Abstract
Importance
Contrast sensitivity (CS) is an important indicator of visual function that impacts daily life, including mobility, visually intensive tasks, safety, and autonomy. Understanding risk factors for CS impairment could lead to prevention of decreases in visual function.
Objective
Determine the incidence of CS impairment in a large cohort, and investigate factors potentially associated with incidence, including cadmium (Cd) and lead (Pb) levels.
Design
The Beaver Dam Offspring Study (BOSS, baseline 2005–2008) was conducted in the adult children of the participants of the population-based Epidemiology of Hearing Loss Study. Follow-up examinations occurred in 2010–2013 and 2015–2017.
Setting
Longitudinal cohort study
Participants
1983 participants free of CS impairment at baseline. Particpants were primarily non-hispanic white, 51.8% women, mean age 48 years (standard deviation=9.3).
Main Outcome
CS was measured using Pelli-Robson Letter Sensitivity Charts, and incident impairment was defined as a log CS score less than 1.55 in either eye at any follow-up examination. Cd and Pb levels were measured in whole blood, using inductively coupled plasma mass spectrometry. Associations between baseline characteristics and CS impairment incidence were examined using Cox proportional hazard models and quantified as hazard ratios (HR) with 95% confidence intervals (CI).
Results
The 10-year cumulative incidence of CS impairment was 24.8%, was similar in women and men, and was highest in the oldest age group (64–84 years) at 66.3%. In multivariable models, a Cd level in the highest quintile (Q5 vs. Q1–4) (HR=1.35, CI=1.02, 1.78), age (per 5 years) (HR=1.34, CI=1.25,1.44), waist circumference (per 5 cm) (HR=1.06, CI=1.02,1.10), and number of plaque sites (1–3 vs. 0, HR=1.37, CI=1.03,1.81; 4–6 vs. 0, HR=2.63, CI=1.26,5.48) were associated with increased risk, while male sex (HR=0.77, CI=0.60,0.98) and any alcohol consumption (vs. none, HR=0.54, CI=0.39, 0.76) were associated with decreased risk. Results were similar when smoking status replaced Cd in the models. Lead level was not associated with increased risk.
Conclusions and Relevance
Incident CS impairment was common in the 10-year follow-up. Cd, but not Pb, was associated with increased risk. Other modifiable risk factors were associated with risk implying that changes in behavior could reduce future incident impairment.
Introduction
Contrast sensitivity (CS) is an important indicator of visual function that measures aspects of vision not captured by the more commonly measured and reported distance visual acuity (VA). CS is a measure of how well an object is seen against its background, and low contrast conditions simulate low light, fog, or glare. As a result CS may be diminished even in those with good VA.1, 2
The prevalence of CS impairment varies by age and is more common in older adults. In the primarily middle-aged Beaver Dam Offspring Study (BOSS) (mean age 49 years) the prevalence of CS impairment was 7.8% whereas in the Beaver Dam Eye Study (BDES) (mean age 65 years) the prevalence was 26%.3–4 CS impacts the ability to function in daily life and may be an important factor in safety and autonomy as well. In earlier studies, poorer CS was associated with lower scores on the Activities of Daily Vision Scale (ADVS) independent of visual acuity and glare sensitivity, and poorer performance on tasks of everyday life, including mobility, inserting keys into locks, and reading.5–6 Similarly, the BDES found poorer CS was associated with worse self-reported general visual function, more limitations with vision dependent activities such as reading small print, and a slower gait time.3,7 In the Longitudinal Aging Study Amsterdam (LASA), investigators found that those with decreased CS had a higher probability of recurrent falls.8 Studies aiming to determine the effects of VA and CS on driving performance found that CS, but not VA, was associated with a driver’s recognition abilities, those with better CS were more likely to drive at night, and older drivers with impaired CS had a 42% increased risk of motor vehicle collision compared to drivers without impairment.9–11 CS has also been associated with other disorders including Alzheimer’s Disease,12 cognitive function and impairment,4, 13 diabetes,14 and multiple sclerosis.15–16
Cadmium (Cd) and lead (Pb) are neurotoxic heavy metals with multiple points of exposure including the home environment. Cd exposure typically occurs through cigarette smoke exposure, and by consuming green leafy vegetables, rice, and shellfish. Pb exposure is frequently from air pollution and old paint or water pipes.17 Both Cd and Pb are associated with impairments in multiple sensory systems and accumulate in ocular tissues including the retina during aging.18–22 The neurotoxic effects of Cd and Pb may play a role in the development of CS impairment, through multiple mechanisms including increased oxidative stress,19, 23 neuronal apoptosis,24 increased inflammation, disruption of metabolism of critical elements, such as zinc and copper,17, 25–27 and interference of cell signaling.28 Cd and Pb are implicated in the pathogenesis of age-related macular degeneration18, 29–34 and cataract formation.35–38 The potential effects of these heavy metals on CS are relatively unknown.
Little is known about other potential risk factors for CS impairment. In a study of older adults, smoking, not consuming any alcohol in the past year, and sedentary behavior were associated with larger decreases in VA over a 20 year period.39 Similar associations may exist for behavioral factors in development of CS impairment. The effect of atherosclerosis on CS is unknown, though it affects other sensory systems and cognition, which may indicate a negative impact on neuronal health and signaling between sensory organs and the brain.40–42 Inflammation may play a similar role and have negative impact on sensory health, including vision. Inflammation is associated with incident age-related macular degeneration (AMD), and may similarly affect CS.39
Given the importance of CS to vision and everyday function, it is important to try to understand what causes a decrease in this measure of vision. Studying potential risk factors in middle-aged adults may identify opportunities for early intervention to preserve good visual function in aging populations.
Methods
Subjects
Recruitment details of the BOSS were previously reported. 43 Briefly, the BOSS is an ongoing cohort study of aging in the adult children of the participants in the population-based Epidemiology of Hearing Loss Study. 44 Baseline data collection occurred during 2005–2008 with participants aged 21–84 years. Two follow-up exam periods occurred at five-year intervals (2010–2013 and 2015–2017). There were 1983 participants at risk of CS impairment at baseline with at least 1 follow-up examination. Study approval was granted by the Institutional Review Board of the University of Wisconsin-Madison. Informed written consent was obtained from all participants prior to each examination.
CS Measurement
CS testing was conducted using the Pelli-Robson Letter Sensitivity Chart.45 Charts were viewed at a distance of one meter, and participants were tested monocularly, wearing trial frames containing the appropriate distance correction, determined by Grand-Seiko autorefractor (WR-5001K; Grand Seiko, Hiroshima, Japan) readings and refined by subjective refraction when VA was worse than 20/40. The charts consist of 16 letter triplets where the contrast in each successive triplet decreases by a factor of 0.15 log units. Participants were encouraged to progress as far as possible, making a best guess if unsure about a particular letter. The last triplet where the participant correctly identified at least 2 of three letters was used to assign a log CS score. A log CS score less than 1.55 was considered impaired and cases were defined when either eye was impaired at follow-up.
Cd and Pb Measurement
Cd and Pb were measured in whole blood samples obtained during the BOSS baseline examination. Blood samples were stored at −80 C until testing in 2015–2016 by the Wisconsin State Lab of Hygiene (WSLH). Inductively coupled plasma mass spectrometry was used to measure both metals. Limits of detection for Cd and Pb, respectively, were 0.21 ug/L and 0.20 ug/dL. Samples used for quality control had to be within 10% of the target value to be considered acceptable and 10% of samples were retested to ensure they met acceptability criteria.
Covariates
Baseline factors potentially associated with risk of cumulative incidence of impaired CS were evaluated. Blood pressure, height, weight, and waist circumference were measured following standard protocols. Hypertension was defined as a measured systolic blood pressure ≥140 mmHg, diastolic BP ≥90 mmHg, or physician diagnosis with current blood pressure medication. Body Mass Index was calculated (kg/m2) and classified as normal (<25), overweight (25–29), or obese (30+). Retinal photographs were taken using a Canon Dgi-45NM fundus camera, slit-lamp lens images were taken using a TopCon SL-D7 slitlamp and TopCon DG-1 camera back, and retroillumination lens images were taken using a Neitz CT-S. Presence of age-related macular degeneration (AMD) was determined by fundus image grading by the University of Wisconsin Ocular Epidemiology Reading Center using the Wisconsin Age-Related Maculopathy Grading System, and presence of cataract (cortical, nuclear sclerosis, or posterior subcapsular) by slit lamp and retro-illumination lens image grading.46–47 Visual acuity was measured monocularly using the ETDRS charts and protocol. Impaired visual acuity was defined as an equivalent Snellen value of 20/40 or worse. Carotid artery ultrasound scans were used to measure intima-media thickness (IMT; mean of up to 12 wall thicknesses in the carotid arteries) and count of plaque in the carotid arteries (0 to 6 sites: common carotid, carotid bulb, and internal carotid, right and left sides). 48 Whole blood glycated hemoglobin (HbA1C) was measured using an automated high performance liquid chromatography method on the Tosoh A1C G7 Glycohemoglobin Analyzer (Tosoh Medics, Inc., San Francisco CA). Diabetes mellitus was defined by an A1C ≥6.5 or a physician diagnosis or of borderline diabetes with current treatment. Inflammatory markers were measured in stored serum samples by the University of Minnesota Advanced Research and Diagnostic Lab. Interleukin 6 (IL-6), tumor necrosis factor alpha (TNFa), Intercellular adhesion molecule 1 (ICAM-1), and soluble vascular cell adhesion molecule 1 (VCAM-1) were measured by a quantitative sandwich enzyme technique using the ELISA QuantiKine High Sensitivity kit, and the Human TNF-a, sICAM-1, and sVCAM-1 high sensitivity QuantiKine immunoassays, respectively, from R&D Systems (Minneapolis, MN). C-reactive protein (CRP) was measured using a latex particle enhanced immunoturbidimetric assay from Roche Diagnostics (Indianapoilis, IN). Age, sex, socio-economic status (household income and education), smoking status (never, past, or current), household information (urban/rural and source of drinking water), exercise (at least once a week), employment (professional/managerial/technical/sales versus farming/forestry/production/fabrication/labor) and work related exposures (heavy metals/solvents), and alcohol consumption (any in past year) were assessed by in-person interview or a self-administered questionnaire following standard protocols. Medication use was documented including statin and multivitamin use.
Statistical analysis
All analyses were completed with the SAS System version 9.4 (SAS Institute, Inc., Gary, NC). Participants who were free of CS impairment in both eyes at baseline were included in the present study (N=1983). Cd and Pb levels were divided into quintiles and exposure was modeled with quintiles 1 through 4 as the reference group. A dose response relationship was also investigated using indicator variables for Cd and Pb levels, and by analyzing the effect of doubling of levels. Potential risk factors were first assessed with age- and sex-adjusted Cox proportional hazards models. Multivariable models were built with a manual backwards elimination approach beginning with variables that were associated in age- and sex-adjusted models. Variables which remained associated in the larger multivariable model or were suggestive of association were considered for the final model which was confirmed using a stepwise selection procedure. Because smoking is a major source of Cd exposure, models were also constructed with one or the other excluded and a sensitivity analysis was conducted among non-smokers to examine this relationship. Additionally, the final cumulative incidence models were repeated excluding all participants with AMD, cataract, or impairment of VA at any examination to check consistency of results, as these conditions are known to have a strong impact on CS.49
Results
Participants had a mean age of 48 years at baseline and 1028 (51.8%) were women. The 10-year cumulative CS impairment incidence was 24.8% (95% confidence interval (CI)=22.9, 26.8) and was similar in women and men, 24.9% and 24.6%, respectively. Figure 1 displays incidence by sex and age group. Incidence was highest in the oldest age group, 66.3%. More than 87% of incident cases occurred in participants who did not have a measured visual acuity worse than 20/40 at any time point.
Figure 1:
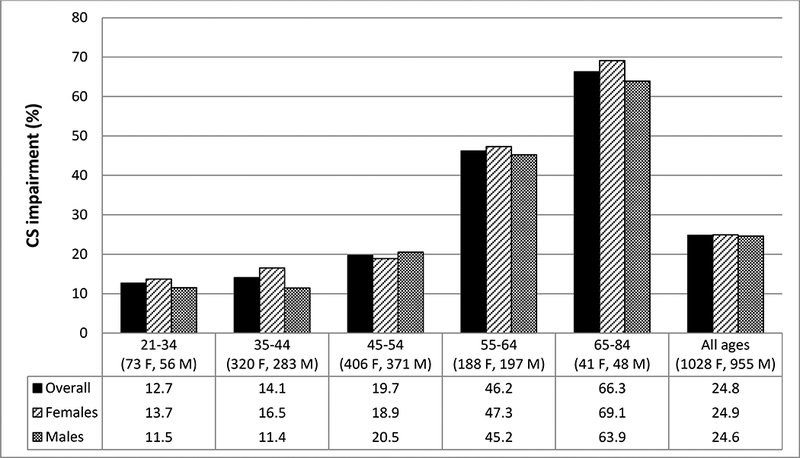
10-year cumulative incidence of contrast sensitivity impairment by sex and baseline age (years)
Age- and sex- adjusted hazard ratios (HR) for baseline characteristics and incident CS impairment can be found in Table 1. Blood Cd in Q5 was associated with an increased risk of CS impairment (HR=1.40, CI=1.09, 1.81), though a similar association did not exist for Pb (HR=0.91, CI=0.69, 1.18). There was no evidence of a dose-response relationship for Cd in age- and sex- adjusted or multivariable models (data not presented). Additionally, lower household income (HR=1.34, CI=1.07, 1.67), current smoking (HR=1.55, CI=1.18, 2.03), more carotid artery sites with plaque (1–3: HR=1.55, CI=1.20, 2.00; 4–6: HR=2.67, CI=1.36, 5.23), thicker IMT (HR=1.19, CI=1.10, 1.29), higher IL-6 (T3: HR=1.54, CI=1.19, 2.01), higher CRP (>3mg/L: HR=1.46, CI=1.11, 1.91), cataract (HR=1.97, CI=1.27, 3.07), VA impairment (HR=2.58, CI=1.32, 5.01), diabetes (HR=2.06, CI=1.37, 3.10), and larger waist circumference (HR=1.07, CI=1.04, 1.11) were associated with increased risk of developing CS impairment. Consumption of alcohol in the previous year was the only factor inversely associated with incident CS impairment (HR=0.62, CI=0.46, 0.85), though history of heavy drinking (4+ drinks per day) was not associated with incident impairment (HR=0.99, CI=0.76, 1.31).
Table 1:
Risk of incident contrast sensitivity (CS) impairment by baseline characteristics
| Baseline Characteristics | Incident CS impairment N (%) | Age- and Sex-Adjusted Hazard Ratio (HR) (95%CI) | |
|---|---|---|---|
| No 1517 (76.5%) | Yes 466 (23.5%) | ||
| Heavy Metals | |||
| Cadmium (μg/L) | |||
| Q1–4 (<0.52) | 1143 (81.6) | 331 (76.1) | Reference |
| Q5 (0.52+) | 257 (18.4) | 104 (23.9) | 1.40 (1.09, 1.81) |
| Lead (μg/dL) | |||
| Q1–4 (<2.06) | 1125 (80.4) | 338 (77.7) | Reference |
| Q5 (2.06+) | 275 (19.6) | 97 (22.3) | 0.91 (0.69, 1.18) |
| Demographics | |||
| Education | |||
| <16 years | 963 (63.8) | 312 (67.2) | Reference |
| 16+ years | 546 (36.2) | 152 (32.8) | 0.96 (0.77, 1.19) |
| Household Income | |||
| <$50,000 | 415 (28.1) | 168 (37.8) | 1.34 (1.07, 1.67) |
| $50,000+ | 1064 (71.9) | 277 (62.2) | Reference |
| Home environment | |||
| Location of Home | |||
| Town/City | 1041 (68.6) | 307 (65.9) | Reference |
| Country | 476 (31.4) | 159 (34.1) | 0.99(0.79, 1.22) |
| Municipal Drinking Water | |||
| No | 538 (35.5) | 173 (37.1) | Reference |
| Yes | 979 (64.5) | 293 (62.9) | 1.0(0.84, 1.28) |
| Employment | |||
| Farming/forestry/production/fabrication/labor | |||
| No | 1006 (75.3) | 281 (77.4) | Reference |
| Yes | 330 (24.7) | 82 (22.6) | 0.88(0.66, 1.18) |
| Metal exposure at Work | |||
| No | 1421 (94.4) | 447 (96.5) | Reference |
| Yes | 85 (5.6) | 16 (3.5) | 0.68(0.40, 1.15) |
| Behavioral factors | |||
| Regular Exercise (at least 1 time/week) | |||
| No | 556 (36.7) | 190 (40.9) | Reference |
| Yes | 959 (63.3) | 275 (59.1) | 0.96(0.78, 1.20) |
| Current smoker | |||
| No | 1267 (83.6) | 378 (81.1) | Reference |
| Yes | 249 (16.4) | 88 (18.9) | 1.55 (1.18, 2.03) |
| Alcohol consumption in previous year | |||
| None | 127 (8.4) | 67 (14.4) | Reference |
| Any | 1389 (91.6) | 389 (85.6) | 0.62 (0.46, 0.85) |
| Medication use | |||
| Multivitamins | |||
| No | 805 (53.1) | 235 (50.4) | Reference |
| Yes | 712 (46.9) | 231 (49.6) | 0.85 (0.69, 1.05) |
| Statins | |||
| No | 1340 (88.3) | 378 (81.1) | Reference |
| Yes | 177 (11.7) | 88 (18.9) | 1.02 (0.77, 1.36) |
| Vascular factors | |||
| Hypertension | |||
| No | 1064 (70.2) | 263 (56.4) | Reference |
| Yes | 452 (29.8) | 203 (43.5) | 1.19 (0.95, 1.48) |
| Number of plaque sites (0–6) | |||
| 0 | 1189 (82.5) | 276 (64.6) | Reference |
| 1–3 | 239 (16.6) | 134 (31.4) | 1.55 (1.20, 2.00) |
| 4–6 | 13 (0.9) | 17 (4.0) | 2.67 (1.36, 5.23) |
| Carotid intima-media thickness (mm)a | |||
| Mean (SD) | 0.63 (0.12) | 0.71 (0.18) | 1.19 (1.10, 1.29)b |
| Inflammatory markers | |||
| Interleukin-6 (pg/mL) | |||
| T1 (<1.27) | 541 (37.5) | 118 (26.6) | Reference |
| T2 (1.27-<2.28) | 501 (34.7) | 140 (31.6) | 0.99 (0.76, 1.30) |
| T3 (2.28+) | 400 (27.7) | 185 (41.8) | 1.54 (1.19, 2.01) |
| Intercellular adhesion molecule-1 (ng/mL) | |||
| T1 (<190.1) | 504 (34.9) | 139 (31.2) | Reference |
| T2 (190.1-<238.5) | 505 (34.9) | 155 (34.7) | 1.00 (0.77, 1.29) |
| T3 (238.5+) | 436 (30.2) | 152 (34.1) | 1.04 (0.80, 1.35) |
| C-reactive protein (mg/L) | |||
| <1.0 | 620 (42.8) | 150 (33.3) | Reference |
| 1.0–3.0 | 537 (37.1) | 175 (38.8) | 1.10 (0.86, 1.41) |
| >3.0 | 292 (20.1) | 126 (27.9) | 1.46 (1.11, 1.91) |
| Tumor necrosis factor-alpha (pg/mL) | |||
| T1 (<.358) | 515 (35.5) | 138 (30.6) | Reference |
| T2 (.358-<.613) | 479 (33.1) | 161 (35.7) | 1.12 (0.87, 1.45) |
| T3 (.613+) | 455 (31.4) | 152 (33.7) | 1.10 (0.85, 1.43) |
| Vascular cell adhesion molecule-1 (ng/mL) | |||
| T1 (<497) | 520 (35.9) | 143 (31.7) | Reference |
| T2 (497-<635) | 491 (33.9) | 142 (31.5) | 0.92 (0.71, 1.19) |
| T3 (635+) | 438 (30.2) | 166 (36.8) | 1.05 (0.81, 1.37) |
| Visual Health | |||
| Age-related macular degenerationc | |||
| No | 1459 (97.1) | 433 (95.8) | Reference |
| Yes | 44 (2.9) | 19 (4.2) | 1.12 (0.65, 1.92) |
| Cataractd | |||
| No | 1459 (97.8) | 410 (90.9) | Reference |
| Yes | 32 (2.2) | 41 (9.1) | 1.97 (1.27, 3.07) |
| Visual acuity impairment (worse eye) | |||
| No | 1499 (98.8) | 452 (97.0) | Reference |
| Yes | 18 (1.2) | 14 (3.0) | 2.58 (1.32, 5.01) |
| Other health | |||
| Diabetes | |||
| No | 1436 (96.8) | 414 (90.6) | Reference |
| Yes | 47 (3.2) | 43 (9.4) | 2.06(1.37, 3.10) |
| Body mass index (Kg/m2) | |||
| <25.0 | 350 (23.3) | 78 (16.9) | Reference |
| 25.0-<30.0 | 532 (35.4) | 141 (30.5) | 0.98 (0.72, 1.34) |
| 30.0+ | 622 (41.4) | 243 (52.6) | 1.28 (0.96, 1.72) |
| Waist circumference (cm)a | |||
| Mean (SD) | 98.2 (15.8) | 103.5 (17.2) | 1.07(1.04, 1.11) |
Presented as mean and standard deviation (SD) where noted.
Parameter estimate based on increase in carotid-IMT of 0.1 mm
Based on graded retinal fundus images
Based on graded slit-lamp and retro-illumination lens images
In the multivariable model, older age (HR=1.36, CI=1.25, 1.47), larger waist circumference (HR=1.06, CI=1.01, 1.11), more carotid plaque sites (1–3: HR=1.43, CI=1.07, 1.92; 4–6: HR=2.75, CI=1.26, 6.05), VA impairment (HR=3.61, CI=1.61, 8.10), and cataract (HR=1.99, CI=1.21, 3.28) were associated with greater risk of CS impairment incidence, while any alcohol consumption in the past year was associated with decreased risk (HR=0.61, CI=0.43, 0.88) (see Table 2). In this model, neither Cd nor smoking was associated with CS impairment incidence. Due to a strong collinear relationship between smoking and Cd, as 75% of smokers were in Q5 and 65.5% of Q5 were smokers, reduced models were run with Cd or smoking separately. In these models, estimates for most covariates remained unchanged, though the effects of Q5 Cd (HR=1.35, CI=1.02, 1.78) and smoking (HR=1.46, CI=1.09, 1.95) strengthened in their respective models (see Table 2). In the sensitivity analysis among non-smokers, the effect of Q5 Cd was attenuated (HR=1.10, CI=0.72, 1.70).
Table 2:
Multivariable models of the risk of incident contrast sensitivity impairment. Hazard Ratio (95% Confidence Interval)
| All Participants | Sensitivity Analysisa (N=1434) | ||||
|---|---|---|---|---|---|
| Characteristic | Full Model | Reduced Model (with smoking) | Reduced Model (with cadmium) | Reduced Model (with smoking) | Reduced Model (with cadmium) |
| Age (per 5 years) | 1.36 (1.25, 1.47) | 1.36 (1.26, 1.46) | 1.34 (1.25, 1.44) | 1.31 (1.21, 1.42) | 1.28 (1.18, 1.39) |
| Male Sex | 0.79 (0.61, 1.04) | 0.76 (0.60, 0.97) | 0.77 (0.60, 0.98) | 0.64 (0.49, 0.84) | 0.65 (0.49, 0.86) |
| Household Income <$50K | 1.16 (0.89, 1.51) | ||||
| Current smoker | 1.23 (0.81, 1.85) | 1.46 (1.09, 1.95) | 1.73 (1.26, 2.39) | ||
| Any Alcohol Consumption | 0.61 (0.43, 0.88) | 0.61 (0.44, 0.85) | 0.54 (0.39, 0.76) | 0.59 (0.40, 0.86) | 0.56 (0.38, 0.83) |
| Current Multivitamin Use | 1.05 (0.82, 1.34) | ||||
| Hypertension | 0.89 (0.67, 1.17) | ||||
| Diabetes | 1.40 (0.82, 2.37) | ||||
| Waist (per 5 cm) | 1.06 (1.01, 1.11) | 1.06 (1.02, 1.10) | 1.06 (1.02, 1.10) | 1.07 (1.03, 1.11) | 1.06 (1.01, 1.10) |
| Number of Plaque Sites (0–6) | |||||
| 1–3 | 1.43 (1.07, 1.92) | 1.43 (1.10, 1.87) | 1.37 (1.03, 1.81) | 1.40 (1.02, 1.91) | 1.33 (0.96, 1.85) |
| 4–6 | 2.75 (1.26, 6.05) | 2.59 (1.25, 5.35) | 2.63 (1.26, 5.48) | 1.67 (0.65, 4.31) | 1.79 (0.69, 4.65) |
| Cadmium (Q5 vs. all others) | 1.14 (0.79, 1.65) | 1.35 (1.02, 1.78) | 1.72 (1.26, 2.35) | ||
| Interleukin-6 | |||||
| Q1 (<1.27) | Ref | ||||
| Q2 (1.27-<2.28) | 0.74 (0.54, 1.02) | ||||
| Q3 (2.28+) | 0.94 (0.65, 1.36) | ||||
| C-reactive protein | |||||
| <1 mg/L | Ref | ||||
| 1–3 mg/L | 1.01 (0.75, 1.35) | ||||
| >3 mg/L | 1.04 (0.72, 1.52) | ||||
| Impaired Visual Acuity | 3.61 (1.61, 8.10) | 3.32 (1.59, 6.93) | 3.05 (1.42, 6.52) | ||
| Cataract | 1.99 (1.21, 3.28) | 2.11 (1.34, 3.33) | 2.08 (1.30, 3.34) | ||
| AMD | 0.91 (0.50, 1.67) | 0.92 (0.51, 1.66) | 0.97 (0.53, 1.75) | ||
Sensitivity analysis excludes participants with visual acuity impairment, cataract, or age-related macular degeneration (AMD).
In the sensitivity analysis, excluding those with ocular comorbidities, estimates for age, sex, alcohol consumption, and waist circumference were similar (See Table 2). The increased risk from Q5 Cd (HR=1.72, CI=1.26, 2.35) and smoking (HR=1.73, CI=1.26, 2.39) was higher in the group without AMD, cataract, or VA impairment. The association between carotid artery plaque and CS impairment incidence was inconsistent in these reduced models with fewer participants.
Discussion
Nearly a quarter of BOSS participants developed CS impairment in the 10-year follow-up period, showing CS impairment is relatively common among aging adults. Previous studies found that poor CS occurs in individuals without ocular comorbidities and in those with good VA. More than 87% of incident cases had normal VA in the BOSS. CS impairment has been found to cause problems with daily activities, lower autonomy including driving, and pose higher risk for falls.3, 5–11 With a large proportion of middle-aged adults experiencing a decrease in CS, better understanding of risk factors for and potentially preventing this decrease is an important target for public health.
Cd and smoking were associated with an increased risk of CS impairment in the BOSS cohort in separate models. As smoking is a main source of Cd exposure, these two risk factors had a high level of collinearity and as a result it was not possible to discern which was ultimately responsible for the increased risk in this study. In analysis limited to non-smokers, the effect of Cd was attenuated. This analysis greatly reduced the number in the highest quintile of exposure, and the result could be due to a lack of power rather than a lack of association. It is also possible some other components of cigarette smoke are involved in development of CS impairment and Cd and smoking could be acting as proxies for that unmeasured exposure. It remains, however, that blood Cd and smoking were associated with increased risk.
Although this is the first study to find Cd to be associated with CS, previous studies reported Cd and smoking are related to other ocular pathology as they were shown to be associated with age-related macular degeneration and cataracts.18, 29–38, 50 Both of these pathologies have in turn been shown to be associated with lower CS.51–55 A study using NHANES data found more than 50% of the total effect of smoking on the risk of cataract could be attributed to Cd’s indirect effects.36 The same may be true of smoking and Cd’s relationship to incident CS impairment. While the biological mechanism by which Cd causes CS impairment cannot be discerned in this study, potential mechanisms include increases in inflammation, reactive oxygen species, apoptosis, and disruption of metabolism of key elements.17, 19, 23–28
In the present study, cataract and visual acuity impairment at baseline displayed strong associations with CS impairment incidence, justifying the need for a sensitivity analysis. While AMD did not, it should be noted that this is a relatively young cohort and baseline cases of AMD were likely to be in early stages. A recent study found CS may not differ between AMD patients in early stages and healthy controls.55 In the follow-up period, these baseline cases would be expected to progress and could confound the association. As a result, these were also excluded from the sensitivity analysis, in order to measure the effect of Cd in eyes without these comorbidities. In this sensitivity analysis, excluding anyone with AMD, cataract, or impaired VA during follow-up, Cd and smoking remained significantly associated with CS impairment. This suggests the mechanism by which Cd and smoking affect CS could be independent of the mechanisms by which the comorbid eye conditions impact it. In addition to Cd and smoking, the associations between CS and measures of adiposity, IMT, plaque, and alcohol consumption demonstrate that the risk is potentially modifiable.
Pb levels were not associated with increased risk of CS impairment in the BOSS cohort. However, exposure was generally low in this population as only 29 participants displayed a level ≥5 μg/dL, the cut-point currently considered to indicate elevated blood Pb in adults, and only 6 had a level at or above 10 μg/dL.56 Although no level of circulating Pb is considered safe, this means the majority of those in Q5 still had relatively low levels. If a higher Pb toxicity is required before changes begin to occur in the retina, the population would not have had the exposure necessary to detect a difference in rate of CS impairment.
Strengths of the current analysis include the large sample size, standardized measurement of key variables, and the longitudinal nature of the study. The sample size provides the power to detect potential differences in risk, the standardized measurements allow for certainty in found associations, and the longitudinal design means exposure proceeds disease. A limitation of this study is that the population is racially and ethnically homogenous. As such the direct generalizability of these findings to other racial and ethnic groups is limited. However, it is unlikely the mechanism by which heavy metals impact vision, specifically CS, would differ among racial or ethnic groups. As previously noted, Cd exposure and cigarette smoking were closely linked and as such no definitive conclusions can be drawn as to whether one or both are responsible for the observed increased risk of CS impairment. Further study is needed to better understand this relationship. Finally, Cd and Pb were measured in blood samples and heavy metals in blood are generally accepted to indicate recent acute exposure. However, overall body burden also contributes to higher circulating levels.17, 57
In conclusion, CS impairment incidence was high with about 1 in 4 developing impairment in the 10 years of follow-up of the BOSS. Cd, but not Pb, was associated with an increased risk of incident CS impairment, though the observed association may be due to some other component of cigarette smoke exposure. Changes in modifiable factors, including exposure to Cd and cigarette smoke and improvement of adiposity or vascular factors could potentially reduce the burden of CS impairment in the population.
KEY POINTS.
Question: What is the relationship between blood cadmium and lead levels and the 10-year incidence of contrast sensitivity (CS) impairment in the Beaver Dam Offspring Study (BOSS), a cohort of middle-aged adults?
Findings: CS impairment incidence was high in this cohort at nearly 25%. Cadmium, but not lead, was associated with increased risk of development of CS impairment in the 10-year follow-up period.
Meaning: Reducing exposure to cadmium, smoking, or both may reduce the burden of CS impairment in middle-aged adults.
Acknowledgments
Funding/Support: This work was supported by Award Number R01AG021917 from the National Institute on Aging and the National Eye Institute (Dr. Cruickshanks) and an unrestricted grant from Research to Prevent Blindness. The content is solely the responsibility of the authors and does not necessarily reflect the official views of the National Institute on Aging or the National Institutes of Health.
Role of Funder/Sponsor: The funding organizations had no role in the design and conduct of the study; collection, management, analysis, and interpretation of the data; preparation, review or approval of the manuscript; and decision to submit the manuscript for publication.
Footnotes
Conflict of Interest Disclosures: All authors have completed and submitted the ICMJE Form for Disclosure of Potential Conflicts of Interest and none were reported.
Publisher's Disclaimer: Disclaimer: The content is solely the responsibility of the authors and does not necessarily represent the official views of the National Institute on Aging, the National Eye Institute, or Research to Prevent Blindness.
References
- 1.Pelli DG, Bex P. Measuring contrast sensitivity. Vision Res. 2013; 90:10–14. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Rubin GS, West SK, Munoz B, et al. A Comprehensive Assessment of Visual Impairment in a Population of Older Americans: The SEE Project. Invest Ophthalmol Vis Sci. 1997; 38(3): 557–568. [PubMed] [Google Scholar]
- 3.Klein BEK, Klein R, Lee KE, Cruickshanks KJ. Associations of performance-based and self-reported measures of visual function. The Beaver Dam Eye Study. Ophthalmic Epidemiol. 1999; 6(1): 49–60. [DOI] [PubMed] [Google Scholar]
- 4.Schubert CR, Cruickshanks KJ, Fischer ME, et al. Sensory Impairments and Cognitive Function in Middle-Aged Adults. J Gerontol A Bio Sci Med Sci. 2017; 72(8): 1087–1090. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Rubin GS, Bandeen-Roche K, Huang GH, et al. The Association of Multiple Visual Impairments with Self-Reported Visual Disability: SEE Project. Invest Ophthalmol Vis Sci. 2001; 42(1): 64–72. [PubMed] [Google Scholar]
- 6.West SK, Rubin GS, Broman AT, Munoz B, Bandeen-Roche K, Turano K. How Does Visual Impairment Affect Performance on Tasks of Everyday Life? Arch Ophthalmol.. 2002; 120: 774–780. [DOI] [PubMed] [Google Scholar]
- 7.Klein BEK, Klein R, Lee KE, Cruickshanks KJ. Performance-based and Self-assessed Measures of Visual Function as Related to History of Falls, Hip Fractures, and Measured Gait Time: The Beaver Dam Eye Study. Ophthalmology, 1998; 105(1): 160–164. [DOI] [PubMed] [Google Scholar]
- 8.De Boer MR, Pluijm SMF, Lips P, et al. Different Aspects of Visual Impairment as Risk Factors for Falls and Fractures in Older Men and Women. J Bone MinerRes. 2004; 19:1539–1547. [DOI] [PubMed] [Google Scholar]
- 9.Wood JM, Owens DA. Standard Measures of Visual Acuity Do Not Predict Drivers’ Recognition Performance Under Day or Night Conditions. Optom Vis Sci. 2005; 82(8): 698–705. [DOI] [PubMed] [Google Scholar]
- 10.Kaleem MA, Munoz BE, Munro CA, Gower EW, West SK. Visual Characteristics of Elderly Night Drivers in the Salisbury Eye Evaluation Driving Study. Invest Ophthalmol Vis Sci. 2012; 53(9): 5161–5167. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Green KA, McGwin G Jr., Owsley C. Associations between Visual, Hearing, and Dual Sensory Impairments and History of Motor Vehicle Collision Involvement by Older Drivers. J Am Geriatr Soc. 2013; 61(2): 252–257. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Risacher SL, WuDunn D, Pepin SM, et al. Visual contrast sensitivity in AD, MCI, & older adults with cognitive complaints. Neurobiol Aging. 2013; 34(4): 1133–1144. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Fischer ME, Cruickshanks KJ, Schubert CR, et al. Age-Related Sensory Impairments and Risk of Cognitive Impairment. J Am Geriatr Soc. 2016; 64(10): 1981–1987. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Gella L, Raman R, Pal SS, Ganesan S, Sharma T. Contrast sensitivity and its determinants in people with diabetes: SN-DREAMS-II, Report No 6. Eye. 2017; 31: 460–466. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Baier ML, Cutter GR, Rudick RA, et al. Low-contrast letter acuity testing captures visual dysfunction in patients with multiple sclerosis. Neurology. 2005; 64:992–995 [DOI] [PubMed] [Google Scholar]
- 16.Mowry EM, Loguidice MJ, Daniels AB, et al. Vision related quality of life in multiple sclerosis: correlation with new measures of low and high contrast letter acuity. J Neurol Neurosurg Psychiatry. 2009; 80: 767–772. [DOI] [PubMed] [Google Scholar]
- 17.Chowdhury BA, Chandra RK. Biological and Health Implications of Toxic Heavy Metal and Essential Trace Element Interactions. Prog Food and Nutr Sci. 1987; 11:55–113. [PubMed] [Google Scholar]
- 18.Erie JC, Good JA, Butz JA. Excess Lead in the Neural Retina in Age-Related Macular Degeneration. Am J Ophthalmol. 2009; 148: 890–894. [DOI] [PubMed] [Google Scholar]
- 19.Wills NK, Sadagopa Ramanujam VM, Chang J, et al. Cadmium accumulation in the human retina: Effects of age, gender, and cellular toxicity. Exp Eye Res. 2008; 86: 41–51. [DOI] [PubMed] [Google Scholar]
- 20.Shiue I Urinary environmental chemical concentrations and vitamin D are associated with vision, hearing, and balance disorders in the elderly. Environ Int. 2013; 53: 41–46. [DOI] [PubMed] [Google Scholar]
- 21.Choi YH, Hu H, Mukherjee B, Miller J, Park SK. Environmental cadmium and lead exposures and hearing loss in U.S. adults: the National Health and Nutrition Examination Survey, 1999 to 2004. Environ Health Perspect. 2012; 120(11): 1544–1550. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Min KB, Lee KJ, Park JB, Min JY. Lead and Cadmium Levels and Balance and Vestibular Dysfunction among Adult Participants in the National Health and Nutrition Examination Survey (NHANES) 1999–2004. Environ Health Perspect. 2012; 120(3): 413–417. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Wang Y, Fang J, Leonard SS, Rao KMK. Cadmium Inhibits The Electron Transfer Chain And Induces Reactive Oxygen Species. Free Radic Biol Med. 2004; 36(11): 1434–1443. [DOI] [PubMed] [Google Scholar]
- 24.Chen L, Xu B, Liu L, et al. Cadmium induction of reactive oxygen species activates mTOR pathway, leading to neuronal cell death. Free Radic Biol Med. 2011; 50(5): 624–632. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Wills NK, Kalariya N, Ramanujam VMS, Lewis JR, Abdollahi SH. Human retinal cadmium accumulation as a factor in the etiology of age-related macular degeneration. Exp Eye Res. 2009; 89: 79–87. [DOI] [PubMed] [Google Scholar]
- 26.Ugarte M, Osborne NN, Brown LA, Bishop PN. Iron, zinc, and copper in retinal physiology and disease. Surv Ophthalmol. 2013; 58: 585–609. [DOI] [PubMed] [Google Scholar]
- 27.Goyer RA. Nutrition and metal toxicity. Am J Clin Nutr. 1995; 61(suppl): 646S–650S. [DOI] [PubMed] [Google Scholar]
- 28.Rana SVS. Metals and apoptosis: Recent developments. J Trace Elem Med Bio. 2008; 22:262–284. [DOI] [PubMed] [Google Scholar]
- 29.Erie JC, Good JA, Butz JA, Hodge DO, Pulido JS. Urinary Cadmium and Age-related Macular Degeneration. Am J Ophthalmol. 2007; 144(3): 414–418. [DOI] [PubMed] [Google Scholar]
- 30.Hwang HS, Lee SB, Jee D. Association between Blood Lead Levels and Age-Related Macular Degeneration. PLoS ONE. 2015; 10(8); e0134338. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Kim EC, Cho E, Jee D. Association Between Blood Cadmium Level and Age-Related Macular Degeneration in a Representative Korean Population. Invest Ophthalmol Vis Sci. 2014; 55(9): 5702–5710. [DOI] [PubMed] [Google Scholar]
- 32.Park SJ, Lee JH, Woo SJ, Kang SW, Park KH. Five Heavy Metallic Elements and Age-Related Macular Degeneration: Korean National Health and Nutrition Examination Survey, 2008–2011. Ophthalmology. 2015; 122: 129–137. [DOI] [PubMed] [Google Scholar]
- 33.Wu EW, Schaumberg DA, Park SK. Environmental cadmium and lead exposures and age-related macular degeneration in US adults: the National Health and Nutrition Examination Survey 2005 to 2008. Environ Res. 2014; 133: 178–184. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Kim MH, Zhao D, Cho J, Guallar E. Cadmium exposure and age-related macular degeneration. J Expo Sci Environ Epidemiol. 2016; 26: 214–218. [DOI] [PubMed] [Google Scholar]
- 35.Kalariya NM, Nair B, Kalariya DK, Wills NK, van Kuijk FJGM. Cadmium-induced induction of cell death in human lens epithelial cells: Implications to smoking associated cataractogenesis. Toxicol Lett. 2010; 198: 56–62. [DOI] [PubMed] [Google Scholar]
- 36.Wang W, Schaumberg DA, Park SK. Cadmium and lead exposure and risk of cataract surgery in U.S. adults. Int J Hyg Environ Health. 2016; 219(8): 850–856. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Langford-Smith A, Tilakaratna V, Lythgoe PR, Clark SJ, Bishop N, Day AJ. Age and Smoking Related Changes in Metal Ion Levels in Human Lens: Implications for Cataract Formation. PLoS ONE. 2016; 11(1): e0147576. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Schaumberg DA, Mendes F, Balaram M, Reza Dana M, Sparrow D, Hu H. Accumulated Lead Exposure and Risk of Age-Related Cataract in Men. JAMA. 2004; 292(22): 2750–2754. [DOI] [PubMed] [Google Scholar]
- 39.Klein R, Lee KE, Gangnon RE, Klein BEK. Relation of Smoking, Drinking and Physical Activity to Changes in Vision Over a 20-Year Period: The Beaver Dam Eye Study. Ophthalmolgy. 2014; 121(6): 1220–1228. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Fischer ME, Schubert CR, Nondahl DM, et al. Subclinical atherosclerosis and increased risk of hearing impairment. Atherosclerosis. 2015; 238: 344–349. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Zhong W, Cruickshanks KJ, Schubert CR, et al. Carotid atherosclerosis and 10-year changes in cognitive function. Atherosclerosis. 2012; 224: 506–510. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Schubert CR, Cruickshanks KJ, Fischer ME, et al. Carotid Intima Media Thickness, Atherosclerosis, and 5-Year Decline in Odor Identification: The Beaver Dam Offspring Study. J Geron A Biol Sci Med Sci. 2015; 70(7): 879–884. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Nash SD, Cruickshanks KJ, Klein R, Klein BEK, Nieto FJ, et al. The prevalence of hearing impairment and associated risk factors: The Beaver Dam Offspring Study. Arch Otolaryngol Head Neck Surg. 2011; 137(5), 432–439. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Cruickshanks KJ, Wily TL, Tweed TS, et al. Prevalence of hearing loss in older adults in Beaver Dam, Wisconsin: The Epidemiology of Hearing Loss Study. Am J Epidemiol. 1998; 148(9), 879–886. [DOI] [PubMed] [Google Scholar]
- 45.Pelli DG, Robson JG, Wilkins AJ. The design of a new letter chart for measuring contrast sensitivity. Clin Vision Sci.1988; 2(3): 187–199. [Google Scholar]
- 46.Klein R, Davis MD, Magli YL, Segal P, Klein BE, Hubbard L. The Wisconsin Age-Related Maculopathy Grading System. Ophthalmology. 1991; 98(7): 1128–1134. [DOI] [PubMed] [Google Scholar]
- 47.Klein BE, Klein R, Linton KL, Magli YL, Neider MW. Assessment of cataracts from photographs in the Beaver Dam Eye Study. Ophthalmology. 1990; 97(11): 1428–1433. [DOI] [PubMed] [Google Scholar]
- 48.Zhong W, Cruickshanks KJ, Huang GH, et al. Carotid atherosclerosis and cognitive function in midlife: the beaver dam offspring study. Atherosclerosis. 2011; 219(1): 330–333. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Pelli DG, Bex P. Measuring contrast Sensitivity. Vision Res. 2013; 90: 10–14. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Cekic O Effect of cigarette smoking on copper, lead, and cadmium accumulation in human lens. Br J Ophthalmol. 1998; 82: 186–188. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Wood JM, Black AA, Mallon K, Kwan AS, Owsley C. Effects of Age-Related Macular Degeneration on Driving Performance. Invest Ophthalmol Vis Sci. 2018; 59(1): 273–279. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Gupta L, Cvintal V, Delvadia R, Sun Y, Erdem E, et al. SPARCS and Pelli-Robson contrast sensitivity testing in normal controls and patients with cataract. 2017. Eye, 31:753–761. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Barteselli G, Gomez ML, Doede AL, et al. Visual function assessment in simulated real-life situations in patients with age-related macular degeneration compared to normal subjects. Eye. 2014; 28: 1231–1238. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Gehrs KM, Anderson DH, Johnson LV, Hageman GS. Age-related macular degeneration – emerging pathogenetic and therapeutic concepts. Ann Med. 2006; 38(7): 450–471. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Owsley C, Huisingh C, Clark ME, Jackson GR, McGwin G Jr. Comparison of Visual Function in Older Eyes in the Earliest Stages of Age-Related Macular Degeneration to Those in Normal Macular Health. Curr Eye Res. 2016; 41(2): 266–272. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Morbidity and Mortality Weekly. Adult Blood Lead Epidemiology and Surveillance – United States 2008–2009. MMWR Morb Mortal Wkly Rep. 2011, July 1; 60(25): 841–845. [PubMed] [Google Scholar]
- 57.Adams SV, Newcomb PA. Cadmium blood and urine concentrations as measures of exposure: NHANES 1999–2010. J Expo Sci Environ Epidemiol. 2014; 24(2): 163–170. [DOI] [PMC free article] [PubMed] [Google Scholar]


