Abstract
Approximately one-third of women experience hysterectomy, or the surgical removal of the uterus, by 60 years of age, with most surgeries occurring prior to the onset of natural menopause. The ovaries are retained in about half of these surgeries, whereas for the other half hysterectomy occurs concurrently with oophorectomy. The dogma is that the nonpregnant uterus is dormant. There have been no preclinical assessments of surgical variations in menopause, including hysterectomy, with and without ovarian conservation, on potential endocrine and cognitive changes. We present a novel rat model of hysterectomy alongside sham, ovariectomy (Ovx), and Ovx-hysterectomy groups to assess effects of surgical menopause variations. Rats without ovaries learned the working memory domain of a complex cognitive task faster than did those with ovaries. Moreover, uterus removal alone had a unique detrimental impact on the ability to handle a high-demand working memory load. The addition of Ovx, that is, Ovx-hysterectomy, prevented this hysterectomy-induced memory deficit. Performance did not differ amongst groups in reference memory–only tasks, suggesting that the working memory domain is particularly sensitive to variations in surgical menopause. Following uterus removal, ovarian histology and estrous cycle monitoring demonstrated that ovaries continued to function, and serum assays indicated altered ovarian hormone and gonadotropin profiles by 2 months after surgery. These results underscore the critical need to further study the contribution of the uterus to the female phenotype, including effects of hysterectomy with and without ovarian conservation, on the trajectory of brain and endocrine aging to decipher the impact of common variations in gynecological surgery in women. Moreover, findings demonstrate that the nonpregnant uterus is not dormant, and indicate that there is an ovarian-uterus-brain system that becomes interrupted when the reproductive tract has been disrupted, leading to alterations in brain functioning.
One of the most common gynecological surgical interventions in women, second only to cesarean section, is hysterectomy, or the surgical removal of the uterus (1, 2). Approximately half of the women who undergo hysterectomy retain their ovaries. This approach is taken to alleviate adverse symptoms associated with benign uterine conditions prompting hysterectomy while maintaining the ovarian tissue, thus preventing an abrupt menopause onset and other deleterious health effects when the woman is of reproductive age at the time of surgery (3). Despite the number of hysterectomy surgeries declining in recent years, it is estimated that currently, by 60 years of age, one-third of women have undergone hysterectomy, with most women undergoing hysterectomy before 50 years of age (4). Thus, most hysterectomies are performed before the average onset of natural menopause, which typically occurs between ages 51 and 52 (5, 6). Some research in premenopausal women suggests that hysterectomy disrupts normal ovarian function and initiates ovarian failure earlier than transitionally menopausal women who maintain their uterus, potentially due to localized ovarian blood flow disruption (7–11), whereas others report little to no change in ovarian function following hysterectomy (12–14). Of note, the measures used to operationally define normal ovarian function vs ovarian failure are not always consistent. Within the primary operational definition of menopause—amenorrhea for 12 consecutive months (5, 6)—women who undergo hysterectomy with ovarian conservation could be considered menopausal in clinical practice despite continued ovarian function following surgery. Thus, determining the onset of the menopause transition in women who have undergone hysterectomy can be challenging, and it may be defined as the onset of menopausal symptoms (e.g., hot flashes) rather than menstrual irregularity [for discussion, see Chalmers et al. (12)], both of which may not occur simultaneously, or may not occur in a sequential fashion where one precedes the other, in reproductively intact women (15). The timing and etiology of menopause may prove to be critical to aging outcomes, as well as for determining an optimal time point for initiating individualized hormone therapy intervention to obtain the most favorable quality of life outcomes.
Premenopausal and postmenopausal circulating reproductive hormone profiles are significantly different from one another. There is abundant evidence that endogenous ovarian hormones, particularly estrogens, have neuroprotective properties as well as beneficial effects on the health and functioning of several body systems, including cardiovascular, skin, bone, and urogenital systems (5). Disruptions to the reproductive hormone feedback loop via gynecological surgery likely have differential effects on the function of these systems depending on age and menopause status at the time of surgery, as well as whether the ovaries are retained. Both human and preclinical animal research indicate that an abrupt loss of circulating ovarian hormones from oophorectomy [the surgical removal of the ovaries; in nonhuman animals, termed ovariectomy (Ovx)] prior to natural reproductive senescence can be detrimental to many aspects of health, including cognition (16–25). Animal model studies of surgical menopause via Ovx confirm a significant decrease in serum ovarian steroid hormones and an increase in gonadotropin levels following surgical ovary removal (20, 26). When ovaries are conserved during hysterectomy, they have the potential to function normally following hysterectomy when the surgery occurs during the premenopausal years. Although there has been limited study of ovarian structure and function following hysterectomy in women, some reports indicate that hysterectomy with ovarian conservation does not result in similar drastic changes in circulating ovarian hormones and gonadotropins as occurs with oophorectomy, because the steroid-producing ovaries remain intact (12, 27). Nonetheless, whether ovarian structure and function are altered following hysterectomy, particularly when the surgery occurs during reproductive years, has been understudied. There is evidence that transient posthysterectomy steroid hormone changes can occur. Vuorento et al. (28) assessed daily salivary progesterone levels preoperatively and postoperatively in premenopausal women who underwent hysterectomy with ovarian conservation, and they reported that 39% of the women had decreased progesterone levels during the luteal phase in at least one menstrual cycle within 6 months of the surgery. Of these women, most irregular serum progesterone levels occurred 1 month after hysterectomy; most of these women returned to preoperative progesterone levels by 6 months after surgery, suggesting that alterations in ovulatory function and steroidogenesis following hysterectomy could be transient (28). Other clinical studies have reported no changes in circulating ovarian hormone levels up to 21 years after hysterectomy when the ovaries were conserved (14, 29), or variable estrogen levels up to 10 years after hysterectomy, which result in inconclusive evidence for altered hormone profiles following the surgical removal of the uterus alone (7). Collectively, there remains a lack of comprehensive, longitudinal assessments in the clinical literature to conclusively determine the short-term or long-term impact of hysterectomy on ovarian structure and function.
The uterus and ovaries are part of an intricately connected system including the hypothalamus, pituitary, and female reproductive tract. During the past few decades, it has become clear that this system, as well as the hormone milieu it produces, influences domains reaching far beyond reproduction alone with actions on many body systems, including the brain [for review, see Souza et al. (30) and Turgeon et al. (31)]. For example, research in animal models shows that experimental manipulation to the endogenous ovarian hormone milieu impacts cognition (32–36). Although the steroid-producing ovaries have been the main focus of these and most related basic science investigations, the uterus is also tightly linked to the hypothalamic–pituitary–ovarian axis loop and the potentially wide-reaching effects of this system. Indeed, despite the long-held dogma that the nonpregnant uterus is a dormant and “useless organ” (37), there is evidence that the uterus contains gonadotropin and steroid hormone receptors (38–40), as well as direct sensory and autonomic innervation from the central and peripheral nervous systems (41–43), even in a nonpregnant state. Thus, it is biologically plausible, and in fact likely, that hysterectomy itself, with or without ovarian conservation, sufficiently alters the hypothalamic–pituitary–female reproductive tract system and, as a result, plays a role in altering the brain and cognition. Along these lines, several recent retrospective clinical studies have reported an increased relative risk of early-onset dementia for women who underwent hysterectomy compared with women with no history of hysterectomy, particularly when the surgery occurred prior to menopause onset (17, 18, 44). In contrast, another group reported a small decrease in the relative risk for developing Alzheimer’s disease associated with oophorectomy, hysterectomy, and oophorectomy with hysterectomy; notably, most women in this study were >51 years of age at the time of surgery, and they were therefore likely postmenopausal (45), with the studies collectively suggesting that menopause status at the time of gynecological surgery could impact cognitive outcomes. To elucidate the impact of variations in surgical menopause on the brain and cognition during aging, we must acknowledge and appreciate the complexity of this multifaceted system and include the examination of the influence of uterine tissues into experimental models.
Few studies have assessed hysterectomy in a preclinical animal model. A handful of endocrine studies using hysterectomy in a rodent model demonstrated that hysterectomy with ovarian conservation resulted in unique endocrine changes, such as increased ovarian aromatase activity, elevated FSH levels, and potentially accelerated ovarian failure via follicle atresia (46–49). Hysterectomy provides a novel model for investigating variations in surgical menopause in the context of studying cognition. Specifically, the additional factor of the presence or absence of uterine tissue provides an ancillary perspective to Ovx menopause models by more closely modeling the surgical procedures that occur most often in clinical practice. Indeed, it is not common practice to remove only the ovaries in women, as does the classic Ovx model used in basic science research.
The cognitive effects of hysterectomy with and without ovarian conservation have not yet been fully explored in a systematic experimental context. Given the large number of women who undergo variations in gynecological surgeries, it is essential to understand how ovarian morphology and function may be altered following hysterectomy, as well as to elucidate how hysterectomy with and without ovarian preservation relates to the trajectory of brain aging and cognitive decline. For a truly translational approach, it is crucial to establish and methodically assess a preclinical rodent model of the most common surgical practices performed in women to obtain a complete understanding of how these surgical manipulations affect endocrine, as well as cognitive and brain, aging. Additionally, using a rodent model allows for controlled manipulation of the type of surgical procedures and the age at which they occur, which is one of the primary complexities in interpreting outcomes when evaluating these phenomena in humans. The rodent reproductive system is well-defined and is in many ways functionally similar to that of humans; as such, the rat provides an excellent preclinical model to investigate the impact of hysterectomy on the brain and cognition. Of note, the uterine body of the rat bifurcates into two uterine horns, which are collectively referred to as the uterus hereafter. We herein aimed to methodically investigate how hysterectomy in adulthood (i.e., prior to the onset of reproductive senescence), with and without ovarian conservation, impacts cognition using a rat model. Utilizing this novel surgical model of hysterectomy, we evaluated spatial learning and memory performance in adult rats that received variations in surgical menopause, including Ovx, hysterectomy, Ovx plus hysterectomy, and sham operations (Fig. 1). Our systematic experimental design also allowed for simultaneous evaluation of endocrine and ovarian profiles known to change following variations in gynecological surgery, including putative biomarkers of menopause. Specifically, we assessed serum hormone levels of ovarian-derived hormones and the gonadotropins and ovarian follicle morphology, as well as monitored estrous cyclicity and body weight changes, to gain a comprehensive understanding of the far-reaching impact that variations in gynecological surgeries have on the body’s reproductive anatomy, physiology, and function, and how these factors ultimately may lead to cognitive changes.
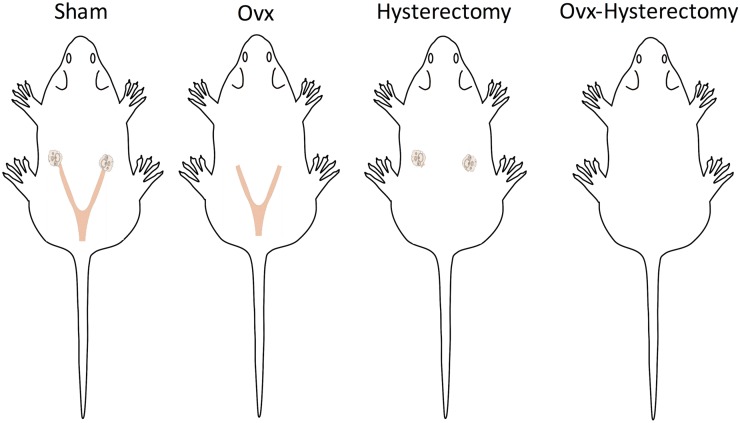
Figure 1.
Diagram of the surgical manipulations performed. Sham-treated rats had an intact uterus and ovaries, Ovx-treated rats had an intact uterus, hysterectomy-treated rats had intact ovaries, and Ovx-hysterectomy–treated rats had both the uterus and ovaries removed.
Methods
Subjects
Sixty virgin, reproductively intact female Fischer-344 (CDF) rats were obtained from the National Institute on Aging colony at Charles Rivers Laboratories (Raleigh, NC). Subjects arrived at the animal facility at 5 months of age, were provided food and water ad libitum, and were maintained on a 12-hour light/12-hour dark cycle for the duration of the study. Rats were given 1 week to acclimate to the vivarium prior to beginning the experiment. All procedures in this experiment were approved by the Arizona State University Institutional Animal Care and Use Committee and adhered to National Institutes of Health standards.
Surgical procedures
Rats were randomly assigned to one of four treatment groups: sham, Ovx, hysterectomy, or Ovx plus hysterectomy (Ovx-hysterectomy). Surgeries were performed 1 week after arrival. All rats were anesthetized with inhaled isoflurane and received 1.0 mg/kg meloxicam (nonsteroidal anti-inflammatory drug), 1.2 mg/kg buprenorphine SR-LAB, and 5.0 mL of an isotonic solution used to ensure postsurgical hydration. All subjects received a ventral midline incision through the skin and peritoneum. The sham surgery group received skin and peritoneum incisions only. In the Ovx group, both ovaries were exposed, ligated along with the oviducts and tips of the uterine horns, and excised. In the hysterectomy group, each uterine horn was ligated and cut below the ovary and oviduct. The uterus was then separated from the adjacent fat, and the uterocervical junction was ligated and cut above the cervix, at the base of the uterine body. In the Ovx-hysterectomy group, the ovaries and uterus were separated from the internal fat, and the uterocervical junction was ligated and cut above the cervix. All groups’ muscle incisions were sutured with dissolvable Vicryl suture, and bupivacaine (Marcaine; Pfizer Pharmaceutical, Hospira Inc., Lake Forest, IL) was applied to the muscle incision prior to skin closure for all subjects. The skin incision was closed with surgical staples. Rats were allowed to recover under a heat lamp and were single-housed for 7 days following the surgery. Two rats died after surgery. As a result, one hysterectomy rat and one Ovx rat were single-housed; the remaining subjects were pair-housed for the entirety of the study. The final numbers per group used in the statistical analyses are as follows (unless otherwise noted): sham, n = 15; hysterectomy, n = 14; Ovx, n = 14; and Ovx-hysterectomy, n = 15.
Weights and vaginal cytology
Baseline weights were recorded at surgery, and weekly weights were recorded until behavior testing began. Two weeks after surgery, daily vaginal smears were evaluated for 8 consecutive days to monitor estrous cyclicity following surgery. Smears were classified according to Goldman et al. (50) and Mennenga and Bimonte-Nelson (51) as proestrus, estrus, metestrus, or diestrus phases. Proestrus was characterized by the presence of round epithelial cells and some cornified cells. Estrus was classified by the presence of primarily cornified cells. Metestrus included the presence of cornified cells, round cells, needle-like cells, and leukocytes. Diestrus was characterized by leukocytes with or without cornified cells.
Behavior
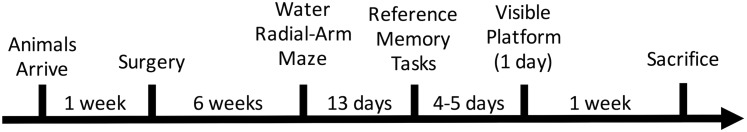
Figure 2 illustrates the timeline for the experiment.
Figure 2.
Study timeline. Rats were tested on a battery of spatial working and RM tasks 6 wk following surgical manipulations. One wk following the last behavioral assay, subjects were euthanized.
Water radial-arm maze
Behavior testing began 6 weeks after surgery. The water radial-arm maze (WRAM) is a win-shift, eight-arm water escape task that tests spatial working and reference memory (RM) in rodents (19, 52–57). The maze had eight evenly spaced arms radiating out from the circular center (each arm was 38.1 × 12.7 cm). Salient spatial cues were located around the room to assist in spatial navigation. Room temperature water (maintained at 18°C to 20°C) was made opaque with black nontoxic powdered paint. Platforms were hidden in the ends of four of the eight arms, submerged just beneath the water surface. Each subject was assigned a unique set of platform locations, which remained constant for that animal across all days of testing. Platform combinations varied between subjects and treatment groups. Each subject had four trials per day. At the beginning of each trial, the rat was placed in the platform-free start arm of the maze and had 3 minutes to locate a platform. Once the rat climbed onto a platform, the rat remained on it for 15 seconds to localize to its spatial location before being returned to a heated testing cage for a 30-second intertrial interval (ITI). During the ITI, the experimenter removed the just-located platform from the WRAM. Once found, platforms were not replaced within a daily testing session. The experimenter then removed any debris and stirred the water to distribute olfactory cues prior to the start of the next trial. If the rat did not find the platform within the allotted 3-minute trial time, the experimenter guided the rat to the nearest platform. Rats received four trials per day for 12 days. On day 13, a 6-hour delay was implemented between trials two and three to evaluate delayed memory retention. Errors, defined as an entry into an arm that did not contain a platform, were recorded for each trial; an arm entry was defined as the rat’s snout passing a line 11 cm into the arm (only visible on the outside of the maze, but not to the rat). Three error types were quantified. Working memory correct (WMC) errors were entries into an arm on trials two through four that previously contained a platform within a day. RM errors were first entries into an arm that never contained a platform. Working memory incorrect (WMI) errors were repeated entries within a day into an arm that never contained a platform.
Morris water maze
After 1 day of rest following the WRAM delay day, a subset of subjects began testing on the Morris water maze (MM), a task that assesses spatial RM. The apparatus was a large round tub (188 cm in diameter) filled with water maintained at 18°C to 20°C and made opaque with nontoxic black powdered paint. One platform (11 cm in diameter) was hidden just below the surface of the water in the northeast quadrant of the maze. The location of the platform remained constant across all days and trials. Salient spatial cues were present around the room to aid in spatial navigation to the platform (58). Rats received four trials per day for 5 consecutive days. At the beginning of each trial, rats were dropped off from one of four starting points (north, south, east, or west). The order in which the rats were exposed to the drop-off points changed across days, but it was the same for all rats within a day. Rats were given a maximum trial time of 60 seconds to locate the platform before the experimenter terminated the trial and led the rat to the platform. Once the platform was found, the rat remained on it for 15 seconds to allow for spatial localization before the experimenter returned the rat to its heated testing cage for an ITI of ∼8 to 10 minutes. On the last day of the MM, a probe trial was given. Following the four regular trials, rats were administered a fifth trial in which the hidden platform was completely removed from the maze, and rats swam freely for the standard allotted trial time of 60 seconds. The probe trial was implemented to evaluate whether the rats had spatially localized to the platform by quantifying the percentage of total swim distance in the target quadrant vs the opposite quadrant. A video camera and tracking system (Ethovision; Noldus Instruments, Wageningen, Netherlands) were used to measure each rat’s swim path (distance in centimeters) across all days and trials, including the probe trial.
Delayed match-to-sample win-stay task
The remaining subset of rats was tested on a delayed match-to-sample (DMS) win-stay task that evaluated spatial RM. The WRAM apparatus was used for this task, but rats were tested in different rooms and with novel spatial cues compared with the WRAM task. One platform was placed in an arm of the WRAM, where it remained across all days and trials. Rats received six trials per day for 4 days. For each trial, subjects were dropped off from one of the six different start arm locations (excluding the platform-containing arm and the arm directly across from the platform). The order in which the drop-off locations occurred varied across days, but it stayed the same within a day for all rats. There was no consistent pattern of left or right turns from the start arm to the platform within or across days. Once the rat found the platform, it was allowed to remain on it and spatially localize for 15 seconds before being returned to its heated testing cage for a 30-second ITI. There was a 90-second maximum trial time; if the rat did not find the platform within the allotted time, the experimenter led the rat to the platform. An arm entry was quantified when the snout of the rat passed an 11-cm mark denoted on each arm of the maze that was visible to the experimenter, but not to the rat. The dependent variable for this task was the total number of errors committed on each trial. Total errors were defined as any entry into a nonplatformed arm within a trial prior to locating the platform.
Visible platform
Following the last day of the RM tasks, all subjects were tested on the visible platform (VP) task to confirm that rats could perform the visual and motoric components of water maze tasks. The apparatus was a rectangular tub (100 × 60 cm) filled with clear water (18°C to 20°C). A black platform (10 cm in diameter) was placed in the tub and protruded about 4 cm above the water’s surface. Opaque curtains were hung in a circular fashion around the room to block potential spatial or geometric cues. All rats received six trials in 1 day. For each trial, the rat was dropped off from a fixed start location. The VP escape location varied semirandomly in three possible locations across trials. The maximum trial time was 90 seconds, and once the rat located the VP, it was allowed to remain on it for 15 seconds before being returned to its heated testing cage for an ITI of 8 to 10 minutes. Latency (in seconds) from drop off to the platform was recorded for each trial.
Euthanization
Rats were euthanized 1 week after the completion of the behavioral battery. Subjects were ∼7 months old at euthanization. Rats were deeply anesthetized with inhaled isoflurane. Blood was collected via cardiocentesis and allowed to clot at 4°C (Vacutainer 367986; Becton Dickinson and Company, Franklin Lakes, NJ). Blood samples were then centrifuged at 2000 rpm at 4°C for 20 minutes. Serum was collected and stored at −20°C until analysis. Ovaries from the sham and hysterectomy groups were collected, trimmed of excess fat, and fixed in 10% buffered formalin for 48 hours before being transferred to 70% ethanol until analysis. Uteri were collected from the sham and Ovx groups, trimmed of excess fat, and wet weight was obtained.
Serum hormone assays
A double antibody liquid-phase RIA (Beckman Coulter, Brea, CA) was used to determine 17β-estradiol levels. This RIA used estradiol-specific antibodies with an [125I]-labeled estradiol as the tracer. Interassay coefficients of variation for this assay averaged 10% at a mean value of 28 pg/mL. Functional sensitivity for this assay was 5 pg/mL. Androstenedione was assessed using an ELISA assay (ALPCO, Salem, NH), based on the typical competitive binding scenario between unlabeled antigen (present in standards, controls, and unknowns) and the enzyme-labeled antigen (conjugate) for a limited number of antibody binding sites on the microwell plate. Interassay coefficients of variation for this assay averaged 9% at a mean value of 0.5 ng/mL. Functional sensitivity of the assay was 0.1 ng/mL. Progesterone levels were determined using an ELISA assay (ALPCO, Salem, NH). Interassay coefficients of variation for the progesterone assay averaged 13% at a mean value of 2.6 ng/mL. Functional sensitivity of this assay was 0.3 ng/mL.
A sensitive two-site sandwich immunoassay was used to measure serum LH (59, 60) using monoclonal antibodies against bovine LH (no. 581B7; RRID: AB_2665514) (61) and against the human LH β subunit (no. 5303: Medix, Kauniainen, Finland; RRID: AB_2665513) (62), as previously described (60). The tracer antibody (no. 518B7) was provided by Dr. Janet Roser (Department of Animal Science, University of California, Davis) (61, 63), iodinated by the chloramine T method, and purified on Sephadex G-50 columns. The capture antibody (no. 5303) (62) was biotinylated and immobilized on avidin-coated polystyrene beads (7 mm; Epitope Diagnostics, San Diego, CA). The standard was a mouse LH reference prep (AFP5306A; provided by Dr. A. F. Parlow and the National Hormone and Peptide Program). The assay had a sensitivity of 0.04 ng/mL.
RIA was used to assay serum FSH levels utilizing reagents provided by Dr. A. F. Parlow and the National Hormone and Peptide Program, as described previously (64). Mouse FSH reference preparation AFP5308D was used for assay standards. The primary antibody was a mouse FSH antiserum (guinea pig; AFP-1760191; RRID: AB_2665512) (65) diluted to a final concentration of 1:400,000. The secondary antibody was purchased from Equitech-Bio and diluted to a final concentration of 1:25. The assay had a sensitivity of 2.0 ng/mL and <0.5% cross-reactivity with other pituitary hormones.
A commercially available ELISA kit (AL-113; ANSH Laboratories, Webster, TX) was used to determine anti-Müllerian hormone (AMH) levels. The sensitivity of this assay was 0.011 ng/mL, and the reportable range spanned from 3.36 to 215.0 ng/mL.
Ovarian follicle counts
One ovary from each sham and hysterectomy subject (i.e., all rats that had ovaries at the end of the experiment) was randomly selected for histological evaluation and quantification of healthy primordial, primary, secondary, and antral follicles, as well as corpora lutea. Following fixation, ovarian tissues were paraffin embedded and sectioned at 5 μm. Every 10th section was mounted on a slide and stained with hematoxylin and eosin Y/phloxine B. Primordial, primary, secondary, and antral follicles were counted at ×20 magnification for every 20th section. Corpora lutea were counted at a lower magnification (Pannoramic DESK; 3DHistech, Budapest, Hungary). The following formula was used to calculate the total number of follicles in the ovary: Nt = (N0 × St × ts)/(S0 × d0), where Nt represents the total calculated number of follicles, N0 indicates the number of follicles observed in the ovary, St represents the total number of sections in the ovary, ts indicates the thickness of the section (µm), S0 indicates the total number of sections observed, and d0 represents the mean diameter of the nucleus (66). Ovarian follicles were classified using criteria from Haas et al. (67). Primordial cells were counted when there was a single layer of squamous granulosa cells surrounding the oocyte. Primary follicles contained a single layer of cuboidal granulosa cells. Secondary follicles required several granulosa cell layers surrounding the oocyte. Antral follicles were counted when the follicle contained two or more layers of granulosa cells and had a fluid-filled antral space in the follicle (67).
Statistical analyses
Data analyses were completed using StatView software. The independent variable for all analyses was surgery type (sham, Ovx, hysterectomy, and Ovx-hysterectomy). Repeated measures ANOVAs were used for WRAM data, with errors as the dependent variable, and trials within days as the repeated measure. Separate repeated measures analyses were completed for each error type (WMC, WMI, and RM errors). WRAM data were blocked into three 4-day blocks, as we have previously published (68). Block 1 (days 1 to 4) was the early acquisition phase, block 2 (days 5 to 8) was the late acquisition phase, and block 3 (days 9 to 12) was the asymptotic phase. For MM data, swim distance to platform (cm) across repeated days and trials was evaluated. In the DMS win-stay task, total errors were evaluated, repeated across days and trials. VP data were evaluated for latency to platform (seconds), repeated across trials. Serum hormone, ovarian follicle, body weight, and uterine weight data were analyzed using ANOVA, with surgery as the independent variable and serum hormone concentration, follicle estimate, body weight, and uterine weight as the dependent variables, respectively. All analyses were two-tailed with an α level set to 0.05; results were deemed marginal when the P value fell between 0.05 and 0.10. Effect size for repeated measures ANOVA analyses were reported as generalized η2 (ηG2) (69, 70). For all other ANOVAs (nonrepeated measures), effect sizes were reported as η2. Effect sizes were interpreted using Cohen general guidelines for η2, where 0.02 is a small effect, 0.13 is a medium effect, and 0.26 is a large effect (70, 71). All effect sizes for post hoc tests were reported using Cohen d and are interpreted by Cohen standard guidelines, where 0.2 indicates a small effect, 0.5 indicates a medium effect, and 0.8 indicates a large effect (71).
Results
Vaginal cytology
Vaginal smears were performed for 8 consecutive days, beginning 2 weeks after surgery. Rats that received Ovx or Ovx-hysterectomy showed blank or diestrus-like smears, indicating a lack of estrogen stimulation of the vaginal epithelium and therefore successful surgical removal of the ovaries. Sham and hysterectomy groups showed normal 4- to 5-day estrous cycles, suggesting continued ovarian function and ovulatory patterns following surgery (Fig. 3).
Figure 3.
Representative images from consecutive days of vaginal cytology monitoring. Rats in the sham and hysterectomy groups (i.e., rats with intact ovaries) showed normal 4- to 5-d estrous cycling when examined 2 wk after surgery. Ovx and Ovx-hysterectomy rats (i.e., rats that experienced surgical removal of the ovaries) showed mostly blank vaginal smear cytology 2 wk after surgery, indicating successful removal of the ovaries.
WRAM
Early acquisition phase (days 1 to 4)
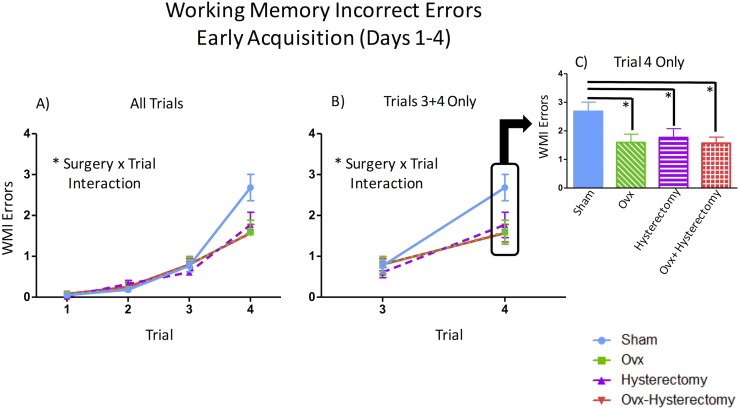
The early acquisition phase best captures initial task-rule acquisition and learning, as well as its associated exploratory behavior in the WRAM. During the early acquisition phase, a main effect of surgery [F(3,54) = 2.90, P < 0.05, ηG2 = 0.02] was observed for WMC errors. Fisher protected least significant difference (PLSD) indicated that Ovx rats made fewer WMC errors than did sham rats (P < 0.01, d = 0.33), and Ovx-hysterectomy rats tended to make fewer WMC errors than did sham rats (P = 0.06, d = 0.20) (Fig. 4A). For this block of testing, there was also a surgery × trial interaction [F(6,108) = 2.84, P < 0.01, ηG2 = 0.03]. Given that previous studies in our laboratory have shown age-, hormone-, and menopause-mediated differences in memory when working memory load is taxed (19, 26, 54–56, 68, 72–74), we further probed this effect and assessed WMC errors for trials 3 and 4 only, which are the high working memory load trials. There was a main effect of surgery [F(3,54) = 2.84, P < 0.05, ηG2 = 0.03] such that sham rats made more errors than did Ovx rats (P < 0.01, d = 0.42) and Ovx-hysterectomy rats (P < 0.05, d = 0.28) on the high working memory load trials (Fig. 4B). There was also a surgery × trial interaction for WMC errors on high working memory load trials [F(3,54) = 3.15, P < 0.05, ηG2 = 0.03] (Fig. 4C). Thus, we further probed this interaction. There were no differences among groups on trial 3. However, there was a main effect of surgery on trial 4, the maximum working memory load trial [F(3,54) = 3.76, P < 0.05, ηG2 = 0.07], with the post hoc analysis again indicating the same effect wherein sham rats made more errors than did Ovx rats (P < 0.01, d = 0.70) and Ovx-hysterectomy rats (P < 0.05, d = 0.45) (Fig. 4D). For WMI errors, there was a surgery × trial interaction [F(9,162) = 3.10, P < 0.01, ηG2 = 0.03] (Fig. 5A). Upon probing the high working memory load trials (trials 3 and 4), a marginal main effect of surgery [F(3,54) = 2.51, P = 0.07, ηG2 = 0.02] and a significant surgery × trial interaction [F(3,54) = 3.02, P < 0.05, ηG2 = 0.02] (Fig. 5B) were revealed. Post hoc analyses indicated that sham rats made more errors than did the Ovx (P < 0.05, d = 0.26), hysterectomy (P < 0.05, d = 0.27), and Ovx-hysterectomy (P < 0.05, d = 0.30) groups. Because there was a significant surgery × trial interaction for the high working memory load trials, we further investigated these effects. There were no differences among groups during trial 3, but there was a main effect of surgery during trial 4 [F(3,54) = 3.45, P < 0.05, ηG2 = 0.05], with sham-treated rats making more errors than did the Ovx (P < 0.01, d = 0.47), hysterectomy (P < 0.05, d = 0.38), and Ovx-hysterectomy (P < 0.01, d = 0.54) groups (Fig. 5C). There were no main effects or interactions with surgery for RM errors during the early acquisition phase.
Figure 4.
WMC errors during the early acquisition phase of the WRAM (days 1 to 4). (A) There was a main effect of surgery across all days and trials within the early acquisition block. (B) There was a main effect of surgery across days 1 to 4 on the high working memory load trials (trials 3 and 4). (C) There was a surgery × trial interaction on high working memory load trials (trials 3 and 4), with (D) a main effect of surgery evident on trial 4 alone across days 1 to 4. *P < 0.05; ^P < 0.10.
Figure 5.
WMI errors during the early acquisition phase of the WRAM (days 1 to 4). (A) A surgery × trial interaction was present across days 1 to 4 for WMI errors. (B) There was a surgery × trial interaction on the high working memory load trials (trials 3 and 4), with (C) a main effect of surgery on trial 4 alone. *P < 0.05.
Late acquisition phase (days 5 to 8)
The late acquisition phase captures midtask learning when rats tend to decrease in errors compared with the early acquisition phase, but they continue to exhibit variable performance as they learn to handle the working memory load associated with the WRAM task. There were no main effects or interactions with surgery during the late acquisition phase. However, there was a marginal main effect of day for WMC errors [F(3,162) = 2.30, P = 0.08, ηG2 = 0.01] and a main effect of day for WMI [F(3,162) = 5.66, P < 0.001, ηG2 = 0.02] and RM errors [F(3,162) = 3.25, P < 0.05, ηG2 = 0.01] such that errors decreased across days, suggesting learning of the task.
Asymptotic phase (days 9 to 12)
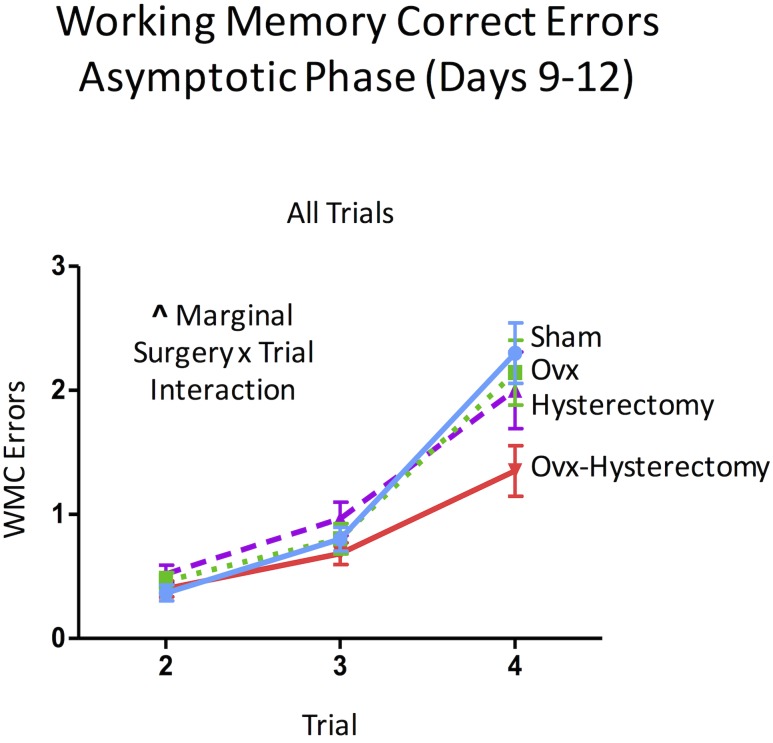
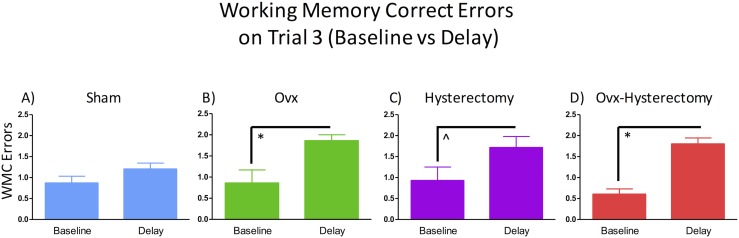
The asymptotic phase of the WRAM is most crucial to understanding spatial working memory performance, as this phase occurs when the rats have learned the rules of this win-shift task and are approaching peak memory performance, where errors are decreased compared with earlier phases and remain at a consistent level of performance within the block of days, here days 9 to 12. For WMC errors in the asymptotic phase, there was a marginal surgery × trial interaction [F(6,108) = 1.93, P = 0.08, ηG2 = 0.02] (Fig. 6). For WMI errors, there was a main effect of surgery [F(3,54) = 3.06, P < 0.05, ηG2 = 0.03]. Fisher PLSD indicated that the hysterectomy group made significantly more WMI errors than did the sham (P < 0.05, d = 0.27), Ovx (P < 0.05, d = 0.28), and Ovx-hysterectomy (P < 0.01, d = 0.40) groups (Fig. 7A). There was also a marginal surgery × trial interaction for WMI errors [F(9,162) = 1.75, P = 0.08, ηG2 = 0.02] (Fig. 7B). When only high working memory load trials (trials 3 and 4) were probed, a main effect of surgery [F(3,54) = 2.93, P < 0.05, ηG2 = 0.05] was again shown. Fisher PLSD showed that the hysterectomy group made significantly more WMI errors than did the sham group (P < 0.05, d = 0.37), such that hysterectomy alone (i.e., with ovarian conservation) impaired memory compared with reproductively unaltered rats. Rats that had only their uterus removed made more WMI errors than any group of rats that had their ovaries removed, whether or not that ovarian removal was concomitant with uterus removal, as indicated by the hysterectomy group making more errors than the Ovx group (P < 0.05, d = 0.39) and the Ovx-hysterectomy group (P < 0.01, d = 0.56) when working memory load was taxed in the asymptotic phase of WRAM (Fig. 7C). Notably, once the ovaries were removed, the additional removal of the uterus had no further impact on cognitive effects (Fisher PLSD Ovx vs Ovx-hysterectomy: P = 0.64). There was no interaction between surgery and trial for this analysis; thus, effects were not further probed. These findings suggest that, 6 weeks after surgery, the surgical removal of the uterus alone, conserving the ovaries, impairs memory when working memory load is taxed in the latter half of testing. Additionally, this detrimental impact of hysterectomy with ovarian conservation appears to be prevented when Ovx accompanies the hysterectomy. There were no main effects or interactions with surgery for RM errors during the asymptotic phase, indicating that the spatial memory deficit observed for the hysterectomy group is likely specific to working memory.
Figure 6.
WMC errors during the asymptotic phase of the WRAM (days 9 to 12). A marginal surgery × trial interaction was present for WMC errors. ^P < 0.10.
Figure 7.
WMI errors during the asymptotic phase of the WRAM (days 9 to 12). (A) A main effect of surgery was evident across all days and trials within the asymptotic phase block. (B) A marginal surgery × trial interaction was found across all days and trials in the asymptotic phase. (C) A main effect of surgery was shown for the high working memory load trials (trials 3 and 4) for the asymptotic phase block. *P < 0.05; ^P < 0.10.
Six-hour delay
On day 13 of WRAM testing, a 6-hour delay was given between trials 2 and 3 to test delayed memory retention. Each treatment group’s performance was assessed separately using a repeated measures ANOVA to evaluate group performance on trial 3 of the last day of baseline testing (day 12) compared with trial 3 following the 6-hour delay on day 13. Sham-treated rats did not show impaired performance between baseline and delay testing, suggesting that there was not a delay-induced impairment (Fig. 8A). There was a main effect of delay day for WMC errors on trial 3 for Ovx rats [F(1,13) = 7.00, P < 0.05, ηG2 = 0.35] (Fig. 8B). For hysterectomy-treated rats, there was a marginal main effect of delay day for WMC errors on trial 3 [F(1,13) = 3.96, P = 0.07, ηG2 = 0.23], such that WMC errors somewhat increased as a result of a 6-hour memory retention delay (Fig. 8C). There was also a main effect of delay day for WMC errors on trial 3 for Ovx-hysterectomy rats [F(1,14) = 47.25, P < 0.0001, ηG2 = 0.77] (Fig. 8D). Thus, both groups without ovaries showed significantly impaired delayed memory retention following a delay in WRAM trials, an effect our laboratory has previously reported (75, 76).
Figure 8.
WRAM delay data. (A–D) WMC errors of each surgery group on trial 3 of the last day of baseline testing (day 12) compared with the number of WMC errors committed on trial 3 of the delay day (first post-delay trial on day 13). *P < 0.05; ^P < 0.10.
RM tasks
A subset of rats (n = 5 per group) underwent training in the spatial RM MM task. All rats learned across days [F(4,64) = 33.48, P < 0.0001, ηG2 = 0.38], with no differences in learning among surgery types. Furthermore, there was a main effect of quadrant on the probe trial [F(1,16) = 129.08, P < 0.0001, ηG2 = 0.87] and no interaction with surgery type, suggesting that all rats successfully spatially localized to the target (previously platformed) quadrant.
All other subjects were trained on a win-stay version of the DMS spatial RM task. Again, there was a main effect of day [F(3,102) = 69.25, P < 0.0001, ηG2 = 0.16] and no interaction with surgery type, such that all rats learned to navigate to the platform and decreased in the total number of errors committed across the 4 days of the task. Given that no differences in RM performance were found on either of these RM-only tasks, nor the RM measure of the WRAM, findings support the tenet that the memory impairment induced by hysterectomy is specific to the working memory domain.
Visible platform
There was a main effect of trial [F(5,270) = 5.53, P < 0.0001, ηG2 = 0.07] that did not interact with surgery type, such that all rats decreased escape latency to the VP across six trials. The average escape latency on trial 1 was 13.55 seconds, and averaged 6.91 seconds on trial 6. This control task verifies that all subjects could perform the procedural components of water maze tasks, including visual and motoric capacities.
Body weights
Two weeks after surgery, there was a main effect of surgery on body weight [F(3,54) = 11.92, P < 0.0001, η2 = 0.40]. Fisher post hoc analyses revealed that Ovx-treated rats weighed more than sham rats (P < 0.0001, d = 1.88) and hysterectomy rats (P < 0.0001, d = 1.61); Ovx-hysterectomy rats also weighed more than sham rats (P < 0.0001 d = 1.48) and hysterectomy rats (P < 0.0001, d = 1.25). This indicates that surgical removal of the ovaries increased body weight compared to rats that retained their ovaries. This difference in body weight between subjects with and without their ovaries continued to diverge across week 3 [F(3,54) = 21.37, P < 0.0001, η2 = 0.54], week 4 [F(3,54) = 37.35, P < 0.0001, η2 = 0.68], week 5 [F(3,54) = 44.02, P < 0.0001, η2 = 0.71], and through the euthanization time point [F(3,54) = 43.77, P < 0.0001, η2 = 0.71]. At euthanization, Ovx and Ovx-hysterectomy rats weighed more than sham rats (Ovx vs sham: P < 0.0001, d = 3.62; Ovx-hysterectomy vs sham: P < 0.0001, d = 2.47). Ovx and Ovx-hysterectomy rats also weighed more than hysterectomy rats (Ovx vs hysterectomy: P < 0.0001, d = 3.82; Ovx-hysterectomy vs hysterectomy: P < 0.0001, d = 2.61) (Fig. 9).
Figure 9.
Weekly body weights were collected beginning on the date of surgery (baseline) and continued until euthanization. Body weights began to significantly diverge between rats with ovaries (i.e., sham and hysterectomy groups) vs rats without intact ovaries (i.e., Ovx and Ovx-hysterectomy groups) beginning 2 wk after surgical manipulation. *P < 0.05.
Uterine weights
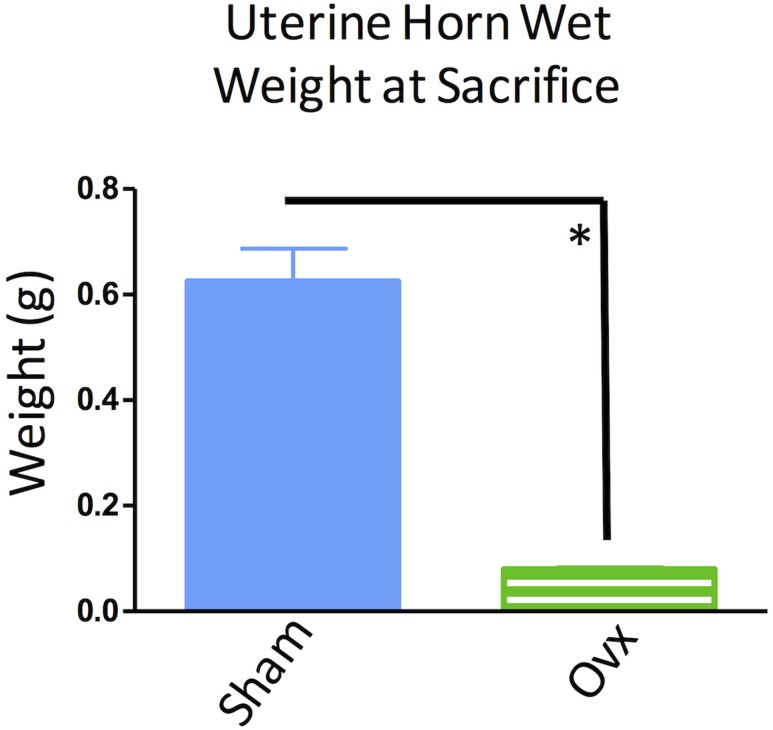
Uterine weights of sham and Ovx rats (i.e., subjects with uterine conservation) were assessed at euthanasia. Ovx rats had decreased uterine weight compared with sham-operated rats [F(1,27) = 72.61, P < 0.0001, η2 = 0.73] (Fig. 10). This confirms that Ovx was successful and that the uteri of sham rats continued to receive stimulation from endogenously circulating estrogen.
Figure 10.
Uterine wet weights from rats that had an intact uterus throughout the experiment (sham and Ovx groups). Uteri from sham-operated rats weighed significantly more than those of the Ovx group, indicating successful removal of the ovaries in the Ovx group and a lack of ovarian hormone stimulation of the uterus. *P < 0.05.
Ovarian follicle counts
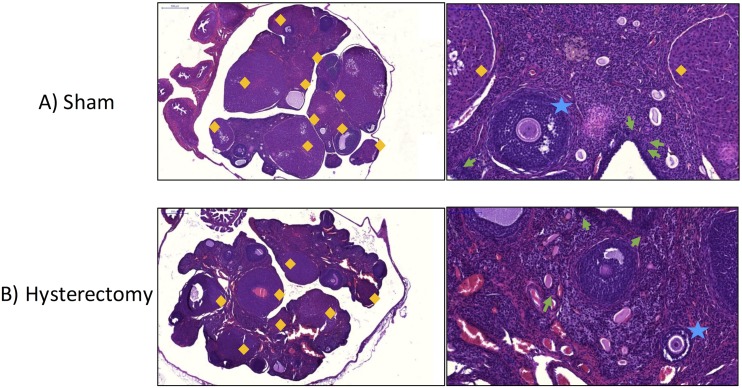
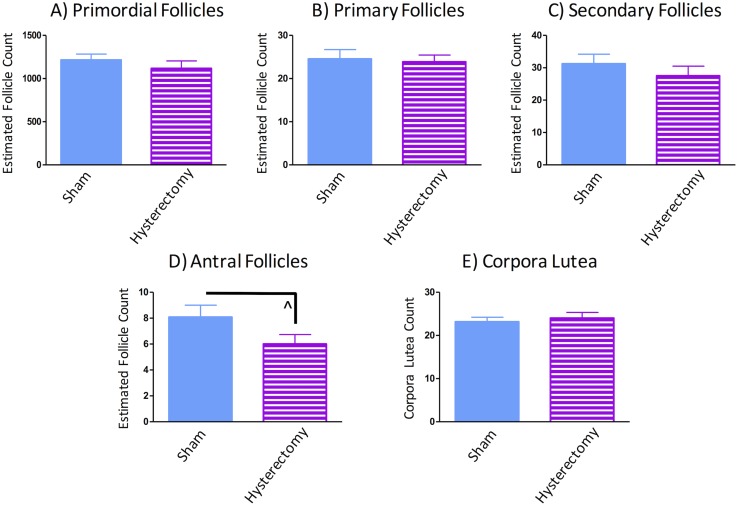
Ovaries were analyzed in rats within the sham and hysterectomy groups; that is, the rats that retained their ovaries throughout the experiment. Figure 11 depicts ovary micrographs of representative subjects from the sham (Fig. 11A) and hysterectomy (Fig. 11B) groups. No differences in early-stage follicles were found between sham and hysterectomy groups (primordials: F(1,25) = 0.39, P = NS, η2 = 0.03, Fig. 12A; primaries: F(1,25) = 0.80, P = NS, η2 = 0.003, Fig. 12B; secondaries: F(1,25) = 0.38, P = NS, η2 = 0.03, Fig. 12C). Although there was a trend toward hysterectomy subjects having fewer antral follicles as compared with sham-treated counterparts, this analysis did not reach significance [F(1,25) = 3.12, P = 0.09, η2 = 0.11] (Fig. 12D). Furthermore, corpora lutea counts were not different from each other [F(1,25) = 0.63, P = NS, η2 = 0.01] (Fig. 12E), suggesting that following hysterectomy surgery, the ovaries of hysterectomy rats did not exhibit morphological changes in healthy follicle counts compared with sham rats, at least up to 2 months after surgical intervention. Two subjects were excluded from the follicle and corpora lutea analyses owing to comprised tissue quality after processing (sham, n = 13; hysterectomy, n = 14).
Figure 11.
Representative ovarian micrographs stained with hematoxylin and eosin Y-phloxine B from (A) a sham-operated rat and (B) a hysterectomy-operated rat. Low-magnification images are original magnification ×3.45 (scale bar, 500 μm). High-magnification images are at original magnification ×20 (scale bar, 100 μm). Corpora lutea are designated by orange diamonds. Primordial follicles are denoted with a green arrow. Secondary follicles are marked with blue stars.
Figure 12.
Healthy ovarian follicle count estimates from groups that had intact ovaries throughout the experiment (sham and hysterectomy groups). Primordial, primary, and secondary follicles did not differ between groups. Hysterectomy-operated rats had marginally fewer antral follicles compared to sham-operated rats. Corpora lutea counts did not differ between groups. ^P < 0.10.
Serum hormone levels
The 17β-estradiol analysis revealed a main effect of surgery [F(3,53) = 15.30, P < 0.0001, η2 = 0.46]. Fisher PLSD indicated that the Ovx group had lower 17β-estradiol levels than did the sham group (P < 0.0001, d = 2.85) and the hysterectomy group (P < 0.001, d = 1.74); the Ovx-hysterectomy group also had lower 17β-estradiol levels than did the sham group (P < 0.0001, d = 2.79) and the hysterectomy group (P < 0.001, d = 1.70), indicating successful surgical removal of the ovaries in rats that were Ovx. Additionally, the hysterectomy group trended toward having lower 17β-estradiol levels compared with the sham group (P = 0.06, d = 0.52) (Fig. 13A). One serum sample was excluded from the 17β-estradiol analysis owing to an issue with the sample during processing (sham, n = 15; Ovx, n = 14; hysterectomy, n = 14; Ovx-hysterectomy, n = 14) .
Figure 13.
Circulating serum ovarian hormone levels from blood collected at euthanization. (A) Sham-operated rats had more 17β-estradiol compared with Ovx and Ovx-hysterectomy groups, and marginally more 17β-estradiol compared with the hysterectomy group. (B) Ovx and Ovx-hysterectomy groups had nondetectable levels of androstenedione at euthanization. Hysterectomy-operated rats had less circulating androstenedione compared with sham-operated rats. (C) Ovx and Ovx-hysterectomy groups had less progesterone than did sham and hysterectomy groups. Hysterectomy-operated rats had more progesterone present than did sham-operated rats. (D) Ovx and Ovx-hysterectomy groups had undetectable levels of AMH at euthanization. AMH levels in hysterectomy and sham rats did not differ from each other. (E) Both groups without ovaries had increased LH levels compared with sham and hysterectomy groups, indicating successful ovary removal. LH levels did not differ between sham and hysterectomy groups. (F) Both groups without ovaries had increased FSH levels compared with sham and hysterectomy groups, indicating successful ovary removal. The hysterectomy group had increased FSH levels compared with the sham group. *P < 0.05, ^P < 0.10.
For androstenedione, there was a main effect of surgery [F(3,54) = 94.23, P < 0.0001, η2 = 0.84]. Fisher PLSD revealed that sham rats had higher androstenedione levels than did Ovx (P < 0.0001, d = 6.08) and Ovx-hysterectomy (P < 0.0001, d = 6.08) groups. Hysterectomy rats also had higher androstenedione levels than did Ovx (P < 0.001, d = 6.42) and Ovx-hysterectomy (P < 0.0001, d = 6.42) groups; indeed, neither Ovx nor Ovx-hysterectomy rats had detectable levels of androstenedione at euthanization. The hysterectomy group had lower androstenedione levels compared with the sham-operated group (P < 0.01, d = 0.70) (Fig. 13B).
Progesterone analyses revealed a main effect of surgery [F(3,54) = 8.79, P < 0.0001, η2 = 0.33], and Fisher PLSD indicated that sham rats had higher progesterone levels compared with Ovx rats (P < 0.05, d = 1.09) and Ovx-hysterectomy rats (P < 0.05, d = 1.23). The hysterectomy group had higher progesterone levels compared with the Ovx group (P < 0.05, d = 1.37) and the Ovx-hysterectomy group (P < 0.05, d = 1.45). Additionally, the hysterectomy group had elevated progesterone levels compared with the sham group (P < 0.05, d = 0.68) (Fig. 13C).
Analysis of AMH, which is produced by ovarian granulosa cells and is considered to be a correlate of ovarian follicle reserve (77, 78), revealed a main effect of surgery [F(3,53) = 93.61, P < 0.0001, η2 = 0.84]. Fisher PLSD showed that sham rats had higher AMH levels than did Ovx rats (P < 0.0001, d = 5.26) and Ovx-hysterectomy rats (P < 0.0001, d = 5.26). Hysterectomy rats also had higher AMH levels than did Ovx rats (P < 0.0001, d = 10.01) and Ovx-hysterectomy rats (P < 0.0001, d = 10.01). Indeed, AMH levels were undetectable in both the Ovx and Ovx-hysterectomy groups. Because AMH is produced by the ovarian follicles, the ovary-intact groups (i.e., sham and hysterectomy groups) were compared with each other in an additional analysis. No statistical difference was seen for AMH levels between sham and hysterectomy rats, corroborating ovarian follicle count results wherein there were no differences in primordial follicle count, the ovarian follicle reserve (Fig. 13D).
LH analyses showed a main effect of surgery [F(3,53) = 27.51, P < 0.0001, η2 = 0.61], with Fisher PLSD indicating that Ovx rats had higher LH levels than did sham rats (P < 0.0001, d = 2.50) and hysterectomy rats (P < 0.0001, d = 2.42). Ovx-hysterectomy rats also had higher LH levels than did sham rats (P < 0.0001, d = 7.09) and hysterectomy rats (P < 0.0001, d = 6.39). These results indicate that surgical removal of the ovaries resulted in higher circulating LH levels, as has been previously observed in rodents (79, 80) and women (81). LH levels were compared between ovary-intact groups only (sham and hysterectomy rats) separately; no differences were observed between ovary-intact groups, suggesting that hysterectomy with ovarian conservation does not alter serum LH levels, at least up to 2 months after surgery as tested here (Fig. 13E).
FSH analyses also revealed a main effect of surgery [F(3,53) = 535.57, P < 0.0001, η2 = 0.97], with Fisher PLSD indicating that Ovx rats had higher FSH levels than did sham rats (P < 0.0001, d = 14.16) and hysterectomy rats (P < 0.0001, d = 12.92); Ovx-hysterectomy rats had higher FSH levels than did sham rats (P < 0.0001, d = 11.372) and hysterectomy rats (P < 0.0001, d = 10.49) as well, corresponding with LH results suggesting that Ovx increases circulating FSH levels, an effect previously observed in rats (80, 82) and women (81). FSH levels were compared between ovary-intact groups only; there was a main effect of surgery for ovary-intact groups [F(1,27) = 4.97, P < 0.05, η2 = 0.16], such that FSH levels were increased in hysterectomy rats compared with sham-operated rats, suggesting a response by the hypothalamus–pituitary axis induced by uterus removal (Fig. 13F).
Discussion
To the best of our knowledge, the current report is the first systematic investigation of variations in surgical menopause including hysterectomy in an adult rat model, testing relationships among reproductive profiles and cognition. A multisystems approach simultaneously evaluated ovarian morphology, endocrine physiology, and cognition within the same subjects. Collectively, results showed that common variations in surgical menopause yield distinct effects on spatial working memory performance across early acquisition to asymptotic phases, and that hysterectomy with ovarian conservation—a novel surgical model—has unique, detrimental effects on spatial working memory 2 months after surgery.
During the early acquisition phase of the WRAM, wherein animals were acquiring task rules through maze exploration, rats with Ovx alone made fewer WMC errors compared with sham rats across all trials. Ovx-hysterectomy rats also made fewer errors than did sham rats on high working memory load trials. For WMI errors, the sham group made more errors during the early acquisition phase compared with all other experimental groups. These findings may be interpreted as enhanced WRAM task rule acquisition for subjects without their ovaries. This enhancement during acquisition could be related to maze-solving strategy or attentional processes. For example, estrogen milieu can impact learning strategies, as shown with exogenous ovarian hormone administration (83) and across estrous cycle phases (84). In accordance with these findings, our laboratory has reported Ovx-induced spatial memory enhancements for aged rats tested on the WRAM as compared with sham-operated rats (55, 56), and other laboratories have concordant findings showing that long-term Ovx yields benefits for spatial memory compared with age-matched reproductively-intact controls in rhesus macaques (85) and mice (86). Of note, there are reports of no effects of Ovx in middle-aged rats, and some work has shown memory impairments after Ovx in young adult and middle-aged rats, effects that occurred after animals had learned the task or after extended temporal delays to evaluate high-demand mnemonic retention, indicating that task phase and difficulty impact outcomes of Ovx (87). Another interpretation of the increased errors in the sham group during the early acquisition phase is related to an increase in exploratory behavior or activity levels in this group, rather than a relative learning impairment per se. Indeed, previous studies have found that Ovx decreases activity levels (88) and increases anxiogenic behaviors (89) in the open field, and that that presence of ovarian hormones restores these activity levels and decreases anxiogenic behavior (88, 89). Thus, it is possible that the rats without ovaries are making fewer arm entries during the first several days of the task, which is operationally defined as better performance, due to an artifact of decreased exploratory or locomotor activity during task acquisition.
During the asymptotic phase of the WRAM (days 9 to 12) when rats have learned the task rules, hysterectomy with ovarian conservation had a unique, detrimental effect on spatial memory when working memory load was taxed compared with all other variations in surgical menopause tested in the present study, as well as compared with sham-operated rats. Interestingly, concomitant surgical removal of the ovaries with the uterus (i.e., Ovx-hysterectomy) prevented the detrimental memory effects of hysterectomy alone on spatial working memory. Rats that received sham surgery or Ovx surgery with uterine conservation did not show cognitive impairments at a high working memory load, suggesting that the presence or absence of ovaries alone does not dictate the observed working memory effects. Taken together, these results indicate that during the asymptotic phase of WRAM, when subjects are performing to the best of their ability, there is a unique impact of uterus removal alone that detrimentally impacts the spatial working memory domain.
Following the WRAM, all subjects were exposed to one of two win-stay RM tasks: the MM or the DMS win-stay task. Compared with the WRAM, these are both lower cognitive demand tasks that do not involve a working memory load component. In each RM task, all subjects learned the task, effectively decreasing swim distance to the platform in the MM or decreasing errors committed across days in the DMS task, regardless of surgery condition. This lack of difference among surgery conditions is notable in that the effects of these variations in surgical menopause are likely a domain-specific, rather than a global, change in cognitive function. Translationally, this is a crucial factor to keep in mind, as deficits in a particular cognitive area may not lead to an overall global decline in cognitive function. Furthermore, the order in which the rats were exposed to the task is likely important when interpreting cognitive outcomes. Specifically, following a high cognitive demand task such as the WRAM, lower cognitive demand tasks may be easier to acquire since the subjects had experienced a taxing spatial memory task initially. In the future, it will be important to explore whether maze task order matters for spatial learning and memory in the context of hormones and aging.
It remains controversial whether the ovaries continue to function normally in the long term following hysterectomy in the premenopausal state. Some evidence from human literature where women in their reproductive years underwent hysterectomy with ovarian conservation implies that the ovaries continue to function normally for many years (12–14); this is evidenced in that these women are not prescribed estrogen-containing hormone therapy following their surgery until they may opt to take it in midlife, around the average age of natural menopause onset, when circulating ovarian hormone levels become erratic and symptoms of menopause, such as hot flashes, begin to present (8). However, other clinical literature reports that women who undergo hysterectomy with ovarian conservation during reproductive years may transition to menopause earlier in life than women who have an intact uterus and ovaries throughout the menopause transition (7, 9, 10). Whether this is directly related to the hysterectomy procedure, or whether these women may be predisposed to an earlier menopause related to a comorbid condition, remains uncertain. Furthermore, recent evidence suggesting that a younger age at hysterectomy is associated with an increased relative risk of developing dementia compared with age-matched intact women (17, 18, 44) points to a crucial role for uterine tissue in a trajectory of healthy aging. The data presented in the current study reveal that rats that received a hysterectomy with ovarian conservation had normal estrous cyclicity 2 weeks after surgery, gained body mass at a rate similar to that of sham-operated controls, and did not show alterations in ovarian follicle morphology 2 months after surgery. However, alterations in circulating ovarian steroid hormones and gonadotropin levels were apparent 2 months after surgery. Whether changes in ovarian function and steroidogenesis are a transient effect of hysterectomy, or whether these are long-term changes in ovarian function, remains an open, but critical, question.
Altered ovarian- and pituitary-derived hormone synthesis and release following hysterectomy are likely key factors to understanding the progression of morphological, physiological, and cognitive changes associated with hysterectomy surgery. It is notable, however, that serum AMH, a putative marker of ovarian follicle reserve, is not different between sham and hysterectomy rats, suggesting that hysterectomy with ovarian conservation did not significantly impact ovarian follicle reserve, at least in this short-term 2 month time point after surgery. Furthermore, serum LH levels, the gonadotropin that regulates ovulation and corpus luteum function, were not different between hysterectomy rats and ovary-intact sham rats; indeed, low LH levels in the hysterectomy group indicate continued ovarian function following surgery, supporting the idea that localized ovarian dysfunction is not the primary contributing factor to the cognitive detriments seen in the hysterectomy rats 6 weeks after surgery, and that uterus itself likely has a unique impact on cognition, with or without additional impacts or interactions from other factors not yet determined. Notably, 2 months after surgery, FSH levels were significantly increased in hysterectomy rats compared with ovary-intact sham rats. Given that FSH is currently the gold standard in clinical settings to determine a woman’s menopause status and has been previously reported to increase following hysterectomy with ovarian conservation in women (7, 11), it is of particular interest to further investigate this increase in FSH in hysterectomy rats in relation to hypothalamic–pituitary–reproductive tract communication and dysfunction. That FSH was one of the hormones altered 2 months after hysterectomy with ovarian conservation in our study implicates a potential accelerated disruption of hypothalamic–pituitary–reproductive tract communication when the uterus alone is removed, which, when considering translational implications, could yield an earlier menopause in women who undergo hysterectomy. One potential contributing factor to this specific change in FSH is altered secretion of inhibins, which are peptide hormones belonging to the TGF-β family produced by growing ovarian follicles. FSH stimulates inhibin production, and, in turn, inhibins selectively regulate FSH release and feedback from the pituitary gland across the female reproductive cycle (90, 91). The only ovarian change we found in our current evaluations was a marginal decrease in antral follicles, which are one of the primary sources of inhibin-B secretion (92). Indeed, declines in inhibins have been proposed as an early marker of ovarian follicular depletion following hysterectomy in women (92). The role of inhibins is of interest to explore in future studies. In our present study, hysterectomy alone yielded changes in several circulating hormone levels produced by the ovaries and pituitary, which could be a result of altered hormone biosynthesis processes, changes in bioenergetics metabolism, and/or steroid release from the ovary prior to any gross morphological changes in the ovarian follicle structure or onset of follicular atresia, which may occur following a longer period after surgical intervention.
We would be remiss if we did not also acknowledge that sex steroid hormones can be derived in nonnegligible quantities from extraovarian sources, including adipose tissue, skin, hair follicles, liver, and the adrenal glands (93), and that changes in these nonreproductive systems could also be induced by the surgical removal of the uterus. As such, the observed reproductive hormone changes following surgery can currently be interpreted as a potential mediating factor of cognitive change, rather than the primary cause. Overall, it is crucial to evaluate the long-term effects of hysterectomy with ovarian conservation on ovary structure and function to better understand how a local change such as removing the uterus can ultimately result in a systemic alteration of neurobiological factors important for learning and memory. Given recent findings indicating autonomic and sensory innervation to the nonpregnant rat uterus (41), one possibility is that disturbance to this uterine innervation via hysterectomy is sufficient to alter neuroendocrine and neurotransmitter signaling in the central and peripheral nervous systems, in turn disrupting hormone production and secretion involved in brain–ovary–uterine communication. Furthermore, there is evidence that adrenergic innervation of the ovaries can influence ovarian hormone secretion during the estrous cycle in the rat (42), and several neurotransmitters within the catecholamine class have been shown to induce androgen production in cultured ovarian theca-interstitial cells (43). These collected findings implicate a direct role of central and peripheral nervous system regulation of the nonpregnant uterus and the ovaries, and they provide a starting point to investigate neurobiological mechanisms associated with the observed cognitive changes in future studies. Additionally, it is imperative to investigate how exogenous estrogen therapy affects this variant of surgical menopause in a rat model, as researchers in the field have historically investigated cognitive effects of estrogen-containing hormone therapy in an Ovx model, where ovaries are removed, but the uterus remains intact. In sum, these findings are a fundamental next step in elucidating how variations in surgical menopause can impact cognitive and brain aging. Specifically, to our knowledge, this is the first preclinical study to methodically investigate the impact of hysterectomy with and without ovarian conservation on learning and memory. Furthermore, using this novel experimental design, we report that the nonpregnant uterus itself is not a quiescent organ. Rather, uterus removal with or without concomitant ovarian removal can have significant effects on physiology and cognition, opening new doors for future investigations into the role of the uterus in behavioral outcomes across the lifespan. In the future, it will be critical to experimentally evaluate the role of age at surgery and time since surgical manipulation to systematically decipher how variations in surgical menopause impact brain aging. Translationally, these findings are impactful in that they can inform clinical understandings of, and lead to additional human studies testing, the intricate connections between the brain and the female reproductive system. This will provide fundamental stepping stones to initiate further exploration into how common variations in gynecological surgery impact quality of life, as well as cognitive and brain aging, in women throughout their lifetimes.
Acknowledgments
We gratefully acknowledge Dr. Julia Files, Dr. Javier Magrina, and Dr. Kristina Butler for invaluable discussion of the hysterectomy model. Steroid hormone assays were determined by the Core Endocrine Laboratory at Pennsylvania State University, for which we acknowledge Dr. Laurence Demers with gratitude. LH, FSH, and AMH assays were determined by the Ligand Assay and Analysis Core of the Center for Research in Reproduction at the University of Virginia School of Medicine, for which we thank Dr. Daniel Haisenleder and Aleisha Schoenfelder. The University of Virginia Center for Research in Reproduction Ligand Assay and Analysis Core is supported by the Eunice Kennedy Shriver National Institute of Child Health and Human Development/National Institutes of Health (NCTRI) Grant P50-HD28934.
Financial Support: H.A.B.-N. is supported by the following: National Institute on Aging Grant AG028084; state of Arizona, Arizona Department of Health Services Grant ADHS14-052688; National Institutes of Health Alzheimer’s Disease Core Center Grant P30AG019610; and by funding from Arizona State University Office of Knowledge Enterprise Development, College of Liberal Arts and Sciences, and Department of Psychology. S.V.K. is supported by the following: National Institute on Aging Grant 1F31AG056110-01A1.
Disclosure Summary: The authors have nothing to disclose.
Glossary
Abbreviations:
- AMH
anti-Müllerian hormone
- DMS
delayed match-to-sample
- ITI
intertrial interval
- MM
Morris water maze
- Ovx
ovariectomy
- PLSD
protected least significant difference
- RM
reference memory
- VP
visible platform
- WMC
working memory correct
- WMI
working memory incorrect
- WRAM
water radial-arm maze
- ηG2
generalized η2
References
- 1. Centers for Disease Control and Prevention Number of first-listed diagnoses for discharges from short-stay hospitals, by ICD-9-CM code, sex, age, and geographic region: United States, 2010. Available at: https://www.cdc.gov/nchs/data/nhds/4procedures/2010pro4_numberprocedureage.pdf. Accessed 2 February 2015.
- 2. Carlson KJ, Nichols DH, Schiff I. Indications for hysterectomy. N Engl J Med. 1993;328(12):856–860. [DOI] [PubMed] [Google Scholar]
- 3. Whiteman MK, Hillis SD, Jamieson DJ, Morrow B, Podgornik MN, Brett KM, Marchbanks PA. Inpatient hysterectomy surveillance in the United States, 2000–2004. Am J Obstet Gynecol. 2008;198(1):34.e1–34.e7. [DOI] [PubMed] [Google Scholar]
- 4. Wright JD, Herzog TJ, Tsui J, Ananth CV, Lewin SN, Lu YS, Neugut AI, Hershman DL. Nationwide trends in the performance of inpatient hysterectomy in the United States. Obstet Gynecol. 2013;122(2 Pt 1):233–241. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Hoffman BL, Schorge JO, Schaffer JI, Halvorson KD, Bradshaw FG, Cunningham LE. Williams Gynecology. 2nd ed.New York, NY: McGraw-Hill Education; 2012. [Google Scholar]
- 6. North American Menopause Society Menopause Practice: A Clinician’s Guide. 5th ed.Mayfield Heights, OH: North American Menopause Society; 2014. [Google Scholar]
- 7. Kaiser R, Kusche M, Würz H. Hormone levels in women after hysterectomy. Arch Gynecol Obstet. 1989;244(3):169–173. [DOI] [PubMed] [Google Scholar]
- 8. Kritz-Silverstein D, Goldani Von Mühlen D, Barrett-Connor E. Prevalence and clustering of menopausal symptoms in older women by hysterectomy and oophorectomy status. J Womens Health Gend Based Med. 2000;9(7):747–755. [DOI] [PubMed] [Google Scholar]
- 9. Moorman PG, Myers ER, Schildkraut JM, Iversen ES, Wang F, Warren N. Effect of hysterectomy with ovarian preservation on ovarian function. Obstet Gynecol. 2011;118(6):1271–1279. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Read MD, Edey KA, Hapeshi J, Foy C. The age of ovarian failure following premenopausal hysterectomy with ovarian conservation. Menopause Int. 2010;16(2):56–59. [DOI] [PubMed] [Google Scholar]
- 11. Chan CC, Ng EH, Ho PC. Ovarian changes after abdominal hysterectomy for benign conditions. J Soc Gynecol Investig. 2005;12(1):54–57. [DOI] [PubMed] [Google Scholar]
- 12. Chalmers C, Lindsay M, Usher D, Warner P, Evans D, Ferguson M. Hysterectomy and ovarian function: levels of follicle stimulating hormone and incidence of menopausal symptoms are not affected by hysterectomy in women under age 45 years. Climacteric. 2002;5(4):366–373. [PubMed] [Google Scholar]
- 13. Findley AD, Siedhoff MT, Hobbs KA, Steege JF, Carey ET, McCall CA, Steiner AZ. Short-term effects of salpingectomy during laparoscopic hysterectomy on ovarian reserve: a pilot randomized controlled trial. Fertil Steril. 2013;100(6):1704–1708. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Beavis EL, Brown JB, Smith MA. Ovarian function after hysterectomy with conservation of the ovaries in pre-menopausal women. J Obstet Gynaecol Br Commonw. 1969;76(11):969–978. [DOI] [PubMed] [Google Scholar]
- 15. Wise PM, Smith MJ, Dubal DB, Wilson ME, Krajnak KM, Rosewell KL. Neuroendocrine influences and repercussions of the menopause. Endocr Rev. 1999;20(3):243–248. [DOI] [PubMed] [Google Scholar]
- 16. Farrag AK, Khedr EM, Abdel-Aleem H, Rageh TA. Effect of surgical menopause on cognitive functions. Dement Geriatr Cogn Disord. 2002;13(3):193–198. [DOI] [PubMed] [Google Scholar]
- 17. Rocca WA, Bower JH, Maraganore DM, Ahlskog JE, Grossardt BR, de Andrade M, Melton LJ III. Increased risk of cognitive impairment or dementia in women who underwent oophorectomy before menopause. Neurology. 2007;69(11):1074–1083. [DOI] [PubMed] [Google Scholar]
- 18. Rocca WA, Grossardt BR, Shuster LT, Stewart EA. Hysterectomy, oophorectomy, estrogen, and the risk of dementia. Neurodegener Dis. 2012;10(1–4):175–178. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Bimonte HA, Denenberg VH. Estradiol facilitates performance as working memory load increases. Psychoneuroendocrinology. 1999;24(2):161–173. [DOI] [PubMed] [Google Scholar]
- 20. Gibbs RB, Johnson DA. Sex-specific effects of gonadectomy and hormone treatment on acquisition of a 12-arm radial maze task by Sprague Dawley rats. Endocrinology. 2008;149(6):3176–3183. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. Talboom JS, Williams BJ, Baxley ER, West SG, Bimonte-Nelson HA. Higher levels of estradiol replacement correlate with better spatial memory in surgically menopausal young and middle-aged rats. Neurobiol Learn Mem. 2008;90(1):155–163. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. Rocca WA, Shuster LT, Grossardt BR, Maraganore DM, Gostout BS, Geda YE, Melton LJ III. Long-term effects of bilateral oophorectomy on brain aging: unanswered questions from the Mayo Clinic Cohort Study of Oophorectomy and Aging. Womens Health (Lond). 2009;5(1):39–48. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23. Rocca WA, Grossardt BR, Shuster LT. Oophorectomy, menopause, estrogen treatment, and cognitive aging: clinical evidence for a window of opportunity. Brain Res. 2011;1379:188–198. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24. Nappi RE, Sinforiani E, Mauri M, Bono G, Polatti F, Nappi G. Memory functioning at menopause: impact of age in ovariectomized women. Gynecol Obstet Invest. 1999;47(1):29–36. [DOI] [PubMed] [Google Scholar]
- 25. Wallace M, Luine V, Arellanos A, Frankfurt M. Ovariectomized rats show decreased recognition memory and spine density in the hippocampus and prefrontal cortex. Brain Res. 2006;1126(1):176–182. [DOI] [PubMed] [Google Scholar]
- 26. Acosta JI, Mayer LP, Braden BB, Nonnenmacher S, Mennenga SE, Bimonte-Nelson HA. The cognitive effects of conjugated equine estrogens depend on whether menopause etiology is transitional or surgical. Endocrinology. 2010;151(8):3795–3804. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27. Corson SL, Levinson CJ, Batzer FR, Otis C. Hormonal levels following sterilization and hysterectomy. J Reprod Med. 1981;26(7):363–370. [PubMed] [Google Scholar]
- 28. Vuorento T, Mäenpää J, Huhtaniemi I. Follow-up of ovarian endocrine function in premenopausal women after hysterectomy by daily measurements of salivary progesterone. Clin Endocrinol (Oxf). 1992;36(5):505–510. [DOI] [PubMed] [Google Scholar]
- 29. Souza AZ, Fonseca AM, Izzo VM, Clauzet RM, Salvatore CA. Ovarian histology and function after total abdominal hysterectomy. Obstet Gynecol. 1986;68(6):847–849. [PubMed] [Google Scholar]
- 30. Dubal DB, Wise PM. Estrogen and neuroprotection: from clinical observations to molecular mechanisms. Dialogues Clin Neurosci. 2002;4(2):149–161. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31. Turgeon JL, Carr MC, Maki PM, Mendelsohn ME, Wise PM. Complex actions of sex steroids in adipose tissue, the cardiovascular system, and brain: Insights from basic science and clinical studies. Endocr Rev. 2006;27(6):575–605. [DOI] [PubMed] [Google Scholar]
- 32. Daniel JM. Estrogens, estrogen receptors, and female cognitive aging: the impact of timing. Horm Behav. 2013;63(2):231–237. [DOI] [PubMed] [Google Scholar]
- 33. Frick KM. Molecular mechanisms underlying the memory-enhancing effects of estradiol. Horm Behav. 2015;74:4–18. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34. Koebele SV, Bimonte-Nelson HA. Trajectories and phenotypes with estrogen exposures across the lifespan: what does Goldilocks have to do with it? Horm Behav. 2015;74:86–104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35. Korol DL, Pisani SL. Estrogens and cognition: friends or foes?: an evaluation of the opposing effects of estrogens on learning and memory. Horm Behav. 2015;74:105–115. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36. Luine VN. Estradiol and cognitive function: past, present and future. Horm Behav. 2014;66(4):602–618. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37. Navot D, Williams MC. The uterus without ovaries In: Altchek A, Deligdisch L, eds. The Uterus: Pathology, Diagnosis, and Management. 1st ed.New York, NY: Springer-Verlag; 1991:294–299. [Google Scholar]
- 38. Lessey BA, Killam AP, Metzger DA, Haney AF, Greene GL, McCarty KS Jr. Immunohistochemical analysis of human uterine estrogen and progesterone receptors throughout the menstrual cycle. J Clin Endocrinol Metab. 1988;67(2):334–340. [DOI] [PubMed] [Google Scholar]
- 39. Stilley JA, Christensen DE, Dahlem KB, Guan R, Santillan DA, England SK, Al-Hendy A, Kirby PA, Segaloff DL. FSH receptor (FSHR) expression in human extragonadal reproductive tissues and the developing placenta, and the impact of its deletion on pregnancy in mice. Biol Reprod. 2014;91(3):74. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40. Reshef E, Lei ZM, Rao CV, Pridham DD, Chegini N, Luborsky JL. The presence of gonadotropin receptors in nonpregnant human uterus, human placenta, fetal membranes, and decidua. J Clin Endocrinol Metab. 1990;70(2):421–430. [DOI] [PubMed] [Google Scholar]
- 41. Gnanamanickam GJ, Llewellyn-Smith IJ. Innervation of the rat uterus at estrus: a study in full-thickness, immunoperoxidase-stained whole-mount preparations. J Comp Neurol. 2011;519(4):621–643. [DOI] [PubMed] [Google Scholar]
- 42. Aguado LI, Ojeda SR. Ovarian adrenergic nerves play a role in maintaining preovulatory steroid secretion. Endocrinology. 1984;114(5):1944–1946. [DOI] [PubMed] [Google Scholar]
- 43. Dyer CA, Erickson GF. Norepinephrine amplifies human chorionic gonadotropin-stimulated androgen biosynthesis by ovarian theca-interstitial cells. Endocrinology. 1985;116(4):1645–1652. [DOI] [PubMed] [Google Scholar]
- 44. Phung TK, Waltoft BL, Laursen TM, Settnes A, Kessing LV, Mortensen PB, Waldemar G. Hysterectomy, oophorectomy and risk of dementia: a nationwide historical cohort study. Dement Geriatr Cogn Disord. 2010;30(1):43–50. [DOI] [PubMed] [Google Scholar]
- 45. Imtiaz B, Tuppurainen M, Tiihonen M, Kivipelto M, Soininen H, Hartikainen S, Tolppanen AM. Oophorectomy, hysterectomy, and risk of Alzheimer’s disease: a nationwide case-control study. J Alzheimers Dis. 2014;42(2):575–581. [DOI] [PubMed] [Google Scholar]
- 46. Bíró J, Ritzén EM, Hall K, Eneroth P. Effects of hysterectomy and uterine extracts on the endocrine system of adult, nonpregnant rats. J Steroid Biochem. 1984;20(1):343–345. [DOI] [PubMed] [Google Scholar]
- 47. Özdamar S, Ülger H, Sorkun HC, Müderris I. Effects of hysterectomy on ovarian morphology and serum FSH level in rats. Maturitas. 2005;52(1):60–64. [DOI] [PubMed] [Google Scholar]
- 48. Tanaka M, Kumai T, Watanabe M, Matsumoto C, Hirai M, Kobayashi S. Effects of hysterectomy on ovulation and related ovarian functions in regular estrous cycle rats. Life Sci. 1994;55(3):237–243. [DOI] [PubMed] [Google Scholar]
- 49. Tapisiz OL, Gungor T, Aytan H, Zergeroglu S, Mulazimoglu B, Bilge U, Mollamahmutoglu L. Does hysterectomy affect ovarian function? Histopathologic evaluation and serum FSH, inhibin A, and inhibin B levels in an experimental rat model. Eur J Obstet Gynecol Reprod Biol. 2008;140(1):61–66. [DOI] [PubMed] [Google Scholar]
- 50. Goldman JM, Murr AS, Cooper RL. The rodent estrous cycle: characterization of vaginal cytology and its utility in toxicological studies. Birth Defects Res B Dev Reprod Toxicol. 2007;80(2):84–97. [DOI] [PubMed] [Google Scholar]
- 51. Mennenga SE, Bimonte-Nelson HA. The importance of incorporating both sexes and embracing hormonal diversity when conducting rodent behavioral assays In: Bimonte-Nelson HA, ed. The Maze Book: Theories, Practice, and Protocols for Testing Rodent Cognition. 1st ed.New York, NY: Springer US; 2015:299–321. [Google Scholar]
- 52. Bimonte HA, Hyde LA, Hoplight BJ, Denenberg VH. In two species, females exhibit superior working memory and inferior reference memory on the water radial-arm maze. Physiol Behav. 2000;70(3–4):311–317. [DOI] [PubMed] [Google Scholar]
- 53. Bimonte HA, Granholm AC, Seo H, Isacson O. Spatial memory testing decreases hippocampal amyloid precursor protein in young, but not aged, female rats. Neurosci Lett. 2002;328(1):50–54. [DOI] [PubMed] [Google Scholar]
- 54. Bimonte HA, Nelson ME, Granholm AC. Age-related deficits as working memory load increases: relationships with growth factors. Neurobiol Aging. 2003;24(1):37–48. [DOI] [PubMed] [Google Scholar]
- 55. Bimonte-Nelson HA, Singleton RS, Hunter CL, Price KL, Moore AB, Granholm AC. Ovarian hormones and cognition in the aged female rat: I. Long-term, but not short-term, ovariectomy enhances spatial performance. Behav Neurosci. 2003;117(6):1395–1406. [DOI] [PubMed] [Google Scholar]
- 56. Bimonte-Nelson HA, Singleton RS, Williams BJ, Granholm AC. Ovarian hormones and cognition in the aged female rat: II. progesterone supplementation reverses the cognitive enhancing effects of ovariectomy. Behav Neurosci. 2004;118(4):707–714. [DOI] [PubMed] [Google Scholar]
- 57. Bimonte-Nelson HA, Daniel JM, Koebele SV. The mazes In: Bimonte-Nelson HA, ed. The Maze Book: Theories, Practice, and Protocols for Testing Rodent Cognition. 1st ed.New York, NY: Springer US; 2015:37–72. [Google Scholar]
- 58. Morris RG, Garrud P, Rawlins JN, O’Keefe J. Place navigation impaired in rats with hippocampal lesions. Nature. 1982;297(5868):681–683. [DOI] [PubMed] [Google Scholar]
- 59. Fallest PC, Trader GL, Darrow JM, Shupnik MA. Regulation of rat luteinizing hormone β gene expression in transgenic mice by steroids and a gonadotropin-releasing hormone antagonist. Biol Reprod. 1995;53(1):103–109. [DOI] [PubMed] [Google Scholar]
- 60. Haavisto AM, Pettersson K, Bergendahl M, Perheentupa A, Roser JF, Huhtaniemi I. A supersensitive immunofluorometric assay for rat luteinizing hormone. Endocrinology. 1993;132(4):1687–1691. [DOI] [PubMed] [Google Scholar]
- 61.RRID:AB_2665514.
- 62.RRID:AB_2665513.
- 63. Matteri RL, Roser JF, Baldwin DM, Lipovetsky V, Papkoff H. Characterization of a monoclonal antibody which detects luteinizing hormone from diverse mammalian species. Domest Anim Endocrinol. 1987;4(3):157–165. [DOI] [PubMed] [Google Scholar]
- 64. Gay VL, Midgley AR Jr, Niswender GD. Patterns of gonadotrophin secretion associated with ovulation. Fed Proc. 1970;29(6):1880–1887. [PubMed] [Google Scholar]
- 65.RRID:AB_2665512.
- 66. Gougeon A, Chainy GB. Morphometric studies of small follicles in ovaries of women at different ages. J Reprod Fertil. 1987;81(2):433–442. [DOI] [PubMed] [Google Scholar]
- 67. Haas JR, Christian PJ, Hoyer PB. Effects of impending ovarian failure induced by 4-vinylcyclohexene diepoxide on fertility in C57BL/6 female mice. Comp Med. 2007;57(5):443–449. [PubMed] [Google Scholar]
- 68. Mennenga SE, Koebele SV, Mousa AA, Alderete TJ, Tsang CW, Acosta JI, Camp BW, Demers LM, Bimonte-Nelson HA. Pharmacological blockade of the aromatase enzyme, but not the androgen receptor, reverses androstenedione-induced cognitive impairments in young surgically menopausal rats. Steroids. 2015;99(Pt A):16–25. [DOI] [PMC free article] [PubMed]
- 69. Olejnik S, Algina J. Generalized eta and omega squared statistics: measures of effect size for some common research designs. Psychol Methods. 2003;8(4):434–447. [DOI] [PubMed] [Google Scholar]
- 70. Bakeman R. Recommended effect size statistics for repeated measures designs. Behav Res Methods. 2005;37(3):379–384. [DOI] [PubMed] [Google Scholar]
- 71. Cohen J. A power primer. Psychol Bull. 1992;112(1):155–159. [DOI] [PubMed] [Google Scholar]
- 72. Mennenga SE, Gerson JE, Koebele SV, Kingston ML, Tsang CW, Engler-Chiurazzi EB, Baxter LC, Bimonte-Nelson HA. Understanding the cognitive impact of the contraceptive estrogen ethinyl estradiol: tonic and cyclic administration impairs memory, and performance correlates with basal forebrain cholinergic system integrity. Psychoneuroendocrinology. 2015;54:1–13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73. Koebele SV, Mennenga SE, Hiroi R, Quihuis AM, Hewitt LT, Poisson ML, George C, Mayer LP, Dyer CA, Aiken LS, Demers LM, Carson C, Bimonte-Nelson HA. Cognitive changes across the menopause transition: a longitudinal evaluation of the impact of age and ovarian status on spatial memory. Horm Behav. 2017;87:96–114. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74. Bimonte-Nelson HA, Hunter CL, Nelson ME, Granholm AC. Frontal cortex BDNF levels correlate with working memory in an animal model of Down syndrome. Behav Brain Res. 2003;139(1–2):47–57. [DOI] [PubMed] [Google Scholar]
- 75. Hiroi R, Weyrich G, Koebele SV, Mennenga SE, Talboom JS, Hewitt LT, Lavery CN, Mendoza P, Jordan A, Bimonte-Nelson HA. Benefits of hormone therapy estrogens depend on estrogen type: 17β-estradiol and conjugated equine estrogens have differential effects on cognitive, anxiety-like, and depressive-like behaviors and increase tryptophan hydroxylase-2 mRNA levels in dorsal raphe nucleus subregions. Front Neurosci. 2016;10:517. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76. Prakapenka AV, Hiroi R, Quihuis AM, Carson C, Patel S, Berns-Leone C, Fox C, Sirianni RW, Bimonte-Nelson HA. Contrasting effects of individual versus combined estrogen and progestogen regimens as working memory load increases in middle-aged ovariectomized rats: one plus one does not equal two. Neurobiol Aging. 2018;64:1–14. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77. Hansen KR, Hodnett GM, Knowlton N, Craig LB. Correlation of ovarian reserve tests with histologically determined primordial follicle number. Fertil Steril. 2011;95(1):170–175. [DOI] [PubMed] [Google Scholar]
- 78. Hansen KR. Predicting reproductive age with biomarkers of ovarian reserve—how (and what) are we measuring? Semin Reprod Med. 2013;31(6):416–426. [DOI] [PubMed] [Google Scholar]
- 79. Gay VL, Midgley AR Jr. Response of the adult rat to orchidectomy and ovariectomy as determined by LH radioimmunoassay. Endocrinology. 1969;84(6):1359–1364. [DOI] [PubMed] [Google Scholar]
- 80. Wise PM, Ratner A. Effect of ovariectomy on plasma LH, FSH, estradiol, and progesterone and medial basal hypothalamic LHRH concentrations old and young rats. Neuroendocrinology. 1980;30(1):15–19. [DOI] [PubMed] [Google Scholar]
- 81. Yen SS, Tsai CC. The effect of ovariectomy on gonadotropin release. J Clin Invest. 1971;50(5):1149–1153. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82. Elias KA, Blake CA. Effects of acute ovariectomy on anterior pituitary gland follicle-stimulating hormone and luteinizing hormone secretion in the metestrous rat. Biol Reprod. 1983;28(5):1107–1113. [DOI] [PubMed] [Google Scholar]
- 83. Korol DL, Kolo LL. Estrogen-induced changes in place and response learning in young adult female rats. Behav Neurosci. 2002;116(3):411–420. [DOI] [PubMed] [Google Scholar]
- 84. Korol DL, Malin EL, Borden KA, Busby RA, Couper-Leo J. Shifts in preferred learning strategy across the estrous cycle in female rats. Horm Behav. 2004;45(5):330–338. [DOI] [PubMed] [Google Scholar]
- 85. Lacreuse A, Herndon JG, Moss MB. Cognitive function in aged ovariectomized female rhesus monkeys. Behav Neurosci. 2000;114(3):506–513. [DOI] [PubMed] [Google Scholar]
- 86. Heikkinen T, Puoliväli J, Tanila H. Effects of long-term ovariectomy and estrogen treatment on maze learning in aged mice. Exp Gerontol. 2004;39(9):1277–1283. [DOI] [PubMed] [Google Scholar]
- 87. Bimonte-Nelson HA, Acosta JI, Talboom JS. Neuroscientists as cartographers: mapping the crossroads of gonadal hormones, memory and age using animal models. Molecules. 2010;15(9):6050–6105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 88. Blizard DA, Lippman HR, Chen JJ. Sex differences in open-field behavior in the rat: the inductive and activational role of gonadal hormones. Physiol Behav. 1975;14(5):601–608. [DOI] [PubMed] [Google Scholar]
- 89. Hiroi R, Neumaier JF. Differential effects of ovarian steroids on anxiety versus fear as measured by open field test and fear-potentiated startle. Behav Brain Res. 2006;166(1):93–100. [DOI] [PubMed] [Google Scholar]
- 90. Norris DO, Lopez KH. The endocrinology of the mammalian ovary In: Norris DO, Lopez KH, eds. Hormones and Reproduction of Vertebrates–Mammals. Vol 5 San Diego, CA: Academic Press; 2011:59–72. [Google Scholar]
- 91. Welt C, Sidis Y, Keutmann H, Schneyer A. Activins, inhibins, and follistatins: from endocrinology to signaling. A paradigm for the new millennium. Exp Biol Med (Maywood). 2002;227(9):724–752. [DOI] [PubMed] [Google Scholar]
- 92. Nahás E, Pontes A, Traiman P, NahásNeto J, Dalben I, De Luca L. Inhibin B and ovarian function after total abdominal hysterectomy in women of reproductive age. Gynecol Endocrinol. 2003;17(2):125–131. [PubMed] [Google Scholar]
- 93. Rosen M, Cedars MI. Female reproductive endocrinology and infertility In: Gardner D, Shoback D, eds. Greenspan’s Basic and Clinical Endocrinology. 8th ed.New York, NY: McGraw-Hill Education; 2007:502–561. [Google Scholar]