Abstract
Natriuretic peptides (NPs) exert diverse effects on several biological and physiological systems, such as kidney function, neural and endocrine signaling, energy metabolism, and cardiovascular function, playing pivotal roles in the regulation of blood pressure (BP) and cardiac and vascular homeostasis. NPs are collectively known as anti-hypertensive hormones and their main functions are directed toward eliciting natriuretic/diuretic, vasorelaxant, anti-proliferative, anti-inflammatory, and anti-hypertrophic effects, thereby, regulating the fluid volume, BP, and renal and cardiovascular conditions. Interactions of NPs with their cognate receptors display a central role in all aspects of cellular, biochemical, and molecular mechanisms that govern physiology and pathophysiology of BP and cardiovascular events. Among the NPs atrial and brain natriuretic peptides (ANP and BNP) activate guanylyl cyclase/natriuretic peptide receptor-A (GC-A/NPRA) and initiate intracellular signaling. The genetic disruption of Npr1 (encoding GC-A/NPRA) in mice exhibits high BP and hypertensive heart disease that is seen in untreated hypertensive subjects, including high BP and heart failure. There has been a surge of interest in the NPs and their receptors and a wealth of information have emerged in the last four decades, including molecular structure, signaling mechanisms, altered phenotypic characterization of transgenic and gene-targeted animal models, and genetic analyses in humans. The major goal of the present review is to emphasize and summarize the critical findings and recent discoveries regarding the molecular and genetic regulation of NPs, physiological metabolic functions, and the signaling of receptor GC-A/NPRA with emphasis on the BP regulation and renal and cardiovascular disorders.
Keywords: cardiovascular hemostasis, cGMP, gene-targeting, hypertension, natriuretic peptide receptor, receptor signaling
INTRODUCTION
Almost 37 yr ago, the pioneering discovery by de Bold and his colleagues (35) demonstrated that atrial extracts contained natriuretic and diuretic activities, which led them to isolate and characterize “atrial natriuretic factor/peptide (ANF/ANP)” and to establish the field of natriuretic peptides (NPs) hormone family. The NPs are a group of peptide hormones that play important roles in the control of blood pressure (BP) by affecting renal, cardiovascular, neuronal, and endocrine homeostasis (92, 123, 154, 155, 198, 199, 212, 229). ANP has been shown to be present in various organs including heart, brain, kidney, and gonads; however, it is mainly secreted from the cardiac atrium that elicits natriuretic, diuretic, vasorelaxant, antimitogenic, antihypertrophic, and anti-inflammatory effects, all of which are predominantly directed to the reduction of body fluid volume and BP homeostasis (16, 53, 61, 62, 105, 154). Later, two other members of NPs, namely brain natriuretic peptide (BNP) and C-type natriuretic peptide (CNP), were identified and characterized with structural properties similar to ANP, but each of them are encoded from a separate gene (177). BNP was isolated and identified from the brain, but largely synthesized in the cardiac ventricles, secreted in the plasma, and exhibits the greater variability in peptide structure. It is considered that CNP is largely synthesized in the endothelial cells of vasculature and to some extent in the central nervous system, which is remarkably conserved between the species. All three NPs contain a ring structure of 17-amino acid residues and joined by a single disulfide-bridge but deviate from each other in the amino- and carboxyl -terminal flanking sequences (123). The primary structure of the ANP precursor derived from the cDNA sequence contained the biologically active peptide sequence in its carboxyl-terminal region and indicated that circulating ANP is a 28-amino acid molecule, which exhibits biological and physiological activities (123).
Plasma membrane guanylyl cyclase (GC) represents the biological active form of ANP receptor (NPR), which was cloned and sequenced from rat brain (26, 186), human placenta (20), mouse testis (164), and rat adrenal gland (41). The molecular cloning and nucleotide sequence analyses have identified three types of NPRs (164, 186). The NP receptors with intrinsic GC catalytic activities have been named as natriuretic peptide receptor-A and -receptor B (NPRA and NPRB), also recognized as GC-A/NPRA and GC-B/NPRB, respectively (56, 86, 97, 154, 164, 186). A third NP receptor lacks the GC catalytic domain and has been termed by default as a natriuretic peptide clearance receptor (NPRC), which is considered to clear some of the NPs from the circulation (55, 118). However, the studies using NPRC gene-knockout mice, suggested that NPRC could modulate local effects of NPs, and unexpectedly homozygous null mutant mice (Npr3−/−) exhibited skeletal abnormalities (120). The NPRA is activated by ANP and BNP, while NPRB is activated by CNP, both receptors generate second messenger cGMP, which triggers the biological and physiological functions of NPs (Fig. 1). On the other hand, all NPs (ANP, BNP, and CNP) bind to NPRC, which does not produce cGMP (154). The primary sequence structure of NPRA contains four main regions, including an extracellular ligand-binding region, a single plasma membrane spanning domain, and the cytoplasmic protein kinase-like homology (protein-KHD) and GC catalytic active-site regions (97, 154, 157, 164). The NPRB has overall domain structure like NPRA with the selective binding specificity to CNP (185, 186). NPRC contains a short (35 residues) intracellular cytoplasmic tail, which is devoid of GC domain, apparently not effective to generate cGMP (2, 3, 55, 69). NPRA is the dominant form of the all NP receptors found in most of the peripheral organs including kidneys, adrenal glands, vascular beds, brain, heart, and gonadal tissues (105, 154, 158, 160, 197). The cell and tissue distribution of NPRA, NPRB, and NPRC and their gene-knockout phenotypes is presented in Table 1.
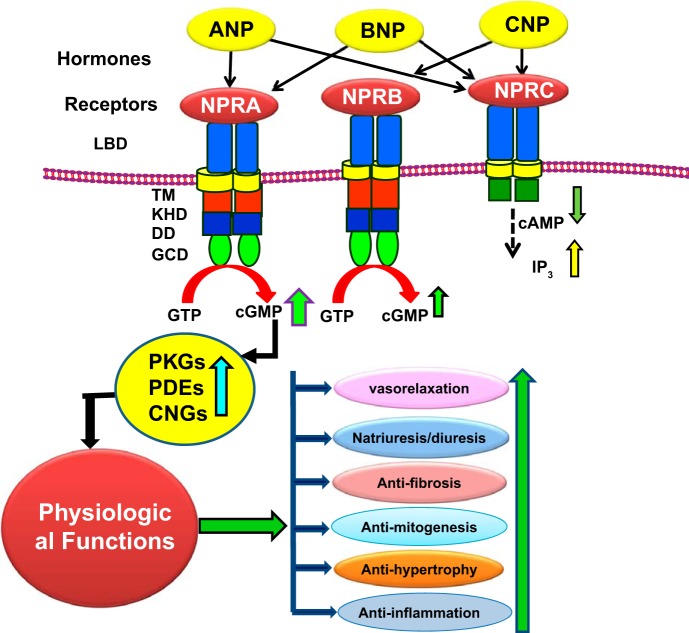
Fig. 1.
Diagrammatic representation of ligand-dependent activation and physiological functions of NPRA, NPRB, and NPRC. ANP-binding activates NPRA, which leads to enhanced production of second messenger cGMP with stimulation and activation of ANP-dependent cellular and physiological responsiveness. CNP activates NPRB, and all three NPs activate NPRC. ANP, atrial natriuretic peptide; BNP, brain natriuretic peptide; CNP, C-type natriuretic peptide; LBD, ligand binding domain; TM, transmembrane domain; KHD, kinase like homology domain; DD, dimerization domain; GCD, guanylyl cyclase catalytic domain; IP3, inositol trisphosphate.
Table 1.
Disease-specific phenotypes of mice with gene disruption of natriuretic peptides and their specific receptors
| Peptide/Protein Nomenclature | Gene Nomenclature | Gene-disrupted Phenotype in Mouse |
|---|---|---|
| ANP/ANF | Nppa | High blood pressure, salt sensitivity, fibrosis (81, 113, 126, 209) |
| BNP | Nppb | Hypertension, vascular complication, fibrosis (149, 217) |
| CNP | Nppc | Dwarfism, reduced bone growth, impaired endochondral ossification (29, 82, 233, 244) |
| NPRA | Npr1 | Volume overload, high blood pressure, salt sensitivity hypertension, cardiac hypertrophy, cardiac fibrosis, inflammation, and reduced testosterone levels (46, 91, 116, 150, 151, 163, 173, 203, 227) |
| NPRB | Npr2 | Seizures, dwarfism, female sterility, decreased adiposity (103, 216) |
| NPRC | Npr3 | Bone deformation with long bone overgrowth (120) |
Nomenclature of peptides/proteins and genes with gene-disrupted phenotypes are indicated in capital letters and italics, respectively. ANP/ANF, atrial natriuretic factor/peptide; BNP, brain natriuretic peptide; CNP, C-type natriuretic peptide; Nppa (coding for pro-ANP); Nppb (coding for pro-BNP); Nppc (coding for pro-CNP); NPRA, natriuretic peptide receptor-A; NPRB, natriuretic peptide receptor-B; NPRC, natriuretic peptide receptor-C; Npr1 (coding for NPRA); Npr2, (coding for NPRB); Npr3 (coding for NPRC).
DISRUPTION OF Nppa AND Npr1 TRIGGERS PATHOPHYSIOLOGY OF HYPERTENSION AND CARDIOVASCULAR DYSFUNCTION
Hypertension is a heritable quantitative trait, which is influenced by both genetic and environmental factors. Molecular and genetic approaches have greatly advanced our understanding of the interindividual variations in BP, influenced by polymorphisms in the genomic sequences of Nppa (encoding pro-ANP), Nppb (encoding pro-BNP), and Npr1 (45, 83, 106, 107, 111, 146, 229). Studies with ANP-deficient mice have demonstrated that a decreased level of ANP can cause hypertension (81, 126, 156, 159). The BP of mutant animals was elevated by 8–16 mmHg when they were fed with standard- or intermediate-salt diets. Moreover, mice with overexpression of ANP exhibited ~30 mmHg lower systolic BP (SBP) compared with nontransgenic siblings (96, 126, 209). Somatic delivery of Nppa in spontaneously hypertensive rats (SHR) showed a continuous reduction of SBP (113). Similarly, ANP gene therapy showed a regulatable reduction in BP in hypertensive animals (181). The results of those previous studies provided the concept that ANP gene therapy could be used for the treatment of high BP and related disorders. Genetic studies have revealed that the ablation of Npr1 in mice triggers BP by ~40 mmHg higher as compared with control animals (150, 201, 227). It has been demonstrated that complete absence of NPRA causes hypertension in mice and leads to altered renin and angiotensin II (ANG II) levels, reduced renal function, cardiac hypertrophy, and lethal vascular events similar to untreated hypertensive human subjects (33, 34, 150, 184, 203, 227, 249). In contrast, Npr1 gene duplication in mutant mice showed a significant reduction in BP and produced the intracellular second messenger cGMP, proportionately to the increasing number of Npr1 gene copies (151, 163, 203, 226, 228, 249). Previous studies have demonstrated that plasma and heart tissues of hypertensive heart patients exhibit very high levels of ANP and BNP (23, 51, 173, 222, 236). Studies in humans and experimental animal models have shown that Nppa and Nppb genes are overexpressed during the conditions of cardiac hypertrophy and heart failure, indicating that autocrine and/or paracrine signaling might be involved in the cardiac protective effects (46, 51, 72, 95, 226, 227, 242).
NPRA Regulates Volume Overload, Sodium Excretion, and BP
The previous studies have examined the specific contribution of NPRA in mediating the signaling mechanisms responsible for natriuretic and diuretic responses to nondilutional intravascular volume expansion (203). In wild-type (2-copy; Npr1+/+) and Npr1 gene-duplicated (4-copy; Npr1++/++) animals, the excretion of Na+ and urine flow were proportionately associated with increases in glomerular filtration rate (GFR), but the SBP was not significantly increased. The homozygous null mutant (0-copy; Npr1−/−) mice exhibited the highest SBP, whereas the gene-duplicated 4-copy mice showed the lowest SBP compared with wild-type mice (203). During the volume expansion period, the mean arterial BP was increased in all three animal genotypes (0-copy, 2-copy, and 4-copy), but it remained significantly lower in gene-duplicated 4-copy mice and was greatly higher in 0-copy null mutant mice compared with wild-type animals (Fig. 2). The renal hemodynamic parameters, including GFR and renal plasma flow (RPF), were significantly lower in Npr1−/− mice and higher in Npr1++/++ mice compared with Npr1+/+ animals (33, 203). The urinary cGMP concentrations were significantly reduced in 0-copy mice but greatly increased in 4-copy mice compared with 2-copy animals during the entire period of volume expansion. It is remarkable to note that Npr1++/++ mice exhibited significantly higher GFR values and cGMP concentrations compared with significantly lower responses to the volume expansion in Npr1−/− mutant mice (Table 2).
Fig. 2.
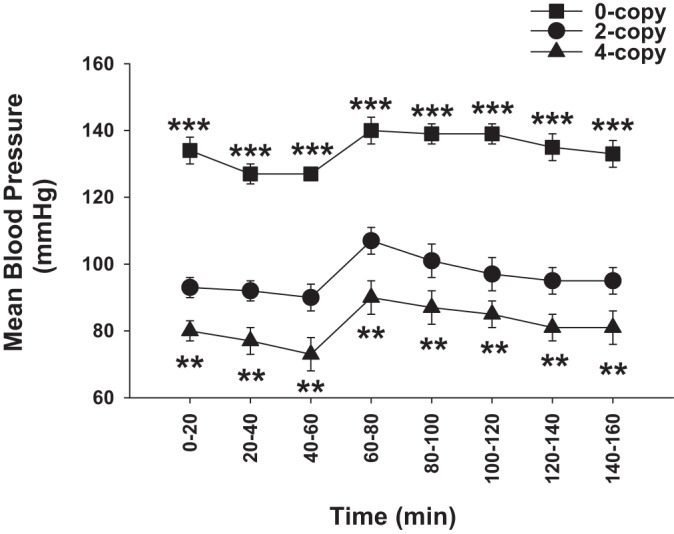
Effect of volume expansion on mean blood pressures in Npr1 homozygous mutant and gene-duplicated mice. Mean blood pressures by cannulated carotid arterial methods were continuously recorded for 160 min throughout the duration of the experiment. **P < 0.01; ***P < 0.001. The figure has been reproduced and adapted with permission from Shi et al. (203).
Table 2.
Effect of volume expansion on glomerular filtration rate in Npr1 gene-targeted mice
| GFR, ml·min−1·g kidney weight−1 | |||
|---|---|---|---|
| Genotype | Before VE | VE | After VE |
| 0-Copy | 0.49 + 0.03* | 0.58 + 0.04† | 0.49 + 0.05* |
| 2-Copy | 0.63 + 0.03 | 0.82 + 0.09 | 0.68 + 0.06 |
| 4-Copy | 0.81 + 0.04 | 1.19 + 0.12† | 0.87 + 0.08* |
Glomerular filtration rate (GFR) was determined before, during, and after pure blood volume expansion (VE) in Npr1 null mutant (0-copy), wild-type (0-copy), and Npr1 gene-duplicated (4-copy) mice. GFR was measured in the three consecutive periods at 0–60 min before VE, 60–80 min during VE, and 80–160 min after VE. n = 9; 0-copy vs. 2-copy.
P < 0.05; 0-copy vs. 2-copy and 4-copy vs 2-copy;
P < 0.01.
Previous studies from our laboratory have demonstrated that during the blood volume expansion, urine flow and sodium excretion were greatly increased in 2-copy and 4-copy animals; however, in 0-copy Npr1 mice only a minor change in urine flow and sodium excretion occurred despite highly elevated BP (203). It should be noted that gene-duplicated Npr1++/++ mice exhibited significantly higher urinary sodium excretion and urine flow compared with 2-copy wild-type animals. Moreover, after blood volume expansion, there was no significant change in potassium excretion in all Npr1 mice genotypes, including 0-copy, 2-copy, or 4-copy mice either before, during, or after blood volume expansion protocol (203).
The Npr1−/− mice exhibit harmfully elevated BP resembling untreated human hypertensive subjects (33, 34, 46, 150, 201, 203, 226, 227). In contrast, increased expression of Npr1 gene (4 copies) in mice significantly lowered SBP and greatly increased the intracellular accumulation of cGMP proportionately to Npr1 gene copy numbers (151, 163, 226, 249). The cascade of ANP/NPRA/cGMP signaling lowers SBP through its direct effects of natriuretic, diuretic, and vasodilatory responses (123, 128, 203). It also lowers BP indirectly by antagonizing the vasoactive pressure hormones, such as ANG II and vasopressin (142, 213). It is considered that the expression of Nppa and Nppb is greatly increased in hypertensive and hypertrophic failing heart patients, but it is still unclear whether ANP/NPRA system is activated to exert a protective effect by antagonizing the action of pressure hormones and decreasing the adverse effects of high BP due to retention of sodium and fluid volume. Moreover, it could also be a consequence of the detrimental cardiac remodeling due to altered levels of gene expression (16, 46, 92, 125, 155). It has been estimated that BP decreases progressively with increasing number of functional Npr1 gene copies with a change in mean arterial pressure of ~8 mmHg per gene copy and proportional increases in GC activity and accumulation of intracellular cGMP levels (151, 163, 203, 226). There is evidence to support the concept that ANP-BNP/NPRA signaling in the kidneys provides a renoprotective role especially under conditions of elevated BP levels (65).
Role of Npr1 in Counteracting the Effects of Renin-Angiotensin-Aldosterone System and BP Regulation
Our previous findings have indicated that the kidney renin content in newborn Npr1−/− pups (2 days after birth) was 2.5-fold higher than in Npr1+/+ control pups, but the plasma renin concentrations in adult Npr1−/− null mutant mice were one-fourth of the wild-type Npr1+/+ animals (201). However, adult (16 wk) hypertensive Npr1−/− mutant animals exhibited 65–70% lower kidney renin contents as compared with Npr1+/+ animals (201). In contrast, the adrenal renin contents were twofold higher in Npr1−/− adult mice than in Npr1+/+ control mice. It should be noted that the effect of increased Npr1 gene-dosage on the renin activity and expression levels remains still unclear. It is possible, however, that the synthesis and expression of kidney renin mRNA and renin content in adult Npr1−/− mice are suppressed due to volume expansion, elevated BP, and/or activation of baroreceptors (156, 201, 203). It is also not yet clear whether the synthesis of intracellular renin in juxtaglomerular and connecting tubule cells is altered in Npr1 mice in a gene dose-dependent manner, which could be due to elevated BP, reflecting a decrease in plasma renin concentrations.
The renal ANG II levels seem to be regulated by ANP-NPRA/cGMP signaling and by additional factors, including salt diet, BP, and renin-angiotensin-aldosterone system (RAAS) itself (11, 220, 240, 246, 247, 249). Variations in dietary sodium intake are closely associated with changes in renal renin contents and plasma renin concentrations (143). Our previous studies have suggested that plasma ANG II levels were decreased in Npr1−/− mice as compared with Npr1+/+ controls; nevertheless, plasma aldosterone concentrations were greatly increased in these mutant animals (249). It is considered that the increased plasma aldosterone levels might be due to the increases in the adrenal renin contents (201). Elevated BP is known to decrease the plasma ANG II levels by inhibiting the synthesis and release of renin that seems to act as a negative feedback mechanism in the Npr1 gene-disrupted mice but not in wild-type mice (201). On the other hand, decreased plasma ANG II levels in Npr1 gene-duplicated mice as compared with wild-type mice seem to be due to a consequence of the antagonistic effects of ANP-BNP/NPRA signaling to RAAS. It is believed that elevations in the renal ANG II concentrations cause reductions in renal function and Na+ excretion that contribute to the sustained hypertension and lead to renal and vascular injury (144, 248). Increased excretion of sodium and urine output with decreased arterial BP in Npr1 gene-duplicated mice might attenuate the counteractive effects of the high-salt diet on the plasma ANG II and aldosterone concentrations (203). Altogether, NPRA signaling seems to exert renal protective effect on the blood volume and BP homeostasis in Npr1 gene-knockout mice.
Our previous studies have suggested that 1-copy haplotype Npr1+/− female mice receiving high-salt diet exhibited increased systolic BP compared with Npr1+/+ animals (247, 249). Moreover, Npr1 ablation led to chronic elevation of BP in mutant mice in a salt-sensitive manner (151). However, an earlier provocative report has indicated that BP remained unchanged in response to either low- or high-salt diets in Npr1 gene-knockout mice (carrying a simple insertion of a neomycin-resistance gene into exon 4 of Npr1 construct), with a similar degree of hypertension (116). Those earlier studies indicated that the ablation of Npr1 did not seem to exhibit salt-sensitive hypertension in mice. However, gene-knockout studies of Nppa have suggested that the deficiency of ANP exhibited its effect on BP in a salt-sensitive manner (81, 127). Interestingly, ANP-BNP/NPRA signaling opposes most of the effect of ANG II in both cellular and physiological environments (Table 3).
Table 3.
Antagonistic actions of ANG II and ANP
| ANG II | ANP/BNP |
|---|---|
| Cellular and whole body effects | |
| 1. Stimulates blood vessel and cell constriction (102, 129) | 1. Inhibits blood vessels constriction (4, 57, 190) |
| 2. Stimulates aldosterone release (238, 245) | 2. Inhibits aldosterone release (7, 10, 175) |
| 3. Stimulates vasopressin release (70, 169, 241) | 3. Inhibits vasopressin release (147, 174) |
| 4. Inhibits renin release (24, 64, 138) | 4. Inhibits renin release (17, 148, 201) |
| 5. Stimulates cell growth proliferation (13, 36, 77, 135, 139, 170, 239) | 5. Inhibits cell growth and proliferation (1, 4, 100, 194) |
| 6. Inhibits apoptosis (130, 170, 239) | 6. Induces apoptosis (74, 239) |
| 7. Enhances glomerulosclerosis (167) | 7. Inhibits glomerulosclerosis (167) |
| 8. Facilitates neurotransmission (52, 71) | 8. Inhibits neurotransmission (39, 40, 75, 140, 141) |
| 9. Causes vascular injury (9, 63, 237) | 9. Protects against vascular injury (16, 105) |
| 10. Increases blood pressure (9, 60, 176, 193, 202, 253, 254) | 10. Reduces blood pressure (16, 35, 81, 113, 203) |
| Biochemical Effects | |
| 1. Stimulates/activation of protein kinase C (100) | 1. Inhibits activation of protein kinase C (100, 101) |
| 2. Increases Ca2+ concentrations (16, 44, 235) | 2. Decreases Ca2+ concentrations (67, 114) |
| 3. Stimulates activation of MAP kinases (42, 77, 162, 171, 224, 252) | 3. Inhibits activation of MAP kinases (134, 161, 194, 221) |
| 4. Stimulates phosphoinositide metabolism (215, 218) | 4. Inhibits phosphoinositide metabolism (85, 86) |
| 5. Interacts with G protein-coupled receptors (88, 172, 200) | 5. Not actively involved with G proteins (85) |
| 6. Activates IKK/NF-κB inflammatory pathways (117, 179) | 6. Inhibits IKK/NF-κB inflammatory pathways (34, 98, 227) |
ANG II, angiotensin II; ANP, atrial natriuretic peptide.
It has been shown that low-salt diet increases the adrenal ANG II and aldosterone concentrations in Npr1−/− and Npr1+/− mutant mice as compared with the levels seen in mice fed with a normal-salt diet (247, 249). However, a high-salt diet lowered the adrenal ANG II and aldosterone concentrations in Npr1+/− and Npr1+/+ mice but not in Npr1++/+ and Npr1++/++ mice as compared with the levels in mice fed with a normal-salt diet. Those previous studies showed that the Npr1 gene copy numbers affect adrenal aldosterone level, especially with a low-salt diet. Sodium restriction increases the adrenal renin activity, ANG II levels, and aldosterone production (27, 38, 122). It was concluded that adrenal aldosterone concentrations were also affected in a Npr1 gene-dose-dependent manner, indicating that the release of adrenal aldosterone levels in 1-copy and 2-copy mice was suppressed in response to the high-salt diet as compared with mice fed the normal-salt diet. However, high-salt diet did not suppress adrenal aldosterone levels in Npr1 gene-duplicated mice (249). The underlying mechanism may be related to the elevated Na+ excretion and urine output that occurs with relatively low BP in Npr1 gene-duplicated 3-copy and 4-copy mice than in wild-type mice; thus, the inhibitory effect of the high-salt diet on adrenal aldosterone concentrations was attenuated in Npr1 gene-duplicated mice. The adrenal gland is more sensitive to a low-salt diet than to a high-salt diet, which is consistent with the fact that the adrenal gland functions to retain salt to regulate blood volume and BP homeostasis. Also, it is well known that a low-salt diet increases the plasma ANG II and aldosterone levels, whereas a high-salt diet inhibits these components of RAAS in the circulation (104, 136). Overall, ANP exerts inhibitory effects on both the synthesis and release of adrenal aldosterone levels (7, 16, 105, 154, 175).
Implication of ANP/NPRA Gene Polymorphisms in the Regulation of BP and Cardiovascular Disorders
ANP and BNP act on the kidneys to induce natriuresis and diuresis, thereby lowering the blood volume and BP (50, 105, 123, 154, 229). Previously, a small population has been identified in which hypertension is the consequence of single gene defects that are inherited in a Mendelian fashion; however, in the most of individuals, BP is determined by the action of many quantitative genes (68, 79, 110, 111, 204, 205). It is believed that genetic analysis provides a more accurate and faster approach to evaluate physiological functions. If the gene of interest is quantitative, such as BP and cardiovascular events, it requires optimum function in maintaining a uniform genetic background. Moreover, gene-targeting technology (gene-knockout and gene-duplication) has provided a unique strategy to study the physiological function in a gene-dose-dependent manner in genetically modified experimental animal models (87, 163, 206, 214, 247).
Genetic polymorphisms of Nppa, Nppb, and Npr1 exist in relation to high BP and cardiovascular disorders in humans (146, 178, 188, 229, 234, 242). A relationship of gene polymorphism in the Nppa promoter region has also been shown with the left ventricular hypertrophy in the Italian hypertensive subjects (178, 229). Those previous findings indicated that individuals carrying Nppa variant alleles exhibited significantly decreased expression of Nppa and reduced levels of circulation ANP, which were associated with the cardiac hypertrophy and congestive heart failure (178, 229). Similarly, an association of a microsatellite marker in the Npr1 promoter region exhibited ventricular hypertrophy, suggesting that the ANP-BNP/NPRA system might contribute to cardiac remodeling in essential hypertensive subjects (178, 229, 242). Moreover, the relationship between hypertension and cardiovascular dysfunction has been noted that even the small increases in BP contributes toward cardiac harmful effects (146, 178, 188).
Previous findings have also indicated the prevalence of high BP with cardiovascular risks factors that have led to a belief in a role for genetic factors (106). A common genetic polymorphism in Nppa and Nppb was discovered to be associated with plasma levels of ANP and BNP, thus contributing toward interindividual variations in high BP and hypertension in humans (146). Those previous findings showed that single nucleotide polymorphisms in Nppa and Nppb were linked with increased plasma ANP/BNP levels and high BP. The contribution of gene mutations has been previously positively correlated for the monogenic forms of high BP, hypertension, and cardiovascular disorders in humans (25, 80, 111, 180, 219). Several pathways, including RAAS, have been suggested to regulate high BP and hypertension; however, the genetic determinants in these pathways contributing to interindividual differences in BP regulation have not been yet completely established. Therefore, an association of common polymorphisms in Nppa and Nppb loci with plasma levels of NPs contributing to interindividual variations in hypertension and the regulation of high BP seems to be a novel mechanism and needs further investigations. A polymorphism in 3′-noncoding region of Npr1 also seems to be linked with an increased expression of NH2-terminal-proBNP (NT-proBNP) in humans (234). Individuals with mutations in the Npr1 promoter region exhibit significantly higher levels of NT-proBNP. The intrinsic mechanisms for this effect could be due to mRNA instability of Npr1, contributing to a decreased translational level of the NPRA (94). The repressed effect of ANP-BNP/NPRA signaling due to a defect in the Npr1 might lead to a compensatory response to an increased expression and secretion of NPs. The positive co-relationship has been noted with Nppa, Nppb, and Npr1 gene disruption in relation with essential hypertension, high BP, and cardiac hypertrophy in the mutant mice (Table 1).
NPRA PREVENTS RENAL INJURY, HYPERTROPHIC GROWTH, AND INFLAMMATION
High BP seems to contribute in initiating and sustaining the fibrotic and hypertrophic remodeling of the kidneys. Several growth factors, ANG II, cytokines, and matrix metalloproteinases (MMPs) are considered to play important roles in the pathogenesis of glomerular and tubulointerstitial fibrosis and hypertrophy of in the kidneys (68, 73, 90). The ANP/NPRA system facilitates renal hemodynamic functions (GFR and RPF) and has been implicated in preventing the renal injury and hypertrophic growth (65, 112). Renal fibrosis and glomerular inflammation exhibit complex pathophysiological etiology involving excessive generation of proinflammatory cytokines, growth factors, imbalanced synthesis/degradation of MMPs, and extracellular matrix (ECM) proteins (78, 124, 132). Mice lacking functional Npr1 exhibit renal fibrosis and hypertrophic growth by altered expression of MMPs, ECM proteins, and proinflammatory cytokines and chemokines (33, 34). It is considered that fibrosis plays a critical role in all types of sustained kidney disease, regardless of etiology (12, 43). Usually, the renal fibrosis is initiated from the excessive synthesis of collagen in mesangial cells, interstitial myofibroblasts, and vascular smooth muscle cells that seem to maintain the stimulated pathophysiological inflammation and fibrosis in the kidneys.
Our previous findings have indicated that altered levels of MMPs play a pivotal role in the initiation and development of the fibrotic growth of the kidneys and heart (33, 165, 227). Under normal conditions, the activity of MMPs is controlled by tissue-specific MMP inhibitors (TIMPs), which deactivate and/or inhibit MMPs during the posttranslational regulatory mechanisms (19, 33, 59, 227). The disruption of the balance between MMPs and TIMPs contributes to the pathophysiological processes of renal fibrosis and hypertrophic growth (166, 189, 223). Usually, TIMPs bind to the active site of MMPs, thereby blocking their access to ECM substrate protein molecules (109). Thus, the altered levels of MMPs and TIMPs could be critical in promoting the renal remodeling of ECM proteins that could contribute to the abnormal mesangial and tubulointerstitial cell architecture and organization. The regulated control of ECM homeostasis is maintained, in part, by a balance between MMPs and TIMPs. Progressive disorders in renal function seems to be associated with the development of glomerulosclerosis and also with renal fibrosis, which is characterized by increased accumulation of ECM proteins and structural rearrangements that involve cellular hypertrophy, hyperplasia, and proliferation (14, 33, 34, 93). The kidney sections stained with Masson’s trichrome showed progressive increases in collagen synthesis and deposition in the interstitial spaces in Npr1−/− and Npr1+/− mice compared with Npr1+/+ mice (33, 34).
Disruption of Npr1 and Altered Expression of Proinflammatory Cytokines in the Kidneys
Increased production of proinflammatory cytokines is a hallmark feature of progressive fibrosis and hypertrophic growth in the kidney and other organs (21, 30, 153, 183, 207, 250). Previous studies have shown that several of the proinflammatory and profibrotic molecules, such as interferon-γ (IFN-γ), interleukins-1, -2, -6, and -17 (Il-1, Il-2, Il-6, and Il-17), tumor necrosis factor-α (TNF-α), and transforming growth factor-beta 1 (TGF-β1) serve to amplify the inflammatory and fibrotic responsiveness in the kidneys of Npr1−/− mice (33, 34, 98, 99). Specifically, blocking the expression and function of proinflammatory cytokines before and/or during the progression of the inflammatory process has been considered a valuable tool in determining the contribution of these molecules in the initiation and progression of the inflammation and fibrotic disease states. However, it is possible that blockade of a single cytokine in the inflammatory cascade might be compensated by the enhanced expression of other cytokines to promote the proinflammatory functions (5, 6, 243). Therefore, the strategy to block the multiple cytokines should be adapted to gain therapeutic advantage to prevent renal inflammation and disease states. Both in vitro and in vivo studies have shown that IL-1 receptor antagonist (IL-1 Ra) treatment suppresses the expression and synthesis of several other proinflammatory cytokines such as IFN-γ, IL-1, IL-2, IL-6, and TNF-α (28, 152, 192, 225). Moreover, our recent studies have indicated that TGF-β1 antagonizes the transcriptional machinery of Npr1 in the cultured mesangial and vascular smooth muscle cells (190).
Nuclear factor-κB (NF-κB) serves as a multifunctional proinflammatory signaling molecule that plays a pivotal role in cell survival, inflammation, and apoptosis (8, 32, 58). NF-κB also enhances the transcriptional activation of multiple genes, including various proinflammatory cytokines, MMPs, and ECM proteins that contribute to increased BP and renal damage in Npr1 gene-disrupted mice (33, 34). The activation and nuclear translocation of NF-κB have been shown to enhance the expression of various proinflammatory cytokines, growth factors, and ECM proteins, leading to increased BP and renal damage in Npr1−/− mice (Fig. 3). It has been suggested that NF-κB activates the transcriptional machinery of many proinflammatory genes in both chronic and acute conditions of inflammatory responses in the kidneys (89, 108). The sustained induction of NF-κB has been shown in experimental animal models and human disease conditions (8, 32, 54, 58, 227). Modulation of NF-κB activation and signaling might be an important strategy to reduce cellular injury and inflammation in the absence of ANP-BNP/NPRA signaling (Fig. 3). There is increasing evidence to suggest that NF-κB is a critical mediator in the pathophysiology of hypertension and cardiovascular diseases, which are characterized by elevated levels of inflammatory cytokines, MMPs, and ECM proteins (34, 115, 226, 227). Our previous findings have suggested that expression and synthesis of monocyte chemoattractant protein-1 (MCP-1) are induced in the renal macrophages in hypertensive disease states and play a critical role in the recruitment of monocytes during kidney inflammation and injury (33, 47). However, in the absence of ANP/NPRA/cGMP signaling cascade, the expression of both proinflammatory and profibrotic cytokine genes is significantly enhanced to contribute to the renal fibrosis and hypertrophy in Npr1−/− mice, independently of elevated BP (33, 34).
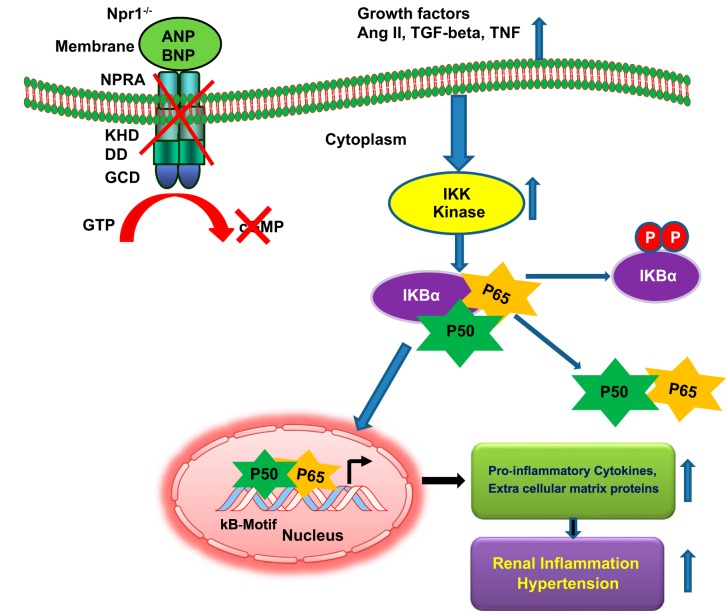
Fig. 3.
Diagrammatic representation of the Npr1 gene-disruption on renal remodeling, and blood pressure. Disruption of Npr1 gene leads to impaired renal functions, which triggers specific structural and molecular changes in 0-copy mice kidney. Activated inhibitory kappa kinase (IKK) induces the phosphorylation of IκBα, which then undergoes enzymatic degradation. The reduction of cytosolic IκBα favors the constitutive activation of NF-κB, which translocates into the nucleus and activates the transcription of proinflammatory cytokines and extracellular matrix protein genes in the kidneys of Npr1 gene-disrupted mice. These molecular changes lead to increase in the inflammatory responses and high blood pressure in Npr1 gene-disrupted mutant mice.
Ablation of Npr1 Causes Renal Fibrosis and End-organ Damage
Previous studies have suggested that renal tubular damage occurs in Npr1−/− and Npr1+/− mice compared with Npr1+/+ mice (33). This damage was characterized by tubular dilation with flattened epithelium and progressive expansion of the interstitial spaces usually referred to as matrix mesangial expansion (MME), which was remarkably pronounced in the kidneys of Npr1 gene-knockout mice. Significant increases were observed in the immunoreactive expression of proliferating cell nuclear antigen (PCNA) and alpha-smooth muscle actin (α-SMA) in the glomeruli, proximal and distal tubules, and arterioles of 0-copy and 1-copy mice compared with 2-copy mice (34, 99). The expression of TGF-β1 was also increased by three- to fourfold in the kidneys of homozygous null mutant Npr1−/− mice compared with wild-type animals (33, 34). TGF-β1 contributes to the genesis of renal fibrosis in a gene-dose-dependent manner (68, 84). The Npr1 gene-disruption causes the renal fibrosis, hypertrophy, and ECM remodeling in mice with decreasing Npr1 gene copy numbers. There were significant increases in the kidney weight/body weight ratio, urinary albumin excretion, plasma creatinine concentrations, and reduced creatinine clearance rate, suggesting an increased incidence of renal pathology in Npr1−/− mice. Increased plasma creatinine was associated with the kidney pathologies of tubulointerstitial fibrosis, nephropathy, and polycystic chronic kidney disease conditions (66, 119, 168, 230).
It has been noted that the expression of both monocytes and macrophages is greatly enhanced in chronic kidney disorders, leading to renal fibrosis (76). The onset of glomerular hypertrophy with the thickening of the basement membrane leads to the enlargement of glomerulus with the onset of MME in chronic renal disease conditions (22, 121, 208, 210). The proinflammatory molecule such as TNF-α has been shown to contribute to chronic kidney inflammation and proliferation of mesangial cells that usually precede interstitial matrix deposition in the obstruction-induced renal injury and fibrosis (30, 124, 145). The previous studies have also shown that an increased expression of TGF-β1 exerts a regulatory effect on the development of MME in the kidneys; however, the inhibition by its antibodies effectively blocked the glomerular enlargement and matrix expression (195, 196, 251).
The ANP/NPRA system has been shown to inhibit the proliferation of mesangial and vascular smooth muscle cells in the kidneys by increased production of second messenger cGMP (18, 157, 162, 163, 194, 221). An enhanced expression of PCNA in kidney tissues reflected the renal hypertrophy in Npr1−/− mice, although the percentages of glomeruli with PCNA-positive cells were increased in the kidneys of both homozygous and heterozygous Npr1 gene-knockout mice. The severity of these increases was largely pronounced in Npr1−/− null mutant animals. Our previous findings have demonstrated that both histological and functional changes in the kidneys correlate with the progressive hypertrophy and fibrosis in Npr1 gene-knockout mice. Nevertheless, the normalization of BP showed only a partial effect in reversing renal fibrosis in Npr1−/− mice (33). It is considered that α-SMA-positive cells serve as a major source of profibrotic cytokine TGF-β1 and have been implicated in the development of tubulointerstitial renal fibrotic lesions (211). Simultaneously, treatment with captopril and losartan has also shown a partial attenuation in immunoexpression of α-SMA in Npr1−/− mice (33). However, those previous studies from our laboratory indicated that the administration of bendroflumethiazide significantly lowered BP but did not produce any beneficial effect in either reversing the kidney fibrosis or decreasing the expression of α-SMA in Npr1−/− mice. Moreover, the residual renal damage seems to occur, despite treatment of Npr1 null mutant mice with three different antihypertensive drugs.
NPRA SIGNALING IN DIABETES COMPLICATIONS
The evidence suggests that reduced levels of ANP seem to be associated with insulin resistance, obesity, and metabolic syndrome in humans (231, 232). However, studies have also shown that ANP/NPRA/cGMP signaling promotes muscle mitochondrial biogenesis and fat oxidation by involving the activation of peroxisome proliferator-activated receptor-γ (PPAR-γ) coactivator-1α (PGC-1α) to prevent the glucose intolerance (131, 133). Moreover, murine adipocytes have been shown to exhibit predominance of NPRC and the lipolytic effect of NPs, which was totally salvaged in Npr3 (encoding NPRC) gene-disrupted mice (15, 191). The ANP/NPRA system has been shown to enhance the browning of white adipocytes in humans, which is considered physiologically beneficial (48, 133). Mice exposed to low temperatures exhibited increased release of circulating ANP with enhanced NPRA/cGMP signaling but showed reduced expression levels of NPRC in both brown and white adipose tissues (15). It has been suggested that the ANP/NPRA system might play a pivotal role in enhancing the expression of PGC-1α, which stimulates oxidative phosphorylation in human skeletal muscle tissues (49). Those previous findings indicated that NPRA signaling enhances and promotes the mitochondrial oxidative metabolism and fat oxidation, which might contribute to the enhanced ability of exercise-induced fat oxidation and energy use. Thus, under aerobic conditions in response to exercise, ANP/NPRA/cGMP signaling has been suggested to promote energy expenditure, which has a central role in regulating the supply of fatty acids for metabolic utilization in both cardiac and skeletal muscle cells (182). Studies in humans and experimental mouse models have suggested that there seems to be a pathophysiological link between obesity, Type 2 diabetes, and insulin resistance with defective NPRA signaling in the skeletal muscle cells (31, 137). Those previous findings suggested that increased plasma ANP/BNP concentrations in diabetic mice and a high-fat diet led to improved blood glucose levels, enhanced insulin signaling, increased lipid oxidation, and reduced lipotoxic effects. It was also demonstrated that NPs increased the lipid oxidation, which accelerated the induction of PGC-1α, independently of PPAR-δ activation (31). Similarly, studies have also shown that NPRA/cGMP-dependent signaling enhanced PGC-1α gene expression in white fat and skeletal muscle cells, suggesting a role of NPRA in diabetes (15, 49). Interestingly, a recent finding has provided the provocative evidence that the ANP/NPRA system might contribute toward the regulatory control of fat mass accumulation in humans (37). On the other hand, defective ANP/NPRA signaling could lead to malfunctional disorders, leading to increased hyperglycemia and hyperinsulinemia, and thereby could provoke high BP, increased lipid accumulation, decreased mitochondrial function, and thus increased renal, cardiac, and vascular dysfunction (Fig. 4).
Fig. 4.
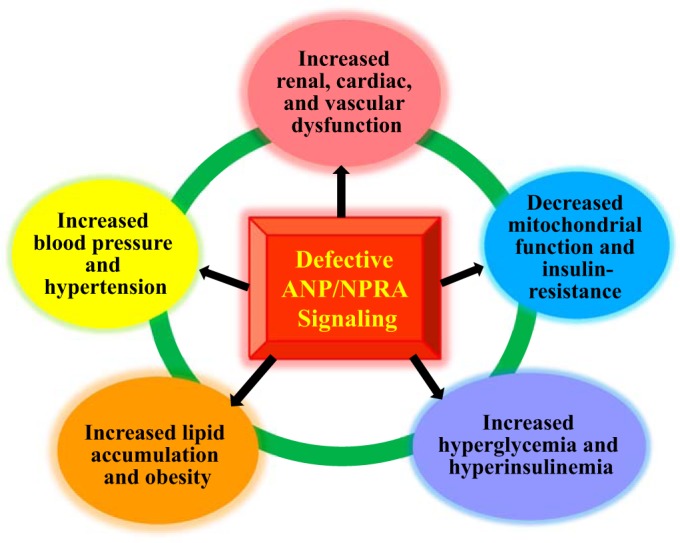
Defective ANP-BNP/NPRA signaling could provoke renal, cardiac, and vascular dysfunction. The defects in ANP-BNP/NPRA signaling cascade are usually associated with hypertension, heart failure, vascular disease, and metabolic syndrome. Thus, defective ANP-BNP/NPRA/cGMP signaling could lead to renal, cardiac, and vascular dysfunction.
CONCLUSIONS AND FUTURE PERSPECTIVES
Recent molecular and genetic studies have greatly contributed toward the understanding of the functional impact of Nppa, Nppb, and Npr1 with emphasis on the kidney disease, hypertension, and cardiovascular dysfunction. The investigative growth in the studies of ANP/NPRA signaling system have advanced our understanding of volume overload, BP, and renal and cardiovascular disorders. Studies utilizing genetically modified mouse models have helped delineate the pathophysiological conditions and physiological functions by overexpression or underexpression of Nppa, Nppb, and Npr1 gene. Specifically, gene-targeting strategies (gene knockout and gene duplication) have greatly helped to produce mouse models of altered Nppa, Nppb, and Npr1 genes to define their precise roles in hypertension and cardiovascular diseases. Using global gene-targeted mice, investigators have been able to delineate the impact of Npr1 expression on BP and other cardiovascular events governed by decreasing or increasing number of Npr1 gene copies in a gene-dose-dependent manner. Future investigations using Npr1 gene-targeted mice in a cell-specific manner in target organs, especially in the kidney, will continue to have enormous potential in studies of hypertension and cardiovascular diseases by altering the number of Npr1 gene copies and thus the product levels of both NPs and NPRA in an identical genetic background. Thus far, the results of animal experimental studies have introduced novel tools for examining the beneficial impact of altered ANP/NPRA signaling cascade in hypertension and cardiovascular disease states.
Although impressive progress has been made, much remains to be learned and discovered about the ANP/NPRA/cGMP system in terms of its signaling, activation, mechanisms, and physiological and pathophysiological roles in hypertension by coordinated function in different organ systems. Furthermore, the molecular events that determine the activation and termination of signals in the ANP-BNP/NPRA system are not yet completely understood. Information regarding the roles of NPs and NPRA at the molecular levels in specific disease conditions, including hypertension, renal disease, and cardiovascular events, is still lacking. Similarly, the paradigm shift of molecular, cellular, and genetic mechanisms of coordinated actions of ANP-BNP/NPRA system is still incompletely understood. Future studies should greatly help in resolving the physiological complexities related to high BP and renal and cardiovascular dysfunction. Current and future investigations should be directed at achieving unique perspectives for determining molecular and genetic aspects of Nppa, Nppb, and Npr1 regulation, expression, and function in physiological and pathophysiological milieu. Future research in the field of the ANP-BNP/NPRA/cGMP system might identify new therapeutic loci for the treatment and prevention of high BP and renal and cardiovascular diseases in humans.
GRANTS
This work was supported by National Heart, Lung, and Blood Institute Grants R01HL-057531 and R01HL-062147.
DISCLOSURES
No conflicts of interest, financial or otherwise, are declared by the author.
AUTHOR CONTRIBUTIONS
K.N.P. conceived and designed research; interpreted results of experiments; prepared figures; drafted manuscript; edited and revised manuscript; and approved final version of manuscript.
ACKNOWLEDGMENTS
The author expresses sincere gratitude to past and present laboratory personnel and collaborators for their immense contributions related to this work. The author also thanks Kamala Pandey for the help in the preparation of this manuscript.
REFERENCES
- 1.Abell TJ, Richards AM, Ikram H, Espiner EA, Yandle T. Atrial natriuretic factor inhibits proliferation of vascular smooth muscle cells stimulated by platelet-derived growth factor. Biochem Biophys Res Commun 160: 1392–1396, 1989. doi: 10.1016/S0006-291X(89)80158-5. [DOI] [PubMed] [Google Scholar]
- 2.Anand-Srivastava MB. Natriuretic peptide receptor-C signaling and regulation. Peptides 26: 1044–1059, 2005. doi: 10.1016/j.peptides.2004.09.023. [DOI] [PubMed] [Google Scholar]
- 3.Anand-Srivastava MB, Trachte GJ. Atrial natriuretic factor receptors and signal transduction mechanisms. Pharmacol Rev 45: 455–497, 1993. [PubMed] [Google Scholar]
- 4.Appel RG. Mechanism of atrial natriuretic factor-induced inhibition of rat mesangial cell mitogenesis. Am J Physiol Endocrinol Metab 259: E312–E318, 1990. doi: 10.1152/ajpendo.1990.259.3.E312. [DOI] [PubMed] [Google Scholar]
- 5.Arend WP. The balance between IL-1 and IL-1Ra in disease. Cytokine Growth Factor Rev 13: 323–340, 2002. doi: 10.1016/S1359-6101(02)00020-5. [DOI] [PubMed] [Google Scholar]
- 6.Arend WP, Malyak M, Guthridge CJ, Gabay C. Interleukin-1 receptor antagonist: role in biology. Annu Rev Immunol 16: 27–55, 1998. doi: 10.1146/annurev.immunol.16.1.27. [DOI] [PubMed] [Google Scholar]
- 7.Atarashi K, Mulrow PJ, Franco-Saenz R, Snajdar R, Rapp J. Inhibition of aldosterone production by an atrial extract. Science 224: 992–994, 1984. doi: 10.1126/science.6326267. [DOI] [PubMed] [Google Scholar]
- 8.Baldwin HS. Early embryonic vascular development. Cardiovasc Res 31, Suppl 1: E34–E45, 1996. doi: 10.1016/S0008-6363(95)00215-4. [DOI] [PubMed] [Google Scholar]
- 9.Barhoumi T, Kasal DA, Li MW, Shbat L, Laurant P, Neves MF, Paradis P, Schiffrin EL. T regulatory lymphocytes prevent angiotensin II-induced hypertension and vascular injury. Hypertension 57: 469–476, 2011. doi: 10.1161/HYPERTENSIONAHA.110.162941. [DOI] [PubMed] [Google Scholar]
- 10.Barrett PQ, Isales CM. The role of cyclic nucleotides in atrial natriuretic peptide-mediated inhibition of aldosterone secretion. Endocrinology 122: 799–808, 1988. doi: 10.1210/endo-122-3-799. [DOI] [PubMed] [Google Scholar]
- 11.Bassett MH, White PC, Rainey WE. The regulation of aldosterone synthase expression. Mol Cell Endocrinol 217: 67–74, 2004. doi: 10.1016/j.mce.2003.10.011. [DOI] [PubMed] [Google Scholar]
- 12.Becker GJ, Hewitson TD. The role of tubulointerstitial injury in chronic renal failure. Curr Opin Nephrol Hypertens 9: 133–138, 2000. doi: 10.1097/00041552-200003000-00006. [DOI] [PubMed] [Google Scholar]
- 13.Bhaskaran M, Reddy K, Radhakrishanan N, Franki N, Ding G, Singhal PC. Angiotensin II induces apoptosis in renal proximal tubular cells. Am J Physiol Renal Physiol 284: F955–F965, 2003. doi: 10.1152/ajprenal.00246.2002. [DOI] [PubMed] [Google Scholar]
- 14.Border WA, Noble NA, Yamamoto T, Harper JR, Yamaguchi Y, Pierschbacher MD, Ruoslahti E. Natural inhibitor of transforming growth factor-beta protects against scarring in experimental kidney disease. Nature 360: 361–364, 1992. doi: 10.1038/360361a0. [DOI] [PubMed] [Google Scholar]
- 15.Bordicchia M, Liu D, Amri EZ, Ailhaud G, Dessì-Fulgheri P, Zhang C, Takahashi N, Sarzani R, Collins S. Cardiac natriuretic peptides act via p38 MAPK to induce the brown fat thermogenic program in mouse and human adipocytes. J Clin Invest 122: 1022–1036, 2012. doi: 10.1172/JCI59701. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Brenner BM, Ballermann BJ, Gunning ME, Zeidel ML. Diverse biological actions of atrial natriuretic peptide. Physiol Rev 70: 665–699, 1990. doi: 10.1152/physrev.1990.70.3.665. [DOI] [PubMed] [Google Scholar]
- 17.Burnett JC Jr, Granger JP, Opgenorth TJ. Effects of synthetic atrial natriuretic factor on renal function and renin release. Am J Physiol Renal Fluid Electrolyte Physiol 247: F863–F866, 1984. doi: 10.1152/ajprenal.1984.247.5.F863. [DOI] [PubMed] [Google Scholar]
- 18.Cao L, Gardner DG. Natriuretic peptides inhibit DNA synthesis in cardiac fibroblasts. Hypertension 25: 227–234, 1995. doi: 10.1161/01.HYP.25.2.227. [DOI] [PubMed] [Google Scholar]
- 19.Cawston TE, Curry VA, Clark IM, Hazleman BL. Identification of a new metalloproteinase inhibitor that forms tight-binding complexes with collagenase. Biochem J 269: 183–187, 1990. doi: 10.1042/bj2690183. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Chang MS, Lowe DG, Lewis M, Hellmiss R, Chen E, Goeddel DV. Differential activation by atrial and brain natriuretic peptides of two different receptor guanylate cyclases. Nature 341: 68–72, 1989. doi: 10.1038/341068a0. [DOI] [PubMed] [Google Scholar]
- 21.Chen G, Goeddel DV. TNF-R1 signaling: a beautiful pathway. Science 296: 1634–1635, 2002. doi: 10.1126/science.1071924. [DOI] [PubMed] [Google Scholar]
- 22.Chen G, Paka L, Kako Y, Singhal P, Duan W, Pillarisetti S. A protective role for kidney apolipoprotein E. Regulation of mesangial cell proliferation and matrix expansion. J Biol Chem 276: 49142–49147, 2001. doi: 10.1074/jbc.M104879200. [DOI] [PubMed] [Google Scholar]
- 23.Chen HH, Burnett JC Jr. The natriuretic peptides in heart failure: diagnostic and therapeutic potentials. Proc Assoc Am Physicians 111: 406–416, 1999. doi: 10.1111/paa.1999.111.5.406. [DOI] [PubMed] [Google Scholar]
- 24.Chen L, Kim SM, Eisner C, Oppermann M, Huang Y, Mizel D, Li L, Chen M, Sequeira Lopez ML, Weinstein LS, Gomez RA, Schnermann J, Briggs JP. Stimulation of renin secretion by angiotensin II blockade is Gsalpha-dependent. J Am Soc Nephrol 21: 986–992, 2010. doi: 10.1681/ASN.2009030307. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Chen MA. Heart failure with preserved ejection fraction in older adults. Am J Med 122: 713–723, 2009. doi: 10.1016/j.amjmed.2009.01.038. [DOI] [PubMed] [Google Scholar]
- 26.Chinkers M, Garbers DL, Chang MS, Lowe DG, Chin HM, Goeddel DV, Schulz S. A membrane form of guanylate cyclase is an atrial natriuretic peptide receptor. Nature 338: 78–83, 1989. doi: 10.1038/338078a0. [DOI] [PubMed] [Google Scholar]
- 27.Chiou CY, Williams GH, Kifor I. Study of the rat adrenal renin-angiotensin system at a cellular level. J Clin Invest 96: 1375–1381, 1995. doi: 10.1172/JCI118172. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Christadoss P, Goluszko E. Treatment of experimental autoimmune myasthenia gravis with recombinant human tumor necrosis factor receptor Fc protein. J Neuroimmunol 122: 186–190, 2002. doi: 10.1016/S0165-5728(01)00473-8. [DOI] [PubMed] [Google Scholar]
- 29.Chusho H, Tamura N, Ogawa Y, Yasoda A, Suda M, Miyazawa T, Nakamura K, Nakao K, Kurihara T, Komatsu Y, Itoh H, Tanaka K, Saito Y, Katsuki M, Nakao K. Dwarfism and early death in mice lacking C-type natriuretic peptide. Proc Natl Acad Sci USA 98: 4016–4021, 2001. doi: 10.1073/pnas.071389098. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Cooker LA, Peterson D, Rambow J, Riser ML, Riser RE, Najmabadi F, Brigstock D, Riser BL. TNF-alpha, but not IFN-gamma, regulates CCN2 (CTGF), collagen type I, and proliferation in mesangial cells: possible roles in the progression of renal fibrosis. Am J Physiol Renal Physiol 293: F157–F165, 2007. doi: 10.1152/ajprenal.00508.2006. [DOI] [PubMed] [Google Scholar]
- 31.Coué M, Badin PM, Vila IK, Laurens C, Louche K, Marquès MA, Bourlier V, Mouisel E, Tavernier G, Rustan AC, Galgani JE, Joanisse DR, Smith SR, Langin D, Moro C. Defective Natriuretic Peptide Receptor Signaling in Skeletal Muscle Links Obesity to Type 2 Diabetes. Diabetes 64: 4033–4045, 2015. doi: 10.2337/db15-0305. [DOI] [PubMed] [Google Scholar]
- 32.Courtois G, Gilmore TD. Mutations in the NF-kappaB signaling pathway: implications for human disease. Oncogene 25: 6831–6843, 2006. doi: 10.1038/sj.onc.1209939. [DOI] [PubMed] [Google Scholar]
- 33.Das S, Au E, Krazit ST, Pandey KN. Targeted disruption of guanylyl cyclase-A/natriuretic peptide receptor-A gene provokes renal fibrosis and remodeling in null mutant mice: role of proinflammatory cytokines. Endocrinology 151: 5841–5850, 2010. doi: 10.1210/en.2010-0655. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Das S, Periyasamy R, Pandey KN. Activation of IKK/NF-κB provokes renal inflammatory responses in guanylyl cyclase/natriuretic peptide receptor-A gene-knockout mice. Physiol Genomics 44: 430–442, 2012. doi: 10.1152/physiolgenomics.00147.2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.de Bold AJ, Borenstein HB, Veress AT, Sonnenberg H. A rapid and potent natriuretic response to intravenous injection of atrial myocardial extract in rats. Life Sci 28: 89–94, 1981. doi: 10.1016/0024-3205(81)90370-2. [DOI] [PubMed] [Google Scholar]
- 36.De Ciuceis C, Amiri F, Brassard P, Endemann DH, Touyz RM, Schiffrin EL. Reduced vascular remodeling, endothelial dysfunction, and oxidative stress in resistance arteries of angiotensin II-infused macrophage colony-stimulating factor-deficient mice: evidence for a role in inflammation in angiotensin-induced vascular injury. Arterioscler Thromb Vasc Biol 25: 2106–2113, 2005. doi: 10.1161/01.ATV.0000181743.28028.57. [DOI] [PubMed] [Google Scholar]
- 37.Dinas PC, Nintou E, Psychou D, Granzotto M, Rossato M, Vettor R, Jamurtas AZ, Koutedakis Y, Metsios GS, Flouris AD. Association of fat mass profile with natriuretic peptide receptor alpha in subcutaneous adipose tissue of medication-free healthy men: A cross-sectional study. F1000 Res 7: 327, 2018. doi: 10.12688/f1000research.14198.2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Doi Y, Atarashi K, Franco-Saenz R, Mulrow PJ. Effect of changes in sodium or potassium balance, and nephrectomy, on adrenal renin and aldosterone concentrations. Hypertension 6: I124–I129, 1984. doi: 10.1161/01.HYP.6.2_Pt_2.I124. [DOI] [PubMed] [Google Scholar]
- 39.Drewett J, Marchand G, Ziegler R, Trachte G. Atrial natriuretic factor inhibits norepinephrine release in an adrenergic clonal cell line (PC12). Eur J Pharmacol 150: 175–179, 1988. doi: 10.1016/0014-2999(88)90765-0. [DOI] [PubMed] [Google Scholar]
- 40.Drewett JG, Trachte GJ, Marchand GR. Atrial natriuretic factor inhibits adrenergic and purinergic neurotransmission in the rabbit isolated vas deferens. J Pharmacol Exp Ther 248: 135–142, 1989. [PubMed] [Google Scholar]
- 41.Duda T, Goraczniak RM, Sharma RK. Site-directed mutational analysis of a membrane guanylate cyclase cDNA reveals the atrial natriuretic factor signaling site. Proc Natl Acad Sci USA 88: 7882–7886, 1991. doi: 10.1073/pnas.88.17.7882. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Duff JL, Berk BC, Corson MA. Angiotensin II stimulates the pp44 and pp42 mitogen-activated protein kinases in cultured rat aortic smooth muscle cells. Biochem Biophys Res Commun 188: 257–264, 1992. doi: 10.1016/0006-291X(92)92378-B. [DOI] [PubMed] [Google Scholar]
- 43.Eddy AA. Molecular basis of renal fibrosis. Pediatr Nephrol 15: 290–301, 2000. doi: 10.1007/s004670000461. [DOI] [PubMed] [Google Scholar]
- 44.Eguchi S, Numaguchi K, Iwasaki H, Matsumoto T, Yamakawa T, Utsunomiya H, Motley ED, Kawakatsu H, Owada KM, Hirata Y, Marumo F, Inagami T. Calcium-dependent epidermal growth factor receptor transactivation mediates the angiotensin II-induced mitogen-activated protein kinase activation in vascular smooth muscle cells. J Biol Chem 273: 8890–8896, 1998. doi: 10.1074/jbc.273.15.8890. [DOI] [PubMed] [Google Scholar]
- 45.Ehret GB, Munroe PB, Rice KM, Bochud M, Johnson AD, Chasman DI, Smith AV, Tobin MD, Verwoert GC, Hwang SJ, Pihur V, Vollenweider P, O’Reilly PF, Amin N, Bragg-Gresham JL, Teumer A, Glazer NL, Launer L, Zhao JH, Aulchenko Y, Heath S, Sõber S, Parsa A, Luan J, Arora P, Dehghan A, Zhang F, Lucas G, Hicks AA, Jackson AU, Peden JF, Tanaka T, Wild SH, Rudan I, Igl W, Milaneschi Y, Parker AN, Fava C, Chambers JC, Fox ER, Kumari M, Go MJ, van der Harst P, Kao WH, Sjögren M, Vinay DG, Alexander M, Tabara Y, Shaw-Hawkins S, Whincup PH, Liu Y, Shi G, Kuusisto J, Tayo B, Seielstad M, Sim X, Nguyen KD, Lehtimäki T, Matullo G, Wu Y, Gaunt TR, Onland-Moret NC, Cooper MN, Platou CG, Org E, Hardy R, Dahgam S, Palmen J, Vitart V, Braund PS, Kuznetsova T, Uiterwaal CS, Adeyemo A, Palmas W, Campbell H, Ludwig B, Tomaszewski M, Tzoulaki I, Palmer ND, Aspelund T, Garcia M, Chang YP, O’Connell JR, Steinle NI, Grobbee DE, Arking DE, Kardia SL, Morrison AC, Hernandez D, Najjar S, McArdle WL, Hadley D, Brown MJ, Connell JM, Hingorani AD, Day IN, Lawlor DA, Beilby JP, Lawrence RW, Clarke R, Hopewell JC, Ongen H, Dreisbach AW, Li Y, Young JH, Bis JC, Kähönen M, Viikari J, Adair LS, Lee NR, Chen MH, Olden M, Pattaro C, Bolton JA, Köttgen A, Bergmann S, Mooser V, Chaturvedi N, Frayling TM, Islam M, Jafar TH, Erdmann J, Kulkarni SR, Bornstein SR, Grässler J, Groop L, Voight BF, Kettunen J, Howard P, Taylor A, Guarrera S, Ricceri F, Emilsson V, Plump A, Barroso I, Khaw KT, Weder AB, Hunt SC, Sun YV, Bergman RN, Collins FS, Bonnycastle LL, Scott LJ, Stringham HM, Peltonen L, Perola M, Vartiainen E, Brand SM, Staessen JA, Wang TJ, Burton PR, Soler Artigas M, Dong Y, Snieder H, Wang X, Zhu H, Lohman KK, Rudock ME, Heckbert SR, Smith NL, Wiggins KL, Doumatey A, Shriner D, Veldre G, Viigimaa M, Kinra S, Prabhakaran D, Tripathy V, Langefeld CD, Rosengren A, Thelle DS, Corsi AM, Singleton A, Forrester T, Hilton G, McKenzie CA, Salako T, Iwai N, Kita Y, Ogihara T, Ohkubo T, Okamura T, Ueshima H, Umemura S, Eyheramendy S, Meitinger T, Wichmann HE, Cho YS, Kim HL, Lee JY, Scott J, Sehmi JS, Zhang W, Hedblad B, Nilsson P, Smith GD, Wong A, Narisu N, Stančáková A, Raffel LJ, Yao J, Kathiresan S, O’Donnell CJ, Schwartz SM, Ikram MA, Longstreth WT Jr, Mosley TH, Seshadri S, Shrine NR, Wain LV, Morken MA, Swift AJ, Laitinen J, Prokopenko I, Zitting P, Cooper JA, Humphries SE, Danesh J, Rasheed A, Goel A, Hamsten A, Watkins H, Bakker SJ, van Gilst WH, Janipalli CS, Mani KR, Yajnik CS, Hofman A, Mattace-Raso FU, Oostra BA, Demirkan A, Isaacs A, Rivadeneira F, Lakatta EG, Orru M, Scuteri A, Ala-Korpela M, Kangas AJ, Lyytikäinen LP, Soininen P, Tukiainen T, Würtz P, Ong RT, Dörr M, Kroemer HK, Völker U, Völzke H, Galan P, Hercberg S, Lathrop M, Zelenika D, Deloukas P, Mangino M, Spector TD, Zhai G, Meschia JF, Nalls MA, Sharma P, Terzic J, Kumar MV, Denniff M, Zukowska-Szczechowska E, Wagenknecht LE, Fowkes FG, Charchar FJ, Schwarz PE, Hayward C, Guo X, Rotimi C, Bots ML, Brand E, Samani NJ, Polasek O, Talmud PJ, Nyberg F, Kuh D, Laan M, Hveem K, Palmer LJ, van der Schouw YT, Casas JP, Mohlke KL, Vineis P, Raitakari O, Ganesh SK, Wong TY, Tai ES, Cooper RS, Laakso M, Rao DC, Harris TB, Morris RW, Dominiczak AF, Kivimaki M, Marmot MG, Miki T, Saleheen D, Chandak GR, Coresh J, Navis G, Salomaa V, Han BG, Zhu X, Kooner JS, Melander O, Ridker PM, Bandinelli S, Gyllensten UB, Wright AF, Wilson JF, Ferrucci L, Farrall M, Tuomilehto J, Pramstaller PP, Elosua R, Soranzo N, Sijbrands EJ, Altshuler D, Loos RJ, Shuldiner AR, Gieger C, Meneton P, Uitterlinden AG, Wareham NJ, Gudnason V, Rotter JI, Rettig R, Uda M, Strachan DP, Witteman JC, Hartikainen AL, Beckmann JS, Boerwinkle E, Vasan RS, Boehnke M, Larson MG, Järvelin MR, Psaty BM, Abecasis GR, Chakravarti A, Elliott P, van Duijn CM, Newton-Cheh C, Levy D, Caulfield MJ, Johnson T, International Consortium for Blood Pressure Genome-Wide Association Studies; CARDIoGRAM consortium; CKDGen Consortium; KidneyGen Consortium; EchoGen consortium; CHARGE-HF consortium . Genetic variants in novel pathways influence blood pressure and cardiovascular disease risk. Nature 478: 103–109, 2011. doi: 10.1038/nature10405. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Ellmers LJ, Scott NJ, Piuhola J, Maeda N, Smithies O, Frampton CM, Richards AM, Cameron VA. Npr1-regulated gene pathways contributing to cardiac hypertrophy and fibrosis. J Mol Endocrinol 38: 245–257, 2007. doi: 10.1677/jme.1.02138. [DOI] [PubMed] [Google Scholar]
- 47.Elmarakby AA, Quigley JE, Pollock DM, Imig JD. Tumor necrosis factor alpha blockade increases renal Cyp2c23 expression and slows the progression of renal damage in salt-sensitive hypertension. Hypertension 47: 557–562, 2006. doi: 10.1161/01.HYP.0000198545.01860.90. [DOI] [PubMed] [Google Scholar]
- 48.Enerbäck S. Human brown adipose tissue. Cell Metab 11: 248–252, 2010. doi: 10.1016/j.cmet.2010.03.008. [DOI] [PubMed] [Google Scholar]
- 49.Engeli S, Birkenfeld AL, Badin PM, Bourlier V, Louche K, Viguerie N, Thalamas C, Montastier E, Larrouy D, Harant I, de Glisezinski I, Lieske S, Reinke J, Beckmann B, Langin D, Jordan J, Moro C. Natriuretic peptides enhance the oxidative capacity of human skeletal muscle. J Clin Invest 122: 4675–4679, 2012. doi: 10.1172/JCI64526. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Espiner EA. Physiology of natriuretic peptides: cardiovascular function, in Contemporary Endocrinology Natriuretic Peptides in Health and Disease (Samson WK, Levin ER, editors). Totowa, NJ: Humana, 1997, p. 123–146. doi: 10.1007/978-1-4612-3960-4_8. [DOI] [Google Scholar]
- 51.Felker GM, Petersen JW, Mark DB. Natriuretic peptides in the diagnosis and management of heart failure. CMAJ 175: 611–617, 2006. doi: 10.1503/cmaj.060236. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Ferguson AV, Washburn DL, Latchford KJ. Hormonal and neurotransmitter roles for angiotensin in the regulation of central autonomic function. Exp Biol Med (Maywood) 226: 85–96, 2001. doi: 10.1177/153537020122600205. [DOI] [PubMed] [Google Scholar]
- 53.Forssmann WG, Richter R, Meyer M. The endocrine heart and natriuretic peptides: histochemistry, cell biology, and functional aspects of the renal urodilatin system. Histochem Cell Biol 110: 335–357, 1998. doi: 10.1007/s004180050295. [DOI] [PubMed] [Google Scholar]
- 54.Frantz S, Fraccarollo D, Wagner H, Behr TM, Jung P, Angermann CE, Ertl G, Bauersachs J. Sustained activation of nuclear factor kappa B and activator protein 1 in chronic heart failure. Cardiovasc Res 57: 749–756, 2003. doi: 10.1016/S0008-6363(02)00723-X. [DOI] [PubMed] [Google Scholar]
- 55.Fuller F, Porter JG, Arfsten AE, Miller J, Schilling JW, Scarborough RM, Lewicki JA, Schenk DB. Atrial natriuretic peptide clearance receptor. Complete sequence and functional expression of cDNA clones. J Biol Chem 263: 9395–9401, 1988. [PubMed] [Google Scholar]
- 56.Garbers DL. Guanylyl cyclase receptors and their endocrine, paracrine, and autocrine ligands. Cell 71: 1–4, 1992. doi: 10.1016/0092-8674(92)90258-E. [DOI] [PubMed] [Google Scholar]
- 57.Garcia R, Thibault G, Cantin M, Genest J. Effect of a purified atrial natriuretic factor on rat and rabbit vascular strips and vascular beds. Am J Physiol Regul Integr Comp Physiol 247: R34–R39, 1984. doi: 10.1152/ajpregu.1984.247.1.R34. [DOI] [PubMed] [Google Scholar]
- 58.Gerondakis S, Grumont R, Gugasyan R, Wong L, Isomura I, Ho W, Banerjee A. Unravelling the complexities of the NF-kappaB signalling pathway using mouse knockout and transgenic models. Oncogene 25: 6781–6799, 2006. doi: 10.1038/sj.onc.1209944. [DOI] [PubMed] [Google Scholar]
- 59.Goldberg GI, Marmer BL, Grant GA, Eisen AZ, Wilhelm S, He CS. Human 72-kilodalton type IV collagenase forms a complex with a tissue inhibitor of metalloproteases designated TIMP-2. Proc Natl Acad Sci USA 86: 8207–8211, 1989. doi: 10.1073/pnas.86.21.8207. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Gratze P, Dechend R, Stocker C, Park JK, Feldt S, Shagdarsuren E, Wellner M, Gueler F, Rong S, Gross V, Obst M, Plehm R, Alenina N, Zenclussen A, Titze J, Small K, Yokota Y, Zenke M, Luft FC, Muller DN. Novel role for inhibitor of differentiation 2 in the genesis of angiotensin II-induced hypertension. Circulation 117: 2645–2656, 2008. doi: 10.1161/CIRCULATIONAHA.107.760116. [DOI] [PubMed] [Google Scholar]
- 61.Gutkowska J, Antunes-Rodrigues J, McCann SM. Atrial natriuretic peptide in brain and pituitary gland. Physiol Rev 77: 465–515, 1997. doi: 10.1152/physrev.1997.77.2.465. [DOI] [PubMed] [Google Scholar]
- 62.Gutkowska J, Jankowski M, Sairam MR, Fujio N, Reis AM, Mukaddam-Daher S, Tremblay J. Hormonal regulation of natriuretic peptide system during induced ovarian follicular development in the rat. Biol Reprod 61: 162–170, 1999. doi: 10.1095/biolreprod61.1.162. [DOI] [PubMed] [Google Scholar]
- 63.Guzik TJ, Hoch NE, Brown KA, McCann LA, Rahman A, Dikalov S, Goronzy J, Weyand C, Harrison DG. Role of the T cell in the genesis of angiotensin II induced hypertension and vascular dysfunction. J Exp Med 204: 2449–2460, 2007. doi: 10.1084/jem.20070657. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Hackenthal E, Aktories K, Jakobs KH. Mode of inhibition of renin release by angiotensin II. J Hypertens Suppl 3: S263–S265, 1985. [PubMed] [Google Scholar]
- 65.Hannken T, Schroeder R, Stahl RA, Wolf G. Atrial natriuretic peptide attenuates ANG II-induced hypertrophy of renal tubular cells. Am J Physiol Renal Physiol 281: F81–F90, 2001. doi: 10.1152/ajprenal.2001.281.1.F81. [DOI] [PubMed] [Google Scholar]
- 66.Haseyama T, Fujita T, Hirasawa F, Tsukada M, Wakui H, Komatsuda A, Ohtani H, Miura AB, Imai H, Koizumi A. Complications of IgA nephropathy in a non-insulin-dependent diabetes model, the Akita mouse. Tohoku J Exp Med 198: 233–244, 2002. doi: 10.1620/tjem.198.233. [DOI] [PubMed] [Google Scholar]
- 67.Hassid A. Atriopeptin II decreases cytosolic free Ca in cultured vascular smooth muscle cells. Am J Physiol Cell Physiol 251: C681–C686, 1986. doi: 10.1152/ajpcell.1986.251.5.C681. [DOI] [PubMed] [Google Scholar]
- 68.Hathaway CK, Gasim AM, Grant R, Chang AS, Kim HS, Madden VJ, Bagnell CR Jr, Jennette JC, Smithies O, Kakoki M. Low TGFβ1 expression prevents and high expression exacerbates diabetic nephropathy in mice. Proc Natl Acad Sci USA 112: 5815–5820, 2015. doi: 10.1073/pnas.1504777112. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.He XL, Dukkipati A, Wang X, Garcia KC. A new paradigm for hormone recognition and allosteric receptor activation revealed from structural studies of NPR-C. Peptides 26: 1035–1043, 2005. doi: 10.1016/j.peptides.2004.08.035. [DOI] [PubMed] [Google Scholar]
- 70.Henrich WL, Walker BR, Handelman WA, Erickson AL, Arnold PE, Schrier RW. Effects of angiotensin II on plasma antidiuretic hormone and renal water excretion. Kidney Int 30: 503–508, 1986. doi: 10.1038/ki.1986.214. [DOI] [PubMed] [Google Scholar]
- 71.Henry M, Grob M, Mouginot D. Endogenous angiotensin II facilitates GABAergic neurotransmission afferent to the Na+-responsive neurons of the rat median preoptic nucleus. Am J Physiol Regul Integr Comp Physiol 297: R783–R792, 2009. doi: 10.1152/ajpregu.00226.2009. [DOI] [PubMed] [Google Scholar]
- 72.Horio T, Nishikimi T, Yoshihara F, Matsuo H, Takishita S, Kangawa K. Inhibitory regulation of hypertrophy by endogenous atrial natriuretic peptide in cultured cardiac myocytes. Hypertension 35: 19–24, 2000. doi: 10.1161/01.HYP.35.1.19. [DOI] [PubMed] [Google Scholar]
- 73.Huang Z, Wen Q, Zhou SF, Yu XQ. Differential chemokine expression in tubular cells in response to urinary proteins from patients with nephrotic syndrome. Cytokine 42: 222–233, 2008. doi: 10.1016/j.cyto.2008.02.005. [DOI] [PubMed] [Google Scholar]
- 74.Hutchinson HG, Trindade PT, Cunanan DB, Wu CF, Pratt RE. Mechanisms of natriuretic-peptide-induced growth inhibition of vascular smooth muscle cells. Cardiovasc Res 35: 158–167, 1997. doi: 10.1016/S0008-6363(97)00086-2. [DOI] [PubMed] [Google Scholar]
- 75.Inagami T, Nakamaru M, Pandey KN, Naruse M, Naruse K, Misono K, Okamura T, Kawamura M. Intracellular action of renin, angiotensin production and release. J Hypertens Suppl 4: S11–S16, 1986. [PubMed] [Google Scholar]
- 76.Isbel NM, Hill PA, Foti R, Mu W, Hurst LA, Stambe C, Lan HY, Atkins RC, Nikolic-Paterson DJ. Tubules are the major site of M-CSF production in experimental kidney disease: correlation with local macrophage proliferation. Kidney Int 60: 614–625, 2001. doi: 10.1046/j.1523-1755.2001.060002614.x. [DOI] [PubMed] [Google Scholar]
- 77.Ishizawa K, Izawa Y, Ito H, Miki C, Miyata K, Fujita Y, Kanematsu Y, Tsuchiya K, Tamaki T, Nishiyama A, Yoshizumi M. Aldosterone stimulates vascular smooth muscle cell proliferation via big mitogen-activated protein kinase 1 activation. Hypertension 46: 1046–1052, 2005. doi: 10.1161/01.HYP.0000172622.51973.f5. [DOI] [PubMed] [Google Scholar]
- 78.Javaid B, Quigg RJ. Treatment of glomerulonephritis: will we ever have options other than steroids and cytotoxics? Kidney Int 67: 1692–1703, 2005. doi: 10.1111/j.1523-1755.2005.00266.x. [DOI] [PubMed] [Google Scholar]
- 79.Jeunemaitre X, Soubrier F, Kotelevtsev YV, Lifton RP, Williams CS, Charru A, Hunt SC, Hopkins PN, Williams RR, Lalouel JM, Corvol P. Molecular basis of human hypertension: role of angiotensinogen. Cell 71: 169–180, 1992. doi: 10.1016/0092-8674(92)90275-H. [DOI] [PubMed] [Google Scholar]
- 80.Ji W, Foo JN, O’Roak BJ, Zhao H, Larson MG, Simon DB, Newton-Cheh C, State MW, Levy D, Lifton RP. Rare independent mutations in renal salt handling genes contribute to blood pressure variation. Nat Genet 40: 592–599, 2008. doi: 10.1038/ng.118. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81.John SW, Krege JH, Oliver PM, Hagaman JR, Hodgin JB, Pang SC, Flynn TG, Smithies O. Genetic decreases in atrial natriuretic peptide and salt-sensitive hypertension. Science 267: 679–681, 1995. doi: 10.1126/science.7839143. [DOI] [PubMed] [Google Scholar]
- 82.Kake T, Kitamura H, Adachi Y, Yoshioka T, Watanabe T, Matsushita H, Fujii T, Kondo E, Tachibe T, Kawase Y, Jishage K, Yasoda A, Mukoyama M, Nakao K. Chronically elevated plasma C-type natriuretic peptide level stimulates skeletal growth in transgenic mice. Am J Physiol Endocrinol Metab 297: E1339–E1348, 2009. doi: 10.1152/ajpendo.00272.2009. [DOI] [PubMed] [Google Scholar]
- 83.Kearney PM, Whelton M, Reynolds K, Muntner P, Whelton PK, He J. Global burden of hypertension: analysis of worldwide data. Lancet 365: 217–223, 2005. doi: 10.1016/S0140-6736(05)70151-3. [DOI] [PubMed] [Google Scholar]
- 84.Khan S, Jena G. Sodium butyrate, a HDAC inhibitor ameliorates eNOS, iNOS and TGF-β1-induced fibrogenesis, apoptosis and DNA damage in the kidney of juvenile diabetic rats. Food Chem Toxicol 73: 127–139, 2014. doi: 10.1016/j.fct.2014.08.010. [DOI] [PubMed] [Google Scholar]
- 85.Khurana ML, Pandey KN. Catalytic activation of guanylate cyclase/atrial natriuretic factor receptor by combined effects of ANF and GTP gamma S in plasma membranes of Leydig tumor cells: involvement of G-proteins. Arch Biochem Biophys 316: 392–398, 1995. doi: 10.1006/abbi.1995.1052. [DOI] [PubMed] [Google Scholar]
- 86.Khurana ML, Pandey KN. Receptor-mediated stimulatory effect of atrial natriuretic factor, brain natriuretic peptide, and C-type natriuretic peptide on testosterone production in purified mouse Leydig cells: activation of cholesterol side-chain cleavage enzyme. Endocrinology 133: 2141–2149, 1993. doi: 10.1210/endo.133.5.8404664. [DOI] [PubMed] [Google Scholar]
- 87.Kim HS, Krege JH, Kluckman KD, Hagaman JR, Hodgin JB, Best CF, Jennette JC, Coffman TM, Maeda N, Smithies O. Genetic control of blood pressure and the angiotensinogen locus. Proc Natl Acad Sci USA 92: 2735–2739, 1995. doi: 10.1073/pnas.92.7.2735. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 88.Kim J, Ahn S, Rajagopal K, Lefkowitz RJ. Independent beta-arrestin2 and Gq/protein kinase Czeta pathways for ERK stimulated by angiotensin type 1A receptors in vascular smooth muscle cells converge on transactivation of the epidermal growth factor receptor. J Biol Chem 284: 11953–11962, 2009. doi: 10.1074/jbc.M808176200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 89.Kim JH, Ha IS, Hwang CI, Lee YJ, Kim J, Yang SH, Kim YS, Cao YA, Choi S, Park WY. Gene expression profiling of anti-GBM glomerulonephritis model: the role of NF-kappaB in immune complex kidney disease. Kidney Int 66: 1826–1837, 2004. doi: 10.1111/j.1523-1755.2004.00956.x. [DOI] [PubMed] [Google Scholar]
- 90.Kim S, Iwao H. Molecular and cellular mechanisms of angiotensin II-mediated cardiovascular and renal diseases. Pharmacol Rev 52: 11–34, 2000. [PubMed] [Google Scholar]
- 91.Kishimoto I, Dubois SK, Garbers DL. The heart communicates with the kidney exclusively through the guanylyl cyclase-A receptor: acute handling of sodium and water in response to volume expansion. Proc Natl Acad Sci USA 93: 6215–6219, 1996. doi: 10.1073/pnas.93.12.6215. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 92.Kishimoto I, Tokudome T, Nakao K, Kangawa K. Natriuretic peptide system: an overview of studies using genetically engineered animal models. FEBS J 278: 1830–1841, 2011. doi: 10.1111/j.1742-4658.2011.08116.x. [DOI] [PubMed] [Google Scholar]
- 93.Kisseleva T, Brenner DA. Mechanisms of fibrogenesis. Exp Biol Med (Maywood) 233: 109–122, 2008. doi: 10.3181/0707-MR-190. [DOI] [PubMed] [Google Scholar]
- 94.Knowles JW, Erickson LM, Guy VK, Sigel CS, Wilder JC, Maeda N. Common variations in noncoding regions of the human natriuretic peptide receptor A gene have quantitative effects. Hum Genet 112: 62–70, 2003. doi: 10.1007/s00439-002-0834-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 95.Knowles JW, Esposito G, Mao L, Hagaman JR, Fox JE, Smithies O, Rockman HA, Maeda N. Pressure-independent enhancement of cardiac hypertrophy in natriuretic peptide receptor A-deficient mice. J Clin Invest 107: 975–984, 2001. doi: 10.1172/JCI11273. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 96.Koh GY, Klug MG, Field LJ. Atrial natriuretic factor and transgenic mice. Hypertension 22: 634–639, 1993. doi: 10.1161/01.HYP.22.4.634. [DOI] [PubMed] [Google Scholar]
- 97.Koller KJ, de Sauvage FJ, Lowe DG, Goeddel DV. Conservation of the kinaselike regulatory domain is essential for activation of the natriuretic peptide receptor guanylyl cyclases. Mol Cell Biol 12: 2581–2590, 1992. doi: 10.1128/MCB.12.6.2581. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 98.Kumar P, Gogulamudi VR, Periasamy R, Raghavaraju G, Subramanian U, Pandey KN. Inhibition of HDAC enhances STAT acetylation, blocks NF-κB, and suppresses the renal inflammation and fibrosis in Npr1 haplotype male mice. Am J Physiol Renal Physiol 313: F781–F795, 2017. doi: 10.1152/ajprenal.00166.2017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 99.Kumar P, Periyasamy R, Das S, Neerukonda S, Mani I, Pandey KN. All-trans retinoic acid and sodium butyrate enhance natriuretic peptide receptor a gene transcription: role of histone modification. Mol Pharmacol 85: 946–957, 2014. doi: 10.1124/mol.114.092221. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 100.Kumar R, Cartledge WA, Lincoln TM, Pandey KN. Expression of guanylyl cyclase-A/atrial natriuretic peptide receptor blocks the activation of protein kinase C in vascular smooth muscle cells. Role of cGMP and cGMP-dependent protein kinase. Hypertension 29: 414–421, 1997. doi: 10.1161/01.HYP.29.1.414. [DOI] [PubMed] [Google Scholar]
- 101.Kumar R, von Geldern TW, Calle RA, Pandey KN. Stimulation of atrial natriuretic peptide receptor/guanylyl cyclase- A signaling pathway antagonizes the activation of protein kinase C-alpha in murine Leydig cells. Biochim Biophys Acta 1356: 221–228, 1997. doi: 10.1016/S0167-4889(96)00168-1. [DOI] [PubMed] [Google Scholar]
- 102.Kushibiki M, Yamada M, Oikawa K, Tomita H, Osanai T, Okumura K. Aldosterone causes vasoconstriction in coronary arterioles of rats via angiotensin II type-1 receptor: influence of hypertension. Eur J Pharmacol 572: 182–188, 2007. doi: 10.1016/j.ejphar.2007.06.017. [DOI] [PubMed] [Google Scholar]
- 103.Langenickel TH, Buttgereit J, Pagel-Langenickel I, Lindner M, Monti J, Beuerlein K, Al-Saadi N, Plehm R, Popova E, Tank J, Dietz R, Willenbrock R, Bader M. Cardiac hypertrophy in transgenic rats expressing a dominant-negative mutant of the natriuretic peptide receptor B. Proc Natl Acad Sci USA 103: 4735–4740, 2006. doi: 10.1073/pnas.0510019103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 104.Laragh J, Sealey J. The renin-angiotensin-aldosterone system in hypertensive disorders: a key to two forms of arteriolar constriction and a possible clue to risk of vascular injury (heart attack and stroke and prognosis). New York: Raven Press, 1990, p. 1329–1348. [Google Scholar]
- 105.Levin ER, Gardner DG, Samson WK. Natriuretic peptides. N Engl J Med 339: 321–328, 1998. doi: 10.1056/NEJM199807303390507. [DOI] [PubMed] [Google Scholar]
- 106.Levy D, DeStefano AL, Larson MG, O’Donnell CJ, Lifton RP, Gavras H, Cupples LA, Myers RH. Evidence for a gene influencing blood pressure on chromosome 17. Genome scan linkage results for longitudinal blood pressure phenotypes in subjects from the Framingham heart study. Hypertension 36: 477–483, 2000. doi: 10.1161/01.HYP.36.4.477. [DOI] [PubMed] [Google Scholar]
- 107.Levy D, Ehret GB, Rice K, Verwoert GC, Launer LJ, Dehghan A, Glazer NL, Morrison AC, Johnson AD, Aspelund T, Aulchenko Y, Lumley T, Köttgen A, Vasan RS, Rivadeneira F, Eiriksdottir G, Guo X, Arking DE, Mitchell GF, Mattace-Raso FU, Smith AV, Taylor K, Scharpf RB, Hwang SJ, Sijbrands EJ, Bis J, Harris TB, Ganesh SK, O’Donnell CJ, Hofman A, Rotter JI, Coresh J, Benjamin EJ, Uitterlinden AG, Heiss G, Fox CS, Witteman JC, Boerwinkle E, Wang TJ, Gudnason V, Larson MG, Chakravarti A, Psaty BM, van Duijn CM. Genome-wide association study of blood pressure and hypertension. Nat Genet 41: 677–687, 2009. doi: 10.1038/ng.384. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 108.Li XC, Zhuo JL. Nuclear factor-kappaB as a hormonal intracellular signaling molecule: focus on angiotensin II-induced cardiovascular and renal injury. Curr Opin Nephrol Hypertens 17: 37–43, 2008. doi: 10.1097/MNH.0b013e3282f2903c. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 109.Li YY, McTiernan CF, Feldman AM. Interplay of matrix metalloproteinases, tissue inhibitors of metalloproteinases and their regulators in cardiac matrix remodeling. Cardiovasc Res 46: 214–224, 2000. doi: 10.1016/S0008-6363(00)00003-1. [DOI] [PubMed] [Google Scholar]
- 110.Lifton RP. Molecular genetics of human blood pressure variation. Science 272: 676–680, 1996. doi: 10.1126/science.272.5262.676. [DOI] [PubMed] [Google Scholar]
- 111.Lifton RP, Gharavi AG, Geller DS. Molecular mechanisms of human hypertension. Cell 104: 545–556, 2001. doi: 10.1016/S0092-8674(01)00241-0. [DOI] [PubMed] [Google Scholar]
- 112.Lin KF, Chao J, Chao L. Atrial natriuretic peptide gene delivery attenuates hypertension, cardiac hypertrophy, and renal injury in salt-sensitive rats. Hum Gene Ther 9: 1429–1438, 1998. doi: 10.1089/hum.1998.9.10-1429. [DOI] [PubMed] [Google Scholar]
- 113.Lin KF, Chao J, Chao L. Human atrial natriuretic peptide gene delivery reduces blood pressure in hypertensive rats. Hypertension 26: 847–853, 1995. doi: 10.1161/01.HYP.26.6.847. [DOI] [PubMed] [Google Scholar]
- 114.Lincoln TM, Komalavilas P, Cornwell TL. Pleiotropic regulation of vascular smooth muscle tone by cyclic GMP-dependent protein kinase. Hypertension 23: 1141–1147, 1994. doi: 10.1161/01.HYP.23.6.1141. [DOI] [PubMed] [Google Scholar]
- 115.Liu SF, Malik AB. NF-kappa B activation as a pathological mechanism of septic shock and inflammation. Am J Physiol Lung Cell Mol Physiol 290: L622–L645, 2006. doi: 10.1152/ajplung.00477.2005. [DOI] [PubMed] [Google Scholar]
- 116.Lopez MJ, Wong SK, Kishimoto I, Dubois S, Mach V, Friesen J, Garbers DL, Beuve A. Salt-resistant hypertension in mice lacking the guanylyl cyclase-A receptor for atrial natriuretic peptide. Nature 378: 65–68, 1995. doi: 10.1038/378065a0. [DOI] [PubMed] [Google Scholar]
- 117.Luther JM, Gainer JV, Murphey LJ, Yu C, Vaughan DE, Morrow JD, Brown NJ. Angiotensin II induces interleukin-6 in humans through a mineralocorticoid receptor-dependent mechanism. Hypertension 48: 1050–1057, 2006. doi: 10.1161/01.HYP.0000248135.97380.76. [DOI] [PubMed] [Google Scholar]
- 118.Maack T. Receptors of atrial natriuretic factor. Annu Rev Physiol 54: 11–27, 1992. doi: 10.1146/annurev.ph.54.030192.000303. [DOI] [PubMed] [Google Scholar]
- 119.Mankhey RW, Wells CC, Bhatti F, Maric C. 17beta-Estradiol supplementation reduces tubulointerstitial fibrosis by increasing MMP activity in the diabetic kidney. Am J Physiol Regul Integr Comp Physiol 292: R769–R777, 2007. doi: 10.1152/ajpregu.00375.2006. [DOI] [PubMed] [Google Scholar]
- 120.Matsukawa N, Grzesik WJ, Takahashi N, Pandey KN, Pang S, Yamauchi M, Smithies O. The natriuretic peptide clearance receptor locally modulates the physiological effects of the natriuretic peptide system. Proc Natl Acad Sci USA 96: 7403–7408, 1999. doi: 10.1073/pnas.96.13.7403. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 121.Mauer SM, Steffes MW, Ellis EN, Sutherland DE, Brown DM, Goetz FC. Structural-functional relationships in diabetic nephropathy. J Clin Invest 74: 1143–1155, 1984. doi: 10.1172/JCI111523. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 122.Mazzocchi G, Malendowicz LK, Markowska A, Albertin G, Nussdorfer GG. Role of adrenal renin-angiotensin system in the control of aldosterone secretion in sodium-restricted rats. Am J Physiol Endocrinol Metab 278: E1027–E1030, 2000. doi: 10.1152/ajpendo.2000.278.6.E1027. [DOI] [PubMed] [Google Scholar]
- 123.McGrath MF, de Bold AJ. Determinants of natriuretic peptide gene expression. Peptides 26: 933–943, 2005. doi: 10.1016/j.peptides.2004.12.022. [DOI] [PubMed] [Google Scholar]
- 124.Meldrum KK, Misseri R, Metcalfe P, Dinarello CA, Hile KL, Meldrum DR. TNF-alpha neutralization ameliorates obstruction-induced renal fibrosis and dysfunction. Am J Physiol Regul Integr Comp Physiol 292: R1456–R1464, 2007. doi: 10.1152/ajpregu.00620.2005. [DOI] [PubMed] [Google Scholar]
- 125.Melo LG, Steinhelper ME, Pang SC, Tse Y, Ackermann U. ANP in regulation of arterial pressure and fluid-electrolyte balance: lessons from genetic mouse models. Physiol Genomics 3: 45–58, 2000. doi: 10.1152/physiolgenomics.2000.3.1.45. [DOI] [PubMed] [Google Scholar]
- 126.Melo LG, Veress AT, Ackermann U, Steinhelper ME, Pang SC, Tse Y, Sonnenberg H. Chronic regulation of arterial blood pressure in ANP transgenic and knockout mice: role of cardiovascular sympathetic tone. Cardiovasc Res 43: 437–444, 1999. doi: 10.1016/S0008-6363(99)00104-2. [DOI] [PubMed] [Google Scholar]
- 127.Melo LG, Veress AT, Chong CK, Pang SC, Flynn TG, Sonnenberg H. Salt-sensitive hypertension in ANP knockout mice: potential role of abnormal plasma renin activity. Am J Physiol 274: R255–R261, 1998. [DOI] [PubMed] [Google Scholar]
- 128.Meyer M, Forssmann WG. Renal actions of atrial natriuretic peptide, in Contemporary Endocrinology: Natriuretic Peptides in Health and Disease (Samson WK, Levin ER, editors). Totawa, NJ: Humana Press, 1997, p. 147–170. doi: 10.1007/978-1-4612-3960-4_9. [DOI] [Google Scholar]
- 129.Michel F, Ambroisine ML, Duriez M, Delcayre C, Levy BI, Silvestre JS. Aldosterone enhances ischemia-induced neovascularization through angiotensin II-dependent pathway. Circulation 109: 1933–1937, 2004. doi: 10.1161/01.CIR.0000127112.36796.9B. [DOI] [PubMed] [Google Scholar]
- 130.Min LJ, Mogi M, Li JM, Iwanami J, Iwai M, Horiuchi M. Aldosterone and angiotensin II synergistically induce mitogenic response in vascular smooth muscle cells. Circ Res 97: 434–442, 2005. doi: 10.1161/01.RES.0000180753.63183.95. [DOI] [PubMed] [Google Scholar]
- 131.Mitsuishi M, Miyashita K, Itoh H. cGMP rescues mitochondrial dysfunction induced by glucose and insulin in myocytes. Biochem Biophys Res Commun 367: 840–845, 2008. doi: 10.1016/j.bbrc.2008.01.017. [DOI] [PubMed] [Google Scholar]
- 132.Miyajima A, Chen J, Lawrence C, Ledbetter S, Soslow RA, Stern J, Jha S, Pigato J, Lemer ML, Poppas DP, Vaughan ED Jr, Felsen D. Antibody to transforming growth factor-beta ameliorates tubular apoptosis in unilateral ureteral obstruction. Kidney Int 58: 2301–2313, 2000. doi: 10.1046/j.1523-1755.2000.00414.x. [DOI] [PubMed] [Google Scholar]
- 133.Miyashita K, Itoh H, Tsujimoto H, Tamura N, Fukunaga Y, Sone M, Yamahara K, Taura D, Inuzuka M, Sonoyama T, Nakao K. Natriuretic peptides/cGMP/cGMP-dependent protein kinase cascades promote muscle mitochondrial biogenesis and prevent obesity. Diabetes 58: 2880–2892, 2009. doi: 10.2337/db09-0393. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 134.Molloy CJ, Taylor DS, Weber H. Angiotensin II stimulation of rapid protein tyrosine phosphorylation and protein kinase activation in rat aortic smooth muscle cells. J Biol Chem 268: 7338–7345, 1993. [PubMed] [Google Scholar]
- 135.Montezano AC, Callera GE, Yogi A, He Y, Tostes RC, He G, Schiffrin EL, Touyz RM. Aldosterone and angiotensin II synergistically stimulate migration in vascular smooth muscle cells through c-Src-regulated redox-sensitive RhoA pathways. Arterioscler Thromb Vasc Biol 28: 1511–1518, 2008. doi: 10.1161/ATVBAHA.108.168021. [DOI] [PubMed] [Google Scholar]
- 136.Morgan T. Interactions between sodium and angiotensin. Clin Exp Pharmacol Physiol 28: 1070–1073, 2001. doi: 10.1046/j.1440-1681.2001.03577.x. [DOI] [PubMed] [Google Scholar]
- 137.Moro C. Natriuretic peptides and fat metabolism. Curr Opin Clin Nutr Metab Care 16: 645–649, 2013. doi: 10.1097/MCO.0b013e32836510ed. [DOI] [PubMed] [Google Scholar]
- 138.Müller DN, Luft FC. Direct renin inhibition with aliskiren in hypertension and target organ damage. Clin J Am Soc Nephrol 1: 221–228, 2006. doi: 10.2215/CJN.01201005. [DOI] [PubMed] [Google Scholar]
- 139.Nakajima M, Hutchinson HG, Fujinaga M, Hayashida W, Morishita R, Zhang L, Horiuchi M, Pratt RE, Dzau VJ. The angiotensin II type 2 (AT2) receptor antagonizes the growth effects of the AT1 receptor: gain-of-function study using gene transfer. Proc Natl Acad Sci USA 92: 10663–10667, 1995. doi: 10.1073/pnas.92.23.10663. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 140.Nakamaru M, Inagami T. Atrial natriuretic factor inhibits norepinephrine release evoked by sympathetic nerve stimulation in isolated perfused rat mesenteric arteries. Eur J Pharmacol 123: 459–461, 1986. doi: 10.1016/0014-2999(86)90724-7. [DOI] [PubMed] [Google Scholar]
- 141.Nakamaru M, Takayanagi R, Inagami T. Effect of atrial natriuretic factor on central angiotensin II-induced responses in rats. Peptides 7: 373–375, 1986. doi: 10.1016/0196-9781(86)90239-1. [DOI] [PubMed] [Google Scholar]
- 142.Navar LG. The kidney in blood pressure regulation and development of hypertension. Med Clin North Am 81: 1165–1198, 1997. doi: 10.1016/S0025-7125(05)70573-3. [DOI] [PubMed] [Google Scholar]
- 143.Navar LG. The intrarenal renin-angiotensin system in hypertension. Kidney Int 65: 1522–1532, 2004. doi: 10.1111/j.1523-1755.2004.00539.x. [DOI] [PubMed] [Google Scholar]
- 144.Navar LG, Kobori H, Prieto-Carrasquero M. Intrarenal angiotensin II and hypertension. Curr Hypertens Rep 5: 135–143, 2003. doi: 10.1007/s11906-003-0070-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 145.Negri AL. Prevention of progressive fibrosis in chronic renal diseases: antifibrotic agents. J Nephrol 17: 496–503, 2004. [PubMed] [Google Scholar]
- 146.Newton-Cheh C, Larson MG, Vasan RS, Levy D, Bloch KD, Surti A, Guiducci C, Kathiresan S, Benjamin EJ, Struck J, Morgenthaler NG, Bergmann A, Blankenberg S, Kee F, Nilsson P, Yin X, Peltonen L, Vartiainen E, Salomaa V, Hirschhorn JN, Melander O, Wang TJ. Association of common variants in NPPA and NPPB with circulating natriuretic peptides and blood pressure. Nat Genet 41: 348–353, 2009. doi: 10.1038/ng.328. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 147.Obana K, Naruse M, Inagami T, Brown AB, Naruse K, Kurimoto F, Sakurai H, Demura H, Shizume K. Atrial natriuretic factor inhibits vasopressin secretion from rat posterior pituitary. Biochem Biophys Res Commun 132: 1088–1094, 1985. doi: 10.1016/0006-291X(85)91918-7. [DOI] [PubMed] [Google Scholar]
- 148.Obana K, Naruse M, Naruse K, Sakurai H, Demura H, Inagami T, Shizume K. Synthetic rat atrial natriuretic factor inhibits in vitro and in vivo renin secretion in rats. Endocrinology 117: 1282–1284, 1985. doi: 10.1210/endo-117-3-1282. [DOI] [PubMed] [Google Scholar]
- 149.Ogawa Y, Itoh H, Tamura N, Suga S, Yoshimasa T, Uehira M, Matsuda S, Shiono S, Nishimoto H, Nakao K. Molecular cloning of the complementary DNA and gene that encode mouse brain natriuretic peptide and generation of transgenic mice that overexpress the brain natriuretic peptide gene. J Clin Invest 93: 1911–1921, 1994. doi: 10.1172/JCI117182. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 150.Oliver PM, Fox JE, Kim R, Rockman HA, Kim HS, Reddick RL, Pandey KN, Milgram SL, Smithies O, Maeda N. Hypertension, cardiac hypertrophy, and sudden death in mice lacking natriuretic peptide receptor A. Proc Natl Acad Sci USA 94: 14730–14735, 1997. doi: 10.1073/pnas.94.26.14730. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 151.Oliver PM, John SW, Purdy KE, Kim R, Maeda N, Goy MF, Smithies O. Natriuretic peptide receptor 1 expression influences blood pressures of mice in a dose-dependent manner. Proc Natl Acad Sci USA 95: 2547–2551, 1998. doi: 10.1073/pnas.95.5.2547. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 152.Ortiz LA, Dutreil M, Fattman C, Pandey AC, Torres G, Go K, Phinney DG. Interleukin 1 receptor antagonist mediates the antiinflammatory and antifibrotic effect of mesenchymal stem cells during lung injury. Proc Natl Acad Sci USA 104: 11002–11007, 2007. doi: 10.1073/pnas.0704421104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 153.Ozawa Y, Kobori H, Suzaki Y, Navar LG. Sustained renal interstitial macrophage infiltration following chronic angiotensin II infusions. Am J Physiol Renal Physiol 292: F330–F339, 2007. doi: 10.1152/ajprenal.00059.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 154.Pandey KN. Biology of natriuretic peptides and their receptors. Peptides 26: 901–932, 2005. doi: 10.1016/j.peptides.2004.09.024. [DOI] [PubMed] [Google Scholar]
- 155.Pandey KN. The functional genomics of guanylyl cyclase/natriuretic peptide receptor-A: perspectives and paradigms. FEBS J 278: 1792–1807, 2011. doi: 10.1111/j.1742-4658.2011.08081.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 156.Pandey KN. Guanylyl cyclase/atrial natriuretic peptide receptor-A: role in the pathophysiology of cardiovascular regulation. Can J Physiol Pharmacol 89: 557–573, 2011. doi: 10.1139/y11-054. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 157.Pandey KN. Guanylyl cyclase/natriuretic peptide receptor-A signaling antagonizes phosphoinositide hydrolysis, Ca(2+) release, and activation of protein kinase C. Front Mol Neurosci 7: 75, 2014. doi: 10.3389/fnmol.2014.00075. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 158.Pandey KN. Internalization and trafficking of guanylyl cyclase/natriuretic peptide receptor-A. Peptides 26: 985–1000, 2005. doi: 10.1016/j.peptides.2004.12.020. [DOI] [PubMed] [Google Scholar]
- 159.Pandey KN. Physiology of Natriuretic Peptides and Their Receptors, in Hypertension and Hormone Mechanisms (Carey RM, editor). Totowa, NJ: Humana Press, 2007, p. 277–305. doi: 10.1007/978-1-59259-987-5_17. [DOI] [Google Scholar]
- 160.Pandey KN. Vascular Action: Natriuretic Peptide Receptors, in Endocrinology of the Vasculature (Sowers JR, editor). Totowa, NJ: Humana Press, 1996, p. 255–267. doi: 10.1007/978-1-4612-0231-8_19. [DOI] [Google Scholar]
- 161.Pandey KN, Kumar R, Li M, Nguyen H. Functional domains and expression of truncated atrial natriuretic peptide receptor-A: the carboxyl-terminal regions direct the receptor internalization and sequestration in COS-7 cells. Mol Pharmacol 57: 259–267, 2000. [PubMed] [Google Scholar]
- 162.Pandey KN, Nguyen HT, Li M, Boyle JW. Natriuretic peptide receptor-A negatively regulates mitogen-activated protein kinase and proliferation of mesangial cells: role of cGMP-dependent protein kinase. Biochem Biophys Res Commun 271: 374–379, 2000. doi: 10.1006/bbrc.2000.2627. [DOI] [PubMed] [Google Scholar]
- 163.Pandey KN, Oliver PM, Maeda N, Smithies O. Hypertension associated with decreased testosterone levels in natriuretic peptide receptor-A gene-knockout and gene-duplicated mutant mouse models. Endocrinology 140: 5112–5119, 1999. doi: 10.1210/endo.140.11.7121. [DOI] [PubMed] [Google Scholar]
- 164.Pandey KN, Singh S. Molecular cloning and expression of murine guanylate cyclase/atrial natriuretic factor receptor cDNA. J Biol Chem 265: 12342–12348, 1990. [PubMed] [Google Scholar]
- 165.Pandey KN, Vellaichamy E. Regulation of cardiac angiotensin-converting enzyme and angiotensin AT1 receptor gene expression in Npr1 gene-disrupted mice. Clin Exp Pharmacol Physiol 37: e70–e77, 2010. doi: 10.1111/j.1440-1681.2009.05315.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 166.Parks WC, Wilson CL, López-Boado YS. Matrix metalloproteinases as modulators of inflammation and innate immunity. Nat Rev Immunol 4: 617–629, 2004. doi: 10.1038/nri1418. [DOI] [PubMed] [Google Scholar]
- 167.Paul RV, Ferguson T, Navar LG. ANF secretion and renal responses to volume expansion with equilibrated blood. Am J Physiol Renal Fluid Electrolyte Physiol 255: F936–F943, 1988. doi: 10.1152/ajprenal.1988.255.5.F936. [DOI] [PubMed] [Google Scholar]
- 168.Philbrick DJ, Bureau DP, Collins FW, Holub BJ. Evidence that soyasaponin Bb retards disease progression in a murine model of polycystic kidney disease. Kidney Int 63: 1230–1239, 2003. doi: 10.1046/j.1523-1755.2003.00869.x. [DOI] [PubMed] [Google Scholar]
- 169.Phillips MI, Heininger F, Toffolo S. The role of brain angiotensin in thirst and AVP release induced by hemorrhage. Regul Pept 66: 3–11, 1996. doi: 10.1016/0167-0115(96)00088-2. [DOI] [PubMed] [Google Scholar]
- 170.Pollman MJ, Yamada T, Horiuchi M, Gibbons GH. Vasoactive substances regulate vascular smooth muscle cell apoptosis. Countervailing influences of nitric oxide and angiotensin II. Circ Res 79: 748–756, 1996. doi: 10.1161/01.RES.79.4.748. [DOI] [PubMed] [Google Scholar]
- 171.Prins BA, Weber MJ, Hu RM, Pedram A, Daniels M, Levin ER. Atrial natriuretic peptide inhibits mitogen-activated protein kinase through the clearance receptor. Potential role in the inhibition of astrocyte proliferation. J Biol Chem 271: 14156–14162, 1996. doi: 10.1074/jbc.271.24.14156. [DOI] [PubMed] [Google Scholar]
- 172.Rautureau Y, Paradis P, Schiffrin EL. Cross-talk between aldosterone and angiotensin signaling in vascular smooth muscle cells. Steroids 76: 834–839, 2011. doi: 10.1016/j.steroids.2011.02.015. [DOI] [PubMed] [Google Scholar]
- 173.Reinhart K, Meisner M, Brunkhorst FM. Markers for sepsis diagnosis: what is useful? Crit Care Clin 22: 503–519, 2006. doi: 10.1016/j.ccc.2006.03.003. [DOI] [PubMed] [Google Scholar]
- 174.Reis WL, Giusti-Paiva A, Ventura RR, Margatho LO, Gomes DA, Elias LL, Antunes-Rodrigues J. Central nitric oxide blocks vasopressin, oxytocin and atrial natriuretic peptide release and antidiuretic and natriuretic responses induced by central angiotensin II in conscious rats. Exp Physiol 92: 903–911, 2007. doi: 10.1113/expphysiol.2007.037911. [DOI] [PubMed] [Google Scholar]
- 175.Richards AM, McDonald D, Fitzpatrick MA, Nicholls MG, Espiner EA, Ikram H, Jans S, Grant S, Yandle T. Atrial natriuretic hormone has biological effects in man at physiological plasma concentrations. J Clin Endocrinol Metab 67: 1134–1139, 1988. doi: 10.1210/jcem-67-6-1134. [DOI] [PubMed] [Google Scholar]
- 176.Rodríguez-Iturbe B, Pons H, Quiroz Y, Gordon K, Rincón J, Chávez M, Parra G, Herrera-Acosta J, Gómez-Garre D, Largo R, Egido J, Johnson RJ. Mycophenolate mofetil prevents salt-sensitive hypertension resulting from angiotensin II exposure. Kidney Int 59: 2222–2232, 2001. doi: 10.1046/j.1523-1755.2001.00737.x. [DOI] [PubMed] [Google Scholar]
- 177.Rosenzweig A, Seidman CE. Atrial natriuretic factor and related peptide hormones. Annu Rev Biochem 60: 229–255, 1991. doi: 10.1146/annurev.bi.60.070191.001305. [DOI] [PubMed] [Google Scholar]
- 178.Rubattu S, Bigatti G, Evangelista A, Lanzani C, Stanzione R, Zagato L, Manunta P, Marchitti S, Venturelli V, Bianchi G, Volpe M, Stella P. Association of atrial natriuretic peptide and type a natriuretic peptide receptor gene polymorphisms with left ventricular mass in human essential hypertension. J Am Coll Cardiol 48: 499–505, 2006. doi: 10.1016/j.jacc.2005.12.081. [DOI] [PubMed] [Google Scholar]
- 179.Ruiz-Ortega M, Lorenzo O, Rupérez M, Blanco J, Egido J. Systemic infusion of angiotensin II into normal rats activates nuclear factor-kappaB and AP-1 in the kidney: role of AT(1) and AT(2) receptors. Am J Pathol 158: 1743–1756, 2001. doi: 10.1016/S0002-9440(10)64130-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 180.Sangaralingham SJ, Burnett JC Jr, McKie PM, Schirger JA, Chen HH. Rationale and design of a randomized, double-blind, placebo-controlled clinical trial to evaluate the efficacy of B-type natriuretic peptide for the preservation of left ventricular function after anterior myocardial infarction. J Card Fail 19: 533–539, 2013. doi: 10.1016/j.cardfail.2013.06.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 181.Schillinger KJ, Tsai SY, Taffet GE, Reddy AK, Marian AJ, Entman ML, Oka K, Chan L, O’Malley BW. Regulatable atrial natriuretic peptide gene therapy for hypertension. Proc Natl Acad Sci USA 102: 13789–13794, 2005. doi: 10.1073/pnas.0506807102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 182.Schlueter N, de Sterke A, Willmes DM, Spranger J, Jordan J, Birkenfeld AL. Metabolic actions of natriuretic peptides and therapeutic potential in the metabolic syndrome. Pharmacol Ther 144: 12–27, 2014. doi: 10.1016/j.pharmthera.2014.04.007. [DOI] [PubMed] [Google Scholar]
- 183.Schnaper HW, Hayashida T, Hubchak SC, Poncelet AC. TGF-beta signal transduction and mesangial cell fibrogenesis. Am J Physiol Renal Physiol 284: F243–F252, 2003. doi: 10.1152/ajprenal.00300.2002. [DOI] [PubMed] [Google Scholar]
- 184.Schreier B, Börner S, Völker K, Gambaryan S, Schäfer SC, Kuhlencordt P, Gassner B, Kuhn M. The heart communicates with the endothelium through the guanylyl cyclase-A receptor: acute handling of intravascular volume in response to volume expansion. Endocrinology 149: 4193–4199, 2008. doi: 10.1210/en.2008-0212. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 185.Schulz S. C-type natriuretic peptide and guanylyl cyclase B receptor. Peptides 26: 1024–1034, 2005. doi: 10.1016/j.peptides.2004.08.027. [DOI] [PubMed] [Google Scholar]
- 186.Schulz S, Singh S, Bellet RA, Singh G, Tubb DJ, Chin H, Garbers DL. The primary structure of a plasma membrane guanylate cyclase demonstrates diversity within this new receptor family. Cell 58: 1155–1162, 1989. doi: 10.1016/0092-8674(89)90513-8. [DOI] [PubMed] [Google Scholar]
- 188.Sciarretta S, Marchitti S, Bianchi F, Moyes A, Barbato E, Di Castro S, Stanzione R, Cotugno M, Castello L, Calvieri C, Eberini I, Sadoshima J, Hobbs AJ, Volpe M, Rubattu S. C2238 atrial natriuretic peptide molecular variant is associated with endothelial damage and dysfunction through natriuretic peptide receptor C signaling. Circ Res 112: 1355–1364, 2013. doi: 10.1161/CIRCRESAHA.113.301325. [DOI] [PubMed] [Google Scholar]
- 189.Seals DF, Courtneidge SA. The ADAMs family of metalloproteases: multidomain proteins with multiple functions. Genes Dev 17: 7–30, 2003. doi: 10.1101/gad.1039703. [DOI] [PubMed] [Google Scholar]
- 190.Sen A, Kumar P, Garg R, Lindsey SH, Katakam PV, Bloodworth M, Pandey KN. Transforming growth factor β1 antagonizes the transcription, expression and vascular signaling of guanylyl cyclase/natriuretic peptide receptor A - role of δEF1. FEBS J 283: 1767–1781, 2016. doi: 10.1111/febs.13701. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 191.Sengenès C, Zakaroff-Girard A, Moulin A, Berlan M, Bouloumié A, Lafontan M, Galitzky J. Natriuretic peptide-dependent lipolysis in fat cells is a primate specificity. Am J Physiol Regul Integr Comp Physiol 283: R257–R265, 2002. doi: 10.1152/ajpregu.00453.2001. [DOI] [PubMed] [Google Scholar]
- 192.Shahani R, Klein LV, Marshall JG, Nicholson S, Rubin BB, Walker PM, Lindsay TF. Hemorrhage-induced alpha-adrenergic signaling results in myocardial TNF-alpha expression and contractile dysfunction. Am J Physiol Heart Circ Physiol 281: H84–H92, 2001. doi: 10.1152/ajpheart.2001.281.1.H84. [DOI] [PubMed] [Google Scholar]
- 193.Shao J, Nangaku M, Miyata T, Inagi R, Yamada K, Kurokawa K, Fujita T. Imbalance of T-cell subsets in angiotensin II-infused hypertensive rats with kidney injury. Hypertension 42: 31–38, 2003. doi: 10.1161/01.HYP.0000075082.06183.4E. [DOI] [PubMed] [Google Scholar]
- 194.Sharma GD, Nguyen HT, Antonov AS, Gerrity RG, von Geldern T, Pandey KN. Expression of atrial natriuretic peptide receptor-A antagonizes the mitogen-activated protein kinases (Erk2 and P38MAPK) in cultured human vascular smooth muscle cells. Mol Cell Biochem 233: 165–173, 2002. doi: 10.1023/A:1015882302796. [DOI] [PubMed] [Google Scholar]
- 195.Sharma K, Jin Y, Guo J, Ziyadeh FN. Neutralization of TGF-beta by anti-TGF-beta antibody attenuates kidney hypertrophy and the enhanced extracellular matrix gene expression in STZ-induced diabetic mice. Diabetes 45: 522–530, 1996. doi: 10.2337/diab.45.4.522. [DOI] [PubMed] [Google Scholar]
- 196.Sharma K, Ziyadeh FN, Alzahabi B, McGowan TA, Kapoor S, Kurnik BR, Kurnik PB, Weisberg LS. Increased renal production of transforming growth factor-beta1 in patients with type II diabetes. Diabetes 46: 854–859, 1997. doi: 10.2337/diab.46.5.854. [DOI] [PubMed] [Google Scholar]
- 197.Sharma RK. Evolution of the membrane guanylate cyclase transduction system. Mol Cell Biochem 230: 3–30, 2002. doi: 10.1023/A:1014280410459. [DOI] [PubMed] [Google Scholar]
- 198.Sharma RK. Membrane guanylate cyclase is a beautiful signal transduction machine: overview. Mol Cell Biochem 334: 3–36, 2010. doi: 10.1007/s11010-009-0336-6. [DOI] [PubMed] [Google Scholar]
- 199.Sharma RK, Duda T. Membrane guanylate cyclase, a multimodal transduction machine: history, present, and future directions. Front Mol Neurosci 7: 56, 2014. doi: 10.3389/fnmol.2014.00056. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 200.Shenoy SK, Lefkowitz RJ. Angiotensin II-stimulated signaling through G proteins and beta-arrestin. Sci STKE 2005: cm14, 2005. doi: 10.1126/stke.3112005cm14. [DOI] [PubMed] [Google Scholar]
- 201.Shi SJ, Nguyen HT, Sharma GD, Navar LG, Pandey KN. Genetic disruption of atrial natriuretic peptide receptor-A alters renin and angiotensin II levels. Am J Physiol Renal Physiol 281: F665–F673, 2001. doi: 10.1152/ajprenal.2001.281.4.F665. [DOI] [PubMed] [Google Scholar]
- 202.Shi SJ, Preuss HG, Abernethy DR, Li X, Jarrell ST, Andrawis NS. Elevated blood pressure in spontaneously hypertensive rats consuming a high sucrose diet is associated with elevated angiotensin II and is reversed by vanadium. J Hypertens 15: 857–862, 1997. doi: 10.1097/00004872-199715080-00009. [DOI] [PubMed] [Google Scholar]
- 203.Shi SJ, Vellaichamy E, Chin SY, Smithies O, Navar LG, Pandey KN. Natriuretic peptide receptor A mediates renal sodium excretory responses to blood volume expansion. Am J Physiol Renal Physiol 285: F694–F702, 2003. doi: 10.1152/ajprenal.00097.2003. [DOI] [PubMed] [Google Scholar]
- 204.Smithies O. Many little things: one geneticist’s view of complex diseases. Nat Rev Genet 6: 419–425, 2005. doi: 10.1038/nrg1605. [DOI] [PubMed] [Google Scholar]
- 205.Smithies O, Kim HS, Takahashi N, Edgell MH. Importance of quantitative genetic variations in the etiology of hypertension. Kidney Int 58: 2265–2280, 2000. doi: 10.1046/j.1523-1755.2000.00411.x. [DOI] [PubMed] [Google Scholar]
- 206.Smithies O, Maeda N. Gene targeting approaches to complex genetic diseases: atherosclerosis and essential hypertension. Proc Natl Acad Sci USA 92: 5266–5272, 1995. doi: 10.1073/pnas.92.12.5266. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 207.Spurgeon KR, Donohoe DL, Basile DP. Transforming growth factor-beta in acute renal failure: receptor expression, effects on proliferation, cellularity, and vascularization after recovery from injury. Am J Physiol Renal Physiol 288: F568–F577, 2005. doi: 10.1152/ajprenal.00330.2004. [DOI] [PubMed] [Google Scholar]
- 208.Steffes MW, Osterby R, Chavers B, Mauer SM. Mesangial expansion as a central mechanism for loss of kidney function in diabetic patients. Diabetes 38: 1077–1081, 1989. doi: 10.2337/diab.38.9.1077. [DOI] [PubMed] [Google Scholar]
- 209.Steinhelper ME, Cochrane KL, Field LJ. Hypotension in transgenic mice expressing atrial natriuretic factor fusion genes. Hypertension 16: 301–307, 1990. doi: 10.1161/01.HYP.16.3.301. [DOI] [PubMed] [Google Scholar]
- 210.Stockand JD, Sansom SC. Regulation of filtration rate by glomerular mesangial cells in health and diabetic renal disease. Am J Kidney Dis 29: 971–981, 1997. doi: 10.1016/S0272-6386(97)90476-5. [DOI] [PubMed] [Google Scholar]
- 211.Strutz F, Zeisberg M, Ziyadeh FN, Yang CQ, Kalluri R, Müller GA, Neilson EG, Renziehausen A, Sisic Z. Role of basic fibroblast growth factor-2 in epithelial-mesenchymal transformation. Kidney Int 61: 1714–1728, 2002. doi: 10.1046/j.1523-1755.2002.00333.x. [DOI] [PubMed] [Google Scholar]
- 212.Suga S, Nakao K, Itoh H, Komatsu Y, Ogawa Y, Hama N, Imura H. Endothelial production of C-type natriuretic peptide and its marked augmentation by transforming growth factor-beta. Possible existence of “vascular natriuretic peptide system”. J Clin Invest 90: 1145–1149, 1992. doi: 10.1172/JCI115933. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 213.Takahashi N, Hagaman JR, Kim HS, Smithies O. Minireview: computer simulations of blood pressure regulation by the renin-angiotensin system. Endocrinology 144: 2184–2190, 2003. doi: 10.1210/en.2002-221045. [DOI] [PubMed] [Google Scholar]
- 214.Takahashi N, Smithies O. Human genetics, animal models and computer simulations for studying hypertension. Trends Genet 20: 136–145, 2004. doi: 10.1016/j.tig.2004.01.004. [DOI] [PubMed] [Google Scholar]
- 215.Takahashi T, Taniguchi T, Konishi H, Kikkawa U, Ishikawa Y, Yokoyama M. Activation of Akt/protein kinase B after stimulation with angiotensin II in vascular smooth muscle cells. Am J Physiol Heart Circ Physiol 276: H1927–H1934, 1999. [DOI] [PubMed] [Google Scholar]
- 216.Tamura N, Doolittle LK, Hammer RE, Shelton JM, Richardson JA, Garbers DL. Critical roles of the guanylyl cyclase B receptor in endochondral ossification and development of female reproductive organs. Proc Natl Acad Sci USA 101: 17300–17305, 2004. doi: 10.1073/pnas.0407894101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 217.Tamura N, Ogawa Y, Chusho H, Nakamura K, Nakao K, Suda M, Kasahara M, Hashimoto R, Katsuura G, Mukoyama M, Itoh H, Saito Y, Tanaka I, Otani H, Katsuki M, Nakao K. Cardiac fibrosis in mice lacking brain natriuretic peptide. Proc Natl Acad Sci USA 97: 4239–4244, 2000. doi: 10.1073/pnas.070371497. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 218.Teitelbaum I, Strasheim A, Berl T. Epidermal growth factor-stimulated phosphoinositide hydrolysis in cultured rat inner medullary collecting tubule cells. Regulation by G protein, calcium, and protein kinase C. J Clin Invest 85: 1044–1050, 1990. doi: 10.1172/JCI114534. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 219.Thomas S, Rich MW. Epidemiology, pathophysiology, and prognosis of heart failure in the elderly. Clin Geriatr Med 23: 1–10, 2007. doi: 10.1016/j.cger.2006.08.001. [DOI] [PubMed] [Google Scholar]
- 220.Tremblay A, Parker KL, Lehoux JG. Dietary potassium supplementation and sodium restriction stimulate aldosterone synthase but not 11 beta-hydroxylase P-450 messenger ribonucleic acid accumulation in rat adrenals and require angiotensin II production. Endocrinology 130: 3152–3158, 1992. doi: 10.1210/endo.130.6.1597135. [DOI] [PubMed] [Google Scholar]
- 221.Tripathi S, Pandey KN. Guanylyl cyclase/natriuretic peptide receptor-A signaling antagonizes the vascular endothelial growth factor-stimulated MAPKs and downstream effectors AP-1 and CREB in mouse mesangial cells. Mol Cell Biochem 368: 47–59, 2012. doi: 10.1007/s11010-012-1341-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 222.Tsutamoto T, Kanamori T, Morigami N, Sugimoto Y, Yamaoka O, Kinoshita M. Possibility of downregulation of atrial natriuretic peptide receptor coupled to guanylate cyclase in peripheral vascular beds of patients with chronic severe heart failure. Circulation 87: 70–75, 1993. doi: 10.1161/01.CIR.87.1.70. [DOI] [PubMed] [Google Scholar]
- 223.Tyagi SC, Kumar S, Voelker DJ, Reddy HK, Janicki JS, Curtis JJ. Differential gene expression of extracellular matrix components in dilated cardiomyopathy. J Cell Biochem 63: 185–198, 1996. doi:. [DOI] [PubMed] [Google Scholar]
- 224.Ushio-Fukai M, Alexander RW, Akers M, Griendling KK. p38 Mitogen-activated protein kinase is a critical component of the redox-sensitive signaling pathways activated by angiotensin II. Role in vascular smooth muscle cell hypertrophy. J Biol Chem 273: 15022–15029, 1998. doi: 10.1074/jbc.273.24.15022. [DOI] [PubMed] [Google Scholar]
- 225.Vallejo JG, Nemoto S, Ishiyama M, Yu B, Knuefermann P, Diwan A, Baker JS, Defreitas G, Tweardy DJ, Mann DL. Functional significance of inflammatory mediators in a murine model of resuscitated hemorrhagic shock. Am J Physiol Heart Circ Physiol 288: H1272–H1277, 2005. doi: 10.1152/ajpheart.01003.2003. [DOI] [PubMed] [Google Scholar]
- 226.Vellaichamy E, Das S, Subramanian U, Maeda N, Pandey KN. Genetically altered mutant mouse models of guanylyl cyclase/natriuretic peptide receptor-A exhibit the cardiac expression of proinflammatory mediators in a gene-dose-dependent manner. Endocrinology 155: 1045–1056, 2014. doi: 10.1210/en.2013-1416. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 227.Vellaichamy E, Khurana ML, Fink J, Pandey KN. Involvement of the NF-kappa B/matrix metalloproteinase pathway in cardiac fibrosis of mice lacking guanylyl cyclase/natriuretic peptide receptor A. J Biol Chem 280: 19230–19242, 2005. doi: 10.1074/jbc.M411373200. [DOI] [PubMed] [Google Scholar]
- 228.Vellaichamy E, Sommana NK, Pandey KN. Reduced cGMP signaling activates NF-kappaB in hypertrophied hearts of mice lacking natriuretic peptide receptor-A. Biochem Biophys Res Commun 327: 106–111, 2005. doi: 10.1016/j.bbrc.2004.11.153. [DOI] [PubMed] [Google Scholar]
- 229.Volpe M, Rubattu S, Burnett J Jr. Natriuretic peptides in cardiovascular diseases: current use and perspectives. Eur Heart J 35: 419–425, 2014. doi: 10.1093/eurheartj/eht466. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 230.Wang JJ, Zhang SX, Mott R, Knapp RR, Cao W, Lau K, Ma JX. Salutary effect of pigment epithelium-derived factor in diabetic nephropathy: evidence for antifibrogenic activities. Diabetes 55: 1678–1685, 2006. doi: 10.2337/db05-1448. [DOI] [PubMed] [Google Scholar]
- 231.Wang TJ, Larson MG, Keyes MJ, Levy D, Benjamin EJ, Vasan RS. Association of plasma natriuretic peptide levels with metabolic risk factors in ambulatory individuals. Circulation 115: 1345–1353, 2007. doi: 10.1161/CIRCULATIONAHA.106.655142. [DOI] [PubMed] [Google Scholar]
- 232.Wang TJ, Larson MG, Levy D, Benjamin EJ, Leip EP, Wilson PW, Vasan RS. Impact of obesity on plasma natriuretic peptide levels. Circulation 109: 594–600, 2004. doi: 10.1161/01.CIR.0000112582.16683.EA. [DOI] [PubMed] [Google Scholar]
- 233.Wang Y, de Waard MC, Sterner-Kock A, Stepan H, Schultheiss HP, Duncker DJ, Walther T. Cardiomyocyte-restricted over-expression of C-type natriuretic peptide prevents cardiac hypertrophy induced by myocardial infarction in mice. Eur J Heart Fail 9: 548–557, 2007. doi: 10.1016/j.ejheart.2007.02.006. [DOI] [PubMed] [Google Scholar]
- 234.Webber MA, Marder SR. Better pharmacotherapy for schizophrenia: what does the future hold? Curr Psychiatry Rep 10: 352–358, 2008. doi: 10.1007/s11920-008-0056-8. [DOI] [PubMed] [Google Scholar]
- 235.Wehling M, Neylon CB, Fullerton M, Bobik A, Funder JW. Nongenomic effects of aldosterone on intracellular Ca2+ in vascular smooth muscle cells. Circ Res 76: 973–979, 1995. doi: 10.1161/01.RES.76.6.973. [DOI] [PubMed] [Google Scholar]
- 236.Wei CM, Heublein DM, Perrella MA, Lerman A, Rodeheffer RJ, McGregor CG, Edwards WD, Schaff HV, Burnett JC Jr. Natriuretic peptide system in human heart failure. Circulation 88: 1004–1009, 1993. doi: 10.1161/01.CIR.88.3.1004. [DOI] [PubMed] [Google Scholar]
- 237.Wenzel P, Knorr M, Kossmann S, Stratmann J, Hausding M, Schuhmacher S, Karbach SH, Schwenk M, Yogev N, Schulz E, Oelze M, Grabbe S, Jonuleit H, Becker C, Daiber A, Waisman A, Münzel T. Lysozyme M-positive monocytes mediate angiotensin II-induced arterial hypertension and vascular dysfunction. Circulation 124: 1370–1381, 2011. doi: 10.1161/CIRCULATIONAHA.111.034470. [DOI] [PubMed] [Google Scholar]
- 238.Wisgerhof M, Brown RD, Hogan MJ, Carpenter PC, Edis AJ. The plasma aldosterone response to angiotensin II infusion in aldosterone-producing adenoma and idiopathic hyperaldosteronism. J Clin Endocrinol Metab 52: 195–198, 1981. doi: 10.1210/jcem-52-2-195. [DOI] [PubMed] [Google Scholar]
- 239.Wu CF, Bishopric NH, Pratt RE. Atrial natriuretic peptide induces apoptosis in neonatal rat cardiac myocytes. J Biol Chem 272: 14860–14866, 1997. doi: 10.1074/jbc.272.23.14860. [DOI] [PubMed] [Google Scholar]
- 240.Wu Z, Zheng W, Sandberg K. Estrogen regulates adrenal angiotensin type 1 receptors by modulating adrenal angiotensin levels. Endocrinology 144: 1350–1356, 2003. doi: 10.1210/en.2002-221100. [DOI] [PubMed] [Google Scholar]
- 241.Xu Z, Glenda C, Day L, Yao J, Ross MG. Osmotic threshold and sensitivity for vasopressin release and fos expression by hypertonic NaCl in ovine fetus. Am J Physiol Endocrinol Metab 279: E1207–E1215, 2000. doi: 10.1152/ajpendo.2000.279.6.E1207. [DOI] [PubMed] [Google Scholar]
- 242.Xue H, Wang S, Wang H, Sun K, Song X, Zhang W, Fu C, Han Y, Hui R. Atrial natriuretic peptide gene promoter polymorphism is associated with left ventricular hypertrophy in hypertension. Clin Sci (Lond) 114: 131–137, 2008. doi: 10.1042/CS20070109. [DOI] [PubMed] [Google Scholar]
- 243.Yang H, Tüzün E, Alagappan D, Yu X, Scott BG, Ischenko A, Christadoss P. IL-1 receptor antagonist-mediated therapeutic effect in murine myasthenia gravis is associated with suppressed serum proinflammatory cytokines, C3, and anti-acetylcholine receptor IgG1. J Immunol 175: 2018–2025, 2005. doi: 10.4049/jimmunol.175.3.2018. [DOI] [PubMed] [Google Scholar]
- 244.Yasoda A, Komatsu Y, Chusho H, Miyazawa T, Ozasa A, Miura M, Kurihara T, Rogi T, Tanaka S, Suda M, Tamura N, Ogawa Y, Nakao K. Overexpression of CNP in chondrocytes rescues achondroplasia through a MAPK-dependent pathway. Nat Med 10: 80–86, 2004. doi: 10.1038/nm971. [DOI] [PubMed] [Google Scholar]
- 245.Yatabe J, Yoneda M, Yatabe MS, Watanabe T, Felder RA, Jose PA, Sanada H. Angiotensin III stimulates aldosterone secretion from adrenal gland partially via angiotensin II type 2 receptor but not angiotensin II type 1 receptor. Endocrinology 152: 1582–1588, 2011. doi: 10.1210/en.2010-1070. [DOI] [PubMed] [Google Scholar]
- 246.Ye P, Kenyon CJ, MacKenzie SM, Seckl JR, Fraser R, Connell JM, Davies E. Regulation of aldosterone synthase gene expression in the rat adrenal gland and central nervous system by sodium and angiotensin II. Endocrinology 144: 3321–3328, 2003. doi: 10.1210/en.2003-0109. [DOI] [PubMed] [Google Scholar]
- 247.Zhao D, Das S, Pandey KN. Interactive roles of NPR1 gene-dosage and salt diets on cardiac angiotensin II, aldosterone and pro-inflammatory cytokines levels in mutant mice. J Hypertens 31: 134–144, 2013. doi: 10.1097/HJH.0b013e32835ac15f. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 248.Zhao D, Pandey KN, Navar LG. ANP-mediated inhibition of distal nephron fractional sodium reabsorption in wild-type and mice overexpressing natriuretic peptide receptor. Am J Physiol Renal Physiol 298: F103–F108, 2010. doi: 10.1152/ajprenal.00479.2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 249.Zhao D, Vellaichamy E, Somanna NK, Pandey KN. Guanylyl cyclase/natriuretic peptide receptor-A gene disruption causes increased adrenal angiotensin II and aldosterone levels. Am J Physiol Renal Physiol 293: F121–F127, 2007. doi: 10.1152/ajprenal.00478.2006. [DOI] [PubMed] [Google Scholar]
- 250.Zhuo JL. Monocyte chemoattractant protein-1: a key mediator of angiotensin II-induced target organ damage in hypertensive heart disease? J Hypertens 22: 451–454, 2004. doi: 10.1097/00004872-200403000-00003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 251.Ziyadeh FN, Hoffman BB, Han DC, Iglesias-De La Cruz MC, Hong SW, Isono M, Chen S, McGowan TA, Sharma K. Long-term prevention of renal insufficiency, excess matrix gene expression, and glomerular mesangial matrix expansion by treatment with monoclonal antitransforming growth factor-beta antibody in db/db diabetic mice. Proc Natl Acad Sci USA 97: 8015–8020, 2000. doi: 10.1073/pnas.120055097. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 252.Zohn IE, Yu H, Li X, Cox AD, Earp HS. Angiotensin II stimulates calcium-dependent activation of c-Jun N-terminal kinase. Mol Cell Biol 15: 6160–6168, 1995. doi: 10.1128/MCB.15.11.6160. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 253.Zou LX, Hymel A, Imig JD, Navar LG. Renal accumulation of circulating angiotensin II in angiotensin II-infused rats. Hypertension 27: 658–662, 1996. doi: 10.1161/01.HYP.27.3.658. [DOI] [PubMed] [Google Scholar]
- 254.Zou LX, Imig JD, von Thun AM, Hymel A, Ono H, Navar LG. Receptor-mediated intrarenal angiotensin II augmentation in angiotensin II-infused rats. Hypertension 28: 669–677, 1996. doi: 10.1161/01.HYP.28.4.669. [DOI] [PubMed] [Google Scholar]