Abstract
Childhood trauma (CT) has repeatedly been associated with cognitive deficits in patients with psychosis but many inconsistencies have been reported so that the nature of the relationship remains unclear. The purpose of this review was to better characterize the contribution of CT to cognitive deficits by considering the type, severity and frequency of childhood traumatic events and their relationships with psychosis at all stages.
Relevant studies were identified via electronic and manual literature searches and included original studies that investigated the relationship between CT and higher cognitive performance or social cognitive performance in patients with schizophrenia, bipolar disorder and psychosis at all stages of the illness stages (i.e. ultra-high risk, first episode or chronic phase).
Overall, a majority of studies reported that patients who experienced CT displayed deficits in general cognitive ability compared to patients with psychosis without such a history. Associations between CT and other cognitive function were more mixed. When comparing patient groups, the association between CT and cognitive function was more inconsistent in patients with chronic schizophrenia than in healthy participants, ultra-high risk individuals, first-episode patients and patients with chronic bipolar disorder.
In understanding the variability in the reported relationships between CT and cognition across study populations, we highlight the variety of questionnaires used and discuss the likelihood of there being differences in cognitive function based on specific stressors, severity and frequency. Finally, we consider future research steps that may shed light on psychobiological mechanisms underlying CT and cognitive performance in patients with psychosis.
Keywords: Childhood trauma, Schizophrenia, Psychosis, Cognitive function, Social cognitive function
Abbreviations
- CAQ
Childhood Adversity Questionnaire
- CATS
Child Abuse and Trauma Scale
- CECA-Q
Childhood Experience of Care Abuse Questionnaire
- CT
Childhood trauma
- CTQ
Childhood Trauma Questionnaire
- ETI
Early Trauma Inventory
- IQ
Intelligence Quotient
- MACE Scale
Maltreatment and Abuse Chronology of Exposure Scale
- RFQ
Risky Families Questionnaire
1. Introduction
1.1. Stress and cognition in schizophrenia and bipolar disorder
Cognitive deficits are highly predictive of social and occupational function in schizophrenia (Green, 2016; Green et al., 2012) (Horan et al., 2012) and related psychotic disorders, such as bipolar disorder with psychotic features (Vlad et al., 2018) and psychosis (Lysaker et al., 2018). These deficits often precede the emergence of clinical symptoms, and are relatively stable during the course of schizophrenia and bipolar disorder (Hill et al., 2008; Kuswanto et al., 2015a; Kuswanto et al., 2015b). Epidemiological and clinical studies have also consistently highlighted the impact of stress-related environmental risk factors, including childhood trauma (CT) or childhood adversity (including physical abuse, physical neglect, emotional abuse, emotional neglect and sexual abuse) (Day et al., 1987; Lataster et al., 2012; Mayo et al., 2017; Holtzman et al., 2013; Bechdolf et al., 2010; Gershon et al., 2013; Thompson et al., 2009; van Os et al., 2010; Walder et al., 2014), urbanicity (McGrath et al., 2004; Newbury et al., 2017), and cannabis use (Murray et al., 2017) in the development of psychosis (Aiello et al., 2012; Fusar-Poli et al., 2017; Misiak et al., 2017).
The potential predisposition of genetic risk factors to both CT and cognitive deficits in psychosis has received growing attention in recent years, in particular with the onset of candidate gene studies. Functional polymorphisms of the Catechol-O-Methyltransferase (COMT) genotype (Green et al., 2014), the FK506 binding protein 5 gene (Green et al., 2015) and brain-derived neurotrophic factor (BDNF) Val66Met polymorphism (Theleritis et al., 2014; Aas et al., 2013) have been associated to cognitive deficits after the experience of CT.
A relationship between CT exposure and cognitive deficits in psychosis has previously been hypothesized (Gilbertson et al., 2002; Cancel et al., 2015; Belanoff et al., 2001; Millan et al., 2012). Recent reviews have discussed the role of CT and cognitive function in psychosis in terms of (1) cognitive function and first-episode of psychosis (Aas et al., 2014) and (2) biological stress pathways in schizophrenia (Ruby et al., 2014). Despite these compelling relationships between CT and cognitive function in patients with psychosis due to inconsistent findings it is not known whether these relationships differ according to the type of psychotic disorder (i.e. schizophrenia, bipolar disorder and psychosis) and the phase of the disorder. The investigation of associations between CT and cognitive function in psychosis across these psychotic disorders is warranted given the similarities in genetic impact (Cardno and Owen, 2014) on the one hand, and evidence of differentiation between the disorders based on cognitive performance (Aas et al., 2012b) and brain structure abnormalities (Liberg et al., 2016) on the other hand. Furthermore, evidence of differences in cognitive function, brain structure and brain function over the course of the psychotic disorders (Lewandowski et al., 2011) - from the stages of ultra-high risk, first episode, to chronic illness - which is supported by evidence showing that CT changes brain development (Teicher et al., 2016).
In addition, it has been suggested that stress experience and stress response are probable factors to explain the relationship between CT and cognitive deficits in patients with psychosis and healthy participants (Sapolsky et al., 1986; Wolf, 2003). However, studies vary widely on the investigation of CT ranging from general CT (as measured with total CT scores) and specific CT events (for example, physical abuse or physical neglect) in patients with schizophrenia, bipolar disorder or psychosis. The differentiation between general versus specific CT across psychotic disorders is warranted after evidence of specific CT events, such as emotional abuse, relating to bipolar disorder has been reported (Etain et al., 2010; Etain et al., 2008), whereas physical abuse appears to be more prevalent in schizophrenia (Etain et al., 2010, Etain et al., 2008).
The objective of this systematic review was to summarize the evidence for an association between CT and cognitive function in patients with psychosis by considering three major points: (1) Types of psychosis, (2) Phases of the psychotic disorders, and (3) Comparison between the general versus specific events of CT. To this end, we reviewed original studies that investigated the role of CT on cognitive or social cognitive performance in patients with schizophrenia, bipolar disorder and psychosis across all phases of the illness in two steps: Firstly, we reviewed the general role of CT on general cognitive ability, memory function and executive function as well as social cognitive function (such as emotion recognition and Theory of Mind). Secondly, we considered detailed information of specific stressors, and frequency and severity of CT. We discussed whether patients with different types of psychotic disorders at different stages can be differentiated based on these relationships which could improve clinical diagnosis. Finally, we considered future research steps that can advance our insights into psychobiological mechanisms by embracing the diversity of CT experience as one of the crucial components to understand the complex relationships between CT and cognitive function in psychosis.
2. Materials and methods
2.1. Literature search strategy
We conducted a systematic literature search using PubMed and PsycINFO for original papers studying the relationship between adverse childhood events and general cognitive and social cognitive performance in patients with schizophrenia, bipolar disorder, and psychosis, published between 1993 and December 2017. We used combinations of the following key terms: ((childhood trauma OR childhood adverse OR childhood adversity OR childhood maltreatment OR developmental trauma OR early life stress OR social stress OR chronic stress OR physical abuse OR sexual abuse OR emotional abuse OR neglect) AND (cognition OR cognitive OR higher cognition OR social cognition) AND (schizophrenia OR bipolar disorder OR psychosis)). Adverse childhood events, such as loss of a family member, divorce of parents, or migration were not included in the literature search. We included only studies that assessed the direct relationship between CT and higher cognition (e.g. general cognitive ability, working memory) and/or between childhood trauma and social cognition (e.g. theory of mind, emotion recognition) in adult patients with schizophrenia, bipolar disorder and psychosis. Neuroimaging studies were excluded. Studies were also searched through references of selected articles. Authors have been contacted for detailed information if not reported in the manuscript.
2.2. Inclusion criteria
Identified studies were screened and selected for inclusion using the following criteria: (1) Original and peer-reviewed articles published in an English language journal, (2) Adult patients with schizophrenia, bipolar disorder or psychosis, and healthy participants were included in the study; (3) Data on chronic stress were reported; (4) Data on higher cognitive or social cognitive performance were reported.
2.3. Study characteristics
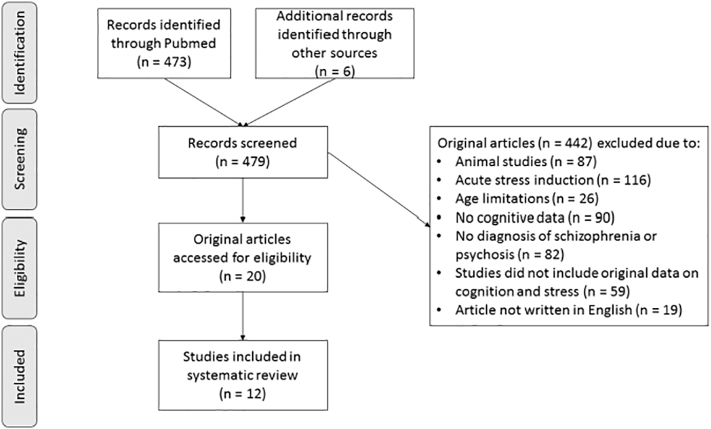
The literature search identified 473 studies in total, of which eight studies were original studies that matched the study criteria. Six additional studies were identified through review of references of these papers. The PRISMA flow diagram is presented in Fig. 1 which displays the number of included and excluded studies as well as the reasons for exclusion. After the initial 12 studies were included, we re-ran the literature search as described above and identified nine additional studies in which a healthy participant group was missing but all other inclusion criteria were reported as outlined above. These nine additional studies were included in the systematic research. Therefore, in total 21 studies were included.
Fig. 1.
Flow diagram selection of study process.
These 21 studies were grouped into studies that addressed higher cognitive function or social cognitive function. For higher cognitive function, three studies were conducted in individuals at ultra-high risk of psychosis (Ucok et al., 2015) or in patients with first-episode psychosis (Aas et al., 2011; Aas et al., 2012a), three studies included patients with chronic bipolar disorder (Poletti et al., 2017; Bucker et al., 2013; Marshall et al., 2016), nine studies were conducted in patients with chronic schizophrenia (Green et al., 2015; Green et al., 2014; McCabe et al., 2012; Kelly et al., 2016; Shannon et al., 2011; Schenkel et al., 2005; Lysaker et al., 2002; Ruby et al., 2017; Li et al., 2017) and three studies in patients with mixed psychosis group – consisting primarily of patients with chronic schizophrenia (van Os et al., 2017; Aas et al., 2012b; Mansueto et al., 2017).
For social cognitive function, three studies examined whether the experience of CT was associated with social cognitive performance in: (1) patients with first-episode psychosis and healthy participants (Garcia et al., 2016), (2) patients with psychosis and healthy participants (Schalinski et al., 2017), (3) and patients at either ultra-high risk of psychosis, patients with first-episode psychosis, patients with chronic bipolar disorder or schizophrenia, and healthy participants (Palmier-Claus et al., 2016).
3. Results
3.1. Childhood trauma and cognitive function in individuals at ultra-high risk of psychosis and patients with first-episode psychosis
A range of neuropsychological tests was administered to measure general cognitive ability, memory function, and executive function in the three included studies of individuals at ultra-high risk of psychosis and patients with first-episode psychosis (Table 1). Of these, two studies administered the Childhood Experience of Care Abuse Questionnaire (CECA-Q) (Aas et al., 2011; Aas et al., 2012a), and one study used the Childhood Trauma Questionnaire (CTQ) (Ucok et al., 2015). The CTQ and the CECA-Q are self-report questionnaires designed to assess childhood trauma occurring before the age of 17 years (for the CECA-Q) and 18 years (for the CTQ). For the CTQ, five subscales of childhood trauma are assessed (i.e. physical abuse, physical neglect, emotional abuse, emotional neglect, sexual abuse). Typically, pre-defined cut-offs are used to determine frequency and severity of childhood trauma for each of the five subscales according to the manual (Bernstein et al., 1994). Similarly for the CECA-Q, pre-determined criteria and cut-offs are applied to assess severity on each of the subscales for traumatic childhood events (i.e. lack of parental care, physical abuse and sexual abuse) (Bifulco et al., 2005).
Table 1.
Childhood trauma and cognitive function in patients at ultra-high risk of psychosis, patients with first-episode psychosis and healthy participants.
| Study | Participants (Patients/Healthy participants) N |
Gender (Patients/Healthy participants) Male : Female |
Age (Patients/Healthy participants) Mean/SD |
Stress questionnaire | Cognitive domains and tests | Main findings – CT and cognitive performance |
|---|---|---|---|---|---|---|
| Patients at ultra-high risk of psychosis | ||||||
| Ucok et al., 2015 | UHR = 53 | UHR = 39 : 14 | UHR = 21.10 ± 4.80 | CTQ (28-item version) |
|
|
| Patients with first-episode psychosis and healthy participants | ||||||
| Aas et al., 2012a | FEP = 83 HP = 63 |
FEP = 52 : 32 HP = 26 : 37 |
FEP = 27.40 ± 7.90 HP = 28.00 ± 7.70 |
CECA-Q |
|
|
| Aas et al., 2011 | FEP = 138 HP = 138 |
FEP= 73 : 65 HP = 64 : 74 |
FEP = 30.6 ± 10.9 HP = 32.2 ± 9.3 |
CECA-Q |
|
|
Abbreviations: CECA-Q, Childhood Experience of Care Abuse Questionnaire; CT, Childhood trauma; CTQ, Childhood Trauma Questionnaire; FEP, patients with first-episode psychosis; HP, healthy participants; IQ, intelligence quotient; NART, National Adult Reading Test; UHR, Ultra – high risk subjects of schizophrenia; WAIS-III, Wechsler Adult Intelligence Scale - III; WAIS-R, Wechsler Adult Intelligence Scale - Revised; WCST, Wisconsin Card Sorting Test; WMS-III, Wechsler Memory Scale; WMS-R, Wechsler Memory Scale – Revised; WTAR, Wechsler Test of Adult Reading.
One out of three studies reported a total severity score which was based on the CTQ (Ucok et al., 2015). While no study reported frequency findings directly, two out of three studies dichotomized frequency/severity of CT experience on the basis of absent/low versus high levels (Aas et al., 2012a; Aas et al., 2011), and one study did so on the basis of low versus high levels (Ucok et al., 2015) (Online Resource 1).
3.1.1. General role and stressor-specific roles of childhood trauma on cognitive function
In the only study that tested individuals at ultra-high risk for psychosis, the authors reported two significant associations between CT and cognitive function. Firstly, individuals with experience of physical trauma performed worse on executive function than individuals without such exposure. Secondly, a significant negative association between increased physical neglect exposure and poorer performance on working memory was also reported (Ucok et al., 2015). No associations with either emotional or sexual abuse, physical or emotional neglect and any cognitive measure were reported. Finally, when experience of CT as a general measure was combined into a total score, no significant associations were observed.
In patients with first-episode psychosis, both studies reported significant negative correlations between greater CT experience and reduced performance on measures of either general cognitive ability (Aas et al., 2011; Aas et al., 2012a) or executive function (Aas et al., 2012a). Of note, comparable CT levels between patients with first-episode psychosis and healthy participants were reported, in contrast to the higher rate of CT levels in individuals at the ultra-high risk phase (Ucok et al., 2015). Each of these studies reported associations with the general CT score only and not individual types of CT.
3.2. Childhood trauma and cognitive function in patients with chronic schizophrenia, bipolar disorder or psychosis
In the 15 studies reviewed, a variety of neuropsychological tasks were used to measure general cognitive ability, memory function and executive function (Table 2). Across these studies, three different questionnaires and one standardized interview were used for the retrospective subjective assessment of CT: (1) the Risky Families Questionnaire (RFQ; (Taylor et al., 2006)), (2) the CTQ, (3) the Childhood Adversity Questionnaire (CAQ; (Rosenman and Rodgers, 2004)), and (4) the Early Trauma Inventory (ETI; (Bremner et al., 2000)). Instead of questionnaires, one additional study based their assessment of childhood adversity on information retrieved from medical charts and unstructured patient interviews (Online Resource 2). The RFQ assesses severity of adverse childhood events, including harsh parenting and overt family conflict (Taylor et al., 2006). The CAQ measures the frequency of childhood maltreatment across 21 items (Rosenman and Rodgers, 2004). The ETI is a 56-item clinical interview for the assessment of physical abuse, emotional abuse, and sexual abuse before and after the age of 18 years. In addition, general traumatic events are measured ranging from parental loss to natural disasters. Frequency is assessed based on the developmental stage, onset and termination of the events, perpetrator, and effect on the individual (Bremner et al., 2000).
Table 2.
Childhood trauma and cognitive function in patients with established bipolar disorder, schizophrenia, psychosis and healthy participants.
| Study | Participants (Patients/Healthy participants) N |
Gender (Patients/Healthy participants) Male : Female |
Age (Patients/Healthy participants) Mean/SD |
Stress questionnaire | Cognitive domains and tests | Main findings – CT and cognitive performance |
|---|---|---|---|---|---|---|
| Patients with established bipolar disorder and healthy participants | ||||||
| Poletti et al., 2017 | BD = 76 HP = 90 |
BD = 51 : 51 HP = 24 : 33 |
BD = 47.75 ± 11.58 HP = 29.60 ± 10.55 |
RFQ |
|
|
| Bucker et al., 2013 | BP = 64 HP = 28 |
BP = 28 : 36 HP = 11 : 17 |
BP = 22.89 ± 4.36 HP = 22.78 ± 4.90 |
CTQ (28 – item version) |
|
|
| Marshall et al., 2016 | BP = 233 HP = 90 |
BP w CT = 32 : 85 BP w/o CT = 42 : 74 HP w CT = 8 : 9 HP w/o CT = 28 : 45 |
BP w CT = 40.90 ± 11.40 BP w/o CT = 38.70 ± 13.10 HP w CT = 36.50 ± 14.20 HP w/o CT = 37.40 ± 14.10 |
CTQ (28 – item version) |
|
|
| Patients with established schizophrenia and bipolar disorder | ||||||
| Aas et al., 2012b | SZ = 239 BP = 167 |
SZ + BP = 214 : 192 | SZ + BP = 30.07 ± 3.00 | CTQ (28 – item version) |
|
|
| Patients with psychosis and healthy participants | ||||||
| van Os et al., 2017 | PSY = 1119 (SZ = 940) Siblings of PSYa = 1059 HP = 586 |
PSY = 850 : 269 Siblings of PSY1 = 487 : 572 HP = 270 : 316 |
PSY = 27.57 ± 7.95 Siblings of PSY1 = 27.83 ± 8.27 HP = 30.42 ± 10.58 |
CTQ (25-item version) |
|
|
| Patients with psychosis | ||||||
| Mansueto et al., 2017b | PSY = 532 | PSY = 402 : 130 | PSY = 27.61 (7.61) | CTQ (25-item version) |
|
|
| Patients with established schizophrenia and healthy participants | ||||||
| Green et al., 2015 | SZ = 617 HP = 659 |
SZ = 415 : 202 HP = 291 : 368 |
SZ = 39.65 ± 10.82 HP = 42.48 ± 13.58 |
CAQ (20-item version) |
|
|
| Green et al., 2014 | SZ = 617 HP = 659 |
SZ = 415 : 202 HP = 291 : 368 |
SZ = 39.65 ± 10.82 HP = 42.48 ± 13.58 |
CAQ (20-item version) |
|
|
| McCabe et al., 2012 | SZ = 408 HP = 267 |
SZ = 65.7 % male HP = 43.4 % male |
SZ = 40.72 ± 11.07 HP = 39.27 ± 13.70 |
CAQ (20 item version) |
|
|
| Patients with established schizophrenia | ||||||
| Ruby et al., 2017 | SZ = 28 | SZ = 20 : 8 | SZ = 31.50 (9.70) | ETI |
|
|
| Li et al., 2017 | SZ = 162 | SZ = 58 : 104 | SZ = 37.82 ± 10.16 | CTQ (28 – item version) |
|
|
| Kelly et al., 2016 | SZ = 80 | SZ = 56 : 24 Females with PA = 10 Females w/o PA = 14 Males with PA = 11 Males w/o PA = 45 |
Females with PA = 37.80 ± 10.80 Females w/o PA = 32.60 ± 11.90 Males with PA = 30.90 ± 7.70 Males w/o PA = 31.6 ± 9.80 |
CTQ (28-item version) |
|
|
| Shannon et al., 2011 | SZ = 85 | SZ = 67 : 18 | SZ = 41.10 ± 11.70 | CTQ (28-item version) |
|
|
| Schenkel et al., 2005 | SZ SZ = 40 | SZ = 25 : 15 SZ with ACE = 10 : 8 SZ w/o ACE = 15 : 7 |
SZ = 41.90 ± 10.70 | Medical charts and interview |
|
|
| Lysaker et al., 2002 | SZ = 36 | SZ = 36 : 0 | SZ = 46.00 ± 10.00 | CAQ |
|
|
Abbreviations: BACS, Brief Assessment of Cognition in Schizophrenia; BD, patients with bipolar disorder; CANTAB, Cambridge Neuropsychological Automated Test Battery; CATS, The Child Abuse and Trauma Scale, CAQ, Childhood Adversity Questionnaire; COWAT, Controlled Oral Word Association Test; CT, Childhood trauma; CTQ, Childhood Trauma Questionnaire; CVLT, California Verbal Learning Test; ETI, Early Trauma Inventory; HP, healthy participants; IQ, intelligence quotient; NART, National Adult Reading Test; PSY, patients with psychosis; RBANS, Repeated Battery for Assessment of Neuropsychological Status; RFQ, Risky Families Questionnaire; RST, Response Shifting Task; SD, Standard Deviation; SZ, Patients with schizophrenia; TMT, Trail Making Task; WASI, Wechsler Abbreviated Scale of Intelligence; WAIS-III, Wechsler Adult Intelligence Scale - III; WCST, Wisconsin Card Sorting Test; WSM-III, Wechsler Memory Scale; WTAR, Wechsler Test of Adult Reading.
No psychiatric diagnosis.
Only baseline data reported.
Five studies reported total severity scores, which were based on the RFQ (Poletti et al., 2017), the CAQ (Green et al., 2014), the CTQ (Marshall et al., 2016; Mansueto et al., 2017), and information retrieved from medical charts and non-standardized interviews (Schenkel et al., 2005). Thirteen studies used a composite score of severity and frequency and used dichotomous variables of CT exposure of (1) low versus high levels (Poletti et al., 2017; Aas et al., 2012b; Shannon et al., 2011; Mansueto et al., 2017), (2) absent versus high levels (Bucker et al., 2013; Kelly et al., 2016), (3) absent versus existent levels (van Os et al., 2017; Green et al., 2014; Green et al., 2015; McCabe et al., 2012; Schenkel et al., 2005) or (iv) below versus above the median split (Aas et al., 2012b) for the subscales or total scores. However not all studies reported mean group-level data for these measures. One study also utilized CT severity and frequency as a continuous variable (van Os et al., 2017).
3.2.1. General versus stressor-specific roles of childhood trauma on cognitive function in patients with chronic bipolar disorder
For patients with bipolar disorder, four studies found evidence of an association between greater CT experience and poorer cognitive performance (Bucker et al., 2013; Poletti et al., 2017; Aas et al., 2012b; Marshall et al., 2016) (Table 2). This association was observed for measures of general cognitive ability (Poletti et al., 2017; Aas et al., 2012b), memory function (Bucker et al., 2013) and executive function (Marshall et al., 2016). A similar negative association was also observed in a healthy participant group in the study by Bucker et al. (2013) and in a combined patient/healthy participant group in the study by Poletti et al. (2017) (Bucker et al., 2013; Poletti et al., 2017). Of these studies, only Aas et al. (2012b) reported stressor-specific roles, which provided the basis for a significant association between increased levels of physical abuse, sexual abuse and physical neglect which was linked with reduced general cognitive ability in patients with bipolar disorder but not in patients with schizophrenia (Aas et al., 2012b). No significant associations between other specific stressors, such as emotional neglect were observed for general cognitive ability, memory or executive function.
3.2.2. General versus stressor-specific roles of childhood trauma on cognitive function in patients with chronic schizophrenia
In studies of patients with schizophrenia, patients generally showed significantly higher rates of CT and reduced cognitive performance compared to healthy participants. Five studies reported significant negative associations between increased CT exposure and poorer cognitive performance, one study reported significant positive associations between increased CT exposure and improved cognitive performance, and five studies reported no significant associations between CT exposure and cognitive performance (see Table 2).
Among the studies that reported an association between increased CT levels and poorer cognitive performance, two studies reported that increased CT levels were associated with poorer general cognitive ability in both patients and healthy participants (McCabe et al., 2012; Green et al., 2015). Four further studies showed significant negative associations between increased CT history and reduced cognition in patients only, either in terms of memory function (Shannon et al., 2011; Mansueto et al., 2017; Li et al., 2017), executive function (Li et al., 2017) or general cognitive ability (Schenkel et al., 2005). In the study by Shannon et al. (2011), however, results were equivocal as increased CT experience was also associated with improved general cognitive ability and working memory function.
In four additional studies, no associations between CT and cognitive function were observed (Green et al., 2014; Lysaker et al., 2002; Kelly et al., 2016; Ruby et al., 2017). In one study by van Os et al. (2017), separate within-group analyses in patients and healthy participants identified a relationship between higher rates of CT and reduced general cognitive ability in healthy participants only but not in patients with schizophrenia (van Os et al., 2017). Differences between the studies cannot be easily accounted for in terms of differences in gender frequency or samples sizes.
Three studies focused their investigation on the role of specific CT types and either general cognitive ability (Kelly et al., 2016; Schenkel et al., 2005), memory function (Kelly et al., 2016; Li et al., 2017) or executive function (Li et al., 2017) in patients with schizophrenia rather than exploring general adversity relationships. Evidence for a relationship between increased levels of physical abuse, sexual abuse and physical neglect on either reduced memory or executive function was observed in two studies (Schenkel et al., 2005; Li et al., 2017), while one study failed to find evidence of a relationship between these stressors and cognitive function (Kelly et al., 2016).
3.3. Childhood trauma and social cognitive function
In three studies that included a measure of social cognition, the measures used included Theory of Mind and emotion recognition (Palmier-Claus et al., 2016), and the MATRICS emotional self-regulation subtest (Garcia et al., 2016; Schalinski et al., 2017) (see Table 3).
Table 3.
Childhood trauma and social cognitive function in patients at ultra-high risk of psychosis, patients with first episode of psychosis, patients with established schizophrenia and healthy participants.
| Study | Participants (Patients/Healthy participants) N |
Gender (Patients/Healthy participants) Male : Female |
Age (Patients/Healthy participants) Mean/SD |
Stress questionnaire | Cognitive domains and tests | Main findings – CT and cognitive performance |
|---|---|---|---|---|---|---|
| Schalinski et al., 2017 | PSY = 168 (SZ = 134) HP = 50 |
PSY = 112 : 56 HP = 28 : 22 |
PSY = 27.90 ± 8.40 HP = 26.80 ± 7.90 |
MACE Scale |
|
|
| Palmier-Claus et al., 2016 | SZ = 20 FEP = 20 UHR = 14 HP = 120 |
SZ = 13 : 7 FEP = 16 : 4 UHR = 6 : 8 HP = 35 : 85 |
SZ = 39.6 ± 8.9 FEP = 24.6 ± 5.2 UHR = 22.6 ± 5.2 HP = 20.1 ± 2.5 |
CTQ (28-item version) |
|
|
| Garcia et al., 2016 | FEP = 79 HP = 58 |
FEP = 48 : 31 HP = 30 : 28 |
FEP (M) = 24.4 ± 4.5 FEP (F) = 26.8 ± 5.9 HP (M) = 24.0 ± 4.8 HP (F) = 23.9 ± 4.2 |
CTQ (28-item version) |
|
|
Abbreviations: CANTAB, Cambridge Neuropsychological Automated Test Battery; CTQ, Childhood Trauma Questionnaire; CT, Childhood trauma; HP, healthy participants; MACE Scale, Maltreatment and Abuse Chronology of Exposure; NART, National Adult Reading Test.
Two of the three studies used the original version of the CTQ to assess frequency and severity of CT experience (Garcia et al., 2016; Palmier-Claus et al., 2016), while Schalinski et al. (2017) assessed CT with the Maltreatment and Abuse Chronology of Exposure (MACE) Scale (Teicher and Parigger, 2015; Schalinski et al., 2017) (Online Resource 3). The 75-item MACE Scale assesses ten subscales of CT until the age of 18 years, such as physical abuse, verbal abuse, non-verbal emotional abuse, witnessing inter-parental abuse and abuse of siblings, peer-related verbal abuse, physical bullying, intra-familial, extra-familial and peer-related sexual abuse, emotional neglect and physical neglect. Severity scores were calculated based on dichotomized CT exposure of absent versus present experience for each year. In addition, multiplicity scores were defined as a number of different stressful event types (Teicher and Parigger, 2015). Group data for severity of CT was reported in two studies (Palmier-Claus et al., 2016; Schalinski et al., 2017). No further data for frequency or the composite score for severity and frequency for comparison between studies were available.
3.3.1. General versus stressor-specific roles of childhood trauma on social cognitive function
Two studies reported significant associations between greater CT exposure and poorer social cognitive performance, one in female patients with first-episode psychosis only (Garcia et al., 2016) and one in patients with psychosis more broadly (Schalinski et al., 2017). In contrast, Palmier-Claus et al. (2016) found no evidence of an association between CT levels and social cognitive performance for either Theory of Mind or emotion recognition in either patients or healthy participants (Palmier-Claus et al., 2016).
Schalinski et al. (2017) is the only study that investigated the association between specific stressful events and social cognition, reporting a significant negative association between increased levels of physical neglect at age eleven with poorer performance on social cognition in patients with psychosis only (Schalinski et al., 2017).
4. Discussion
4.1. Childhood trauma and cognitive function in schizophrenia, bipolar disorder and other psychotic disorders
The purpose of this systematic review was to summarize and discuss findings on relationships between CT and cognitive function in patients with schizophrenia, bipolar disorder with psychotic features and psychosis across all phases. In addition, we contrasted general versus specific events of CT on cognitive function. Significantly reduced cognitive function following the experience of CT was observed in patients with bipolar disorder and schizophrenia as well as healthy participants. Across study groups, associations between CT experience and cognitive performance were more variable in patients with chronic schizophrenia than in for either healthy participants, ultra-high-risk individuals, first-episode patients, or patients with chronic bipolar disorder, possibly owing to the high levels of cognitive heterogeneity in this group. In addition, associations between increased CT levels and reduced cognitive function varied considerably according to the self-reported CT severity in the studies – for example, whether the group comparisons were based on patients and healthy participants with present (i.e. low and high CT severity), only low CT severity or only high CT severity. The severity level is important for the interpretation of the findings which encompass resilience, susceptibility and compensation. For social cognition, CT studies were too few and equivocal to allow conclusions to be drawn.
An important focus of our review was to examine the general versus specific roles of CT on cognitive function. Across all patient and healthy participant groups, general cognitive ability was the domain most frequently associated with increased CT experience. Based on this data, CT may be understood as representing a moderator of general cognitive ability, both in patients with psychosis and healthy participants when CT frequency/severity is high. An equally important focus of our review was to assess whether specific types of CT differed in their cognitive effects. Here we found that the most consistently reported association between CT and cognition was for physical trauma, physical neglect or physical abuse which was significantly negatively correlated with both general and domain specific aspects of cognition.
This systematic review complements and substantially expands evidence covered by previous reviews (Aas et al., 2014; Millan et al., 2012; Ruby et al., 2014) by synthesizing the ever growing number of studies focused on the effects of CT on cognitive function in patients with psychosis at different stages of the illnesses. It highlights the variability in cognitive performance that is observed following general versus specific stressor types and severity/frequency. Furthermore, the evidence we present for deficits in general cognitive ability in healthy participants taking part in psychosis studies is consistent with evidence in non-psychiatric cognitive studies of children, adolescents (Pechtel and Pizzagalli, 2011) and healthy adults following the experience of CT (Majer et al., 2010; Pechtel and Pizzagalli, 2011; Nakao et al., 2013; Fuge et al., 2014; Philip et al., 2016; Lovallo et al., 2016; Lovallo et al., 2013).
4.2. Variability in cognitive effects following childhood trauma
While making these inferences, the evidence of inconsistencies between studies is also noteworthy. Potential reasons for the inconsistent findings of both decreased and increased cognitive performance in patients with schizophrenia, bipolar disorder and psychosis include the high variability of CT measurement and analysis approaches used. Three major points have been identified: Firstly, different stress questionnaires were administered that varied based on the assessment of a general CT score and specific stressful event type, CT severity and CT frequency. Secondly, use of dichotomous variables of CT scores differed between studies (i.e. total CT score; absent versus present CT scores); and thirdly, group data on severity and frequency were partly lacking. The inconsistent utilization of both CT assessment and data reporting across studies did not allow a comparison despite the application of standardized questionnaires and scoring of frequency/severity levels. However, the fact that the same measurement inconsistencies were found across all groups makes it difficult to explain the schizophrenia group-related inconsistencies purely on this basis.
We further discuss differences in individual stress sensitivities that could explain both increased and decreased cognitive function: While healthy individuals with low exposure to CT may be able to overcome these challenges so that they only become evident following more severe CT exposure, the same may not be true for patients. However, the lack of reported data on CT frequency/severity on an individual level or a subgroup of study participants does not allow us to prove or disprove this hypothesis. Recent findings strongly suggest though that the role of recent stress in an individual's life (Allott et al., 2015; Reininghaus et al., 2016a; Reininghaus et al., 2016b) and the potentially stressful testing situation (Muehlhan et al., 2011; Philip et al., 2016) impact on the performance and stress coping mechanism for patients with psychosis and healthy participants. However, those cumulative stress factors have only begun to be researched in the context of CT and cognitive function in patients with psychosis (Trotta et al., 2015; Ajnakina et al., 2016; Peters et al., 2016).
The inconsistent findings regarding the association between CT and cognition in this review may be also explained by the well documented variability in cognitive performance in schizophrenia. Variability in cognitive performance is partly explained by genetic risk in schizophrenia (Blokland et al., 2016), although at least some of these genetic risk factors are likely to influence also cognitive function in other psychotic disorders (Van Assche et al., 2017; Parellada et al., 2017; Ranlund et al., 2018). Secondary environmental factors, such a low occupational level and social isolation, have also been associated with cognitive performance in chronic schizophrenia and, to a lesser extent, in bipolar disorder (Karpov et al., 2017). Above and beyond the relationships between these genetic and environmental factors on cognition, these factors may also be important for the degree to which individuals can compensate for the deleterious outcomes of CT. Emerging research suggests that gender could be implicated in the relationship between CT and cognitive function in patients with psychosis (Pruessner et al., 2017a; Kocsis-Bogar et al., 2018).
4.3. Limitations and suggestions for further investigations
One limitation of this systematic review is the small number of studies available for each diagnostic group across the different illness stages. This limited our ability to make inferences about whether there are reliable distinctions about the roles of different illness stages regarding the relationships between CT and cognitive functions. In addition, other factors in the association between CT and cognition that we were unable to consider in this systematic review include biological factors such as endocrine processes, inflammatory processes and genetics. These factors were not included in this systematic review due to the very small number of available studies in psychosis for a systematic review.
Significant decreases in IL-6 levels and increases in HPA–axis hormone concentrations after acute stress have been repeatedly reported in healthy participants (Marsland et al., 2017; Steptoe et al., 2007; Slavish et al., 2015) and patients with first episode of psychosis (Pruessner et al., 2017b; Capuzzi et al., 2017). Similarly, increased levels of IL-6 after acute stress in healthy participants with experience of CT and increased hypothalamus-pituitary-adrenal (HPA)-axis reactivity in female healthy participants with prenatal exposures were found (Carpenter et al., 2010; Carpenter et al., 2017). Importantly, endocrine alterations have been shown to affect brain structure and function in the general population (Sapolsky et al., 1986; McEwen et al., 2015) and in psychosis (Ruby et al., 2014; Phillips et al., 2006; Quide et al., 2017; Cancel et al., 2017; Cancel et al., 2015) which is further supported by animal work (Bergink et al., 2014).
Data from CT studies are likely to be somewhat skewed by the lower prevalence of CT exposure in healthy participants than in patients with psychosis. In addition, many healthy participants have not experienced any CT events, whereas those with CT exposure have often experienced several types of stressful events with varying degrees of frequency and severity (Aas et al., 2012b; Shannon et al., 2011; Schenkel et al., 2005) which is rarely considered in the analysis and interpretation of data. Support for the relevance of severity levels on brain function comes from a recent review suggesting that these factors impact differently on brain function and functional networks (Teicher et al., 2016).
5. Conclusion
To our knowledge, this is the first systematic review to highlight the relevance of differentiating between a general role of CT or specific CT types and the varying factors for severity and frequency on cognitive function in individuals with schizophrenia, bipolar disorder and psychosis across all phases. Evidence of a deleterious role of CT on cognition is shown and gaps in our knowledge are highlighted which is based on the inconsistent use of CT assessment, data reporting and lack of a healthy control group in some studies.
Addressing these limitations in our current understanding about the relationship between CT and cognition has the potential to significantly improve our insights into the psychobiological pathways underlying cognitive deficits after the CT experience in patients with schizophrenia, bipolar disorder and psychosis. Optimized measurement of specific CT events and consistent reporting of CT severity is essential to elucidate these psychological mechanisms, as previously suggested for major depressive disorder (Steine et al., 2017). Furthermore, we suggest to study different CT severity levels in schizophrenia, bipolar disorder and psychosis because different CT severity levels allow a more detailed interpretation of reduced cognitive function in the context of resilient, susceptible and compensatory mechanisms which may necessitate different individualized treatment approaches. Additionally, we propose that longitudinal study designs for investigating cognitive function after CT experience should be integrated with the measurement at least one of the following stress markers: Daily stress levels, endocrine concentrations, immunological concentrations, and genetic markers to gain a better biological understanding of the long-term consequences changes of CT on adults. We promote a better understanding of relationships between CT experience and cognitive function in schizophrenia, bipolar disorder and psychosis across all phases of the disorders for optimized prevention and treatment approaches for coping after CT experience, cognitive function and psychosis.
Acknowledgments
Acknowledgements
Funding
This work was supported by the European Research Council (ERC-2015-STG-677467) and Science Foundation Ireland (SFI-16/ERCS/3787) awarded to GD.
Conflict of interest
The authors declare that they have no conflict of interest.
Footnotes
Supplementary data to this article can be found online at https://doi.org/10.1016/j.scog.2018.11.001.
Contributor Information
Maria R. Dauvermann, Email: maria.dauvermann@nuigalway.ie.
Gary Donohoe, Email: gary.donohoe@nuigalway.ie.
Appendix A. Supplementary data
Supplementary tables
References
- Aas M., Dazzan P., Fisher H.L., Morgan C., Morgan K., Reichenberg A., Zanelli J., Fearon P., Jones P.B., Murray R.M., Pariante C.M. Childhood trauma and cognitive function in first-episode affective and non-affective psychosis. Schizophr. Res. 2011;129:12–19. doi: 10.1016/j.schres.2011.03.017. [DOI] [PubMed] [Google Scholar]
- Aas M., Navari S., Gibbs A., Mondelli V., Fisher H.L., Morgan C., Morgan K., Maccabe J., Reichenberg A., Zanelli J., Fearon P., Jones P.B., Murray R.M., Pariante C.M., Dazzan P. Is there a link between childhood trauma, cognition, and amygdala and hippocampus volume in first-episode psychosis? Schizophr. Res. 2012;137:73–79. doi: 10.1016/j.schres.2012.01.035. [DOI] [PubMed] [Google Scholar]
- Aas M., Steen N.E., Agartz I., Aminoff S.R., Lorentzen S., Sundet K., Andreassen O.A., Melle I. Is cognitive impairment following early life stress in severe mental disorders based on specific or general cognitive functioning? Psychiatry Res. 2012;198:495–500. doi: 10.1016/j.psychres.2011.12.045. [DOI] [PubMed] [Google Scholar]
- Aas M., Haukvik U.K., Djurovic S., Bergmann O., Athanasiu L., Tesli M.S., Hellvin T., Steen N.E., Agartz I., Lorentzen S., Sundet K., Andreassen O.A., Melle I. BDNF val66met modulates the association between childhood trauma, cognitive and brain abnormalities in psychoses. Prog. Neuro-Psychopharmacol. Biol. Psychiatry. 2013;46:181–188. doi: 10.1016/j.pnpbp.2013.07.008. [DOI] [PubMed] [Google Scholar]
- Aas M., Dazzan P., Mondelli V., Melle I., Murray R.M., Pariante C.M. A systematic review of cognitive function in first-episode psychosis, including a discussion on childhood trauma, stress, and inflammation. Front. Psychol. 2014;4:182. doi: 10.3389/fpsyt.2013.00182. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Aiello G., Horowitz M., Hepgul N., Pariante C.M., Mondelli V. Stress abnormalities in individuals at risk for psychosis: a review of studies in subjects with familial risk or with "at risk" mental state. Psychoneuroendocrinology. 2012;37:1600–1613. doi: 10.1016/j.psyneuen.2012.05.003. [DOI] [PubMed] [Google Scholar]
- Ajnakina O., Trotta A., Oakley-Hannibal E., Di Forti M., Stilo S.A., Kolliakou A., Gardner-Sood P., Gaughran F., David A.S., Dazzan P., Pariante C., Mondelli V., Morgan C., Vassos E., Murray R.M., Fisher H.L. Impact of childhood adversities on specific symptom dimensions in first-episode psychosis. Psychol. Med. 2016;46:317–326. doi: 10.1017/S0033291715001816. [DOI] [PubMed] [Google Scholar]
- Allott K.A., Rapado-Castro M., Proffitt T.M., Bendall S., Garner B., Butselaar F., Markulev C., Phassouliotis C., Mcgorry P.D., Wood S.J., Cotton S.M., Phillips L.J. The impact of neuropsychological functioning and coping style on perceived stress in individuals with first-episode psychosis and healthy controls. Psychiatry Res. 2015;226:128–135. doi: 10.1016/j.psychres.2014.12.032. [DOI] [PubMed] [Google Scholar]
- Bechdolf A., Thompson A., Nelson B., Cotton S., Simmons M.B., Amminger G.P., Leicester S., Francey S.M., Mcnab C., Krstev H., Sidis A., Mcgorry P.D., Yung A.R. Experience of trauma and conversion to psychosis in an ultra-high-risk (prodromal) group. Acta Psychiatr. Scand. 2010;121:377–384. doi: 10.1111/j.1600-0447.2010.01542.x. [DOI] [PubMed] [Google Scholar]
- Belanoff J.K., Gross K., Yager A., Schatzberg A.F. Corticosteroids and cognition. J. Psychiatr. Res. 2001;35:127–145. doi: 10.1016/s0022-3956(01)00018-8. [DOI] [PubMed] [Google Scholar]
- Bergink V., Gibney S.M., Drexhage H.A. Autoimmunity, inflammation, and psychosis: a search for peripheral markers. Biol. Psychiatry. 2014;75:324–331. doi: 10.1016/j.biopsych.2013.09.037. [DOI] [PubMed] [Google Scholar]
- Bernstein D.P., Fink L., Handelsman L., Foote J., Lovejoy M., Wenzel K., Sapareto E., Ruggiero J. Initial reliability and validity of a new retrospective measure of child abuse and neglect. Am. J. Psychiatry. 1994;151:1132–1136. doi: 10.1176/ajp.151.8.1132. [DOI] [PubMed] [Google Scholar]
- Bifulco A., Bernazzani O., Moran P.M., Jacobs C. The childhood experience of care and abuse questionnaire (CECA.Q): validation in a community series. Br. J. Clin. Psychol. 2005;44:563–581. doi: 10.1348/014466505X35344. [DOI] [PubMed] [Google Scholar]
- Blokland G.A., Mesholam-Gately R.I., Toulopoulou T., Del Re E.C., Lam M., Delisi L.E., Donohoe G., Walters J.T., Consortium G., Seidman L.J., Petryshen T.L. Heritability of neuropsychological measures in schizophrenia and nonpsychiatric populations: A systematic review and meta-analysis. Schizophr. Bull. 2016;43(4):788–800. doi: 10.1093/schbul/sbw146. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bremner J.D., Vermetten E., Mazure C.M. Development and preliminary psychometric properties of an instrument for the measurement of childhood trauma: the Early Trauma Inventory. Depress. Anxiety. 2000;12:1–12. doi: 10.1002/1520-6394(2000)12:1<1::AID-DA1>3.0.CO;2-W. [DOI] [PubMed] [Google Scholar]
- Bucker J., Kozicky J., Torres I.J., Kauer-Sant'anna M., Silveira L.E., Bond D.J., Lam R.W., Yatham L.N. The impact of childhood trauma on cognitive functioning in patients recently recovered from a first manic episode: data from the Systematic Treatment Optimization Program for Early Mania (STOP-EM) J. Affect. Disord. 2013;148:424–430. doi: 10.1016/j.jad.2012.11.022. [DOI] [PubMed] [Google Scholar]
- Cancel A., Comte M., Truillet R., Boukezzi S., Rousseau P.F., Zendjidjian X.Y., Sage T., Lazerges P.E., Guedj E., Khalfa S., Azorin J.M., Blin O., Fakra E. Childhood neglect predicts disorganization in schizophrenia through grey matter decrease in dorsolateral prefrontal cortex. Acta Psychiatr. Scand. 2015;132:244–256. doi: 10.1111/acps.12455. [DOI] [PubMed] [Google Scholar]
- Cancel A., Comte M., Boutet C., Schneider F.C., Rousseau P.F., Boukezzi S., Gay A., Sigaud T., Massoubre C., Berna F., Zendjidjian X.Y., Azorin J.M., Blin O., Fakra E. Childhood trauma and emotional processing circuits in schizophrenia: a functional connectivity study. Schizophr. Res. 2017;184:69–72. doi: 10.1016/j.schres.2016.12.003. [DOI] [PubMed] [Google Scholar]
- Capuzzi E., Bartoli F., Crocamo C., Clerici M., Carra G. Acute variations of cytokine levels after antipsychotic treatment in drug-naive subjects with a first-episode psychosis: a meta-analysis. Neurosci. Biobehav. Rev. 2017;77:122–128. doi: 10.1016/j.neubiorev.2017.03.003. [DOI] [PubMed] [Google Scholar]
- Cardno A.G., Owen M.J. Genetic relationships between schizophrenia, bipolar disorder, and schizoaffective disorder. Schizophr. Bull. 2014;40:504–515. doi: 10.1093/schbul/sbu016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Carpenter L.L., Gawuga C.E., Tyrka A.R., Lee J.K., Anderson G.M., Price L.H. Association between plasma IL-6 response to acute stress and early-life adversity in healthy adults. Neuropsychopharmacology. 2010;35:2617–2623. doi: 10.1038/npp.2010.159. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Carpenter T., Grecian S.M., Reynolds R.M. Sex differences in early-life programming of the hypothalamic-pituitary-adrenal axis in humans suggest increased vulnerability in females: a systematic review. J. Dev. Orig. Health Dis. 2017;8:244–255. doi: 10.1017/S204017441600074X. [DOI] [PubMed] [Google Scholar]
- Day R., Nielsen J.A., Korten A., Ernberg G., Dube K.C., Gebhart J., Jablensky A., Leon C., Marsella A., Olatawura M. Stressful life events preceding the acute onset of schizophrenia: a cross-national study from the World Health Organization. Cult. Med. Psychiatry. 1987;11:123–205. doi: 10.1007/BF00122563. [DOI] [PubMed] [Google Scholar]
- Etain B., Henry C., Bellivier F., Mathieu F., Leboyer M. Beyond genetics: childhood affective trauma in bipolar disorder. Bipolar Disord. 2008;10:867–876. doi: 10.1111/j.1399-5618.2008.00635.x. [DOI] [PubMed] [Google Scholar]
- Etain B., Mathieu F., Henry C., Raust A., Roy I., Germain A., Leboyer M., Bellivier F. Preferential association between childhood emotional abuse and bipolar disorder. J. Trauma. Stress. 2010;23:376–383. doi: 10.1002/jts.20532. [DOI] [PubMed] [Google Scholar]
- Fuge P., Aust S., Fan Y., Weigand A., Gartner M., Feeser M., Bajbouj M., Grimm S. Interaction of early life stress and corticotropin-releasing hormone receptor gene: effects on working memory. Biol. Psychiatry. 2014;76:888–894. doi: 10.1016/j.biopsych.2014.04.016. [DOI] [PubMed] [Google Scholar]
- Fusar-Poli P., Tantardini M., De Simone S., Ramella-Cravaro V., Oliver D., Kingdon J., Kotlicka-Antczak M., Valmaggia L., Lee J., Millan M.J., Galderisi S., Balottin U., Ricca V., Mcguire P. Deconstructing vulnerability for psychosis: meta-analysis of environmental risk factors for psychosis in subjects at ultra high-risk. Eur. Psychiatry. 2017;40:65–75. doi: 10.1016/j.eurpsy.2016.09.003. [DOI] [PubMed] [Google Scholar]
- Garcia M., Montalvo I., Creus M., Cabezas A., Sole M., Algora M.J., Moreno I., Gutierrez-Zotes A., Labad J. Sex differences in the effect of childhood trauma on the clinical expression of early psychosis. Compr. Psychiatry. 2016;68:86–96. doi: 10.1016/j.comppsych.2016.04.004. [DOI] [PubMed] [Google Scholar]
- Gershon A., Johnson S.L., Miller I. Chronic stressors and trauma: prospective influences on the course of bipolar disorder. Psychol. Med. 2013;43:2583–2592. doi: 10.1017/S0033291713000147. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gilbertson M.W., Shenton M.E., Ciszewski A., Kasai K., Lasko N.B., Orr S.P., Pitman R.K. Smaller hippocampal volume predicts pathologic vulnerability to psychological trauma. Nat. Neurosci. 2002;5:1242–1247. doi: 10.1038/nn958. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Green M.F. Impact of cognitive and social cognitive impairment on functional outcomes in patients with schizophrenia. J. Clin. Psychiatry. 2016;77(Suppl. 2):8–11. doi: 10.4088/JCP.14074su1c.02. [DOI] [PubMed] [Google Scholar]
- Green M.F., Bearden C.E., Cannon T.D., Fiske A.P., Hellemann G.S., Horan W.P., Kee K., Kern R.S., Lee J., Sergi M.J., Subotnik K.L., Sugar C.A., Ventura J., Yee C.M., Nuechterlein K.H. Social cognition in schizophrenia, part 1: performance across phase of illness. Schizophr. Bull. 2012;38:854–864. doi: 10.1093/schbul/sbq171. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Green M.J., Chia T.Y., Cairns M.J., Wu J., Tooney P.A., Scott R.J., Carr V.J., Australian Schizophrenia Research, B Catechol-O-methyltransferase (COMT) genotype moderates the effects of childhood trauma on cognition and symptoms in schizophrenia. J. Psychiatr. Res. 2014;49:43–50. doi: 10.1016/j.jpsychires.2013.10.018. [DOI] [PubMed] [Google Scholar]
- Green M.J., Raudino A., Cairns M.J., Wu J., Tooney P.A., Scott R.J., Carr V.J., Australian Schizophrenia Research, B Do common genotypes of FK506 binding protein 5 (FKBP5) moderate the effects of childhood maltreatment on cognition in schizophrenia and healthy controls? J. Psychiatr. Res. 2015;70:9–17. doi: 10.1016/j.jpsychires.2015.07.019. [DOI] [PubMed] [Google Scholar]
- Hill S.K., Harris M.S., Herbener E.S., Pavuluri M., Sweeney J.A. Neurocognitive allied phenotypes for schizophrenia and bipolar disorder. Schizophr. Bull. 2008;34:743–759. doi: 10.1093/schbul/sbn027. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Holtzman C.W., Trotman H.D., Goulding S.M., Ryan A.T., MacDonald A.N., Shapiro D.I., Brasfield J.L., Walker E.F. Stress and neurodevelopmental processes in the emergence of psychosis. Neuroscience. 2013;249:172–191. doi: 10.1016/j.neuroscience.2012.12.017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Horan W.P., Green M.F., Degroot M., Fiske A., Hellemann G., Kee K., Kern R.S., Lee J., Sergi M.J., Subotnik K.L., Sugar C.A., Ventura J., Nuechterlein K.H. Social cognition in schizophrenia, part 2: 12-month stability and prediction of functional outcome in first-episode patients. Schizophr. Bull. 2012;38:865–872. doi: 10.1093/schbul/sbr001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Karpov B., Joffe G., Aaltonen K., Suvisaari J., Baryshnikov I., Naatanen P., Koivisto M., Melartin T., Oksanen J., Suominen K., Heikkinen M., Isometsa E. Level of functioning, perceived work ability, and work status among psychiatric patients with major mental disorders. Eur. Psychiatry. 2017;44:83–89. doi: 10.1016/j.eurpsy.2017.03.010. [DOI] [PubMed] [Google Scholar]
- Kelly D.L., Rowland L.M., Patchan K.M., Sullivan K., Earl A., Raley H., Liu F., Feldman S., Mcmahon R.P. Schizophrenia clinical symptom differences in women vs. men with and without a history of childhood physical abuse. Child Adolesc. Psychiatry Ment. Health. 2016;10:5. doi: 10.1186/s13034-016-0092-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kocsis-Bogar K., Meszaros V., Perczel-Forintos D. Gender differences in the relationship of childhood trauma and the course of illness in schizophrenia. Compr. Psychiatry. 2018;82:84–88. doi: 10.1016/j.comppsych.2018.01.007. [DOI] [PubMed] [Google Scholar]
- Kuswanto C., Chin R., Sum M.Y., Sengupta S., Fagiolini A., Mcintyre R.S., Vieta E., Sim K. Shared and divergent neurocognitive impairments in adult patients with schizophrenia and bipolar disorder: Whither the evidence? Neurosci. Biobehav. Rev. 2015;61:66–89. doi: 10.1016/j.neubiorev.2015.12.002. [DOI] [PubMed] [Google Scholar]
- Kuswanto C.N., Sum M.Y., Qiu A., Sitoh Y.Y., Liu J., Sim K. The impact of genome wide supported microRNA-137 (MIR137) risk variants on frontal and striatal white matter integrity, neurocognitive functioning, and negative symptoms in schizophrenia. Am. J. Med. Genet. B Neuropsychiatr. Genet. 2015;168B:317–326. doi: 10.1002/ajmg.b.32314. [DOI] [PubMed] [Google Scholar]
- Lataster J., Myin-Germeys I., Lieb R., Wittchen H.U., van OS J. Adversity and psychosis: a 10-year prospective study investigating synergism between early and recent adversity in psychosis. Acta Psychiatr. Scand. 2012;125:388–399. doi: 10.1111/j.1600-0447.2011.01805.x. [DOI] [PubMed] [Google Scholar]
- Lewandowski K.E., Cohen B.M., Ongur D. Evolution of neuropsychological dysfunction during the course of schizophrenia and bipolar disorder. Psychol. Med. 2011;41:225–241. doi: 10.1017/S0033291710001042. [DOI] [PubMed] [Google Scholar]
- Li X.B., Bo Q.J., Zhang G.P., Zheng W., Wang Z.M., Li A.N., Tian Q., Liu J.T., Tang Y.L., Wang C.Y. Effect of childhood trauma on cognitive functions in a sample of Chinese patients with schizophrenia. Compr. Psychiatry. 2017;76:147–152. doi: 10.1016/j.comppsych.2017.04.010. [DOI] [PubMed] [Google Scholar]
- Liberg B., Rahm C., Panayiotou A., Pantelis C. Brain change trajectories that differentiate the major psychoses. Eur. J. Clin. Investig. 2016;46:658–674. doi: 10.1111/eci.12641. [DOI] [PubMed] [Google Scholar]
- Lovallo W.R., Farag N.H., Sorocco K.H., Acheson A., Cohoon A.J., Vincent A.S. Early life adversity contributes to impaired cognition and impulsive behavior: studies from the Oklahoma Family Health Patterns Project. Alcohol. Clin. Exp. Res. 2013;37:616–623. doi: 10.1111/acer.12016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lovallo W.R., Enoch M.A., Acheson A., Cohoon A.J., Sorocco K.H., Hodgkinson C.A., Vincent A.S., Goldman D. Early-life adversity interacts with FKBP5 genotypes: altered working memory and cardiac stress reactivity in the Oklahoma Family Health Patterns Project. Neuropsychopharmacology. 2016;41:1724–1732. doi: 10.1038/npp.2015.347. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lysaker P.H., Wright D.E., Clements C.A., Plascak-Hallberg C.D. Neurocognitive and psychosocial correlates of hostility among persons in a post-acute phase of schizophrenia spectrum disorders. Compr. Psychiatry. 2002;43:319–324. doi: 10.1053/comp.2002.33493. [DOI] [PubMed] [Google Scholar]
- Lysaker P.H., Hamm J.A., Hasson-Ohayon I., Pattison M.L., Leonhardt B.L. Promoting recovery from severe mental illness: implications from research on metacognition and metacognitive reflection and insight therapy. World J. Psychiatry. 2018;8:1–11. doi: 10.5498/wjp.v8.i1.1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Majer M., Nater U.M., Lin J.M., Capuron L., Reeves W.C. Association of childhood trauma with cognitive function in healthy adults: a pilot study. BMC Neurol. 2010;10:61. doi: 10.1186/1471-2377-10-61. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mansueto G., Van Nierop M., Schruers K., Investigators G., Alizadeh B.Z., Bartels-Velthuis A.A., Van Beveren N.J., Bruggeman R., Cahn W., De Haan L., Delespaul P., Meijer C.J., Myin-Germeys I., Kahn R.S., Schirmbeck F., Simons C.J.P., Van Haren N.E.M., van Os J., Van Winkel R. The role of cognitive functioning in the relationship between childhood trauma and a mixed phenotype of affective-anxious-psychotic symptoms in psychotic disorders. Schizophr. Res. 2017;192:262–268. doi: 10.1016/j.schres.2017.04.003. [DOI] [PubMed] [Google Scholar]
- Marshall D.F., Passarotti A.M., Ryan K.A., Kamali M., Saunders E.F., Pester B., Mcinnis M.G., Langenecker S.A. Deficient inhibitory control as an outcome of childhood trauma. Psychiatry Res. 2016;235:7–12. doi: 10.1016/j.psychres.2015.12.013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Marsland A.L., Walsh C., Lockwood K., John-Henderson N.A. The effects of acute psychological stress on circulating and stimulated inflammatory markers: a systematic review and meta-analysis. Brain Behav. Immun. 2017;64:208–219. doi: 10.1016/j.bbi.2017.01.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mayo D., Corey S., Kelly L.H., Yohannes S., Youngquist A.L., Stuart B.K., Niendam T.A., Loewy R.L. The role of trauma and stressful life events among individuals at clinical high risk for psychosis: a review. Front. Psychol. 2017;8:55. doi: 10.3389/fpsyt.2017.00055. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McCabe K.L., Maloney E.A., Stain H.J., Loughland C.M., Carr V.J. Relationship between childhood adversity and clinical and cognitive features in schizophrenia. J. Psychiatr. Res. 2012;46:600–607. doi: 10.1016/j.jpsychires.2012.01.023. [DOI] [PubMed] [Google Scholar]
- McEwen B.S., Gray J.D., Nasca C. 60 years of neuroendocrinology: redefining neuroendocrinology: stress, sex and cognitive and emotional regulation. J. Endocrinol. 2015;226:T67–T83. doi: 10.1530/JOE-15-0121. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McGrath J., Saha S., Welham J., El Saadi O., Maccauley C., Chant D. A systematic review of the incidence of schizophrenia: the distribution of rates and the influence of sex, urbanicity, migrant status and methodology. BMC Med. 2004;2:13. doi: 10.1186/1741-7015-2-13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Millan M.J., Agid Y., Brune M., Bullmore E.T., Carter C.S., Clayton N.S., Connor R., Davis S., Deakin B., Derubeis R.J., Dubois B., Geyer M.A., Goodwin G.M., Gorwood P., Jay T.M., Joels M., Mansuy I.M., Meyer-Lindenberg A., Murphy D., Rolls E., Saletu B., Spedding M., Sweeney J., Whittington M., Young L.J. Cognitive dysfunction in psychiatric disorders: characteristics, causes and the quest for improved therapy. Nat. Rev. Drug Discov. 2012;11:141–168. doi: 10.1038/nrd3628. [DOI] [PubMed] [Google Scholar]
- Misiak B., Krefft M., Bielawski T., Moustafa A.A., Sasiadek M.M., Frydecka D. Toward a unified theory of childhood trauma and psychosis: a comprehensive review of epidemiological, clinical, neuropsychological and biological findings. Neurosci. Biobehav. Rev. 2017;75:393–406. doi: 10.1016/j.neubiorev.2017.02.015. [DOI] [PubMed] [Google Scholar]
- Muehlhan M., Lueken U., Wittchen H.U., Kirschbaum C. The scanner as a stressor: evidence from subjective and neuroendocrine stress parameters in the time course of a functional magnetic resonance imaging session. Int. J. Psychophysiol. 2011;79:118–126. doi: 10.1016/j.ijpsycho.2010.09.009. [DOI] [PubMed] [Google Scholar]
- Murray R.M., Englund A., Abi-Dargham A., Lewis D.A., Di Forti M., Davies C., Sherif M., Mcguire P., D'souza D.C. Cannabis-associated psychosis: neural substrate and clinical impact. Neuropharmacology. 2017;124:89–104. doi: 10.1016/j.neuropharm.2017.06.018. [DOI] [PubMed] [Google Scholar]
- Nakao T., Matsumoto T., Morita M., Shimizu D., Yoshimura S., Northoff G., Morinobu S., Okamoto Y., Yamawaki S. The degree of early life stress predicts decreased medial prefrontal activations and the shift from internally to externally guided decision making: an exploratory NIRS study during resting state and self-oriented task. Front. Hum. Neurosci. 2013;7:339. doi: 10.3389/fnhum.2013.00339. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Newbury J., Arseneault L., Caspi A., Moffitt T.E., Odgers C.L., Fisher H.L. Cumulative effects of neighborhood social adversity and personal crime victimization on adolescent psychotic experiences. Schizophr. Bull. 2017;44(2):348–358. doi: 10.1093/schbul/sbx060. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Palmier-Claus J., Berry K., Darrell-Berry H., Emsley R., Parker S., Drake R., Bucci S. Childhood adversity and social functioning in psychosis: exploring clinical and cognitive mediators. Psychiatry Res. 2016;238:25–32. doi: 10.1016/j.psychres.2016.02.004. [DOI] [PubMed] [Google Scholar]
- Parellada M., Gomez-Vallejo S., Burdeus M., Arango C. Developmental differences between schizophrenia and bipolar disorder. Schizophr. Bull. 2017;43:1176–1189. doi: 10.1093/schbul/sbx126. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pechtel P., Pizzagalli D.A. Effects of early life stress on cognitive and affective function: an integrated review of human literature. Psychopharmacology. 2011;214:55–70. doi: 10.1007/s00213-010-2009-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Peters E., Ward T., Jackson M., Morgan C., Charalambides M., Mcguire P., Woodruff P., Jacobsen P., Chadwick P., Garety P.A. Clinical, socio-demographic and psychological characteristics in individuals with persistent psychotic experiences with and without a "need for care". World Psychiatry. 2016;15:41–52. doi: 10.1002/wps.20301. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Philip N.S., Sweet L.H., Tyrka A.R., Carpenter S.L., Albright S.E., Price L.H., Carpenter L.L. Exposure to childhood trauma is associated with altered n-back activation and performance in healthy adults: implications for a commonly used working memory task. Brain Imaging Behav. 2016;10:124–135. doi: 10.1007/s11682-015-9373-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Phillips L.J., Mcgorry P.D., Garner B., Thompson K.N., Pantelis C., Wood S.J., Berger G. Stress, the hippocampus and the hypothalamic-pituitary-adrenal axis: implications for the development of psychotic disorders. Aust. N. Z. J. Psychiatry. 2006;40:725–741. doi: 10.1080/j.1440-1614.2006.01877.x. [DOI] [PubMed] [Google Scholar]
- Poletti S., Aggio V., Brioschi S., Dallaspezia S., Colombo C., Benedetti F. Multidimensional cognitive impairment in unipolar and bipolar depression and the moderator effect of adverse childhood experiences. Psychiatry Clin. Neurosci. 2017;71:309–317. doi: 10.1111/pcn.12497. [DOI] [PubMed] [Google Scholar]
- Pruessner M., Bechard-Evans L., Pira S., Joober R., Collins D.L., Pruessner J.C., Malla A.K. Interplay of hippocampal volume and hypothalamus-pituitary-adrenal axis function as markers of stress vulnerability in men at ultra-high risk for psychosis. Psychol. Med. 2017;47:471–483. doi: 10.1017/S0033291716002658. [DOI] [PubMed] [Google Scholar]
- Pruessner M., Cullen A.E., Aas M., Walker E.F. The neural diathesis-stress model of schizophrenia revisited: an update on recent findings considering illness stage and neurobiological and methodological complexities. Neurosci. Biobehav. Rev. 2017;73:191–218. doi: 10.1016/j.neubiorev.2016.12.013. [DOI] [PubMed] [Google Scholar]
- Quide Y., Ong X.H., Mohnke S., Schnell K., Walter H., Carr V.J., Green M.J. Childhood trauma-related alterations in brain function during a Theory-of-Mind task in schizophrenia. Schizophr. Res. 2017;189:162–168. doi: 10.1016/j.schres.2017.02.012. [DOI] [PubMed] [Google Scholar]
- Ranlund S., Calafato S., Thygesen J.H., Lin K., Cahn W., Crespo-Facorro B., De Zwarte S.M.C., Diez A., Di Forti M., GROUP, Iyegbe C., Jablensky A., Jones R., Hall M.H., Kahn R., Kalaydjieva L., Kravariti E., Mcdonald C., Mcintosh A.M., Mcquillin A., PEIC, Picchioni M., Prata D.P., Rujescu D., Schulze K., Shaikh M., Toulopoulou T., Van Haren N., van Os J., Vassos E., Walshe M., WTCCC, Lewis C., Murray R.M., Powell J., Bramon E. A polygenic risk score analysis of psychosis endophenotypes across brain functional, structural, and cognitive domains. Am. J. Med. Genet. B Neuropsychiatr. Genet. 2018;177:21–34. doi: 10.1002/ajmg.b.32581. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Reininghaus U., Gayer-Anderson C., Valmaggia L., Kempton M.J., Calem M., Onyejiaka A., Hubbard K., Dazzan P., Beards S., Fisher H.L., Mills J.G., Mcguire P., Craig T.K., Garety P., van Os J., Murray R.M., Wykes T., Myin-Germeys I., Morgan C. Psychological processes underlying the association between childhood trauma and psychosis in daily life: an experience sampling study. Psychol. Med. 2016;46:2799–2813. doi: 10.1017/S003329171600146X. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Reininghaus U., Kempton M.J., Valmaggia L., Craig T.K., Garety P., Onyejiaka A., Gayer-Anderson C., So S.H., Hubbard K., Beards S., Dazzan P., Pariante C., Mondelli V., Fisher H.L., Mills J.G., Viechtbauer W., Mcguire P., van Os J., Murray R.M., Wykes T., Myin-Germeys I., Morgan C. Stress sensitivity, aberrant salience, and threat anticipation in early psychosis: an experience sampling study. Schizophr. Bull. 2016;42:712–722. doi: 10.1093/schbul/sbv190. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rosenman S., Rodgers B. Childhood adversity in an Australian population. Soc. Psychiatry Psychiatr. Epidemiol. 2004;39:695–702. doi: 10.1007/s00127-004-0802-0. [DOI] [PubMed] [Google Scholar]
- Ruby E., Polito S., Mcmahon K., Gorovitz M., Corcoran C., Malaspina D. Pathways associating childhood trauma to the neurobiology of Schizophrenia. Front Psychol. Behav. Sci. 2014;3:1–17. [PMC free article] [PubMed] [Google Scholar]
- Ruby E., Rothman K., Corcoran C., Goetz R.R., Malaspina D. Influence of early trauma on features of schizophrenia. Early Interv. Psychiatry. 2017;11:322–333. doi: 10.1111/eip.12239. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sapolsky R.M., Krey L.C., McEwen B.S. The neuroendocrinology of stress and aging: the glucocorticoid cascade hypothesis. Endocr. Rev. 1986;7:284–301. doi: 10.1210/edrv-7-3-284. [DOI] [PubMed] [Google Scholar]
- Schalinski I., Teicher M.H., Carolus A.M., Rockstroh B. Defining the impact of childhood adversities on cognitive deficits in psychosis: an exploratory analysis. Schizophr. Res. 2017;192:351–356. doi: 10.1016/j.schres.2017.05.014. [DOI] [PubMed] [Google Scholar]
- Schenkel L.S., Spaulding W.D., Dilillo D., Silverstein S.M. Histories of childhood maltreatment in schizophrenia: relationships with premorbid functioning, symptomatology, and cognitive deficits. Schizophr. Res. 2005;76:273–286. doi: 10.1016/j.schres.2005.03.003. [DOI] [PubMed] [Google Scholar]
- Shannon C., Douse K., Mccusker C., Feeney L., Barrett S., Mulholland C. The association between childhood trauma and memory functioning in schizophrenia. Schizophr. Bull. 2011;37:531–537. doi: 10.1093/schbul/sbp096. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Slavish D.C., Graham-Engeland J.E., Smyth J.M., Engeland C.G. Salivary markers of inflammation in response to acute stress. Brain Behav. Immun. 2015;44:253–269. doi: 10.1016/j.bbi.2014.08.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Steine I.M., Winje D., Krystal J.H., Bjorvatn B., Milde A.M., Gronli J., Nordhus I.H., Pallesen S. Cumulative childhood maltreatment and its dose-response relation with adult symptomatology: findings in a sample of adult survivors of sexual abuse. Child Abuse Negl. 2017;65:99–111. doi: 10.1016/j.chiabu.2017.01.008. [DOI] [PubMed] [Google Scholar]
- Steptoe A., Hamer M., Chida Y. The effects of acute psychological stress on circulating inflammatory factors in humans: a review and meta-analysis. Brain Behav. Immun. 2007;21:901–912. doi: 10.1016/j.bbi.2007.03.011. [DOI] [PubMed] [Google Scholar]
- Taylor S.E., Eisenberger N.I., Saxbe D., Lehman B.J., Lieberman M.D. Neural responses to emotional stimuli are associated with childhood family stress. Biol. Psychiatry. 2006;60:296–301. doi: 10.1016/j.biopsych.2005.09.027. [DOI] [PubMed] [Google Scholar]
- Teicher M.H., Parigger A. The 'Maltreatment and Abuse Chronology of Exposure' (MACE) scale for the retrospective assessment of abuse and neglect during development. PLoS One. 2015;10 doi: 10.1371/journal.pone.0117423. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Teicher M.H., Samson J.A., Anderson C.M., Ohashi K. The effects of childhood maltreatment on brain structure, function and connectivity. Nat. Rev. Neurosci. 2016;17:652–666. doi: 10.1038/nrn.2016.111. [DOI] [PubMed] [Google Scholar]
- Theleritis C., Fisher H.L., Shafer I., Winters L., Stahl D., Morgan C., Dazzan P., Breedvelt J., Sambath I., Vitoratou S., Russo M., Reichenberg A., Falcone M.A., Mondelli V., O'connor J., David A., Mcguire P., Pariante C., Di Forti M., Murray R.M., Bonaccorso S. Brain derived Neurotropic Factor (BDNF) is associated with childhood abuse but not cognitive domains in first episode psychosis. Schizophr. Res. 2014;159:56–61. doi: 10.1016/j.schres.2014.07.013. [DOI] [PubMed] [Google Scholar]
- Thompson J.L., Kelly M., Kimhy D., Harkavy-Friedman J.M., Khan S., Messinger J.W., Schobel S., Goetz R., Malaspina D., Corcoran C. Childhood trauma and prodromal symptoms among individuals at clinical high risk for psychosis. Schizophr. Res. 2009;108:176–181. doi: 10.1016/j.schres.2008.12.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Trotta A., Murray R.M., Fisher H.L. The impact of childhood adversity on the persistence of psychotic symptoms: a systematic review and meta-analysis. Psychol. Med. 2015;45:2481–2498. doi: 10.1017/S0033291715000574. [DOI] [PubMed] [Google Scholar]
- Ucok A., Kaya H., Ugurpala C., Cikrikcili U., Ergul C., Yokusoglu C., Bulbul O., Direk N. History of childhood physical trauma is related to cognitive decline in individuals with ultra-high risk for psychosis. Schizophr. Res. 2015;169:199–203. doi: 10.1016/j.schres.2015.08.038. [DOI] [PubMed] [Google Scholar]
- Van Assche L., Morrens M., Luyten P., Van De Ven L., Vandenbulcke M. The neuropsychology and neurobiology of late-onset schizophrenia and very-late-onset schizophrenia-like psychosis: a critical review. Neurosci. Biobehav. Rev. 2017;83:604–621. doi: 10.1016/j.neubiorev.2017.08.024. [DOI] [PubMed] [Google Scholar]
- van Os J., Kenis G., Rutten B.P. The environment and schizophrenia. Nature. 2010;468:203–212. doi: 10.1038/nature09563. [DOI] [PubMed] [Google Scholar]
- van Os J., Marsman A., Van Dam D., Simons C.J., Investigators, G Evidence that the impact of childhood trauma on IQ is substantial in controls, moderate in siblings, and absent in patients with psychotic disorder. Schizophr. Bull. 2017;43:316–324. doi: 10.1093/schbul/sbw177. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vlad M., Raucher-Chene D., Henry A., Kaladjian A. Functional outcome and social cognition in bipolar disorder: is there a connection? Eur. Psychiatry. 2018;52:116–125. doi: 10.1016/j.eurpsy.2018.05.002. [DOI] [PubMed] [Google Scholar]
- Walder D.J., Faraone S.V., Glatt S.J., Tsuang M.T., Seidman L.J. Genetic liability, prenatal health, stress and family environment: risk factors in the Harvard Adolescent Family High Risk for schizophrenia study. Schizophr. Res. 2014;157:142–148. doi: 10.1016/j.schres.2014.04.015. [DOI] [PubMed] [Google Scholar]
- Wolf O.T. HPA axis and memory. Best Pract. Res. Clin. Endocrinol. Metab. 2003;17:287–299. doi: 10.1016/s1521-690x(02)00101-x. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Supplementary tables