Abstract
Background
Endometrial cancer is the second most common cancer among female cancer survivors in the United States. Cardiovascular disease is the leading cause of death among endometrial cancer survivors. Studies that examine long-term cardiovascular outcomes among endometrial cancer survivors are critical.
Methods
Cohorts of 2648 endometrial cancer survivors diagnosed between 1997 and 2012 and 10 503 age-matched women from the general population were identified. Cardiovascular disease diagnoses were identified from electronic medical records and statewide ambulatory surgery and statewide inpatient data. Cox regression models were used to estimate hazard ratios (HRs) at one to five years, more than five to 10 years, and more than 10 years after cancer diagnosis.
Results
Between one and five years after diagnosis, increased cardiovascular risks among endometrial cancer survivors were observed for phlebitis, thrombophlebitis, and thromboembolism (HR = 2.07, 99% confidence interval [CI] = 1.57 to 2.72), pulmonary heart disease (HR = 1.74, 99% CI = 1.26 to 2.40), and atrial fibrillation (HR = 1.50, 99% CI = 1.07 to 2.11). At more than five to 10 years, some elevated risk persisted for cardiovascular diseases. Compared with patients who had surgery, patients who additionally had radiation therapy and/or chemotherapy were at increased risk for heart and circulatory system disorders between one and five years after cancer diagnosis. Older age and obesity were also risk factors for hypertension and heart disease among endometrial cancer survivors.
Conclusions
Endometrial cancer survivors are at higher risk for various adverse long-term cardiovascular outcomes compared with women from the general population. This study suggests that increased monitoring for cardiovascular diseases may be necessary for endometrial cancer patients for 10 years after cancer diagnosis.
Endometrial cancer is the fourth most commonly diagnosed cancer among women in the United States and the second most common cancer among female cancer survivors (1,2). Incidence rates among women younger than age 50 years have been increasing by 1.3% per year since 1988 and by 1.9% among women older than age 50 years since 2005 (3). It was the sixth most common cause of death from cancer among women in the United States in 2017, with an estimated 10 920 deaths (2). As of 2017, there were an estimated 757 200 endometrial cancer survivors in the United States (1).
Previous studies of long-term health effects among endometrial cancer survivors have focused largely on quality of life, mental health, obesity, and adverse sexual side effects (4–13), though there have been several studies that have examined long-term cardiovascular outcomes among endometrial cancer survivors (14–16). A study among long-term survivors of breast, prostate, colorectal, ovarian, and endometrial cancers reported that endometrial cancer survivors (n = 194) were diagnosed with an average of 2.4 comorbid conditions after their cancer diagnosis, which was second only to breast cancer survivors (with 2.9 comorbid conditions) (14). In addition, 21.2% of endometrial cancer survivors experienced at least one adverse cardiovascular outcome after cancer diagnosis, which was higher than any of the other cancer sites.
Based on Surveillance, Epidemiology, and End Results (SEER) data, cardiovascular disease was the leading cause of death more than five years after cancer diagnosis among 33 232 endometrial cancer survivors diagnosed between 1973 and 1988 (15). The proportion of women diagnosed with endometrial cancer who died of cardiovascular disease in this population (42.1%) was higher than that of all women during that same time period (35%). Another study using SEER data for endometrial cancer survivors diagnosed between 1988 and 2002 reported that cardiovascular disease remained the leading cause of death among endometrial cancer survivors between five and ten years after diagnosis (16).
Obesity is a risk factor for both endometrial cancer and cardiovascular disease (17). The proportion of the American population that is obese has more than doubled since 1960, from 15.8% to 36.6%, and the proportion that is extremely obese is more than six times greater than in 1960, from 1.4% to 8.6% (18). This increase in the prevalence of obesity in conjunction with changes in other endometrial cancer risk factors (19–23) may contribute to the increased incidence of endometrial cancer observed over the past several decades.
Many of the studies that have examined long-term outcomes among endometrial cancer survivors had small sample sizes, lacked a comparison group, or relied on patient-reported outcomes (4–13). Studies that examine risk for long-term cardiovascular outcomes among endometrial cancer survivors are becoming increasingly more critical because of the high overall survival rate among individuals diagnosed with endometrial cancer, the large number of endometrial cancer survivors, the projected increase in the number of endometrial cancer diagnoses (3), the introduction of more complex therapies, and the high mortality due to cardiovascular disease among endometrial cancer survivors.
Methods
Data Collection
An initial cohort of 3621 endometrial cancer survivors was identified using the Utah Population Database. Diagnosis data were available from the statewide SEER Utah Cancer Registry for women age 18 years and older diagnosed with invasive first primary endometrial cancer between 1997 and 2012 in the state of Utah (SEER ICD-O-3 codes: C54.0-C55.9). Endometrial cancer histological subtypes adenocarcinoma, endometrioid, mucinous adenocarcinoma, and adenocarcinoma with squamous differentiation were classified as type I (ICD-O-3 morphology codes: 8140, 8260, 8380, 8382, 8480, 8482, 8560, and 8570), and clear cell carcinomas and papillary serous carcinomas as type II (ICD-O-3 morphology codes: 8310, 8441, and 8460) (24). Endometrial cancer survivors were matched on birth year and birth state with up to five women from the general population. Studies using Utah Population Database (UPDB) data have been approved by the University of Utah’s Resource for Genetic and Epidemiologic Research and its Institutional Review Board.
Outcome data used for this study included statewide ambulatory and inpatient data from the Utah Department of Health and electronic medical record data from Intermountain Health Care and the University of Utah Health Sciences Center. Utah is considered to have a minimal percentage of residents who seek health care out of the state, based on a report by the National Association of Health Data Organizations that reviewed interstate exchange of nonresident data for health research and public health purposes (25). Additionally, according to the US Census Bureau’s state-to-state migration flow data for 2016, approximately 2.9% of Utahns left the state; thus the out-migration rate is fairly low (26). Data from the Utah Population Database included records from the Utah Cancer Registry, Utah driver’s licenses, vital records, and the Utah Department of Health. We also identified tobacco smokers with the ICD-9 code for “tobacco use disorders” 305.1, ICD-10 codes for nicotine dependence, and with CPT codes for tobacco cessation counseling based on the American Academy of Family Physicians coding guidelines (27).
A total of 153 endometrial cancer patients were excluded because their cancer was not staged, 470 because grade was missing, 285 because follow-up time did not exceed one year, and 65 because their Utah residence did not exceed one year. Stage and grade were necessary for our sample because we were interested in their potential role in risk for cardiovascular outcomes. There were 2648 endometrial cancer survivors and 10 503 women from the general population included in the final sample.
Outcome data included all available ICD-9 diagnosis codes and diagnosis dates. The Clinical Classification Software developed by the Health Cost and Utilization Project was used to categorize ICD-9 codes into four levels of specificity (levels 1–4) (additional details are provided in the Supplementary Methods, available online; ICD codes are provided in Supplementary Table 1, available online) (28). Long-term cardiovascular outcomes were identified from one to five years, more than five to 10 years, and 10 or more years after endometrial cancer diagnosis. Follow-up time for incident cases of each outcome was calculated from the endometrial cancer survivor’s initial cancer diagnosis to the date of diagnosis, last date of follow-up, or date of death. Individuals who did not have that outcome were censored at the date of last follow-up if that date fell within the analysis time period. Level 3 to 4 outcomes diagnosed prior to the start of each analysis time period were considered prevalent cases of those outcomes, and individuals were excluded from the relevant models. Level 2 outcomes were broader and contained multiple disparate conditions; thus we did not exclude prevalent diagnoses.
Statistical Analysis
Chi-square tests were used to compare baseline characteristics between the endometrial cancer survivor and general population cohorts. Multivariable Cox proportional hazard models were used to calculate hazard ratios for long-term cardiovascular outcomes from one to five years, more than five to 10 years, and 10 or more years after endometrial cancer diagnosis. We used 99% confidence intervals to account for multiple testing due to the large number of outcomes. Multivariable models were adjusted for matching factors, baseline body mass index (BMI), baseline Charlson Comorbidity Index (CCI) (29), and race. Cox proportional hazard models were also used to investigate risk factors such as treatment type, stage, grade, age at diagnosis, year of diagnosis, race, BMI, smoking, family history of cardiovascular disease, and baseline hypercholesterolemia for hypertension, heart disease, arterial diseases, and diseases of the veins and lymphatics among endometrial cancer survivors. We adjusted for tobacco smoking because smoking has been shown to be inversely associated with endometrial cancer (30) and smoking is a risk factor for cardiovascular disease (31).
The proportional hazards assumption was checked for each model using a test for nonzero slope of the Schoenfeld residuals vs time. Models that were in violation of the proportional hazards assumption were then tested with flexible parametric survival models with restricted cubic splines. Hazard ratios from the Cox proportional hazard models were reported where there were no substantive differences.
Baseline BMI values at least one year prior to endometrial cancer diagnosis were calculated from the driver’s license records for both cohorts. For individuals missing BMI, values were imputed using a linear regression model that included cancer diagnosis, baseline CCI, and age at endometrial cancer diagnosis as covariates. Models were run with and without the imputed values to assure that the inferences did not change due to the imputation of BMI.
All statistical tests were two-sided, and a P value of less than .05 was considered statistically significant, except for the results in Tables 3–5, for which a P value of less than .01 was considered statistically significant.
Table 3.
Hypertension and cerebrovascular disease risk at 1–5 and >5–10 years after cancer diagnosis among endometrial cancer survivors in comparison with a general population cohort of women*
| Diseases | 1–5 y | >5–10 y | ||||
|---|---|---|---|---|---|---|
| Patients | General population |
Patients | General population |
|||
| No. (%) | No. (%) | HR (99% CI) | No. (%) | No. (%) | HR (99% CI) | |
| Hypertension | 1237 (46.7) | 3431 (32.7) | 1.52 (1.37 to 1.68) | 874 (33) | 2906 (27.7) | 1.25 (1.11 to 1.42) |
| Essential hypertension | 202 (17.3) | 1165 (16.1) | 0.98 (0.77 to 1.25) | 137 (14.2) | 814 (13.5) | 0.93 (0.67 to 1.28) |
| Hypertension with comp./secondary hypertension | 106 (4.2) | 241 (2.4) | 1.73 (1.22 to 2.45) | 101 (4.2) | 241 (2.4) | 1.45 (0.99 to 2.12) |
| Hypertensive heart and/or renal disease | 90 (3.5) | 213 (2.1) | 1.67 (1.14 to 2.43) | 95 (3.9) | 218 (2.2) | 1.53 (1.03 to 2.28) |
| Cerebrovascular disease | 145 (5.5) | 560 (5.3) | 1.05 (0.81 to 1.37) | 124 (4.7) | 494 (4.7) | 1.05 (0.77 to 1.42) |
| Acute cerebrovascular disease (acute stroke) | 56 (2.2) | 212 (2.1) | 1.22 (0.80 to 1.87) | 47 (1.9) | 203 (2) | 1.07 (0.66 to 1.74) |
| Occlusion of cerebral arteries | 32 (1.2) | 125 (1.2) | 1.05 (0.60 to 1.84) | 32 (1.7) | 101 (1.3) | 1.01 (0.56 to 1.83) |
| Occlusion or stenosis of precerebral arteries | 27 (1) | 127 (1.2) | 0.82 (0.45 to 1.51) | 26 (1) | 112 (1.1) | 0.85 (0.42 to 1.73) |
| Transient cerebral ischemia (transient ischemic attack) | 35 (1.4) | 149 (1.5) | 0.81 (0.48 to 1.39) | 34 (1.3) | 106 (1.1) | 1.33 (0.73 to 2.43) |
Models adjusted for race, baseline body mass index, baseline Charlson Comorbidity Index, and smoking. The following outcomes were evaluated, but no elevated risk was observed: other hypertensive complications, intracranial hemorrhage, acute but ill-defined cerebrovascular accident, other and ill-defined cerebrovascular disease, late effects of cerebrovascular disease. CI = confidence interval; HR = hazard ratio.
Results
The endometrial cancer survivors cohort had a higher proportion of obese individuals (44.2% vs 19.2%) than the general population cohort (P < .001) (Table 1). Approximately 81.5% of the endometrial cancer survivors were diagnosed with low-grade tumors, and 80.3% with local-stage disease (Table 2).
Table 1.
Demographic characteristics among endometrial cancer survivor and general population cohorts
| Characteristics | Endometrial cancer | General population | P* |
|---|---|---|---|
| (n = 2648) | (n = 10 503) | ||
| No. (%) | No. (%) | ||
| Birth year | |||
| <1920 | 116 (4.4) | 473 (4.5) | |
| 1920–1929 | 310 (11.7) | 1192 (11.4) | |
| 1930–1939 | 541 (20.4) | 2015 (19.2) | |
| 1940–1949 | 787 (29.7) | 3085 (29.4) | |
| 1950–1959 | 591 (22.3) | 2444 (23.3) | |
| >1960 | 303 (11.4) | 1294 (12.3) | .54 |
| Race | |||
| White | 2525 (95.4) | 9617 (91.6) | |
| Black | 10 (0.4) | 29 (0.3) | |
| American Indian/Alaskan Native | 111 (1.1) | 32 (1.2) | |
| Asian | 277 (2.6) | 19 (0.7) | |
| Pacific Islander | 60 (0.6) | 48 (1.8) | |
| Unknown | 14 (0.5) | 409 (3.9) | <.001 |
| Vital status | |||
| Alive | 1924 (72.7) | 8906 (84.8) | |
| Dead | 724 (27.3) | 1597 (15.2) | <.001 |
| Baseline body mass index, kg/m2 | |||
| <18.5 | 18 (0.7) | 307 (2.9) | |
| 18.5–24.9 | 645 (24.4) | 4994 (47.6) | |
| 25–29.0 | 814 (30.7) | 3190 (30.7) | |
| >30 | 1171 (44.2) | 2012 (19.2) | <.001 |
| Age attained at the end of follow-up, y | |||
| <50 | 161 (6.1) | 697 (6.6) | |
| 50–59 | 389 (14.7) | 1697 (16.2) | |
| 60–69 | 826 (31.2) | 3142 (29.9) | |
| 70–79 | 692 (26.1) | 2637 (25.1) | |
| 80–89 | 442 (16.7) | 1787 (17) | |
| 90+ | 138 (5.2) | 543 (5.2) | .3 |
| Follow-up period, y | |||
| 1–5 | 726 (27.4) | 2742 (26.1) | |
| >5–10 | 1028 (38.8) | 4062 (38.7) | |
| >10–15 | 595 (22.5) | 2576 (24.5) | |
| 15+ | 299 (11.3) | 1123 (10.7) | .12 |
| Smoking status | |||
| No | 2281 (86.1) | 9045 (86.1) | |
| Yes | 367 (13.9) | 1458 (13.9) | .98 |
| Family history of heart disease in first-degree relatives | |||
| No | 1375 (51.9) | 5320 (50.7) | |
| Yes | 1273 (48.1) | 5183 (49.4) | .24 |
| Previous hypercholesterolemia | |||
| No | 2168 (81.9) | 9008 (85.8) | <.001 |
| Yes | 480 (18.1) | 1495 (14.2) | |
| Previous cardiovascular disease | |||
| No | 1183 (44.7) | 5620 (53.5) | <.001 |
| Yes | 1465 (55.3) | 4883 (46.5) | |
Two-sided chi-square test.
Table 2.
Clinical characteristics among endometrial cancer survivors (n = 2648)
| Characteristic | No. (%) |
|---|---|
| Diagnosis year | |
| 1997–2000 | 563 (21.3) |
| 2001–2003 | 471 (17.8) |
| 2004–2006 | 483 (18.2) |
| 2007–2009 | 572 (21.6) |
| 2010–2012 | 559 (21.1) |
| Age at diagnosis, y | |
| <40 | 140 (5.3) |
| 40–49 | 307 (11.6) |
| 50–59 | 786 (29.7) |
| 60–69 | 758 (28.6) |
| 70–79 | 451 (17) |
| 80+ | 206 (7.8) |
| Grade | |
| Grade I (well differentiated) | 1314 (49.6) |
| Grade II (moderately differentiated) | 845 (31.9) |
| Grade III (poorly differentiated) | 421 (15.9) |
| Grade IV (undifferentiated) | 68 (2.6) |
| Stage | |
| Local | 2121 (80.3) |
| Regional | 437 (16.4) |
| Advanced | 90 (3.3) |
| Histology | |
| Endometrioid adenocarcinoma | 1853 (70) |
| Adenocarcinoma with squamous differentiation | 56 (2.1) |
| Serous adenocarcinoma | 87 (3.3) |
| Clear cell adenocarcinoma | 17 (0.6) |
| Mixed cell adenocarcinoma | 47 (1.8) |
| Mucinous adenocarcinoma | 45 (1.7) |
| Carcinosarcoma | 26 (1) |
| Stromal sarcoma | 44 (1.7) |
| Leiomyosarcoma | 41 (1.6) |
| Other | 432 (16.3) |
| Endometrial cancer type | |
| Type I | 2300 (86.9) |
| Type II | 87 (3.3) |
| Unknown | 261 (9.9) |
| Treatment type | |
| Surgery only | 1813 (68.5) |
| Surgery and radiation | 579 (21.9) |
| Surgery and chemotherapy | 84 (3.2) |
| Surgery, radiation, and chemotherapy | 124 (4.7) |
| No available treatment information | 48 (1.8) |
Elevated risks for hypertension were observed among endometrial cancer survivors compared with the general population cohort (Table 3) between one and five years (hazard ratio [HR] = 1.52, 99% confidence interval [CI] = 1.37 to 1.68) and between more than five and 10 years (HR = 1.25, 99% CI = 1.11 to 1.42) after diagnosis. Increased risks for multiple circulatory system diseases were observed one to five years after cancer diagnosis (Table 4). Endometrial cancer survivors were more likely to be diagnosed with arterial diseases during both time periods. Elevated risk during the one to five–year time period was observed for peripheral and vascular atherosclerosis (HR = 1.81, 99% CI = 1.28 to 2.55), hypotension (HR = 1.86, 99% CI = 1.30 to 2.66), and phlebitis, thrombophlebitis, thromboembolism (HR = 2.07, 99% CI = 1.57 to 2.72). We estimated the hazard ratios for 10 or more years after cancer diagnosis, but very few associations were observed. The associations we observed were for hypotension (HR = 1.71, 99% CI = 1.02 to 2.88), diseases of veins and lymphatics (HR = 1.46, 99% CI = 1.10 to 1.95), and other diseases of veins and lymphatics (HR = 2.48, 99% CI = 1.24 to 4.96) (data not shown).
Table 4.
Circulatory system disease risks at 1–5 and >5–10 years after diagnosis among endometrial cancer survivors in comparison with a general population cohort of women*
| Diseases | 1–5 y | >5–10 y | ||||
|---|---|---|---|---|---|---|
| Patients | General population |
Patients | General population |
|||
| No. (%) | No. (%) | HR (99% CI) | No. (%) | No. (%) | HR (99% CI) | |
| Diseases of arteries, arterioles, and capillaries | 495 (18.7) | 1366 (13) | 1.47 (1.26 to 1.72) | 372 (14.1) | 1244 (11.8) | 1.29 (1.07 to 1.55) |
| Peripheral and visceral atherosclerosis | 108 (4.2) | 256 (2.5) | 1.81 (1.28 to 2.55) | 64 (2.6) | 233 (2.3) | 1.4 (0.89 to 2.20) |
| Atherosclerosis of arteries of extremities | 17 (0.6) | 52 (0.5) | 1.72 (0.70 to 4.21) | 14 (0.5) | 37 (0.4) | 1.45 (0.49 to 4.28) |
| Peripheral vascular disease unspecified | 39 (1.5) | 103 (1) | 1.9 (1.08 to 3.33) | 22 (0.9) | 93 (0.9) | 0.91 (0.42 to 1.96) |
| Other peripheral and visceral atherosclerosis | 70 (2.7) | 163 (1.6) | 1.73 (1.13 to 2.65) | 46 (1.8) | 161 (1.6) | 1.4 (0.82 to 2.41) |
| Aortic, peripheral, and visceral artery aneurysms | 17 (0.7) | 63 (0.6) | 1.15 (0.51 to 2.59) | 12 (0.5) | 61 (0.6) | 1.01 (0.36 to 2.87) |
| Aortic and peripheral arterial embolism or thrombosis | 10 (0.4) | 24 (0.2) | 1.69 (0.56 to 5.09) | 7 (0.3) | 20 (0.2) | 0.94 (0.22 to 3.97) |
| Other circulatory disease | 262 (12.7) | 779 (8.4) | 1.54 (1.24 to 1.92) | 175 (9.7) | 671 (7.9) | 1.26 (0.96 to 1.65) |
| Hypotension | 99 (3.9) | 237 (2.3) | 1.86 (1.30 to 2.66) | 73 (3) | 227 (2.3) | 1.43 (0.95 to 2.17) |
| Other and unspecified circulatory disease | 223 (10.5) | 681 (7.3) | 1.39 (1.10 to 1.75) | 144 (7.6) | 593 (6.8) | 1.14 (0.85 to 1.52) |
| Diseases of veins and lymphatics | 615 (23.2) | 1380 (13.1) | 1.87 (1.63 to 2.15) | 380 (14.4) | 1098 (10.5) | 1.45 (1.22 to 1.72) |
| Phlebitis, thrombophlebitis, and thromboembolism | 134 (5.7) | 248 (2.5) | 2.07 (1.57 to 2.72) | 64 (2.9) | 205 (2.1) | 1.53 (1.08 to 2.17) |
| Phlebitis and thrombophlebitis | 53 (2.1) | 82 (0.8) | 3.16 (1.86 to 5.37) | 18 (0.7) | 64 (0.6) | 1.95 (0.85 to 4.44) |
| Other venous embolism and thrombosis | 124 (5.2) | 225 (2.2) | 2.01 (1.52 to 2.66) | 55 (2.4) | 183 (1.9) | 1.37 (0.96 to 1.97) |
| Hemorrhoids | 206 (9.1) | 680 (7.4) | 1.27 (1.02 to 1.61) | 125 (6) | 481 (5.7) | 1.15 (0.86 to 1.55) |
| Other diseases of veins and lymphatics | 120 (4.8) | 119 (1.2) | 4.55 (3.08 to 6.73) | 45 (1.9) | 120 (1.2) | 1.66 (0.95 to 2.88) |
Models adjusted for race, baseline body mass index, baseline Charlson Comorbidity Index, and smoking. The following outcomes were evaluated, but no elevated risk was observed: abdominal aortic aneurysm, without rupture, other aneurysm, arterial embolism and thrombosis of lower extremity artery, other arterial embolism and thrombosis, varicose veins of lower extremity. CI = confidence interval; HR = hazard ratio.
Approximately 25.7% of cancer survivors were diagnosed with diseases of the heart more than five to 10 years after cancer diagnosis (Table 5). Endometrial cancer survivors were 47% more likely to be diagnosed with a disease of the heart between one and five years after cancer diagnosis and 33% more likely between more than five and 10 years after diagnosis. Elevated risks among endometrial cancer survivors were observed for pulmonary heart disease (HR = 1.74, 99% CI = 1.26 to 2.40) and atrial fibrillation (HR = 1.50, 99% CI = 1.07 to 2.11).
Table 5.
Heart disease risk at 1–5 and >5–10 years after diagnosis among endometrial cancer survivors in comparison with a general population cohort of women*
| Diseases | 1–5 y | >5–10 y | ||||
|---|---|---|---|---|---|---|
| Patients | General population |
Patients | General population |
|||
| No. (%) | No. (%) | HR (99% CI) | No. (%) | No. (%) | HR (99% CI) | |
| Diseases of the heart | 962 (36.3) | 2742 (26.1) | 1.47 (1.31 to 1.64) | 680 (25.7) | 2275 (21.7) | 1.33 (1.16 to 1.52) |
| Heart valve disorders | 129 (5.5) | 425 (4.3) | 1.24 (0.92 to 1.67) | 100 (4.5) | 346 (3.7) | 1.2 (0.85 to 1.71) |
| Peri-, endo-, and myocarditis, cardiomyopathy | 48 (1.9) | 157 (1.5) | 1.34 (0.82 to 2.20) | 44 (1.7) | 123 (1.2) | 1.31 (0.74 to 2.33) |
| Cardiomyopathy | 28 (1.1) | 104 (1) | 1.09 (0.58 to 2.05) | 31 (1.2) | 91 (0.9) | 1.18 (0.57 to 2.42) |
| Other peri-, endo-, and myocarditis | 25 (1.9) | 69 (0.7) | 2.16 (1.04 to 4.47) | 20 (0.8) | 41 (0.4) | 2.21 (1.94 to 5.23) |
| Acute myocardial infarction | 41 (1.6) | 113 (1.1) | 1.59 (0.92 to 2.76) | 26 (1) | 104 (1) | 0.76 (0.34 to 1.70) |
| Coronary atherosclerosis and other heart disease | 130 (5.5) | 454 (4.7) | 1.35 (1.00 to 1.83) | 98 (4.4) | 367 (4) | 1.04 (1.71 to 1.52) |
| Angina pectoris | 27 (1.1) | 114 (1.1) | 0.82 (0.43 to 1.57) | 34 (1.3) | 99 (1) | 1.2 (0.61 to 2.36) |
| Coronary atherosclerosis | 114 (4.7) | 381 (3.9) | 1.36 (0.99 to 1.88) | 85 (3.6) | 298 (3.1) | 1.05 (0.69 to 1.59) |
| Nonspecific chest pain | 222 (10.6) | 724 (8.3) | 1.27 (1.02 to 1.60) | 131 (7) | 527 (6.6) | 1.19 (1.88 to 1.60) |
| Pulmonary heart disease | 130 (5.3) | 266 (2.6) | 1.74 (1.26 to 2.40) | 63 (2.7) | 245 (2.5) | 1.11 (1.73 to 1.71) |
| Other and ill-defined heart disease | 113 (4.6) | 319 (3.1) | 1.25 (0.90 to 1.73) | 97 (4.2) | 293 (3) | 1.43 (0.98 to 2.08) |
| Conduction disorders | 62 (2.5) | 204 (2) | 1.33 (0.86 to 2.06) | 48 (1.9) | 208 (2.1) | 0.97 (0.58 to 1.63) |
| Cardiac dysrhythmias | 225 (10.8) | 704 (7.7) | 1.55 (1.23 to 1.97) | 160 (8.6) | 572 (6.8) | 1.41 (1.06 to 1.88) |
| Paroxysmal supraventricular tachycardia | 16 (0.6) | 57 (0.6) | 1.13 (0.50 to 2.52) | 11 (0.4) | 37 (0.4) | 0.74 (0.24 to 2.33) |
| Paroxysmal ventricular tachycardia | 17 (0.7) | 46 (0.4) | 2.16 (0.82 to 5.66) | 17 (0.7) | 54 (0.5) | 1.41 (0.61 to 3.26) |
| Atrial fibrillation | 101 (4.1) | 295 (2.9) | 1.5 (1.07 to 2.11) | 75 (3.2) | 296 (3) | 1.09 (1.74 to 1.62) |
| Atrial flutter | 28 (1.1) | 70 (0.7) | 1.48 (0.76 to 2.89) | 24 (0.9) | 78 (0.8) | 1.06 (0.51 to 2.20) |
| Premature beats | 49 (1.9) | 142 (1.4) | 1.54 (0.92 to 2.57) | 36 (1.4) | 123 (1.2) | 1.46 (0.80 to 2.68) |
| Other cardiac dysrhythmias | 194 (8.7) | 566 (6) | 1.53 (1.19 to 1.97) | 142 (6) | 444 (5) | 1.63 (1.21 to 2.21) |
| Cardiac arrest and ventricular fibrillation | 17 (0.7) | 47 (0.5) | 2.06 (0.83 to 5.11) | 16 (0.6) | 49 (0.5) | 0.97 (0.37 to 2.57) |
| Congestive heart failure, nonhypertensive | 127 (5.2) | 382 (3.8) | 1.56 (1.14 to 2.13) | 100 (4.3) | 326 (3.4) | 1.47 (1.00 to 2.16) |
Models adjusted for baseline body mass index, baseline Charlson Comorbidity Index, and race. Models in violation of the proportional hazards assumption that were evaluated using flexible parametric survival models with restricted cubic splines. The following outcomes were evaluated, but no elevated risk was observed: chronic rheumatic disease of the heart valves, nonrheumatic mitral valve disorders, other heart valve disorders, unstable angina (intermediate coronary syndrome), other forms of chronic heart disease, atrioventricular block, bundle branch block, anomalous atrioventricular block, sinoatrial node dysfunction, heart failure. CI = confidence interval; HR = hazard ratio.
Among endometrial cancer survivors, 68.5% were treated with surgery alone, 21.9% with surgery and radiotherapy, 3.2% with surgery and chemotherapy, and 4.7% with surgery, radiotherapy, and chemotherapy (Table 2). Among individuals treated with radiotherapy and/or chemotherapy in addition to surgery, elevated risks were observed for diseases of arteries, diseases of veins/lymphatics, and heart disease at one to five years compared with those treated with surgery alone (Table 6).
Table 6.
Risk factors for hypertension, heart disease, and vascular diseases among endometrial cancer survivors
| Potential risk factors | Hypertension |
Heart disease |
Diseases of the arteries |
Diseases of the veins/lymphatics |
||||
|---|---|---|---|---|---|---|---|---|
| 1–5 y | >5–10 y | 1–5 y | >5–10 y | 1–5 y | >5–10 y | 1–5 y | >5–10 y | |
| HR (95% CI) | HR (95% CI) | HR (95% CI) | HR (95% CI) | HR (95% CI) | HR (95% CI) | HR (95% CI) | HR (95% CI) | |
| Treatment type* | ||||||||
| Surgery only | 1.00 (reference) | 1.00 (reference) | 1.00 (reference) | 1.00 (reference) | 1.00 (reference) | 1.00 (reference) | 1.00 (reference) | 1.00 (reference) |
| Surgery + radiation | 1.10 (0.96 to 1.26) | 1.05 (0.89 to 1.24) | 1.05 (0.9 to 1.23) | 1.05 (0.87 to 1.26) | 1.24 (1.00 to 1.53) | 0.99 (0.77 to 1.28) | 1.24 (1.03 to 1.49) | 1.09 (0.86 to 1.39) |
| Surgery + chemotherapy | 1.28 (0.91 to 1.81) | 1.13 (0.6 to 2.13) | 2.34 (1.66 to 3.31) | 1.24 (0.61 to 2.51) | 3.42 (2.24 to 5.21) | 1.30 (0.53 to 3.20) | 2.08 (1.38 to 3.12) | 1.34 (0.6 to 3.03) |
| Surgery, radiation, + chemotherapy | 1.12 (0.85 to 1.48) | 1.12 (0.75 to 1.68) | 1.81 (1.36 to 2.41) | 1.17 (0.74 to 1.87) | 1.92 (1.31 to 2.83) | 1.14 (0.62 to 2.1) | 2.33 (1.71 to 3.16) | 1.01 (0.57 to 1.81) |
| Stage† | ||||||||
| Local | 1.00 (reference) | 1.00 (reference) | 1.00 (reference) | 1.00 (reference) | 1.00 (reference) | 1.00 (reference) | 1.00 (reference) | 1.00 (reference) |
| Regional | 1.00 (0.86 to 1.17) | 1.06 (0.87 to 1.29) | 1.06 (0.89 to 1.26) | 1.07 (0.86 to 1.34) | 1.25 (0.99 to 1.58) | 1.10 (0.81 to 1.48) | 1.31 (1.06 to 1.6) | 1.15 (0.87 to 1.53) |
| Advanced | 0.93 (0.63 to 1.35) | 0.67 (0.27 to 1.62) | 1.85 (1.32 to 2.61) | 1.99 (0.97 to 4.05) | 2.80 (1.82 to 4.31) | 1.15 (0.41 to 3.18) | 3.34 (2.32 to 4.81) | 1.89 (0.83 to 4.31) |
| Grade† | ||||||||
| I | 1.00 (reference) | 1.00 (reference) | 1.00 (reference) | 1.00 (reference) | 1.00 (reference) | 1.00 (reference) | 1.00 (reference) | 1.00 (reference) |
| II | 0.95 (0.84 to 1.08) | 0.95 (0.82 to 1.10) | 1.07 (0.87 to 1.22) | 1.24 (1.01 to 1.53) | 1.24 (1.01 to 1.53) | 1.01 (0.80 to 1.27) | 1.14 (0.95 to 1.36) | 1.15 (0.93 to 1.42) |
| III | 1.02 (0.86 to 1.20) | 0.77 (0.61 to 0.97) | 1.50 (1.26 to 1.78) | 0.95 (0.74 to 1.22) | 1.82 (1.43 to 2.32) | 0.93 (0.67 to 1.31) | 1.54 (1.24 to 1.92) | 0.92 (0.65 to 1.29) |
| IV | 1.02 (0.69 to 1.51) | 0.71 (0.33 to 1.50) | 2.22 (1.54 to 3.21) | 0.66 (0.27 to 1.61) | 3.31 (2.09 to 5.23) | 0.53 (0.13 to 2.14) | 1.65 (1.02 to 2.67) | 0.23 (0.03 to 1.65) |
| Year of diagnosis‡ | ||||||||
| 1997–2000 | 1.00 (reference) | 1.00 (reference) | 1.00 (reference) | 1.00 (reference) | 1.00 (reference) | 1.00 (reference) | 1.00 (reference) | 1.00 (reference) |
| 2001–2004 | 1.28 (1.08 to 1.51) | 1.20 (1.01 to 1.43) | 1.01 (0.84 to 1.21) | 0.98 (0.81 to 1.18) | 1.12 (0.86 to 1.47) | 1.09 (0.84 to 1.41) | 1.00 (0.78 to 1.27) | 1.11 (0.86 to 1.43) |
| 2005–2010 | 1.17 (1.00 to 1.36) | 0.84 (0.71 to 1.00) | 0.86 (0.73 to 1.02) | 0.73 (0.60 to 0.88) | 1.10 (0.86 to 1.4) | 0.89 (0.68 to 1.16) | 1.30 (1.05 to 1.6) | 1.06 (0.82 to 1.36) |
| 2011–2012 | 1.12 (0.91 to 1.37) | 0.33 (0.05 to 2.35) | 0.73 (0.58 to 0.93) | 1.08 (0.78 to 1.5) | 0.98 (0.73 to 1.33) | |||
| Age at diagnosis, y§ | ||||||||
| <50 | 1.00 (reference) | 1.00 (reference) | 1.00 (reference) | 1.00 (reference) | 1.00 (reference) | 1.00 (reference) | 1.00 (reference) | 1.00 (reference) |
| 50–59 | 1.69 (1.36 to 2.09) | 1.68 (1.33 to 2.12) | 1.54 (1.21 to 1.98) | 1.45 (1.12 to 1.89) | 1.63 (1.15 to 2.30) | 1.58 (1.09 to 2.28) | 1.44 (1.11 to 1.86) | 1.05 (0.79 to 1.4) |
| 60–69 | 2.60 (2.11 to 3.21) | 2.46 (1.95 to 3.10) | 2.31 (1.82 to 2.94) | 2.13 (1.65 to 2.76) | 2.02 (1.44 to 2.84) | 2.35 (1.64 to 3.36) | 1.52 (1.17 to 1.97) | 1.05 (0.79 to 1.41) |
| 70–79 | 3.29 (2.63 to 4.12) | 2.88 (2.23 to 3.72) | 3.04 (2.36 to 3.91) | 2.78 (2.09 to 3.69) | 3.21 (2.26 to 4.57) | 2.60 (1.75 to 3.86) | 1.56 (1.17 to 2.09) | 1.09 (0.77 to 1.55) |
| 80+ | 4.17 (3.22 to 5.40) | 3.63 (2.63 to 5.02) | 5.17 (3.91 to 6.84) | 4.88 (3.49 to 6.83) | 4.66 (3.15 to 6.89) | 2.93 (1.78 to 4.83) | 1.47 (1.01 to 2.13) | 0.65 (0.36 to 1.19) |
| Charlson Comorbidity Index‖ | ||||||||
| 0 | 1.00 (reference) | 1.00 (reference) | 1.00 (reference) | 1.00 (reference) | 1.00 (reference) | 1.00 (reference) | 1.00 (reference) | 1.00 (reference) |
| 1 | 1.58 (1.37 to 1.83) | 1.78 (1.50 to 2.10) | 1.74 (1.48 to 2.05) | 1.67 (1.38 to 2.02) | 1.84 (1.46 to 2.32) | 2.2 (1.70 to 2.83) | 1.12 (0.91 to 1.38) | 1.68 (1.31 to 2.14) |
| 2+ | 3.02 (2.63 to 3.47) | 3.15 (2.64 to 3.75) | 3.17 (2.72 to 3.71) | 3.27 (2.70 to 3.96) | 2.96 (2.37 to 3.68) | 3.81 (2.92 to 4.96) | 1.47 (1.19 to 1.80) | 1.90 (1.43 to 2.52) |
| Baseline BMI, kg/m2¶ | ||||||||
| <18.5 | 0.94 (0.42 to 2.11) | 1.86 (0.82 to 4.21) | 0.54 (0.2 to 1.45) | 0.85 (0.27 to 2.69) | 0.75 (0.18 to 3.04) | 2.25 (0.82 to 6.15) | 0.49 (0.12 to 1.98) | 2.41 (0.97 to 5.98) |
| 18.5–24.9 | 1.00 (reference) | 1.00 (reference) | 1.00 (reference) | 1.00 (reference) | 1.00 (reference) | 1.00 (reference) | 1.00 (reference) | 1.00 (reference) |
| <25–29.9 | 1.62 (1.37 to 1.91) | 1.37 (1.13 to 1.67) | 1.25 (1.04 to 1.49) | 1.20 (0.97 to 1.49) | 1.17 (0.91 to 1.52) | 1.29 (0.98 to 1.70) | 1.00 (0.80 to 1.25) | 1.27 (0.95 to 1.70) |
| >30 | 1.74 (1.49 to 2.04) | 1.64 (1.36 to 1.97) | 1.20 (1.01 to 1.42) | 1.14 (0.93 to 1.39) | 1.31 (1.03 to 1.67) | 0.93 (0.71 to 1.22) | 1.19 (0.97 to 1.46) | 1.28 (0.97 to 1.68) |
| Smoke¶ | ||||||||
| No | 1.00 (reference) | 1.00 (reference) | 1.00 (reference) | 1.00 (reference) | 1.00 (reference) | 1.00 (reference) | 1.00 (reference) | 1.00 (reference) |
| Yes | 1.15 (0.98 to 1.34) | 1.14 (0.94 to 1.38) | 1.61 (1.36 to 1.90) | 1.47 (1.19 to 1.8) | 2.14 (1.73 to 2.64) | 1.85 (1.44 to 2.39) | 1.11 (0.89 to 1.38) | 1.09 (0.83 to 1.43) |
| Family history CVD# | ||||||||
| No | 1.00 (reference) | 1.00 (reference) | 1.00 (reference) | 1.00 (reference) | 1.00 (reference) | 1.00 (reference) | 1.00 (reference) | 1.00 (reference) |
| Yes | 0.98 (0.87 to 1.10) | 1.13 (0.98 to 1.29) | 1.14 (1.00 to 1.30) | 1.08 (0.93 to 1.27) | 0.88 (0.74 to 1.06) | 0.96 (0.78 to 1.19) | 1.04 (0.88 to 1.21) | 1.01 (0.83 to 1.23) |
| Baseline hypercholesterolemia** | ||||||||
| No | 1.00 (reference) | 1.00 (reference) | 1.00 (reference) | 1.00 (reference) | 1.00 (reference) | 1.00 (reference) | 1.00 (reference) | 1.00 (reference) |
| Yes | 1.88 (1.67 to 2.12) | 1.98 (1.71 to 2.29) | 1.22 (1.06 to 1.39) | 1.31 (1.11 to 1.54) | 1.14 (0.95 to 1.38) | 1.31(1.11 to 1.54) | 1.24 (1.04 to 1.46) | 1.30 (1.05 to 1.60) |
Models adjusted for CCI, BMI, race, smoking, year of diagnosis, and age at diagnosis. BMI = body mass index; CCI = Charlson comorbidity index; CI = confidence interval; HR = hazard ratio.
Models adjusted for CCI, BMI, race, smoking, histology, year of diagnosis, and age at diagnosis.
Models adjusted for CCI, BMI, race, smoking, endometrial cancer type.
Models adjusted for CCI, BMI, race, smoking, stage, histology diagnosis year.
Models adjusted for age at diagnosis, diagnosis year, race, smoking.
Models adjusted for CCI, age at diagnosis, race.
Models adjusted for CCI, BMI, race, smoking, endometrial cancer type, year of diagnosis, and age at diagnosis.
Models adjusted for CCI, BMI, race, smoking, age at diagnosis.
Higher BMI was an important risk factor for hypertension and heart disease among endometrial cancer survivors, with both overweight and obese individuals having higher risk (Table 6). Obese individuals also had elevated risk of diseases of arteries at one to five years. Risk for hypertension, heart disease, and diseases of arteries, arterioles, and capillaries increased with each 10-year interval of age at diagnosis compared with those diagnosed prior to age 50 years. This association was observed for diseases of the veins and lymphatics at one to five years but not at more than five to 10 years. Stage, grade, and Charlson Comorbidity Index at baseline were important risk factors for these diseases among endometrial cancer survivors.
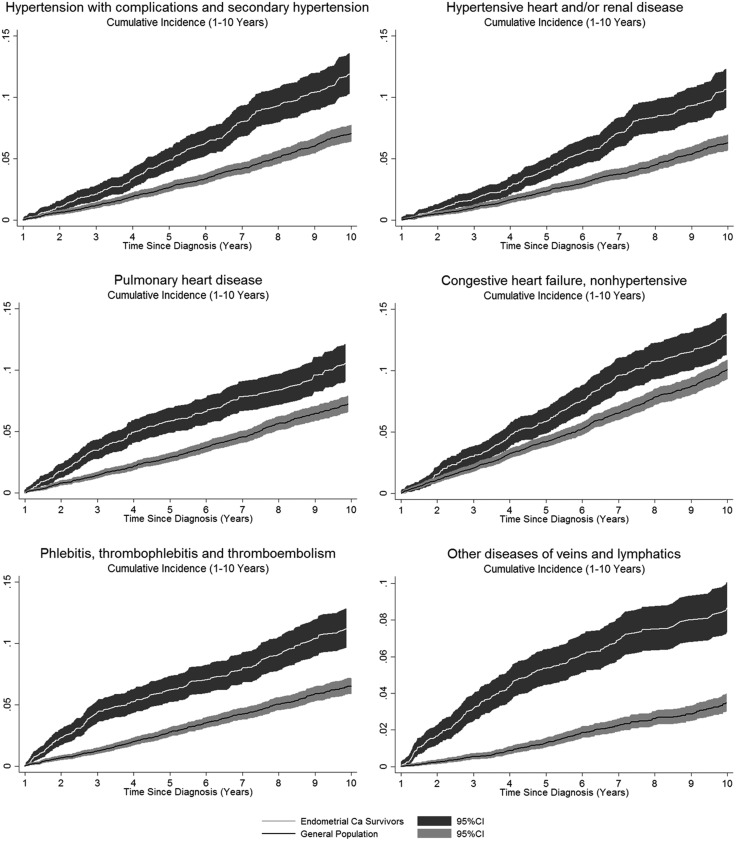
The comparison of cumulative incidence curves for newly diagnosed pulmonary heart disease, congestive heart failure, phlebitis and thrombophlebitis, and secondary hypertension over the span of one to 10 years after endometrial cancer diagnosis clearly show higher incidence among endometrial cancer survivors compared with the general population cohort (Figure 1). Cumulative incidence was greater than 10% for many of these cardiovascular diseases among endometrial cancer patients 10 years after cancer diagnosis.
Figure 1.
Cumulative incidence plots for select cardiovascular disease outcomes. White line and confidence intervals show cumulative incidence for endometrial cancer survivors; black line and confidence interval bands show cumulative incidence for the general population cohort. CI = confidence interval.
Discussion
This study is the first to examine risk for all available cardiovascular outcomes in the electronic medical records of several thousand endometrial cancer survivors and matched individuals from the general population in a large cohort study. Endometrial cancer survivors were at higher risk for hypertension, diseases of the arteries, arterioles, and capillaries, diseases of the veins and lymphatics, and diseases of the heart at one to five years after cancer diagnosis, with some risk persisting at five to 10 years after cancer diagnosis. Cerebrovascular disease was the only major category for which no increased risk was observed among endometrial cancer survivors. Among endometrial cancer survivors, our results suggest that risk for heart disease is elevated among individuals treated with chemotherapy compared with those who were treated with surgery alone. Elevated risk was observed for circulatory system disorders among patients treated with radiation therapy and/or chemotherapy in conjunction with surgery compared with patients treated with surgery alone.
Prior studies have reported similar proportions of endometrial cancer survivors who have hypertension diagnoses (43%–51% vs 46.7% in our study) (32–35), but this study is the first to quantify risk for hypertension after cancer diagnosis among endometrial cancer survivors compared with the general population. Among endometrial cancer survivors in our study, the strongest predictors for increased risk of hypertension were being overweight or obese, increased age, and higher Charlson Comorbidity Index. Risk did not vary by treatment type, stage, or grade. Our findings provide further evidence for the strong association between shared risk factors for both endometrial cancer and hypertension.
A twofold increase in the risk of phlebitis, thrombophlebitis, and thromboembolism was detected among endometrial cancer survivors in this study. A previous study of 827 endometrial cancer patients identified 72 patients who developed venous thromboembolism (VTE; 8.7%), which was associated with decreased survival (36). A randomized phase II trial of temsirolimus alone or as combination therapy was suspended early because five events of deep venous thrombosis and two pulmonary emboli occurred in the combination arm (37). In another study of 1123 gynecologic oncology patients, 3.3% developed VTE and 92% of gynecologic oncology patients were in the high-risk category according to the Caprini risk assessment model for VTE risk because they underwent surgery (38). Although risk of VTE is fairly established for cancer patients, the risk among endometrial cancer patients relative to a general population cohort had not previously been estimated, to our knowledge. Surgery for endometrial cancer is a potential risk factor for phlebitis, thrombophlebitis, and thromboembolism, but shared risk factors for this diagnosis and endometrial cancer include older age, obesity, and inactivity (39).
Hypotension may be a risk factor for cardiovascular and cerebrovascular diseases (40), and the elevated risk of hypotension among endometrial cancer survivors in this study may be related to the increased heart disease risks. Individuals who have hypertension are also at risk for hypotension when they take medication for hypertension (40). We observed an increased risk of both hypertension and hypotension among endometrial cancer survivors.
For circulatory system disorders, it is possible that radiation damage to the endothelial cells of the vascular system could be a potential mechanism (41). Pelvic radiotherapy targets the “gross disease, the lower common iliacs, external iliacs, internal iliacs, parameteria, upper vagina/para-vaginal tissue, and presacral lymph nodes” (45). Endometrial cancer treatment includes surgery for 93% of patients, radiation therapy for 30%, and chemotherapy for 8% (42). There has been an increasing trend to use vaginal brachytherapy (∼7 Gy) instead of beam radiation therapy (∼45 Gy), resulting in lower radiation exposure (43) and an increasing trend of chemotherapy use (44). Chemotherapy treatment is not common as it is generally only used to treat stage III or IV patients, who comprise a small proportion of all endometrial cancer patients (<20%). Chemotherapy agents include cisplatin, doxorubicin, paclitaxel, carboplatin, ifosfamide, and docetaxel (45), some of which may have potential cardiotoxic effects (46). Targeted drugs of interest under clinical trial testing for endometrial cancer include bevacizumab, everolimus, and nivolumab or ipilimumab (47).
For diseases of the heart, individuals diagnosed with endometrial cancer were approximately 50% more likely to be diagnosed with cardiac dysrhythmias and congestive heart failure than individuals in the general population at both one to five years and more than five to 10 years after cancer diagnosis. Both have well-established associations with obesity and a number of additional cardiovascular diseases, including hypertension, myocarditis, myocardial infarction, and cardiomyopathy (48,49). While cardiomyopathy and myocardial infarction risk was similar to that of the general population, other peri/endo/myocarditis diseases were more common in endometrial cancer survivors. Cardiac dysrhythmias and congestive heart failure as a result of cancer treatment have more often been associated with pharmacologic interventions than with radiation (50,51), and the increased risk for heart disease overall among endometrial cancer survivors treated with chemotherapy, either in conjunction with surgery or radiation and surgery, compared with those treated with surgery alone, support this evidence. Our results suggest that cancer treatment increasing the risk of cardiovascular disease was largely confined to one to five years after cancer diagnosis and that treatment with chemotherapy was an important factor.
This study has a number of important strengths. The large sample provides a study population that is sufficiently powered to examine a large number of outcomes without being overburdened by the penalty imposed by multiple comparisons. This is a critical feature in a study intended to encapsulate the experience of endometrial cancer survivors over long periods of time. The data used in this study incorporate medical records from the state’s two largest health care providers as well as statewide ambulatory surgery and inpatient data, which provide comprehensive medical record data for a large number of individuals. In addition to the large sample size, these data contain a large amount of follow-up time for individuals in both cohorts. The mean follow-up time among endometrial cancer survivors is 8.5 years. In contrast to cancer survivor studies that rely on self-reports of disease, which are susceptible to survival bias, our study is less susceptible to survival bias because we used long-term health records as the source of disease diagnoses.
This study also has a number of limitations. While this study utilized comprehensive electronic medical record data from the two largest health care systems in the state, as well as statewide ambulatory surgery and inpatient data, there is the possibility that study participants could have been diagnosed with cardiovascular outcomes in hospitals and clinics not covered by the data sources. However, approximately 99.6% of cancer patients and 98.5% of the general population cohort did have records in these data sources. Although 33.8% of the endometrial cancer patients had follow-up for 10+ years after cancer diagnosis, the number of cardiovascular events was small, and we did not have had adequate statistical power to detect increased risks. However, with the UPDB as a data source, we are able to update our analysis on a regular basis. Another limitation of this study is that some of the subjects were missing baseline BMI data, which was addressed by imputation of BMI values. It was required that baseline BMI be recorded at least one year prior to the survivor’s cancer diagnosis to minimize temporal issues. We assured that the inferences for our results did not change whether we used only those with BMI included or those whose BMI was imputed.
Treatment data were limited to broad categories and did not include radiation dosage, specific chemotherapy agents, and duration of treatment. However, the treatment data that were available did provide evidence that risks for several cardiovascular outcomes vary by treatment type and obtaining more detailed treatment data is possible with medical chart abstraction in the future. Medication information would also have been informative but is currently not available. Although we did adjust for tobacco smoking, we did not have detailed frequency or duration of smoking habits to adjust for pack-years of smoking. However, we clearly showed that tobacco smoking was a risk factor for heart disease, as expected.
Cancer patients are expected to be under increased medical surveillance, particularly in the first several years after cancer diagnosis. Follow-up is recommended every two to three months for two to three years for endometrial cancer patients according to the National Comprehensive Cancer Network guidelines (45). Our results for five to 10 years after cancer diagnosis would be less likely to be subject to surveillance bias, and increased risks were observed in this time period. For the risk factor analysis among endometrial cancer patients only, we would expect the disease misclassification to be nondifferential in the comparison groups of potential risk factors. Our estimates for risk factors for cardiovascular disease could be subject to bias toward the null, but we still observed increased risks for various factors such as age and obesity.
In conclusion, endometrial cancer survivors in this cohort were at higher risk for a number of long-term cardiovascular outcomes. Many of the conditions examined in this study have shared risk factors with endometrial cancer. But after adjusting for baseline BMI, baseline Charlson Comorbidity Index, and race, it is clear from these results that survivors of endometrial cancer in this cohort experienced a high burden of cardiovascular events. These results highlight the importance of placing greater emphasis on survivorship and that increased monitoring and risk management for cardiovascular disease for 10 years among endometrial cancer survivors is warranted.
Funding
This work was supported by a grant from the National Cancer Institute at the National Institutes of Health (NIH; R21 CA185811), the Marian Bishop Award from the Department of Family and Preventive Medicine at the University of Utah and the National Center for Research Resources grant “Sharing Statewide Health Data for Genetic Research” (R01 RR021746, G. Mineau, PI), with additional support from the Utah State Department of Health and the University of Utah.
Notes
Affiliations of authors: Division of Public Health, Department of Family and Preventive Medicine (SS, JS, AAS, MH), Department of Radiation Oncology (DG), Department of Surgery (HAH), Department of Dermatology (YPW), and Division of Oncology, Department of Medicine (TLW), University of Utah School of Medicine, Salt Lake City, UT; Pedigree and Population Resources, Population Sciences (YW, AF, KS, HAH), Huntsman Cancer Institute (SS, DG, YPW, AAS, TLW, MH), Salt Lake City, UT; Department of Health Policy and Management, UCLA Fielding School of Public Health, Los Angeles, CA (PAG); Intermountain Healthcare, Salt Lake City, UT (KR, JS); University of Utah Health Sciences Center, Salt Lake City, UT (VD, MN); Utah Cancer Registry, Salt Lake City, UT (KH); Department of Preventive Medicine, Keck School of Medicine of USC, Los Angeles, CA (VWS).
The funders had no role in the design of the study; the collection, analysis, or interpretation of the data; the writing of the manuscript; or the decision to submit the manuscript for publication.
The authors have no conflicts of interest to disclose.
We thank the Pedigree and Population Resource of the Huntsman Cancer Institute, University of Utah (funded in part by the Huntsman Cancer Foundation), for its role in the ongoing collection, maintenance, and support of the Utah Population Database. We thank the University of Utah Center for Clinical and Translational Science (funded by NIH Clinical and Translational Science Awards), the Pedigree and Population Resource, University of Utah Information Technology Services and Biomedical Informatics Core for establishing the Master Subject Index between the Utah Population Database, the University of Utah Health Sciences Center, and Intermountain Healthcare.
Supplementary Material
References
- 1. American Cancer Society. Cancer Treatment and Survivorship Facts and Figures, 2016-2017. Atlanta, GA: American Cancer Society; 2016. [Google Scholar]
- 2. American Cancer Society. Cancer Facts & Figures 2017. Atlanta, GA: American Cancer Society; 2017. [Google Scholar]
- 3.Surveillance, Epidemiology, and End Results (SEER) Program. SEER*Stat Database: Incidence - SEER 9 Regs Research Data, Nov 2016 Sub (1973-2014). https://seer.cancer.gov/data-software/documentation/seerstat/nov2016/. Accessed April 2017.
- 4. Shisler R, Sinnott JA, Wang V, et al. Life after endometrial cancer: A systematic review of patient-reported outcomes. Gynecol Oncol. 2018;1482:403–413. [DOI] [PubMed] [Google Scholar]
- 5. Dobrzycka B, Terlikowski R, Kulesza-Brończyk B, et al. Quality of life in long-term survivors of early stage endometrial cancer. Ann Agric Environ Med. 2017;243:513–516. [DOI] [PubMed] [Google Scholar]
- 6. Rossi A, Garber CE, Kaur G, et al. Physical activity-related differences in body mass index and patient-reported quality of life in socioculturally diverse endometrial cancer survivors. Support Care Cancer. 2017;257:2169–2177. [DOI] [PubMed] [Google Scholar]
- 7. Gao H, Xiao M, Bai H, Zhang Z.. Sexual function and quality of life among patients with endometrial cancer after surgery. Int J Gynecol Cancer. 2017;273:608–612. [DOI] [PubMed] [Google Scholar]
- 8. de Boer SM, Nout RA, Jürgenliemk-Schulz IM, et al. Long-term impact of endometrial cancer diagnosis and treatment on health-related quality of life and cancer survivorship: Results from the randomized PORTEC-2 trial. Int J Radiat Oncol Biol Phys. 2015;934:797–809. [DOI] [PubMed] [Google Scholar]
- 9. Damast S, Alektiar K, Eaton A, et al. Comparative patient-centered outcomes (health state and adverse sexual symptoms) between adjuvant brachytherapy versus no adjuvant brachytherapy in early stage endometrial cancer. Ann Surg Oncol. 2014;218:2740–2754. [DOI] [PubMed] [Google Scholar]
- 10. Vaz AF, Pinto-Neto AM, Conde DM, et al. Quality of life and menopausal and sexual symptoms in gynecologic cancer survivors: A cohort study. Menopause. 2011;186:662–669. [DOI] [PubMed] [Google Scholar]
- 11. Beekers N, Husson O, Mols F, et al. Symptoms of anxiety and depression are associated with satisfaction with information provision and internet use among 3080 cancer survivors: Results of the PROFILES Registry. Cancer Nurs. 2015;385:335–342. [DOI] [PubMed] [Google Scholar]
- 12. Fader AN, Frasure HE, Gil KM, Berger NA, von Gruenigen VE.. Quality of life in endometrial cancer survivors: What does obesity have to do with it? Obstet Gynecol Int. 2011;2011:308609. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Bradley S, Rose S, Lutgendorf S, Costanzo E, Anderson B.. Quality of life and mental health in cervical and endometrial cancer survivors. Gynecol Oncol. 2006;1003:479–486. [DOI] [PubMed] [Google Scholar]
- 14. Leach CR, Weaver KE, Aziz NM, et al. The complex health profile of long-term cancer survivors: Prevalence and predictors of comorbid conditions. J Cancer Surviv. 2015;92:239–251. [DOI] [PubMed] [Google Scholar]
- 15. Ward KK, Shah NR, Saenz CC, et al. Cardiovascular disease is the leading cause of death among endometrial cancer patients. Gynecol.Oncol. 2012;126:176–179. [DOI] [PubMed] [Google Scholar]
- 16. Felix AS, Bower JK, Pfeiffer RM, et al. High cardiovascular disease mortality after endometrial cancer diagnosis: Results from the Surveillance, Epidemiology, and End Results (SEER) Database. Int J Cancer. 2017;1403:555–564. [DOI] [PubMed] [Google Scholar]
- 17. Aune D, Navarro Rosenblatt DA, Chan DS, et al. Anthropometric factors and endometrial cancer risk: A systematic review and dose-response meta-analysis of prospective studies. Ann Oncol. 2015;268:1635–1648. [DOI] [PubMed] [Google Scholar]
- 18. Fryar CD, Carroll MD, Ogden CL.. Prevalence of overweight, obesity, and extreme obesity among adults: United States, 1960–1962 through 2011–2012. National Center for Health Statistics, 2011. http://www.cdc.gov/nchs/data/hestat/obesity_adult_11_12/obesity_adult_11_12.htm. [Google Scholar]
- 19. Vrachnis N, Iavazzo C, Iliodromiti Z, et al. Diabetes mellitus and gynecologic cancer: Molecular mechanisms, epidemiological, clinical and prognostic perspectives. Arch Gynecol Obstet. 2016;2932:239–246. [DOI] [PubMed] [Google Scholar]
- 20. La Vecchia C1, Negri E, Franceschi S, et al. Long-term impact of reproductive factors on cancer risk. Int J Cancer. 1993;532:215–219. [DOI] [PubMed] [Google Scholar]
- 21. Benshushan A, Paltiel O, Rojansky N, et al. IUD use and the risk of endometrial cancer. Eur J Obstet Gynecol Reprod Biol. 2002;1052:166–169. [DOI] [PubMed] [Google Scholar]
- 22. Beining RM, Dennis LK, Smith EM, et al. Meta-analysis of intrauterine device use and risk of endometrial cancer. Ann Epidemiol. 2008;186:492–499. [DOI] [PubMed] [Google Scholar]
- 23. Marjoribanks J, Farquhar C, Roberts H, et al. Long-term hormone therapy for perimenopausal and postmenopausal women. Cochrane Database Syst Rev. 2017;1:CD004143. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24. Felix AS, Weissfeld JL, Stone RA, et al. Factors associated with type I and type II endometrial cancer. Cancer Causes Control. 2010;2111:1851–1856. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25. The National Association of Health Data Organizations. Next Steps for the InterState Exchange of Nonresident Data Between State Health Data Organizations. Salt Lake City, Utah: The National Association of Health Data Organizations; 2009. [Google Scholar]
- 26. US Census Bureau. State-to-state migration flows. 2017. Retrieved from https://www.census.gov/data/tables/time-series/demo/geographic-mobility/state-to-state-migration.html. Accessed December 2017.
- 27. https://www.aafp.org/patient-care/public-health/tobacco-nicotine/coding-reference.html. Accessed January 2017.
- 28. Healthcare Cost and Utilization Project. Clinical classification software. https://www.hcup-us.ahrq.gov/tools_software.jsp. Accessed January 2017.
- 29. Charlson ME, Pompei P, Ales KL, et al. A new method of classifying prognostic comorbidity in longitudinal studies: Development and validation. J Chronic Dis. 1987;405:373–383. [DOI] [PubMed] [Google Scholar]
- 30. Setiawan VW, Yang HP, Pike MC, et al. Type I and II endometrial cancers: Have they different risk factors? J Clin Oncol. 2013;3120:2607–2618. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31. Morris PB, Ference BA, Jahangir E, et al. Cardiovascular effects of exposure to cigarette smoke and electronic cigarettes: Clinical perspectives from the Prevention of Cardiovascular Disease Section Leadership Council and Early Career Councils of the American College of Cardiology. J Am Coll Cardiol. 2015;6612:1378–1391. [DOI] [PubMed] [Google Scholar]
- 32. von Gruenigen VE, Waggoner SE, Frasure HE, et al. Lifestyle challenges in endometrial cancer survivorship. Obstet Gynecol. 2011;1171:93–100. [DOI] [PubMed] [Google Scholar]
- 33. Nicholas Z, Hu N, Ying J, et al. Impact of comorbid conditions on survival in endometrial cancer. Am J Clin Oncol. 2014;372:131–134. [DOI] [PubMed] [Google Scholar]
- 34. Felix AS, Blair CK, Lehman A, et al. Cardiovascular disease mortality among women with endometrial cancer in the Iowa Women's Health Study. Cancer Causes Control. 2017;2810:1043–1051. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35. Yin XH, Jia HY, Xue XR, et al. Clinical analysis of endometrial cancer patients with obesity, diabetes, and hypertension. Int J Clin Exp Med. 2014;73:736–743. [PMC free article] [PubMed] [Google Scholar]
- 36. Machida H, Hom MS, Maeda M, et al. Signs and symptoms of venous thromboembolism and survival outcome of endometrial cancer. Int J Gynecol Cancer. 2016;265:924–932. [DOI] [PubMed] [Google Scholar]
- 37. Fleming GF, Filiaci VL, Marzullo B, et al. Temsirolimus with or without megestrol acetate and tamoxifen for endometrial cancer: A gynecologic oncology group study. Gynecol Oncol. 2014;1323:585–592. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38. Bazzett-Matabele L, Straughn JM Jr, Rocconi RP.. Validation of a venous thromboembolism risk assessment model in gynecologic oncology. Gynecol Oncol. 2014;1341:160–163. [DOI] [PubMed] [Google Scholar]
- 39. Khalil J, Bensaid B, Elkacemi H, et al. Venous thromboembolism in cancer patients: An underestimated major health problem. World J Surg Oncol. 2015;13:204. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40. Ricci F, De Caterina R, Fedorowski A.. Orthostatic hypotension: Epidemiology, prognosis, and treatment. J Am Coll Cardiol. 2015;667:848–860. [DOI] [PubMed] [Google Scholar]
- 41. Puukila S, Lemon JA, Lees SJ, Tai TC, Boreham DR, Khaper N.. Impact of ionizing radiation on the cardiovascular system: A review. Radiat Res. 2017;188(4.2):539–546. [DOI] [PubMed] [Google Scholar]
- 42. Modh A, Ghanem AI, Burmeister C, Rasool N, Elshaikh MA.. Trends in the utilization of adjuvant vaginal brachytherapy in women with early-stag endometrial carcinoma: Results of an updated period analysis of SEER data. Brachytherapy. 2016;155:554–561. [DOI] [PubMed] [Google Scholar]
- 43. Trimble EL, Harlan LC, Clegg LX, Stevens JL.. Pre-operative imaging, surgery and adjuvant therapy for women diagnosed with cancer of the corpus uteri in community practice in the United States. Gynecol Oncol. 2005;963:741–748. [DOI] [PubMed] [Google Scholar]
- 44. Pereira EB, De B, Kolev V, et al. Survey of current practice patterns in the treatment of early-stage endometrial cancer. Int J Gynecol Cancer. 2016;262:341–347. [DOI] [PubMed] [Google Scholar]
- 45. National Comprehensive Cancer Network. NCCN Clinical Practice Guidelines in Oncology: Uterine Neoplasms. Version 1.2018. Fort Washington, PA: National Comprehensive Cancer Network; 2017. [Google Scholar]
- 46. Albini A, Pennesi G, Donatelli F, Cammarota R, De Flora S, Noonan DM.. Cardiotoxicity of anticancer drugs: The need for cardio-oncology and cardio-oncological prevention. J Natl Cancer Inst. 2010;1021:14–25. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47. Morice P, Leary A, Creutzberg C, Abu-Rustum N, Darai E.. Endometrial cancer. Lancet. 2016;387;1094–1108. [DOI] [PubMed] [Google Scholar]
- 48. LaMori JC, Mody SH, Gross HJ, et al. Burden of comorbidities among patients with atrial fibrillation. Ther Adv Cardiovasc Dis. 2013;72:53–62. [DOI] [PubMed] [Google Scholar]
- 49. Shakir DK, Rasul KI.. Chemotherapy induced cardiomyopathy: Pathogenesis, monitoring and management. J Clin Med Res. 2009;11:8–12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50. Ewer MS, Benjamin RS, Yeh ET.. Cardiac dysrhythmia in the cancer patient In: Kufe DW, Pollock RE, Weichselbaum RR, et al. , eds. Holland-Frei Cancer Medicine .6th ed Hamilton, ON, Canada: BC Decker; 2003. [Google Scholar]
- 51. Yeh ET, Tong AT, Lenihan DJ, et al. Cardiovascular complications of cancer therapy: Diagnosis, pathogenesis, and management. Circulation. 2004;10925:3122–3131. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.