Summary
The present study was designed to examine the potential cellular antiseizure mechanisms of everolimus, a mechanistic target of rapamycin (mTOR) pathway blocker, in pediatric epilepsy cases. Cortical tissue samples obtained from pediatric patients (n = 11, ages 0.67–6.75 years) undergoing surgical resections for the treatment of their pharmacoresistant epilepsy were examined electrophysiologically in ex vivo slices. The cohort included mTOR‐mediated pathologies (tuberous sclerosis complex [TSC] and severe cortical dysplasia [CD]) as well as non–mTOR‐mediated pathologies (tumor and perinatal infarct). Bath application of everolimus (2 μm) had practically no effect on spontaneous inhibitory postsynaptic activity. In contrast, long‐term application of everolimus reduced spontaneous excitatory postsynaptic activity, burst discharges induced by blockade of γ‐aminobutyric acid A (GABAA) receptors, and epileptiform activity generated by 4‐aminopyridine, a K+ channel blocker. The antiseizure effects were more pronounced in TSC and CD cases, whereas in non–mTOR‐mediated pathologies, the effects were subtle at best. These results support further clinical trials of everolimus in mTOR pathway–mediated pathologies and emphasize that the effects require sustained exposure over time.
Keywords: Everolimus, Pediatric epilepsy surgery, mTOR pathway, Ex vivo, Mechanisms
Key Points.
Everolimus demonstrated antiseizure effects in mTOR‐mediated pathologies; effects in non‐mTOR‐mediated pathologies were negligible
Everolimus antiseizure effects required sustained exposure over time
The mechanism whereby everolimus reduces epileptic activity involves reduction in glutamate receptor–mediated excitatory activity
More extensive clinical studies on the antiseizure effects of everolimus in mTOR‐mediated pathologies are warranted
Sirolimus, also known as rapamycin, is a naturally occurring molecule first isolated from Streptomyces hygroscopicus on Easter Island in 1964.1 It selectively inhibits the mechanistic target of rapamycin (mTOR) pathway, which plays a key role in cell survival, proliferation, metabolism, and growth.2 Sirolimus has antibiotic, antiproliferative, and immunosuppressive actions. Everolimus, marketed as Afinitor, is a derivative of sirolimus that inhibits the mTOR pathway via similar mechanisms. Yet, both inhibitors result in different clinical profiles.3 Everolimus differs structurally from sirolimus by the addition of a hydroxyethyl ester group at carbon 40, which is thought to give everolimus its shorter half‐life and greater oral bioavailability.3, 4 There also is evidence that, even though both compounds inhibit the mTOR pathway at comparable doses, only everolimus can distribute to the mitochondria in the brain.5
Everolimus was first approved by the U.S. Food and Drug Administration (FDA) in 2009 and is currently used primarily to treat breast cancer in postmenopausal women, to prevent rejection of liver or kidney transplant, and to slow the growth of certain gastrointestinal and metastatic pancreatic neuroendocrine tumors.6, 7 In tuberous sclerosis complex (TSC), an autosomal dominant multisystem disorder that results from mutations in either the TSC1 or TSC2 genes,8 everolimus has been used to reduce the size of subependymal giant cell astrocytoma (SEGA).9 Clinically, only everolimus is approved to treat TSC, mainly because of a lack of phase III studies of sirolimus for TSC, although several small studies showed sirolimus to be safe and effective for TSC.3, 10 Other clinical trials have been designed to examine the effects of everolimus on other TSC‐related lesions, such as angiomyolipoma formation in the kidneys and lymphangioleiomyomatosis.11, 12
A hyperactive mTOR pathway plays a key role in the pathophysiology and seizure development of TSC13 and has been linked to an increase in α‐amino‐3‐hydroxy‐5‐methyl‐4‐isoxazolepropionic acid (AMPA) and N‐methyl‐d‐aspartate (NMDA) glutamate receptor expression, and to the presence of giant cells, reactive astrocytes, and dysplastic neurons.14, 15 Recently everolimus has been shown to have some antiseizure effects.16 In a prospective, open‐label, phase I/II trial in children with TSC and refractory epilepsy,17 as well as in a double‐blind, placebo‐controlled and randomized, global phase III trial in children and adults with TSC and refractory epilepsy,18 preliminary data showed a dose‐dependent reduction in median seizure frequency with an acceptable safety profile.18 Although some potential antiseizure mechanisms of mTOR inhibitors, such as increased γ‐aminobutyric acid A (GABAA) receptor–mediated synaptic activity,19 have been gleaned from studies in TSC animal models, it is not known which mechanisms are at work in humans and these could be unique due to species and genetic mutation differences. Using a clinical and basic research infrastructure at the University of California Los Angeles (UCLA),20 we set out to study the potential antiseizure mechanisms of everolimus in an ex vivo model utilizing freshly excised human cortical tissue from children with medically refractory focal epilepsy, with or without a known TSC mutation, undergoing surgical resection of the epileptogenic zone.
Methods
Standard protocol approvals
The UCLA Institutional Review Board approved the use of human subjects for research purposes, and parents or responsible persons signed informed consents and Health Insurance Portability and Accountability Act (HIPAA) authorizations. Because this study was not a clinical trial, it is not registered in any public registry. Children undergoing resective surgery with the UCLA Pediatric Epilepsy Program to help control their medically refractory focal epilepsy were sequentially recruited between January 2014 and January 2017. We targeted children ~6 years of age or younger based on previous studies from our group indicating that excised cortical tissue samples are in general easier to process and study than tissue from older patients.21 For this preliminary study, cortical tissue samples from the following 3 groups of etiologies were included: (1) TSC, with known mTOR pathway involvement (n = 4); (2) cortical dysplasia (CD) and hemimegalencephaly (HME) with implicated PI3K/AKT/mTOR pathway activation22 (n = 4); and (3) non‐mTOR pathway etiologies, such as tumor (n = 1) and perinatal infarct (n = 2).
Electrocorticography and surgical resection
The site and margin of the surgical resection were based on recommendations from a multidisciplinary meeting after careful consideration of the presurgical evaluation of each patient, as reported previously.21 For all 3 groups of etiologies, we strived for complete resection of the epileptogenic zone chiefly defined by noninvasive testing20, 23, 24 including video–electroencephalography (EEG) capturing ictal events, high‐resolution magnetic resonance imaging (MRI), and 18‐fluorodeoxyglucose–positron emission tomography (FDG‐PET), as well as magnetic source imaging25 and co‐registration of MRI and FDG‐PET, as reported previously when the initial battery of tests was inconclusive.26, 27
In the operating room we routinely utilized brief intraoperative pre‐resection electrocorticography (ECoG) to confirm and/or refine the ultimate margins of the surgical resection. With this brief intraoperative ECoG, high‐frequency oscillations, specifically the fast ripple bandwidth (250–500 Hz), were identified28 in the operating room before resection began.29 The cortical tissue displaying fast ripple generation was considered the most epileptogenic portion of the total resection and was the cortical tissue further processed for this study.
Specimen transport, processing, and recording
The fast ripple–generating cortex was resected with no use of electrocautery. This tissue sample was immediately immersed in ice‐cold artificial cerebrospinal fluid (ACSF) enriched with sucrose for better preservation and then expeditiously hand‐carried out of the operating room and transported directly to the laboratory within 5–10 min after resection. The high sucrose‐based slicing solution contained (in mm): 26 NaHCO3, 1.25 NaH2PO4, 208 sucrose, 10 glucose, 2.5 KCl, 1.3 MgCl2, 8 MgSO4. Coronal slices (300 μm) were cut and transferred to an incubating chamber containing ACSF (in mm): 130 NaCl, 3 KCl, 1.25 NaH2PO4, 26 NaHCO3, 2 MgCl2, 2 CaCl2, and 10 glucose oxygenated with 95% O2‐5% CO2 (pH 7.2–7.4, osmolality 290–310 mOsm/L, 32–34°C). Slices were allowed to recover for an additional 60 min at room temperature prior to recording. All recordings were performed at room temperature using an upright microscope (Olympus BX51WI) equipped with infrared‐differential interference contrast (IR‐DIC) optics.
Whole‐cell patch‐clamp recordings in voltage‐ or current‐clamp modes were obtained from cortical pyramidal neurons (layers II–V) visualized with IR‐DIC.30 The patch pipette (3–5 MΩ resistance) contained a cesium‐based internal solution (in mm): 125 Cs‐methanesulfonate, 4 NaCl, 1 MgCl2, 5 MgATP, 9 ethylene glycol‐bis(?‐aminoethyl ether)‐N,N,N',N'‐tetraacetic acid (EGTA), 8 4‐(2‐hydroxyethyl)‐1‐piperazineethanesulfonic acid (HEPES), 1 Guanosine‐5′‐triphosphate (GTP)‐Tris, 10 phosphocreatine, and 0.1 leupeptin (pH 7.2 with CsOH, 270–280 mOsm/L) for voltage clamp recordings. K‐gluconate–based solution containing (in mm): 112.5 K‐gluconate, 4 NaCl, 17.5 KCl, 0.5 CaCl2, 1 MgCl2, 5 ATP, 1 NaGTP, 5 EGTA, 10 HEPES, pH 7.2 (270–280 mOsm/L) was used for current clamp recordings. After breaking through the membrane, cell properties (capacitance, input resistance, and decay time constant) were recorded while the membrane potential was held at −70 mV. Electrode access resistances during whole‐cell recordings were <25 MΩ.
After characterizing membrane properties, neuronal excitability was determined in current clamp mode before and after addition of everolimus (Tocris, 200 nm and 2 μm in 0.1% Dimethyl sulfoxide). In voltage clamp, spontaneous excitatory and inhibitory postsynaptic currents (sEPSCs and sIPSCs) were measured to determine changes after bath application of everolimus. Amplitude, frequency, and kinetics of spontaneous synaptic currents were assessed using MiniAnalysis software. Because spontaneous epileptic activity does not occur frequently in slices, cellular hyperexcitability was induced by bath application of proconvulsant agents such as K+ channel blocker 4‐aminopyridine (4‐AP, 100 μm) and/or the GABAA receptor antagonist bicuculline (BIC, 20 μm).31 In this exploratory study, different experimental conditions were used to maximize data collection. In most cases, the effects of everolimus on membrane oscillations induced by 4‐AP were examined in voltage clamp mode (+10 or −70 mV holding potential). After a stable baseline of epileptiform discharges was obtained (usually within 5–7 min) everolimus was applied either acutely (10–15 min) or for longer periods (>15 min). In a few cases, slices were pre‐incubated for 1–2 h in everolimus. The effects of everolimus on 4‐AP membrane oscillations were then correlated with the 3 groups of etiologies mentioned earlier, namely TSC, CD etiologies with mTOR involvement, and non‐mTOR etiologies.
Statistics
In the text and figures, results are expressed as mean ± standard error of the mean (SEM). For group comparisons, we used one‐way analysis of variance (ANOVA; with Bonferroni correction) or ANOVA of Ranks (Dunn's method) tests. To evaluate the effects of everolimus, a paired Student's t‐test or a chi‐square test was used. Statistical significance was set at p < 0.05.
Results
Cohort and number of cells recorded
Eleven patients were recruited for the 3 types of etiologies: 4 children with TSC, 4 children with CD/HME, and 3 children with non‐mTOR etiologies (one postresection for tumor and 2 perinatal infarct cases). The CD cases included CD type Ic (n = 2), CD type IIa/HME (n = 1), and CD type IIb (n = 1), as defined by the International League Against Epilepsy (ILAE) classification of CDs.32 In the present cohort, 2 of the patients were female (both with non‐mTOR pathologies). There was a statistically significant interaction in mean age values among groups (p = 0.044, one‐way ANOVA), with the non‐mTOR group (5.4 ± 1 year) being older than the CD/HME (2.5 ± 0.8 year) and TSC (2.1 ± 0.4 year), consistent with our previous studies.20 However, post hoc (Bonferroni t‐test) comparisons between groups did not yield significant differences. If non‐mTOR cases (5.4 ± 1 year, n = 3) were compared with cases involving mTOR activation (CD + TSC, 2.3 ± 0.5 year, n = 8), the age difference became statistically significant (p = 0.01, Student's t‐test). The clinical findings of these 11 children are detailed in Table 1.
Table 1.
Cohort characteristics
| Pt no. | Pathology | Age at surgery (year) | Gender | No. AEDs at surgery | Area of resection | Area of FR studied |
|---|---|---|---|---|---|---|
| 1 | CD type IIa/HEM | 0.67 | M | 2 | LH | LFP |
| 2 | CD type Ic/Heterotopia | 1.50 | M | 2 | RH | RTP |
| 3 | CD type Ic | 2.33 | M | 4 | LH | LFT |
| 4 | CD type IIb | 2.33 | M | 2 | LTP | LP |
| 5 | TSC | 1.16 | M | 3 | LF | LF |
| 6 | TSC | 2.16 | M | 4 | RF | RF |
| 7 | TSC | 2.45 | M | 3 | LF | LF |
| 8 | TSC | 2.75 | M | 3 | RF | RF |
| 9 | Teratoma | 3.58 | F | 1 | RH | RF |
| 10 | Perinatal infarct | 5.82 | M | 2 | LH | LF |
| 11 | Perinatal infarct | 6.75 | F | 3 | RH | RTP |
AED, antiepileptic drug; FR, fast ripples; TSC, tuberous sclerosis complex; M, male; F, female; L, left; R, right; H, hemisphere; F, frontal; T, temporal; P, parietal.
Each cortical resection had between 2 and 6 individual neurons studied, with and without everolimus. In total, 66 neurons were recorded, and of those 45 were tested for everolimus and/or pro‐convulsant drugs under different experimental conditions (TSC cases n = 18 cells, CD/HME cases n = 12 cells, and non‐mTOR cases n = 15 cells). Of those, 42 were tested with everolimus and 3 were only tested with 4‐AP for comparison. Recorded cells were pyramidal neurons that included a majority of normal‐appearing pyramidal neurons (n = 39), immature (n = 1 from a TSC case), and dysmorphic cytomegalic pyramidal neurons (n = 5, 4 from 2 TSC cases and 1 from a CD type IIa/HEM case), as defined by the ILAE classification of CD32. Balloon cells were seen occasionally but were not tested with everolimus. Regardless of treatment, cells were filled with biocytin during recordings and then processed histologically. Some typical examples of pyramidal neurons recorded in this cohort are shown in Figure 1A.
Figure 1.
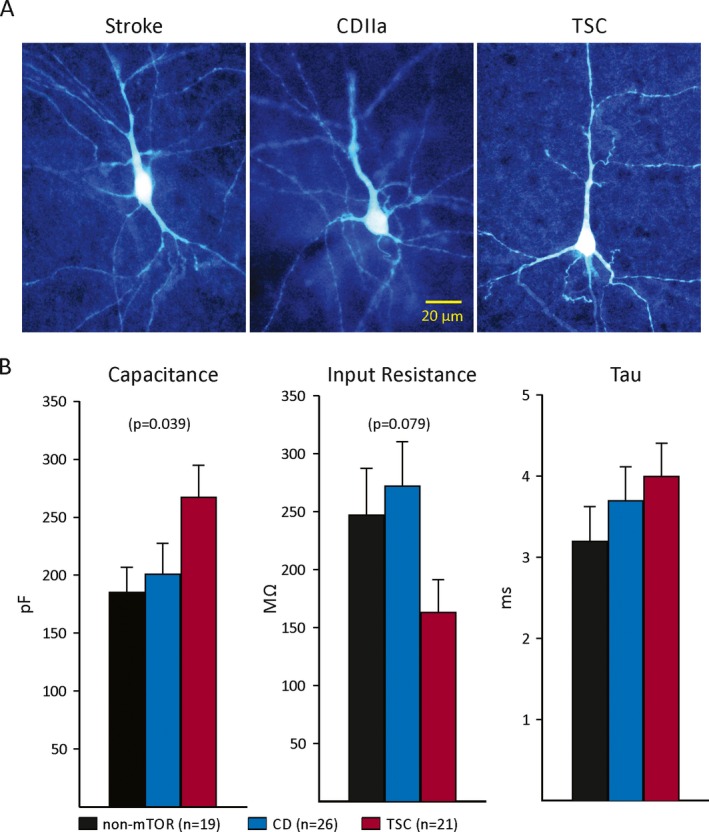
A, Examples of pyramidal‐shaped neurons that were recorded electrophysiologically, tested for everolimus, and filled with biocytin to examine morphology. Notice that these cells displayed signs of atrophy including sparse dendritic spines and blebbing. Calibration in the middle panel applies to all. B, Basic membrane properties in the 3 groups. Cell capacitance was significantly different among groups, with cells from TSC cases being larger than those from non‐mTOR and CD cases. There was a trend for input resistance to be different among groups. Decay time constant was not significantly different among groups (p = 0.31).
Biophysical membrane properties
Basic membrane properties (cell capacitance, input resistance, and decay time constant) were calculated in voltage clamp mode by applying a 10 mV depolarizing voltage command from a holding voltage (Vh) of −70 mV and using the pClamp software. There was a statistically significant difference in cell membrane capacitance among groups (p = 0.039, one‐way ANOVA on ranks). The average cell capacitance was larger in TSC compared with CD and non‐mTOR groups. This probably reflects the fact that in TSC cases some pyramidal neurons are larger as a result of mTOR pathway activation. In contrast, we observed a strong, albeit nonsignificant trend (p = 0.079), for membrane input resistance to be decreased in neurons from TSC cases, in general agreement with the observation that these cells were larger. The decay time constant was not significantly different among neurons from the 3 groups (Fig. 1B).
Effects of everolimus on spontaneous synaptic activity
A potential mechanism of antiepileptic effects for any given drug is re‐establishment of the synaptic excitatory/inhibitory balance. For example, everolimus could exert antiepileptic effects by increasing GABAergic activity. Thus, we tested the effects of everolimus on sIPSC frequency by holding the membrane at +10 mV. At this potential, synaptic currents are mediated by GABAA receptors.31 In 5 neurons examined, bath application of everolimus produced inconsistent effects, with some cells showing small increases (n = 2) and other cells showing small decreases (n = 3) (Fig. S1). On average, the frequency was reduced by about 10%, suggesting that everolimus does not affect GABAergic neurotransmission onto pyramidal neurons in a significant way. Furthermore, kinetic analysis of sIPSCs revealed no changes in rise or decay times (Table 2 and Fig. S2).
Table 2.
(A) sEPSC kinetics and (B) sIPSC kinetics
| Rise time (msec) | Half‐amplitude duration (msec) | Decay time (msec) | Area (pA/msec) | Amplitude (pA) | |
|---|---|---|---|---|---|
| (A) | |||||
| Control | 0.9 ± 0.1 | 6.0 ± 0.4 | 6.0 ± 0.6 | 106.3 ± 8.7 | −7.7 ± 0.6 |
| Everolimus | 0.9 ± 0.1 | 5.3 ± 0.2 | 5.0 ± 0.4 | 112.1 ± 10.3 | −7.6 ± 0.6 |
| (B) | |||||
| Control | 3.5 ± 0.6 | 25.6 ± 4.4 | 32.3 ± 6.3 | 952.1 ± 134.2 | 22.7 ± 1.0 |
| Everolimus | 3.3 ± 0.5 | 28.1 ± 4.8 | 39.4 ± 6.8 | 1,032 ± 195.7 | 22.7 ± 2.3 |
Antiepileptic effects may also occur via reduction of excitatory inputs. To test this possibility, we bath applied everolimus after recording baseline spontaneous synaptic activity (2–3 min) at a holding potential of −70 mV. At this potential, synaptic activity is mediated primarily by glutamate AMPA receptors. To fully isolate glutamatergic synaptic currents, the GABAA receptor antagonist BIC (20 μm) was added to the external solution. Long‐term (>15 min) application of everolimus (n = 8 cells from 2 TSC cases and one stroke case) produced a significant reduction in the frequency of sEPSCs (Fig. 2A,B). Furthermore, synaptic bursts and large‐amplitude events commonly observed after BIC application also were reduced. The average percent reduction in frequency after everolimus was 35%. The most dramatic effects were seen in one cell (TSC, 1.16‐year‐old) that had been incubated in everolimus for >1 h. In this case, the frequency in 2 cells that received no treatment was 4.1 ± 1.1 Hz, whereas in the cell incubated in everolimus, the sEPSC frequency was 0.8 Hz. Kinetic analysis of sEPSCs revealed no changes in rise or decay times (Table 2 and Fig. S2). This suggested that one possible mechanism of antiepileptic activity is via reduction of glutamate AMPA receptor–mediated synaptic activity.
Figure 2.
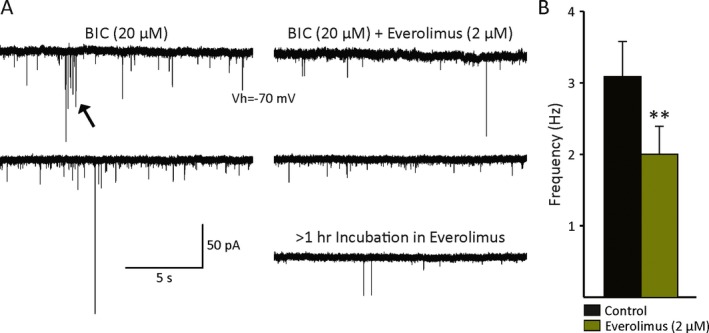
A, Example traces showing the effects of everolimus on the frequency of sEPSCs recorded from 2 TSC cases. Cells were held at −70 mV to minimize the contribution of inhibitory synaptic activity and BIC (20 μm) was added to the ACSF. Long‐term (>15 min) application of everolimus reduced the frequency of sEPSCs and also decreased bursting activity (arrow), as well as the occurrence of large‐amplitude synaptic events. B, The bar graph indicates there was a statistically significant change in sEPSC frequency after everolimus (p = 0.01, paired t‐test, n = 8 cells).
Effects of everolimus on pharmacologically induced paroxysmal activity
Spontaneous epileptiform activity rarely occurs in ex vivo conditions even when the sample is from the epileptogenic zone. This is probably because of interruption of long‐range excitatory circuits caused by the slicing procedure. Epileptiform activity in cortical tissue can be induced by 4‐AP (a compound that blocks the A‐type K+ current, thereby facilitating neurotransmitter release) and/or by blocking inhibitory transmission with the GABAA receptor antagonist BIC.
Within 2–4 min after bath application of 4‐AP (100 μm, with or without BIC), spontaneous rhythmic epileptiform discharges occurred in most pyramidal neurons. The frequency of these membrane oscillations remained relatively constant, but the amplitude tended to decrease progressively. The average frequency of 4‐AP oscillations was 9.4/min (n = 16 cells), with no difference among groups (TSC, 7.8 ± 2/min, CD 10 ± 4/min and non‐mTOR 9.9 ± 1/min, p = 0.79). Initial pilot studies tested 2 doses of everolimus: 200 nm and 2 μm. However, it soon became evident that the lower dose produced no detectable effects (n = 3 cells from 2 TSC cases) (not shown). The effects of everolimus (2 μm) on 4‐AP oscillations were tested in 3 conditions: (1) simultaneous application of everolimus and 4‐AP; (2) everolimus added soon after induction of steady 4‐AP oscillations (about 4–5 min after drug application) and kept in the bath for more than 15 min; and (3) after long‐term pre‐incubation (1–2 h) of the slices in everolimus. Simultaneous application of 4‐AP, BIC, and everolimus did not affect the frequency of epileptiform discharges (n = 2) (not shown). Most cells were tested with acute, prolonged application of the drug in the bath solution.
In cells from TSC cases, everolimus demonstrated antiepileptic effects in 8 cells and no clear effects in one cell. This effect was manifested by a reduction in the frequency and/or amplitude of 4‐AP oscillations (Fig. 3A and Fig. S3). Remarkably, incubation in everolimus for 1–2 h fully prevented the occurrence of epileptiform activity (Fig. 3B). Only an increase in the frequency and amplitude of spontaneous synaptic events occurred. In CD/HME cases, everolimus also displayed antiepileptic effects. Everolimus reduced the frequency of 4‐AP‐induced paroxysmal discharges in 4 cells (Fig. 4A) and had no effect in 2 cells. In current clamp mode, epileptiform activity induced by BIC and 4‐AP led to membrane oscillations and burst firing at depolarized potentials. After everolimus, membrane oscillations subsided and the firing pattern became regular instead of bursting (Fig. 4B). Finally, in pyramidal neurons from non‐mTOR pathologies, the effects of everolimus were subtle at best (n = 3), and in most cases no effects were observed (n = 4), even after long‐term incubation in the drug (Fig. 5). There was a statistically significant difference in everolimus effects between the TSC and the non‐mTOR group (p = 0.049, chi‐square test, n = 9 and 7 cells, respectively), whereas the difference between mTOR (TSC and CD combined) and non‐mTOR pathologies almost reached statistical significance (p = 0.08, chi‐square test). On average, in mTOR pathologies everolimus reduced the frequency of membrane oscillations from 7.7 ± 2/min to 5.7 ± 2/min (p = 0.002, paired t‐test). In non‐mTOR pathologies, the effect was subtle and not statistically significant. On average, the frequency of membrane oscillations changed from 7.3 ± 0.5/min to 6.3 ± 1/min (p = 0.423, paired t‐test).
Figure 3.
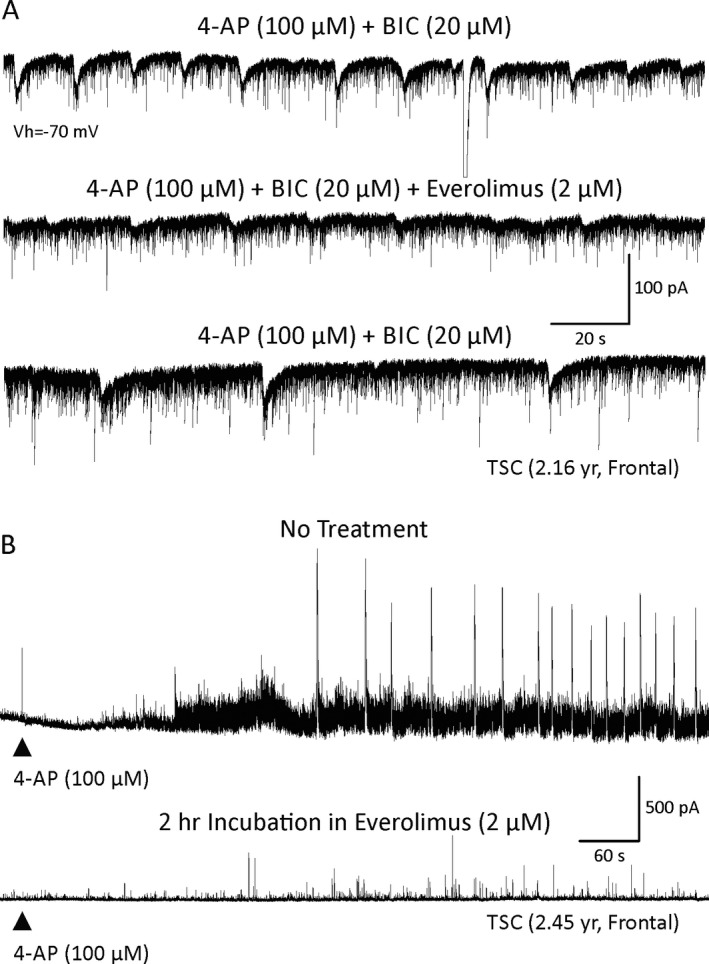
A, Epileptiform activity was induced by bath application of 4‐AP and BIC in a pyramidal neuron from a TSC case. The cell was held at −70 mV. Addition of everolimus reduced the amplitude and frequency of epileptiform activity. This effect partially washed out after removal of everolimus. B, The most dramatic effects were observed with pre‐incubation of slices in everolimus. Top trace shows the effect of 4‐AP application in a cell from a slice incubated in ACSF only (TSC case). Oscillations and paroxysmal discharges were induced. In a slice incubated for 2 h in everolimus, the effects of 4‐AP were significantly reduced and no paroxysmal discharges were observed.
Figure 4.
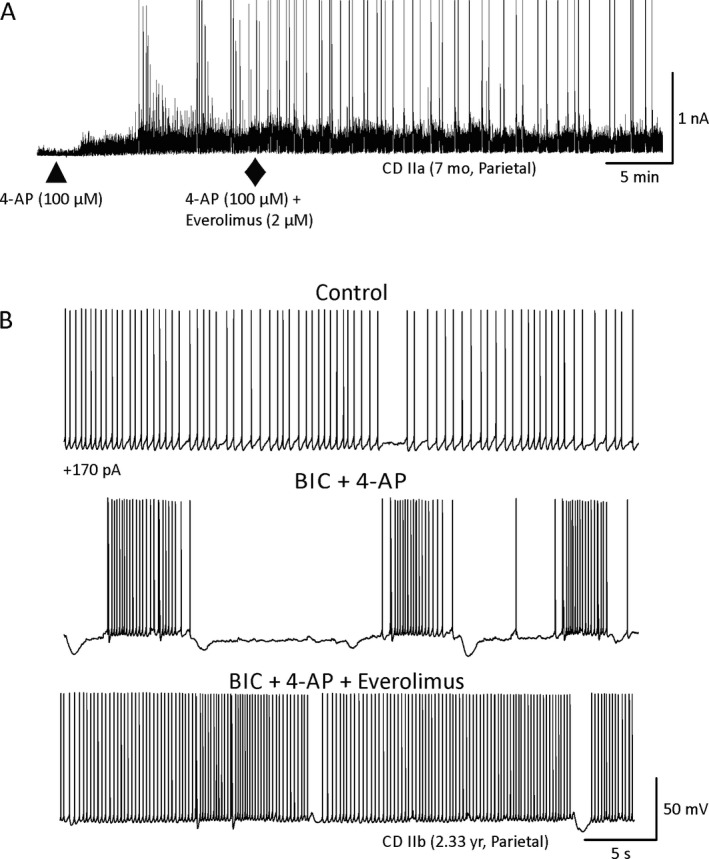
A, In CD cases, similar to the effects observed in TSC cases, everolimus reduced the frequency of 4‐AP membrane oscillations. B, Effects of everolimus on action potential firing induced by depolarizing current (+170 pA). In control conditions (ACSF), regular firing was evoked by current injection. After addition of BIC and 4‐AP, membrane oscillations and burst firing occurred. Everolimus reduced membrane oscillations and bursting activity.
Figure 5.
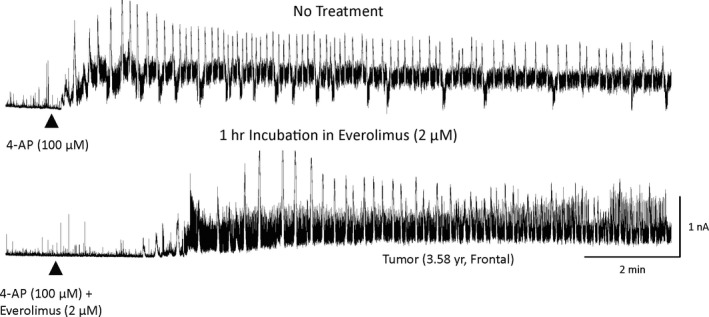
Effects of everolimus in a non‐mTOR case (tumor). In a cell recorded in a slice that received no treatment, 4‐AP rapidly induced membrane oscillations. In the cell recorded in a slice incubated for 1 h in everolimus, besides an increase in latency and slightly lower frequency of membrane oscillations, the effects were only mild compared with those observed in mTOR cases.
Discussion
Significant advances have been made in recent years toward understanding the mechanism of action of everolimus. Yet, the details of its antiseizure properties remain to be elucidated. In this study, comparing ex vivo brain slices from TSC, CD/HME, and non‐mTOR pathologies, we demonstrate that everolimus had clear and robust anti‐seizure effects in the TSC and CD/HME groups. In the non–mTOR‐mediated posttumor and postinfarct etiologies the effects of everolimus were mild or nonexistent. These findings are similar to those of previous reports from our laboratory showing antiseizure effects of rapamycin in TSC and CD cases but not in non‐CD pathologies.33, 34 The results from these studies further validate the potential antiseizure effects of rapamycin and everolimus in pathologies involving hyperactivation of the mTOR pathway.
Because this study selected the most epileptogenic cortical tissue with ECoG, specifically with high‐frequency oscillations, the removal of which has been highly associated with postoperative seizure freedom,28, 29 the results would likely closely resemble the antiseizure effects of mTOR inhibitors in a clinical scenario. These findings are encouraging for a pilot clinical trial in children with CD or HME, and medically refractory epilepsy, to test the efficacy and safety of everolimus in these 2 mTOR‐mediated conditions.
It is interesting to note that only the higher everolimus concentration demonstrated antiseizure effects, whereas the lower everolimus concentration did not. This observation is consistent with the better efficacy found with the higher everolimus arm of the EXamining everolimus In a Study of Tuberous sclerosis complex (EXIST‐3) epilepsy trial in TSC.18 This higher dose was better tolerated in children 6 years of age and younger,18 which notably is the same age group as in this ex vivo study. The significantly younger age of the TSC and CD/HME groups at the time of surgery, compared with the non‐mTOR group, reflects the highly epileptogenic and medically refractory nature of these mTOR‐mediated conditions. Indeed, cortical malformations constitute the most prevalent pathologic diagnosis for children undergoing epilepsy surgery, comprising 25–40% of the refractory childhood epilepsies.35 Approximately 75% of patients with cortical malformations will have epilepsy at some point in their lifetime.36
Cell membrane properties were different among pathologies. TSC cells were larger than in other pathologies. This was expected due to mTOR activation. Although a few cytomegalic neurons were observed in CD cases, the average size was not increased, probably because the CD group included CD type I and type II/HME cases. In addition to the pathology, time of exposure to the drug determined the intensity of the effect. When applied simultaneously with 4‐AP, everolimus had no effect. When applied acutely but for a long time, a decrease in epileptiform activity was observed. However, everolimus was most effective after long‐time (1–2 h) incubation. Under these conditions, the latency to paroxysmal events was delayed and their frequency and amplitude were drastically reduced. Similarly, everolimus reduced the frequency of sEPSCs with long‐term application.
To date, it is still unclear how everolimus has antiseizure properties in TSC because it is also unclear how exactly tubers contribute to seizure activity. Cortical tubers have been shown to have decreased GABAA receptor levels and possess cytomegalic cells that may indicate altered maturation and enhanced excitability.30, 37, 38 It also has been shown that neurons in the immediate area of the tubers have increased excitability.39, 40 The timing of everolimus administration in the course of the disease seems relevant to its effect, suggesting that its role in inhibiting the mTOR pathway may alter the aberrant growth of neurons and contribute to its therapeutic effect.33
With regard to the possible mechanism(s) by which everolimus produces antiepileptic effects and based on the fact that long‐term exposure to the drug reduced the frequency of sEPSCs, we could speculate that one potential mechanism could involve a decrease in cell membrane surface expression of glutamate AMPA receptors. Indeed, in cell models it has been shown that the mTOR pathway regulates the surface expression of AMPA receptors41 and in animal models rapamycin decreases glutamatergic synaptic transmission.42 Thus, long‐term exposure to everolimus, at least in cortical pyramidal neurons, could internalize AMPA receptors, thereby decreasing glutamate‐induced membrane depolarization. We also found that in current clamp mode, everolimus changed the firing pattern from bursting to continuous firing, suggesting that bursting activity and synchrony are disrupted by drug application. Another potential mechanism involves direct effects of mTOR inhibitors on astrocytes and GLT‐1 glutamate transporter expression. For example, recent evidence indicates that rapamycin can upregulate GLT‐1 expression.43, 44, 45, 46
Significance and Limitations
The present study has a number of limitations inherent to the use of human pathologic brain tissue. First, all patients were taking antiseizure medication and this may have affected the different outcomes. Second, the use of an ex vivo model, which is a reduced preparation,47 does not allow examination of the role of long‐range projections. Third, due to the scarcity of pediatric epilepsy surgical tissue, a more‐detailed quantitative analysis was not feasible. By design, this was primarily an exploratory study on everolimus antiseizure effects. Thus, we had to rely on convergent evidence from different experimental conditions to provide a glimpse into drug mechanisms and draw some tentative conclusions. Finally, we only examined everolimus effects on neuronal cells and we know that mTOR‐mediated abnormalities, including epileptogenesis, are not only restricted to neurons but also to non‐neuronal populations. For example, mouse models of TSC can display seizures when the Tsc1 gene is removed from glial cells only.48, 49 Thus, future work should also study the effects of everolimus on non‐neuronal populations. Despite these limitations, studying the electrophysiologic effects of everolimus in human tissue from patients with epilepsy represents a good model that closely resembles physiologic conditions in a controlled environment. The present results shed some light on cellular mechanisms of drug actions and support the need for additional studies, in particular examination of surface expression of AMPA receptors before and after everolimus treatment, as well as its effect on glutamate transporters.
Disclosure
None of the authors has any conflict of interest to disclose. We confirm that we have read the Journal's position on issues involved in ethical publication and affirm that this report is consistent with those guidelines.
Supporting information
Figure S1. Example traces showing the effects of everolimus on the frequency of sIPSCs. Cells were held at +10 mV to minimize the contribution of excitatory synaptic activity. Acute bath application of everolimus (2 μm for about 15 min) did not affect IPSC frequency significantly. On average, there was only a small, nonsignificant decrease.
Figure S2. Everolimus did not affect sEPSC or sIPSC kinetics. Traces are averages of total spontaneous synaptic events recorded in one cell. Average values are shown in Table 2.
Figure S3. Top trace shows the effect of 4‐AP application in a cell from a slice incubated in ACSF only. Oscillations and paroxysmal discharges accompanied by large inward currents were induced. In a slice incubated for 70 min in everolimus, 4‐AP oscillations were significantly reduced and no paroxysmal discharges were observed.
Acknowledgments
This study was supported by an investigator‐initiated study between JYW and Novartis Pharmaceutical Inc. (study number RAD001MUS246T). GWM was supported by the Davies/Crandall Endowed Chair for epilepsy research at UCLA. We would like to thank the patients and their parents for allowing the use of resected specimens for experimentation. We also thank the UCLA Hospital Pediatric Neurology staff for their assistance. Ms. My N. Huynh did the biocytin processing.
Biography
Dr. Carlos Cepeda is a professor of psychiatry & biobehavioral sciences at the University of California Los Angeles.

References
- 1. Crino PB. Rapamycin and tuberous sclerosis complex: from Easter Island to epilepsy. Ann Neurol 2008;63:415–417. [DOI] [PubMed] [Google Scholar]
- 2. Manning BD. Game of TOR – the target of rapamycin rules four kingdoms. N Engl J Med 2017;377:1297–1299. [DOI] [PubMed] [Google Scholar]
- 3. MacKeigan JP, Krueger DA. Differentiating the mTOR inhibitors everolimus and sirolimus in the treatment of tuberous sclerosis complex. Neuro Oncol 2015;17:1550–1559. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Choi J, Chen J, Schreiber SL, et al. Structure of the FKBP12‐rapamycin complex interacting with the binding domain of human FRAP. Science 1996;273:239–242. [DOI] [PubMed] [Google Scholar]
- 5. Klawitter J, Gottschalk S, Hainz C, et al. Immunosuppressant neurotoxicity in rat brain models: oxidative stress and cellular metabolism. Chem Res Toxicol 2010;23:608–619. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Schramm A, Friedl TW, Schochter F, et al. Therapeutic intervention based on circulating tumor cell phenotype in metastatic breast cancer: concept of the DETECT study program. Arch Gynecol Obstet 2016;293:271–281. [DOI] [PubMed] [Google Scholar]
- 7. Yao JC, Fazio N, Singh S, et al. Everolimus for the treatment of advanced, non‐functional neuroendocrine tumours of the lung or gastrointestinal tract (RADIANT‐4): a randomised, placebo‐controlled, phase 3 study. Lancet 2016;387:968–977. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Hasbani DM, Crino PB. Tuberous sclerosis complex. Handb Clin Neurol 2018;148:813–822. [DOI] [PubMed] [Google Scholar]
- 9. Franz DN, Belousova E, Sparagana S, et al. Efficacy and safety of everolimus for subependymal giant cell astrocytomas associated with tuberous sclerosis complex (EXIST‐1): a multicentre, randomised, placebo‐controlled phase 3 trial. Lancet 2013;381:125–132. [DOI] [PubMed] [Google Scholar]
- 10. Franz DN, Leonard J, Tudor C, et al. Rapamycin causes regression of astrocytomas in tuberous sclerosis complex. Ann Neurol 2006;59:490–498. [DOI] [PubMed] [Google Scholar]
- 11. Bissler JJ, Kingswood JC, Radzikowska E, et al. Everolimus for angiomyolipoma associated with tuberous sclerosis complex or sporadic lymphangioleiomyomatosis (EXIST‐2): a multicentre, randomised, double‐blind, placebo‐controlled trial. Lancet 2013;381:817–824. [DOI] [PubMed] [Google Scholar]
- 12. Goldberg HJ, Harari S, Cottin V, et al. Everolimus for the treatment of lymphangioleiomyomatosis: a phase II study. Eur Respir J 2015;46:783–794. [DOI] [PubMed] [Google Scholar]
- 13. Lasarge CL, Danzer SC. Mechanisms regulating neuronal excitability and seizure development following mTOR pathway hyperactivation. Front Mol Neurosci 2014;7:18. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Curatolo P. Mechanistic target of rapamycin (mTOR) in tuberous sclerosis complex‐associated epilepsy. Pediatr Neurol 2015;52:281–289. [DOI] [PubMed] [Google Scholar]
- 15. Talos DM, Kwiatkowski DJ, Cordero K, et al. Cell‐specific alterations of glutamate receptor expression in tuberous sclerosis complex cortical tubers. Ann Neurol 2008;63:454–465. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Curatolo P, Moavero R, van Scheppingen J, et al. mTOR dysregulation and tuberous sclerosis‐related epilepsy. Expert Rev Neurother 2018;18:185–201. [DOI] [PubMed] [Google Scholar]
- 17. Krueger DA, Wilfong AA, Holland‐Bouley K, et al. Everolimus treatment of refractory epilepsy in tuberous sclerosis complex. Ann Neurol 2013;74:679–687. [DOI] [PubMed] [Google Scholar]
- 18. French JA, Lawson JA, Yapici Z, et al. Adjunctive everolimus therapy for treatment‐resistant focal‐onset seizures associated with tuberous sclerosis (EXIST‐3): a phase 3, randomised, double‐blind, placebo‐controlled study. Lancet 2016;388:2153–2163. [DOI] [PubMed] [Google Scholar]
- 19. Hauptman JS, Cepeda C, Antonios J, et al. The effects of mTOR modulation on the excitability of neocortical neurons and networks. In: Neuroscience Meeting Planner San Diego, CA, 2010: No. 657.13.
- 20. Lerner JT, Salamon N, Hauptman JS, et al. Assessment and surgical outcomes for mild type I and severe type II cortical dysplasia: a critical review and the UCLA experience. Epilepsia 2009;50:1310–1335. [DOI] [PubMed] [Google Scholar]
- 21. Cepeda C, Hurst RS, Flores‐Hernandez J, et al. Morphological and electrophysiological characterization of abnormal cell types in pediatric cortical dysplasia. J Neurosci Res 2003;72:472–486. [DOI] [PubMed] [Google Scholar]
- 22. D'Gama AM, Geng Y, Couto JA, et al. Mammalian target of rapamycin pathway mutations cause hemimegalencephaly and focal cortical dysplasia. Ann Neurol 2015;77:720–725. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23. Hemb M, Velasco TR, Parnes MS, et al. Improved outcomes in pediatric epilepsy surgery: the UCLA experience, 1986–2008. Neurology 2010;74:1768–1775. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24. Wu JY, Salamon N, Kirsch HE, et al. Noninvasive testing, early surgery, and seizure freedom in tuberous sclerosis complex. Neurology 2010;74:392–398. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25. Wu JY, Sutherling WW, Koh S, et al. Magnetic source imaging localizes epileptogenic zone in children with tuberous sclerosis complex. Neurology 2006;66:1270–1272. [DOI] [PubMed] [Google Scholar]
- 26. Hauptman JS, Salamon N, Mathern GW. Neuroimaging in the definition and organization of the epilepsies: we're not there yet. Epilepsia 2012;53(Suppl 2):22–27. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27. Salamon N, Kung J, Shaw SJ, et al. FDG‐PET/MRI coregistration improves detection of cortical dysplasia in patients with epilepsy. Neurology 2008;71:1594–1601. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28. Wu JY, Sankar R, Lerner JT, et al. Removing interictal fast ripples on electrocorticography linked with seizure freedom in children. Neurology 2010;75:1686–1694. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29. Hussain SA, Mathern GW, Sankar R, et al. Prospective and “live” fast ripple detection and localization in the operating room: impact on epilepsy surgery outcomes in children. Epilepsy Res 2016;127:344–351. [DOI] [PubMed] [Google Scholar]
- 30. Cepeda C, Andre VM, Hauptman JS, et al. Enhanced GABAergic network and receptor function in pediatric cortical dysplasia Type IIB compared with Tuberous Sclerosis Complex. Neurobiol Dis 2012;45:310–321. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31. Cepeda C, Chen JY, Wu JY, et al. Pacemaker GABA synaptic activity may contribute to network synchronization in pediatric cortical dysplasia. Neurobiol Dis 2014;62:208–217. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32. Blumcke I, Thom M, Aronica E, et al. The clinicopathologic spectrum of focal cortical dysplasias: a consensus classification proposed by an ad hoc Task Force of the ILAE Diagnostic Methods Commission. Epilepsia 2011;52:158–174. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33. Galanopoulou AS, Gorter JA, Cepeda C. Finding a better drug for epilepsy: the mTOR pathway as an antiepileptogenic target. Epilepsia 2012;53:1119–1130. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34. Cepeda C, André V, Hauptman J, et al. Differential sensitivity of cortical neurons to 4‐aminopyridine and rapamycin in diverse forms of pediatric epilepsy. In: Neuroscience Meeting Planner San Diego, CA, 2010: No. 657.9.
- 35. Guerrini R, Holthausen H, Parmeggiani L, et al. Epilepsy and malformations of the cerebral cortex In Roger J, Bureau M, Dravet C. (Eds) Epileptic syndromes in infancy, childhood and adolescence. 3rd Ed London: John Libbey, 2002:457–479. [Google Scholar]
- 36. Leventer RJ, Phelan EM, Coleman LT, et al. Clinical and imaging features of cortical malformations in childhood. Neurology 1999;53:715–722. [DOI] [PubMed] [Google Scholar]
- 37. Boer K, Troost D, Jansen F, et al. Clinicopathological and immunohistochemical findings in an autopsy case of tuberous sclerosis complex. Neuropathology 2008;28:577–590. [DOI] [PubMed] [Google Scholar]
- 38. Mori K, Mori T, Toda Y, et al. Decreased benzodiazepine receptor and increased GABA level in cortical tubers in tuberous sclerosis complex. Brain Dev 2012;34:478–486. [DOI] [PubMed] [Google Scholar]
- 39. Wang Y, Greenwood JS, Calcagnotto ME, et al. Neocortical hyperexcitability in a human case of tuberous sclerosis complex and mice lacking neuronal expression of TSC1. Ann Neurol 2007;61:139–152. [DOI] [PubMed] [Google Scholar]
- 40. Feliciano DM, Lin TV, Hartman NW, et al. A circuitry and biochemical basis for tuberous sclerosis symptoms: from epilepsy to neurocognitive deficits. Int J Dev Neurosci 2013;31:667–678. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41. Wang Y, Barbaro MF, Baraban SC. A role for the mTOR pathway in surface expression of AMPA receptors. Neurosci Lett 2006;401:35–39. [DOI] [PubMed] [Google Scholar]
- 42. Weston MC, Chen H, Swann JW. Multiple roles for mammalian target of rapamycin signaling in both glutamatergic and GABAergic synaptic transmission. J Neurosci 2012;32:11441–11452. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43. Ji YF, Zhou L, Xie YJ, et al. Upregulation of glutamate transporter GLT‐1 by mTOR‐Akt‐NF‐small ka, CyrillicB cascade in astrocytic oxygen‐glucose deprivation. Glia 2013;61:1959–1975. [DOI] [PubMed] [Google Scholar]
- 44. Chen LL, Wu JC, Wang LH, et al. Rapamycin prevents the mutant huntingtin‐suppressed GLT‐1 expression in cultured astrocytes. Acta Pharmacol Sin 2012;33:385–392. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45. Abousaab A, Uzcategui NL, Elsir B, et al. Up‐regulation of the excitatory amino acid transporters EAAT1 and EAAT2 by mammalian target of rapamycin. Cell Physiol Biochem 2016;39:2492–2500. [DOI] [PubMed] [Google Scholar]
- 46. Zhang Y, He X, Wu X, et al. Rapamycin upregulates glutamate transporter and IL‐6 expression in astrocytes in a mouse model of Parkinson's disease. Cell Death Dis 2017;8:e2611. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47. Bregestovski P, Bernard C. Excitatory GABA: how a correct observation may turn out to be an experimental artifact. Front Pharmacol 2012;3:65. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48. Zhao X, Liao Y, Morgan S, et al. Noninflammatory changes of microglia are sufficient to cause epilepsy. Cell Rep 2018;22:2080–2093. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49. Uhlmann EJ, Wong M, Baldwin RL, et al. Astrocyte‐specific TSC1 conditional knockout mice exhibit abnormal neuronal organization and seizures. Ann Neurol 2002;52:285–296. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Figure S1. Example traces showing the effects of everolimus on the frequency of sIPSCs. Cells were held at +10 mV to minimize the contribution of excitatory synaptic activity. Acute bath application of everolimus (2 μm for about 15 min) did not affect IPSC frequency significantly. On average, there was only a small, nonsignificant decrease.
Figure S2. Everolimus did not affect sEPSC or sIPSC kinetics. Traces are averages of total spontaneous synaptic events recorded in one cell. Average values are shown in Table 2.
Figure S3. Top trace shows the effect of 4‐AP application in a cell from a slice incubated in ACSF only. Oscillations and paroxysmal discharges accompanied by large inward currents were induced. In a slice incubated for 70 min in everolimus, 4‐AP oscillations were significantly reduced and no paroxysmal discharges were observed.


