The biochemical processes and enzymes responsible for glycolysis and alcoholic fermentation by the yeast S. cerevisiae have long been the subject of scientific research. Nevertheless, the factors determining fermentation performance in vivo are not fully understood. As a result, the industrial breeding of yeast strains has required empirical characterization of fermentation by screening numerous mutants through laborious fermentation tests. To establish a rational and efficient breeding strategy, key regulators of alcoholic fermentation need to be identified. In the present study, we focused on how sake yeast strains of S. cerevisiae have acquired high alcoholic fermentation performance. Our findings provide a rational molecular basis to design yeast strains with optimal fermentation performance for production of alcoholic beverages and bioethanol. In addition, as the evolutionarily conserved TORC1-Greatwall-PP2AB55δ pathway plays a major role in the glycolytic control, our work may contribute to research on carbohydrate metabolism in higher eukaryotes.
KEYWORDS: Cdc55p, Greatwall, PP2AB55δ, Rim15p, Saccharomyces cerevisiae, Schizosaccharomyces pombe, TORC1, alcoholic fermentation, sake yeast
ABSTRACT
Saccharomyces cerevisiae sake yeast strain Kyokai no. 7 (K7) and its relatives carry a homozygous loss-of-function mutation in the RIM15 gene, which encodes a Greatwall family protein kinase. Disruption of RIM15 in nonsake yeast strains leads to improved alcoholic fermentation, indicating that the defect in Rim15p is associated with the enhanced fermentation performance of sake yeast cells. In order to understand how Rim15p mediates fermentation control, we here focused on target-of-rapamycin protein kinase complex 1 (TORC1) and protein phosphatase 2A with the B55δ regulatory subunit (PP2AB55δ), complexes that are known to act upstream and downstream of Rim15p, respectively. Several lines of evidence, including our previous transcriptomic analysis data, suggested enhanced TORC1 signaling in sake yeast cells during sake fermentation. Fermentation tests of the TORC1-related mutants using a laboratory strain revealed that TORC1 signaling positively regulates the initial fermentation rate in a Rim15p-dependent manner. Deletion of the CDC55 gene, encoding B55δ, abolished the high fermentation performance of Rim15p-deficient laboratory yeast and sake yeast cells, indicating that PP2AB55δ mediates the fermentation control by TORC1 and Rim15p. The TORC1-Greatwall-PP2AB55δ pathway similarly affected the fermentation rate in the fission yeast Schizosaccharomyces pombe, strongly suggesting that the evolutionarily conserved pathway governs alcoholic fermentation in yeasts. It is likely that elevated PP2AB55δ activity accounts for the high fermentation performance of sake yeast cells. Heterozygous loss-of-function mutations in CDC55 found in K7-related sake strains may indicate that the Rim15p-deficient phenotypes are disadvantageous to cell survival.
IMPORTANCE The biochemical processes and enzymes responsible for glycolysis and alcoholic fermentation by the yeast S. cerevisiae have long been the subject of scientific research. Nevertheless, the factors determining fermentation performance in vivo are not fully understood. As a result, the industrial breeding of yeast strains has required empirical characterization of fermentation by screening numerous mutants through laborious fermentation tests. To establish a rational and efficient breeding strategy, key regulators of alcoholic fermentation need to be identified. In the present study, we focused on how sake yeast strains of S. cerevisiae have acquired high alcoholic fermentation performance. Our findings provide a rational molecular basis to design yeast strains with optimal fermentation performance for production of alcoholic beverages and bioethanol. In addition, as the evolutionarily conserved TORC1-Greatwall-PP2AB55δ pathway plays a major role in the glycolytic control, our work may contribute to research on carbohydrate metabolism in higher eukaryotes.
INTRODUCTION
Sake, an alcoholic beverage made from fermented rice, typically has a higher alcohol content than beer or wine. During sake fermentation, saccharification by hydrolytic enzymes of Aspergillus oryzae and alcoholic fermentation by Saccharomyces cerevisiae sake yeast are the major bioconversions. Thus, the high alcohol content of sake is at least partly attributable to the unique characteristics of sake yeast. Sake yeast strains have long been selected based on the high fermentation performance as well as the balanced production of aroma and flavor compounds (1, 2). Our previous comparative genomic and transcriptomic analyses revealed that a representative sake yeast, strain Kyokai no. 7 (K7), and its relatives carry a loss-of-function mutation in RIM15 (rim155054_5055insA), a gene of a highly conserved Greatwall family protein kinase (3–5). Disruption of the RIM15 gene in nonsake yeast strains, such as laboratory, beer, and bioethanol strains, leads to an increase in the fermentation rate (5–9), demonstrating that Rim15p inhibits alcoholic fermentation. Thus, the rim155054_5055insA mutation appears to be associated with the enhanced fermentation property of K7. Nevertheless, this loss-of-function mutation cannot be solely responsible for the sake yeast’s improved fermentation, because expression of the functional RIM15 gene does not suppress alcoholic fermentation in K7 (5). To better understand this phenomenon, the Rim15p-mediated fermentation control needs to be further dissected through comparative analysis between sake and nonsake yeast strains.
While Rim15p has been identified as a key inhibitor of alcoholic fermentation, involvement of the upstream regulators of Rim15p (Fig. 1) in fermentation control has not yet been fully examined. In S. cerevisiae, Rim15p activity is under the control of several nutrient-sensing signaling protein kinases, including protein kinase A (PKA), the phosphate-sensing cyclin and cyclin-dependent protein kinase (CDK) complex termed Pho80p-Pho85p, and target-of-rapamycin protein kinase complex 1 (TORC1) (10, 11). Thus, inactivation of these kinases under nutrient starvation or other stress conditions may trigger Rim15p-dependent inhibition of alcoholic fermentation. Activation of TORC1 is mediated by the heterodimeric Rag GTPases (Gtr1p-Gtr2p in S. cerevisiae), which are negatively regulated by the Seh1p-associated protein complex inhibiting TORC1 (SEACIT) subcomplex (Iml1p-Npr2p-Npr3p in S. cerevisiae), which acts as a GTPase-activating protein for Gtr1p (12–14). Active TORC1 phosphorylates multiple targets, including Sch9p, the yeast orthologue of the mammalian serum and glucocorticoid-regulated kinase (SGK) (15). Direct phosphorylation of Rim15p at Ser1061 by Sch9p contributes to sequestration of Rim15p in the cytoplasm, thereby inhibiting Rim15p functions (16). Recently, it was reported that the TORC1-Sch9p-Rim15p pathway is conserved and present in the evolutionarily distant fission yeast Schizosaccharomyces pombe (17), although it remains to be determined if the pathway affects the fermentation performance in this yeast species. In contrast, mammalian TORC1 (mTORC1) positively regulates glycolysis by the induction of glycolytic gene expression through hypoxia-inducible factor 1α (HIF1α) (18).
FIG 1.
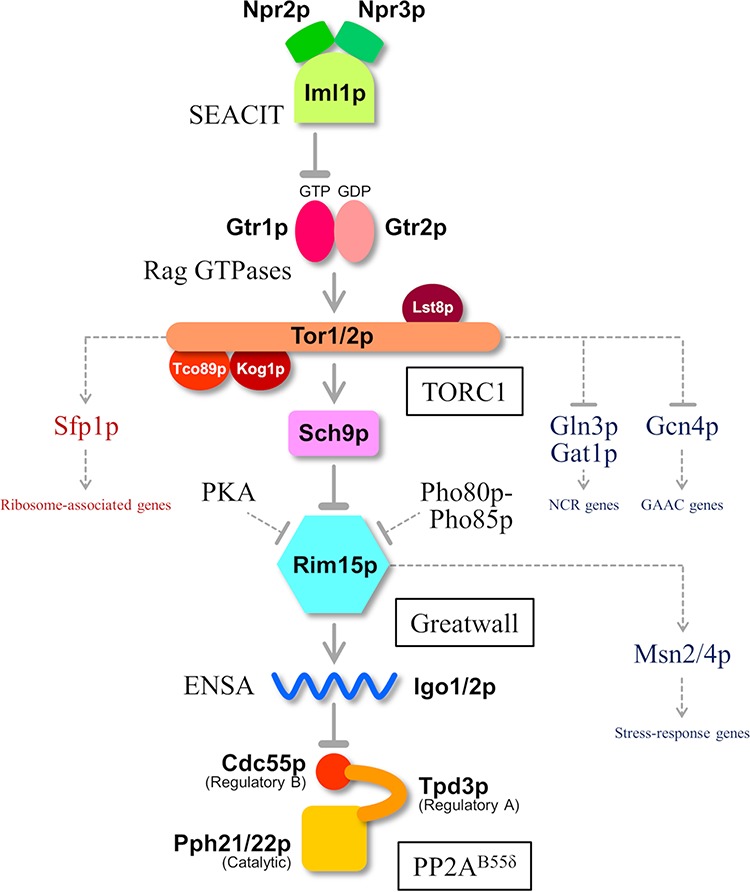
The TORC1-Greatwall-PP2AB55δ pathway and its associated factors in S. cerevisiae. Increased Sfp1p-targeted gene expression and decreased Gln3p-, Gat1p-, Gcn4p-, and Msn2/4p-targeted gene expression were observed in sake yeast cells, suggesting high TORC1 activity. TORC1, target of rapamycin complex 1; PP2AB55δ, protein phosphatase 2A complexed with a regulatory subunit B55δ; SEACIT, Seh1p-associated protein complex inhibiting TORC1; PKA, protein kinase A; NCR, nitrogen catabolite repression; GAAC, general amino acid control.
In S. cerevisiae, Rim15p targets the redundant transcription factors Msn2p and Msn4p (Msn2/4p) to mediate entry into the quiescent state (19, 20). In the context of fermentation control, Rim15p and Msn2/4p are required for the transcriptional induction of the UDP-glucose pyrophosphorylase-encoding gene UGP1, which switches the mode of glucose metabolism from glycolysis (a catabolic mode) to UDP-glucose synthesis (an anabolic mode) (7). However, no orthologue of Msn2/4p has been found in other organisms, and the role of the Greatwall family protein kinases in carbohydrate metabolism is unknown in S. pombe or higher eukaryotes. The Greatwall protein kinase was originally identified as a potential cell cycle activator in Drosophila (21). In animals, Greatwall directly phosphorylates a small protein called α-endosulfine (ENSA), which inhibits the activity of protein phosphatase 2A accompanied by the regulatory subunit B55δ (PP2AB55δ) (22, 23). Due to the antimitotic activity of PP2AB55δ, Greatwall is required for maintenance of mitosis. More recently, the Greatwall-ENSA-PP2AB55δ pathway was reported to be conserved in S. cerevisiae; Rim15p phosphorylates ENSA orthologues Igo1/2p to inhibit PP2A with the Cdc55p regulatory subunit (24–26). The orthologous pathway has also been found in S. pombe, and it plays a pivotal role in TORC1-mediated cell cycle control (17). However, the effect of PP2AB55δ on fermentation performance was unknown.
In the present study, we tested whether the TORC1-Greatwall-PP2AB55δ pathway participates in the control of alcoholic fermentation in S. cerevisiae and S. pombe. Our results provide new insights into how yeast cells determine the mode of glucose metabolism, especially in the context of the enhanced fermentation performance of sake yeast strains.
RESULTS
TORC1-associated transcriptomic profiles during alcoholic fermentation in laboratory and sake yeast strains.
Our previous comparative transcriptomic analysis indicated that the expression of the Rim15p- and Msn2/4p-targeted genes was attenuated in K701 (a strain derived from K7) compared to that in the laboratory strain X2180 early in a 20-day sake fermentation test (3). This may be attributed not only to the sake yeast-specific loss-of-function mutation in the RIM15 gene (rim155054_5055insA; see also reference 5) but also to higher TORC1 activity in the sake strains. TORC1 activity induces the ribosomal genes and the ribosome biogenesis genes, while it represses the general amino acid control (GAAC) and nitrogen catabolite repression (NCR) genes, as well as the Rim15p- and Msn2/4p-dependent stress response genes (27) (Fig. 1). We revisited our previous transcriptomic data indicating that among all differences in gene expression during sake fermentation, K701 shows statistically significantly stronger expression of ribosome-associated genes with an RNA polymerase A and C (PAC) motif (under the control of Sfp1p) and weaker expression of GAAC genes with a Gcn4p-responsive element (GCRE) and stress response genes with an Msn2/4p-responsive element (STRE) than X2180 (3). Comparison of the gene expression profiles (see Fig. S1 in the supplemental material) indicated that the Sfp1p-dependent genes are rapidly downregulated, while GAAC and NCR genes are transiently upregulated early in sake fermentation in X2180. These results suggest that TORC1 activity is decreased after the onset of alcoholic fermentation using X2180. In contrast, these transcriptomic traits were less clearly observed in K701, implying a slower decline in TORC1 activity during the initial stage of alcoholic fermentation by K701.
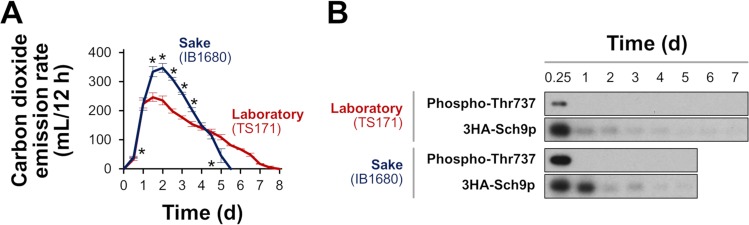
To directly monitor TORC1 activity, an antibody against phospho-Thr737 of Sch9p (28) was used, as this TORC1-dependent phosphorylation of Sch9p is known to mediate signaling to Rim15p. Laboratory yeast and K701 lineage sake yeast cells engineered to overexpress 3HA-tagged Sch9p from a glycolytic gene promoter were sampled during alcoholic fermentation in YPD20 medium (1% yeast extract, 2% peptone, and 20% glucose). The sake strain exhibited a higher rate of carbon dioxide emission and completed alcoholic fermentation more rapidly than the laboratory strain (Fig. 2A). Phosphorylation of Sch9p Thr737 was detected only at the initial stage (at 6 h from the onset of alcoholic fermentation) and was more prominent in the sake strain than in the laboratory strain (Fig. 2B). The signals decayed quickly over time in both strains, suggesting that TORC1 activity is highest at the onset of alcoholic fermentation. It should be noted that the glycolytic promoter used in this study was inactivated after the completion of logarithmic phase (>12 to 24 h) and that 3HA-Sch9p was expressed at only low levels after 2 days. It is also worth noting that the abundance of 3HA-Sch9p may suggest that the glycolytic promoter activity remains longer in sake yeast cells than in laboratory yeast cells, which may be another plausible explanation of the high fermentation performance of sake yeast cells.
FIG 2.
Phosphorylation levels of the Thr737 residue of Sch9p, comparing laboratory and sake strains during an 8-day fermentation test in YPD20. (A) Carbon dioxide emission rates of a laboratory strain (TS171; red) and a sake strain (IB1680; blue). Data represent the mean ± standard deviation (SD) from three (for TS171) or four (for IB1680) independent experiments. *, significant difference (t test, P < 0.05) compared to the laboratory strain at the respective time point. (B) The phosphorylation status of the Thr737 residue of Sch9 in a laboratory strain (TS171, upper panels) and a sake strain (IB1680, lower panels) was analyzed by Western blotting with an anti-phospho-T737-Sch9 antibody, and the total protein level of 3HA-Sch9p was assayed with an anti-HA antibody, as previously described (28). An equal amount of protein from the whole-cell lysates was analyzed by Western blot analyses.
Effects of the TORC1-Greatwall-PP2AB55δ pathway on fermentation performance.
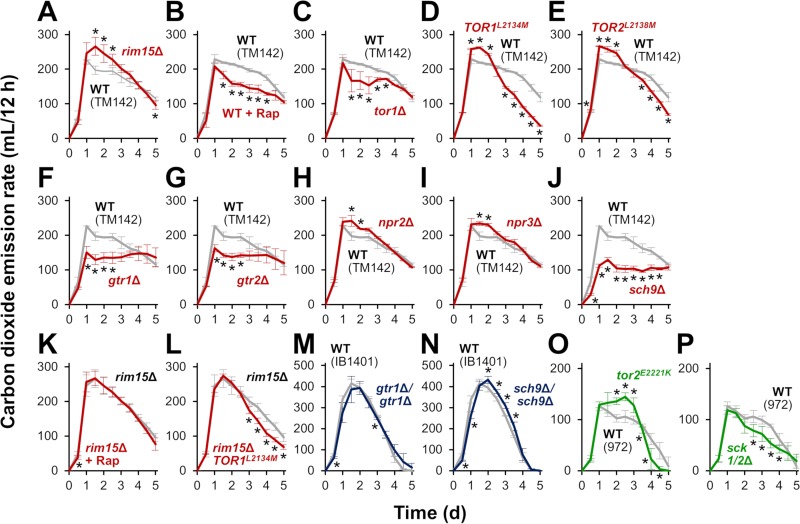
In S. cerevisiae laboratory strains, loss of Rim15p leads to an increase in the initial rate of carbon dioxide emission during alcoholic fermentation (5, 7) (Fig. 3A). To examine whether TORC1 acts as a negative regulator of Rim15p activity in fermentation control, we tested the effect of altered TORC1 signaling on fermentation performance in a laboratory strain (Fig. 3, red graphs). Addition of a low concentration (1 nM) of the TORC1 inhibitor rapamycin to the medium led to a decrease in the rate of carbon dioxide emission from day 1.5 to 4 (Fig. 3B). Since cell growth was not severely affected by 1 nM rapamycin (data not shown), the observed attenuation of carbon dioxide production was most likely indicative of reduced cellular fermentation performance. Deletion of the TOR1 gene, which encodes a nonessential catalytic subunit of TORC1, also decreased carbon dioxide emission from day 1.5 to 3.5 (Fig. 3C). Deletion of TOR2, which encodes a second TOR kinase that can also serve as a catalytic subunit of TORC1, was not tested in this study because Tor2p is essential for cell viability in S. cerevisiae. In contrast, the hyperactive TOR1 and TOR2 alleles (TOR1L2134M and TOR2L2138M) (28) increased carbon dioxide emission around day 1 to 2 (Fig. 3D and E). Strains harboring either of these hyperactive alleles exhibited drastic decreases in the rate of carbon dioxide emission toward the end of the fermentation tests. Excess TORC1 activity might prevent yeast cells from acquiring stress tolerance, leading to cell death caused by higher concentrations of ethanol on later days. These results suggested that TORC1 activity correlates with fermentation performance during the initial stage of the process. Indeed, deletion of GTR1 or GTR2, activators of TORC1 signaling, decreased carbon dioxide emission (Fig. 3F and G). In addition, disruption of NPR2 or NPR3, which encode the components of the SEACIT subcomplex that inhibit TORC1 signaling, resulted in increased carbon dioxide emission around day 1.5 to 2 (Fig. 3H and I), corroborating the role of TORC1 as a positive regulator of alcoholic fermentation. On the other hand, loss of Sch9p, which mediates signaling between TORC1 and Rim15p (Fig. 1), markedly decreased carbon dioxide emission (Fig. 3J).
FIG 3.
Effects of modification of the TORC1-Greatwall pathway on fermentation progression. Fermentation was monitored by measuring carbon dioxide emission. (A) Fermentation profiles of strain TM142 (wild type; gray) and its rim15Δ disruptant (red). (B) Fermentation profiles of strain TM142 in YPD20 medium in the absence (wild type; gray) or presence (red) of 1 nM rapamycin. (C to J) Fermentation profiles of strain TM142 (wild type; gray) and its tor1Δ (C), TOR1L2134M (D), TOR2L2138M (E), gtr1Δ (F), gtr2Δ (G), npr2Δ (H), npr3Δ (I), or sch9Δ (J) mutant (red). (K) Fermentation profiles of strain TM142 rim15Δ in YPD20 medium in the absence (gray) or presence (red) of 1 nM rapamycin. (L) Fermentation profiles of strain TM142 rim15Δ (gray) and its TOR1L2134M mutant (red). (M and N) Fermentation profiles of strain IB1401 (wild type; gray) and its gtr1Δ/gtr1Δ (M) or sch9Δ/sch9Δ (N) disruptant (blue). (O and P) Fermentation profiles of the wild-type S. pombe strain (gray) and its tor2E2221K (O) or sck1/2Δ (P) mutant (green). Fermentation tests were performed in YPD20 medium (A to N) or in YPD10 medium (O and P) at 30°C for 5 days. Values represent the mean ± SD of data from two or more independent experiments. *, significantly different from the value for the control experiment (t test, P < 0.05). Note that the data from experiments using laboratory, sake, and fission yeast strains are indicated in red, blue, and green, respectively. WT, wild type; Rap, rapamycin.
Next, the effects of TORC1 on fermentation performance were examined in the Rim15p-deficient strains from both laboratory and sake yeast backgrounds. In rim15Δ cells of the laboratory strain, 1 nM rapamycin did not affect carbon dioxide emission (Fig. 3K). We confirmed that the growth of rim15Δ cells was not affected by 1 nM rapamycin (data not shown). In the rim15Δ background, the hyperactive TOR1L2134M allele did not increase the initial rate of carbon dioxide emission (1 to 2 days) but caused a decrease in the later stage of fermentation (>3 days) (Fig. 3L). These data suggested that Rim15p is required for the TORC1-triggered fermentation control, specifically in the early stage of alcoholic fermentation. Considering that the sake strains carry a homozygous loss-of-function mutation, rim155054_5055insA (5), it was predicted that the fermentation performance of the sake strain is not affected by TORC1 signaling. As expected, deletion of the GTR1 or SCH9 gene in the sake strain did not change the maximum rate of carbon dioxide emission (Fig. 3M and N, blue graphs), although alcoholic fermentation was slightly delayed in both cases, probably due to slower cell growth. Is the TORC1-dependent fermentation control evolutionarily conserved among different yeast species? To address this point, we also assessed whether the conserved TORC1 pathway affects fermentation performance in the fission yeast S. pombe (Fig. 3, green graphs). As observed in budding yeast, an activated allele of tor2+ (encoding a catalytic subunit of TORC1 in S. pombe), tor2E2221K (29), brought about increased carbon dioxide emission in fission yeast (Fig. 3O). Furthermore, deletion of the redundant Sch9p-orthologous genes, sck1+ and sck2+, resulted in decreased carbon dioxide emission (Fig. 3P).
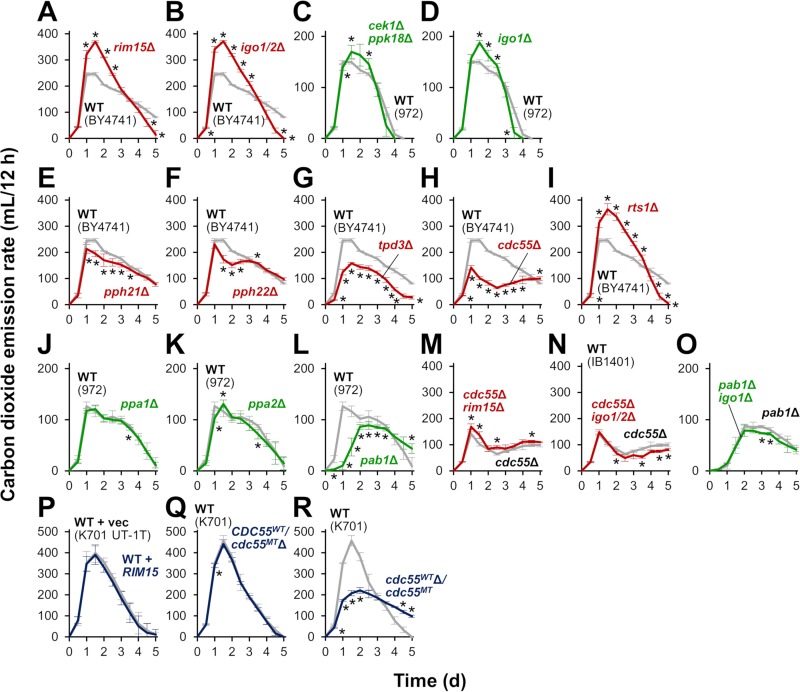
Does Greatwall-triggered signaling to PP2AB55δ play a role in the control of alcoholic fermentation? To address this, we tested the effect of the altered Greatwall-ENSA-PP2AB55δ pathway on fermentation performance in a laboratory strain (Fig. 4, red graphs) and a sake strain (blue graphs) of S. cerevisiae, as well as in S. pombe (green graphs). Deletion of the redundant ENSA-encoding genes IGO1 and IGO2 (IGO1/2), which mediate the signaling between Greatwall and PP2AB55δ in S. cerevisiae (Fig. 1), led to an increased rate of carbon dioxide emission, as did deletion of RIM15 (Fig. 4A and B). Similarly, in S. pombe, both the cek1Δ ppk18Δ and igo1Δ strains, which lack Greatwall and ENSA, respectively (17), exhibited higher rates of carbon dioxide emission than did the wild type (Fig. 4C and D). PP2A is a heterotrimeric enzyme complex composed of structural (A), regulatory (B), and catalytic (C) subunits. In budding yeast, the loss of both C subunit-encoding genes PPH21 and PPH22 leads to cell death, but disruption of either gene alone only weakly decreased carbon dioxide emission (Fig. 4E and F). In addition, deletion of the A subunit-encoding TPD3 gene inhibited alcoholic fermentation (Fig. 4G). Moreover, deletion of CDC55, which encodes a B55δ family regulatory subunit, severely decreased the rate of carbon dioxide emission throughout the duration of fermentation, whereas deletion of RTS1, which encodes a B56 family regulatory subunit, promoted alcoholic fermentation (Fig. 4H and I). In S. pombe, loss of the ppa1+ or ppa2+ gene, which encode C subunit isoforms, did not appear to affect alcoholic fermentation; however, loss of the pab1+ gene, encoding a B55δ subunit, impaired alcoholic fermentation (Fig. 4J to L). Together, these data suggested that the Greatwall-ENSA-PP2AB55δ pathway is involved in the control of alcoholic fermentation in both S. cerevisiae and S. pombe. When combined with the Greatwall or ENSA defects, deletion of the CDC55 (S. cerevisiae) or pab1+ (S. pombe) gene almost fully canceled high fermentation performance (Fig. 4M to O). Thus, PP2AB55δ is likely the major target of Greatwall and ENSA in the control of alcoholic fermentation in both yeasts. Based on these data, it was hypothesized that PP2AB55δ is responsible for the high fermentation performance of sake yeast strains.
FIG 4.
Effects of modification of the Greatwall-PP2AB55δ pathway on fermentation progression. Fermentation was monitored by measuring carbon dioxide emission. (A and B) Fermentation profiles of strain BY4741 (wild type; gray) and its rim15Δ (A) or igo1/2Δ (B) disruptant (red). (C and D) Fermentation profiles of the wild-type S. pombe strain (gray) and its cek1Δ/ppk18Δ (C) or igo1Δ (D) disruptant (green). (E to I) Fermentation profiles of strain BY4741 (wild type; gray) and its pph21Δ (E), pph22Δ (F), tpd3Δ (G), cdc55Δ (H), or rts1Δ (I) disruptant (red). (J to L) Fermentation profiles of the wild-type S. pombe strain (gray) and its ppa1Δ (J), ppa2Δ (K), or pab1Δ (L) disruptant (green). (M and N) Fermentation profiles of strain BY4741 cdc55Δ (gray) and its rim15Δ (M) or igo1/2Δ (N) disruptant (red). (O) Fermentation profiles of the S. pombe pab1Δ strain (gray) and its igo1Δ disruptant (green). (P) Fermentation profiles of strain K701 UT-1T with an empty vector (wild type; gray) and with a functional RIM15-expressing plasmid (blue). (Q and R) Fermentation profiles of strain K701 (wild type; gray) and its CDC55WT/cdc55MTΔ (Q) or cdc55WTΔ/cdc55MT (N) disruptant (blue). Fermentation tests were performed in YPD20 medium (A, B, E to I, M, N, and P to R) or in YPD10 medium (C, D, J to L, and O) at 30°C for 5 days. Values represent the mean ± SD of data from two or more independent experiments. *, significantly different from the value for the control experiment (t test, P < 0.05). Note that the data from experiments using laboratory, sake, and fission yeast strains are indicated by red, blue, and green, respectively. WT, wild type.
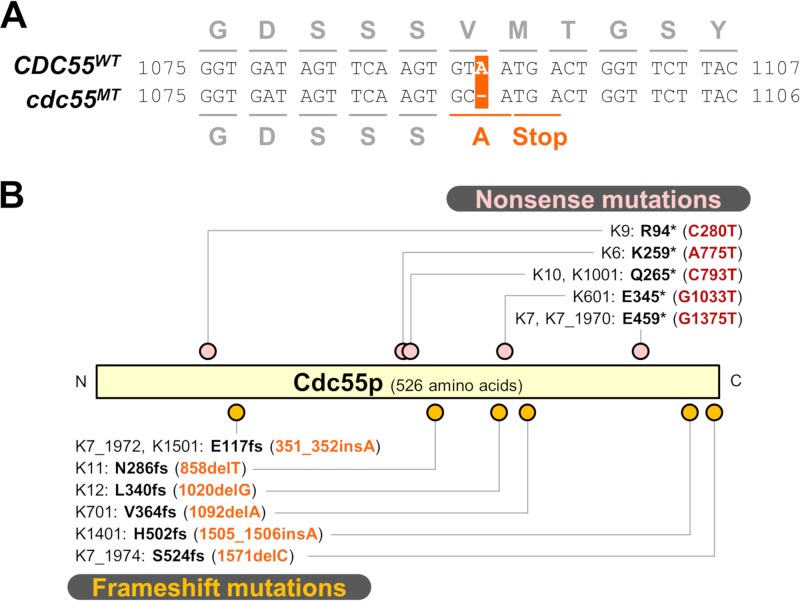
Consistent with a previous report (5), expression of the functional RIM15 gene derived from a laboratory strain did not attenuate alcoholic fermentation in the sake strain (Fig. 4P). Therefore, we next evaluated the role of PP2AB55δ downstream of Rim15p in the high fermentation performance of sake yeast cells. Interestingly, we found that the diploid sake strain K701 is heterozygous for the deletion of a single adenine nucleotide at position 1092 of the CDC55 gene (designated the cdc55MT allele), resulting in a frameshift and premature polypeptide termination (Fig. 5A); thus, K701 carries only one functional CDC55 allele (designated the CDC55WT allele). To directly test the role of PP2AB55δ in the sake yeast, the K701 strain was mutagenized by introduction of a CDC55-disrupting construct, yielding 12 heterozygous disruptants. Direct sequencing of the CDC55 loci amplified from genomic DNA revealed that the cdc55MT allele was disrupted in six of the heterozygous disruptants, while the CDC55WT allele was disrupted in the other six heterozygous disruptants. The former class, in which the CDC55WT allele remains intact, exhibited fermentation characteristics similar to those of the parental K701 strain (Fig. 4Q), while the latter class, with no functional CDC55 gene, exhibited markedly lower carbon dioxide emission, especially in the initial stage of fermentation (0.5 to 2 days) (Fig. 4R). These results indicated that the CDC55WT allele is required for the high fermentation performance of the K701 sake yeast strain.
FIG 5.
Heterozygous nonsense or frameshift mutations found in the CDC55 genes of K7-related sake strains. (A) The cdc551092delA (also known as cdc55MT) mutation unique to K701. In this loss-of-function allele of K701, deletion of a single adenine nucleotide at open reading frame (ORF) nucleotide 1092 causes a premature stop codon. (B) Mutation sites of the CDC55 gene of K7-related sake strains. Nonsense and frameshift mutation sites are indicated by pink and orange dots, respectively. fs, frameshift.
Heterozygous nonsense or frameshift mutations in the CDC55 gene of the sake yeast strains.
As mentioned above, we identified a heterozygous loss-of-function mutation (cdc551092delA) in diploid K701 (Fig. 5A). To test whether this mutation is conserved among the sake strains, we analyzed the sequences of the CDC55 genes in 17 K7-related Kyokai sake strains, i.e., K6, K601, K7, K701, K9, K901, K10, K1001, K11, K12, K13, K14, K1401, K1501, K1601, K1701, and K1801. (Note that the numbering corresponds to the sequential isolation of these strains. The “01” suffix is used to indicate foamless variants that do not generate thick foam layers during sake fermentation; for instance, K701 is the foamless variant of K7 [30].) As shown in Fig. 5B, the cdc551092delA mutation is unique to K701, and 65% (11 of 17) of the tested strains contain other nonsense or frameshift mutations in the open reading frame of the CDC55 gene. Notably, the three most recently isolated strains, K1601, K1701, and K1801, have neither a nonsense mutation nor a frameshift mutation in this locus. Although there are a few lineage-specific mutations, such as cdc55C793T in K10 and K1001 and cdc55351_352insA in K7 and K1501 (2), closely associated strains do not always contain the same mutation (e.g., K6 versus K601, K7 versus K701, or K7 versus K11). Each year, every Kyokai sake yeast strain was selected from clone stocks before distribution by the Brewing Society of Japan; notably, the K7 strains from three different years (1970, 1972, and 1974) carry distinct cdc55 mutations. The K7 strain used for whole-genome analysis (4) harbors a cdc55 mutation identical to the cdc551571delC allele in K7_1970. Thus, it appears that most of the cdc55 mutations represent independent events that occurred after the establishment of the individual sake strains. While the cdc551571delC mutation in K7_1974 results in an additional 27 amino acid residues at the carboxyl terminus of the encoded protein, each of the other frameshift mutations leads to a premature stop codon that truncates the carboxyl terminus. Since all of the identified mutations are heterozygous, the effects of the cdc55 loss-of-function mutations may be masked by the functional CDC55 allele, as observed in K701.
Effects of PP2AB55δ on the intracellular levels of glycolytic intermediates.
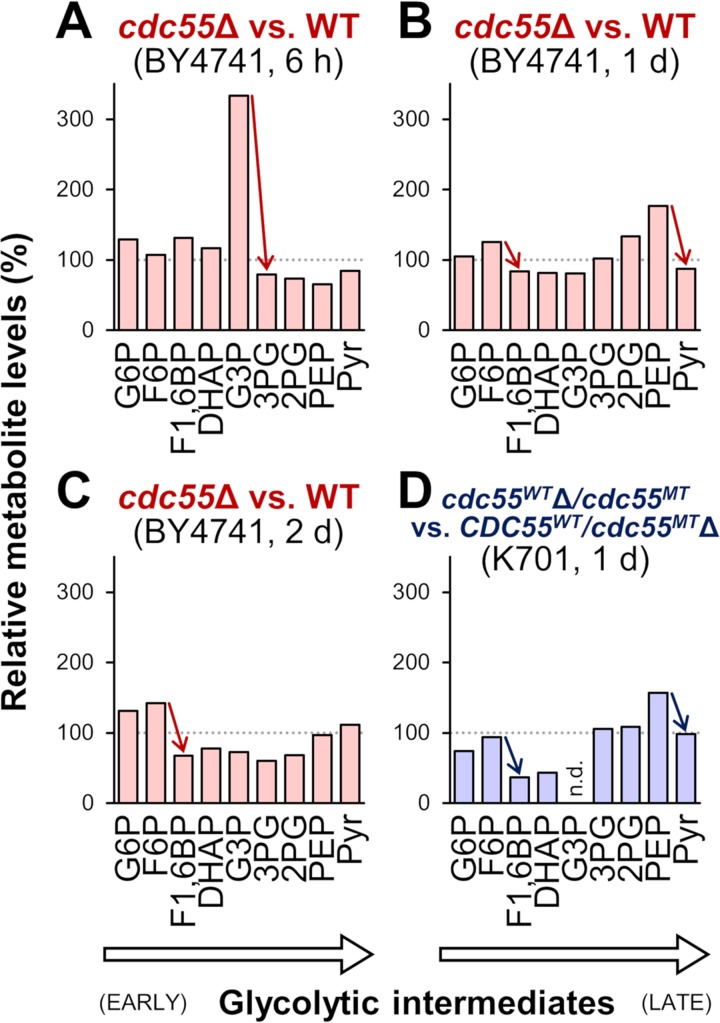
Since PP2AB55δ dephosphorylates many cellular substrates (31), it is difficult to infer how PP2AB55δ controls alcoholic fermentation. However, it may be worth examining whether PP2AB55δ regulates the activities of carbon metabolic enzymes through protein dephosphorylation, as several recent studies have shed light on posttranslational modifications as regulatory mechanisms for metabolic flux in vivo (32, 33). In the present study, we adopted a metabolomic approach to explore the glycolytic reactions that may be affected by the loss of PP2AB55δ function. Metabolites were extracted from cells sampled at the early stages (6 h, 1 day, or 2 days) of alcoholic fermentation in YPD20 medium. Relative metabolite levels at 6 h indicated that the pools of early glycolytic intermediates (glucose 6-phosphate [G6P], fructose 6-phosphate [F6P], fructose 1,6-bisphosphte [F1,6BP], and dihydroxyacetone phosphate [DHAP]) were slightly increased by deletion of the CDC55 gene in the laboratory strain BY4741 (Fig. 6A). In contrast, the level of glyceraldehyde 3-phosphate (G3P) accumulated in cdc55Δ cells was 3-fold higher than that in wild-type cells, while the intracellular pools of 3-phosphoglyceric acid (3PG) and the ensuing glycolytic intermediates were smaller in cdc55Δ cells. These data suggest that, at 6 h, the metabolic steps between G3P and 3PG are specifically compromised by deletion of the CDC55 gene. We noted that 1,3-bisphosphoglyceric acid (1,3BPG), an intermediate between G3P and 3PG in the glycolytic pathway, was not detected in either wild-type or cdc55Δ cells in the present analysis. At 1 day, similar accumulations were observed for F6P and phosphoenolpyruvic acid (PEP) in cdc55Δ cells (Fig. 6B); the accumulation of F6P remained even at 2 days (Fig. 6C). These data suggested that, at 1 to 2 days, the metabolic steps between F6P and F1,6BP, and between PEP and pyruvic acid, are disturbed by deletion of the CDC55 gene. In the sake strain K701 at 1 day from the onset of alcoholic fermentation, the accumulations of F6P and PEP were observed in CDC55WT-deficient cells (cdc55WTΔ/cdc55MT) (Fig. 6D), consistent with the results obtained using laboratory yeast cells. Based on these results, it is possible to hypothesize that the enzymatic activity of phosphofructokinase (F6P to F1,6BP) is reproducibly negatively affected by the loss of PP2AB55δ function in both laboratory and sake strains when the fermentation rates reach their maxima.
FIG 6.
Effects of Cdc55p on glycolytic intermediate levels in the early stage of alcoholic fermentation. (A to C) Intracellular metabolite levels of laboratory strain BY4741 cdc55Δ at 6 h (A), 1 day (B), and 2 days (C) from the onset of alcoholic fermentation; values are normalized to those of the wild-type BY4741 at the respective time point. (D) Intracellular metabolite levels of sake strain K701 cdc55WTΔ/cdc55MT at 1 day from the onset of alcoholic fermentation; values are normalized to those of K701 CDC55WT/cdc55MTΔ. Red and blue arrows indicate notable differences between adjacent metabolites. Each datum provided is from a single experiment from a representative fermentation test. G6P, glucose 6-phosphate; F6P, fructose 6-phosphate; F1,6BP, fructose 1,6-bisphosphate; DHAP, dihydroxyacetone phosphate; G3P, glyceraldehyde 3-phosphate; 3PG, 3-phosphoglyceric acid; 2PG, 2-phosphoglyceric acid; PEP, phosphoenolpyruvic acid; Pyr, pyruvic acid; n.d., not determined. Note that G3P was not detected in K701 cdc55WTΔ/cdc55MT and that 1,3-bisphosphoglyceric acid (1,3BPG) was not detected in any of the samples.
DISCUSSION
Although the genes and enzymes of the glycolysis and alcoholic fermentation pathways have been thoroughly studied in S. cerevisiae, the mechanisms by which intracellular signaling pathways regulate carbohydrate metabolism in response to extracellular cues are still not fully elucidated. We previously identified a loss-of-function mutation in the RIM15 gene (rim155054_5055insA) that is present in K7 and shared among the associated sake yeast strains, suggesting that this mutation is associated with enhanced fermentation performance (5, 7). In the present work, we showed that sake yeast cells exhibit elevated TORC1 activity during alcoholic fermentation in comparison to laboratory strains. TORC1 upregulates the Sfp1p-targeted genes encoding ribosome-associated proteins and downregulates members of the NCR and GAAC regulons in a Rim15p-independent manner (27). These attributes (see Fig. S1 in the supplemental material), as well as the observed defect in induction of the Msn2/4p-mediated stress-response genes (3), suggest enhanced activation of TORC1 in sake yeast cells (compared to laboratory strains). The high level of phosphorylated Thr737 of Sch9p observed in sake yeast cells (Fig. 2) is consistent with this idea. As previously reported, TORC1 activity is not fully attenuated in K7 cells even under nitrogen limitation (34). Therefore, elevated TORC1 activity can be regarded as a novel hallmark of the sake yeast cells. In general, nutritional limitation and environmental stresses rapidly inactivate TORC1 in yeast, resulting in inhibition of cell growth and proliferation. We postulate that the maintenance of high TORC1 activity in sake yeast cells may facilitate cellular metabolic activity even under fermentative conditions. Among the components of TORC1, only Tor1p contains missense mutations (R167Q and T1456I) in K7 and its relatives. Further studies will be needed to evaluate the roles of the mutations in TOR1 and those in other genes to be discovered in sake yeast strains.
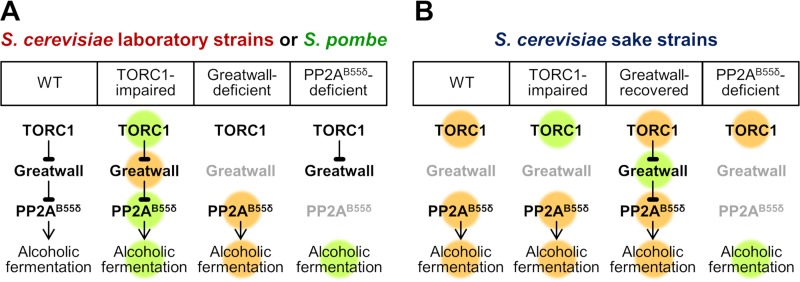
In the present study, we demonstrated that the conserved TORC1-Greatwall-PP2AB55δ pathway is key to the control of alcoholic fermentation (Fig. 7A). In S. cerevisiae laboratory strains and S. pombe, altered TORC1 activities led to changes in fermentation performance, specifically at the early stage of alcoholic fermentation. However, in laboratory yeast cells deficient in Greatwall, the initial rate of alcoholic fermentation was maintained and was not affected by changes in TORC1. It should be also noted that Greatwall does not affect the fermentation performance in a TORC1-hyperactivated strain (compare the fermentation profiles of TOR1L2134M in Fig. 3D and rim15Δ TOR1L2134M in Fig. 3L). In contrast, in PP2AB55δ-deficient laboratory yeast cells, the fermentation rate was strikingly low and was not enhanced even by a loss of Greatwall or ENSA. The observed strong epistasis suggested that the Greatwall-PP2AB55δ pathway, among numerous downstream effector proteins of TORC1, is the primary mediator of fermentation control. This epistasis also indicated that PP2AB55δ is the major regulator of the alcoholic fermentation machinery.
FIG 7.
A hypothetical model of the regulation of fermentation control by the TORC1-Greatwall-PP2AB55δ pathway. Orange and green indicate activities higher and lower, respectively, than those of S. cerevisiae wild-type laboratory strains. (A) In S. cerevisiae laboratory strains and S. pombe, changes in the activity of TORC1, Greatwall, or PP2AB55δ may lead to altered alcoholic fermentation performance. (B) In S. cerevisiae sake strains, both the high TORC1 activity and the loss of Rim15p may contribute to the constitutively high PP2AB55δ activity. Thus, PP2AB55δ must be disrupted to impair the fermentation performance in these strains.
In our hypothesis, both high TORC1 activity and loss of Rim15p contribute to the activation of PP2AB55δ and the subsequent enhancement of the cellular fermentation performance in the K7-related sake strains (Fig. 7B). Indeed, neither impairment of TORC1 nor recovery of Rim15p is sufficient to attenuate PP2AB55δ activity in these cells. Presumably, even if TORC1 activity is decreased, the change in TORC1 signaling may not be conveyed downstream due to the loss of Rim15p (Fig. 1). On the other hand, if a functional RIM15 gene is restored, the hyperactivated TORC1 can inhibit the functions of Rim15p, resulting in elevated PP2AB55δ activity. Our data indicated that the high fermentation performance of sake yeast cells was abrogated only when the functional CDC55 (B55δ-encoding) gene was disrupted (Fig. 4R). Consequently, the two changes (in TORC1 and Rim15p) observed in the TORC1-Greatwall-PP2AB55δ pathway of sake yeast cells may mutually ensure the robust phenotype of these strains in the context of alcoholic fermentation.
Why do multiple sake yeast strains possess putative loss-of-function mutations (i.e., nonsense mutations and frameshift mutations [Fig. 5B]) in the CDC55 gene? Since diploid sake yeast strains contain two copies of the CDC55 gene, heterozygosity for a loss-of-function mutation at the loci may not yield apparent effects on alcoholic fermentation. In fact, the most recently isolated strains, K1601, K1701, and K1801, which do not contain any nonsense or frameshift mutations, exhibit high fermentation performance as the other sake strains do. PP2AB55δ regulates not only carbohydrate metabolism but also cell cycle progression. In S. cerevisiae, PP2AB55δ is the key inhibitor of the entry into quiescence (G0 phase). Loss of Rim15p decreases the expression of stress response genes and shortens chronological life span, and cdc55Δ is able to suppress such Rim15p-deficient phenotypes (24). The heterozygous loss-of-function mutations in CDC55 in the sake strains may reduce the dosage of functional Cdc55p, thereby serving as weak suppressors of the long-term survival defect associated with the rim155054_5055insA mutation (see Fig. S2 in the supplemental material). Thus, the individual sake strains may have independently acquired and maintained the heterozygous cdc55 mutations during decades of adaptation. If another mutation in the functional CDC55 allele or a loss-of-heterozygosity (LOH) event occurs, the lack of Cdc55p function further enhances cell viability but severely impairs fermentation performance. Based on our model, the heterozygous cdc55 mutations may decrease the genetic stability for high fermentation performance and thus should be eliminated to facilitate the development of genetically stable sake yeast strains.
Comparison of the glycolytic intermediate pools between wild-type and cdc55Δ cells suggested that the loss of PP2AB55δ negatively affects the metabolic reactions responsible for the conversion of (i) F6P to F1,6BP, (ii) G3P to 3PG, and (iii) PEP to pyruvic acid during the initial stage of alcoholic fermentation (Fig. 6). We presume that these defects are at least partially responsible for the low fermentation performance of cdc55Δ cells. Intriguingly, PP2AB55δ appears to control individual glycolytic reactions in a fermentation phase-specific manner; only the defect in conversion of G3P to 3PG was observed at 6 h from the onset of alcoholic fermentation in a laboratory strain, whereas the defects in conversion of F6P to F1,6BP and/or of PEP to pyruvic acid were observed from 1 day to 2 days. Thus, these results imply that the activities of glycolytic enzymes are separately regulated during alcoholic fermentation and that the pleiotropic functions of PP2AB55δ contribute to the optimal glycolytic flux. Among the glycolytic enzymes, phosphofructokinase and pyruvate kinase catalyze irreversible and rate-limiting reactions, i.e., conversion of F6P to F1,6BP and of PEP to pyruvic acid, respectively, in glycolysis. Recent integrated phosphoproteomics data for budding yeast indicate that Pfk1p and Pfk2p (the α and β subunits of phosphofructokinase, respectively) and Cdc19p (the main pyruvate kinase isozyme) form phosphorylation hubs, suggesting that multiple protein kinases phosphorylate these enzymes to modulate their activity, intracellular localization, or protein degradation (32, 33). For example, it has been reported that phosphorylation of residue Ser163 of Pfk2p inhibits the phosphofructokinase activity in vivo under gluconeogenic conditions (35). The protein phosphatase activity of PP2AB55δ may directly regulate glycolytic enzymes by counteracting such inhibitory phosphorylation. In fact, Pfk1p and Pfk2p are listed as putative PP2AB55δ-dephosphorylated proteins (31). The 3PG kinase Pgk1p, which is involved in the conversion of G3P to 3PG, also is a putative PP2AB55δ target. The phosphorylation status and the activities of the candidate enzymes should be compared between wild-type and cdc55Δ cells during alcoholic fermentation. Since the glycolytic pathway and the posttranslational modifications of the glycolytic enzymes are often conserved evolutionarily, our study may also offer clues to identify novel key mechanisms of protein phosphorylation-mediated glycolytic control by the TORC1-Greatwall-PP2AB55δ pathway.
MATERIALS AND METHODS
Yeast strains.
The yeast strains used in this study are listed in Table 1. Saccharomyces cerevisiae laboratory strain BY4741 and its single-deletion mutants were obtained from the European Saccharomyces cerevisiae Archive for Functional Analysis (Euroscarf), Germany. Another S. cerevisiae laboratory strain, X2180, and Schizosaccharomyces pombe wild-type strain 972 were obtained from the American Type Culture Collection (ATCC), USA. Sake yeast strains Kyokai no. 7 (K7) and its relatives (K6, K601, K701, K9, K901, K10, K1001, K11, K12, K13, K14, K1401, K1501, K1601, K1701, and K1801) were provided by the Brewing Society of Japan (BSJ). S. pombe strain ED666 cek1Δ::kanMX (h+ ade6-M210 ura4-D18 leu1-32 cek1Δ::kanMX) was obtained from Bioneer (Korea).
TABLE 1.
Yeast strains used in this study
| Species and strain | Genotype | Source or referencea |
|---|---|---|
| S. cerevisiae | ||
| Laboratory strains | ||
| BY4741 background with RIM15WT and CDC55WT | ||
| BY4741 | MATa leu2Δ0 his3Δ1 ura3Δ0 met15Δ0 | Euroscarf |
| BY4741 rim15Δ | BY4741 rim15Δ::kanMX | Euroscarf |
| BY4741 igo1Δ | BY4741 igo1Δ::kanMX | Euroscarf |
| BY4741 igo1/2Δ | BY4741 igo1Δ::kanMX igo2Δ::hphNT | This study |
| BY4741 pph21Δ | BY4741 pph21Δ::kanMX | Euroscarf |
| BY4741 pph22Δ | BY4741 pph22Δ::kanMX | Euroscarf |
| BY4741 tpd3Δ | BY4741 tpd3Δ::kanMX | Euroscarf |
| BY4741 cdc55Δ | BY4741 cdc55Δ::natNT | This study |
| BY4741 rts1Δ | BY4741 rts1Δ::kanMX | Euroscarf |
| BY4741 cdc55Δ rim15Δ | BY4741 cdc55Δ::natNT rim15Δ::kanMX | This study |
| BY4741 cdc55Δ igo1/2Δ | BY4741 cdc55Δ::natNT igo1Δ::kanMX igo2Δ::hphNT | This study |
| X2180 | MATa/α, wild type | ATCC |
| TM142 background with RIM15WT and CDC55WT | ||
| TM142 | MATα leu2Δ1 his3Δ200 trp1Δ63 ura3-52 | 28 |
| TM142 rim15Δ | TM142 rim15Δ::kanMX | This study |
| TM142 tor1Δ (MY007) | TM142 tor1Δ::kanMX | This study |
| TM142 TOR1L2134M (MY010) | TM142 TOR1L2134M | This study |
| TM142 TOR1L2134M rim15Δ | TM142 TOR1L2134M rim15Δ::kanMX | This study |
| TM142 TOR2L2138M (MY013) | TM142 TOR2L2138M | This study |
| TM142 gtr1Δ (TS084) | TM142 gtr1Δ::kanMX | This study |
| TM142 gtr2Δ (TS087) | TM142 gtr2Δ::kanMX | This study |
| TM142 npr2Δ (TS232) | TM142 npr2Δ::kanMX | 38 |
| TM142 npr3Δ (TS236) | TM142 npr3Δ::kanMX | 38 |
| TM142 sch9Δ (TS858) | TM142 sch9Δ::kanMX | This study |
| TM142 PGPD-3HA-SCH9 (TS171) | TM142 (natNT)PGPD-3HA-SCH9 | 28 |
| Sake strains | ||
| K701 background with rim155054_5055insA/rim155054_5055insA
and CDC55WT/cdc551092delA |
||
| K701 | MATa/α, wild type; foamless variant of K7 | BSJ |
| K701 CDC55WT/cdc55MTΔ | K701 CDC55WT/cdc55MTΔ::natNT | This study |
| K701 cdc55WTΔ/cdc55MT | K701 cdc55WTΔ::natNT/cdc55MT | This study |
| UT-1T | K701 TRP1/trp1 ura3/ura3 | 46 |
| UT-1T + vector | K701 UT-1T(pAUR112) | 5 |
| UT-1T + RIM15 | K701 UT-1T(pAUR112-ScRIM15) | 5 |
| IB1401 | K701 leu2Δ::loxP/leu2Δ::loxP his3Δ::loxP/his3Δ::loxP trp1/trp1 ura3/ura3 | 47 |
| K701 gtr1Δ/gtr1Δ (IB1663) | K701 leu2Δ::loxP/leu2Δ::loxP trp1/trp1 ura3/ura3 gtr1Δ::CgLEU2/gtr1Δ::CgTRP1 | 34 |
| IB1401 sch9Δ/sch9Δ (IB1536) | IB1401 sch9Δ::CgHIS3/sch9Δ::CgTRP1 | This study |
| IB1401 PGPD-3HA-SCH9 (IB1680) | IB1401(p416-3HA-SCH9) | This study |
| Other backgrounds with rim155054_5055insA/rim155054_5055insA | ||
| K6 | BSJ | |
| K601 | Foamless variant of K6 | BSJ |
| K7 | BSJ | |
| K9 | BSJ | |
| K901 | Foamless variant of K9 | BSJ |
| K10 | BSJ | |
| K1001 | Foamless variant of K10 | BSJ |
| K11 | BSJ | |
| K12 | BSJ | |
| K13 | BSJ | |
| K14 | BSJ | |
| K1401 | Foamless variant of K14 | BSJ |
| K1501 | BSJ | |
| K1601 | BSJ | |
| K1701 | BSJ | |
| K1801 | BSJ | |
| S. pombe | ||
| 972 | h−, wild-type | ATCC |
| 972 tor2E2221K (CA8829) | h− tor2E2221K(kanMX) | 48 |
| 972 sck1/2Δ (CA7568) | h− his7-366 ura4Δ18 sck1Δ::his7+ sck2Δ::ura4+ | This study |
| 972 cek1Δ ppk18Δ | h− cek1Δ::natMX ppk18Δ::hphMX | This study |
| 972 igo1Δ | h− igo1Δ::kanMX | This study |
| 972 ppa1Δ (CA13052, CA13053) | h− ura4-D18 ppa1Δ::ura4+ | This study |
| 972 ppa2Δ (CA13054, CA13055) | h− ura4-D18 ppa2Δ::ura4+ | This study |
| 972 pab1Δ (CA13214, CA13215) | h− ura4-D18 pab1Δ::ura4+ | This study |
| 972 pab1Δ igo1Δ (HT741) | h− ura4-D18 pab1Δ::ura4+ igo1Δ::kanMX | This study |
aEuroscarf, European Saccharomyces cerevisiae Archive for Functional Analysis (Germany); ATCC, American Type Culture Collection (United States); BSJ, Brewing Society of Japan (Japan).
Disruption of the IGO2 gene in BY4741 igo1Δ was performed using a PCR-based method (36) with the gene-specific primer pair IGO2-DF (5′-CAT ATA AAC AAA AAT ACG TAC CAA AGA AGT GTT ATA AAA GTG ATA TAA CTC GGA TCC CCG GGT TAA TTA A-3′) and IGO-DR (5′-TAG TGA TGA TAA AAA AAA AGT AAT ACC ACA TAA TAT CAT TCC TTC ATT AGG AAT TCG AGC TCG TTT AAA C-3′) and plasmid pFA6a-hphNT (37) as the template to generate BY4741 igo1Δ::kanMX igo2Δ::hphNT (BY4741 igo1/2Δ). Disruption of the CDC55 gene in wild-type BY4741, BY4741 rim15Δ, and BY4741 igo1/2Δ was performed using a PCR-based method (36) with the gene-specific primer pair CDC55-DF (5′-AGG TCA AAC TGG AGA GAT CTT ACG CAT AAA GAA ATA TAA TAT AGC GCA CAC GGA TCC CCG GGT TAA TTA A-3′) and CDC55-DR (5′-GGG AAG ATA TGG GAT AAA AAA AAG TAA GGG AAA ATA AGG AAT TAT TAT AAG AAT TCG AGC TCG TTT AAA C-3′) and plasmid pFA6a-natNT (37) as the template to generate BY4741 cdc55Δ::natNT (BY4741 cdc55Δ), BY4741 cdc55Δ::natNT rim15Δ::kanMX (BY4741 cdc55Δ rim15Δ), and BY4741 cdc55Δ::natNT igo1Δ::kanMX igo2Δ::hphNT (BY4741 cdc55Δ igo1/2Δ), respectively.
The TOR1L2134M mutation was previously reported as a hyperactive point mutation in the kinase domain of Tor1p (28). Since the mutation site was conserved in the TOR2 gene, the corresponding mutation was also introduced to generate TOR2L2138M. Disruption of the RIM15 gene in wild-type TM142 or in TM142 TOR1L2134M was performed using a PCR-based method (36) with the gene-specific primer pair RIM15-DF (5′-TTT CTC TTG CCT CAT TTG ATA GAA TAG ATA AGC CCA GTA GAG GAA GAC AGC GGA TCC CCG GGT TAA TTA A-3′) and RIM15-DR (5′-CAA AGT TTT TAT TCA GTT ATT TTT TTT AAT TAT CTT TAT CTT AAA ATT TAG AAT TCG AGC TCG TTT AAA C-3′) and plasmid pFA6a-kanMX (37) as the template to generate TM142 rim15Δ::kanMX (TM142 rim15Δ) and TM142 TOR1L2134M rim15Δ::kanMX (TM142 TOR1L2134M rim15Δ), respectively. TM142 gtr1Δ::kanMX (TM142 gtr1Δ; TS084), TM142 gtr2Δ::kanMX (TM142 gtr2Δ; TS087), and TM142 sch9Δ::kanMX (TM142 sch9Δ; TS858) were from the laboratory stock (28, 38).
Heterozygous disruption of the CDC55 gene in K701 was performed using a PCR-based method (36) with the gene-specific primer pair CDC55-DF and CDC55-DR and plasmid pFA6a-natNT (37) as the template. Correct disruption of the CDC55WT or cdc55MT allele was confirmed by genomic PCR and direct DNA sequencing of the PCR product. Homozygous disruption of the SCH9 gene in IB1401 was performed according to a previous report (34). To overexpress 3HA-tagged Sch9p from a glycolytic gene promoter in IB1401, plasmid p416-3HA-SCH9 (kindly gifted from Kevin Morano from the University of Texas, USA) was introduced into IB1401.
Disruption of the ppk18+ and igo1+ genes in wild-type strain 972 was performed using a PCR-based method (36) with a gene-specific primer pair and plasmid pFA6a-kanMX (37) as the template to generate 972 ppk18Δ::kanMX and 972 igo1Δ::kanMX (972 igo1Δ), respectively. The kanMX genes in ED666 cek1Δ::kanMX and 972 ppk18Δ::kanMX were replaced with natMX and hphMX, respectively, using a one-step marker switch (39) to generate ED666 cek1Δ::natMX and 972 ppk18Δ::hphMX, respectively. Both strains were mated and sporulated to generate the prototrophic double mutant 972 cek1Δ::natMX ppk18Δ::hphMX (972 cek1Δ ppk18Δ). To construct the prototrophic mutants 972 sck1Δ::his7+ sck2Δ::ura4+ (972 sck1Δ sck2Δ), 972 ppa1Δ::ura4+ (ppa1Δ), ppa2Δ::ura4+ (972 ppa2Δ), and 972 pab1Δ::ura4+ (972 pab1Δ), a suitable wild-type strain was mated with JX766 (40), MY1121, MY1122 (41), and MY7214 (42), respectively, and sporulated. The pab1Δ and igo1Δ strains were mated and sporulated to generate the prototrophic double mutant 972 pab1Δ::ura4+ igo1Δ::kanMX (972 pab1Δ igo1Δ).
Yeast cells were routinely grown in liquid YPD medium (1% yeast extract, 2% peptone, and 2% glucose) at 30°C, unless stated otherwise.
Sequencing of the CDC55 gene.
To analyze the CDC55 sequence, the gene was amplified by PCR with the primer pair CDC55-(-150)-F (5′-GGC AGC TTA ATA CGA TTA CCC C-3′) and CDC55-(+1906)-R (5′-TGG TGA AGT GAT GAA AGA AGT CC-3′), using genomic DNA from the strain of interest as the template. The PCR product was sequenced directly using a BigDye Terminator v3.1 cycle sequencing kit (Thermo Fisher Scientific) and primers CDC55-seq2 (5′-TCG AGG TCA AAC TGG AGA GA-3′), CDC55-seq3 (5′-AAA ATC ATT GCT GCC ACC CC-3′), and CDC55-seq4 (5′-TGA TAC CTA TGA AAA CGA TGC GA-3′) on a 3130xl Genetic Analyzer (Applied Biosystems); sequencing was performed at Fasmac Co., Ltd. (Japan).
Fermentation tests.
For measurements of fermentation rates, yeast cells were precultured in YPD medium at 30°C overnight, inoculated into 50 ml of YPD20 medium (1% yeast extract, 2% peptone, and 20% glucose) for S. cerevisiae or YPD10 medium (1% yeast extract, 2% peptone, and 10% glucose) for S. pombe at a final optical density at a wavelength of 600 nm (OD600) of 0.1, and then further incubated at 30°C without shaking. Fermentation progression was continuously monitored by measuring the volume of evolved carbon dioxide gas using a Fermograph II apparatus (Atto) (43).
Analysis of intracellular metabolite profiles.
During the fermentation tests in YPD20 medium, yeast cells corresponding to an OD600 of 20 were collected at 6 h, 1 day, or 2 days from the onset of the fermentation tests. All pretreatment procedures for the samples were performed according to the protocols provided by Human Metabolic Technologies, Inc. Briefly, each sample of yeast cells was washed twice with 1 ml ice-cold Milli-Q water, suspended in 1.6 ml methanol containing 5 μM internal standard solution 1 (Human Metabolic Technologies), and then sonicated for 30 s at room temperature. Cationic compounds were measured in the positive mode of capillary electrophoresis-time of flight mass spectrometry (CE-TOFMS), and anionic compounds were measured in the positive and negative modes of capillary electrophoresis-tandem mass spectrometry (CE-MS/MS) (44). Peaks detected by CE-TOFMS and CE-MS/MS were extracted using automatic integration software (MasterHands [Keio University] [45] and MassHunter Quantitative Analysis B.06.00 [Agilent Technologies], respectively) to obtain peak information, including m/z, migration time, and peak area. The peaks were annotated with putative metabolites from the HMT metabolite database (Human Metabolic Technologies) based on their migration times in CE and m/z values as determined by TOFMS and MS/MS. Metabolite concentrations were calculated by normalizing the peak area of each metabolite with respect to the area of the internal standard and by using standard curves, which were obtained from three-point calibrations.
Supplementary Material
ACKNOWLEDGMENTS
The Japan Society for the Promotion of Science (JSPS) provided funding to D.W. under grant number 16K18676, to S.I. under grant number 17H03795, and to T.M. under grant numbers 25291042 and 17H03802. The Public Foundation of Elizabeth Arnold-Fuji provided funding to D.W. The Foundation for the Nara Institute of Science and Technology provided funding to D.W. The Project of the NARO Bio-oriented Technology Research Advancement Institution (Research Program on Development of Innovative Technology) provided funding to H.T. under grant number 30017B.
We declare no conflicts of interest.
Footnotes
Supplemental material for this article may be found at https://doi.org/10.1128/AEM.02083-18.
REFERENCES
- 1.Kitagaki H, Kitamoto K. 2013. Breeding research on sake yeasts in Japan: history, recent technological advances, and future perspectives. Annu Rev Food Sci Technol 4:215–235. doi: 10.1146/annurev-food-030212-182545. [DOI] [PubMed] [Google Scholar]
- 2.Ohnuki S, Okada H, Friedrich A, Kanno Y, Goshima T, Hasuda H, Inahashi M, Okazaki N, Tamura H, Nakamura R, Hirata D, Fukuda H, Shimoi H, Kitamoto K, Watanabe D, Schacherer J, Akao T, Ohya Y. 2017. Phenotypic diagnosis of lineage and differentiation during sake yeast breeding. G3 (Bethesda) 7:2807–2820. doi: 10.1534/g3.117.044099. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Watanabe D, Wu H, Noguchi C, Zhou Y, Akao T, Shimoi H. 2011. Enhancement of the initial rate of ethanol fermentation due to dysfunction of yeast stress response components Msn2p and/or Msn4p. Appl Environ Microbiol 77:934–941. doi: 10.1128/AEM.01869-10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Akao T, Yashiro I, Hosoyama A, Kitagaki H, Horikawa H, Watanabe D, Akada R, Ando Y, Harashima S, Inoue T, Inoue Y, Kajiwara S, Kitamoto K, Kitamoto N, Kobayashi O, Kuhara S, Masubuchi T, Mizoguchi H, Nakao Y, Nakazato A, Namise M, Oba T, Ogata T, Ohta A, Sato M, Shibasaki S, Takatsume Y, Tanimoto S, Tsuboi H, Nishimura A, Yoda K, Ishikawa T, Iwashita K, Fujita N, Shimoi H. 2011. Whole-genome sequencing of sake yeast Saccharomyces cerevisiae Kyokai no. 7. DNA Res 18:423–434. doi: 10.1093/dnares/dsr029. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Watanabe D, Araki Y, Zhou Y, Maeya N, Akao T, Shimoi H. 2012. A loss-of-function mutation in the PAS kinase Rim15p is related to defective quiescence entry and high fermentation rates of Saccharomyces cerevisiae sake yeast strains. Appl Environ Microbiol 78:4008–4016. doi: 10.1128/AEM.00165-12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Inai T, Watanabe D, Zhou Y, Fukada R, Akao T, Shima J, Takagi H, Shimoi H. 2013. Rim15p-mediated regulation of sucrose utilization during molasses fermentation using Saccharomyces cerevisiae strain PE-2. J Biosci Bioeng 116:591–594. doi: 10.1016/j.jbiosc.2013.05.015. [DOI] [PubMed] [Google Scholar]
- 7.Watanabe D, Zhou Y, Hirata A, Sugimoto Y, Takagi K, Akao T, Ohya Y, Takagi H, Shimoi H. 2016. Inhibitory role of Greatwall-like protein kinase Rim15p in alcoholic fermentation via upregulating the UDP-glucose synthesis pathway in Saccharomyces cerevisiae. Appl Environ Microbiol 82:340–351. doi: 10.1128/AEM.02977-15. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Oomuro M, Kato T, Zhou Y, Watanabe D, Motoyama Y, Yamagishi H, Akao T, Aizawa M. 2016. Defective quiescence entry promotes the fermentation performance of bottom-fermenting brewer's yeast. J Biosci Bioeng 122:577–582. doi: 10.1016/j.jbiosc.2016.04.007. [DOI] [PubMed] [Google Scholar]
- 9.Watanabe D, Kaneko A, Sugimoto Y, Ohnuki S, Takagi H, Ohya Y. 2017. Promoter engineering of the Saccharomyces cerevisiae RIM15 gene for improvement of alcoholic fermentation rates under stress conditions. J Biosci Bioeng 123:183–189. doi: 10.1016/j.jbiosc.2016.08.004. [DOI] [PubMed] [Google Scholar]
- 10.Pedruzzi I, Dubouloz F, Cameroni E, Wanke V, Roosen J, Winderickx J, De Virgilio C. 2003. TOR and PKA signaling pathways converge on the protein kinase Rim15 to control entry into G0. Mol Cell 12:1607–1613. doi: 10.1016/S1097-2765(03)00485-4. [DOI] [PubMed] [Google Scholar]
- 11.Wanke V, Pedruzzi I, Cameroni E, Dubouloz F, De Virgilio C. 2005. Regulation of G0 entry by the Pho80-Pho85 cyclin-CDK complex. EMBO J 24:4271–4278. doi: 10.1038/sj.emboj.7600889. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Panchaud N, Péli-Gulli MP, De Virgilio C. 2013. Amino acid deprivation inhibits TORC1 through a GTPase-activating protein complex for the Rag family GTPase Gtr1. Sci Signal 6:ra42. doi: 10.1126/scisignal.2004112. [DOI] [PubMed] [Google Scholar]
- 13.Nicastro R, Sardu A, Panchaud N, De Virgilio C. 2017. The architecture of the Rag GTPase signaling network. Biomolecules 7:48. doi: 10.3390/biom7030048. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Tatebe H, Shiozaki K. 2017. Evolutionary conservation of the components in the TOR signaling pathways. Biomolecules 7:77. doi: 10.3390/biom7040077. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Urban J, Soulard A, Huber A, Lippman S, Mukhopadhyay D, Deloche O, Wanke V, Anrather D, Ammerer G, Riezman H, Broach JR, De Virgilio C, Hall MN, Loewith R. 2007. Sch9 is a major target of TORC1 in Saccharomyces cerevisiae. Mol Cell 26:663–674. doi: 10.1016/j.molcel.2007.04.020. [DOI] [PubMed] [Google Scholar]
- 16.Wanke V, Cameroni E, Uotila A, Piccolis M, Urban J, Loewith R, De Virgilio C. 2008. Caffeine extends yeast lifespan by targeting TORC1. Mol Microbiol 69:277–285. doi: 10.1111/j.1365-2958.2008.06292.x. [DOI] [PubMed] [Google Scholar]
- 17.Chica N, Rozalén AE, Pérez-Hidalgo L, Rubio A, Novak B, Moreno S. 2016. Nutritional control of cell size by the Greatwall-endosulfine-PP2A·B55 pathway. Curr Biol 26:319–330. doi: 10.1016/j.cub.2015.12.035. [DOI] [PubMed] [Google Scholar]
- 18.Düvel K, Yecies JL, Menon S, Raman P, Lipovsky AI, Souza AL, Triantafellow E, Ma Q, Gorski R, Cleaver S, Vander Heiden MG, MacKeigan JP, Finan PM, Clish CB, Murphy LO, Manning BD. 2010. Activation of a metabolic gene regulatory network downstream of mTOR complex 1. Mol Cell 39:171–183. doi: 10.1016/j.molcel.2010.06.022. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Cameroni E, Hulo N, Roosen J, Winderickx J, De Virgilio C. 2004. The novel yeast PAS kinase Rim15 orchestrates G0-associated antioxidant defense mechanisms. Cell Cycle 3:462–468. [PubMed] [Google Scholar]
- 20.Lee P, Kim MS, Paik SM, Choi SH, Cho BR, Hahn JS. 2013. Rim15-dependent activation of Hsf1 and Msn2/4 transcription factors by direct phosphorylation in Saccharomyces cerevisiae. FEBS Lett 587:3648–3655. doi: 10.1016/j.febslet.2013.10.004. [DOI] [PubMed] [Google Scholar]
- 21.Yu J, Fleming SL, Williams B, Williams EV, Li Z, Somma P, Rieder CL, Goldberg ML. 2004. Greatwall kinase: a nuclear protein required for proper chromosome condensation and mitotic progression in Drosophila. J Cell Biol 164:487–492. doi: 10.1083/jcb.200310059. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Mochida S, Maslen SL, Skehel M, Hunt T. 2010. Greatwall phosphorylates an inhibitor of protein phosphatase 2A that is essential for mitosis. Science 330:1670–1673. doi: 10.1126/science.1195689. [DOI] [PubMed] [Google Scholar]
- 23.Gharbi-Ayachi A, Labbé JC, Burgess A, Vigneron S, Strub JM, Brioudes E, Van-Dorsselaer A, Castro A, Lorca T. 2010. The substrate of Greatwall kinase, Arpp19, controls mitosis by inhibiting protein phosphatase 2A. Science 330:1673–1677. doi: 10.1126/science.1197048. [DOI] [PubMed] [Google Scholar]
- 24.Bontron S, Jaquenoud M, Vaga S, Talarek N, Bodenmiller B, Aebersold R, De Virgilio C. 2013. Yeast endosulfines control entry into quiescence and chronological life span by inhibiting protein phosphatase 2A. Cell Rep 3:16–22. doi: 10.1016/j.celrep.2012.11.025. [DOI] [PubMed] [Google Scholar]
- 25.Moreno-Torres M, Jaquenoud M, De Virgilio C. 2015. TORC1 controls G1-S cell cycle transition in yeast via Mpk1 and the greatwall kinase pathway. Nat Commun 6:8256. doi: 10.1038/ncomms9256. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Moreno-Torres M, Jaquenoud M, Péli-Gulli MP, Nicastro R, De Virgilio C. 2017. TORC1 coordinates the conversion of Sic1 from a target to an inhibitor of cyclin-CDK-Cks1. Cell Discov 3:17012. doi: 10.1038/celldisc.2017.12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Conrad M, Schothorst J, Kankipati HN, Van Zeebroeck G, Rubio-Texeira M, Thevelein JM. 2014. Nutrient sensing and signaling in the yeast Saccharomyces cerevisiae. FEMS Microbiol Rev 38:254–299. doi: 10.1111/1574-6976.12065. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Takahara T, Maeda T. 2012. Transient sequestration of TORC1 into stress granules during heat stress. Mol Cell 47:242–252. doi: 10.1016/j.molcel.2012.05.019. [DOI] [PubMed] [Google Scholar]
- 29.Urano J, Sato T, Matsuo T, Otsubo Y, Yamamoto M, Tamanoi F. 2007. Point mutations in TOR confer Rheb-independent growth in fission yeast and nutrient-independent mammalian TOR signaling in mammalian cells. Proc Natl Acad Sci U S A 104:3514–3519. doi: 10.1073/pnas.0608510104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Ouchi K, Akiyama H. 1971. Non-foaming mutants of sake yeasts selection by cell agglutination method and by froth flotation method. Agric Biol Chem 35:1024–1032. doi: 10.1080/00021369.1971.10860043. [DOI] [Google Scholar]
- 31.Baro B, Játiva S, Calabria I, Vinaixa J, Bech-Serra JJ, de LaTorre C, Rodrigues J, Hernáez ML, Gil C, Barceló-Batllori S, Larsen MR, Queralt E. 2018. SILAC-based phosphoproteomics reveals new PP2A-Cdc55-regulated processes in budding yeast. Gigascience 7:giy047. doi: 10.1093/gigascience/giy047. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Tripodi F, Nicastro R, Reghellin V, Coccetti P. 2015. Post-translational modifications on yeast carbon metabolism: regulatory mechanisms beyond transcriptional control. Biochim Biophys Acta 1850:620–627. doi: 10.1016/j.bbagen.2014.12.010. [DOI] [PubMed] [Google Scholar]
- 33.Chen Y, Nielsen J. 2016. Flux control through protein phosphorylation in yeast. FEMS Yeast Res 16:fow096. doi: 10.1093/femsyr/fow096. [DOI] [PubMed] [Google Scholar]
- 34.Nakazawa N, Sato A, Hosaka M. 2016. TORC1 activity is partially reduced under nitrogen starvation conditions in sake yeast Kyokai no. 7, Saccharomyces cerevisiae. J Biosci Bioeng 121:247–252. doi: 10.1016/j.jbiosc.2015.07.002. [DOI] [PubMed] [Google Scholar]
- 35.Oliveira AP, Ludwig C, Picotti P, Kogadeeva M, Aebersold R, Sauer U. 2012. Regulation of yeast central metabolism by enzyme phosphorylation. Mol Syst Biol 8:623. doi: 10.1038/msb.2012.55. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Goldstein AL, McCusker JH. 1999. Three new dominant drug resistance cassettes for gene disruption in Saccharomyces cerevisiae. Yeast 15:1541–1553. doi:. [DOI] [PubMed] [Google Scholar]
- 37.Janke C, Magiera MM, Rathfelder N, Taxis C, Reber S, Maekawa H, Moreno-Borchart A, Doenges G, Schwob E, Schiebel E, Knop M. 2004. A versatile toolbox for PCR-based tagging of yeast genes: new fluorescent proteins, more markers and promoter substitution cassettes. Yeast 21:947–962. doi: 10.1002/yea.1142. [DOI] [PubMed] [Google Scholar]
- 38.Tanigawa M, Maeda T. 2017. An in vitro TORC1 kinase assay that recapitulates the Gtr-independent glutamine-responsive TORC1 activation mechanism on yeast vacuoles. Mol Cell Biol 37:e00075-17. doi: 10.1128/MCB.00075-17. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Sato M, Dhut S, Toda T. 2005. New drug-resistant cassettes for gene disruption and epitope tagging in Schizosaccharomyces pombe. Yeast 22:583–591. doi: 10.1002/yea.1233. [DOI] [PubMed] [Google Scholar]
- 40.Fujita M, Yamamoto M. 1998. S. pombe sck2+, a second homologue of S. cerevisiae SCH9 in fission yeast, encodes a putative protein kinase closely related to PKA in function. Curr Genet 33:248–254. doi: 10.1007/s002940050333. [DOI] [PubMed] [Google Scholar]
- 41.Kinoshita N, Ohkura H, Yanagida M. 1990. Distinct, essential roles of type 1 and 2A protein phosphatases in the control of the fission yeast cell division cycle. Cell 63:405–415. doi: 10.1016/0092-8674(90)90173-C. [DOI] [PubMed] [Google Scholar]
- 42.Kinoshita K, Nemoto T, Nabeshima K, Kondoh H, Niwa H, Yanagida M. 1996. The regulatory subunits of fission yeast protein phosphatase 2A (PP2A) affect cell morphogenesis, cell wall synthesis and cytokinesis. Genes Cells 1:29–45. doi: 10.1046/j.1365-2443.1996.02002.x. [DOI] [PubMed] [Google Scholar]
- 43.Watanabe D, Ota T, Nitta F, Akao T, Shimoi H. 2011. Automatic measurement of sake fermentation kinetics using a multi-channel gas monitor system. J Biosci Bioeng 112:54–57. doi: 10.1016/j.jbiosc.2011.03.007. [DOI] [PubMed] [Google Scholar]
- 44.Soga T, Heiger DN. 2000. Amino acid analysis by capillary electrophoresis electrospray ionization mass spectrometry. Anal Chem 72:1236–1241. doi: 10.1021/ac990976y. [DOI] [PubMed] [Google Scholar]
- 45.Sugimoto M, Wong DT, Hirayama A, Soga T, Tomita M. 2010. Capillary electrophoresis mass spectrometry-based saliva metabolomics identified oral, breast and pancreatic cancer-specific profiles. Metabolomics 6:78–95. doi: 10.1007/s11306-009-0178-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Kitamoto K, Oda K, Gomi K, Takahashi K. 1990. Construction of uracil and tryptophan auxotrophic mutants from sake yeasts by disruption of URA3 and TRP1 genes. Agric Biol Chem 54:2979–2987. doi: 10.1271/bbb1961.54.2979. [DOI] [Google Scholar]
- 47.Nakazawa N, Abe K, Koshika Y, Iwano K. 2010. Cln3 blocks IME1 transcription and the Ime1-Ume6 interaction to cause the sporulation incompetence in a sake yeast, Kyokai no. 7. J Biosci Bioeng 110:1–7. doi: 10.1016/j.jbiosc.2010.01.006. [DOI] [PubMed] [Google Scholar]
- 48.Chia KH, Fukuda T, Sofyantoro F, Matsuda T, Amai T, Shiozaki K. 2017. Ragulator and GATOR1 complexes promote fission yeast growth by attenuating TOR complex 1 through Rag GTPases. eLIFE 6:e30880. doi: 10.7554/eLife.30880. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.