Abstract
The airway epithelial cell (AEC) response to allergens helps initiate and propagate allergic inflammation in asthma. CARMA3 is a scaffold protein that mediates G protein–coupled receptor–induced NF-κB activation in airway epithelium. In this study, we demonstrate that mice with CARMA3-deficient AECs have reduced airway inflammation, as well as reduced type 2 cytokine levels in response to Alternaria alternata. These mice also have reduced production of IL-33 and IL-25, and reduced numbers of innate lymphoid cells in the lung. We also show that CARMA3-deficient human AECs have decreased production of proasthmatic mediators in response to A. alternata. Finally, we show that CARMA3 interacts with inositol 1,4,5-trisphosphate receptors in AECs, and that inhibition of CARMA3 signaling reduces A. alternata–induced intracellular calcium release. In conclusion, we show that CARMA3 signaling in AECs helps mediate A. alternata–induced allergic airway inflammation, and that CARMA3 is an important signaling molecule for type 2 immune responses in the lung.
Keywords: CARMA3, Alternaria alternata, asthma, allergic lung inflammation
Asthma is a complex disease defined by inflammation of the airways together with airway hyperresponsiveness (AHR) and mucus hypersecretion (1). In the majority of cases, the airway inflammation associated with asthma results from an allergic-type reaction to an inhaled substance from the environment (2). In response to inhaled stimuli, signals from airway epithelial cells (AECs) help determine whether a particular allergen will trigger an inflammatory response (3–6). In particular, the production of cytokines, such as thymic stromal lymphopoietin (TSLP), granulocyte–macrophage colony–stimulating factor (GM-CSF), IL-33, IL-25, and CCL20/macrophage inflammatory protein (MIP)-3α by epithelial cells leads to activation and maturation of airway dendritic cells (DCs) and for the migration of DCs and T cells to the airways (7). These inflammatory mediators are upregulated in the airways of subjects with asthma in response to inhaled allergens known to induce allergic airway inflammation (7). In addition, type 2 innate lymphoid cells (ILC2s), which are important sources of IL-5 and IL-13 in the lung and for initiation of allergic airway inflammation, can also be activated by IL-25 and IL-33 (7, 8).
The transcription factor, NF-κB, regulates TSLP, GM-CSF, and CCL20/MIP-3α expression (9–11). Consequently, it is an attractive therapeutic target for inhibiting the production of these important mediators. CARMA3 is an intracellular scaffold protein that mediates NF-κB activation through interactions with Bcl10, mucosa-associated lymphoid tissue lymphoma translocation protein 1 (MALT1), and NF-κB essential modulator (NEMO)/IKKγ (12, 13). It is expressed in nonhematopoietic cells in the heart, liver, kidney, and lung (14, 15). Previous work has shown that CARMA3 mediates proinflammatory NF-κB activation in response to G protein–coupled receptor (GPCR) stimulation in parenchymal cells (16–18). Furthermore, our laboratory has shown that CARMA3 is expressed in AECs, and is critical for the production of TSLP, GM-CSF, and CCL20/MIP-3α in response to GPCR ligands that have been shown to be elevated in the lungs of subjects with asthma (18–20).
Allergic sensitivity to fungal allergens is an important cause of allergic asthma. Sensitivity to the fungus, Alternaria alternata, has been shown to correlate with asthma severity and asthma exacerbations (21–23). Fungal allergens, such as A. alternata, have intrinsic proteolytic properties that have the potential to act as adjuvants in driving airway inflammation (24, 25). A. alternata–specific serine protease activity has been demonstrated to cause NF-κB activation through protease-activated receptor (PAR) 2 and increases in intracellular calcium in AECs, leading to airway inflammation (26). In addition, this protease activity promotes the release of IL-33, which then drives the release of type 2 cytokines (27). A. alternata extracts have also been shown to stimulate production of IL-6, IL-8, and GM-CSF (28), and to disrupt barrier integrity of the airway epithelium (29). Consistent with this, when administered to the lungs of mice, A. alternata extracts induce type 2 lung inflammation (30).
Recently, we have shown that CARMA3 signaling in AECs is vital for the development of allergic airway inflammation in two T cell–dependent murine models of asthma (20). In those studies, we demonstrated that production of AEC-derived DC maturation signals in response to inhaled ovalbumin and house dust mite are dependent on CARMA3 signaling, and that the activation of allergen-specific T cells requires intact AEC CARMA3. In the present study, we demonstrate that CARMA3 expression in AECs is also important for the development of innate immune responses and allergic inflammation in an acute model of asthma exacerbations that is not T cell dependent. Mice with deletion of CARMA3 in AECs did not develop as much inflammation as wild-type mice in response to inhalation of A. alternata. We also demonstrate that CARMA3 is necessary for AEC production of several key cytokines in response to A. alternata, and that CARMA3-deficient mice have reduced numbers of ILC2 in the airways after exposure to A. alternata. Finally, we demonstrate a novel role for CARMA3 in early intracellular calcium release in response to GPCR stimulation in AECs with evidence that CARMA3 interacts with the inositol 1,4,5-trisphosphate (IP3) receptor (ITPR) family of proteins, which mediate intracellular calcium release from endoplasmic reticulum.
Methods
Reagents
A. alternata was purchased from Greer Laboratories.
Mice
All protocols and studies were approved by the Massachusetts General Hospital Subcommittee on Research and Animal Care. Surfactant protein C (SPC)Cre/CARMA3+/+ and SPCCre/CARMA3F/F mice were generated as previously described (20). Mice were used at 6–8 weeks of age, and were sex matched for all studies.
Asthma Models
Allergic airway inflammation was induced with A. alternata as previously described (31). Briefly, 100 μg total weight of A. alternata (2 mg/ml) in 50 μl PBS was administered intranasally on Days 1–4. Mice were harvested for analysis on Day 5, 24 hours after the last inhalation. In addition, mice were administered a single dose of 100 μg A. alternata and harvested 24 hours later. Control mice received 50 μl PBS intranasally.
Mouse Harvest and Analysis
BAL, harvest of the lungs and thoracic lymph nodes (TLN) and cytospins were performed as previously described (18).
Epithelial Cell Stimulation and CARMA3 Knockdown
CARMA3 expression was knocked down in normal human bronchial epithelial (NHBE) cells cultured on an air–liquid interface (ALI), as previously described (20). The cells were then stimulated with A. alternata (100 μg) for either 0.5, 1, 2, or 6 hours for RNA extraction and protein collection.
Quantitative PCR
RNA extractions and RT-PCR were performed as previously described (20).
Protein Quantification
Supernatants from ALI were collected and then used in commercial ELISA kits for human IL-33 (R&D Systems) according to the manufacturer’s protocol.
Measurement of Lung Function in Mice
Lung function was measured using the Flexivent system (Scireq), as previously described (32).
CARMA3 Protein Work and Colocalization Studies
T-RExTM-293 (Invitrogen) tetracycline-inducible cell line was stably transfected with a FLAG-CARMA3 construct and treated with 100 ng/ml tetracycline for 2 days before cell harvest. Cell lysates prepared from the samples were used for tandem affinity purification, as described previously (33). CARMA3 and associated proteins were separated by SDS-PAGE. The protein bands were then cut from the gel and sent for mass spectrometry analysis and identification of isolated proteins.
Coimmunoprecipitation and Western Blot Analysis
GFP-tagged human CARMA3 was expressed in HEK293T cells and immunoprecipitated with an anti-GFP antibody according to the manufacturer’s instructions (Thermofisher) Western blot probed with rabbit anti-ITPR3 (ab78556; Abcam).
Immunofluorescence and Microscopy
NHBE cells were fixed on Transwell inserts with 4% paraformaldehyde for 10 minutes at room temperature. Membranes were incubated with primary antibodies and then with secondary antibodies, and counterstained with DAPI. The primary antibodies used were mouse anti-CARMA3 (1:100; sc-271849; Santa Cruz Biotechnology), rabbit anti-mouse ITPR1 (ab5804; Abcam); anti-mouse ITPR2 (ab5805; Abcam); and anti-mouse ITPR3 (ab113292; Abcam).
Proximal Ligation In Situ Assay
The in situ Detection Reagents Red kit (DUO92008; Millipore Sigma) was used on fixed and permeabilized NHBE cells according to the manufacturer’s protocol. The following primary antibodies were used in this experiment: mouse anti-mouse Carma3 (SC-271849; Santa Cruz Biotechnology); rabbit anti-mouse Carma3 (PA5-19997; Invitrogen); rabbit anti-mouse ITPR1 (ab5804; Abcam); anti-mouse ITPR2 (ab5805; Abcam); anti-mouse ITPR3 (ab113292; Abcam).
Calcium Flux
NHBEs cells were resuspended in Ca2 + /Mg2 + PBS, and fluorescence was filtered through the 530/30 nm band pass filter and collected in FL-1/FITC. Baseline calcium levels were recorded for 1 minute on the Accuri C6 (BD Biosciences) and were followed by the addition of either 2.5 μM A23187 (positive control/Ca2 + ionophore; Sigma-Aldrich) or 100 μg A. alternata to each sample.
Statistical Analysis
Data are expressed as mean (±SEM). Differences between means were tested for statistical significance using unpaired t tests as appropriate to the experiment. For multiple comparisons, a two-way ANOVA test was used for lung function analysis. From such comparisons, differences yielding P less than 0.05 were judged to be significant.
Results
Deletion of CARMA3 in AECs Reduces Allergic Airway Inflammation in a Murine Model of Asthma
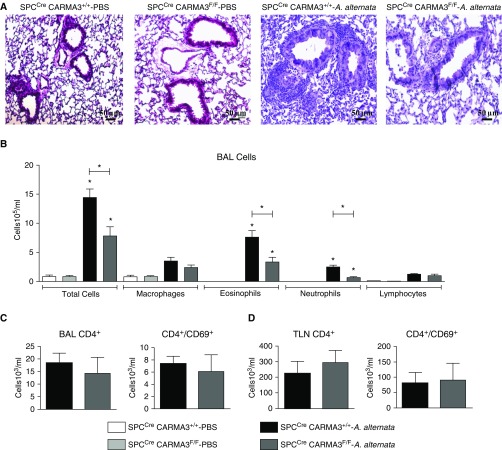
We have previously described the generation of mice with disruption of CARMA3 expression in AECs (SPCCre/CARMA3F/F) (20). SPCCre/CARMA3F/F and SPCCre/CARMA3+/+ littermate control mice were administered 100 μg A. alternata intranasally on Days 1–4 and then analyzed for airway inflammation on Day 5. Lung histology showed less airway inflammation in the SPCCre/CARMA3F/F mice compared with the SPCCre/CARMA3+/+ mice (Figure 1A). BAL total cell counts, as well as eosinophil and neutrophil counts, were reduced in SPCCre/CARMA3F/F mice compared with control mice (Figure 1B). However, the numbers of CD4+ and CD4+/CD69+ cells recovered from the BAL and TLN did not differ between SPCCre/CARMA3+/+ and SPCCre/CARMA3F/F mice (Figures 1C and 1D). These data demonstrate that CARMA3 expression in AECs has an important role in A. alternata–induced allergic airway inflammation in vivo. Specifically, AEC CARMA3 is critical for eosinophil and neutrophil recruitment after A. alternata exposure.
Figure 1.
Attenuation of airway inflammation in Alternaria alternata–treated SPCCre/CARMA3F/F mice. SPCCre/CARMA3+/+ and SPCCre/CARMA3F/F mice received either PBS or 100 μg A. alternata intranasally on Days 1–4 and harvested on Day 5. (A) Histopathologic analysis of lung sections stained with hematoxylin and eosin from SPCCre/CARMA3+/+–PBS–, SPCCre/CARMA3F/F–PBS–, SPCCre/CARMA3+/+–A. alternata–, and SPCCre/CARMA3F/F–A. alternata–treated mice. Scale bars = 50 μm. (B) Total cells, macrophages, eosinophils, neutrophils, and lymphocytes were enumerated in BAL fluid. CD4+ and CD4+/CD69+ cells were determined in (C) BAL fluid and (D) thoracic lymph nodes (TLN) by flow cytometry. Data are means ± SEM of eight mice per group from two experiments. *P < 0.05 (A. alternata treatment compared with the same genotype that was treated with PBS or SPCCre/CARMA3+/+–A. alternata compared with SPCCre/CARMA3F/F–A. alternata).
Deletion of CARMA3 in AEC Does Not Attenuate AHR after A. Alternata
We assessed whether deletion of CARMA3 expression in AECs would attenuate AHR. Airway resistance and airway compliance were measured using a forced oscillation technique, as described previously (20). Although A. alternata–treated mice showed increased airway resistance and decreased airway compliance compared with PBS controls, there was no difference in lung function between A. alternata–treated SPCCre/CARMA3+/+ and SPCCre/CARMA3F/F mice (see Figures E1A–E1D in the data supplement). These results demonstrate that CARMA3 signaling is not critical for A. alternata–induced AHR. This is in agreement with our previous findings, in which deletion of CARMA3 in AECs in vivo attenuated ovalbumin-induced airway inflammation, but not AHR (20).
AEC CARMA3 Deficiency Impairs Lung Cytokine Production
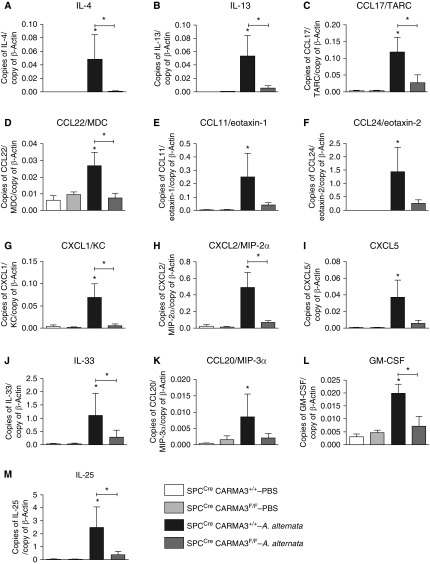
We measured the RNA expression of a group of asthma-relevant cytokines in the lungs of SPCCre/CARMA3F/F mice and SPCCre/CARMA3+/+ mice after PBS or A. alternata exposure. The SPCCre/CARMA3F/F mice had reduced levels of several type 2 cytokines and chemokines, as well as the neutrophil-specific chemokines, CXCL1/keratinocyte chemoattractant (KC), CXCL2/MIP-2α, and CXCL5 (Figures 2A–2I). We also found reduced RNA levels of the AEC-derived cytokines, IL-33, CCL20/MIP-3α, GM-CSF, and IL-25 (Figures 2J–2M). TSLP and IL-5 RNA were not detected in the lungs of mice. These data suggest that CARMA3 is necessary for asthma-associated inflammatory cytokine responses to A. alternata.
Figure 2.
Reduced production of proinflammatory mediators in A. alternata–treated SPCCre/CARMA3F/F mice. SPCCre/CARMA3+/+ and SPCCre/CARMA3F/F mice received either PBS or 100 μg A. alternata intranasally on Days 1–4 and harvested on Day 5. RNA levels of (A) IL-4, (B) IL-13, (C) CCL17/thymus- and activation-regulated chemokine (TARC), (D) CCL22/macrophage-derived chemokine (MDC), (E) CCL11/eotaxin-1, (F) CCL24/eotaxin-2, (G) CXCL1/keratinocyte chemoattractant (KC), (H) CXCL2/macrophage inflammatory protein (MIP)-2α, (I) CXCL5, (J) IL-33, (K) CCL20/MIP-3α, (L) granulocyte–macrophage colony–stimulating factor (GM-CSF), and (M) IL-25 in the lung quantified by quantitative PCR. β-actin levels were used to normalize the values of genes tested. Data are means ± SEM of six to eight mice per group from two experiments. *P < 0.05 (A. alternata treatment compared with the same genotype that was treated with PBS or SPCCre/CARMA3+/+–A. alternata compared with SPCCre/CARMA3F/F–A. alternata).
AEC CARMA3 Deficiency Impairs IL-33 Release
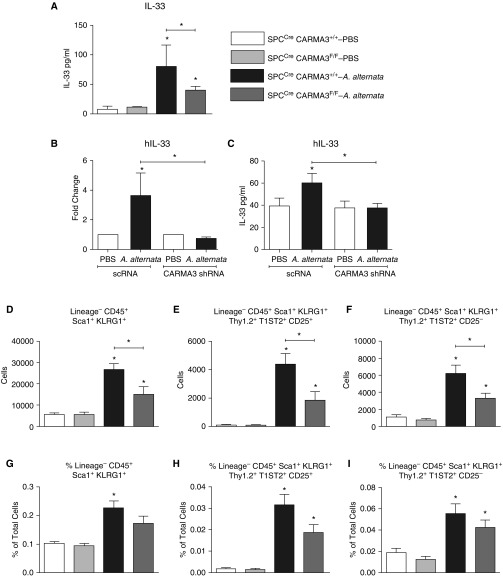
Although we saw reduced levels of IL-33 RNA in the lung, IL-33 is preformed in AECs and quickly released after allergen challenge (34). Thus, we measured the protein levels of IL-33 in the BAL of CARMA3-deficient mice 24 hours after a single dose of 100 μg A. alternata (Figure 3A). IL-33 protein levels were lower in SPCCre/CARMA3F/F mice compared with controls. To correlate the results in the mouse model to human AECs, we used primary NHBE cells cultured to form an ALI. Expression of CARMA3 was reduced in these cells after infection with a lentivirus, which expressed an shRNA specific to CARMA3, as previously described (20). CARMA3 knockdown reduced cell RNA levels at 6 hours and protein levels of IL-33 released into the media 30 minutes after A. alternata (100 μg) exposure (Figures 3B and 3C). Interestingly, protein levels at 1 and 2 hours after A. alternata exposure were similar in NHBE cells with CARMA3 knockdown and controls (data not shown). These data suggest that CARMA3 is necessary for both IL-33 production and immediate release after allergen exposure.
Figure 3.
Reduced numbers of type 2 innate lymphoid cells (ILC2s) isolated from SPCCre/CARMA3F/F mice. (A) SPCCre/CARMA3+/+ and SPCCre/CARMA3F/F mice received a single dose of either PBS or 100 μg A. alternata intranasally and were harvested 24 hours later. Protein levels of IL-33 in the BAL quantified by ELISA. Data are means ± SEM of eight mice per group from two experiments. *P < 0.05 (A. alternata treatment compared with the same genotype that was treated with PBS or SPCCre/CARMA3+/+–A. alternata compared with SPCCre/CARMA3F/F–A. alternata). (B–C) Normal human bronchial epithelial (NHBE) cells were grown on an air–liquid interface postinfection with lentivirus containing a nontargeting shRNA (scRNA) or a CARMA3-targeting shRNA. These cells were then stimulated with either media or 100 μg A. alternata for 6 hours for RNA analysis (B) or 0.5 hour for protein (C). Values are the means ± SEM of three to six samples. This experiment was repeated twice. *P < 0.05 (A. alternata treatment compared with the same targeting shRNA that was treated with media or scRNA–A. alternata compared with CARMA3 shRNA–A. alternata). SPCCre/CARMA3+/+ and SPCCre/CARMA3F/F mice received either PBS or 100 μg A. alternata intranasally on Days 1–4. (D–F) On Day 5, the lungs were isolated and the number of lineage− CD45+ Sca-1+ KLRG1+ ILC2s, lineage− CD45+ Sca-1+ KLRG1+ Thy1.2+ T1ST2+ CD25+ ILC2s, and lineage− CD45+ Sca-1+ KLRG1+ Thy1.2+ T1ST2+ CD25− ILC2s were determined by flow cytometry. (G–I) The percentage of total lung cells of lineage− CD45+ Sca-1+ KLRG1+ ILC2s, lineage− CD45+ Sca-1+ KLRG1+ Thy1.2+ T1ST2+ CD25+ ILC2s, and lineage− CD45+ Sca-1+ KLRG1+ Thy1.2+ T1ST2+ CD25− ILC2s were determined by flow cytometry. Data are means ± SEM of eight mice per group from two experiments. *P < 0.05 (A. alternata treatment compared with the same genotype that was treated with PBS or SPCCre/CARMA3+/+–A. alternata compared with SPCCre/CARMA3F/F–A. alternata).
Attenuated ILC2 Responses in AEC CARMA3–Deficient Mice
ILC2s are an important early source of IL-13, and have been shown to be critical for the immune response to A. alternata (35). ILC2s are recruited into the airways and are stimulated to release cytokines by IL-33 and IL-25 (36). Because we found reduced levels of IL-13, IL-33, and IL-25 in SPCCre/CARMA3F/F mice, we analyzed ILC2 numbers in vivo using a recently published gating strategy (37). There were no baseline differences in the number of lung ILC2s in AEC CARMA3–deficient mice compared with control mice. However, the total number and percentage of ILC2s were reduced in the lungs of AEC CARMA3–deficient mice after challenge with A. alternata (Figures 3D–3I). Representative flow plots for ILC2s are shown in Figures E2A–E2D. These data suggest that AEC CARMA3 helps mediate ILC2 accumulation in the lung after allergen challenge, and that this may be one mechanism by which CARMA3 deficiency in AECs attenuates type 2 cytokine production in the lung and airway inflammation. This may then impair ILC2 recruitment and cytokine production, which contributes to the overall reduction in airway inflammation observed in SPCCre/CARMA3F/F mice in response to A. alternata.
AEC CARMA3 Is Required for A. alternata–induced Calcium Flux
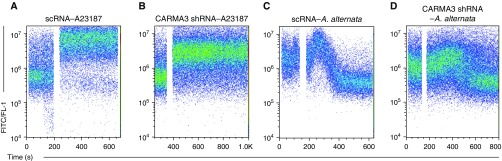
A. alternata has also been shown to lead to a rapid increase in cytosolic calcium in human AECs that is also mediated through PAR2 (26, 38). The release of IL-33 in response to A. alternata is very rapid, and known to be calcium dependent, suggesting that CARMA3 may also signal via an NF-κB–independent pathway in parallel (16, 39, 40). Thus, we decided to investigate the role of CARMA3 in the A. alternata–induced calcium response in AECs. CARMA3 expression was knocked down in NHBE cells with the lentiviral CARMA3-targeting shRNA, as described previously here. The cells were then incubated with 3 μM Fluo-4 and baseline calcium levels were recorded for 60 seconds. The calcium ionophore, A23187, was added and induced calcium flux in CARMA3-deficient cells as well as controls (Figures 4A and 4B). We also observed calcium flux approximately 30 seconds after stimulation in control cells when 100 μg of A. alternata was added (Figure 4C). However, A. alternata–induced calcium flux was delayed and attenuated in NHBE cells that had knockdown of CARMA3 with a reduction in the relative peak fluorescence compared with baseline (2.52 vs. 1.46 a.u. for control and CARMA3-shRNA respectively; P = 0.042; Figure 4D).
Figure 4.
Attenuated calcium flux in NHBEs with reduced CARMA3 expression. NHBEs were grown in T-75 flasks and infected with either a lentivirus containing a nontargeting shRNA (scRNA) or a CARMA3-targeting shRNA. NHBEs were trypsinized and incubated with 3 μM Fluo-4. Baseline calcium levels were recorded for 1 minute and were followed by the addition of either (A and B) 2.5 μM A23187 (positive control/Ca2 + ionophore; Sigma-Aldrich) or (C and D) 100 μg A. alternata. Representative plots are shown from three independent experiments.
CARMA3 Binds ITPRs in AECs
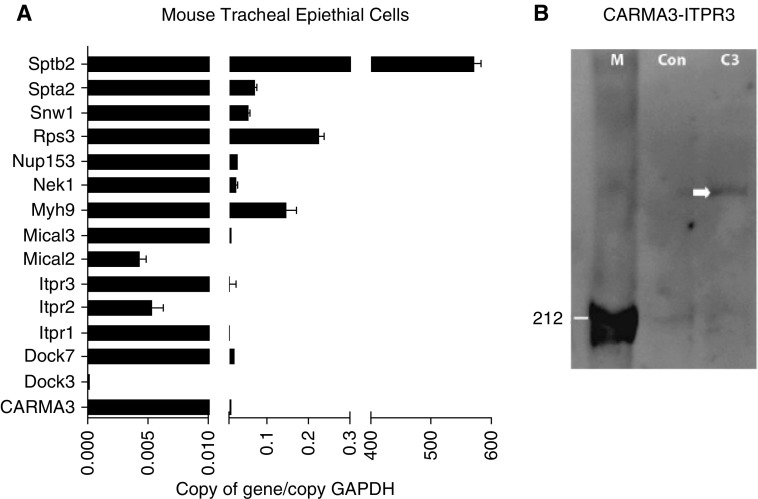
To investigate potential molecular mechanisms for the reduced calcium flux in NHBE cells with knockdown of CARMA3 after A. alternata exposure, we performed a protein–protein interaction screen for CARMA3. We overexpressed CARMA3 in HEK293T cells and then performed tandem affinity purification with CARMA3 pulldown and mass-spectrometric identification of CARMA3-interacting proteins. Along with the known CARMA3 binding proteins, Bcl10 and MALT1, we identified a number of novel proteins that bind to CARMA3, which have not been assigned to any previously known CARMA3 signaling pathways. We then used quantitative real-time PCR to detect mRNA expression of a subset of these novel interacting proteins in unstimulated mouse tracheal epithelial cells (Figure 5A). The diverse nature of these novel binding partners suggested that, apart from the already-described CARMA3 signaling mechanisms, CARMA3 could be associated with several other intracellular signaling cascades in AECs, including ITPR-mediated calcium flux. AECs express all three ITPRs (41), and binding of these receptors by regulatory proteins in the cytoplasm is a prerequisite for the release of calcium. To validate our protein–protein interaction screen, GFP-tagged human CARMA3 was expressed in HEK293T cells and was IP with an anti-GFP antibody. We then probed the IP with an antibody to the ITPR3. There was a band consistent with ITPR3 at 300 kD (Figure 5B) found in the co-IP lysates from cells transfected with GFP-CARMA3, which was not seen in co-IP lysates from nontransfected cells.
Figure 5.
CARMA3 interacts with inositol 1,4,5-trisphosphate receptor (ITPR) 3 in airway epithelial cells (AECs). (A) mRNA expression of novel CARMA3-interacting proteins in naive, unstimulated mouse tracheal epithelial cells assessed by real-time quantitative PCR. Data are means ± SEM of copy numbers relative to GAPDH. (B) Western blot probed with an ITPR3 antibody. C3 = CARMA3 protein pulled down from cells transfected with a construct containing CARMA3-GFP; Con = CARMA3-GFP protein pulled down from nontransfected control cells; M = markers; white arrow marks positive band.
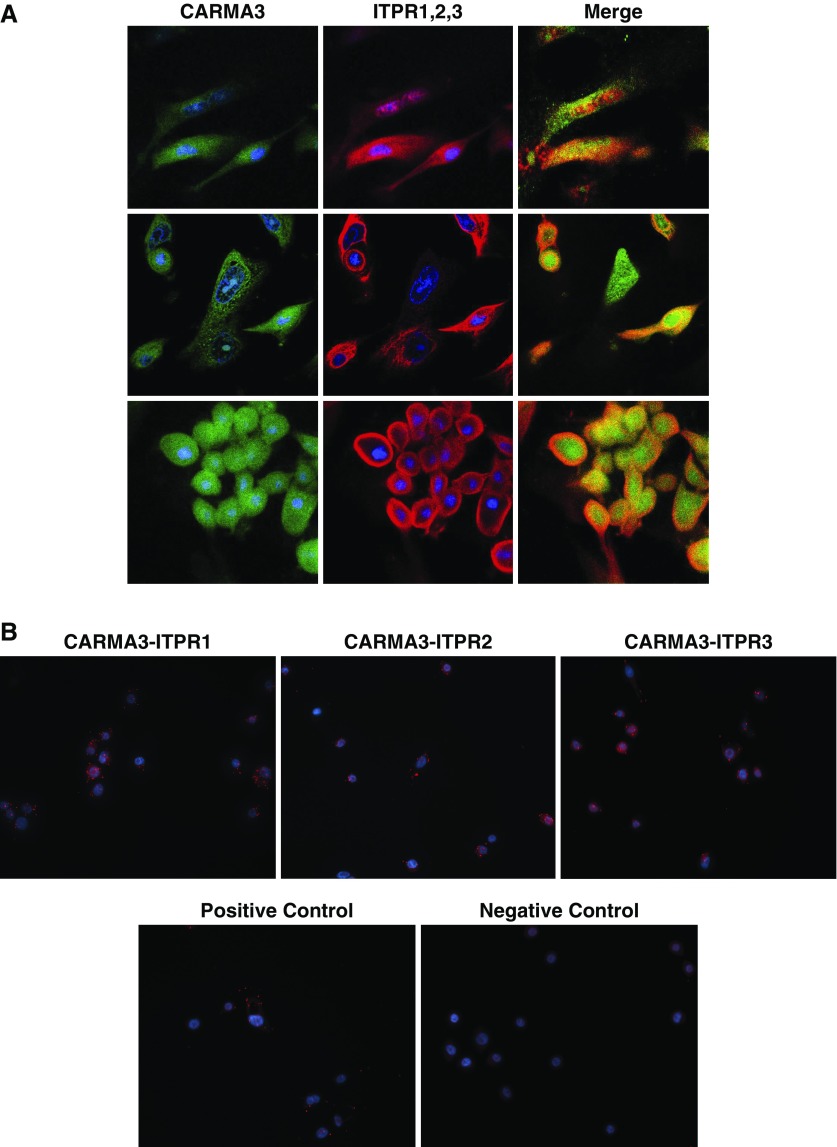
Immunofluorescent staining of NHBEs with antibodies to CARMA3 and ITPR3 disclosed close approximation of these proteins in the cytoplasm (Figure 6A). We also used an in situ proximal ligation assay (PLA; Duolink; Sigma-Aldrich) to further define the localization of CARMA3 and the ITPRs. Staining with antibodies to CARMA3 and ITPR1, -2, or -3 resulted in multiple fluorescent signals in NHBE cells, indicating that CARMA3 and the ITPR proteins are within 40 nm of each other in the cytoplasm of the cells and suggesting that they are interacting (Figure 6B).
Figure 6.
CARMA3 and the ITPRs colocalize in AECs. (A) Immunofluorescence of NHBEs stained with antibodies (Abs) against CARMA3 and ITPR1 (top row), ITPR2 (middle row), and ITPR3 (bottom row). All images were taken using the same exposure at ×60 magnification. (B) In situ proximal ligation assay on NHBEs stained with Abs against CARMA3 and ITPR1 (left), ITPR2 (middle), and ITPR3 (right). The negative control includes a nonspecific Ab instead of the ITPR Abs and the positive control is done with two Abs against CARMA3. All images were taken using the same exposure at ×40 magnification.
Discussion
CARMA3 is a molecular scaffold that mediates the assembly of multiprotein complexes involved in the activation of NF-κB, a transcription factor that directs inflammation and immunity. Our laboratory has shown that CARMA3 is expressed in AECs and is necessary for production of TSLP, GM-CSF, and CCL20/MIP-3α in response to several GPCR ligands elevated in the lungs of subjects with asthma (18–20). In addition, we have also described a vital role for AEC CARMA3 in bridging the innate and adaptive immune response and promoting airway inflammation in a murine model of allergic asthma (20). Results of the present study show an important relationship between CARMA3 signaling and the development of A. alternata–induced airway inflammation. A. alternata–induced eosinophilic and neutrophilic airway inflammation is reduced in mice with CARMA3-deficient AECs. Furthermore, proinflammatory cytokine and chemokine production, innate cytokine production, and ILC2 numbers are attenuated in mice deficient in CARMA3. In addition, we provide evidence of a novel interaction between the ITPRs and CARMA3, as well as a role for CARMA3 in mediating calcium flux in AECs in response to A. alternata. Taken together, these results suggest a critical role for CARMA3 in the development of airway inflammation associated with exacerbations of allergic asthma.
An association exists between sensitivity to A. alternata and asthma severity (21–23). In addition, A. alternata has been shown to cause allergic inflammation in mice, independent of adaptive immunity (30, 42). Fungal allergens, such as A. alternata, possess intrinsic proteolytic activities that act as adjuvants in driving airway inflammation (24, 25). A. alternata–specific serine proteases can drive proinflammatory lung inflammation via PAR2 on epithelial cells, which leads to CARMA3-mediated NF-κB activation (43). A. alternata can also lead to increased intracellular calcium in AECs via PAR2-mediated phospholipase C activity and IP3 release (26, 44, 45). This can then lead to rapid release of preformed pools of IL-33 from AECs in response to intracellular calcium (16, 39, 40). In this article, we have used an in vivo model of allergic airways disease whereby mice are exposed to a whole extract of A. alternata in the absence of peripheral sensitization, as previously described (31). In this model, the airway epithelium is the first point of contact with A. alternata, thereby mimicking the scenario in humans. A. alternaria is used both in vitro and in vivo to model asthma exacerbations and innate responses to allergens (27, 31), and we show a fundamental role for AEC-CARMA3 signaling in the innate responses to A. alternata.
The airway epithelium is central to asthma pathogenesis and innate responses to allergens. Of particular importance is the early release of cytokines and mediators that facilitate sensitization to allergens and promote airway inflammation. ILC2s have been discovered as an important source of type 2 cytokines in the lung, and ILC2s can be activated by IL-25 and IL-33 (8). The reduction of A. alternata–induced production of IL-33 and IL-25 in CARMA3-deficient AECs and mice suggested that ILC2 numbers in SPCCre/CARMA3F/F mice may also be reduced. Indeed, this was seen when ILC2 numbers were quantified by flow cytometry in the lung tissue after A. alternata administration. Thus, it is likely that the attenuated numbers of ILC2s in the lungs of SPCCre/CARMA3F/F mice, and consequent reduction of type 2 cytokine production, contributes to the overall reduction in airway inflammation observed in response to A. alternata. It was also interesting to observe that the airway inflammatory response to A. alternata in SPCCre/CARMA3F/F mice was attenuated, but not completely abrogated. This suggests that mediators, such as IL-33, may still be released from AECs, independent of GPCR-CARMA3 signaling, in response to cell stress and damage caused by allergens (46). The residual production of IL-33 and other cytokines in CARMA3-deficient mice likely still drives a reduced inflammatory response to A. alternata.
A. alternata has been shown to induce a rapid increase in cytosolic calcium concentration in human AECs via activation of PAR2 and increased IP3 (26, 44, 45). We observed that A. alternata–stimulated rapid release of IL-33 from AECs (30 min) was attenuated in SPCCre/CARMA3F/F mice. Because this is too rapid to be mediated by NF-κB signaling, this suggests that CARMA3 may signal via an NF-κB–independent pathway to mediate this release. Indeed, we found that A. alternata induced rapid calcium mobilization in NHBEs, and that knockdown of CARMA3 expression in NHBEs reduced and delayed the peak calcium flux in response to A. alternata. This suggests that CARMA3 helps mediate A. alternata–stimulated calcium mobilization in AECs, and inhibition of CARMA3 may alter the source or mechanisms of intracellular calcium release. After a protein–protein interaction screen for CARMA3, we identified the ITPRs as a group of novel proteins that bind to CARMA3. The ITPRs are expressed on the endoplasmic reticulum in AECs (47), bind IP3, and facilitate the release of calcium from the endoplasmic reticulum lumen into the cytosol. Binding of ITPR by regulatory proteins in the cytoplasm and endoplasmic reticulum lumen is a prerequisite for the release of intracellular calcium (48). We also found that, in NHBEs, CARMA3 colocalized with ITPR, and thus we believe that this may be a novel protein–protein interaction that helps facilitate the immediate responses of AECs to A. alternata.
The airway epithelium is at the interface between the lung and the environment, and represents the first line of defense against infections and toxins (49). AECs are in constant contact with the environment, and express many receptors to detect and rapidly respond to pathogen-associated, as well as damage-associated, molecules released upon tissue damage, cell death, or cellular stress. GPCRs, such as the PARs, line the airway epithelium and are critical to innate responses to allergens, such as A. alternata (27, 38). In addition, it is likely that additional GPCRs may also contribute to this process, including the P2Y family of receptors responding to ATP, which can be released in response to A. alternata and cell damage (50). Previously, we have shown that P2Y1, P2Y2, and P2Y6 are expressed on AECs and that CARMA3 deficiency abrogates cytokine responses to ATP (20). Thus, we believe that CARMA3 likely signals downstream of multiple GPCRs and proinflammatory pathways. Given the increasing prevalence of fungal aeroallergens, we believe that the GPCR–CARMA3 axis could be targeted therapeutically in asthma.
In this article, we present data that demonstrate that CARMA3 signaling in AECs promotes allergic airway inflammation in response to A. alternate, and could be a significant contributor to driving the innate immune responses associated with asthma. We also describe a novel CARMA3-mediated signaling pathway, independent of NF-κB that contributes to proinflammatory mediator production. In summary, this study demonstrates that CARMA3 signaling in AECs drives allergic airway inflammation, and could be a promising therapeutic target to reduce airway inflammation in asthma.
Footnotes
This work was supported by Department of Defense grant PR150903 (B.D.M.), and by a Massachusetts General Hospital Executive Committee on Research Interim Support award (B.D.M.).
Author Contributions: Conception and design of study—B.C., R.J.X., J.L.C., and B.D.M.; analysis and interpretation—B.C., A.P.-S., J.G., K.D., T.K., J.R., J.L.C., and B.D.M.; drafting the manuscript—B.C., A.P.-S., J.G., and B.D.M.
This article has a data supplement, which is accessible from this issue’s table of contents at www.atsjournals.org.
Originally Published in Press as DOI: 10.1165/rcmb.2017-0181OC on June 29, 2018
Author disclosures are available with the text of this article at www.atsjournals.org.
References
- 1.Busse WW, Lemanske RF., Jr Asthma. N Engl J Med. 2001;344:350–362. doi: 10.1056/NEJM200102013440507. [DOI] [PubMed] [Google Scholar]
- 2.Lloyd CM, Hessel EM. Functions of T cells in asthma: more than just T(H)2 cells. Nat Rev Immunol. 2010;10:838–848. doi: 10.1038/nri2870. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Eisenbarth SC, Piggott DA, Bottomly K. The master regulators of allergic inflammation: dendritic cells in Th2 sensitization. Curr Opin Immunol. 2003;15:620–626. doi: 10.1016/j.coi.2003.09.003. [DOI] [PubMed] [Google Scholar]
- 4.Herrick CA, Bottomly K. To respond or not to respond: T cells in allergic asthma. Nat Rev Immunol. 2003;3:405–412. doi: 10.1038/nri1084. [DOI] [PubMed] [Google Scholar]
- 5.Huh JC, Strickland DH, Jahnsen FL, Turner DJ, Thomas JA, Napoli S, et al. Bidirectional interactions between antigen-bearing respiratory tract dendritic cells (DCs) and T cells precede the late phase reaction in experimental asthma: DC activation occurs in the airway mucosa but not in the lung parenchyma. J Exp Med. 2003;198:19–30. doi: 10.1084/jem.20021328. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Piggott DA, Eisenbarth SC, Xu L, Constant SL, Huleatt JW, Herrick CA, et al. MyD88-dependent induction of allergic Th2 responses to intranasal antigen. J Clin Invest. 2005;115:459–467. doi: 10.1172/JCI22462. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Hammad H, Lambrecht BN. Barrier epithelial cells and the control of type 2 immunity. Immunity. 2015;43:29–40. doi: 10.1016/j.immuni.2015.07.007. [DOI] [PubMed] [Google Scholar]
- 8.Kita H. ILC2s and fungal allergy. Allergol Int. 2015;64:219–226. doi: 10.1016/j.alit.2015.04.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Kao CY, Huang F, Chen Y, Thai P, Wachi S, Kim C, et al. Up-regulation of CC chemokine ligand 20 expression in human airway epithelium by IL-17 through a JAK-independent but MEK/NF-κB–dependent signaling pathway. J Immunol. 2005;175:6676–6685. doi: 10.4049/jimmunol.175.10.6676. [DOI] [PubMed] [Google Scholar]
- 10.Lee HC, Ziegler SF. Inducible expression of the proallergic cytokine thymic stromal lymphopoietin in airway epithelial cells is controlled by NF-κB. Proc Natl Acad Sci USA. 2007;104:914–919. doi: 10.1073/pnas.0607305104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Schreck R, Baeuerle PA. NF-κ B as inducible transcriptional activator of the granulocyte–macrophage colony–stimulating factor gene. Mol Cell Biol. 1990;10:1281–1286. doi: 10.1128/mcb.10.3.1281. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Stilo R, Liguoro D, Di Jeso B, Formisano S, Consiglio E, Leonardi A, et al. Physical and functional interaction of CARMA1 and CARMA3 with Iκ kinase γ–NF-κB essential modulator. J Biol Chem. 2004;279:34323–34331. doi: 10.1074/jbc.M402244200. [DOI] [PubMed] [Google Scholar]
- 13.Wang L, Guo Y, Huang WJ, Ke X, Poyet JL, Manji GA, et al. Card10 is a novel caspase recruitment domain/membrane-associated guanylate kinase family member that interacts with BCL10 and activates NF-κB. J Biol Chem. 2001;276:21405–21409. doi: 10.1074/jbc.M102488200. [DOI] [PubMed] [Google Scholar]
- 14.Jordan CT, Cao L, Roberson ED, Pierson KC, Yang CF, Joyce CE, et al. PSORS2 is due to mutations in CARD14. Am J Hum Genet. 2012;90:784–795. doi: 10.1016/j.ajhg.2012.03.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Wegener E, Krappmann D. CARD-Bcl10-Malt1 signalosomes: missing link to NF-κB. Sci STKE. 2007;2007:pe21. doi: 10.1126/stke.3842007pe21. [DOI] [PubMed] [Google Scholar]
- 16.Grabiner BC, Blonska M, Lin PC, You Y, Wang D, Sun J, et al. CARMA3 deficiency abrogates G protein–coupled receptor–induced NF-κB activation. Genes Dev. 2007;21:984–996. doi: 10.1101/gad.1502507. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.McAllister-Lucas LM, Jin X, Gu S, Siu K, McDonnell S, Ruland J, et al. The CARMA3-Bcl10-MALT1 signalosome promotes angiotensin II–dependent vascular inflammation and atherogenesis. J Biol Chem. 2010;285:25880–25884. doi: 10.1074/jbc.C110.109421. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Medoff BD, Landry AL, Wittbold KA, Sandall BP, Derby MC, Cao Z, et al. CARMA3 mediates lysophosphatidic acid–stimulated cytokine secretion by bronchial epithelial cells. Am J Respir Cell Mol Biol. 2009;40:286–294. doi: 10.1165/rcmb.2008-0129OC. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Georas SN, Berdyshev E, Hubbard W, Gorshkova IA, Usatyuk PV, Saatian B, et al. Lysophosphatidic acid is detectable in human bronchoalveolar lavage fluids at baseline and increased after segmental allergen challenge. Clin Exp Allergy. 2007;37:311–322. doi: 10.1111/j.1365-2222.2006.02626.x. [DOI] [PubMed] [Google Scholar]
- 20.Causton B, Ramadas RA, Cho JL, Jones K, Pardo-Saganta A, Rajagopal J, et al. Carma3 is critical for the initiation of allergic airway inflammation. J Immunol. 2015;195:683–694. doi: 10.4049/jimmunol.1402983. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Agarwal R. Severe asthma with fungal sensitization. Curr Allergy Asthma Rep. 2011;11:403–413. doi: 10.1007/s11882-011-0217-4. [DOI] [PubMed] [Google Scholar]
- 22.Dales RE, Cakmak S, Burnett RT, Judek S, Coates F, Brook JR. Influence of ambient fungal spores on emergency visits for asthma to a regional children’s hospital. Am J Respir Crit Care Med. 2000;162:2087–2090. doi: 10.1164/ajrccm.162.6.2001020. [DOI] [PubMed] [Google Scholar]
- 23.Neukirch C, Henry C, Leynaert B, Liard R, Bousquet J, Neukirch F. Is sensitization to Alternaria alternata a risk factor for severe asthma? A population-based study. J Allergy Clin Immunol. 1999;103:709–711. doi: 10.1016/s0091-6749(99)70247-2. [DOI] [PubMed] [Google Scholar]
- 24.Porter P, Susarla SC, Polikepahad S, Qian Y, Hampton J, Kiss A, et al. Link between allergic asthma and airway mucosal infection suggested by proteinase-secreting household fungi. Mucosal Immunol. 2009;2:504–517. doi: 10.1038/mi.2009.102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Porter PC, Yang T, Luong A, Delclos GL, Abramson SL, Kheradmand F, et al. Proteinases as molecular adjuvants in allergic airway disease. Biochim Biophys Acta. 2011;1810:1059–1065. doi: 10.1016/j.bbagen.2011.04.019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Boitano S, Flynn AN, Sherwood CL, Schulz SM, Hoffman J, Gruzinova I, et al. Alternaria alternata serine proteases induce lung inflammation and airway epithelial cell activation via PAR2. Am J Physiol Lung Cell Mol Physiol. 2011;300:L605–L614. doi: 10.1152/ajplung.00359.2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Snelgrove RJ, Gregory LG, Peiro T, Akthar S, Campbell GA, Walker SA, et al. Alternaria-derived serine protease activity drives IL-33–mediated asthma exacerbations. J Allergy Clin Immunol. 2014;134:583–592.e6. doi: 10.1016/j.jaci.2014.02.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Knight DA, Lim S, Scaffidi AK, Roche N, Chung KF, Stewart GA, et al. Protease-activated receptors in human airways: upregulation of PAR-2 in respiratory epithelium from patients with asthma. J Allergy Clin Immunol. 2001;108:797–803. doi: 10.1067/mai.2001.119025. [DOI] [PubMed] [Google Scholar]
- 29.Reed CE, Kita H. The role of protease activation of inflammation in allergic respiratory diseases. J Allergy Clin Immunol. 2004;114:997–1008; quiz, p. 1009. doi: 10.1016/j.jaci.2004.07.060. [DOI] [PubMed] [Google Scholar]
- 30.Kim HK, Lund S, Baum R, Rosenthal P, Khorram N, Doherty TA. Innate type 2 response to Alternaria extract enhances ryegrass-induced lung inflammation. Int Arch Allergy Immunol. 2014;163:92–105. doi: 10.1159/000356341. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Maazi H, Patel N, Sankaranarayanan I, Suzuki Y, Rigas D, Soroosh P, et al. ICOS:ICOS–ligand interaction is required for type 2 innate lymphoid cell function, homeostasis, and induction of airway hyperreactivity. Immunity. 2015;42:538–551. doi: 10.1016/j.immuni.2015.02.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Ramadas RA, Ewart SL, Medoff BD, LeVine AM. Interleukin-1 family member 9 stimulates chemokine production and neutrophil influx in mouse lungs. Am J Respir Cell Mol Biol. 2011;44:134–145. doi: 10.1165/rcmb.2009-0315OC. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Cao Z, Huett A, Kuballa P, Giallourakis C, Xavier RJ. DLG1 is an anchor for the E3 ligase MARCH2 at sites of cell–cell contact. Cell Signal. 2008;20:73–82. doi: 10.1016/j.cellsig.2007.08.019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Divekar R, Kita H. Recent advances in epithelium-derived cytokines (IL-33, IL-25, and thymic stromal lymphopoietin) and allergic inflammation. Curr Opin Allergy Clin Immunol. 2015;15:98–103. doi: 10.1097/ACI.0000000000000133. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Martinez-Gonzalez I, Steer CA, Takei F. Lung ILC2s link innate and adaptive responses in allergic inflammation. Trends Immunol. 2015;36:189–195. doi: 10.1016/j.it.2015.01.005. [DOI] [PubMed] [Google Scholar]
- 36.Halim TYF, McKenzie ANJ. New kids on the block: group 2 innate lymphoid cells and type 2 inflammation in the lung. Chest. 2013;144:1681–1686. doi: 10.1378/chest.13-0911. [DOI] [PubMed] [Google Scholar]
- 37.Moro K, Ealey KN, Kabata H, Koyasu S. Isolation and analysis of group 2 innate lymphoid cells in mice. Nat Protoc. 2015;10:792–806. doi: 10.1038/nprot.2015.047. [DOI] [PubMed] [Google Scholar]
- 38.Matsuwaki Y, Wada K, White T, Moriyama H, Kita H. Alternaria fungus induces the production of GM-CSF, interleukin-6 and interleukin-8 and calcium signaling in human airway epithelium through protease-activated receptor 2. Int Arch Allergy Immunol. 2012;158(suppl 1):19–29. doi: 10.1159/000337756. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Uchida M, Anderson EL, Squillace DL, Patil N, Maniak PJ, Iijima K, et al. Oxidative stress serves as a key checkpoint for IL-33 release by airway epithelium. Allergy. 2017;72:1521–1531. doi: 10.1111/all.13158. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Hsu CL, Bryce PJ. Inducible IL-33 expression by mast cells is regulated by a calcium-dependent pathway. J Immunol. 2012;189:3421–3429. doi: 10.4049/jimmunol.1201224. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Newton CL, Mignery GA, Südhof TC. Co-expression in vertebrate tissues and cell lines of multiple inositol 1,4,5-trisphosphate (InsP3) receptors with distinct affinities for InsP3. J Biol Chem. 1994;269:28613–28619. [PubMed] [Google Scholar]
- 42.Doherty TA, Khorram N, Lund S, Mehta AK, Croft M, Broide DH. Lung type 2 innate lymphoid cells express cysteinyl leukotriene receptor 1, which regulates TH2 cytokine production. J Allergy Clin Immunol. 2013;132:205–213. doi: 10.1016/j.jaci.2013.03.048. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Delekta PC, Apel IJ, Gu S, Siu K, Hattori Y, McAllister-Lucas LM, et al. Thrombin-dependent NF-κB activation and monocyte/endothelial adhesion are mediated by the CARMA3·Bcl10·MALT1 signalosome. J Biol Chem. 2010;285:41432–41442. doi: 10.1074/jbc.M110.158949. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Son GY, Son A, Yang YM, Park W, Chang I, Lee JH, et al. Airborne allergens induce protease activated receptor-2–mediated production of inflammatory cytokines in human gingival epithelium. Arch Oral Biol. 2016;61:138–143. doi: 10.1016/j.archoralbio.2015.10.015. [DOI] [PubMed] [Google Scholar]
- 45.Matsuwaki Y, Wada K, White T, Moriyama H, Kita H. Alternaria fungus induces the production of GM-CSF, interleukin-6 and interleukin-8 and calcium signaling in human airway epithelium through protease-activated receptor 2. Int Arch Allergy Immunol. 2012;158:19–29. doi: 10.1159/000337756. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Cayrol C, Girard JP. IL-33: an alarmin cytokine with crucial roles in innate immunity, inflammation and allergy. Curr Opin Immunol. 2014;31:31–37. doi: 10.1016/j.coi.2014.09.004. [DOI] [PubMed] [Google Scholar]
- 47.Sugiyama T, Yamamoto-Hino M, Wasano K, Mikoshiba K, Hasegawa M. Subtype-specific expression patterns of inositol 1,4,5-trisphosphate receptors in rat airway epithelial cells. J Histochem Cytochem. 1996;44:1237–1242. doi: 10.1177/44.11.8918898. [DOI] [PubMed] [Google Scholar]
- 48.Choe CU, Ehrlich BE. The inositol 1,4,5-trisphosphate receptor (IP3R) and its regulators: sometimes good and sometimes bad teamwork. Sci STKE. 2006;2006:re15. doi: 10.1126/stke.3632006re15. [DOI] [PubMed] [Google Scholar]
- 49.Xiao C, Puddicombe SM, Field S, Haywood J, Broughton-Head V, Puxeddu I, et al. Defective epithelial barrier function in asthma. J Allergy Clin Immunol. 2011;128:549–556.e1–12. doi: 10.1016/j.jaci.2011.05.038. [DOI] [PubMed] [Google Scholar]
- 50.Kouzaki H, Iijima K, Kobayashi T, O’Grady SM, Kita H. The danger signal, extracellular ATP, is a sensor for an airborne allergen and triggers IL-33 release and innate Th2-type responses. J Immunol. 2011;186:4375–4387. doi: 10.4049/jimmunol.1003020. [DOI] [PMC free article] [PubMed] [Google Scholar]