Highlights
-
•
Low dose TSEB of 12 Gy is an effective treatment option with therapeutic results more than acceptable.
-
•
Comparable treatment results of Low Dose TSEB with Standard Full Dose scheme of 36 Gy.
-
•
Less recorded toxicity of low dose TSEB than Standard Full Dose scheme of 36 Gy.
Abstract
Background & purpose
Although rare, cutaneous lymphomas represent a separate entity in hematologic oncology. T cell origin lymphomas are most common, with Mycosis Fungoides (MF) accounting for about 50–70% of cases. Sezary Syndrome (SS), which represents the leukemic varian of MF, accounts for 3% of Cutaneous T Cell Lymphomas (CTCL). Total Skin Electron Beam Therapy (TSEB) is included at the mainstream of treatment choices for CTCL. The scope of this study is to evaluate the effectiveness and toxicity of two treatment schedules of TSEB.
Methods and materials
We report our experience with TSEB in the management of MF and SS, as of 14 patients treated in our institution from 2011 to 2015. 8 patients received the 12 Gy (low dose) scheme while 6 patients were managed with 36 Gy (standard or full dose scheme) according to six dual field Stanford technique. The endpoints were overall response rate, duration of response and toxicity of treatment.
Results
After a median follow up of 2.5 years we noted excellent treatment outcome, with both schemes being well tolerated and resulting in comparable response rates. The overall response rate for both treatment regimens was over 87.5%. Treatment was well tolerated with mild toxicity.
Conclusion
The role of TSEB in the management of MF and SS is well established. The low dose TSEB schedule of 12 Gy is an effective treatment option, since therapeutic results are more than acceptable, compliance is excellent and toxicity is minimal. Moreover, the evidence that it can be repeated safely makes it more attractive than the standard 36 Gy scheme, when a patient is referred to radiation treatment according to treatment guidelines.
1. Introduction
Cutaneous T cell lymphomas (CTCL) represent a rare but separate entity in hematologic oncology. Accounting for about 4 percent of Non-Hodgkin Lymphomas (NHL), cutaneous lymphomas are a group of disorders characterized by their epidermotropic behavior, primarily affecting the skin. Mycosis fungoides (MF) and Sezary syndrome (SS) are the most common types of primary cutaneous T-cell lymphomas, with an incidence rate of approximately 4–6 new cases per million people [1], [2].
Their dominant characteristic is the epidermotropic behavior of T cells affecting primary skin. MF is characterized by patches, plaques and in more advanced stages by tumorous lesions and visceral involvement while SS is primarily characterized by general erythema with blood involvement. The history of disease varies from the indolent long history of MF to the poor prognosis of SS [2], [3].
Several studies have demonstrated that the main prognostic factor of MF is stage at presentation, while large cell transformation, folliculotropic type and levels of lactate dehydrogenase (LDH) are also of prognostic significance [3].
2. Patients and methods
2.1. Study cohort
We prospectively analyzed the therapeutic results and toxicity of TSEB in 14 patients treated in our institution from 2011 until 2015. Patients were diagnosed with MF or SS and initially staged IB to IV after clinical evaluation according to International Society for Cutaneous Lymphomas/European Organization for Research and Treatment of Cancer (ISCL/EORTC) criteria [4]. 6 patients were treated with the full or standard dose scheme of 36 Gy while 8 patients were managed with the low dose scheme of 12 Gy.
Patients included in our study had failed to at least one prior therapy, skin directed and/or systemic and had good performance status of ECOG 2 or lower. Patients were assessed weekly during treatment, every two months for the next 6 months and every 3 months thereafter. The primary endpoint was response to treatment defined as complete response (CR) with no visible lesions, partial response (PR) with remission in skin lesions of at least 50% from baseline or no response (NR). The secondary endpoints were: duration of response in patients with CR or PR, duration of clinical benefit (DCB) defined as the duration from initial response until the initiation of any other systemic treatment, Overall Survival (OS) and finally acute and chronic toxicity of treatment. Assessment was performed according to the consensus statement for the clinical end points and response criteria of the International Society for Cutaneous Lymphomas (ISCL), the United States Cutaneous Lymphoma Consortium (USCLC), and the Cutaneous Lymphoma Task Force of the European Organization for Research and Treatment of Cancer (EORTC) using mSwat score to assess skin disease burden [5], [6]. The recorded toxicity was assessed according to Common Terminology Criteria for Adverse Events v.4.0 [7]
All our patients were referred and assessed by a team of experts in a multidisciplinary approach in terms of a local tumor board, including a hematologist, dermatologist and a radiation oncologist [8].
2.2. TSEB technique
All patients were treated at ATTIKON University Hospital of Athens. The implemented technique was “six-dual-field” technique introduced and developed originally at Sanford University and in accordance to the described procedure in AAPM (American Association of Physicists in Medicine) Report No 23 [9], [10]. The implementation of TSEB technique at our institution begun in 2009 and was described in detail in relative, previous publications [11], [12]. We used a uniform and sufficient large field of 200 to 80 cm. The SSD was set to 3.8 m and treatment was delivered via symmetrical electron beams of 6 MeV energy via a Varian 2100C linac accelerator. Patients received either 36 Gy with fractions of 2 Gy/cycle over 9 weeks or 12 Gy of 2 Gy/cycle over a period of 3 weeks. Boost or supplemented radiation dose via electron fields was administered to tumorous or underdosed sites. The most frequent site of underdosage after TLD dosimetry was found to be the perineum region, a frequent site of underdosage according to data from other studies [13].
3. Results
3.1. Patient characteristics
We prospectively analyzed the therapeutic results and toxicity of TSEB in 14 patients treated in our institution from 2011 until 2015. Patients were randomly assigned to either 36 Gy or 12 Gy of TSEB by using a random number generator software (odd for the first scheme and even for the second scheme). 6 patients were treated with the standard dose scheme of 36 Gy while 8 patients were managed with the low dose scheme of 12 Gy. All patients completed treatment schedule without interruptions. Median age was 64 years (52–76). 9 patients were male and 5 females. 11 patients were diagnosed with MF and 3 patients with SS. MF patients were clinically staged as follows: 2 patients stage IB, 1 stage IIA, 5 stage IIB and 3 stage IIIA. Median duration of follow up was 33 months (5–61 months). Patients’ characteristics are summarized in Table 1.
Table 1.
Patient characteristics.
| Number of patients | 14 |
| Male | 9 (64%) |
| Female | 5 (36%) |
| Median age (y) | 64 (52–76) |
| MF | |
| T2 (IB) | 2 (14%) |
| T2N1 (IIA) | 1 (7%) |
| T3 (IIB) | 5 (37%) |
| T4 (IIIA) | 3 (21%) |
| Sezary Syndrome | 3 (21%) |
| Median no. of prior therapies | 2 |
3.2. Therapeutic results
Both schemes showed excellent overall response rate: 100% for the 36 Gy group and 87.5% for the 12 Gy group. For the group of patients received 36 Gy, 4 experienced complete response (67%) and the rest 2 partial response (33%). For the low dose scheme of 12 Gy, 2 patients showed complete response (25%), 5 partial (62.5%) while one patient did not respond to treatment.
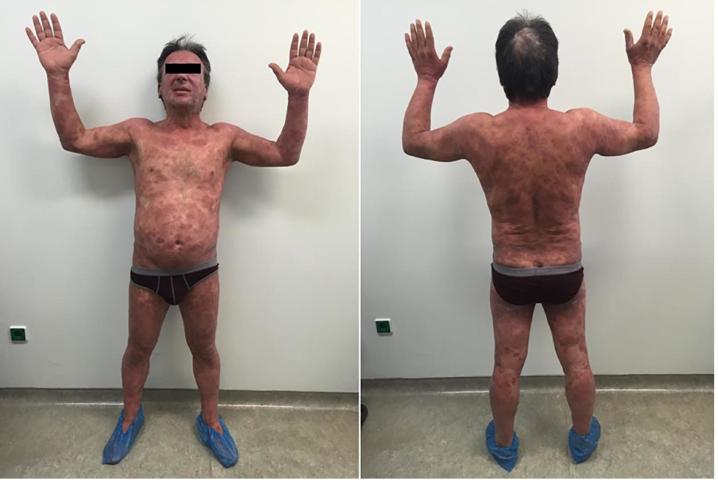
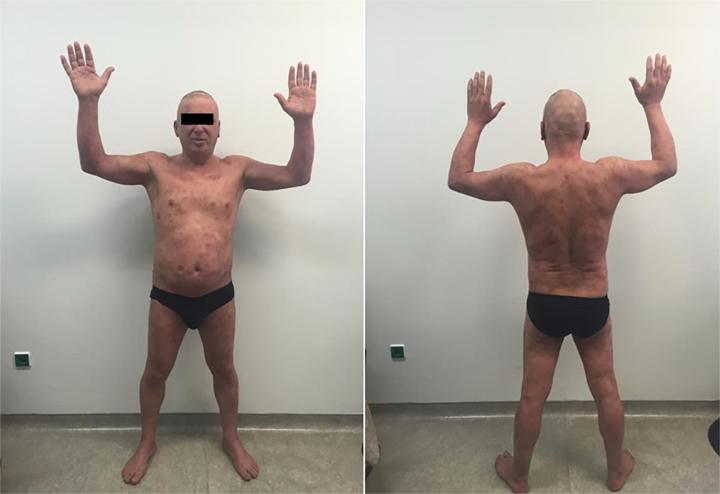
For the low dose scheme 2 patients showed CR (stage IB), 5 patients showed PR (1 patient stage IIA, 2 patients stage IIB, 1 patient stage IIIA, 1 patient with SS) and 1 patient showed no response (stage IIIA). For the full dose scheme 4 patients showed CR (2 patients stage IIB, 1 patient IIIA, 1 patient with SS) while 2 patients (1 stage IIIA and 1 with SS) showed PR. Response rate by stage is summarized in Table 2. Representative photos of a patient treated with low dose TSEB with response to treatment are shown in Fig. 1a, Fig. 1b.
Table 2.
Results of TSEB.
| 36 Gy | 12 Gy | |
|---|---|---|
| Overall response | 100% (6/6) | 87.5% (7/8) |
| Complete response | 67% (4/6)
|
25% (2/8)
|
| Partial response | 33% (2/6)
|
62.5% (5/8)
|
| No response | – | 12.5% (1/8)
|
| Median duration of response | 10.5 months | 9.25 months |
| Median duration of clinical benefit | 11.5 months | 10.1 months |
Fig. 1a.
Patient at the beginning of TSEB treatment 12 Gy.
Fig. 1b.
Patient 1 month after completion of treatment.
The duration of response, which was the secondary endpoint of our study, did not differ significantly between the two groups. The median duration of overall response rate was 10.5 months for the 36 Gy group and 9.25 months for the scheme of 12 Gy.
As far as DCB defined as the time interval from TSEB treatment to the initiation of any other treatment either skin directed or systemic our results where slightly improved as the median duration of response was 11.5 months for the full dose scheme and 10.1 months for the low dose scheme.
For the entire cohort group, low dose TSEB was repeated in one patient 13 months after completion of first treatment course due to relapse. Patient had been treated initially with the low dose scheme. The toxicity recorded during the second course was more profound with alopecia and generalized erythema.
3.3 Toxicity of treatment
The recorded toxicity was mild for the entire cohort, except one case of Grade 3 erythema in the 36 Gy group. Two patients developed limb edema and 3 patients blisters in low extremities, all in the 36 Gy scheme group of patients. Four patients in the 12 Gy group experienced reversible alopecia while all patients who received 36 Gy developed permanent hair loss. No skin infections were recorded. Table 3 summarizes the recorded toxicity, according to Common Terminology Criteria for Adverse Events v4.03.
Table 3.
Toxicity of treatment.
| 36 Gy/6 patients |
12 Gy/8 patients |
|||||||
|---|---|---|---|---|---|---|---|---|
| Toxicity | all grades | Gr1 | Gr2 | Gr3 | all grades | Gr1 | Gr2 | Gr3 |
| Dermatitis | 4 (66%) | 1 (17%) | 2 (33%) | 1 (17%) | 1 (12.5%) | 1 (12.5%) | – | – |
| Alopecia | 6 (100%) | 6 (100%) | – | – | 4 (50%) | 4 (50%) | – | – |
| Skin infection | – | – | – | – | – | – | – | – |
| Skin pain | 2 (33%) | 2 (33%) | – | – | – | – | – | – |
| Nail disorders | 2 (33%) | 1 (17%) | 1 (17%) | – | 1 (12.5%) | 1 (12.5%) | – | – |
| Limb edema | 2 (33%) | 2 (33%) | – | – | – | – | – | – |
| Blisters low extremities | 3 (50%) | 2 (33%) | 1 (17%) | – | – | – | – | – |
| Xerosis | 1 (17%) | 1 (17%) | – | – | 1 (12.5%) | 1 (12.5%) | – | – |
4. Discussion
From the previous century and very early after the introduction of X-rays, researchers have documented the effectiveness of radiation to skin lesions relative to cutaneous lymphomas. Radiation treatment with TSEB technique is among the many skin directed treatment options for the disease. Due mostly to the high radio- sensitivity of lymphoma cells it is well established that radiation therapy is the single most efficient treatment modality in the management of MF [14]. It takes advantage of special characteristics of particle radiation by electrons that can deliver therapeutic dose to patient’s skin without damaging subjacent healthy organs.
Several studies have demonstrated that there is a dose response relationship between doses of TSEB and response rates. Doses in the region of 30–36 Gy are more efficacious in the management of MF by means of achieving complete response (CR) rates up to 90%. Data from the University of Stanford showed this exact dose response relationship: CR was 18% for doses less than 10 Gy, 55% for doses between 10 and 20 Gy, 75% for doses between 25 and 30 Gy and 94% for doses of 30 Gy or greater. Duration of response was also another parameter that showed a direct relationship to administered dose as patients receiving more than 25 Gy remained disease free longer time compared with those that were treated with doses less than 25 Gy [15].
A meta analysis confirmed previous results and showed that CR rates are associated with stage of disease, energy of the electron beam and total dose at skin surface area. The CR rates were 96% in stages IA, IB, and IIA, 36% in stage IIB, and 60% in stage II with doses of 32–36 Gy [16]. The consensus guidelines from the EORTC are in accordance with these data and suggest that optimal dose of TSEB for the management of MF should be in the range of 30–36 Gy [17].
Unfortunately, the main issue in the management of MF is that despite the proven effectiveness of radiation therapy even with doses in the range of 30–36 Gy, the disease regress and one of the main goals of treatment is the prolongation of the overall response duration time. To address this issue, several agents either topical or systemic have been employed as maintenance treatment but even so, recurrence is the rule in the natural course of the disease [18], [19], [20]. New treatment strategies with combination agents like vorinostat, are under investigation some of them in well-designed randomized trials but mature data are still lacking [21].
Taking into consideration both the natural course of the disease and its high radio-sensitivity, many investigators examined lower treatment regimens in order to evaluate the therapeutic effectiveness of lower doses of TSEB that could probably be repeated in case of progression or relapse. Recent data suggest that doses lower than standard full dose scheme can achieve acceptable and comparable treatment results.
In the pooled analysis from Hoppe et al. 33 patients with MF were managed with 12 Gy of 1 Gy per fraction over a period of 3 weeks. They reported excellent and durable overall response rates of 88% with mild toxicity. They concluded that although a higher CR rate may be preferable, the low dose scheme is of considerable benefit as: it achieves a significant reduction in the mSWAT score (median 93.5% from baseline), it is short (3 weeks), is less toxic than the standard scheme and can be repeated safely more than one times [22].
A study from Denmark by Kamstrup et al. evaluated the low dose TSEB by means of overall response rate, complete response or very good partial response rate and duration of response. 21 patients were treated with 10 Gy over a short period of time (2.5 weeks). After a median follow up of 15.7 months the authors reported 95% overall response rate and nearly 60% of complete or very good partial response. The median duration of response was 6 months. Given the fact that the 10 Gy regimen is very safe and can be repeated more than two times in conjunction with the long natural history of disease with the need of multiple treatments during a life time, they concluded that low dose TSEB offers the benefit of re-irradiation at times of relapse or progression [23].
In a very recent study from UK cutaneous lymphoma group by Morris et al, the results of low dose TSEB over 2 weeks in 103 patients with MF were documented after a median follow up of 20.6 months. They reported excellent overall response rate of 87% (18% complete response rate and 69% partial response). Median response duration was 11.8 months while PFS was 13.2 months. They concluded that low dose TSEB is well tolerated with lower toxicity than higher dose treatment schedules [24].
Finally, a recent study from Germany conducted a comparative analysis for the toxicity profile of low dose TSEB compared to conventional doses. They recorded statistically significant lower Grade 2 toxicity (33 vs. 79%) and lower Grade 3 events (6 vs. 15%) for patients treated with the low dose scheme of 12 Gy [25].
Following previous publications reporting on clinical applications of TSEB, our study also evaluated the effectiveness and toxicity of low dose scheme compared to the standard dose of 36 Gy [26], [27], [28]. Even with the very small cohort of 14 patients we demonstrated that the overall response rate for both treatment schedules was excellent (93%). The 36 Gy scheme resulted in a very good complete response rate of 67% while the rate of complete responders for the 12 Gy regimen was as expected lower (25%). The toxicity was minimal as Grade 3 events occurred only in one patient received 36 Gy (7% for the entire cohort).
Further analysis of the 12 Gy group of patients, which was an interesting topic as low dose TSEB is gaining more and more attention due to the reasons mentioned above, showed that our results were in accordance with the data from relevant literature. Although the rate of complete response was 25%, the overall response rate was 87.5% as 7 out of 8 patients showed improvement in skin disease burden according to mSwat evaluation and symptom relief. The overall response rate recorded in the studies of Hoppe et al., Kamstrup et al. and the UK study were 88%, 95% and 87% respectively [23], [24], [25].
Several other recent studies have evaluated the emerging role of low dose TSEB in the management of MF and have showed comparable results to those from the previously mentioned studies [29], [30], [31].
It seems that the aspect of assigning 12 Gy TSEB remains a clinical decision related to the potential need of re-irradiation. According to our opinion the radiation oncologist should prescribe the low dose scheme which seems equivalent and reevaluate the need for 36 Gy in cases of disease that seems not clinically responsive during the administration of low dose scheme. Eventually the therapeutic decision should be individualized.
5. Conclusions
The role of TSEB in the management of MF and SS is well established. The low dose TSEB schedule of 12 Gy is an effective treatment option, since therapeutic results are more than acceptable, compliance is excellent and toxicity is minimal. The fact that it can be repeated safely makes it more attractive than the standard 36 Gy scheme when a patient is referred to radiation treatment according to treatment guidelines.
Conflict of interest
None.
Source of financial support/Funding statement
This research did not receive any specific grant from funding agencies in the public, commercial, or not-for-profit sectors.
References
- 1.Kim Y.H., Hoppe R.T. Mycosis fungoides and the Sezary Syndrome. Semin Oncol. 1999;26(3):276–289. [PubMed] [Google Scholar]
- 2.Willemze R., Jaffe E.S., Burg G. WHO – EORTC classification for cutaneous lymphomas. Blood. 2005;105:3768–3785. doi: 10.1182/blood-2004-09-3502. [DOI] [PubMed] [Google Scholar]
- 3.Kim Y.H., Liu H.L., Mraz-Gernhard S. Long-term outcome of 525 patients with mycosis fungoides and Sézary syndrome: clinical prognostic factors and risk for disease progression. Arch Dermatol. 2003;139:857–866. doi: 10.1001/archderm.139.7.857. [DOI] [PubMed] [Google Scholar]
- 4.Olsen E., Vonderheid E., Pimpinelli N. Revisions to the staging and classification of mycosis fungoides and Sézary syndrome: a proposal of the International Society for Cutaneous Lymphomas (ISCL) and the cutaneous lymphoma task force of the European Organization of Research and Treatment of Cancer (EORTC) Blood. 2007;110:1713–1722. doi: 10.1182/blood-2007-03-055749. [DOI] [PubMed] [Google Scholar]
- 5.Olsen E., Whittaker S., Kim Y.H. Clinical end points and response criteria in mycosis fungoides and Sezary Syndrome: a Consensus Statement of the International Society for Cutaneous Lymphomas, the United States Cutaneous Lymphoma Consortium, and the Cutaneous Lymphoma Task Force of the European Organisation for Research and Treatment of Cancer. J Clin Oncol. 2011;29:2598–2607. doi: 10.1200/JCO.2010.32.0630. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Stevens S.R., Ke M.S., Parry E.J. Quantifying skin disease burden in mycosis fungoides type cutaneous T cell lymphomas. Arch Derm. 2002;138:42–48. doi: 10.1001/archderm.138.1.42. [DOI] [PubMed] [Google Scholar]
- 7.Common Terminology Criteria for Adverse Effects https://www.eortc.be/services/doc/ctc/CTCAE_4.03_2010-06-14_QuickReference_5x7.pdf.
- 8.Zygogianni A., Syrigos K., Mistakidou K., Fotineas A., Kyrgias G., Ferendouros V. Structure and function of the oncologic boards in Greece. Description of the institutional and scientific frame; objective problems and difficulties. J BUON. 2013;1:281–288. [PubMed] [Google Scholar]
- 9.Cox R.S., Heck R.J., Fessenden P. Development of total skin electron therapy at two energies. Int J Radiat Oncol Biol Phys. 1990;18:659–669. doi: 10.1016/0360-3016(90)90075-u. [DOI] [PubMed] [Google Scholar]
- 10.Karzmack CJ, Anderson J, Fessenden P, Svensson G, Buffa A, Khan F, et al. AAPM report No.23, total skin electron therapy: technique and dosimetry. Report of Group 30. Radiation therapy Committee AAPM; 1987.
- 11.Platoni K., Diamantopoulos S., Panayiotakis G. First application of total skin electron beam irradiation in Greece: setup, measurements and dosimetry. Physica Med. 2012;28:174–182. doi: 10.1016/j.ejmp.2011.03.007. [DOI] [PubMed] [Google Scholar]
- 12.Diamantopoulos S., Platoni K., Dilvoi M. Clinical implementation of total skin electron beam (TSEB) therapy: a review of the relevant literature. Physica Med. 2011;27:62–68. doi: 10.1016/j.ejmp.2010.09.001. [DOI] [PubMed] [Google Scholar]
- 13.Elsayad K., Moustakis C., Simonsen M. In-vivo dosimetric analysis in total skin electron beam therapy. Phys Imaging Radiat Oncol. 2018;6:61–65. doi: 10.1016/j.phro.2018.05.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Hoppe R. Mycosis fungoides: radiation therapy. Dermatol Ther. 2003;16:347–354. doi: 10.1111/j.1396-0296.2003.01647.x. [DOI] [PubMed] [Google Scholar]
- 15.Hoppe R.T., Fuks Z., Bagshaw M.A. The rationale of curative radiotherapy in mycosis fungoides. Int J Radiat Oncol Biol Phys. 1977;2:843–885. doi: 10.1016/0360-3016(77)90182-1. [DOI] [PubMed] [Google Scholar]
- 16.Jones G.W., Hoppe R.T., Glatstein E. Electron beam treatment for cutaneous T-cell lymphoma. Hematol Oncol Clin North Am. 1995;9:1057–1076. [PubMed] [Google Scholar]
- 17.Jones G.W., Kacinski B.M., Wilson L.D. Total skin electron radiation in the management of mycosis fungoides: consensus of the European Organization for Research and Treatment of Cancer (EORTC) Cutaneous Lymphoma Project Group. J Am Acad Dermatol. 2002;47:364–370. doi: 10.1067/mjd.2002.123482. [DOI] [PubMed] [Google Scholar]
- 18.Roberge D., Muanza T., Blake G., Shustik C., Vuong T., Freeman C.R. Does adjuvant alpha-interferon improve outcome when combined with total skin irradiation for mycosis fungoides? Br J Dermatol. 2007;156:57–61. doi: 10.1111/j.1365-2133.2006.07559.x. [DOI] [PubMed] [Google Scholar]
- 19.Wilson L.D., Jones G.W., Kim D. Experience with total skin electron beam therapy in combination with extracorporeal photopheresis in the management of patients with erythrodermic (T4) mycosis fungoides. J Am Acad Dermatol. 2000;43:54–60. doi: 10.1067/mjd.2000.105510. [DOI] [PubMed] [Google Scholar]
- 20.Braverman I.M., Yager N.B., Chen M. Combined total body electron beam irradiation and chemotherapy for mycosis fungoides. J Am Acad Dermatol. 1987;16:45–60. doi: 10.1016/s0190-9622(87)70004-8. [DOI] [PubMed] [Google Scholar]
- 21.A Multicenter, Open-label, Randomized, Phase I/II Study Evaluating the Safety and Efficacy of Low-dose (12 Gy) Total Skin Electron Beam Therapy (TSEBT) combined with vorinostat versus low-dose TSEBT monotherapy in Mycosis Fungoides (MF) https://ClinicalTrials.gov/show/NCT01187446.
- 22.Hoppe R., Harrison C., Tavallaee M. Low-dose total skin electron beam therapy as an effective modality to reduce disease burden in patients with mycosis fungoides: results of a pooled analysis from 3 phase-II clinical trials. J Am Acad Dermatol. 2015;72:286–292. doi: 10.1016/j.jaad.2014.10.014. [DOI] [PubMed] [Google Scholar]
- 23.Kamstrup M., Gniadecki R., Iversen L. Low-dose (10-Gy) total skin electron beam therapy for cutaneous T-cell lymphoma: an open clinical study and pooled data analysis. Int J Radiat Oncol Biol Phys. 2015;92:138–143. doi: 10.1016/j.ijrobp.2015.01.047. [DOI] [PubMed] [Google Scholar]
- 24.Morris S., Scarisbrick J., Frew J., eta, al. The results of Low Dose Total Skin Electron Beam Radiotherapy (TSEB), in patients with mycosis fungoides from the UK cutaneous lymphoma group. Int J Radiat Oncol Biol Phys. 2017;99:627–633. doi: 10.1016/j.ijrobp.2017.05.052. [DOI] [PubMed] [Google Scholar]
- 25.Kroeger K., Elsayad K., Moustakis C. Low-dose total skin electron beam therapy for cutaneous lymphoma. Strahlenther Onkol. 2017;193:1024–1030. doi: 10.1007/s00066-017-1188-8. [DOI] [PubMed] [Google Scholar]
- 26.Platoni K., Diamantopoulos S., Dilvoi M., Delinikolas P., Kypraiou E., Efstathopoulos E. First application of hemi-body electron beam irradiation for Kaposi sarcoma at the lower extremities. J BUON. 2018;23:268–272. [PubMed] [Google Scholar]
- 27.Delinikolas P., Patatoukas G., Kouloulias V., Dilvoi M., Plousi A., Efstathopoulos E. A novel Hemi-Body Irradiation technique using electron beams (HBIe) Phys Med. 2018;46:16–24. doi: 10.1016/j.ejmp.2017.12.022. [DOI] [PubMed] [Google Scholar]
- 28.Diamantopoulos S., Kantemiris I., Patatoukas G., Dilvoi M., Efstathopoulos E., Kouloulias V. Theoretical and experimental determination of scaling factors in electron dosimetry for 3D-printed polylactic acid. Med Phys. 2018;45:1708–1714. doi: 10.1002/mp.12790. [DOI] [PubMed] [Google Scholar]
- 29.Elsayad K., Kriz J., Moustakis C. Total skin electron beam for primary cutaneous T-cell lymphoma. Int J Radiat Oncol Biol Phys. 2015;93:1077–1086. doi: 10.1016/j.ijrobp.2015.08.041. [DOI] [PubMed] [Google Scholar]
- 30.Chowdhary M., Kabbani A.A., Rimtepathip P. Rapidly progressive stage IVB mycosis fungoides treated with low-dose total skin electron beam therapy. Oncol Targets Ther. 2015;8:1597–1601. doi: 10.2147/OTT.S87219. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Gamble M., Tocci E., DeSimone J. Low dose total skin electron beam radiation in cutaneous T-Cell lymphoma: review. J Cancer Ther. 2014;5:1372–1379. [Google Scholar]