We compared stool toxin concentrations in adult inpatients with symptomatic Clostridioides difficile infection (CDI) vs asymptomatic carriage. Median concentrations differed only when CDI was defined by detectable stool toxin (vs positive nucleic acid amplification test); concentration did not differentiate an individual with CDI from a carrier.
Keywords: C. difficile, toxin, Simoa, carriage, diarrhea
Abstract
Background
We used an ultrasensitive, quantitative single molecule array (Simoa) immunoassay to test whether concentrations of Clostridioides (formerly Clostridium) difficile toxins A and/or B in the stool of adult inpatients with C. difficile infection (CDI) were higher than in asymptomatic carriers of toxinogenic C. difficile.
Methods
Patients enrolled as CDI-NAAT had clinically significant diarrhea and a positive nucleic acid amplification test (NAAT), per US guidelines, and received CDI treatment. Potential carriers had recently received antibiotics and did not have diarrhea; positive NAAT confirmed carriage. Baseline stool samples were tested by Simoa for toxin A and B.
Results
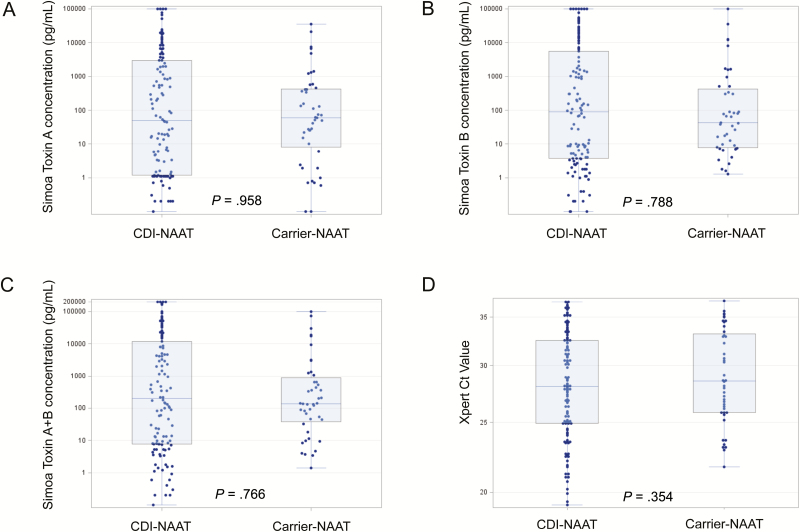
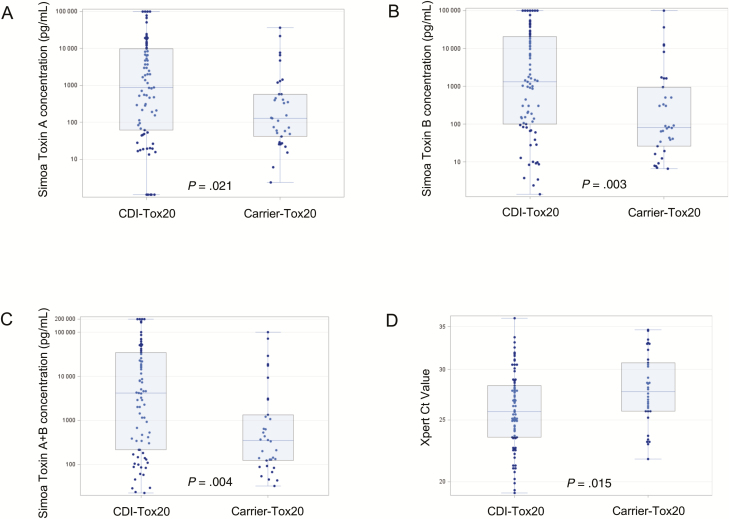
Stool toxin concentrations in both CDI-NAAT (n = 122) and carrier-NAAT (n = 44) cohorts spanned 5 logs (0 pg/mL to >100000 pg/mL). Seventy-nine of 122 (65%) CDI-NAAT and 34 of 44 (77%) carrier-NAAT had toxin A + B concentration ≥20 pg/mL (clinical cutoff). Median toxin A, toxin B, toxin A + B, and NAAT cycle threshold (Ct) values in CDI-NAAT and carrier-NAAT cohorts were similar (toxin A, 50.6 vs 60.0 pg/mL, P = .958; toxin B, 89.5 vs 42.3 pg/mL, P = .788; toxin A + B, 197.2 vs 137.3 pg/mL, P = .766; Ct, 28.1 vs 28.6, P = .354). However, when CDI/carrier cohorts were limited to those with detectable toxin, respective medians were significantly different (A: 874.0 vs 129.7, P = .021; B: 1317.0 vs 81.7, P = .003, A + B, 4180.7 vs 349.6, P = .004; Ct, 25.8 vs 27.7, P = .015).
Conclusions
Toxin concentration did not differentiate an individual with CDI from one with asymptomatic carriage. Median stool toxin concentrations in groups with CDI vs carriage differed, but only when groups were defined by detectable stool toxin (vs positive NAAT).
The international medical community has not reached consensus regarding optimal strategies for diagnosis of Clostridioides (formerly Clostridium) difficile infection (CDI) [1]. The wide variability in clinical presentation and outcomes of CDI (ranging from mild diarrhea to severe colitis and death) [2], combined with the potential for asymptomatic carriage (presence of toxinogenic C. difficile in the colon but no symptoms of CDI), have generated considerable diagnostic confusion. Despite decades of investigation and debate regarding the relative merits of tests capable of detecting either toxinogenic C. difficile bacteria (eg, culture or nucleic acid amplification test [NAAT]) or the toxins it can produce (cytotoxicity assay [CTA] or enzyme immunoassay [EIA]) [2], it remains unclear which tests or algorithmic combinations of tests provide highest diagnostic utility for CDI [1, 3]. Meanwhile, CDI marches forward as a top etiology of nosocomial bacterial infection in the United States, with high cumulative morbidity, mortality, and cost [3, 4].
Some experts recommend prioritizing toxin detection to guide treatment decisions, based on data suggesting that patients with detectable toxin in stool (compared to patients whose stool is NAAT positive for a toxin gene, but is toxin negative) are at highest risk for poor outcomes [5, 6]. However, current toxin detection tests are considered either too analytically insensitive (EIA) or too cumbersome and slow (CTA) to provide optimal utility. Importantly, the arguments in support of the prognostic value of toxin detection are confounded by studies that show that toxin can sometimes be detected in the stool of asymptomatic individuals [7, 8]. However, no study thus far has, to our knowledge, quantified and directly compared stool toxin concentrations in parallel cohorts of untreated patients who are carriers of (asymptomatic) vs infected with (symptomatic) toxinogenic C. difficile. In part, this knowledge gap remains because there previously was no assay capable of highly sensitive, quantitative, and separate measurements of C. difficile toxins A and B over the necessary concentration ranges.
We have recently developed an ultrasensitive assay for detection and quantification of C. difficile toxins utilizing single molecule array (Simoa) technology [9, 10]. This assay is capable of separately detecting and quantifying C. difficile toxins A and B over a 5-log range of concentrations, starting from an analytical cutoff of approximately 1 pg/mL and clinical cutoff of approximately 20 pg/mL in diluted stool samples. Given the current impasse in the field regarding detection of toxin vs organism, we set out to use a next-generation Simoa assay to directly compare concentrations of toxins A and B in the stool of NAAT-positive adult inpatients with CDI (with or without the presence of detectable toxin) vs those with asymptomatic C. difficile carriage. Our a priori primary hypothesis was that concentrations of C. difficile toxin A and/or B would be higher in the stool of individuals with CDI as compared to symptomless carriers of toxinogenic C. difficile.
METHODS
CDI-NAAT Cohort
Eligible patients were inpatients ≥18 years old with positive clinical stool NAAT result (below), were initiating CDI therapy, and had diarrhea, defined as follows: (1) documentation of ≥3 unformed bowel movements during any 24 hours in the 48 hours before stool collection, OR (2) persistent diarrhea in the 48 hours before stool collection per medical notes. Whenever possible, definition 1 was applied. Assessment for the presence of diarrhea included review of nursing input/output logs for number and consistency of stools, consultation with treating clinicians, and detailed chart review (requiring mention of “diarrhea,” “loose stools,” and/or increased frequency, in notes written by multiple providers). Patients for whom there was any doubt about the presence of diarrhea, or who had chronic diarrhea, were excluded. The diagnostic clinical stool sample (submitted for routine C. difficile testing) was captured as a discarded sample. Patients were excluded if the diagnostic specimen was of insufficient volume or >72 hours old, if they had received CDI treatment for >24 hours prior to stool collection, or if they had a colostomy. Administration of any laxative in the 72 hours prior to sample collection was recorded. Peak white blood cell (WBC) count/creatinine and nadir albumin values within 5 days before to 2 days after stool collection were recorded. Intensive care unit (ICU) admission, colectomy, and death rates were assessed during the 40 days after enrollment.
Asymptomatic Carrier-NAAT Cohort
Eligible patients were inpatients ≥18 years old who were admitted for at least 72 hours, had received at least 1 dose of an antibiotic within the past 7 days, and had absence of diarrhea, defined as no report of diarrhea by patient or nurse in the 48 hours prior to enrollment (absence of diarrhea in the preceding 48 hours was reconfirmed at time of stool specimen receipt, if delayed relative to enrollment.). Assessment for the absence of diarrhea included detailed chart review, review of nursing input/output logs, and conversations with nurses and patients. Patients with ≥2 loose stools within a 24-hour period were excluded; patients with 1 loose stool were included only if providers had recently administered a laxative. Patients were excluded if they were unable to provide a stool sample, had a colostomy, had received oral or intravenous metronidazole, oral vancomycin, oral rifaximin, and/or oral fidaxomicin for >24 hours within the past 7 days, had been diagnosed with CDI in the past 6 months, or had tested negative for C. difficile within the past 7 days. Recording of laxative receipt, laboratory measurements, and outcomes mirrored those for the CDI-NAAT cohort. Subjects were followed for 40 days after stool collection for possible CDI occurrence. Stool samples were collected prospectively under verbal informed consent. NAAT (Xpert C. difficile/Epi) was performed on all samples, and positive samples retained as the carrier-NAAT cohort.
Details of sample handling, Simoa assay, NAAT, cytotoxicity assay, and statistical analysis are shown in Supplementary Materials.
RESULTS
For the CDI-NAAT cohort, 122 samples met all inclusion/exclusion criteria. Forty-four of 382 (11.5%) stool samples from asymptomatic subjects were NAAT positive (carrier-NAAT cohort). The CDI-NAAT and carrier-NAAT cohorts were demographically similar (Table 1), with no significant differences in baseline WBC, creatinine, or albumin values, nor rates of ICU admission, all-cause mortality, or colectomy within 40 days of enrollment. CDI-NAAT patients were more likely than carrier-NAAT patients to test positive for a NAP-1 strain (12.3% vs 4.6%, P = .24; Table 1). Seventy-nine of 122 (65%) CDI-NAAT and 34 of 44 (77%) carrier-NAAT subjects had total toxin (toxin A + toxin B) concentrations above a clinical cutoff of 20 pg/mL (see Methods); these subgroups were designated as “CDI-Tox20” and “carrier-Tox20” cohorts, respectively. CDI-Tox20 and carrier-Tox20 cohorts were also similar to each other across all parameters (Table 1) with 2 exceptions: Albumin levels were significantly lower in the CDI-Tox20 group (2.9 vs 3.4 mg/dL, P = .017), and more CDI-Tox20 subjects tested positive for NAP-1 (19.0% vs 5.9%, P = .09).
Table 1.
Comparison of Demographics, Baseline Laboratory Values, and Clinical Outcomes for Clostridioides difficile Infection and Carrier Cohorts
| Variable | CDI-NAAT (n = 122) |
Carrier-NAAT (n = 44) |
P Value | CDI-Tox20 (n = 79) |
Carrier-Tox20 (n = 34) |
P Value |
|---|---|---|---|---|---|---|
| Age, y, median (IQR) | 67.0 (55.0–76.0) | 64.5 (49.0–73.5) | .365 | 68.0 (57.0–77.0) | 65.5 (52.0–77.0) | .353 |
| Sex | .860 | .682 | ||||
| Female | 67/122 (54.9) | 23/44 (52.3) | 44/79 (55.7) | 17/34 (50.0) | ||
| Male | 55/122 (45.1) | 21/44 (47.7) | 35/79 (44.3) | 17/34 (50.0) | ||
| Ethnicity | .454 | .435 | ||||
| Hispanic | 8/111 (7.2) | 1/36 (2.8) | 7/71 (9.9) | 1/28 (3.6) | ||
| Non-Hispanic | 103/111 (92.8) | 35/36 (97.2) | 64/71 (90.1) | 27/28 (96.4) | ||
| Race | .323 | .553 | ||||
| White | 97/122 (79.5) | 31/35 (88.6) | 64/79 (81.0) | 24/27 (88.9) | ||
| Other | 25/122 (20.5) | 4/35 (11.4) | 15/79 (19.0) | 3/27 (11.1) | ||
| Laboratory results | ||||||
| WBC, ×109 cells/μL, median (IQR) | 10.6 (6.9–17.4), n = 120 | 11.2 (6.9–14.8), n = 44 | .724 | 10.9 (8.1–17.4), n = 78 | 10.6 (6.6–14.9), n = 34 | .349 |
| WBC ≥15, ×109 cells/L | 41/120 (34.2) | 10/44 (22.7) | .161 | 28/78 (35.9) | 8/34 (23.5) | .272 |
| Creatinine, mg/dL, median (IQR) | 1.0 (0.8–1.7), n = 120 | 1.2 (0.8–1.9), n = 44 | .407 | 1.0 (0.8–1.7), n = 78 | 1.1 (0.8–1.8), n = 34 | .358 |
| Creatinine ≥1.5, mg/dL | 38/120 (31.7) | 14/44 (31.8) | 1.0 | 22/78 (28.2) | 9/34 (26.5) | 1.0 |
| Albumin, mg/dL, median (IQR) | 2.9 (2.5–3.6), n = 108 | 3.4 (3.1–3.8), n = 21 | .078 | 2.9 (2.6–3.5), n = 71 | 3.4 (3.2–3.8), n = 16 | .017 |
| ICU admissions | 16/122 (13.1) | 4/44 (9.1) | .596 | 8/79 (10.1) | 2/34 (5.9) | .721 |
| Colectomy | 0/122 (0.0) | 0/44 (0.0) | NA | 0/79 (0.0) | 0/34 (0.0) | NA |
| Death in 30 d | 3/122 (2.5) | 4/44 (9.1) | .080 | 1/79 (1.3) | 1/34 (2.9) | .513 |
| Death in 40 d | 10/122 (8.2) | 5/44 (11.4) | .546 | 5/79 (6.3) | 2/34 (5.9) | 1.0 |
| 027-NAP1-BI | 15/122 (12.3) | 2/44 (4.6) | .244 | 15/79 (19.0) | 2/34 (5.9) | .090 |
Data are presented as No. (%) unless otherwise indicated. CDI-NAAT and carrier-NAAT cohorts were defined by positive results on stool NAAT, while CDI-Tox20 and carrier-Tox20 cohorts included only the subjects that were positive by both NAAT and single molecule array (Simoa; Toxin A + B ≥20 pg/mL). Laboratory values were from time of enrollment; ICU admission, colectomy, and death rates were assessed within 40 days of enrollment.
Abbreviations: CDI, Clostridioides difficile infection; ICU, intensive care unit; IQR, interquartile range; NA, not applicable; NAAT, nucleic acid amplification test; Tox20, stool toxin A + B ≥20 pg/mL; WBC, white blood count.
Toxin A and B concentrations as measured by Simoa in diluted stool (21-fold dilution factor) were distributed over a 5-log range (0 to >100000 pg/mL). Stool toxin concentrations (as measured by Simoa) and cycle threshold (Ct) values (as measured by Xpert NAAT) in CDI-NAAT vs carrier-NAAT cohorts are shown in Figure 1. Median values for toxin A concentration, toxin B concentration, and toxin A + B concentration were not significantly different in the CDI-NAAT and carrier-NAAT cohorts (Table 2). In contrast, when the CDI-Tox20 and carrier-Tox20 cohorts were compared, all median toxin concentration values (A, B, and A + B) were significantly higher in the CDI-Tox20 cohort (Figure 2 and Table 2). CTA was performed on all samples; 60 of 122 (49%) CDI-NAAT and 17 of 44 (39%) carrier-NAAT samples tested positive (designated CDI-CTA and carrier-CTA cohorts). Fifty-five of 59 (93%) CDI-NAAT and 13 of 14 (93%) carrier-NAAT samples with Simoa toxin B concentrations ≥100 pg/mL had positive CTA results, whereas the majority of samples with toxin B concentrations <100 pg/mL tested negative by CTA (90% overall). Fifty of 122 (41%) CDI-NAAT and 11 of 44 (25%) carrier-NAAT patients had total toxin concentrations >1000 pg/mL (as a proxy for EIA limit of detection [LOD]; designated CDI-Tox1000 and carrier-Tox1000 cohorts). CDI-CTA/carrier-CTA and CDI-Tox1000/carrier-Tox1000 groups were similar to each other across all parameters (Supplementary Table 1), with the exception that the percentage of CDI-CTA patients with WBC ≥15 (×109 cells/μL) was significantly higher than in the carrier-CTA group (45.0% vs 17.7%, P = .051). Median toxin concentrations were higher in CDI-CTA (vs carrier-CTA) and CDI-Tox1000 (vs carrier-Tox1000) cohorts (Supplementary Table 2), though differences were not statistically significant.
Figure 1.
Dot plots showing distribution of toxin concentrations (measured by Simoa) and Ct values (measured by Xpert NAAT) in symptomatic (CDI-NAAT) vs asymptomatic (carrier-NAAT) cohorts (defined by positive stool NAAT result). A, Simoa toxin A concentration. B, Simoa toxin B concentration. C, Simoa toxin A + B concentration. D, Xpert Ct value. The bottom and top edges of the boxes for each cohort indicate the interquartile range, the horizontal line bisecting the box indicates the median value, and the whiskers represent maximum and minimum values. P values for comparison of the respective medians are shown. Abbreviations: CDI, Clostridioides difficile infection; Ct, cycle threshold; NAAT, nucleic acid amplification test; Simoa, single molecule array.
Table 2.
Comparison of Median Toxin Concentrations and Nucleic Acid Amplification Test Cycle Threshold Values in Clostridioides difficile Infection and Carrier Cohorts
| Variable | CDI-NAAT | Carrier-NAAT | P Value | CDI-Tox20 | Carrier-Tox20 | P Value |
|---|---|---|---|---|---|---|
| Simoa Toxin A concentration, pg/mL, median (IQR) | 50.6 (1.2–2995.0), n = 122 | 60.0 (8.1–428.9), n = 44 | .958 | 874.0 (62.8–9738.0), n = 79 | 129.7 (41.8–584.1), n = 34 | .021 |
| Simoa Toxin B concentration, pg/mL, median (IQR) | 89.5 (3.7–5551.0), n = 122 | 42.3 (7.8–417.0), n = 44 | .788 | 1317.0 (99.5–20229.0), n = 79 | 81.7 (26.6–940.6), n = 34 | .003 |
| Simoa Toxin A + B concentration, pg/mL, median (IQR) | 197.2 (7.8–11762.0), n = 122 | 137.3 (38.2–862.4), n = 44 | .766 | 4180.7 (215.0–34916.0), n = 79 | 349.6 (123.0–1342.0), n = 34 | .004 |
| Xpert Toxin B Ct, median (IQR) | 28.1 (24.9–32.5), n = 120 | 28.6 (25.8–33.2), n = 44 | .354 | 25.8 (23.50–28.30), n = 78 | 27.7 (25.80–30.7), n = 34 | .015 |
CDI-NAAT and carrier-NAAT cohorts were defined by positive results on stool NAAT, while CDI-Tox20 and carrier-Tox20 cohorts included only the subjects that were positive by both NAAT and single molecule array (Simoa; Toxin A + B ≥20 pg/mL).
Abbreviations: CDI, Clostridioides difficile infection; Ct, cycle threshold; IQR, interquartile range; NAAT, nucleic acid amplification test; Tox20, stool toxin A + B ≥20 pg/mL.
Figure 2.
Dot plots showing distribution of toxin concentrations (measured by Simoa) and Ct values (measured by Xpert NAAT) in symptomatic (CDI-Tox20) vs asymptomatic (carrier-Tox20) cohorts (defined by positive stool NAAT result and stool toxin A + B ≥20 pg/mL by Simoa). A, Simoa toxin A concentration. B, Simoa toxin B concentration. C, Simoa toxin A + B concentration. D, Xpert Ct value. The bottom and top edges of the boxes for each cohort indicate the interquartile range, the horizontal line bisecting the box indicates the median value, and the whiskers represent maximum and minimum values. P values for comparison of the respective medians are shown. Abbreviations: CDI, Clostridioides difficile infection; Ct, cycle threshold; NAAT, nucleic acid amplification test; Simoa, single molecule array; Tox20, stool toxin A + B ≥20 pg/mL.
There was no toxin A + B concentration cutoff value that yielded useful sensitivity and specificity for distinction of CDI-NAAT vs carrier-NAAT; the area under the receiver operating curve (AUC) was 0.515. Similarly, no cutoff adequately distinguished between CDI-Tox20 and carrier-Tox20 (AUC = 0.677), CDI-CTA and carrier-CTA (AUC = 0.651), or CDI-Tox1000 and carrier-Tox1000 (AUC = 0.631) groups.
Within 40 days of stool collection, 3 of 44 (6.8%) of the carrier-NAAT patients were suspected clinically to have CDI. However, only 1 fulfilled our study diagnostic criteria for CDI (see Supplementary Results for details). None of the remaining carrier-NAAT patients were suspected to have CDI during the follow-up period, including 10 patients with toxin A + B concentrations >1000 pg/mL (4 of whom had concentrations >10000 pg/mL).
Median toxin A, toxin B, and toxin A + B values were compared for groups of stool samples of different consistencies (formed, semi-formed, liquid) in the CDI-NAAT and carrier-NAAT cohorts (Table 3). All median toxin concentrations trended up as stool consistency varied from formed to semi-formed to liquid, and Ct values trended down (Table 3). Proportions of each subgroup who had received any laxative within the 72 hours prior to stool collection are also noted in Table 3.
Table 3.
Median Toxin Concentration, Median Cycle Threshold Value, and Percentage With Laxative Ingestion in Clostridioides difficile Infection and Carrier Nucleic Acid Amplification Test Cohorts, Categorized by Stool Consistency
| Group | No. | Laxative | Simoa Toxin A | Simoa Toxin B | Simoa Toxin A + B | Cycle Threshold |
|---|---|---|---|---|---|---|
| CDI | 122 | |||||
| Formed | 0 | NA | NA | NA | NA | NA |
| Semi-formed | 48 | 22/48 (46%) | 18.8 (0.8–2436.7) | 28.1 (3.0–7136.3) | 113.6 (6.2–9820.2) | 28.8 (25.1–33.8) |
| Liquid | 74 | 19/74 (26%) | 65.6 (1.5–3670.0) | 108.7 (4.10–5551.0) | 258.9 (8.3–11762.0) | 27.6 (23.6–31.5) |
| P = .41 | P = .46 | P = .35 | P = .09 | |||
| Carrier | 44 | |||||
| Formed | 16 | 9/16 (56%) | 10.7 (1.45–62.0) | 14.45 (7.35–42.3) | 48.8 (8.8–130.2) | 31.7 (26.9–34.3) |
| Semi-formed | 24 | 16/24 (67%) | 129.7 (28.3–951.1) | 87.6 (18.4–1626.7) | 349.6 (88.0–3054.0) | 28.3 (25.7–31.7) |
| Liquid | 4 | 3/4 (75%) | 899 (292.5–3945.5) | 253.5 (5.3–6275.5) | 1152.5 (543.3–9975.5) | 27.1 (25.0–32.1) |
| P < .01 | P = .06 | P < .01 | P = .36 |
Laxative data refer to any dose of a relevant oral agent given within the 72 hours prior to the baseline stool collection. For toxin concentrations and cycle threshold values, data presented are median (interquartile range).
Abbreviations: CDI, Clostridioides difficile infection; NA, not applicable; Simoa, single molecule array.
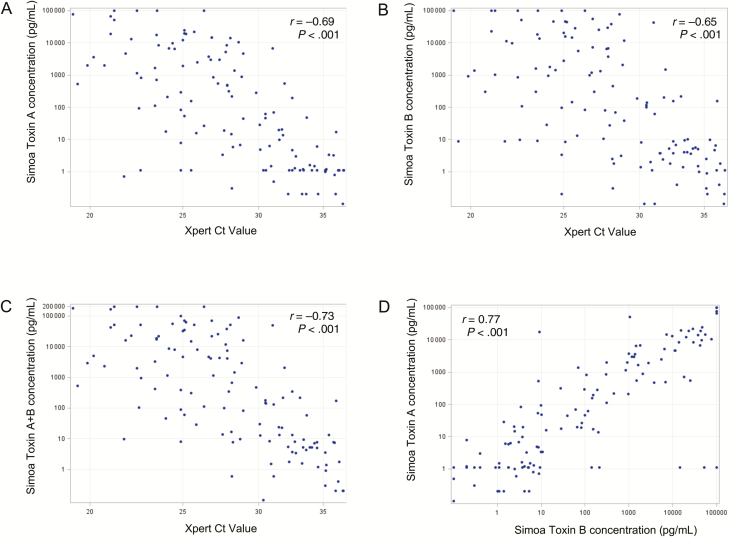
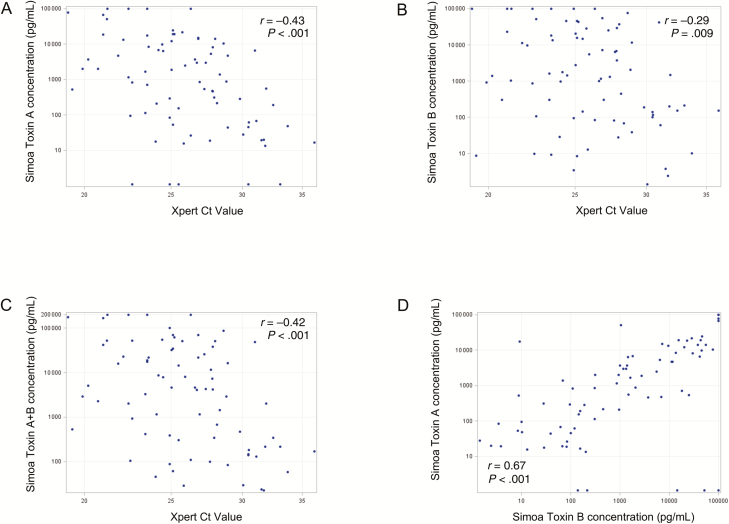
Median NAAT Ct values were not significantly different in the CDI-NAAT vs carrier-NAAT cohorts, but significantly lower in the CDI-Tox20 vs the carrier-Tox20 cohort (Figures 1D and 2D; Table 2). Differences between NAAT Ct values in CDI-CTA/carrier-CTA and CDI-Tox1000/carrier-Tox1000 cohorts did not reach statistical significance (Supplementary Table 2). Xpert Ct values correlated moderately with toxin A, toxin B, and toxin A + B concentrations, and toxin A and toxin B concentrations correlated moderately with each other, in CDI-NAAT, carrier-NAAT, CDI-Tox20, and carrier-Tox20 cohorts (Supplementary Table 3). Plots of these correlations for the CDI-NAAT and CDI-Tox-20 cohorts are shown in Figures 3 and 4, respectively.
Figure 3.
Correlations between toxin concentrations (measured by Simoa) and Ct values (measured by Xpert NAAT) and between toxin A and toxin B in the symptomatic cohort defined by positive stool NAAT result (CDI-NAAT). A, Simoa toxin A concentration vs Xpert Ct value. B, Simoa toxin B concentration vs Xpert Ct value. C, Simoa toxin A + B concentration vs Xpert Ct value. D, Simoa toxin A concentration vs Simoa toxin B concentration. Spearman r values and P values for each correlation are shown. Abbreviations: CDI, Clostridioides difficile infection; Ct, cycle threshold; NAAT, nucleic acid amplification test; Simoa, single molecule array.
Figure 4.
Correlations between toxin concentrations (measured by Simoa) and Ct values (measured by Xpert NAAT) and between toxin A and toxin B in the symptomatic cohort defined by positive stool NAAT result and stool toxin A + B concentration ≥20 pg/mL by Simoa (CDI-Tox20). A, Simoa toxin A concentration vs Xpert Ct value. B, Simoa toxin B concentration vs Xpert Ct value. C, Simoa toxin A + B concentration vs Xpert Ct value. D, Simoa toxin A concentration vs Simoa toxin B concentration. Spearman r values and P values for each correlation are shown. Abbreviations: CDI, Clostridioides difficile infection; Ct, cycle threshold; NAAT, nucleic acid amplification test; Simoa, single molecule array; Tox20, stool toxin A + B ≥20 pg/mL.
DISCUSSION
Symptoms and signs consistent with CDI are highly variable, ranging from mild diarrhea to severe colitis, colectomy, or death [2]. Provider concern about severe CDI-related outcomes is high enough that even patients with minimal or self-resolving diarrhea are sometimes tested and treated for CDI (see, eg, [11]). Confusing the matter further, patients with no diarrhea whatsoever can still be colonized with toxinogenic C. difficile, and can transmit that organism to others who might be more susceptible to disease [12, 13]. It is no surprise that the medical community is struggling to decide between diagnostic algorithms focused on the most sensitive detection of toxinogenic organism in stool and algorithms focused on detection of actual C. difficile toxins in stool [1].
While the medical community has a shared goal—to detect patients who should be treated for CDI to prevent severe outcomes—there is disagreement about which patients who test positive for toxinogenic C. difficile should be treated. US guidelines focus on sensitive detection of toxinogenic organism (NAAT or glutamate dehydrogenase [GDH]/toxin EIAs) [3, 14]. For GDH-positive/toxin-negative EIA results, follow-up NAAT has been recommended [3, 15], suggesting that NAAT-positive results in symptomatic patients are to be considered diagnostic of CDI, even if results of currently available toxin tests are negative. In contrast, guidelines from Europe [16] prioritize detection of toxin, based on data suggesting that toxin-positive patients are at higher risk for bad outcomes [5, 6].
To date, the fact that current commercially available toxin immunoassays have limited analytical sensitivity (typical LOD, ~1 ng/mL [17, 18]) has significantly confounded diagnostic algorithms for CDI; importantly, it is clear that detection of toxin by EIA does not capture all patients at risk for severe CDI and its complications [19, 20]. This diagnostic gap led our group to develop ultrasensitive rapid toxin detection assays based on single molecule array (Simoa) technology. With this technology, we were previously able to separately detect and quantify toxins A and B in stool with analytical LODs of <1 pg/mL and clinical cutoffs of approximately 20 pg/mL in stool [9, 10]. In those studies, approximately 25% of NAAT-positive clinical samples had toxin B concentration <20 pg/mL by Simoa, but subjects were not rigorously screened to confirm that they had diarrhea. Here, we have demonstrated the application of Simoa assays to compare toxin concentrations in 2 rigorously defined NAAT-positive cohorts of adult inpatients within 1 hospital: patients with diarrhea consistent with CDI vs those who have no diarrhea or other clinical evidence of colitis. The hypothesis underlying this comparison was that patients with CDI (as diagnosed by NAAT, in combination with diarrhea) would have significantly higher stool toxin levels than asymptomatic carriers, and furthermore, that the majority of asymptomatic carriers would have minimal or undetectable stool toxin. Secondarily, we compared toxin concentrations in the subsets of these cohorts who had detectable stool toxin (A + B ≥20 pg/mL by Simoa). Our results have surprisingly but clearly demonstrated that stool toxin A and B concentration alone cannot distinguish a patient with CDI (diagnosed by either NAAT or toxin detection) from an asymptomatic carrier, because concentration distributions in both types of patients overlap substantially. However, our results also demonstrate that when considered as a group, toxin concentrations are significantly higher (and Ct values significantly lower) in CDI patients than in carriers—but this difference is seen only when CDI is diagnosed by toxin detection, and not when CDI is diagnosed by NAAT. Similarly, comparison of CDI and carrier cohorts defined by positive CTA, or by toxin A + B ≥1000 pg/mL by Simoa (as a proxy for the LOD of EIA), showed that toxin concentrations were higher (and Ct values lower) in CDI cohorts, though reduced sample sizes likely affected the statistical significance. Our findings add strength to the argument that detection of toxin is more clinically relevant than detection of the toxin gene. While a prior study [21] did compare mean Ct values in symptomatic vs asymptomatic adult inpatients and find that they were similar, the symptomatic patients in that study were not evaluated to confirm that they actually had diarrhea consistent with CDI and, importantly, no toxin tests were performed.
There is recent interest in whether NAAT Ct values correlate with the presence or absence of detectable toxin (by EIA or CTA) in stool from symptomatic patients [22–24], potentially allowing use of NAAT results to predict which patients have elevated stool toxin levels and should thus be prioritized for treatment; other studies have investigated whether Ct value can be used to predict clinical outcome [25]. Senchyna et al [22] estimated that an Xpert Ct cutoff of 26.4 had a negative predictive value of 97.1% for excluding the presence of toxin in stool; however, as shown in Figures 3 and 4, multiple patients in our CDI-NAAT and CDI-Tox20 cohorts with Xpert Ct values >26.4 had detectable stool toxin by Simoa, including above analytical thresholds for EIA (~1000 pg/mL) and CTA (toxin B of ~100 pg/mL).
Given these findings, we may assume that neither stool toxin concentration nor Ct value can reliably distinguish a symptomatic patient with CDI from a symptomatic patient who is colonized and whose diarrhea is actually due to another cause.
Our findings beg the critical question of why some patients with high concentrations of C. difficile toxins in their stool are symptomatic and others are not. The demographics of our symptomatic and asymptomatic patients were not significantly different. In terms of bacterial virulence, a higher percentage of the symptomatic patients with CDI had NAP-1 strains in stool, but at most, NAP-1 strains accounted for 19% of the strains in the CDI-Tox20 cohort. Given the likelihood that multiple factors in addition to toxin influence symptomatic expression of CDI, a full investigation of possible explanations will need to include analysis of host blood and stool inflammatory markers, blood and stool antitoxin immunoglobulin levels, stool metabolites, multiplexed testing for other stool pathogens, and/or microbiome analyses to attempt to define key determinants of CDI vs the carrier state.
One potential limitation of our study was our requirement that asymptomatic patients have received at least 1 dose of antibiotic prior to enrollment, which was intended to increase the proportion of C. difficile carriers (given that antibiotic use is a risk factor for C. difficile colonization [26]). However, it is possible that this requirement selected for a population that was closer on the spectrum to the disease state (CDI) than a completely randomly selected group of inpatients might have been. While we are certain that subjects enrolled as potential carriers did not have clinical diarrhea (Methods), we did observe that 28 of 44 (64%) of the carrier study stools were semi-formed or liquid (though we also note that the majority of the cohort had received laxatives; Table 3). Interestingly, stool consistency appeared to correlate with toxin concentration, with higher median toxin concentrations (and, to a lesser extent, lower Ct values) trending with less-formed stool. It is thus possible that some of the carrier patients had subclinical toxin-associated colitis, leading to higher water content in stool. Notably, however, only 1 of 44 carrier subjects progressed to probable CDI within 40 days of follow-up. Thus, our findings suggest yet another, previously unrecognized, category in the clinical spectrum of CDI (ie, subclinical colitis). Another limitation was that we were not able to fully control sample temperature between provision of a sample by the patient and the sample’s arrival in the microbiology laboratory (at which point it was refrigerated), which may have impacted toxin concentration. However, sample handling was the same for both CDI and carrier cohorts, and mirrored institutional sample handling for clinical C. difficile testing. Finally, because it was not possible to perform all tests in real time, we performed all testing (other than Xpert NAAT performed for initial clinical diagnosis) on frozen sample aliquots. We were fastidious in our refrigeration, mixing of samples prior to aliquoting, and weighing stool to ensure precise Simoa measurements despite variation in stool consistency [21], and all samples from both cohorts were processed in parallel using identical protocols and procedures. Finally, we recognize that results from a single center may not be generalizable.
Given that the presence of toxin is necessary, though not sufficient, for CDI, ultrasensitive measurement of stool toxin (rather than toxinogenic organism, by NAAT) may provide benefit by excluding CDI in symptomatic patients who are NAAT positive but do not have detectable toxin, and potentially by supporting diagnosis and outcome prediction (currently under study) by providing an actual toxin concentration. However, if an additional institutional goal is to detect all patients who have C. difficile infection OR carriage to optimize infection control measures (as colonized patients can still transmit the organism to others [12, 13]), NAAT would likely be required, as there are NAAT-positive patients who do not have detectable stool toxin by Simoa. Ultimately, it may be that an accurate diagnosis of CDI will require both toxin measurement and additional testing for host biomarkers that can be shown to be specific to CDI; it is clear that clinically symptomatic CDI is the result of an interplay of multiple factors including host immune responses. Regardless, our data strongly reinforce the conclusion that accurate diagnosis of CDI cannot be done without rigorous confirmation of CDI symptoms—and consideration of all alternative explanations for those symptoms—prior to testing and treatment.
Supplementary Data
Supplementary materials are available at Clinical Infectious Diseases online. Consisting of data provided by the authors to benefit the reader, the posted materials are not copyedited and are the sole responsibility of the authors, so questions or comments should be addressed to the corresponding author.
Supplementary Material
Notes
Financial support. This work was supported by grants from the National Institutes of Health, National Institute of Allergy and Infectious Diseases (grant number 1R01AI116596-01 to N. R. P. and C. P. K.) and Institut Mérieux (to N. R. P. and C. P. K.). Simoa assays were performed by bioMérieux.
Acknowledgments. The authors thank all patients who participated in this study, as well as Carolyn Alonso, Javier Villafuerte Gálvez, and the technologists in the Beth Israel Deaconess Medical Center Clinical Microbiology Laboratory for their help with sample collection.
Potential conflicts of interest. A. B., B. R., A. L., and M. A. M. are employees of bioMérieux. C. P. K. has acted as a paid consultant to Artugen, Facile Therapeutics, First Light Biosciences, Finch, Matrivax, Merck, Seres Health, and Vedanta and has received grant support from Merck. X. C. has received grants from Ostrigen and Merck. All other authors report no potential conflicts of interest. All authors have submitted the ICMJE Form for Disclosure of Potential Conflicts of Interest. Conflicts that the editors consider relevant to the content of the manuscript have been disclosed.
References
- 1. Fang FC, Polage CR, Wilcox MH. Point-counterpoint: what is the optimal approach for detection of Clostridium difficile infection?J Clin Microbiol 2017; 55:670–80. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2. Burnham CA, Carroll KC. Diagnosis of Clostridium difficile infection: an ongoing conundrum for clinicians and for clinical laboratories. Clin Microbiol Rev 2013; 26:604–30. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. McDonald LC, Gerding DN, Johnson S, et al. . Clinical practice guidelines for Clostridium difficile infection in adults and children: 2017 update by the Infectious Diseases Society of America (IDSA) and Society for Healthcare Epidemiology of America (SHEA). Clin Infect Dis 2018; 66:987–94. [DOI] [PubMed] [Google Scholar]
- 4. Lessa FC, Mu Y, Bamberg WM, et al. . Burden of Clostridium difficile infection in the United States. N Engl J Med 2015; 372:825–34. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Polage CR, Gyorke CE, Kennedy MA, et al. . Overdiagnosis of Clostridium difficile infection in the molecular test era. JAMA Intern Med 2015; 175:1792–801. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Planche TD, Davies KA, Coen PG, et al. . Differences in outcome according to Clostridium difficile testing method: a prospective multicentre diagnostic validation study of C difficile infection. Lancet Infect Dis 2013; 13:936–45. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Kyne L, Warny M, Qamar A, Kelly CP. Asymptomatic carriage of Clostridium difficile and serum levels of IgG antibody against toxin A. N Engl J Med 2000; 342:390–7. [DOI] [PubMed] [Google Scholar]
- 8. McFarland LV, Elmer GW, Stamm WE, Mulligan ME. Correlation of immunoblot type, enterotoxin production, and cytotoxin production with clinical manifestations of Clostridium difficile infection in a cohort of hospitalized patients. Infect Immun 1991; 59:2456–62. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Pollock NR, Song L, Zhao M, et al. . Differential immunodetection of toxin B from highly virulent Clostridium difficile BI/NAP-1/027. J Clin Microbiol 2015; 53:1705–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Song L, Zhao M, Duffy DC, et al. . Development and validation of digital enzyme-linked immunosorbent assays for ultrasensitive detection and quantification of Clostridium difficile toxins in stool. J Clin Microbiol 2015; 53:3204–12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Truong CY, Gombar S, Wilson R, et al. . Real-time electronic tracking of diarrheal episodes and laxative therapy enables verification of Clostridium difficile clinical testing criteria and reduction of Clostridium difficile infection rates. J Clin Microbiol 2017; 55:1276–84. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Riggs MM, Sethi AK, Zabarsky TF, Eckstein EC, Jump RL, Donskey CJ. Asymptomatic carriers are a potential source for transmission of epidemic and nonepidemic Clostridium difficile strains among long-term care facility residents. Clin Infect Dis 2007; 45:992–8. [DOI] [PubMed] [Google Scholar]
- 13. Longtin Y, Paquet-Bolduc B, Gilca R, et al. . Effect of detecting and isolating Clostridium difficile carriers at hospital admission on the incidence of C difficile infections: a quasi-experimental controlled study. JAMA Intern Med 2016; 176:796–804. [DOI] [PubMed] [Google Scholar]
- 14. Surawicz CM, Brandt LJ, Binion DG, et al. . Guidelines for diagnosis, treatment, and prevention of Clostridium difficile infections. Am J Gastroenterol 2013; 108:478–98; quiz 499. [DOI] [PubMed] [Google Scholar]
- 15. Brecher SM, Novak-Weekley SM, Nagy E. Laboratory diagnosis of Clostridium difficile infections: there is light at the end of the colon. Clin Infect Dis 2013; 57:1175–81. [DOI] [PubMed] [Google Scholar]
- 16. Crobach MJ, Planche T, Eckert C, et al. . European Society of Clinical Microbiology and Infectious Diseases: update of the diagnostic guidance document for Clostridium difficile infection. Clin Microbiol Infect 2016; 22:S63–81. [DOI] [PubMed] [Google Scholar]
- 17. ImmunoCard Toxins A & B [package insert]. Cincinnati, OH: Meridian Bioscience, Inc; December, 2016. [Google Scholar]
- 18. C. difficile Tox A/B II [package insert]. Blacksburg, VA: TechLab, Inc. June, 2017.
- 19. Guerrero DM, Chou C, Jury LA, Nerandzic MM, Cadnum JC, Donskey CJ. Clinical and infection control implications of Clostridium difficile infection with negative enzyme immunoassay for toxin. Clin Infect Dis 2011; 53:287–90. [DOI] [PubMed] [Google Scholar]
- 20. Origüen J, Corbella L, Orellana MÁ, et al. . Comparison of the clinical course of Clostridium difficile infection in glutamate dehydrogenase-positive toxin-negative patients diagnosed by PCR to those with a positive toxin test. Clin Microbiol Infect 2018; 24:414–21. [DOI] [PubMed] [Google Scholar]
- 21. Truong C, Schroeder LF, Gaur R, et al. . Clostridium difficile rates in asymptomatic and symptomatic hospitalized patients using nucleic acid testing. Diagn Microbiol Infect Dis 2017; 87:365–70. [DOI] [PubMed] [Google Scholar]
- 22. Senchyna F, Gaur RL, Gombar S, Truong CY, Schroeder LF, Banaei N. Clostridium difficile PCR cycle threshold predicts free toxin. J Clin Microbiol 2017; 55:2651–60. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23. Kim HN, Kim H, Moon HW, Hur M, Yun YM. Toxin positivity and tcdB gene load in broad-spectrum Clostridium difficile infection. Infection 2018; 46:113–7. [DOI] [PubMed] [Google Scholar]
- 24. Crobach MJT, Duszenko N, Terveer EM, Verduin CM, Kuijper EJ. Nucleic acid amplification test quantitation as predictor of toxin presence in Clostridium difficile infection. J Clin Microbiol 2018; 56. doi: 10.1128/JCM.01316-17. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25. Reigadas E, Alcalá L, Valerio M, Marín M, Martin A, Bouza E. Toxin B PCR cycle threshold as a predictor of poor outcome of Clostridium difficile infection: a derivation and validation cohort study. J Antimicrob Chemother 2016; 71:1380–5. [DOI] [PubMed] [Google Scholar]
- 26. Crobach MJT, Vernon JJ, Loo VG, et al. . Understanding Clostridium difficile colonization. Clin Microbiol Rev 2018; 31:e00021-17. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.