Main Text
The search for strategic molecular targets among a myriad of cell signaling pathways has long been a cornerstone for both molecular cancer research and gene therapy research, leading to the development of novel cancer therapies that act at the level of the genome (DNA), the transcriptome (RNA), and/or the proteome (protein). In this article, we focus on a pivotal and therapeutically accessible locus of executive cell cycle checkpoint control—focusing specifically on the cyclin G1 protein (CCNG1 proto-oncogene product) and the associated oncogenic drivers arrayed along the aberrant biochemical pathways that promote and ensure uncontrolled cell proliferation, resulting in oncogenesis, increasingly aggressive metastasis, and chemotherapeutic refractoriness.
Molecular Drivers of the Cell Division Cycle: Overriding Normal Cell Cycle Checkpoint Control
Basic research into the primal executive mechanisms governing the cell division cycle have identified site-specific (primary sequence specific) protein phosphorylation to be a major regulatory theme that governs the transition phases of the cell cycle—that is, the orderly “activation” of quiescent stem cells at the G0 to G1 boundary to become “capable” of cell proliferation (competence promoting factor), followed by the initiation of DNA synthesis (S-phase promoting factor), and followed by genomic proofreading and the physical partitioning into daughter cells via the elegant biomechanics of mitosis (M-phase promoting factor). Conceptually, this family of executive cell cycle control enzymes are site-specific protein kinases (phosphotransferases; aka cyclin-dependent kinases or CDKs), which recognize specific structural features or target sequences, characterized by Gordon et al.,1 arrayed along major cell-cycle and gene-regulatory proteins. Thus, a canonical “cyclin” is thought of as an oscillating, positive-acting “regulatory subunit” of an identified CDK, which both “activates” the catalytic subunit and physically “targets” the otherwise inactive and otherwise undiscerning (blind) kinase to the cognate phosphorylation site(s) of the targeted cell cycle regulatory proteins.
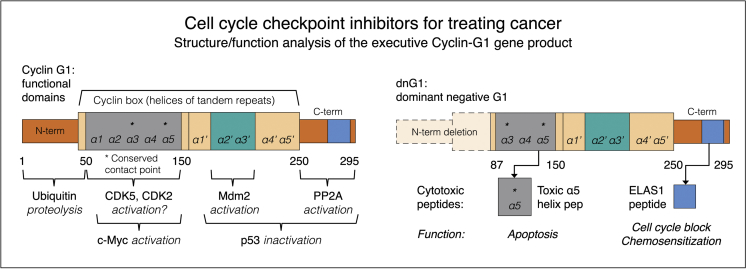
Named alphabetically in order of discovery-molecular characterization, the so-called canonical “cyclins” are positive-acting regulatory targeting subunits of the CDK holoenzymes that are periodically expressed, assembled, activated, and catabolized in strict accordance with the discrete phases of the cell division cycle. Working backward in cell time, from the massive global kinase activity associated with mitosis (M-phase promoting factor = cyclin B+CDC2), to the decisive executor-tumor suppressor functions associated with S-phase entry (S-phase promoting factor = cyclin A+CDK2, or CDC2), to the heightened metabolic pathways associated with sustained G1 or growth phase (cyclins D1, D2, D3, E + respective kinase partners), the identification of cyclin G1 (CCNG1 proto-oncogene), an early riser, and the assessment of its constitutive expression, as well as its essential function, in human cancer cells, represented somewhat of a conundrum at the time. It looked very much like a canonical “cyclin” protein, in terms of primary structure of its telltale “cyclin box” (see Figure 1), while it lacked (1) an identifiable kinase partner, (2) an identifiable phospho-acceptor “target protein,” and (3) the cyclical behavior of the so-called “canonical cyclins” that agreeably and accordingly marked the major phase transitions of the mammalian cell division cycle.
Figure 1.
Structure and Function Analysis of the Executive Cyclin G1 Gene Product
Left: cyclin G1 functional domains. Cyclin G1 physically binds to the ser/thr protein phosphatase subunit designated 2A (PP2A) to activate a key regulatory oncoprotein, Mdm2. The Mdm2 oncoprotein forms a physical complex with the p53 tumor suppressor, thus inactivating its tumor suppressor function while additionally acting as a specific E3 ubiquitin ligase that is responsible for the ubiquitination and degradation of the p53 tumor suppressor protein.4 This dephosphorylation event is cyclin G1-dependent. Cyclin G1 also activates CDK5 and CDK2 to target and activate the c-Myc onco-protein. Right: dnG1 dominant-negative G1 (Killer Gene). The experimentally optimized cyclin G1 inhibitor, a cytocidal dominant-negative mutant construct of cyclin G1, is devoid of the “ubiquitinated” N terminus (proteolytic processing) as well as the first two helical segments (α1 and α 2) of the definitive Cyclin Box: characteristically arrayed in cyclins as a tandem set of helical segments, including two highly-conserved residues (asterisks) essential for cyclin-dependent kinase (Cdk) binding. The cytocidal dnG1 protein—which induces apoptosis in proliferative cells—retains the presumptive CDK contact points (helix α 3*, α 5*) and the structural domains attributed to PP2A, β’, and Mdm2 binding. Remarkably, small synthetic peptides (e.g., ELAS1 and 5 helix peptides) derived from structures or homologous interfaces contained within the cytocidal dnG1 protein have been reported to induce cell cycle blockade and apoptosis, respectively.
Fortunately, what was hidden from the wise in terms of rigid canonical considerations was revealed to the experimentalists and physician-scientists who looked beyond the meager definitions to explore the actual structure and function relations of the cyclin G1 protein (by genetic engineering of CCNG1) in the context of cancer gene therapy, long before cyclin G1 was determined to be the prime molecular driver of the elusive cell competence factor, the pivotal executive component of the Cyclin G1/p53/Mdm2 Axis governing cell cycle checkpoint control and, perhaps, a most strategic target for new precision molecular and genetic cancer therapies as well as chemo-sensitization.1
Biochemically, cyclin G1 (CCNG1 gene product) was a “non-canonical” yet demonstrably essential and potentially oncogenic “cyclin-like” protein whose first appearance (expression) and executive action is on the earliest cell cycle events that drive the quiescent stem cell from G0 to enter G1 phase. Mechanistically, cyclin G1 physically binds to a major cellular ser/thr protein phosphatase subunit designated 2A (PP2A), thereby “targeting” the otherwise undiscerning phosphatase activity to a cyclin G1-targeted protein, which happens to be Mdm2 (oncogene product). The Mdm2 protein, in turn, targets, inhibits, and degrades the p53 tumor suppressor, an often-lost, yet vitally important, substrate (guardian of DNA fidelity with executioner functions) in the normal regulation of the cell division cycle. This oncogenic pathway (i.e., the Cyclin G1/Mdm2/p53 axis) is distinguishable from the set of canonical oncogenic G1 cyclins (D1, D2, D3, cyclin E, cyclin A) that target CDK complexes cyclically and precisely to pRB (and Rb-related) tumor suppressor proteins, whose inhibition releases E2F transcription factors that drive cells to irreversibly enter the S-phase of the division cycle (G1 to S) (Cyclin/CDK/Rb/E2F axis).1
The biochemical “activation” of the Mdm2 oncoprotein by the oncogenic cyclin G1 is a crucial link in the emerging cyclin G1/Mdm2/p53 axis. The Mdm2 gene itself is amplified and/or overexpressed in numerous human cancers, including soft tissue sarcoma, osteosarcoma, and esophageal carcinoma.2, 3 The Mdm2 oncoprotein is known to form a physical complex with the p53 tumor suppressor, thus inhibiting its transcriptional “tumor suppressor” function. In addition, Mdm2 acts as a specific E3 ubiquitin ligase, responsible for the ubiquitination and ultimate degradation of the p53 tumor suppressor protein.4 Thus, the oncogenic potential of Mdm2 to override the decisive-protective tumor-suppressive functions of wild-type p53 is uniquely, if not entirely, cyclin G1-dependent. Additional support for the executive role of cyclin G1—in relation to the pivotal Cyclin G1/Mdm2/p53 axis—came from high-throughput screening for regulatory microRNA species that are commonly lost with the development of human cancers. Apparently, the major species (∼70% of the total population) of regulatory microRNAs that are commonly lost in the pathogenesis and stage-wise progression of hepatocellular carcinoma is miR-122, which physically “targets” the CCNG1 (cyclin G1) gene for suppression and thus appears to be a natural growth suppressive-microRNA focused on limiting the expression of cyclin G1 in the quiescent stem cells of this potentially proliferative, highly regenerative organ.5 Turning to virology, a renewed appreciation of the oncogenic potential of dysregulated CCNG1 gene expression—in terms of both persistent stem cell activation (cell competence) and overriding p53-mediated checkpoint control (thus driving cell survival over DNA fidelity)—came to light when it was discovered that the carcinogenic hepatitis B virus (HBV) produces a protein, the HBx-protein, that specifically, directly, perhaps strategically, downregulates the normal expression of miR-122,6 which results in increased CCNG1 gene expression; raising cyclin G1 to sufficient levels that cyclin-G/PP2A complexes activate Mdm2 and ultimately override the executor-suppressor functions of wild-type p53, thereby abolishing the well-known p53-mediated inhibition of HBV replication as well.6
Finally, the curiously non-canonical cyclin G1 was formally ushered (at least experimentally) into the ballroom, with the prize of the fair as its cellular target—that is, the illustrious c-Myc oncogene, long considered to be the most “desirable” molecular locus for clinical intervention in all of cancer therapy, and yet it was, until now, considered to be among the “least druggable” of all the cancer targets.1, 7 The recent discovery that the once-non-canonical cyclin G1 partners with CDK2 (and CDK5, on occasion) to physically target and site-specifically phosphorylate (activate) the c-Myc oncoprotein which, in turn, provides the transcriptional drive for selective protein synthesis at the very threshold of the G0 to G1 transition, is both informative and important. In that this newfound cyclin G1/CDk2/c-Myc axis of stem cell activation represents the necessary biochemical linkage to the theoretical competence promoting factor, which appears whenever quiescent stem cells regain cell competence—competence to proliferate as needed for normal tissue repair, and when it comes to cancer, competence to proliferate continuously—it is in this remarkable association with c-Myc that the biochemical contingencies for canonization of cyclin G1 are finally met: (1) cyclin G1 gains two attractive CDK partners; (2) cyclin G1 gains a critical substrate target protein, the elusive c-Myc oncoprotein, and (3) the absence of cyclical oscillations in the levels of cyclin G1 protein expressed in cancer cells, in cadence with the discrete phases cell division cycle, is readily explainable by the provocative notion that cancer cells are constitutively competent in terms of this first-and-rate-limiting oncogenic cyclin driver, CCNG1. The clinical upside of this provocative notion is that the strategic blockade of cyclin G1 function, by experimental suppression of CCNG1 gene expression or the molecular blockade of cyclin G1-dependent pathways (Figure 1), is invariably fatal to the cancer cell, thus clinically effective, in a number of human cancers.1
Mechanistically, c-Myc is a critical platelet-derived growth factor (PDGF)-inducible “competence gene” that activates diverse cellular processes associated with entry into and progression through the cell cycle, including the synthesis of cellular components in preparation for growth, DNA synthesis, and cell division. It is in this manner, by activating and selectively targeting Cdk5 kinase activity to activate and stabilize the c-Myc oncoprotein, that the overexpression of CCNG1 enables cancer cells to overcome radiation-induced (i.e., DNA-damage-induced) cell cycle arrest.1, 7 Although the transcriptional targets of c-Myc include a number of DNA repair genes, thereby coupling DNA replication to the pathways and processes that preserve the integrity of the genome,8 the net effect of CCNG1 function in association with Cdk5 (or Cdk2) is to abrogate DNA-fidelity checkpoint controls to promote cell survival, cell competence, and cell cycle progression at the ‘peril’ of increasing error-prone DNA synthesis, as is often found in cancers.
Cyclin G1 Pathway Inhibitor Therapy: Genetic Engineering of a Killer Gene Product
The first tumor-targeted gene therapy product that is based on the strategic blockade of cyclin G1-dependent pathways is DeltaRex-G (former names: Rexin-G and Mx-dnG1), which encodes a dominant negative mutant construct of the CCNG1 gene (designated dnG1 protein) that is devoid of the ubiquitinated N terminus (proteolytic processing) as well as the first two helical segments (α1 and α2) of the definitive cyclin box, characteristically arrayed in “cyclins” as a tandem set of helical segments, including two highly-conserved residues essential for CDK binding.1 The cytocidal dnG1 protein, which induces apoptosis in proliferative cells, retains the presumptive CDK contact points (helix α3*, α5*) and the complete structural domains attributed to PP2A, β’, and Mdm2 binding. Recently, new therapeutic synthetic peptides (e.g., ELAS1 and α5 helix peptides, see Gordon et al.1) derived from structures and/or homologous interfaces contained within the dnG1 protein are themselves reported to induce cell cycle blockade and apoptosis, respectively (Figure 1). Suppressing CCNG1 expression with miR-122, as well as CCNG1 silencing, increases the sensitivity of hepatocellular carcinoma (HCC) cells to doxorubicin,9 thereby establishing the rational basis for combined gene- chemo- and miRNA-based therapies for HCC, based on the suppression or blockade of cyclin G1-dependent pathways.
Meanwhile, there is increasing clinical evidence that innovative tumor-targeted cancer therapies—based on the progress made in discovering, characterizing, and elaborating the structure and function relationships of cyclin G1—may indeed be uniquely effective in managing aggressive metastatic cancers, such that repeated infusions of DeltaRex-G, a tumor-targeted dnG1 expression construct, were determined to be potentially curative, even when standard chemotherapies had previously failed.1 In addition to statistically significant gains in patient overall survival, a considerable number of advanced-stage, chemotherapy-resistant cancer patients treated with repeated infusions of DeltaRex-G as monotherapy (i.e., single-agent efficacy), including metastatic pancreatic cancer, osteosarcoma, and soft tissue sarcoma patients, remain cancer-free or without active disease progression 10 years after the initiation of DeltaRex-G treatment.10 Table 1 lists and summarizes the results of 5 U.S.-based phase 1/2 clinical trials and one phase 2 study with long-term survivors that have resulted in orphan drug designations of DeltaRex-G for pancreatic cancer, soft tissue sarcoma, and osteosarcoma and fast track designation of DeltaRex-G for pancreatic cancer (S.P.C., H. Bruckner, M.A. Morse, N. Assudani, F.L.H., E.M. Gordon, unpublished data).11, 12, 13, 14, 15, 16 Hence, the development of DeltaRex-G, which, by itself, induces apoptosis in cancer cells and tumor-associated vasculature (in the presence or absence of a functional p53 gatekeeper), may be a powerful new clinical application in terms of applied cell cycle checkpoint control, which merits conscientious clinical development. Our ongoing studies have confirmed that CCNG1 expression is predictably elevated in many types of cancers,17 which suggests that monitoring CCNG1 expression in tumors, as well as its associated oncogenic effectors, may identify patients who will benefit from CCNG1 inhibitor therapy.
Table 1.
Clinical Trial NCT Number, Site, Principal Investigator(s), Phase of Trial, Cancer Type and Treatment Outcome Using DeltaRex-G as Monotherapy for Chemoresistant Solid Malignancies
| Clinical Trial NCT No. | Clinical Site | Principal Investigator(s) | Phase | Cancer Type | No. of Patients | Treatment Outcome |
|---|---|---|---|---|---|---|
|
NCT00121745 Dose level: minus 3–minus 111 |
Rochester, MN | E. Galanis | phase 1 | pancreatic adenocarcinoma, gemcitabine resistant |
12 | RECIST v1.0: 1 SD, 11 PD 0% 1-year OS |
|
NCT00504998a Dose level 1–3 (S.P.C., H. Bruckner, M.A. Morse, N. Assudani, F.L.H., E.M. Gordon, unpublished data)12, 13, 14 |
Santa Monica, CA | S.P. Chawla | phase 1/2 | pancreatic adenocarcinoma, gemcitabine resistant |
20 | RECIST v1.0: 1CR, 2 PR, 12 SD 28.6% 1-year OS 21.4% 1.5-year OS 1 alive in sustained remission, 10 years N.B.: gained orphan drug and fast track designation for pancreatic adenocarcinoma from the FDA |
| Manhattan, NY | H.W. Bruckner | |||||
| Durham, NC | M.A. Morse | |||||
|
NCT00505713a Dose level 1–410, 15, 16 |
Santa Monica, CA | S.P. Chawla | phase 1/2 | bone and soft tissue sarcoma, chemotherapy resistant |
36 | 38.5% 1-year OS; 31% 2-year OS 2 alive, with no active disease, 10 years; NB: gained orphan drug designation for soft tissue sarcoma from the FDA |
|
NCT00505271a Dose level 1–4 (unpublished data) |
Santa Monica, CA | S.P. Chawla | phase 1/2 | breast cancer, chemotherapy resistant |
20 | 60% 1-year OS 1 alive, 10 years |
| Manhattan, NY | H.W. Bruckner | |||||
|
NCT00572130a Dose level 1–215 |
Santa Monica, CA | S.P. Chawla | phase 2 | osteosarcoma, chemotherapy resistant |
22 | 27.3% 1-year OS 22.7% 2-year OS 1 alive in sustained remission, 10 years; N.B.: gained orphan drug designation for osteosarcoma from the FDA |
cfu, colony forming units; OS, overall survival; CR, complete remission; PR, partial response; SD, stable disease.
Dose level 1, 1 × 10e11 cfu 2–3 times per week; dose level 2, 2 × 10e11 cfu 3 times per week; dose level 3, 3 × 10e11 cfu 3 times per week; dose level 4, 4 × 10e11 cfu 3 times per week.
Perspectives on New Combinatorial Approaches to Cancer Management
Targeted cancer therapies are likely to be more effective and less toxic to normal cells than standard chemotherapeutic agents and radiation therapy.18 These therapies are commonly used alone, in combination with other targeted therapies, and in combination with other cancer treatments, such as chemotherapy. Targeted cancer therapies approved for clinical use include drugs that block cell growth signaling (e.g., tyrosine kinase and serine-threonine kinase inhibitors), drugs that disrupt tumor blood vessel development (e.g., bevacizumab), evoke apoptosis or programed death of specific cancer cells (e.g., trabectedin), activate the immune system to recognize tumor neoantigens and destroy specific cancer cells (e.g., cancer vaccines and immune checkpoint inhibitors), and/or deliver cytotoxic toxic drugs (e.g., nab-paclitaxel) to cancer cells. Based on observations and reports of chemo-sensitization, we theorize that combinatorial therapies using DeltaRex-G, a cyclin G1 inhibitor, and other molecular targets along the CCNG1 pathway, including Mdm2, PP2A, p53, Rb, and c-Myc, may exert additive, complementary, and/or synergistic effects in the treatment of advanced metastatic cancers. Drugs that are already US Food and Drug Administration (FDA) approved or are currently in clinical trials include the following: the Mdm2 inhibitor (e.g., AMG232 and Nutlin 3a),19 the CDK4/CDK6/Rb inhibitor Palbociclib (PD0332991, Ibrance),20 and the mutated p53 inhibitor SAHA (Vorinostat).21 While c-Myc is overexpressed in many kinds of cancer, strategies to effectively modulate c-Myc activity (outside of modulating its targeting and activation by cyclin G1) do not yet exist; however, the small molecule anticancer agent APTO-253 appears to inhibit c-Myc expression to some degree, while inducing cell cycle arrest and apoptosis in certain hematologic malignancies.22
It is possible that repeated cyclin G1 inhibition alone, such as by DeltaRex-G—by suppressive genetic strategies and/or selective biochemical blockades—will turn out to be a necessary and sufficient treatment regimen in terms of cancer gene therapy. However, at this reflective point in time, it would also be prudent to monitor the associated pharmacological effects on the other major oncogenic drivers within these newly characterized cyclin-G1 dependent pathways. Evaluating the safety and efficacy of DeltaRex-G combination regimens, that being cyclin G1 inhibitor therapy combined with modulating one or more of the executor proteins (CDK2/5, PP2A, p53, c-Myc) in the CCNG1 pathway, represents a new opportunity for advancement of cell cycle checkpoint inhibitors in the field of cancer medicine. On the other hand, DeltaRex-G is cytocidal to cancer cells, tumor-associated vasculature, and malignant stromal fibroblasts and may well prime the recruitment and/or entry of cytokines, immune modulators,23, 24, 25 and, potentially, chemotherapeutic, anti-angiogenic, and targeted therapies into the tumor microenvironment. For instance, in a phase 1/2 study using DeltaRex-G + Reximmune-C, a tumor-targeted gene vector encoding a human granulocyte-macrophage colony-stimulating factor (GM-CSF) gene, the reported 1-year overall survival was 86% in chemoresistant solid tumors and B cell lymphoma.24, 25 Hence, combinatorial therapies external to the CCNG1 inhibitor pathway may include DeltaRex-G plus (1) immune-modulatory monoclonal antibodies, including FDA-approved immune checkpoint inhibitors; (2) cytotoxic chemotherapies, such as doxorubicin and trabectedin; (3) anti-angiogenesis agents, such as bevacizumab; (4) selective tyrosine kinase inhibitors; and/or (5) monoclonal antibodies directed against specific features of the evolving metastatic cancer cells (e.g., panitumumab, cetuximab). Viewed from a biochemical perspective, which teaches that “the-first-and-rate-limiting-step” of a given biochemical pathway is often the most important as it is often leveraged in terms of regulatory cause-and-effect, the strategic blockade of cyclin G1 function—its competence-promoting function and its pro-survival function in the face of increasing genetic instability—sets the stage for the new clinical applications and optimization of combinatorial therapies with renewed assurance that cyclin G1 itself is a strategic therapeutic locus indeed.
Acknowledgments
The authors are grateful to Heather Gordon for outstanding graphic illustration.
References
- 1.Gordon E.M., Ravicz J.R., Liu S., Chawla S.P., Hall F.L. Cell cycle checkpoint control: The cyclin G1/Mdm2/p53 axis emerges as a strategic target for broad-spectrum cancer gene therapy - A review of molecular mechanisms for oncologists. Mol. Clin. Oncol. 2018;9:115–134. doi: 10.3892/mco.2018.1657. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Piette J., Neel H., Maréchal V. Mdm2: keeping p53 under control. Oncogene. 1997;15:1001–1010. doi: 10.1038/sj.onc.1201432. [DOI] [PubMed] [Google Scholar]
- 3.Momand J., Jung D., Wilczynski S., Niland J. The MDM2 gene amplification database. Nucleic Acids Res. 1998;26:3453–3459. doi: 10.1093/nar/26.15.3453. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Momand J., Zambetti G.P., Olson D.C., George D., Levine A.J. The mdm-2 oncogene product forms a complex with the p53 protein and inhibits p53-mediated transactivation. Cell. 1992;69:1237–1245. doi: 10.1016/0092-8674(92)90644-r. [DOI] [PubMed] [Google Scholar]
- 5.Gramantieri L., Ferracin M., Fornari F., Veronese A., Sabbioni S., Liu C.G., Calin G.A., Giovannini C., Ferrazzi E., Grazi L.G. Cyclin G1 is a target of miR-122a, a microRNA frequently down-regulated in human hepatocellular carcinoma. Cancer Res. 2007;67:6092–6099. doi: 10.1158/0008-5472.CAN-06-4607. [DOI] [PubMed] [Google Scholar]
- 6.Bandopadhyay M., Sarkar N., Datta S., Das D., Pal A., Panigrahi R., Banerjee A., Panda C.K., Das C., Chakrabarti S., Chakravarty R. Hepatitis B virus X protein mediated suppression of miRNA-122 expression enhances hepatoblastoma cell proliferation through cyclin G1-p53 axis. Infect. Agent. Cancer. 2016;11:40. doi: 10.1186/s13027-016-0085-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Seo H.R., Kim J., Bae S., Soh J.W., Lee Y.S. Cdk5-mediated phosphorylation of c-Myc on Ser-62 is essential in transcriptional activation of cyclin B1 by cyclin G1. J. Biol. Chem. 2008;283:15601–15610. doi: 10.1074/jbc.M800987200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Menssen A., Hermeking H. Characterization of the c-MYC-regulated transcriptome by SAGE: identification and analysis of c-MYC target genes. Proc. Natl. Acad. Sci. USA. 2002;99:6274–6279. doi: 10.1073/pnas.082005599. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Fornari F., Gramantieri L., Giovannini C., Veronese A., Ferracin M., Sabbioni S., Calin G.A., Grazi G.L., Croce C.M., Tavolari S. MiR-122/cyclin G1 interaction modulates p53 activity and affects doxorubicin sensitivity of human hepatocarcinoma cells. Cancer Res. 2009;69:5761–5767. doi: 10.1158/0008-5472.CAN-08-4797. [DOI] [PubMed] [Google Scholar]
- 10.Kim S., Federman N., Gordon E.M., Hall F.L., Chawla S.P. Rexin-G®, a tumor-targeted retrovector for malignant peripheral nerve sheath tumor: A case report. Mol. Clin. Oncol. 2017;6:861–865. doi: 10.3892/mco.2017.1231. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Galanis E., Carlson S.K., Foster N.R., Lowe V., Quevedo F., McWilliams R.R., Grothey A., Jatoi A., Alberts S.R., Rubin J. Phase I trial of a pathotropic retroviral vector expressing a cytocidal cyclin G1 construct (Rexin-G) in patients with advanced pancreatic cancer. Mol. Ther. 2008;16:979–984. doi: 10.1038/mt.2008.29. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Chawla S.P., Chua V.S., Fernandez L., Quon D., Blackwelder W.C., Gordon E.M., Hall F.L., Hall F.L. Advanced phase I/II studies of targeted gene delivery in vivo: intravenous Rexin-G for gemcitabine-resistant metastatic pancreatic cancer. Mol. Ther. 2010;18:435–441. doi: 10.1038/mt.2009.228. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Hall F.L., Levy J.P., Reed R.A., Petchpud W.N., Chua V.S., Chawla S.P., Gordon E.M. Pathotropic targeting advances clinical oncology: tumor-targeted localization of therapeutic gene delivery. Oncol. Rep. 2010;24:829–833. doi: 10.3892/or.2010.829. [DOI] [PubMed] [Google Scholar]
- 14.Bruckner H., Chawla S.P., Chua V.S., Quon D.V., Fernandez L., Saralou A., Fisher M., Gordon E.M., Hall F.L. Phase I and II studies of intravenous Rexin-G as monotherapy for stage IVb gemcitabine-resistant pancreatic cancer. J Clin Oncol. 2010;28(15_suppl) 4149-4149. [Google Scholar]
- 15.Chawla S.P., Chua V.S., Fernandez L., Quon D., Saralou A., Blackwelder W.C., Hall F.L., Gordon E.M. Phase I/II and phase II studies of targeted gene delivery in vivo: intravenous Rexin-G for chemotherapy-resistant sarcoma and osteosarcoma. Mol. Ther. 2009;17:1651–1657. doi: 10.1038/mt.2009.126. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Chawla S.P., Chawla N.S., Quon D., Chua-Alcala V., Blackwelder W.C., Hall F.L., Gordon E.M. An advanced phase 1/2 Study using an XC-targeted gene therapy vector for chemotherapy resistant sarcoma. Sarcoma Res-Int. 2016;3:1–7. [Google Scholar]
- 17.Ravicz J., Liu S., Andrali S.S., Reddy S., Sellappan S., Leong B., Chawla S.P., Hall F.L., Gordon E.M. Differential expression of human cyclin G1 (CCNG1) in cancer, a novel biomarker in development for CCNG1 inhibitor therapy. J Clin Oncol. 2018 Published online June 1, 2018. [Google Scholar]
- 18.Gerber D.E. Targeted therapies: a new generation of cancer treatments. Am. Fam. Physician. 2008;77:311–319. [PubMed] [Google Scholar]
- 19.Uehling D.E., Harris P.A. Recent progress on MAP kinase pathway inhibitors. Bioorg Med Chem Lett. 2015;25:4047–4056. doi: 10.1016/j.bmcl.2015.07.093. [DOI] [PubMed] [Google Scholar]
- 20.Lu J. Palbociclib: a first-in-class CDK4/CDK6 inhibitor for the treatment of hormone-receptor positive advanced breast cancer. J. Hematol. Oncol. 2015;8:98. doi: 10.1186/s13045-015-0194-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Richon V.M. Cancer biology: mechanism of anti-tumour action of vorinostat (suberoylanilide hydroxamic acid), a novel histone deacetylase inhibitor. Br. J. Cancer. 2006;95(Suppl 1):S2–S6. [Google Scholar]
- 22.Rice W.G., Lightfoot J., Zhang H., Cheng T., Vellanki A., Peralta R., Howell S.B., Local A., Chau F., Esquivies L. Clinical Pharmacokinetics of Apto-253 Support Its Use as a novel agent for the treatment of relapsed or refractory hematologic malignancies. Blood. 2015;126:4934. [Google Scholar]
- 23.Stendahl Dy P., Chawla S.P., Hall F.L., Gordon E.M. Immune cell trafficking in the tumor microenvironment of human cyclin G1 (CCNG1) inhibitor-treated tumors. Brit J Cancer Res. 2018;1:202–207. [Google Scholar]
- 24.Ignacio J.G., Bruckner H., Manalo R.E., San Juan F.S., Baniqued L., Madamba A., Gordon E.M., Hall F.L. Tumor-targeted cancer vaccination (GeneVieve Protocol): A phase I/II study of intravenous Rexin-G and Reximmune-C for chemotherapy-resistant cancers. J Clin Oncol. 2011;29(suppl_15) 2589–2589. [Google Scholar]
- 25.Ignacio J.G., San Juan F., Manalo R.A., Soheili Nategh E., Tamhane J., Kantamneni L., Chawa S.P., Hall F.L., Gordon E.M. The Genevieve Protocol: Phase I/II evaluation of a dual targeted approach to cancer gene therapy/immunotherapy. Clin. Oncol. 2018;3:1537. [Google Scholar]