Abstract
Riboflavin (RF), is essential for normal cellular metabolism/function. Intestinal RF absorption occurs via a specific carrier-mediated process that involves the apical transporter RFVT-3 (SLC52A3) and the basolateral RFVT-1 (SLC52A1). Previously, we characterized different cellular/molecular aspects of the intestinal RF uptake process, but nothing is known about the effect of proinflammatory cytokines on the uptake event. We addressed this issue using in vitro, ex vivo, and in vivo models. First, we determined the level of mRNA expression of the human (h)RFVT-3 and hRFVT-1 in intestinal tissue of patients with inflammatory bowel disease (IBD) and observed a markedly lower level compared with controls. In the in vitro model, exposing Caco-2 cells to tumor necrosis factor-α (TNF-α) led to a significant inhibition in RF uptake, an effect that was abrogated upon knocking down TNF receptor 1 (TNFR1). The inhibition in RF uptake was associated with a significant reduction in the expression of hRFVT-3 and -1 protein and mRNA levels, as well as in the activity of the SLC52A3 and SLC52A1 promoters. The latter effects appear to involve Sp1 and NF-κB sites in these promoters. Similarly, exposure of mouse small intestinal enteroids and wild-type mice to TNF-α led to a significant inhibition in physiological and molecular parameters of intestinal RF uptake. Collectively, these findings demonstrate that exposure of intestinal epithelial cells to TNF-α leads to inhibition in RF uptake and that this effect is mediated, at least in part, via transcriptional mechanism(s). These findings may explain the significantly low RF levels observed in patients with IBD.
Keywords: intestinal transport, riboflavin; SLC52A3, SLC52A1; TNF-α
INTRODUCTION
Riboflavin (RF), a member of the water-soluble family of vitamins, is required for normal cellular metabolism, proliferation, and function. In its biologically active forms [flavin mononucleotide (FMN) and flavin adenine dinucleotide (FAD)], the vitamin assumes a key role in the transfer of electrons in oxidation-reduction reactions involving carbohydrate, lipid, and amino acid metabolism as well as in the metabolism of certain water-soluble vitamins (pyridoxine and folate) (6, 28). A role for RF in protein folding in the endoplasmic reticulum (42), in the regulation of cellular energy utilization (10), and in normal function of the immune system have also been reported (18, 32). The vitamin also appears to possess antioxidant and anti-inflammatory properties (12, 33). Thus, it is not surprising that deficiency of RF leads to a variety of clinical abnormalities, including degenerative changes in the nervous system, anemia, skin lesions, cataract, and growth retardation (22, 28); it also appears to be associated with increased susceptibility to cancer (21). Such deficiency/suboptimal levels occur in patients with inflammatory bowel disease (IBD) (7, 16), diabetes mellitus (13), and those with chronic alcoholism (3); it also occurs in patients with Brown-Vialetto Van Laere and Fazio Londe syndromes [rare neurological disorders caused by mutations in human RF transporters 2 and 3 (hRFVT-2 and hRFVT-3)] (5, 9). In contrast to the deleterious effect of RF deficiency, optimizing RF status appears to be associated with reduction in the risk of esophageal squamous cell carcinoma (44 and references therein).
Humans (and all other mammals) cannot synthesize RF endogenously, and thus must obtain the vitamin from exogenous sources (diet and colonic microbiota) via absorption in the intestine (11, 30). Thus, the intestine plays a central role in regulating normal body homeostasis of RF, and disturbance in this function leads to RF deficiency/suboptimal levels. The mechanism of absorption of RF in the small and large intestine has been studied by us and others using a variety of human and animal intestinal sample preparations (24, 25, 27, 29, 41). Studies have shown transport of RF in the small and large intestine to be similar and to occur via an efficient and specific carrier-mediated mechanism (24–27, 29, 41). Other studies have shown that the RF transport system that operates at the apical membrane domain of the polarized absorptive cells is RFVT-3 (product of the SLC52A3 gene), while that at the basolateral membrane domain of these cells is RFVT-1 (product of the SLC52A1 gene) (39, 46, 48). A critical role for RFVT-3 in the intestinal RF absorption process has also been demonstrated in studies utilizing in vitro gene-silencing (39) and in vivo conditional (intestinal-specific) RFVT-3 (SLC52A3) knockout (37) approaches. Knowledge about transcriptional regulation of the intestinal RF uptake process and the transport systems involved has also been emerging with the cloning/characterization of the 5′-regulatory regions of the SLC52A3 and SLC52A1 genes and the identification of the minimal regions needed for basal activity of the involved promoters and the cis-regulatory elements (e. g., Sp1) involved (8, 23).
The intestinal mucosa is constantly exposed to potential harmful/antigenic substances that trigger inflammatory response. When this response is not properly controlled (as in the case of IBD), chronic inflammation occurs that is associated with excessive levels of proinflammatory cytokines such as tumor necrosis factor-α (TNF-α) and interferon-γ (IFN-γ) in both the gut mucosa and the circulation (40, 43). Prolonged exposure of the intestine to such elevated levels of proinflammatory cytokines has been shown to lead to significant changes in epithelial physiology including its absorptive and secretory functions (1, 2, 4, 19, 38). There is nothing currently known about the effect of prolonged exposure of intestinal epithelial cells to proinflammatory cytokines on RF transport. We address this issue in this study using in vitro (Caco-2 cells), ex vivo (mouse enteroides), and in vivo (mouse) model systems. Our results showed that exposure of intestinal epithelial cells to TNF-α leads to a significant inhibition in RF uptake and that this inhibition is mediated, at least in part, via transcriptional mechanism(s) involving the RFVT-3 and RFVT-1 systems.
MATERIALS AND METHODS
Materials
Caco-2 cells were obtained from American Type Culture Collection (ATCC, Manassas, VA). [3H]RF (specific activity 12.5 Ci/mmol; radiochemical purity 97%) was purchased from Moravek Biochemicals (Brea, CA). All other chemicals and reagents were purchased from commercial sources and were of analytic/molecular biology grade. RT-qPCR primers (Table 1) used in this study were purchased from Sigma Genosys (Woodlands, TX). hRFVT-3 rabbit polyclonal antibodies were custom synthesized from Alpha Diagnostics International (San Antonio, TX). Human anti-RFVT-1 (GTX87668) antibody was from GeneTex (Irvine, CA), anti-Sp1 (sc-14027), and anti-mouse β-actin (sc-47778) antibodies from Santa Cruz Biotechnology (Santa Cruz, CA), mouse anti-RFVT-3 (bs-4164R) from Bioss (Bioss Antibodies, Woburn, MA), anti-RFVT-2 (AP55250SU-N) from Origene (Rockville, MD), and anti-rabbit β-actin (MAS-15739) from ThermoFisher Scientific (Grand Island, NY). The secondary antibodies, anti-rabbit IRDye-800 and anti-mouse IRDye-680, were purchased from LI-COR Bioscience (Lincoln, NE). The recombinant human and mouse TNF-α were purchased from Invitrogen (Carlsbad, CA) and Peprotech (Rocky Hill, NJ), respectively. TNFR1 siRNA was purchased from ThermoFisher Scientific. Celastrol was obtained from Invivogen (San Diego, CA). The TissueScan cDNA Array I (Cat. No. CCRT101) was obtained from Origene (Rockville, MD).
Table 1.
Primers used for RT-qPCR analysis
| Gene Name | Forward and Reverse Primers (5′–3′) |
|---|---|
| hRFVT-3 | CTGGTCTGCGTCTTCGGAATG; ACCACCGTGAGGTAGGAGG |
| hRFVT-1 | TGGCCTGTTGTACCTCTAATGT; CCCACACCTTGCACTAGGG |
| hSp1 | CCATACCCCTTAACCCCG; GAATTTTCACTAATGTTTCCCACC |
| hTBP | TATAATCCCAAGCGGTTTGC; GCTGGAAAACCCAACTTCTG |
| hTNFR1 | TCACCGCTTCAGAAAACCACC; GGTCCACTGTGCAAGAAGAGA |
| hβ-Actin | CATCCTGCGTCTGGACCT; TAATGTCACGCACGATTTCC |
| mRFVT-3 | GGACAGCATAGTGTGGCCTTC; CACAGGTGGTGATACCGGAG |
| mRFVT-2 | CCTCCCTTCCTACCTCTCTG; TAGGAAGGCCACTGAGTACG |
| mβ-Actin | ATCCTCTTCCTCCCTGGA; TTCATGGATGCCACAGGA |
h, human; m, murine; RF, riboflavin; RFVT, RF transporter; TBP, TATA-box binding protein; TNFR1, tumor necrosis factor receptor 1.
Cell Culture and RF Uptake Studies
Human-derived intestinal epithelial Caco-2 cells were grown in complete Eagle’s minimum essential medium (EMEM) at 37°C in 5% CO2 environment. Postconfluent (3–4 days, differentiated) Caco-2 cell monolayers were used to investigate the effect of proinflammatory cytokines on RF uptake and determine the protein and mRNA expression levels of RF transporters and Sp1, and SLC52A3 and SLC52A1 promoter activity. To assess RF uptake in vitro, postconfluent Caco-2 cells were treated with either 20 ng/ml TNF-α, 30 ng/ml IFN-γ, or TNF-α + celastrol (100 nM) for 48 h. Uptake was then performed using Krebs-Ringer (KR) buffer containing (in mM): 123 NaCl, 4.93 KCl, 1.23 MgSO4, 0.85 CaCl2, 5 glucose, 5 glutamine, 10 HEPES, and 10 MES (pH 7.4) as well as [3H]RF (0.3 µCi/ml) for 5 min at 37°C. The reaction was terminated after 5 min (initial rate of uptake) (36, 39) by the addition of ice-cold KR buffer. Subsequently, cells were lysed with 1 ml of 1 N NaOH, and neutralized with 10 N HCl. Radioactivity was assessed in a liquid scintillation counter. The protein content of cell digests was measured using the Dc protein assay kit (Bio-Rad, Hercules, CA). For in vivo RF uptake, mice (C57BL/6) were injected with TNF-α (15 µg/mouse) intraperitoneally, and control mice were given PBS as described before (35). After 48 h, mice were euthanized, and the jejunum sheets (~1 cm) were incubated in vitro in KR buffer containing [3H]RF in the presence or absence of unlabeled 1 mM RF for 5 min and then processed for radioactivity measurement. The animal protocol used in this study was approved by the Institutional Animal Care and Use Committee (IACUC), University of California, Irvine, CA.
Mouse Small Intestinal Enteroids Preparation and TNF-α Treatment
For ex vivo investigations, we used 8- to 10-wk-old C57BL/6 mice (Jackson Laboratory, Bar Harbor, ME) to generate small intestinal enteroids by using an established protocol (31, 38). The enteroids were treated with mouse recombinant TNF-α (20 ng/ml) for 48 h and used to determine the expression of both RFVT-3 and RFVT-1 at the protein and mRNA levels.
Quantitative Real-Time PCR Analysis
Total RNA isolated from Caco-2 cells, mouse enteroids and mouse jejunum treated with TNF-α were subjected to reverse transcription (RT) using the iScript cDNA synthesis kit (Bio-Rad, Hercules, CA). PCR was performed using human and mouse gene-specific primers (Table 1), and the PCR conditions were as previously described (17). Data normalized to β-actin were quantified using a relative relationship method supplied by the iCycler manufacturer (Bio-Rad). Levels of expression of the hRFVT-3 and hRFVT-1 mRNA in cDNA samples of patients with IBD (ulcerative colitis) and control subjects were determined using the Origene TissueScan Array (CCRT101). This array plate contained cDNA samples prepared from the intestinal tissue of four normal subjects and twelve patients with ulcerative colitis. The cDNAs in each well were normalized with β-actin by the vendor (Origene).
siRNA Transfection
TNFR1 (30 pmol)-specific small interfering RNA (siRNA; ThermoScientific) was transfected into Caco-2 cells at 60–80% confluence using Lipofectamine RNAiMAX (Life Technologies) according to the manufacturer’s protocol. Twenty-four hours posttransfection, cells were used for TNF-α treatment for 48 h followed by RF uptake.
Transfection and Firefly Luciferase Assay
SLC52A3 and SLC52A1 wild-type (WT) and mutant promoter constructs (3 µg/ml), along with 100 ng of Renilla luciferase-thymidine kinase (pRL-TK) plasmid (Promega, Madison, WI), were transiently transfected in Caco-2 cells using Lipofectamine 2000 reagent (Life Technologies) for 24 h. Cells were subsequently treated with either TNF-α or TNF-α + celastrol. After 48 h, cells were lysed, and Renilla-normalized firefly luciferase activity was measured using the dual-luciferase assay system (Promega).
Isolation of Protein and Western Blot Analysis
TNF-α-treated and untreated Caco-2 cells or mouse enteroids or mouse jejunum were lysed in radioimmunoprecipitation assay buffer (Sigma) containing Complete protease inhibitor cocktail (Roche, NJ). The soluble protein fraction was isolated, followed by centrifugation (12,000 rpm, 20 min). Protein concentrations were measured using a Dc protein assay kit (Bio-Rad). To determine the level of RFVTs protein expression, an equal amount of protein (40 µg) was loaded onto 4–12% Bis-Tris gradient minigels (Invitrogen) and transferred to Immobilon polyvinylidene difluoride membrane (Fisher Scientific). Subsequently, the blot was probed with hRFVT-3 (1:200), hRFVT-1 (1:200), hSp1 (1: 200), and hβ-actin (1:3,000), mRFVT-3 (1:200), mRFVT-2 (1: 200) and mβ-actin (1:3,000) antibodies. The secondary antibodies used were anti-rabbit IRDye-800 and anti-mouse IRDye-680 (both at 1:30,000 dilutions). The specific immunoreactive bands were detected using the Odyssey infrared imaging system (LI-COR Bioscience, Lincoln, NE), and their densities were quantified using LI-COR software.
Statistical Analysis
Uptake data presented in this study represent the mean ± SE of at least three to four independent experiments with multiple determinants and are expressed as a percentage relative to simultaneously performed controls. The RT-qPCR, Western blotting, and firefly luciferase assays were determined from at least three to four independent sample preparations. Student’s t-test was used for statistical analysis, and P < 0.05 was considered statistically significant.
RESULTS
Expression of hRFVT-3 and hRFVT-1 mRNA in Intestinal Tissue of IBD Patients and Control Subjects
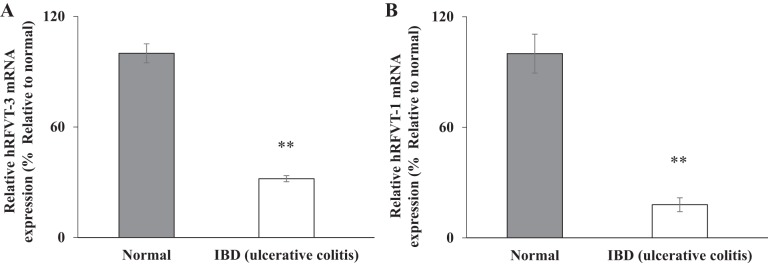
As mentioned earlier, patients with IBD have markedly reduced levels of RF (7, 16). In this study, we sought to determine whether the expression of the intestinal RF transporters is negatively impacted in IBD patient samples. To examine this, hRFVT-3 and hRFVT-1 mRNA expression levels were determined using the TissueScan cDNA Array I. This array plate contains cDNA from four control adults and twelve adult patients with ulcerative colitis. To determine the level of expression of RF transporters, we performed RT-qPCR analysis, and the results showed a significantly (P < 0.01 for both) lower level of expression of hRFVT-3 (Fig. 1A) and hRFVT-1 (Fig. 1B) mRNA in ulcerative colitis patient samples compared with control samples.
Fig. 1.
Level of expression of riboflavin (RF) transporters in intestinal tissue of inflammatory bowel disease (IBD, i.e., ulcerative colitis) patients and control subjects. Levels of expression of the human RF transporters hRFVT-3 and hRFVT-1 mRNA were determined by RT-qPCR using cDNA prepared from intestinal tissue of patients with IBD and control subjects (see materials and methods). Data are means ± SE; samples were from 4 normal subjects and 12 patients with ulcerative colitis. **P < 0.01.
Effect of Proinflammatory Cytokines on Physiological/Molecular Parameters of Intestinal RF Uptake Process
In vitro studies utilizing Caco-2 cells.
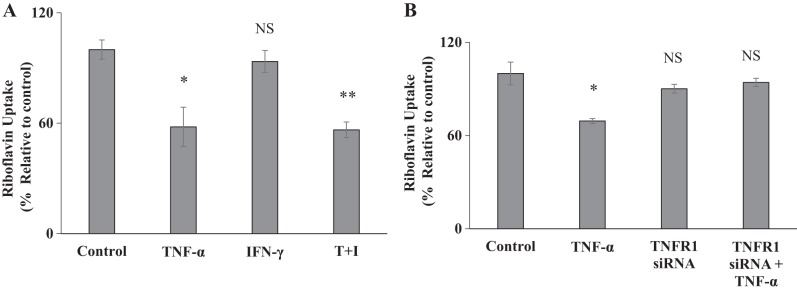
In this study, we examined the effect of the proinflammatory cytokine TNF-α (as well as that of IFN-γ) on RF uptake by confluent Caco-2 monolayers. For this, cells were treated with 20 ng/ml TNF-α (or with 30 ng/ml of IFN-γ) for 48 h followed by examination of carrier-mediated RF uptake. The result showed a significant (P < 0.05) inhibition in RF uptake by Caco-2 cells treated with TNF-α (but not those treated with IFN-γ) (Fig. 2A) [note: IFN-γ did not increase the degree of inhibition caused by TNF-α in RF uptake when added together (Fig. 2A)].
Fig. 2.
Effect of proinflammatory cytokines on riboflavin (RF) uptake. A: Caco-2 cells were treated with tumor necrosis factor-α (TNF-α; 20 ng/ml), interferon-γ (IFN-γ; 30 ng/ml), or a combination of both for 48 h followed by determination of carrier-mediated RF uptake. B: Caco-2 cells were transiently transfected with TNF receptor 1 (TNFR1) siRNA for 24 h followed by treatment with TNF-α (20 ng/ml for 48 h); RF uptake was then determined. Data are means ± SE of at least 3 different sets of experiments with multiple determination. **P < 0.01, *P < 0.05; NS, not significant.
TNF-α transduces its signals via TNF receptor 1 (TNFR1) and TNF receptor 2 (TNFR2). Since TNFR1 is expressed at a higher level in intestinal epithelial cells (2, 19), we examined the effect of knocking down this receptor with gene-specific siRNA on the effect of TNF-α on RF uptake. The results showed that knocking down the TNFR1 (the knockdown was confirmed by RT-qPCR; data not shown) led to abrogation in the inhibitory effect of TNF-α on vitamin uptake (Fig. 2B).
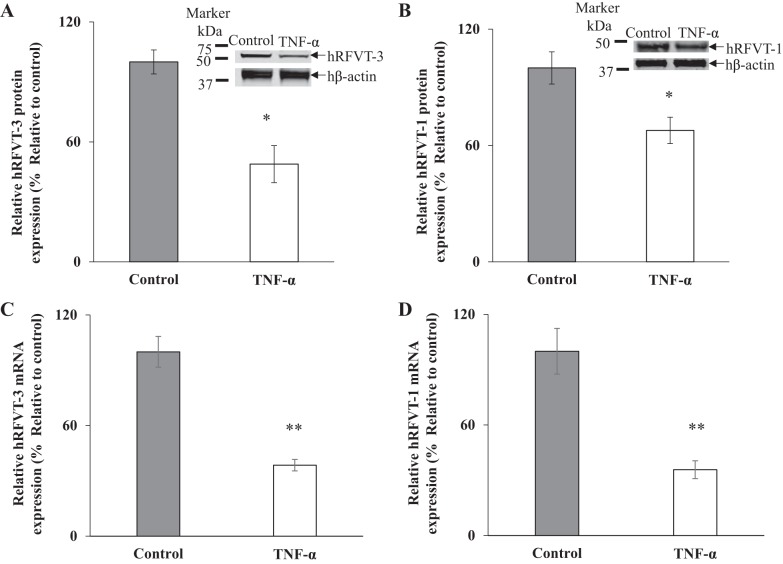
In another study, we examined the effect of prolonged (48 h) exposure of Caco-2 cells to TNF-α on the level of expression of hRFVT-3 and hRFVT-1 proteins. This was done by means of Western blot analysis using specific polyclonal antibodies against these transporters. The results showed a significant (P < 0.05 for both) reduction in the level of expression of hRFVT-3 and hRFVT-1 proteins in cells exposed to TNF-α compared with controls (Fig. 3, A and B).
Fig. 3.
Effect of tumor necrosis factor-α (TNF-α) on level of expression of human riboflavin (RF) transporters hRFVT-3 and hRFVT-1 protein and mRNA in Caco-2 cells. Cells were treated with TNF-α (20 ng/ml for 48 h) followed by determination of hRFVT-3 (A) and hRFVT-1 (B) protein levels by Western blotting. RT-qPCR was performed to determine the level of hRFVT-3 (C) and hRFVT-1 (D) mRNA expression using TNF-α-treated and untreated RNA samples. Data are means ± SE of at least 3 different sets of independent experiments. **P < 0.01, *P < 0.05.
We also examined the effect of prolonged exposure of Caco-2 cells to TNF-α on the level of expression of hRFVT-3 and hRFVT-1 mRNA. This was done by RT-qPCR using specific primers for these transporters. The results showed a significant (P < 0.01 for both) reduction in the level of expression of both hRFVT-3 and hRFVT-1 mRNA in Caco-2 cells exposed to TNF-α compared with controls (Fig. 3, C and D).
Ex vivo studies utilizing mouse small intestinal enteroids.
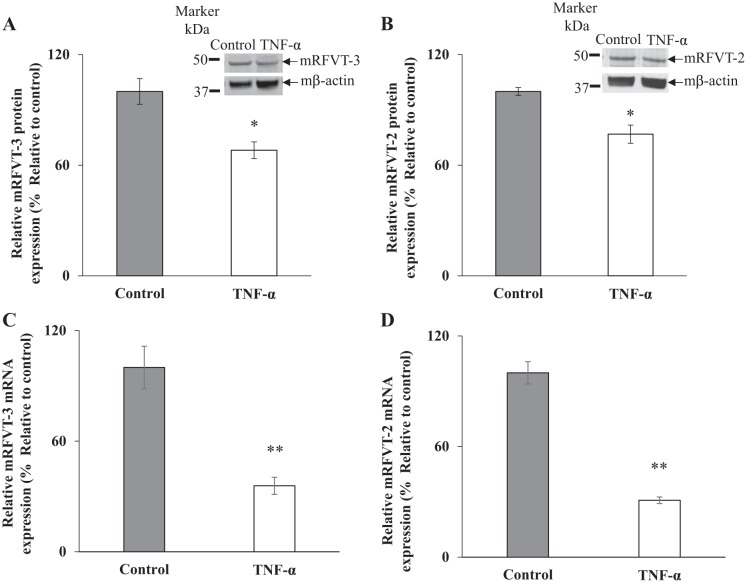
In these studies, we utilized the ex vivo mouse small intestinal enteroids model to confirm our in vitro findings with Caco-2 cells on the effect of TNF-α on level of expression of the intestinal RF transport systems. [Note: the mouse intestine, like its human counterpart, expresses the apical hRFVT-3 (mRFVT-3); however, it expresses the so-called mRFVT-2 at its basolateral membrane. The mRFVT-2 shares a high degree of homology with the basolateral hRFVT-1 (45, 47)]. To determine the effect of TNF-α on the level of mRFVT-3 and mRFVT-2 expression, mouse small intestinal enteroids were treated with murine (m)TNF-α (20 ng/ml; 48 h) followed by analysis of protein and mRNA expression of these RF transporters by Western blot analysis and RT-qPCR, respectively. The results showed that such treatment led to a significant decrease in the level of expression of mRFVT-3 and mRFVT-2 (P < 0.05 for both) proteins (Fig. 4, A and B) and mRNA (P < 0.01 for both) levels compared with controls (Fig. 4, C and D).
Fig. 4.
Effect of tumor necrosis factor-α (TNF-α) on level of expression of murine riboflavin (RF) transporters mRFVT-3 and mRFVT-1 protein and mRNA in mouse jejunal enteroids. Enteroids were treated with mouse recombinant TNF-α (20 ng/ml for 48 h) followed by determination of the level of mRFVT-3 (A) and mRFVT-2 (B) proteins by Western blotting. Levels of mRFVT-3 (C) and mRFVT-2 (D) mRNA were determined by RT-q PCR. Data are means ± SE of at least 3 independent sets of experiments. **P < 0.01, *P < 0.05.
In vivo studies utilizing mouse intestinal preparations.
To extend the findings of the effect of TNF-α on intestinal RF uptake in the in vivo situation, we examined the effect of injecting the TNF-α into the wild-type mice (injection, 15 µg/mouse ip) on physiological and molecular parameters of intestinal RF uptake (all assays were performed 48 h after TNF-α injection). The results showed that such treatment led to a significant (P < 0.01) inhibition in mouse jejunal carrier-mediated RF uptake (Fig. 5A). We also examined the level of expression of mRFVT-3 and mRFVT-2 proteins in mice treated with TNF-α by Western blot analysis. The result showed a significant reduction in the level of expression of mRFVT-3 (P < 0.01) and mRFVT-2 (P < 0.05) proteins in mice treated with TNF-α (Fig. 5, B and C). In another study, we measured the expression level of mRFVT-3 and mRFVT-2 mRNA by RT-qPCR, and the result showed that the mRNA expression of both mRFVT-3 (P < 0.01) and mRFVT-2 (P < 0.05) were significantly reduced in mice treated with TNF-α compared with untreated controls (Fig. 5, D and E).
Fig. 5.
Effect of tumor necrosis factor-α (TNF-α) on riboflavin (RF) uptake and RF transporter expression in native mouse jejunum tissue. A: 8- to 10-wk-old male C57BL/6 mice were injected intraperitoneally with mTNF-α (15 µg/mouse) for 48 h, and RF uptake was determined in jejunum sheet. Western blotting was performed to determine the expression level of murine RF transporter 3 (mRFVT-3; B) and mRFVT-2 (C) proteins using TNF-α-treated and untreated mouse jejunum protein samples. RT-qPCR was performed to determine the level of mRFVT-3 (D) and mRFVT-2 (E) mRNA expression using TNF-α-treated and untreated mouse jejunum RNA samples. Data are means ± SE of at least from 3 pairs of mice. **P < 0.01, *P < 0.05.
Involvement of Transcriptional Mechanisms in the Effect of TNF-α on Intestinal RF Uptake Process
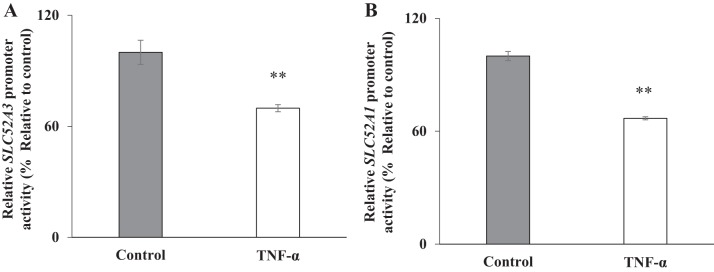
Changes in the level of mRNA expression of a particular gene by external factors/stimuli could be achieved via different mechanisms. One such prominent mechanism is alteration in the transcription rate of the involved gene. To determine the possibility that such a mechanism is involved in the effect of TNF-α on expression of the intestinal RF transporters, we examined the effect of exposure to the cytokine of Caco-2 cells transfected with the individual SLC52A3 and SLC52A1 promoter constructs. The results showed that TNF-α treatment of such cells led to a significant (P < 0.01 for both) reduction in the activity of SLC52A3 and SLC52A1 promoters (Fig. 6, A and B). These findings demonstrated that TNF-α-arbitrated effects on the levels of expression of hRFVT-3 and hRFVT-1 are mediated, at least in part, via transcriptional mechanisms of their responsive genes.
Fig. 6.
Effect of tumor necrosis factor-α (TNF-α) on activity of the SLC52A3 and SLC52A1 promoters. SLC52A3 (A) and SLC52A1 (B) promoter constructs in pGL3 basic were transiently transfected into Caco-2 cells for 24 h, followed by treatment with TNF-α (20 ng/ml for 48 h) and determination of promoter activity by luciferase assay. Values are means ± SE of at least 3 independent experiments. **P < 0.01.
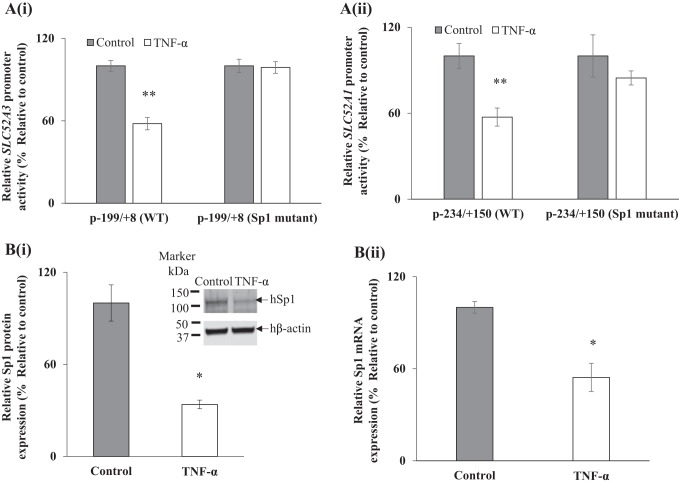
Previous studies from our laboratory have shown that the minimal promoter regions of the SLC52A3 and SLC52A1 promoters contain Sp1 cis-regulatory elements (−74/−71 for SLC52A3 and −47/−41 for SLC52A1) that are important for their activity (8, 23). Thus, we examined the effect of mutations at these sites on the inhibitory effect of TNF-α on activity of these promoters. The WT and mutant constructs of both SLC52A3 (p-199/+8) and SLC52A1 (p-234/+150) (generated and characterized previously in our lab; 8, 23) were transiently transfected into Caco-2 cells followed by exposure of cells to TNF-α (20 ng/ml, 48 h). The results showed that mutating these Sp1 binding sites led to disappearance of the inhibitory effect of TNF-α on activity of the SLC52A3 and SLC52A1 promoters (Fig. 7, Ai and Aii).
Fig. 8.
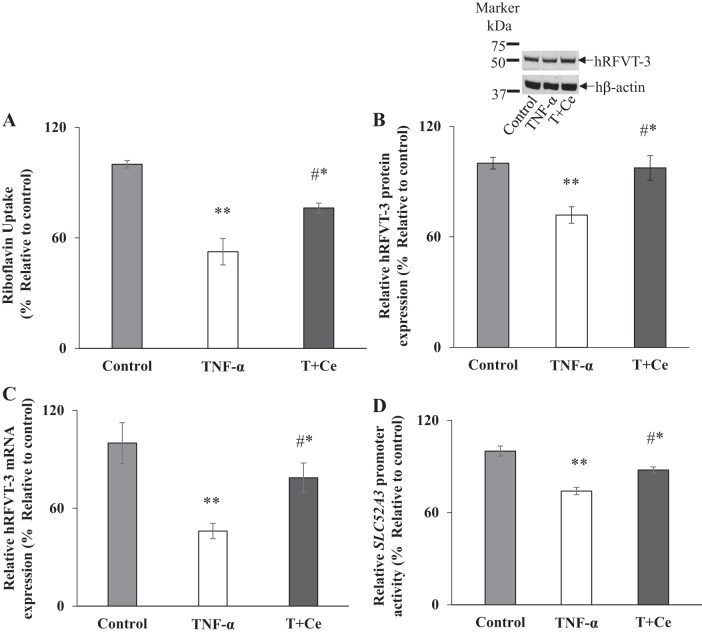
Role of the NF-κB signaling pathway in mediating the effect of tumor necrosis factor-α (TNF-α) on riboflavin (RF) uptake, expression of human RF transporter 3 (hRFVT-3), and activity of the SLC52A3 promoter. Caco-2 cells were pretreated with celastrol (100 nM) for 5 h before TNF-α treatment (20 ng/ml for 48 h), followed by determination of carrier-mediated RF uptake (A), levels of expression of the hRFVT-3 protein and mRNA (B and C), and activity of the SLC52A3 promoter (D). T, TNF-α; C, celastrol. Data are means ± SE of at least 3 independent experiments. **P < 0.01, *P < 0.05 (#significantly recovered vs. TNF-α treated).
Fig. 7.
A: Role of Sp1 sites in the SLC52A3 and SLC52A1 promoters in mediating the effect of tumor necrosis factor-α (TNF-α) on promoter activity. Wild-type (WT) and mutant construct of SLC52A3 (i) and SLC52A1 (ii) were transfected into Caco-2 cells, treated with TNF-α (20 ng/ml for 48 h), followed by performance of luciferase assay. B: effect of TNF-α treatment (20 ng/ml for 48 h) on level of expression of Sp1 protein (i) and mRNA (ii). Protein and mRNA levels were determined by Western blotting and RT-qPCR, respectively. Data are means ± SE of at least 3 independent sets of experiments. **P < 0.01, *P < 0.05.
In another study, we examined whether TNF-α treatment of Caco-2 cells also affects the level of expression of the transcription factor Sp1 itself (which is necessary for driving basal activity of both the SLC52A3 and SLC52A1 promoters). The results showed that exposure of Caco-2 cells to TNF-α to led to a significant (P < 0.05 for both) reduction in levels of expression of the Sp1 at the protein and mRNA levels (Fig. 7, Bi and Bii).
Role of the NF-κB Signaling Pathway in Mediating the TNF-α Effect on Intestinal RF Uptake
The intracellular NF-κB signaling pathway is a major pathway through which TNF-α exerts its effects on cellular events, including gene expression (15, 19, 20). To determine whether the NF-κB signaling pathway is involved in mediating the effect of TNF-α on intestinal RF uptake and the expression of hRFVT-3 as well as SLC52A3 promoter activity [we focused on the hRFVT-3 transporter because it is the predominant RF transporter expressed in the gut and plays an important role in RF absorption (39); also, the promoter of the SLC52A3 gene contains two NF-κB sites (−95/−92 and −49/−46) (8)], we examined the ability of the specific NF-κB pharmacological inhibitor celastrol [a novel triterpene, that blocks NF-κB signaling by inhibiting IkBα phosphorylation and prevents IkBα degradation (34)] in preventing the effects of TNF-α on physiological/molecular parameters of the intestinal RF uptake process. For this, we treated Caco-2 cells with TNF-α for 48 h in the presence and absence of celastrol (100 nM). The results showed that treatment with celastrol led to a significant (P < 0.05) reversal of the inhibitory effect of TNF-α on RF uptake and level of expression of the hRFVT-3 protein and mRNA (Fig. 8, A–C), as well as reversal of the degree of inhibition in the activity of the SLC52A3 promoter (Fig. 8D). These findings suggest that the NF-κB signaling pathway is involved in mediating the effect of TNF-α on the physiology and molecular biology of intestinal RF uptake.
As mentioned above, the SLC52A3 promoter has two NF-κB binding sites; thus, we sought to examine the possible involvement of these sites in mediating the TNF-α effect on activity of this promoter. We used NF-κB binding site mutated promoter constructs that have been previously generated/characterized in our lab (8). For this, the SLC52A3 WT and NF-κB mutant constructs [p-199/+8 (mutant 1) and p-199/+8 (mutant 2)] were transiently transfected into Caco-2 cells, and promoter activity was measured after 48 h of TNF-α treatment. The results showed that mutating the NF-κB binding sites in the SLC52A3 promoter led to disappearance of the inhibitory effect of TNF-α on promoter activity (Fig. 9). This finding further confirms the involvement of the NF-κB pathway in mediating the effect of TNF-α on the physiology/molecular biology of the intestinal RF uptake and hRFVT-3 expression.
Fig. 9.
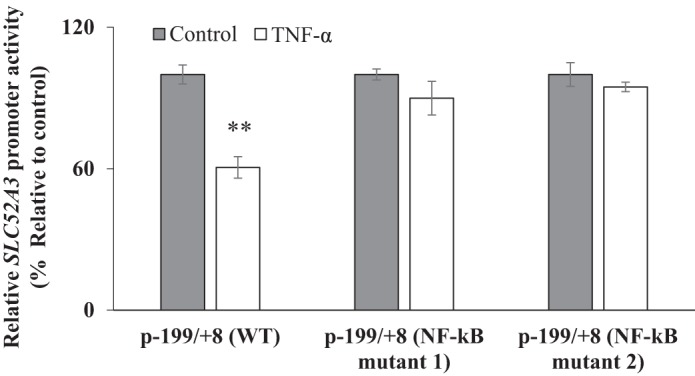
Role of the NF-κB sites in the SLC52A3 promoter in mediating the effect of tumor necrosis factor-α (TNF-α) on promoter activity. Caco-2 cells were transiently transfected with wild-type (WT) or mutated promoter constructs for 24 h, treated with TNF-α (20 ng/ml for 48 h), followed by determination of promoter activity by luciferase assay. Data are means ± SE of at least 3 different sets of experiments. **P < 0.01.
DISCUSSION
The aim of this study was to examine the effects of proinflammatory cytokines on intestinal absorption of vitamin B2, RF. Our interest in addressing this issue stems from the fact that recent studies have shown that prolonged exposure of the gut to elevated levels of proinflammatory cytokines (like TNF-α) has profound effects on its absorptive and secretory functions (1, 2, 4, 19, 38). The level of such proinflammatory cytokines in gut mucosa (and in circulation) increases markedly in conditions of chronic inflammation (as in IBD) and following prolonged bacterial infection (14, 40, 43). What effect such a prolonged exposure of the intestine to elevated levels of proinflammatory cytokines has on its ability to absorb the micronutrient RF is not known. In addition to its pathophysiological relevance, addressing this issue is of nutritional value in light of findings that IBD patients have significantly low levels of RF (7, 16). We addressed this issue using three complementary models: an in vitro (Caco-2 cells), an ex vivo (mouse jejunal enteroids), and an in vivo (wild-type mice) models.
Our studies with the in vitro model (confluent Caco-2 cells) showed prolonged exposure of the cells to TNF-α (but not to INFγ) to lead to a significant inhibition in carrier-mediated RF uptake. This inhibitory effect of TNF-α on RF uptake was found to be mediated via the apically expressed TNFR1 (2), as knocking down this receptor with the use of gene-specific siRNA led to disappearance of the inhibitory effect. The inhibition in intestinal RF uptake by TNF-α was associated with a significant reduction in the level of expression of the apical RF uptake system, i.e., hRFVT-3, at the protein and mRNA levels. Similar findings were obtained with the ex vivo and in vivo models used in this study, where exposure to the proinflammatory cytokine TNF-α led to significant inhibition in RF uptake and in level of expression of the mRFVT-3 protein and mRNA. Interestingly, in all the three models used in this study, exposure of intestinal epithelial cells to TNF-α also led to a significant inhibition in the level of expression of the basolateral RF transporter (i. e., hRFVT-1 in the case of the human intestinal cells and mRFVT-2 in the case of the mouse preparations). These findings demonstrate that TNF-α affects the intestinal RF absorption process at the apical as well as the basolateral membrane domain levels.
The inhibitory effect of TNF-α on the level of expression of hRFVT-3 and hRFVT-1 appears to be mediated at the level of transcription of the SLC52A3 and SLC52A1 genes. This conclusion is based on the observations that treating Caco-2 cells expressing the promoter of these genes with TNF-α led to a significant inhibition in activity of both promoters. Since the transcription factor Sp1 drives the basal activity of the SLC52A3 and SLC52A1 promoters in intestinal epithelial cells, as shown by us previously (8, 23), we also investigated the possible involvement of the Sp1 sites in mediating the TNF-α effects on activity of the SLC53A3 and SLC53A1 promoters. This was done by examining the effect of mutating the Sp1 binding sites in these promoters on the TNF-α effect, with the results showing a significant reversal in the TNF-α inhibition in promoter activity. These findings suggest that the Sp1 sites in the SLC53A3 and SLC53A1 promoters are involved in mediating the inhibitory effect of TNF-α on promoter activity. It is interesting to mention here that treatment of Caco-2 cells with TNF-α was associated with a significant reduction in the level of expression of Sp1. This finding further suggests the involvement of this transcription factor in mediating the effect of this proinflammatory cytokine on expression of the intestinal RF transporters.
As mentioned earlier, the NF-κB pathway is a major signaling pathway through which TNF-α exerts its effects on cells (15, 19, 38). Our findings in this study, that the specific NF-κB pharmacological inhibitor celastrol causes a marked reversal in the inhibitory effect of TNF-α on RF uptake, on the level of expression of the hRFVT-3 protein and mRNA, and on the activity of the SLC52A3 promoter, support the involvement of this pathway in mediating the effect of this proinflammatory cytokine on intestinal RF uptake physiology and molecular biology. Further support for the latter conclusion comes from the observations that mutating the two NF-κB sites in the SLC52A3 promoter led to disappearance of the inhibitory effect of TNF-α on promoter activity.
Finally, the findings that the level of expression of the hRFVT-3 and hRFVT-1 mRNA in intestinal tissue of patients with IBD is significantly lower than that in control subjects further highlight the translational relevance of the current findings. In summary, our studies show that prolonged exposure of the intestine to TNF-α negatively impacts the physiology and molecular biology of RF absorption and that this effect is mediated via transcriptional mechanism(s) involving the SLC52A3 and SLC52A1 genes. These findings may explain the significantly reduced level of RF in patients with chronic intestinal inflammation.
GRANTS
This study was supported by grants from the Department of Veteran Affairs and the National Institutes of Health, DK-58057, DK-56057, AA-018071, and DK-107474.
DISCLOSURES
No conflicts of interest, financial or otherwise, are declared by the authors.
AUTHOR CONTRIBUTIONS
K.Y.A., V.S.S., and H.M.S. conceived and designed research; K.Y.A., O.A.A., V.S.S., P.S., and R.K. performed experiments; K.Y.A., V.S.S., P.S., and H.M.S. analyzed data; K.Y.A., V.S.S., P.S., and H.M.S. interpreted results of experiments; K.Y.A. prepared figures; K.Y.A., V.S.S., and H.M.S. drafted manuscript; K.Y.A., O.A.A., V.S.S., P.S., and H.M.S. edited and revised manuscript; K.Y.A., O.A.A., V.S.S., P.S., R.K., and H.M.S. approved final version of manuscript.
REFERENCES
- 1.Aggarwal BB, Gupta SC, Kim JH. Historical perspectives on tumor necrosis factor and its superfamily: 25 years later, a golden journey. Blood 119: 651–665, 2012. doi: 10.1182/blood-2011-04-325225. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Barrenetxe J, Sánchez O, Barber A, Gascón S, Rodríguez-Yoldi MJ, Lostao MP. TNFα regulates sugar transporters in the human intestinal epithelial cell line Caco-2. Cytokine 64: 181–187, 2013. doi: 10.1016/j.cyto.2013.07.004. [DOI] [PubMed] [Google Scholar]
- 3.Bonjour JP. Vitamins and alcoholism. V. Riboflavin, VI. Niacin, VII. Pantothenic acid, and VIII. Biotin. Int J Vitam Nutr Res 50: 425–440, 1980. [PubMed] [Google Scholar]
- 4.Borthakur A, Anbazhagan AN, Kumar A, Raheja G, Singh V, Ramaswamy K, Dudeja PK. The probiotic Lactobacillus plantarum counteracts TNFα-induced downregulation of SMCT1 expression and function. Am J Physiol Gastrointest Liver Physiol 299: G928–G934, 2010. doi: 10.1152/ajpgi.00279.2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Bosch AM, Abeling NG, Ijlst L, Knoester H, van der Pol WL, Stroomer AE, Wanders RJ, Visser G, Wijburg FA, Duran M, Waterham HR. Brown-Vialetto-Van Laere and Fazio Londe syndrome is associated with a riboflavin transporter defect mimicking mild MADD: a new inborn error of metabolism with potential treatment. J Inherit Metab Dis 34: 159–164, 2011. doi: 10.1007/s10545-010-9242-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Cooperman JM, Lopez R. Riboflavin. Handbook of Vitamins: Nutritional, Biochemical, and Clinical Aspects, edited by Machlin LJ. New York: Marcel Dekker, 1984, p. 299–327. [Google Scholar]
- 7.Fernandez-Banares F, Abad-Lacruz A, Xiol X, Gine JJ, Dolz C, Cabre E, Esteve M, Gonzalez-Huix F, Gassull MA. Vitamin status in patients with inflammatory bowel disease. Am J Gastroenterol 84: 744–748, 1989. [PubMed] [Google Scholar]
- 8.Ghosal A, Sabui S, Said HM. Identification and characterization of the minimal 5′-regulatory region of the human riboflavin transporter-3 (SLC52A3) in intestinal epithelial cells. Am J Physiol Cell Physiol 308: C189–C196, 2015. doi: 10.1152/ajpcell.00342.2014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Green P, Wiseman M, Crow YJ, Houlden H, Riphagen S, Lin JP, Raymond FL, Childs AM, Sheridan E, Edwards S, Josifova DJ. Brown-Vialetto-Van Laere syndrome, a ponto-bulbar palsy with deafness, is caused by mutations in c20orf54. Am J Hum Genet 86: 485–489, 2010. doi: 10.1016/j.ajhg.2010.02.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Hino S, Sakamoto A, Nagaoka K, Anan K, Wang Y, Mimasu S, Umehara T, Yokoyama S, Kosai K, Nakao M. FAD-dependent lysine-specific demethylase-1 regulates cellular energy expenditure. Nat Commun 3: 758, 2012. doi: 10.1038/ncomms1755. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Iinuma S. Synthesis of riboflavin by intestinal bacteria [Kyoto]. J Vitaminol (Kyoto) 1: 6–13, 1955. doi: 10.5925/jnsv1954.1.2_6. [DOI] [PubMed] [Google Scholar]
- 12.Iwanaga K, Hasegawa T, Hultquist DE, Harada H, Yoshikawa Y, Yanamadala S, Liao H, Visovatti SH, Pinsky DJ. Riboflavin-mediated reduction of oxidant injury, rejection, and vasculopathy after cardiac allotransplantation. Transplantation 83: 747–753, 2007. doi: 10.1097/01.tp.0000256283.06469.d4. [DOI] [PubMed] [Google Scholar]
- 13.Kodentsova VM, Vrzhesinskaia OA, Trofimenko EV, Sokol’nikov AA, Beketova NA, Blazheevich NV, Isaeva VA, Aleĭnik SI, Trofimenko LS, Dronova VI, Spirichev VB. [Vitamin status of children with diabetes mellitus]. Vopr Med Khim 40: 45–48, 1994. 8160431 [PubMed] [Google Scholar]
- 14.Komatsu M, Kobayashi D, Saito K, Furuya D, Yagihashi A, Araake H, Tsuji N, Sakamaki S, Niitsu Y, Watanabe N. Tumor necrosis factor-alpha in serum of patients with inflammatory bowel disease as measured by a highly sensitive immuno-PCR. Clin Chem 47: 1297–1301, 2001. [PubMed] [Google Scholar]
- 15.Kumar A, Chatterjee I, Gujral T, Alakkam A, Coffing H, Anbazhagan AN, Borthakur A, Saksena S, Gill RK, Alrefai WA, Dudeja PK. Activation of nuclear factor-κB by tumor necrosis factor in intestinal epithelial cells and mouse intestinal epithelia reduces expression of the chloride transporter SLC26A3. Gastroenterology 153: 1338–1350.e3, 2017. doi: 10.1053/j.gastro.2017.08.024. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Kuroki F, Iida M, Tominaga M, Matsumoto T, Hirakawa K, Sugiyama S, Fujishima M. Multiple vitamin status in Crohn’s disease. Correlation with disease activity. Dig Dis Sci 38: 1614–1618, 1993. doi: 10.1007/BF01303168. [DOI] [PubMed] [Google Scholar]
- 17.Livak KJ, Schmittgen TD. Analysis of relative gene expression data using real-time quantitative PCR and the 2(−delta delta C(T)) method. Methods 25: 402–408, 2001. doi: 10.1006/meth.2001.1262. [DOI] [PubMed] [Google Scholar]
- 18.Mazur-Bialy AI, Buchala B, Plytycz B. Riboflavin deprivation inhibits macrophage viability and activity—a study on the RAW 264.7 cell line. Br J Nutr 110: 509–514, 2013. doi: 10.1017/S0007114512005351. [DOI] [PubMed] [Google Scholar]
- 19.Mochizuki T, Satsu H, Shimizu M. Signaling pathways involved in tumor necrosis factor alpha-induced upregulation of the taurine transporter in Caco-2 cells. FEBS Lett 579: 3069–3074, 2005. doi: 10.1016/j.febslet.2005.04.063. [DOI] [PubMed] [Google Scholar]
- 20.Neurath MF, Becker C, Barbulescu K. Role of NF-kappaB in immune and inflammatory responses in the gut. Gut 43: 856–860, 1998. doi: 10.1136/gut.43.6.856. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Pangrekar J, Krishnaswamy K, Jagadeesan V. Effects of riboflavin deficiency and riboflavin administration on carcinogen-DNA binding. Food Chem Toxicol 31: 745–750, 1993. doi: 10.1016/0278-6915(93)90146-P. [DOI] [PubMed] [Google Scholar]
- 22.Rivlin RS. Riboflavin. Encyclopedia of Dietary Supplements, edited by Coates PM, Betz JM, Blackman MR, Cragg GM, Levine M, Moss J, White JD. New York: CRC, 2010, p. 691–699. doi: 10.1201/b14669-80. [DOI] [Google Scholar]
- 23.Sabui S, Ghosal A, Said HM. Identification and characterization of 5′-flanking region of the human riboflavin transporter 1 gene (SLC52A1). Gene 553: 49–56, 2014. doi: 10.1016/j.gene.2014.10.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Said HM, Hollander D, Mohammadkhani R. Uptake of riboflavin by intestinal basolateral membrane vesicles: a specialized carrier-mediated process. Biochim Biophys Acta 1148: 263–268, 1993. doi: 10.1016/0005-2736(93)90138-P. [DOI] [PubMed] [Google Scholar]
- 25.Said HM, Ma TY. Mechanism of riboflavine uptake by Caco-2 human intestinal epithelial cells. Am J Physiol Gastrointest Liver Physiol 266: G15–G21, 1994. [DOI] [PubMed] [Google Scholar]
- 26.Said HM, Mohammadkhani R. Uptake of riboflavin across the brush border membrane of rat intestine: regulation by dietary vitamin levels. Gastroenterology 105: 1294–1298, 1993. doi: 10.1016/0016-5085(93)90131-U. [DOI] [PubMed] [Google Scholar]
- 27.Said HM, Ortiz A, Moyer MP, Yanagawa N. Riboflavin uptake by human-derived colonic epithelial NCM460 cells. Am J Physiol Cell Physiol 278: C270–C276, 2000. doi: 10.1152/ajpcell.2000.278.2.C270. [DOI] [PubMed] [Google Scholar]
- 28.Said HM, Ross C. Riboflavin. Modern Nutrition in Health and Disease, edited by Shils ME, Shike M, Ross AC, Caballero B, Cousins RJ. New York: Lippincott Williams & Wilkins Woters Kluwer, 2011, p. 325–330. [Google Scholar]
- 29.Said HM, Arianas P. Transport of riboflavin in human intestinal brush border membrane vesicles. Gastroenterology 100: 82–88, 1991. doi: 10.1016/0016-5085(91)90586-A. [DOI] [PubMed] [Google Scholar]
- 30.Said HM. Intestinal absorption of water-soluble vitamins in health and disease. Biochem J 437: 357–372, 2011. doi: 10.1042/BJ20110326. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Sato T, Stange DE, Ferrante M, Vries RG, Van Es JH, Van den Brink S, Van Houdt WJ, Pronk A, Van Gorp J, Siersema PD, Clevers H. Long-term expansion of epithelial organoids from human colon, adenoma, adenocarcinoma, and Barrett’s epithelium. Gastroenterology 141: 1762–1772, 2011. doi: 10.1053/j.gastro.2011.07.050. [DOI] [PubMed] [Google Scholar]
- 32.Schramm M, Wiegmann K, Schramm S, Gluschko A, Herb M, Utermöhlen O, Krönke M. Riboflavin (vitamin B2) deficiency impairs NADPH oxidase 2 (Nox2) priming and defense against Listeria monocytogenes. Eur J Immunol 44: 728–741, 2014. doi: 10.1002/eji.201343940. [DOI] [PubMed] [Google Scholar]
- 33.Seekamp A, Hultquist DE, Till GO. Protection by vitamin B2 against oxidant-mediated acute lung injury. Inflammation 23: 449–460, 1999. doi: 10.1023/A:1021965026580. [DOI] [PubMed] [Google Scholar]
- 34.Sethi G, Ahn KS, Pandey MK, Aggarwal BB. Celastrol, a novel triterpene, potentiates TNF-induced apoptosis and suppresses invasion of tumor cells by inhibiting NF-kappaB-regulated gene products and TAK1-mediated NF-kappaB activation. Blood 109: 2727–2735, 2007. doi: 10.1182/blood-2006-10-050807. [DOI] [PubMed] [Google Scholar]
- 35.Staelens J, Wielockx B, Puimège L, Van Roy F, Guénet JL, Libert C. Hyporesponsiveness of SPRET/Ei mice to lethal shock induced by tumor necrosis factor and implications for a TNF-based antitumor therapy. Proc Natl Acad Sci USA 99: 9340–9345, 2002. doi: 10.1073/pnas.142293699. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Subramanian VS, Ghosal A, Subramanya SB, Lytle C, Said HM. Differentiation-dependent regulation of intestinal vitamin B2 uptake: studies utilizing human-derived intestinal epithelial Caco-2 cells and native rat intestine. Am J Physiol Gastrointest Liver Physiol 304: G741–G748, 2013. doi: 10.1152/ajpgi.00018.2013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Subramanian VS, Lambrecht N, Lytle C, Said HM. Conditional (intestinal-specific) knockout of the riboflavin transporter-3 (RFVT-3) impairs riboflavin absorption. Am J Physiol Gastrointest Liver Physiol 310: G285–G293, 2016. doi: 10.1152/ajpgi.00340.2015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Subramanian VS, Sabui S, Subramenium GA, Marchant JS, Said HM. Tumor necrosis factor-α reduces intestinal vitamin C uptake: a role for NF-κB-mediated signaling. Am J Physiol Gastrointest Liver Physiol 315: G241–G248, 2018. doi: 10.1152/ajpgi.00071.2018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Subramanian VS, Subramanya SB, Rapp L, Marchant JS, Ma TY, Said HM. Differential expression of human riboflavin transporters -1, -2, and -3 in polarized epithelia: a key role for hRFT-2 in intestinal riboflavin uptake. Biochim Biophys Acta 1808: 3016–3021, 2011. doi: 10.1016/j.bbamem.2011.08.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Sulzbach De Oliveira HS, Biolchi V, Richardt Medeiros HR, Bizerra Gandor Jantsch DB, Knabben DE Oliveira Becker Delving LK, Reckziegel R, Goettert MI, Brum IS, Pozzobon A. Effect of Helicobacter pylori on NFKB1, p38α and TNFα mRNA expression levels in human gastric mucosa. Exp Ther Med 11: 2365–2372, 2016. doi: 10.3892/etm.2016.3213. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Tomei S, Yuasa H, Inoue K, Watanabe J. Transport functions of riboflavin carriers in the rat small intestine and colon: site difference and effects of tricyclic-type drugs. Drug Deliv 8: 119–124, 2001. doi: 10.1080/107175401316906874. [DOI] [PubMed] [Google Scholar]
- 42.Tu BP, Ho-Schleyer SC, Travers KJ, Weissman JS. Biochemical basis of oxidative protein folding in the endoplasmic reticulum. Science 290: 1571–1574, 2000. doi: 10.1126/science.290.5496.1571. [DOI] [PubMed] [Google Scholar]
- 43.Vitale S, Strisciuglio C, Pisapia L, Miele E, Barba P, Vitale A, Cenni S, Bassi V, Maglio M, Del Pozzo G, Troncone R, Staiano A, Gianfrani C. Cytokine production profile in intestinal mucosa of paediatric inflammatory bowel disease. PLoS One 12: e0182313, 2017. doi: 10.1371/journal.pone.0182313. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Wang LD, Zhou FY, Li XM, Sun LD, Song X, Jin Y, Li JM, Kong GQ, Qi H, Cui J, Zhang LQ, Yang JZ, Li JL, Li XC, Ren JL, Liu ZC, Gao WJ, Yuan L, Wei W, Zhang YR, Wang WP, Sheyhidin I, Li F, Chen BP, Ren SW, Liu B, Li D, Ku JW, Fan ZM, Zhou SL, Guo ZG, Zhao XK, Liu N, Ai YH, Shen FF, Cui WY, Song S, Guo T, Huang J, Yuan C, Huang J, Wu Y, Yue WB, Feng CW, Li HL, Wang Y, Tian JY, Lu Y, Yuan Y, Zhu WL, Liu M, Fu WJ, Yang X, Wang HJ, Han SL, Chen J, Han M, Wang HY, Zhang P, Li XM, Dong JC, Xing GL, Wang R, Guo M, Chang ZW, Liu HL, Guo L, Yuan ZQ, Liu H, Lu Q, Yang LQ, Zhu FG, Yang XF, Feng XS, Wang Z, Li Y, Gao SG, Qige Q, Bai LT, Yang WJ, Lei GY, Shen ZY, Chen LQ, Li EM, Xu LY, Wu ZY, Cao WK, Wang JP, Bao ZQ, Chen JL, Ding GC, Zhuang X, Zhou YF, Zheng HF, Zhang Z, Zuo XB, Dong ZM, Fan DM, He X, Wang J, Zhou Q, Zhang QX, Jiao XY, Lian SY, Ji AF, Lu XM, Wang JS, Chang FB, Lu CD, Chen ZG, Miao JJ, Fan ZL, Lin RB, Liu TJ, Wei JC, Kong QP, Lan Y, Fan YJ, Gao FS, Wang TY, Xie D, Chen SQ, Yang WC, Hong JY, Wang L, Qiu SL, Cai ZM, Zhang XJ. Genome-wide association study of esophageal squamous cell carcinoma in Chinese subjects identifies susceptibility loci at PLCE1 and C20orf54. Nat Genet 42: 759–763, 2010. [Erratum in Nat Genet 46: 1041, 2014.] doi: 10.1038/ng.648. [DOI] [PubMed] [Google Scholar]
- 45.Wu AM, Dedina L, Dalvi P, Yang M, Leon-Cheon J, Earl B, Harper PA, Ito S. Riboflavin uptake transporter Slc52a2 (RFVT2) is upregulated in the mouse mammary gland during lactation. Am J Physiol Regul Integr Comp Physiol 310: R578–R585, 2016. doi: 10.1152/ajpregu.00507.2015. [DOI] [PubMed] [Google Scholar]
- 46.Yamamoto S, Inoue K, Ohta KY, Fukatsu R, Maeda JY, Yoshida Y, Yuasa H. Identification and functional characterization of rat riboflavin transporter 2. J Biochem 145: 437–443, 2009. doi: 10.1093/jb/mvn181. [DOI] [PubMed] [Google Scholar]
- 47.Yao Y, Yonezawa A, Yoshimatsu H, Omura T, Masuda S, Matsubara K. Involvement of riboflavin transporter RFVT2/Slc52a2 in hepatic homeostasis of riboflavin in mice. Eur J Pharmacol 714: 281–287, 2013. doi: 10.1016/j.ejphar.2013.07.042. [DOI] [PubMed] [Google Scholar]
- 48.Yonezawa A, Masuda S, Katsura T, Inui K. Identification and functional characterization of a novel human and rat riboflavin transporter, RFT1. Am J Physiol Cell Physiol 295: C632–C641, 2008. doi: 10.1152/ajpcell.00019.2008. [DOI] [PubMed] [Google Scholar]