False penicillin allergies are depriving many patients of β-lactam antibiotic therapy, with adverse consequence on healthcare costs and treatment outcomes. Debunking false penicillin allergies through confirmatory testing may be an important component of antimicrobial stewardship practice.
Keywords: penicillin allergy, clinical outcomes, innate immunity, β-lactams, allergy testing
Abstract
The majority of patients with reported penicillin allergy are not allergic when tested or challenged. Penicillin allergy testing has been shown to significantly reduce annual healthcare expenditures. Data have emerged showing β-lactams have multidimensional antibacterial effects in vivo, far beyond what is appreciated in standard bacteriological susceptibility testing media. These include enhancing bacterial killing by the innate immune system. Supporting the clinical relevance of these secondary underappreciated effects are recent clinical and pharmacoeconomic analyses that show worse outcomes in patients with reported penicillin allergies who receive non-β-lactam antibiotics when compared to their non-penicillin-allergic counterparts. This is particularly relevant in the treatment of Staphylococcus aureus bacteremia. This article reviews the tremendous advantages offered by β-lactam therapy and makes a strong case that the debunking of false penicillin allergies through a detailed allergy history and penicillin allergy testing should be a vital component of antimicrobial stewardship practices.
In the history of medicine, antibiotics are the class of medications with the greatest impact on human health, reflected in a 2-decade increase in life expectancy in developed countries since the dawn of the antibiotic era in the 1940s. Antibiotics control common infectious diseases such as pneumonia, urinary tract infections, and skin infections through microbial killing, and support the success of many modern clinical therapeutic modalities such as cancer chemotherapy, surgery, and transplantation where infectious risk is increased [1].
The β-lactam class of antibiotics has its origin in Sir Alexander Fleming’s serendipitous observation of the antibacterial properties of the Penicillium mold and the subsequent discovery of penicillin [1]. Approaching the 90th anniversary of this historic event in September 2018, our understanding of the therapeutic properties of β-lactam drugs beyond their direct activities in bacteriological media is still unfolding. The goals of this commentary are 2-fold. We will first review how β-lactam drugs possess numerous adjunctive pleural pharmacodynamics activities, not exhibited by other antimicrobial classes, that modulate bacterial interaction with, and susceptibility to, the innate immune system—all in a manner that benefits patients. Second, we will explore the negative consequences of withholding β-lactam antibiotics in favor of other drug classes in patients with purported (but unproven) penicillin allergies, and how penicillin allergy testing can prove to be a very cost-effective element of a successful antimicrobial stewardship initiative.
THE NATURE OF PENICILLIN “ALLERGIES”
A sensitivity to penicillin is the most common “allergy” noted among patients in the United States, self-reported by 10% of adult patients, thus accounting for approximately 25 million people [2, 3]. However, as many as 98% of these patients are deemed nonallergic by subsequent penicillin allergy testing, and tolerate future β-lactams with only a slightly greater risk than average individuals [4–6]. Allergies to penicillin may have become part of the medical record because the reaction recalled by the patient was due to another medication, the reaction was nonimmunologic (eg, gastrointestinal upset, nausea, diarrhea), or the patient was simply told of a reaction by their family decades earlier, without any recollection of details. Likewise, one cannot discount the possibility of a rash produced by an intercurrent (usually viral) infection or, as exemplified by the diffuse rash experienced by some patients receiving amoxicillin during acute mononucleosis, a transient immunologic reaction rather than a true allergy [7]. Whatever their story, the vast majority of patients with such purported allergies in their medical records are destined to receive alternative classes of antibiotics, with some direct and indirect adverse consequences to treatment and outcomes, which we review below.
ADVERSE OUTCOMES IN PATIENTS ALLERGIC TO PENICILLIN
In a matched retrospective cohort study, Macy and Contreras [8] found several adverse consequences among patients with reported penicillin allergy matched to similar patients without reported allergies. The “penicillin-allergic” patients experienced significantly longer hospital stays, a 23% increase in Clostridium difficile infection, 14% more methicillin-resistant Staphylococcus aureus (MRSA) infections, and 30% more vancomycin-resistant Enterococcus (VRE) infections. The authors concluded that increases in opportunistic infections may have been driven by significantly higher usage of vancomycin, clindamycin, and fluoroquinolone, as vancomycin exposure is associated with VRE, and clindamycin and fluoroquinolones are the antimicrobial classes posing the greatest risk for C. difficile [8]. In another study, 31%–51% of vancomycin use was attributed to alternative therapy in patients with penicillin allergy [9].
In patients with severe invasive methicillin-susceptible S. aureus (MSSA) infections such as bacteremia and endocarditis, resorting to vancomycin to avoid a purported penicillin allergy may have serious impact on outcomes, given consistent and reproducible clinical evidence pointing to vancomycin’s inferior performance in these patient settings [10–16]. For example, in a large retrospective cohort of 5633 Veterans Affairs patients with MSSA bacteremia, patients who received definitive therapy with a β-lactam had 35% lower mortality, and patients receiving cefazolin or an antistaphylococcal penicillin a 43% lower mortality, compared with patients who received vancomycin [16]. Indeed, the 2-fold increased mortality in patients with MRSA bacteremia compared to those with MSSA bacteremia may be largely attributable to the fallback use of vancomycin against MRSA [17].
Based on the preponderance of evidence, vancomycin use should be avoided in the treatment of MSSA bacteremia. Alternatives to vancomycin have emerged in the last 2 decades for the penicillin-allergic patient, with daptomycin being the first US Food and Drug Administration–approved alternative therapy in MSSA bacteremia, showing noninferiority to β-lactams in a prospective randomized trial [18]. A recent study of patients with reported penicillin allergies and MSSA bacteremia compared outcomes of patients that either (1) were given vancomycin without any assessment, (2) received cefazolin if anaphylaxis was ruled out by history, or (3) had a complete allergy evaluation including penicillin skin testing [19]. They found as expected that vancomycin yielded the lowest cure rates (67%) and highest rates of recurrence (15%), in contrast to those who received cefazolin, where cure rates were approximately 84% and relapse only 9%. The vancomycin-treated group also had the highest rates of allergic and other adverse drug reactions. This study makes it very clear that taking a stated penicillin allergy at face value and avoiding it with vancomycin use is providing suboptimal care in MSSA bacteremia [19]. The same researchers showed in a subsequent study of MSSA bacteremia that optimal and adequate antimicrobial therapy was hampered by a history of a penicillin allergy [20].
Further evidence of the negative consequences of purported penicillin allergies continues to accumulate. Compared to patients without allergy, reported penicillin-allergic patients have 50% increased odds of getting a surgical site infection, attributable to more frequent receipt of second-line agents such as vancomycin (35% vs 3%) or clindamycin (49% vs 3%), and less likely receipt of cefazolin (12% vs 92%) [21]. Another large multicenter prospective cohort study found that patients with reported allergies who did not receive preferred β-lactam therapy had an adjusted odds ratio of 3 for an adverse event, compared to those without allergy or those that received an alternative β-lactam agent [22]. In a retrospective study at a Veterans Affairs hospital, patients with a penicillin allergy had a significantly longer time to receipt of first antibiotic dose (236 minutes vs 187 minutes, P = .03), and were more likely to receive a carbapenem or fluoroquinolone antibiotic (P < .00001), which may have implications on selection of more antimicrobial resistance or C. difficile infections [23]. From an economic standpoint, a recent comprehensive meta-analysis showed that reported penicillin-allergic patients had higher outpatient and inpatient drug costs and inpatient hospitalizations that cost on average $1145–$4254 more per patient [24].
In summary, consistent data have emerged pointing toward possible shortcomings in clinical outcomes and medical care costs when utilizing non-β-lactam antibiotics in patients with penicillin allergies. These studies are not definitive and cannot rule out the possibility that penicillin allergy may be a surrogate marker of a suboptimal response to infection. Until more definitive clinical or immunological studies can be done, another approach is to determine if β-lactams offer any additional adjunctive properties that are not seen in other antibiotic classes. These are discussed in the next section.
INDIRECT ANTIMICROBIAL PROPERTIES OF β-LACTAMS: BEYOND THE STANDARD MINIMUM INHIBITORY CONCENTRATION
Delay in therapy as discussed above may be one contributing factor toward inferior outcomes in patients with penicillin allergies [23]. However, significant data have emerged in the last few years indicating that β-lactams display unique antibacterial properties that are not appreciated in our standard in vitro susceptibility testing assays. Antistaphylococcal β-lactams (eg. nafcillin, oxacillin, flucloxacillin) were for many years considered clinically irrelevant against MRSA due to absence of activity in standard minimum inhibitory concentration (MIC) testing in bacteriologic media such as Mueller-Hinton broth. However, these agents have recently been shown to render MRSA more vulnerable to killing by antimicrobial peptides and other components of the innate immune system [25, 26]. These effects have recently been employed in combinatorial treatment regimens against MRSA, wherein reinstitution of various β-lactam drugs with either daptomycin or vancomycin led to more rapid bloodstream clearance and successful salvage of refractory MRSA bacteremia [25–28]. Similar effects were identified with ampicillin-resistant VRE, wherein the addition of ampicillin, which alone demonstrated no activity, markedly potentiated the activity of daptomycin and host innate peptides in the killing of the pathogen [29]. Ceftaroline and other β-lactams exhibit similar properties for sensitizing VRE to cationic peptides and immune clearance [30, 31]. VRE isolates with a daptomycin MIC of 4 mg/L, considered susceptible under the current Clinical and Laboratory Standards Institute breakpoint, have been associated with increases in daptomycin treatment failure in bacteremia, stimulating calls to lower the daptomycin enterococcal susceptibility breakpoint to ≤2 mg/L [32]. β-Lactam plus daptomycin combination therapy restores clinical success rates in VRE bacteremia against isolates with daptomycin MIC of 4 mg/L to rates similar to those seen for isolates with lower MICs [33].
Cationic antimicrobial peptides such as cathelicidins, defensins, and platelet microbicidal proteins (PMPs) are key front-line elements of human innate immune defense against systemic infection [34]. Bacterial strains may evolve resistance to bactericidal activity of these peptides, sometimes as an unintended consequence of exposure to structurally similar pharmaceutical antibiotics that we administer [35], or simply by chronic persistence in vivo whereby they may be exposed to the peptides at sublethal doses, for example, in high-inoculum infections with poor surgical source control [36, 37]. Resistance to these peptides may impact clinical outcomes. For example, PMP resistance in staphylococci and streptococci is associated with endocarditis and metastatic infection [38–40], and MRSA isolates from patients with persistent bacteremia were more resistant to PMP killing than those from resolved bacteremia [41]. It appears that β-lactams, by enhancing killing of S. aureus, Enterococcus species, and potentially other organisms, provide a means by which bacteremia may be cleared more efficiently by boosting the activity of cationic peptides of the innate immune system or, when used in a combination regimen, potentiation of cell wall–active antibiotics such as daptomycin or vancomycin.
It is beyond the scope of this article to discuss recognition of S. aureus and other bacteria by innate immunity; thus, we refer readers to comprehensive reviews on the subject [42]. A poor proinflammatory cytokine response by leukocytes exposed to S. aureus is associated with worse outcomes in mice challenged with S. aureus [43]. Rose et al examined cytokine expression on the day of clinical presentation in patients with S. aureus bloodstream infection, and found that a low interleukin 1β (IL-1β) response was a significant marker for persistent bacteremia, whereas elevated concentrations of IL-10 was a predictor of increased patient mortality [44]. IL-1β is a potent inducer of T lymphocytes and neutrophils and augments the production of several proinflammatory cytokines in response to S. aureus and other pathogens [42, 45]. Recent work suggests that β-lactams may enhance S. aureus expression of its pore-forming α-toxin [46, 47], modulate O-acetyl transferase-mediated lysozyme resistance [48, 49], or influence other exotoxin expression through PBP1 binding [50]. These steps may enhance inflammasome activation and host IL- 1β production, thereby promoting more rapid bacterial clearance from the bloodstream. Furthermore, MRSA bacterial cell wall synthesized in the presence of β-lactams exhibits a reduction in cross-linking and generates a stronger IL-1β response by macrophages [51]. Enhanced IL- 1β production may exacerbate inflammation and clinical symptomsin certain exotoxin- driven S. aureus infections (eg, pneumonia, soft tissue infection). However, scientific data suggests that the β-lactam induction of IL- 1β signaling may counteract establishment of infection or enhance clearance of endovascular S. aureus infection [52]. Indeed, increased expression of α- toxin, a known IL- 1β inducer, attenuates S. aureus virulence in a rabbit endocarditis model [53].
Another key adjunctive property of β-lactams in MRSA treatment is synergy with cationic host defense peptides that is not seen with vancomycin [25]. In line with these immunological synergies, MRSA grown in 1/50th MIC of nafcillin showed significant reduction in skin lesions when injected subcutaneously into mice, in contrast to untreated controls or MRSA grown in 1/4th MIC of vancomycin [25]. Compared to cefazolin, use of vancomycin as surgical prophylaxis increases the risk of MSSA surgical infection. Conversely, vancomycin may not outperform cefazolin in MRSA prophylaxis [54], although a meta-analysis on this topic that shows a benefit of vancomycin as surgical prophylaxis in preventing MRSA postoperative infection leaves this topic controversial [55].
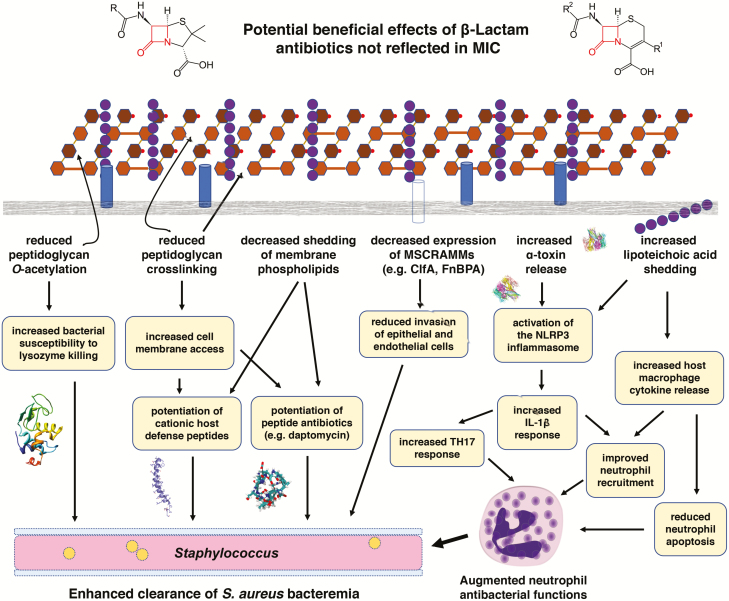
Laboratory science is building evidence that β-lactams play an important role as “immunoadjuvants” in addition to antibiotics in S. aureus bacteremia. A summary diagram demonstrating the mechanisms of enhancement of immune system killing of S. aureus by β-lactams, including what was discussed above [44, 56–59], is depicted schematically in Figure 1. Patients who receive non–β-lactam antibiotics in response to purported penicillin allergy may be missing out on these significant effects that enhance treatment or prevent infection, as reflected in the negative clinical and medical economic data associated with penicillin-allergic patients reviewed above.
Figure 1.
β-Lactams exhibit conservable impacts on the relationship between bacterial pathogens and the human host. This has been studied in detail with Staphylococcus aureus, whereby the effects on bacteria render them more vulnerable to clearance by the innate immune system through multiple mechanisms outlined above. Abbreviations: ClfA, clumping factor A; FnBPA, fibronectin binding protein A; IL-1β, interleukin 1β; MIC, minimum inhibitory concentration; MSCRAMMs, microbial surface components recognizing adhesive matrix molecules; TH17, T-helper 17 cell.
It is important to point out that β-lactams are not the “be-all and end-all” of antimicrobial agents with respect to host inflammation. As β-lactams induce α-toxin and other exotoxin expression to enhance IL-1β (a benefit in bacteremia), the same property may generate more local tissue inflammation and thereby more damage and worse clinical outcomes in infection types driven by exotoxin expression, such as pneumonia [60] and soft tissue infection [61]. This rationale is behind the recommendation of concomitant β-lactam and clindamycin therapy for extreme examples of these types of infections such as necrotizing fasciitis, presumably whereby the clindamycin reduces superantigen expression induced by the β-lactams. These types of studies make it clear that the assessment of antimicrobial therapy likely needs to extend to including pharmacodynamic interactions not just of drugs between each other, but also drugs with the innate immune system.
ALTERNATIVE β-LACTAM OPTIONS: CROSS-ALLERGIES ACROSS THE β-LACTAM SUBCLASSES
In patients with an unconfirmed penicillin allergy, cross-reactivity to other β-lactams has been a historical concern. While early research incorrectly implied a penicillin-cephalosporin cross-reactivity of up to 10%, this is now recognized to be a gross overestimation [62]. Unfortunately, these false data have populated medical practice, resulting in the propagation of the 10% cross-allergy myth across decades. In contrast, more careful recent studies demonstrate clinically significant immunologically mediated cross-reactivity of <1% [63–67]. These low rates have been incorporated into guidelines within the anaphylaxis practice parameter. They state that approximately 4% of patients who have positive penicillin skin tests also react to cephalosporins, whereas patients who have negative penicillin skin tests can receive cephalosporins without allergy concerns [68].
Penicillins and carbapenems both have a bicyclic core rendering them similar in structure. The bicyclic core is composed of a 5-membered ring, which is attached to the β-lactam ring. Since the inception of the first carbapenem drug approval, there has been a theoretical concern for cross-reactivity of a drug in this class in a patient with a verified history of a penicillin allergy. As with cephalosporins, early studies overestimated the cross-allergy risk between penicillin, while more recent studies place the risk at 0.9%–11%. Currently, there are experts in the field stating that the avoidance of carbapenem use in patients with a history of penicillin allergy should be reconsidered [69]. In 2015, researchers tested patients with a positive penicillin skin test with 3 different carbapenems (imipenem-cilastatin, meropenem, and ertapenem) in 211 subjects [70]. None tested positive to any of the carbapenems. A subsequent study examining the cross-allergy of a confirmed penicillin immunoglobulin E (IgE)–mediated hypersensitivity to ertapenem found the cross-reactivity was only 1 of 36 (2.8%) to full-dose systemic exposure to ertapenem [71]. These authors concluded that the practice of avoiding carbapenems in patients with β-lactam allergy histories should now be abandoned.
With respect to monobactams (ie, aztreonam), the risk of cross-allergy to penicillin appears to be negligible. A 2016 study tested 214 subjects who had a positive penicillin skin test (T-cell–mediated hypersensitivity) with an aztreonam skin test [71]. No subjects had a positive skin test to aztreonam. One hundred seventy of the patients (all with negative aztreonam skin tests) accepted to be further challenged with systemic exposure. No subjects had clinical signs or symptoms of an adverse drug reaction [72].
Consistent with cephalosporins, immunological studies indicate that the side chain of aztreonam determines cross-reactivity, rather than the monobactam ring itself [73]. Of note, the aztreonam side-chain is identical to the side-chain of ceftazidime. Hence, the risk of cross-reactivity between aztreonam and ceftazidime is predicted to be much higher than the other cephalosporins and penicillin derivatives [73]. Consequently, clinical practice of using aztreonam in patients with a history of penicillin allergy is supported by the joint Task Force on Practice Parameters [68], stating concern only for patients with ceftazidime allergies.
THE ROLE OF PENICILLIN ALLERGY TESTING IN ANTIMICROBIAL STEWARDSHIP
Although it is common for a patient or family member to declare a drug allergy, treating clinicians must verify the allergy by taking a thorough history and, if deemed necessary, test for a type 1 reaction. Often, obtaining a thorough history by interviewing the patient has proven effective in removing the purported allergy from the patient’s medical profile [65, 74].
The current reference standard test to confirm acute penicillin-class antibiotic tolerance is an oral amoxicillin 250 mg challenge with 1 hour of observation. Delayed-onset tolerance is confirmed by the lack of a rash over the next 5 days. Direct oral challenges have been safely used in children and adults with low-risk histories [75].
Skin tests may also be used to diagnose a type I (immediate) IgE-mediated drug hypersensitivity, especially in the inpatient setting. However, skin testing, prior to an oral challenge, is often not necessary to safely rule out type I (immediate) IgE-mediated hypersensitivity in individuals with low-risk drug allergy histories. Some clinicians in the acute care setting are opposed to taking the time for confirmation of tolerance testing, because it may delay initiation of antibiotics. In individuals with a low-risk history, taking 1 hour to confirm tolerance may be an hour well spent [63, 76–78].
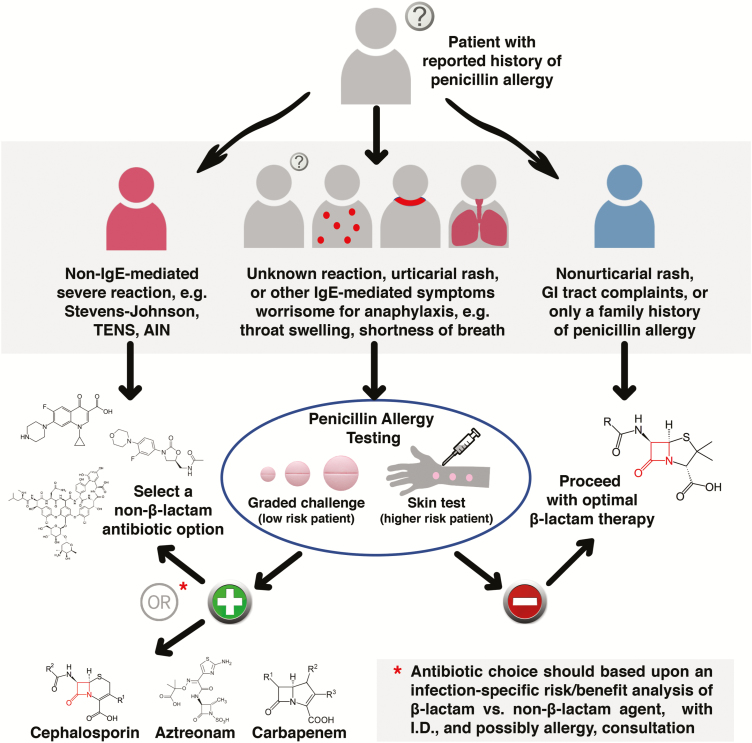
Based on the evidence favoring β-lactam therapy for some serious infections (eg, S. aureus bacteremia), poor outcomes in patients with purported penicillin allergies, and the fact that purported allergies are rarely true allergies, we advocate a combinatorial scheme of a detailed history algorithm supplemented with penicillin allergy testing, as was conducted by Ramsey and Staicu in their recent study [79]. Figure 2 outlines an approach to the hospitalized patient with penicillin allergy requiring antimicrobial therapy. An initial history stratifies patients into those in whom a purported allergy is dismissed by history alone (eg, gastrointestinal complaints, candidiasis), those in whom a documented life-threatening non-IgE-mediated reaction eliminates β-lactam use (eg, Stevens-Johnson toxic epidermal necrolysis syndrome, acute interstitial nephritis), and those in whom penicillin testing can help delineate therapy. For the latter group, a negative allergy test (anticipated for the majority, especially if the urticarial rash history is remote) would place them into the nonallergy group, where they stand to receive the greatest benefit from this intervention.
Figure 2.
A proposed algorithm for approaching hospitalized patients with purported penicillin allergy, using a combination of detailed clinical history and skin testing. Abbreviations: AIN, acute interstitial nephritis; GI, gastrointestinal; ID, infectious diseases; IgE, immunoglobulin G; TENS, toxic epidermal necrolysis syndrome.
Complex clinical decision making occurs in patients who test skin positive but have more severe infections where β-lactams have proven advantageous. While those patients would not be challenged with penicillins, except in rare situations where there would be no other therapeutic options and therefore must be dealt with through desensitization, the low likelihood of cross-allergy to other β-lactam groups would have to be weighed against the significant benefit of using this group of drugs. A very common example where this would arise would be the potential choice of cefazolin instead of vancomycin for MSSA bacteremia. The clinical advantages of cefazolin over vancomycin would likely outweigh the <5% risk of cross-allergy, especially in patients with complex infections. Daptomycin would be an alternative that appears to offer similar outcomes to β-lactam therapy, but is currently considerably more costly [18]. Such cases should be handled with the assistance of an infectious disease consultant, and perhaps with further input from an allergy-immunology specialist, especially in light of emerging data that this approach markedly improves outcomes [80, 81].
In sum, a coordinated and resolute approach to de-label false allergies, with the assistance of infectious disease physicians and infectious disease pharmacists, may lead to improved patient outcomes and reduction in healthcare costs [82, 83].
Notes
Financial support. This research was funded by the National Institutes of Health (grant numbers 1U54HD090259 and 1U01AI124316 to G. S. and V. N.) and supported by Sharp Healthcare (San Diego, CA).
Potential conflicts of interest. G. S. has received speaking honoraria from Allergan, Sunovion, and The Medicines Company; has received consulting fees from Allergan and Paratek Pharmaceuticals; and is on the scientific advisory board of Cidara Therapeutics and Arsanis Pharmaceuticals. V. N. has received research grant support from Roche Pharma and is on the scientific advisory board of Cidara Therapeutics and InhibRx. M. G. reports no potential conflicts of interest. All authors have submitted the ICMJE Form for Disclosure of Potential Conflicts of Interest. Conflicts that the editors consider relevant to the content of the manuscript have been disclosed.
References
- 1. Aminov RI. A brief history of the antibiotic era: lessons learned and challenges for the future. Frontiers in Microbiol 2010; 1:1–7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2. Macy E. The clinical evaluation of penicillin allergy: what is necessary, sufficient and safe given the materials currently available?Clin Exp Allergy 2011; 41:1498–501. [DOI] [PubMed] [Google Scholar]
- 3. Solensky R. Hypersensitivity reactions to beta-lactam antibiotics. Clin Rev Allergy Immunol 2003; 24:201–20. [DOI] [PubMed] [Google Scholar]
- 4. Joint Task Force on Practice Parameters; American Academy of Allergy, Asthma and Immunology; American College of Allergy, Asthma, and Immunology; Joint Council of Allergy, Asthma and Immunology. Drug allergy: an updated practice parameter. Ann Allergy Asthma Immunol 2010; 105:259–73. [DOI] [PubMed] [Google Scholar]
- 5. Macy E, Ngor EW. Safely diagnosing clinically significant penicillin allergy using only penicilloyl-poly-lysine, penicillin, and oral amoxicillin. J Allergy Clin Immunol Pract 2013; 1:258–63. [DOI] [PubMed] [Google Scholar]
- 6. Macy E, Schatz M, Lin C, Poon KY. The falling rate of positive penicillin skin tests from 1995 to 2007. Perm J 2009; 13:12–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Renn CN, Straff W, Dorfmüller A, Al-Masaoudi T, Merk HF, Sachs B. Amoxicillin-induced exanthema in young adults with infectious mononucleosis: demonstration of drug-specific lymphocyte reactivity. Br J Dermatol 2002; 147:1166–70. [DOI] [PubMed] [Google Scholar]
- 8. Macy E, Contreras R. Health care use and serious infection prevalence associated with penicillin “allergy” in hospitalized patients: a cohort study. J Allergy Clin Immunol 2014; 133:790–6. [DOI] [PubMed] [Google Scholar]
- 9. Solensky R. Penicillin allergy as a public health measure. J Allergy Clin Immunol 2014; 133:797–8. [DOI] [PubMed] [Google Scholar]
- 10. Schweizer ML, Furuno JP, Harris AD, et al. . Comparative effectiveness of nafcillin or cefazolin versus vancomycin in methicillin-susceptible Staphylococcus aureus bacteremia. BMC Infect Dis 2011; 11:279. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Li J, Echevarria KL, Hughes DW, Cadena JA, Bowling JE, Lewis JS 2nd. Comparison of cefazolin versus oxacillin for treatment of complicated bacteremia caused by methicillin-susceptible Staphylococcus aureus. Antimicrob Agents Chemother 2014; 58:5117–24. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Kim SH, Kim KH, Kim HB, et al. . Outcome of vancomycin treatment in patients with methicillin-susceptible Staphylococcus aureus bacteremia. Antimicrob Agents Chemother 2008; 52:192–7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Chang FY, Peacock JE Jr, Musher DM, et al. . Staphylococcus aureus bacteremia: recurrence and the impact of antibiotic treatment in a prospective multicenter study. Medicine (Baltimore) 2003; 82:333–9. [DOI] [PubMed] [Google Scholar]
- 14. Chan KE, Warren HS, Thadhani RI, et al. . Prevalence and outcomes of antimicrobial treatment for Staphylococcus aureus bacteremia in outpatients with ESRD. J Am Soc Nephrol 2012; 23:1551–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Stryjewski ME, Szczech LA, Benjamin DK Jr, et al. . Use of vancomycin or first-generation cephalosporins for the treatment of hemodialysis-dependent patients with methicillin-susceptible Staphylococcus aureus bacteremia. Clin Infect Dis 2007; 44:190–6. [DOI] [PubMed] [Google Scholar]
- 16. McDanel JS, Perencevich EN, Diekema DJ, et al. . Comparative effectiveness of beta-lactams versus vancomycin for treatment of methicillin-susceptible Staphylococcus aureus bloodstream infections among 122 hospitals. Clin Infect Dis 2015; 61:361–7. [DOI] [PubMed] [Google Scholar]
- 17. Cosgrove S, Sakoulas G, Perencevich EN, Schwaber MJ, Karchmer AW, Carmeli Y. Mortality related to methicillin-resistant Staphylococcus aureus compared to methicillin-susceptible S. aureus: a meta-analysis. Clin Infect Dis 2003; 36:53–9. [DOI] [PubMed] [Google Scholar]
- 18. Fowler VG Jr, Boucher HW, Corey GR, et al. . S. aureus Endocarditis and Bacteremia Study Group Daptomycin versus standard therapy for bacteremia and endocarditis caused by Staphylococcus aureus. N Engl J Med 2006; 355:653–65. [DOI] [PubMed] [Google Scholar]
- 19. Blumenthal KG, Parker RA, Shenoy ES, Walensky RP. Improving clinical outcomes in patients with methicillin-sensitive Staphylococcus aureus bacteremia and reported penicillin allergy. Clin Infect Dis 2015; 61:741–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Blumenthal KG, Shenoy ES, Huang M, et al. . The impact of reporting a prior penicillin allergy on the treatment of methicillin-sensitive Staphylococcus aureus bacteremia. PLoS One 2016; 11:e0159406. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. Blumenthal KG, Ryan EE, Li Y, Lee H, Kuhlen JL, Shenoy ES. The impact of a reported penicillin allergy on surgical site infection risk. Clin Infect Dis 2017; 66:329–36. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. MacFadden DR, LaDelfa A, Leen J, et al. . Impact of reported beta-lactam allergy on inpatient outcomes: a multicenter prospective cohort study. Clin Infect Dis 2016; 63:904–10. [DOI] [PubMed] [Google Scholar]
- 23. Conway EL, Lin K, Sellick JA, et al. . Impact of penicillin allergy on time to first dose of antimicrobial therapy and clinical outcomes. Clin Ther 2017; 39:2276–83. [DOI] [PubMed] [Google Scholar]
- 24. Mattingly TJ, Fulton A, Lumish RA, et al. . The cost of self-reported penicillin allergy: a systematic review. J Allergy Clin Immunol Pract 2018. doi: 10.1016/j.jaip.2017.12.033. [DOI] [PubMed] [Google Scholar]
- 25. Sakoulas G, Okumura CY, Thienphrapa W, et al. . Nafcillin enhances innate immune-mediated killing of methicillin-resistant Staphylococcus aureus. J Mol Med (Berl) 2014; 92:139–49. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26. Dhand A, Bayer AS, Pogliano J, et al. . Use of antistaphylococcal beta-lactams to increase daptomycin activity in eradicating persistent bacteremia due to methicillin-resistant Staphylococcus aureus: role of enhanced daptomycin binding. Clin Infect Dis 2011; 53:158–63. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27. Davis JS, Sud A, O’Sullivan MVN, Robinson JO, et al. . Combination antibiotics for methicillin resistant Staphylococcus aureus (CAMERA) study group; combination of vancomycin and β-lactam therapy for methicillin-resistant Staphylococcus aureus bacteremia: a pilot multicenter randomized controlled trial. Clin Infect Dis 2016; 62:173–80. [DOI] [PubMed] [Google Scholar]
- 28. Sakoulas G, Moise PA, Casapao AM, et al. . Antimicrobial salvage therapy for persistent staphylococcal bacteremia using daptomycin plus ceftaroline. Clin Ther 2014; 36:1317–33. [DOI] [PubMed] [Google Scholar]
- 29. Sakoulas G, Bayer AS, Pogliano J, et al. . Ampicillin enhances daptomycin- and cationic host defense peptide-mediated killing of ampicillin- and vancomycin-resistant Enterococcus faecium. Antimicrob Agents Chemother 2012; 56:838–44. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30. Sakoulas G, Rose W, Nonejuie P, et al. . Ceftaroline restores daptomycin activity against daptomycin-nonsusceptible vancomycin-resistant Enterococcus faecium. Antimicrob Agents Chemother 2014; 58:1494–500. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31. Smith JR, Barber KE, Raut A, Aboutaleb M, Sakoulas G, Rybak MJ. β-Lactam combinations with daptomycin provide synergy against vancomycin-resistant Enterococcus faecalis and Enterococcus faecium. J Antimicrob Chemother 2015; 70:1738–43. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32. Shukla BS, Shelburne S, Reyes K, et al. . Influence of minimum inhibitory concentration in clinical outcomes of Enterococcus faecium bacteremia treated with daptomycin: is it time to change the breakpoint?Clin Infect Dis 2016; 62:1514–20. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33. Moise PA, Sakoulas G, McKinnell JA, et al. . Effects of concomitant beta-lactam antibiotics on daptomycin treatment outcomes in vancomycin-resistant Enterococcus bacteremia. Clin Ther 2015; 37:1443– 53. [DOI] [PubMed] [Google Scholar]
- 34. Nizet V. Antimicrobial peptide resistance mechanisms of human bacterial pathogens. Curr Issues Mol Biol 2006; 8:11–26. [PubMed] [Google Scholar]
- 35. Sakoulas G, Eliopoulos GM, Fowler VG Jr, et al. . Reduced susceptibility of Staphylococcus aureus to vancomycin and platelet microbicidal protein correlates with defective autolysis and loss of accessory gene regulator (agr) function. Antimicrob Agents Chemother 2005; 49:2687–92. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36. Kullar R, McKinnell JA, Sakoulas G. Avoiding the perfect storm: the biologic and clinical case for reevaluating the 7-day expectation for methicillin-resistant Staphylococcus aureus bacteremia before switching therapy. Clin Infect Dis 2014; 59:1455–61. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37. Mishra NN, Yang SJ, Chen L, et al. . Emergence of daptomycin resistance in daptomycin-naive rabbits with methicillin-resistant Staphylococcus aureus prosthetic joint infection is associated with resistance to host defense cationic peptides and mprF polymorphisms. PLoS One 2013; 8:e71151. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38. Wu T, Yeaman MR, Bayer AS. In vitro resistance to platelet microbicidal protein correlates with endocarditis source among bacteremic staphylococcal and streptococcal isolates. Antimicrob Agents Chemother 1994; 38:729–32. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39. Bayer AS, Cheng D, Yeaman MR, et al. . In vitro resistance to thrombin-induced platelet microbicidal protein among clinical bacteremic isolates of Staphylococcus aureus correlates with an endovascular infectious source. Antimicrob Agents Chemother 1998; 42:3169–72. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40. Fowler VG Jr, Sakoulas G, McIntyre LM, et al. . Persistent bacteremia due to methicillin-resistant Staphylococcus aureus infection is associated with agr dysfunction and low-level in vitro resistance to thrombin-induced platelet microbicidal protein. J Infect Dis 2004; 190:1140–9. [DOI] [PubMed] [Google Scholar]
- 41. Mishra NN, Bayer AS, Moise PA, Yeaman MR, Sakoulas G. Reduced susceptibility to host-defense cationic peptides and daptomycin coemerge in methicillin-resistant Staphylococcus aureus from daptomycin-naive bacteremic patients. J Infect Dis 2012; 206:1160–7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42. Thammavongsa V, Kim HK, Missiakas D, Schneewind O. Staphylococcal manipulation of host immune responses. Nat Rev Microbiol 2015; 13:529–43. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43. Kielian T, Bearden ED, Baldwin AC, Esen N. IL-1 and TNF-alpha play a pivotal role in the host immune response in a mouse model of Staphylococcus aureus-induced experimental brain abscess. J Neuropathol Exp Neurol 2004; 63:381–96. [DOI] [PubMed] [Google Scholar]
- 44. Rose WE, Eickhoff JC, Shukla SK, et al. . Elevated serum interleukin-10 at time of hospital admission is predictive of mortality in patients with Staphylococcus aureus bacteremia. J Infect Dis 2012; 206:1604–11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45. Miller LS, Pietras EM, Uricchio LH, et al. . Inflammasome-mediated production of IL-1beta is required for neutrophil recruitment against Staphylococcus aureus in vivo. J Immunol 2007; 179:6933–42. [DOI] [PubMed] [Google Scholar]
- 46. Stevens DL, Ma Y, Salmi DB, McIndoo E, Wallace RJ, Bryant AE. Impact of antibiotics on expression of virulence-associated exotoxin genes in methicillin-sensitive and methicillin-resistant Staphylococcus aureus. J Infect Dis 2007; 195:202–11. [DOI] [PubMed] [Google Scholar]
- 47. Craven RR, Gao X, Allen IC, et al. . Staphylococcus aureus alpha-hemolysin activates the NLRP3-inflammasome in human and mouse monocytic cells. PLoS One 2009; 4:e7446. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48. Qoronfleh MW, Wilkinson BJ. Effects of growth of methicillin-resistant and -susceptible Staphylococcus aureus in the presence of beta-lactams on peptidoglycan structure and susceptibility to lytic enzymes. Antimicrob Agents Chemother 1986; 29:250–7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49. Shimada T, Park BG, Wolf AJ, et al. . Staphylococcus aureus evades the lysozyme-based digestion of peptidoglycan that links phagocytosis and macrophage IL-1beta secretion. Cell Host Microb 2010; 21:38. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50. Dumitrescu O, Choudhury P, Boisset S, et al. . Beta-lactams interfering with PBP1 induce Panton-Valentine leukocidin expression by triggering sarA and rot global regulators of Staphylococcus aureus. Antimicrob Agents Chemother 2011; 55:3261–71. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51. Müller S, Wolf AJ, Iliev ID, Berg BL, Underhill DM, Liu GY. Poorly cross-linked peptidoglycan in MRSA due to mecA induction activates the inflammasome and exacerbates immunopathology. Cell Host Microbe 2015; 18:604–12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52. Kebaier C, Chamberland RR, Allen IC, et al. . Staphylococcus aureus α-hemolysin mediates virulence in a murine model of severe pneumonia through activation of the NLRP3 inflammasome. J Infect Dis 2012; 205:807–17. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53. Bayer AS, Ramos MD, Menzies BE, Yeaman MR, Shen AJ, Cheung AL. Hyperproduction of alpha-toxin by Staphylococcus aureus results in paradoxically reduced virulence in experimental endocarditis: a host defense role for platelet microbicidal proteins. Infect Immun 1997; 65:4652–60. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54. Finkelstein R, Rabino G, Mashiah T, et al. . Vancomycin versus cefazolin prophylaxis for cardiac surgery in the setting of a high prevalence of methicillin-resistant staphylococcal infections. J Thorac Cardiovasc Surg 2002; 123:326–32. [DOI] [PubMed] [Google Scholar]
- 55. Schweizer M, Perencevich E, McDanel J, et al. . Effectiveness of a bundled intervention of decolonization and prophylaxis to decrease gram positive surgical site infections after cardiac or orthopedic surgery: systematic review and meta-analysis. BMJ 2013; 346:f2743. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56. Schennings T, Heimdahl A, Coster K, Flock JI. Immunization with fibronectin binding protein from Staphylococcus aureus protects against experimental endocarditis in rats. Microb Pathog 1993; 15:227–36. [DOI] [PubMed] [Google Scholar]
- 57. Rennermalm A, Li YH, Bohaufs L, et al. . Antibodies against a truncated Staphylococcus aureus fibronectin-binding protein protect against dissemination of infection in the rat. Vaccine 2001; 19:3376–83. [DOI] [PubMed] [Google Scholar]
- 58. Vernachio J, Bayer AS, Le T, et al. . Anti-clumping factor A immunoglobulin reduces the duration of methicillin-resistant Staphylococcus aureus bacteremia in an experimental model of infective endocarditis. Antimicrob Agents Chemother 2003; 47:3400–6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59. Ricci-Tam C, Newton GL, Sakoulas G, Nizet V, Pogliano K, Pogliano J. Effects of beta-lactams on the proteome of daptomycin susceptible and nonsusceptible methicillin-resistant Staphylococcus aureus. In: American Society of Microbiology Annual Meeting, Denver, CO, 18–21 May 2013. [Google Scholar]
- 60. Bonesso MF, Yeh AJ, Villaruz AE, et al. . Key role of α-toxin in fatal pneumonia caused by Staphylococcus aureus sequence type 398. Am J Respir Crit Care Med 2016; 193:217–20. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61. Le VT, Tkaczyk C, Chau S, et al. . Acute bacterial skin and skin structure infection: critical role of alpha-toxin and protective effects of its neutralization by a human antibody. Antimicrob Agents Chemother 2016; 60:5640–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62. Herbert ME, Brewster GS, Lanctot-Herbert M. Medical myth: ten percent of patients who are allergic to penicillin will have serious reactions if exposed to cephalosporins. West J Med 2000; 172:341. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63. Albin S, Agarwal S. Prevalence and characteristics of reported penicillin allergy in an urban outpatient adult population. Allergy Asthma Proc 2014; 35:489–94. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64. Goodman EJ, Morgan MJ, Johnson PA, Nichols BA, Denk N, Gold BB. Cephalosporins can be given to penicillin-allergic patients who do not exhibit an anaphylactic response. J Clin Anesth 2001; 13:561–4. [DOI] [PubMed] [Google Scholar]
- 65. Daulat S, Solensky R, Earl HS, Casey W, Gruchalla RS. Safety of cephalosporin administration to patients with histories of penicillin allergy. J Allergy Clin Immunol 2004; 113:1220–2. [DOI] [PubMed] [Google Scholar]
- 66. Park MA, Koch CA, Klemawesch P, Joshi A, Li JT. Increased adverse drug reactions to cephalosporins in penicillin allergy patients with positive penicillin skin test. Int Arch Allergy Immunol 2010; 153:268–73. [DOI] [PubMed] [Google Scholar]
- 67. Ahmed KA, Fox SJ, Frigas E, Park MA. Clinical outcome in the use of cephalosporins in pediatric patients with a history of penicillin allergy. Int Arch Allergy Immunol 2012; 158:405–10. [DOI] [PubMed] [Google Scholar]
- 68. Lieberman P, Nicklas RA, Oppenheimer J, et al. . The diagnosis and management of anaphylaxis practice parameter: 2010 update. J Allergy Clin Immunol 2010; 126:477–80.e1–42. [DOI] [PubMed] [Google Scholar]
- 69. Frumin J, Gallagher JC. Allergic cross-sensitivity between penicillin, carbapenem, and monobactam antibiotics: what are the chances?Ann Pharmacother 2009; 43:304–15. [DOI] [PubMed] [Google Scholar]
- 70. Gaeta F, Valluzzi RL, Alonzi C, Maggioletti M, Caruso C, Romano A. Tolerability of aztreonam and carbapenems in patients with IgE-mediated hypersensitivity to penicillins. J Allergy Clin Immunol 2015; 135:972–6. [DOI] [PubMed] [Google Scholar]
- 71. Buonomo A, Pascolini L, Rizzi A, et al. . Cross-reactivity and tolerability of ertapenem in patients with IgE-mediated hypersensitivity to β-Lactams. J Investig Allergol Clin Immunol 2016; 26:100–5. [DOI] [PubMed] [Google Scholar]
- 72. Romano A, Gaeta F, Valluzzi RL, Maggioletti M, Caruso C, Quaratino D. Cross-reactivity and tolerability of aztreonam and cephalosporins in subjects with a T cell-mediated hypersensitivity to penicillins. J Allergy Clin Immunol 2016; 138:179–86. [DOI] [PubMed] [Google Scholar]
- 73. Adkinson NF Jr, Swabb EA, Sugerman AA. Immunology of the monobactam aztreonam. Antimicrob Agents Chemother 1984; 25:93–7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74. Torda A, Chan V. Antibiotic allergy labels-the impact of taking a clinical history. Int J Clin Pract 2018; 72:e13058. [DOI] [PubMed] [Google Scholar]
- 75. Mill C, Primeau MN, Medoff E, et al. . Assessing the diagnostic properties of a graded oral provocation challenge for the diagnosis of immediate and nonimmediate reactions to amoxicillin in children. JAMA Pediatr 2016; 170:e160033. [DOI] [PubMed] [Google Scholar]
- 76. Leis JA, Palmay L, Ho G, et al. . Point-of-care β-lactam allergy skin testing by antimicrobial stewardship programs: a pragmatic multicenter prospective evaluation. Clin Infect Dis 2017; 65:1059–65. [DOI] [PubMed] [Google Scholar]
- 77. del Real GA, Rose ME, Ramirez-Atamoros MT, et al. . Penicillin skin testing in patients with a history of beta-lactam allergy. Ann Allergy Asthma Immunol 2007; 98:355–9. [DOI] [PubMed] [Google Scholar]
- 78. King EA, Challa S, Curtin P, Bielory L. Penicillin skin testing in hospitalized patients with β-lactam allergies: effect on antibiotic selection and cost. Ann Allergy Asthma Immunol 2016; 117:67–71. [DOI] [PubMed] [Google Scholar]
- 79. Ramsey A, Staicu ML. Use of a penicillin allergy screening algorithm and penicillin skin testing for transitioning hospitalized patients to first-line antibiotic therapy. J Allergy Clin Immunol Pract 2017. doi: 10.1016/j.jaip.2017.11.012. [DOI] [PubMed] [Google Scholar]
- 80. Vogel M, Schmitz RP, Hagel S, et al. . Infectious disease consultation for Staphylococcus aureus bacteremia—a systematic review and meta-analysis. J Infect 2016; 72:19–28. [DOI] [PubMed] [Google Scholar]
- 81. Bai AD, Showler A, Burry L, et al. . Impact of infectious disease consultation on quality of care, mortality, and length of stay in Staphylococcus aureus bacteremia: results from a large multicenter cohort study. Clin Infect Dis 2015; 60:1451–61. [DOI] [PubMed] [Google Scholar]
- 82. Beekman SE, Worth LJ, Polgreen PM, et al. . Improving antimicrobial stewardship by antibiotic allergy delabeling: evaluation of knowledge, attitude, and practices through the emerging infections network. Open Forum Infect Dis 2016; 3:ofw153. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 83. Sigona NS, Steele JM, Miller CD. Impact of a pharmacist-driven beta-lactam allergy interview on inpatient antimicrobial therapy: a pilot project. J Am Pharm Assoc (2003) 2016; 56:665–9. [DOI] [PubMed] [Google Scholar]