(5,130)=55.18, p<0.0001, pη2=0.680; just before and after Performance phase greatest]. There was also a Time main effect [F(1,26)=47.54, p<0.0001, Pre- >Post-treatment, pη2=0.646] and an Order × Time interaction [F(5,130)=5.48, p=.0013, pη2=0.174]. However, there was no Arm main effect or interactions (all p’s >0.37).
4. Discussion
The current pilot study did not find support for our primary hypotheses that post exposure naps, provided after key exposure sessions during cognitive behavioral therapy for SAD, would augment treatment related reductions in social anxiety symptoms or reduce blink startle EMG reactivity during anticipation of a social stressor. Although social anxiety symptoms measured by the primary clinical outcome measure, LSAS, diminished significantly from pre- to post-treatment, indicating that treatment was effective, this reduction did not significantly differ between Nap versus Wake arms. Similarly, none of the secondary clinical variables showed evidence of superior improvement in the Nap arm (Table S1, Supplementary Results).
Despite the lack of support for a nap-intervention effect on the SAD-symptom and EMG-startle primary outcomes, there were some signals suggestive of a potential nap effect in secondary and follow up exploratory analyses. For example, secondary physiological indices of sympathetic activation and stress, viz., SCR to loud tones during anticipation of the stressor, SCL during the stressor, and stressor-induced cortisol rise, showed significant pre- to post-treatment reduction in the Nap but not the Wake arm (Figures 3, 4). A similar, albeit weaker effect was also suggested for another indicator of sympathetic arousal, the LF/HF ratio of heart-rate variability (Supplementary Results). Additionally, if the declines in SUDS ratings across the exposure exercise in therapy sessions 4 and 5 are considered a proxy measure of short-term therapeutic gain in the sessions that followed the nap interventions, then this may be an additional indication of better response to treatment in the Nap arm.
Why some of the psychophysiological and endocrine measures indicated that post-exposure naps enhanced reduction in social reactivity with therapy whereas no additional improvement in subjectively reported social anxiety symptoms occurred is not entirely clear. Nonetheless, in studies using the TSST, discrepancies between physiological measures and subjective responses are extremely common (Campbell and Ehlert, 2012), especially when subjective measures are obtained retrospectively (Hellhammer and Schubert, 2012). Such dissociation also has appeared examining changes over repeated presentations of the TSST (Boesch et al., 2014). Correlation between physiological measures and subjective responses only occurred in 25% of 358 TSST studies reviewed by Campbell and Ehlert (2012) who suggest that physiological response and subjective experience may represent dissociable components of an individual’s response to stress.
One possibility is that biomarkers of emotional responses, such as those expressed by the autonomic nervous system, provide a more sensitive index of sleep-enhanced extinction but may not be consciously perceived, and are thereby not reflected in measures that require cognitive appraisal. It is also possible that autonomic and self-report measures reflect different underlying processes. An interesting parallel to this possibility has been reported for d-cycloserine (DCS), also given in temporal proximity to exposure therapy to enhance extinction (Otto et al., 2016). DCS enhances extinction in rodents and in the treatment of individuals with anxiety disorders, but does not enhance experimental extinction in healthy subjects (Grillon, 2009). Grillon has suggested that there are two complementary fear conditioning and extinction systems in humans: a low-level, phylogenetically primitive system that operates outside of conscious awareness and is pathologically recruited in anxiety disorders, and a higher-level system that requires conscious awareness and cognition. Thus, sleep, like DCS, may influence the former, enhancing plasticity supporting autonomic and endocrine responses, but may be less effective in changing conscious appraisals.
4.1. Limitations
Consistent with its exploratory nature, the current pilot study has a number of limitations. Low statistical power resulting from a small sample size increased the possibility of Type-2 error. Available resources allowed study of only this limited sample in this initial test of the effect of naps after exposure sessions. Moreover, multiple outcome measures inflated the chance of Type-1 error. Because the Arm × Time interactions for physiological variables reached only trend-level significance, improvement across treatment in the Nap arm alone should be interpreted cautiously. Nonetheless, as a pilot study, these multiple outcome measures provide the ability to generate multiple hypotheses for more rigorous examination in future larger trials. Follow-up evaluations taking place at greater intervals following treatment would be necessary to assess whether physiological responses to stress might precede clinical response. However, because there were no indications of even a tendency for LSAS ratings to show greater improvement in the Nap arm, it is likely that more robust post-exposure sleep interventions (e.g., a full night’s sleep) might also be required in order to influence self-reported clinical symptoms. The two 2-h post-exposure nap opportunities likely represent a limited intervention in the context of other influences on treatment response during 5 weeks of treatment. Specifically, the current nap intervention may have been insufficient to reach a threshold (floor) of total sleep or of specific sleep stages individually or in combination above which post-exposure sleep could produce a detectable effect on clinical measures such as the LSAS. Future studies might strive for more robust interventions such as an architecturally complete night of normal sleep following exposure that contains greater amounts of REM (e.g., Pace-Schott et al., 2012a). For example, among the 7 individuals who achieved REM in at least 1 nap, the association between change in total LSAS score and total minutes of REM in both naps showed a large effect size (R=0.635), but this association did not reach significance (p=0.13). It is also possible that daytime sleep disturbed nocturnal sleep for those in the Nap arm, thereby counteracting any beneficial sleep-related effect on clinical symptoms. Indeed, mean actigraph and diary sleep parameters for nights following naps differed from their grand mean values in the expected direction (lower TST, longer SOL, lower SE), although not reaching significance (all p’s ≥ 0.10) except for diary reported SOL (p=0.045). Future study designs with a full-night consolidation period would prevent potential confounding effects of naps on nocturnal sleep quality.
5. Conclusions
A brief exposure-based treatment for SAD significantly reduced self-report indices of social anxiety, but reductions were not enhanced by post-exposure naps. However, some secondary physiological indices of stress evoked by a social stressor decreased more in participants who napped suggesting potential beneficial effects of post-exposure naps on physiological responses to social stress.
Supplementary Material
Highlights.
Sleep following exposure sessions might strengthen therapeutic extinction memory
Participants with Social Anxiety Disorder completed 5-session group exposure therapy
17 napped and 15 remained awake following 2 intensive social exposure sessions
Naps did not improve pre-post therapy outcomes on the Liebowitz Social Anxiety Scale
Naps enhanced pre-post therapy reduction in physiological reactivity to social stressor
Acknowledgements
Supported by NIH/NIMH R21MH103484. The authors thank the volunteers who served as “audience” for the mpTSST and Margaret Merlino, RSPGT for sleep laboratory assistance.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- Abel T, Havekes R, Saletin JM, Walker MP, 2013. Sleep, plasticity and memory from molecules to whole-brain networks. Curr. Biol 23 (17), R774–788. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Allen AP, Kennedy PJ, Cryan JF, Dinan TG, Clarke G, 2014. Biological and psychological markers of stress in humans: focus on the Trier Social Stress Test. Neurosci. Biobehav. Rev 38, 94–124. [DOI] [PubMed] [Google Scholar]
- Allen AP, Kennedy PJ, Dockray S, Cryan JF, Dinan TG, Clarke G, 2016. The Trier Social Stress Test: principles and practice. Neurobiol. Stress 6, 113–126. [DOI] [PMC free article] [PubMed] [Google Scholar]
- APA, 2013. Diagnostic and Statistical Manual of Mental Disorders (Fifth ed.). American Psychiatric Publishing, Arlington, VA. [Google Scholar]
- Beesdo K, Bittner A, Pine DS, Stein MB, Hofler M, Lieb R, et al. , 2007. Incidence of social anxiety disorder and the consistent risk for secondary depression in the first three decades of life. Arch. Gen. Psychiatry 64 (8), 903–912. [DOI] [PubMed] [Google Scholar]
- Boesch M, Sefidan S, Ehlert U, Annen H, Wyss T, Steptoe A, et al. ,, 2014. Mood and autonomic responses to repeated exposure to the Trier Social Stress Test for Groups (TSST-G). Psychoneuroendocrinology 43, 41–51. [DOI] [PubMed] [Google Scholar]
- Buhlmann U, Wilhelm S, Deckersbach T, Rauch SL, Pitman RK, Orr SP, 2007. Physiologic responses to loud tones in individuals with obsessive-compulsive disorder. Psychosom. Med 69 (2), 166–172. [DOI] [PubMed] [Google Scholar]
- Campbell J, Ehlert U, 2012. Acute psychosocial stress: does the emotional stress response correspond with physiological responses? Psychoneuroendocrinology 37 (8), 1111–1134. [DOI] [PubMed] [Google Scholar]
- Craske MG, Kircanski K, Zelikowsky M, Mystkowski J, Chowdhury N, Baker A, 2008. Optimizing inhibitory learning during exposure therapy. Behav. Res. Ther 46 (1), 5–27. [DOI] [PubMed] [Google Scholar]
- da Costa Souza A, Ribeiro S, 2015. Sleep Deprivation and Gene Expression. Curr. Top. Behav. Neurosci 25:65–90. [DOI] [PubMed] [Google Scholar]
- Frisch JU, Hausser JA, Mojzisch A, 2015. The Trier Social Stress Test as a paradigm to study how people respond to threat in social interactions. Front. Psychol 6, 14. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Grillon C, 2009. D-cycloserine facilitation of fear extinction and exposure-based therapy might rely on lower-level, automatic mechanisms. Biol. Psychiatry 66 (7), 636–641. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Grillon C, Baas J, 2003. A review of the modulation of the startle reflex by affective states and its application in psychiatry. Clin Neurophysiol. 114 (9), 1557–1579. [DOI] [PubMed] [Google Scholar]
- Heimberg RG, 1991. Cognitive behavioral treatment of social phobia in a group setting: A treatment manual (2nd ed.). Center for Stress and Anxiety Disorders, State University of New York, Albany, NY. [Google Scholar]
- Hellhammer J, Schubert M, 2012. The physiological response to Trier Social Stress Test relates to subjective measures of stress during but not before or after the test. Psychoneuroendocrinology 37 (1), 119–124. [DOI] [PubMed] [Google Scholar]
- Hofmann SG, 2004. Exposure Therapy for Social Anxiety Disorder. (unpublished treatment manual). [Google Scholar]
- Hofmann SG, Meuret AE, Smits JA, Simon NM, Pollack MH, Eisenmenger K, et al. , 2006. Augmentation of exposure therapy with D-cycloserine for social anxiety disorder. Arch. Gen. Psychiatry 63 (3), 298–304. [DOI] [PubMed] [Google Scholar]
- Hofmann SG, Smits JA, 2008. Cognitive-behavioral therapy for adult anxiety disorders: a meta-analysis of randomized placebo-controlled trials. J. Clin. Psychiatry 69 (4), 621–632. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Iber C, et al. , 2007. The AASM Manual for the Scoring of Sleep and Associated Events: Rules, Terminology and Technical Specification American Academy of Sleep Medicine, Westchester, IL. [Google Scholar]
- Jorstad-Stein EC, Heimberg RG, 2009. Social phobia: an update on treatment. Psychiatr. Clin. North Am 32 (3), 641–663. [DOI] [PubMed] [Google Scholar]
- Kessler RC, Berglund P, Demler O, Jin R, Merikangas KR, Walters EE, 2005. Lifetime prevalence and age-of-onset distributions of DSM-IV disorders in the National Comorbidity Survey Replication. Arch. Gen. Psychiatry 62 (6), 593–602. [DOI] [PubMed] [Google Scholar]
- Kirschbaum C, Hellhammer DH, 1994. Salivary cortisol in psychoneuroendocrine research: recent developments and applications. Psychoneuroendocrinology. 19 (4), 313–333. [DOI] [PubMed] [Google Scholar]
- Kirschbaum C, Pirke KM, Hellhammer DH, 1993. The 'Trier Social Stress Test'--a tool for investigating psychobiological stress responses in a laboratory setting. Neuropsychobiology 28 (1-2), 76–81. [DOI] [PubMed] [Google Scholar]
- Kleim B, Wilhelm FH, Temp L, Margraf J, Wiederhold BK, Rasch B, 2014. Sleep enhances exposure therapy. Psychol. Med 44 (7), 1511–1519. [DOI] [PubMed] [Google Scholar]
- Krause AJ, Simon EB, Mander BA, Greer SM, Saletin JM, Goldstein-Piekarski AN, et al. ,, 2017. The sleep-deprived human brain. Nat. Rev. Neurosci 18 (7), 404–418. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liebowitz MR, 1987. Social anxiety. Mod. Probl. Pharmacopsychiatry 22, 141–173. [DOI] [PubMed] [Google Scholar]
- Lopez-Duran NL, Mayer SE, Abelson JL, 2014. Modeling neuroendocrine stress reactivity in salivary cortisol: adjusting for peak latency variability. Stress 17 (4), 285–295. [DOI] [PubMed] [Google Scholar]
- Marshall AJ, Acheson DT, Risbrough VB, Straus LD, Drummond SP, 2014. Fear conditioning, safety learning, and sleep in humans. J. Neurosci 34 (35), 11754–11760. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Menz MM, Rihm JS, Buchel C, 2016. REM sleep is causal to successful consolidation of dangerous and safety stimuli and reduces return of fear after extinction. J. Neurosci 36 (7), 2148–2160. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Milad MR, Quirk GJ, 2012. Fear extinction as a model for translational neuroscience: ten years of progress. Annu. Rev. Psychol. 63, 129–151. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Morris SE, Cuthbert BN, 2012. Research Domain Criteria: cognitive systems, neural circuits, and dimensions of behavior. Dialogues Clin. Neurosci 14 (1), 29–37. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Morrison AS, Heimberg RG, 2013. Social anxiety and social anxiety disorder. Annu. Rev. Clin. Psychol 9, 249–274. [DOI] [PubMed] [Google Scholar]
- Norrholm SD, Anderson KM, Olin IW, Jovanovic T, Kwon C, Warren VT, et al. , 2011. Versatility of fear-potentiated startle paradigms for assessing human conditioned fear extinction and return of fear. Front. Behav. Neurosci 5, 77. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Otto MW, Kredlow MA, Smits JA, Hofmann SG, Tolin DF, de Kleine RA et al. , 2016. Enhancement of psychosocial treatment with d-cycloserine: models, moderators, and future directions. Biol. Psychiatry 80 (4), 274–283. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pace-Schott EF, Germain A, Milad MR, 2015a. Effects of sleep on memory for conditioned fear and fear extinction. Psychol. Bull 141 (4), 835–857. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pace-Schott EF, Germain A, Milad MR, 2015b. Sleep and REM sleep disturbance in the pathophysiology of PTSD: the role of extinction memory. Biol. Mood Anxiety Disord 5, 3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pace-Schott EF, Milad MR, Orr SP, Rauch SL, Stickgold R, Pitman RK, 2009. Sleep promotes generalization of extinction of conditioned fear. Sleep 32 (1), 19–26. [PMC free article] [PubMed] [Google Scholar]
- Pace-Schott EF, Shepherd E, Spencer RM, Marcello M, Tucker M, Propper RE, et al. , 2011. Napping promotes inter-session habituation to emotional stimuli. Neurobiol. Learn. Mem 95 (1), 24–36. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pace-Schott EF, Tracy LE, Rubin Z, Mollica AG, Ellenbogen JM, Bianchi MT, et al. , 2014. Interactions of time of day and sleep with between-session habituation and extinction memory in young adult males. Exp. Brain Res 232 (5), 1443–1458. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pace-Schott EF, Verga PW, Bennett TS, Spencer RM, 2012a. Sleep promotes consolidation and generalization of extinction learning in simulated exposure therapy for spider fear. Journal of Psychiatric Research 46, 1036–1044. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pace-Schott EF, Verga PW, Bennett TS, Spencer RM, 2012b. Sleep promotes consolidation and generalization of extinction learning in simulated exposure therapy for spider fear. J. Psychiatr. Res 46 (8), 1036–1044. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ponniah K, Hollon SD, 2008. Empirically supported psychological interventions for social phobia in adults: a qualitative review of randomized controlled trials. Psychol. Med 38 (1), 3–14. [DOI] [PubMed] [Google Scholar]
- Rasch B, Born J, 2013. About sleep's role in memory. Physiol. Rev 93 (2), 681–766. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Richardson JTE, 2011. Eta squared and partial eta squared as measurements of effect size in educational research. Educ. Res. Rev 6, 135–147. [Google Scholar]
- Smits JA, Rosenfield D, Davis ML, Julian K, Handelsman PR, Otto MW et al. , 2014. Yohimbine enhancement of exposure therapy for social anxiety disorder: a randomized controlled trial. Biol. Psychiatry 75 (11), 840–846. [DOI] [PubMed] [Google Scholar]
- Spoormaker VI, Schroter MS, Andrade KC, Dresler M, Kiem SA, Goya-Maldonado, et al. , 2012. Effects of rapid eye movement sleep deprivation on fear extinction recall and prediction error signaling. Hum Brain Mapp 33 (10), 2362–2376. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stein MB, Kean YM, 2000. Disability and quality of life in social phobia: epidemiologic findings. Am. J. Psychiatry 157 (10), 1606–1613. [DOI] [PubMed] [Google Scholar]
- Stein MB, Torgrud LJ, Walker JR, 2000. Social phobia symptoms, subtypes, and severity: findings from a community survey. Arch. Gen. Psychiatry 57 (11), 1046–1052. [DOI] [PubMed] [Google Scholar]
- Straus LD, Acheson DT, Risbrough VB, Drummond SPA, 2017. Sleep deprivation disrupts recall of conditioned fear extinction. Biol. Psychiatry Cogn. Neurosci. Neuroimaging 2 (2), 123–129. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Thabane L, Hopewell S, Lancaster GA, Bond CM, Coleman CL, Campbell MJ, et al. , 2016. Methods and processes for development of a CONSORT extension for reporting pilot randomized controlled trials. Pilot Feasibility Stud. 2, 25. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zorn JV, Schur RR, Boks MP, Kahn RS, Joels M, Vinkers CH, 2017. Cortisol stress reactivity across psychiatric disorders: A systematic review and meta-analysis. Psychoneuroendocrinology 77, 25–36. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.


