Abstract
Emerging literature indicates individual and contextual differences impact response to oxytocin (OT). Intimate partner violence (IPV) is one chronic stressor that may moderate OT response. To test the hypothesis that IPV moderates the association between OT and reactivity to a dyadic conflict task, data from a larger randomized controlled study was collected from heterosexual couples (N=60 individuals; 30 couples) at high risk for IPV due to substance misuse. Partners within each dyad completed a 10-minute dyadic conflict task in the laboratory, and then self-administered a single dose of OT (40 IU) or placebo. Forty-five minutes later, participants completed another 10-minute dyadic conflict task. Stress reactivity was measured before and after the second conflict task using neuroendocrine (i.e., salivary cortisol), physiological (i.e., skin conductance), and subjective responses. Couple conflict behaviors were observed during the conflict tasks and assessed using a validated coding system. Among women, physical IPV modulated skin conductance in those administered OT, and OT interacted with physical and psychological IPV to yield less positive subjective and behavioral responses. No main or moderating effects were found for men. Findings support emerging literature on sex differences in response to OT. Future research is needed to effectively translate OT into therapeutic intervention.
Keywords: Oxytocin, intimate partner violence, couples, substance use, sex differences
1. Introduction
Evidence suggests desirable effects of oxytocin (OT) on social and health behaviors, but emerging research has identified individual and contextual differences that modulate OT response (Bartels, 2012; Hurlemann and Scheele, 2016). For example, sex differences in OT response have been extensively documented (Ditzen et al., 2013; Flanagan et al., 2018a; Lynn et al., 2014; Rilling et al., 2014). Research in healthy participants suggests that OT is associated with increased activity in brain areas with high numbers of OT receptors, and lower emotional arousal and more positive behaviors during a couple conflict task in men; however, none of these associations have been found in women (Ditzen et al., 2013; Flanagan et al., 2018a; Rilling et al., 2014).
Studies have also found that OT effectively attenuates neurobiological and behavioral stress reactivity among individuals with psychological or social vulnerabilities and maladaptive stress responses, as compared to healthy individuals (Bartz et al., 2010; Flanagan et al., 2015; Quirin et al., 2011). Thus, OT may not selectively enhance prosocial behavior, but rather amplify one’s social tendencies (Shamay-Tsoory and Abu-Akel, 2016). This hypothesis explains, in part, findings that OT is associated with magnified negative affect and maladaptive behaviors in certain studies (Bartz et al., 2011; Bertsch et al., 2013; DeWall et al., 2014; Flanagan et al., 2018a).
The beneficial effects of OT on social and health behaviors have been commonly attributed to its ability to attenuate hypothalamic–pituitary–adrenal (HPA) axis dysregulation. HPA axis regulation is often measured in the form of cortisol reactivity, and increased cortisol production during stress is normative among healthy populations (Kirschbaum et al., 1993). However, individuals who encounter chronic stress or trauma, such as intimate partner violence (IPV) victimization, may have overactive or blunted reactivity (Lovallo, 2006; Yehuda et al., 2015).
Addressing the impact of IPV, specifically, on OT response is a critical addition to the translational OT literature. Abundant literature has identified substance misuse as both a precipitant to IPV perpetration and a consequence of IPV victimization (Afifi et al., 2012; Smith et al., 2012). Indeed, individuals with substance use disorder (SUD) are at 1.4 to 8.5 greater odds of perpetrating IPV and at 1.5 to 6.0 greater odds of experiencing IPV victimization (Afifi et al., 2012). Dyadic interventions are highly efficacious in the treatment of SUD (McCrady Barbara et al., 2016; Powers et al., 2008), and research has found that OT may reduce symptoms associated with SUD (Eidelman-Rothman et al., 2015; Flanagan et al., 2018b; Lee and Weerts, 2016; McGregor and Bowen, 2012). Because IPV is associated with SUD, examining the interaction between OT and IPV perpetration and victimization in couples is an integral step in advancing the therapeutic potential of OT.
Addressing the impact of IPV on OT response also represents advancement in the field of IPV. The IPV literature is becoming more interdisciplinary with increased attention to physiological outcomes, including cortisol response and skin conductance. However, findings in this preliminary literature are mixed. Some studies suggest that IPV victimization is associated with lower cortisol levels, perhaps due to habituation of stressful situations, while others find that IPV victimization is linked with higher cortisol levels (Basu et al., 2013; Pinna et al., 2014; Pinto et al., 2016). Likewise, some studies find that individuals who are exposed to or perpetrate IPV demonstrate lower skin conductance reactivity while others show higher reactivity or no association (Babcock et al., 2005; Freed and D’Andrea, 2015; Romero-Martínez et al., 2013).
The literature examining the effects of OT among couple’s subjective responding and conflict behaviors is also mixed. Some studies find OT increases positive communication and reduces HPA axis dysregulation in normative samples (Algoe et al., 2017; Ditzen et al., 2013; Ditzen et al., 2009; Gouin et al., 2010; Kruger et al., 2018). However, other studies with couples have resulted in null findings (Behnia et al., 2014) or have found that OT has undesirable effects on couple behaviors. Notably, using the current data, Flanagan et al. (2018a) found OT administration was associated with fewer relationship enhancing attributions for men and women, and increased distress-maintaining attributions for women during a conflict task. Another study of undergraduate students found OT was associated with increased subjective aggression among individuals with higher trait physical aggression (DeWall et al., 2014).
Previous research found that OT is associated with attenuated cortisol reactivity in women and some communication behaviors in both men and women but not other measures of stress reactivity (i.e., positive subjective reactivity, skin conductance) (Flanagan et al., 2018a; Solomon et al., in press). In the current study, we build upon these findings to assess whether psychological and physical IPV perpetration and victimization moderate the effects of OT on cortisol response, skin conductance, subjective reactivity, and dyadic conflict behaviors in a sample of couples who are at high risk for IPV due to their substance misuse. It was hypothesized that: 1) there would be a main effect of psychological and physical IPV perpetration and victimization, such that participants with greater IPV perpetration and/or victimization would show diminished stress reactivity responses and less adaptive conflict behaviors (i.e., fewer relationship-enhancing attributions and more distress-maintaining attributions); 2) psychological and physical IPV perpetration and victimization would moderate OT response, such that participants randomized to the OT condition who report less severe IPV perpetration and victimization would show more adaptive responses relative to participants in the placebo condition.
2. Methods
2. 1. Participants
Participants were recruited from advertisements on the internet, in treatment clinics, and around the community. Thirty-three couples (66 individual participants) enrolled in the study between 2014 and 2015. Participants were required to be 18–65 years of age. Within each dyad, one or both partners must have engaged in hazardous drinking (i.e., 4 or more standard drinks for women, 6 or more for men on one occasion) or illicit drug use during the past 60 days.
Participants were excluded from enrollment if they: 1) were pregnant or breastfeeding; 2) had a history of or current physical or psychiatric diagnosis known to impact HPA axis function;had a BMI ≥ 39; 4) used prescribed medications that interfere with activity in the HPA axis; 4) had active suicidal or homicidal ideation and intent. We also excluded participants who had severe, unilateral IPV in the past year as determined by the Revised Conflict Resolution Tactics Scale (CTS-2; Straus et al., 1996) in order to ensure safety of the participants during and following their laboratory visit. Two same-sex female couples (n=4) enrolled in the study; the remainder of the participants were opposite-sex couples. Given the small number of same-sex couples, there was not adequate power to test effects of sex constellations within couples (i.e., same-sex female compared to opposite-sex couples), thus the same-sex couples were excluded from the current analyses. One couple was excluded due to questionable reliability of the data. The final sample was 30 opposite-sex couples. Participants were aged 32.1 years (SD=9.90) on average, and over half identified as African American (53.3%). Most couples were cohabitating (83.3%) and not married. Participants randomized to OT did not differ from those randomized to the placebo condition on age, race, relationship status, psychological IPV perpetration, psychological IPV victimization, physical IPV perpetration, nor physical IPV victimization.
2. 2. Measures
2.2.1. Cortisol response.
To assess cortisol response, unstimulated salivary samples were collected from participants in polypropylene vials and stored on ice at seven different time points (see ‘Laboratory Procedures’ below). Saliva samples were divided into 1.8 nunc tubes and frozen at −70°C until assayed. Using a high sensitivity salivary cortisol enzyme immunoassay kit (intra-assay precision of 3.35%–3.65%, lower sensitivity limit of <0.003 μg/dL; Salimetrics, LLC), saliva samples were assay twice. Samples were then analyzed simultaneously with a PowerWave HT Microplate Spectrophotometer and a Precision Series Automated Liquid Handling System (BioTek Instruments, Inc.).
2.2.2. Skin conductance.
Skin conductance was assessed with an eight-channel biofeedback encoder (ProComp Infiniti) with sensors placed on index and middle fingers. The biofeedback encoder sampled continuously at a rate of 256 Hz and skin conductance was measured in microsiemens. Participants’ average skin conductance at each time point was calculated.
2.2.3. Positive subjective reactivity.
Participants rated their feelings toward their partner at seven time points before, during, and after the conflict resolution tasks. Ratings were made on a 10-point scale from 1 (not at all) to 10 (extremely). To assess positive subjective reactivity toward a partner, ratings of: “How warmly do you currently feel toward your partner?”; “How close do you currently feel toward your partner’’ and “How angry are you currently feeling toward your partner?” (reverse coded) were combined and summed at each time point. Higher scores reflect more positive emotions; Cronbach’s αs ranged from 0.74 to 0.87.
2.2.4. Conflict resolution behaviors.
Couples completed two 10-minute, video recorded conflict resolution tasks. The task involved each partner identifying three relationship problems. In cases where partners’ most important topic was not the same, a coin flip determined which partner’s topic was discussed. The same problem was discussed during both tasks to ensure that study outcomes were not confounded by variability in the topic. The couple was asked to discuss the topic with one another and work toward its resolution. As detailed elsewhere (see Flanagan et al., 2018a), recorded behaviors were coded using the Rapid Marital Interaction Coding Scheme (RMICS; Heyman, 2004; Heyman et al., 1995) by its developers who were blind to treatment condition. In the present analyses, two coded conflict behaviors were examined, given that these behaviors were associated with OT delivery in the main outcomes analyses (see Flanagan et al., 2018a): 1) distress-maintaining attributions (e.g., statements of blame or negative attributions, denying responsibility) and 2) relationship-enhancing attributions (e.g., statements exempting partner from blame for a negative event, positive attributions). Change in each partner’s conflict behaviors was computed by subtracting the frequency of the behavior in first conflict task from the second task.
2.2.5. Intimate partner violence.
Psychological and physical IPV perpetration and victimization were assessed with the CTS-2 (Straus et al., 1996). The psychological aggression subscale (α = 0.84 for men; α = 0.79 for women) measured how many times participants were perpetrators or victims of aggressive acts (e.g., insults or swearing, destroying something belonging to partner). The physical assault subscale (α = 0.83 for men; α = 0.89 for women) measured if participants had perpetrated or been on the receiving end of physical acts of violence (e.g., pushing/shoving, kicking, choking). Psychological and physical IPV scores were calculated by summing the frequency of aggressive acts that had occurred within the last year to yield a continuous measure of IPV severity. Higher scores reflect a greater number of acts of aggression.
2. 3. Procedures
2.3.1. Baseline procedures.
To control for variation HPA axis functioning, all participants were scheduled to arrive for the study at 8:00am and visits were scheduled during the luteal phase of women’s menstrual cycles. Upon arriving at the office, participants read and signed a consent form, approved by the local Institutional Review Board, before study procedures occurred. Couples were separated from their partner to complete informed consent and the baseline assessment. Women were required to complete a urine pregnancy test, and if their test was negative both partners completed breathalyzer tests and urine drug screens.
2.3.2. Laboratory procedures.
Baseline saliva samples (Time 1) were collected at approximately 9:00am. Afterward, participants were given a 10-minute acclamation period, followed by the first 10-minute conflict resolution task (9:30am). Participants provided a saliva sample immediately following the task (Time 2). Next, participants were randomly assigned in a double-blind manner (1:1) to receive intranasal OT (40 IU) or placebo. Partners within a couple were randomized to the same drug condition. OT nasal spray or matching placebo (i.e., saline) were dispensed by the research pharmacy, and participants self-administered the spray at approximately 9:35am. Participants were then given a 45-minute resting period. At approximately 10:20am (Time 3), participants provided another saliva sample and engaged in the second conflict resolution task. Immediately following the completion of the second conflict task, at 10:35am (Time 4), data, including saliva samples were collected from participants. These data were collected again at 15- (Time 5), 30- (Time 6), and 60-minutes (Time 7) after the conflict task. Participants were debriefed and compensated.
2. 4. Data analytic plan
Multilevel growth curve models, run in SPSS v. 24, tested whether psychological and physical IPV victimization and perpetration moderated the association between drug condition (OT, coded as 1; placebo, coded as 0) and measures of physiological and subjective stress reactivity across individuals. These models accounted for the nested nature of the data (i.e., repeated measures within individuals). Rather than using a dyadic data analytic approach, analyses were run separately in men and women to preserve statistical power.
Independent models examined the effect of psychological IPV perpetration, psychological IPV victimization, physical IPV perpetration, and psychological IPV victimization on physiological and stress reactivity. All models contained random intercepts. For physiological measures of stress reactivity (i.e., cortisol and skin conductance) the typical inverted U-curve containing linear and quadratic effects was modeled starting with Time 3 (i.e., the beginning of the second conflict task, after drug administration). Mean-centered baseline cortisol/skin conductance (mean of Time 1 and Time 2) was placed in the model as a covariate to control for baseline variation between participants, and drug condition, and interaction effects for drug condition were included as predictors. For our subjective measure of stress reactivity, preliminary analysis suggested modeling linear effects was adequate, starting with Time 3. Again, mean-centered baseline subjective emotional reactivity (mean of Time 1 and Time 2) was placed in the model as a covariate. Predictor variables included drug condition, IPV victimization/perpetration, and interaction effects.
Change in conflict behaviors were computed as single variables, and not measured repeatedly over time. Thus, independent linear regression models examined change in conflict behaviors (i.e., relationship enhancing attributions and distress-maintaining attributions) as a function of drug condition and IPV. The regression models were also run separately for men and women to preserve statistical power. These models contained experimental drug condition, IPV perpetration/victimization, and interaction effects with drug condition as predictors. For all models, p-values< 0.05 are considered statistically significant.
3. Results
3. 1. Baseline characteristics.
Men in the sample had mean scores of 28.87 (SD=26.85) and 30.03 (SD=26.54) on psychological perpetration and victimization, respectively. They had mean scores of 4.44 (SD=6.14) and 11.11 (SD=20.96) on physical perpetration and victimization, respectively. All psychological and physical IPV scores were significantly correlated with one another (r’s ranged from 0.51 to 0.99, p’s<0.001). Mean scores on distress maintaining attributions were −0.31 (SD=2.29) and on relationship enhancing attributions were −0.93 (SD=2.62).
Women’s mean scores were 29.07 (SD=20.96) for psychological perpetration and 33.77 (SD=31.01) for psychological victimization. Their mean scores on physical perpetration and victimization were 6.78 (SD=10.56) and 7.13 (SD=16.17), respectively. Physical and psychological IPV scores were significantly correlated with one another (r’s ranged from 0.44 to 0.90, p’s<0.001). Mean scores were 0.21 (SD=2.70) for distress maintaining attributions and −0.28 (SD=3.07) for relationship enhancing attributions.
3. 2. Cortisol reactivity.
Among men, baseline cortisol levels acted as a significant covariate, but no other significant main effects or interactions were found on cortisol reactivity for models examining psychological and physical IPV perpetration/victimization, respectively (Table 1). For women, baseline cortisol also acted as a significant covariate on all models. In addition, for the model examining physical IPV victimization as a moderator, there was a significant time × drug condition interaction, such that as time progressed those in the OT condition had cortisol levels that stayed low relative to those in the placebo. For the model examining physical IPV perpetration as the moderator, significant 2-way interactions emerged. As shown in Figure 1a, women who received OT had lower cortisol levels across time relative to those matched in IPV severity who received placebo; further, those who reported greater physical IPV perpetration against their partner showed attenuated cortisol reactivity as time progressed relative to those with who reported low levels IPV perpetration. There were no other significant main effects or interactions for any of the other models.
Table 1.
Cortisol reactivity to a conflict resolution task by drug condition and level of intimate partner violence (IPV)
| Model | Men | Women | ||||
|---|---|---|---|---|---|---|
| Psychological Victimization | B | SE | p | B | SE | p |
| Intercept | 0.10 | 0.03 | 0.000 | 0.04 | 0.05 | 0.357 |
| Time | −0.02 | 0.01 | 0.018 | −0.01 | 0.01 | 0.479 |
| Time2 | 0.00 | 0.00 | 0.188 | 0.00 | 0.00 | 0.714 |
| Baseline Cortisol | 0.54 | 0.05 | 0.000 | 0.79 | 0.10 | 0.000 |
| Drug Condition (Placebo v. OT) | −0.03 | 0.03 | 0.314 | −0.05 | 0.05 | 0.296 |
| Time × Drug Condition | 0.02 | 0.01 | 0.125 | −0.02 | 0.01 | 0.127 |
| Time2 × Drug Condition | 0.00 | 0.00 | 0.411 | 0.00 | 0.00 | 0.148 |
| IPV | 0.00 | 0.00 | 0.220 | 0.00 | 0.00 | 0.676 |
| Drug Condition × IPV | 0.00 | 0.00 | 0.465 | 0.00 | 0.00 | 0.749 |
| Time × IPV | 0.00 | 0.00 | 0.607 | 0.00 | 0.00 | 0.873 |
| Time2 × IPV | 0.00 | 0.00 | 0.591 | 0.00 | 0.00 | 0.775 |
| Time × Drug Condition × IPV | 0.00 | 0.00 | 0.224 | 0.00 | 0.00 | 0.671 |
| Time2 × Drug Condition × IPV | 0.00 | 0.00 | 0.558 | 0.00 | 0.00 | 0.900 |
| Psychological Perpetration | ||||||
| Intercept | 0.09 | 0.03 | 0.001 | 0.04 | 0.05 | 0.422 |
| Time | −0.02 | 0.01 | 0.018 | −0.01 | 0.01 | 0.254 |
| Time2 | 0.00 | 0.00 | 0.149 | 0.00 | 0.00 | 0.797 |
| Baseline Cortisol | 0.55 | 0.05 | 0.000 | 0.79 | 0.10 | 0.000 |
| Drug Condition (Placebo v. OT) | −0.02 | 0.03 | 0.514 | −0.04 | 0.05 | 0.386 |
| Time × Drug Condition | 0.02 | 0.01 | 0.201 | −0.02 | 0.01 | 0.206 |
| Time2 × Drug Condition | 0.00 | 0.00 | 0.408 | 0.00 | 0.00 | 0.162 |
| IPV | 0.00 | 0.00 | 0.704 | 0.00 | 0.00 | 0.804 |
| Drug Condition × IPV | 0.00 | 0.00 | 0.952 | 0.00 | 0.00 | 0.899 |
| Time × IPV | 0.00 | 0.00 | 0.591 | 0.00 | 0.00 | 0.456 |
| Time2 × IPV | 0.00 | 0.00 | 0.693 | 0.00 | 0.00 | 0.880 |
| Time × Drug Condition × IPV | 0.00 | 0.00 | 0.192 | 0.00 | 0.00 | 0.477 |
| Time2 × Drug Condition × IPV | 0.00 | 0.00 | 0.374 | 0.00 | 0.00 | 0.907 |
| Physical Victimization | ||||||
| Intercept | 0.08 | 0.02 | 0.001 | 0.03 | 0.03 | 0.392 |
| Time | −0.02 | 0.01 | 0.004 | 0.00 | 0.01 | 0.658 |
| Time2 | 0.00 | 0.00 | 0.037 | 0.00 | 0.00 | 0.843 |
| Baseline Cortisol | 0.54 | 0.05 | 0.000 | 0.80 | 0.10 | 0.000 |
| Drug Condition (Placebo v. OT) | −0.01 | 0.02 | 0.757 | −0.04 | 0.03 | 0.289 |
| Time × Drug Condition | 0.01 | 0.01 | 0.477 | −0.02 | 0.01 | 0.014 |
| Time2 × Drug Condition | 0.00 | 0.00 | 0.562 | 0.00 | 0.00 | 0.106 |
| IPV | 0.00 | 0.00 | 0.322 | 0.00 | 0.00 | 0.899 |
| Drug Condition × IPV | 0.00 | 0.00 | 0.313 | 0.00 | 0.00 | 0.992 |
| Time × IPV | 0.00 | 0.00 | 0.499 | 0.00 | 0.00 | 0.345 |
| Time2 × IPV | 0.00 | 0.00 | 0.950 | 0.00 | 0.00 | 0.976 |
| Time × Drug Condition × IPV | 0.00 | 0.00 | 0.872 | 0.00 | 0.00 | 0.586 |
| Time2 × Drug Condition × IPV | 0.00 | 0.00 | 0.734 | 0.00 | 0.00 | 0.787 |
| Physical Perpetration | ||||||
| Intercept | 0.09 | 0.02 | 0.000 | 0.04 | 0.03 | 0.281 |
| Time | −0.02 | 0.01 | 0.001 | 0.00 | 0.01 | 0.607 |
| Time2 | 0.00 | 0.00 | 0.046 | 0.00 | 0.00 | 0.363 |
| Baseline Cortisol | 0.54 | 0.05 | 0.000 | 0.81 | 0.09 | 0.000 |
| Drug Condition (Placebo v. OT) | −0.02 | 0.02 | 0.356 | −0.05 | 0.04 | 0.211 |
| Time × Drug Condition | 0.00 | 0.01 | 0.893 | −0.03 | 0.01 | 0.003 |
| Time2 × Drug Condition | 0.00 | 0.00 | 0.967 | 0.00 | 0.00 | 0.046 |
| IPV | 0.00 | 0.00 | 0.945 | 0.00 | 0.00 | 0.418 |
| Drug Condition × IPV | 0.00 | 0.00 | 0.829 | 0.00 | 0.00 | 0.544 |
| Time × IPV | 0.00 | 0.00 | 0.926 | 0.00 | 0.00 | 0.023 |
| Time2 × IPV | 0.00 | 0.00 | 0.939 | 0.00 | 0.00 | 0.206 |
| Time × Drug Condition × IPV | 0.00 | 0.00 | 0.473 | 0.00 | 0.00 | 0.075 |
| Time2 × Drug Condition × IPV | 0.00 | 0.00 | 0.800 | 0.00 | 0.00 | 0.357 |
Note. Statistically significant effects at p < 0.05 are bolded. Effects that trend toward significance at p < 0.10 are bolded and italicized.
Figure 1.
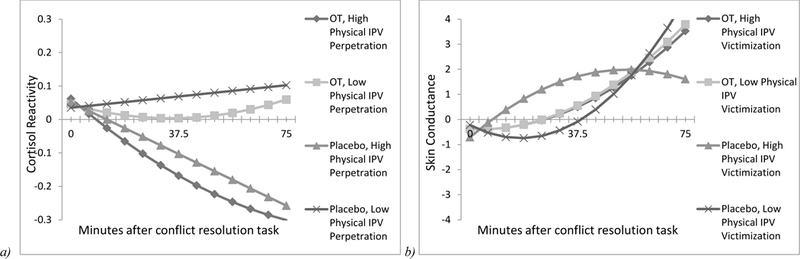
a) Women’s cortisol reactivity to a conflict resolution task by drug condition and level of physical intimate partner violence (IPV) perpetration; b) Women’s skin conductance to a conflict resolution task by drug condition and level of physical intimate partner violence (IPV) perpetration All statistically significant effects (p < 0.05) are represented, and values are plotted at ± 1 SD above and below the mean. OT = oxytocin.
3. 3. Skin conductance.
Models examining skin conductance in men produced similar findings to models exploring cortisol reactivity. Baseline levels of skin conductance were a significant covariate in all models but no main effects or interactions were found (Table 2). Likewise, baseline skin conductance was a significant covariate for women in each model and models examining physical IPV victimization yielded significant interactions. As seen in Figure 1b, the pattern of skin conductance was most differentiated in participants who received placebo and reported higher levels of physical IPV victimization. For this group, skin conductance increased rapidly over time and then decreased. For all other groups, skin conductance steadily increased over time without decreasing.
Table 2.
Skin conductance reactivity to a conflict resolution task by drug condition and level of intimate partner violence (IPV)
| Model | Men | Women | ||||
|---|---|---|---|---|---|---|
| Psychological Victimization | B | SE | p | B | SE | p |
| Intercept | −0.47 | 0.55 | 0.399 | 0.08 | 1.10 | 0.944 |
| Time | 0.18 | 0.13 | 0.171 | −0.05 | 0.22 | 0.820 |
| Time2 | 0.00 | 0.02 | 0.944 | 0.06 | 0.03 | 0.081 |
| Baseline Cortisol | 1.23 | 0.12 | 0.000 | 0.85 | 0.13 | 0.000 |
| Drug Condition (Placebo v. OT) | −0.18 | 0.71 | 0.801 | 0.67 | 1.22 | 0.586 |
| Time × Drug Condition | −0.14 | 0.20 | 0.503 | 0.29 | 0.30 | 0.332 |
| Time2 × Drug Condition | 0.00 | 0.03 | 0.940 | −0.06 | 0.05 | 0.229 |
| IPV | −0.01 | 0.01 | 0.663 | 0.00 | 0.02 | 0.847 |
| Drug Condition × IPV | 0.00 | 0.02 | 0.793 | −0.01 | 0.03 | 0.637 |
| Time × IPV | 0.00 | 0.00 | 0.455 | 0.00 | 0.01 | 0.784 |
| Time2 × IPV | 0.00 | 0.00 | 0.963 | 0.00 | 0.00 | 0.350 |
| Time × Drug Condition × IPV | 0.00 | 0.01 | 0.405 | 0.00 | 0.01 | 0.544 |
| Time2 × Drug Condition × IPV | 0.00 | 0.00 | 0.999 | 0.00 | 0.00 | 0.325 |
| Psychological Perpetration | ||||||
| Intercept | −0.52 | 0.56 | 0.357 | 0.64 | 1.15 | 0.582 |
| Time | 0.18 | 0.13 | 0.173 | 0.18 | 0.25 | 0.474 |
| Time2 | 0.00 | 0.02 | 0.900 | 0.02 | 0.04 | 0.540 |
| Baseline Cortisol | 1.22 | 0.12 | 0.000 | 0.85 | 0.13 | 0.000 |
| Drug Condition (Placebo v. OT) | −0.06 | 0.70 | 0.930 | 0.01 | 1.27 | 0.996 |
| Time × Drug Condition | −0.12 | 0.20 | 0.536 | 0.03 | 0.32 | 0.918 |
| Time2 × Drug Condition | 0.00 | 0.03 | 0.955 | −0.02 | 0.05 | 0.738 |
| IPV | 0.00 | 0.01 | 0.827 | −0.02 | 0.03 | 0.593 |
| Drug Condition × IPV | 0.00 | 0.02 | 0.979 | 0.01 | 0.03 | 0.741 |
| Time × IPV | 0.00 | 0.00 | 0.467 | −0.01 | 0.01 | 0.373 |
| Time2 × IPV | 0.00 | 0.00 | 0.901 | 0.00 | 0.00 | 0.699 |
| Time × Drug Condition × IPV | 0.00 | 0.00 | 0.464 | 0.01 | 0.01 | 0.548 |
| Time2 × Drug Condition × IPV | 0.00 | 0.00 | 0.866 | 0.00 | 0.00 | 0.831 |
| Physical Victimization | ||||||
| Intercept | −0.55 | 0.48 | 0.261 | −0.40 | 0.84 | 0.637 |
| Time | 0.14 | 0.11 | 0.194 | −0.20 | 0.15 | 0.199 |
| Time2 | 0.00 | 0.02 | 0.856 | 0.07 | 0.02 | 0.004 |
| Baseline Cortisol | 1.23 | 0.11 | 0.000 | 0.88 | 0.13 | 0.000 |
| Drug Condition (Placebo v. OT) | 0.06 | 0.56 | 0.912 | 0.84 | 0.91 | 0.364 |
| Time × Drug Condition | 0.05 | 0.16 | 0.743 | 0.35 | 0.22 | 0.122 |
| Time2 × Drug Condition | −0.02 | 0.02 | 0.501 | −0.05 | 0.03 | 0.128 |
| IPV | −0.01 | 0.04 | 0.796 | 0.14 | 0.08 | 0.078 |
| Drug Condition × IPV | 0.00 | 0.04 | 0.966 | −0.14 | 0.08 | 0.086 |
| Time × IPV | −0.01 | 0.01 | 0.601 | 0.06 | 0.02 | 0.004 |
| Time2 × IPV | 0.00 | 0.00 | 0.802 | −0.01 | 0.00 | 0.001 |
| Time × Drug Condition × IPV | 0.00 | 0.01 | 0.994 | −0.06 | 0.02 | 0.006 |
| Time2 × Drug Condition × IPV | 0.00 | 0.00 | 0.449 | 0.01 | 0.00 | 0.003 |
| Physical Perpetration | ||||||
| Intercept | −0.48 | 0.49 | 0.330 | 0.08 | 0.95 | 0.929 |
| Time | 0.17 | 0.11 | 0.125 | −0.02 | 0.17 | 0.885 |
| Time2 | 0.00 | 0.02 | 0.977 | 0.05 | 0.03 | 0.070 |
| Baseline Cortisol | 1.21 | 0.11 | 0.000 | 0.85 | 0.13 | 0.000 |
| Drug Condition (Placebo v. OT) | −0.07 | 0.58 | 0.912 | 0.51 | 1.05 | 0.634 |
| Time × Drug Condition | 0.07 | 0.16 | 0.677 | 0.19 | 0.25 | 0.458 |
| Time2 × Drug Condition | −0.01 | 0.03 | 0.736 | −0.04 | 0.04 | 0.327 |
| IPV | −0.02 | 0.06 | 0.717 | 0.02 | 0.09 | 0.797 |
| Drug Condition × IPV | 0.01 | 0.08 | 0.930 | −0.04 | 0.10 | 0.720 |
| Time × IPV | −0.02 | 0.02 | 0.346 | 0.01 | 0.02 | 0.830 |
| Time2 × IPV | 0.00 | 0.00 | 0.972 | 0.00 | 0.00 | 0.399 |
| Time × Drug Condition × IPV | −0.01 | 0.02 | 0.793 | −0.01 | 0.03 | 0.807 |
| Time2 × Drug Condition × IPV | 0.00 | 0.00 | 0.640 | 0.00 | 0.00 | 0.399 |
Note. Statistically significant effects at p < 0.05 are bolded. Effects that trend toward significance at p < 0.10 are bolded and italicized.
3. 4. Positive subjective reactivity.
Among men, no significant main effects or interactions were found for models examining positive subjective reactivity as the outcome, though baseline positive subjective reactivity was a significant covariate (Table 3). For women, baseline positive subjective reactivity was a significant covariate in all models. For the model examining psychological IPV victimization as a moderator, significant 2- and 3-way interactions emerged. As shown in Figure 2a, women who received OT showed increases in positive subjective reactivity when they reported low levels of psychological IPV victimization; individuals who received OT and reported higher levels of psychological IPV victimization showed decreases in positive subjective reactivity. Individuals who received placebo showed relatively similar levels of positive subjective reactivity to each other and across time. For models examining physical IPV perpetration and victimization as moderators, significant 2- and 3-way interactions emerged as well. Individuals reporting greater physical IPV severity showed decreases in positive subjective reactivity when they received OT and increases in positive subjective reactivity when they received placebo (Figure 2b-c). In contrast, individuals who reported low levels of physical IPV showed slight increases in positive subjective reactivity when they received OT, and decreases in positive subjective reactivity when they received placebo. No other significant main effects or interactions were found.
Table 3.
Positive subjective reactivity to a conflict resolution task by drug condition and level of intimate partner violence (IPV)
| Model | Men | Women | ||||
|---|---|---|---|---|---|---|
| Psychological Victimization | B | SE | p | B | SE | p |
| Intercept | 1.76 | 2.26 | 0.443 | 2.95 | 3.16 | 0.358 |
| Time | 0.14 | 0.14 | 0.307 | 0.00 | 0.17 | 0.996 |
| Baseline Positive Emotionality | 0.94 | 0.08 | 0.000 | 0.88 | 0.11 | 0.000 |
| Drug Condition (Placebo v. OT) | −0.01 | 1.26 | 0.993 | 1.64 | 1.63 | 0.320 |
| Time ×Drug Condition | 0.08 | 0.20 | 0.678 | 0.47 | 0.22 | 0.034 |
| IPV | 0.00 | 0.03 | 0.892 | 0.02 | 0.03 | 0.547 |
| Drug Condition × IPV | −0.02 | 0.03 | 0.561 | −0.08 | 0.03 | 0.030 |
| Time × IPV | 0.00 | 0.00 | 0.884 | 0.01 | 0.00 | 0.097 |
| Time × Drug Condition × IPV | 0.00 | 0.01 | 0.530 | −0.02 | 0.00 | 0.002 |
| Psychological Perpetration | ||||||
| Intercept | 1.27 | 2.39 | 0.600 | 3.72 | 3.37 | 0.280 |
| Time | 0.16 | 0.14 | 0.249 | 0.17 | 0.19 | 0.358 |
| Baseline Positive Emotionality | 0.96 | 0.08 | 0.000 | 0.87 | 0.11 | 0.000 |
| Drug Condition (Placebo v. OT) | −0.45 | 1.27 | 0.726 | 0.62 | 1.82 | 0.738 |
| Time ×Drug Condition | −0.10 | 0.20 | 0.614 | 0.25 | 0.23 | 0.283 |
| IPV | 0.00 | 0.02 | 0.973 | 0.01 | 0.04 | 0.908 |
| Drug Condition × IPV | 0.00 | 0.03 | 0.891 | −0.05 | 0.05 | 0.280 |
| Time × IPV | 0.00 | 0.00 | 0.753 | 0.00 | 0.01 | 0.772 |
| Time × Drug Condition × IPV | 0.00 | 0.01 | 0.670 | −0.01 | 0.01 | 0.119 |
| Physical Victimization | ||||||
| Intercept | 1.24 | 2.49 | 0.623 | −0.19 | 3.61 | 0.959 |
| Time | 0.14 | 0.12 | 0.231 | 0.07 | 0.12 | 0.557 |
| Baseline Positive Emotionality | 0.96 | 0.08 | 0.000 | 1.00 | 0.13 | 0.000 |
| Drug Condition (Placebo v. OT) | −0.59 | 1.04 | 0.577 | 0.43 | 1.50 | 0.778 |
| Time ×Drug Condition | −0.07 | 0.16 | 0.679 | 0.17 | 0.16 | 0.307 |
| IPV | 0.00 | 0.07 | 0.973 | 0.14 | 0.13 | 0.272 |
| Drug Condition × IPV | 0.00 | 0.07 | 0.970 | −0.19 | 0.14 | 0.172 |
| Time × IPV | 0.00 | 0.01 | 0.869 | 0.04 | 0.02 | 0.004 |
| Time × Drug Condition × IPV | 0.00 | 0.01 | 0.800 | −0.05 | 0.02 | 0.001 |
| Physical Perpetration | ||||||
| Intercept | 1.73 | 2.24 | 0.448 | 0.54 | 3.72 | 0.885 |
| Time | 0.12 | 0.12 | 0.282 | 0.03 | 0.13 | 0.831 |
| Baseline Positive Emotionality | 0.94 | 0.08 | 0.000 | 0.97 | 0.13 | 0.000 |
| Drug Condition (Placebo v. OT) | −0.21 | 1.04 | 0.842 | 0.60 | 1.64 | 0.717 |
| Time ×Drug Condition | 0.04 | 0.17 | 0.815 | 0.26 | 0.18 | 0.149 |
| IPV | −0.01 | 0.12 | 0.927 | 0.12 | 0.15 | 0.422 |
| Drug Condition × IPV | −0.07 | 0.14 | 0.617 | −0.21 | 0.17 | 0.223 |
| Time × IPV | 0.00 | 0.02 | 0.947 | 0.05 | 0.02 | 0.011 |
| Time × Drug Condition × IPV | −0.01 | 0.02 | 0.539 | −0.06 | 0.02 | 0.002 |
Note. Statistically significant effects at p < 0.05 are bolded. Effects that trend toward significance at p < 0.10 are bolded and italicized.
Figure 2.
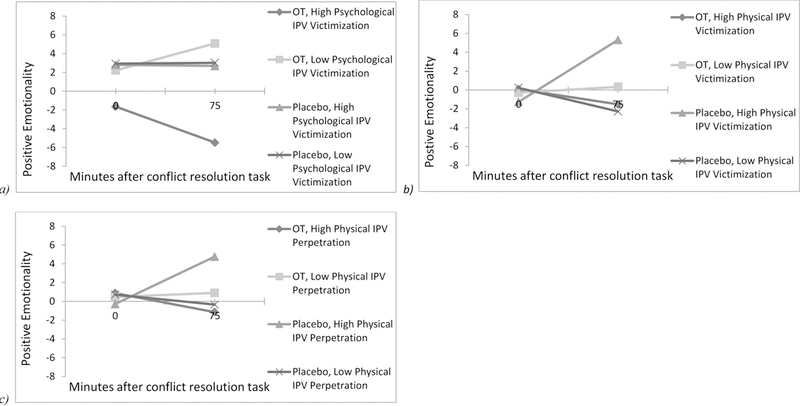
a) Women’s subjective ratings of positive emotionality in response to a conflict resolution task by drug condition and level of psychological intimate partner violence (IPV) victimization; b) Women’s subjective ratings of positive emotionality in response to a conflict resolution task by drug condition and level of physical intimate partner violence (IPV) victimization; c) Women’s subjective ratings of positive emotionality in response to a conflict resolution task by drug condition and level of physical intimate partner violence (IPV) perpetration. All statistically significant effects (p < 0.05) are represented, and values are plotted at ± 1 SD above and below the mean. OT = oxytocin.
3. 5. Conflict behaviors.
As presented in Tables 4 and 5, no significant main effects or interactions emerged for the models examining the relationship enhancing or distress-maintaining attributions outcomes in men. Among women, no statistically significant effects of drug condition, IPV, or the interaction between the two were found on change in relationship enhancing attributions. When examining distress maintaining attributions, main effects of psychological IPV perpetration and psychological IPV victimization were significant. These significant effects suggest that greater psychological IPV severity was associated with increased distress maintaining attributions in the second task relative to the first. No other main effects or interactions were found.
Table 4.
Change in relationship-enhancing attributions from Task 1 to Task 2 by drug condition and level of intimate partner violence (IPV)
| Model | Men | Women | ||||
|---|---|---|---|---|---|---|
| Psychological Victimization | B | SE | p | B | SE | p |
| Intercept | −0.16 | 1.03 | 0.877 | 0.33 | 1.26 | 0.795 |
| Drug Condition (Placebo v. OT) | −1.83 | 1.49 | 0.231 | −0.69 | 1.65 | 0.682 |
| IPV | −0.03 | 0.03 | 0.405 | 0.02 | 0.03 | 0.589 |
| Drug Condition × IPV | 0.05 | 0.04 | 0.191 | −0.04 | 0.04 | 0.295 |
| Psychological Perpetration | ||||||
| Intercept | −0.36 | 1.04 | 0.729 | 1.53 | 1.41 | 0.288 |
| Drug Condition (Placebo v. OT) | −1.43 | 1.49 | 0.346 | −2.05 | 1.74 | 0.250 |
| IPV | −0.01 | 0.02 | 0.582 | −0.03 | 0.05 | 0.555 |
| Drug Condition × IPV | 0.04 | 0.04 | 0.306 | 0.01 | 0.05 | 0.883 |
| Physical Victimization | ||||||
| Intercept | −0.92 | 0.87 | 0.300 | 0.20 | 0.88 | 0.820 |
| Drug Condition (Placebo v. OT) | −0.42 | 1.18 | 0.727 | −1.12 | 1.20 | 0.361 |
| IPV | 0.03 | 0.09 | 0.726 | 0.19 | 0.11 | 0.106 |
| Drug Condition × IPV | −0.01 | 0.09 | 0.887 | −0.21 | 0.12 | 0.081 |
| Physical Perpetration | ||||||
| Intercept | −0.77 | 0.88 | 0.389 | 0.54 | 0.96 | 0.578 |
| Drug Condition (Placebo v. OT) | 0.27 | 1.25 | 0.832 | −1.09 | 1.33 | 0.420 |
| IPV | 0.00 | 0.14 | 0.995 | 0.08 | 0.14 | 0.553 |
| Drug Condition × IPV | −0.10 | 0.18 | 0.561 | −0.15 | 0.15 | 0.316 |
Note. Statistically significant effects at p < 0.05 are bolded. Effects that trend toward significance at p < 0.10 are bolded and italicized.
Table 5.
Change in distress-maintaining attributions from Task 1 to Task 2 by drug condition and level of intimate partner violence (IPV)
| Model | Men | Women | ||||
|---|---|---|---|---|---|---|
| Psychological Victimization | B | SE | p | B | SE | p |
| Intercept | 0.72 | 0.89 | 0.425 | −2.71 | 0.92 | 0.007 |
| Drug Condition (Placebo v. OT) | −1.77 | 1.29 | 0.184 | 2.18 | 1.21 | 0.083 |
| IPV | −0.01 | 0.03 | 0.591 | 0.07 | 0.02 | 0.004 |
| Drug Condition × IPV | 0.02 | 0.03 | 0.575 | −0.03 | 0.03 | 0.254 |
| Psychological Perpetration | ||||||
| Intercept | −0.36 | 1.04 | 0.729 | −2.60 | 1.07 | 0.023 |
| Drug Condition (Placebo v. OT) | −1.43 | 1.49 | 0.346 | 2.05 | 1.32 | 0.133 |
| IPV | −0.01 | 0.02 | 0.582 | 0.08 | 0.04 | 0.025 |
| Drug Condition × IPV | 0.04 | 0.04 | 0.306 | −0.04 | 0.04 | 0.280 |
| Physical Victimization | ||||||
| Intercept | −0.04 | 0.71 | 0.961 | −1.14 | 0.75 | 0.753 |
| Drug Condition (Placebo v. OT) | −1.03 | 0.97 | 0.300 | 1.34 | 1.02 | 1.021 |
| IPV | 0.08 | 0.07 | 0.243 | 0.17 | 0.09 | 0.094 |
| Drug Condition × IPV | −0.07 | 0.07 | 0.335 | −0.11 | 0.10 | 0.099 |
| Physical Perpetration | ||||||
| Intercept | 0.17 | 0.72 | 0.812 | −1.10 | 0.80 | 0.803 |
| Drug Condition (Placebo v. OT) | −1.68 | 1.02 | 0.114 | 0.93 | 1.11 | 1.106 |
| IPV | 0.07 | 0.12 | 0.581 | 0.15 | 0.11 | 0.114 |
| Drug Condition × IPV | 0.05 | 0.14 | 0.731 | −0.04 | 0.12 | 0.124 |
Note. Statistically significant effects at p < 0.05 are bolded. Effects that trend toward significance at p < 0.10 are bolded and italicized.
4. Discussion
With the aim of advancing the translational potential of OT, the current study hypothesized that 1) there would be a main effect of psychological and physical IPV perpetration and victimization on stress reactivity responses and conflict behaviors 2) psychological and physical IPV perpetration and victimization would moderate OT response. Results from the current study partially support hypotheses and highlight the nuanced response of OT in a sample of couples at high risk for IPV due to substance misuse. First, it is worth highlighting the gender differences found in the current study. Significant findings only emerged for women but not men. This may be explained by sex differences in the endogenous OT system or differential responses to laboratory stress paradigms (Back et al., 2005; Macdonald Kai, 2012; Weisman et al., 2013). Men may have been less sensitive to the dyadic conflict task than women. For instance, research shows men are more likely to withdraw during discussion tasks (Christensen and Heavey, 1990). Future research should continue to parse sex differences in OT response and examine under which contexts and for which people OT produces desirable effects.
Specifically, our study found that among women in the sample, there was a main effect of physical IPV perpetration on cortisol reactivity, such that women who reported greater IPV severity showed more attenuated cortisol responses. This finding is consistent with research that finds IPV is associated with lower levels of cortisol (Basu et al., 2013; Johnson et al., 2008; Pinto et al., 2016), suggesting that individuals with interpersonal stressors or traumas have blunted physiological stress reactivity. IPV did not significantly interact with drug, though a three-way interaction of time × drug condition ×physical IPV perpetration approached significance; such an interaction would suggest that more severe IPV modulates cortisol reactivity in individuals delivered OT such that their reactivity is similar to those who received OT and reported low levels of IPV (i.e., cortisol levels decrease then increase over time vs. cortisol levels steadily increasing or decreasing over time). Future studies should model and examine this effect using larger sample sizes.
Findings also suggest that physical IPV victimization moderated the association between OT treatment and skin conductance in women in this sample. Although a previous study by our group found that there were no overall differences in skin conductance by drug condition (Solomon, et al, in press), in the current study, physical IPV was a significant moderator of this relationship in women. Specifically, skin conductance among individuals who were randomized to receive OT and reported high levels of physical IPV more closely matched the skin conductance response of individuals who were delivered OT and reported low physical IPV. OT might temper this specific physiological response among individuals with a history of physical (but perhaps not psychological).
Physical and psychological IPV also moderated women’s dyadic conflict behaviors and positive subjective reactivity in this sample. In general, women with greater IPV severity reported decreases in positive subjective reactivity over time when they received OT, whereas those who reported greater levels of IPV reported increases in positive subjective reactivity when they received placebo. Results also suggest women who reported greater levels of physical IPV victimization showed more distress maintaining and fewer relationship enhancing attributions when delivered OT relative to placebo. Examining these responses in sum, OT decreases positive behaviors and subjective reactivity among women reporting greater IPV severity but not among women reporting low levels of IPV. Indeed, women who reported the highest levels of IPV were generally more likely to report lower levels of relationship functioning. Thus, OT may amplify maladaptive interpersonal patterns in individuals with the most distressed relationships. This finding fits with the hypothesis that OT magnifies one’s social tendencies (Shamay-Tsoory and Abu-Akel, 2016).
Altogether, our results suggest OT may help regulate physiological responses and amplify maladaptive subjective and behavioral responses in women with a history of IPV. These seemingly contradictory findings are surprising, particularly given the literature documenting the anxiolytic and prosocial effects of OT (MacDonald and MacDonald, 2010); however, more recent literature has found that the link between physiological stress reactivity and social behavior may not be direct (see Ditzen et al., 2013). Alternatively, when physiological responses are modulated by OT, women with a history of IPV may endorse more entrenched subjective responses. Thus, future research should examine time-lagged associations between physiological reactivity and behavioral responses to test causal links. In addition, because couples in this study were not subject to a behavioral intervention, future studies should investigate whether pairing OT with a couples intervention or behavioral skills training maximizes the drug’s translational potential.
There are several limitations to this exploratory study. The small sample size limited statistical power and the ability to use more advanced models and modeling techniques, including examining men and women together in the same model, examining additional moderators, and testing partner effects and the effects of partners’ substance use concordance. Further, the current study tested multiple hypotheses, and given the exploratory nature of the investigation, a multiple test adjustment was not used (Bender and Lange, 2001). Findings should be interpreted with caution and replicated in the future studies. These findings are also limited in their generalizability. We did not have a healthy control group of which to compare our sample. Participants were not recruited based on their IPV history; rather, participants were recruited from the community based on reported high levels of substance use, a known correlate of IPV, and related problems. Further, we did not account for substance-related outcomes like craving in this analysis. Thus, findings from this laboratory study might not translate clearly to a treatment study with a repeated OT administration. The dose-response relationship in OT research is still under investigation so outcomes might be different with a different dose (Cardoso et al., 2013; Spengler et al., 2017). Despite controlling for diurnal variations by ensuring that all participants completed the study early in the morning, findings should be interpreted with consideration to the fact that cortisol levels will naturally decrease throughout the day. Despite these limitations, this investigation used a well-controlled laboratory design and was the first to examine the moderating effects IPV on OT among substance misusing couples.
Highlights.
Intimate partner violence (IPV) is a stressor that may impact oxytocin response.
IPV modulated oxytocin on reactivity to a dyadic conflict task in women.
No main or moderating effects of IPV on oxytocin were found in men.
Findings support literature on sex differences in oxytocin response.
Acknowledgments
Funding Information
This manuscript is the result of work supported, in part, by the National Institute on Child Health and Human Development and the Office of Research on Women’s Health (K12HD055885), the National Institute on Alcohol Abuse and Alcoholism (T32AA747430 and K23AA023845), and the National Institute on Drug Abuse (K02DA039229).
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- Afifi TO, Henriksen CA, Asmundson GJG, Sareen J, 2012. Victimization and perpetration of intimate partner violence and substance use disorders in a nationally representative sample. The Journal of Nervous and Mental Disease 200 (8), 684–691. [DOI] [PubMed] [Google Scholar]
- Algoe SB, Kurtz LE, Grewen K, 2017. Oxytocin and Social Bonds: The Role of Oxytocin in Perceptions of Romantic Partners’ Bonding Behavior. Psychol Sci 28 (12), 1763–1772. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Babcock JC, Green CE, Webb SA, Yerington TP, 2005. Psychophysiological Profiles of Batterers: Autonomic Emotional Reactivity as It Predicts the Antisocial Spectrum of Behavior Among Intimate Partner Abusers. Journal of Abnormal Psychology 114 (3), 444–455. [DOI] [PubMed] [Google Scholar]
- Back SE, Brady KT, Jackson JL, Salstrom S, Zinzow H, 2005. Gender differences in stress reactivity among cocaine-dependent individuals. Psychopharmacology 180 (1), 169–176. [DOI] [PubMed] [Google Scholar]
- Bartels A, 2012. Oxytocin and the social brain: beware the complexity. Neuropsychopharmacology 37 (8), 1795–1796. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bartz JA, Simeon D, Hamilton H, Kim S, Crystal S, Braun A, Vicens V, Hollander E, 2011. Oxytocin can hinder trust and cooperation in borderline personality disorder. Social Cognition and Affective Neuroscience 6 (5), 556–563. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bartz JA, Zaki J, Bolger N, Hollander E, Ludwig NN, Kolevzon A, Ochsner KN, 2010. Oxytocin selectively improves empathic accuracy. Psychological Science 21 (10), 1426–1428. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Basu A, Levendosky AA, Lonstein JS, 2013. Trauma Sequelae and Cortisol Levels in Women Exposed to Intimate Partner Violence. Psychodynamic Psychiatry 41 (2), 29p. [DOI] [PubMed] [Google Scholar]
- Behnia B, Heinrichs M, Bergmann W, Jung S, Germann J, Schedlowski M, Hartmann U, Kruger THC, 2014. Differential effects of intranasal oxytocin on sexual experiences and partner interactions in couples. Hormones and Behavior 65 (3), 308–318. [DOI] [PubMed] [Google Scholar]
- Bender R, Lange S, 2001. Adjusting for multiple testing—when and how? Journal of Clinical Epidemiology 54 (4), 343–349. [DOI] [PubMed] [Google Scholar]
- Bertsch K, Gamer M, Schmidt B, Schmidinger I, Walther S, Kästel T, Schnell K, Büchel C, Domes G, Herpertz SC, 2013. Oxytocin and reduction of social threat hypersensitivity in women with borderline personality disorder. American Journal of Psychiatry 170 (10), 1169–1177. [DOI] [PubMed] [Google Scholar]
- Cardoso C, Ellenbogen MA, Orlando MA, Bacon SL, Joober R, 2013. Intranasal oxytocin attenuates the cortisol response to physical stress: a dose-response study. Psychoneuroendocrinology 38 (3), 399–407. [DOI] [PubMed] [Google Scholar]
- Christensen A, Heavey CL, 1990. Gender and social structure in the demand/withdraw pattern of marital conflict. Journal of Personality and Social Psychology 59 (1), 73–81. [DOI] [PubMed] [Google Scholar]
- DeWall CN, Gillath O, Pressman SD, Black LL, Bartz JA, Moskovitz J, Stetler DA, 2014. When the Love Hormone Leads to Violence Oxytocin Increases Intimate Partner Violence Inclinations Among High Trait Aggressive People. Social Psychological and Personality Science 5 (6), 691–697. [Google Scholar]
- Ditzen B, Nater UM, Schaer M, Marca RL, Bodenmann G, Ehlert U, Heinrichs M, 2013. Sex-specific effects of intranasal oxytocin on autonomic nervous system and emotional responses to couple conflict. Social Cognitive and Affective Neuroscience 8(8), 897–902. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ditzen B, Schaer M, Gabriel B, Bodenmann G, Ehlert U, Heinrichs M, 2009. Intranasal oxytocin increases positive communication and reduces cortisol levels during couple conflict. Biological Psychiatry 65 (9), 728–731. [DOI] [PubMed] [Google Scholar]
- Eidelman-Rothman M, Goldstein A, Levy J, Weisman O, Schneiderman I, Mankuta D, Zagoory-Sharon O, Feldman R, 2015. Oxytocin affects spontaneous neural oscillations in trauma-exposed war veterans. Frontiers in Behavioral Neuroscience 9, 165. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Flanagan JC, Baker NL, McRae AL, Brady KT, Moran-Santa Maria M, 2015. Effects of Adverse Childhood Experiences on the Association between Intranasal Oxytocin and Social Stress Reactivity among Individuals with Cocaine Dependence. Psychiatry research 229 (1–2), 94–100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Flanagan JC, Fischer MS, Nietert PJ, Back SE, Maria MM, Snead A, Brady KT, 2018a. Effects of oxytocin on cortisol reactivity and conflict resolution behaviors among couples with substance misuse. Psychiatry Res 260, 346–352. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Flanagan JC, Sippel LM, Wahlquist A, Moran-Santa Maria MM, Back SE, 2018b. Augmenting Prolonged Exposure therapy for PTSD with intranasal oxytocin: A randomized, placebo-controlled pilot trial. J Psychiatr Res 98, 64–69. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Freed S, D’Andrea W, 2015. Autonomic Arousal and Emotion in Victims of Interpersonal Violence: Shame Proneness But Not Anxiety Predicts Vagal Tone. Journal of Trauma & Dissociation 16 (4), 367–383. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gouin J-P, Carter CS, Pournajafi-Nazarloo H, Glaser R, Malarkey WB, Loving TJ, Stowell J, Kiecolt-Glaser JK, 2010. Marital behavior, oxytocin, vasopressin, and wound healing. Psychoneuroendocrinology 35 (7), 1082–1090. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Heyman RE, 2004. Rapid Marital Interaction Coding System Lawrence Erlbaum Associates, Mahwah, NJ. [Google Scholar]
- Heyman RE, Weiss RL, Eddy JM, 1995. Marital interaction coding system: Revision and empirical evaluation. Behaviour research and therapy 33 (6), 737–746. [DOI] [PubMed] [Google Scholar]
- Hurlemann R, Scheele D, 2016. Dissecting the Role of Oxytocin in the Formation and Loss of Social Relationships. Biological Psychiatry 79 (3), 185–193. [DOI] [PubMed] [Google Scholar]
- Johnson DM, Delahanty DL, Pinna K, 2008. The cortisol awakening response as a function of PTSD severity and abuse chronicity in sheltered battered women. Journal of Anxiety Disorders 22 (5), 793–800. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kirschbaum C, Pirke KM, Hellhammer DH, 1993. The ‘Trier Social Stress Test’ – A Tool for Investigating Psychobiological Stress Responses in a Laboratory Setting. Neuropsychobiology 28 (1–2), 76–81. [DOI] [PubMed] [Google Scholar]
- Kruger THC, Deiter F, Zhang Y, Jung S, Schippert C, Kahl KG, Heinrichs M, Schedlowski M, Hartmann U, 2018. Effects of Intranasal Oxytocin Administration on Sexual Functions in Healthy Women: A Laboratory Paradigm. J Clin Psychopharmacol 38 (3), 239–242. [DOI] [PubMed] [Google Scholar]
- Lee MR, Weerts EM, 2016. Oxytocin for the treatment of drug and alcohol use disorders. Behavioural Pharmacology 27 (8), 640–648. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lovallo WR, 2006. Cortisol secretion patterns in addiction and addiction risk. International Journal of Psychophysiology 59 (3), 195–202. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lynn SJ, Hoge EA, Fischer LE, Barrett LF, Simon NM, 2014. Gender Differences in oxytocin-associated disruption of decision bias during emotion perception. Journal of Cognitive Neuroscience 219 (1), 198–203. [DOI] [PMC free article] [PubMed] [Google Scholar]
- MacDonald K, MacDonald TM, 2010. The peptide that binds: A systematic review of oxycotin and its prosocial effects in humans. Harvard Review of Psychiatry 18 (1), 1–21. [DOI] [PubMed] [Google Scholar]
- Macdonald Kai SK, 2012. Sex, receptors, and attachment: a review of individual factors influencing response to oxytocin. Frontiers in Neuroscience 6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McCrady Barbara S, Wilson Adam D, Muñoz Rosa E, Fink Brandi C, Fokas K, Borders A, 2016. Alcohol-Focused Behavioral Couple Therapy. Family Process 55 (3), 443–459. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McGregor IS, Bowen MT, 2012. Breaking the loop: Oxytocin as a potential treatment for drug addiction. Hormones and Behavior 61 (3), 331–339. [DOI] [PubMed] [Google Scholar]
- Pinna KLM, Johnson DM, Delahanty DL, 2014. PTSD, comorbid depression, and the cortisol waking response in victims of intimate partner violence: preliminary evidence. Anxiety, Stress & Coping 27 (3), 253–269. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pinto RJ, Correia-Santos P, Costa-Leite J, Levendosky AA, Jongenelen I, 2016. Cortisol awakening response among women exposed to intimate partner violence. Psychoneuroendocrinology 74 (Supplement C), 57–64. [DOI] [PubMed] [Google Scholar]
- Powers MB, Vedel E, Emmelkamp PMG, 2008. Behavioral couples therapy (BCT) for alcohol and drug use disorders: A meta-analysis. Clinical Psychology Review 28 (6), 952–962. [DOI] [PubMed] [Google Scholar]
- Quirin M, Kuhl J, Düsing R, 2011. Oxytocin buffers cortisol responses to stress in individuals with impaired emotion regulation abilities. Psychoneuroendocrinology 36 (6), 898–904. [DOI] [PubMed] [Google Scholar]
- Rilling JK, DeMarco AC, Hackett PD, Chen X, Gautam P, Stair S, Haroon E, Thompson R, Ditzen B, Patel R, 2014. Sex differences in the neural and behavioral response to intranasal oxytocin and vasopressin during human social interaction. Psychoneuroendocrinology 39, 237–248. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Romero-Martínez A, Lila M, Williams RK, González-Bono E, Moya-Albiol L, 2013. Skin conductance rises in preparation and recovery to psychosocial stress and its relationship with impulsivity and testosterone in intimate partner violence perpetrators. International Journal of Psychophysiology 90 (3), 329–333. [DOI] [PubMed] [Google Scholar]
- Shamay-Tsoory SG, Abu-Akel A, 2016. The Social Salience Hypothesis of Oxytocin. Biological Psychiatry 79 (3), 194–202. [DOI] [PubMed] [Google Scholar]
- Smith PH, Homish GG, Leonard KE, Cornelius JR, 2012. Intimate partner violence and specific substance use disorders: Findings from the National Epidemiologic Survey on Alcohol and Related Conditions. Psychology of Addictive Behaviors 26 (2), 236–245. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Solomon DT, Nietert PJ, Smith DW, Back SE, Barden E, Flanagan JC, in press Effects of oxytocin on emotional and physiological responses to a conflict resolution task in couples with substance misuse [DOI] [PMC free article] [PubMed]
- Spengler FB, Schultz J, Scheele D, Essel M, Maier W, Heinrichs M, Hurlemann R, 2017. Kinetics and Dose Dependency of Intranasal Oxytocin Effects on Amygdala Reactivity. Biol Psychiatry 82 (12), 885–894. [DOI] [PubMed] [Google Scholar]
- Straus MA, Hamby SL, Boney-McCoy S, Sugarman DB, 1996. The Revised Conflict Tactics Scales (CTS2). Journal of Family Issues 17 (3), 283–316. [Google Scholar]
- Weisman O, Zagoory-Sharon O, Schneiderman I, Gordon I, Feldman R, 2013. Plasma oxytocin distributions in a large cohort of women and men and their gender-specific associations with anxiety. Psychoneuroendocrinology 38 (5), 694–701. [DOI] [PubMed] [Google Scholar]
- Yehuda R, Hoge CW, McFarlane AC, Vermetten E, Lanius RA, Nievergelt CM, Hobfoll SE, Koenen KC, Neylan TC, Hyman SE, 2015. Post-traumatic stress disorder. Nat Rev Dis Primers 1, 15057. [DOI] [PubMed] [Google Scholar]


