Abstract
Most kidney stones (KS) are composed of calcium oxalate, and small increases in urine oxalate affect the stone risk. Intestinal oxalate secretion mediated by anion exchanger SLC26A6 (PAT1) plays a crucial role in limiting net absorption of ingested oxalate, thereby preventing hyperoxaluria and related KS, reflecting the importance of understanding regulation of intestinal oxalate transport. We previously showed that ATP and UTP inhibit oxalate transport by human intestinal Caco2-BBE cells (C2). Since ATP is rapidly degraded to adenosine (ADO), we examined whether intestinal oxalate transport is regulated by ADO. We measured [14C]oxalate uptake in the presence of an outward Cl gradient as an assay of Cl-oxalate exchange activity, ≥49% of which is PAT1-mediated in C2 cells. We found that ADO significantly inhibited oxalate transport by C2 cells, an effect completely blocked by the nonselective ADO receptor antagonist 8-p-sulfophenyltheophylline. ADO also significantly inhibited oxalate efflux by C2 cells, which is important since PAT1 mediates oxalate efflux in vivo. Using pharmacological antagonists and A2B adenosine receptor (A2B AR) siRNA knockdown studies, we observed that ADO inhibits oxalate transport through the A2B AR, phospholipase C, and PKC. ADO inhibits oxalate transport by reducing PAT1 surface expression as shown by biotinylation studies. We conclude that ADO inhibits oxalate transport by lowering PAT1 surface expression in C2 cells through signaling pathways including the A2B AR, PKC, and phospholipase C. Given higher ADO levels and overexpression of the A2B AR in inflammatory bowel disease (IBD), our findings have potential relevance to pathophysiology of IBD-associated hyperoxaluria and related KS.
Keywords: A2B adenosine receptor, inflammation, intestinal oxalate transport, phospholipase C, PKC, SLC26A6
INTRODUCTION
Nephrolithiasis is the second most prevalent kidney disease in the United States after hypertension. It is a major source of patient discomfort and disability, lost working days, and health-care costs (~$10 billion annually). Hyperoxaluria is a major risk factor for kidney stones (KS), and ~80% of KS are composed of calcium oxalate (14). Urinary oxalate is an important determinant of supersaturation, and the risk for stone formation is affected by small changes in urine oxalate (18). Of note is that the risk of stone formation begins to rise in both men and women even at urinary oxalate levels traditionally considered to be within the normal range (25–30 mg/day) (18). Importantly, a history of a single KS is associated with a significantly increased risk of poor renal outcome, including advanced chronic kidney disease (CKD) and end-stage renal disease (ESRD) (1). Both CKD and ESRD are associated with significantly higher long term morbidity and mortality, as well as a substantial cost burden.
The mammalian intestine plays a critical role in oxalate homeostasis by serving as a site for dietary oxalate absorption as well as an avenue, together with the kidneys, for oxalate excretion (36). The amount of oxalate excreted in the urine is influenced by dietary oxalate intake, net intestinal absorption, endogenous production, and renal clearance. Intestinal oxalate absorption is predominantly passive through the paracellular pathway, while secretion is active through the transcellular (transepithelial) pathway (42). The following examples reflect the crucial role of the intestine in oxalate homeostasis. Hyperoxaluria and a high incidence of KS are common in inflammatory bowel disease (IBD) patients (11). Hyperoxaluria is a major complication (developing in >50% of patients) of bariatric surgery for obesity and small-bowel resection (62). In addition, anion exchanger SLC26A6 [also known as putative anion exchanger 1 = (PAT1)] null mice have a critical defect in intestinal oxalate secretion leading to enhanced net absorption of ingested oxalate, hyperoxalemia, hyperoxaluria, and a high incidence of calcium oxalate KS (27, 39), indicating that defects in the function or regulation of this key transporter are potential molecular mechanisms predisposing to calcium oxalate KS in humans. Collectively, the molecular mechanisms regulating PAT1 and intestinal oxalate transport are potentially very important for the management of hyperoxaluria and related KS. Better understanding of such molecular regulatory mechanisms could yield valuable information that may lead to the design of novel therapeutic approaches for the prevention and/or treatment of hyperoxaluria and related KS. Of note is that hyperoxaluria remains without specific therapy.
As described above, PAT1 plays a crucial role in small intestinal transcellular oxalate secretion, thereby preventing hyperoxaluria and related KS. Transcellular intestinal oxalate secretion requires oxalate influx into the enterocyte from blood (basolateral side), where anion exchanger SLC26A1 is likely involved, and then its efflux from the luminal side by PAT1 (±other transporters, potentially including SLC26A2). Of note is that PAT1 mediates ≥49% of apical oxalate uptake by human intestinal Caco2-BBE cells (C2) (6, 28) and it functions in the direction of exchanging intracellular oxalate for mucosal Cl during the process of transepithelial intestinal oxalate secretion. However, PAT1 can operate in either direction (40), and we therefore measured its activity by the more convenient assay of cellular oxalate uptake.
We previously reported that the extracellular nucleotides ATP and UTP negatively regulate oxalate transport by lowering PAT1 surface expression in C2 cells through signaling pathways including the P2Y2 purinergic receptor, phospholipase C (PLC), and PKC-δ (3). Importantly, luminal ATP is rapidly degraded to adenosine (ADO) by ectonucleotidases (50). We therefore examined whether adenosinergic signaling regulates intestinal oxalate transport. We find that ADO negatively regulates oxalate transport by lowering PAT1 surface expression in C2 cells through signaling pathways including the A2B AR, PKC, and PLC. These findings have potential importance because intestinal cells are known to be exposed to ADO under physiological and pathological conditions. Of significant interest is that these findings might have a potential relevance to the pathophysiology of the hyperoxaluria seen in association with conditions characterized by chronic intestinal inflammation [where significantly higher luminal intestinal ADO concentrations as well as overexpression of the A2B AR were reported (4)], including IBD, celiac disease, obesity, and diabetes mellitus (DM).
MATERIALS AND METHODS
Cell culture.
Human intestinal Caco2-BBE cells (C2) were grown and maintained as we previously reported (3). The oxalate flux and other studies described below were performed using confluent cells grown for 5–13 days postplating on 6-well or 24-well plastic supports and/or on 0.4-µm collagen-coated polystyrene transwell membrane filters (Corning, Corning, New York) in 12- and/or 24-mm inserts. The transepithelial resistance (TER) was measured using EVOM2 Epithelial Voltohmmeter (World Precision Instruments).
Radioactive flux studies.
[14C]oxalate flux studies in C2 cells were performed following our previously published methods (3, 34). Briefly, we assessed Cl-oxalate exchange activity, the predominant transport mode of PAT1, by imposing an outward Cl gradient by removing extracellular Cl (Cli > Clo). This has been accomplished by performing the uptake experiments in a Cl-free uptake buffer by replacing KCL with K-gluconate. [14C]oxalate (20 μM) was added to the Cl-free buffer, and the influx of [14C]oxalate in exchange for intracellular Cl (i.e., Cl-oxalate exchange activity) was measured for 6 min. This 6-min influx period was chosen because it falls within the linear range of oxalate uptake by these cells. We previously showed that imposing an outward Cl gradient by removing extracellular Cl (Cli > Clo) caused significant stimulation of [14C]oxalate uptake, which is greatly inhibited (>80%) by external Cl (Clo > Cli), consistent with Cl-oxalate exchange (34). We also demonstrated that 100 µM DIDS (anion exchange inhibitor) significantly inhibited (>93%) this Cl-oxalate exchange activity, indicating that the observed oxalate uptake is mediated by one or more of the involved anion exchanger(s) in C2 cells (3). Importantly, we and others have shown that PAT1 mediates ≥49% of the above described Cl-oxalate exchange activity in C2 cells (6, 28). The uptake of [14C]oxalate was terminated by two to three rapid washes of the cell monolayers with ice-cold Cl-free solution. The cells were then solubilized in 0.2 N NaOH followed by neutralization with an equivalent amount of 0.2 N HCl. The solubilized cells were then transferred to vials with scintillation fluid (Opti-Fluor; Packard), and the radioactivity was measured by scintillation spectrometry. Flux studies on cells grown on transwells were similarly performed as above, with the flux solution added to the apical side of the transwell when the unidirectional apical influx was assessed. The influx of [14C]oxalate was terminated by two to three rapid washes of the cell monolayers with ice-cold Cl-free solution, and the transwells were then placed upside down and were allowed to dry for several minutes. Membrane filters containing the cells were cut from the support and placed into vials with scintillation fluid, and radioactivity was similarly measured following overnight solubilization.
For [14C]oxalate efflux studies, C2 cells were first preloaded with radioisotope by incubating for 6 min in Cl-free buffer containing 20 µM [14C]oxalate. The [14C]oxalate preloading was terminated by two to three rapid washes of the cell monolayers with the Cl-free solution. The C2 cells were then treated with vehicle (Control) or ADO for 2 min, followed by two rapid washes of the cell monolayers with the Cl-free solution. The C2 cells were then reincubated for 10 min in the Cl-free buffer without (Control/No Cl) or isotonic replacement of gluconate with 10 mM Cl (Control + Cl and ADO + Cl). The C2 [14C]oxalate contents were measured at the end of the 6-min preloading period (Initial) and after the 10-min reincubation period (Control/No Cl, Control + Cl, and ADO + Cl), and net efflux was calculated as the difference between the initial C2 [14C]oxalate contents and that remaining after 10-min reincubation.
A2B AR knockdown in C2 cells.
To knock down the A2B AR expression in C2 cells, a specific A2B AR short interfering RNA (siRNA) oligonucleotide (catalog no. 29642) was purchased from Santa Cruz Biotechnology (Santa Cruz, CA). A scrambled artificial sequence oligonucleotide was used as a negative control siRNA (catalog no. sc-37007). C2 cells were untransfected (UT) or transfected with the negative control siRNA (NC siRNA) or the A2B AR specific siRNA (A2B AR siRNA) according to the manufacturer’s protocol. We assessed the A2B AR mRNA expression using real-time PCR and total protein expression using immunoblotting to evaluate the efficiency of the silencing procedure. We observed that the 72-h time point (compared with 48 h and 96 h) following transfection gave the highest and consistent reduction in the A2B AR mRNA expression in pilot experiments, and therefore, the 72-h time point was used for the reported results.
Real-time PCR.
RNA was extracted from C2 cells using E.Z.N.A. Total RNA Kit (Omega Bio-tek, Norcross, GA). RNA was reverse transcribed and amplified with Power SYBR Green PCR Master Mix kit (Applied Biosystems). Human A2B AR was amplified with gene-specific primers (forward primer, 5′-TTCTGGCCGTGGCAGTC-3′; reverse primer, 5′-AGGACAGCAATGACCCCT-3′). Human GAPDH was amplified as an internal control using gene-specific primers (forward primer, 5′-TCCCTGAGCTGAACGGGAAG-3′; reverse primer, 5′-GGAGGAGTGGGTGTCGCTGT-3′). Relative A2B AR mRNA level was expressed as a percentage of UT normalized to GAPDH.
SDS-PAGE, Western blotting, and surface biotinylation.
SDS-PAGE and Western blotting was performed as we previously reported (3). Briefly, equal amounts of protein lysates were separated by SDS-PAGE using 7.5% polyacrylamide gels, with subsequent electrotransfer to polyvinylidene difluoride (Immobilon-P, Millipore). All immunoblots were stained with Ponceau S solution [0.1% Ponceau S (wt/vol) in 5% acetic acid (vol/vol) following transfer. Immunoblots were incubated overnight at 4°C with anti-SLC26A6 antibody (catalog no. sc-26728; Santa Cruz Biotechnology; 1:200 dilution), anti-alkaline phosphatase antibody (catalog no. sc-271431; Santa Cruz Biotechnology; 1:250 dilution), anti-A2B AR antibody (catalog no. sc-28996; Santa Cruz; 1:400 dilution), or anti-GAPDH antibody (catalog no. sc-32233; Santa Cruz Biotechnology; 1:10,000 dilution). The strips then were washed in blotto and incubated for 1 h with horseradish peroxidase-conjugated secondary antibodies. Antibody reactivity was detected with the enhanced chemiluminescence system (SuperSignal West; Thermo Scientific) according to the manufacturer’s protocol.
Surface biotinylation studies were performed as previously described (3). Briefly, C2 cells grown on six-well plates were treated with vehicle (Control) or 100 μM adenosine (ADO) for 2 min in the culture medium followed by washing ×1 in ice-cold PBS. C2 cells were incubated with 2 mg/ml of the biotinylation reagent Sulfo-NHS-SS-Biotin (Thermo Scientific) in PBS for two 30-min washes (3, 34, 35) to biotinylate surface membrane proteins (with all steps performed on ice and/or in a cold room), followed by washing ×2 in ice-cold PBS. The cells were then incubated in PBS containing 100 mM glycine for 15 min to quench excess biotin, and subsequently washed ×2 with PBS. Total protein lysates were prepared as described above and normalized samples were incubated overnight with 150–200 μl of streptavidin agarose beads (Thermo Scientific). Biotinylated proteins were dissociated from the beads with sample buffer (10% SDS, 2% β-mercaptoethanol, 20% glycerol, 5 mM Tris·HCl, pH 6.8) containing 100 mM DTT, as well as by boiling for 2 min. Following separation by SDS-PAGE, proteins were transferred to immunoblots and probed with the anti-SLC26A6 or the anti-alkaline phosphatase antibody as above.
Materials.
U73343, PP2, SB 202190, and Gö6983 were purchased from Calbiochem. Adenosine (ADO), 8-p-sulfophenyltheophylline (8-SPT), and DIDS were purchased from Sigma. The A2B AR selective antagonist N-(4-cyanophenyl)-2-[4-(2,3,6,7-tetrahydro-2,6-dioxo-1,3-dipropyl-1H-purin-8-yl)phenoxy]-acetamide (MRS-1754) was purchased from Tocris Bioscience. U73122 was purchased from Enzo Life Sciences. U0126 and LY 294002 were purchased from Promega. [14C]oxalate was purchased from Vitrax (specific activity: 54 mCi/mmol). Of note is that the concentration of each drug as well as the incubation periods for the different protein kinase inhibitors were selected based on published literature (references provided in results). In addition, we routinely test several concentrations and preincubation time periods in pilot experiments.
Statistical analysis.
Experimental data are presented as a percentage of the respective control (100%) value and are expressed as means ± SE. Data were analyzed by one-way ANOVA followed by Bonferroni or Student-Newman-Keuls post hoc test or by Student’s t-test for paired or unpaired samples when comparing two groups. P < 0.05 was considered statistically significant.
RESULTS
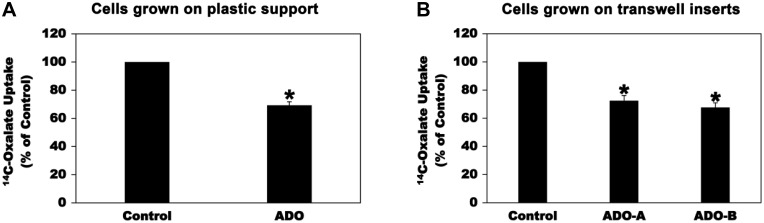
We had previously established C2 cells as a useful model to study regulation of intestinal oxalate transport (3, 6). We found that the extracellular nucleotides ATP and UTP negatively regulate oxalate transport by C2 cells by reducing PAT1 surface expression (3). Since ATP is rapidly degraded to ADO by ectonucleotidases (50), we therefore examined whether intestinal oxalate transport is subject to regulation by adenosinergic signaling. To this end, we assessed oxalate uptake by C2 cells by imposing an outward Cl gradient by removing extracellular Cl (Cli > Clo) and measuring DIDS-sensitive influx of radioactive [14C]oxalate in exchange for intracellular Cl (i.e., Cl-oxalate exchange activity) as we previously reported (3). Of note is that PAT1 mediates ≥ 49% of apical oxalate uptake by C2 (6, 28). As shown in Fig. 1A, preincubation of C2 cells grown on plastic support with 100 µM ADO (37, 61) for 2 min caused significant inhibition of [14C]oxalate uptake (by 31%). Importantly, 100 µM ADO [apical (ADO-A) or basolateral (ADO-B)] for 2 min also similarly inhibited [14C]oxalate uptake (by 28% apically and 32% basolaterally) by C2 cells grown on transwell inserts (Fig. 1B). ADO has no effect on the TER [mean TER in Ω·cm2: 325.3 ± 2.1, 318.0 ± 4.5, 322.8 ± 3.8, and 338 ± 6.3 for untreated cells, and cells treated with vehicle, ADO-A, and ADO-B, respectively], indicating that ADO did not affect the paracellular permeability. These results indicate that adenosinergic signaling negatively regulates oxalate transport by C2 cells.
Fig. 1.
Effect of adenosine (ADO) on [14C]oxalate uptake by Caco2-BBE (C2) cells. A: C2 cells grown on plastic support were preincubated with vehicle (Control) or 100 µM ADO for 2 min in the culture medium, and then [14C]oxalate uptake was measured as described in materials and methods. Values are means ± SE of 7 independent experiments each of which was done in triplicate and was normalized to the respective Control value. ADO significantly reduced [14C]oxalate uptake (*P < 0.00002, two-tailed t-test). B: C2 cells grown on transwell inserts were preincubated with vehicle (Control) or 100 µM ADO [apically (ADO-A) or basolaterally (ADO-B)] for 2 min in the culture medium, and then [14C]oxalate uptake was measured as described in materials and methods. Values are means ± SE of 3 independent experiments each of which was done in triplicate and was normalized to the respective Control value. ADO significantly reduced [14C]oxalate uptake (*P < 0.01 and < 0.001 for Control compared with ADO-A and ADO-B, respectively, by ANOVA).
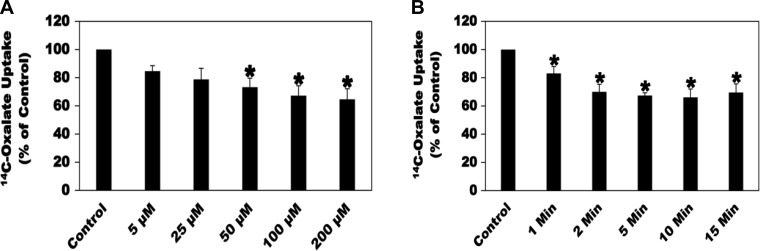
To generate an ADO dose response, the effects of 5, 25, 50, 100, and 200 µM ADO for 2 min on [14C]oxalate uptake by C2 cells were evaluated. As shown in Fig. 2A, ADO inhibited oxalate transport by C2 cells in a dose-dependent manner, with the 100 and 200 µM concentrations giving the maximum inhibition of oxalate transport and to a similar extent. Using a fit model in Excel, an IC50 (the ADO concentration at which 50% inhibition of oxalate transport by C2 cells can be achieved) of 9.2 µM are calculated. To determine over what time course the inhibitory effects of ADO occur, C2 cells were treated with 100 µM ADO for 1, 2, 5, 10, and 15 min before measurement of [14C]oxalate uptake. ADO significantly inhibited oxalate transport by C2 cells by 17, 30, 33, 34, and 31% for 1, 2, 5, 10, and 15 min, respectively (Fig. 2B), with no significant difference in the inhibition observed at 2, 5, 10, and 15 min. Based on the results of the dose response and time course, 100 µM ADO for 2 min was chosen for the study.
Fig. 2.
Effect of different concentrations of adenosine (ADO; A) and different preincubation periods (B) on [14C]oxalate uptake by Caco2-BBE (C2) cells. A: C2 cells were preincubated with vehicle (Control) or different concentrations of ADO (5, 25, 50, 100, and 200 µM) for 2 min in the culture medium, and then [14C]oxalate uptake was measured as described in materials and methods. Values are means ± SE of 6–7 independent experiments each of which was done in triplicate and was normalized to the respective Control value. 50, 100, and 200 µM ADO significantly reduced [14C]oxalate uptake (*P < 0.05, < 0.01, and < 0.01 for Control compared with 50, 100, and 200 µM, respectively, by ANOVA). B: to determine over what time course does the inhibitory effects of ADO occur, C2 cells were preincubated with vehicle (Control) or 100 µM ADO for 1, 2, 5, 10, and 15 min in the culture medium, and then [14C]oxalate uptake was measured as described in materials and methods. Values are means ± SE of 6–9 independent experiments each of which was done in triplicate and was normalized to the respective Control value. ADO significantly reduced [14C]oxalate uptake over all time points (*P < 0.05, < 0.001, < 0.001, < 0.001, and < 0.001 for Control compared with 1, 2, 5, 10, and 15 min, respectively, by ANOVA).
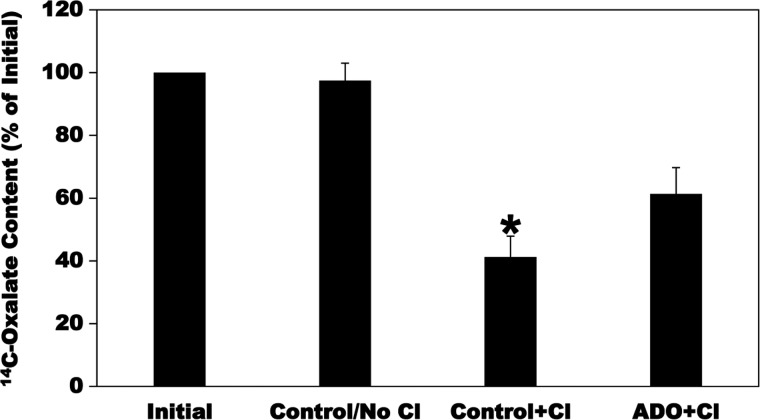
Since PAT1 operates in the direction of exchanging intracellular oxalate for mucosal Cl and/or other anion(s) (oxalate efflux) during the process of transepithelial intestinal oxalate secretion, we assessed whether ADO also affects oxalate efflux (secretion) as observed with oxalate uptake (i.e., influx). C2 cells were first preloaded with [14C]oxalate as described in materials and methods and were then treated with vehicle (Control) or 100 µM ADO for 2 min. The C2 cells were then reincubated for 10 min in Cl-free buffer without (Control/No Cl) or isotonic replacement of gluconate with 10 mM Cl (Control + Cl and ADO + Cl). The C2 [14C]oxalate contents were measured at the end of the preloading period (Initial) and after the 10-min reincubation period, and net efflux was calculated as described in materials and methods. As seen in Fig. 3, there is no detectable [14C]oxalate efflux in the absence of Cl in the external medium (Control/No Cl). The addition of external Cl (Control + Cl) significantly stimulated [14C]oxalate efflux (by 59%), reflecting Cl-oxalate exchange. Importantly, ADO significantly reduced (by 34%) Cl-induced [14C]oxalate efflux (ADO + Cl) compared with Control + Cl. These results show that ADO similarly inhibits oxalate efflux as observed with oxalate uptake, which is very important since PAT1 mediates oxalate efflux under in vivo conditions (27, 39).
Fig. 3.
Effect of adenosine (ADO) on [14C]oxalate efflux by Caco2-BBE (C2) cells. C2 cells were first preloaded with radioisotope by incubating for 6 min in Cl-free uptake buffer containing 20 µM [14C]oxalate. The [14C]oxalate preloading was terminated by 2–3 rapid washes of the cell monolayers with Cl-free solution. The C2 cells were then treated with vehicle (Control) or ADO for 2 min, followed by 2 rapid washes of the cell monolayers with Cl-free solution. The C2 cells were then reincubated for 10 min in the Cl-free buffer without (Control/No Cl) or isotonic replacement of gluconate with 10 mM Cl (Control + Cl and ADO + Cl). The C2 [14C]oxalate contents were measured at the end of the 6-min preloading period (Initial) and after 10-min reincubation, and net efflux was calculated as described in materials and methods. Values are means ± SE of 4 independent experiments each of which was done in duplicate or triplicate and was normalized to the Initial value. ADO significantly reduced the Cl-induced [14C]oxalate efflux (*P < 0.001, < 0.001, and < 0.05 for Control + Cl compared with Initial, Control/No Cl, and ADO + Cl, respectively, by ANOVA).
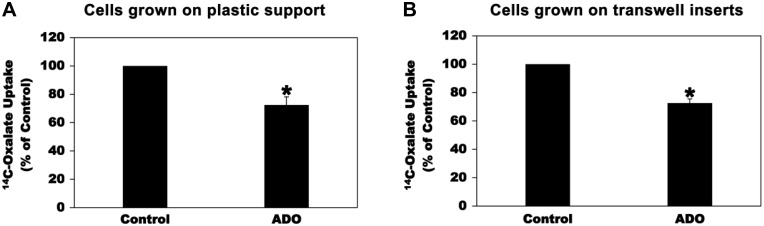
We similarly examined the effects of ADO on oxalate transport by the human colonic cell line T84 to make sure that the effects of the adenosinergic signaling on intestinal oxalate transport are not cell-line specific. We previously showed that PAT1 mediates most of oxalate transport by T84 cells (34). Preincubation with 100 µM ADO for 2 min significantly inhibited [14C]oxalate uptake by T84 cells grown on plastic support (Fig. 4A; by 28%) or transwell inserts (Fig. 4B; by 27%). These results show that the effects of the adenosinergic signaling on oxalate transport by intestinal epithelial cells are not cell-line specific.
Fig. 4.
Effect of adenosine (ADO) on [14C]oxalate uptake by T84 cells. A: T84 cells grown on plastic support were preincubated with vehicle (Control) or 100 µM ADO for 2 min in the culture medium, and then [14C]oxalate uptake was measured as described in materials and methods. Values are means ± SE of 4 independent experiments each of which was done in triplicate and was normalized to the respective Control value. ADO significantly reduced [14C]oxalate uptake (*P < 0.02, two-tailed t-test). B: T84 cells grown on transwell inserts were preincubated apically with vehicle (Control) or 100 µM ADO for 2 min in the culture medium, and then [14C]oxalate uptake was measured as described in materials and methods. Values are means ± SE of 3 independent experiments each of which was done in triplicate and was normalized to the respective Control value. ADO significantly reduced [14C]oxalate uptake (*P < 0.02, two-tailed t-test).
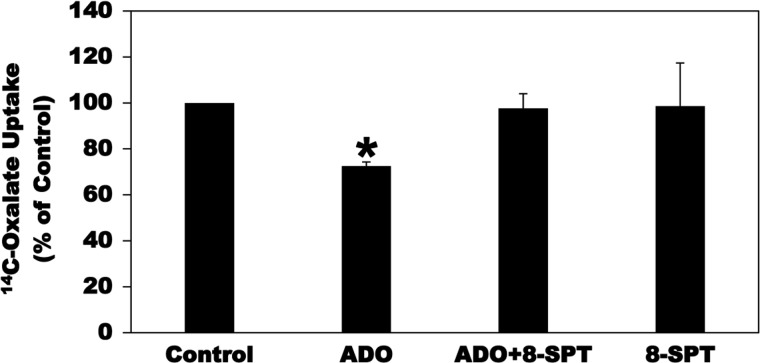
ADO modulates cell function by activating cell surface receptors that are coupled to intracellular signaling transduction cascades (24, 26). ADO signals though four G-protein-coupled-receptors (GPCRs): A1, A2A, A2B, and A3 adenosine receptors (24, 26). To characterize the AR(s) mediating the inhibitory action of ADO on [14C]oxalate uptake by C2 cells, we first tested the effect of the nonspecific AR antagonist 8-SPT. As shown in Fig. 5, preincubation of C2 cells with 8-SPT (100 µM, 15 min) before incubation with ADO completely and significantly blocked the inhibitory action of ADO on [14C]oxalate uptake by C2 cells, whereas 8-SPT had no significant effect on baseline transport. These results suggest the involvement of one or more of the four ARs described above.
Fig. 5.
Effect of the nonselective adenosine receptor antagonist 8-p-sulfophenyltheophylline (8-SPT) on adenosine (ADO)-induced inhibition of [14C]oxalate uptake by Caco2-BBE (C2) cells. C2 cells were preincubated with vehicle (Control) or 100 µM ADO for 2 min in the culture medium, and then [14C]oxalate uptake was measured as described in materials and methods. [14C]oxalate uptake was also measured in the presence of 8-SPT (100 µM) for 15 min followed by 100 µM ADO with continued presence of 8-SPT (ADO + 8-SPT) for 2 min, or 100 µM 8-SPT (8-SPT) alone for 17 min. Values are means ± SE of 5–6 independent experiments each of which was done in triplicate and was normalized to the respective Control value. 8-p-Sulfophenyltheophylline (8-SPT) completely and significantly blocked the inhibition induced by ADO (*P < 0.001 and < 0.01 for ADO compared with Control and ADO + 8-SPT, respectively, by ANOVA).
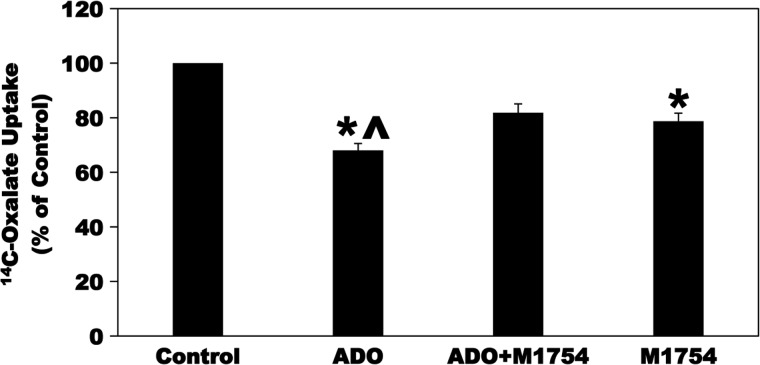
Caco-2 cells express higher levels of the A2B AR mRNA compared with the A2A AR and A3 AR (31). In addition, the A2B AR is the predominant AR expressed by mouse epithelial cells as well as it is the predominant AR expressed in the cecum and colon in the intact human colonic mucosa (29, 67, 71). Moreover, apical expression of the A2B AR was reported in both the small and large intestines of humans (4). We therefore evaluated whether ADO acts through the A2B AR to inhibit oxalate transport by C2 cells. Toward this end, C2 cells were preincubated with the A2B AR selective antagonist MRS-1754 (M1754; 100 µM × 5 min) before incubation with ADO. As shown in Fig. 6, preincubation of C2 cells with M1754 before incubation with ADO significantly reduced the ADO-induced inhibition of [14C]oxalate uptake by C2 cells despite the observation that M1754 alone caused a significant reduction in [14C]oxalate uptake by C2 cells. It is possible that M1754 would have completely blocked the ADO-mediated inhibition of oxalate transport if it did not inhibit baseline oxalate transport. These results indicate that ADO most likely signals through the A2B AR to inhibit oxalate transport by C2 cells.
Fig. 6.
Effect of the selective A2B adenosine receptor (A2B AR) antagonist MRS-1754 (M1754) on adenosine (ADO)-induced inhibition of [14C]oxalate uptake by Caco2-BBE (C2) cells. C2 cells were preincubated with vehicle (Control) or 100 µM ADO for 2 min in the culture medium, and then [14C]oxalate uptake was measured as described in materials and methods. [14C]oxalate uptake was also measured in the presence of M1754 (100 µM) for 5 min followed by 100 µM ADO with continued presence of M1754 (ADO + M1754) for 2 min or 100 µM M1754 alone for 7 min. Values are means ± SE of 4 independent experiments each of which was done in triplicate and was normalized to the respective Control value. M1754 significantly reduced the inhibition induced by ADO (*P < 0.001 for ADO and M1754 compared with Control; ^P < 0.05 for ADO compared with ADO + M1754, by ANOVA).
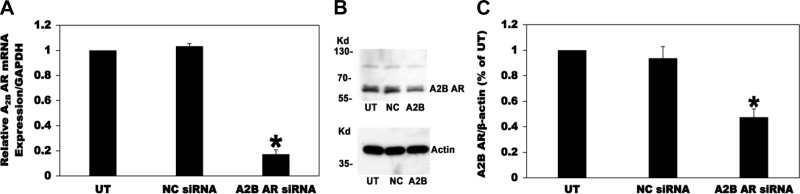
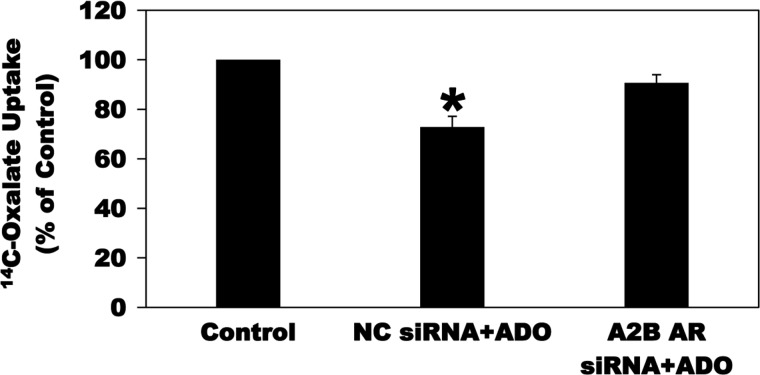
Since absolute selectivity is always an issue with pharmacological antagonists, we therefore used siRNA to knockdown the A2B AR expression in C2 cells to confirm its role in mediating the ADO-induced inhibition of oxalate transport by C2 cells. To this end, C2 cells were UT or transfected with a negative control siRNA (NC siRNA) or a specific A2B AR siRNA (A2B AR siRNA). Using real-time PCR and as shown in Fig. 7A, siRNA knockdown of the A2B AR significantly reduced its mRNA expression (by 83%), whereas the NC siRNA had no effect, indicating that the observed reduction in A2B AR mRNA expression is not due to a general effect of the silencing procedure. Using immunoblotting, we also showed that the A2B AR siRNA knockdown significantly reduced its total protein expression (by 53%), whereas the NC siRNA had no effect (Fig. 7, B and C). Interestingly, and as seen in Fig. 8, silencing the A2B AR completely and significantly blocked the ADO-induced inhibition of [14C]oxalate uptake by C2 cells. These results confirm that ADO acts through the A2B AR to negatively regulate [14C]oxalate uptake by C2 cells.
Fig. 7.
A2B adenosine receptor (A2B AR) knockdown in Caco2-BBE (C2) cells using siRNA. A: C2 cells were untransfected (UT) or transfected with a negative control siRNA (NC siRNA) or a specific A2B AR siRNA (A2B AR siRNA), and then total RNA was isolated 72 h later for real-time PCR analysis. Values are means ± SE of 5 independent experiments each of which was done in triplicate. Relative A2B AR mRNA expression level is expressed as a percentage of UT normalized to GAPDH. siRNA knockdown of the A2B AR significantly reduced its mRNA expression level (*P < 0.001 for A2B AR siRNA compared with UT and NC siRNA, by ANOVA). B: a representative Western blot analysis of A2B AR total protein expression. A2B AR protein expression was evaluated in C2 cell lysate (30 µg protein/lane; NC, C2 cells transfected with the negative control siRNA; A2B, C2 cells transfected with the siRNA targeting the A2B AR). The lower half of the same blot was probed with an anti-β-actin antibody to normalize loading of protein in each lane (bottom). C: densitometry of immunoblot results. Western blot band density was quantified using ImageJ software. Values are means ± SE for 4 independent experiments of relative A2B AR abundance to β-actin and are presented as a percentage of the UT value. siRNA knockdown of A2B AR significantly reduced A2B AR total protein expression (*P < 0.001 and < 0.01 for A2B AR siRNA compared with UT and NC siRNA, respectively, by ANOVA).
Fig. 8.
Effect of the A2B adenosine receptor (A2B AR) knockdown on adenosine (ADO)-induced inhibition of [14C]oxalate uptake by C2 cells. C2 cells transfected with the negative control siRNA (NC siRNA) or A2B AR siRNA (A2B AR siRNA) were preincubated with vehicle (Control) or 100 µM ADO (NC siRNA + ADO) or (A2B AR siRNA + ADO) for 2 min in the culture medium, and then [14C]oxalate uptake was measured as described in materials and methods. Values are means ± SE of 5 independent experiments each of which was done in triplicate and was normalized to the respective Control value. siRNA knockdown of the A2B AR completely and significantly blocked the inhibition induced by ADO (*P < 0.001 and < 0.01 for NC siRNA + ADO compared with Control and A2B AR siRNA + ADO, respectively, by ANOVA).
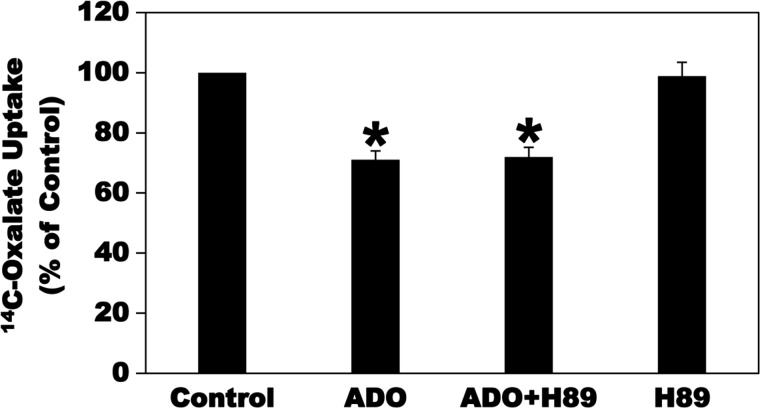
Activation of the A2A and A2B ARs stimulates adenylyl cyclase (AC), leading to an increase in cAMP levels (24). Increases in the concentration of the second messenger cAMP can lead to activation of the cAMP-dependent enzyme PKA (57, 75). To evaluate whether ADO inhibits oxalate transport in C2 cells by activating the PKA signaling pathway, the effect of the PKA inhibitor H89 on the ADO-induced inhibition was evaluated. As shown in Fig. 9, preincubation of C2 cells with H89 (40 µM × 30 min) before incubation with ADO had no significant effect on the ADO-induced inhibition of [14C]oxalate uptake by C2 cells. H89 also had no significant effect on baseline transport (Fig. 9). Of note is that we also tried preincubation with H89 at 20 and 40 µM (5, 60, 70) for 5–15 min and observed no significant effect on the ADO-induced inhibition of oxalate transport. These results indicate that the PKA signaling pathway is unlikely to be involved in the ADO-induced signal transduction cascade leading to inhibition of [14C]oxalate uptake by C2 cells.
Fig. 9.
Effect of the PKA inhibitor H89 on the adenosine (ADO)-induced inhibition of [14C]oxalate uptake by Caco2-BBE (C2) cells. C2 cells were preincubated with vehicle (Control) or 100 µM ADO for 2 min in the culture medium, and then [14C]oxalate uptake was measured as described in materials and methods. [14C]oxalate uptake was also measured in the presence of H89 (40 µM) for 30 min followed by 100 µM ADO with continued presence of H89 (ADO + H89) for 2 min or 40 µM H89 (H89) alone for 32 min. Values are means ± SE of 4 independent experiments each of which was done in triplicate and was normalized to the respective Control value. H89 had no significant effect on the inhibition induced by ADO (*P < 0.001 for ADO and ADO + H89 compared with Control, by ANOVA).
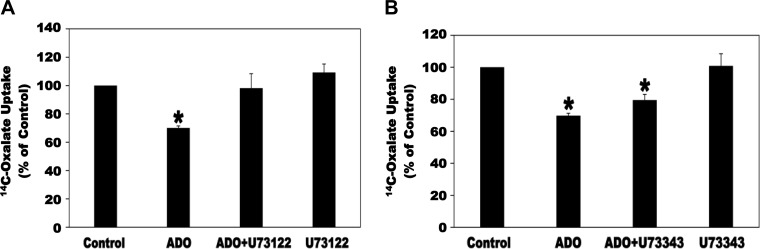
The A2B AR activates PLC through Gq proteins (8, 26). We therefore examined whether PLC is involved in the signaling cascade leading to inhibition of oxalate transport by ADO. To this end, C2 cells were preincubated with the PLC inhibitor U73122 (5 µM × 30 min) before incubation with ADO and then [14C]oxalate uptake was measured. As shown in Fig. 10A, U73122 completely and significantly blocked the ADO-induced inhibition of [14C]oxalate uptake by C2 cells, whereas it had no significant effect on baseline transport. Importantly, under the same conditions, its inactive analog U73343 (5 µM) had no significant effect on the observed inhibition or on baseline transport (Fig. 10B). These findings indicate that PLC is involved in the signaling transduction cascade leading to inhibition of oxalate transport by ADO.
Fig. 10.
Effect of the phospholipase C inhibitor U73122 (A) and its inactive analog U73343 (B) on the adenosine (ADO)-induced inhibition of [14C]oxalate uptake by Caco2-BBE (C2) cells. A: C2 cells were preincubated with vehicle (Control), 100 µM ADO (ADO) for 2 min, 5 µM U73122 for 30 min followed by 100 µM ADO with continued presence of U73122 (ADO + U73122) for 2 min, or 5 µM U73122 (U73122) alone for 32 min and then [14C]oxalate uptake was measured as described in materials and methods. Values are means ± SE of 5 independent experiments each of which was done in triplicate and was normalized to the respective Control value. U73122 completely and significantly blocked the inhibition induced by ADO (*P < 0.05 for ADO compared with Control and ADO + U73122, by ANOVA). B: C2 cells were preincubated with vehicle (Control), 100 µM ADO (ADO) for 2 min, 5 µM U73343 for 30 min followed by 100 µM ADO with continued presence of U73343 (ADO + U73343) for 2 min, or 5 µM U73343 (U73343) alone for 32 min and then [14C]oxalate uptake was measured as described in materials and methods. Values are means ± SE of 5 independent experiments each of which was done in triplicate and was normalized to the respective Control value. U73343 had no significant effect on the inhibition induced by ADO. *P < 0.001 and < 0.05 for Control compared with ADO and ADO + U73343, respectively, by ANOVA.
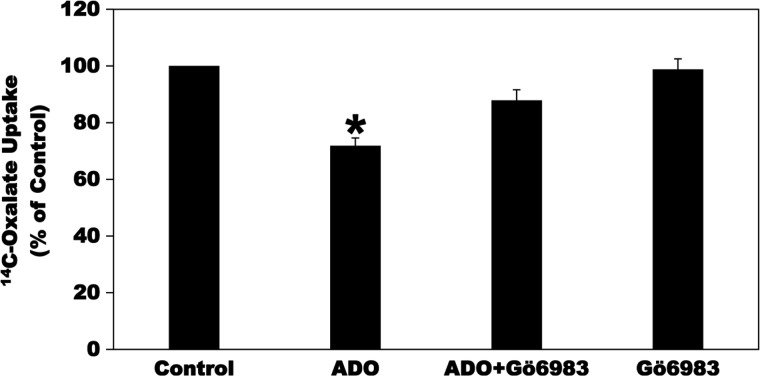
ADO signals through the A2B AR to stimulate IL-6 production in murine primary microglia cells via PKC activation (58). To evaluate whether ADO inhibits [14C]oxalate uptake by C2 cells through signaling pathways including PKC activation, we tested the effects of the PKC inhibitor Gö6983 on ADO-induced reduction of [14C]oxalate transport by C2 cells. As shown in Fig. 11, preincubation of C2 cells with Gö6983 (5 µM × 30 min) before incubation with ADO significantly reduced the ADO-induced inhibition of [14C]oxalate uptake by C2 cells, whereas it had no significant effect on baseline transport. These results indicate that ADO inhibits [14C]oxalate uptake by C2 cells through activation of a Gö6983-sensitive PKC signaling pathway. Interestingly, this is similar to our previous observations with the PKC activator phorbol 12-myristate 13-acetate-induced inhibition of the activities of mouse PAT1 expressed in Xenopus oocytes (35) and endogenous PAT1 in T84 and C2 cells (3, 34).
Fig. 11.
Effect of the PKC inhibitor Gö6983 on the adenosine (ADO)-induced inhibition of [14C]oxalate uptake by Caco2-BBE (C2) cells. C2 cells were preincubated with vehicle (Control) or 100 µM ADO (ADO) for 2 min in the culture medium, and then [14C]oxalate uptake was measured as described in materials and methods. [14C]oxalate uptake was also measured in the presence of Gö6983 (5 µM) for 30 min followed by 100 µM ADO with continued presence of Gö6983 (ADO + Gö6983) for 2 min or 5 µM Gö6983 alone for 32 min. Values are means ± SE of 5 independent experiments each of which was done in duplicate or triplicate and was normalized to the respective Control value. Gö6983 significantly reduced the inhibition induced by ADO (*P < 0.001 and < 0.01 for ADO compared with Control and ADO + Gö6983, respectively, by ANOVA).
In addition to activating AC, PLC, and PKC, ADO has been shown to mediate its effects on target cells through signaling pathways including the MAP kinases ERK1/2, phosphatidylinositol 3-kinase (PI3K), and p38 (8, 58). In addition, we also examined the potential role of Src kinases. To evaluate whether one or more of these signaling pathway(s) might be involved in the ADO-induced inhibition of oxalate transport by C2 cells, C2 cells were preincubated with the ERK1/2 inhibitor U0126 (10–20 µM; Ref. 77), the p38 inhibitor SB 202190 (10–20 µM; Ref. 66), the PI3K inhibitor LY294002 (10, 20, and 30 µM), the general tyrosine kinase inhibitor genistein (5–10 µM), and the specific Src family kinase inhibitor PP2 (10, 20, and 30 µM; Ref. 12, 34) for different time periods before incubation with ADO and then [14C]oxalate uptake was measured. We observed that all of these inhibitors [used at concentrations and for time periods known to effectively block these signaling pathways in Caco2 and T84 intestinal cells (5, 12, 70, 77) had no significant effect on the ADO-induced inhibition of oxalate transport by C2 cells (data not shown). These findings indicate that ERK1/2, PI3K, p38, and Src kinases are unlikely to be involved in the ADO-induced signaling pathway leading to inhibition of [14C]oxalate uptake by C2 cells.
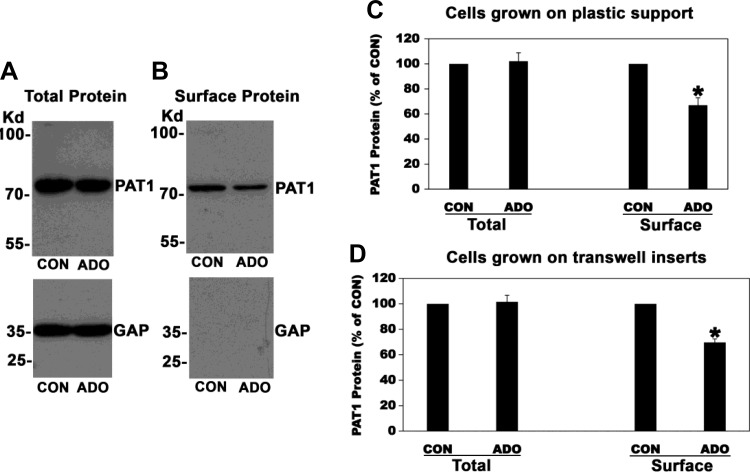
To define the molecular mechanism(s) underlying ADO-induced inhibition of oxalate transport by C2 cells, we examined whether the observed negative regulation is due to ADO-mediated reduction in PAT1 surface expression, as previously seen with PKC, cholinergic, and purinergic inhibition of mouse PAT1 expressed in Xenopus oocytes and endogenous PAT1 in T84 and C2 cells, respectively (3, 34, 35). To this end, surface biotinylation studies were performed. Control (CON) or ADO-treated cells were incubated with the surface biotinylation reagent Sulfo-NHS-SS-Biotin, biotinylated proteins were precipitated with streptavidin, and then immunoblots were prepared and probed with an anti-PAT1 antibody to assess surface PAT1 expression. In addition, immunoblots of C2 cell lysates were prepared and probed to assess total PAT1 expression. Representative immunoblots showing total and surface PAT1 protein are shown in Fig. 12, A and B, respectively, and the scanned data are presented in Fig. 12C (plastic-grown cells) and 12D (transwell-grown cells). The data are shown as the ratio of the densitometry of the total PAT1 protein band to the respective GAPDH protein band from the same lane of the same gel and then presented as a percentage of the CON (100%) value. As shown in Fig. 12, ADO significantly reduced (30–33%) the amount of surface PAT1 protein available to biotinylation in C2 cells grown on plastic support or transwell inserts, which is in general agreement with the observed ADO-induced inhibition of oxalate transport by C2 cells. However, ADO had no significant effect on total PAT1 protein expression. Equal loading was verified by probing the lower half of the same blot with an anti-GAPDH antibody and observing no significant difference (Fig. 12A). Taken together, these results strongly support PAT1 redistribution from the surface membrane without a change in total protein expression as the molecular mechanism underlying ADO-induced suppression of oxalate transport by C2 cells.
Fig. 12.
Effect of adenosine (ADO) on total (A) and surface (B) PAT1 protein expression in Caco2-BBE (C2) cells. A: a representative Western blot analysis of total PAT1 protein. C2 cells were preincubated with vehicle (CON) or 100 µM ADO for 2 min in the culture medium, and then PAT1 protein expression was evaluated in the cell lysate (30 µg protein/lane). The lower half of the same blot was probed with an anti-GAPDH (GAP) antibody to verify equal loading of protein in each lane (lower panel). B: A representative Western blot analysis of surface PAT1 protein. C2 cells were preincubated with vehicle (CON) or 100 µM ADO for 2 min in the culture medium, and then PAT1 surface protein expression was evaluated after streptavidin precipitation of surface biotinylated proteins from 2,500 µg of initial cell lysate. C: densitometry of immunoblot results. Western blot band density was quantified using ImageJ software. Values are means ± SE for 6 independent experiments (plastic-grown cells) of relative total PAT1 abundance to GAPDH and biotinylated PAT1 and are presented as a percentage of the respective control (CON) value. ADO significantly reduced the amount of PAT1 protein available to surface biotinylation (*P < 0.003, by paired t-test). D: densitometry of immunoblot results. Western blot band density was quantified using ImageJ software. Values are means ± SE for 5 independent experiments (transwell-grown cells) of relative total PAT1 abundance to GAPDH and biotinylated PAT1 and are presented as a percentage of the respective control (CON) value. ADO significantly reduced the amount of PAT1 protein available to surface biotinylation (*P < 0.0004, by paired t-test).
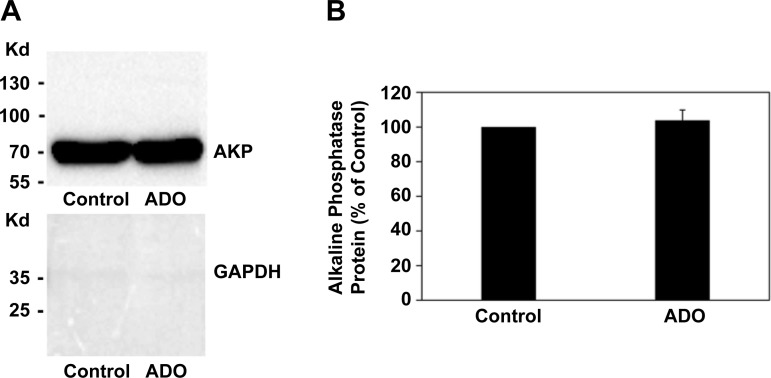
To address the specificity of ADO-induced internalization of PAT1, we examined the effect of ADO on the surface expression of the apical membrane protein alkaline phosphatase (AKP). To this end, C2 cells were treated with vehicle (Control) or ADO for 2 min and then surface biotinylation studies were performed as described above. As shown in Fig. 13, ADO has no significant effect on AKP surface protein expression, reflecting the specificity of PAT1 internalization by ADO.
Fig. 13.
Effect of adenosine (ADO) on surface alkaline phosphatase (AKP) protein expression in Caco2-BBE (C2) cells. A: a representative Western blot analysis of surface AKP protein expression. C2 cells were preincubated with vehicle (Control) or 100 µM ADO for 2 min in the culture medium, and then AKP surface protein expression was evaluated after streptavidin precipitation of surface biotinylated proteins from 300 µg of initial cell lysate. The lower half of the same blot was probed with an anti-GAPDH antibody. B: densitometry of immunoblot results. Western blot band density was quantified using ImageJ software. Values are means ± SE for 3 independent experiments and are presented as a percentage of the respective Control value. ADO has no significant effect on AKP surface protein expression.
DISCUSSION
In this study we used the well characterized human intestinal epithelial cell line C2 to examine whether adenosinergic signaling regulates intestinal oxalate transport. We found that oxalate transport by C2 cells is negatively regulated by ADO. ADO also similarly inhibits oxalate efflux by C2 cells, which is very important since PAT1 mediates oxalate efflux under in vivo conditions. Experiments using the A2B AR-selective antagonist M1754 suggest that ADO acts through the A2B AR to inhibit oxalate transport by C2 cells, and this is confirmed by siRNA knockdown studies of the A2B AR. Utilizing selective pharmacological inhibitors we demonstrated that ADO signals through activation of PLC and PKC to inhibit oxalate transport by C2 cells. In addition, we have shown reduced PAT1 surface membrane expression to be the molecular mechanism underlying ADO-induced inhibition of oxalate transport by C2 cells using surface biotinylation studies. The specificity of ADO-induced internalization of PAT1 is shown by the observation that ADO has no effect on the surface expression of the apical membrane protein AKP.
The purine nucleoside ADO is released by almost all cells, as well as generated in the extracellular space as a degradation product of released ATP breakdown by a number of ectoenzymes, including 5-nucleotidase (CD73) and apyrase (CD39) (79). Sources for intestinal extracellular ATP include the following: mechanical stimulation by food, water, and stool, lysis of apoptotic enterocytes, intestinal bacterial invasion and metabolism, physiologic stimuli such as receptor stimulation, membrane stress, and changes in cell volume (16, 48). Extracellular ADO is either recycled (e.g., through dipyridamole-sensitive carriers) or interacts with cell surface ARs in an autocrine or paracrine manner (53). Extracellular ADO concentration significantly increases (up to micromolar levels) in pathophysiological conditions characterized by ischemia, hypoxia, and inflammation, as a result for example of the transport and/or diffusion of intracellular ADO that is developed from the large pools of intracellular ATP in hypoxic conditions in inflamed tissues (69). ADO regulates a wide variety of physiological processes by interacting with four GPCRs: A1, A2A, A2B, and A3 ARs, which belong to the P1 class of purinergic receptors (25). In particular, A1 and A3 ARs act through Gi proteins to inhibit AC activity, whereas A2A and A2B ARs act through Gs proteins to stimulate AC activity (24). ADO plays an important role in the regulation of epithelial ion and fluid transport (4). The ADO agonist NECA inhibits VIP- and prostaglandin-E2-stimulated jejunal and ileal fluid secretion in rats, with the A2B AR suggested to be the mediator of this inhibitory effect (17, 33). In addition, studies in A1 null mice and their controls indicate that ADO plays an important role in intestinal Cl secretion (32). A significant reduction in ADO-induced jejunal Cl secretion was observed in A1 null mice, while ADO-mediated jejunal Cl secretion was abolished in control mice in the presence of the A1 AR antagonist DPCPX (32). We now find that ADO inhibits PAT1-mediated Cl-oxalate exchange activity in C2 cells (including oxalate efflux) by reducing PAT1 surface membrane expression through signaling pathways including the A2B AR, PLC, and PKC. We anticipate that adenosinergic signaling similarly regulates intestinal oxalate transport in native tissues in vivo based on the fact that C2 cells are highly polarized and exhibit several functional properties resembling the native intestinal epithelium (59, 65).
Patients with IBD have up to 100-fold increased risk of KS (64), with the commonly observed enteric hyperoxaluria being the major risk factor (7, 11, 38). The IBD-associated hyperoxaluria has been largely attributed to fat malabsorption (10), where malabsorbed fat complexes with calcium leaving oxalate free and easily absorbed. Importantly, malabsorption occurs in Crohn’s disease but not in ulcerative colitis, but hyperoxaluria is also seen in ulcerative colitis (30). Therefore, malabsorption might not be the only important risk factor predisposing to hyperoxaluria and KS in IBD, and it is possible that other contributing factor(s) exist(s). As indicated above, PAT1-mediated intestinal oxalate secretion plays a crucial role in limiting net absorption of ingested oxalate, thereby preventing hyperoxaluria and related KS (39). A role for adenosinergic signaling in the regulation of intestinal oxalate transport has not been previously recognized. Our finding that ADO inhibits PAT1-mediated Cl-oxalate exchange activity in C2 cells suggests a potential role for adenosinergic signaling in the regulation of oxalate homeostasis as outlined below. Large amounts of ATP are rapidly released into the extracellular space (i.e., intestinal lumen in the setting of IBD) at sites of inflammation due to cell and tissue damage, hypoxia, and low pH (21, 55). In addition to intestinal epithelial cells, activated leukocytes, platelets, and smooth muscle cells also secrete ATP in IBD (21, 55). Interestingly, the leading edge of a migrating neutrophil releases ATP, with 1,000-fold higher ATP levels seen during inflammation (13, 46, 52). Moreover, inflammatory processes (including colitis) lead to a significant increase in the expression and activity of the ectoenzyme CD73, which in turns leads to rapid conversion of ATP into ADO (54, 63). Furthermore, during active inflammation ADO is formed in the intestinal lumen from neutrophil-derived 5′-AMP (56). Importantly, significant upregulation of the A1, A2A, and A2B ARs was reported in pathological conditions (including intestinal inflammation) (4). Worthy of mentioning is that the A2B AR mRNA and protein expression are upregulated in murine and human colitis (44). Therefore, it is highly possible that sustained supraphysiological concentrations of luminal intestinal ADO could be seen in the setting of chronic inflammation such as IBD. In view of our finding that ADO signals through the A2B AR to inhibit PAT1-mediated oxalate transport by C2 cells, we hypothesize that signaling through the overexpressed A2B AR leads to sustained ADO-induced inhibition of PAT1-mediated intestinal oxalate secretion, thereby potentially contributing to the IBD-associated hyperoxaluria and high incidence of related KS in IBD patients. Of interest in this regard is that murine colitis is attenuated in the A2B AR null mice and that the selective A2B AR antagonist ATL-801 was shown to significantly improve inflammation in animal models of colitis (45, 47). Therefore, it will be of significant interest to evaluate the therapeutic potential of ATL-801 and other selective A2B AR antagonists on the IBD-associated hyperoxaluria and related KS in future studies.
A case of subclinical celiac disease with significant hyperoxaluria leading to CKD was reported (9). Interestingly, this patient had significantly reduced small intestinal PAT1 apical expression compared with a controlled subject, which likely contributed to reduced PAT1-mediated active small intestinal oxalate secretion and therefore to the reported hyperoxaluria (9). Chronic intestinal inflammation is seen in celiac disease, and this could potentially lead to significantly higher luminal intestinal ATP and ADO levels as described above with IBD. Mild to moderate hyperoxaluria is frequently seen in obese stone formers and a positive correlation between increased body size and elevated urinary oxalate excretion has been reported in population-based studies (41, 51, 72). Obesity is characterized by increased intestinal and systemic inflammation in animals and humans (19, 22, 49, 73, 74). In addition, diabetic patients [who also have increased intestinal mucosal and systemic inflammation (20, 78)] excrete 2 mg/day more urinary oxalate than those without diabetes (72). Moreover, diabetic KS formers excrete significantly higher urine oxalate (>6 mg/day) than nondiabetic stone formers (23). Similar to IBD, the obesity- and DM-associated intestinal inflammation could potentially lead to significantly higher luminal intestinal ATP and ADO levels. Taken together, it is possible that the expected high luminal intestinal ADO concentrations inhibit PAT1-mediated intestinal oxalate secretion, thereby potentially contributing to the hyperoxaluria seen in association with celiac disease, obesity, and/or DM.
ADO released in intestinal lumen during active inflammation was shown to induce IL-6 secretion into the apical compartment, which then permits activation of transmigrating neutrophils and enhances their microbicidal activity (68). This is of significant interest since we recently found that the proinflammatory cytokines TNF-α, IFN-γ, and IL-6 significantly inhibit (by ~30–33%) oxalate transport by C2 cells through mechanisms including reduced PAT1 mRNA and total protein expression (2). TNF-α also significantly reduced mouse jejunal transcellular oxalate secretion, converting oxalate transport from net secretion in vehicle-treated tissues to net absorption in TNF-α-treated tissues (2). Of interest in this regard is that significantly reduced PAT1 mRNA expression was reported in a mouse model for mild ileocolonic inflammation (76). Therefore, in addition to directly inhibiting PAT1-mediated intestinal oxalate transport, ADO could also potentially suppresses intestinal oxalate transport by stimulating IL-6 secretion. Based on our current and previous findings that ADO and ATP (including other nucleotides such as UTP) act through the A2B AR and the P2Y2 purinergic receptor, respectively, to inhibit oxalate transport in C2 cells by reducing PAT1 surface expression, as well as our observation that TNF-α, IFN-γ, and IL-6 also suppress oxalate transport by C2 cells, we propose the following model. The IBD-associated high luminal intestinal ADO and ATP concentrations signal through the overexpressed A2B AR and P2Y2 purinergic receptor, respectively, and lead to sustained inhibition of PAT1-mediated intestinal oxalate secretion, thereby potentially contributing to the pathogenesis of the IBD-associated hyperoxaluria and related KS. In addition, the IBD-associated elevated TNF-α, IFN-γ, and IL-6 levels could also cause sustained suppression of PAT1-mediated intestinal oxalate secretion, and therefore, they might similarly contribute to the pathogenesis of the IBD-associated hyperoxaluria and related KS. Future studies will concentrate on evaluating the therapeutic potential of combined blockade of these pathways in vivo in a mouse model that we had already established for the IBD-associated hyperoxaluria (characterized by 2-fold hyperoxaluria and significantly reduced distal ileal PAT1 mRNA and total protein expression compared with controls) to see if such therapeutic strategy will resolve or improve the associated hyperoxaluria. It is possible that the combination of ADO, ATP, TNF-α, IFN-γ, and/or IL-6 also act together in other conditions characterized by chronic intestinal inflammation (including celiac disease, obesity, and DM) to inhibit PAT1-mediated intestinal oxalate secretion, and therefore potentially contribute to the hyperoxaluria seen in association with these conditions.
A role for CFTR in regulating intestinal oxalate secretion was recently reported (43). Defective PAT1-mediated duodenal transcellular oxalate secretion was reported in CFTR null mice, and this might contribute to the hyperoxalemia and hyperoxaluria observed in these mice (43). In addition, coexpression of CFTR in Xenopus oocytes stimulates PAT1-mediated Cl-oxalate exchange activity (43). CF patients have a high incidence of hyperoxaluria and related KS, and it is possible that defective PAT1-mediated intestinal oxalate secretion might contribute to this hyperoxaluria. As described above, the A2B ARs act through Gs proteins to stimulate AC activity (24), and ADO acting through the A2B AR is known to regulate airway surface liquid volume through its activation of CFTR (15). Moreover, the A2B AR can also activate PLC and PKC as mentioned above. Therefore, depending on the dominant pathway, ADO can act through PLC/PKC or PKA/CFTR (less likely since H89 did not block the ADO-induced inhibition of oxalate transport by C2 cells) to inhibit or stimulate PAT1-mediated inhibit intestinal secretion, respectively. Furthermore, the ADO/A2B AR regulatory system can potentially lead to inhibition of PAT1-mediated intestinal secretion through its effects on intestinal inflammation. It is potentially possible that the ADO/A2B AR/PKA/CFTR pathway might mitigate/neutralize the inhibitory effects of the ADO/A2B AR/PKC/inflammatory pathway on PAT1-mediated intestinal oxalate secretion. However, since oxalate has no known physiologic function in the body, this is less likely to be the case since the body does not need oxalate. This assumption will change if future studies identify a physiologic function for oxalate [i.e., the body need oxalate to accomplish specific physiologic function(s)].
In summary, we have shown that adenosinergic signaling inhibits oxalate transport by reducing PAT1 surface protein expression in C2 cells through signaling pathways including the A2B AR, PLC, and PKC. These findings suggest that adenosinergic regulation of intestinal oxalate transport might play an important role in overall oxalate homeostasis and thereby could affect urinary oxalate excretion and stone risk. Our findings have a potential relevance to the pathophysiology of the hyperoxaluria seen in association with conditions characterized by chronic intestinal inflammation, including IBD, celiac disease, obesity, and DM.
GRANTS
This work was supported by National Institute of Diabetes and Digestive and Kidney Diseases Grants K08-DK-067245 and K08-DK-067245-S1 (to H. A. Hassan) and P30-DK-42086 (to the Digestive Disease Research Center of the University of Chicago).
DISCLOSURES
H. A. Hassan is Founder and Chief Scientific Officer for Oxalo Therapeutics. None of the other authors have any conflicts of interest, financial or otherwise, to disclose.
AUTHOR CONTRIBUTIONS
D.J. and H.H. conceived and designed research; D.J., A.A., S.R., M.B., M.R.A., S.J., J.S., S.S., W.A., and H.H. performed experiments; D.J., A.A., S.R., M.B., M.R.A., S.J., J.S., and H.H. analyzed data; D.J., A.A., S.R., M.M., and H.H. interpreted results of experiments; H.H. prepared figures; H.H. drafted manuscript; S.S., M.M., and H.H. edited and revised manuscript; D.J., A.A., S.R., M.B., M.R.A., S.J., J.S., S.S., W.A., M.M., and H.H. approved final version of manuscript.
ACKNOWLEDGMENTS
We thank Malik Malik, Donna Arvans, Sara Abdelaziz, Faisal Sakeen, and Rashda Norui for technical assistance.
REFERENCES
- 1.Alexander RT, Hemmelgarn BR, Wiebe N, Bello A, Morgan C, Samuel S, Klarenbach SW, Curhan GC, Tonelli M; Alberta Kidney Disease Network . Kidney stones and kidney function loss: a cohort study. BMJ 345: e5287, 2012. doi: 10.1136/bmj.e5287. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Amin R, Asplin J, Jung D, Bashir M, Alshaikh A, Ratakonda S, Sharma S, Jeon S, Granja I, Matern D, Hassan H. Reduced active transcellular intestinal oxalate secretion contributes to the pathogenesis of obesity-associated hyperoxaluria. Kidney Int 93: 1098–1107, 2018. doi: 10.1016/j.kint.2017.11.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Amin R, Sharma S, Ratakonda S, Hassan HA. Extracellular nucleotides inhibit oxalate transport by human intestinal Caco-2-BBe cells through PKC-δ activation. Am J Physiol Cell Physiol 305: C78–C89, 2013. doi: 10.1152/ajpcell.00339.2012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Antonioli L, Fornai M, Colucci R, Ghisu N, Tuccori M, Del Tacca M, Blandizzi C. Regulation of enteric functions by adenosine: pathophysiological and pharmacological implications. Pharmacol Ther 120: 233–253, 2008. doi: 10.1016/j.pharmthera.2008.08.010. [DOI] [PubMed] [Google Scholar]
- 5.Ao M, Venkatasubramanian J, Boonkaewwan C, Ganesan N, Syed A, Benya RV, Rao MC. Lubiprostone activates Cl− secretion via cAMP signaling and increases membrane CFTR in the human colon carcinoma cell line, T84. Dig Dis Sci 56: 339–351, 2011. doi: 10.1007/s10620-010-1495-8. [DOI] [PubMed] [Google Scholar]
- 6.Arvans D, Jung YC, Antonopoulos D, Koval J, Granja I, Bashir M, Karrar E, Roy-Chowdhury J, Musch M, Asplin J, Chang E, Hassan H. Oxalobacter formigenes-derived bioactive factors stimulate oxalate transport by intestinal epithelial cells. J Am Soc Nephrol 28: 876–887, 2017. doi: 10.1681/ASN.2016020132. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Böhles H, Beifuss OJ, Brandl U, Pichl J, Akçetin Z, Demling L. Urinary factors of kidney stone formation in patients with Crohn’s disease. Klin Wochenschr 66: 87–91, 1988. doi: 10.1007/BF01774220. [DOI] [PubMed] [Google Scholar]
- 8.Borea PA, Varani K, Vincenzi F, Baraldi PG, Tabrizi MA, Merighi S, Gessi S. The A3 adenosine receptor: history and perspectives. Pharmacol Rev 67: 74–102, 2015. doi: 10.1124/pr.113.008540. [DOI] [PubMed] [Google Scholar]
- 9.Capolongo G, Abul-Ezz S, Moe OW, Sakhaee K. Subclinical celiac disease and crystal-induced kidney disease following kidney transplant. Am J Kidney Dis 60: 662–667, 2012. doi: 10.1053/j.ajkd.2012.02.342. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Caspary WF, Tönissen J. [Enteric hyperoxaluria. I. Intestinal oxalate absorption in gastrointestinal diseases (author’s transl)]. Klin Wochenschr 56: 607–615, 1978. doi: 10.1007/BF01477009. [DOI] [PubMed] [Google Scholar]
- 11.Caudarella R, Rizzoli E, Pironi L, Malavolta N, Martelli G, Poggioli G, Gozzetti G, Miglioli M. Renal stone formation in patients with inflammatory bowel disease. Scanning Microsc 7: 371–379, 1993. [PubMed] [Google Scholar]
- 12.Chaturvedi LS, Marsh HM, Shang X, Zheng Y, Basson MD. Repetitive deformation activates focal adhesion kinase and ERK mitogenic signals in human Caco-2 intestinal epithelial cells through Src and Rac1. J Biol Chem 282: 14–28, 2007. doi: 10.1074/jbc.M605817200. [DOI] [PubMed] [Google Scholar]
- 13.Chen Y, Corriden R, Inoue Y, Yip L, Hashiguchi N, Zinkernagel A, Nizet V, Insel PA, Junger WG. ATP release guides neutrophil chemotaxis via P2Y2 and A3 receptors. Science 314: 1792–1795, 2006. doi: 10.1126/science.1132559. [DOI] [PubMed] [Google Scholar]
- 14.Coe FL, Evan A, Worcester E. Kidney stone disease. J Clin Invest 115: 2598–2608, 2005. doi: 10.1172/JCI26662. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Com G, Clancy JP. Adenosine receptors, cystic fibrosis, and airway hydration. Handb Exp Pharmacol 193: 363–381, 2009. doi: 10.1007/978-3-540-89615-9_12. [DOI] [PubMed] [Google Scholar]
- 16.Corriden R, Insel PA. Basal release of ATP: an autocrine-paracrine mechanism for cell regulation. Sci Signal 3: re1, 2010. doi: 10.1126/scisignal.3104re1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Coupar IM, Hancock DL. The adenosine agonist NECA inhibits intestinal secretion and peristalsis. J Pharm Pharmacol 46: 801–804, 1994. doi: 10.1111/j.2042-7158.1994.tb03733.x. [DOI] [PubMed] [Google Scholar]
- 18.Curhan GC, Taylor EN. 24-h uric acid excretion and the risk of kidney stones. Kidney Int 73: 489–496, 2008. doi: 10.1038/sj.ki.5002708. [DOI] [PubMed] [Google Scholar]
- 19.Das UN. Is obesity an inflammatory condition? Nutrition 17: 953–966, 2001. doi: 10.1016/S0899-9007(01)00672-4. [DOI] [PubMed] [Google Scholar]
- 20.de Kort S, Keszthelyi D, Masclee AA. Leaky gut and diabetes mellitus: what is the link? Obes Rev 12: 449–458, 2011. doi: 10.1111/j.1467-789X.2010.00845.x. [DOI] [PubMed] [Google Scholar]
- 21.Di Virgilio F, Chiozzi P, Ferrari D, Falzoni S, Sanz JM, Morelli A, Torboli M, Bolognesi G, Baricordi OR. Nucleotide receptors: an emerging family of regulatory molecules in blood cells. Blood 97: 587–600, 2001. doi: 10.1182/blood.V97.3.587. [DOI] [PubMed] [Google Scholar]
- 22.Duparc T, Naslain D, Colom A, Muccioli GG, Massaly N, Delzenne NM, Valet P, Cani PD, Knauf C. Jejunum inflammation in obese and diabetic mice impairs enteric glucose detection and modifies nitric oxide release in the hypothalamus. Antioxid Redox Signal 14: 415–423, 2011. doi: 10.1089/ars.2010.3330. [DOI] [PubMed] [Google Scholar]
- 23.Eisner BH, Porten SP, Bechis SK, Stoller ML. Diabetic kidney stone formers excrete more oxalate and have lower urine pH than nondiabetic stone formers. J Urol 183: 2244–2248, 2010. doi: 10.1016/j.juro.2010.02.007. [DOI] [PubMed] [Google Scholar]
- 24.Fredholm BB. Adenosine receptors as targets for drug development. Drug News Perspect 16: 283–289, 2003. doi: 10.1358/dnp.2003.16.5.829316. [DOI] [PubMed] [Google Scholar]
- 25.Fredholm BB, IJzerman AP, Jacobson KA, Klotz KN, Linden J. International Union of Pharmacology. XXV. Nomenclature and classification of adenosine receptors. Pharmacol Rev 53: 527–552, 2001. [PMC free article] [PubMed] [Google Scholar]
- 26.Fredholm BB. Fredholm BB, IJzerman AP, Jacobson KA, Linden J, Muller CE. International Union of Basic and Clinical Pharmacology. LXXXI. Nomenclature and classification of adenosine receptors–an update. Pharmacol Rev 63: 1–34, 2011. doi: 10.1124/pr.110.003285. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Freel RW, Hatch M, Green M, Soleimani M. Ileal oxalate absorption and urinary oxalate excretion are enhanced in Slc26a6 null mice. Am J Physiol Gastrointest Liver Physiol 290: G719–G728, 2006. doi: 10.1152/ajpgi.00481.2005. [DOI] [PubMed] [Google Scholar]
- 28.Freel RW, Morozumi M, Hatch M. Parsing apical oxalate exchange in Caco-2BBe1 monolayers: siRNA knockdown of SLC26A6 reveals the role and properties of PAT-1. Am J Physiol Gastrointest Liver Physiol 297: G918–G929, 2009. doi: 10.1152/ajpgi.00251.2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Frick JS, MacManus CF, Scully M, Glover LE, Eltzschig HK, Colgan SP. Contribution of adenosine A2B receptors to inflammatory parameters of experimental colitis. J Immunol 182: 4957–4964, 2009. doi: 10.4049/jimmunol.0801324. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Fukushima T, Ishiguro N, Matsuda Y, Takemura H, Tsuchiya S. Clinical and urinary characteristics of urolithiasis in ulcerative colitis. Am J Gastroenterol 77: 238–242, 1982. [PubMed] [Google Scholar]
- 31.Gessi S, Merighi S, Varani K, Cattabriga E, Benini A, Mirandola P, Leung E, Mac Lennan S, Feo C, Baraldi S, Borea PA. Adenosine receptors in colon carcinoma tissues and colon tumoral cell lines: focus on the A(3) adenosine subtype. J Cell Physiol 211: 826–836, 2007. doi: 10.1002/jcp.20994. [DOI] [PubMed] [Google Scholar]
- 32.Ghanem E, Lövdahl C, Daré E, Ledent C, Fredholm BB, Boeynaems JM, Van Driessche W, Beauwens R. Luminal adenosine stimulates chloride secretion through A1 receptor in mouse jejunum. Am J Physiol Gastrointest Liver Physiol 288: G972–G977, 2005. doi: 10.1152/ajpgi.00346.2004. [DOI] [PubMed] [Google Scholar]
- 33.Hancock DL, Coupar IM. Functional characterization of the adenosine receptor mediating inhibition of intestinal secretion. Br J Pharmacol 114: 152–156, 1995. doi: 10.1111/j.1476-5381.1995.tb14919.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Hassan HA, Cheng M, Aronson PS. Cholinergic signaling inhibits oxalate transport by human intestinal T84 cells. Am J Physiol Cell Physiol 302: C46–C58, 2012. doi: 10.1152/ajpcell.00075.2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Hassan HA, Mentone S, Karniski LP, Rajendran VM, Aronson PS. Regulation of anion exchanger Slc26a6 by protein kinase C. Am J Physiol Cell Physiol 292: C1485–C1492, 2007. doi: 10.1152/ajpcell.00447.2006. [DOI] [PubMed] [Google Scholar]
- 36.Hatch M, Freel RW. Intestinal transport of an obdurate anion: oxalate. Urol Res 33: 1–16, 2005. doi: 10.1007/s00240-004-0445-3. [DOI] [PubMed] [Google Scholar]
- 37.Hayashi M, Inagaki A, Novak I, Matsuda H. The adenosine A2B receptor is involved in anion secretion in human pancreatic duct Capan-1 epithelial cells. Pflugers Arch 468: 1171–1181, 2016. doi: 10.1007/s00424-016-1806-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Hueppelshaeuser R, von Unruh GE, Habbig S, Beck BB, Buderus S, Hesse A, Hoppe B. Enteric hyperoxaluria, recurrent urolithiasis, and systemic oxalosis in patients with Crohn’s disease. Pediatr Nephrol 27: 1103–1109, 2012. doi: 10.1007/s00467-012-2126-8. [DOI] [PubMed] [Google Scholar]
- 39.Jiang Z, Asplin JR, Evan AP, Rajendran VM, Velazquez H, Nottoli TP, Binder HJ, Aronson PS. Calcium oxalate urolithiasis in mice lacking anion transporter Slc26a6. Nat Genet 38: 474–478, 2006. doi: 10.1038/ng1762. [DOI] [PubMed] [Google Scholar]
- 40.Jiang Z, Grichtchenko II, Boron WF, Aronson PS. Specificity of anion exchange mediated by mouse Slc26a6. J Biol Chem 277: 33963–33967, 2002. doi: 10.1074/jbc.M202660200. [DOI] [PubMed] [Google Scholar]
- 41.Kleinman JG. Bariatric surgery, hyperoxaluria, and nephrolithiasis: a plea for close postoperative management of risk factors. Kidney Int 72: 8–10, 2007. doi: 10.1038/sj.ki.5002284. [DOI] [PubMed] [Google Scholar]
- 42.Knauf F, Ko N, Jiang Z, Robertson WG, Van Itallie CM, Anderson JM, Aronson PS. Net intestinal transport of oxalate reflects passive absorption and SLC26A6-mediated secretion. J Am Soc Nephrol 22: 2247–2255, 2011. doi: 10.1681/ASN.2011040433. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Knauf F, Thomson RB, Heneghan JF, Jiang Z, Adebamiro A, Thomson CL, Barone C, Asplin JR, Egan ME, Alper SL, Aronson PS. Loss of cystic fibrosis transmembrane regulator impairs intestinal oxalate secretion. J Am Soc Nephrol 28: 242–249, 2017. doi: 10.1681/ASN.2016030279. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Kolachala V, Asamoah V, Wang L, Obertone TS, Ziegler TR, Merlin D, Sitaraman SV. TNF-alpha upregulates adenosine 2b (A2b) receptor expression and signaling in intestinal epithelial cells: a basis for A2bR overexpression in colitis. Cell Mol Life Sci 62: 2647–2657, 2005. doi: 10.1007/s00018-005-5328-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Kolachala V, Ruble B, Vijay-Kumar M, Wang L, Mwangi S, Figler H, Figler R, Srinivasan S, Gewirtz A, Linden J, Merlin D, Sitaraman S. Blockade of adenosine A2B receptors ameliorates murine colitis. Br J Pharmacol 155: 127–137, 2008. doi: 10.1038/bjp.2008.227. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Kolachala VL, Bajaj R, Chalasani M, Sitaraman SV. Purinergic receptors in gastrointestinal inflammation. Am J Physiol Gastrointest Liver Physiol 294: G401–G410, 2008. doi: 10.1152/ajpgi.00454.2007. [DOI] [PubMed] [Google Scholar]
- 47.Kolachala VL, Vijay-Kumar M, Dalmasso G, Yang D, Linden J, Wang L, Gewirtz A, Ravid K, Merlin D, Sitaraman SV. A2B adenosine receptor gene deletion attenuates murine colitis. Gastroenterology 135: 861–870, 2008. doi: 10.1053/j.gastro.2008.05.049. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Lazarowski ER, Boucher RC, Harden TK. Mechanisms of release of nucleotides and integration of their action as P2X- and P2Y-receptor activating molecules. Mol Pharmacol 64: 785–795, 2003. doi: 10.1124/mol.64.4.785. [DOI] [PubMed] [Google Scholar]
- 49.Lee YH, Pratley RE. The evolving role of inflammation in obesity and the metabolic syndrome. Curr Diab Rep 5: 70–75, 2005. doi: 10.1007/s11892-005-0071-7. [DOI] [PubMed] [Google Scholar]
- 50.Leipziger J. Control of epithelial transport via luminal P2 receptors. Am J Physiol Renal Physiol 284: F419–F432, 2003. doi: 10.1152/ajprenal.00075.2002. [DOI] [PubMed] [Google Scholar]
- 51.Lemann J Jr, Pleuss JA, Worcester EM, Hornick L, Schrab D, Hoffmann RG. Urinary oxalate excretion increases with body size and decreases with increasing dietary calcium intake among healthy adults. Kidney Int 49: 200–208, 1996. doi: 10.1038/ki.1996.27. [DOI] [PubMed] [Google Scholar]
- 52.Linden J. Cell biology. Purinergic chemotaxis. Science 314: 1689–1690, 2006. doi: 10.1126/science.1137190. [DOI] [PubMed] [Google Scholar]
- 53.Linden J. Molecular approach to adenosine receptors: receptor-mediated mechanisms of tissue protection. Annu Rev Pharmacol Toxicol 41: 775–787, 2001. doi: 10.1146/annurev.pharmtox.41.1.775. [DOI] [PubMed] [Google Scholar]
- 54.Louis NA, Robinson AM, MacManus CF, Karhausen J, Scully M, Colgan SP. Control of IFN-alphaA by CD73: implications for mucosal inflammation. J Immunol 180: 4246–4255, 2008. doi: 10.4049/jimmunol.180.6.4246. [DOI] [PubMed] [Google Scholar]
- 55.Luttikhuizen DT, Harmsen MC, de Leij LF, van Luyn MJ. Expression of P2 receptors at sites of chronic inflammation. Cell Tissue Res 317: 289–298, 2004. doi: 10.1007/s00441-004-0939-x. [DOI] [PubMed] [Google Scholar]
- 56.Madara JL, Patapoff TW, Gillece-Castro B, Colgan SP, Parkos CA, Delp C, Mrsny RJ. 5′-adenosine monophosphate is the neutrophil-derived paracrine factor that elicits chloride secretion from T84 intestinal epithelial cell monolayers. J Clin Invest 91: 2320–2325, 1993. doi: 10.1172/JCI116462. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Meinkoth JL, Alberts AS, Went W, Fantozzi D, Taylor SS, Hagiwara M, Montminy M, Feramisco JR. Signal transduction through the cAMP-dependent protein kinase. Mol Cell Biochem 127-128: 179–186, 1993. doi: 10.1007/BF01076769. [DOI] [PubMed] [Google Scholar]
- 58.Merighi S, Bencivenni S, Vincenzi F, Varani K, Borea PA, Gessi S. A2B adenosine receptors stimulate IL-6 production in primary murine microglia through p38 MAPK kinase pathway. Pharmacol Res 117: 9–19, 2017. doi: 10.1016/j.phrs.2016.11.024. [DOI] [PubMed] [Google Scholar]
- 59.Merlin D, Steel A, Gewirtz AT, Si-Tahar M, Hediger MA, Madara JL. hPepT1-mediated epithelial transport of bacteria-derived chemotactic peptides enhances neutrophil-epithelial interactions. J Clin Invest 102: 2011–2018, 1998. doi: 10.1172/JCI4179. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Nduati V, Yan Y, Dalmasso G, Driss A, Sitaraman S, Merlin D. Leptin transcriptionally enhances peptide transporter (hPepT1) expression and activity via the cAMP-response element-binding protein and Cdx2 transcription factors. J Biol Chem 282: 1359–1373, 2007. doi: 10.1074/jbc.M604267200. [DOI] [PubMed] [Google Scholar]
- 61.Neary JT, van Breemen C, Forster E, Norenberg LO, Norenberg MD. ATP stimulates calcium influx in primary astrocyte cultures. Biochem Biophys Res Commun 157: 1410–1416, 1988. doi: 10.1016/S0006-291X(88)81032-5. [DOI] [PubMed] [Google Scholar]
- 62.Nelson WK, Houghton SG, Milliner DS, Lieske JC, Sarr MG. Enteric hyperoxaluria, nephrolithiasis, and oxalate nephropathy: potentially serious and unappreciated complications of Roux-en-Y gastric bypass. Surg Obes Relat Dis 1: 481–485, 2005. doi: 10.1016/j.soard.2005.07.002. [DOI] [PubMed] [Google Scholar]
- 63.Noji T, Karasawa A, Kusaka H. Adenosine uptake inhibitors. Eur J Pharmacol 495: 1–16, 2004. doi: 10.1016/j.ejphar.2004.05.003. [DOI] [PubMed] [Google Scholar]
- 64.Pardi DS, Tremaine WJ, Sandborn WJ, McCarthy JT. Renal and urologic complications of inflammatory bowel disease. Am J Gastroenterol 93: 504–514, 1998. doi: 10.1111/j.1572-0241.1998.156_b.x. [DOI] [PubMed] [Google Scholar]
- 65.Peterson MD, Mooseker MS. Characterization of the enterocyte-like brush border cytoskeleton of the C2BBe clones of the human intestinal cell line, Caco-2. J Cell Sci 102: 581–600, 1992. [DOI] [PubMed] [Google Scholar]
- 66.Pham H, Vincenti R, Slice LW. COX-2 promoter activation by AT1R-Gq-PAK-p38beta signaling in intestinal epithelial cells. Biochim Biophys Acta 1779: 408–413, 2008. doi: 10.1016/j.bbagrm.2008.05.004. [DOI] [PubMed] [Google Scholar]
- 67.Rivkees SA, Reppert SM. RFL9 encodes an A2b-adenosine receptor. Mol Endocrinol 6: 1598–1604, 1992. doi: 10.1210/mend.6.10.1333049. [DOI] [PubMed] [Google Scholar]
- 68.Sitaraman SV, Merlin D, Wang L, Wong M, Gewirtz AT, Si-Tahar M, Madara JL. Neutrophil-epithelial crosstalk at the intestinal lumenal surface mediated by reciprocal secretion of adenosine and IL-6. J Clin Invest 107: 861–869, 2001. doi: 10.1172/JCI11783. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Sitkovsky MV, Ohta A. The ‘danger’ sensors that STOP the immune response: the A2 adenosine receptors? Trends Immunol 26: 299–304, 2005. doi: 10.1016/j.it.2005.04.004. [DOI] [PubMed] [Google Scholar]
- 70.Srivastava V, Dey I, Leung P, Chadee K. Prostaglandin E2 modulates IL-8 expression through formation of a multiprotein enhanceosome in human colonic epithelial cells. Eur J Immunol 42: 912–923, 2012. doi: 10.1002/eji.201141965. [DOI] [PubMed] [Google Scholar]
- 71.Strohmeier GR, Reppert SM, Lencer WI, Madara JL. The A2b adenosine receptor mediates cAMP responses to adenosine receptor agonists in human intestinal epithelia. J Biol Chem 270: 2387–2394, 1995. doi: 10.1074/jbc.270.5.2387. [DOI] [PubMed] [Google Scholar]
- 72.Taylor EN, Curhan GC. Determinants of 24-hour urinary oxalate excretion. Clin J Am Soc Nephrol 3: 1453–1460, 2008. doi: 10.2215/CJN.01410308. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Trayhurn P. Adipose tissue in obesity–an inflammatory issue. Endocrinology 146: 1003–1005, 2005. doi: 10.1210/en.2004-1597. [DOI] [PubMed] [Google Scholar]
- 74.Veilleux A, Grenier E, Marceau P, Carpentier AC, Richard D, Levy E. Intestinal lipid handling: evidence and implication of insulin signaling abnormalities in human obese subjects. Arterioscler Thromb Vasc Biol 34: 644–653, 2014. doi: 10.1161/ATVBAHA.113.302993. [DOI] [PubMed] [Google Scholar]
- 75.Walsh DA, Van Patten SM. Multiple pathway signal transduction by the cAMP-dependent protein kinase. FASEB J 8: 1227–1236, 1994. doi: 10.1096/fasebj.8.15.8001734. [DOI] [PubMed] [Google Scholar]
- 76.Xiao F, Juric M, Li J, Riederer B, Yeruva S, Singh AK, Zheng L, Glage S, Kollias G, Dudeja P, Tian DA, Xu G, Zhu J, Bachmann O, Seidler U. Loss of downregulated in adenoma (DRA) impairs mucosal HCO3(-) secretion in murine ileocolonic inflammation. Inflamm Bowel Dis 18: 101–111, 2012. doi: 10.1002/ibd.21744. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77.Yoo BK, He P, Lee SJ, Yun CC. Lysophosphatidic acid 5 receptor induces activation of Na+/H+ exchanger 3 via apical epidermal growth factor receptor in intestinal epithelial cells. Am J Physiol Cell Physiol 301: C1008–C1016, 2011. doi: 10.1152/ajpcell.00231.2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78.Zhong HJ, Yuan Y, Xie WR, Chen MH, He XX. Type 2 Diabetes Mellitus Is Associated with More Serious Small Intestinal Mucosal Injuries. PLoS One 11: e0162354, 2016. doi: 10.1371/journal.pone.0162354. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79.Zimmermann H. Extracellular metabolism of ATP and other nucleotides. Naunyn Schmiedebergs Arch Pharmacol 362: 299–309, 2000. doi: 10.1007/s002100000309. [DOI] [PubMed] [Google Scholar]