Abstract
Li-Fraumeni syndrome (LFS) is a rare hereditary cancer disorder with highly variable clinical outcomes that results from germline mutations in the TP53 gene. Here we report that the quaternary structure of p53 is an important factor affecting cellular functions and the clinical outcomes of LFS patients (n = 87). Specifically, carriers of monomeric p53 mutants (n = 56) exhibited complete penetrance, with a 2.11-fold greater risk of cancer-related death (95% confidence interval [CI] = 1.07 to 4.30) and a statistically significantly lower median survival age as compared with carriers of multimeric (dimeric or tetrameric, n = 31) p53 mutants (33 years, 95% CI = 30 to 50, vs 51 years, 95% CI = 40 to NA, respectively, two-sided P = .03), who presented incomplete penetrance. Cellular functional assays using p53-null H1299 cells expressing clinically relevant p53 mutants confirmed that the cellular effects observed upon loss of p53 oligomerization are associated with clinical outcomes of LFS patients. The association between p53 oligomeric state and clinical phenotype suggests that TP53 mutations are not all equivalent and supports the implementation of new genotype-adapted guidelines for the management of LFS patients with TP53 mutations in the oligomerization domain.
Mutations in the TP53 gene that encodes the major tumor suppressor protein p53 are the most common underlying cause of Li-Fraumeni syndrome (LFS), a rare hereditary disorder characterized by a high risk for developing a wide spectrum of early-onset cancers (1,2). Importantly, different TP53 mutations confer varying degrees of penetrance and considerable phenotypic heterogeneity, which makes disease management a major clinical challenge for LFS patients (3,4). Most mutations within the oligomerization domain (OD) of p53, which are found among 5.2% of LFS patients (excluding the Brazilian founder mutation R337H), have not been characterized (Figure 1A). Missense mutations are the predominant alteration in the OD of LFS patients (61.25%, excluding R337H), followed by nonsense (36.25%) and silent mutations (2.5%) (5).
Figure 1.
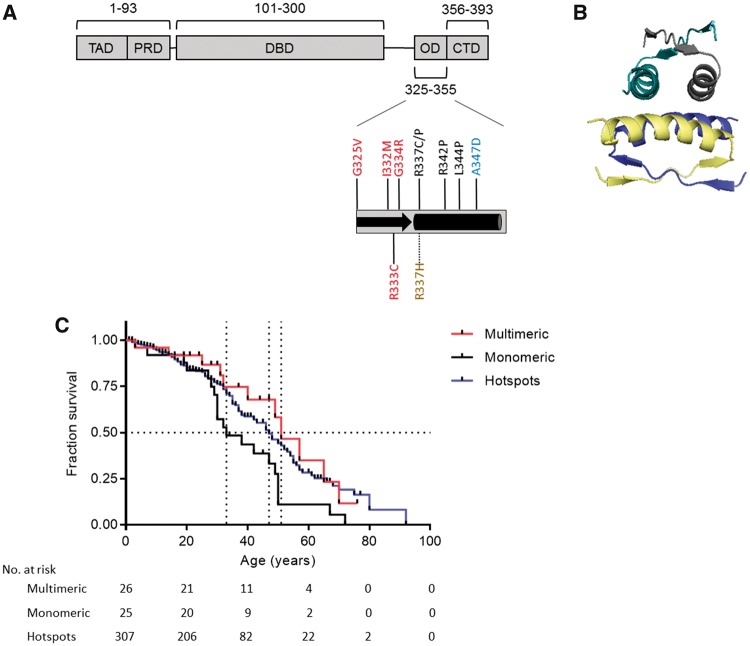
Survival analysis of patients carrying germline TP53 missense mutations in the oligomerization domain stratified by mutant p53 oligomeric status. A) The p53 oligomerization domain (OD; residues 325–355) is composed of a short β-strand (black arrow) followed by an α-helix (black rectangle). Cancer-related germline missense mutations within the C-terminal OD of the p53 protein were stratified into subgroups based on the oligomeric status of mutant p53 proteins. All TP53 mutations analyzed in this study are depicted by their location in the OD structural domain, where monomeric mutants in black and a dimeric mutant in blue are mostly located in the OD α-helical region, whereas tetrameric mutants in red typically occur in the OD β-strand segment. The pH-dependent tetrameric mutant R337H is colored gold. B) A ribbon model of the predicted structure of a tetrameric arrangement of p53 ODs is displayed to highlight that their α-helical regions are clustered to generate the tetramer-interacting interface (PDB ID: 1PES) (8). C) Kaplan-Meier survival curves comparing the lifetime cancer survival of Li-Fraumeni syndrome patients stratified by the ability of mutant p53 to form multimers (dimeric and tetrameric mutant p53 carriers) vs monomeric p53 carriers and carriers of well-known hotspot p53 mutants (missense mutations at codons 175, 245, 248, 273, and 282) for comparison. Survival was defined as the age at cancer-related death. Patients who did not have an event were censored at the age of their last follow-up. P values were calculated using two-sided log-rank tests. Dotted lines indicate median survival times, and vertical lines on the Kaplan-Meier curves indicate patients who were censored.
The p53 protein is a transcription factor that is fully active when it oligomerizes into its tetrameric state—an event that is induced by cellular stress (6,7). Oligomerization of p53 occurs through its 30-amino acid OD, a structural element consisting of a β-strand and an α-helix (Figure 1B) (8). Mutations within the OD can result in p53 mutants that cannot form tetramers, remaining monomeric or assembling only into dimers, whereas other mutations in the OD do not affect the ability of p53 to form tetramers (9). The aim of this study was to characterize the functional and clinical consequences of TP53 OD mutations and to determine whether alterations in the oligomeric status of p53 affect clinical outcomes in LFS.
Data sets from LFS patients harboring TP53 missense mutations in residues 325–355 of p53 were obtained from the latest versions of the International Agency for Research on Cancer (IARC) TP53 database R18 and cBioPortal 1.4.2 (5,10). A total of 87 germline carriers and 73 patients with sporadic tumors were identified. Four patients from The Hospital for Sick Children (Toronto, ON, Canada) were added to this analysis, of whom two patients have been previously described (Supplementary Table 1, available online) (11). All participants provided informed consent. The Hospital for Sick Children research ethics board approved use of this data (0019910602). Hazard ratios and P values comparing Kaplan-Meier survival curves were calculated using log-rank tests. All P values were based on a two-sided hypothesis, and statistical significance was set at a P value of less than .05. Detailed statistical analyses and methods for all in vitro assays are provided in the Supplementary Methods (available online).
Each cancer-related germline TP53 mutation within the OD reported in the IARC TP53 database (Figure 1A) was generated to determine their functional consequences in H1299 p53-null lung cancer cells (Supplementary Table 2, available online). These reported mutations either maintain the ability of p53 to form tetramers (G325V, I332M, R333C, G334R, and R337H) or lack the ability to tetramerize, forming only dimeric p53 species (A347D) or monomeric p53 species (R337C, R337P, R342P, and L344P) based on cross-linking experiments (Supplementary Figure 1A, available online). The cellular expression of all monomeric p53 mutants associated with a severe reduction in tumor suppression functions (increased colony formation, decreased growth arrest, and death induction) (Supplementary Figure 1, B–F, available online). In contrast, all p53 OD mutants that maintain tetramer-forming ability had comparable activities to wild-type p53. The dimeric mutant retained partial tumor suppressor function with the ability to suppress cellular growth by inhibiting DNA synthesis, supporting a previous report that dimeric p53 species induce cytostasis (12). All p53 mutants properly localized to the nucleus, regardless of their oligomeric status (Supplementary Figure 1G, available online). Additionally, oligomeric mutants with the ability to form multimers (dimers or tetramers) tended to have enhanced transactivation function in comparison with monomeric mutants (Supplementary Figure 2, available online) (13).
LFS patients were stratified according to the oligomeric status of their mutant p53 proteins (monomeric or multimeric p53 subgroups) (Table 1; Supplementary Figure 3 and Table 3, available online). In total, the cohort included 56 carriers of monomeric mutants from 10 separate families, 14 carriers of dimeric mutants from three separate families, and 17 carriers of tetrameric mutants from seven separate families (Table 1). Carriers of the pH-dependent, tetrameric R337H mutant p53 have been classified separately from other mutation clusters located in the OD in view of its unique biochemical and genetic characteristics (14–16).
Table 1.
Stratification of patients carrying germline TP53 mutations into subgroups according to p53 oligomeric status
| TP53 mutation subgroup | n | Alleles | Mean age of first tumor onset (median), y | No. affected by cancer, No. (%) | Multiple primary tumors, No. (%) | Cumulative survival*, No. (%) | Median survival time, age (95% CI) | HR (95% CI)† | P† |
|---|---|---|---|---|---|---|---|---|---|
| Monomeric | 56 | L344P, R342P, R337C, R337P | 31.3 (30.0) | 56 (100) | 9 (16.1) | 9 (16.1) | 33 (30 to 50) | — | — |
| Multimeric | 31 | A347D, G334R, R333C, I332M, G325V | 30.0 (29.0) | 25 (80.6) | 5 (16.1) | 16 (51.6) | 51 (40 to NA) | 2.11 (1.07 to 4.30) | .03 |
| Hotspots | 504 | All missense mutations at codons 175, 245, 248, 273, and 282 | 26.9 (26.0) | 472 (93.6) | 132 (26.2) | 233 (46.2) | 47 (43 to 53) | 1.76 (1.17 to 3.68) | .01 |
Cumulative survival was calculated as follows: (total living patients ÷ total patients) × 100. CI = confidence interval; HR = hazard ratio.
Hazard ratios and P values comparing Kaplan-Meier survival curves in Figure 1C were calculated using two-sided log-rank tests in comparison with the monomeric group.
The cumulative survival among multimeric p53 mutant carriers was considerably more favorable as compared with the survival among monomeric p53 mutant carriers (51.6% vs 16.1%, respectively, P = .001, proportion test) (Table 1). A Kaplan-Meier survival analysis highlighted differences between lifetime cancer survival outcomes of LFS patients relative to the oligomeric status of their mutant p53 and, also in comparison with carriers of hotspot TP53 mutations, identified carriers of monomeric mutants as a high-risk group (Figure 1C). Specifically, there was a 2.11-fold (95% confidence interval [CI] = 1.07 to 4.30) lower risk of cancer-related death and a substantially extended median lifespan in carriers of partially functional p53 mutants that can multimerize (n = 26) as compared with the monomeric p53 group (n = 25, 51 years, 95% CI = 40 to NA years, vs 33 years, 95% CI = 30 to 50 years, respectively, P = .03) (Figure 1C). Importantly, the same survival trend was observed in an analysis of 73 cancer patients with sporadic TP53 mutations in the p53 OD (P < .001), an event that occurs in one of 450 sporadic tumors (Supplementary Figure 4, Supplementary Tables 3 and 4, and Supplementary Methods, available online). In comparison with germline carriers of well-known hotspot TP53 mutations (missense mutations in codons 175, 245, 248, 273, and 282), monomeric p53 carriers had lower cumulative survival (16.1% vs 46.2%, P < .001, proportion test), with a 1.76-fold (95% CI = 1.17 to 3.68) increased risk of cancer-related death (Table 1). Carriers of hotspot mutants (n = 307) had lifespans 14 years longer as compared with monomeric p53 carriers (47 years, 95% CI = 43 to 53 years, vs 33 years, 95% CI = 30 to 50 years, respectively, P = .01) (Figure 1C).
LFS patients with monomeric p53 alleles displayed complete disease penetrance (100% developed cancer), in contrast to carriers of multimeric or hotspot p53 mutant alleles, who presented incomplete penetrance (80.6% and 93.6%, P = .003 and P = .1, proportion test, respectively). There were no statistical differences between the frequency of individuals affected by multiple primary tumors (proportion test) or between the ages of first tumor onset (Kruskal-Wallis test) across the oligomeric subgroups or as compared with hotspot mutation carriers (Table 1). A wide distribution of tumor sites was observed among each subgroup, a common trait seen in LFS patients. Interestingly, oligomeric subgroups displayed tissue-specific tumor manifestations that were primarily conserved when comparing germline and sporadic data sets of patients harboring TP53 OD mutations, with breast cancer being predominant among patients with monomeric and dimeric p53 mutants (Supplementary Figure 5, available online).
Despite the limitation of sample size, statistical significance was obtained between survival outcomes from multiple unrelated families, primarily due to the large effect size between the oligomeric subgroups. We expect that the increased use of next-generation sequencing across the entire TP53 locus will identify more OD mutations (exons 9 and 10) that will strengthen this association.
In conclusion, TP53 mutations in the OD result in a broad spectrum of functional loss that can be delineated according to p53 oligomeric status. Mutations resulting in the loss of p53 oligomerization are associated with loss of tumor suppressor functions in cells and more severe disease outcomes in LFS patients. Knowledge of this genotype–phenotype relationship might be considered for clinical surveillance and counseling of these specific carriers.
Funding
This work was supported by the Canadian Institutes of Health Research (148556 to JG, 143234 to DM) and the Terry Fox Research Institute (1046 to DM).
Notes
Affiliations of authors: Department of Medical Biophysics (NWF, AP, JT, DM, JG), Genetics and Genomic Biology Program (DM), and Department of Pharmaceutical Sciences (JG), University of Toronto, Toronto, Ontario, Canada, University of Toronto, Toronto, Ontario, Canada; Physical Sciences, Sunnybrook Research Institute, Toronto, Ontario, Canada (NWF, AP, JG); Division of Hematology/Oncology, Department of Pediatrics, The Hospital for Sick Children, Toronto, Ontario, Canada (JT, DM).
The funders had no role in the design of the study; the collection, analysis, or interpretation of the data; the writing of the manuscript; or the decision to submit the manuscript for publication. There are no potential conflicts of interest to disclose.
Supplementary Material
References
- 1. Li FP, Fraumeni JF, Mulvihill JJ, et al. A cancer family syndrome in twenty-four kindreds. Cancer Res. 1988;4818:5358–5362. [PubMed] [Google Scholar]
- 2. Malkin D, Li FP, Strong LC, et al. Germ line p53 mutations in a familial syndrome of breast cancer, sarcomas, and other neoplasms. Science. 1990;2504985:1233–1238. [DOI] [PubMed] [Google Scholar]
- 3. Chompret A, Brugières L, Ronsin M, et al. P53 germline mutations in childhood cancers and cancer risk for carrier individuals. Br J Cancer. 2000;8212:1932–1937. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Wu CC, Shete S, Amos CI, Strong LC.. Joint effects of germ-line p53 mutation and sex on cancer risk in Li-Fraumeni syndrome. Cancer Res. 2006;6616:8287–8292. [DOI] [PubMed] [Google Scholar]
- 5. Bouaoun L, Sonkin D, Ardin M, et al. TP53 variations in human cancers: New lessons from the IARC TP53 database and genomics data. Hum Mutat. 2016;379:865–876. [DOI] [PubMed] [Google Scholar]
- 6. Gaglia G, Guan Y, Shah JV, Lahav G.. Activation and control of p53 tetramerization in individual living cells. Proc Natl Acad Sci U S A. 2013;11038:15497–15501. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Chène P. The role of tetramerization in p53 function. Oncogene. 2001;2021:2611–2617. [DOI] [PubMed] [Google Scholar]
- 8. Lee W, Harvey TS, Yin Y, Yau P, Litchfield D, Arrowsmith CH.. Solution structure of the tetrameric minimum transforming domain of p53. Nat Struct Biol. 1994;112:877–890. [DOI] [PubMed] [Google Scholar]
- 9. Kawaguchi T, Kato S, Otsuka K, et al. The relationship among p53 oligomer formation, structure and transcriptional activity using a comprehensive missense mutation library. Oncogene. 2005;2446:6976–6981. [DOI] [PubMed] [Google Scholar]
- 10. Gao J, Aksoy BA, Dogrusoz U, et al. Integrative analysis of complex cancer genomics and clinical profiles using the cBioPortal. Sci Signal. 2013;6269:pl1.. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Villani A, Shore A, Wasserman JD, et al. Biochemical and imaging surveillance in germline TP53 mutation carriers with Li-Fraumeni syndrome: 11 year follow-up of a prospective observational study. Lancet Oncol. 2016;179:1295–1305. [DOI] [PubMed] [Google Scholar]
- 12. Fischer NW, Prodeus A, Malkin D, Gariépy J.. p53 oligomerization status modulates cell fate decisions between growth, arrest and apoptosis. Cell Cycle. 2016;1523:3210–3219. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Kato S, Han SY, Liu W, et al. Understanding the function-structure and function-mutation relationships of p53 tumor suppressor protein by high-resolution missense mutation analysis. Proc Natl Acad Sci U S A. 2003;10014:8424–8429. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. DiGiammarino EL, Lee AS, Cadwell C, et al. A novel mechanism of tumorigenesis involving pH-dependent destabilization of a mutant p53 tetramer. Nat Struct Biol. 2002;91:12–16.11753428 [Google Scholar]
- 15. Achatz MI, Olivier M, Le Calvez F, et al. The TP53 mutation, R337H, is associated with Li-Fraumeni and Li-Fraumeni-like syndromes in Brazilian families. Cancer Lett. 2007;245(1-2):96–102. [DOI] [PubMed] [Google Scholar]
- 16. Wasserman JD, Novokmet A, Eichler-Jonsson C, et al. Prevalence and functional consequence of TP53 mutations in pediatric adrenocortical carcinoma: A Children’s Oncology Group study. J Clin Oncol. 2015;336:602–609. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.