Abstract
Key points
This is the first study to demonstrate an altered circadian phase shifting response in a circadian rhythm sleep disorder.
Patients with delayed sleep–wake phase disorder (DSWPD) demonstrate greater sensitivity of the circadian system to the phase‐delaying effects of light.
Increased circadian sensitivity to light is associated with later circadian timing within both control and DSWPD groups.
DSWPD patients had a greater sustained pupil response after light exposure.
Treatments for DSWPD should consider sensitivity of the circadian system to light as a potential underlying vulnerability, making patients susceptible to relapse.
Abstract
Patients with delayed sleep–wake phase disorder (DSWPD) exhibit delayed sleep–wake behaviour relative to desired bedtime, often leading to chronic sleep restriction and daytime dysfunction. The majority of DSWPD patients also display delayed circadian timing in the melatonin rhythm. Hypersensitivity of the circadian system to phase‐delaying light is a plausible physiological basis for DSWPD vulnerability. We compared the phase shifting response to a 6.5 h light exposure (∼150 lux) between male patients with diagnosed DSWPD (n = 10; aged 20.8 ± 2.3 years) and male healthy controls (n = 11; aged 22.4 ± 3.3 years). Salivary dim light melatonin onset (DLMO) was measured under controlled conditions in dim light (<3 lux) before and after light exposure. Correcting for the circadian time of the light exposure, DSWPD patients exhibited 31.5% greater phase delay shifts than healthy controls. In both groups, a later initial melatonin phase was associated with a greater magnitude phase shift, indicating that increased circadian sensitivity to light may be a factor that contributes to delayed phase, even in non‐clinical groups. DSWPD patients also had reduced pupil size following the light exposure, and showed a trend towards increased melatonin suppression during light exposure. These findings indicate that, for patients with DSWPD, assessment of light sensitivity may be an important factor that can inform behavioural therapy, including minimization of exposure to phase‐delaying night‐time light.
Keywords: DSPD, light, Circadian rhythm, phase shift
Key points
This is the first study to demonstrate an altered circadian phase shifting response in a circadian rhythm sleep disorder.
Patients with delayed sleep–wake phase disorder (DSWPD) demonstrate greater sensitivity of the circadian system to the phase‐delaying effects of light.
Increased circadian sensitivity to light is associated with later circadian timing within both control and DSWPD groups.
DSWPD patients had a greater sustained pupil response after light exposure.
Treatments for DSWPD should consider sensitivity of the circadian system to light as a potential underlying vulnerability, making patients susceptible to relapse.
Introduction
Delayed sleep–wake phase disorder (DSWPD) is a circadian rhythm sleep disorder in which patients experience a delay in their major sleep episode relative to desired or socially optimal timing (American Academy of Sleep Medicine, 2014). Patients with DSWPD often have inappropriate alignment of their circadian clock with the natural 24 h light–dark cycle (Czeisler et al. 1981), with circadian rhythms delayed by 2–6 h relative to their desired bedtime (Micic et al. 2015). This delay is associated with increased sleep onset latency and, combined with early school or work times, often results in chronic sleep restriction and poor sleep quality, as sleep is attempted at times when the circadian clock is promoting wake. Patients with DSWPD also have high rates of depressive symptoms (Alvarez et al. 1992; Vandeputte & de Weerd, 2003; Murray et al. 2017), and experience significant daytime dysfunction, including lower academic performance (Lack, 1986), increased irritability and increased daytime sleepiness (Alvarez et al. 1992; Kripke et al. 2008).
A potential mechanism for the development of delayed circadian timing in DSWPD patients is an abnormal response to environmental light cues. The circadian system endogenously generates rhythms that have a period near to, but not exactly 24 h and therefore environmental time cues are required to synchronize these rhythms with the external 24 h day. Light is the most powerful synchronizing agent for the human circadian clock, with light exposure in the early biological night causing a phase delay and light exposure in the late biological night a phase advance (Minors et al. 1991; Zeitzer et al. 2000; Khalsa et al. 2003; St Hilaire et al. 2012). While the ability of the circadian system to shift its phase is essential for synchronization to the 24 h day, abnormal sensitivity to light, combined with use of artificial sources of light in the early biological night, could result in pathological misalignment. It has been proposed that patients with DSWPD may be hypersensitive to the effects of light exposure (Czeisler et al. 1981; Dagan & Eisenstein, 1999).
The only known study that directly compared light sensitivity in DSWPD (then referred to as Delayed Sleep Phase Syndrome; DSPS) to a healthy control group found that patients exhibited a 15% increase in melatonin suppression during 2 h of 1000 lux (cool‐white fluorescent lights), compared to controls (Aoki et al. 2001). In addition, greater melatonin suppression in response to 1.5 h of 500 lux blue light (fluorescent with blue filter) has recently been associated with later circadian phase in a subclinical group of young adults complaining of a delayed sleep schedule (Moderie et al. 2017), suggesting greater light sensitivity may play a causal role in the development of persistent delays in circadian phase.
Although a moderate increase in the suppression of melatonin to light in DSWPD has been demonstrated previously, it has not yet been determined whether DSWPD patients exhibit an increased circadian phase resetting response to light relative to healthy controls. Additionally, sensitivity to normal room‐light levels has not been examined in DSWPD patients. To address these issues, we studied the phase shifting response to a 6.5 h normal room light exposure (∼150 lux at eye level) in DSWPD patients and healthy controls. Given that DSWPD patients exhibit delayed circadian rhythms, we specifically investigated sensitivity in the delay region of the phase response curve by assessing the effect of light in the early biological night. In addition to being a normally occurring indoor lighting level (Scheuermaier et al. 2010) with known effects on melatonin suppression (Gooley et al. 2011), 150 lux was chosen due to its position in the active region of the dose–response curve to light (far below saturating light levels) during this circadian time (Zeitzer et al. 2000). We hypothesized that DSWPD patients would exhibit a greater phase delay and greater melatonin suppression in response to night‐time light exposure, compared to healthy controls.
Methods
Ethical approval
The current study was approved by the Monash University Human Research Ethics Committee and participants gave written informed consent prior to participation (Project No. 6027). This study was in line with the standards set by the Declaration of Helsinki (revision no. 7), except for registration in a database.
Participants
Participants were recruited from the community via posters, online, radio and train advertisements. A total of 24 male participants completed the study: 12 patients with diagnosed DSWPD (aged 21.3 ± 3.0 years) and 12 healthy controls (aged 22.5 ± 3.1 years). Previous research has suggested sex differences in sensitivity of the circadian system to light in both animals and humans (Davis et al. 1983; Monteleone et al. 1995; Cain et al. 2010). Therefore, only male participants were selected to reduce variability in light sensitivity within groups due to sex.
Phase 1: preliminary screening and consent
Participants were selected through three extensive screening phases (see Fig. 1 for the participant flowchart). In Phase 1, participants completed a range of online and over‐the‐phone questions to assess preliminary eligibility for the study. This included determining whether DSWPD patients exhibited sleep–wake behaviour consistent with a diagnosis, and that healthy controls demonstrated a healthy sleep–wake cycle. Participants were deemed eligible at this stage if: they had a BMI within the normal range of 18–30 kg m−2, they were Caucasian, they were fluent in English (first language or equivalent), they had no medical conditions or psychological/psychiatric history that might influence sleep or circadian rhythms, they were not on any medication that would influence sleep or circadian rhythms, they were a non‐smoker, daily caffeine consumptions was <300 mg, weekly alcohol consumption was <14 standard drinks, and they had not undertaken any transmeridian travel (>2 time zones) or participated in any shift work in the past 3 months. Healthy control participants had to have a self‐reported sleep onset occurring between 22.00 and 00.00 h, had a sleep length of 7–9 h, did not regularly undertake naps more than once a week, and had no sleep complaints or sleep pathology. DSWPD patients were required to have sleep onset time occurring after 01.00 h, a desire to sleep earlier than their current habitual bedtime (but not earlier than 22.00 h), an inability to fall asleep at their desired bedtime and no sleep pathology other than DSWPD. Participants were additionally excluded if their Beck Depression Inventory II (BDI‐II; Beck et al. 1996) score was above 13 for healthy controls to ensure their healthy control status, or above 18 for DSWPD patients to allow for mild depression symptoms, a common co‐morbidity of DSWPD (Alvarez et al. 1992; Vandeputte & de Weerd, 2003; Murray et al. 2017). Additional questionnaires completed by participants used for describing the sample included the Morning–Eveningness Questionnaire (MEQ; Horne & Östberg, 1976); Pittsburgh Sleep Quality Index (PSQI; Buysse et al. 1989); Insomnia Severity Index (ISI; Bastien et al. 2001); Depression, Anxiety and Stress Scale (DASS‐21; Lovibond & Lovibond, 1995; Henry & Crawford, 2005); and the General Anxiety Disorder 7‐item scale (GAD‐7; Spitzer et al. 2006).
Figure 1.
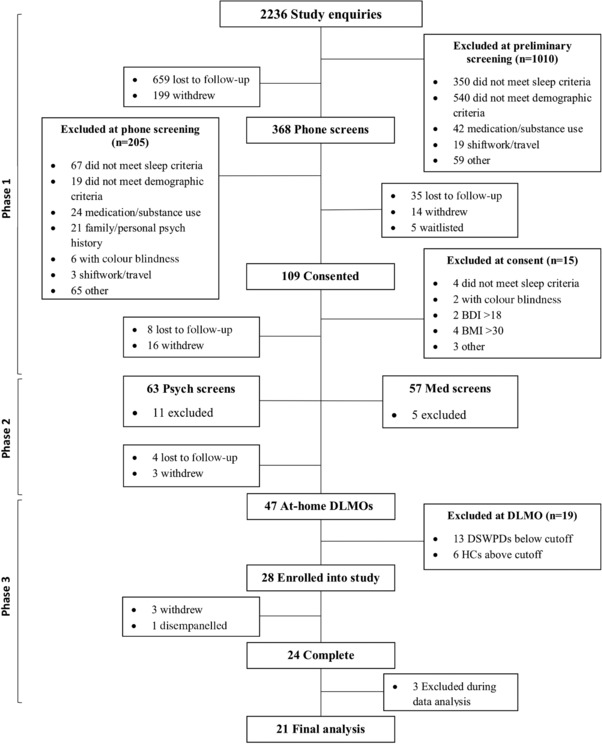
Flow chart of participant numbers and excluded participants at each step of the screening process
Phase 2: medical and psychiatric screening
Participants underwent a comprehensive examination with a registered sleep physician to confirm a diagnosis of DSWPD in the patient group (according to the criteria of the International Classification of Sleep Disorders (ICSD‐3); American Academy of Sleep Medicine, 2014), and a lack of any diagnoses in healthy controls. DSWPD diagnosis criteria A–C and E from the ICSD‐3 were confirmed by the sleep physician and criterion D (sleep log and actigraphy for at least 7 days demonstrating a delay in the timing of the habitual sleep period) was measured and confirmed by researchers prior to participation. Participants also underwent the Structured Clinical Interview for DSM‐5 (SCID‐5) to assess any personal or familial history of psychological or psychiatric disorders. This interview was conducted by a provisional psychologist under the supervision of a fully registered psychologist. Healthy control participants were deemed ineligible if they had any personal or familial history of any Axis‐1 disorders. DSWPD patients were excluded for any personal history of Axis‐1 disorders or any familial history which could result in increased risk associated with the sleep deprivation protocol (e.g. psychosis, bipolar disorder).
Phase 3: at‐home dim light melatonin onset measurement
Prior to admission to the study, participants underwent an at‐home assessment of dim light melatonin onset (DLMO) to confirm normal circadian timing in control participants, and a circadian delay in DSWPD patients. Participants were seated in a dimly lit room (<5 lux, measured at eye level, standing and seated, using a calibrated photometer) and were instructed to collect hourly saliva samples using salivettes (Sarstedt, Nümbrecht, Germany) from 5 h prior to their habitual bedtime until 2 h after (a total of 7 samples). A light sensor (Hobo, Onset, Bourne, MA, USA) was pinned to each participant's clothing to ensure compliance to the lighting conditions after researchers left the premises. Participants were instructed to avoid posture change, movement, food, and drink from 30 min prior to collection of each saliva sample. Participants were also asked to refrain from caffeine, alcohol and mouthwash on the day of collection. Healthy controls were deemed eligible for further study if they had a DLMO time of earlier than 22.00 h and a normal phase relationship (i.e. DLMO had to occur 1–3 h before habitual bedtime). DSWPD patients were deemed eligible for further study if their DLMO time was later than 23.00 h, and they were classified as having ‘circadian DSWPD’ (Murray et al. 2017), defined as having a DLMO within 30 min before desired bedtime or after desired bedtime (Murray et al. 2017). For example, if desired bedtime was midnight, DLMO was required to be 23.30 h or later to be considered delayed.
In‐home sleep–wake monitoring
During in‐home DLMO assessment, participants were provided with a wrist‐worn device (Actiwatch Spectrum Plus, Philips Respironics, Bend, OR, USA) to record activity and light levels until admission to the in‐lab protocol. Participants also completed a daily sleep diary to confirm bed and wake times. Participants were required to maintain a sleep–wake cycle consistent with the inclusion characteristics of their group (i.e. DSWPD participants needed to have an average bedtime past 01.00 h, and healthy controls needed to sleep on average 7–9 h starting between 22.00 and 00.00 h), to be admitted to the in‐lab protocol. Participant characteristics are presented in Table 1.
Table 1.
Demographic information and questionnaire scores for the healthy control and DSWPD groups
| Control | DSWPD | P | |
|---|---|---|---|
| Age (years) | 22.36 (3.26) | 20.80 (2.35) | 0.23 |
| Body mass index (kg m−2) | 22.62 (2.45) | 22.22 (4.19) | 0.79 |
| Bedtime | 23:23 (00:49) | 3:10 (01:13) | <0.001 |
| At‐home DLMO time | 20:50 (00.17) | 01:05 (01.24) | <0.001 |
| Phase angle (h) | 2.45 (0.73) | 1.49 (1.53) | 0.09 |
| Morningness–Eveningness Questionnaire | 56.64 (4.30) | 39.00 (4.72) | <0.001 |
| Pittsburgh Sleep Quality Index (range = 0–12) | 2.40 (1.51) | 7.22 (2.54) | <0.001 |
| Insomnia Severity Index (0–18) | 1.55 (1.51) | 12.33 (3.28) | <0.001 |
| Beck Depression Inventory – II (0–11) | 1.09 (1.14) | 6.00 (3.00) | <0.001 |
| DASS‐Depression (0–7) | 0.80 (0.63) | 4.25 (2.38) | <0.001 |
| DASS‐Anxiety (0–4) | 0.40 (0.70) | 1.63 (1.30) | 0.02 |
| DASS‐Stress (0–11) | 1.20 (1.99) | 4.88 (3.44) | 0.01 |
| General Anxiety Disorder 7‐item scale (0–10) | 0.80 (1.48) | 4.50 (2.78) | 0.002 |
Data are presented as mean and SD. Times are given as hours and minutes. Average bedtime was determined using the pre‐lab actigraphy. Dim light melatonin onset (DLMO) was measured using a 4 pg/ml cutoff (Carskadon et al. 1998). Phase angle is the time difference (h) between DLMO and actual bedtime (not desired bedtime). DASS, Depression, Anxiety and Stress Scale.
In‐laboratory protocol
Approximately 2 weeks after the at‐home DLMO collection (mean = 13.5 days, SD = 4.7 days, range = 7–24 days), participants completed a 6‐day stay in a completely time‐cue‐free (no windows, watches, TV, internet, phone or other indicators of time of day) and temperature‐regulated environment (see Fig. 2 for a representative raster plot). Participants were required to abstain from all caffeine, alcohol and medication for at least a week prior to the study, and were self‐reported to be free from shift work, nicotine, prescription medication and illicit drugs for at least 3 months on the day of admission. Urine toxicology screening was performed upon admission to confirm no recent use of illicit drugs (amphetamines, THC, cocaine, opiates and phencyclidine).
Figure 2. Raster plot of an example study protocol from home DLMO to discharge based on a HC participant with a bedtime of ∼23.00 h and DLMO of ∼20.00 h.
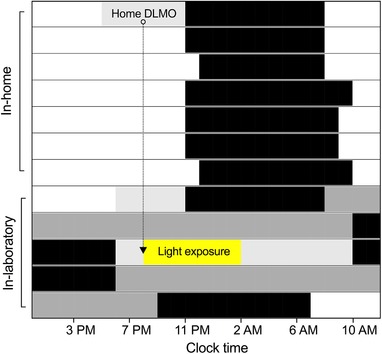
Sleep periods are denoted in black, dim‐light conditions in light grey and constant routine procedures in dark grey; ○ denotes home DLMO time, which was used to time the start of the in‐laboratory light exposure. [Color figure can be viewed at wileyonlinelibrary.com]
A constant room temperature of ∼22–23°C was maintained across the 6 day protocol. Day 1 of the study was a baseline day where saliva samples were collected, beginning from 5 h prior up until the participants’ habitual bedtime, as assessed through sleep diaries and actigraphy.
Following the baseline night, participants were given an 8 h sleep opportunity starting at their habitual bedtime. Upon awakening, they commenced a 27 h constant routine protocol (CR1). Participants were kept awake in a semi‐recumbent posture in dim light (<3 lux) and fed room‐temperature identical isocaloric snacks consisting of a sandwich quarter, 30 ml of juice and 80 ml of water every hour for the full 27 h CR1 period. Saliva samples for measurement of melatonin were taken hourly throughout the protocol, immediately prior to the small snack. Participants completed toilet opportunities using a bedpan or urinal to minimize postural changes. At the conclusion of CR1, participants were given an 8 h sleep opportunity, before waking to a 16 h day during which a 6.5 h light exposure was delivered. Melatonin profiles from CR1 were evaluated and two DSWPD patients, and one healthy control were excluded due to failing to meet the DLMO and sleep timing eligibility criteria for their respective groups. The final eligible participant sample was 21 male participants: 10 DSWPD patients (aged 22.4 ± 3.3 years) and 11 healthy controls (aged 22.4 ± 2.4 years).
The 6.5 h light exposure was delivered beginning at each individual participant's pre‐lab DLMO time, timed to occur in the delay region of the human phase response curve (PRC) to light from Khalsa et al. (2003). Preceding and following the 6.5 h light exposure, participants remained in lighting of <3 lux. Static pupil size was measured before, during and after this 6.5 h light exposure. Participants primarily remained seated, but were permitted to stand for bathroom breaks. After the light exposure day, participants were given another 8 h sleep opportunity prior to completing the second CR (CR2), which was identical to the first. After CR2, participants were given an extended recovery sleep of 10 h, then discharged on day 6 of the study protocol.
Lighting
Ambient lighting was generated using fluorescent lamps (Philips, Eindhoven, The Netherlands) and neutral density filters (Lee, Andover, UK), which delivered broad spectrum lighting with a correlated colour temperature of ∼4029 K and spectral peak of 544 nm (MK350N spectrometer, UPRTek, Zhunan, Taiwan). Lighting was measured approximately every 8 h throughout the CRs and approximately every hour from angle of gaze during the light exposure, to ensure the stability of lighting within and between participants (Tektronix J17 Luma Colour; Beaverton, OR, USA). During periods of <3 lux, measurements taken at a height of 1.2 m on a vertical plane were approximately 3.27 lux throughout the suite and measurements taken on the horizontal plane were approximately 1.44 lux.
During the light exposure, participants were seated in front of a white wall to reduce variation in the reflection of light and therefore reduce the effects of movement on retinal light exposure. Participants were required to maintain a fixed gaze for 10 min intervals towards a point in front of them that was measured to reflect ∼150 lux at angle of gaze, alternating with 10 min intervals of free gaze. During the 150 lux lighting condition, measurements taken at a height of 1.2 m on a vertical plane averaged 203 lux throughout the suite, and measurements taken on the horizontal plane averaged 91 lux. Measurements at the angle of gaze averaged 150 lux (SD = 2.66 lux). Effective illuminances for human photopigments (Lucas et al. 2014) during the light exposure condition (∼150 lux) are as follows: irradiance, 44.83 μW cm−2; photopic, 150.66 lux; cyanopic, 73.61 lux; melanopic, 79.35 lux; rhodopic, 102.94 lux; chloropic, 131.20 lux; and erythropic, 141.49 lux.
Salivary melatonin assays
Saliva samples were taken hourly throughout the in‐lab study. Participants were required to maintain a constant posture and to refrain from eating and drinking for 30 min prior to each saliva sample. Samples were frozen at −80°C, before being shipped to the Adelaide University Research Assay Facility. The samples were analysed by double antibody radioimmunoassay, using standards and reagents supplied by Buhlmann Laboratories (RKDSM‐2, Buhlmann Laboratories AG, Schönenbuch, Switzerland). This assay is based on the Voultsios et al. (1997) G280 anti‐melatonin antibody and 2‐[125I]iodomelatonin as the radioligand and follows the protocol provided by Buhlmann. The sensitivity of the assay was 4.3 pM. Saliva samples were assayed in duplicate (200 μl per duplicate). The intra‐assay coefficient of variation of the at‐home sample assays was 6.5%. The interassay coefficient of variation of the low concentration quality control was 13.2%, and the interassay coefficient of variation of the high concentration quality control was 8.0%. The intra‐assay coefficient of variation of the in‐lab sample assays was 6.7%. The interassay coefficient of variation of the low concentration quality control was 7.6%, and the interassay coefficient of variation of the high concentration quality control was 8.6%.
Pupillometry
Static pupil size was measured during and after the light exposure using an infrared pupilometer (Neuroptics PLR‐2000; Irvine, CA, USA). Measurements were taken at 10 min before lights on, 1 min after lights on, and then hourly for the first 3 h of the light exposure. Following the light exposure, measurements were taken at 30 min intervals for 2 h, with a final measurement 4 h after lights off. Measurements were ∼30 s in duration, and the average pupil size during each measurement was calculated after excluding any missing data due to eye blinks. Due to device malfunction, data were not available for three healthy control participants.
Statistics
Melatonin phase, melatonin amplitude, phase shift and relative phase shift
Melatonin phase shift was calculated as the difference in the timing of salivary DLMO between CR1 and CR2 (i.e. before and after the light exposure). Salivary DLMO was defined using a linear interpolation with a threshold of 25% of the peak melatonin concentration from a three‐harmonic least‐squares fit to the data. Salivary DLMOff was computed with the same threshold for the descending portion of the curve, and timing of melatonin midpoint was calculated as the average of DLMO and DLMOff times. Melatonin amplitude for both CR1 and CR2 was defined as the difference between the average and peak melatonin concentration from the above three‐harmonic least‐squares fit.
As described above, pre‐lab DLMO timing was used as our estimate for what the participant's DLMO timing would be during CR1, with lights‐on targeted to occur at CR1 DLMO time. However, due to high intraindividual variability within the DSWPD group (see Fig. 3), there was significant spread in the timing of light relative to CR1 DLMO (range was from 95 min before to 122 min after DLMO), meaning light stimuli frequently missed the intended timing (in 5 DSWPD patients and 1 healthy control it was more than 60 min off target). Different individuals therefore received light at different points on the PRC, hindering any direct comparisons of raw phase shifts between individuals or groups.
Figure 3.
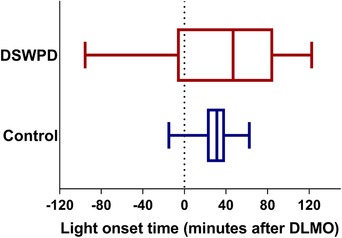
The range of light onset timing compared to the CR 1 DLMO for both healthy controls and DSWPD patients [Color figure can be viewed at wileyonlinelibrary.com]
To adjust for wide variability in the circadian timing of light exposure, we compared the observed phase shift for each individual to the phase shift predicted by the PRC in Khalsa et al. (2003), who used similar light exposure procedures and light sources (both brand and colour temperature). The relative phase shift (i.e. fraction of the predicted phase shift) was computed for each individual, providing an estimate of that individual's relative sensitivity to light. To derive the relative phase shift, we first computed a predicted phase shift, using the melatonin midpoint, from the fitted curve in Khalsa et al. (2003). Since our light level was lower than the level used by Khalsa et al. (2003) (∼150 lux vs. ∼10,000 lux), we divided the predicted phase shift by the ratio of the dose–response curve values for 150 lux vs. 10,000 lux found by Zeitzer et al. (2000), who used 6.5 h light pulses and similar lights (both brand and colour temperature) to induce circadian phase shifting. The ratio computed was 1.61 (i.e. 10,000 lux is 1.61 times more effective than 150 lux in shifting circadian phase for the same duration light pulse). Finally, the relative phase shift was computed by dividing an individual's observed phase shift by the predicted phase shift. A value of 1 represents the average expected response from these previous studies in healthy young adults.
Melatonin suppression
The area under the curve (AUC) was calculated using the trapezoidal method for the light exposure period, and the corresponding CR1 circadian time. AUC suppression for the light exposure compared to the CR1 dark control was then calculated by determining the percentage reduction in melatonin concentration between the AUC of CR1 and the AUC of the 6.5 h light exposure.
Analyses
Group differences in the mean relative phase shift between DSWPD patients and healthy controls were analysed using an independent samples t test. Differences in melatonin suppression levels between groups over the 6.5 h light exposure were analysed using an independent samples t test. To investigate the relationship between an individual's light sensitivity (relative phase shift) and his circadian timing (melatonin midpoint), we used a linear mixed‐effects model to predict melatonin midpoint. The model assumed fixed effects for group (nominal variable) and relative phase shift (continuous variable), and random effects for participants to account for individual differences. An interaction of group with relative phase shift was tested for significance.
Pupil size data were analysed using a linear mixed‐effects model. The model assumed fixed effects for group (nominal variable) and time point (nominal variable), an interaction of group with time point, and random effects for participants to account for individual differences, treating the DSWPD pre‐light as the reference. Time points that were 60 min or longer after lights off were grouped, because no temporal dynamics were observed in either group beyond this point.
Results
Relative phase shift
To assess the difference in overall light sensitivity between groups, the average relative phase shift was calculated for each group (n = 21, shown in Fig. 4) and compared using an independent samples t test. DSWPD patients had a significantly higher relative phase shift than the healthy controls (1.21 ± 0.28 vs. 0.92 ± 0.18, P = 0.02, Cohen's d = 1.02), indicating significantly greater sensitivity to light. DSWPD patients showed 31.5% greater relative phase‐shift compared to healthy controls.
Figure 4. Larger circadian phase shifting is observed in DSWPD group than control group.
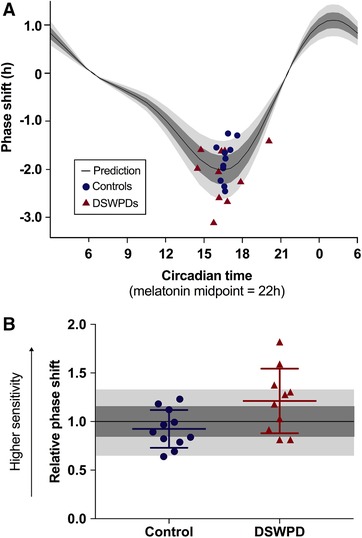
A, individual datapoints for phase shift (y‐axis) vs. circadian timing of the light exposure based on DLMO (x‐axis), showing Control (blue circles) and DSWPD (red triangles). The black curve shows the predicted phase shift, using the phase response curve (PRC) from Khalsa et al. (2003), rescaled by a factor of 1.61 to match our lower brightness stimulus (150 vs. 9500 lux), based on the human dose response curve to light published in Zeitzer et al. (2000). Plotting uses the convention from Khalsa et al. (2003), where circadian time of 22.00 h represents the melatonin midpoint. B, relative phase shifts are plotted for each individual, defined as the actual phase shift divided by the predicted phase shift from panel A. A significant group difference is found between DSWPD and controls (P < 0.05). In both panels, dark grey shading represents within 15% of predicted and light grey shading represents within 30% of predicted. [Color figure can be viewed at wileyonlinelibrary.com]
A linear mixed‐effects model analysis was used to evaluate the ability to predict melatonin midpoint from relative phase shift (a measure of light sensitivity) and whether this was different between groups (see Fig. 5). The estimated effect of relative phase shift was 1.66 h (P = 0.02, 95% CI: 0.28–3.04 h), meaning that for an increase of 0.1 in the relative phase shift (i.e. 10% of the predicted response), there was a 0.16 h delay in melatonin midpoint. No significant interaction of relative phase shift with group was detected, meaning we assume a fixed slope in both groups. The effect of group was 4.10 h (P < 10−8, 95% CI: 3.28–4.91 h), meaning that for the same relative phase shift (i.e. light sensitivity), a DSWPD participant was on average 4.10 h later in his melatonin midpoint than a healthy control participant.
Figure 5. Relationships between melatonin midpoint and relative phase shift for both DSWPD patients and healthy controls.
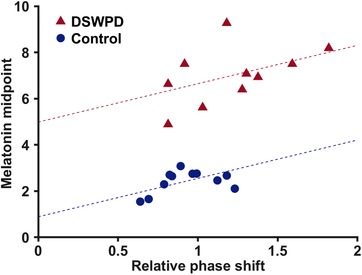
Dashed lines show predictions from the mixed‐effects model. [Color figure can be viewed at wileyonlinelibrary.com]
Melatonin suppression
Figure 6 shows the AUC suppression for each participant (n = 21) during the 6.5 h light exposure. The DSWPD patients (mean = 77.03, SD = 21.48) showed 12.6% more melatonin suppression on average than the healthy control participants (mean = 64.40, SD = 21.27), but this difference was not statistically significant (P = 0.19, Cohen's d = 0.59, independent samples t test). Based on the dose–response curve of Zeitzer et al. (2000) in healthy participants, the expected suppression level at 150 lux is approximately 75%. A larger proportion of DSWPD patients demonstrated suppression above this level (60%) than healthy controls (18%), but this difference in proportion did not reach statistical significance (P = 0.08, OR: 0.15, 95% CI: 0.02–1.08, Fisher's exact test).
Figure 6. The difference in percentage of melatonin suppression between groups.
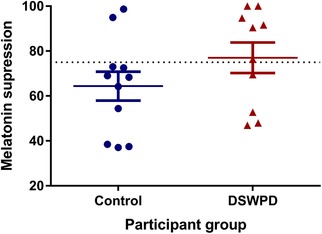
The dotted line denotes average suppression to 150 lux lighting from Zeitzer et al. (2000). [Color figure can be viewed at wileyonlinelibrary.com]
Melatonin amplitude
Independent samples t tests were used to assess if there were any intra‐ and interindividual and group differences in melatonin amplitude between CR1 and CR2. There was no significant difference in average melatonin amplitude between healthy controls (mean = 10.09, SD = 3.31) and DSWPD patients (mean = 10.67, SD = 4.69) during CR1 (P = 0.75, Cohen's d = 0.14), or healthy controls (mean = 8.73, SD = 2.88) and DSWPD patients (mean = 10.40, SD = 4.91) during CR2 (P = 0.35, Cohen's d = 0.41). There were also no significant differences between CR1 and CR2 within healthy control participants (P = 0.07, Cohen's d = 0.44) or CR1 and CR2 within DSWPD patients (P = 0.56, Cohen's d = 0.06).
Pupil size
Static pupil sizes were compared between groups (n = 19, 10 DSWPD patients, 9 healthy controls) and across time points using a linear mixed‐effects model. As shown in Fig. 7, there was no significant effect of group in the reference level (i.e. pre‐light). There were significant effects of time compared to the reference level (i.e. pre‐light) at Light on + 60 min (−0.95 mm, 95% CI: −1.61 to −0.28 mm, P < 0.01), Light on + 120 min (−1.04 mm, 95% CI: −1.71 to −0.38 mm, P < 0.01), Light on + 180 min (−0.89 mm, 95% CI: −1.55 to −0.23 mm, P < 0.01), and Light off + 30 min (−0.71 mm, 95% CI: −1.37 to −0.04 mm, P < 0.05), indicating smaller pupil diameter for both groups during and shortly after the light exposure. There was additionally a significant interaction effect of group with time at Light off + 60 min onwards (0.82 mm, 95% CI: 0.03–1.61 mm, P <0.05), indicating that after the light exposure, DSWPD patients had significantly smaller pupil diameter relative to healthy controls.
Figure 7. The mean and SEM of static pupil size for each group before, during and after the 6.5 h light exposure period.
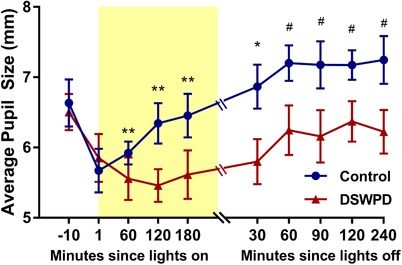
Statistical significance: * P < 0.05 and ** P <0.01 for time effect, #P < 0.05 for the time × group effect. [Color figure can be viewed at wileyonlinelibrary.com]
Discussion
This was the first study to directly examine phase shifting responses to light in DSWPD. DSWPD patients showed a 31.5% larger phase‐shifting response to the moderate intensity evening light exposure (∼150 lux) compared to healthy controls, demonstrating increased sensitivity of the circadian system to light. Notably, this was shown using a normal indoor light level, typically experienced during the night. This finding highlights the importance of light sensitivity in disease vulnerability.
We examined differences in the phase shifting response between DSWPD patients and healthy controls to evening light exposure. Due to high interindividual variability in the circadian timing of light exposure, we applied a novel PRC‐based method to correct for individual differences in timing of the light stimulus. We found that those with DSWPD had a greater phase shifting response than controls, demonstrating increased sensitivity to phase‐delaying light. This increased sensitivity creates a physiological vulnerability to light at night causing delays (e.g. artificial light sources). This physiological mechanism is consistent with changes in sleep–wake behaviour alone failing to alleviate symptoms in DSWPD; over 90% of DSWPD patients regress to pre‐treatment symptoms within 1 year of ceasing treatments to correct circadian phase, with over a quarter relapsing within 1 week (Dagan et al. 2009). Such high treatment failure rates demonstrate that aligning sleep–wake behaviour to an earlier time has only transient benefits in the vast majority of patients. Without addressing the increased phase‐delaying response to evening light, regression to abnormally late timing is likely to recur. A focus on blocking or avoiding even moderate light levels may be of particular importance in treatment efficacy.
Within both healthy control and DSWPD patient groups, we found that later melatonin timing was associated with larger phase shifts (i.e. increased light sensitivity). This indicates that increased sensitivity predicts later circadian timing, even in the absence of sleep pathology. Light sensitivity may therefore be an important determinant factor for an individual's chronotype in addition to other circadian or sleep homeostatic factors (Phillips et al. 2010; Van der Meijden et al. 2016). The DSWPD phenotype may be a pathological manifestation of the relationship between light sensitivity and chronotype.
If light sensitivity alone were the mechanism behind DSWPD development, we would expect minimal overlap in light sensitivity between groups. Instead, however, we observed DSWPD patients with relatively early circadian timing who had similar light sensitivity to healthy controls with relatively late circadian timing. Moreover, we observed healthy controls with typical circadian timing and high light sensitivity, as well as DSWPD patients with abnormally late timing and low light sensitivity. These observations indicate that factors other than light sensitivity must play an important role in the development of delayed circadian phase and DSWPD. The DSWPD patients have a later circadian phase by ∼4 h than would be predicted given their light sensitivity, implying that behavioural light exposure patterns (e.g. self‐selected exposure to artificial sources of light) also play an important role in the aetiology of DSWPD. This is supported by studies demonstrating increased night‐time light exposure in DSWPD patients (Auger et al. 2011; Van der Maren et al. 2018), as well as modelling results that show increased use of artificial light sources amplifies individual differences in circadian and sleep timing (Skeldon et al. 2017; Swaminathan et al. 2017). Therefore, it is likely that delayed timing in DSWPD is a result of both physiological and behavioural differences.
We found a trend towards greater melatonin suppression in DSWPD patients compared to healthy controls, with comparable difference in suppression (12.6%) to the 15% difference between DSWPD patients and controls reported by Aoki et al. (2001). Greater melatonin suppression may contribute to a decreased signal for sleep and increased alertness, contributing to the inability to initiate sleep at a desired time. Melatonin may also work to antagonize phase shifting directly (Hunt et al. 2001) or produce advances that counteract the delaying response to light (Wright et al. 1986; Dahlitz et al. 1991). Thus, a greater melatonin suppression response could plausibly contribute to the greater phase shifting response in DSWPD.
It has been suggested that patients with DSWPD may be sleeping through the advance portion of their PRC and therefore not receiving sufficient morning light, perpetuating delays in circadian phase (Ozaki et al. 1996). While this may occur during times when patients are trying to catch up on lost sleep (e.g. on weekends), it does not appear to explain the circadian delays seen in our patient sample. Given the strict criteria in this study for DSWPD patients to have normal phase angles and regular average bedtimes, participants typically woke ∼10 h (range = 7.5–12 h) after melatonin onset, during the advance region of their PRC and therefore were likely to receive comparable light exposure during this time to healthy controls. Hypersensitivity to evening light is likely to be a contributor to DSWPD, being perpetuated by behavioural factors such as unstable and delayed sleep schedules and night‐time light exposure. This would be consistent with the recent finding that light patterns in individuals with irregular, delayed sleep patterns predict differences in circadian timing (Phillips et al. 2017).
We found that DSWPD patients had reduced pupil size following the experimental light exposure, relative to healthy controls. At no point during or after exposure did DSWPD patients have larger pupil size. This indicates that our observed differences in phase shifting response between groups were not simply due to more light reaching the retina in DSWPD patients. It has been shown that a larger pupil diameter during light exposure is related to higher melatonin suppression (Higuchi et al. 2008); therefore, based on pupil size alone, our healthy control participants would be expected to have larger phase shifts than DSWPD patients. The larger phase shifts seen in our DSWPD patients are likely due to physiological differences at the level of the retina, retinohypothalamic tract, or the suprachiasmatic nucleus (SCN). Sustained pupil constriction is driven by the activation of melanopsin‐containing intrinsically photosensitive retinal ganglion cells (ipRGCs; Gooley et al. 2012; Adhikari et al. 2015). Differences in ipRGC function (as measured by sustained pupil responses) should relate to the ability of light to impact the circadian clock. Supporting this, it was found that greater sustained pupil constriction associated with later preferred and habitual sleep timing (Van der Meijden et al. 2016). Additionally, a single nucleotide polymorphism on the melanopsin gene (OPN4) was shown to relate to pupil size during light exposure (Higuchi et al. 2013). The potentially sustained constriction seen in our DSWPD patients may indicate an underlying increase in light signalling to the SCN after the light stimulus has ended, and therefore may be one of the physiological mechanisms underlying the greater phase shifting response seen in this group.
There are two notable limitations to our study. First, this study only examined differences in the delay region of the PRC. The assumption used for our analysis is that the shape of the PRC is locally similar in the delay region between DSWPD and healthy controls, differing primarily in amplitude between the groups. This assumption does not preclude potential differences in other aspects of the PRC. For example, the advance region of the PRC (occurring in the morning) could be blunted, or the delay region could be different in width in DSWPD compared to healthy controls. Full PRCs comparing DSWPD and healthy controls would be needed to resolve this question. Second, given potential differences between men and women in light sensitivity (Monteleone et al. 1995), our study only included male participants. Further study will be needed in women with DSWPD to determine whether alternative mechanisms underlie this disorder in women.
Our findings have important implications for the treatment and mitigation of DSWPD. Current treatments typically focus on restoring normal sleep–wake timing. These treatments require a high level of adherence and consistency from patients. Furthermore, these treatments fail to address any underlying physiological mechanisms that may be perpetuating circadian delays. It was recently demonstrated that as many as 43% of patients diagnosed with DSWPD do not exhibit a delay in circadian phase, indicating that the cause of their sleep disturbance is not circadian in nature (Murray et al. 2017). Despite our selection criteria requiring a late DLMO time, we still found a wide range in levels of circadian light sensitivity within our DSWPD group. This finding indicates that both circadian timing and circadian light sensitivity provide independently useful clinical information. For example, some patients may exhibit late timing in the absence of increased sensitivity, and therefore may benefit from a Cognitive Behavioral Therapy for Insomnia (CBT‐I) intervention. Others may present with both late timing and increased sensitivity and therefore may benefit from strict light–dark scheduling with ‘light hygiene’ practices (avoidance of light at night, use of filters on devices, etc.). It is therefore essential that clinically viable measures of these two related but distinct physiological characteristics (circadian timing and light sensitivity) are developed to inform individualized treatment practices. Information derived from such measures could also be input to mathematical models of the circadian system that predict circadian and sleep–wake timing in terms of underlying physiology (Phillips et al. 2010), enabling improved predictions of individual‐level responses to therapeutic interventions.
Although various studies have suggested altered sensitivity to light may play a role in the aetiology of circadian rhythm sleep disorders, this study was the first to examine differences in the phase shifting response to light between a patient group and healthy controls. Our findings will inform the development of mechanism‐based interventions for DSWPD, which could include targeting light sensitivity or tailoring behavioural modifications to reduce or increase light exposure at appropriate times of day.
Additional information
Competing interests
L.A.W., A.J.K.P., I.T.H., E.M.M. and S.W.C. report no conflicts of interest. C.A. has no conflicts of interests related to the results reported in this paper. In the interest of full disclosure, she has received contract research support from VicRoads, Rio Tinto Coal Australia, and BHP Mining. She has received lecturing fees from Ausmed, Healthmed, and TEVA. C.A. is a Theme Leader in the Cooperative Research Centre for Alertness, Safety and Productivity. L.C.L. reports that he is a shareholder in Re‐time Pty. Ltd, a company that markets light devices capable of re‐timing circadian rhythms. S.W.L. has no conflicts of interests related to the research or results reported in this paper. In the interests of full disclosure, commercial interests from the last 3 years (2015–2018) are listed below. S.W.L. has received consulting fees from the Atlanta Falcons, Atlanta Hawks, Pegasus Capital Advisors LP, Serrado Capital, Slingshot Insights; and has current consulting contracts with Akili Interactive, Consumer Sleep Solutions, Delos Living LLC, Headwaters Inc., Hintsa Performance AG, Light Cognitive, Lighting Science Group Corporation, Mental Workout, PlanLED, OpTerra Energy Services Inc., Six Senses, Wyle Integrated Science and Engineering. S.W.L. has received unrestricted equipment gifts from Biological Illuminations LLC, Bionetics Corporation, and F.Lux Software LLC; has equity in iSLEEP, Pty; advance author payment and/or royalties from Oxford University Press; honoraria plus travel, accommodation and/or meals for invited seminars, conference presentations or teaching from BHP Billiton, Lightfair, Informa Exhibitions (USGBC), Teague; travel, accommodation and/or meals only (no honoraria) for invited seminars, conference presentations or teaching from DIN, FASEB, Lightfair, SLTBR, and USGBC. S.W.L. has completed an investigator‐initiated research grant from Biological Illumination LLC and has an ongoing investigator‐initiated grant from F. Lux Software LLC. S.W.L. holds a process patent for ‘Systems and methods for determining and/or controlling sleep quality’, which is assigned to the Brigham and Women's Hospital per Hospital policy. S.W.L. has also served as a paid expert for legal proceedings related to light, sleep, and health. S.W.L. is also a Program Leader for the CRC for Alertness, Safety and Productivity, Australia. S.M.W.R. reports that he has served as a consultant through his institution to Vanda Pharmaceuticals, Philips Respironics, EdanSafe, The Australian Workers’ Union, National Transport Commission, Transport Accident Commission, New South Wales Department of Education and Communities, and has through his institution received research grants and/or unrestricted educational grants from Vanda Pharmaceuticals, Shell, Teva Pharmaceuticals, Rio Tinto, Seeing Machines, Takeda Pharmaceuticals North America, Philips Lighting, Philips Respironics, Cephalon, and ResMed Foundation, and reimbursements for conference travel expenses from Vanda Pharmaceuticals. His institution has received equipment donations or other support from Optalert, Compumedics, and Tyco Healthcare. He has served as an expert witness and/or consultant to shift work organizations. SMWR also serves as a Program Leader in the Cooperative Research Centre for Alertness, Safety and Productivity.
Author contributions
S.W.C. conceived of the study. S.W.C., S.W.R., S.W.L., L.C.L. and C.A. contributed to study design. L.A.W., I.T.H. and E.M.M. performed the experiments. L.A.W., A.J.K.P. and S.W.C. analysed data. L.A.W., A.J.K.P. and S.W.C. wrote the manuscript. L.A.W., A.J.K.P., I.T.H., E.M.M., C.A., L.C.L., S.W.L., S.M.W.R. and S.W.C. revised the manuscript. All authors have read and approved the final version of this manuscript and agree to be accountable for all aspects of the work in ensuring that questions related to the accuracy or integrity of any part of the work are appropriately investigated and resolved. All persons designated as authors qualify for authorship, and all those who qualify for authorship are listed.
Funding
This work was supported by a National Health and Medical Research Council Project Grant to S.W.C. (1087665). L.A.W. and E.M.M. receive financial support from the Australian Government through Research Training Program (RTP) Scholarships.
Biography
Lauren Watson is a PhD candidate in the School of Psychological Sciences at Monash University, Australia and Monash Institute of Cognitive and Clinical Neursosciences. Her research interests lie in circadian rhythms and sensitivity of the circadian system to light in humans, particularly those with delayed sleep–wake phase disorder. Lauren has a Bachelor of Arts with Honours (Psychology) from Monash University.

Edited by: Ole Paulsen & William Taylor
This is an Editor's Choice article from the 15 December 2018 issue.
References
- Adhikari P, Zele AJ & Feigl B (2015). The post‐illumination pupil response (PIPR). Invest Ophthalmol Vis Sci 56, 3838–3849. [DOI] [PubMed] [Google Scholar]
- Alvarez B, Dahlitz M, Vignau J & Parkes JD (1992). The delayed sleep phase syndrome: clinical and investigative findings in 14 subjects. J Neurol Neurosurg Psychiatry 55, 665–670. [DOI] [PMC free article] [PubMed] [Google Scholar]
- American Academy of Sleep Medicine (2014). The International Classification of Sleep Disorders, Diagnostic and Coding Manual, 3rd edn. American Academy of Sleep Medicine, Westchester, IL, USA. [Google Scholar]
- Aoki H, Ozeki Y & Yamada N (2001). Hypersensitivity of melatonin suppression in response to light in patients with delayed sleep phase syndrome. Chronobiol Int 18, 263–271. [DOI] [PubMed] [Google Scholar]
- Auger RR, Burgess HJ, Dierkhising RA, Sharma RG & Slocumb NL (2011). Light exposure among adolescents with delayed sleep phase disorder: a prospective cohort study. Chronobiol Int 28, 911–920. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bastien CH, Vallières A & Morin CM (2001). Validation of the Insomnia Severity Index as an outcome measure for insomnia research. Sleep Med 2, 297–307. [DOI] [PubMed] [Google Scholar]
- Beck AT, Steer RA & Brown GK (1996). Manual for the Beck Depression Inventory – II. Psychological Corporation, San Antonio, TX, USA. [Google Scholar]
- Buysse DJ, Reynolds CF, Monk TH, Berman SR & Kupfer DJ (1989). The Pittsburgh sleep quality index: A new instrument for psychiatric practice and research. Psychiatry Res 28, 193–213. [DOI] [PubMed] [Google Scholar]
- Cain SW, Dennison CF, Zeitzer JM, Guzik AM, Khalsa SB, Santhi N, Schoen MW, Czeisler CA & Duffy JF (2010). Sex differences in phase angle of entrainment and melatonin amplitude in humans. J Biol Rhythms 25, 288–296. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Carskadon MA, Wolfson AR, Acebo C, Tzischinsky O & Seifer R (1998). Adolescent sleep patterns, circadian timing, and sleepiness at a transition to early school days. Sleep 21, 871–881. [DOI] [PubMed] [Google Scholar]
- Czeisler CA, Richardson GS, Coleman RM, Zimmerman JC, Moore‐Ede MC, Dement WC & Weitzman ED (1981). Chronotherapy: resetting the circadian clocks of patients with delayed sleep phase insomnia. Sleep 4, 1–21. [DOI] [PubMed] [Google Scholar]
- Dagan Y & Eisenstein M (1999). Orcadian rhythm sleep disorders: toward a more precise definition and diagnosis. Chronobiol Int 16, 213–222. [DOI] [PubMed] [Google Scholar]
- Dagan Y, Yovel I, Hallis D, Eisenstein M & Raichik I (2009). Evaluating the role of melatonin in the long‐term treatment of delayed sleep phase syndrome (DSPS). Chronobiol Int 15, 181–190. [DOI] [PubMed] [Google Scholar]
- Dahlitz M, Alvarez B, Vignau J, English J, Arendt J & Parkes JD (1991). Delayed sleep phase syndrome response to melatonin. The Lancet 337, 1121–1124. [DOI] [PubMed] [Google Scholar]
- Davis FC, Darrow JM & Menaker M (1983). Sex differences in the circadian control of hamster wheel‐running activity. Am J Physiol 244, R93–R105. [DOI] [PubMed] [Google Scholar]
- Gooley JJ, Chamberlain K, Smith KA, Khalsa SB, Rajaratnam SM, Van Reen E, Zeitzer JM, Czeisler CA & Lockley SW (2011). Exposure to room light before bedtime suppresses melatonin onset and shortens melatonin duration in humans. J Clin Endocrinol Metab 96, E463–E472. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gooley JJ, Ho Mien I, St Hilaire MA, Yeo SC, Chua EC, van Reen E, Hanley CJ, Hull JT, Czeisler CA & Lockley SW (2012). Melanopsin and rod‐cone photoreceptors play different roles in mediating pupillary light responses during exposure to continuous light in humans. J Neurosci 32, 14242–14253. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Henry JD & Crawford JR (2005). The short‐form version of the Depression Anxiety Stress Scales (DASS‐21): Construct validity and normative data in a large non‐clinical sample. Br J Clin Psychol 44, 227–239. [DOI] [PubMed] [Google Scholar]
- Higuchi S, Hida A, Tsujimura S, Mishima K, Yasukouchi A, Lee SI, Kinjyo Y & Miyahira M. (2013) Melanopsin gene polymorphism I394T is associated with pupillary light responses in a dose‐dependent manner. PLoS One 8, e60310. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Higuchi S, Ishibashi K, Aritake S, Enomoto M, Hida A, Tamura M, Kozaki T, Motohashi Y & Mishima K (2008). Inter‐individual difference in pupil size correlates to suppression of melatonin by exposure to light. Neurosci Lett 440, 23–26. [DOI] [PubMed] [Google Scholar]
- Horne JA & Östberg O (1976). A self‐assessment questionnaire to determine morningness‐eveningness in human circadian rhythms. Int J Chronobiol 4, 97–110. [PubMed] [Google Scholar]
- Hunt AE, Al‐Ghoul WM, Gillette MU & Dubocovich ML (2001). Activation of MT2 melatonin receptors in rat suprachiasmatic nucleus phase advances the circadian clock. Am J Physiol Cell Physiol 280, C110–C118. [DOI] [PubMed] [Google Scholar]
- Khalsa SB, Jewett ME, Cajochen C & Czeisler CA (2003). A phase response curve to single bright light pulses in human subjects. J Physiol 549, 945–952. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kripke DF, Rex KM, Ancoli‐Israel S, Nievergelt CM, Klimecki W & Kelsoe JR (2008). Delayed sleep phase cases and controls. J Circadian Rhythms 6, 6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lack LC (1986). Delayed sleep and sleep loss in university students. J Am Coll Health 35, 105–110. [DOI] [PubMed] [Google Scholar]
- Lovibond SH & Lovibond PF (1995). Manual for the Depression Anxiety Stress Scales, 2nd edn Psychology Foundation, Sydney, Australia. [Google Scholar]
- Lucas RJ, Peirson SN, Berson DM, Brown TM, Cooper HM, Czeisler CA, Figueiro MG, Gamlin PD, Lockley SW, O'Hagan JB, Price LLA, Provencio I, Skene DJ & Brainard GC (2014). Measuring and using light in the melanopsin age. Trends Neurosci 37, 1–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Micic G, Lovato N, Gradisar M, Burgess HJ, Ferguson SA, Kennaway DJ & Lack L (2015). Nocturnal melatonin profiles in patients with delayed sleep‐wake phase disorder and control sleepers. J Biol Rhythms 30, 437–448. [DOI] [PubMed] [Google Scholar]
- Minors DS, Waterhouse JM & Wirz‐Justice A (1991). A human phase‐response curve to light. Neurosci Lett 133, 36–40. [DOI] [PubMed] [Google Scholar]
- Moderie C, Van der Maren S & Dumont M (2017). Circadian phase, dynamics of subjective sleepiness and sensitivity to blue light in young adults complaining of a delayed sleep schedule. Sleep Med 34, 148–155. [DOI] [PubMed] [Google Scholar]
- Monteleone P, Esposito G, La Rocca A & Maj M (1995). Does bright light suppress nocturnal melatonin secretion more in women than men? J Neural Transm 102, 75–80. [DOI] [PubMed] [Google Scholar]
- Murray JM, Sletten TL, Magee M, Gordon C, Lovato N, Bartlett DJ, Kennaway DJ, Lack L, Grunstein RR, Lockley SW & Rajaratnam SM (2017). Prevalence of circadian misalignment and its association with depressive symptoms in delayed sleep phase disorder. Sleep 40, zsw002. [DOI] [PubMed] [Google Scholar]
- Ozaki S, Uchiyama M, Shirakawa S & Okawa M (1996). Prolonged interval from body temperature nadir to sleep offset in patients with delayed sleep phase syndrome. Sleep 19, 36–40. [PubMed] [Google Scholar]
- Phillips AJK, Chen PY & Robinson PA (2010). Probing the mechanisms of chronotype using quantitative modeling. J Biol Rhythms 25, 217–227. [DOI] [PubMed] [Google Scholar]
- Phillips AJK, Clerx WM, O'Brien CS, Sano A, Barger LK, Picard RW, Lockley SW, Klerman EB & Czeisler CA (2017). Irregular sleep/wake patterns are associated with poorer academic performance and delayed circadian and sleep/wake timing. Sci Rep 7, 3216. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Scheuermaier K, Laffan AM & Duffy JF (2010). Light exposure patterns in healthy older and young adults. J Biol Rhythms 25, 113–122. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Skeldon AC, Phillips AJK & Dijk DJ (2017). The effects of self‐selected light‐dark cycles and social constraints on human sleep and circadian timing: a modeling approach. Sci Rep 7, 45158. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Spitzer RL, Kroenke K, Williams JW & Löwe B (2006). A brief measure for assessing generalized anxiety disorder: The GAD‐7. Arch Intern Med 166, 1092–1097. [DOI] [PubMed] [Google Scholar]
- St Hilaire MA, Gooley JJ, Khalsa SB, Kronauer RE, Czeisler CA & Lockley SW (2012). Human phase response curve to a 1 h pulse of bright white light. J Physiol 590, 3035–3045. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Swaminathan K, Klerman EB & Phillips AJK (2017). Are individual differences in sleep and circadian timing amplified by use of artificial light sources? J Biol Rhythms 32, 165–176. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Van der Maren S, Moderie C, Duclos C, Paquet J, Daneault V & Dumont M (2018). Daily profiles of light exposure and evening use of light‐emitting devices in young adults complaining of a delayed sleep schedule. J Biol Rhythms 33, 192–202. [DOI] [PubMed] [Google Scholar]
- Van der Meijden WP, Van Someren JL, Te Lindert BH, Bruijel J, van Oosterhout F, Coppens JE, Kalsbeek A, Cajochen C, Bourgin P & Van Someren EJ (2016). Individual differences in sleep timing relate to melanopsin‐based phototransduction in healthy adolescents and young adults. Sleep 39, 1305–1310. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vandeputte M & de Weerd A (2003). Sleep disorders and depressive feelings: a global survey with the Beck depression scale. Sleep Med 4, 343–345. [DOI] [PubMed] [Google Scholar]
- Voultsios A, Kennaway DJ & Dawson D (1997). Salivary melatonin as a circadian phase marker: validation and comparison to plasma melatonin. J Biol Rhythms 12, 457–466. [DOI] [PubMed] [Google Scholar]
- Wright J, Aldhous M, Franey C, English J & Arendt J (1986). The effects of exogenous melatonin on endocrine function in man. Clin Endocrinol 24, 375–382. [DOI] [PubMed] [Google Scholar]
- Zeitzer JM, Dijk D‐J, Kronauer RE, Brown EN & Czeisler CA (2000). Sensitivity of the human circadian pacemaker to nocturnal light: melatonin phase resetting and suppression. J Physiol 526, 695–702. [DOI] [PMC free article] [PubMed] [Google Scholar]


