Abstract
Adolescent stress can impact health and well-being not only during adulthood of the exposed individual but even in future generations. To investigate the molecular mechanisms underlying these long-term effects, we exposed adolescent males to stress and measured anxiety behaviors and gene expression in the amygdala – a critical region in the control of emotional states - in their progeny for two generations, offspring and grandoffspring. Male C57BL/6 mice underwent chronic unpredictable stress (CUS) for two-weeks during adolescence and were used to produce two generations of offspring. Male and female offspring and grandoffspring were tested in behavioral assays to measure affective behavior and stress reactivity. Remarkably, transgenerational inheritance of paternal stress exposure produced a protective phenotype in the male, but not the female lineage. RNA-Seq analysis of the amygdala from male offspring and grandoffspring identified differentially expressed genes in mice derived from fathers exposed to CUS. The differentially expressed genes clustered into numerous pathways, and the ‘notch signaling’ pathway was the most significantly altered in male grandoffspring. Therefore, we show that paternal stress exposure impacts future generations which manifest in behavioral changes and molecular adaptations.
Introduction
Stress exposure directly impacts the exposed individual, but remarkably can even affect the health of subsequent generations of offspring (for review of current literature see [1, 2] Adolescent stress can predispose individuals to increased anxiety and depression in adulthood, and furthermore, traumatic events experienced by parents can impact offspring behavior and physiology [3]. Rodent studies have recapitulated some clinical findings, showing inheritance of stress exposure in first and second generation offspring [4–9].
In humans, parental exposure to stressors that produce long-term changes in individual behavior, including the development of neuropsychiatric disorders, is a risk factor for altered physiology and behavior in offspring [10]. The children of undernourished mothers have an increased incidence of coronary heart disease [11], glucose tolerance, and body mass index [12] later in life. These data support the “developmental origins of health and disease” or DOHaD hypothesis [13]. Paternal transmission of exposure to the Dutch famine has been associated with the incidence of metabolic and psychiatric disorders in offspring (5), and there are additional reports of other traumatic events experienced by parents that contribute to offspring risk for psychiatric disorders [14].
The amygdala is a key brain region in mediating stress response that undergoes significant remodeling during adolescence, rendering it susceptible to early-life stress exposure [15]. Other labs have conducted transcriptomic analysis in the offspring of stressed fathers from other brain regions, including the paraventricular nucleus and hippocampus [8, 16, 17]. While the intergenerational and transgenerational effects of parental stress exposure on offspring anxiety, depression, and response to stress have been characterized [4, 8, 9, 18], a direct link between the transgenerational effects of stress on affective behaviors and the transcriptional changes of the amygdala has not been reported. We conducted a chronic unpredictable stress paradigm in mice and evaluated the transgenerational effects of stress exposure by examining the transcriptome and behavioral consequences on subsequent generations. We found an association between altered amygdala transcriptomes and affective behavior in male grandoffspring.
Methods and Materials
Animals
Male C57BL/6NTac mice (6–8 weeks of age, 20–30 g) obtained from Taconic Farms (Hudson, NY) were group housed in open cages with a filter top, maintained on a 12-h light/dark cycle, with the light cycle from 6am to 6pm. Food and water were available ad libitum in accordance with the University of Pennsylvania Animal Care and Use Committee. Mice were bred for two generations to generate the F0 stress-exposed mice used in the current study, all mice were weaned at 4 weeks of age. In-house breeding decreased the impact of prior housing and transportation stress on the mice and allowed us to isolate the effects of CUS in our facility on several generations of offspring.
Adolescent chronic unpredictable stress (CUS) exposure
Male mice underwent chronic unpredictable stress (CUS) during adolescence for 12 days, starting at post-natal day 28 (PND28). Mice were randomly assigned to stress or no stress groups with all mice in a given cage assigned to the same treatment group. The CUS paradigm was adapted from previous studies that induced anhedonia immediately following exposure [19] and has been used successfully in our laboratory [20] (for details see Supplementary Table S1). Briefly, animals were exposed to three stressors a day for 12 consecutive days and returned to the animal colony between stressors and after the final stressor.
Mating and offspring generation
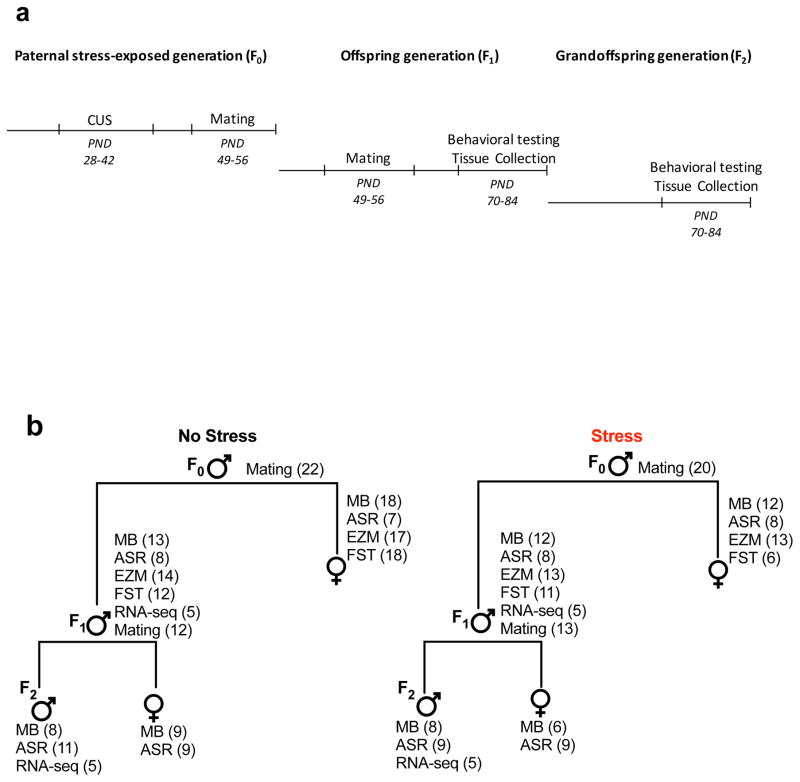
One week following CUS exposure male mice (PND49) were placed with age matched naive females for 7 days, which allowed for elimination of any sperm that may have developed prior to CUS exposure. Male offspring (PND56) were then mated with non-stressed females and removed when a vaginal plug was found. No-stress control males underwent the same mating protocol in each generation. Figure 1a summarizes our experimental timeline, and Figure 1b lists the mice used for behavior, mating, and RNA-sequencing.
Figure 1. Study design and timeline.
Schematic representation of the experimental design. C57/B6 male mice were exposed to adolescent stress (F0) and then used for mating to generate the offspring. Both male and female offspring were tested for affective behaviors, and only males were used tissue collection for RNA-sequencing and mating to generate the grandoffspring (a). Diagram representation of the mice used for behavioral testing, mating, or RNA-sequencing in the stress-exposed mice, offspring, and grandoffspring (b). All mice were used only for one behavioral test, and mice used for mating or for RNA-sequencing were not evaluated in behavioral tests. Figure 1a describes the exact number of mice that were used in each behavioral test.
Behavioral testing
All experimental testing sessions were conducted during the light cycle between 8:00 a.m. and 5:00 p.m. Behavioral testing was conducted in male and female offspring and grandoffspring between PND70-84. Mice were only used for one behavioral test, and mice used for mating were not evaluated in behavioral tests. Offspring males and females were tested in MB, ASR, EZM, FST tests. Grandoffspring males and females were tested in the MB and ASR tests. Figure 1a describes the exact number of mice that were used in each behavioral test.
Marble Burying (MB)
The marble burring (MB) test was performed as described [21]. After a 1-hour acclimation period to the testing room (lighting=40 lux), mice were placed individually in a test cage that resembled their home cage (26 × 20 × 14 cm). Twenty marbles were distributed evenly in the cages in 5 rows of 4 on top of mouse bedding (5 cm in depth) and a clear lid placed on the cage. Animals were left undisturbed for 15 minutes, after which the number of marbles buried, distinguished by being 75% or more submerged under bedding, was quantified by a trained observer blinded to experimental groups.
Acoustic Startle Response (ASR)
The reflexive response to an unexpected tone was assessed using the acoustic startle response (ASR) [22]. Animals were placed in acoustic startle chambers (SR-Labs, San Diego, CA, USA) which consisted of a light- and sound-attenuating outer plastic box and an inner non-restrictive plastic cylinder chamber affixed to a stage platform. Broadband acoustic startle tones were emitted from a high frequency speaker mounted above the mouse chamber. Startle reflexes were measured by a piezo electronics monitor mounted under the stage platform. Each testing session lasted 30 minutes. Animals were habituated to the startle chamber with a 67-decibel sound pressure level (dB SPL) background white-noise for five minutes. After habituation, animals were presented with 10 rounds of 5 pseudo-random startle tones differing in dB SPL (75 to 120dB SPL). Pseudo-random inter-stimulus intervals (ISIs) were generated by the Startle Response software (SR-Labs; San Diego, CA). ISIs consisted of 26, 28, 30, 32, and 34 seconds. Immediately after each startle tone presentation, the startle amplitude was measured as the average voltage emitted by the piezo electric pickup per each millisecond for the 100 ms response window. Data collected at the 120dB tones was used for statistical analysis.
Elevated Zero Maze (EZM)
The elevated zero maze (EZM) was used as a second test for changes in anxiety during adulthood. Following a 1-hour period of acclimation to the testing room (lighting - 40 lux) mice were placed in the maze consisting of two open arms and two closed arms elevated 24 inches off the ground and left undisturbed for 5 minutes. Mice were video recorded for the duration of testing and time spent in the open arms was quantified by a trained observer blind to experimental groups.
Forced Swim Test (FST)
Behavioral immobility was evaluated using the forced swim test (FST). Mice were placed into Plexiglas cylinders filled with water (25°C; 30 – 38 cm high) for 6 minutes while being video recorded. The time spent immobile during the swim session was recorded by an observer blinded to treatment groups. A mouse was considered immobile when making only those movements necessary to keep its head above water.
RNA-sequencing (RNA-Seq)
Brains were rapidly removed from a subset of behaviorally naïve male offspring and grandoffspring, from both stressed and non-stressed lineages (n=5 mice per group), at PND84 and sectioned in a brain block. 200 μm coronal brain sections were used to collect 1.2 mm punches of the amygdala bilaterally. Total RNA was isolated from amygdala punches of the offspring and grandoffspring using the Stratagene Nanoprep RNA isolation kit. 100 ng total of RNA was used to construct cDNA libraries with TruSeq® Stranded mRNA kit poly-A enrichment (Illumina, RS 122-2101), according to the manufacturer’s protocol. Each library was prepared with a unique adapter index to allow for multiplexing during sequencing. Molarity of libraries was quantified using the KAPA library quantification assay (Kapa Biosystems, Cat. No. KK4873). All libraries were pooled together and sequenced as a single group on an Illumina HiSeq5000 by the University of Pennsylvania Next-Generation Sequencing Core (NGSC).
Raw RNA-Seq reads were aligned to the GRCm38 build of the mouse genome using STAR v2.5.3a [23]. STAR was provided with gene models from the Ensembl v90 genome annotation [24]. The Pipeline of RNA-Seq Transformations (PORT) v0.8.4-beta (https://github.com/itmat/Normalization) was used to perform gene-level normalization and quantification of the aligned data. PORT is an implementation of the re-sampling approach for normalization proposed by Li and Tibshirani [25]. Briefly, PORT accounts for potential confounding factors like reads mapping to rRNA and mitochondrial DNA. Next, PORT determines the input samples with the fewest number of gene-mapping reads and re-samples all datasets to the same number of reads. Lastly, PORT quantifies the normalized, gene-level reads counts for each sample. Differentially expressed genes between stress and no-stress lineages were identified separately for the offspring and grandoffspring using the limma (v3.34.0) software package [26]. Prior to DE testing, genes with less than 5 reads across all samples in the stress or no-stress conditions were filtered out. DAVID v6.8 [27, 28] was used to perform functional annotation clustering of the differentially expressed genes. For DAVID analyses and heatmaps, differentially expressed genes (DEGs) in offspring and grandoffspring were identified using limma FDR cutoffs of 0.45 and 0.2, respectively. These FDR cutoffs were selected to yield a sufficient number of DEGs (offspring = 1679; grandoffspring = 1913) for pathway and enrichment analyses. The background lists consisted of all genes that passed expression filtering that were included in the DE analysis. Sequencing data are available in the Gene Expression omnibus repository (GSE108452) https://www.ncbi.nlm.nih.gov/geo/query/acc.cgi?acc=GSE108452
qPCR Array
RNA was obtained by pooling 20ng of 5 RNA samples used for sequencing studies. cDNA was generated with 100ng RNA with the RT2 First Strand Kit (Qiagen Cat no. 330401). Notch signaling pathway-related genes were evaluated using the Qiagen RT2 profiler PCR Array for mouse notch signaling (Cat no. 330231 PAMM-059ZA). One 96-well plate array was used for detection of genes from the stress derived lineage and one 96-well plate array was used for detection of genes from the no stress derived lineage.
Statistical analysis
All data are presented as the mean ± standard error of the mean (SEM). A one-way ANOVA was used to evaluate statistical changes in our behavioral groups. Statistical analyses were performed using Graphpad Prism 7 (Graphpad Software, La Jolla, CA), with the threshold for statistical significance set as P < 0.05.
Results
Paternal adolescent CUS exposure produces no changes in affective behavior in offspring
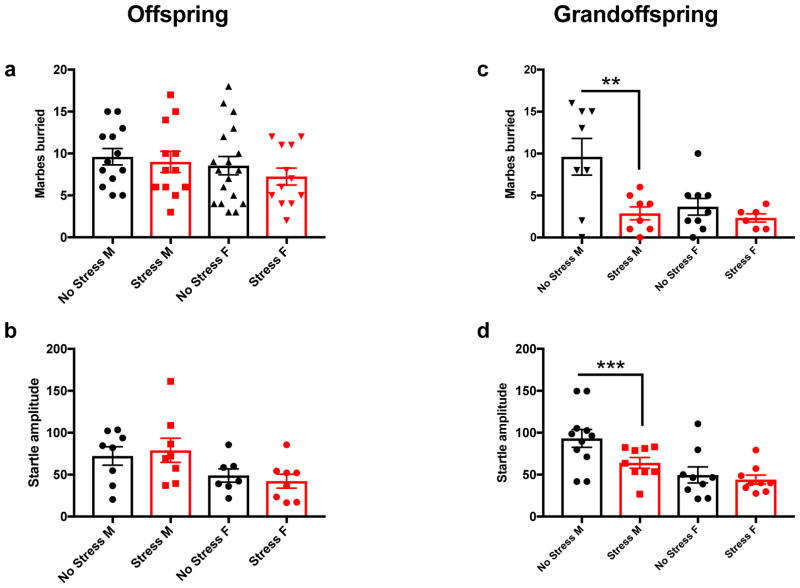
We tested male and female offspring to determine if paternal CUS exposure could impact affective behavior. The MB assay was conducted on 13 males and 18 females from no stress derived controls and 12 males and 12 females in the paternal stress derived group. The startle response to an acoustic tone (ASR) was evaluated in 8 males and 7 females from no stress derived controls and 8 males and 8 females from the paternal stress derived group. The EZM assay was conducted on 14 males and 17 females no stress derived controls, and 13 male and 13 female paternal stress derived mice. FST was conducted on 12 males and 18 females no stress derived controls and 11 male and 6 female paternal stress derived mice. A one-way ANOVA was used to evaluate statistical changes. There was no significant difference in the number of marbles buried (Fig. 2a), the startle response to an acoustic tone (Figure 2b) the time spent in the open arm (Supplemental Fig. S1a) or time spent immobile (Supplemental Fig. S1b) in the paternal derived stress lineage versus the no stress lineage in the offspring.
Figure 2. Paternal adolescent CUS exposure reduces male grandoffspring affective behavior and startle response.
Male and female offspring were evaluated in the MB (a) and ASR (b) tests, and no behavioral changes between the mice derived from paternal stress or no stress controls were observed. Male and female grandoffspring were evaluated in the MB and ASR tests, male grandoffspring derived from the stress lineage (n=8) buried fewer marbles than those derived from the non-stress lineage (n=8) (c), and also had reduced startle amplitude (n=9) compared to controls (n=11) (d). This effect was not observed in female mice. MB no stress (n=9) stress (n=6), and ASR no stress (n= 9) and stress (n=9). **p = 0.0023, * p = 0.0398.
Paternal adolescent CUS exposure produces sex-dependent decreases in marble burying and blunted startle response in grandoffspring
We evaluated male and female grandoffspring in the MB test and ASR to determine if paternal CUS exposure impacts these behaviors two generations after the direct stress exposure. In the MB assay, one-way ANOVA revealed a significant effect [F (3,27) =6.281, p=0.0023]. Using a pair wise Bonferroni post hoc comparison we found male grandoffspring derived from paternal stress (n=8) buried significantly fewer marbles in the MB test compared to control males (n=8), (p=0.0025). This effect was not observed in females from the paternal stress derived group (n=6) versus controls (n=9), (p=>0.9999) (Figure 2c). The female no stress group in our study buried fewer marbles compared to the male counterpart, therefore, it is possible that we could not capture a reduction in the number of marbles buried due to a floor effect.
Males showed a significant effect in ASR using a one-way ANOVA [F (3,34) =6.938, p=0.0009]. Bonferroni post hoc pair wise comparison showed that male mice from the stress derived mouse line (n=9) had a blunted startle amplitude at the 120-dB tone compared with controls (n=11) (p=0.0402). Female siblings showed no differences (n=9) compared to controls (n=9) (p>0.999) (Figure 2d). Thus, we observed a transgenerational effect of paternal CUS, resulting in decreased anxiety-like and blunted startle behavior specifically in male grandoffspring.
Differential gene expression in the amygdala of male offspring and grandoffspring from a stress-derived lineage
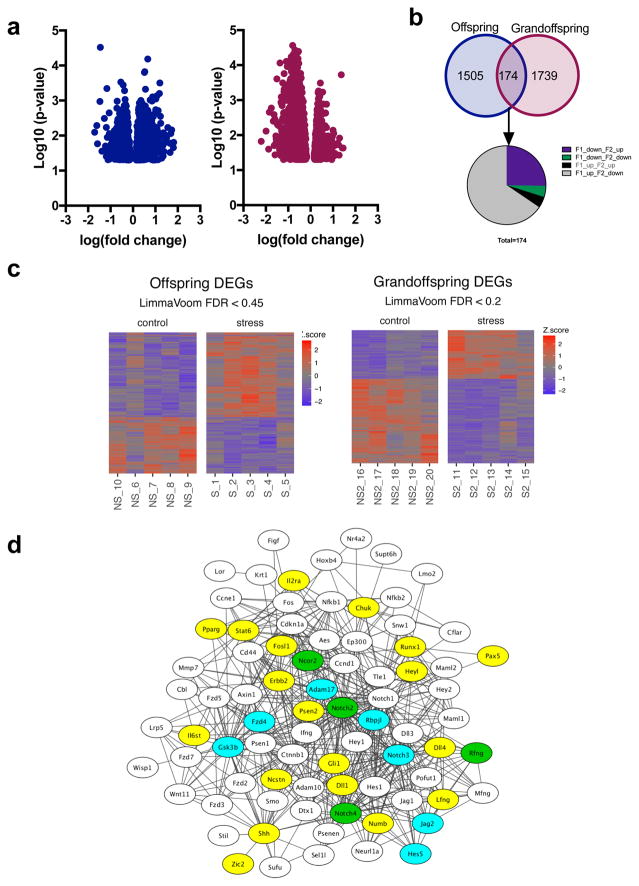
Based on our sex-specific behavioral results, we assessed the transcriptomic profile of the amygdala in the offspring and grand offspring of stress-exposed males. Sequencing was performed on the amygdala of five animals per treatment group, with an average sequencing depth of approximately 20 million reads per sample and 91% alignment to the mouse genome (Supplementary Table S2). As expected, the vast majority of steady-state gene levels were similar between stress and no-stress lineages, and between the offspring and grandoffspring, with the majority of gene expression changes of less than 4-fold between comparisons (Fig. 3a). Differentially expressed genes (DEG) between mice from stressed and non-stressed lineages were identified in both the offspring and grandoffspring (Fig. 3b). Our heat maps of 5 mice per sequencing group shows consistent patterns of gene expression changes in animals belonging to a given group.
Figure 3. DEGs identified from RNA-sequencing of the amygdala from male offspring and grandoffspring.
Volcano plots of the DEGs identified in the offspring and grandoffspring (a). Venn Diagram of the DEGs identified individually in the offspring or the grandoffspring and common between the two generations. Of the 174 common genes, a majority of the genes that are upregulated in offspring are downregulated in grandoffspring, and vice versa (b). Heat maps of DEGs identified in the 5 samples identified in each experimental group: stress derived male offspring vs. no stress derived male offspring, and stress derived male grandoffspring vs no stress derived male offspring (c). Network analysis of notch signaling pathway (d). DEGs identified in RNA-seq only are blue, genes with over 1.5fold differential expression in qPCR are yellow, and overlapping genes are green.
When we performed DEG analysis to determine the intergenerational effect of CUS in the offspring, we identified 1,679 genes (FDR < 0.45) in the amygdalae of the stress derived male offspring. Of these genes, 1,002 were increased due to stress inheritance and 677 were decreased. DEG analysis conducted on males of the grandoffspring identified 1,913 affected genes (FDR < 0.2) of which 707 activated and 1,206 were repressed in the stress-exposure lineage.
A majority of the differentially expressed genes were identified in only the offspring or the grandoffspring; however, 174 DEGs were common to both generations. A majority of the common genes were differentially regulated between generations; 114 were upregulated in offspring, but downregulated in grandoffspring, while 44 were downregulated in offspring, but upregulated in grandoffspring (Fig. 3c).
Gene ontology (GO) clustering revealed significant enrichment of GO sets populated by the DEGs identified in male offspring and grandoffspring
Functional annotation was performed using DAVID 6.8 to gain insight into the intergenerational and transgenerational effects of stress and implications on biological and cellular functions. The common DEGs (174 total) identified in the amygdala from both offspring and grandoffspring clustered into 64 GO annotation groups. Supplementary Table S3 lists the most significant (p<0.01) GO association terms into three broad categories: biological process, cellular component, and molecular function. KEGG pathway analysis clustered the DEGs into 3 significantly affected pathways (Table 1). Functional annotation results show that even the common DEGs between the offspring and grandoffspring, for the most part, differentially regulated. Additionally, the pathways identified can have broad molecular and cellular effects, which could have behavioral implications that extend beyond the analyses we performed.
Table 1. List of KEGG pathways that were enriched for DEGs identified in the offspring and grandoffspring.
DEGs identified as having a q < 0.45 in the offspring, and q < 0.2 in the grandoffspring were used to conduct functional annotation with David 6.8 was to determine the KEGG pathways associated with DEGs identified in the amygdala of offspring and grandoffspring individually, and the common DEGs in the offspring and grandoffspring.
| Offspring DEGs | ||
|---|---|---|
| KEGG Pathway | # of genes | p value |
| Ribosome | 49 | 3.90E-22 |
| Oxidative phosphorylation | 39 | 1.30E-14 |
| Parkinson’s disease | 39 | 1.50E-13 |
| Huntington’s disease | 43 | 6.20E-12 |
| Alzheimer’s disease | 39 | 4.50E-11 |
| Non-alcoholic fatty liver disease (NAFLD) | 33 | 6.30E-09 |
| Cardiac muscle contraction | 17 | 3.20E-05 |
| Axon guidance | 19 | 1.90E-03 |
| Morphine addiction | 15 | 2.90E-03 |
| Retrograde endocannabinoid signaling | 16 | 2.90E-03 |
| Grandoffspring DEGs | ||
| KEGG Pathway | # of genes | p value |
| Notch signaling pathway | 17 | 2.10E-06 |
| Protein export | 9 | 2.70E-03 |
| Ubiquitin mediated proteolysis | 24 | 2.70E-03 |
| Protein processing in endoplasmic reticulum | 27 | 2.80E-03 |
| Proteasome | 11 | 4.70E-03 |
| Spliceosome | 22 | 5.50E-03 |
| Endocytosis | 38 | 6.20E-03 |
| Focal adhesion | 30 | 7.20E-03 |
| Signaling pathways regulating pluripotency of stem cells | 22 | 8.30E-03 |
| Common Offspring and Grandoffspring DEGs | ||
| KEGG Pathway | # of genes | p value |
| ECM-receptor interaction | 6 | 2.90E-04 |
Functional annotation and qPCR array in the DEGs from male grandoffspring identified uniquely affected pathways
Given that our behavioral tests showed reduced affective behavior and startle reactivity only in the grandoffspring, we focused further analysis on the genes that were differentially expressed in the grandoffspring, and those that were differentially expressed in the opposite direction in the offspring vs grandoffspring. The most significant (p<0.01) GO association terms are listed in Supplementary Table S4, and include wide-spread associations with important molecular and cellular homeostasis functions. KEGG pathway analysis identified 27 pathways that were affected as a result of the transgenerational effects of paternal derived CUS (Table 1). The pathway with the largest number of associated genes was “metabolic pathways”, while the pathway with the most significant enrichment of genes was the “notch signaling pathway”, with 17 of 49 pathway-associated genes found to be significantly differentially regulated in the grandoffspring.
To confirm the significance of the notch signaling pathway in transgenerational stress, we performed a qPCR array on the no stress- and stress- derived grandoffspring. Indeed, we found that 19 genes in the notch signaling pathway were differentially regulated (at least 1.5 fold) in the stress group compared to the no stress controls. We used the STRING Database [29], which provides known and predicted protein-protein interactions to identify the likelihood of interactions between proteins. The genes tested in the qPCR array and genes determined by KEGG to be associated with the notch signaling pathway were uploaded to the STRING Database to generate known and putative protein-protein interactions. Network interactions from DEGs and upregulated genes determined by qPCR were analyzed using Cytoscape software [30] (Fig. 3d).
Discussion
Adolescence is a sensitive window for stress exposure. Physical, social, or a combination of physical and social stressors such as CUS, during this critical developmental window results in altered anxiety, and depression in adulthood in mice [9, 18, 30] and rats [31–33]. Early life experiences can induce epigenetic changes in germ cells which can influence the development and wellbeing of subsequent generations. We have previously shown that adolescent CUS exposure increases anxiety and depression-like behavior, and blunts startle reactivity during adulthood (26). In the current study, we investigated the intergenerational and transgenerational effects of paternal adolescent stress exposure. Remarkably, and important for our understanding of paternal-derived transgenerational inheritance, we observed a sex-specific blunted startle reactivity, and decreased marble burying two generations removed from the direct stress exposure. Furthermore, we found that transgenerational stress exposure produces more significant differential gene expression in the amygdala of the grandoffspring versus the offspring.
Decreased anxiety in grandoffspring from paternal stress has been reported in the elevated plus maze and light dark box assays [34], and we report here reductions in number of marbles buried in the MB test, a phenotype reminiscent of anxiolytic compounds [35, 36]. While a decrease in anxiety may be considered beneficial under some circumstances, it is important to note that these investigations measured basal anxiety levels. We did not measure anxiety following an acute or chronic stressor in either the offspring or grandoffspring where a reduction might be protective. Indeed, several studies have demonstrated the beneficial or protective outcome of intergenerational or transgenerational inheritance on a variety of outcomes including cocaine self-administration [37], wound-healing [38], and hepatic gluconeogenesis [39].
We did not observe behavioral changes in the offspring of mice directly exposed to stress. This is in contrast to reports that have shown transmission of stress to the first generation of offspring using different forms of early-life and adolescent stress exposure paradigms resulting in aberrant anxiety behaviors [4, 8, 9, 18, 34, 40]. There are several differences in the experimental design of our study compared to other reports. Most notably, we imposed both physical and social stressors during adolescence, while other studies have utilized variations of social stress, including social instability and maternal separation. In addition, the difference in the age at which stressors are conducted could be critical. Maternal separation occurs from PND1-PND14, while our CUS protocol was conducted from PND28 to PND40. It is possible that stress exposure during sensitive developmental windows play an important role in the transmission of a stress phenotype. These variations bring to light the impact of different stressors, and age of stress exposure; they highlight the need for further studies to consider differences in social stress, physical stressors, the age of exposure, and ultimately the impact on subsequent generations.
Our data identify differentially expressed genes that are common and unique to the amygdala of the offspring and grandoffspring. Given our sex-specific behavioral findings, we conducted RNA sequencing on male offspring and grandoffspring only. The amygdala was selected for transcriptome analysis because it is functionally primed, through its substructure nuclear organization and connectivity to several regions throughout the brain, to integrate responses to external sensory information and mediate behaviors [41]. Furthermore, the amygdala has overlapping roles in anxiety and acoustic startle reactivity [42]. Transcriptional analysis has been conducted in the amygdala in a prenatal exposure to inflammation [43] and other brain regions have been evaluated in the offspring of stressed fathers [8, 16, 17]. Our RNA-sequencing study in the amygdala represents the first whole-genome transcriptional analysis comparison of offspring and grandoffspring of parental stress exposure.
We found 1913 DEGs in the male grandoffsping with a FDR < 0.2. However, we see no changes in the offspring compared to the grandoffspring at the same statistical threshold. In order to conduct a comparison between the offspring and grandoffspring, we used a more lenient cutoff, FDR < 0.45, on the offspring so that we could use a similar number of DEGs in our analysis. This comparison revealed 174 common DEGs between the two generations. Most of these transcripts were upregulated in the offspring, but downregulated in the grandoffspring, who overall had more downregulated DEGs than upregulated DEGs. These RNA-sequencing results indicate that the grandoffspring had greater transcriptional changes in the amygdala than the offspring, which is in agreement with the behavioral data where we measured effects in the grandoffspring and not in the offspring. This information may provide clues as to why stress effects seem to skip a generation and what types of genes and pathways may contribute reduced anxiety and startle response in offspring.
Top KEGG pathways identified in the amygdala of male offspring include generalized diseases and common cellular function. Of interest, significantly affected pathways include Alzheimer’s diseases, axon guidance, and morphine addiction. It would be interesting to see if transcriptional changes in the amygdala lead to changes in cognitive behaviors, and given the prevalence of the comorbidity of anxiety, depression, and substance use disorder, further investigation on how transgenerational stress effects the addictive behaviors of these offspring should be conducted.
We conducted functional annotation of the DEGs identified in the grandoffspring and those that were differentially affected in the opposite direction between the offspring and grandoffspring, as they are most likely functionally related to the transgenerationally inherited behavioral patterns. Due to a limitation in the samples collected from this study, we only used RNA-sequencing data for analysis and therefore focused our efforts on identifying broad pathways that could depict the reasons for the beneficial effect on anxiety and affective behaviors in the grandoffspring. Of the 9 KEGG pathways identified, the most significantly affected was the notch signaling pathway, with differential expression in 35% of genes in the stress-derived grandoffspring. The notch signaling pathway is a key mechanism of cellular communication and transcriptional activation. It has an essential role in central nervous system development, including cell differentiation and neurite outgrowth [44]. It is also important in cellular plasticity, including cortical neuron remodeling and dendrite branching [45]. Among the many genes changing in the notch signaling pathway, we found that the central receptors, Notch 1, 3, and 4, were differentially expressed in the grandoffspring from the stress-derived lineage. Others have shown that notch receptor subtypes and other components of the notch signaling pathway are downregulated in the amygdala after fear conditioning in rodents [46], in suicide victims [47], Alzheimer’s disease, and individuals with mild cognitive impairment [48]. Functional annotation of DEGs identified that the notch signaling pathway was significantly affected by transgenerational stress, and network analysis of all genes profiled for qPCR combined with the DEGs further confirmed changes in the notch signaling pathway due to transgenerational stress. This analysis suggests that changes in the notch signaling pathway due to transgenerational stress could affect neuronal plasticity in the grandoffspring. Future work should confirm changes in select genes from the notch signaling pathway and conduct an investigation of the structure, architecture, and connectivity of neurons from the amygdala of grandoffspring. Our study was conducted in adult mice, however, given the role of the notch pathway in development, it may be important to determine transgenerational transcriptional changes prior to adulthood, when additional developmental factors may also have changes in expression.
In summary, results from the present study demonstrate that male mice exposed to CUS transmit different behavioral responses and transcriptional regulation in the amygdala, a brain area involved in stress response and emotional processing. The present study adds valuable insight into stress interactions across generations that affect behavior, and contribute substantially to the growing literature of across-generation transmission of the parental environment by providing potential targets for regulation of phenotypic inheritance.
Supplementary Material
EZM (a) and FST (b) were used to evaluate anxiety and depressive like behaviors in male and female offspring derived from the stress and no stress lineages. No changes were observed between groups.
Table S1: Stressors and lengths of exposure for CUS paradigm
Table S2: RNA-sequencing read alignment and mapping
Table S3: List of gene ontologies associated with differentially expressed genes in the amygdala of offspring and grandoffspring mice derived from F0 adolescent stress. David 6.8 was used to determine the GO categories and terms associated with the genes identified as differentially expressed in the amygdala of both offspring and grandoffspring mice. The top 10 gene ontology terms in each of three broad GO categories and the number of genes associated with each term is listed with the p-value. GO terms are organized by lowest p-value.
Table S4: List of gene ontologies associated with genes differentially expressed in the amygdala of stress-derived grandoffspring. David 6.8 was used to determine the GO categories and terms associated with the differentially expressed genes identified in the amygdala of only grandoffspring, and DEGs in the opposite direction of offspring. The top 10 gene ontology terms in each of three broad GO categories and the number of genes associated with each term is listed with the p-value. GO terms are organized by lowest p-value.
Acknowledgments
This work was supported by the National Institute on Drug Abuse [R01DA033646 (J.A.B.), T32MH14654 (M.T.M) and, T32DA28874 (N.L.Y)]. We thank Kenneth Bisson and Jenna Hebert for experimental assistance.
References
- 1.Heard E, Martienssen RA. Transgenerational epigenetic inheritance: myths and mechanisms. Cell. 2014:95–109. doi: 10.1016/j.cell.2014.02.045. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Gapp K, Bohacek J. Epigenetic germline inheritance in mammals: looking to the past to understand the future. Genes, Brain and Behavior. 2017 doi: 10.1111/gbb.12407. [DOI] [PubMed] [Google Scholar]
- 3.Matthews SG, Phillips DI. Transgenerational inheritance of stress pathology. Experimental Neurology. 2012:95–101. doi: 10.1016/j.expneurol.2011.01.009. [DOI] [PubMed] [Google Scholar]
- 4.Franklin TB, et al. Epigenetic Transmission of the Impact of Early Stress Across Generations. Biological Psychiatry. 2010:408–415. doi: 10.1016/j.biopsych.2010.05.036. [DOI] [PubMed] [Google Scholar]
- 5.Franklin TB, et al. PLoS ONE. Public Library of Science; 2011. Influence of early stress on social abilities and serotonergic functions across generations in mice; p. e21842. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Dietz DM, et al. Paternal transmission of stress-induced pathologies. Biological Psychiatry. 2011:408–414. doi: 10.1016/j.biopsych.2011.05.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Leshem M, Schulkin J. Dev Psychobiol. Wiley Subscription Services, Inc., A Wiley Company; 2012. Transgenerational effects of infantile adversity and enrichment in male and female rats; pp. 169–186. [DOI] [PubMed] [Google Scholar]
- 8.Rodgers AB, et al. J Neurosci. Society for Neuroscience; 2013. Paternal stress exposure alters sperm microRNA content and reprograms offspring HPA stress axis regulation; pp. 9003–9012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Saavedra-Rodríguez L, Feig LA. Chronic social instability induces anxiety and defective social interactions across generations. Biological Psychiatry. 2013:44–53. doi: 10.1016/j.biopsych.2012.06.035. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Yehuda R, et al. Vulnerability to posttraumatic stress disorder in adult offspring of Holocaust survivors. Am J Psychiatry. 1998:1163–1171. doi: 10.1176/ajp.155.9.1163. [DOI] [PubMed] [Google Scholar]
- 11.Barker DJ. BMJ. BMJ Group; 1990. The fetal and infant origins of adult disease; p. 1111. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Roseboom TJ, et al. Effects of prenatal exposure to the Dutch famine on adult disease in later life: an overview. Molecular and cellular endocrinology. 2001;185(1):93–98. doi: 10.1016/s0303-7207(01)00721-3. [DOI] [PubMed] [Google Scholar]
- 13.Barker DJP. J Intern Med. Blackwell Publishing Ltd; 2007. The origins of the developmental origins theory; pp. 412–417. [DOI] [PubMed] [Google Scholar]
- 14.Yehuda R, et al. Maternal, not paternal, PTSD is related to increased risk for PTSD in offspring of Holocaust survivors. Journal of Psychiatric Research. 2008:1104–1111. doi: 10.1016/j.jpsychires.2008.01.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Andersen SL, Teicher MH. Delayed effects of early stress on hippocampal development. Neuropsychopharmacology. 2004;29(11):1988. doi: 10.1038/sj.npp.1300528. [DOI] [PubMed] [Google Scholar]
- 16.Crews D, et al. Proc Natl Acad Sci USA. National Acad Sciences; 2012. Epigenetic transgenerational inheritance of altered stress responses; pp. 9143–9148. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Bohacek J, et al. Transgenerational Epigenetic Effects on Brain Functions. Biological Psychiatry. 2013:313–320. doi: 10.1016/j.biopsych.2012.08.019. [DOI] [PubMed] [Google Scholar]
- 18.Weiss IC, et al. Inheritable Effect of Unpredictable Maternal Separation on Behavioral Responses in Mice. Front Behav Neurosci. 2011 doi: 10.3389/fnbeh.2011.00003. Frontiers. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Schmidt HD, Duman RS. Peripheral BDNF produces antidepressant-like effects in cellular and behavioral models. Neuropsychopharmacology. 2010:2378–2391. doi: 10.1038/npp.2010.114. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Yohn NL, Blendy JA. Adolescent Chronic Unpredictable Stress Exposure is a Sensitive Window for Long-Term Changes in Adult Behavior in Mice. Neuropsychopharmacology. 2017:42. doi: 10.1038/npp.2017.11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Njung'e K, Handley SL. Evaluation of marble-burying behavior as a model of anxiety. Pharmacol Biochem Behav. 1991:63–67. doi: 10.1016/0091-3057(91)90590-x. [DOI] [PubMed] [Google Scholar]
- 22.Davis M. Neurochemical modulation of sensory-motor reactivity: Acoustic and tactile startle reflexes. Neurosci Biobehav Rev. 1980:241–263. doi: 10.1016/0149-7634(80)90016-0. [DOI] [PubMed] [Google Scholar]
- 23.Dobin A, et al. STAR: ultrafast universal RNA-seq aligner. Bioinformatics. 2013;29(1):15–21. doi: 10.1093/bioinformatics/bts635. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Aken BL, et al. Ensembl 2017. Nucleic acids research. 2016;45(D1):D635–D642. doi: 10.1093/nar/gkw1104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Li J, Tibshirani R. Finding consistent patterns: a nonparametric approach for identifying differential expression in RNA-Seq data. Statistical methods in medical research. 2013;22(5):519–536. doi: 10.1177/0962280211428386. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Ritchie ME, et al. limma powers differential expression analyses for RNA-sequencing and microarray studies. Nucleic acids research. 2015;43(7):e47–e47. doi: 10.1093/nar/gkv007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Huang DW, Sherman BT, Lempicki RA. Nucl Acids Res. Oxford University Press; 2009. Bioinformatics enrichment tools: paths toward the comprehensive functional analysis of large gene lists; pp. 1–13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Huang DW, Sherman BT, Lempicki RA. Systematic and integrative analysis of large gene lists using DAVID bioinformatics resources. Nat Protoc. 2009:44–57. doi: 10.1038/nprot.2008.211. [DOI] [PubMed] [Google Scholar]
- 29.Szklarczyk D, et al. The STRING database in 2017: quality-controlled protein–protein association networks, made broadly accessible. Nucleic acids research. 2017;45(D1):D362–D368. doi: 10.1093/nar/gkw937. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Schmidt MV, et al. High susceptibility to chronic social stress is associated with a depression-like phenotype. Psychoneuroendocrinology. 2010:635–643. doi: 10.1016/j.psyneuen.2009.10.002. [DOI] [PubMed] [Google Scholar]
- 31.Tsoory M, Cohen H, Richter-Levin G. Juvenile stress induces a predisposition to either anxiety or depressive-like symptoms following stress in adulthood. Eur Neuropsychopharmacol. 2007:245–256. doi: 10.1016/j.euroneuro.2006.06.007. [DOI] [PubMed] [Google Scholar]
- 32.McCormick CM, Smith C, Mathews IZ. Effects of chronic social stress in adolescence on anxiety and neuroendocrine response to mild stress in male and female rats. Behavioural Brain Research. 2008:228–238. doi: 10.1016/j.bbr.2007.09.005. [DOI] [PubMed] [Google Scholar]
- 33.Eiland L, et al. Chronic juvenile stress produces corticolimbic dendritic architectural remodeling and modulates emotional behavior in male and female rats. Psychoneuroendocrinology. 2012:39–47. doi: 10.1016/j.psyneuen.2011.04.015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Gapp K, et al. Implication of sperm RNAs in transgenerational inheritance of the effects of early trauma in mice. Nat Neurosci. 2014:667–669. doi: 10.1038/nn.3695. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Davis M, et al. Fear-potentiated startle: a neural and pharmacological analysis. Behavioural Brain Research. 1993:175–198. doi: 10.1016/0166-4328(93)90102-v. [DOI] [PubMed] [Google Scholar]
- 36.Hijzen TH, et al. Predictive validity of the potentiated startle response as a behavioral model for anxiolytic drugs. Psychopharmacology. 1995:150–154. doi: 10.1007/BF02245833. [DOI] [PubMed] [Google Scholar]
- 37.Vassoler FM, et al. Epigenetic inheritance of a cocaine-resistance phenotype. Nat Neurosci. 2013:42–47. doi: 10.1038/nn.3280. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Zeybel M, et al. Multigenerational epigenetic adaptation of the hepatic wound-healing response. Nature medicine. 2012;18(9):1369. doi: 10.1038/nm.2893. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Wu L, et al. Paternal psychological stress reprograms hepatic gluconeogenesis in offspring. Cell metabolism. 2016;23(4):735–743. doi: 10.1016/j.cmet.2016.01.014. [DOI] [PubMed] [Google Scholar]
- 40.Rodgers AB, et al. Proc Natl Acad Sci USA. National Acad Sciences; 2015. Transgenerational epigenetic programming via sperm microRNA recapitulates effects of paternal stress; pp. 13699–13704. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Rodrigues SM, LeDoux JE, Sapolsky RM. The influence of stress hormones on fear circuitry. Annu Rev Neurosci. 2009:289–313. doi: 10.1146/annurev.neuro.051508.135620. [DOI] [PubMed] [Google Scholar]
- 42.Davis M. The role of the amygdala in fear and anxiety. Annu Rev Neurosci. 1992 doi: 10.1146/annurev.ne.15.030192.002033. [DOI] [PubMed] [Google Scholar]
- 43.Weber-Stadlbauer U, et al. Transgenerational transmission and modification of pathological traits induced by prenatal immune activation. Molecular psychiatry. 2017;22(1):102. doi: 10.1038/mp.2016.41. [DOI] [PubMed] [Google Scholar]
- 44.Šestan N, Artavanis-Tsakonas S, Rakic P. Contact-dependent inhibition of cortical neurite growth mediated by notch signaling. Science. 1999;286(5440):741–746. doi: 10.1126/science.286.5440.741. [DOI] [PubMed] [Google Scholar]
- 45.Bonini SA, et al. Nuclear factor κB-dependent neurite remodeling is mediated by Notch pathway. Journal of Neuroscience. 2011;31(32):11697–11705. doi: 10.1523/JNEUROSCI.1113-11.2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Dias BG, et al. Amygdala-dependent fear memory consolidation via miR-34a and Notch signaling. Neuron. 2014;83(4):906–918. doi: 10.1016/j.neuron.2014.07.019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Monsalve EM, et al. Abnormal expression pattern of Notch receptors, ligands, and downstream effectors in the dorsolateral prefrontal cortex and amygdala of suicidal victims. Molecular neurobiology. 2014;49(2):957–965. doi: 10.1007/s12035-013-8570-z. [DOI] [PubMed] [Google Scholar]
- 48.De Strooper B. Lessons from a failed γ-secretase Alzheimer trial. Cell. 2014;159(4):721–726. doi: 10.1016/j.cell.2014.10.016. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
EZM (a) and FST (b) were used to evaluate anxiety and depressive like behaviors in male and female offspring derived from the stress and no stress lineages. No changes were observed between groups.
Table S1: Stressors and lengths of exposure for CUS paradigm
Table S2: RNA-sequencing read alignment and mapping
Table S3: List of gene ontologies associated with differentially expressed genes in the amygdala of offspring and grandoffspring mice derived from F0 adolescent stress. David 6.8 was used to determine the GO categories and terms associated with the genes identified as differentially expressed in the amygdala of both offspring and grandoffspring mice. The top 10 gene ontology terms in each of three broad GO categories and the number of genes associated with each term is listed with the p-value. GO terms are organized by lowest p-value.
Table S4: List of gene ontologies associated with genes differentially expressed in the amygdala of stress-derived grandoffspring. David 6.8 was used to determine the GO categories and terms associated with the differentially expressed genes identified in the amygdala of only grandoffspring, and DEGs in the opposite direction of offspring. The top 10 gene ontology terms in each of three broad GO categories and the number of genes associated with each term is listed with the p-value. GO terms are organized by lowest p-value.