Abstract
Background
Radiotherapy after breast conservation has become the standard of care. Prior meta-analyses on effects of radiotherapy predated availability of gene expression profiling (GEP) to assess recurrence risk and/or did not include all relevant outcomes. This analysis used GEP information with pooled individual-level data to evaluate the impact of omitting radiotherapy on recurrence and mortality.
Methods
We considered trials that evaluated or administered radiotherapy after lumpectomy in women with low-risk breast cancer. Women included had undergone lumpectomy and were treated with hormonal therapy for stage I, ER+ and/or PR+, HER2− breast cancer with Oncotype scores no greater than 18. Recurrence-free interval (RFI), type of RFI (locoregional or distant), and breast cancer–specific and overall survival were compared between no radiotherapy and radiotherapy using adjusted Cox models. All statistical tests were two-sided.
Results
The final sample included 1778 women from seven trials. Omission of radiotherapy was associated with an overall adjusted hazard ratio of 2.59 (95% confidence interval [CI] = 1.38 to 4.89, P = .003) for RFI. There was a statistically significant increase in any first locoregional recurrence (P = .001), but not distant recurrence events (P = .90), or breast cancer–specific (P = .85) or overall survival (P = .61). Five-year RFI rate was high (93.5% for no radiotherapy vs 97.9% for radiotherapy; absolute reduction = 4.4%, 95% CI = 0.7% to 8.1%, P = .03). The effects of radiotherapy varied across subgroups, with lower RFI rates for those with Oncotype scores of less than 11 (vs 11–18), older (vs younger), and ER+/PR+ status (vs other).
Conclusions
Omission of radiotherapy in hormone-sensitive patients with low recurrence risk may lead to a modest increase in locoregional recurrence event rates, but does not appear to increase the rate of distant recurrence or death.
Over the last two decades, breast cancer survival has steadily improved, in part due to early detection, use of new treatment agents, and more intensive regimens (1). With increasing knowledge of molecular heterogeneity, there is a growing recognition that certain subgroups of breast cancer patients may have comparable benefits and lower burden with less, rather than more, treatment (2–4).
A 2011 meta-analysis of 17 randomized clinical trials conducted by the Early Breast Cancer Trialists’ Collaborative Group (EBCTCG) found that although radiotherapy after breast-conserving surgery reduced overall recurrence rates in node-negative patients, it only provided a modest survival benefit, and that benefit appeared confined to subsets of patients who experienced a 10% or greater reduction in local recurrence with radiotherapy (2). Additionally, reduction in recurrence varied by age and tumor characteristics (2). For instance, one of the trials that enrolled women aged 70 years and older concluded that radiotherapy might be safely omitted in older patients with hormone-sensitive breast cancer who are willing to accept a slightly higher risk of local recurrence (3).
However, understanding factors associated with recurrence risk has dramatically evolved since the 2011 EBCTCG analyses. Molecular tumor markers and gene-expression profiling (GEP), especially for hormone-sensitive breast cancer, are now the standard of care for chemotherapy decision making. Further, GEP is increasingly demonstrated as prognostic for locoregional recurrence, supporting its potential to identify patients at the lowest risk of recurrence where radiotherapy might be reasonably omitted. A recent retrospective study of tumor genomic profiling of specimens from two older trials demonstrated a statistically significant association between locoregional recurrence rates and recurrence-risk scores (5). Unfortunately, that study did not examine the effects of radiotherapy and other outcomes important in treatment decision making, such as distant recurrence and mortality. It has become increasingly difficult to conduct new randomized trials to compare broad outcomes related to radiotherapy in all clinically relevant subgroups, given the large sample sizes required with low event rates, increasingly effective hormonal therapy regimens, and the long follow-up required to capture any late distant events (3).
To help fill current gaps in clinical practice, this study determined the effect of radiotherapy on locoregional and distant recurrence and breast cancer–specific and all-cause mortality conditional on genomic risk assessments in a pooled analysis of breast cancer patients from seven clinical trials (3,6–11). Our results are intended to inform clinical debates about care for groups that might consider omission of radiotherapy and to highlight considerations for the efficient design of future clinical trials.
Methods
Data Sources
Members of the Cancer Intervention and Surveillance Modeling Network (CISNET) Breast Cancer Working Group conducted this study in collaboration with the National Cancer Institute (NCI) Breast Cancer Steering Committee’s Breast Oncology Local Disease (BOLD) Task Force, and clinical trial investigators from the seven clinical trials. The study was considered exempt by the Georgetown University Oncology Institutional Review Board because it used de-identified secondary data released for research purposes under NCI data-sharing policies.
Study Design and Population
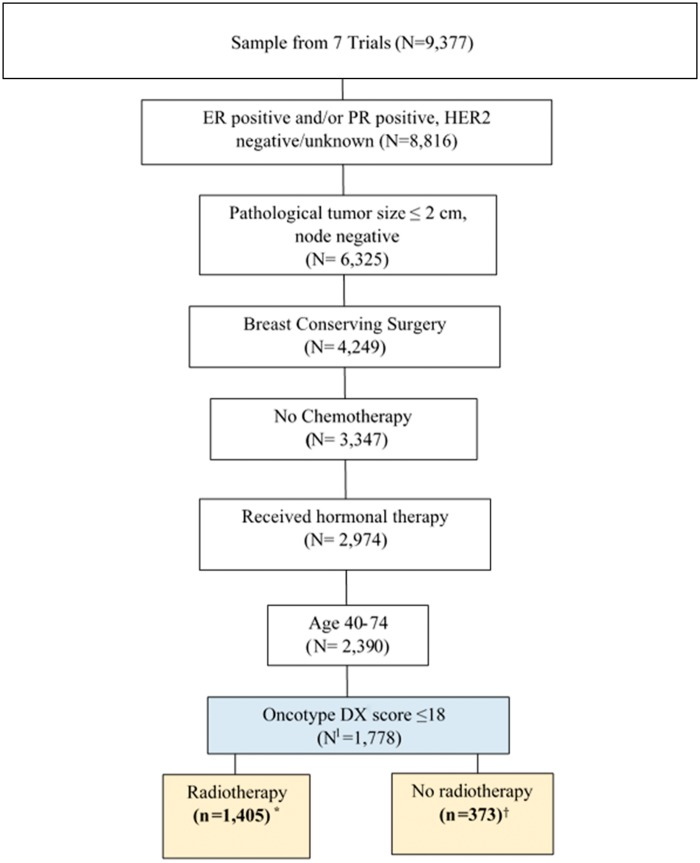
We adapted the inclusion criteria from a recent clinical trial proposed by NRG Oncology to evaluate omission of radiotherapy after breast conservation among patients aged 40–74 years with low-risk breast cancers with planned hormonal therapy and no chemotherapy. “Low-risk” cancers were defined as AJCC (version 6) primary invasive stage I disease (≤2 cm pathological tumor size, pathologically/clinically node-negative) (12), estrogen receptor (ER)-positive and/or progesterone receptor (PR)-positive, and human epidermal growth factor-2 (HER2) negative or unknown. An additional eligibility criterion was Oncotype DX score of no more than 18 (Figure 1).
Figure 1.
Selection of patients with stage I, lymph node–negative, ER and/or PR+, HER2− breast cancer with Oncotype DX score ≤ 18, treated with breast conservation and hormonal therapy. *Randomly assigned to radiotherapy N = 304, Radiotherapy given per treatment protocol N = 1101; †Randomly assigned to no radiotherapy N = 312, Radiotherapy not given per treatment protocol N = 61. Assumed 100% compliance to protocol. ER = estrogen receptor; PR = progesterone receptor; HER2− = human epidermal growth factor-2 negative.
Data Collection
Seventeen trials were included in the 2011 Oxford meta-analysis of the effects of radiotherapy (2). Of these, we considered the seven trials categorized as “evaluating the need for radiotherapy after lumpectomy in low-risk patients” (3,6–11,13–15). Detailed information about the trials is included in Supplementary Table 1 (available online).
Study Endpoints
The primary endpoint was recurrence-free interval (RFI) (16,17), and included time from random assignment/enrollment to any occurrence of local (invasive), regional, or distant recurrence, or breast cancer death. This endpoint was censored at the time of contralateral breast cancer, other second primary cancers, or other-cause death.
Secondary endpoints were RFI by type of recurrence (locoregional or distant), overall survival (time from random assignment/enrollment to death), and breast cancer–specific survival (time from random assignment/enrollment to breast cancer death). The patients were followed up to 10 years because many trials did not follow patients for recurrence beyond this period. Published TAILORx data were truncated at five years (8), so we utilized the same length of follow-up for that trial.
Intervention and Covariates
Radiotherapy (yes/no) was the intervention of interest. Other factors examined as potential covariates or subgroups were patient age, tumor grade (low/moderate/high/unknown), ER and PR status (ER+ and PR+/other), HER2 (negative/unknown), initial hormonal treatment (tamoxifen/aromatase inhibitors/other), tumor size (<1 cm/>1 cm), trial, and Oncotype DX score.
Statistical Analysis
The primary analysis was conducted by combining data from the trials into a summary effect for radiotherapy without weighting (18,19). Prior to pooling the data, the study-specific effects of radiotherapy were estimated, and homogeneity was examined using the Cochran Q test. Because the trials were not homogeneous in effect, trial was included as a covariate in subsequent analyses.
The TAILORx trial included Oncotype data and was published after the 2011 Oxford overview (2,8). Therefore, except for patients in the TAILORx study, Oncotype scores (0–100) were imputed for the other six trials using a deterministic regression–based multiple imputation approach (20) and a population-based donor dataset with Oncotype scores (Supplementary Methods, available online) (21,22). The imputation model included age, tumor size, grade, radiation, and ER/PR and HER2 status. Coefficients and standard errors from all analyses were adjusted for variability between imputations according to the combination rules outlined by Rubin (1987) (20).
We examined the distribution of patient characteristics overall, by radiotherapy and study. After individual data were pooled and study-eligible patients selected, the women in the final analytic sample were no longer randomly assigned to radiotherapy. This resulted in an imbalance in baseline characteristics between the no-RT vs RT groups. Therefore, multivariable Cox proportional hazards models were used to examine study endpoints and derive survival plots by radiotherapy, adjusting for other covariates. The primary test statistic for multiple imputation estimates was a Student t test with the degrees of freedom depending on the number of imputations and the increase in variance of estimates due to missing data; the results were considered statistically significant if the two-sided P value was less than .05. All models consistently supported the assumption of proportional hazards and Cox–Snell residuals indicated good model fit. Analyses were repeated to determine the effects of radiotherapy on secondary outcomes. The adjusted five- and 10-year RFI and breast cancer–specific and all-cause survival rates were calculated using adjusted Cox proportional hazards models. All statistical tests were two-sided.
Subgroup Analyses
Exploratory analyses were conducted to evaluate the effects of radiotherapy within subgroups based on age at random assignment/enrollment (<60/60–70/70+ years), hormone receptor status (ER+ and PR+/other), Oncotype (0–10/11–18), grade (low and intermediate/high), and tumor size (≤1 cm/>1 cm).
Sensitivity Analyses
We evaluated RFI, locoregional RFI, and distant RFI using competing risk models to estimate subdistribution hazard ratios and 95% confidence intervals considering other-cause mortality as a competing event using the methods described by Gray (23). In competing risk analyses, differences in endpoints were adjusted for potential risk factors with statistical significance determined using a two-sided Wald test.
We also performed a propensity score analysis of radiotherapy effect on RFI and type of RFI. A logistic regression model estimated the propensity to receive radiotherapy, given patient characteristics and study and interaction terms (Supplementary Methods, available online). The results were then used to calculate inverse-probability-of-treatment-weighted estimates in Cox models.
We examined the effects of radiotherapy on endpoints excluding women from NSABP B-14, B-20, and TAILORx trials, the three trials where receipt of radiotherapy was not randomized. A second sensitivity analysis examined the effect of including older women (74 years and older). Adjusted RFI hazard ratios (HRs) were also estimated with data truncated at five and nine years.
Results
Trials and Patient Characteristics
Of the trials (3,6–11,13–15) considered for the analysis, we excluded women if chemotherapy was included in the trial arm (n = 1) (15), information on ER/PR status was not provided (n = 1) (14), and/or the study could not provide individual-level data (n = 1) (13). Four of the included trials randomized radiotherapy: Cancer and Leukemia Group-B [CALGB]-9343 (3), National Surgical Adjuvant Breast and Bowel Project [NSABP] B-21 (9), Toronto/Vancouver trial (6), and the German Breast Cancer Study Group [GBSG-V]) (7). One trial allowed radiotherapy to be assigned based on clinical decisions (Trial Assigning Individualized Options for Treatment [TAILORx]) (8), and two (NSABP B-14/B-20) (10,11) gave radiotherapy after breast conservation to all patients as part of the treatment protocol.
The Oncotype imputation model and validation results are provided in Supplementary Tables 2–5 and Supplementary Figures 1–2 (available online). The imputation model showed good fit, and the distributions of the estimated and actual Oncotype scores were similar at the cohort level.
The final sample included 1778 women from seven trials. The mean age of women in the sample was 59 years (median = 59, range = 40–74 years), with 50.8% younger than 60 years (Table 1); 64.4% received tamoxifen. Women who did not receive radiotherapy were older or had smaller tumor sizes than those who received radiotherapy. The mean Oncotype DX score among eligible women was 10 (median = 10, SD = 4, range = 0–18). The five-year RFI rates across the trials included in the analysis were low and comparable (range = 92.3–98.9%) (Supplementary Table 6, available online).
Table 1.
Sample characteristics
| Characteristic | Total (%) (N = 1778) | % | Radiotherapy (n = 1405) |
No radiotherapy (n = 373) |
P* | ||
|---|---|---|---|---|---|---|---|
| No. | Col % | No. | Col % | ||||
| Mean age (SD), y | 1778 | 59 (9) | 1405 | 58 (9) | 373 | 63 (9) | <.001 |
| Age at random assignment/enrollment | |||||||
| <60 years | 903 | 50.8 | 773 | 55.1 | 130 | 34.8 | <.001 |
| 60-70 years | 296 | 16.6 | 174 | 12.3 | 122 | 32.7 | |
| >70 years | 579 | 32.6 | 458 | 32.6 | 121 | 32.5 | |
| Tumor grade | |||||||
| Low | 439 | 24.7 | 341 | 24.3 | 98 | 26.3 | <.001 |
| Intermediate | 765 | 43.0 | 610 | 43.4 | 155 | 41.6 | |
| High | 157 | 8.8 | 121 | 8.6 | 36 | 9.6 | |
| Missing/Unknown | 417 | 23.5 | 333 | 23.7 | 84 | 22.5 | |
| Pathological tumor size, cm | |||||||
| ≤ 1 | 518 | 29.1 | 357 | 25.4 | 161 | 43.1 | <.001 |
| >1 | 1005 | 56.5 | 904 | 64.3 | 101 | 27.1 | |
| Unknown (but stage I or ≤2 cm per protocol) | 255 | 14.4 | 144 | 10.3 | 111 | 29.8 | |
| Hormone status | |||||||
| ER+ and PR+ | 1361 | 76.5 | 1143 | 81.3 | 218 | 58.4 | <.001 |
| Other (ER+ or PR+ or one unknown and the other +) | 417 | 23.5 | 262 | 18.7 | 155 | 41.6 | |
| HER2 status | |||||||
| Known negative | 986 | 55.5 | 835 | 59.5 | 151 | 40.5 | <.001 |
| Unknown | 792 | 44.5 | 570 | 40.5 | 222 | 59.5 | |
| Initial endocrine therapy | |||||||
| Tamoxifen | 1145 | 64.4 | 815 | 58 | 330 | 88.5 | <.001 |
| AI† | 434 | 24.4 | 403 | 28.7 | 31 | 8.3 | |
| AI and Tamoxifen | 161 | 9.1 | 152 | 10.9 | 9 | 2.4 | |
| Ovarian suppression (+/- AI or Tam) | 38 | 2.1 | 35 | 2.5 | 3 | 0.8 | |
| Study | |||||||
| NSABP B-21 | 216 | 12.2 | 103 | 7.4 | 113 | 30.3 | <.001 |
| CALGB 9343 | 135 | 7.6 | 68 | 4.8 | 67 | 17.9 | |
| Toronto/Vancouver Trial | 177 | 10 | 87 | 6.2 | 90 | 24.1 | |
| GBSG V | 88 | 4.9 | 46 | 3.3 | 42 | 11.3 | |
| TAILORx | 809 | 45.5 | 748 | 53.2 | 61 | 16.4 | |
| NSABP B-14 | 208 | 11.7 | 208 | 14.8 | −‡ | −‡ | |
| NSABP B-20 | 145 | 8.1 | 145 | 10.3 | −‡ | −‡ | |
| Years of diagnosis | |||||||
| 1980-1989 | 353 | 19.8 | 353 | 25.1 | −‡ | −‡ | <.001 |
| 1990-2000 | 616 | 34.7 | 304 | 21.7 | 312 | 83.7 | |
| 2001 or later | 809 | 45.5 | 748 | 53.2 | 61 | 16.3 | |
| Overall median length of follow-up (range), y | 1778 | 5(0.1–23) | 1405 | 5(0.1–23) | 373 | 8(0.2–18) | <.001 |
| Radiotherapy randomized | |||||||
| Yes | 616 | 34.7 | 304 | 21.6 | 312 | 83.7 | <.001 |
| No | 1162 | 65.3 | 1101 | 78.4 | 61 | 16.3 | |
| Oncotype DX risk group | |||||||
| 0-10 | 1120 | 63.0 | 959 | 68.3 | 161 | 43.1 | <.001 |
| 11-18 | 658 | 37.0 | 446 | 31.7 | 212 | 56.9 | |
Two-sided P values are based on χ2 or Student t tests. ER = estrogen receptor; PR = progesterone receptor; AI = aromatase inhibitor; NSABP = National Surgical Adjuvant Breast and Bowel Project; CALGB = Cancer And Leukemia Group B; TAILORx = Trial Assigning IndividuaLized Options for Treatment (Rx); GBSG V = German Breast Cancer Study Group V.
Approximately 20% women younger than age 60 years and 30% women aged 60 years and older received aromatase inhibitors only.
Radiotherapy was given to women per treatment protocol. Assumed 100% compliance to protocol.
Primary Endpoint-RFI
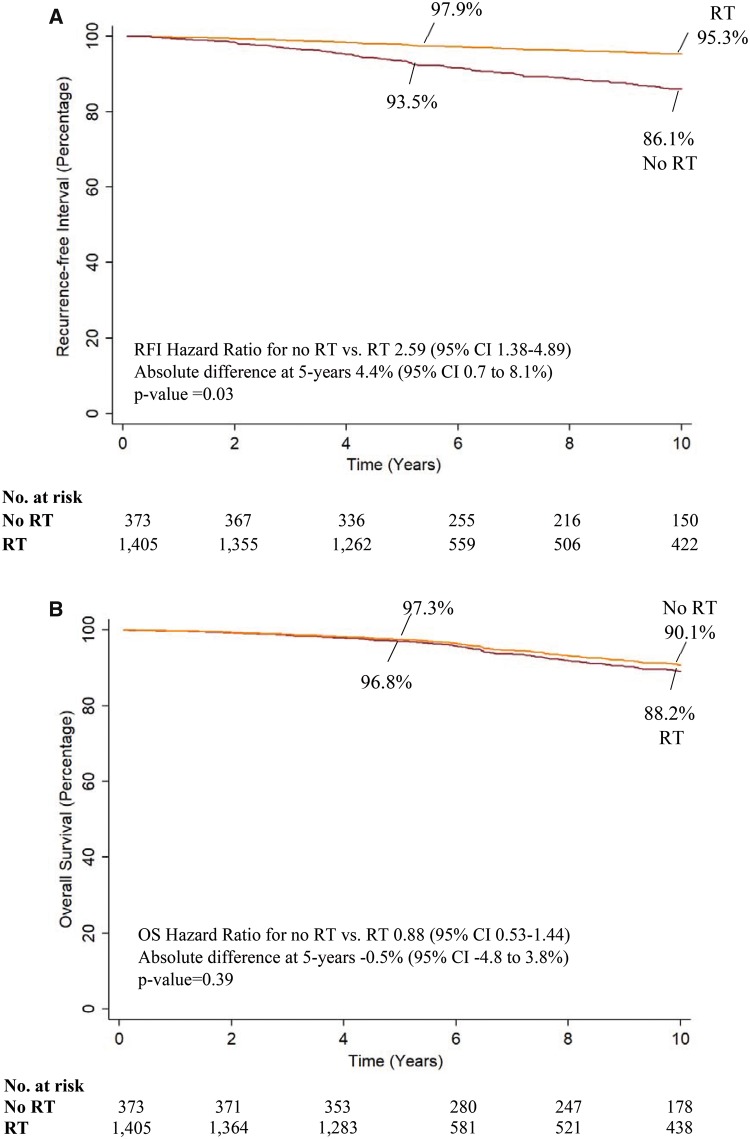
Omission of radiotherapy was associated with approximately a 2.5-fold increase of any occurrence of locoregional, distant recurrence or breast cancer death (adjusted RFI HR = 2.59, 95% CI = 1.38 to 4.89; P = .003) (Table 2). The adjusted five-year RFI rate with radiotherapy was 97.9% compared with 93.5% without radiotherapy (absolute difference = 4.4%, 95% CI = 0.7% to 8.1%; P = .03) (Figure 2A). The adjusted 10-year RFI rate with radiotherapy was 95.3% and 86.1% without radiotherapy (absolute difference = 9.2%, 95% CI = 1.8% to 16.6%, P = .02).
Table 2.
Hazard ratios for recurrence-free interval and overall and breast cancer–specific survival for omission vs receipt of radiotherapy
| Study endpoints | No. | No. of events/Total No. of women |
HR (95% CI) | P* | |
|---|---|---|---|---|---|
| No RT | RT | ||||
| RFI | |||||
| Unweighted (no RT vs RT)† | 1778 | 53/373 | 91/1405 | 2.59 (1.38 to 4.89) | .003 |
| Propensity weighted (no RT vs RT) ‡ | 1778 | 2.75 (1.67 to 4.54) | <.001 | ||
| Competing risk model (no RT vs RT)† | 1778 | 2.63 (1.40 to 4.92) | .003 | ||
| Locoregional RFI§ | |||||
| Unweighted (no RT vs RT) † | 1741 | 38/366 | 30/1375 | 3.91 (1.81 to 8.45) | .001 |
| Propensity weighted (no RT vs RT) ‡ | 1741 | 3.92 (1.87 to 8.20) | <.001 | ||
| Competing risk model (no RT vs RT) † | 1741 | 3.97 (1.85 to 8.50) | <.001 | ||
| Distant RFI‖ | |||||
| Unweighted (no RT vs RT) † | 1710 | 7/335 | 30/1375 | 0.90 (0.26 to 3.10) | .90 |
| Propensity weighted (no RT vs RT)‡ | 1710 | 0.84 (0.30 to 2.34) | .81 | ||
| Competing risk model (no RT vs RT) † | 1710 | 0.82 (0.24 to 2.75) | .75 | ||
| Mortality outcomes | |||||
| All-cause mortality † | 1778 | 44/373 | 104/1405 | 0.88 (0.53 to 1.44) | .61 |
| Breast cancer–specific mortality † | 1778 | 6/373 | 19/1405 | 1.14 (0.30 to 4.43) | .85 |
Two-sided P values are based on Student t test. RFI = recurrence-free interval; CI = confidence interval; RT = radiotherapy.
Adjusted for patient age, tumor grade (low, moderate, high, or unknown), ER/PR status (ER+ and PR+ or other), HER2 (negative or unknown), initial hormonal treatment (tamoxifen, aromatase inhibitors or other), tumor size, trial, and Oncotype DX recurrence score.
Inverse probability of treatment weighted (IPTW) analysis.
Excluding distant recurrence events.
Excluding locoregional recurrence events.
Figure 2.
Recurrence-free interval (RFI) and overall survival (OS) by receipt of radiotherapy (RT). Recurrence-free interval (A) and overall survival (B) were determined using a Cox model adjusted for patient age, tumor grade (low, moderate, high, or unknown), ER/PR status (ER+ and PR+ or other), HER2 (negative or unknown), type of initial hormonal treatment (tamoxifen, aromatase inhibitors, or other), tumor size, trial, and Oncotype DX recurrence score. Evaluated at mean age, tumor size, Oncotype DX recurrence score, and tamoxifen hormonal treatment, low grade, ER+/PR+ status, and in studies where radiotherapy was randomized. P values for rate differences are based on a two-tailed Z test.
Omission of radiotherapy statistically significantly increased the risk of locoregional recurrence events (adjusted HR = 3.91, 95% CI = 1.81 to 8.45, P = .001), but not the risk of distant recurrence (P = .90) (Table 2). The adjusted five-year locoregional RFI rate with radiotherapy was 98.6% compared with 93.7% without radiotherapy (absolute difference = 4.9%, 95% CI = 2.5% to 7.2%, P < .001), whereas the 10-year adjusted rate with radiotherapy was 96.6% and 85.5% without radiotherapy (absolute difference = 11.1%, 95% CI = 6.8% to 15.4%, P < .001). The results were unchanged in propensity score weighted and competing risk analyses (Table 2).
Overall and Breast Cancer–Specific Survival
Radiotherapy was not statistically significantly associated with overall (P = .61) or breast cancer–specific survival (P = .85) (Table 2). The adjusted five- and 10-year overall survival rates were 96.8% and 88.2% with radiotherapy compared with 97.3% and 90.1% without radiotherapy, respectively (absolute difference at five years = −0.5%, 95% CI = −4.8% to 3.8%, P = .39, 10 years = −1.9%, 95% CI = −16.5% to 12.7%, P = .39) (Figure 2B). The adjusted five-year breast cancer–specific survival rate with radiotherapy was 99.2% and 99.1% without radiotherapy (absolute difference = 0.1%, 95% CI = −1.3% to 1.5%, P = .40).
Subgroup Analyses
The adjusted RFI hazard ratios and five-year rates by radiotherapy tended to vary by patient characteristics (Table 3). The differences in RFI rates for omission of radiotherapy followed a similar pattern, with smaller absolute differences in RFI rates in the lower risk subgroups compared to the higher risk subgroups.
Table 3.
Hazard ratios and five-year recurrence-free interval rates for omission vs receipt of radiotherapy for patient subgroups
| Characteristics | N | No. of events/Total No. of women |
HR (95% CI) | 5-year RFI rates, % |
|||
|---|---|---|---|---|---|---|---|
| No RT | RT | No RT | RT | Absolute differences, % (95% CI) | |||
| Overall* | 1778 | 53/373 | 91/1405 | 2.59 (1.38 to 4.89) | 93.5 | 97.9 | 4.4 (0.7 to 8.1) |
| Hormone status† | |||||||
| ER+ and PR+ | 1361 | 25/218 | 39/1143 | 2.47(0.92 to 6.68) | 94.5 | 98.0 | 3.5 (−1.3 to 8.3) |
| Other | 417 | 20/155 | 22/262 | 2.55 (1.15 to 5.66) | 92.0 | 97.7 | 5.7 (0.4 to 11.8) |
| Oncotype DX recurrence score‡ | |||||||
| 0-10 | 1120 | 10/161 | 16/959 | 2.39 (0.87 to 6.58) | 94.7 | 98.6 | 3.9 (−1.2 to 8.9) |
| 11-18 | 658 | 19/212 | 25/446 | 2.62 (1.10 to 6.28) | 92.0 | 97.3 | 5.3 (0.3 to 11.0) |
| Age Group, at random assignment/enrollment§ | |||||||
| <60 y | 903 | 17/130 | 42/773 | 2.45 (1.00 to 7.82) | 90.0 | 95.4 | 5.4 (2.9 to 13.7) |
| 60-70 y | 296 | 13/122 | 12/174 | 2.37 (0.89 to 10.35) | 93.4 | 98.4 | 5.0 (1.0 to 9.0) |
| >70 y | 579 | 14/121 | 6/458 | 1.99 (0.29 to 13.47) | 95.1 | 98.1 | 3.0 (−0.9 to 6.9) |
| Grade ‖ | |||||||
| Low | 439 | 6/98 | 9/341 | 2.33 (0.59 to 9.20) | 99.0 | 99.8 | 0.8 (−1.4 to 2.9) |
| Intermediate/High | 922 | 20/191 | 23/731 | 2.16 (0.90 to 5.18) | 91.6 | 96.1 | 4.5 (−1.9 to 10.9) |
| Tumor size¶ | |||||||
| ≤ 1 cm | 635 | 24/209 | 19/426 | 2.37 (1.07 to 5.26) | 92.8 | 96.4 | 3.6 (−1.6 to 8.7) |
| > 1 cm | 1143 | 20/164 | 42/979 | 2.74 (1.42 to 5.32) | 92.0 | 96.8 | 4.8 (−2.6 to 12.2) |
Cox models adjusted for patient age, tumor grade (low, moderate, high, or unknown), ER/PR status (ER+ and PR+ or other), HER2 (negative or unknown), initial hormonal treatment (tamoxifen, aromatase inhibitors or other), tumor size, trial, and Oncotype DX recurrence score. RFI rates evaluated at mean age, tumor size, Oncotype DX recurrence score, and tamoxifen hormonal treatment, low grade, ER+/ PR+ status and in studies where radiotherapy was randomized. RFI = recurrence-free interval; CI = confidence interval; HR = hazard ratio; RT = radiotherapy.
Cox models adjusted for patient age, tumor grade (low, moderate, high, or unknown), tumor size, trial type, and Oncotype DX recurrence score.
Cox models adjusted for patient age, tumor grade (low, moderate, high, or unknown), ER/PR status (ER+ and PR+ or other), tumor size, trial type, and Oncotype DX® recurrence score.
Cox models adjusted for patient age, tumor grade (low/moderate, high, or unknown), ER/PR status (ER+ and PR+ or other), tumor size, trial type and Oncotype DX® recurrence score.
Cox models adjusted for patient age, ER/PR status (ER+ and PR+ or other), tumor size, trial type, and Oncotype DX® recurrence score.
Cox models adjusted for patient age, ER/PR status (ER+ and PR+ or other), and Oncotype DX recurrence score.
Sensitivity Analyses
Exclusion of the three trials where radiotherapy was not randomized (B-14/B-20/TAILORx trials), exclusion of the TAILORx trial only, use of different study follow-up, or differences in ages included in the sample did not substantially change the results (Table 4).
Table 4.
Sensitivity analyses
| Study endpoints | N | No. of events/Total No. of women |
HR* (95% CI) | P† | |
|---|---|---|---|---|---|
| No RT | RT | ||||
| Excluding NSABP B-14, B-20, and TAILORx (radiotherapy not randomized) | |||||
| RFI | 616 | 43/312 | 17/304 | 2.70 (1.36 to 5.36) | .005 |
| Locoregional RFI | 603 | 37/306 | 10/297 | 4.11 (1.78 to 9.47) | .001 |
| Distant RFI | 570 | 6/275 | 7/295 | 0.95 (0.26 to 3.48) | .94 |
| Excluding TAILORx only | |||||
| RFI | 969 | 44/312 | 54/657 | 2.66 (1.36 to 5.23) | .005 |
| Locoregional RFI | 935 | 37/305 | 27/630 | 3.97 (1.72 to 9.10) | .001 |
| Distant RFI | 906 | 6/275 | 28/631 | 0.99 (0.27 to 3.64) | .98 |
| Including ages 40 to 75 years and older | |||||
| RFI | 2080 | 54/524 | 76/1556 | 2.01 (1.18 to 3.45) | .01 |
| Locoregional RFI | 1994 | 35/501 | 23/1493 | 3.81 (1.88 to 7.72) | <.001 |
| Distant RFI | 1974 | 10/472 | 41/1502 | 0.65 (0.26 to 1.60) | .40 |
| 5-year study duration | |||||
| RFI | 1778 | 22/373 | 37/1405 | 2.55 (1.06 to 6.18) | .04 |
| Locoregional RFI | 1742 | 19/367 | 19/1375 | 3.69 (1.36 to 10.03) | .01 |
| Distant RFI | 1710 | ||||
| 9-year study duration | |||||
| RFI | 1778 | 22/373 | 37/1405 | 2.64 (1.35 to 5.15) | .005 |
| Locoregional RFI | 1742 | 41/367 | 55/1375 | 4.25 (1.58 to 9.81) | .001 |
| Distant RFI | 1710 | 6/335 | 28/1375 | 0.90 (0.25 to 3.19) | .87 |
Cox models adjusted for patient age, tumor grade (low, moderate, high, or unknown), ER/PR status (ER+ and PR+ or other), HER2 (negative or unknown), type of initial hormonal treatment (tamoxifen, aromatase inhibitors or other), tumor size, study type, and Oncotype DX recurrence score. RFI = recurrence-free interval; CI = confidence interval; HR = hazard ratio; NSABP = National Surgical Adjuvant Breast and Bowel Project; TAILORx = Trial Assigning IndividuaLized Options for Treatment (Rx).
Two-sided P values are based on Student t test.
Discussion
This study extends prior research to examine the effects of omitting radiotherapy in breast cancer patients aged 40–74 years who are at low risk of recurrence based on information about molecular subtypes and tumor gene expression profiles. Although the absolute differences in RFI rates were small, results indicated that omitting radiotherapy would increase the risk of recurrence, even in a group defined as low risk by gene expression profile. This result is largely due to a statistically significant increase in locoregional recurrence events with omission of radiotherapy, with no statistically significant effect on distant recurrences or death. However, there may be patient subgroups within the low-risk population where it may be reasonable to consider omitting radiotherapy, such as those with the lowest Oncotype scores or low tumor grade. Finally, there was no statistically significant effect of radiotherapy on distant recurrence, breast cancer–specific mortality, or all-cause mortality, suggesting that this is an appropriate population for the investigation of radiotherapy omission among women willing to tolerate a modest increased risk of locoregional recurrence.
The recent EBCTCG meta-analyses of 10 801 patients from 17 trials demonstrated that omission of radiotherapy increased the five-year risk of any breast cancer recurrence in node-negative women from 10.6% to 22.5% (absolute difference = 11.9%) (2). In contrast, by only focusing on hormone-sensitive breast cancers with favorable prognosis further defined by estimated Oncotype scores, we found that omission of radiotherapy increased the 5-year risk of any recurrence by a more modest absolute difference of 4.4%. Further, our analysis showed that the vast majority of recurrences prevented by radiotherapy are clinically salvageable locoregional events rather than distant metastases (5,13). The effect of radiotherapy on the absolute differences of RFI and locoregional RFI increased with increasing follow-up. Locoregional recurrences are known to occur later in follow-up especially among ER-positive tumors. Therefore, the benefits of radiotherapy on reducing the risk of locoregional recurrence may increase with even longer-term follow-up than included in this analysis. Radiation-induced harms will also be more likely to be observed with longer follow-up.
The pooled analysis included seven clinical trials relevant for evaluating the risk of omitting radiotherapy in low-risk breast cancer. In contrast to previous studies, the majority of women included in the current analysis were younger (<60 years) and diagnosed after year 2000, allowing us to evaluate the likely effects of omitting radiotherapy in younger women with low Oncotype scores. Although studies were selected for using modern radiotherapy regimens and most of the women included in this study were diagnosed after the year 2000, medical care and breast cancer treatment have changed over the past 20 years, potentially limiting the generalizability of the study findings to current populations.
The results of the subgroup analyses for RFI with omission of radiotherapy were consistent with past studies showing that certain subgroups have sufficiently low recurrence risks to potentially opt out of radiation (2). Although the rate of events observed was low and the original studies were not powered to detect subgroup effects, the trends in absolute differences in the pooled data suggest that there may be lower risk categories within subgroups defined by age, Oncotype, tumor size, hormonal status, and/or grade where the increase in recurrence is sufficiently small (and primarily locoregional) without radiotherapy. In these cases, decisions about use of radiotherapy might be preference-based. These results suggest that future trials should consider including sufficiently large samples in subgroups to detect relevant differences in primary endpoints and to collect data on patient preferences for study outcomes.
The results of this pooled data analysis were robust, but several caveats should be considered in evaluating the findings. First, when data were combined, radiotherapy was either no longer randomly assigned or may not have been randomly assigned in the original trial. The selection of patients to radiotherapy and/or imbalance of any unmeasured covariates by radiotherapy group could have biased results. However, results of the propensity-weighted analyses were similar to unweighted analyses and findings from the pooled sample were similar to prior published studies (7,14,24) and the Oxford Overview (2) for comparable populations, so the results are not likely to be spurious. Second, we could not assess the effect of radiotherapy among postmenopausal women receiving aromatase inhibitors. Future research might consider whether aromatase inhibitors lower the risk of recurrence sufficiently more than tamoxifen such that omission of radiotherapy might not lead to an elevated risk of recurrence events. Here, 64% of women younger than 60 years received tamoxifen; and there were too few patients aged 40–49 years to estimate radiotherapy effects separately for premenopausal patients. The younger women were predominantly from the TAILORx trial and systematically different to other patients in this age group from older trials. Next, we could not differentiate clinically from pathologically determined node status among women enrolled in certain trials. However, given that the majority of women included in the trials required or included pathologically determined node-negative status (52%), we do not expect our findings to differ by clinical vs pathological node status. Finally, we did not impute missing HER2 information for this analysis, assuming that HER2 was missing at random and independent of time-to-events. The magnitude and direction of potential biases introduced by missing HER2 data are unknown.
Another consideration is that Oncotype results were imputed because they were not available in the original trials (except for TAILORx) (8). We employed a population-based donor dataset (22), and a deterministic regression–based multiple imputation approach (20), to estimate missing Oncotype scores. Although the distribution of observable patient characteristics between the population-based donor dataset and the pooled clinical trial dataset were comparable, women eligible for clinical trials could differ from population-based data in terms of eligibility, compliance, and other unobservable characteristics. As a result, even though our imputation was robust, it may have not removed all bias due to missing data. Additionally, women receiving Oncotype testing in the population-based registry mostly included women with low- and intermediate-risk cancers (21,22). However, women enrolled in these trials were selected to participate in the trials without knowledge of Oncotype; therefore they may have had higher or lower scores than the source population from which they were drawn. Hence it is not possible to infer the impact of the Oncotype score imputation on misclassification and bias.
Finally, even in this large pooled sample there were few events, limiting power to detect statistically significant differences in radiotherapy effects in subgroups. However, the trends in absolute differences in RFI rates were consistent with clinical expectations. The trials included did not explicitly consider any excess deaths due to radiotherapy-induced lung cancer or cardiovascular disease, so we could not directly assess the impact of radiation-induced harms (4). However, these would have likely been captured in the analysis as other-cause deaths, and radiotherapy did not decrease overall survival. Although these effects are uncommon, especially among nonsmokers (4), it will be important to specifically capture these events in future trials to more completely understand the balance of benefits and harms of radiotherapy in low-risk patients.
Overall, the results of this pooled analysis suggest that omission of radiotherapy after breast conservation in hormone-sensitive, low-risk patients could lead to higher relative differences, but small absolute differences in RFI rates. Clinical decision making is usually based on absolute differences. Moreover, omission of radiotherapy does not appear to increase distant recurrences or early death in this low-risk population. Future trial design with modern clinical management should consider focusing on subgroups within the population presently considered low risk for recurrence to determine if there are those who can safely omit radiotherapy after breast conservation.
Funding
This work was supported by the National Institutes of Health under National Cancer Institute (NCI) Grants U01CA12958, U10CA180868, UG1CA189867, and U10CA180822. Supplemental funding was provided by NCI’s Coordinating Center For Clinical Trials and a Lombardi Comprehensive Cancer Center American Cancer Society Young Investigator Award (ACS IRG 92–152-20) and the Cancer Prevention Research Fellowship sponsored by the American Society of Preventive Oncology and Breast Cancer Research Foundation (ASPO-17–001) to Dr. Jayasekera.
Notes
Affiliations of authors: Department of Oncology, Georgetown University Medical Center and Cancer Prevention and Control Program, Georgetown-Lombardi Comprehensive Cancer Center, Washington, DC (JJ, JM); Departments of Family and Social Medicine and Epidemiology and Population Health, Albert Einstein College of Medicine, Bronx, NY (CS); Department of Radiation Oncology, University of Michigan, Ann Arbor, MI (RJ); The James Department of Radiation Oncology, The Ohio State University Comprehensive Cancer Center, Columbus, OH (JW); Canadian Cancer Trials Group, Queen’s University, Kingston, ON, Canada (JAWC-Retired); Research Department of Oncology, Division of Radiation Oncology, McMaster University, Hamilton, ON, Canada (TW); Montefiore Medical Center, Albert Einstein College of Medicine, Bronx, NY (JAS); NRG Oncology, and the Department of Biostatistics, University of Pittsburgh Graduate School of Public Health, Pittsburgh, PA (SJA); Radiation Medicine Program, Princess Margaret Cancer Centre, Department of Radiation Oncology, University of Toronto, Toronto, ON, Canada (AWF); Institute for Medical Biometry and Statistics, Faculty of Medicine and Medical Center - University of Freiburg, Freiburg, Germany (WS); Department of Radiation Oncology, Indiana University, Bloomington, IN (RCZ); Department of Biostatistics, Division of Quantitative Sciences, The University of Texas MD Anderson Cancer Center, Houston, TX (YL, JS, XH, DB); NRG Oncology, and The Division of Breast Surgical Oncology, Allegheny General Hospital, Allegheny Health Network, Pittsburgh, PA (TBJ); Division of Cancer Control and Population Sciences, National Cancer Institute, Bethesda, MD (EJF); Department of Biostatistics, Bioinformatics and Biomathematics, Georgetown-Lombardi Comprehensive Cancer Center, Georgetown University Medical Center, Washington, DC (GL).
The funders had no role in the design of the study; the collection, analysis, and interpretation of the data; the writing of the manuscript; and the decision to submit the manuscript for publication.
Jinani Jayasekera, Yisheng Li, Clyde Schechter, Reshma Jagsi, Juhee Song, George Luta, Judith-Anne Chapman, Eric Feuer, Richard Zellars, Thomas Julian, Timothy Whelan, Xuelin Huang, Stewart Anderson, Anthony Fyles, Willi Sauerbrei, and Jeanne Mandelblatt have nothing to disclose.
Julia White received honoraria from Qfix. Joseph Sparano owns stock in Metastat, has served in an advisory role for Genentech/Roche, Novartis, AstraZeneca, Celgene, Lilly, Celldex, Pfizer, Prescient Therapeutics, Juno Therapeutics, and Merrimack, and has received research funding from Prescient Therapeutics, Deciphera, Genentech/Roche, Merck, Novartis, and Merrimack. Donald Berry is co-owner of Berry Consultants, LLC, a company that designs adaptive Bayesian clinical trials for pharmaceutical and medical device companies, NIH cooperative groups, international consortia, and patient advocacy groups.
Jinani Jayasekera was responsible for conception, design, data analyses, interpretation of results, manuscript preparation, and approval of the final manuscript.
Clyde Schechter was responsible for conception, design, data analyses, interpretation of results, manuscript preparation, and approval of the final manuscript.
Reshma Jagsi was responsible for conception, interpretation of results, and approval of the final manuscript.
Joseph A. Sparano was responsible for conception, interpretation of results, and approval of the final manuscript.
Juhee Song was responsible for data analyses and approval of the final manuscript.
Yisheng Li was responsible for data analyses and approval of the final manuscript.
Julia White was responsible for conception, interpretation of results, and approval of the final manuscript.
George Luta was responsible for data analyses, interpretation of results, and approval of the final manuscript.
Judith-Anne Chapman was responsible for interpretation of results and approval of the final manuscript.
Eric J. Feuer was responsible for conception, provision of data, interpretation of results, and approval of the final manuscript.
Richard C. Zellars was responsible for interpretation of results and approval of the final manuscript.
Thomas B. Julian was responsible for conception, interpretation of results and approval of the final manuscript.
Timothy Whelan was responsible for interpretation of results and approval of the final manuscript.
Xuelin Huang was responsible for data analyses, interpretation of results, and approval of the final manuscript.
Stewart J. Anderson was responsible for provision of data, interpretation of results, and approval of the final manuscript.
Anthony W. Fyles was responsible for provision of data, interpretation of results, and approval of the final manuscript.
Willi Sauerbrei was responsible for provision of data, interpretation of results, and approval of the final manuscript.
Donald Berry was responsible for conception, design, data analyses, interpretation of results, manuscript preparation, and approval of the final manuscript.
Jeanne Mandelblatt was responsible for conception, design, data analyses, interpretation of results, manuscript preparation, and approval of the final manuscript.
We acknowledge the generous funding from the National Institutes of Health under National Cancer Institute Grant U01CA12958, U10CA180868, UG1CA189867, U10CA180822, and supplemental funding provided by NCI’s Coordinating Center for Clinical Trials as well as by the Georgetown-Lombardi American Cancer Society Young Investigator Award (ACS IRG 92–152-20) and the Cancer Prevention Research Fellowship sponsored by the American Society of Preventive Oncology and Breast Cancer Research Foundation (ASPO-17–001) to Dr Jayasekera.
We are grateful to the researchers who undertook the trials and shared their data: Anthony Fyles (Toronto/Vancouver Trial); Stewart J. Anderson, Joseph P. Constantino (NSABP Trials); Donald Berry (CALGB 9343); Joseph A. Sparano, Robert Gray (TAILORx); and Willi Sauerbrei (GBSG V). We also thank Genomic Health for provision of de-identified, locked NSABP-Genomic Health data. Finally, we thank Jo Anne Zujewski and National Cancer Institute staff for assistance facilitating data requests and Chris Cadham for assistance during manuscript preparation.
Supplementary Material
References
- 1. Miller KD, Siegel RL, Lin CC, et al. Cancer treatment and survivorship statistics, 2016. CA Cancer J Clin. 2016;664:271–289. [DOI] [PubMed] [Google Scholar]
- 2. Darby S, McGale P, Correa C, et al. Effect of radiotherapy after breast-conserving surgery on 10-year recurrence and 15-year breast cancer death: meta-analysis of individual patient data for 10,801 women in 17 randomised trials. Lancet. 2011;3789804:1707–1716. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Hughes KS, Schnaper LA, Bellon JR, et al. Lumpectomy plus tamoxifen with or without irradiation in women age 70 years or older with early breast cancer: Long-term follow-up of CALGB 9343. J Clin Oncol. 2013;3119:2382–2387. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Taylor C, Correa C, Duane FK, et al. Estimating the risks of breast cancer radiotherapy: evidence from modern radiation doses to the lungs and heart and from previous randomized trials. J Clin Oncol. 2017; doi:10.1200/JCO.2016.72.0722. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Mamounas EP, Tang G, Fisher B, et al. Association between the 21-gene recurrence score assay and risk of locoregional recurrence in node-negative, estrogen receptor–positive breast cancer: results from NSABP B-14 and NSABP B-20. J Clin Oncol. 2010;2810:1677–1683. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Fyles AW, McCready DR, Manchul LA, et al. Tamoxifen with or without breast irradiation in women 50 years of age or older with early breast cancer. N Engl J Med. 2004;35110:963–970. [DOI] [PubMed] [Google Scholar]
- 7. Winzer KJ, Sauerbrei W, Braun M, et al. Radiation therapy and tamoxifen after breast-conserving surgery: updated results of a 2 x 2 randomised clinical trial in patients with low risk of recurrence. Eur J Cancer. 2010;461:95–101. [DOI] [PubMed] [Google Scholar]
- 8. Sparano JA, Gray RJ, Makower DF, et al. Prospective validation of a 21-gene expression assay in breast cancer. N Engl J Med. 2015;37321:2005–2014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Fisher B, Bryant J, Dignam JJ, et al. Tamoxifen, radiation therapy, or both for prevention of ipsilateral breast tumor recurrence after lumpectomy in women with invasive breast cancers of one centimeter or less. J Clin Oncol. 2002;2020:4141–4149. [DOI] [PubMed] [Google Scholar]
- 10. Fisher B, Costantino J, Redmond C, et al. A Randomized clinical trial evaluating tamoxifen in the treatment of patients with node-negative breast cancer who have estrogen-receptor–positive tumors. N Engl J Med. 1989;3208:479–484. [DOI] [PubMed] [Google Scholar]
- 11. Fisher B, Dignam J, Wolmark N, et al. Tamoxifen and chemotherapy for lymph node-negative, estrogen receptor-positive breast cancer. J Natl Cancer Inst. 1997;8922:1673–1682. [DOI] [PubMed] [Google Scholar]
- 12. American Cancer Society, American Joint Committee on Cancer. AJCC Cancer Staging Handbook: From the AJCC Cancer Staging Manual. 6th ed New York, NY: Springer; 2002. [Google Scholar]
- 13. Kunkler IH, Williams LJ, Jack WJ, et al. Breast-conserving surgery with or without irradiation in women aged 65 years or older with early breast cancer (PRIME II): a randomised controlled trial. Lancet Oncol. 2015;163:266–273. [DOI] [PubMed] [Google Scholar]
- 14. Blamey RW, Bates T, Chetty U, et al. Radiotherapy or tamoxifen after conserving surgery for breast cancers of excellent prognosis: British Association of Surgical Oncology (BASO) II trial. Eur J Cancer. 2013;4910:2294–2302. [DOI] [PubMed] [Google Scholar]
- 15. Potter R, Gnant M, Kwasny W, et al. Lumpectomy plus tamoxifen or anastrozole with or without whole breast irradiation in women with favorable early breast cancer. Int J Radiat Oncol Biol Phys. 2007;682:334–340. [DOI] [PubMed] [Google Scholar]
- 16. Hudis CA, Barlow WE, Costantino JP, et al. Proposal for standardized definitions for efficacy end points in adjuvant breast cancer trials: the STEEP system. J Clin Oncol. 2007;2515:2127–2132. [DOI] [PubMed] [Google Scholar]
- 17. Trimble EL, Abrams JS, Meyer RM, et al. Improving cancer outcomes through international collaboration in academic cancer treatment trials. J Clin Oncol. 2009;2730:5109–5114. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Bravata DM, Olkin I.. Simple pooling versus combining in meta-analysis. Eval Health Prof. 2001;242:218–230. [DOI] [PubMed] [Google Scholar]
- 19. Friedenreich CM. Methods for pooled analyses of epidemiologic studies. Epidemiology. 1993;44:295–302. [DOI] [PubMed] [Google Scholar]
- 20. Rubin DB. Multiple Imputation for Nonresponse in Surveys. Hoboken, NJ: John Wiley & Sons; 1987. [Google Scholar]
- 21. Petkov VI, Miller DP, Howlader N, et al. Breast-cancer-specific mortality in patients treated based on the 21-gene assay: A SEER population-based study. NPJ Breast Cancer. 2016;21:16017.. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. Mahadevia PJ, Fleisher LA, Frick KD, et al. Lung cancer screening with helical computed tomography in older adult smokers: a decision and cost-effectiveness analysis. JAMA. 2003;2893:313–322. [DOI] [PubMed] [Google Scholar]
- 23. Marshall D, Simpson KN, Earle CC, et al. Potential cost-effectiveness of one-time screening for lung cancer (LC) in a high risk cohort. Lung Cancer. 2001;323:227–236. [DOI] [PubMed] [Google Scholar]
- 24. Holli K, Hietanen P, Saaristo R, et al. Radiotherapy after segmental resection of breast cancer with favorable prognostic features: 12-year follow-up results of a randomized trial. J Clin Oncol. 2009;276:927–932. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.