Abstract
Background
Previous studies have reported conflicting information regarding the prognostic role of p16 in nonoropharyngeal head and neck squamous cell carcinoma (HNSCC).
Methods
Using the US Veterans Affairs database, we analyzed 1448 patients with locoregionally advanced HNSCC and known p16 status diagnosed between 2005 and 2015 and treated with surgery, radiotherapy, or chemoradiotherapy. Tumor p16 status was determined through manual review of pathology reports of primary tumor specimens. Oropharyngeal (n = 1061) or nonoropharyngeal (n = 387; hypopharyngeal, laryngeal, or oral cavity) tumor site was determined from tumor registry data and manually reviewed for accuracy. We used multivariable Cox regression to analyze the effect of p16 status on overall survival (OS), cancer-specific survival (CSS), and competing mortality (CM) for oropharyngeal or nonoropharyngeal tumor sites. All statistical tests were two-sided.
Results
In multivariable models adjusting for treatment, stage, age, comorbidity, and body mass index, patients with p16-positive tumors had improved OS, CSS, and CM compared with patients with p16-negative tumors in both oropharyngeal (OS: hazard ratio [HR] = 0.53, 95% confidence interval [CI] = 0.40 to 0.71, P < .001; CSS: HR = 0.50, 95% CI = 0.35 to 0.73, P < .001; CM: HR = 0.59, 95% CI = 0.38 to 0.93, P = .02) and nonoropharyngeal primary sites (OS: HR = 0.41, 95% CI = 0.25 to 0.69, P < .001; CSS: HR = 0.37, 95% CI = 0.18 to 0.77, P = .008; CM: HR = 0.46, 95% CI = 0.23 to 0.95, P = .04). The prognostic impact of p16 status did not statistically significantly differ by primary tumor site for OS, CSS, or CM (Pinteraction > .05).
Conclusions
Our findings support the hypothesis that p16 has a similar prognostic role in both nonoropharyngeal and oropharyngeal cancer. Consideration should be given to increased testing for p16 in laryngeal, hypopharyngeal, and oral cavity primaries.
Multiple studies have found that p16 (INK4A) is an important prognostic biomarker in oropharyngeal head and neck squamous cell carcinoma (HNSCC) (1–7). The prognostic role of p16 in nonoropharyngeal HNSCC, however, is uncertain. Several studies have concluded that p16 has no prognostic significance in nonoropharyngeal HNSCC (7–13), whereas others have concluded the opposite (14–20). As a result, practice guidelines generally recommend routine testing for p16 in oropharyngeal but not nonoropharyngeal HNSCC (21).
Many prior studies examining the prognostic role of p16 in nonoropharyngeal HNSCC have been limited by small sample sizes and low rates of p16 testing, potentially explaining the lack of an observed prognostic role of p16 testing in specific contexts. Other studies have been constrained by the lack of key prognostic information, such as comorbidity status, which may confound analyses of effects on overall survival (22,23). Furthermore, many studies omit analyses of effects of p16 on cause-specific events, which can result in misleading inferences (24,25). Therefore, we sought to investigate further the potential prognostic role of p16 in nonoropharyngeal vs oropharyngeal HNSCC, with particular attention to estimating adjusted effects on cancer-specific mortality and competing mortality.
Methods
Population, Data Source, and Sampling Methods
From the US national Veterans Affairs (VA) database, we identified patients with locoregionally advanced (AJCC stage III or IV) nonmetastatic HNSCC of the oropharynx, oral cavity, hypopharynx, or larynx diagnosed between 2005 and 2015 and treated with either surgery, radiation therapy (RT), or chemoradiotherapy (CRT). The Veterans Affairs Informatics and Computing Infrastructure (VINCI) collects detailed information regarding baseline demographic information, tumor- and treatment-related factors, and outcomes for US veterans from VA hospitals nationwide (26). The VINCI system includes longitudinal data regarding diagnoses, procedures, medications, labs, physiologic measurements, text notes, and reports, while tumor registry data are gathered at individual VA medical centers by trained registrars according to standard protocols issued from the American College of Surgeons (27). As such, VINCI serves as a rich and unique data source for outcomes research with substantial potential to inform clinical practice, particularly for diseases such as HNSCC that are prevalent among US veterans. We excluded patients with missing covariate data (n = 613; eight with p16 positive [p16+] nonoropharyngeal cancer). The 9447-patient sample represents patients from 120 VA hospitals in the United States. This study was approved by the local institutional review board, and the need for informed consent was waived.
Definitions of Tumor Site, p16 Status, Treatment, and Covariates
Tumor p16 status was identified through manual review and verification of all pathology reports. Tumors were categorized as p16 positive (p16+) if the pathology report referred to widespread, diffuse, or strong staining for p16, described staining in 70% or more of tumor cells (1,14), or indicated that the tumor was positive for p16 without other qualifiers. Tumors were categorized as p16 negative (p16-) if the p16 staining was noted as “negative,” less than 70%, or staining was only focally positive. These subgroups were combined based on their similar prognosis (Supplementary Figure 1, available online). We excluded patients whose tumor p16 status was determined only by neck lymph node biopsy or fine needle aspiration. In situ hybridization (ISH) results for high-risk human papillomavirus (HPV) subtypes were also available in a subset of patients with known p16 status. Primary site was manually verified for accuracy among the subset of patients with known p16 status. Patients with unclear tumor site or tumors that overlapped multiple subsites were excluded.
Covariates collected from tumor registry data included tumor stage (binary, T3-4 vs T1-2), nodal stage (binary, N0 vs any N), anatomic site (categorical), age at diagnosis (continuous, per 10 years), race (categorical, black/white/other), sex (binary, male vs female), year of diagnosis (continuous, per year), employment (binary, employed/unemployed), marital status (binary, married/unmarried), current tobacco use (binary, yes/no), treatment type (categorical; upfront surgery, definitive chemoradiotherapy, or definitive radiotherapy), and body mass index (BMI; continuous, per 5 kg/m2). Concurrent chemoradiotherapy was defined as any systemic therapy regimen starting two weeks before or after the radiation start date (including targeted therapies, such as cetuximab). The Charlson comorbidity index (CCI) was constructed using ICD-9/10 diagnoses in the year before diagnosis and was coded in multivariable models as a binary variable (0–1 vs ≥2) (28,29). Zip code–level median income and education (percentage with high school diploma) were obtained from the 2015 American Community Survey five-year estimates and were coded as continuous variables in multivariable models.
Outcomes
Outcomes included overall survival, cancer-specific mortality, and noncancer (ie, competing) mortality. Cancer-specific mortality was defined as death attributed to HNSCC, whereas competing mortality was defined as death attributed to any other cause. Cause of death was obtained via the National Death Index for deaths through 2014, and tumor registry data for deaths after 2014. Cause of death data from the National Death Index are widely considered reliable (30,31). Patients were censored at the last follow-up with a VA provider, current through March 2017. Survival time was measured from the date of first definitive surgical or radiation treatment; we did not observe any differences in the lag time between diagnosis and treatment across tumor site or p16 group (mean = 52 days overall; P = .91 for oropharyngeal vs nonoropharyngeal tumor site; P = .29 for p16-positive vs p16-negative; two-sided t test).
Statistical Analysis
Baseline patient characteristics were compared using chi-square tests for categorical variables and t tests or one-way analysis of variance for continuous variables. Survival outcomes were compared between p16+ and p16- groups using univariate and multivariable Cox proportional hazards regression models (32). The “full” Cox model included all covariates. To reduce overfitting in the setting of a limited number of mortality events, we also used a parsimonious Cox model in which we dropped the least clinically and/or statistically significant covariates (race, tobacco use, marital status, employment, zip code income, zip code, and education). Supplementary Table 1 (available online) shows the degrees of freedom (DF) and number of events for each model; note that all but two models had at least five events per DF. The proportional hazards assumption for the p16 term was verified for the p16 term using cumulative sums of martingale residuals and the supremum test. For the analysis of cause-specific events, we also performed a Fine-Gray competing risk regression to test effects on subdistribution hazards (33).
We tested the hypothesis that p16 status has a differential prognostic effect in oropharyngeal and nonoropharyngeal tumors using an interaction term in the parsimonious (reduced) multivariable Cox regression models. Finally, we used multivariable logistic regression to identify the main predictors of patients having known (“tested”) vs unknown (“untested”) tumor p16 status. All statistical tests were two-sided, and a P value of less than .05 was considered statistically significant. The statistical analysis was performed with SAS v9.4 (SAS Institute, Cary, NC) and R v3.3 (R Core Team, Vienna, Austria).
Results
The sample included 9447 patients, of whom 1448 (15.3%) had known tumor p16 status (387 nonoropharyngeal and 1061 oropharyngeal tumors) (Table 1; Supplementary Tables 2 and 3, available online). Of the 1448 patients with known p16 status, nonoropharyngeal patients tended to have greater comorbidity, lower BMI, higher unmarried rates, and higher rates of upfront surgery (Table 1). Nonoropharyngeal patients also tended to have more advanced tumor stage (67.7% T3 or T4 vs 40.5% for oropharyngeal) but lower nodal stage (29.7% N0 vs 6.7% for oropharyngeal) at presentation. Among nonoropharyngeal patients with known p16 status, laryngeal tumors were most common (47.0%), followed by oral cavity (33.1%) and hypopharynx (19.9%).
Table 1.
Characteristics of the sample with known p16 status, by tumor site
| Covariate | Non-OPX | OPX | P* |
|---|---|---|---|
| Sample size, No. (%) | 387 | 1061 | |
| Age at diagnosis, mean (SD), y | 64.9 (8.2) | 63.5 (7.9) | .005 |
| Male, No. (%) | 382 (98.7) | 1053 (99.2) | .34 |
| Race, No. (%) | |||
| White | 323 (83.5) | 901 (84.9) | .05 |
| Black | 59 (15.2) | 127 (11.9) | |
| Other | 5 (1.3) | 33 (3.1) | |
| Year of diagnosis, No. (%) | |||
| 2005–2008 | 12 (3.1) | 20 (1.8) | .35 |
| 2009–2012 | 138 (35.6) | 395 (37.2) | |
| 2013–2015 | 237 (61.2) | 646 (60.8) | |
| Charlson comorbidity index, No. (%) | |||
| 0 | 144 (37.2) | 584 (55.0) | <.001 |
| 1 | 76 (19.6) | 213 (20.1) | |
| 2 | 69 (17.8) | 132 (12.4) | |
| ≥3 | 98 (25.3) | 132 (12.4) | |
| Treatment | |||
| Chemo RT | 166 (42.9) | 724 (68.2) | <.001 |
| RT | 47 (12.1) | 89 (8.4) | |
| Upfront surgery | 174 (44.9) | 248 (23.4) | |
| Body mass index, mean (SD), mg/kg2 | 23.2 (5.7) | 25.0 (5.5) | <.001 |
| Employed, No. (%) | 42 (10.8) | 162 (15.3) | .03 |
| Married, No. (%) | 127 (32.8) | 426 (40.2) | .01 |
| Current tobacco use, No. (%) | 244 (63.0) | 433 (40.8) | <.001 |
| Zip code income in $1000, mean (SD) | 51.7 (18) | 53.5 (20) | .11 |
| Zip code % with high school diploma, mean (SD) | 85 (9.6) | 87 (7.6) | .002 |
| p16 tumor status | |||
| Negative | 255 (65.9) | 171 (16.1) | <.001 |
| Focally positive | 54 (13.9) | 42 (3.9) | |
| Strong/diffuse | 23 (5.9) | 398 (37.5) | |
| Positive NOS | 55 (14.2) | 450 (42.4) | |
| Tumor stage, No. (%) | |||
| 1 | 32 (8.3) | 252 (23.7) | <.001 |
| 2 | 93 (24.0) | 379 (35.7) | |
| 3 | 133 (34.4) | 223 (21.0) | |
| 4 | 129 (33.3) | 207 (19.5) | |
| Nodal stage, No. (%) | |||
| 0 | 115 (29.7) | 71 (6.7) | <.001 |
| 1 | 71 (18.3) | 167 (15.7) | |
| 2 | 198 (51.2) | 785 (73.9) | |
| 3 | 3 (0.8) | 38 (3.6) | |
| Tumor site, No. (%) | |||
| Hypopharynx | 77 (19.9) | –† | |
| Larynx | 182 (47.0) | – | |
| Oral cavity | 128 (33.1) | – |
P values were calculated with two-sample t test for continuous variables and chi-square test for categorical variables. All tests were two-sided. OPX = oropharyngeal site.
All patients in this group had oropharyngeal tumors.
The median follow-up was 3.0 years for the subset with known p16 status. Five-year overall survival was statistically significantly higher among p16+ compared with p16- patients for both oropharyngeal (76.8%, 95% CI = 72.3% to 80.8%, vs 50.2%, 95% CI = 41.6% to 58.9%, P < .001) and nonoropharyngeal tumors (75.6%, 95% CI = 65.2% to 86.0%, vs 46.4%, 95% CI = 38.5% to 54.2%, P < .001) (Figure 1A). Both cancer-specific mortality (Figure 1B) and competing mortality (Figure 1C) were lower in p16+ compared with p16- tumors, regardless of site. In unadjusted and adjusted regression models, p16+ tumors were strongly associated with improved overall survival and reduced hazards for cancer-specific and competing mortality in both the oropharyngeal (OS: hazard ratio [HR] = 0.53, 95% CI = 0.40 to 0.71, P < .001; CSS: HR = 0.50, 95% CI = 0.35 to 0.73, P < .001; CM: HR = 0.59, 95% CI = 0.38 to 0.93, P = .02) and nonoropharyngeal groups (OS: HR = 0.41, 95% CI = 0.25 to 0.69, P < .001; CSS: HR = 0.37, 95% CI = 0.18 to 0.77, P = .008; CM: HR = 0.46, 95% CI = 0.23 to 0.95, P = .04) (Table 2). In the multivariable Cox model including both oropharyngeal and nonoropharyngeal groups for effects on overall survival, cancer-specific mortality, and competing mortality, the interaction terms between p16 status and anatomic site were not statistically significant (P = .37, P = .45, and P = .55, respectively). Results were similar using the Fine-Gray model, with a statistically significant independent association between p16+ status and improved cancer-specific survival in both oropharyngeal and nonoropharyngeal tumors.
Figure 1.
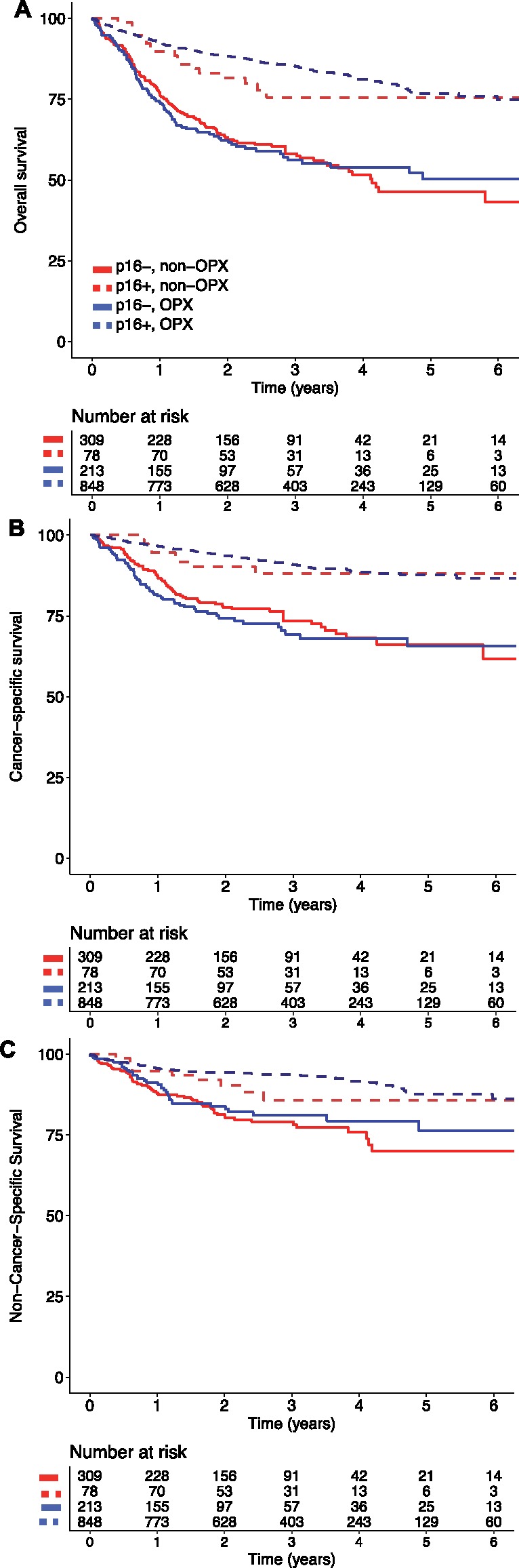
Survival outcomes by tumor site and tumor p16 status. Overall survival (A), cancer-specific survival (B), and competing mortality (C) by tumor site and tumor p16 status. Solid lines show p16- tumors, and dotted lines show p16+. OPX = oropharyngeal tumor.
Table 2.
Unadjusted and adjusted effects of p16 status on outcomes
| Oropharyngeal |
Nonoropharyngeal |
||||
|---|---|---|---|---|---|
| Outcome | Model | HR or SDHR* for p16+ (95% CI) | P† | HR or SDHR for p16+ (95% CI) | P† |
| OS | Unadjusted Cox | 0.32 (0.25 to 0.42) | <.001 | 0.42 (0.26 to 0.70) | <.001 |
| Full Cox‡ | 0.56 (0.42 to 0.76) | <.001 | 0.42 (0.25 to 0.71) | .001 | |
| Reduced Cox | 0.53 (0.40 to 0.71) | <.001 | 0.41 (0.25 to 0.69) | <.001 | |
| CSS | Unadjusted Cox | 0.27 (0.19 to 0.38) | <.001 | 0.36 (0.17 to 0.75) | .006 |
| Full Cox | 0.51 (0.35 to 0.75) | <.001 | 0.35 (0.17 to 0.75) | .006 | |
| Reduced Cox | 0.50 (0.35 to 0.73) | <.001 | 0.37 (0.18 to 0.77) | .008 | |
| Fine-Gray | 0.55 (0.37 to 0.80) | .002 | 0.41 (0.20 to 0.86) | .02 | |
| Competing mortality | Unadjusted Cox | 0.41 (0.27 to 0.62) | <.001 | 0.50 (0.25 to 1.01) | .05 |
| Full Cox | 0.69 (0.43 to 1.10) | .12 | 0.48 (0.23 to 1.00) | .049 | |
| Reduced Cox | 0.59 (0.38 to 0.93) | .02 | 0.46 (0.23 to 0.95) | .04 | |
| Fine-Gray | 0.70 (0.44 to 1.11) | .13 | 0.56 (0.27 to 1.13) | .11 | |
Hazard ratios are reported for Cox models, and subdistribution hazard ratios are reported for the Fine-Gray model. A ratio of less than 1 indicates reduced hazard (or subdistribution hazard) for the event in the p16+ group. CI = confidence interval; CSS = cancer-specific survival; HR = hazard ratio; OS = overall survival; SDHR = subdistribution hazard ratio.
Two-sided P values were calculated based on a Wald chi-square statistic.
The full Cox models adjusted for p16 status, race, Charlson comorbidity index, current tobacco use, tumor site, treatment modality, tumor stage, nodal stage, marital status, employment status, age at diagnosis, body mass index, and zip code education and income. The reduced Cox model adjusted for all of these except race, current tobacco use, marital status, employment status, and zip code education and income. The Fine-Gray model used the reduced set of covariates.
Among nonoropharyngeal tumors, the percentage of tumors that were p16+ were close to 20% in all subsites (larynx: 19.8%; hypopharynx: 20.8%; oral cavity: 20.3%). In unadjusted and adjusted Cox models, p16+ tumors were associated with improved cancer-specific survival in each nonoropharyngeal subsite, though the effects generally were not statistically significant, likely due to small sample sizes and a limited number of events in the nonoropharyngeal subsites (larynx: unadjusted HR = 0.40, 95% CI = 0.12 to 1.33, P = .13, adjusted HR = 0.43, 95% CI = 0.13 to 1.44, P = .17; oral cavity: unadjusted HR = 0.23, 95% CI = 0.05 to 0.96, P = .04, adjusted HR = 0.26, 95% CI = 0.06 to 1.11, P = .07; hypopharynx: unadjusted HR = 0.52, 95% CI = 0.15 to 1.77, P = .29, adjusted HR = 0.68, 95% CI = 0.18 to 2.60, P = .57).
A subset of the sample with known p16 status also had HPV in situ hybridization results reported for high-risk subtypes (163 of 1448 patients, 11.3%; 131 oropharyngeal, 32 nonoropharyngeal) (Supplementary Table 4, available online). Overall, five of 32 nonoropharyngeal patients were positive for high-risk HPV by in situ hybridization (15.6%) vs 62 of 131 (47.3%) for oropharyngeal patients. Discordance rates between HPV in situ hybridization and p16 results were higher in the oropharyngeal group, though this difference was not statistically significant (29.8%, 95% CI = 21.9% to 37.6%, for the oropharyngeal group vs 15.6%, 95% CI = 6.4% to 28%, discordant for the nonoropharyngeal group, P = .11).
We next investigated the characteristics of patients who were not tested for p16 status. We noted an increase in the utilization of p16 testing throughout the study period, with the percentage of oropharyngeal tumors tested for p16 increasing from 2.8% in 2008 to 67.1% in 2015 (Figure 2A). The percentage of nonoropharyngeal tumors tested for p16 similarly increased from 1.5% in 2008 to 30.8% in 2015. Patients who were not tested for p16 had inferior cancer-specific survival compared with tested patients, for both oropharyngeal (75.1%, 95% CI = 73.6% to 76.6%, vs 83.5%, 95% CI = 80.6% to 86.4%, at five years, P < .001 by log-rank test) and nonoropharyngeal cancer (61.9%, 95% CI = 60.1% to 63.7%, vs 70.7%, 95% CI = 64.1% to 77.2%, P < .001) (Figure 2B). The five-year overall survival estimates among untested oropharyngeal and nonoropharyngeal tumors were 53.1% (95% CI = 51.4% to 54.7%) and 34.5% (95% CI = 32.9% to 35.9%), respectively. On univariate analysis, compared with p16-positive patients, untested patients tended to be diagnosed earlier in the study period, to be current tobacco users, to have higher tumor stage and lower nodal stage, and to have nonoropharyngeal tumors (Supplementary Table 3, available online). In the multivariable logistic regression model, the most statistically significant predictors of increased odds of receiving p16 testing were younger age at diagnosis, more recent year of diagnosis, oropharyngeal tumor location, higher body mass index, and higher zip code–level income (Table 3).
Figure 2.
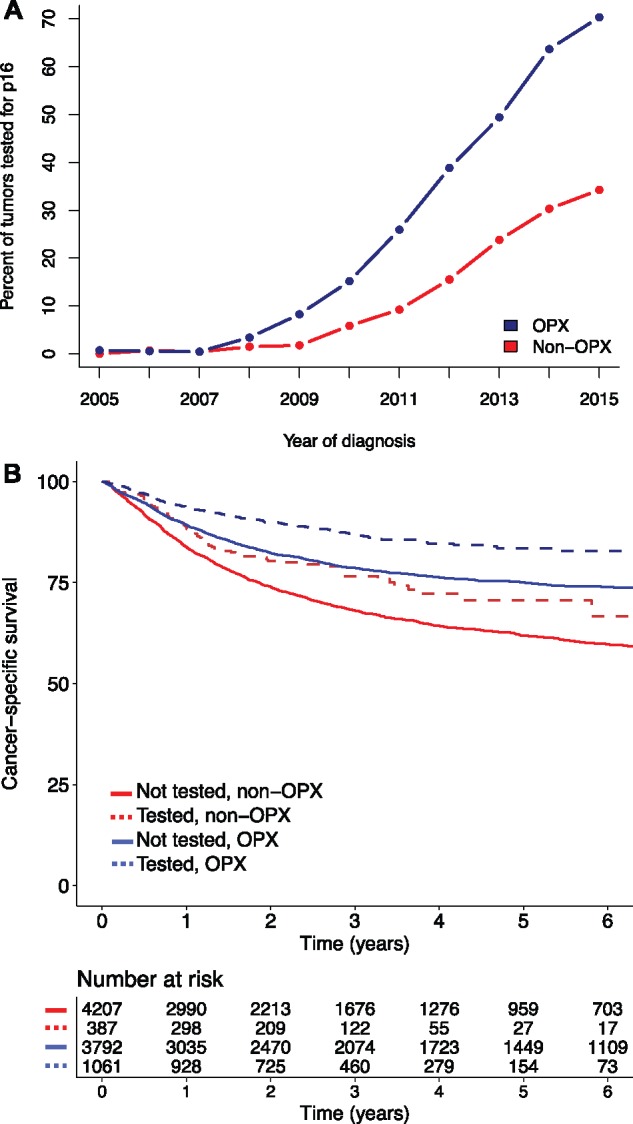
Comparison of patients tested or not tested for p16 status. A) Percentage of all tumors that were tested for p16 from 2005 to 2015. B) Cancer-specific survival for patients with oropharyngeal or nonoropharyngeal tumors who were either tested for p16 (dotted) or not tested (solid). Patients who were tested for p16 had improved survival for both tumor locations. OPX = oropharyngeal tumor.
Table 3.
Results of logistic regression for predictors of receiving p16 testing
| Covariate | OR (95% CI)* | P† |
|---|---|---|
| Age at diagnosis (per 10 y) | 0.89 (0.82 to 0.97) | .02 |
| Race | ||
| White | 1.00(ref) | |
| Black | 0.99 (0.83 to 1.19) | .94 |
| Other | 0.99 (0.75 to 1.31) | .96 |
| Female | 0.61 (0.32 to 1.19) | .15 |
| Body mass index (per 5 kg/m2) | 1.07 (1.01 to 1.14) | .03 |
| Charlson comorbidity index | ||
| 0–1 | 1.00 (ref) | |
| ≥ 2 | 0.91 (0.79 to 1.06) | .24 |
| Current tobacco use | 0.98 (0.85 to 1.13) | .81 |
| Zip code mean income (per $10 000) | 1.15 (1.10 to 1.20) | <.001 |
| Zip code % with high school diploma (per 10%) | 0.93 (0.84 to 1.03) | .15 |
| Employed | 1.12 (0.91 to 1.37) | .29 |
| Married | 0.92 (0.80 to 1.06) | .26 |
| Year of diagnosis (per year) | 1.79 (1.73 to 1.85) | <.001 |
| Tumor stage | ||
| 1–2 | 1.00 (ref) | |
| 3–4 | 0.98 (0.84 to 1.13) | .76 |
| Nodal stage | ||
| 0 | 1.00(ref) | |
| ≥1 | 1.04 (0.84 to 1.29) | .70 |
| Tumor site | ||
| Oropharynx | 1.00 (ref) | |
| Hypopharynx | 0.93 (0.76 to 1.15) | .53 |
| Larynx | 0.52 (0.44 to 0.61) | <.001 |
| Oral cavity | 0.94 (0.78 to 1.13) | .49 |
| Treatment | ||
| Upfront surgery | 1.00 (ref) | |
| ChemoRT | 0.94 (0.84 to 1.04) | .21 |
| RT | 0.91 (0.78 to 1.06) | .24 |
An odds ratio greater than one indicates a higher odds of receiving p16 testing. chemoRT = chemoradiotherapy; CI = confidence interval; OR = odds ratio; RT = radiotherapy.
Two-sided P values were calculated based on a Wald chi-square statistic.
Discussion
Our findings support the hypothesis that p16 has a similar prognostic role in oropharyngeal and nonoropharyngeal tumors, in contrast to the conclusions from other recent studies. In particular, we found no evidence to support the conclusion that p16 has a different effect on survival or cancer-specific mortality after adjusting for background competing events, treatment, and potential confounders such as stage, age, comorbidity, and BMI. Our findings provide motivation to increase testing for p16 status in oropharyngeal and nonoropharyngeal HNSCC alike.
This is the first study, to our knowledge, to conduct a thorough analysis of effects of p16 status on cause-specific events in HNSCC. As is clear in Figure 1 and Table 2, the prognostic effect of p16 positivity on survival in both oropharyngeal and nonoropharyngeal HNSCC is partially attributable to a lower hazard for cancer-specific mortality, indicating a more indolent natural history for p16+ cancers, and partially attributable to a lower hazard for competing mortality, indicating that patients with p16+ disease are healthier and less likely to die from competing causes (even after controlling for several known predictors). Investigations of treatments and prognostic factors that rely solely on overall survival, progression-free survival, and other event-free survival end points are potentially misleading, due to confounding from effects on nonspecific events, including death from noncancer causes (24,25,34,35). This can make conclusions highly susceptible to variation in the background incidence of competing events, undermining the inferences from such studies.
Several studies, including a recent publication by Fakhry et al. (8), have previously concluded that neither HPV status (determined by in situ hybridization) nor p16 (determined by immunohistochemistry) has a prognostic role in nonoropharyngeal HNSCC. Another important study by Chung et al. (14) examined effects of both HPV and p16 for HNSCC patients treated on Radiation Therapy Oncology Group trials and concluded that only the latter was prognostically significant, albeit less so than in oropharyngeal cancer. Possible reasons for the contrast between our findings and other studies should be considered.
Our study addresses a large population representing 120 VA centers nationwide. The VA database provides a rich source of both treatment and tumor-related prognostic factors and known predictors of competing events, with greater power to estimate adjusted effects within subgroups. As such, our sample is likely to be more generalizable and representative of community practice patterns than single or oligo-institutional or trial-based data. The total number of p16+ nonoropharyngeal tumors was comparatively higher in our study, increasing power to detect effects, especially in multivariable models. Our study also examined the cause-specific effects of p16 status, which provides different interpretations regarding its prognostic role.
Unlike other studies, however, due to selective and low frequency of testing in nonoropharyngeal cancer patients, we are unable to comment on the prognostic role of HPV, which has higher rates of discordance with p16 in nonoropharyngeal compared with oropharyngeal cancer (14). Our cohort was also more heterogeneous in terms of treatment. Though we controlled for treatment modality in our analyses, it is possible that the prognostic value of p16 may interact with treatment in more subtle ways. Other key differences are the exclusion of nasopharyngeal tumors from our study, in contrast to Fakhry et al., and the inclusion of surgical patients, in contrast to Chung et al. Additionally, p16 status was not centrally reviewed in this study. While this could introduce misclassification if pathologists did not apply uniform definitions of p16 positivity, we would expect any such discordance or misclassification only to attenuate our ability to detect associations between p16 and outcomes, biasing our results toward the null. Moreover, the rate of discordance between peripheral/community and central determinations of p16 status by immunohistochemistry is quite low (Richard Jordan, personal communication). Of note, the proportion of nonoropharyngeal tumors in our study that were p16+ (20%) was higher than Fakhry et al. (10%) and similar to Chung et al. (20%). Lastly, it should also be noted that our sample represents an almost exclusively male population; effects in females could well be different than what we observed (8).
A general problem affecting many studies on this subject is the low rate of routine testing in nonoropharyngeal HNSCC. The nonoropharyngeal tumors that were tested are likely to be highly selected, perhaps due to ambiguity in the primary site, patient risk factors, and/or variations in providers’ practice. Thus, the true prevalence of p16 positivity in nonoropharyngeal HNSCC could not be accurately assessed in our study and may be lower than what we observed. Additionally, both patients with p16+ and p16- disease had lower cancer-specific mortality than untested patients, further indicating that tested patients are likely a selected subpopulation with unmeasured characteristics that favor improved survival. However, the presence of selection bias in p16 testing does not necessarily imply a different prognostic value of p16 in the untested population. Although we do not consider it likely that patients with p16+ disease were more likely to be tested than p16- patients with the same risk factors, or that this would differ by primary site, we cannot exclude this possibility. Future studies using population-based sampling methods in subjects undergoing routine testing would be desirable to further illuminate the prognostic role of p16 in nonoropharyngeal HNSCC, including the nasopharynx and other subsites.
HPV is widely known for driving tumorigenesis in the oropharynx, as opposed to the oral cavity, larynx, or hypopharynx, where the majority of cancers are still caused by classical risk factors like smoking and alcohol use. There has been controversy over whether the presence of p16 in nonoropharyngeal tumors represents a truly oncogenic HPV infection, a bystander (nononcogenic) HPV infection, or an entirely unrelated process. However, p16 can be a useful prognostic tool irrespective of its relationship (or lack thereof) to HPV. Even if less likely to be positive in nonoropharyngeal compared with oropharyngeal HNSCC, p16 may still be equally valuable as a prognostic or predictive biomarker.
In conclusion, our findings support the hypothesis that p16 has a similar prognostic role in nonoropharyngeal (specifically laryngeal, hypopharyngeal, and oral cavity) and oropharyngeal HNSCC, arguing for increased testing in the nonoropharyngeal HNSCC population to guide future research.
Funding
This work was supported by the National Institutes of Health (grant number TL1TR001443) and the Center for Translational Radiation Medicine and Imaging in La Jolla, California.
Notes
Affiliations of authors: Department of Radiation Medicine and Applied Sciences (AKB, EJS, LV, KZ, JDM, LKM), Division of Biostatistics and Bioinformatics, Department of Family Medicine and Public Health (HS), Moores Cancer Center (CN), Division of Otolaryngology, Department of Surgery (JAC), and Division of Hematology and Oncology, Department of Medicine (EEWC), University of California San Diego, La Jolla, CA.
The funders had no role in the design of the study; the collection, analysis, or interpretation of the data; the writing of the manuscript; or the decision to submit the manuscript for publication.
The authors have no conflicts of interest to disclose.
Supplementary Material
References
- 1. Ang KK, Harris J, Wheeler R, et al. Human papillomavirus and survival of patients with oropharyngeal cancer. N Engl J Med. 2010;3631:24–35. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2. Gillison ML, D'Souza G, Westra W, et al. Distinct risk factor profiles for human papillomavirus type 16-positive and human papillomavirus type 16-negative head and neck cancers. J Natl Cancer Inst. 2008;1006:407–420. [DOI] [PubMed] [Google Scholar]
- 3. Ragin CC, Taioli E.. Survival of squamous cell carcinoma of the head and neck in relation to human papillomavirus infection: Review and meta-analysis. Int J Cancer. 2007;1218:1813–1820. [DOI] [PubMed] [Google Scholar]
- 4. Fakhry C, Westra WH, Li S, et al. Improved survival of patients with human papillomavirus-positive head and neck squamous cell carcinoma in a prospective clinical trial. J Natl Cancer Inst. 2008;1004:261–269. [DOI] [PubMed] [Google Scholar]
- 5. Ndiaye C, Mena M, Alemany L, et al. HPV DNA, E6/E7 mRNA, and p16INK4a detection in head and neck cancers: A systematic review and meta-analysis. Lancet Oncol. 2014;1512:1319–1331. [DOI] [PubMed] [Google Scholar]
- 6. O'Sullivan B, Huang SH, Su J, et al. Development and validation of a staging system for HPV-related oropharyngeal cancer by the International Collaboration on Oropharyngeal cancer Network for Staging (ICON-S): A multicentre cohort study. Lancet Oncol. 2016;174:440–451. [DOI] [PubMed] [Google Scholar]
- 7. D'Souza G, Westra WH, Wang SJ, et al. Differences in the prevalence of human papillomavirus (HPV) in head and neck squamous cell cancers by sex, race, anatomic tumor site, and HPV detection method. JAMA Oncol. In press. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Fakhry C, Westra WH, Wang SJ, et al. The prognostic role of sex, race, and human papillomavirus in oropharyngeal and nonoropharyngeal head and neck squamous cell cancer. Cancer. 2017;1239:1566–1575. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Young RJ, Urban D, Angel C, et al. Frequency and prognostic significance of p16(INK4A) protein overexpression and transcriptionally active human papillomavirus infection in laryngeal squamous cell carcinoma. Br J Cancer. 2015;1126:1098–1104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Combes JD, Franceschi S.. Role of human papillomavirus in nonoropharyngeal head and neck cancers. Oral Oncol. 2014;505:370–379. [DOI] [PubMed] [Google Scholar]
- 11. Meshman J, Wang PC, Chin R, et al. Prognostic significance of p16 in squamous cell carcinoma of the larynx and hypopharynx. Am J Otolaryngol. 2017;381:31–37. [DOI] [PubMed] [Google Scholar]
- 12. Wilson DD, Rahimi AS, Saylor DK, et al. p16 not a prognostic marker for hypopharyngeal squamous cell carcinoma. Arch Otolaryngol Head Neck Surg. 2012;1386:556–561. [DOI] [PubMed] [Google Scholar]
- 13. Lai K, Killingsworth M, Matthews S, et al. Differences in survival outcome between oropharyngeal and oral cavity squamous cell carcinoma in relation to HPV status. J Oral Pathol Med. 2017;468:574–582. [DOI] [PubMed] [Google Scholar]
- 14. Chung CH, Zhang Q, Kong CS, et al. p16 protein expression and human papillomavirus status as prognostic biomarkers of nonoropharyngeal head and neck squamous cell carcinoma. J Clin Oncol. 2014;3235:3930–3938. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Sivars L, Bersani C, Grun N, et al. Human papillomavirus is a favourable prognostic factor in cancer of unknown primary in the head and neck region and in hypopharyngeal cancer. Mol Clin Oncol. 2016;56:671–674. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Satgunaseelan L, Virk SA, Lum T, et al. p16 expression independent of human papillomavirus is associated with lower stage and longer disease-free survival in oral cavity squamous cell carcinoma. Pathology. 2016;485:441–448. [DOI] [PubMed] [Google Scholar]
- 17. Alsidawi S, Westin GFM, Chintakuntlawar AV, et al. The impact of HPV infection on survival of patients with nonoropharyngeal head and neck cancer. J Clin Oncol. 2017;35(15_suppl):6048–6048. [Google Scholar]
- 18. Wookey V, Appiah AK, Kallam A, et al. HPV status and survival in nonoropharyngeal squamous cell carcinoma of the head and neck. J Clin Oncol. 2017;35(15_suppl):6046–6046. [Google Scholar]
- 19. Feinstein AJ, Shay SG, Chang E, et al. Treatment outcomes in veterans with HPV-positive head and neck cancer. Am J Otolaryngol. 2017;382:188–192. [DOI] [PubMed] [Google Scholar]
- 20. Silva SD, Nonogaki S, Soares FA, et al. p16 (INK4a) has clinicopathological and prognostic impact on oropharynx and larynx squamous cell carcinoma. Braz J Med Biol Res. 2012;4512:1327–1333. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.NCCN. National Comprehensive Cancer Network guidelines. Version 2.2017. Head and neck cancers. https://www.nccn.org/professionals/physician_gls/pdf/head-and-neck.pdf. Accessed February 9, 2018. [DOI] [PubMed]
- 22. Rose BS, Jeong JH, Nath SK, et al. Population-based study of competing mortality in head and neck cancer. J Clin Oncol. 2011;2926:3503–3509. [DOI] [PubMed] [Google Scholar]
- 23. Piccirillo JF, Tierney RM, Costas I, et al. Prognostic importance of comorbidity in a hospital-based cancer registry. JAMA. 2004;29120:2441–2447. [DOI] [PubMed] [Google Scholar]
- 24. Mell LK, Jeong JH.. Pitfalls of using composite primary end points in the presence of competing risks. J Clin Oncol. 2010;2828:4297–4299. [DOI] [PubMed] [Google Scholar]
- 25. Mell LK, Carmona R, Gulaya S, et al. Cause-specific effects of radiotherapy and lymphadenectomy in stage I-II endometrial cancer: A population-based study. J Natl Cancer Inst. 2013;10521:1656–1666. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Veterans Affairs Health Services research and development: VA Informatics and Computing Infrastructure (VINCI). https://www.hsrd.research.va.gov/for_researchers/vinci/. Accessed February 9, 2018.
- 27. Facility Oncology Registry Data Standards. American College of Surgeons; 2016. https://www.facs.org/∼/media/files/quality%20programs/cancer/ncdb/fords%202016.ashx. Accessed February 9, 2018.
- 28. Charlson ME, Pompei P, Ales KL, et al. A new method of classifying prognostic comorbidity in longitudinal studies: Development and validation. J Chronic Dis. 1987;405:373–383. [DOI] [PubMed] [Google Scholar]
- 29. Quan H, Sundararajan V, Halfon P, et al. Coding algorithms for defining comorbidities in ICD-9-CM and ICD-10 administrative data. Med Care. 2005;4311:1130–1139. [DOI] [PubMed] [Google Scholar]
- 30. Sathiakumar N, Delzell E, Abdalla O.. Using the National Death Index to obtain underlying cause of death codes. J Occup Environ Med. 1998;409:808-813. [DOI] [PubMed] [Google Scholar]
- 31. Cowper D, Kubal J, Maynard C, et al. A primer and comparative review of major U.S. mortality databases. Ann Epidemiol. 2002;12:462–468. [DOI] [PubMed] [Google Scholar]
- 32. Cox DR. Regression Models and Life-Tables. J R Stat Soc Series B Stat Methodol. 1972;342:187–220. [Google Scholar]
- 33. Fine J, Gray R.. A proportional hazards model for the subdistribution of a competing risk. J Am Stat Assoc. 1999;94:496–509. [Google Scholar]
- 34. Nout RA, Fiets WE, Struikmans H, et al. The in- or exclusion of non-breast cancer related death and contralateral breast cancer significantly affects estimated outcome probability in early breast cancer. Breast Cancer Res Treat. 2008;1093:567–572. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35. Mell LK, Zakeri K, Rose BS.. On lumping, splitting, and the nosology of clinical trial populations and end points. J Clin Oncol. 2014;3210:1089–1090. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.


