Abstract
Pulmonary lymphangioleiomyomatosis (LAM) is a slow-progressing metastatic disease that is driven by mutations in the tumor suppressor tuberous sclerosis complex 1/2 (TSC1/2). Rapamycin inhibits LAM cell proliferation and is the only approved treatment, but it cannot cause the regression of existing lesions and can only stabilize the disease. However, in other cancers, immunotherapies such as checkpoint blockade against PD-1 and its ligand PD-L1 have shown promise in causing tumor regression and even curing some patients. Thus, we asked whether PD-L1 has a role in LAM progression. In vitro, PD-L1 expression in murine Tsc2-null cells is unaffected by mTOR inhibition with torin but can be upregulated by IFN-γ. Using immunohistochemistry and single-cell flow cytometry, we found increased PD-L1 expression both in human lung tissue from patients with LAM and in Tsc2-null lesions in a murine model of LAM. In this model, PD-L1 is highly expressed in the lung by antigen-presenting and stromal cells, and activated T cells expressing PD-1 infiltrate the affected lung. In vivo treatment with anti–PD-1 antibody significantly prolongs mouse survival in the model of LAM. Together, these data demonstrate that PD-1/PD-L1–mediated immunosuppression may occur in LAM, and suggest new opportunities for therapeutic targeting that may provide benefits beyond those of rapamycin.
Keywords: human, mTOR, PD-1, torin, TSC2
Clinical Relevance
We present the novel finding that human lymphangioleiomyomatosis (LAM) lungs express the molecule PD-L1, a key player in T-cell inhibition, and this finding was recapitulated in our immunocompetent mouse model of LAM. We demonstrate that large numbers of innate and adaptive immune cells infiltrated the lungs but failed to eradicate the disease, in part owing to immune evasion via PD-L1. Importantly, treatment of a mouse LAM model with anti–PD-1 antibody dramatically improved survival. Our exciting new data suggest the potential for repurposing cancer immunotherapies as novel treatments in LAM, especially for patients with LAM who are unresponsive to rapamycin.
Pulmonary lymphangioleiomyomatosis (LAM), a rare genetic lung disease that affects primarily women of reproductive age, is characterized by the growth of atypical smooth muscle–like LAM cells forming microscopic lesions within the lungs and axial lymphatics (1). LAM is characterized by cystic destruction of the lung interstitium, obstruction of pulmonary lymphatics, and progressive loss of lung function. Investigators have made marked progress in understanding the etiology of LAM by linking inactivating mutations of the tumor suppressor genes TSC1 and TSC2 to the constitutive activation of mammalian/mechanistic target of rapamycin complex 1 (mTORC1) with neoplastic LAM cell growth (2–5). Successful preclinical and clinical studies targeting mTORC1 culminated in Food and Drug Administration approval of the mTOR inhibitor rapamycin (sirolimus) as the first treatment for LAM in 2015 (6). However, rapamycin only slows disease progression, and incomplete responses are common (7, 8). Thus, there remains an urgent need to identify new targets for the development of curative LAM treatments.
Recently, immunotherapies have emerged as promising treatments for various diseases, including neoplastic tumors (9, 10). These treatments seek to reactivate antitumor immune responses that have been shut off by immune-evasive mechanisms induced by the tumors (9). Various mechanisms have been found in tumors to evade the immune system, including 1) downregulation or loss of neoantigens, 2) enhancement of resistance to immune cell–induced cytotoxicity via antiapoptotic mechanisms, 3) inhibition of T-cell entry, 4) suppression of cytotoxic functions of T cells through recruitment of antiinflammatory cells (e.g., myeloid-derived suppressor cells), and 5) induction of T-cell anergy or exhaustion by activating checkpoint molecules such as programmed cell death protein 1 (PD-1) and cytotoxic T lymphocyte–associated antigen 4 (CTLA-4) (10–14). Although many studies have focused on understanding the tumor immune microenvironment, our knowledge and understanding of immunity in LAM are very limited (1). An important unanswered question is how LAM cells escape detection and elimination by the immune system (14). Studies have demonstrated that LAM cells express melanoma antigens such as ganglioside D3 and gp100 (15, 16), suggesting that LAM cells may be susceptible to cytotoxic lymphocytes. LAM cells have also been shown to express the pro-oncogenic transcription factor STAT3, sustaining their survival (17, 18). However, it is unclear how LAM cells escape immune surveillance. Interestingly, type II IFN-γ, which is known to suppress cancer cell growth, was shown to have a very limited inhibitory effect on uncontrolled LAM cell growth in culture (17), suggesting that LAM cells can develop a resistance to immune cytotoxicity mediated by IFN-γ. LAM lesions may also change their microenvironment by recruiting tumor-associated macrophages (19) and stromal fibroblasts (20). In addition, elevated levels of natural killer (NK) cell–activating receptors and their ligands in LAM tissue and serum suggest a role for the innate immune system in LAM (21). However, the potential clinical application of immunomodulatory approaches for treatment of LAM requires further investigation into the role of immunity in LAM.
In cancer, checkpoint blockade has proven to be among the most effective immunotherapeutic treatments (10–13). Among these, the most clinically validated are antibodies that block PD-1 or PD-1 ligand (PD-L1 or B7 homolog 1 [B7-H1]) axis. PD-L1 is upregulated in some tumor microenvironments (on tumor cells, stromal cells, and/or antigen-presenting cells [APCs]), and the interaction of PD-L1 with PD-1 on T cells is a potent driver of T-cell anergy and exhaustion (10). Thus, therapeutic blockade of these molecules (e.g., with blocking antibodies) can stimulate antitumor immunity (10, 12). Anti–PD-1 therapy has proven particularly effective in subsets of patients with non–small cell lung cancers, metastatic melanoma, Hodgkin’s lymphoma, and bladder cancer (12). Furthermore, unlike cell-based therapies, engineered T cells, or dendritic cell vaccines, checkpoint blockade is far easier to administer in nonspecialized centers, and thus has been the subject of more clinical trials than any other immunotherapy (22, 23). In this study, we examined the expression patterns of PD-L1 protein in lung tissue from patients with LAM, in TSC2-null cells, and in an immunocompetent murine model of LAM. Furthermore, we assessed T-cell infiltration in our murine model and investigated the potential of using immunotherapies (here, anti–PD-1 treatment) as novel treatments for LAM.
Methods
Immunohistochemical/Immunofluorescence Analysis
Lung tissue samples from six patients with advanced LAM disease and four control subjects were obtained from the National Disease Research Interchange and University of Texas Health Science Center in compliance with University of Pennsylvania Review Board–approved procedures. Immunohistochemistry/immunofluorescence was performed using antibodies against human PD-L1 (E1L3N) XP (Cell Signaling Technology) or murine PD-L1 (Novus Biologicals), phospho-ribosomal protein S6 (pS6) (Cell Signaling Technology), smooth muscle (SM) α-actin (Sigma Chemical Co.), and prospero homeobox protein 1 (Prox1) (Abcam, ab199359 clone Epr19273). Images were analyzed using Aperio ImageScope software (Leica Biosystems Imaging) and quantified using FIJI deconvolution software.
Immunocompetent Mouse Model of LAM
To study the immune involvement in LAM, we developed a metastatic model of LAM in immunocompetent C57BL/6 mice. We examined lung lesion formation induced by Tsc2-null, kidney-derived epithelial tumor TTJ cells derived from Tsc2−/+ C57BL/6 mice, first in immunodeficient nude BALB/c mice and then in C57BL/6 mice. As controls, we used Tsc2-expressing mouse kidney tubular epithelial TM1 cells and Lewis lung carcinoma cells, an established model of mouse lung cancer (24). A detailed description of how the TTJ and TM1 cells were generated and the immunocompetent mouse model of LAM was established is included in the data supplement. All animal procedures were performed according to a protocol approved by the University of Pennsylvania Animal Care and Use Committee.
In Vivo Treatment with Anti–PD-1 Antibody
Mice were treated either with 300 μg/mouse anti-mouse PD-1 antibody (CD279) (RMP1-14) (BioXCell) (n = 20) or with 300 μg/mouse anti-mouse control IgG2a (BioXCell) (n = 10) by intraperitoneal injection twice a week. Treatment started at 10 days after tail vein injection of 106 TTJ cells into C57BL/6 mice. We observed the animals by monitoring their weights, and they were killed when they exhibited an ∼20% loss of body weight, in accordance with our Animal Care and Use Committee–approved protocol. The lungs were inflated under 25 cm2 H2O pressure and fixed for morphological and immunohistochemical analyses as described previously (19).
Flow Cytometry
In human LAM lungs, the expression of PD-L1 and CD14 was analyzed by flow cytometry of single-cell suspensions prepared as previously described (25, 26). Mouse lungs were digested into single-cell suspensions and cells were stained with antibodies for the appropriate markers to identify cell types, including T cells, stromal cells, and APCs. Antibodies were purchased from Biolegend or eBiosciences (ThermoFisher Scientific).
Data Analysis
Data points from individual assays represent the mean ± SE. Statistically significant differences among groups were assessed by ANOVA (with the Bonferroni-Dunn correction), Kaplan-Meier (with log-sum rank analysis), or Student’s t test, and P values < 0.05 were considered sufficient to reject the null hypothesis for all analyses.
For further details regarding the methods used in this work (including cell culture, the immunocompetent mouse model of LAM, flow cytometry, Western blotting, and qRT-PCR), see the data supplement.
Results
PD-L1 Expression and T-Cell Infiltration in Human LAM Lung Lesions
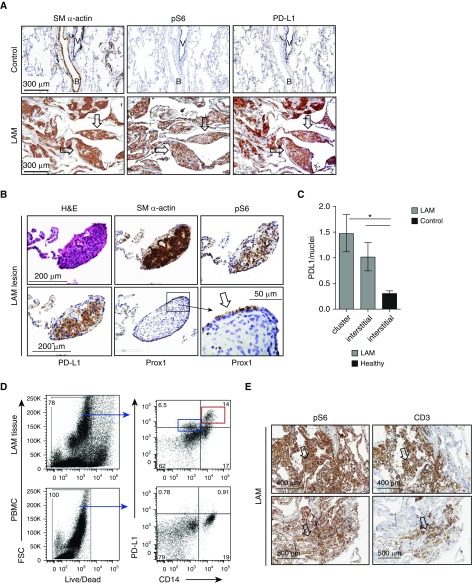
We performed immunohistochemistry (IHC) staining of tissue sections from distal lungs obtained from patients with LAM who had undergone transplantation to determine whether LAM cells expressing smooth muscle (SM) α-actin and phospho-ribosomal protein S6 (pS6), a biomarker of TSC2 loss and mTORC1 activation, also express PD-L1. IHC-specific staining of control lung tissue (Figure 1A, upper panels) compared with LAM lung tissue (Figure 1A, lower panels) highlighted typical LAM cell nodules (arrows). Marked reactivity for PD-L1 was also detected in some LAM nodules (Figure 1A, lower panel), whereas PD-L1 staining was significantly lower in lung sections from healthy control subjects (Figures 1A, upper panel, and 1B). To determine the specificity of PD-L1 staining for LAM disease, we performed an IHC analysis of lung sections from patients with cystic fibrosis, and smooth-muscle cells of the aorta and bronchus. PD-L1 was not detected in these samples (data not shown).
Figure 1.
Upregulation of programmed death ligand-1 (PD-L1) in human lymphangioleiomyomatosis (LAM) lungs. (A and B) Representative images of immunohistochemistry (IHC) analysis of PD-L1 in human LAM lungs. LAM cells were detected with smooth muscle (SM) α-actin and phospho-ribosomal protein S6 (pS6), and lymphatic endothelial cells were detected with prospero homebox protein 1 (Prox1). (A) Lung specimens from healthy human subjects (control subjects, n = 4; upper panels) and LAM lungs (lower panels). Scale bars: 300 μm. (B) Tissue samples from human LAM lungs (n = 6). Arrows indicate LAM nodules. B = bronchus; H&E = hematoxylin and eosin; V = vessel. Scale bars: 200 μm and 50 μm. (C) PD-L1 expression in images was quantified as PD-L1–positive pixels/hematoxylin (nuclei) using FIJI ImageJ deconvolution software, and LAM lungs (n = 6) were compared to control lungs (n = 4). Statistics were performed using Student’s t test; *P < 0.05. (D) Flow-cytometric analysis of PD-L1 expression in CD14+ versus CD14− cells from single-cell lung suspensions obtained from enzymatically digested LAM lung tissue (right column) (n = 2); peripheral blood monocytic cells (PBMCs) served as controls (n = 2). Red box: a high level of PD-L1 was associated with CD14+ monocytes/macrophages in LAM tissue compared with peripheral blood CD14+ cells. Blue box: expression of PD-L1 at a lower level was found on other CD14− cells, consistent with PD-L1 expression in LAM lung nodules. FSC = forward scatter. (E) Representative images of CD3+ cell infiltration of pS6+ human LAM lesions (n = 4). Scale bars: 400 μm.
In addition to the LAM cell nodules found in LAM lungs, metastasizing LAM cell clusters have been detected in chylous pleural fluid and in the lumen of the thoracic duct of patients with LAM (27). These clusters are covered by vascular endothelial growth factor receptor 3–positive lymphatic endothelial cells (28), which might prevent immune detection of LAM cells, facilitating their uncontrolled growth, survival, and metastasis (29, 30). In our study, we identified LAM cell nodules positive for pS6 and SM α-actin, which also showed immunoreactivity for PD-L1 and the lymphatic endothelial marker Prox-1 (Figure 1B). A quantitative analysis of IHC staining using FIJI deconvolution software demonstrated statistically significant PD-L1 upregulation in LAM nodules and LAM lung interstitium compared with control lung interstitium (Figure 1C).
PD-L1 upregulation was further demonstrated by flow-cytometry analysis of single-cell suspensions obtained by enzymatic digestion of LAM lungs. LAM lung cells, gated on all live cells (left column), demonstrated elevated expression of PD-L1 in both CD14+ and CD14− populations compared with peripheral blood monocytic cells from the same patients with LAM used as controls (Figure 1D). A high level of PD-L1 was associated with CD14+ monocytes/macrophages in LAM tissue compared with peripheral blood CD14+ cells (Figure 1D, red box). Expression of PD-L1 at a lower level was also found on other CD14− cells (Figure 1D, blue box), consistent with PD-L1 expression in LAM lungs.
Because PD-1-/PD-L1–based immune suppression requires a direct interaction between cells expressing PD-L1 and T cells expressing PD-1, we assessed colocalization of these cells in LAM lungs. We show that CD3+ T cells infiltrate pS6+ LAM lesions (Figure 1E; images are consecutive slices), indicating that T-cell–LAM-cell interactions may occur and could modulate the T-cell response in LAM.
PD-L1 Upregulation in Tsc2-Null TTJ Lesions in the Immunocompetent Mouse Model of LAM
Preclinical testing of anti–PD-1/PD-L1 antibodies for potential immune-targeting therapies for LAM requires an immunocompetent mouse model of LAM. Because genetic deletion of either Tsc2 or Tsc1 is embryonically lethal, and heterozygous animals do not recapitulate LAM disease (31), we focused our efforts on generating a Tsc2-null lung tumor model similar to LAM. A mouse model of experimental metastasis of Tsc2-null cells to the lung could recapitulate the proposed metastatic model of LAM, which postulates that in some patients, cells with TSC2 mutations from the kidney metastasize to the lung (24). Our initial efforts to generate a xenograft model in severe combined immunodeficiency mice using primary human LAM cells showed that when cells were injected into the tail vein, only a few LAM cells were detected in the lung after months of observations with no evidence of progressive growth (unpublished data). These observations suggest that primary human LAM cells do not grow in the lungs of even immunocompromised mice. It is possible that LAM cells can trigger innate immune tumor suppression even in an adaptive immune compromised environment.
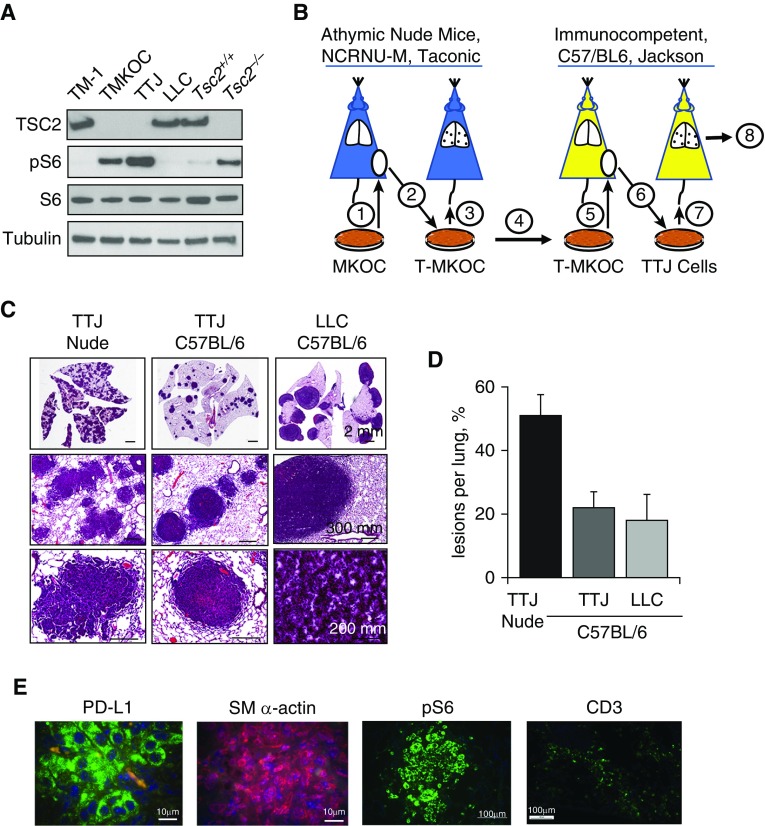
To develop an experimental metastatic model of LAM with Tsc2-null lung lesions in immunocompetent C57BL/6 mice, we used Tsc2-null TTJ cells, which show mTORC1 activation (Figure 2A). TTJ cells were generated by successive propagation of the original Tsc2-null MKOC cells derived from mouse kidney lesions that spontaneously develop in heterozygous Tsc2+/− C57BL/6 mice (31, 32) (schematically represented in Figure 2B and described in the data supplement). The original Tsc2-null MKOC cells underwent in vivo immune editing, producing TTJ cells by sequential propagation in immunodeficient nude BALB/c background and immunocompetent C57BL/6 mice. This approach allows selection for growth of tumor variants that escape from immune suppression (14).
Figure 2.
PD-L1 expression in Tsc2-null lung lesions in a metastatic immunocompetent mouse model of LAM. (A) Immunoblot analysis of Tsc2-expressing cells (TM1, Lewis lung carcinoma [LLC], and Tsc2+/+ mouse embryonic fibroblasts [MEFs]) and Tsc2-deficient cells (TMKOC, TTJ, and Tsc2−/− MEFs) with antibody against TSC2, pS6, S6, and tubulin. (B) Schematic representation of experimental procedures used to establish the Tsc2-null immunocompetent mouse LAM model. See text and the data supplement for details. (C) Representative H&E staining of lungs from nude mice injected with Tsc2-null TTJ cells, and C57BL/6 mice injected with either Tsc2-null TTJ or LLC cells. Animals were killed 18–21 days after injection, and lungs were inflated and stained with H&E. Scale bars: 2 mm, 200 mm, and 300 mm. (D) H&E images of the lungs were analyzed for the percentage of lesions per lung using Student’s t test for comparisons (n = 5 per each group, mean value ± SE), with P = 0.04 for TTJ nude versus TTJ C57BL/6, and P = 0.03 for TTJ nude versus LLC C57BL/6. (E) IHC analysis of pS6, SM α-actin, PD-L1 expression, and CD3+ T cells in C57BL/6 mouse lungs with Tsc2-null lung lesions (n = 5). Scale bars: 10 μm and 100 μm.
We first sought to test whether, when cultured in vitro, mouse MKOC and TTJ cells (compared with Tsc2-expressing M1 cells and Tsc2−/−- and Tsc2+/+ mouse embryonic fibroblasts [MEFs]) express PD-L1 and major histocompatability complex I (MHCI) molecules, which would allow for direct interaction with the T-cell receptor and subsequent suppression of T cells along the PD-1/PD-L1 axis. In addition, because it is well established that IFN-γ produced by cytotoxic T cells prevents tumor development by immune evasion, we also sought to determine whether IFN-γ stimulation would enhance PD-L1 and MHCI expression similarly to what is seen in stromal cells (33). Our analysis demonstrated that both Tsc2-expressing cells (M1 and Tsc2+/+ MEFs) and Tsc2-deficient cells (MKOC, TTJ, and Tsc2−/− MEFs) expressed low levels of PD-L1 and MHCI at baseline, and that this expression was significantly potentiated by stimulation with IFN-γ (Figure E2). Interestingly, the Tsc2-null cells derived from mouse kidney lesions (MKOC and TTJ) had approximately four- to fivefold higher PD-L1 expression upon IFN-γ stimulation compared with Tsc2-expressing M1 cells (Figure E2A). Expression patterns were minimally affected or increased by mTORC1 inhibition with torin in mouse cells, both at baseline and after IFN-γ stimulation (Figures E2B, E2D, and E2F). Interestingly, in MEFs, mTORC1 inhibition with torin significantly increased MHCI expression upon IFN-γ stimulation (Figure E2H), a phenomenon we are now investigating. PD-L1 expression was confirmed by qRT-PCR (Figure E3).
When we tail-vein–injected Tsc2-null TTJ cells to form a mouse model of metastatic LAM, we saw multiple lesions covering 50.7% ± 6.6% of the total lung in nude mice (Figures 2C and 2D). In contrast, the same number of TTJ cells injected into C57BL/6 mice formed significantly fewer lesions (21.5% ± 4.6%) (Figures 2C, 2D, and E4), likely due to both the very different phenotypes of BALB/c-derived nude mice and C57BL/6 mice and the increased immune suppression of Tsc2-null lung tumors in immunocompetent C57BL/6 mice. Immunohistochemical staining showed mTORC1 activation as detected by pS6 immunoreactivity and SM α-actin and PD-L1 expression in Tsc2-null TTJ lesions in lungs from C57BL/6 mice (Figures 2E and E5). CD3+ T-cell infiltrates were also detected near Tsc2-null TTJ lesions (Figure 2E), suggesting that an interaction between T cells and Tsc2-null cells may modulate T-cell activation and tumor growth.
APCs Infiltrate Mouse Lungs with Tsc2-Null Lesions and Express High Levels of PD-L1 in a Murine Model of LAM
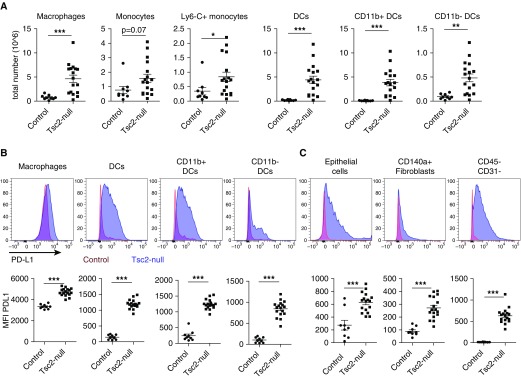
We investigated lung infiltration of APCs, which are known for their interactions with lymphocytes, and their PD-L1 expression in the metastatic mouse model of LAM with Tsc2-null lung lesions. We found marked increases in dendritic cells, both CD11b+ and CD11b− subtypes, monocytes, and macrophages in mouse lungs with Tsc2-null lesions compared with controls (Figure 3A). PD-L1 expression was increased in the various APC populations in the lungs from the mouse model of LAM (Figure 3B, upper panels), as evident in the shift of fluorescence intensity (representative histograms are shown in Figure 3B, upper panels; blue: LAM model with Tsc2-null lesions; control: control lung), and this difference was statistically significant when quantified via flow cytometry (Figure 3B, lower panels). Interestingly, PD-L1 expression was also significantly increased in CD45− stromal cell populations, including CD140a+ fibroblasts, epithelial cells, and CD31− cells (including Tsc2-null TTJ cells in the mouse model of LAM) (Figure 3C). Overall, our data indicate that high levels of PD-L1 expression occur in stromal cells (including Tsc2-null TTJ cells) as well as immune cells (particularly APCs) that can modulate T-cell activation and respond to Tsc2-null TTJ cells.
Figure 3.
Antigen-presenting cells infiltrate lungs with Tsc2-null lesions and highly express PD-L1. (A) Lungs with Tsc2-null lesions taken from C57BL/6 mice killed ∼3 weeks after injection had increased numbers of macrophages, dendritic cells (CD11b+ and CD11b−), and monocytes (activated Ly6C+ and nonactivated Ly6C−). (B) Representative histograms show a shift in PD-L1 expression in lungs with Tsc2-null lesions (blue) compared with control lungs (red) in antigen-presenting cells, and quantification of PD-L1 expression via median fluorescence intensity (MFI) in these cell populations. (C) PD-L1 expression in CD45− stromal cell populations, including CD140a+ fibroblasts, epithelial cells, and CD31− cells (including Tsc2-null TTJ cells) in the mouse model of LAM. Statistical analysis was performed using Student’s t test with significance at *P < 0.05 (**P < 0.01, ***P < 0.001, n ≥ 5 mice, n = 2 experiments). DC = dendritic cell.
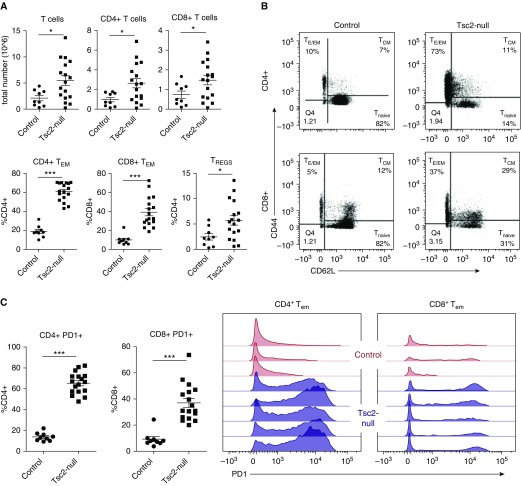
T Cells Expressing High Levels of PD-1 Infiltrate the Lungs with Tsc2-Null Lesions
In our previous study, we identified the infiltration of innate immune cells such as macrophages, eosinophils, and neutrophils in lungs from the mouse model of LAM on a BALB/c-derived nude background (19). However, little is known about the T-cell response in LAM lungs. We used our mouse model of LAM in immunocompetent mice to explore the adaptive immune response to LAM. Here, we found that in addition to innate cell infiltration, total T-cell numbers (including both CD4+ and CD8+ T cells) were increased (Figure 4A). Interestingly, we found that the distribution of T-cell subsets differed between mice with Tsc2-null lesions and control mice. Thus, Tsc2-null lung lesions had significantly higher numbers of effector memory T cells (both CD8+ and CD4+) compared with controls, and also showed increased numbers of regulatory T cells (Figures 4A and 4B). Furthermore, CD4+ and CD8+ effector memory T cells in Tsc2-null lungs had significantly higher expression of PD-1, the binding partner for PD-L1, compared with control lungs (Figure 4C).
Figure 4.
T cells infiltrate lungs with Tsc2-null lesions and highly express PD-1. (A) Lungs with Tsc2-null lesions taken from C57BL/6 mice killed ∼3 weeks after injection have increased CD4+ and CD8+ T cells, CD4+ and CD8+ effector/effector memory T (TEM) cells, and regulatory T cells (TREGS), presented as a percentage of total CD4+ and CD8+ T cells. (B) Representative flow-cytometry diagrams illustrate the increase in CD4+ and CD8+ TEM cells in lungs with Tsc2-null lesions compared with controls. (C) Quantification of overall PD-1+ CD4+ and CD8+ T cells, and representative plots showing PD-1 expression on TEM cells in control lungs (red) compared with lungs with Tsc2-null lesions (blue). Statistical analysis was performed using Student’s t test with significance at *P < 0.05 (***P < 0.001, n > 5 mice, n = 2 experiments).
Treatment with Anti–PD-1 Antibody Improves Survival in the Mouse Model of LAM
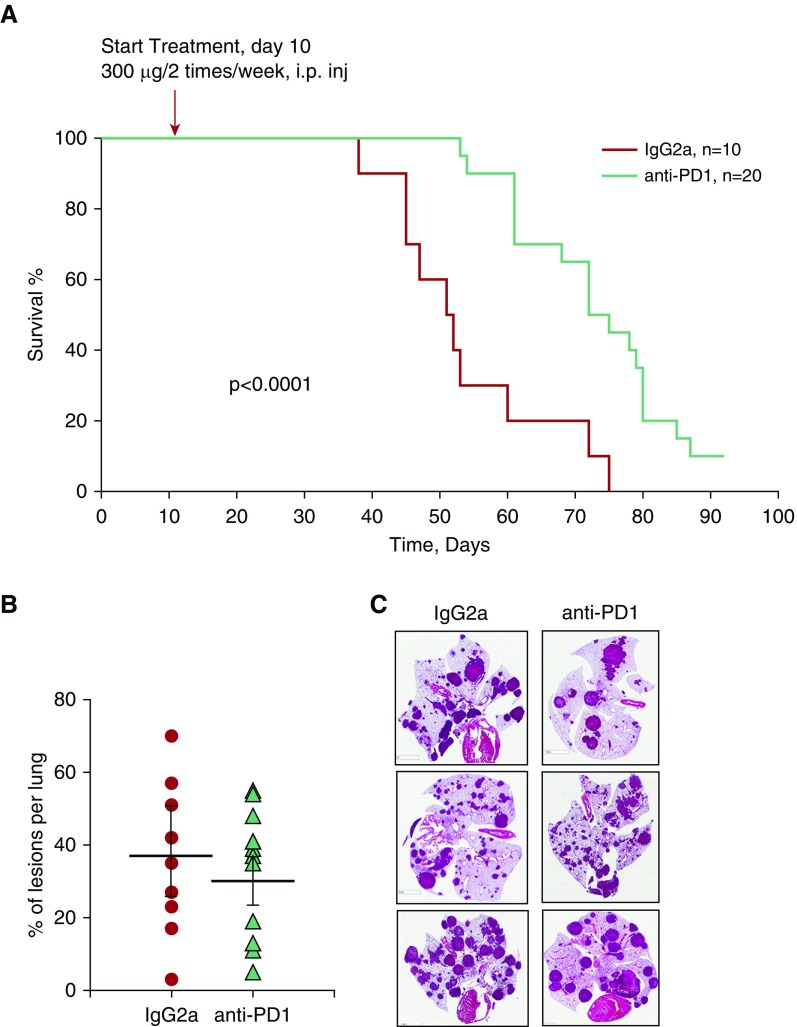
To assess whether immunotherapeutic targeting of the PD-1/PD-L1 axis in mice with Tsc2-null lung lesions suppressed LAM, we performed a survival study comparing the effects of treatment with anti–PD-1 antibody and a control isotype antibody. As shown in Figure 5A, survival was significantly increased by anti–PD-1 treatment. At day 55, ∼70% of the anti–PD-1-treated mice survived, compared with only ∼30% of the isotype-treated mice. Furthermore, at day 75, more than 45% of the anti–PD-1-treated mice still survived, whereas none of the isotype-treated mice did. A morphometric analysis of lungs taken when the animals were killed (i.e., when they had lost ∼20% of their body weight) surprisingly demonstrated that the percentage of lesions per total lung was not significantly different between the groups (Figure 5B). However, there was great heterogeneity in the size and morphology of the lesions in both anti–PD-1–treated and IgG-treated lungs (Figure 5C). Collectively, our data demonstrate a significant increase of survival in mice with Tsc2-null lesions by treatment with anti–PD-1 antibody.
Figure 5.
Anti–PD-1 antibody treatment enhances survival in a mouse model of LAM. (A) Kaplan-Meier analysis of survival in mice with Tsc2-null lung lesions. Mice were treated with either anti–PD-1 antibody (green) (n = 20) or isotype control IgG2a antibody (red) (n = 10). Animal survival was assessed as 20% loss of body weight. Statistical significance was determined for mice treated with anti–PD-1 antibody versus IgG2a antibody by log-sum rank test; P < 0.0001. (B) Percentage of lung lesions per lung in isotype control IgG2a- and anti–PD-1–treated mice at the time they were killed. Statistical analysis was performed using Student’s t test with P = 0.24. (C) Representative H&E images of lungs with Tsc2-null lesions from mice treated with either anti–PD-1 antibody or IgG2a at the time they were killed. Scale bars: 5 mm.
Discussion
Cancer immunotherapies require an existing pool of T cells that can respond to antigens specific to a particular type of cancer. These cancer antigen-specific T cells are suppressed by the immune microenvironment but subsequently can be activated by immunotherapy (34–36). In LAM, several antigens have been identified that are also found in melanoma and neuroendocrine tumors. These include ganglioside D3 and gp100, both of which are potential targets for NKT and cytotoxic T cells (15, 16). Indeed, studies by Klarquist and colleagues and Gilbert and colleagues demonstrated that LAM cells are susceptible to NKT-cell and cytotoxic lymphocyte–mediating immune targeting (15, 16). These studies also identified that despite expression of GD3, there was no NKT-cell recruitment to LAM lungs. This suggests that enhancing NKT-cell responses through immunization targeting GD3 may provide new therapeutic LAM treatments. However, clinical application of this approach for treatment of LAM has not been investigated (37).
Our studies have focused on classic CD8+/CD4+ T-cell responses in LAM, and we have found that in our mouse model, significantly more T cells infiltrate lungs with Tsc2-null lesions compared with lungs of controls. In addition, we have found that these infiltrating T cells highly express PD-1, suggesting that T cells may have been shifted toward tolerance or anergy by PD-1–PD-L1 interactions (38).
Recent evidence in cancer has demonstrated that the therapeutic efficacy of treatments such as the anti–PD-1 therapy we used in the present studies is highly dependent on T-cell infiltration into tumors (10, 39, 40). Indeed, patients with “hot tumors,” or tumors with significant immune cell infiltrates, respond better to immune checkpoint blockade, whereas patients with “cold tumors” often fall in the nonresponder group. Further advances have demonstrated that the immune infiltrates are likely due to increased chemokine production in some tumors that attract immune cells (39, 41). For this reason, efforts to enhance chemokine production in tumors to transform previously “cold” tumors into “hot” tumors have shown significant promise. Chemokines such as CCL21, which attracts CCR7+ naive and central memory T cells along with other immune cells, have been combined with other immunotherapies as effective treatments for tumors lacking immune infiltration (42–45). Strategies such as these could potentially be applied in LAM to enhance immune infiltration into the lungs. When combined with checkpoint inhibition or other immunotherapeutic strategies, this may further enhance treatment efficacy.
The present study identifies PD-L1 as a potential novel therapeutic target in LAM. Identification of PD-L1 upregulation in LAM cell nodules demonstrates one potential mechanism of immunomodulation that may enable LAM cells to escape immune detection and destruction. Activation of Akt and mTOR has been linked to PD-L1 upregulation in experimental animal models and in glioma (46, 47). In LAM, mutational inactivation of TSC1/2 uncouples mTORC1 from upstream control by growth factors and nutrients, leading to uncontrolled growth (1, 3, 29, 30). Our study adds to the pathobiology of LAM by demonstrating PD-L1 upregulation in LAM lungs. In addition, we show that Tsc2-null lesions in immunocompetent mice have increased levels of both innate and adaptive immune cells. Furthermore, stromal cells, epithelial cells, fibroblasts, and T cells have upregulated expression of PD-L1. In this immunocompetent mouse model of metastatic LAM, treatment with anti–PD-1 antibody significantly extended mouse survival. Several important caveats with regard to this model should be noted. First, the cell of origin for the TTJ lung lesions in our model is a kidney-derived tumor. In human LAM, the origin of the LAM lung cells is still unclear, although kidney angiomyolipomas are common in this patient population. Second, the subcutaneous injection of Tsc2-null TTJ cells and their passaging through BALB/c-derived nude and C57BL/6 mice may have selected for particularly immune evasive cells that may respond well to immunotherapy, such as anti–PD-1. Third, Tsc2-null lung tumor growth in the mouse is much more aggressive than lung lesion growth in patients with LAM, so immunotherapy may have different effects in human LAM cells because of their slower proliferation kinetics. Further studies are needed to identify specific molecular and cellular mechanisms of PD-L1 upregulation and its role in immunomodulation in LAM. Because blockage of the immune checkpoint axis PD-1/PD-L1 is among the most promising approaches in cancer immunotherapy, our findings also provide a new opportunity for therapeutic targeting that extends beyond the stabilization benefits of rapamycin. Such targeting may also provide an approach for patients who are unresponsive to rapamycin treatment and those with advanced LAM disease.
Acknowledgments
Acknowledgment
We thank Dr. Jilly Evans for a critical reading of the manuscript, Mrs. Li-Ping Wang (Pathology Clinical Service Center, University of Pennsylvania) for expert technical assistance with immunohistochemical staining of human tissue, and Ms. Jackie Yun Ying and Minmin Lu (Histology Core, Penn Center for Pulmonary Biology and the Cardiovascular Institute) for expert assistance with immunohistochemical staining of mouse tissue. Note: The manuscript (on related topic) by Liu, H.-J. et al. entitled “TSC2-deficient tumors have evidence of T cell exhaustion and respond to anti–PD-1/anti–CTLA-4 immunotherapy” had been published in JCI Insight 3, doi:10.1172/jci.insight.98674 (2018).
Footnotes
Supported by National Institutes of Health/National Heart, Lung, and Blood Institute and National Institute for Allergy and Infectious Diseases grants R01 HL114085 (V.P.K.), R01 HL131626 (V.P.K.), R21 AI130821 (M.A.S.), T32 HL07605 (K.M.), F32 HL134251 (K.M.), and P01 HL098067 (T.N.W.); LAM Foundation Pilot Award LAM0121P01-17 (V.P.K.); and The New Zealand LAM Charitable Trust (M.J.M.).
Author Contributions: Conception and design, K.M., M.J.M., E.N.A.-V., L.L., K.O., M.A.S., and V.P.K. Acquisition of data: K.M., M.J.M., E.N.A.-V., L.L., K.O., R.R., N.Z., L.F.A., I.K., and E.E. Data analysis and interpretation: K.M., M.J.M., E.N.A.-V., L.L., K.O., A.N.V., A.J.G., I.K., T.N.W., E.E., M.A.S., and V.P.K. Drafting of the manuscript for important intellectual content: K.M., M.J.M., E.E., M.A.S., and V.P.K. Final approval of the manuscript: all authors.
This article has a data supplement, which is accessible from this issue’s table of contents at www.atsjournals.org.
Originally Published in Press as DOI: 10.1165/rcmb.2018-0123OC on August 10, 2018
Author disclosures are available with the text of this article at www.atsjournals.org.
References
- 1.Krymskaya VP, McCormack FX. Lymphangioleiomyomatosis: a monogenic model of malignancy. Annu Rev Med. 2017;68:69–83. doi: 10.1146/annurev-med-050715-104245. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Carsillo T, Astrinidis A, Henske EP. Mutations in the tuberous sclerosis complex gene TSC2 are a cause of sporadic pulmonary lymphangioleiomyomatosis. Proc Natl Acad Sci USA. 2000;97:6085–6090. doi: 10.1073/pnas.97.11.6085. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Goncharova EA, Goncharov DA, Eszterhas A, Hunter DS, Glassberg MK, Yeung RS, et al. Tuberin regulates p70 S6 kinase activation and ribosomal protein S6 phosphorylation. A role for the TSC2 tumor suppressor gene in pulmonary lymphangioleiomyomatosis (LAM) J Biol Chem. 2002;277:30958–30967. doi: 10.1074/jbc.M202678200. [DOI] [PubMed] [Google Scholar]
- 4.Goncharova EA, Goncharov DA, Lim PN, Noonan D, Krymskaya VP. Modulation of cell migration and invasiveness by tumor suppressor TSC2 in lymphangioleiomyomatosis. Am J Respir Cell Mol Biol. 2006;34:473–480. doi: 10.1165/rcmb.2005-0374OC. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Crooks DM, Pacheco-Rodriguez G, DeCastro RM, McCoy JP, Jr, Wang JA, Kumaki F, et al. Molecular and genetic analysis of disseminated neoplastic cells in lymphangioleiomyomatosis. Proc Natl Acad Sci USA. 2004;101:17462–17467. doi: 10.1073/pnas.0407971101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.McCormack FX, Inoue Y, Moss J, Singer LG, Strange C, Nakata K, et al. National Institutes of Health Rare Lung Diseases Consortium; MILES Trial Group. Efficacy and safety of sirolimus in lymphangioleiomyomatosis. N Engl J Med. 2011;364:1595–1606. doi: 10.1056/NEJMoa1100391. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.McCormack FX, Gupta N, Finlay GR, Young LR, Taveira-DaSilva AM, Glasgow CG, et al. ATS/JRS Committee on Lymphangioleiomyomatosis. Official American Thoracic Society/Japanese Respiratory Society clinical practice guidelines: lymphangioleiomyomatosis diagnosis and management. Am J Respir Crit Care Med. 2016;194:748–761. doi: 10.1164/rccm.201607-1384ST. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Krymskaya VP. Therapeutic strategies for treatment of pulmonary lymphangioleiomyomatosis (LAM) Expert Opin Orphan Drugs. 2014;2:1063–1074. doi: 10.1517/21678707.2014.953481. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Hanahan D, Weinberg RA. Hallmarks of cancer: the next generation. Cell. 2011;144:646–674. doi: 10.1016/j.cell.2011.02.013. [DOI] [PubMed] [Google Scholar]
- 10.Pardoll DM. The blockade of immune checkpoints in cancer immunotherapy. Nat Rev Cancer. 2012;12:252–264. doi: 10.1038/nrc3239. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Duraiswamy J, Freeman GJ, Coukos G. Therapeutic PD-1 pathway blockade augments with other modalities of immunotherapy T-cell function to prevent immune decline in ovarian cancer. Cancer Res. 2013;73:6900–6912. doi: 10.1158/0008-5472.CAN-13-1550. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Page DB, Postow MA, Callahan MK, Allison JP, Wolchok JD. Immune modulation in cancer with antibodies. Annu Rev Med. 2014;65:185–202. doi: 10.1146/annurev-med-092012-112807. [DOI] [PubMed] [Google Scholar]
- 13.Roh W, Chen PL, Reuben A, Spencer CN, Prieto PA, Miller JP, et al. Integrated molecular analysis of tumor biopsies on sequential CTLA-4 and PD-1 blockade reveals markers of response and resistance. Sci Transl Med. 2017;9:pii: eaah3560. doi: 10.1126/scitranslmed.aah3560. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Schreiber RD, Old LJ, Smyth MJ. Cancer immunoediting: integrating immunity’s roles in cancer suppression and promotion. Science. 2011;331:1565–1570. doi: 10.1126/science.1203486. [DOI] [PubMed] [Google Scholar]
- 15.Klarquist J, Barfuss A, Kandala S, Reust MJ, Braun RK, Hu J, et al. Melanoma-associated antigen expression in lymphangioleiomyomatosis renders tumor cells susceptible to cytotoxic T cells. Am J Pathol. 2009;175:2463–2472. doi: 10.2353/ajpath.2009.090525. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Gilbert ER, Eby JM, Hammer AM, Klarquist J, Christensen DG, Barfuss AJ, et al. Positioning ganglioside D3 as an immunotherapeutic target in lymphangioleiomyomatosis. Am J Pathol. 2013;183:226–234. doi: 10.1016/j.ajpath.2013.04.002. [DOI] [PubMed] [Google Scholar]
- 17.Goncharova EA, Goncharov DA, Damera G, Tliba O, Amrani Y, Panettieri RA, Jr, et al. Signal transducer and activator of transcription 3 is required for abnormal proliferation and survival of TSC2-deficient cells: relevance to pulmonary lymphangioleiomyomatosis. Mol Pharmacol. 2009;76:766–777. doi: 10.1124/mol.109.057042. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.El-Hashemite N, Kwiatkowski DJ. Interferon-gamma-Jak-Stat signaling in pulmonary lymphangioleiomyomatosis and renal angiomyolipoma: a potential therapeutic target. Am J Respir Cell Mol Biol. 2005;33:227–230. doi: 10.1165/rcmb.2005-0152RC. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Goncharova EA, Goncharov DA, Fehrenbach M, Khavin I, Ducka B, Hino O, et al. Prevention of alveolar destruction and airspace enlargement in a mouse model of pulmonary lymphangioleiomyomatosis (LAM) Sci Transl Med. 2012;4:154ra134. doi: 10.1126/scitranslmed.3003840. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Clements D, Dongre A, Krymskaya VP, Johnson SR. Wild type mesenchymal cells contribute to the lung pathology of lymphangioleiomyomatosis. PLoS One. 2015;10:e0126025. doi: 10.1371/journal.pone.0126025. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Osterburg AR, Nelson RL, Yaniv BZ, Foot R, Donica WR, Nashu MA, et al. NK cell activating receptor ligand expression in lymphangioleiomyomatosis is associated with lung function decline. JCI Insight. 2016;1:e87270. doi: 10.1172/jci.insight.87270. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Sharma P, Allison JP. Immune checkpoint targeting in cancer therapy: toward combination strategies with curative potential. Cell. 2015;161:205–214. doi: 10.1016/j.cell.2015.03.030. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Sharma P, Allison JP. The future of immune checkpoint therapy. Science. 2015;348:56–61. doi: 10.1126/science.aaa8172. [DOI] [PubMed] [Google Scholar]
- 24.Hiratsuka S, Nakamura K, Iwai S, Murakami M, Itoh T, Kijima H, et al. MMP9 induction by vascular endothelial growth factor receptor-1 is involved in lung-specific metastasis. Cancer Cell. 2002;2:289–300. doi: 10.1016/s1535-6108(02)00153-8. [DOI] [PubMed] [Google Scholar]
- 25.Quatromoni JG, Singhal S, Bhojnagarwala P, Hancock WW, Albelda SM, Eruslanov E. An optimized disaggregation method for human lung tumors that preserves the phenotype and function of the immune cells. J Leukoc Biol. 2015;97:201–209. doi: 10.1189/jlb.5TA0814-373. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Eruslanov EB, Bhojnagarwala PS, Quatromoni JG, Stephen TL, Ranganathan A, Deshpande C, et al. Tumor-associated neutrophils stimulate T cell responses in early-stage human lung cancer. J Clin Invest. 2014;124:5466–5480. doi: 10.1172/JCI77053. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Seyama K, Kumasaka T, Kurihara M, Mitani K, Sato T. Lymphangioleiomyomatosis: a disease involving the lymphatic system. Lymphat Res Biol. 2010;8:21–31. doi: 10.1089/lrb.2009.0018. [DOI] [PubMed] [Google Scholar]
- 28.Kumasaka T, Seyama K, Mitani K, Sato T, Souma S, Kondo T, et al. Lymphangiogenesis in lymphangioleiomyomatosis: its implication in the progression of lymphangioleiomyomatosis. Am J Surg Pathol. 2004;28:1007–1016. doi: 10.1097/01.pas.0000126859.70814.6d. [DOI] [PubMed] [Google Scholar]
- 29.Henske EP, McCormack FX. Lymphangioleiomyomatosis—a wolf in sheep’s clothing. J Clin Invest. 2012;122:3807–3816. doi: 10.1172/JCI58709. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Itkin M, McCormack FX. Nonmalignant adult thoracic lymphatic disorders. Clin Chest Med. 2016;37:409–420. doi: 10.1016/j.ccm.2016.04.004. [DOI] [PubMed] [Google Scholar]
- 31.Kwiatkowski DJ. Animal models of lymphangioleiomyomatosis (LAM) and tuberous sclerosis complex (TSC) Lymphat Res Biol. 2010;8:51–57. doi: 10.1089/lrb.2009.0013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Kobayashi T, Minowa O, Kuno J, Mitani H, Hino O, Noda T. Renal carcinogenesis, hepatic hemangiomatosis, and embryonic lethality caused by a germ-line Tsc2 mutation in mice. Cancer Res. 1999;59:1206–1211. [PubMed] [Google Scholar]
- 33.Woo SR, Corrales L, Gajewski TF. Innate immune recognition of cancer. Annu Rev Immunol. 2015;33:445–474. doi: 10.1146/annurev-immunol-032414-112043. [DOI] [PubMed] [Google Scholar]
- 34.Peggs KS, Quezada SA, Korman AJ, Allison JP. Principles and use of anti-CTLA4 antibody in human cancer immunotherapy. Curr Opin Immunol. 2006;18:206–213. doi: 10.1016/j.coi.2006.01.011. [DOI] [PubMed] [Google Scholar]
- 35.Topalian SL, Drake CG, Pardoll DM. Targeting the PD-1/B7-H1(PD-L1) pathway to activate anti-tumor immunity. Curr Opin Immunol. 2012;24:207–212. doi: 10.1016/j.coi.2011.12.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Fankhauser M, Broggi MAS, Potin L, Bordry N, Jeanbart L, Lund AW, et al. Tumor lymphangiogenesis promotes T cell infiltration and potentiates immunotherapy in melanoma. Sci Transl Med. 2017;9:pii: eaal4712. doi: 10.1126/scitranslmed.aal4712. [DOI] [PubMed] [Google Scholar]
- 37.Dilling DF, Gilbert ER, Picken MM, Eby JM, Love RB, Le Poole IC. A current viewpoint of lymphangioleiomyomatosis supporting immunotherapeutic treatment options. Am J Respir Cell Mol Biol. 2012;46:1–5. doi: 10.1165/rcmb.2011-0215TR. [DOI] [PubMed] [Google Scholar]
- 38.Dong H, Strome SE, Salomao DR, Tamura H, Hirano F, Flies DB, et al. Tumor-associated B7-H1 promotes T-cell apoptosis: a potential mechanism of immune evasion. Nat Med. 2002;8:793–800. doi: 10.1038/nm730. [DOI] [PubMed] [Google Scholar]
- 39.Ji RR, Chasalow SD, Wang L, Hamid O, Schmidt H, Cogswell J, et al. An immune-active tumor microenvironment favors clinical response to ipilimumab. Cancer Immunol Immunother. 2012;61:1019–1031. doi: 10.1007/s00262-011-1172-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Tumeh PC, Harview CL, Yearley JH, Shintaku IP, Taylor EJ, Robert L, et al. PD-1 blockade induces responses by inhibiting adaptive immune resistance. Nature. 2014;515:568–571. doi: 10.1038/nature13954. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Harlin H, Meng Y, Peterson AC, Zha Y, Tretiakova M, Slingluff C, et al. Chemokine expression in melanoma metastases associated with CD8+ T-cell recruitment. Cancer Res. 2009;69:3077–3085. doi: 10.1158/0008-5472.CAN-08-2281. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Lin Y, Sharma S, John MS. Ccl21 cancer immunotherapy. Cancers (Basel) 2014;6:1098–1110. doi: 10.3390/cancers6021098. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Yang SC, Hillinger S, Riedl K, Zhang L, Zhu L, Huang M, et al. Intratumoral administration of dendritic cells overexpressing CCL21 generates systemic antitumor responses and confers tumor immunity. Clin Cancer Res. 2004;10:2891–2901. doi: 10.1158/1078-0432.ccr-03-0380. [DOI] [PubMed] [Google Scholar]
- 44.Kirk CJ, Hartigan-O’Connor D, Mulé JJ. The dynamics of the T-cell antitumor response: chemokine-secreting dendritic cells can prime tumor-reactive T cells extranodally. Cancer Res. 2001;61:8794–8802. [PubMed] [Google Scholar]
- 45.Salehi-Rad R, Walser T, Ong S, Park S, Sharma S, Jay L, et al. CCL21 combined with PD-1 blockade cooperatively inhibits tumor growth in KRAS murine model of NSCLC. J Thorac Oncol. 2017;12:S1537. [Google Scholar]
- 46.Parsa AT, Waldron JS, Panner A, Crane CA, Parney IF, Barry JJ, et al. Loss of tumor suppressor PTEN function increases B7-H1 expression and immunoresistance in glioma. Nat Med. 2007;13:84–88. doi: 10.1038/nm1517. [DOI] [PubMed] [Google Scholar]
- 47.Chen J, Zhang XD, Proud C. Dissecting the signaling pathways that mediate cancer in PTEN and LKB1 double-knockout mice. Sci Signal. 2015;8:pe1. doi: 10.1126/scisignal.aac8321. [DOI] [PubMed] [Google Scholar]