Abstract
Objectives
To describe the pharmacokinetics of isoniazid and acetyl-isoniazid in TB/HIV-coinfected patients, and assess the effects of efavirenz co-administration and a 50% increase in the dose of rifampicin on the pharmacokinetic parameters of isoniazid and acetyl-isoniazid.
Methods
TB/HIV-coinfected patients participating in the three-treatment-arm RAFA randomized controlled trial conducted in West Africa were recruited into the pharmacokinetics sub-study. Five serial blood samples were collected on a single visit between 4 and 8 weeks after initiation of antituberculosis treatment. Concentration–time data for isoniazid and acetyl-isoniazid were analysed using non-linear mixed-effects models.
Results
Isoniazid concentrations from 150 patients were available for analysis, and 79 of these (53%) also had concentrations of acetyl-isoniazid. Isoniazid pharmacokinetics was best described with a two-compartment disposition model with lagged first-order absorption and elimination using a semi-mechanistic model describing hepatic extraction. The model identified two elimination pathways, separating formation of acetyl-isoniazid from other routes of metabolism. The predicted AUC0–24 is reduced by 29% in patients who are fast acetylators of isoniazid and receiving efavirenz-based ART (6.73 versus 4.68 mg·h/L). In slow acetylators, efavirenz-based ART had no effect on isoniazid exposure (AUC0–24 = 17.5 mg·h/L).
Conclusions
Efavirenz-based ART affects the acetylation metabolic pathway amongst rapid acetylators, resulting in reduced exposure to isoniazid. Pharmacokinetics of isoniazid and acetyl-isoniazid were not influenced by the 50% increase in rifampicin dose.
Introduction
Isoniazid is part of the multidrug regimen used for the treatment of drug-susceptible TB, which also includes rifampicin, pyrazinamide and ethambutol.1 The currently recommended daily dose of isoniazid is 5 mg/kg (4–6 mg/kg),1 administered with the aim of achieving a Cmax of 3–6 mg/L, which TB patients are expected to tolerate.2 The drug has excellent early bactericidal activity (EBA), mostly against rapidly metabolizing and replicating bacilli.3 In an EBA study, a steady-state AUC of 10.52 mg·h/L achieved 90% of the maximum bactericidal activity.4
The major metabolic pathways for isoniazid include acetylation via N-acetyltransferase 2 (NAT2) to form acetyl-isoniazid and hydrolysis to produce isonicotinic acid.5,6 Polymorphisms in NAT2 confer high interindividual variability in isoniazid exposure and are associated with fast, intermediate and slow metabolizer phenotypes.7 Interindividual variability in isoniazid due to NAT2 polymorphisms may be associated with the emergence of MDR TB, treatment failure or relapse, reduced bactericidal activity, and toxicity.8,9 Variability in isoniazid pharmacokinetics is also attributed to drug–drug interactions, weight, sex, health condition and formulation.10–13 TB/HIV-coinfected patients receiving antituberculosis therapy and ART are at risk of drug–drug interactions, and a study conducted in Mozambique showed a 29% reduction in isoniazid exposure when co-administered with efavirenz-based ART.12 Rifampicin induces a number of drug-metabolizing enzymes and doses higher than the currently recommended 10 mg/kg can potentially affect the pharmacokinetics of co-administered drugs. Therefore, we sought to describe the population pharmacokinetics of isoniazid and acetyl-isoniazid among TB/HIV-coinfected patients recruited in a trial embedded within a routine clinical setting and evaluated the effects of efavirenz-based ART and a 50% increase in dose of rifampicin.
Patients and methods
Study design and patient selection
The RAFA study (PACTR201105000291300) was a three-arm randomized controlled trial designed to assess the effect of timing of ART and high-dose (+50%) rifampicin on patient survival and TB treatment outcomes among TB/HIV-coinfected and treatment-naive patients in Benin, Guinea, and Senegal. Patients were randomized to either a control arm, which was standard of care at the time (standard antitubercular treatment with 10 mg/kg doses of rifampicin and start of ART 8 weeks thereafter), or to early start of ART (2 weeks after initiating antituberculosis treatment), or to receive a high dose of rifampicin in the first 8 weeks of TB treatment (50% dose increase, i.e. 15 mg/kg, and start of ART 8 weeks after initiating antituberculosis treatment). Patients were included in the study if they were aged ≥18 years, had a positive HIV test, had a CD4+ lymphocyte count >50 cells/mm3, were ART-naive, and had a recent TB diagnosis (with bacteriological or molecular evidence). Women were excluded if they were pregnant, lactating or unwilling to use contraception. Other criteria used for exclusion included HIV-2 infection, recreational drug and alcohol abuse that could influence the outcome of the study, and laboratory values outside the normal ranges defined by the NIH, with the exception of patients with up to grade-3 anaemia, who were included.14
Antituberculosis treatment was administered as a four-drug fixed drug combination (FDC) according to WHO weight-band-based guidelines.1 Each tablet contained 75 mg of isoniazid, 150 mg of rifampicin, 400 mg of pyrazinamide and 275 mg of ethambutol. Patients weighing <38 kg, between 38 and 54 kg, between 55 and 70 kg and >70 kg received two, three, four and five tablets, respectively. Patients randomized to high-dose rifampicin received the weight-based number of standard FDCs, plus additional 150 mg tablets of rifampicin only, to attain a dose of 15 mg/kg of body weight. The efavirenz dose was 600 mg regardless of body weight. All patients received co-trimoxazole preventive therapy at the start of TB treatment as recommended by WHO.15
Specimen collection and drug quantification
A subgroup of patients enrolled at clinical sites in Benin and Guinea underwent pharmacokinetic sampling. Between 4 and 8 weeks after antituberculosis treatment initiation, patients were admitted overnight before pharmacokinetic sampling. Five serial blood samples were drawn: pre-dose (∼15 min before a dose) and 2, 3, 6 and 10 h post-dose. Blood samples were processed and plasma was stored immediately at −80°C before transfer on ice to the analytical laboratory (Division of Clinical Pharmacology, University of Cape Town, South Africa), where isoniazid and acetyl-isoniazid were quantified using a validated LC–MS/MS assay developed at the laboratory. Samples were processed with a protein precipitation extraction method using isoniazid-d4 and acetyl-isoniazid-d4 as internal standards, followed by HPLC with MS/MS detection using an AB SCIEX API 3000 instrument. The analyte, metabolite and internal standards were monitored at mass transitions of the protonated precursor ions m/z 138.11, 180.16, 142.21 and 184.21 to the product ions m/z 79.10, 121.10, 83.10 and 83.20 for isoniazid, acetyl-isoniazid, isoniazid-d4 and acetyl-isoniazid-d4, respectively. The calibration curves fitted quadratic (weighted by 1/concentration) regressions over the ranges 0.102–26.0 mg/L for isoniazid and 0.0501–25.6 mg/L for acetyl-isoniazid. The combined accuracy (Nominal) and precision (CV) statistics of the limit of quantification, low medium and high quality control samples (three validation batches, N = 18) for both analyte and metabolite were between 92.2% and 107% and between 2.9% and 10.9%, respectively.
Pharmacokinetic data analysis
Isoniazid and acetyl-isoniazid pharmacokinetics data were interpreted using non-linear mixed-effects modelling in the software NONMEM version 7.3 and the algorithm first-order conditional estimation with η–ε interaction (FOCE-I).16 Perl-Speaks-NONMEM version 4.7.0, Pirana version 2.9.6 and the R package xpose4 version 4.6.0 were used to interact with NONMEM, track model development and evaluate model diagnostics, respectively.17 R version 3.2.3 was used for data manipulation, generating additional plots and post-modelling analysis using RStudio interface version 0.99.903.18,19
Model development was performed in a stepwise manner, starting with the structural pharmacokinetic model for isoniazid and then incorporating acetyl-isoniazid. One- and two-compartment disposition models together with simple first-order elimination or a liver compartment to capture the first-pass effect were explored to describe the pharmacokinetics of isoniazid.20 As individual acetylator genotype was not available, inclusion of a mixture model with two or three sub-populations was investigated to classify patients into different acetylator phenotypes.21 Allometric scaling was applied to all clearance and volume of distribution parameters (exponent fixed to 0.75 for clearance and 1 for volume) for both isoniazid and acetyl-isoniazid to account for the effect of body size using total body weight (TBW), fat-free mass (FFM) or body fat.22 The mixture model on clearance of isoniazid and allometric scaling were included in the early phases of model development, as there is strong evidence supporting their effect on the pharmacokinetics of isoniazid.7,23 Absorption of isoniazid was described using a first-order absorption model, with or without a delay, achieved using a lag time or a chain of transit compartments.24 Clearance of isoniazid was assumed to be either via a single metabolic pathway or two metabolic pathways, with one being responsible for the formation of acetyl-isoniazid. The pharmacokinetics of acetyl-isoniazid was described using either a one- or two-compartment disposition model and first-order elimination from the central compartment. A correction factor was included in the model to adjust for differences in molecular weight at the formation of acetyl-isoniazid (179.18 g/mol for acetyl-isoniazid versus 137.139 g/mol for isoniazid).25
A log-normal distribution was assumed for between-subject variability (BSV) and between-occasion variability (BOV) of the pharmacokinetic parameters. Residual unexplained variability, defined separately for isoniazid and acetyl-isoniazid, comprised both additive and proportional components. Concentration readings below the lower limit of quantification (LLOQ) for both isoniazid and acetyl-isoniazid were provided by the laboratory and included in the analysis. Undetectable concentrations and those <10% of the respective LLOQ value were censored at 10% of the LLOQ, and half the censoring value was imputed, following the implementation of method M6 given by Beal.26 The minimum value of the additive error for both isoniazid and acetyl-isoniazid was fixed to 20% of the respective LLOQ value.
The effect of potential covariates on the pharmacokinetic parameters was investigated in the model, including early initiation of ART, high-dose rifampicin, CLCR (estimated by the Cockcroft–Gault equation27), batch of FDC tablets, ALT and study site. ALT and serum creatinine were measured on the pharmacokinetic sampling day. Covariate effects were retained in the model if physiologically plausible, statistically significant as defined by a drop in objective function value (OFV) of >3.84 for 1 degree of freedom (df), corresponding to a P value of <0.05 for a χ2 distribution, and if they improved goodness-of-fit plots. A non-parametric bootstrap (n = 300, with replacement) was applied to evaluate the robustness of the final parameter estimates and compute 95% CIs. Model-derived individual exposures were extracted from the final model and summarized in different strata of significant covariates, including metabolic status.
Ethics
Ethics approval for the study was provided by the University of Cape Town (reference 153/2011), the London School of Hygiene and Tropical Medicine (reference 5917) and the national ethical committees of Benin (reference 004 31 March 2011) and Guinea (reference 02/CNERS/11). All patients provided written consent before enrolment in the study.
Results
Patient characteristics
Isoniazid concentrations were available in 150 patients, whereas acetyl-isoniazid was only measured in 79 patients. A total of 745 plasma isoniazid concentrations and 390 plasma acetyl-isoniazid concentrations were included in the pharmacokinetic analysis. Concentrations of isoniazid were undetectable (concentrations were less than the sensitivity of the spectrometer) in 12% of the plasma samples, with 83% of these being pre-dose samples, whereas acetyl-isoniazid was detectable in all 390 plasma samples. The median weight, height and FFM in the cohort were 51 kg, 1.70 m and 43.3 kg, respectively (Table 1). Of the 150 patients, 40 (27%) were randomized to the early-ART arm and 70 (47%) to the high-dose rifampicin arm.
Table 1.
Patient characteristics prior to starting antituberculosis treatment
| Characteristic | Value |
|---|---|
| Total number of patients | 150 |
| Females, n (%) | 56 (37.3) |
| Early-ART arm, n (%) | 40 (26.7) |
| High-dose rifampicin arm, n (%) | 70 (46.7) |
| Weight, kg | 51 (33–87) |
| Height, m | 1.7 (1.4–1.94) |
| FFM, kg | 43.3 (24.8–65.3) |
| Age, years | 38 (19–65) |
| CD4+ cells/mm3 | 167 (51–772) |
| Viral load, copies/mL (×103) | 150 (0.08–10000) |
| Serum creatinine, μmol/L | 89 (23–212) |
| Haemoglobin, g/dL | 9.35 (5.2–15.9) |
| ALT, U/L | 26 (3–97) |
| White blood cells, ×109/L | 7 (2.6–26.2) |
| Platelets, ×109/L | 339 (124–832) |
Unless otherwise indicated, median (range) values are presented.
Isoniazid and acetyl-isoniazid pharmacokinetics
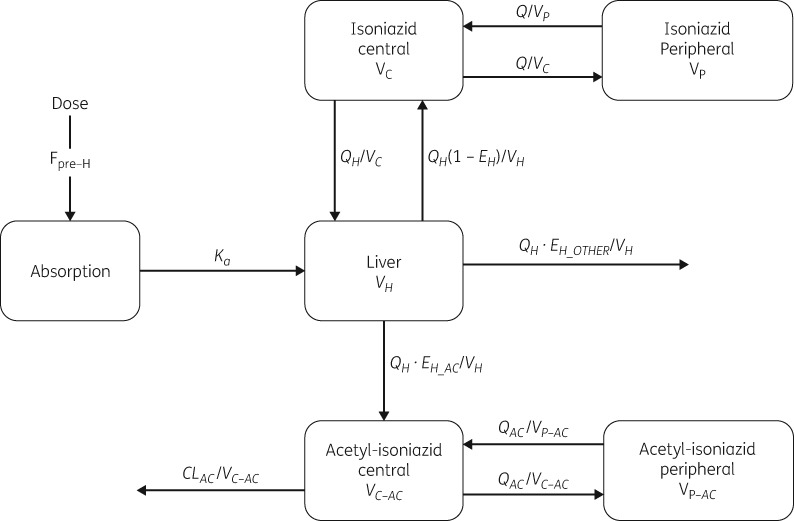
The schematic diagram of the final integrated model for isoniazid and acetyl-isoniazid pharmacokinetics is shown in Figure 1. Isoniazid pharmacokinetics was best described using a two-compartment disposition model. The inclusion of a liver compartment to account for hepatic extraction including a first-pass effect improved the model fit (ΔOFV = −9; no extra parameter was estimated), and so did the inclusion of a lag time (tlag) in absorption (ΔOFV = −22, 1 df, P < 0.001). Our model could separate acetylation, which leads to the formation of acetyl-isoniazid, from other routes of elimination (OFV = −143, 1 df, P < 0.001). The population distribution for isoniazid clearance to acetyl-isoniazid was found to be bimodal, with the proportions of fast and slow acetylators each estimated to be 50%; attempts to characterize trimodality were not supported by the data. The pharmacokinetics of acetyl-isoniazid followed a two-compartment disposition model with first-order elimination.
Figure 1.
Schematic diagram of the model describing the pharmacokinetics of isoniazid and acetyl-isoniazid. Fpre-H, pre-hepatic bioavailability; Ka, absorption rate constant; EH, hepatic extraction; EH_AC, hepatic extraction to acetyl-isoniazid via acetylation; EH_OTHER, hepatic extraction via other routes of metabolism; VC, volume of central compartment for isoniazid; VP, volume of peripheral compartment for isoniazid; VC-AC, volume of central compartment for acetyl-isoniazid; VP-AC, volume of peripheral compartment for acetyl-isoniazid; VH, volume of liver; Q, inter-compartmental clearance for isoniazid; QAC, inter-compartmental clearance for acetyl-isoniazid; QH, hepatic plasma flow; CLAC, clearance for acetyl-isoniazid.
The typical pre-hepatic bioavailability was fixed to a reference value of 1 and stochastic BOV was included (by handling pre- and post-dose samples as separate pharmacokinetic occasions). A subject with a bioavailability of 1.2 means that the subject’s bioavailability is 20% more compared with a typical individual. The typical values for the volume of the liver compartment and hepatic plasma flow were fixed to 1 L and 50 L/h, respectively, and the unbound fraction of isoniazid was fixed to 95%.28,29 Sensitivity analysis was performed on the values chosen for hepatic plasma flow and unbound fraction and they did not affect any of the overall conclusions. Allometric scaling was applied to all clearance and volume parameters including the liver, using FFM, which was superior to total body weight (ΔOFV = −14; no extra parameters estimated).
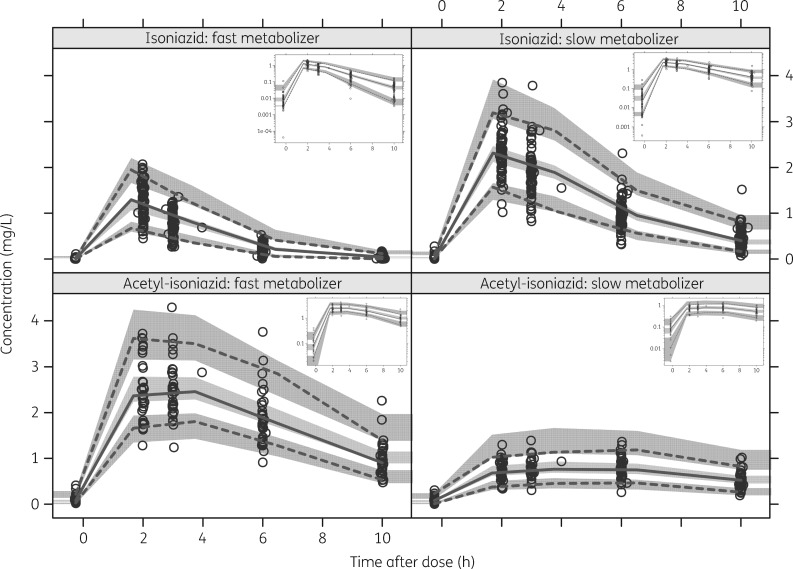
Table 2 shows the parameter estimates from the final model and the associated 95% CIs. The visual predictive check (VPC) displayed in Figure 2 provides evidence that the model describes the observed data adequately. The model estimated that the acetylation intrinsic clearance was on average around 6-fold higher for fast compared with slow metabolizers. This makes acetylation the major route of elimination for fast metabolizers, contributing ∼75% of the total clearance, whereas it represents only ∼30% of the clearance in slow metabolizers. Patients classified as fast acetylators and receiving efavirenz-based ART had 54% higher intrinsic clearance compared with ART-naive patients for the acetylation pathway, which is associated with the formation of acetyl-isoniazid (ΔOFV = −9, 1 df, P < 0.002). No effect of efavirenz-based ART on acetylation clearance of isoniazid was supported in patients classified as slow acetylators [i.e. only a small and non-statistically significant trend was found (P > 0.05; only 5% increase in clearance)] and thus not included in the final model. There was no effect of efavirenz-based ART on the clearance component not associated with acetylation. Inclusion of the effect of high-dose rifampicin on the pharmacokinetics of isoniazid was not significant and did not improve the model fit.
Table 2.
Parameter estimates of the final model
| Parameter | Typical value (95% CI)a | BSV/BOV as %CVb (95% CI)a |
|---|---|---|
| Isoniazid | ||
| clearance (L/h) | ||
| acetylation: fastc,d | 35.9 (30.1–45.1) | 35.6 (28.6–41.8) |
| acetylation: slowc,d | 6.64 (5.55–8.39) | |
| other pathwaysd | 11.4 (9.11–13.3) | 34.6 (17.7–45.4) |
| central volume (L)d | 47.7 (40.8–56.3) | 18.9 (8.29–27.7) |
| inter-compartmental clearance (L/h)d | 4.80 (0.730–11.5) | – |
| peripheral volume (L)d | 8.14 (5.06–247) | – |
| absorption constant (/h) | 1.59 (1.36–2.09) | 36.7 (22.9–54.3) |
| absorption lag (h) | 0.287 (0.202–0.590) | |
| pre-hepatic bioavailabilitye | 1 fixed | 16.9 (12.4–22.8)f |
| proportion of fast acetylators (%) | 50.1 (40.5–59.0) | – |
| efavirenz effect on acetylation clearance in fast acetylators (%) | +54.1 (23.2–97.8) | – |
| drug batch effect on bioavailability (%) | −62.1 (−66.7 to −57.0) | – |
| proportional error (%) | 11.2 (9.55–12.6) | – |
| additive error (mg/L) | 0.02 fixed | – |
| Acetyl-isoniazid | ||
| clearance (L/h)d | 6.77 (5.78–7.87) | 17.5 (13.9–21.3) |
| central volume (L)d | 34.4 (30.5–40.1) | – |
| inter-compartmental clearance (L/h)d | 0.506 (0.323–1.42) | – |
| peripheral volume (L)d | 81.4 (19.8–991) | – |
| proportional error (%) | 5.25 (3.9–6.49) | – |
| additive error (mg/L) | 0.013 (0.01–0.019) | – |
Obtained with a non-parametric bootstrap (n = 300).
Variability is assumed to be log-normally distributed and is reported as approximate %CV.
Intrinsic CL of isoniazid when given without efavirenz.
All CL and volume parameters have been allometrically scaled with FFM, and the typical values reported here refer to the typical patient, with an FFM of 43.3 kg.
Pre-hepatic bioavailability is the fraction of the drug that is absorbed, and crosses the gut wall unchanged, thus entering the portal vein and reaching the liver.
BOV.
Figure 2.
VPCs for isoniazid and acetyl-isoniazid stratified by metabolic status. The lines represent the 2.5th, 50th and 97.5th percentiles of the observed concentrations (circles). The shaded regions are the 95% prediction intervals for the 2.5th, 50th and 97.5th percentiles. The sub-plot in each stratum shows the same VPC with a logarithmic transformation applied to the y-axis.
Additionally, our model detected 62.1% reduced bioavailability in all 28 patients treated with two of the FDC batches (ΔOFV = −106, 1 df, P < 0.001). Of these patients, 70% were in the high-dose rifampicin arm, while 11% and 18% were in the early-ART and control arms, respectively. The proportion of patients receiving tablets from these two batches did not differ by mixture-assigned acetylator status (20% and 18% among slow and fast acetylators, respectively). BSV in clearance attributed to acetylation was slightly higher compared with other routes of metabolism. A large improvement in model fit (ΔOFV = −37, 1 df, P < 0.001) was observed when each route of elimination was allowed to have its own BSV parameter.
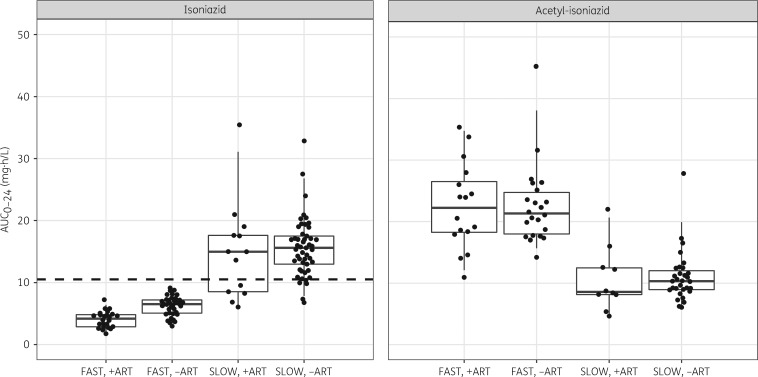
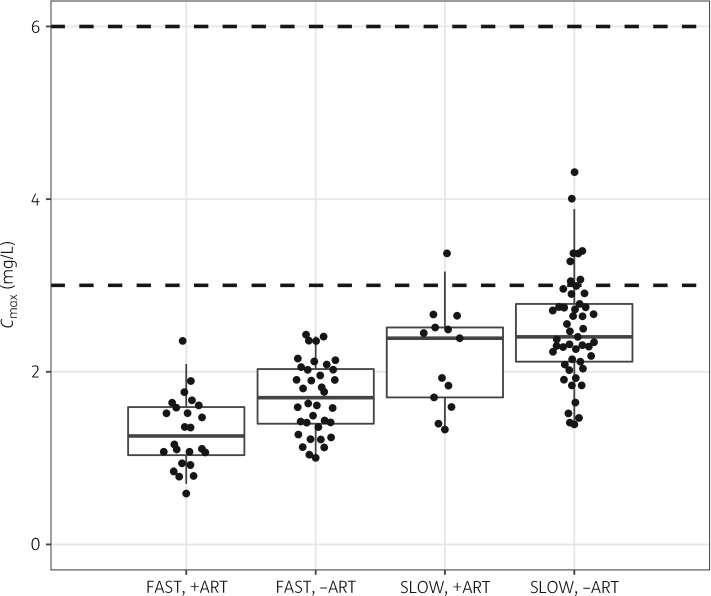
Table 3 shows that the predicted AUC0–24 for a fast acetylator on efavirenz-based ART is 73% lower than a slow acetylator after adjusting for other effects in the model, including the dose. When compared with a fast acetylator not on efavirenz-based ART, the AUC0–24 is reduced by 29% (6.73 versus 4.68 mg·h/L). The post hoc steady-state individual estimates of AUC0–24 and Cmax from the final model are summarized in Table 4 and displayed in Figures 3 and 4, except for the 28 patients receiving the reduced-bioavailability FDC batches, which were excluded. None of the patients classified as fast acetylators achieved the AUC0–24 of 10.52 mg·h/L previously associated with EBA, whereas >75% of slow acetylators achieved this exposure level (Table 4 and Figure 3). Figure 4 depicts the model-derived Cmax on the pharmacokinetic sampling day (equivalent to steady state). Regardless of treatment arm, all patients classified as fast acetylators had Cmax below the range of 3–6 mg/L and >85% of the slow acetylators had Cmax below the range.
Table 3.
Estimates of oral clearance and AUC at steady state for isoniazid and acetyl-isoniazid for slow and fast acetylators when given with or without efavirenz-based ARTa
| Scenario | Intrinsic CL of isoniazid (L/h) | Hepatic extraction of isoniazid (EH) (%) | Oral CL of isoniazid (L/h) | Change in isoniazid oral CL (%) | Isoniazid AUC (mg·h/L)b | Acetyl-isoniazid AUC (mg·h/L)c |
|---|---|---|---|---|---|---|
| Slow acetylator | 18.0 | 26 | 17.1 | reference | 17.5 | 5.44 |
| Fast acetylator not on efavirenz-based ART | 47.3 | 47 | 44.9 | +162 | 6.68 | 20.8 |
| Fast acetylator on efavirenz-based ART | 66.7 | 56 | 63.4 | +270 | 4.73 | 26.8 |
The estimates reported here refer to an individual with an FFM of 43.3 kg (the median in our cohort). These are calculated using population parameter estimates from the fixed effects component of the final model.
AUC for a dose of 300 mg.
Table 4.
Summary of AUC0–24 and Cmax for isoniazid and acetyl-isoniazid stratified by treatment arm [receiving efavirenz (EFV)-based ART versus no ART] and NAT2 metabolizer statusa
| Arm and status | AUC0–24, mg·h/L (90% range) | Cmax, mg/L (90% range) |
|---|---|---|
| Isoniazid (N = 122) | ||
| fast acetylator on EFV-based ART | 4.18 (2.38–5.81) | 1.26 (0.788–1.87) |
| fast acetylator not on EFV-based ART | 6.52 (3.64–8.73) | 1.70 (1.10–2.37) |
| slow acetylator on EFV-based ART | 15.0 (6.53–26.8) | 2.39 (1.37–2.95) |
| slow acetylator not on EFV-based ART | 15.6 (9.88–22.8) | 2.41 (1.49–3.39) |
| Acetyl-isoniazid (N = 79) | ||
| fast acetylator on EFV-based ART | 22.2 (13.3–34.2) | 1.83 (2.87–3.81) |
| fast acetylator not on EFV-based ART | 21.3 (17.0–31.4) | 1.64 (2.29–3.32) |
| slow acetylator on EFV-based ART | 8.59 (4.98–19.3) | 0.489 (0.741–1.24) |
| slow acetylator not on EFV-based ART | 10.3 (6.57–16.9) | 0.61 (0.822–1.36) |
Individual AUC0–24 and Cmax were calculated using the post hoc individual parameter estimates (including fixed effects and BSV and BOV parameters) from the final model. The values are provided for comparison with other studies, but they are not used for statistical inference since they are dependent on the model and affected by statistical shrinkage.
Figure 3.
Boxplots of AUC0–24 for isoniazid and acetyl-isoniazid stratified by treatment arm (receiving efavirenz-based ART versus no ART) and NAT2 metabolizer status. The dots are model-derived individual exposures (steady-state AUC0–24). The dashed line represents the exposure (AUC0–24 = 10.52 mg·h/L) associated with 90% early bactericidal activity of isoniazid.4
Figure 4.
Boxplot of Cmax for isoniazid stratified by treatment arm (receiving efavirenz-based ART versus no ART) and NAT2 acetylator status (fast or slow). The dashed lines indicate the expected minimum and maximum Cmax concentrations for isoniazid.
Discussion
This study described the pharmacokinetics of isoniazid and acetyl-isoniazid in a cohort of TB/HIV-coinfected patients. Using population pharmacokinetic modelling, we showed that co-administration of isoniazid and efavirenz resulted in markedly reduced exposure to isoniazid amongst fast acetylators. The model predicts that for a typical individual the AUC0–24 is decreased by 29% when isoniazid is co-administered with efavirenz-based ART in fast acetylators. Results from a study conducted in Mozambique also showed a 29% reduction in isoniazid exposures in TB/HIV-infected patients.12 However, no categorization into fast and slow acetylators was performed. In our study, we were able to show that the effect of efavirenz is specific for the acetylation metabolic pathway and thus it affects the fast acetylators more. Efavirenz induces a number of enzymes in the cytochrome P450 family,30 but to the best of our knowledge nothing has been reported on the effect of efavirenz on the activity of NAT2. Our results and the results from the study conducted in Mozambique suggest that efavirenz may modulate the activity of NAT2. The effect of efavirenz-based ART on the acetylation pathway also results in higher concentrations of acetyl-isoniazid. Isoniazid-induced hepatotoxicity is related to hydrolysis of isoniazid and acetyl-isoniazid by amidases to form toxic hydrazine and acetyl-hydrazine, respectively.31 Therefore, depending on the affinity of the specific isoform of the amidase for either isoniazid or acetyl-isoniazid, hepatotoxicity patterns in TB patients receiving efavirenz-based antiretroviral therapy may be altered.
The model-predicted 6-fold higher acetylation intrinsic clearance for fast compared with slow acetylators observed in our study is within the range previously reported.32,33 Slow acetylators lack functional NAT2 enzyme,34 and our results show that the clearance reported in other studies for slow acetylators is driven largely by metabolic pathways other than acetylation. Overlapping of the exposure ranges between slow and fast acetylators is not unexpected, since multiple different slow-acetylator genotypes may lead to high pharmacokinetic variability.35–37
Adequacy of exposure to isoniazid on the pharmacokinetic sampling day is evaluated against expected AUC0–24 or Cmax. The Cmax range of 3–6 mg/L was suggested based on concentrations observed in healthy volunteers recruited under controlled Phase I studies and in TB patients.2 However, the Cmax observed in TB/HIV-coinfected patients could be lower than in healthy volunteers and TB patients. Moreover, although Cmax remains a popular target because of its immediacy and ease of interpretation, it suffers from the limitation of being extremely variable even within the same patient on different occasions due to the erratic nature of drug absorption processes.38 Steady-state AUC0–24 is a much more stable exposure index and a value >10.52 mg·h/L has been associated with 90% of the maximum EBA of isoniazid.4 All patients classified as fast acetylators had AUC0–24 <10.52 mg·h/L, and this reflects the extent of metabolism due to NAT2 activity in fast compared with slow acetylators and its overall effect on exposure. This results in widely varying exposure levels between fast and slow acetylators, thereby contributing to differences in treatment outcomes and toxicity. The proportion of patients at risk of drug-induced liver injury was reported to be 78% with the currently recommended dosing (5 mg/kg) compared with 0% in the NAT2-adjusted dose group (2.5 mg/kg for slow acetylators and 7.5 mg/kg for fast acetylators). Among fast acetylators, early treatment failure in the standard dose arm was double that in the NAT2-adjusted dose arm.39 Reduced isoniazid exposure could lead to an inadequate intensive and continuation phase treatment regimen, with patients being effectively only on rifampicin monotherapy during the continuation phase. This might increase the risk of emergence of drug resistance. The effects of reduced exposure to isoniazid due to NAT2 activity could be minimized if point-of-care phenotypic tests are developed to assist dosing in patients with drug-susceptible TB.
The result that a 50% increase in rifampicin dose does not influence the pharmacokinetics of isoniazid is in line with reports by Boeree et al.,40 who investigated the effect of higher doses of rifampicin of up to 35 mg/kg (3.5-fold increase) on pharmacokinetics of the other first-line TB drugs and did not find any difference in their AUC between standard- and higher-dose rifampicin arms. Based on these results, we can conclude that increased doses of rifampicin are unlikely to affect isoniazid exposure.
While a single FDC product was used throughout the study, we found reduced bioavailability in two of the drug batches. This finding underlines the importance of continuous quality control checks on batch-to-batch variability, as well as maintenance of appropriate storage conditions. This result also reflects the challenges associated with conducting clinical trials in routine clinical settings and, most importantly, it provides essential information for monitoring of drug quality in TB treatment programmes. An analysis of the drug content in a sample of tablets from the two batches with reduced bioavailability might have helped to assess the quality of the tablets, but unfortunately there were no remaining tablets from the implicated batches when this effect was detected.
Differences in body size and composition included in our model via allometric scaling using FFM (calculated using weight, height and sex) also explain some variability in the pharmacokinetics of isoniazid in addition to acetylator status.41 This combined effect of weight, height and sex could explain the reported disparity in exposure between males and females,11 since females usually have a larger proportion of body fat, which does not contribute to the size of other body organs such as the liver.42 Recent population pharmacokinetics models for the first-line antituberculosis drugs have shown that FFM is a better size descriptor for scaling clearance and volume compared with total body weight.23,43 We postulate that the implementation of evidence-based dosing by weight band, accounting for the effects of FFM on volume and clearance, would reduce the variability in drug exposure usually observed among TB patients, and this will translate into favourable treatment outcomes and reduced toxicity.
A limitation of our study is that no genotyping has been performed to identify the acetylator status of each patient; however, this will be done in a future study using DNA samples from some patients. Without genetic information, it is difficult to separate homozygous fast-acetylator and heterozygous fast-acetylator status.44 However, the proportion of fast acetylators (50%) in our model is not different from those that have been reported for African populations.32,45
In conclusion, we developed a population pharmacokinetic model for isoniazid and acetyl-isoniazid in a population of TB/HIV-coinfected patients. The model could separate clearance due to acetylation and other routes of metabolism and predicted a 29% reduction in AUC0–24 of isoniazid for fast acetylators receiving efavirenz-based ART compared with no ART. A 50% increase in the dose of rifampicin did not influence the pharmacokinetics of isoniazid and acetyl-isoniazid.
Contributor Information
the RAFA team:
Alimatou N’Diaye, Ibrahima Mariétou Mbaye, Bouke De Jong, Severin Anagonou, Salim Diatema, Ibrahima Khalil Gomina, Severin Gossa, Blanche Tanimomo, Wilfried Bekou, Tatiana Galperine, Andre Furco, Mouctar Diallo, Boubacar Bah, Falilou Bah, Néné Barry, Abdourahmane Barry, Sadjo Barry, Mamadou Telly Barry, Aissatou Bah Sylla, Alpha Mamadou Barry, Marie Sarr, Ndéye Fatou Ngom, Kine Ndiaye, Diama Sakho, Justine Ngom, Fatoumata Ba, Amadou Seck, Andre Furco, Sian Floyd, Keith Branson, Judith Glynn, Dany Phillips, Nadia Oubaya, and Caroline Saint-Martin
Acknowledgements
We wish to thank the participating patients and the members of the RAFA study team. The Division of Clinical Pharmacology at the University of Cape Town gratefully acknowledges Novartis Pharma for support of the development of pharmacometric skills in Africa.
Members of the RAFA study team
Alimatou N’Diaye (project manager); Ibrahima Mariétou Mbaye (clinical monitor); Bouke De Jong (Institute of Tropical Medicine, Belgium); Severin Anagonou, Salim Diatema, Ibrahima Khalil Gomina, Severin Gossa, Blanche Tanimomo, Wilfried Bekou (Programme national de lute anti-TB, Benin); Tatiana Galperine (Hopital Tenon, France); Andre Furco (University College London, UK); Mouctar Diallo, Boubacar Bah, Falilou Bah, Néné Barry, Abdourahmane Barry, Sadjo Barry, Mamadou Telly Barry, Aissatou Bah Sylla, Alpha Mamadou Barry (Service de Pneumo-Phtisiologie, Hopital Ignace Deen, Guinea); Marie Sarr, Ndéye Fatou Ngom, Kine Ndiaye, Diama Sakho, Justine Ngom, Fatoumata Ba, Amadou Seck (Programme national de lute anti-TB, Senegal); Andre Furco (University College London, UK); Sian Floyd, Keith Branson, Judith Glynn, Dany Phillips, Nadia Oubaya, Caroline Saint-Martin (London School of Hygiene and Tropical Medicine, UK).
Funding
The RAFA clinical trial and its PK component were sponsored by the European & Developing Countries Clinical Trials Partnership (PACTR201105000291300). H. M. is supported by the Wellcome Trust (206379/Z/17/Z). M. T. C. is supported by the European & Developing Countries Clinical Trials Partnership (PACTR201105000291300). M. T. C. and H. M. are funded in part by the National Research Foundation of South Africa (grants 95106 and 90729). The drug assays were supported in part by the National Institute of Allergy and Infectious Diseases of the National Institutes of Health (UM1AI068634, UM1 AI068636 and UM1AI106701, U01 AI068632), the Eunice Kennedy Shriver National Institute of Child Health and Human Development, and the National Institute of Mental Health (AI068632).
Transparency declarations
None to declare.
Author contributions
M. T. C. developed the population pharmacokinetic model and prepared the first draft of the manuscript. H. M. was a principal investigator for the project and supervised M. T. C. on modelling and preparation of the manuscript. L. W. coordinated the processing of plasma samples, prepared the laboratory methods section on the manuscript and reviewed the manuscript. D. A. coordinated the project at the Benin site and approved the final version of the manuscript. O. B-S. coordinated the project at the Guinea site and approved the final version of the manuscript. C. M. was a Principal Investigator, coordinated the whole project, reviewed the manuscript and approved the final version. P. D. supervised M. T. C. on modelling and preparation of the manuscript, reviewed and approved final version of the manuscript.
Disclaimer
The authors alone are responsible for the views expressed in this publication and they do not necessarily represent the decisions, policies or views of WHO. The content of this article is solely the responsibility of the authors and does not necessarily represent the official views of their governments.
References
- 1. World Health Organization. Treatment of Tuberculosis: Guidelines 2010. http://www.who.int/tb/publications/2010/9789241547833/en/. [PubMed]
- 2. Alsultan A, Peloquin CA.. Therapeutic drug monitoring in the treatment of tuberculosis: an update. Drugs 2014; 74: 839–54. [DOI] [PubMed] [Google Scholar]
- 3. Donald PR, Sirgel FA, Botha FJ. et al. The early bactericidal activity of isoniazid related to its dose size in pulmonary tuberculosis. Am J Respir Crit Care Med 1997; 156: 895–900. [DOI] [PubMed] [Google Scholar]
- 4. Donald PR, Parkin DP, Seifart HI. et al. The influence of dose and N-acetyltransferase-2 (NAT2) genotype and phenotype on the pharmacokinetics and pharmacodynamics of isoniazid. Eur J Clin Pharmacol 2007; 63: 633–9. [DOI] [PubMed] [Google Scholar]
- 5. Ellard GA, Gammon PT.. Pharmacokinetics of isoniazid metabolism in man. J Pharmacokinet Biopharm 1976; 4: 83–113. [DOI] [PubMed] [Google Scholar]
- 6. Ellard GA. Variations between individuals and populations in the acetylation of isoniazid and its significance for the treatment of pulmonary tuberculosis. Clin Pharmacol Ther 1976; 19: 610–25. [DOI] [PubMed] [Google Scholar]
- 7. Parkin DP, Vandenplas S, Botha FJ. et al. Trimodality of isoniazid elimination: phenotype and genotype in patients with tuberculosis. Am J Respir Crit Care Med 1997; 155: 1717–22. [DOI] [PubMed] [Google Scholar]
- 8. Donald PR, Sirgel FA, Venter A. et al. The influence of human N-acetyltransferase genotype on the early bactericidal activity of isoniazid. Clin Infect Dis 2004; 39: 1425–30. [DOI] [PubMed] [Google Scholar]
- 9. Pasipanodya JG, Srivastava S, Gumbo T.. Meta-analysis of clinical studies supports the pharmacokinetic variability hypothesis for acquired drug resistance and failure of antituberculosis therapy. Clin Infect Dis 2012; 55: 169–77. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Wilkins JJ, Langdon G, McIlleron H. et al. Variability in the population pharmacokinetics of isoniazid in South African tuberculosis patients. Br J Clin Pharmacol 2011; 72: 51–62. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. McIlleron H, Rustomjee R, Vahedi M. et al. Reduced antituberculosis drug concentrations in HIV-infected patients who are men or have low weight: implications for international dosing guidelines. Antimicrob Agents Chemother 2012; 56: 3232–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Bhatt NB, Bar C, Amin A. et al. Pharmacokinetics of rifampin and isoniazid in tuberculosis-HIV-coinfected patients receiving nevirapine- or efavirenz-based antiretroviral treatment. Antimicrob Agents Chemother 2014; 58: 3182–90. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Babalik A, Ulus IH, Bakirci N. et al. Plasma concentrations of isoniazid and rifampin are decreased in adult pulmonary tuberculosis patients with diabetes mellitus. Antimicrob Agents Chemother 2013; 57: 5740–2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. National Institute of Allergy and Infectious Diseases. 2007. Division of Microbiology and Infectious Diseases Adult Toxicity Table. https://www.niaid.nih.gov/sites/default/files/dmidadulttox.pdf. [Google Scholar]
- 15. World Health Organization. Antiretroviral Therapy for HIV Infection in Adults and Adolescents: Recommendations for a Public Health Approach: 2010 Revision.http://www.who.int/hiv/pub/arv/adult2010/en/. [PubMed]
- 16. Beal S, Sheiner L, Boeckmann A. et al. NONMEM Users’ Guides (1989–2013). Ellicott City, MD, USA: ICON Development Solutions, 2013. [Google Scholar]
- 17. Keizer RJ, Karlsson MO, Hooker A.. Modeling and simulation workbench for NONMEM: tutorial on Pirana, PsN, and Xpose. CPT Pharmacometrics Syst Pharmacol 2013; 2: e50.. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. R Core Team. R: A Language and Environment for Statistical Computing. Vienna: R Foundation for Statistical Computing, 2017. http://www.r-project.org/. [Google Scholar]
- 19. RStudio Team. RStudio: Integrated Development Environment for R. Boston, MA, USA: RStudio, Inc, 2015. http://www.rstudio.org/. [Google Scholar]
- 20. Gordi T, Xie R, Huong NV. et al. A semiphysiological pharmacokinetic model for artemisinin in healthy subjects incorporating autoinduction of metabolism and saturable first-pass hepatic extraction. Br J Clin Pharmacol 2005; 59: 189–98. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. Frame B. Mixture modeling with NONMEM V In: Ette EI, Williams PJ, eds. Pharmacometrics: The Science of Quantitative Pharmacology. Hoboken, NJ, USA: John Wiley & Sons, Inc, 2007; 723–57. [Google Scholar]
- 22. Anderson BJ, Holford NHG.. Mechanism-based concepts of size and maturity in pharmacokinetics. Annu Rev Pharmacol Toxicol 2008; 59: 303–32. [DOI] [PubMed] [Google Scholar]
- 23. Denti P, Jeremiah K, Chigutsa E. et al. Pharmacokinetics of isoniazid, pyrazinamide, and ethambutol in newly diagnosed pulmonary TB patients in Tanzania. PLoS One 2015; 10: e0141002.. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24. Savic R, Jonker D, Kerbusch T. et al. Implementation of a transit compartment model for describing drug absorption in pharmacokinetic studies. J Pharmacokinet Pharmacodyn 2007; 34: 711–26. [DOI] [PubMed] [Google Scholar]
- 25. Zannikos P, Argenti D.. Analysis of urine excretion data In: Bonate PL, Howard D, eds. Pharmacokinetics in Drug Development: Clinical Study Design and Analysis, Vol. 1 Arlington, VA, USA: AAPS Press, 2004; 267–89. [Google Scholar]
- 26. Beal SL. Ways to fit a PK model with some data below the quantification limit. J Pharmacokinet Pharmacodyn 2001; 28: 481–504. [DOI] [PubMed] [Google Scholar]
- 27. Cockcroft DW, Gault H.. Prediction of creatinine clearance from serum creatinine. Nephron 1976; 16: 31–41. [DOI] [PubMed] [Google Scholar]
- 28. Clemmesen JO, Tygstrup N, Ott P.. Hepatic plasma flow estimated according to Fick’s principle in patients with hepatic encephalopathy: evaluation of indocyanine green and d-sorbitol as test substances. Hepatology 1998; 27: 666–73. [DOI] [PubMed] [Google Scholar]
- 29. Sturkenboom MGG, van der Lijke H, Jongedijk EM. et al. Quantification of isoniazid, pyrazinamide and ethambutol in serum using liquid chromatography-tandem mass spectrometry. J Appl Bioanal 2015; 1: 89–98. [Google Scholar]
- 30. Adkins JC, Noble S.. Efavirenz. Drugs 1998; 56: 1055–64. [DOI] [PubMed] [Google Scholar]
- 31. Wang P, Pradhan K, Zhong X-B. et al. Isoniazid metabolism and hepatotoxicity. Acta Pharm Sin B 2016; 6: 384–92. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32. Ellard GA. The potential clinical significance of the isoniazid acetylator phenotype in the treatment of pulmonary tuberculosis. Tubercle 1984; 65: 211–27. [DOI] [PubMed] [Google Scholar]
- 33. Seng KY, Hee KH, Soon GH. et al. Population pharmacokinetic analysis of isoniazid, acetyl-isoniazid, and isonicotinic acid in healthy volunteers. Antimicrob Agents Chemother 2015; 59: 6791–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34. Hein DW, Doll MA, Fretland AJ. et al. Molecular genetics and epidemiology of the NAT1 and NAT2 acetylation polymorphisms. Cancer Epidemiol Biomarkers Prev 2000; 9: 29–42. [PubMed] [Google Scholar]
- 35. Fretland AJ, Leff MA, Doll MA. et al. Functional characterization of human N-acetyltransferase 2 (NAT2) single nucleotide polymorphisms. Pharmacogenetics 2001; 11: 207–15. [DOI] [PubMed] [Google Scholar]
- 36. Leff MA, Fretland AJ, Doll MA. et al. Novel human N-acetyltransferase 2 alleles that differ in mechanism for slow acetylator phenotype. J Biol Chem 1999; 274: 34519–22. [DOI] [PubMed] [Google Scholar]
- 37. Chen B, Li JH, Xu YM. et al. The influence of NAT2 genotypes on the plasma concentration of isoniazid and acetyl-isoniazid in Chinese pulmonary tuberculosis patients. Clin Chim Acta 2006; 365: 104–8. [DOI] [PubMed] [Google Scholar]
- 38. Devaleenal DB, Ramachandran G, Swaminathan S.. The challenges of pharmacokinetic variability of first-line anti-TB drugs. Expert Rev Clin Pharmacol 2017; 10: 47–58. [DOI] [PubMed] [Google Scholar]
- 39. Azuma J, Ohno M, Kubota R. et al. NAT2 genotype guided regimen reduces isoniazid-induced liver injury and early treatment failure in the 6-month four-drug standard treatment of tuberculosis: a randomized controlled trial for pharmacogenetics-based therapy. Eur J Clin Pharmacol 2013; 69: 1091–101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40. Boeree MJ, Heinrich N, Aarnoutse R. et al. High-dose rifampicin, moxifloxacin, and SQ109 for treating tuberculosis: a multi-arm, multi-stage randomised controlled trial. Lancet Infect Dis 2017; 17: 39–49. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41. Anderson GD. Gender differences in pharmacological response. Int Rev Neurobiol 2008; 83: 1–10. [DOI] [PubMed] [Google Scholar]
- 42. Green B, Duffull SB.. What is the best size descriptor to use for pharmacokinetic studies in the obese? Br J Clin Pharmacol 2004; 58: 119–33. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43. Rockwood N, Meintjes G, Chirehwa M. et al. HIV-1 coinfection does not reduce exposure to rifampin, isoniazid, and pyrazinamide in South African tuberculosis outpatients. Antimicrob Agents Chemother 2016; 60: 6050–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44. Hickman D, Sim E.. N-acetyltransferase polymorphism: comparison of phenotype and genotype in humans. Biochem Pharmacol 1991; 42: 1007–14. [DOI] [PubMed] [Google Scholar]
- 45. Roy PD, Majumder M, Roy B.. Pharmacogenomics of anti-TB drugs-related hepatotoxicity. Pharmacogenomics 2008; 9: 311–21. [DOI] [PubMed] [Google Scholar]