Abstract
Short chain fatty acids (SCFAs) are protective against inflammatory diseases. Free fatty acid receptor 2 (FFA2), is a target of SCFAs however, their selectivity for FFA2 over other FFA receptors is limited. This study aimed to functionally characterise 2-(4-chlorophenyl)-3-methyl-N-(thiazole-2-yl)butanamide (4CMTB) and 4-((4-(2-chlorophenyl)thiazole-2-yl)amino)-4oxo-3-phenylbutanoic acid (2CTAP), and their enantiomers, in modulating FFA2 activity. The racemic mixture (R/S) and its constituents (R-) and (S-) 4CMTB or 2CTAP were used to stimulate human (h)FFA2 in the absence or presence of acetate. Calcium ions (Ca2+), phosphorylated extracellular signal-regulated kinase 1 and 2 (pERK1/2) and cyclic adenosine monophosphate (cAMP) were measured. R/S-4CMTB is a functionally selective ago-allosteric ligand that enhances Ca2+ response to acetate. Both R/S-4CMTB and S-4CMTB are more potent activators of pERK1/2 and inhibitors of forskolin-induced cAMP than acetate. S-4CMTB increased neutrophil infiltration in intestinal ischemia reperfusion injury (IRI). 2CTAP inhibited constitutive Ca2+ levels, antagonised acetate-induced pERK1/2 and prevented damage following IRI. This study characterises enantiomers of functionally selective ligands for FFA2 in cells stably expressing hFFA2. It highlights the novel roles of selective FFA2 enantiomers 4CMTB and 2CTAP on Ca2+, pERK1/2 and cAMP and their roles as allosteric modulators which, may assist in efforts to design novel therapeutic agents for FFA2-driven inflammatory diseases.
Introduction
Short chain fatty acids (SCFAs) impart protective effects in inflammatory diseases such as colitis, asthma and arthritis1. They act on many cell types, including neutrophils, principally by acting on free fatty acid receptors (FFARs). Free fatty acid receptor 2 (FFA2/FFAR2) is a classical G protein-coupled receptor (GPCR) that signals through Gα proteins. It is highly expressed on cells expressing intestinal peptide YY (PYY) and glucagon-like peptide-1 (GLP-1); viz. endocrine L-cells, basal crypts, colonocytes and enterocytes of the small and large intestine2,3. Most studies have probed the role of FFA2 in metabolic disorders4,5. However, the highest expression of FFA2 is found in innate immune cells, especially in neutrophils, eosinophils, monocytes and B-lymphocytes2,6,7. This suggests that the receptor may also have a role in innate immunity and inflammation. This has led to studies of FFA2 in inflammatory disorders such as inflammatory bowel disease8, colon cancer9, asthma10 and arthritis11. The role of FFA2 as a regulator of inflammation is further supported by observations of FFA2-deficient mice (FFA2−/−), which exhibit exacerbated or non-resolving inflammation in colitis, arthritis and asthma models11.
Acetate, propionate and butyrate elicit FFA2-dependent chemotaxis of immune cells, most notably neutrophils12–14. Conversely, FFA2−/− mice are more prone to inflammation in DSS-induced colitis12. These potential discrepancies are likely due to the role of neutrophils in acute versus chronic situations. FFA2 shares 43% similarity with FFA315 and both respond to the same SCFA ligands due to their highly conserved orthosteric sites. The order of potency for FFA2 is acetate (C2) = propionate (C3) > butyrate (C4) > valerate (C5) = formate (C1), and for FFA3 is butyrate (C4) = propionate (C3) = valerate (C5) > acetate (C2) = formate (C1)6. FFA2 and FFA3 have different tissue expression profiles and physiological roles, but there remains insufficient discrimination of SCFA binding to produce adequate drug candidates7. Additionally, SCFAs have poor potency and high μM-mM concentrations are required to elicit FFA2 and FFA3 responses in vitro, which has led to the search for more potent and selective synthetic ligands.
This study fully characterises two FFA2 ligands; R-, S-, R/S- 2-(4-chlorophenyl)-3-methyl-N-(thiazole-2-yl)butanimide (4CMTB) and 4-((4-(2-chlorophenyl)thiazole-2-yl)amino)-4oxo-3-phenylbutanoic acid (2CTAP), and probes their signalling profiles in CHO cells stably expressing the human FFA2 receptor (hFFA2). We demonstrate that racemic 4CMTB is an ago-allosteric ligand, enhancing calcium ion (Ca2+) responses to acetate. R/S-4CMTB and S-4CMTB are more potent activators of phosphorylated extracellular signal-regulated kinase 1 and 2 (pERK1/2) and inhibitors of forskolin-induced cyclic adenosine monophosphate (cAMP), compared to acetate alone. 2CTAP is an inverse agonist that inhibits constitutive Ca2+ levels and antagonises acetate-induced pERK1/2, but has no effect on cAMP. Conversely, at low concentrations both R- and S-4CMTB and R- and S-2CTAP are negative allosteric modulators of Ca2+-induced acetate responses. We report novel roles for the selective FFA2 enantiomers, 4CMTB and 2CTAP, on Ca2+, pERK1/2 and cAMP responses and as allosteric modulators and, highlight their activity in an in vivo intestinal ischemia reperfusion injury (IRI) model.
Results
Characterisation of R/S-4CMTB and R/S-2CTAP in CHO cells stably transfected with FFA2
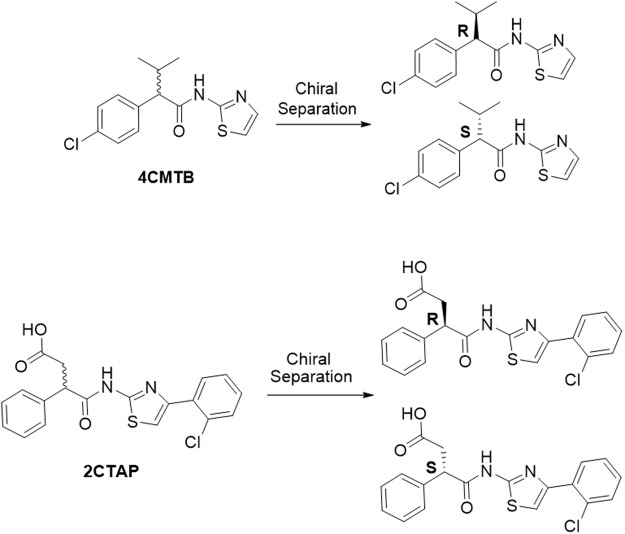
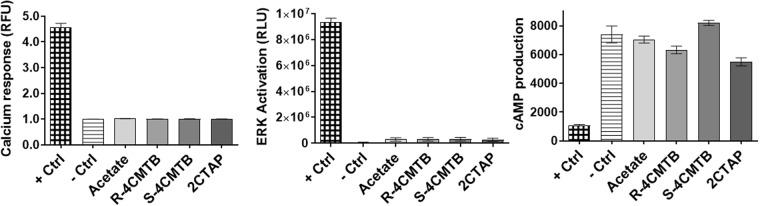
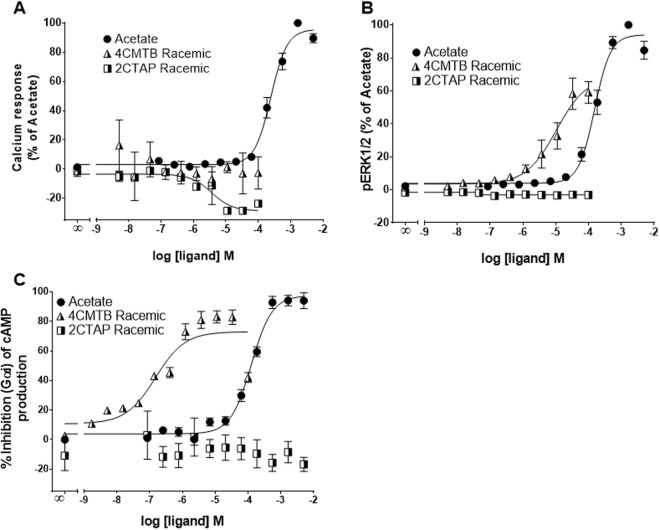
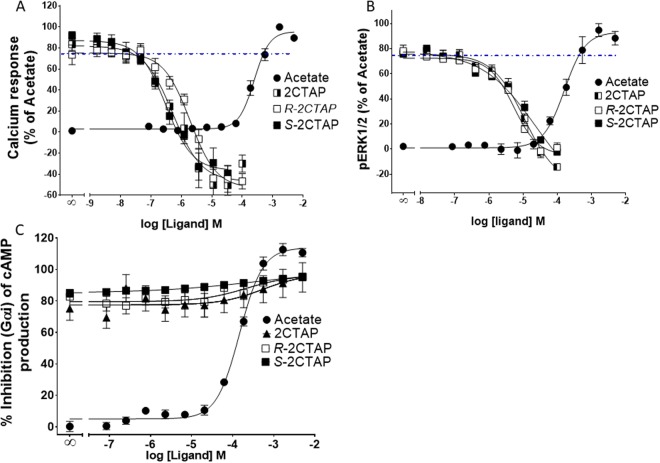
FFA2 signals via Gαq, Gαi2,6,15 and β-arrestin16. To characterise the functional selectivity of 4CMTB and 2CTAP for FFA2, Ca2+, pERK and cAMP assays were carried out, with comparison to the known FFA2 agonist, acetate, in a CHO cell line stably transfected with hFFA2. The chemical structures of racemic 4CMTB (R/S-4CMTB) and 2CTAP (R/S-2CTAP) and chiral separations are shown in Fig. 1. To ensure that the responses seen in these assays were specific for FFA2 and not activating native receptors expressed on the parental CHO-K1 cell line the FFA2 ligands used in this study were continuously tested on the parental CHO-K1 cell line alongside CHO-FFA2 cells. No response was induced by acetate, 4CMTB, 2CTAP or their enantiomers in any of the assays. Responses for the highest concentration of the ligands seen in the parental CHO-K1 cells across Ca2+. pERK1/2 and cAMP are shown in Fig. 2. Acetate increased Ca2+, pERK1/2 and inhibited forskolin-induced cAMP, in a concentration-dependent manner (Fig. 3, Table 1). R/S-4CMTB did not induce a Ca2+ response, however it caused a concentration-dependent increase in pERK1/2 and inhibition of forskolin-induced cAMP, with its effects more potent but less efficacious than acetate (Fig. 3). R/S-2CTAP caused a concentration-dependent decrease in Ca2+, however, had no effect on pERK1/2 or forskolin-induced cAMP (Fig. 3, Table 2).
Figure 1.
Chemical structures of 2-(4-chlorophenyl)-3-methyl-N-(thiazole-2-yl)butanamide (4-CMTB) and 4-((4-(2-chlorophenyl)thiazole-2-yl)amino)-4-oxo-3-phenylbutanoic acid (2CTAP) with resolved enantiomers for each.
Figure 2.
Responses for the highest concentrations of acetate, R-4CMTB, S-4CMTB and 2CTAP on parental CHO-K1 cells from left to right Ca2+ response, pERK1/2 response, Forskolin induced cAMP response. Ligand responses are plotted against positive and negative controls for each assay. Data are mean ± SEM combined from at least three independent experiments.
Figure 3.
Biologic activity of 4CMTB and 2CTAP in Ca2+, ERK and cAMP functional assays in CHO cells stably expressing hFFA2. (A) Ca2+ release was detected using the Fluo-4-direct Ca2+ assay. (B) pERK1/2 response was measured using AlphaScreen SureFire pERK1/2 (Thr202/Tyr204) assay kit. (C) Inhibition of forskolin-induced cAMP measured using the LANCE Ultra cAMP assay. Data were combined from at least three independent experiments and expressed as a percentage of the maximal response from acetate.
Table 1.
pEC50 and Emax values for 4CMTB through Ca2+, ERK and cAMP assays.
| Ligand | Assay | |||||
|---|---|---|---|---|---|---|
| Ca2+ | pERK1/2 | cAMP | ||||
| pEC50 | Emax | pEC50 | Emax | pEC50 | Emax | |
| Acetate | 3.8 | 100 | 3.6 | 100 | 3.9 | 100 |
| R/S-4CMTB | — | — | 5.8 | 85 | 7.0 | 83 |
| R-4CMTB | — | — | 5.0 | 60 | 5.2 | 74 |
| S-4CMTB | 5.7 | 55 | 6.1 | 102 | 6.8 | 99 |
| R/S-4CMTB + PTX | — | — | 4.7 | 20 | ||
| R-4CMTB + PTX | — | — | 4.7 | 16 | ||
| S-4CMTB + PTX | — | — | 4.8 | 22 | ||
| R/S-4CMTB + Acetate | 3.2 | |||||
| R-4CMTB + Acetate | 3.1 | |||||
| S-4CMTB + Acetate | 3.6 | |||||
Table 2.
pEC50 and Emax values for 2CTAP through Ca2+, ERK and cAMP assays.
| Ligand | Assay | ||||||
|---|---|---|---|---|---|---|---|
| Ca2+ | pEC50 | cAMP | |||||
| pEC50 | Emax | pEC50 | Emax | pEC50 | Emax | ||
| Agonist | Acetate | 3.8 | 100 | 3.6 | 100 | 3.9 | 100 |
| Agonist | R/S-2CTAP | — | — | — | — | — | — |
| R-2CTAP | — | — | — | — | — | — | |
| S-2CTAP | — | — | — | — | — | — | |
| Antagonist | R/S-2CTAP | 6.0 | 4.8 | — | |||
| R-2CTAP | 6.6 | 5.6 | — | ||||
| S-2CTAP | 5.7 | 4.4 | — | ||||
| R/S-2CTAP + Acetate | 6.2 | ||||||
| R-2CTAP + Acetate | 6.6 | ||||||
| S-2CTAP + Acetate | 5.8 | ||||||
Characterisation of enantiomers of 4CMTB and 2CTAP in CHO cells stably transfected with FFA2
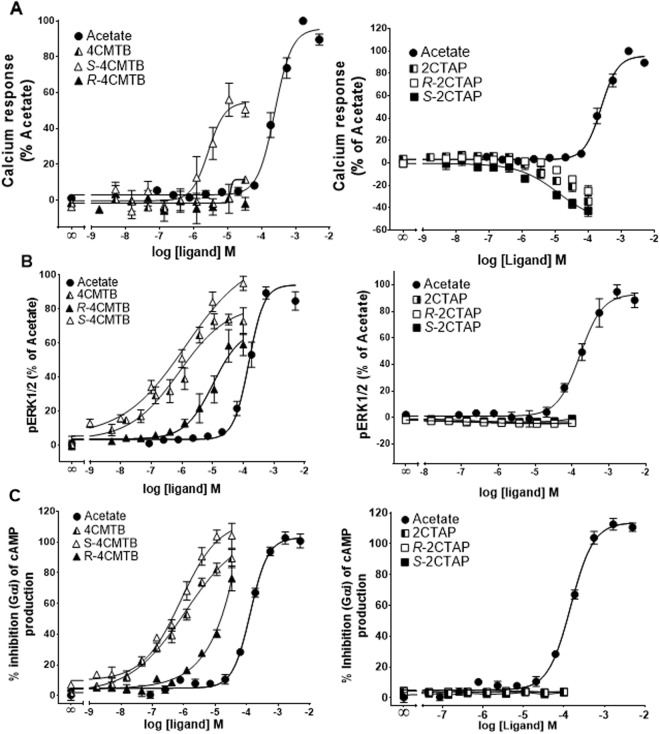
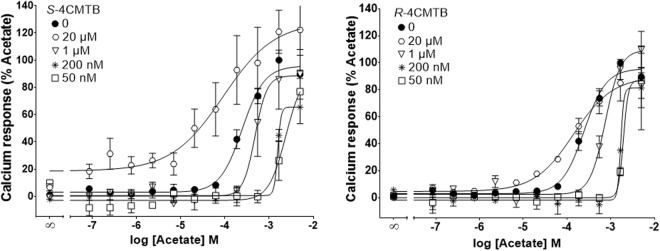
To evaluate the functional selectivity of 4CMTB enantiomers the racemic batch was separated on a Phenomenex Lux Cellulose-2 chiral column. S-4CMTB elicited concentration-dependent increases in Ca2+, which was more potent but less efficacious than acetate, whereas R-4CMTB had no effect on Ca2+ (Fig. 4A). S-4CMTB and R/S-4CMTB had similar potency and efficacy in increasing pERK1/2 (Fig. 4B) and inhibiting forskolin-induced cAMP (Fig. 4C). R-4CMTB was less potent than S-4CMTB and R/S-4CMTB but more potent than acetate. R-2CTAP and S-2CTAP both decreased Ca2+ in a similar concentration dependent manner to R/S-2CTAP (Fig. 4A), however they had no effects on pERK1/2 or cAMP responses (Fig. 4B,C).
Figure 4.
Dose response curves of 4CMTB and 2CTAP enantiomers in CHO cells stably expressing FFA2. (A) Ca2+ responses. (B) pERK1/2 responses. (C) Forskolin-induced cAMP. Data were combined from at least three independent experiments and expressed as a percentage of the maximal response from acetate.
To study the functional selectivity of allosteric effects in a physiological setting, different concentrations of 4CMTB were tested on an acetate dose response curve. In the Fluo-4 direct Ca2+ assay both S and R-4CMTB were found to be negative allosteric modulators below 1 µM. At 20 µM R-4CMTB had a mild effect on the potency of acetate but did not increase the efficacy. At 20 µM S-4CMTB increased both the potency and efficacy of acetate (Fig. 5, Table 1).
Figure 5.
Allosteric activity of 4CMTB enantiomers in a Ca2+ assay. Ca2+ responses were measured in the presence of different concentrations of acetate with varying concentrations of R-4CMTB or S-4CMTB. Data were combined from at least three independent experiments and expressed as a percentage of the maximal response from acetate.
Characterisation of the functional activity of 2CTAP
The functional nature of 2CTAP has not been explored beyond results from a GTPyS assay17. To confirm those findings and further investigate the functional nature of 2CTAP the Fluo-4 direct calcium assay, LANCE Ultra cAMP assay and a pERK1/2 assay, with a CHO cell line stably transfected with hFFA2 were utilised. R/S-2CTAP failed to induce intracellular Ca2+, phosphorylate ERK1/2 or inhibit forskolin-induced cAMP independently (Fig. 3). However, R/S-2CTAP appeared to inhibit constitutive Ca2+ levels. This effect was also seen with the R and S enantiomers with the S enantiomer having slightly higher efficacy over the R enantiomer and the recemate (Fig. 4A). Thus, indicating that 2CTAP could be an inverse agonist through the Gαq pathway. To fully establish the function of 2CTAP as an allosteric modulator we tested the effects of 2CTAP dose response curves on Ca2+ release in the presence of 2 mM acetate (Fig. 6). 2CTAP inhibited acetate induced Ca2+ release in a dose dependent manner and lowered the response below constitutive levels further supporting 2CTAP as an inverse agonist through Gαq (Fig. 6A). Acetate induced phosphorylation of ERK1/2 was also inhibited by 2CTAP in a dose dependent manner (Fig. 6B) suggesting that 2CTAP is an antagonist.
Figure 6.
Allosteric activity of 2CTAP and the enantiomers. (A) Ca2+ responses detected in a dose dependent manner in the presence of 2 mM acetate (dotted line) show that all enantiomers of 2CTAP reduce the signal below that of the basal levels. (B) ERK activation by 2 mM acetate was inhibited by all enantiomers of 2CTAP. (C) Forskolin-induced cAMP was not inhibited by 2CTAP nor either enantiomer. Data were combined from at least three independent experiments and expressed as a percentage of the maximal response from acetate.
The effects of functionally selective ligands in a mouse model of ischemia reperfusion injury (IRI)
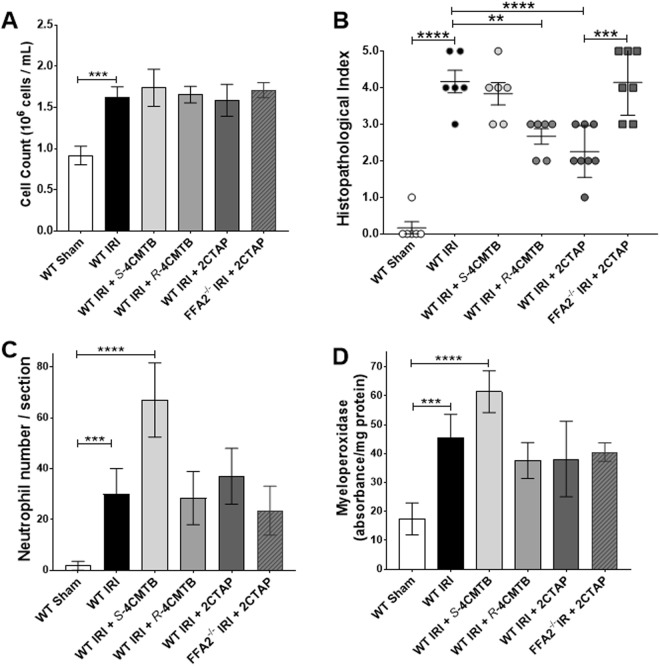
The signalling data indicates that S-4CMTB has a similar profile to acetate but is specific to FFA218 where as 2CTAP appears to be an antagonist and thus could offer different therapeutic advantages in disease. To further advance the understanding of these ligands in disease we tested these ligands in the neutrophil dependent mouse model of superior mesenteric artery occlusion (SMOA) that represents intestinal IRI19. We confirmed an increase in circulating neutrophils in IRI compared to sham (Fig. 7A). Ligands dosed to sham-operated mice did not increase circulating neutrophils (Circulating neutrophil counts for sham-operated groups dosed with R-4CMTB = 0.81 × 106 ± 0.11; S-4CMTB = 0.92 × 106 ± 0.07; 2CTAP = 0.83 × 106 ± 0.09). Circulating neutrophil numbers in IRI groups dosed with R-4CMTB, S-4CMTB and 2CTAP were comparable to vehicle control IRI-groups Fig. 7A. Histological examination of haematoxylin and eosin (H&E) stained ileum sections showed normal mucosal villi in vehicle control and dosed sham-operated mice. In sharp contrast, mice subjected to IRI displayed numerous swollen haemorrhagic villi and extensive development of sub-epithelial spaces as indicated by a high histopathological score. S-4CMTB did not reduce villi damage however, mucosal damage was significantly reduced in WT IRI groups dosed with 2CTAP, and, although to a lesser degree, R-4CMTB (Fig. 7B). Neutrophil infiltration upon reperfusion is considered to significantly contribute to the damage caused during IRI20. Infiltration of neutrophils into the ileum was therefore examined by counting esterase-stained neutrophils and measuring myeloperoxidase (Fig. 7C and D). S-4CMTB increased neutrophil infiltration into the villi (Fig. 7C,D) in line with its previously shown chemotactic properties6,13. R-4CMTB and 2CTAP did not reduce neutrophil infiltration below the IRI group despite their protective effects, suggesting they may rather be modulating neutrophil function at the site of injury. The protective effects of 2CTAP were abolished in the absence of FFA2 indicating that the in vivo activity observed with 2CTAP is specific to FFA2 (Fig. 7).
Figure 7.
The effects of R and S-4CMTB and 2CTAP in a mouse model of intestinal IRI. (A) Serum neutrophil numbers. (B) Quantitative histopathological scores of H&E-stained intestinal sections. (C) Neutrophil infiltration detected by counting esterase stained neutrophils in ileum sections. (D) Neutrophil infiltration into the ileum measured by myeloperoxidase. Data are mean ± SEM. ****p < 0.0001; ***p < 0.001 **p < 0.01. n = 7 ± 1.
Discussion
Selective, allosteric, potent ligands that bind to FFA2 may have potential as therapies for inflammatory diseases. We characterised two reported FFA2 ligands (4CMTB and 2CTAP) and their enantiomers to assess their pharmacological properties at hFFA2. We demonstrated that S-4CMTB is a positive allosteric modulator whereas R-4CMTB is a functionally selective ligand that does not activate Gαq. 2CTAP is a functionally selective inverse agonist that inhibits constitutive Ca2+ levels and antagonises acetate-induced ERK phosphorylation. This study substantially extends the previous knowledge of the activity of 4CMTB and 2CTAP by characterising the enantiomers of 4CMTB and 2CTAP, relative to acetate, in CHO cells stably expressing hFFA2 and in a mouse model of intestinal IRI.
The SCFAs acetate, propionate and butyrate activate FFA2 and FFA3 and have important roles in health and disease. Notably this includes their anti-inflammatory properties that are mediated through the modulation of chemotaxis, reactive oxygen species (ROS) and cytokine production11,14,21. Several allosteric ligands for FFA2 have been reported that could be developed into novel therapies17,18,22. However, these have not been characterised across G protein pathways and assumed function is often based on a single signalling pathway or through binding studies alone. FFA2 is known to signal through Gαi, Gαq and β-arrestin pathways2,15,23. Functional selectivity at orthosteric sites is a well characterised concept that has changed fundamental views on GPCR signalling24,25. Although functional selectivity across allosteric sites is not as well documented, it is hypothesised that allosteric ligands are more likely to have biased signalling properties, which could differ again in the presence of the orthosteric ligand26.
This study describes the functional selectivity of two synthetic molecules for FFA2. The phenylacetamide compound 4CMTB from Amgen is a putative ago-allosteric agonist at FFA218, and the carboxylic acid derivatised variant, 2CTAP from Euroscreen, is a putative allosteric agonist at FFA2. 4CMTB has been studied across different signalling pathways with a focus on binding assays and Gαi and Gαq pathways7,18. It has been shown to be a positive allosteric modulator of FFA227,28. 4CMTB is a direct FFA2 agonist in cAMP and [S35]GTPγS assays with 100- to 1,000-fold higher affinity than acetate and propionate, but with a positive modulatory effect on acetate and propionate in vivo21. Other studies have reported various other effects of 4CMTB including chemotaxis of human6 and mouse13 neutrophils, reduced proliferation of leukaemia cells29, mediation of intracellular signalling in adipocytes via Gαi30 and inhibition of lipolysis in differentiated 3T3L1 cells18. The in vivo observations of 4CMTB suggest that it is an ago-allosteric modulator, but mutational studies have failed to identify the binding sites of 4CMTB18,31.
2CTAP was patented as an allosteric agonist of FFA2 based on a binding assay17 with a maximal response at 60 nM in the GTPγS assay. It also increased glucose uptake in 3T3-L1 cells and inhibited lipolysis in isolated adipocytes in a concentration-dependent manner18. Acetate has been used as an endogenous ligand throughout these studies as it has been shown to be the most specific SCFA agonist of FFA26 with no histone deacetylase activity observed13.
4CMTB did not induce calcium signalling in a Fluo-4 direct Ca2+ assay but did show positive cooperativity with acetate providing greater potency and increased efficacy over acetate alone. Surprisingly, 2CTAP did not have any agonist or ago-allosteric properties in a Ca2+ assay however it inhibited acetate-induced calcium responses in a dose dependent manner, suggesting that 2CTAP is an antagonist of FFA2.
The Gαq pathway and intracellular Ca2+ levels have been reported to promote neutrophil migration32 and the production of ROS33. Neutrophils are a common cause of damage in acute inflammatory diseases and thus blocking Gαq could prevent excessive neutrophil migration32 and reduce ROS production. FFA2 antagonists have already been reported to be able to reduce neutrophil migration17,22,34. The current study demonstrates that R-, S- and R/S-2CTAP can inhibit acetate-induced increases in Ca2+. In the mouse model of intestinal IRI neutrophil infiltration was not prevented by 2CTAP. However, mucosal damage was diminished in the IRI group dosed with 2CTAP and this was negated in FFA2−/− mice. The mechanism for this protection could be through 2CTAP blocking FFA2 induced ROS production although further investigation is required to fully elucidate the specific mechanisms of 2CTAP on neutrophil function.
Interestingly, the allosteric activity of R and S-4CMTB was different through the Gαq pathway. It is well established, unfortunately due to the thalidomide tragedy, that compounds exist as a racemic mixture of (R) and (S) enantiomers which can have different biological activity. The subtle differences in the atomic structure mean that in a calcium assay S-4CMTB is an ago-allosteric ligand where R-4CMTB does not have any intrinsic ability and appears to be a negative allosteric modulator. This is supported by the in vivo data which showed R-4CMTB did not increase neutrophil infiltration and had a protective effect in IRI in contrast to S-4CMTB. Unlike R-4CMTB and the antagonist 2CTAP, S-4CMTB increased neutrophil infiltration corroborating the importance of the Gαq pathway in FFA2 induced chemotaxis.
pERK1/2 and cAMP are key downstream signalling pathways of FFA2. In neutrophils, activation of pERK1/2 can cause the formation of neutrophil extracellular traps35, and cAMP activation leads to neutrophil chemotaxis36. Extracellular traps have been associated with increased damage in heart and hepatic ischemia reperfusion injury37,38. R, S- and R/S-4CMTB have similar potency and efficacy for pERK1/2 and partially inhibit forskolin-induced cAMP suggesting they are Gαi ago allosteric modulators. These responses are more potent than the selective FFA2 agonist acetate. 2CTAP inhibited pERK1/2 but not cAMP. Inhibition of pERK1/2 could contribute to the protective effects seen in vivo. Further investigation is required to fully understand the exact biological activity that these ligands have at each pathway and how this affects neutrophil function. However, we have identified 2CTAP as an antagonist that is specific at FFA2 and R and S-4CMTB which are functionally selective ligands for FFA2 and therefore present valuable tools for elucidating Ca2+, pERK1/2 and cAMP signalling downstream of FFA2 and the effects of FFA2 on neutrophil function.
There have been many challenges surrounding FFA2 biology, including poor potency and selectivity of ligands, altered function due to the allosteric mode of action and the fact that FFA2−/− mice also have reduced expression of FFA3. R-, S- and R/S-4CMTB provide useful ligands to elucidate signalling bias at FFA2 at specific concentrations and R-, S- and R/S-2CTAP provide useful antagonists to elucidate FFA2-induced Ca2+ signalling in disease settings. Further studies assessing the efficacy of these compounds in rodents is warranted as some of the incomplete understanding of FFA2 biology is due to variability in ligand selectivity between human and rodent FFA221,39. However, initial studies in our mouse model of intestinal IRI support the in vitro data carried out on hFFA2 suggesting mouse and human homologues may have similar biological function and thus further investigation of these ligands in other disease models could be beneficial.
There is a need to assess enantiomeric forms of molecules, particularly targeting hFFA2 as activation of distinct signalling pathways may provide different biological effects. 4CMTB, reinforced the often-overlooked potential of enantiomers to impart different signalling properties and potential therapeutics may go unnoticed in a racemic mixture. S- and R/S-4CMTB are agonists at FFA2 showing similar potency and efficacy for pERK1/2 and cAMP signalling, with S-4CMTB more potent than acetate at increasing Ca2+. 4CMTB and 2CTAP therefore provide useful compounds to further elucidate the translational applicability of FFA2 agonists and antagonists into mouse models of disease, and to clarify the discrepancies surrounding FFA2 biology. Unravelling the mechanisms of these pharmacologically selective ligands provides invaluable tools to further FFA2 biology and pharmacology, particularly in neutrophil-driven disease.
Methods
CHO-K1 cells
Chinese Hamster Ovary (CHO-K1) cells were purchased from Cellank Australia as a negative control, as they do not endogenously express FFA2. These cells were cultured in Ham’s F12, 10% FBS and 1% penicillin streptomycin. Cells were passaged when 70–80% confluent using 0.05% trypsin: EDTA in phosphate-buffered saline (PBS), pH 7.4. All cells in culture, once seeded, were incubated at 37 °C with 5% CO2.
CHO-FFA2 cells
CHO-K1, stably expressing human FFA2 (CHO-FFA2) was purchased from DiscoverX Corporation. CHO-FFA2 cells were cultured in a buffer of Ham’s F12, 10% FBS, 1% penicillin streptomycin and 800 μg/mL of G418 (Geneticin) (Invitrogen Life Technologies). Cells were passaged when 70–80% confluent using 0.05% trypsin with 1 mM EDTA in phosphate-buffered saline (PBS), pH 7.4. All cells in culture, once seeded, were incubated at 37 °C with 5% CO2.
FFA2 ligands
Acetate was purchased from Chem-Supply (Gillman, Australia), propionate and butyrate were purchased from Sigma-Aldrich (Missouri, USA). 4CMTB patented by Amgen18 and 2CTAP patented by Euroscreen16 were synthesized as below from purchased components.
Synthesis of 4CMTB
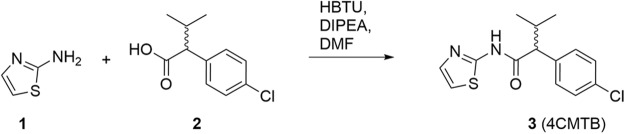
4CMTB was synthesised according to the route shown in Fig. 8. where the commercially available 2-aminothiazole and 2-(4-chlorophenyl)-3-methylbutanoic acid were reacted using standard HBTU coupling with Hünig’s base in DMF. The desired amide 4CMTB was obtained in 70% yield after recrystallization and data are consistent with literature precedent.
Figure 8.
Synthetic route to 4CMTB.
Synthesis of 2CTAP
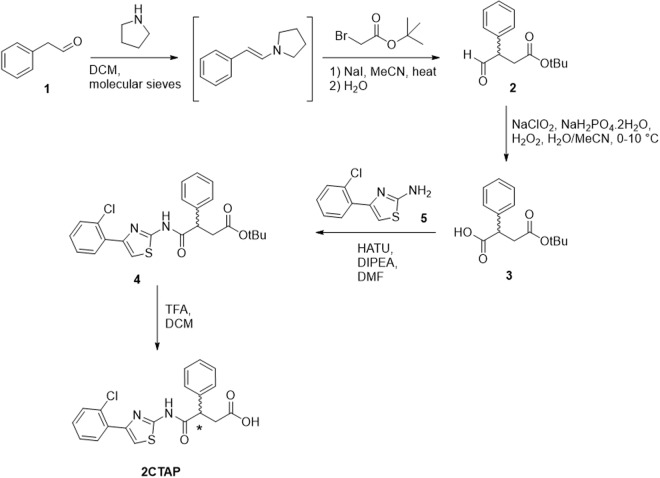
2CTAP was synthesised according to the route shown in Fig. 9. Phenylacetaldehyde (1) was converted to the enamine via reaction with pyrrolidine under anhydrous conditions following the procedure of Belanger et al.40. Subsequent reaction with t-butyl bromoacetate and sodium hydride followed by hydrolysis of the enamine gave the desired aldehyde (2), tert-butyl 4-oxo-3-phenylbutanoate in moderate yield over the two steps. Potassium permanganate was initially used as an oxidant to convert the aldehyde to the corresponding acid (3) however yields were exceptionally low, and reactions did not proceed cleanly. The milder Pinnick oxidation procedure41 gave conversion to 4-(tert-butoxy)-4-oxo-2-phenylbutanoic acid (3) in 59% yield. 4-(2-chlorophenyl)thiazol-2-amine (5) was synthesized using standard Hantsch thiazole synthesis. Coupling of carboxylic acid (3) with the 2-aminothiazole (5) using standard HBTU coupling gave the protected product (4) with 93% yield after purification using column chromatography. Subsequent deprotection using trifluoroacetic acid gave 2CTAP in quantitative yield with spectra in agreement with literature. The racemic 2CTAP was separated into the two enantiomers using chiral column chromatography. Chiral LC-MS analyses were conducted using an Agilent Technologies 1200 Series Instrument with a G1316A UV-Vis detector (λ = 210 and 254 nm), 1200 Series ELSD and 6110 quadrupole ESI-MS, using Phenomenex Lux Cellulose-2 column (4. 6 × 250 mm, 250 Å~4.6 mm, 3 μm, flow rate 1 mL/min, room temperature). The eluents were 0.05% formic acid in water (A) and 0.05% formic acid in ACN (B). Using a 30 min isocratic method set at 60% mobile phase B. The Chiral HPLC purifications were achieved using a Gilson PLC 2020 Personal Purification System using a Phenomenex Lux Cellulose-2 column (21.2 × 150 mm, 150 Å~21.2 mm, 5 μm, flow rate 20 mL/min, room temperature). The eluents were 0.1% formic acid in water (A) and 0.1% formic acid in ACN (B). Using a 30 min isocratic method set at 60% mobile phase B. Samples were dissolved in chloroform and optical rotation was determined on an automatic polarimeter. The enantiomers were formulated as the sodium salt to aid their aqueous solubility in subsequent testing.
Figure 9.
Synthetic route to 2CTAP.
Ca2+ flux assay
Fluo-4 Direct assay was preformed according to manufacturer’s instructions with additional wash steps and read on a Fluorescent Imaging Plate Reader (FLIPR) (Molecular Devices, California). Briefly, 10,000 cells were seeded/well in 10 μL of media in a 384 well block with a clear bottom plate and incubated overnight at 37 °C. An equivalent volume of 2x Flou-4 Direct calcium reagent was added to each well and incubated for 30 min at 37 °C and a further 30 min at room temperature. Ligand addition was carried out on the FLIPR instrument whilst fluorescence was measured at excitation 494 nm and emission at 516 nm for five minutes to enable capture of the fast calcium response.
ERK1/2 phosphorylation assay
To measure pERK1/2 we utilised the high throughput AlphaScreen SureFire pERK1/2 (Thr202/Tyr204) Assay Kit (PerkinElmer)42. Detection of pERK1/2 is enabled by immuno-sandwich capture of endogenous pERK1/2 in cell lysates. Antibody-coated AlphaScreen beads generate a highly amplified signal when near due to binding of pERK1/2. Cells were seeded at 50,000 cells/well in a 96 well tissue culture plate and incubated for 24 hours at 37 °C. Cells were then incubated in serum free media overnight. Test compounds were added to the cells and incubated for 10 min at room temperature before cells were lysed and assayed for pERK1/2 using the AlphaScreen-based detection kit according to the manufacturer’s protocol (PerkinElmer).
cAMP assay
The LANCE Ultra cAMP homogeneous time resolved resonance energy transfer (TR-FRET) immunoassay (PerkinElmer) provides a highly sensitive and robust method for the detection of cAMP. Briefly, cells were re-suspended in stimulation buffer (1 x HBSS, 5 mM HEPES, 0.5 mM IBMX, 0.1% BSA (pH7.4)) and transferred to 384-proxiplate (PerkinElmer) at a density of 2000 cells/well. Cells were stimulated with agonist and forskolin (5 μM), where appropriate, and incubated for 30 min. EucAMP tracer and ULight-anti-cAMP solutions were added to the cells and incubated for 1 hour at room temperature. Time- resolved FRET signals were measured after excitation at 320 nm on the EnVision. All data were normalized to the functional response obtained with 1.67 mM sodium acetate.
Conventional and FFA2 knockout mice
Specific pathogen-free (SPF) conventional C57BL/6J (WT) mice were obtained from the Australian Research Council (ARC) and maintained at The University of Queensland Biological Resources Animal Facilities under specific pathogen-free conditions.
FFA2−/− knockout mice were obtained from Monash University and bred at The University of Queensland Biological Resources Animal Facilities under SPF conditions. Male mice 10–12 weeks weighing 20–30 g were used for all experiments. All experiments were performed in accordance with the guidelines and approval of The University of Queensland Animal Ethics Committee.
Intestinal Ischemia Reperfusion Injury Model
Anesthetized mice underwent laparotomy followed by superior mesenteric artery occlusion by a loop of ligature to induce nontraumatic intestinal ischemia. After 35 minutes the ligature was removed to allow for a reperfusion period of 150 minutes. Sham-operated mice underwent the same surgical procedure except for the superior mesenteric artery occlusion. Mice were orally gavaged with ligand 48 hours, 24 hours and 1 hour prior to surgery. Mice were euthanised and small intestinal tissue or whole blood [collected in 1 mg/mL EDTA and 0.1 mg/mL nafamostat mesylate (FUT-175)] was collected for further analysis.
Data and statistical analysis
All data is expressed as mean ± SEM from at least three independent experiments. Data analysis and curve fitting were carried out using the GraphPad Prism v6.0 software package (GraphPad Software, California). Concentration-response data were fit to four parameter sigmoidal concentration-response curves. All statistical analysis of curve fit parameters was carried out by independently fitting the data from triplicate experiments and comparing the resulting curve fit values by t-test or one-way-ANOVA as appropriate. Statistical differences between curves was analysed using an F-test. All statistical analysis of in vivo work was carried out using one-way anova with Tukey’s multiple comparison.
Acknowledgements
C.D. is supported by an NHMRC Early Career Postdoctoral Fellowship. T.M.W. is supported by an NHMRC Career Development Fellowship. P.M.H. is supported by an NHMRC Principal Research Fellowship and a Brawn Fellowship, Faculty of Health & Medicine, University of Newcastle.
Author Contributions
M.C. conceived the project and directed the study program. Z.V.S. wrote the initial draft manuscript and carried out most of the in vitro assays and all the in vivo work. D.C. carried out some biological assays and contributed to manuscript writing. R.H. contributed to project planning and manuscript writing. A.R. designed the synthesis, wrote the synthesis sections and contributed to manuscript editing. A.R., N.M., E.T. and D.E. conducted the synthesis, purification and characterisation of the ligands. T.M.W. conceived and designed the in vivo experiments and oversaw their completion in his laboratory and contributed to project planning and manuscript editing. M.A.C. and C.D. co-wrote the revisions, P.M.H. contributed to planning and performing in vitro assays and writing and editing of the manuscript.
Data Availability Statement
The datasets generated during and/or analysed during the current study are available from the corresponding author on reasonable request.
Competing Interests
The authors declare no competing interests.
Footnotes
Publisher’s note: Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
References
- 1.Budden KF, et al. Emerging pathogenic links between microbiota and the gut–lung axis. Nat. Rev. Microbiol. 2017;15:55–63. doi: 10.1038/nrmicro.2016.142. [DOI] [PubMed] [Google Scholar]
- 2.Brown AJ, et al. The Orphan G protein-coupled receptors GPR41 and GPR43 are activated by propionate and other short chain carboxylic acids. J. Biol. Chem. 2003;278:11312–9. doi: 10.1074/jbc.M211609200. [DOI] [PubMed] [Google Scholar]
- 3.Karaki S, et al. Expression of the short-chain fatty acid receptor, GPR43, in the human colon. J. Mol. Histol. 2008;39:135–142. doi: 10.1007/s10735-007-9145-y. [DOI] [PubMed] [Google Scholar]
- 4.Swaminath G, et al. Allosteric rescuing of loss-of-function FFAR2 mutations. FEBS Lett. 2010;584:4208–14. doi: 10.1016/j.febslet.2010.09.007. [DOI] [PubMed] [Google Scholar]
- 5.Bjursell M, et al. Improved glucose control and reduced body fat mass in free fatty acid receptor 2-deficient mice fed a high-fat diet. Am. J. Physiol. Endocrinol. Metab. 2011;300:E211–20. doi: 10.1152/ajpendo.00229.2010. [DOI] [PubMed] [Google Scholar]
- 6.Le Poul E, et al. Functional characterization of human receptors for short chain fatty acids and their role in polymorphonuclear cell activation. J. Biol. Chem. 2003;278:25481–9. doi: 10.1074/jbc.M301403200. [DOI] [PubMed] [Google Scholar]
- 7.Schmidt J, et al. Selective orthosteric free fatty acid receptor 2 (FFA2) agonists: identification of the structural and chemical requirements for selective activation of FFA2 versus FFA3. J. Biol. Chem. 2011;286:10628–40. doi: 10.1074/jbc.M110.210872. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Karaki S, et al. Short-chain fatty acid receptor, GPR43, is expressed by enteroendocrine cells and mucosal mast cells in rat intestine. Cell Tissue Res. 2006;324:353–60. doi: 10.1007/s00441-005-0140-x. [DOI] [PubMed] [Google Scholar]
- 9.Comalada M, et al. The effects of short-chain fatty acids on colon epithelial proliferation and survival depend on the cellular phenotype. J. Cancer Res. Clin. Oncol. 2006;132:487–497. doi: 10.1007/s00432-006-0092-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Macia L, et al. Microbial influences on epithelial integrity and immune function as a basis for inflammatory diseases. Immunol. Rev. 2012;245:164–76. doi: 10.1111/j.1600-065X.2011.01080.x. [DOI] [PubMed] [Google Scholar]
- 11.Maslowski KM, et al. Regulation of inflammatory responses by gut microbiota and chemoattractant receptor GPR43. Nature. 2009;461:1282–1286. doi: 10.1038/nature08530. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Sina C, et al. G protein-coupled receptor 43 is essential for neutrophil recruitment during intestinal inflammation. J. Immunol. 2009;183:7514–22. doi: 10.4049/jimmunol.0900063. [DOI] [PubMed] [Google Scholar]
- 13.Vinolo MAR, et al. SCFAs Induce Mouse Neutrophil Chemotaxis through the GPR43 Receptor. PLoS One. 2011;6:e21205. doi: 10.1371/journal.pone.0021205. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Vinolo MAR, et al. Short-chain fatty acids stimulate the migration of neutrophils to inflammatory sites. Clin. Sci. (Lond). 2009;117:331–8. doi: 10.1042/CS20080642. [DOI] [PubMed] [Google Scholar]
- 15.Stoddart LA, Smith NJ, Milligan G. International Union of Pharmacology. LXXI. Free fatty acid receptors FFA1, -2, and -3: pharmacology and pathophysiological functions. Pharmacol. Rev. 2008;60:405–17. doi: 10.1124/pr.108.00802. [DOI] [PubMed] [Google Scholar]
- 16.Lee SU, et al. β-Arrestin 2 Mediates G Protein-Coupled Receptor 43 Signals to Nuclear Factor-κB. Biol. Pharm. Bull. 2013;36:1754–1759. doi: 10.1248/bpb.b13-00312. [DOI] [PubMed] [Google Scholar]
- 17.Hoveyda, H. et al. Compounds, pharmaceu- tical composition and methods for use in treating metabolic disorders. PCT Int. Appl. WO2010066682 (2010).
- 18.Lee T, et al. Identification and Functional Characterization of Allosteric Agonists for the G Protein-Coupled Receptor FFA2. Mol. Pharmacol. 2008;74:1599–1609. doi: 10.1124/mol.108.049536. [DOI] [PubMed] [Google Scholar]
- 19.Wu, M. C. L. et al. The receptor for complement component C3a mediates protection from intestinal ischemia-reperfusion injuries by inhibiting neutrophil mobilization. Proc. Natl. Acad. Sci. USA 1–6, 10.1073/pnas.1218815110 (2013). [DOI] [PMC free article] [PubMed]
- 20.Schofield ZV, Woodruff TM, Halai R, Wu MC-L, Cooper MA. Neutrophils-a key component of ischemia-reperfusion injury. Shock. 2013;40:463–70. doi: 10.1097/SHK.0000000000000044. [DOI] [PubMed] [Google Scholar]
- 21.Ulven T. Short-chain free fatty acid receptors FFA2/GPR43 and FFA3/GPR41 as new potential therapeutic targets. Front. Endocrinol. (Lausanne). 2012;3:111. doi: 10.3389/fendo.2012.00111. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Labéguère, F., Alvey, L., Newsome, G., Laurent, S. & Fletcher, S. Novel dihydropyrimidinoisoquinolinones and pharmaceutical compositions thereof for the treatment of inflammatory disorders (gpr84 antagonists) (2013).
- 23.Lee SU, et al. β-Arrestin 2 Mediates G Protein-Coupled. Receptor 43 Signals to Nuclear. 2013;36:1754–1759. doi: 10.1248/bpb.b13-00312. [DOI] [PubMed] [Google Scholar]
- 24.Kenakin TP. Cellular assays as portals to seven-transmembrane receptor-based drug discovery. Nat. Rev. Drug Discov. 2009;8:617–26. doi: 10.1038/nrd2838. [DOI] [PubMed] [Google Scholar]
- 25.Stallaert, W., Christopoulos, A. & Bouvier, M. Ligand functional selectivity and quantitative pharmacology at G protein-coupled receptors. 811–825 (2011). [DOI] [PubMed]
- 26.May LT, Leach K, Sexton PM, Christopoulos A. Allosteric modulation of G protein-coupled receptors. Annu. Rev. Pharmacol. Toxicol. 2007;47:1–51. doi: 10.1146/annurev.pharmtox.47.120505.105159. [DOI] [PubMed] [Google Scholar]
- 27.Brown AJ, et al. Pharmacological properties of acid N-thiazolylamide FFA2 agonists. Pharmacol. Res. Perspect. 2015;3:e00141. doi: 10.1002/prp2.141. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Hudson BD, et al. Defining the molecular basis for the first potent and selective orthosteric agonists of the FFA2 free fatty acid receptor. J. Biol. Chem. 2013;288:17296–17312. doi: 10.1074/jbc.M113.455337. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Bindels LB, et al. Gut microbiota-derived propionate reduces cancer cell proliferation in the liver. Br. J. Cancer. 2012;107:1–8. doi: 10.1038/bjc.2012.409. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Kimura I, et al. The gut microbiota suppresses insulin-mediated fat accumulation via the short-chain fatty acid receptor GPR43. Nat. Commun. 2013;4:1829. doi: 10.1038/ncomms2852. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Smith NJ, et al. Extracellular loop 2 of the free fatty acid receptor 2 mediates allosterism of a phenylacetamide ago-allosteric modulator. Mol. Pharmacol. 2011;80:163–73. doi: 10.1124/mol.110.070789. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Bindels LB, Dewulf EM, Delzenne NM. GPR43/FFA2: physiopathological relevance and therapeutic prospects. Trends Pharmacol. Sci. 2013;34:226–232. doi: 10.1016/j.tips.2013.02.002. [DOI] [PubMed] [Google Scholar]
- 33.Futosi K, Fodor S, Mócsai A. Neutrophil cell surface receptors and their intracellular signal transduction pathways. Int. Immunopharmacol. 2013;17:638–50. doi: 10.1016/j.intimp.2013.06.034. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Pizzonero, M. et al. Discovery and optimization of an azetidine chemical series as a free fatty acid receptor 2 (FFA2) antagonist: from hit to clinic. J. Med. Chem. 2 (2014). [DOI] [PubMed]
- 35.Hakkim A, et al. Activation of the Raf-MEK-ERK pathway is required for neutrophil extracellular trap formation. Nat. Chem. Biol. 2011;7:75–77. doi: 10.1038/nchembio.496. [DOI] [PubMed] [Google Scholar]
- 36.Harvath L, Robbins JD, Russell AA, Seamon KB. cAMP and human neutrophil chemotaxis. Elevation of cAMP differentially affects chemotactic responsiveness. J. Immunol. 1991;146:224–32. [PubMed] [Google Scholar]
- 37.Ge L, et al. Neutrophil extracellular traps in ischemia-reperfusion injury-induced myocardial no-reflow: therapeutic potential of DNase-based reperfusion strategy. Am. J. Physiol. Circ. Physiol. 2015;308:H500–H509. doi: 10.1152/ajpheart.00381.2014. [DOI] [PubMed] [Google Scholar]
- 38.Arumugam S, Girish Subbiah K, Kemparaju K, Thirunavukkarasu C. Neutrophil extracellular traps in acrolein promoted hepatic ischemia reperfusion injury: Therapeutic potential of NOX2 and p38MAPK inhibitors. J. Cell. Physiol. 2018;233:3244–3261. doi: 10.1002/jcp.26167. [DOI] [PubMed] [Google Scholar]
- 39.Milligan G, Stoddart LA, Smith NJ. Agonism and allosterism: the pharmacology of the free fatty acid receptors FFA2 and FFA3. Br. J. Pharmacol. 2009;158:146–53. doi: 10.1111/j.1476-5381.2009.00421.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Bélanger G, Doré M, Ménard F, Darsigny V. Highly chemoselective formation of aldehyde enamines under very mild reaction conditions. J. Org. Chem. 2006;71:7481–7484. doi: 10.1021/jo0611061. [DOI] [PubMed] [Google Scholar]
- 41.Ebbers EJ, Ariaans GJA, Bruggink A, Zwanenburg B. Controlled racemization and asymmetric transformation of α-substituted carboxylic acids in the melt. Tetrahedron Asymmetry. 1999;10:3701–3718. doi: 10.1016/S0957-4166(99)00387-0. [DOI] [Google Scholar]
- 42.Osmond RIW, et al. GPCR screening via ERK 1/2: a novel platform for screening G protein-coupled receptors. J. Biomol. Screen. 2005;10:730–7. doi: 10.1177/1087057105277968. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
The datasets generated during and/or analysed during the current study are available from the corresponding author on reasonable request.