Multitrophic interactions involving insect pests, their natural enemies, microorganisms, and plant hosts are increasingly being recognized as relevant factors in pest management. In response to herbivory attacks, plants activate a wide range of defenses that aim to mitigate the damage. Attacked plants release herbivore-induced plant volatiles (HIPVs), which can act as priming signals for other plants and attract natural enemies of herbivores, and which may have a direct negative impact on herbivore survival. In the present work, we show that exposure of the insects to the induced volatiles could increase the insects’ susceptibility to the entomopathogens naturally occurring in the plant environment. These findings suggest a novel role for plant volatiles by influencing insect interactions with natural pathogens, probably mediated by alterations in the insect microbiota composition. In addition, this work provides evidence for selectable plant traits (production of secondary metabolites) that can have an influence on the ecology of the pests and could be relevant in the improvement of pest management strategies using natural entomopathogens.
KEYWORDS: Bacillus thuringiensis, baculovirus, entomopathogen, indole, linalool, plant volatiles, plant-microbe interactions
ABSTRACT
In response to insect herbivory, plants mobilize various defenses. Defense responses include the release of herbivore-induced plant volatiles (HIPVs) that can serve as signals to alert undamaged tissues and to attract natural enemies of the herbivores. Some HIPVs can have a direct negative impact on herbivore survival, but it is not well understood by what mechanisms. Here, we tested the hypothesis that exposure to HIPVs renders insects more susceptible to natural pathogens. Exposure of the caterpillars of the noctuid Spodoptera exigua to indole and linalool, but not exposure to (Z)-3-hexenyl acetate, increased the susceptibility to Spodoptera exigua multiple nucleopolyhedrovirus (SeMNPV). We also found that exposure to indole, but not exposure to linalool or (Z)-3-hexenyl acetate, increased the pathogenicity of Bacillus thuringiensis. Additional experiments revealed significant changes in microbiota composition after forty-eight hours of larval exposure to indole. Overall, these results provide evidence that certain HIPVs can strongly enhance the susceptibility of caterpillars to pathogens, possibly through effects on the insect gut microbiota. These findings suggest a novel mechanism by which HIPVs can protect plants from herbivorous insects.
IMPORTANCE Multitrophic interactions involving insect pests, their natural enemies, microorganisms, and plant hosts are increasingly being recognized as relevant factors in pest management. In response to herbivory attacks, plants activate a wide range of defenses that aim to mitigate the damage. Attacked plants release herbivore-induced plant volatiles (HIPVs), which can act as priming signals for other plants and attract natural enemies of herbivores, and which may have a direct negative impact on herbivore survival. In the present work, we show that exposure of the insects to the induced volatiles could increase the insects’ susceptibility to the entomopathogens naturally occurring in the plant environment. These findings suggest a novel role for plant volatiles by influencing insect interactions with natural pathogens, probably mediated by alterations in the insect microbiota composition. In addition, this work provides evidence for selectable plant traits (production of secondary metabolites) that can have an influence on the ecology of the pests and could be relevant in the improvement of pest management strategies using natural entomopathogens.
INTRODUCTION
Plants defend themselves against herbivores through the production of specific metabolites and proteins with toxic, repellent, or antinutritive properties (1). These defense compounds are either produced constitutively or induced in response to herbivore attack (2). Induction is mainly mediated by the insect feeding, and it leads to the activation of multiple signaling pathways that regulate the production of defensive proteins and metabolites (3–5). Herbivores exhibit multiple feeding styles (e.g., chewing, sucking) and differ in the levels of specialization to their host plants. Accordingly, the plant response can vary depending on the type of herbivore and can involve a combination of responses in case of multiple attacks (6, 7). Plant defense responses can also be elicited by other herbivore-related factors, such as oviposition by insects (8, 9), or even by the perception of volatiles emitted by neighboring plants in response to insect attack (10, 11).
Plant-emitted volatiles represent a group of specialized metabolites that play an important role in plant defense against herbivory. Attacked plants release herbivore-induced plant volatiles (HIPVs), which can act as priming signals (10, 12, 13) or attract natural enemies of herbivores (14–16). HIPVs can also have direct benefits for the plant by repelling the herbivore or reducing its growth and survival in the plant (17). For instance, in tomato (Solanum lycopersicum), the green leaf volatile (Z)-3-hexenol from infested neighbor plants was found to be converted to (Z)-3-hexenyl-vicianoside, reducing survival and growth of Spodoptera litura caterpillars (18). More recently, it has also been shown that the HIPV indole increases weight gain but reduces food consumption and survival in Spodoptera littoralis (9).
In a multitrophic context, the eventual outcome of the interaction between plant and herbivore is also modulated by pathogenic microbes, which is assumed to be due to direct as well as indirect effects of toxic phytochemicals on entomopathogen persistence and infectivity (19–21). Although some authors have speculated about the possibility of plants promoting the action or abundance of microbial entomopathogens (22), not much information is available about the impact of HIPVs on the pathogenicity of entomopathogens. So far, only a few studies have reported the influence of certain plant volatiles on the conidial germination rates of entomopathogenic fungi (23, 24). To test this, we investigated the effect of specific HIPVs on the pathogenicity of two types of entomopathogens that naturally infect the beet armyworm, Spodoptera exigua. Larval mortality due to the Spodoptera exigua multiple nucleopolyhedrovirus (SeMNPV) and to Bacillus thuringiensis was measured during exposure of the insect to three of the most common plant volatiles, indole, linalool, and hexenyl-acetate. Additionally, we evaluated the effect of these volatiles on insect cellular immunity and gut microbiota composition. Indeed, in addition to the well-studied direct interactions between pathogens and the insect immune system, it is increasingly evident that the gut mutualistic and commensal communities can enhance resistance or tolerance of insects to pathogens (25–27). The results reveal a novel indirect defensive role for HIPVs by enhancing the pathogenicity of entomopathogens.
(This article was submitted to an online preprint archive [28].)
RESULTS
HIPV effects on susceptibility to viral and bacterial pathogens.
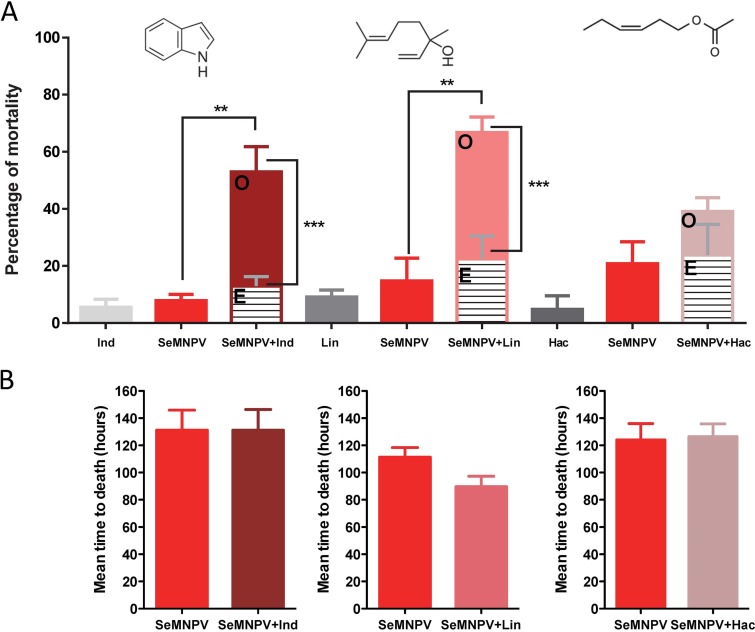
Compared with the control conditions in the absence of HIPVs, a significant increase in mortality due to baculovirus infection was observed when larvae were reared in the presence of indole (F [2, 6] = 13.8, P = 0.006, one-way analysis of variance [ANOVA]; q [0.05, 2, 6] = 6.3, Newman-Keuls posttest) or linalool (F [2, 6] = 12.5, P = 0.007, one-way ANOVA; q [0.05, 2, 6] = 5.8, Newman-Keuls posttest) (Fig. 1A and Fig. S1). No effect on SeMNPV pathogenicity was observed when the larvae were exposed to hexenyl-acetate (F [2, 6] = 2.71, P = 0.40, one-way ANOVA). Significant synergistic interaction was found between the SeMNPV virus and indole (χ2 [1, N = 82] = 14.42, P = 0.001) or linalool (χ2 [1, N = 82] = 23.74, P < 0.0001). At the SeMNPV dose that we used, no increase in virulence (measured as the mean of time to death by the viral infection) was observed for any of the HIPV treatments (Fig. 1B and Fig. S1).
FIG 1.
Effect of the tested HIPVs on S. exigua susceptibility to SeMNPV infection (102 occlusion bodies [OBs]/larvae). Ind (indole 4 mg), Lin (linalool 10%), and Hac (hexenyl acetate 10%). (A) Percentage of larval mortality for the different combinations. Observed mortality (O) and expected mortality (E), assuming the additive model. Statistical analyses were performed using one-way ANOVA with a Newman-Keuls posttest to compare the mortalities, and the chi-square test was used to check whether there is a synergistic or additive effect between the different treatments. (B) Mean time to death produced by baculovirus in the presence/absence of the corresponding HIPV. Values were statistically compared using Student's t test.
The effect of exposure to the indole on the SeMNPV infectivity was also tested at a higher viral dose (5 × 104 occlusion bodies [OBs]/larvae, producing about 80% to 90% mortality). Under these conditions, no additional increase in mortality was observed in the presence of indole; however, a significant increase in virulence of the virus was found, with mortality occurring 20% earlier in the indole-exposed insects (Fig. S2A and B). In a more controlled environment, where indole was released at a similar rate to that produced by maize plants (50 ng/h) (10), we also observed a significant increase in baculovirus virulence (Fig. S2C and D).
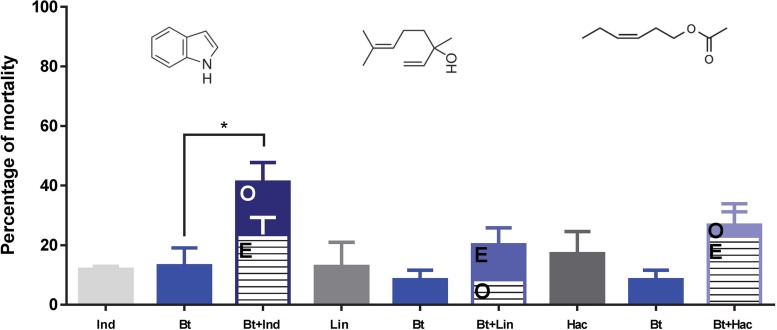
We also evaluated the effects of HIPVs on the insect’s susceptibility to a bacterial pathogen (Fig. 2). Under our experimental conditions, mortality due to B. thuringiensis was affected by the exposure to indole (F [2, 4] = 9.34, P = 0.03, one-way ANOVA; q [0.05, 2, 4] = 5.2, Newman-Keuls posttest) and not affected by exposure to linalool or (Z)-3-hexenyl acetate. In this case, no significant synergistic interaction was found between B. thuringiensis and indole (χ2 [1, N = 74] = 1.62, P = 0.12), and the contribution of indole to the mortality by B. thuringiensis was only additive.
FIG 2.
Effect of the tested HIPVs on S. exigua larvae susceptibility to B. thuringiensis (Xentari). Ind (indole 4 mg), Lin (linalool 0.1%) and Hac (hexenyl acetate 0.1%). Percentage of larval mortality for the different combinations. Observed mortality (O) and expected mortality (E), assuming the additive model. Statistical analyses were performed using one-way ANOVA with a Newman-Keuls posttest to compare the mortalities, and the chi-square test was used to check whether there is a synergistic or additive effect between the different treatments.
Immune status of insects exposed to volatiles.
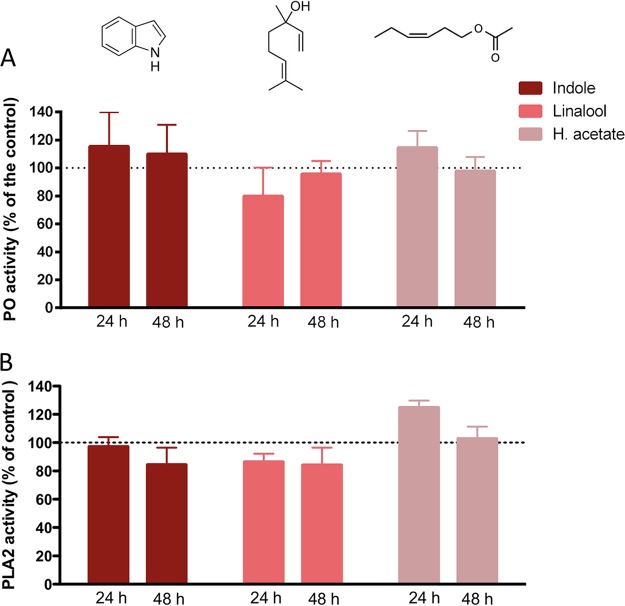
To test if exposure to HIPVs affects the immunological status of S. exigua, we measured the levels of two enzymatic key markers of the cellular immunity in insects, phenoloxidase (PO), and phospholipase A2 (PLA2). PO is involved in the processes of encapsulation and melanization (29), whereas the enzyme PLA2 activates the eicosanoid pathway, which is involved in the cellular immunity in insects (30). Several studies have shown that the inhibition of eicosanoids increases insect susceptibility to baculoviruses (31, 32). PO activity was measured in the hemolymph of L3 larvae exposed to the different HIPVs for 24 and 48 h. Compared to controls, the exposure had no effect on PO activity (Fig. 3A). PLA2 activity was measured on the whole-body extract of L3 larvae exposed to the three HIPVs for 24 and 48 h (Fig. 3B). Again, no effect on enzyme activity was observed for any of the treatments.
FIG 3.
Effect of the tested HIPVs on two enzymatic markers of the cellular immunity of S. exigua. (A) Relative phenoloxidase activity in the hemolymph of insects exposed to selected volatiles at 24 and 48 h after exposure. (B) Relative PLA2 activity in the fat bodies of insects exposed to selected volatiles at 24 and 48 h after exposure. For both markers, the activity is normalized according to the activity obtained for the nonexposed insects.
Changes in midgut microbiota after exposure to indole.
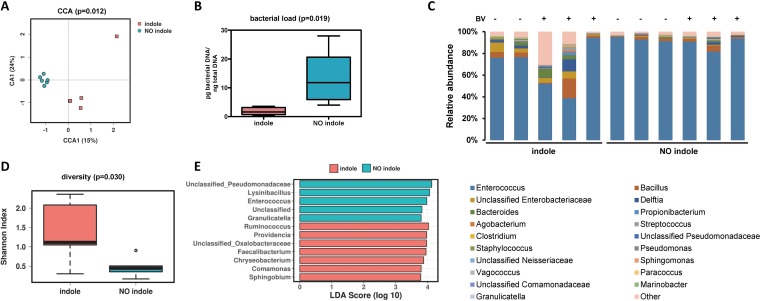
Indole is known to be involved in bacterial processes, either by mediating bacterial communication and quorum sensing (33) or through antimicrobial activity via RNA synthesis inhibition (34). We therefore also evaluated, in side-by-side experiments, the effect of indole exposure, as well as baculovirus infection, on the larval gut microbiota load and composition. No major effect of baculovirus infection on microbiota composition and diversity was observed 48 h postinfection. However, exposure to indole had a significant effect on the microbiota load, alpha diversity, and composition (Fig. 4). A multivariate canonical correspondence analysis (CCA) showed a clearly different microbial profile (P = 0.012) between the indole-exposed and nonexposed groups (Fig. 4A). Forty-eight hours of exposure to indole resulted in a significant decrease in gut bacterial load (P < 0.019; Fig. 4B) and a significant increase in bacterial diversity (P = 0.03; based on the Shannon diversity index) (Fig. 4C and D). The relative abundance in percentages of the top genera in each sample, as depicted in Fig. 4C, suggest that changes in diversity would be associated with reduction in the relative abundance of bacteria of the genus Enterococcus (Fig. 4C). Linear discriminant analysis effect size (LEfSE) confirmed this differential abundance of the genus Enterococcus and revealed specific genera that were differentially enriched in each group (Fig. 4E). Among the most represented genera in the indole-exposed group were Faecalibacterium, Ruminococcus, Comanomonas, Chryseobacterium, Providencia, Sphingobium, and unclassified Oxalobacteriaceae genera, while four different genera were significantly overrepresented in the insects that were not exposed to indole.
FIG 4.
Effect of the exposure to indole on the gut microbiota composition of the S. exigua larvae. (A) Canonical correspondence analysis (CCA) showing the relationship between gut microbiome composition (genus level) in the indole-exposed and nonexposed insects. (B) Bacterial load calculated for the samples from the indole-exposed and nonexposed insects. (C) Relative abundance in percentage of the top genera in samples from the indole-exposed and nonexposed insects. Exposition to the viral infection is indicated as + in the top of the panel. (D) Microbial diversity calculated as the Shannon index in the samples from the indole-exposed and nonexposed insects. (E) LefSE (linear discriminant analysis effect size) results, reporting the more significantly overrepresented taxa for the indole and no-indole groups.
DISCUSSION
HIPVs play multiple roles in plant-herbivore interactions (17, 35, 36). Here, we show that HIPVs, in addition to their already known roles, can also have a role in affecting the susceptibility of insect herbivores to viral and bacterial pathogens. These entomopathogens occur naturally in the ecosystem (37–39), but they are also used as active ingredients in biopesticides (40). Specifically, we found that indole and linalool, two volatiles produced and released by various plant species, such as maize, cotton, rice, tomato, tobacco, etc. in response to herbivory (10, 36, 41–44), have a synergistic effect on SeMNPV infectivity. To a lesser degree, the combination of indole with the bacterium B. thuringiensis boosted mortality caused by the bacteria in an additive manner.
In the case of indole, its effect on the susceptibility of S. exigua to the baculovirus was observed in different experimental settings and at concentrations of the pathogen. Moreover, a synergistic mortality effect was found at a viral dose that caused only sublethal infections in most of the tested insects. At a higher viral dose, which caused mortality in most of the infected insects, the effect of indole exposure was reflected in virus virulence. This increase in virulence in the presence of indole was confirmed under more controlled conditions, where the insects were exposed under a continuous airflow and at a realistic concentration of indole.
Baculovirus infections are very common in natural populations of Lepidoptera (45). In the case of S. exigua, 54% of larvae in the field have a nonlethal infection of their baculovirus, SeMNPV (38). The dynamics of pathogen-host interactions in insects are determined primarily by host and pathogen density, but also by the virulence of the pathogen (impact on infected individuals ranging from slightly debilitating to lethal) (46). Our results suggest that exposure to indole and linalool can increase pathogen virulence of SeMNPV to a degree that normally sublethal doses of the virus become lethal. This may have an important impact in the context of crop protection and could help to significantly decrease pest densities in the field and consequently reduce crop damage. Recent studies have started to provide evidence for selectable plant traits that enhance the ability of pathogens to control insect pests (47). Our data further confirm the potential of plant traits to enhance the efficacy of entomopathogens as biocontrol agents. It is likely that from the extensive arsenal of metabolites produced by plants (48), many others could also synergize the pest management potential of entomopathogens that are naturally found in the ecosystem or artificially released as pest control agents.
We also explored the molecular basis that underlies the effect of HIPVs on susceptibility to entomopathogens. Indole and oxindole have previously been found to be produced by entomopathogenic bacteria and to inhibit the in vitro activity of PLA2, one of the key enzymes from the eicosanoid pathway that is involved in the cellular immunity (49, 50). It has been shown that certain inhibitors of the eicosanoid pathway (including a PLA2 inhibitor), when added to the rearing diet of caterpillars (at concentrations of about 30 to 50 mM), can increase their susceptibility to nucleopolyhedroviruses (31). In our study, however, when we analyzed the effect of HIPV exposure on PLA2 activity of the exposed larvae, we did not detect any reduction in the enzymatic activity for any of the three volatiles. Similarly, no effect on the activity of PO, an enzyme involved in cellular and humoral defense, was observed in the insects exposed to the three HIPVs. Even though we cannot fully exclude the possibility that the volatiles have a direct negative effect on other aspects of the insects’ immune system, these results strongly suggest that another mechanism, different from the direct interference with the insect’s cellular immunity, mediates the enhanced susceptibility after exposure to indole and linalool. One such mechanism could involve changes in the gut microbiota caused by the HIPVs. We and others have previously shown that changes in the gut microbiota composition can affect an insect’s susceptibility to bacterial (26, 51) and viral (52) pathogens. Insect gut microbiota composition and homeostasis depend on its diet (53) and immune system (54), but it also relies on the microbial synthesis and secretion of metabolites and enzymes that contribute to the establishment of the interactions with the host and other microbes (55). Gut microbiota influences in insect development and physiology (56), and, consequently, dysbiosis in microbiota composition may have important effects on gut physiology and homeostasis, leading to enhanced success of viral infections.
The changes that we observed in the gut microbial composition after indole exposure may be caused by direct effects of the indole on the microbiota or by changes in physiological parameters of the larvae that might indirectly affect an insect’s microbiota. Given the known role of indole in microbial processes (57), it is possible that the observed changes are the result of direct exposure of the gut microbes to indole. More than 85 bacterial species (Gram negative as well as Gram positive) can synthesize indole (58). As an intercellular signal molecule, indole controls diverse aspects of bacterial physiology in indole-producing bacteria, such as spore formation, plasmid stability, drug resistance, biofilm formation, and virulence (57, 58). In our measurements, species from the genus Enterococcus were the most dominant in the microbiota community of the S. exigua larvae. It is likely that indole exposure interfered with normal growth of Enterococcus species, thereby possibly promoting the growth of other bacterial species that could affect the insects’ physiology in a way that it lowers their resistance to entomopathogens. Vega et al. (59) have shown that bacterial communication through indole signaling induces persistence, a phenomenon that allows a subset of an isogenic bacterial population to tolerate antibiotic treatment. It is possible that the observed indole-induced increase in microbial diversity involves a similar mechanism, in this case leading to enhanced susceptibility to the pathogen. Indeed, it has been shown that indole has a minor beneficial effect on Escherichia coli when it is cultured with Enterococccus faecalis (60). It would be interesting to test the effect of indole on the growth of specific Enterococcus species isolates from the larval gut.
In summary, our results support a novel role for the HIPVs in plant-insect-microbe interactions. In addition to their function in direct defense, signaling between plant tissues, and multitrophic interactions (61), HIPVs may mediate interactions between insects and their pathogens. These interactions are likely affected by altered gut microbiota composition as a result of indole exposure. The observed increase in susceptibility to viral and bacterial pathogens provides an additional element to the possible application of HIPVs to regulate the abundance and dynamics of insect pests. Further experiments using other insect-pathogen combinations and other HIPVs are needed to determine the prevalence of the phenomenon and to further resolve the underlying genetic and physiological mechanisms.
EXPERIMENTAL PROCEDURES
Insects and chemicals.
The Spodoptera exigua colony was established with eggs that were provided by Andermatt Biocontrol AG (Grossdietwil, Switzerland) and was continuously reared on artificial diet (62) at 25 ± 3°C with 70% ± 5% relative humidity and a photoperiod of light/dark (LD) 16:8 h.
The synthetic volatiles used in the bioassays [indole, linalool and (Z)-3-hexenyl acetate] were purchased from Sigma-Aldrich.
Effect of the HIPVs on SeMNPV infectivity.
For the exposure to selected HIPVs, we prepared a 0.2-ml microcentrifuge tube, to which we added 4 mg of indole powder or 10 µl of 10% of linalool or 10% (Z)-3-hexenyl acetate (in distilled water). After perforating the lid of a tube with a G25 needle, it was placed in a rearing well (2 cm × 2 cm × 2 cm) that contained an individual larva and a piece of artificial diet. The well was then sealed with microperforated adhesive tape (product no. 9074-L; Frontier Agricultural Sciences).
Aiming to assess the effect of the selected HIPVs on the SeMNPV, third instar (first-day) S. exigua larvae were orally infected and reared in the presence or absence of one of the volatiles. For this, larvae were fed individually with diet plugs (about 0.4 mm3) containing different amounts (102 or 5 × 104) of occlusion bodies (OBs) from the SeMNPV. Larvae were kept for 24 h with the virus-contaminated food. After that, larvae that completely consumed the food were selected for the bioassay and fed with virus-free artificial diet. Larval mortality was then recorded every 12 h until the death or pupation of all the larvae. Mortality curves were then assessed using the Kaplan-Meier method and compared using log-rank analysis (Mentel-Cox test) and the GraphPad Prism program (GraphPad Software Inc., San Diego, CA). In addition, and due to the different levels of mortality for each treatment, changes in virulence were estimated by comparison of the mean time to death. The statistical differences were assessed using either the Student's t test or one-way ANOVA with the Newman-Keuls posttest (GraphPad Prism). Three independent replicates were performed, using 16 larvae per treatment and replicate.
In a second experiment, newly molted third instar larvae were exposed to the volatile indole at approximately 50 ng/h, similar to what is released by caterpillar-infested maize plants (9, 10). For this purpose, volatile dispensers that consisted of 2-ml amber glass vials (Supelco, Sigma-Aldrich) supplied with 20 mg of synthetic indole were used. The vials were closed with an open screw cap with rubber septum. The septum was pierced with 2-µl microcaps (Drummond Scientific, Broomall, PA) through which indole diffused at a constant rate. Groups of caterpillars (5 to 6) were placed in individual plastic cages (5-cm diameter and 2-cm height) covered with a nylon mesh and fed with a cube of artificial diet contaminated with 50 µl of 104 OBs/ml, then kept into glass vessels which contained control or indole-releasing dispenser. Purified air entered these vessels via Teflon tubing at a rate of 0.3 l min−1 to avoid indole overaccumulation. The larvae were reared at 22 ± 2°C and supplied with fresh diet every 48 h. Mortality curves and mean time to death were assessed as described above. Three independent replicates were performed using 16 larvae per treatment and replicate.
Effect of the different HIPVs on susceptibility to B. thuringiensis infection.
Effect of the selected HIPVs on the entomopathogenic bacterium Bacillus thuringiensis was tested using the surface contamination bioassay method (63). In these experiments, a formulation of wettable granules containing Bacillus thuringiensis subsp. aizawai (XenTari; Kenogard S.A, Spain) was tested. Surface contamination assays were employed with first instar S. exigua larvae, and the larvae were exposed to the different HIPVs, as described for the first experiments. Briefly, a volume of 50 µl of the bacterial suspension was applied on the surface of the diet in individual wells (0.5 ng/cm2) and left to dry for 30 to 60 min in a flow hood. Then, first instar larvae were placed individually in each well, together with the tube containing the respective volatile, and mortality was recorded after 5 days. Statistical analysis was performed using one-way ANOVA with the Newman-Keuls posttest (GraphPad Prism). Three independent replicates were performed using 16 larvae per treatment and replicate.
Analyses of the interaction of entomopathogens with the different HIPVs.
Possible antagonistic/synergistic interactions between entomopathogens and each of the selected HIPVs were determined using the mortality values at seven and 5 days postinfection for the SeMNPV and B. thuringiensis treatments, respectively. Mortality percentages were corrected using the Abbott correction (64). Then, the expected mortality was calculated with the response addition model (65), which is used to evaluate mixtures of substances that have different modes of action, employing the following equation:
where E(cMIX) is the prediction of a total effect of the mixture (in our case, mortality) and E(cA) and E(cB) are the observed effects caused by individual SeMNPV or B. thuringiensis treatments and the volatile, respectively (65). Significance of the deviations between the observed and expected mortality values was assessed using a chi-square test (GraphPad Prism).
Effect of the HIPVs on insect immunity.
In order to study the effect of the HIPVs on the immune system of S. exigua, the enzymatic activities of the phenoloxidase (PO) and phospholipase A2 (PLA2), two markers of cellular immunity, were measured. For the PO assay, hemolymph of third instar (L3) larvae exposed or not exposed to a volatile (same conditions as above) was extracted 24 and 48 h after exposure and centrifuged at 500 × g for 2 min at 4°C to remove the hemocytes. Four μl of cell-free hemolymph, 46 μl of 1× phosphate-buffered saline (PBS), and 50 μl of the substrate l-dopamine (100 μg/ml in 1× PBS) were added to each well in a 96-well microtiter plate. PO activity was determined by monitoring the increase of absorbance at 492 nm for 30 min using the Infinite 200 Pro multimode plate reader (Tecan Group Ltd., Switzerland). The activity of the enzyme was represented as the initial velocity (V0) of the reaction, measuring the change in absorbance per second. To perform the assay of PLA2 activity, bodies of the L3 larvae mentioned above were homogenized in Tris-HCl 50 mM (pH 7.0) and centrifuged at maximum speed for 5 min at 4°C. The protein concentration was determined using the Bradford assay (72), with bovine serum albumin (BSA) as a standard. The enzymatic reaction was done with 136 μl of Tris-HCl 50 mM (pH 7.0), 1 μl of CaCl2, 150 mM, 1.5 μl of BSA 10%, 10 μl of larval extract, and 1.5 μl of pyrene-labeled substrate [1-hexadecanoyl-2-(1-pyrenedecanoyl)-sn-glycero-3-phosphocholine; 10 mM in ethanol; Thermo Fisher]. A multimode plate reader (Tecan) was used to measure fluorescence intensity by excitation at 345 nm and emission at 398 nm. The activity of PLA2 was then calculated as the changes in fluorescence per second. Due to the intrinsic variability between biological replicates, values for each enzyme and treatment were calculated as the difference in percentage of activity with unexposed insects within each replicate.
Microbiota composition and diversity.
To determine if exposure to indole and/or infection with the baculovirus influence the gut microbiota of S. exigua, third instar (first-day) larvae were exposed to indole and infected with SeMNPV, as described above. After 48 h, larval midguts from each treatment were dissected, pooled by treatment and homogenized in Luria-Bertani (LB) medium supplemented with 10% of glycerol. A fraction of the homogenized guts was used for total DNA extraction using the MasterPure DNA purification kit (Epicentre, Madison, WI). Three replicates were performed using 5 larvae per treatment and replicate, and for each replicate, the different treatments were applied simultaneously in a side-by-side manner. PCR amplification of the 16S rRNA (V3-V4 region) and sequencing were carried out using a 2 × 300-bp paired-end run (MiSeq reagent kit v3) on an Illumina MiSeq sequencing platform at the Foundation for the Promotion of Health and Biomedical Research (FISABIO; Valencia). Quality assessment of obtained reads was done with the prinseq-lite program (66) with defined parameters (i.e., min_length, 50; trim_qual_right, 20; trim_qual_type, mean; trim_qual_window, 20). Paired reads from Illumina sequencing were joined using fastq-join from the ea-tools suite (67). Filtered and demultiplexed sequences were then processed with the open-source software QIIME v.1.9. (68), using default parameters. A total of 12 samples were sequenced. One sequence showed fewer than 1,000 reads and was removed for further analysis. The sequences were then binned into operational taxonomic units (OTUs) using de novo OTU picking based on 97% identity and filtering the unassigned taxa. Bacterial composition was also determined with filtering of the unassigned, Chloroflexi, and Cyanobacteria taxa, and the 20 most abundant genera were represented in a bar graphic using Excel software. Calypso version 8.2 (69) was used with the OTU table data transformed by CSS (cumulative sum scaling) + log with total sum normalization, to generate a canonical correspondence analysis (CCA) plot for multivariate analysis at genus level, with indole exposure as factor. Alpha diversity using the Shannon index and linear discriminant analysis effect size (LEfSE) (70) was determined at genus level, and again with indole exposure as factor.
Total DNA was also used to determine the bacterial load by specific quantitative PCR (qPCR), using universal primers for the 16S rRNA gene (71). The qPCRs were carried out in a StepOnePlus real-time PCR system (Applied Biosystems, Foster City, CA). Reactions were performed using 5× Hot FIREpol EvaGreen qPCR mix Plus (ROX) (Solis BioDyne, Tartu, Estonia) in a total volume of 20 μl. The bacterial concentration in each sample was calculated by comparison with the threshold cycle (CT) values obtained from a standard curve of known bacterial DNA concentration. These were generated using serial 10-fold dilutions of DNA extracted from E. coli bacteria. Bacterial loads were statistically compared with the Student's t test (GraphPad Prism).
Supplementary Material
ACKNOWLEDGMENTS
This study was partially supported by the Spanish Ministry of Economy, Industry and Competitiveness and by European FEDER funds (grant AGL2014-57752-C2-2R). A.F. was the recipient of a Ph.D. grant from the Spanish Ministry of Education, Culture and Sport (grant FPU16/02363).
We thank Rosa Maria González-Martínez for her excellent help with insect rearing and laboratory management and Alejandro Tena (IVIA, Spain) for his suggestions on the data analysis.
We declare that we have no competing interests.
Footnotes
Supplemental material for this article may be found at https://doi.org/10.1128/AEM.01468-18.
REFERENCES
- 1.Jander G, Howe G. 2008. Plant interactions with arthropod herbivores: state of the field. Plant Physiol 146:801–803. doi: 10.1104/pp.104.900247. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Schoonhoven LM, Van Loon JJ, Dicke M. 2005. Insect-plant biology. Oxford University Press, Oxford, UK. [Google Scholar]
- 3.War AR, Paulraj MG, Ahmad T, Buhroo AA, Hussain B, Ignacimuthu S, Sharma HC. 2012. Mechanisms of plant defense against insect herbivores. Plant Signal Behav 7:1306–1320. doi: 10.4161/psb.21663. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Lazebnik J, Frago E, Dicke M, van Loon JJA. 2014. Phytohormone mediation of interactions between herbivores and plant pathogens. J Chem Ecol 40:730–741. doi: 10.1007/s10886-014-0480-7. [DOI] [PubMed] [Google Scholar]
- 5.Mithöfer A, Boland W. 2012. Plant defense against herbivores: chemical aspects. Annu Rev Plant Biol 63:431–450. doi: 10.1146/annurev-arplant-042110-103854. [DOI] [PubMed] [Google Scholar]
- 6.Bautista-Lozada A, Espinosa-García FJ. 2013. Odor uniformity among tomato individuals in response to herbivore depends on insect species. PLoS ONE 8:e77199. doi: 10.1371/journal.pone.0077199. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Ali JG, Agrawal AA. 2012. Specialist versus generalist insect herbivores and plant defense. Trends Plant Sci 17:293–302. doi: 10.1016/j.tplants.2012.02.006. [DOI] [PubMed] [Google Scholar]
- 8.Hilker M, Fatouros NE. 2015. Plant responses to insect egg deposition. Annu Rev Entomol 60:493–515. doi: 10.1146/annurev-ento-010814-020620. [DOI] [PubMed] [Google Scholar]
- 9.Veyrat N, Robert CAM, Turlings TCJ, Erb M. 2016. Herbivore intoxication as a potential primary function of an inducible volatile plant signal. J Ecol 104:591–600. doi: 10.1111/1365-2745.12526. [DOI] [Google Scholar]
- 10.Erb M, Veyrat N, Robert CA, Xu H, Frey M, Ton J, Turlings TC. 2015. Indole is an essential herbivore-induced volatile priming signal in maize. Nature Communications 6 doi: 10.1038/ncomms7273. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Kim J, Felton GW. 2013. Priming of antiherbivore defensive responses in plants. Insect Sci 20:273–285. doi: 10.1111/j.1744-7917.2012.01584.x. [DOI] [PubMed] [Google Scholar]
- 12.Frost CJ, Mescher MC, Carlson JE, De Moraes CM. 2008. Plant defense priming against herbivores: getting ready for a different battle. Plant Physiol 146:818–824. doi: 10.1104/pp.107.113027. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Heil M, Silva Bueno JC. 2007. Within-plant signaling by volatiles leads to induction and priming of an indirect plant defense in nature. Proc Natl Acad Sci U S A 104:5467–5472. doi: 10.1073/pnas.0610266104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Dicke M, Sabelis MW. 1987. How plants obtain predatory mites as bodyguards. Netherlands J Zool 38:148–165. doi: 10.1163/156854288X00111. [DOI] [Google Scholar]
- 15.Turlings TCJ, Tumlinson JH, Lewis WJ. 1990. Exploitation of herbivore-induced plant odors by host-seeking parasitic wasps. Science 250:1251–1253. doi: 10.1126/science.250.4985.1251. [DOI] [PubMed] [Google Scholar]
- 16.De Moraes CM, Lewis WJ, Paré PW, Alborn HT, Tumlinson JH. 1998. Herbivore-infested plants selectively attract parasitoids. Nature 393:570. doi: 10.1038/31219. [DOI] [Google Scholar]
- 17.Maag D, Erb M, Köllner TG, Gershenzon J. 2015. Defensive weapons and defense signals in plants: some metabolites serve both roles. Bioessays 37:167–174. doi: 10.1002/bies.201400124. [DOI] [PubMed] [Google Scholar]
- 18.Sugimoto K, Matsui K, Iijima Y, Akakabe Y, Muramoto S, Ozawa R, Uefune M, Sasaki R, Alamgir KM, Akitake S, Nobuke T, Galis I, Aoki K, Shibata D, Takabayashi J. 2014. Intake and transformation to a glycoside of (Z)-3-hexenol from infested neighbors reveals a mode of plant odor reception and defense. Proc Natl Acad Sci U S A 111:7144–7149. doi: 10.1073/pnas.1320660111. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Cory JS, Hoover K. 2006. Plant-mediated effects in insect-pathogen interactions. Trends Ecol Evol 21:278–286. doi: 10.1016/j.tree.2006.02.005. [DOI] [PubMed] [Google Scholar]
- 20.Shikano I. 2017. Evolutionary ecology of multitrophic interactions between plants, insect herbivores and entomopathogens. J Chem Ecol 43:586–598. doi: 10.1007/s10886-017-0850-z. [DOI] [PubMed] [Google Scholar]
- 21.Shikano I, Shumaker KL, Peiffer M, Felton GW, Hoover K. 2017. Plant-mediated effects on an insect–pathogen interaction vary with intraspecific genetic variation in plant defences. Oecologia 183:1121–1134. doi: 10.1007/s00442-017-3826-3. [DOI] [PubMed] [Google Scholar]
- 22.Elliot SL, Sabelis MW, Janssen A, Van Der G LPS, Beerling EAM, Fransen J. 2000. Can plants use entomopathogens as bodyguards? Ecology Lett 3:228–235. doi: 10.1046/j.1461-0248.2000.00137.x. [DOI] [Google Scholar]
- 23.Lin Y, Hussain M, Avery PB, Qasim M, Fang D, Wang L. 2016. Volatiles from plants induced by multiple aphid attacks promote conidial performance of Lecanicillium lecanii. PLoS One 11:e0151844. doi: 10.1371/journal.pone.0151844. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Brown GC, Prochaska GL, Hildebrand DF, Nordin GL, Jackson DM. 1995. Green leaf volatiles inhibit conidial germination of the entomopathogen Pandora neoaphidis (Entomopthorales: Entomophthoraceae). Environ Entomol 24:1637–1643. doi: 10.1093/ee/24.6.1637. [DOI] [Google Scholar]
- 25.Broderick NA, Raffa KF, Handelsman J. 2006. Midgut bacteria required for Bacillus thuringiensis insecticidal activity. Proc Natl Acad Sci U S A 103:15196–15199. doi: 10.1073/pnas.0604865103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Hernández-Martínez P, Naseri B, Navarro-Cerrillo G, Escriche B, Ferré J, Herrero S. 2010. Increase in midgut microbiota load induces an apparent immune priming and increases tolerance to Bacillus thuringiensis. Environ Microbiol 12:2730–2737. doi: 10.1111/j.1462-2920.2010.02241.x. [DOI] [PubMed] [Google Scholar]
- 27.Van Arnam EB, Currie CR, Clardy J. 2018. Defense contracts: molecular protection in insect-microbe symbioses. Chem Soc Rev 47:1638–1651. doi: 10.1039/c7cs00340d. [DOI] [PubMed] [Google Scholar]
- 28.Gasmi L, Martínez-Solís M, Frattini A, Ye M, Collado MC, Turlings TCJ, Erb M, Herrero S. 2018. Can herbivore-induced volatiles protect plants by increasing the herbivores’ susceptibility to natural pathogens? bioRxiv 10.1101/317560. [DOI] [PMC free article] [PubMed]
- 29.Cerenius L, Soderhall K. 2004. The prophenoloxidase-activating system in invertebrates. Immunol Rev 198:116–126. doi: 10.1111/j.0105-2896.2004.00116.x. [DOI] [PubMed] [Google Scholar]
- 30.Stanley D, Miller J, Tunaz H. 2009. Eicosanoid actions in insect immunity. J Innate Immun 1:282–290. doi: 10.1159/000210371. [DOI] [PubMed] [Google Scholar]
- 31.Stanley DW, Shapiro M. 2009. Eicosanoids influence insect susceptibility to nucleopolyhedroviruses. J Invertebr Pathol 102:245–249. doi: 10.1016/j.jip.2009.09.002. [DOI] [PubMed] [Google Scholar]
- 32.Kim J, Kim Y. 2011. Benzylideneacetone, an eicosanoid biosynthesis inhibitor enhances baculovirus pathogenicity in the diamondback moth, Plutella xylostella. J Invertebr Pathol 106:308–313. doi: 10.1016/j.jip.2010.11.006. [DOI] [PubMed] [Google Scholar]
- 33.Kim J, Park W. 2013. Indole inhibits bacterial quorum sensing signal transmission by interfering with quorum sensing regulator folding. Microbiology 159:2616–2625. doi: 10.1099/mic.0.070615-0. [DOI] [PubMed] [Google Scholar]
- 34.Sundar L, Chang F. 1993. Antimicrobial activity and biosynthesis of indole antibiotics produced by Xenorhabdus nematophilus. J Gen Microbiol 139:3139–3148. doi: 10.1099/00221287-139-12-3139. [DOI] [PubMed] [Google Scholar]
- 35.Arimura G-i, Matsui K, Takabayashi J. 2009. Chemical and molecular ecology of herbivore-induced plant volatiles: proximate factors and their ultimate functions. Plant Cell Physiol 50:911–923. doi: 10.1093/pcp/pcp030. [DOI] [PubMed] [Google Scholar]
- 36.Turlings TCJ, Erb M. 2018. Tritrophic interactions mediated by herbivore-induced plant volatiles: mechanisms, ecological relevance, and application potential. Annu Rev Entomol 63: 433–452. doi: 10.1146/annurev-ento-020117-043507. [DOI] [PubMed] [Google Scholar]
- 37.Hernandez-Rodriguez CS, Ferre J. 2009. Ecological distribution and characterization of four collections of Bacillus thuringiensis strains. JBasic Microbiol 49:152–157. doi: 10.1002/jobm.200800121. [DOI] [PubMed] [Google Scholar]
- 38.Virto C, Navarro D, Tellez MM, Herrero S, Williams T, Murillo R, Caballero P. 2014. Natural populations of Spodoptera exigua are infected by multiple viruses that are transmitted to their offspring. J Invertebr Pathol 122:22–27. doi: 10.1016/j.jip.2014.07.007. [DOI] [PubMed] [Google Scholar]
- 39.Williams T, Virto C, Murillo R, Caballero P. 2017. Covert infection of insects by baculoviruses. Front Microbiol 8:1337. doi: 10.3389/fmicb.2017.01337. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Lacey LA, Grzywacz D, Shapiro-Ilan DI, Frutos R, Brownbridge M, Goettel MS. 2015. Insect pathogens as biological control agents: back to the future. J Invertebr Pathol 132:1–41. doi: 10.1016/j.jip.2015.07.009. [DOI] [PubMed] [Google Scholar]
- 41.Dubouzet JG, Matsuda F, Ishihara A, Miyagawa H, Wakasa K. 2013. Production of indole alkaloids by metabolic engineering of the tryptophan pathway in rice. Plant Biotechnol J 11:1103–1111. doi: 10.1111/pbi.12105. [DOI] [PubMed] [Google Scholar]
- 42.McCall PJ, Turlings TCJ, Loughrin J, Proveaux AT, Tumlinson JH. 1994. Herbivore-induced volatile emissions from cotton (Gossypium hirsutum L.) seedlings. J Chem Ecol 20:3039–3050. doi: 10.1007/BF02033709. [DOI] [PubMed] [Google Scholar]
- 43.van Schie CCN, Haring MA, Schuurink RC. 2007. Tomato linalool synthase is induced in trichomes by jasmonic acid. Plant Mol Biol 64:251–263. doi: 10.1007/s11103-007-9149-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Stenberg JA, Heil M, Åhman I, Björkman C. 2015. Optimizing crops for biocontrol of pests and disease. Trends Plant Sci 20:698–712. doi: 10.1016/j.tplants.2015.08.007. [DOI] [PubMed] [Google Scholar]
- 45.Clem RJ, Passarelli AL. 2013. Baculoviruses: sophisticated pathogens of insects. PLoS Pathog 9:e1003729. doi: 10.1371/journal.ppat.1003729. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Myers JH, Cory JS. 2016. Ecology and evolution of pathogens in natural populations of Lepidoptera. Evol Appl 9:231–247. doi: 10.1111/eva.12328. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Shikano I, Rosa C, Tan C-W, Felton GW. 2017. Tritrophic interactions: microbe-mediated plant effects on insect herbivores. Annu Rev Phytopathol 55:313–331. doi: 10.1146/annurev-phyto-080516-035319. [DOI] [PubMed] [Google Scholar]
- 48.Schwab W. 2003. Metabolome diversity: too few genes, too many metabolites? Phytochemistry 62:837–849. doi: 10.1016/S0031-9422(02)00723-9. [DOI] [PubMed] [Google Scholar]
- 49.Sadekuzzaman M, Park Y, Lee S, Kim K, Jung JK, Kim Y. 2017. An entomopathogenic bacterium, Xenorhabdus hominickii ANU101, produces oxindole and suppresses host insect immune response by inhibiting eicosanoid biosynthesis. J Invertebr Pathol 145:13–22. doi: 10.1016/j.jip.2017.03.004. [DOI] [PubMed] [Google Scholar]
- 50.Seo S, Lee S, Hong Y, Kim Y. 2012. Phospholipase A2 inhibitors synthesized by two entomopathogenic bacteria, Xenorhabdus nematophila and Photorhabdus temperata subsp. temperata. Appl Environ Microbiol 78:3816–3823. doi: 10.1128/AEM.00301-12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Broderick NA, Robinson CJ, McMahon MD, Holt J, Handelsman J, Raffa KF. 2009. Contributions of gut bacteria to Bacillus thuringiensis-induced mortality vary across a range of Lepidoptera. BMC Biol 7:11. doi: 10.1186/1741-7007-7-11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Jakubowska AK, Vogel H, Herrero S. 2013. Increase in gut microbiota after immune suppression in baculovirus-infected larvae. PLoS Pathog 9:e1003379. doi: 10.1371/journal.ppat.1003379. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Robinson CJ, Schloss P, Ramos Y, Raffa K, Handelsman J. 2010. Robustness of the bacterial community in the cabbage white butterfly larval midgut. Microb Ecol 59:199–211. doi: 10.1007/s00248-009-9595-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Buchon N, Broderick NA, Lemaitre B. 2013. Gut homeostasis in a microbial world: insights from Drosophila melanogaster. Nat Rev Microbiol 11:615–626. doi: 10.1038/nrmicro3074. [DOI] [PubMed] [Google Scholar]
- 55.Lee W-J, Hase K. 2014. Gut microbiota-generated metabolites in animal health and disease. Nat Chem Biol 10:416–424. doi: 10.1038/nchembio.1535. [DOI] [PubMed] [Google Scholar]
- 56.Engel P, Moran NA. 2013. The gut microbiota of insects – diversity in structure and function. FEMS Microbiol Rev 37:699–735. doi: 10.1111/1574-6976.12025. [DOI] [PubMed] [Google Scholar]
- 57.Kim J, Park W. 2015. Indole: a signaling molecule or a mere metabolic byproduct that alters bacterial physiology at a high concentration? J Microbiol 53:421–428. doi: 10.1007/s12275-015-5273-3. [DOI] [PubMed] [Google Scholar]
- 58.Lee J-H, Lee J. 2010. Indole as an intercellular signal in microbial communities. FEMS Microbiol Rev 34:426–444. doi: 10.1111/j.1574-6976.2009.00204.x. [DOI] [PubMed] [Google Scholar]
- 59.Vega NM, Allison KR, Khalil AS, Collins JJ. 2012. Signaling-mediated bacterial persister formation. Nat Chem Biol 8:431–433. doi: 10.1038/nchembio.915. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Pringle SL, Palmer KL, McLean RJC. 2017. Indole production provides limited benefit to Escherichia coli during co-culture with Enterococcus faecalis. Arch Microbiol 199:145–153. doi: 10.1007/s00203-016-1289-2. [DOI] [PubMed] [Google Scholar]
- 61.Dicke M. 2016. Plant phenotypic plasticity in the phytobiome: a volatile issue. Curr Opin Plant Biol 32:17–23. doi: 10.1016/j.pbi.2016.05.004. [DOI] [PubMed] [Google Scholar]
- 62.Elvira S, Gorria N, Muñoz D, Williams T, Caballero P. 2010. A simplified low-cost diet for rearing Spodoptera exigua (Lepidoptera: Noctuidae) and its effect on S. exigua nucleopolyhedrovirus production. J Econ Entomol 103:17–24. doi: 10.1603/EC09246. [DOI] [PubMed] [Google Scholar]
- 63.Eberle KE, Wennman JT, Klespies RG, Jehle JA. 2012. Basic techniques in insect virology, p 15–74. In Lacey LA. (ed), Manual of techniques in invertebrate pathology, 2nd ed Academic Press, London, UK. [Google Scholar]
- 64.Abbott WS. 1925. A method of computing the effectiveness of an insecticide. J Economic Entomology 18:265–267. doi: 10.1093/jee/18.2.265a. [DOI] [Google Scholar]
- 65.Finney DJ. 1942. The analysis of toxicity tests on mixtures of Poisons. Ann Applied Biology 29:82–94. doi: 10.1111/j.1744-7348.1942.tb06923.x. [DOI] [Google Scholar]
- 66.Schmieder R, Edwards R. 2011. Quality control and preprocessing of metagenomic datasets. Bioinformatics 27:863–864. doi: 10.1093/bioinformatics/btr026. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Aronesty E. 2011. Command-line tools for processing biological sequencing data, ea-utils. Expression Analysis, Durham, NC. [Google Scholar]
- 68.Caporaso JG, Kuczynski J, Stombaugh J, Bittinger K, Bushman FD, Costello EK, Fierer N, Peña AG, Goodrich JK, Gordon JI, Huttley GA, Kelley ST, Knights D, Koenig JE, Ley RE, Lozupone CA, McDonald D, Muegge BD, Pirrung M, Reeder J, Sevinsky JR, Turnbaugh PJ, Walters WA, Widmann J, Yatsunenko T, Zaneveld J, Knight R. 2010. QIIME allows analysis of high-throughput community sequencing data. Nat Methods 7:335–336. doi: 10.1038/nmeth.f.303. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Zakrzewski M, Proietti C, Ellis JJ, Hasan S, Brion M-J, Berger B, Krause L. 2016. Calypso: a user-friendly web-server for mining and visualizing microbiome–environment interactions. Bioinformatics 33:782–783. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Segata N, Izard J, Waldron L, Gevers D, Miropolsky L, Garrett WS, Huttenhower C. 2011. Metagenomic biomarker discovery and explanation. Genome Biol 12:R60. doi: 10.1186/gb-2011-12-6-r60. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Nadkarni MA, Martin FE, Jacques NA, Hunter N. 2002. Determination of bacterial load by real-time PCR using a broad-range (universal) probe and primers set. Microbiology 148:257–266. doi: 10.1099/00221287-148-1-257. [DOI] [PubMed] [Google Scholar]
- 72.Bradford MM. 1976. A rapid and sensitive method for the quantitation of microgram quantities of protein utilizing the principle of protein-dye binding. Anal Biochem 72:248–254. doi: 10.1016/0003-2697(76)90527-3. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.