Abstract
The anorexigenic effects of oxytocin have been widely documented and accepted; however, no study has yet used the Preferred Reporting Items for Systematic Reviews and Meta-Analysis (PRISMA) guidelines to compile previous findings in a single systematic review and quantitative meta-analysis. The present review aimed to identify published and unpublished studies examining the effects of oxytocin on energy intake in animals and humans, as well as the factors that moderate this effect. Web of Science, Pub Med and Ovid were searched for published and unpublished studies reporting the effects of oxytocin on energy intake in wild-type animals and in humans when administered in the absence of other active drugs or surgery. Two thousand and forty-nine articles were identified through the original systematic literature search, from which 54 articles were identified as being relevant for inclusion in the present review. An additional 3 relevant articles were identified in a later update of the literature search. Overall, a single dose of oxytocin was found to reduce feeding in animals. Despite several individual studies reporting that this effect persists to the end of the third week of chronic administration in rodent models, overall, this anorexigenic effect did not hold in the meta-analyses testing the effects of chronic administration. There was no overall effect of oxytocin on energy intake in humans, although a trend was identified for oxytocin to reduce the consumption of solid foods. The anorexigenic effect of oxytocin is moderated by pregnant status, dose, method of administration and diet composition.
Keywords: animals, energy intake, feeding, humans, oxytocin
1 |. INTRODUCTION
Verbalis et al.1 and Kirchgessner2 first proposed a link between oxytocin and the control of food intake almost 30 years ago, which was subsequently corroborated by the Arletti et al.3 Today, oxytocin has a well-established and well-accepted role in reducing food intake in rodents, although these effects were found to be conditional on several factors.4
The reported inhibitory effect of oxytocin on feeding has taken on new relevance in the face of the high prevalence of obesity in developed countries5 and the recognition of the psychological and functional difficulties faced by individuals with binge-type eating disorders, including bulimia nervosa and binge-eating disorder.6,7 Accordingly, it has been proposed that oxytocin may be a useful supplement to administer to counter overeating and obesity.8,9
In recent years, several narrative reviews have examined the role of oxytocin in a variety of functions related to the homeostasis of energy status, including its effects on feeding, energy expenditure, lipolysis, glucose homeostasis and macronutrient preference,9–12 as well as its potential to regulate disordered eating in humans.13 However, systematic reviews and meta-analyses in the style of the Preferred Reporting Items for Systematic Reviews and Meta-Analysis (PRISMA) guidelines are not commonly used in neurobiology and have not been previously used to estimate the size of the effect of oxytocin on feeding and its possible moderators. This systematic method offers benefits such as reproducibility, accountability of the search methods used and quantitative precision in the measurement of effect size across different samples.14 De Vries et al.15 therefore adapted similar guidelines for systematic reviews of animal intervention studies aiming to bring these same benefits in methodical rigor to animal research.
The present study aimed to use this rigorous methodology to synthesise the effects of oxytocin on feeding. We used PRISMA guidelines to identify all original published and unpublished experiments testing the effects of exogenous oxytocin on energy intake in wild-type animals and in humans, where oxytocin was administered in the absence of other active drugs or surgeries. Subsequently, we identified subsets of experimental designs conducive to synthesis by quantitative meta-analysis. We also aimed to identify relevant moderators of the effects of oxytocin on feeding to clarify the conditions under which these anorexigenic effects hold.
2 |. METHODS
2.1 |. Search strategy and eligibility criteria
Two of the researchers in the present study conducted a systematic literature search to identify original studies that had administered exogenous oxytocin to either animals or humans and compared its effects on energy intake with a placebo condition. This search was conducted in accordance with PRISMA guidelines.14 Reporting guidelines suggested by de Vries et al.15 were followed for pre-clinical intervention studies with respect to the data extraction and reporting methods described below.
The search terms included in the literature search were: “oxytocin” AND (“feed*” OR “food” OR “eat” OR “consum*” OR “intake” OR “hunger” OR “satiety” OR “appetite” OR “meal”). The eligibility criteria for studies included in the systematic review were:
Inclusion criteria:
Original experiment.
Independent variable: Administration of exogenous oxytocin compared to placebo.
Dependent variable: Quantity of food or nutritive substance consumed.
Oxytocin administered in isolation from any other drug or neural stimulation.
Article available in English.
Neurologically typical participants (eg, participants with Prader-Willi syndrome were excluded).
Exclusion criteria:
Studies testing consumption of alcohol/ethanol or methamphetamines.
Studies testing consumption of plain water, saccharin, or sodium solutions.
Studies of breastfeeding neonates.
Studies measuring the effect of oxytocin on conditioned taste aversion.
In March 2016, these terms were included in a title or topic search in the Web of Science (Core Collection) and in a text word search in PubMed. In November 2016, these same search terms were entered into a literature search using the following Ovid resources: International Pharmaceutical Abstracts, Ovid MEDLINE(R) I-Process & Other Non-Indexed Citations, Ovid MEDLINE R, PsycArticles Full Text, PsycInfo, Ovid MEDLINE(R) Epub Ahead of Print. The PubMed and Web of Science searches were then updated by the first authors in December 2016.
The first author then proceeded to screen the reference lists of relevant reviews aiming to identify articles not included in the main search results, and independently contacted authors known to have unpublished eligible studies (identified through individual correspondence, conference attendance, and reference to an unpublished data within a published paper). In March 2017, the second author repeated the literature search among the same databases. The first and second authors discussed discrepancies among the identified search results until a consensus regarding the eligibility of each article was reached. The literature search was once again updated using the same databases in July 2017. Basic study characteristics (including sample information, dose of oxytocin administration and duration of feeding measurement) and a qualitative summary of the findings of each experiment were extracted by a single author.
2.2 |. Meta-regression
Given the wide variety of studies identified by the systematic literature search, we opted to conduct five separate meta-regressions aiming to maximise the homogeneity within each analysis. The five meta-regressions were then conducted amongst each of the following sets of experiment: (i) single-dose animal studies measuring feeding over one hour after a central injection of oxytocin; (ii) single-dose animal studies measuring feeding over one hour after systemic administration of oxytocin; (iii) chronic-dosing animal studies administering central injections of oxytocin; (iv) chronic-dosing animal studies administering systemic injections of oxytocin; and (v) human studies. Effect size data (including raw means and SD or SE) and sample sizes for each study were necessary for each eligible study to be included in a meta-regression. These data were extracted by a single author directly from tables or text within the paper in cases where they were reported. For papers in which these data were not reported, the authors of the paper were contacted with a request for this information via e-mail, or via Research Gate where a current valid e-mail address was unavailable. Where these data were unavailable, the eligible paper has been described in the results of the systematic review but was omitted from the meta-regression by necessity.
Because not all of the studies identified in original systematic review were eligible for inclusion in one of these meta-regressions, the full findings of each study included in the systematic review have been reported in separate tables. The moderating and mediating factors influencing the effects of oxytocin on energy intake have been summarised in a qualitative synthesis, which follows the quantitative results reported below.
Each meta-regression was conducted as a random-effects multi-level analysis with autoregressive structure, where the second-level corresponded to a specific sample of test subjects. The results of the meta-regression are reported in terms of the standardised mean difference between placebo and oxytocin conditions. The meta-regressions were conducted using the escalc and rma.mv commands in the metafor package for R.16 The forest plots and bias plots were generated using COMPREHENSIVE META-ANALYSIS (CMA) software (https://www.meta-analysis.com). In 4 experiments within the meta-analysis for human studies, the within-subject correlation was not available. The average within-subject correlation for human studies (0.61) was therefore imputed for these experiments.
3 |. RESULTS
3.1 |. Systematic review
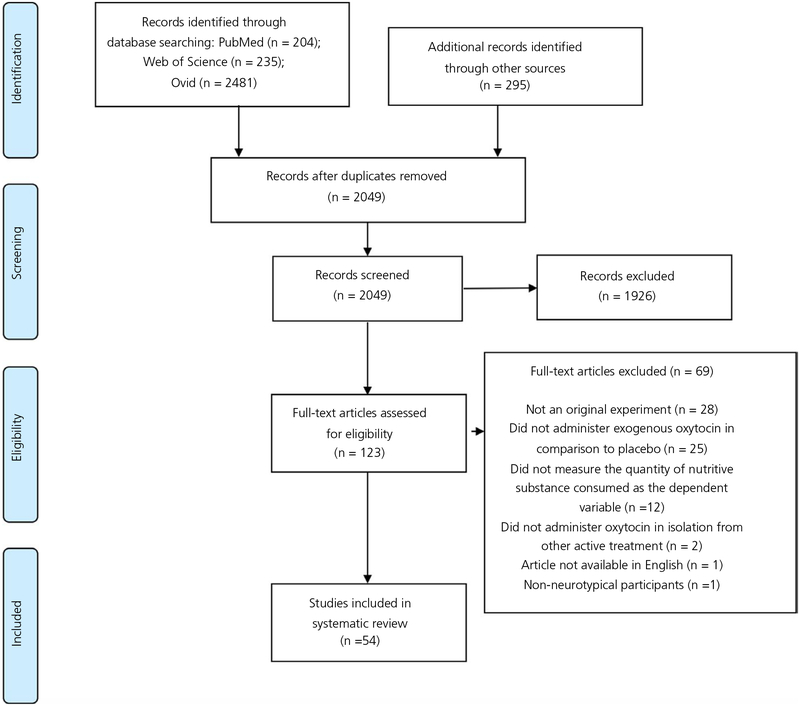
The quantity of papers identified and screened at each step of the PRISMA process during the original systematic literature search is presented in Figure 1. Two additional unique papers were identified through the updated literature search, and one additional paper was identified through author correspondence. The original and updated literature searches together identified a total of 57 relevant papers.
FIGURE 1.
Preferred Reporting Items for Systematic Reviews and Meta-Analysis (PRISMA) flow diagram for the original systematic literature search
Forty-seven papers included at least 1 experiment that measured the effects of a single dose of oxytocin on feeding. The 114 experiments measuring the effects of a single dose of oxytocin are summarised in the Supporting information (Table S1). Eighteen papers included at least 1 experiment that administered a chronic dose of oxytocin. The 56 experiments measuring the effects of chronic oxytocin dosing on feeding are summarised in the Supporting information (Table S2).
3.2 |. Meta-regression
3.2.1 |. Acute central animal studies
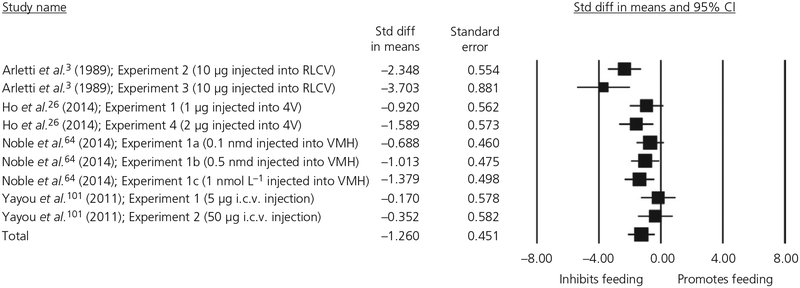
We first conducted the meta-regression for the studies that administered oxytocin centrally, entering moderators for Dose and Body Mass. Because all animals were male and housed individually, we did not include moderators for gender or socialisation. The Cook’s plot generated by this analysis revealed that 1 study by Arletti et al.3 (Experiment 2a) yielded an undue influence on the results. This study was therefore excluded from the final analysis, resulting in a total of 9 experiments with a pooled sample size of 150 observations (note that, because several studies incorporated a within-subjects design and/or repeated experiments using the same animals, the number of total observations does not equal the total number of subjects). The final analysis found a significant main effect of oxytocin, showing that a single dose of centrally-administered oxytocin reduced feeding with a large effect size (d = −1.26, SE = 0.451, P = .005, 95% confidence interval [CI] = −2.149 to −0.380). The forest plot for this meta-regression is shown in Figure 2. Neither of the included moderators was significant: Dose (estimate = −0.003, SE = 0.017, P = .857, 95% CI = −0.036 to 0.030); Body Mass (estimate = 0.000, SE = 0.000, P = .315, 95% CI = −0.000 to 0.000). There was significant heterogeneity: Q8 = 22.94, P = .003. There was significant residual heterogeneity after controlling for Dose (QE7 = 22.94, P = .002 and Body Mass QE7 = 20.49, P = .005.
FIGURE 2.
Forest plot of studies measuring the effect of a single-dose of central oxytocin on energy intake over a 1-h measurement duration in animals. RLCV, right lateral cerebral ventricle; 4V, fourth cerebral ventricle; VMH, ventromedial hypothalamus; CI, confidence interval
3.2.2 |. Acute systemic animal studies
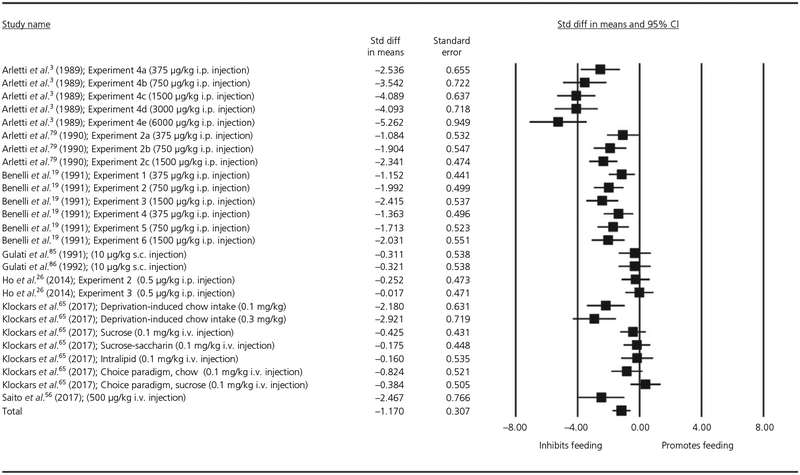
We next repeated the analysis for studies administering a single dose of systemic oxytocin including moderation analyses for Gender, Dose and Body Mass. This meta-regression included findings from 26 experiments and 510 observations. All animals were individually housed; therefore, socialisation was not entered as a moderator. Visual inspection of the Cook’s plot did not reveal an undue influence of any study (all values <0.12). This analysis for single-dose studies administering oxytocin systemically also found that oxytocin significantly reduced feeding with a large effect size (d = −1.17, SE = 0.307, P < .001, 95% CI = −1.776 to −0.574). There was significant heterogeneity in the results: Q25 = 188.38, P < .001. The moderation analyses revealed a small dose-response effect (estimate = −0.002, SE = 0.0005, P < .0001, 95% CI = −0.003 to −0.001). The forest plot for this meta-regression is shown in Figure 3. Neither of the other included moderators was significant: Gender (estimate = 0.48, SE = 1.220, P = .694, 95% CI = −1.912 to 2.872); Body Mass (estimate = 0.008, SE = 0.005, P = .091, 95% CI = −0.001 to 0.017). There was significant residual heterogeneity after controlling for each moderator: Dose (QE24 = 104.31, P < .001); Sex (QE24 = 187.81, P < .001); Body Mass (QE24 = 131.74, P < .001).
FIGURE 3.
Forest plot of studies measuring the effect of a single-dose of systemic oxytocin on energy intake over a 1-h measurement duration in animals. CI, confidence interval
3.2.3 |. Chronic central animal studies
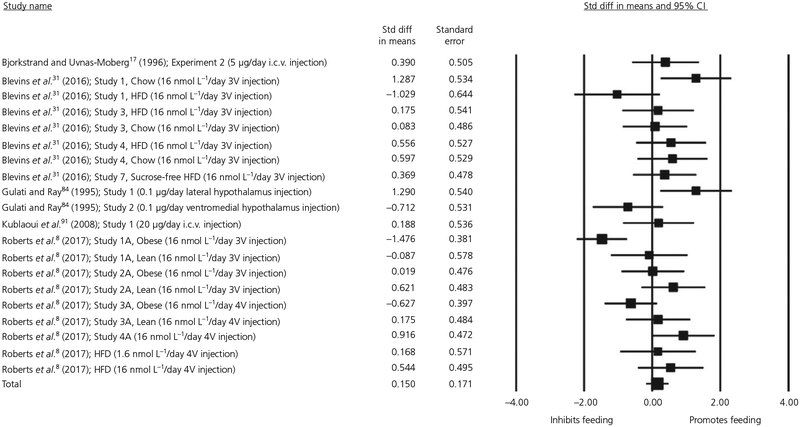
For the meta-regression of animal studies administering repeated central injections of oxytocin, we entered the following moderators: Gender, Dose, Body Mass and Duration of Oxytocin Administration. The quantity of energy intake on the final day of feeding measurement was compared between oxytocin and placebo conditions. Data were drawn from 20 experiments with a total of 349 observations. Because all animals were housed alone, socialisation was not included as a potential moderator. Visual inspection of the Cook’s plot did not reveal an undue influence of any study (all values <0.30). This analysis did not find a significant main effect of oxytocin on feeding when administered centrally in chronic infusions (d = 0.15, SE = 0.171, P = .379, 95% CI = −0.185 to 0.485). The forest plot for this meta-regression is shown in Figure 4. There was significant heterogeneity: Q19 = 43.08, P = .001. None of the included moderators were significant and there was significant residual heterogeneity after controlling for each moderator. The results of all moderation analyses are presented in Table 1.
FIGURE 4.
Forest plot of studies measuring the effect of chronic dosing of central oxytocin on energy intake in animals. 3V, third cerebral ventricle; 4V, fourth cerebral ventricle; CI, confidence interval; HFD, high-fat diet
TABLE 1.
Results of moderator analyses for chronic central animal studies
| Estimate | SE | P | Confidence interval | Residual heterogeneity | |||
|---|---|---|---|---|---|---|---|
| Lower bound | Upper bound | QE | P | ||||
| Gender | −0.16 | 0.580 | .787 | −1.293 | 0.980 | 42.80 | <.001 |
| Dose | −0.004 | 0.025 | .881 | −0.054 | 0.046 | 42.61 | <.001 |
| Body mass | −0.001 | 0.001 | .437 | −0.002 | 0.001 | 40.52 | .002 |
| Duration of administration | −0.01 | 0.022 | .494 | −0.057 | 0.028 | 41.56 | .001 |
P < .05;
P < .001.
3.2.4 |. Chronic systemic animal studies
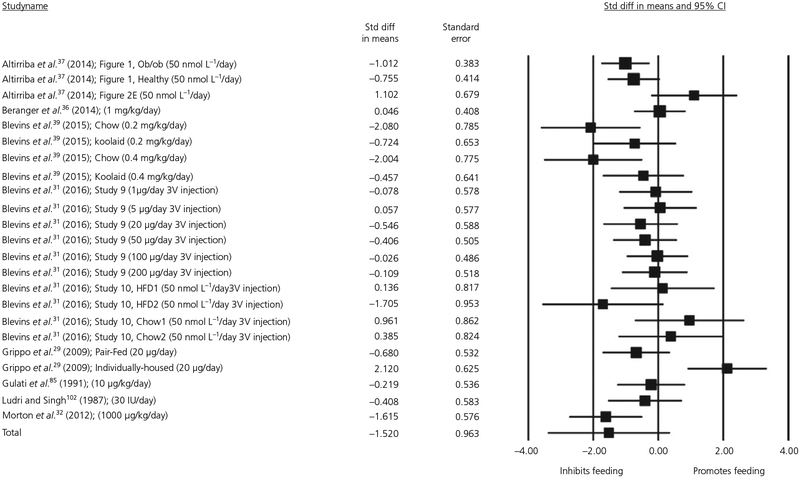
The same analysis was then repeated for animal studies that administered chronic infusions of oxytocin systemically. The quantity of energy intake on the final day of feeding measurement was compared between oxytocin and placebo conditions. Seventeen experiments with a total of 255 observations were included in this meta-regression. We entered the following moderators: Gender, Dose, Body Mass, Duration of Administration and Social Condition. Visual inspection of the Cook’s plot did not reveal an undue influence of any study (all values <0.90). Again, we did not find a significant main effect of oxytocin on feeding (d = −1.52, SE = 0.963, P = .115, 95% CI = −3.407 to 0.369). The forest plot for this meta-regression is shown in Figure 5. The moderators Gender, Dose and Duration of Administration were significant. Oxytocin had a significantly greater anorexigenic effect in males and the inhibitory effect of oxytocin on feeding decreased in magnitude over time. The results for dose indicated a reverse dose-response effect, such that greater dose was associated with less inhibition of oxytocin on feeding; however, the effect size was close to zero (estimate = 0.0002, SE = 0.0001, P = .039, 95% CI = 0.0000 to 0.0003). There was significant heterogeneity in study results (Q22 = 206.88, P < .001) and significant residual heterogeneity after controlling for each moderator. The results of all other moderator analyses are reported in Table 2.
FIGURE 5.
Forest plot of studies measuring the effect of chronic dosing of systemic oxytocin on energy intake in animals; CI, confidence interval
TABLE 2.
Results of moderator analyses for chronic systemic animal studies
| Estimate | SE | P | Confidence interval | Residual heterogeneity | |||
|---|---|---|---|---|---|---|---|
| Lower bound | Upper bound | QE | P | ||||
| Gender | −0.71* | 0.292 | .015 | −1.281 | −0.136 | 200.99 | <.001 |
| Socialisation | −0.28 | 0.275 | .306 | −0.821 | 0.258 | 205.83 | <.001 |
| Body mass | 0.000 | 0.000 | .456 | −0.000 | 0.000 | 101.78 | <.001 |
| Duration of administration | 0.02* | 0.009 | .021 | 0.003 | 0.038 | 201.53 | <.001 |
P < .05.
3.2.5 |. Human studies
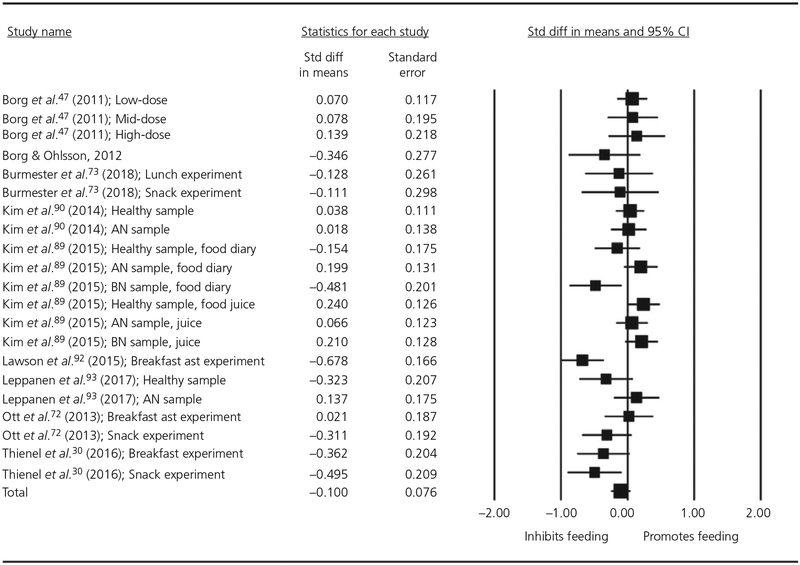
The meta-regression for human studies included 21 experiments with a total of 1020 observations. We entered the following moderators: Liquid-vs-Solid Food, Gender, Dose, Fasted-vs-Full condition, Duration of Feeding and Diagnosis. Visual inspection of the Cook’s plot did not reveal undue influence of any study (all values <0.8). This analysis did not find a significant main effect of oxytocin on feeding (estimate = −0.10, SE = 0.075, P = .194, 95% CI = −0.245 to 0.050). The forest plot for this analysis is shown in Figure 6.
FIGURE 6.
Forest plot of studies measuring the effect of a single-dose of intranasal oxytocin on energy intake in humans. CI, confidence interval; AN = anorexia nervosa; BN = bulimia nervosa.
There was significant heterogeneity between studies: Q = 55.82, d.f. = 20, P < .001. All moderators except for Dose and Diagnosis were found to be significant (Table 3). Oxytocin reduced feeding to a greater degree for solid, rather than liquid foods (eg, nutrient drinks, juice and smoothies). There was a greater inhibitory effect of oxytocin on feeding for males than females, when participants were full, rather than fasted, and when food was presented for a longer period of time (although the effect size was close to zero). There was a marginally significant effect such that oxytocin reduced feeding to a greater degree in obese participants. A greater dose of oxytocin was not associated with quantity of food consumption. There was significant residual heterogeneity after taking each moderator into account. Residual heterogeneity for each moderator is reported in Table 3.
TABLE 3.
Results of moderator analyses for all human studies
| Estimate | SE | P | Confidence interval | Residual heterogeneity | |||
|---|---|---|---|---|---|---|---|
| Lower bound | Upper bound | QE | P | ||||
| Liquid-vs-solid food | −0.30*** | 0.077 | <.001 | −0.456 | −0.153 | 40.46 | .003 |
| Gender | −0.39** | 0.134 | .004 | −0.650 | −0.123 | 47.70 | <.001 |
| Fasted or full condition | −0.14** | 0.047 | .003 | −0.230 | −0.047 | 48.57 | <.001 |
| Duration of food presentation | −0.0002** | 0.0001 | .002 | −0.0003 | −0.0001 | 50.62 | <.001 |
| Diagnosis (Obesity vs AN) | −0.570 | 0.316 | .071 | −1.189 | 0.048 | 49.08 | <.001 |
| Dose | 0.01 | 0.006 | .141 | −0.003 | 0.020 | 53.24 | <.001 |
P < .05;
P < .01;
P < .001.
AN = anorexia nervosa.
Based on the difficulty of interpreting the results given such a range of significant moderators, we then proceeded to repeat the analysis including only studies that had measured the consumption of solid foods. Because all but one of the studies in the meta-regression for solid foods included only female participants, the gender moderator was not included in this analysis.
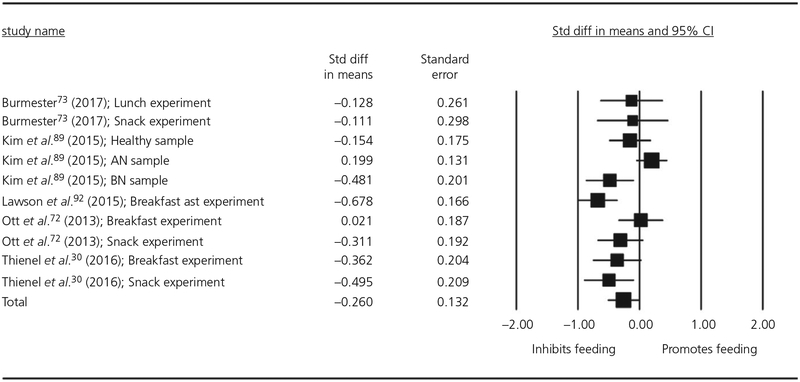
Ten experiments with a total of 486 observations were included in the meta-regression for human studies measuring consumption of solid foods. Visual inspection of the Cook’s plot did not reveal undue influence of any study (all values <0.7). The meta-regression for human studies isolated into the solid food condition found a marginally significant main effect of oxytocin on food consumption with a small effect size (d = −0.25, SE = 0.132, P = .055, 95% CI = −0.510 to 0.006). The forest plot for this meta-regression is shown in Figure 7. None of the included moderators were significant (Table 4). There was significant residual heterogeneity after controlling for Gender, Fasted or Full condition, Duration of Administration and Dose. Although the diagnosis moderator was not significant (QM3 = 3.01, P = .391), there was no longer significant residual heterogeneity after controlling for diagnosis (QE6 = 12.18, P = .058).
FIGURE 7.
Forest plot of studies measuring the effect of a single-dose of intranasal oxytocin on solid food intake in humans. CI, confidence interval
TABLE 4.
Results of moderator analyses for human studies measuring solid food intake
| Estimate | SE | P | Confidence interval | Residual heterogeneity | |||
|---|---|---|---|---|---|---|---|
| Lower bound | Upper bound | QE | P | ||||
| Gender | −0.28 | 0.264 | .300 | −0.791 | 0.241 | 19.36 | .013 |
| Fasted or full condition | −0.06 | 0.057 | .266 | −0.174 | 0.048 | 22.01 | .005 |
| Duration of administration | 0.0002 | 0.0002 | .281 | −0.0002 | 0.0006 | 19.32 | .013 |
| Dose | 0.02 | 0.017 | .297 | −0.015 | 0.050 | 19.36 | .013 |
3.2.6 |. Publication bias
The funnel plots associated with each meta-regression are shown in the Supporting information (Figures S1–S5). The funnel plots indicated that most studies had a moderate degree of precision, with a broad range of effect sizes being reported. These findings do not suggest systematic publication bias towards papers with either a strong or weak effect size.
3.3 |. Mediators and moderators of the anorexigenic effects of oxytocin
Among the studies included in the current systematic review, there were many factors that were found to moderate the effect of oxytocin on feeding in some studies, including sex of the animal, pregnancy, dose, method of administration, setting and dietary factors. Because not all studies met criteria for inclusion in one of the meta-regressions, the results reported below focus on a sample of other identified studies that examined a moderating or mediating factor in a controlled experimental design.
3.3.1 |. Sex differences
Several studies investigated sex differences with respect to the effects of oxytocin on feeding. Bjorkstrand and Uvnas-Moberg17 found that i.c.v. injection of oxytocin (5 μg) increased feeding in female but not male rats. Conversely, Zhou et al.18 found that oxytocin induced a greater reduction in feeding in female rats, with anorexigenic effects being observed using doses of oxytocin of 0.3, 1 and 3 mg kg−1, whereas only the dose of 3 mg kg−1 was effective at reducing feeding in males. To further add to these mixed findings, Benelli et al.19 failed to find any differences in male versus female rat responses to the effects of oxytocin on feeding. Previous studies have demonstrated that feeding varies across differing stages of the oestrous cycle in rodents, nonhuman primates and humans.20–22 The feeding response to hunger-related hormones (including ghrelin) and neurosteroids also varies throughout different stages of the oestrous cycle in female rats.23 It is recommended that future studies continue to investigate the extent to which oestrous cycle phase and fluctuations in ovarian hormones might influence the satiety response to oxytocin in female animal models.
3.3.2 |. Pregnancy
Only one study24 tested the effect of pregnancy as a moderator for the effect of oxytocin on feeding. This study identified that i.c.v. injection of oxytocin (1 μg) decreased feeding in virgin female rats over 1 hour of measurement, whereas it had no continued effect on feeding over 12 hours. However, a different pattern of effects was observed in pregnant rats, with the same dose of oxytocin exerting no effect on feeding over 1 hour, whereas it increased feeding over 12 hours. It was proposed that this may be a result of changes in the expression and binding affinity of central oxytocin receptors during pregnancy.24
3.3.3 |. Dose and method of administration
Most studies testing a dose-response effect of oxytocin found that greater doses of oxytocin were associated with correspondingly lower levels of subsequent feeding. The minimum effective dose required to observe anorexigenic effects of oxytocin depended on the method of administration, with central injections of oxytocin requiring much lower doses than peripheral injections.
Findings of note include those of Ong et al.,25 who reported that 1 μg μL−1 dose of oxytocin injected into the fourth ventricle reduced feeding over 30 minutes only when a dietary preload had been provided and did not continue to affect feeding over 1.5 hours. By contrast, a dose of 0.3 μg of oxytocin injected into the nucleus of the solitary tract (NTS) was found to significantly reduce feeding over 30 minutes, regardless of whether a dietary preload had been provided. This inhibitory effect on feeding persisted over 1.5 hours in the dietary preload condition. These findings appear to indicate a greater sensitivity to the effects of oxytocin on feeding within the NTS than within hindbrain receptors accessed via the fourth cerebral ventricle, suggesting that the NTS may represent a more proximal site mediating the effects of oxytocin on feeding. However, these results contradict those reported by Ho et al.,26 who found that oxytocin injection into the fourth ventricle was effective at reducing feeding in the absence of a preload at a dose of 1 μg. The differential sensitivity of the NTS and fourth ventricle to the anorexigenic effects of oxytocin therefore represents an interesting avenue for future research.
In another study examining the impact of injecting oxytocin into different regions of the central nervous system, Herisson et al.27 demonstrated that doses of oxytocin of 1 and 3 μg injected directly into the nucleus accumbens core reduced both chow intake and the consumption of a 10% sucrose solution in male rats, whereas these inhibitory effects were not observed when the same dose was injected into the nucleus accumbens shell. The administration of oxytocin into the nucleus accumbens core was associated with increased Fos-immunoreactivity in both the nucleus accumbens core itself, as well as the paraventricular nucleus and supraoptic nucleus of the hypothalamus, comprising two regions dense in oxytocin neurones and receptors that are involved in feeding regulation.28 These findings therefore support a potential role of the nucleus accumbens core in mediating the anorexigenic effects of oxytocin, which does not extend to the nucleus accumbens shell.
One point to note is that most studies administered high supraphysiological doses of oxytocin. Although these doses may be required in the case of peripheral administration to increase the chances that some oxytocin will cross the blood-brain barrier, it should be noted that these findings may reflect partial oxytocin binding with vasopressin receptors at high doses that would not occur at physiological levels.
3.3.4 |. Social setting
The social conditions in which animals were housed were also found to impact the effect of oxytocin on feeding. Grippo et al.29 reported that isolating female prairie voles from litter-mates resulted in a decrease in sucrose intake over a period of two weeks in a placebo condition, although this effect was prevented by oxytocin. In the co-housed group of prairie voles, however, there were no changes in sucrose intake over time, nor were there any differences between the oxytocin and placebo conditions. Additionally, Herisson et al.27 found that central oxytocin administration reduced food and sucrose intake in individually-housed male rats, whereas neither of these effects was observed in male rats allowed some social contact with a conspecific. Both of these studies highlight the potential for social housing to prevent oxytocinergic reductions in feeding that would otherwise occur in isolated social settings.
3.3.5 |. Dietary factors
Finally, diet-induced obesity was also identified as an important moderating factor for the effects of oxytocin on feeding in both animals and humans.8,30–34 On the whole, direct comparisons of lean animals consuming standard chow with diet-induced obese animals consuming high-fat diets found that oxytocin had more consistent inhibitory effects on feeding in the dietary-induced obese animals.8,31 Blevins et al.31 showed that this moderating effect persists in high-fat diet-fed rats, even when matched for body mass and adiposity with chow-fed controls, thus indicating that this moderating effect may be more attributable to the fat-content of the diet of an animal than to body composition.
4 |. DISCUSSION
The present review aimed to identify published and unpublished studies testing the effects of exogenous oxytocin on energy intake in wild-type animals and humans, where oxytocin was administered in isolation from other active drugs and surgery. The systematic review and meta-analysis revealed a robust inhibitory effect of oxytocin on energy intake when administered as a single dose in animals, regardless of whether it was administered via a central or peripheral route. Additionally, although several individual experiments did show a continued inhibitory effect of oxytocin for periods of two or more weeks in rats and mice,8,31,35–39 when final-day energy intake was compared between placebo and oxytocin conditions in the quantitative meta-analysis, this inhibitory effect did not hold.
The human studies did not find a main effect of oxytocin on energy intake; however, there was a trend towards a decrease in the consumption of solid foods induced by oxytocin. Additionally, oxytocin had a stronger inhibitory effect on energy intake in male participants, in obese participants, and when participants completed the experiment in the full condition (rather than fasted condition). The specific finding that oxytocin reduced feeding to a greater degree in obese humans30 is consistent with findings from animal studies that have also indicated a greater anorexigenic effect in diet-induced obese mice and rats.8
The moderating effect of liquid versus solid foods was unexpected amongst the human studies, particularly given previous animal research demonstrating the inhibitory effect of oxytocin on the consumption of palatable liquid solutions.27,40,41 The mechanism driving the difference between the effect of oxytocin on liquid and solid foods is unclear. One hypothesis is that oxytocin may reduce gastric motility, thus contributing to the sensation of satiety because solid food remains in the gut for a longer period of time. This hypothesis is supported by research demonstrating that oxytocin can reduce gastric motility in rats42–45 and mice.46 However, Borg et al.47 reported evidence suggesting that oxytocin does not affect gastric emptying rate in humans following consumption of a liquid meal. Further research would be useful to test this hypothesis as it pertains to solid foods, as well as to further investigate the reasons for the greater inhibitory effect of oxytocin for solid versus liquid consumption in humans.
The pattern for the inhibitory effect of oxytocin on feeding to decrease over time was reflected in a significant moderator analysis carried out for the meta-regression of studies that administered systemic oxytocin to animals chronically over time. This meta-regression and the meta-regression for human studies were also significantly moderated by sex, such that the effects of oxytocin on reducing feeding were significantly greater for male animals. This overall moderating effect of sex across studies included in the meta-regression is interesting to observe given the highly mixed findings regarding sex differences reported in individual studies.17–19 A greater density of oxytocin receptors has previously been reported within the spinal cord and ventromedial hypothalamus of male rats,48 which may potentially explain the greater sensitivity to the anorexigenic effects of oxytocin in males. However, the activity of oxytocin and oxytocin receptors also varies across stages of the follicular cycle in female prairie voles.49 Furthermore, levels of endogenous plasma oxytocin vary across stages of the menstrual cycle in humans.50 One can hypothesise that this natural variation may moderate the effects of exogenously-administered oxytocin. Variation in the follicular stage at which oxytocin was administered to female animals may therefore partially account for the mixed findings reported for feeding effects in previous studies. It would be useful to specifically investigate variation in the effect of oxytocin on feeding across different phases of the follicular cycle, as well as associated variation in plasma levels of other hormones (eg, oestrogen).
In terms of the mechanisms explaining the greater effect of oxytocin in obese animals and humans, oxytocin receptors exhibit a higher-affinity binding state in the presence of cholesterol.51 Therefore, it may be that a greater fat-and cholesterol-rich diet at least partially explains the greater anorexigenic effects of oxytocin observed in obese animals and humans.
The mechanisms and neural circuits explaining the overall anorexigenic effects of oxytocin are still somewhat uncertain. Oxytocin is known to mediate the anorexigenic effects of cholecystokinin (CCK), which acts on oxytocin neurones via vagal afferents from the gut.1 This finding has received further support from research demonstrating that injections of oxytocin into the third cerebral ventricle enhance the anorexigenic effects of low doses of CCK-8,31 whereas, conversely, pre-treatment of an oxytocin receptor antagonist into the fourth ventricle suppresses the anorexigenic effects of CCK.52,53 In addition to this role mediating the effects of CCK, oxytocin has also been implicated in mediating the inhibitory effects of leptin54,55 and nesfatin-156 on food intake. The downstream mechanisms by which oxytocin impacts on feeding, however, are less certain.
It is likely that central and peripheral oxytocin exert effects of feeding via different mechanisms. Previous research has identified that only approximately 0.002% of peripheral oxytocin crosses the blood-brain barrier, where it might access central receptors.57 However, the extent to which peripheral oxytocin exerts its effects via central versus peripheral receptors, such as those in the gut,58 may be species-dependent. In mice, the literature supports a role for vagal afferent nerves in mediating the anorexigenic effect of peripheral oxytocin.59,60 This interpretation draws support from research showing that oxytocin receptors are expressed in the no-dose ganglion of the vagus nerve,59 as well as further research that has gone on to demonstrate that vagotomy results in an attenuation of the anorexigenic effect of peripherally-administered oxytocin in mice.60 In rats, however, Ho et al.26 showed that hindbrain receptors accessed via the fourth ventricle are predominantly responsible for mediating the inhibitory effects of peripheral oxytocin on feeding. Further research clarifying which pathway(s) predominate this mediating effect in primates and humans would be useful.
Brain nuclei including the paraventricular nucleus, NTS and arcuate nucleus were implicated as being potentially relevant in mediating the effects of oxytocin on feeding.25,60–63 This evidence originates from studies indicating that direct injection of oxytocin into these areas suppresses food intake,25,61 as well as from immunohistological studies demonstrating that Fos activation of oxytocin neurones in these regions co-occurs with the termination of feeding.60–63 Furthermore, studies have shown that injection of oxytocin directly into the ventral tegmental area, nucleus accumbens core and ventromedial hypothalamus is effective with respect to inhibiting feeding, thereby indicating that these regions may mediate the anorexigenic effects of oxytocin as well.27,41,64 Given that oxytocin is effective in reducing energy intake when administered both centrally and peripherally, it may be the case that oxytocin acts as a central messenger integrating central and peripheral signals. This hypothesis, however, requires further evidence for corroboration.
It has also been proposed that oxytocin may exert inhibitory effects on energy intake via a physiological pathway mediated by reward-based mechanisms.65 This hypothesis is supported by the high density of oxytocin receptors along the pathways connecting the nucleus accumbens and ventral tegmental area,66–68 which are two regions known to be highly involved with processing food rewards.69 Additionally, oxytocin injected directly into the nucleus accumbens has been found to reduce methamphetamine-induced place preference70 and to prevent relapse to methamphetamine-seeking behavior after extinction.71 Taken together, these findings highlight the ability for oxytocin to disrupt reward-related processing in these regions, which may additionally extend to suppressing reward-based feeding behaviour. This hypothesis, if true, may also explain the stronger effects of oxytocin in reducing hedonic, as opposed to hunger-driven, feeding in human studies.30,72,73 Further research in animals and humans would be useful to test this hypothesis and clarify the precise mechanisms of the acute action of oxytocin on energy intake.
The confirmation of the anorexigenic effects of oxytocin when administered as a single dose echoes conventional understanding, at the same time as highlighting the limits of this effect, such as the reverse (orexigenic) effect observed in pregnancy24 and the observations reported when socially-housed animals subsequently undergo separation from litter-mates.29 The null findings revealed in the meta-analysis testing the chronic effects of oxytocin on feeding are disappointing in the context of potential hopes for developing oxytocin supplementation as a new treatment for binge-type eating disorders in humans, and conflict with individual studies that have reported the persistence of the anorexigenic effects of oxytocin over two to three weeks of measurement in rats and mice,8,31,36,38,74,75 as well as for two weeks post-treatment in rhesus monkeys.39 It may be the case that a regime of intermittent oxytocin administration would result in the same anorexigenic effects observed within the course of a single experimental administration, without resulting in the same degree of receptor adaptations. Future research testing different temporal regimes of oxytocin administration is recommended to test this hypothesis.
The diminishing effects of oxytocin are in keeping with the results arising from individual studies making use of the repeated administration of oxytocin8,31,33,36,37 or an oxytocin agonist.76 These findings are also in accordance with social experiments showing that the anxiolytic effects of oxytocin disappear or reverse over time.77 Peters et al.77 found that the reversal of acute anxiolytic effects over chronic dosing was associated with a concurrent reduction in oxytocin receptor binding. Indeed, previous work has shown that oxytocin receptor binding can be reduced by as much as 50% over ten days of chronic administration, driven largely by down-regulation of the oxytocin receptor.78 It is therefore likely that this reduced binding potential may explain the dampening of the effects of oxytocin on feeding.
The null findings generated from the meta-regressions of chronically-administered oxytocin studies should be interpreted with some degree of caution. Although the final-day analyses used for the meta-regressions maximised the number of commensurable studies eligible for inclusion, it may be that noise in the data on the final day data masked smaller effects identified by studies that compared average consumption across several days. It should also be noted that the scope of the present review is limited to the effects of oxytocin on energy intake alone, and that the effects of oxytocin on other metabolic parameters deterring obesity (eg, lipolysis, brown adipose tissue thermogenesis and energy expenditure) may persist with chronic administration.74
The limitations of the present review include some inherent drawbacks of the methodology chosen for the meta-regressions. We aimed to reach a compromise between maximising the homogeneity of studies included in each meta-regression, at the same time as also including the maximum number of experiments. The choice to include only single-dose studies that measured the effects of oxytocin over one hour of feeding therefore constrained these meta-regressions to a reasonable scope and similar effect size. Differences in the exact location of administration and animals included in each experiment, however, may have added to the heterogeneity of effect size observed across studies. Furthermore, the present systematic review did not include findings from non-wild-type animals (eg, Sim-haploinsufficient rats and mice) or animals whose nervous systems were altered by surgery or direct stimulation. Therefore, although the present findings reveal the effects of exogenous oxytocin in wild-type animals, it should be noted that there are further findings reflecting the implication of oxytocin on feeding that were not included within the scope of the present review.
Regarding the clinical implications of these findings, it is particularly encouraging to have observed that the anorexigenic effects of oxytocin were stronger for populations that suffer from over-eating and binge-eating. These findings suggest that, in the short term, oxytocin may reduce the likelihood of binge-eating and overeating for populations with obesity, bulimia nervosa and binge-eating disorders. However, the null findings from the meta-regressions of chronic animal studies cast doubt on the persistence of the acute effects of oxytocin. Testing different dosing schedules of oxytocin would be useful for identifying a potential frequency and dose of administration that maintains the beneficial effects of oxytocin over time, without resulting in a reduction of receptor binding.
In conclusion, the present systematic review has confirmed the anorexigenic effect of a single dose of oxytocin in animals first documented by the Arletti laboratory,3 at the same time as demonstrating that this anorexigenic effect does not persist throughout chronic dosing. There was a trend for intranasal oxytocin to reduce feeding in humans, and this effect was stronger for individuals with obesity, bulimia nervosa, and binge-eating disorders. Future research is needed to further clarify the mechanisms responsible for these effects, and whether different dosing schedules might prevent their attenuation with chronic administration.
Supplementary Material
ACKNOWLEDGEMENTS
The authors declare that they have no conflicts of interest. ML was supported by grants from the Swiss Fund for Anorexia Nervosa, the Guy’s and St Thomas’ NHS Foundation Trust, and a King’s Health Partners Challenge Fund. YP was supported by an Economic and Social Research Council fellowship (grant number ES/K009400/1). JT was supported by the National Institute for Health Research (NIHR) Mental Health Biomedical Research Centre at South London and Maudsley NHS Foundation Trust and King’s College London. JB serves on the scientific advisory board and is a consultant for OXT Therapeutics, Inc.
Funding information
The Swiss Fund for Anorexia Nervosa; The Economic and Social Research Council, Grant/Award Number: ES/K009400/1; The Guy’s and St Thomas’ NHS Foundation Trust; The National Institute for Health Research (NIHR) Mental Health Biomedical Research Centre; The King’s Health Partners Challenge Fund; This material was based upon work supported by the Office of Research and Development, Medical Research Service, Department of Veterans Affairs (VA). JB was supported by the VA Merit Review Awards 1l01BX001213–01A1 and BX004102–01, NIH R01DK115976, and the United States (U.S.) Department of Veterans Affairs Biomedical Laboratory Research and Development Service. JB serves on the scientific advisory board and consultant for OXT Therapeutics, Inc.
Footnotes
SUPPORTING INFORMATION
Additional Supporting Information may be found online in the supporting information tab for this article.
REFERENCES
- 1.Verbalis JG, McCann MJ, McHale CM, Stricker EM. Oxytocin secretion in response to cholecystokinin and food: differentiation of nausea from satiety. Science. 1986;232:1417–1419. [DOI] [PubMed] [Google Scholar]
- 2.Kirchgessner AL, Sclafani A, Nilaver G. Histochemical identification of a PVN-hindbrain feeding pathway. Physiol Behav. 1988;42:529–543. [DOI] [PubMed] [Google Scholar]
- 3.Arletti R, Benelli A, Bertolini A. Influence of oxytocin on feeding behavior in the rat. Peptides. 1989;10:89–93. [DOI] [PubMed] [Google Scholar]
- 4.Olszewski P, Klockars A, Levine A. Oxytocin: a conditional anorexigen whose effects on appetite depend on the physiological, behavioural and social contexts. J Neuroendocrinol. 2016;28 https://doi.org/10.1111/jne.12376 [DOI] [PubMed] [Google Scholar]
- 5.Arroyo-Johnson C, Mincey KD. Obesity epidemiology worldwide. Gastroenterol Clin North Am. 2016;45:571–579. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.American Psychiatric Association. Diagnostic and Statistical Manual of Mental Disorders. Arlington, TX: American Psychiatric Publishing; 2013. [Google Scholar]
- 7.Pawaskar M, Witt EA, Supina D, Herman BK, Wadden TA. Impact of binge eating disorder on functional impairment and work productivity in an adult community sample in the United States. Int J Clin Pract. 2017;71:e12970. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Roberts ZS, Wolden-Hanson T, Matsen ME, et al. Chronic hind-brain administration of oxytocin is sufficient to elicit weight loss in diet-induced obese rats. Am J Physiol Regul Integr Comp Physiol. 2017;313:R357–R371. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Spetter MS, Hallschmid M. Current findings on the role of oxytocin in the regulation of food intake. Physiol Behav. 2017;176:31–39. [DOI] [PubMed] [Google Scholar]
- 10.Lawson EA. The effects of oxytocin on eating behaviour and metabolism in humans. Nat Rev Endocrinol. 2017;13:700–709. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Leng G, Sabatier N. Oxytocin – the sweet hormone? Trends Endocrinol Metab. 2017;28:365–376. [DOI] [PubMed] [Google Scholar]
- 12.Sabatier N, Leng G, Menzies J. Oxytocin, feeding, and satiety. Front Endocrinol (Lausanne). 2013;4:35. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Giel K, Zipfel S, Hallschmid M. Oxytocin and eating disorders: a narrative review on emerging findings and perspectives. Curr Neuropharmacol. 2017; DOI: 10.2174/1570159X15666171128143158. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Moher D, Liberati A, Tetzlaff J, Altman DG; PRISMA Group. Preferred reporting items for systematic reviews and meta-analyses: the PRISMA statement. Ann Intern Med. 2009;151:264–269. [DOI] [PubMed] [Google Scholar]
- 15.Vries R, Hooijmans CR, Langendam MW, et al. A protocol format for the preparation, registration and publication of systematic reviews of animal intervention studies. Evid Based Preclin Med. 2015;2:1–9. [Google Scholar]
- 16.Viechtbauer W Conducting meta-analyses in R with the metafor package. J Stat Softw. 2010;36:1–48. [Google Scholar]
- 17.Bjorkstrand E, Uvnas-Moberg K. Central oxytocin increases food intake and daily weight gain in rats. Physiol Behav. 1996;59:947–952. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Zhou L, Ghee SM, See RE, Reichel CM. Oxytocin differentially affects sucrose taking and seeking in male and female rats. Behav Brain Res. 2015;283:184–190. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Benelli A, Bertolini A, Arletti R. Oxytocin-induced inhibition of feeding and drinking: no sexual dimorphism in rats. Neuropeptides. 1991;20:57–62. [DOI] [PubMed] [Google Scholar]
- 20.Dye L, Blundell J. Menstrual cycle and appetite control: implications for weight regulation. Hum Reprod. 1997;12:1142–1151. [DOI] [PubMed] [Google Scholar]
- 21.Reddy D, Kulkarni S. Sex and estrous cycle-dependent changes in neurosteroid and benzodiazepine effects on food consumption and plus-maze learning behaviors in rats. Pharmacol Biochem Behav. 1999;62:53–60. [DOI] [PubMed] [Google Scholar]
- 22.Buffenstein R, Poppitt SD, McDevitt RM, Prentice AM. Food in-take and the menstrual cycle: a retrospective analysis, with implications for appetite research. Physiol Behav. 1995;58:1067–1077. [DOI] [PubMed] [Google Scholar]
- 23.Sakurazawa N, Mano-Otagiri A, Nemoto T, Shibasaki T. Effects of intracerebroventricular ghrelin on food intake and Fos expression in the arcuate nucleus of the hypothalamus in female rats vary with estrous cycle phase. Neurosci Lett. 2013;541:204–208. [DOI] [PubMed] [Google Scholar]
- 24.Douglas AJ, Johnstone LE, Leng G. Neuroendocrine mechanisms of change in food intake during pregnancy: a potential role for brain oxytocin. Physiol Behav. 2007;91:352–365. [DOI] [PubMed] [Google Scholar]
- 25.Ong ZY, Alhadeff AL, Grill HJ. Medial nucleus tractus solitarius oxytocin receptor signaling and food intake control: the role of gastrointestinal satiation signal processing. Am J Physiol Regul Integr Comp Physiol. 2015;308:R800–R806. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Ho JM, Anekonda VT, Thompson BW, et al. Hindbrain oxytocin receptors contribute to the effects of circulating oxytocin on food intake in male rats. Endocrinology. 2014;155:2845–2857. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Herisson F, Waas JR, Fredriksson R, Schiöth HB, Levine AS, Olszewski PK. Oxytocin acting in the nucleus accumbens core decreases food intake. J Neuroendocrinol. 2016;28.https://doi.org/10.1111/jne.12381 [DOI] [PubMed] [Google Scholar]
- 28.Johnstone LE, Fong TM, Leng G. Neuronal activation in the hypothalamus and brainstem during feeding in rats. Cell Metab. 2006;4:313–321. [DOI] [PubMed] [Google Scholar]
- 29.Grippo AJ, Trahanas DM, Zimmerman RR 2nd, Porges SW, Carter CS. Oxytocin protects against negative behavioral and autonomic consequences of long-term social isolation. Psychoneuroendocrinology. 2009;34:1542–1553. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Thienel M, Fritsche A, Heinrichs M, et al. Oxytocin’s inhibitory effect on food intake is stronger in obese than normal-weight men. Int J Obes. 2016;40:1707–1714. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Blevins JE, Thompson BW, Anekonda VT, et al. Chronic CNS oxytocin signaling preferentially induces fat loss in high-fat diet-fed rats by enhancing satiety responses and increasing lipid utilization. Am J Physiol Regul Integr Comp Physiol. 2016;310:R640–R658. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Morton GJ, Thatcher BS, Reidelberger RD, et al. Peripheral oxytocin suppresses food intake and causes weight loss in diet-induced obese rats. Am J Physiol Endocrinol Metab. 2012;302:E134–E144. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Maejima Y, Iwasaki Y, Yamahara Y, Kodaira M, Sedbazar U, Yada T. Peripheral oxytocin treatment ameliorates obesity by reducing food intake and visceral fat mass. Aging (Albany NY). 2011;3:1169–1177. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Deblon N, Veyrat-Durebex C, Bourgoin L, et al. Mechanisms of the anti-obesity effects of oxytocin in diet-induced obese rats. PLoS ONE. 2011;6:e25565. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Zhang G, Cai D. Circadian intervention of obesity development via resting-stage feeding manipulation or oxytocin treatment. Am J Physiol Endocrinol Metab. 2011;301:E1004–E1012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Beranger GE, Pisani DF, Castel J, et al. Oxytocin reverses ovariectomy-induced osteopenia and body fat gain. Endocrinology. 2014;155:1340–1352. [DOI] [PubMed] [Google Scholar]
- 37.Altirriba J, Poher AL, Caillon A, et al. Divergent effects of oxytocin treatment of obese diabetic mice on adiposity and diabetes. Endocrinology. 2014;155:4189–4201. [DOI] [PubMed] [Google Scholar]
- 38.Balazova L, Krskova K, Suski M, et al. Metabolic effects of sub-chronic peripheral oxytocin administration in lean and obese zucker rats. J Physiol Pharmacol. 2016;67:531–541. [PubMed] [Google Scholar]
- 39.Blevins JE, Graham JL, Morton GJ, et al. Chronic oxytocin administration inhibits food intake, increases energy expenditure, and produces weight loss in fructose-fed obese rhesus monkeys. Am J Physiol Regul Integr Comp Physiol. 2015;308:R431–R438. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Lokrantz C-M, Uvnas-Moberg K, Kaplan JM. Effects of central oxytocin administration on intraoral intake of glucose in deprived and nondeprived rats. Physiol Behav. 1997;62:347–352. [DOI] [PubMed] [Google Scholar]
- 41.Mullis K, Kay K, Williams DL. Oxytocin action in the ventral teg-mental area affects sucrose intake. Brain Res. 2013;1513:85–91. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Wu CL, Hung CR, Chang FY, Pau KY, Wang JL, Wang PS. Involvement of cholecystokinin receptor in the inhibition of gastric emptying by oxytocin in male rats. Pflugers Arch. 2002;445:187–193. [DOI] [PubMed] [Google Scholar]
- 43.Wu CL, Hung CR, Chang FY, Pau KY, Wang PS. Pharmacological effects of oxytocin on gastric emptying and intestinal transit of a non-nutritive liquid meal in female rats. Naunyn Schmiedebergs Arch Pharmacol. 2003;367:406–413. [DOI] [PubMed] [Google Scholar]
- 44.Flanagan LM, Olson BR, Sved AF, Verbalis JG, Stricker EM. Gastric motility in consious rats given oxytocin and an oxytocin antagonist centrally. Brain Res. 1992;578:256–260. [DOI] [PubMed] [Google Scholar]
- 45.Rogers RC, Hermann GE. Oxytocin, oxytocin antagonist, TRH, and hypothalamic paraventricular nucleus stimulation effects on gastric motility. Peptides. 1987;8:505–513. [DOI] [PubMed] [Google Scholar]
- 46.Welch MG, Margolis KG, Li Z, Gershon MD. Oxytocin regulates gastrointestinal motility, inflammation, macromolecular permeability, and mucosal maintenance in mice. Am J Physiol Gastrointest Liver Physiol. 2014;307:G848–G862. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Borg J, Simren M, Ohlsson B. Oxytocin reduces satiety scores without affecting the volume of nutrient intake or gastric emptying rate in healthy subjects. Neurogastroenterol Motil. 2011;23:56-61, e5. [DOI] [PubMed] [Google Scholar]
- 48.Uhl-Bronner S, Waltisperger E, Martínez-Lorenzana G, Condes Lara M, Freund-Mercier MJ. Sexually dimorphic expression of oxytocin binding sites in forebrain and spinal cord of the rat. Neuroscience. 2005;135:147–154. [DOI] [PubMed] [Google Scholar]
- 49.Witt D, Carter C, Lnsel T. Oxytocin receptor binding in female prairie voles: endogenous and exogenous oestradiol stimulation. J Neuroendocrinol. 1991;3:155–161. [DOI] [PubMed] [Google Scholar]
- 50.Salonia A, Nappi RE, Pontillo M, et al. Menstrual cycle-related changes in plasma oxytocin are relevant to normal sexual function in healthy women. Horm Behav. 2005;47:164–169. [DOI] [PubMed] [Google Scholar]
- 51.Gimpl G Interaction of G protein coupled receptors and cholesterol. Chem Phys Lipid. 2016;199:61–73. [DOI] [PubMed] [Google Scholar]
- 52.Olson BR, Drutarosky MD, Stricker EM, Verbalis JG. Brain oxytocin receptor antagonism blunts the effects of anorexigenic treatments in rats: evidence for central oxytocin inhibition of food intake. Endocrinology. 1991;129:785–791. [DOI] [PubMed] [Google Scholar]
- 53.Blevins JE, Eakin TJ, Murphy JA, Schwartz MW, Baskin DG. Oxytocin innervation of caudal brainstem nuclei activated by cholecystokinin. Brain Res. 2003;993:30–41. [DOI] [PubMed] [Google Scholar]
- 54.Blevins JE, Schwartz MW, Baskin DG. Evidence that paraventricular nucleus oxytocin neurons link hypothalamic leptin action to caudal brain stem nuclei controlling meal size. Am J Physiol Regul Integr Comp Physiol. 2004;287:R87–R96. [DOI] [PubMed] [Google Scholar]
- 55.Wu Z, Xu Y, Zhu Y, et al. An obligate role of oxytocin neurons in diet induced energy expenditure. PLoS ONE. 2012;7:e45167. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Saito R, Sonoda S, Ueno H, et al. Involvement of central nesfatin-1 neurons on oxytocin-induced feeding suppression in rats. Neurosci Lett. 2017;655:54–60. [DOI] [PubMed] [Google Scholar]
- 57.Mens WB, Witter A, Greidanus TBVW. Penetration of neurohypophyseal hormones from plasma into cerebrospinal fluid (CSF): half-times of disappearance of these neuropeptides from CSF. Brain Res. 1983;262:143–149. [DOI] [PubMed] [Google Scholar]
- 58.Ohlsson B, Truedsson M, Djerf P, Sundler F. Oxytocin is expressed throughout the human gastrointestinal tract. Regul Pept. 2006;135:7–11. [DOI] [PubMed] [Google Scholar]
- 59.Welch MG, Tamir H, Gross KJ, Chen J, Anwar M, Gershon MD. Expression and developmental regulation of oxytocin (OT) and oxytocin receptors (OTR) in the enteric nervous system (ENS) and intestinal epithelium. J Comp Neurol. 2009;512:256–270. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Iwasaki Y, Maejima Y, Suyama S, et al. Peripheral oxytocin activates vagal afferent neurons to suppress feeding in normal and leptin-resistant mice: a route for ameliorating hyperphagia and obesity. Am J Physiol Regul Integr Comp Physiol. 2015;308:R360–R369. [DOI] [PubMed] [Google Scholar]
- 61.Maejima Y, Sakuma K, Santoso P, et al. Oxytocinergic circuit from paraventricular and supraoptic nuclei to arcuate POMC neurons in hypothalamus. FEBS Lett. 2014;588:4404–4412. [DOI] [PubMed] [Google Scholar]
- 62.Olszewski PK, Klockars A, Olszewska AM, Fredriksson R, Schiöth HB, Levine AS. Molecular, immunohistochemical, and pharmacological evidence of oxytocin’s role as inhibitor of carbohydrate but not fat intake. Endocrinology. 2010;151:4736–4744. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Fenselau H, Campbell JN, Verstegen AMJ, et al. A rapidly acting glutamatergic ARC->PVH satiety circuit postsynaptically regulated by alpha-MSH. Nat Neurosci. 2016;21:21. [DOI] [PubMed] [Google Scholar]
- 64.Noble EE, Billington CJ, Kotz CM, Wang C. Oxytocin in the ventromedial hypothalamic nucleus reduces feeding and acutely increases energy expenditure. Am J Physiol Regul Integr Comp Physiol. 2014;307:R737–R745. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Klockars A, Brunton C, Li L, Levine AS, Olszewski PK. Intravenous administration of oxytocin in rats acutely decreases deprivation-induced chow intake, but it fails to affect consumption of palatable solutions. Peptides. 2017;93:13–19. [DOI] [PubMed] [Google Scholar]
- 66.Shahrokh DK, Zhang TY, Diorio J, Gratton A, Meaney MJ. Oxytocin-dopamine interactions mediate variations in maternal behavior in the rat. Endocrinology. 2010;151:2276–2286. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Mitre M, Marlin BJ, Schiavo JK, et al. A distributed network for social cognition enriched for oxytocin receptors. J Neurosci. 2016;36:2517–2535. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Peris J, MacFadyen K, Smith JA, de Kloet AD, Wang L, Krause EG. Oxytocin receptors are expressed on dopamine and glutamate neurons in the mouse ventral tegmental area that project to nucleus accumbens and other mesolimbic targets. J Comp Neurol. 2017;525:1094–1108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Berridge KC. ‘Liking’and ‘wanting’food rewards: brain substrates and roles in eating disorders. Physiol Behav. 2009;97:537–550. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Baracz SJ, Rourke PI, Pardey MC, Hunt GE, McGregor IS, Cornish JL. Oxytocin directly administered into the nucleus accumbens core or subthalamic nucleus attenuates methamphetamine-induced conditioned place preference. Behav Brain Res. 2012;228:185–193. [DOI] [PubMed] [Google Scholar]
- 71.Baracz SJ, Everett NA, McGregor IS, Cornish JL. Oxytocin in the nucleus accumbens core reduces reinstatement of methamphetamine-seeking behaviour in rats. Addict Biol. 2016;21:316–325. [DOI] [PubMed] [Google Scholar]
- 72.Ott V, Finlayson G, Lehnert H, et al. Oxytocin reduces reward-driven food intake in humans. Diabetes. 2013;62:3418–3425. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Burmester V Intranasal oxytocin reduces hedonic eating in satiated males. In: British Feeding and Drinking Group Annual Conference Wokefield Park, Berkshire; 2017. [Google Scholar]
- 74.Blevins JE, Baskin DG. Translational and therapeutic potential of oxytocin as an anti-obesity strategy: insights from rodents, nonhuman primates and humans. Physiol Behav. 2015;152:438–449. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.Zhang G, Bai H, Zhang H, et al. Neuropeptide exocytosis involving synaptotagmin-4 and oxytocin in hypothalamic programming of body weight and energy balance. Neuron. 2011;69:523–535. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76.Olson BR, Drutarosky MD, Chow MS, Hruby VJ, Stricker EM, Verbalis JG. Oxytocin and an oxytocin agonist administered centrally decrease food intake in rats. Peptides. 1991;12:113–118. [DOI] [PubMed] [Google Scholar]
- 77.Peters S, Slattery DA, Uschold-Schmidt N, Reber SO, Neumann ID. Dose-dependent effects of chronic central infusion of oxytocin on anxiety, oxytocin receptor binding and stress-related parameters in mice. Psychoneuroendocrinology. 2014;42:225–236. [DOI] [PubMed] [Google Scholar]
- 78.Insel TR, Winslow JT, Witt DM. Homologous regulation of brain oxytocin receptors. Endocrinology. 1992;130:2602–2608. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.