Abstract
Subjects maintaining a ≥10% dietary weight loss exhibit decreased circulating concentrations of bioactive thyroid hormones and increased skeletal muscle work efficiency largely due to increased expression of more-efficient myosin heavy chain (MHC) isoforms (MHC I) and significantly mediated by the adipocyte-derived hormone leptin. The primary purpose of this study was to examine the effects of triiodothyronine (T3) repletion on energy homeostasis and skeletal muscle physiology in weight-reduced subjects and to compare these results with the effects of leptin repletion. Nine healthy in-patients with obesity were studied at usual weight (Wtinitial) and following a 10% dietary weight loss while receiving 5 wk of a placebo (Wt−10%placebo) or T3 (Wt−10%T3) in a single-blind crossover design. Primary outcome variables were skeletal muscle work efficiency and vastus lateralis muscle mRNA expression. These results were compared with the effects of leptin repletion in a population of 22 subjects, some of whom participated in a previous study. At Wt−10%placebo, skeletal muscle work efficiency and relative expression of the more-efficient/less-efficient MHC I/MHC II isoforms were significantly increased and the ratio of the less-efficient to the more-efficient sarco(endo)plasmic reticulum Ca2+-ATPase isoforms (SERCA1/SERCA2) was significantly decreased. These changes were largely reversed by T3 repletion to a degree similar to the changes that occurred with leptin repletion. These data support the hypothesis that the effects of leptin on energy expenditure in weight-reduced individuals are largely mediated by T3 and suggest that further study of the possible role of thyroid hormone repletion as adjunctive therapy to help sustain weight loss is needed.
Keywords: energy homeostasis, leptin, muscle, thyroid, weight loss
INTRODUCTION
Maintenance of a ≥10% reduced body weight in obese or lean subjects results in a decline (~300–400 kcal below that predicted by changes in body mass and composition) in energy expenditure (EE) due mostly to increased skeletal muscle work (chemomechanical) efficiency (14, 21). Coordinate changes in energy intake due to delayed satiation, decreased perception of how much food has been eaten, increased hunger, and preference for calorically dense foods (18, 21, 32, 41) result in hypometabolism and hyperphagia that are sufficient to account for the >75% recidivism rate following otherwise successful weight loss (21, 28).
Circulating concentrations of the adipocyte-derived hormone leptin are reduced following weight loss (35), and low-dose leptin repletion at least partially reverses most of these “weight-reduced” phenotypes (4, 18, 33, 35, 36). The weight-reduced state is also accompanied by decreased bioactive thyroid hormones and decreased sympathetic nervous system (SNS) tone, which also favor weight regain (21, 34). The aim of this study was to expand our previous studies of leptin repletion (10 subjects) and determine whether leptin-mediated changes in the hypothalamic-pituitary-thyroid (HPT) axis are the proximate cause of the increase in skeletal muscle work efficiency that occurs during maintenance of a reduced body weight.
The changes in skeletal muscle following weight loss and the associated relative decline in circulating concentrations of bioactive thyroid hormones are similar to those seen in hypothyroid subjects (33, 34). Triiodothyronine (T3) administration to hypothyroid subjects increases the proportion of glycolytic, less chemomechanically efficient myosin heavy chain (MHC) isoforms (MHC II) in skeletal muscle (5, 6) via respective inhibitory and stimulatory response elements in the promoter regions of MHCI and sarco(endo)plasmic reticulum Ca2+-ATPase (SERCA2) (3, 38), similar to the effects of leptin repletion following weight loss. The lack of efficacy of physiological doses of thyroid hormone in promoting weight loss in euthyroid individuals (16) does not preclude this formulation.
The hypothesis was that T3 “repletion” would partially reverse the decreased EE, increased skeletal muscle work efficiency, and changes in vastus lateralis mRNA expression (increased expression of more-efficient MHC and SERCA isoforms) in weight-reduced subjects to a degree similar to leptin repletion (35). This hypothesis implies mechanisms of leptin action on skeletal muscle, and the use of thyroid supplementation following weight loss could be a therapeutic adjunct by reversing the adaptive thermogenesis that otherwise favors weight regain.
MATERIALS AND METHODS
Subjects with obesity [body mass index (BMI) >30 kg/m2] were enrolled at maximum lifetime weight, which they had maintained within a 2-kg range for ≥6 mo (25). Recruitment procedures and exclusion criteria are reported elsewhere (22, 33, 34). All studies were approved by the Columbia University Medical Center Institutional Review Board and are consistent with guiding principles for research involving humans (2). Written informed consent was obtained from all subjects before enrollment. Subject characteristics are presented in Table 1.
Table 1.
Subject characteristics
| Thyroid Repletion Studies [n = 9 (3 M, 6 F)] |
Leptin Repletion Studies [n = 22 (9 M, 13 F)] |
|||||
|---|---|---|---|---|---|---|
| Wtinitial | Wt−10%placebo | Wt−10%T3 | Wtinitial | Wt−10%placebo | Wt−10%leptin | |
| Age, yr | 32.2 (8.6) | 34.2 (8.1) | ||||
| Weight, kg | 97.5 (17.4) | 85.5 (13.8)* | 84.0 (14.0)*† | 114.6 (31.8) | 101.4 (29.6)* | 100.2 (29.0)*† |
| FFM, kg | 54.5 (9.3) | 52.6 (9.9)* | 51.6 (10.0)* | 62.4 (14.3) | 60.1 (14.0)* | 59.1 (14.0)* |
| FM, kg | 43.0 (17.0) | 32.9 (14.2)* | 32.4 (14.3)*† | 51.7 (22.6) | 42.3 (19.7)* | 41.1 (19.2)*† |
| %Body fat | 43.1 (11.6) | 38.0 (16.4)* | 37.1 (16.2)* | 43.4 (11.2) | 39.4 (10.9)* | 39.1 (11.3)* |
| Temperature, °C | 36.6 (0.3) | 36.7 (0.3) | 36.7 (0.3) | 36.5 (0.3) | 36.6 (0.3) | 36.5 (0.2) |
| Pulse, beats/min | 71 (9) | 68 (9)* | 73 (12)† | 72 (8) | 69 (8)* | 71 (8) |
| SBP, mmHg | 112 (9) | 110 (13) | 116 (9) | 113 (10) | 108 (12)* | 112 (11)† |
| DBP, mmHg | 65 (8) | 64 (7) | 67 (8) | 68 (8) | 67 (6) | 68 (7) |
| Serum T3, ng/dl | 101.7 (14.5) (range 84–131) | 93.8 (12.4)*(range 78–110) | 121.1 (12.9)*†(range 101–139 | 105.1 (19.0)(range 79–132) | 92.5 (17.7)*(range 61–127) | 101.9 (18.7)†(range 78–143) |
| Serum T4, μg/dl | 6.15 (1.11)(range 4.84–8.49) | 5.85 (1.12)(range 4.05–7.45) | 3.45 (1.74)*†(range 0.63–6.09) | 7.20 (0.99)(range 5.03–9.42) | 6.68 (0.93)*(range 5.38–9.32) | 7.32 (1.12)†(range 5.50–9.77) |
| Serum TSH, mU/l | 1.95 (0.65)(range 0.76–3.05) | 1.35 (0.74)*(range 0.86–2.84) | 0.51 (0.20)*†(range 0.04–1.71) | 1.63 (1.07)(range 0.71–4.97) | 1.23 (0.93)*(range 0.16–4.13) | 1.26 (1.06)*(range 0.18–4.45) |
| T4/T3 | 0.061 (0.013) | 0.067 (0.021) | 0.028 (0.016)*† | 0.070 (0.016) | 0.074 (0.018) | 0.073 (0.015) |
| Leptin, ng/ml | 39.7 (30.2) | 27.0 (22.9)* | 26.6 (22.3)* | 48.2 (44.3) | 34.9 (31.5)* | 54.4 (39.2)† |
Values are means (SD). FFM, fat-free mass; FM, fat mass; SBP and DBP, systolic and diastolic blood pressure; T3, triiodothyronine; T4, thyroxine; Wtinitial, usual body weight; Wt−10%placebo, body weight after 10% weight loss in subjects receiving 5 wk of placebo treatment; Wt−10%T3, body weight after 10% weight loss in subjects receiving 5 wk of T3 treatment; Wt−10%leptin, body weight after 10% weight loss in subjects receiving 5 wk of leptin treatment.
P < 0.05 vs. Wtinitial;
P < 0.05 vs. Wt−10%placebo.
Subjects were in-patients in the Clinical Research Center at Columbia Presbyterian Medical Center throughout the study. They were weighed daily at 6 AM and were instructed to consume all meals before midnight. As described previously (22), subjects were fed a liquid formula diet [40% of calories as fat (corn oil), 45% as carbohydrate (glucose polymer), and 15% as protein (casein hydrolysate)], plus vitamin and mineral supplements, in quantities sufficient to maintain a stable weight (defined as a mean weight variation of <10 g/day for ≥2 wk). This weight plateau is designated Wtinitial.
Both the T3 and leptin repletion studies were performed using a randomized, single-blind crossover design. Upon enrollment, subjects were randomized according to whether a random number generated was odd (placebo first) or even (T3 or leptin repletion first). After completion of the studies (see below) at Wtinitial, the same liquid formula diet at 800 kcal of energy/day was provided until the subjects had lost ~10% of Wtinitial. The duration of the weight loss phase was 36–58 days. Once a ~10% weight loss had been achieved, intake was adjusted until subjects were again weight-stable, as described above. In a randomized, single-blind, crossover design, subjects in the thyroid repletion studies then received 5 wk of a placebo (designated Wt−10%placebo) or T3 (initially 25 µg/day total, designated Wt−10%T3). Plasma thyroid hormone concentrations were monitored weekly during placebo and T3 administration; during T3 supplementation, doses were titrated when necessary to maintain 8 AM T3 levels within the normal range (80–220 ng/dl) and ≤25% above plasma T3 at Wtinitial. The first three subjects received a single oral daily 25-µg dose of T3, which is ~50% of the “replacement dose” of T3 reported necessary to titrate thyroid-stimulating hormone (TSH) to normal concentrations in athyrotic individuals (8). One subject demonstrated TSH oversuppression to <0.1 mU/l. Divided T3 dosing (8) has been shown to avoid oversuppression of TSH and possible subclinical hyperthyroidism (11), and the initial dosing schedule was switched to 12.5 µg po twice a day for all subsequent subjects, with titration as needed to keep the circulating concentrations of T3 at 8 AM (drawn before the morning placebo or T3 dose) within the “normal range,” as described above. T3 was always administered ≥45 min before breakfast. Subjects remained on isocaloric diets at Wt−10%placebo and Wt−10%T3.
For comparison of the effects of T3 and leptin repletion on EE, data were available from 22 subjects (9 male, 13 female; 19 obese, 3 never-obese), 10 of whom (3 male, 7 female) participated in previous studies in this laboratory (4, 14, 33) (Table 1). These subjects were studied at Wtinitial, Wt−10%placebo, and after 10% dietary weight loss while receiving leptin for 5 wk (Wt−10%leptin) using a randomized single-blind crossover design, as described above.
After weight loss, subjects were weight-stabilized. Once reduced-weight stability was achieved, subjects received twice-daily injections of placebo (saline) or leptin starting at an initial leptin dose of 0.08 mg/kg fat mass at Wt−10%/dose in males and 0.14 mg/kg fat mass at Wt−10%/dose in females. Leptin or placebo (saline) was administered as twice-daily injections in doses titrated to restore circulating leptin concentrations at 8 AM (prior to dosing) to concentrations at Wtinitial, as previously described (33).
Leptin repletion data were collected over a period of 8 yr, from 2003 to 2011. T3 repletion studies were conducted over 3 yr, from 2012 to 2015. Throughout both studies, the same assays, meal structure, and protocols, except the repleted hormone, were used.
Subjects underwent the following studies at Wtinitial, Wt−10%placebo, and Wt−10%T3 (T3 repletion studies) and at Wtinitial, Wt−10%placebo, and Wt−10%leptin (leptin repletion studies). In some cases (see results), measurements were not obtained from all subjects.
Body Composition
Body composition [fat-free mass (FFM) and fat mass (FM)] was measured by dual-photon-beam absorptiometry (30).
Vital Signs
Temperature (oral), pulse, respiratory rate, and blood pressure were measured between 6 and 7 AM (before breakfast). Mean values from the last 10 days of each study period were compared to assess the effects of weight loss and thyroid hormone repletion.
Energy Expenditure
Resting EE (REE) and the thermic effect of feeding (TEF) were measured by indirect calorimetry using a Hood calorimeter (22). Total (24-h) EE (TEE) was measured by caloric titration. Nonresting EE (NREE) was calculated as follows: NREE = TEE − (REE + TEF) (22).
Autonomic Nervous System Activity
Autonomic nervous system (ANS) activity was assessed by 24-h urinary catecholamine excretion (7) and by heart rate changes following serial blockade of the parasympathetic nervous system (PNS) and SNS (33). Briefly, resting PNS and SNS tone were calculated based on ECG R-R interval changes during sequential administration of the muscarinic PNS blocker atropine followed by the cardioselective SNS blocker esmolol. PNS activity was calculated as the R-R interval relative to baseline following atropine. SNS activity was calculated as the change in R-R interval in subjects who received atropine and esmolol compared with those who received atropine alone.
Endocrine Testing
Thyroxine (T4), T3, TSH, and leptin were determined by radioimmunoassay (34) and were measured at 8 AM in all phases of the study and immediately before administration of T3, leptin, or placebo in subjects following weight reduction.
Skeletal Muscle
In vivo studies.
Graded bicycle ergometry measurements were made by indirect calorimetry as resistance was ramped up from 10 to 25 to 50 to 75 W in 4-min increments. Ergometry studies were performed approximately every 2–3 wk during weight stabilization periods, and exercises were prescribed to maintain fitness based on the anaerobic threshold.
31P-NMR spectroscopy to assess muscle contractile efficiency [work/calories consumed and ATP cost/muscle contraction via inorganic phosphate (Pi)-to-phosphocreatine (PCr) ratio during exercise] was performed in six subjects (2 male, 4 female) receiving T3 repletion at rest and while pressing a pedal against 44.4-kPa resistance during each study period. These data were not available for the leptin-repleted subjects.
In vitro studies.
Vastus lateralis muscle biopsies (50–100 mg/sample) were obtained under local anesthesia (1% lidocaine) using a Bergstrom needle and frozen in liquid nitrogen. Samples were used to analyze mRNA expression of MHC I, IIa, and IIx and SERCA1 and SERCA2. TRIzol (Invitrogen) was used to extract RNA from frozen muscle.
RNA extraction and analyses were performed as described previously in similar studies of leptin repletion following weight loss (4).
Calculations and Statistical Analyses
Data are presented as means (SD). Calculations of TEE were based on caloric intake adjusted for changes (compared with placebo) in body mass and composition (energy stores). Chemical energy content was assigned as 9.4 kcal/g wet wt of FM and 0.91 kcal/g wet wt of FFM excluding bone (29). TEE during thyroid or leptin repletion was calculated as follows: [weight maintenance diet calories during the reduced weight run-in phase (TEE at Wt−10%placebo) – energy content of weight change] ÷ number of days receiving T3 repletion at the time body composition was measured. Because regression lines relating EE to body composition and body mass do not have zero intercepts, ratios of energy to mass may be different following weight loss, even if values remain on the same regression line (31). To enable the increased sensitivity of paired testing and to account for the nonzero intercepts described above, regression equations at Wtinitial were used to predict EE at Wt−10%placebo and body weight after 10% weight loss in T3- or leptin-repleted subjects (Wt−10%repleted). To assess whether there were significant plateau effects on the relationship of EE to body mass or composition, the observed − predicted values (residuals) were tested against the null hypothesis that they equaled zero (31). The baseline regression equations at Wtinitial used to calculate residuals were based on both groups (T3 and leptin repletion) combined, and residual values relative to Wtinitial are also presented for each group.
EE during bicycle ergometry was calculated using the Weir equations (43) and expressed as energy expended in kilocalories per minute above REE. Net mechanical efficiency was calculated as [100 × (work done, in kcal/min)/(EE above resting, in kcal/min), i.e., the percentage of energy expended that was accounted for by work (14).
Within-group comparisons were made by ANOVA with repeated measures. Between-groups comparisons (subjects studied before and after weight loss with and without T3 repletion and with and without leptin repletion) were made by ANOVA. In cases where data sets were not complete (see below), comparisons were made for subject demographics (age, sex, weight, BMI, and baseline thyroid, leptin, and EE data) to ascertain whether there were significant differences between subjects for whom data were available and those for whom data were not available. Normality of data distributions was confirmed by Wilk-Shapiro testing.
Data from in vitro studies of skeletal muscle were available for only 6 of 9 subjects receiving T3 repletion and 10 of 22 subjects receiving leptin repletion. Baseline BMI z-scores and changes in BMI z-scores and EE of subjects for whom muscle biopsy data were available were compared with those of all subjects in the same repletion studies (T3 or leptin) for any significant differences from control for missing data.
Statistical significance was prospectively defined as P < 0.05. Because of the smaller number of subjects in the T3 repletion group, values with P < 0.1 are also noted as possible areas for future studies, although they are not considered statistically significant. Data were analyzed using Statistica 6.0 software (39).
RESULTS
Subjects
T3 doses were titrated down to 20 µg/day in two of the first three subjects. No further T3 dosing adjustments were necessary in subjects who began with a 12.5-µg dose of T3 twice a day. Significant additional weight loss with T3 and leptin repletion is shown in Table 1. Comparison of the composition of weight loss during dietary restriction with weight loss during T3 repletion revealed a nonsignificant trend toward greater fractional loss of FFM than FM during T3 repletion (~79% of weight loss was FM between Wtinitial and Wt−10%placebo and ~40% between Wt−10%placebo and Wt−10%T3) in an analysis of covariance including testing order as a covariate. Pulse and blood pressure trended downward following weight loss (Wt−10%placebo vs. Wtinitial) and upward following T3 repletion (Wt−10%T3 vs. Wt−10%placebo).
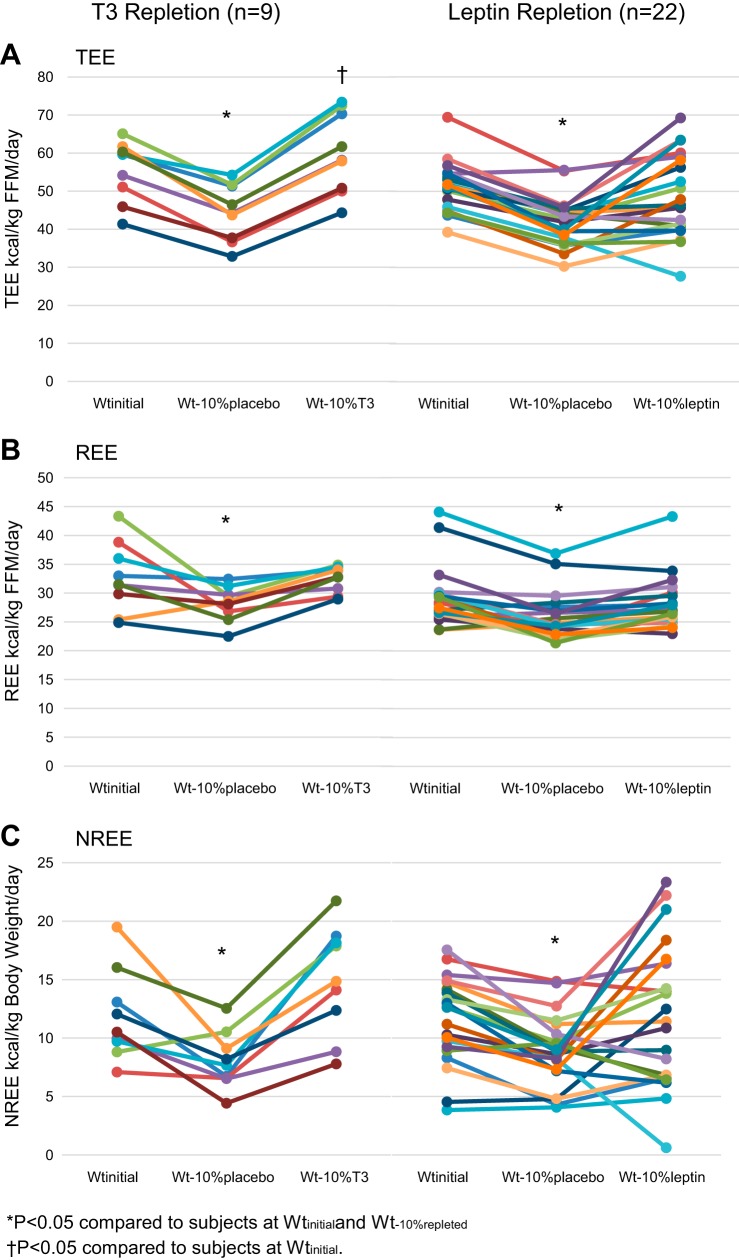
Circulating concentrations of T3 and TSH were significantly diminished at Wt−10%placebo compared with Wtinitial. After T3 repletion, circulating concentrations of T3 were significantly higher than at Wtinitial or Wt−10%placebo but well within the normal physiological range; T4 and TSH levels were suppressed significantly below values at Wtinitial and Wt−10%placebo. T3 administration resulted in significant additional suppression of T4 and the ratio of T4 to T3, as reported in studies of T3 repletion in athyrotic individuals (8), with no significant effect on suppression of circulating leptin concentrations following weight loss. Circulating concentrations of leptin, T3, and T4 were significantly higher at Wt−10%leptin than Wt−10%placebo, with no significant effects of TSH suppression following weight loss. At Wt−10%placebo, TEE, REE, and NREE were significantly suppressed, whether expressed as kilocalories or kilocalories per kilogram of FFM (for TEE and REE) or kilocalories per kilogram of body weight (for NREE) (Fig. 1). T3 and leptin repletion resulted in significant increases in all these categories of EE corrected for FFM or weight, as described above (Table 2).
Fig. 1.
Effects of thyroid [triiodothyronine (T3)] and leptin repletion on energy expenditure. A: total energy expenditure (TEE). B: resting energy expenditure (REE). C: nonresting energy expenditure (NREE). FFM, fat-free mass; Wtinitial, usual body weight; Wt−10%placebo, body weight after 10% weight loss in subjects receiving 5 wk of placebo treatment; Wt−10%T3, body weight after 10% weight loss in subjects receiving 5 wk of T3 treatment; Wt−10%leptin, body weight after 10% weight loss in subjects receiving 5 wk of leptin treatment. Values are means (SD). *P < 0.05 vs. Wtinitial and body weight after 10% weight loss in T3- or leptin-repleted subjects (Wt−10%repleted); †P < 0.05 vs. Wtinitial.
Table 2.
Effects of weight loss and T3 repletion on energy expenditure and the autonomic nervous system
| T3 Repletion (n = 9) |
Leptin Repletion (n = 22) |
|||||
|---|---|---|---|---|---|---|
| Wtinitial | Wt−10%placebo | Wt−10%T3 | Wtinitial | Wt−10%placebo | Wt−10%leptin | |
| TEE, kcal/day | 2,961 (243) | 2,278 (249)* | 2,478 (374)*† | 3,131 (500) | 2,457 (410)* | 2,766 (799)*† |
| REE, kcal/day | 1,753 (375) | 1,538 (279)* | 1,689 (404) | 1,830 (470) | 1,548 (368)* | 1,649 (384)*† |
| NREE, kcal/day | 1,119 (254) | 671 (173)* | 714 (329)* | 1,256 (417) | 864 (304)* | 1,068 (569)*† |
| TEE/FFM, kcal/kg | 55.4 (7.9) | 44.3 (7.4)* | 59.9 (10.5)*† | 51.1 (6.3) | 41.7 (6.2)* | 48.1 (12.7)† |
| REE/FFM, kcal/kg | 32.7 (6.0) | 28.2 (3.0)* | 32.4 (2.2)† | 29.6 (5.3) | 26.0 (3.9)* | 28.5 (5.4)† |
| NREE/body wt, kcal/kg | 11.9 (3.9) | 8.0 (2.4)* | 14.9 (4.7)† | 11.4 (3.7) | 8.7 (3.1)* | 10.8 (7.4)† |
| Urine Norepi, µg/day | 32 (9) | 28 (10)* | 36 (8)*† | 46 (8) | 40 (6)* | 41 (5)* |
| Urine Epi, µg/day | 15 (10) | 14 (13) | 23 (15)*† | 12 (9) | 8 (10)* | 16 (12)*† |
| %Change from Wtinitial [n = 15 (all obese)] | ||||||
| SNS tone | −17.0 (23.6)‡ | 21.6 (39.3)†‡ | −24.7 (24.2)‡ | −9.6 (25.6)† | ||
| PNS tone | 19.3 (20.4)‡ | 20.7 (22.3)‡ | 22.3 (23.1)‡ | 21.9 (20.9)‡ | ||
Values are means (SD). TEE, total energy expenditure, REE, resting energy expenditure; NREE, nonresting energy expenditure; FFM, fat-free mass; Epi, epinephrine; Norepi, norepinephrine; SNS, sympathetic nervous system; PNS parasympathetic nervous system; Wtinitial, usual body weight; Wt−10%placebo, body weight after 10% weight loss in subjects receiving 5 wk of placebo treatment; Wt−10%T3, body weight after 10% weight loss in subjects receiving 5 wk of T3 treatment; Wt−10%leptin, body weight after 10% weight loss in subjects receiving 5 wk of leptin treatment.
P < 0.05 vs. Wtinitial;
P < 0.05 vs. Wt−10%placebo;
P < 0.05 vs. 0.
Skeletal Muscle
In vivo studies.
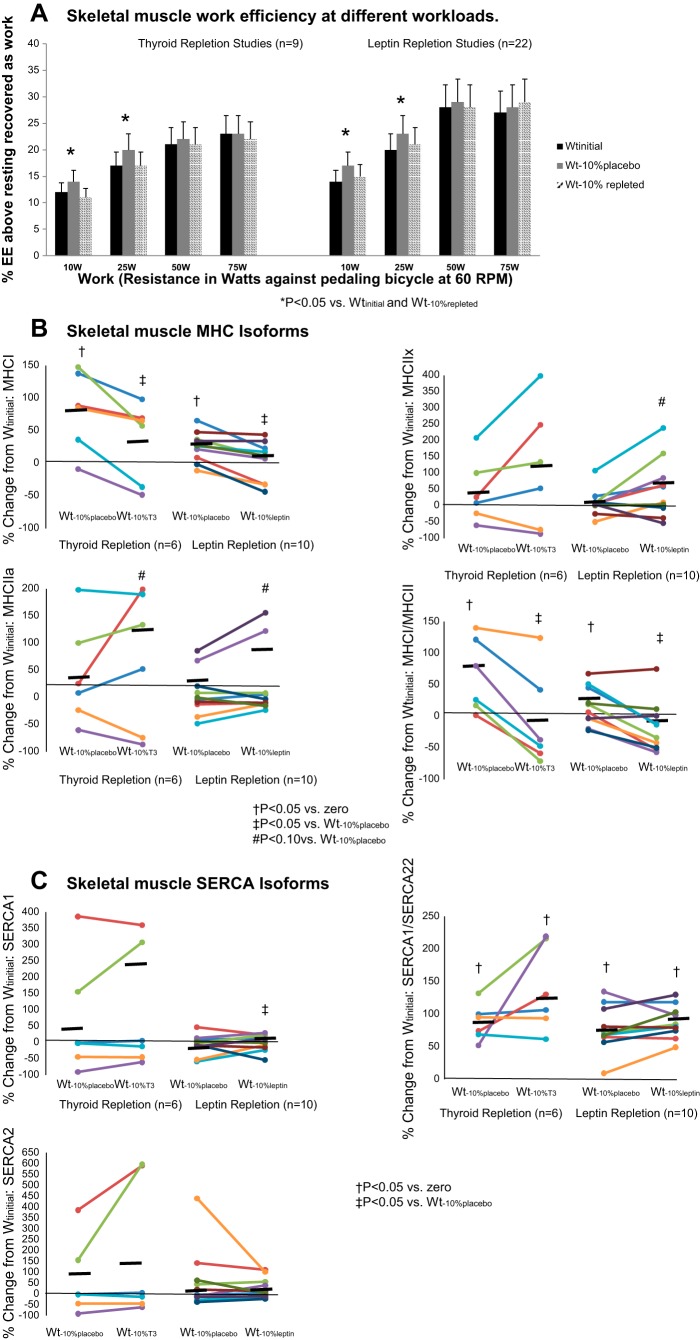
The effects of weight loss on bicycle ergometry measures were evident only at low levels of work, as reported in our earlier studies using similar methods (37). At Wt−10%placebo, EE above resting was significantly reduced during cycling to generate 10 W of power, and net mechanical efficiency was significantly increased during cycling to generate 10 or 25 W of power (Fig. 2A). NMR spectroscopy during low-level exercise (depressing a pedal once every 4 s against 41.4-kPa resistance) showed a nonsignificant decline in the Pi-to-PCr ratio (indicating increased muscle efficiency) from 0.18 (0.06) to 0.12 (0.0 7) in five of six subjects (P = 0.076) and a significant increase in the Pi-to-PCr ratio to 0.31 (0.17) at Wt−10%T3 compared with placebo.
Fig. 2.
A: skeletal muscle efficiency at 10-, 25-, 50-, and 75-W workloads. Values are means (SD). B and C: expression of different isoforms of myosin heavy chain (MHC) and sarco(endo)plasmic reticulum Ca2+-ATPase (SERCA) and ratios of MHC I to MHC II and SERCA1 to SERCA2, which reflect overall balance between more-efficient (MHC I and SERCA2) and less-efficient (MHC II and SERCA1) muscle gene expression. Horizontal black bars, means. Wtinitial, usual body weight; Wt−10%placebo, body weight after 10% weight loss in subjects receiving 5 wk of placebo treatment; Wt−10%T3, body weight after 10% weight loss in subjects receiving 5 wk of T3 treatment; Wt−10%leptin, body weight after 10% weight loss in subjects receiving 5 wk of leptin treatment; Wt−10%repleted, body weight after 10% weight loss in subjects receiving 5 wk of T3 or leptin treatment. *P < 0.05 vs. Wtinitial and body weight after 10% weight loss in T3- or leptin-repleted subjects (Wt−10%repleted); †P < 0.05 vs. 0; ‡P < 0.05 vs. Wt−10%placebo; #P < 0.10 vs. Wt−10%placebo.
In vitro studies.
At Wt−10%placebo, there were significant increases in MHC I expression and the ratio of MHC I to MCH II expression, with a nonsignificant trend toward decreases in expression of MHC IIa and SERCA1 (Fig. 2B, Table 3) and the ratio of SERCA1 to SERCA2 expression (Fig. 2C, Table 3). Administration of T3 or leptin resulted in “reversal” of all these changes, with significant increases in MHC IIa expression and the ratio of SERCA1 to SERCA2 and decreases in the ratio of MHC I to MHC II expression compared with Wt−10%placebo. There was a nonsignificant trend in both groups toward an increase in expression of MHC IIa and MHC IIx following T3 or leptin repletion and a significant increase in expression of SERCA1 following leptin, but not T3, repletion.
Table 3.
Effects of dietary weight loss and T3 repletion on mRNA expression in vastus lateralis muscle
| Wtinitial | Wt−10%placebo | Wt−10%T3 | |
|---|---|---|---|
| MHC I | 19.6 (9.9) | 26.4 (12.1)* | 20.9 (5.0)† |
| MHC IIa | 19.9 (16.4) | 22.4 (17.0) | 29.2 (13.5)*† |
| MHC IIx | 13.0 (10.6) | 13.9 (13.8) | 13.0 (8.9) |
| MHC I/MHC II | 0.60 (0.29) | 0.72 (0.34)* | 0.55 (0.37)† |
| SERCA1 | 26.9 (18.1) | 21.4 (0.21) | 28.8 (13.1) |
| SERCA2 | 14.5 (16.6) | 16.3 (10.5) | 13.1 (5.1) |
| SERCA1/SERCA2 | 1.86 (0.67) | 1.21 (0.60)* | 2.18 (0.89)† |
Values are means (SD), expressed in arbitrary units per milligram of vastus lateralis muscle, in 6 subjects (2 male, 4 female). MHC, myosin heavy chain; SERCA, sarco(endo)plasmic reticulum Ca2+-ATPase; Wtinitial, usual body weight; Wt−10%placebo, body weight after 10% weight loss in subjects receiving 5 wk of placebo treatment; Wt−10%T3, body weight after 10% weight loss in subjects receiving 5 wk of T3 treatment. Leptin repletion data are available elsewhere (4).
P < 0.05 vs. Wtinitial;
P < 0.05 vs. Wt-10%placebo.
Autonomic Nervous System
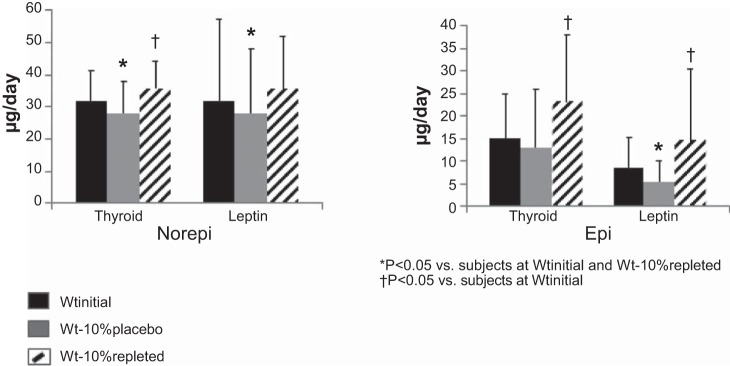
Significant declines in SNS tone measured by sequential pharmacological blockade at Wt−10%placebo were similarly “reversed” by T3 or leptin repletion. A significant increase in PNS tone at Wt−10%placebo was not significantly affected by T3 or leptin repletion (Table 2). Twenty-four-hour urinary norepinephrine excretion was significantly decreased following weight loss (Wt−10%placebo) in both repletion groups, while the reduction in 24-h urinary epinephrine excretion following weight loss reached significance only in the leptin-repleted group (Fig. 3). Both epinephrine and norepinephrine were significantly increased following T3 or leptin repletion compared with Wt−10%placebo. Epinephrine excretion following both T3 and leptin repletion was significantly increased above baseline, while norepinephrine excretion was significantly increased above baseline only in T3-repleted subjects.
Fig. 3.
Effects of thyroid hormone [triiodothyronine (T3), n = 9] and leptin (n = 10) repletion on 24-h urinary excretion of epinephrine (Epi) and norepinephrine (Norepi). Wtinitial, usual body weight; Wt−10%placebo, body weight after 10% weight loss in subjects receiving 5 wk of placebo treatment; Wt−10%T3, body weight after 10% weight loss in subjects receiving 5 wk of T3 treatment; Wt−10%leptin, body weight after 10% weight loss in subjects receiving 5 wk of leptin treatment. Values are means (SD). *P < 0.05 vs. Wtinitial and body weight after 10% weight loss in T3- or leptin-repleted subjects (Wt−10%repleted); †P < 0.05 vs. Wtinitial.
DISCUSSION
The aims of this study were to determine whether T3 repletion in subjects following weight loss would attenuate the declines in EE and increase in skeletal muscle work efficiency that occur following weight loss and whether the magnitude of these effects would be similar to those observed following leptin repletion (36). Skeletal muscle was the major focus of this investigation because of observations regarding the effects of weight loss on skeletal muscle contractile efficiency (14, 21, 34) and the remedial consequences of leptin repletion on this phenotype and on the associated reductions in circulating T3 concentrations and SNS activity (33). The major finding of this study is that T3 administration does “reverse” the effects of weight loss on EE, the SNS, and skeletal muscle chemomechanical efficiency and gene expression to a degree similar to that seen following exogenous leptin repletion.
Repletion of thyroid hormone in hypothyroid states increases the proportion of glycolytic, less chemomechanically efficient MHC II isoforms in skeletal muscle (5, 6) via response elements in the promoter regions of MHCI and SERCA2 (3, 38, 44). The low T3 levels in weight-reduced subjects may be permissive to the significant effects of T3 repletion on skeletal muscle after weight loss. The similarity of the effects of hypothyroidism and weight reduction on skeletal muscle contractile efficiency and gene expression suggests that decreased T3 may be a primary mediator of increased skeletal muscle contractile efficiency and adaptive thermogenesis following weight loss.
Leptin provides a primary signal regarding both long-term energy stores and acute negative energy balance to the hypothalamus (13). Leptin repletion attenuates the decline in circulating concentrations of bioactive hormones and SNS tone that would otherwise occur during fasting (20) and maintenance of a reduced weight (20, 36). The stimulatory effects of leptin on the HPT axis are likely mediated via direct stimulatory effects of leptin on the hypothalamic thyrotropin-releasing hormone promoter (27) and indirectly via leptin effects on proopiomelanocortin and agouti-related protein neurons in the arcuate nucleus (ARC) that project to the thyrotropin-releasing hormone-expressing paraventricular nucleus (PVN) neurons (13). Multiple hypothalamic leptin response regions, including the ARC, PVN, dorsomedial hypothalamus, and ventromedial hypothalamus, modulate activity in the rostral ventral lateral medulla and other brain areas controlling SNS activity (26).
Persistent adaptive thermogenesis and declines in SNS tone and circulating concentrations of bioactive thyroid hormones following dietary (34, 41) weight loss are well documented (24, 41). Baseline concentrations of thyroid hormones expressed as log(TSH) or as the ratio of T4 to TSH [a reciprocal measure of thyroid T4 production in response to TSH (12)] were positively and negatively correlated, respectively, with the loss of FM during caloric restriction, suggesting that individuals with higher baseline TSH levels and evidence of lower T4 production in response to TSH at baseline may be more prone to lose fat during weight loss.
Studies of higher doses of T3 (75 μg/day) given to adults at usual weight have resulted in an approximate doubling of circulating concentrations of T3 to mild hyperthyroid levels (higher than levels in the present study), partial T4 and complete TSH suppression, and increased TEE, REE (measured as sleeping metabolic rate), and exercise EE, similar to the effects observed in the present study (23). A meta-analysis of the effects of T3 administration to subjects during weight loss was inconclusive regarding promotion of weight loss (16). The potential effect of “reversing” the decline in T3 levels that occurs as a result of weight reduction in subjects in a state of energy balance after weight loss has not been examined previously.
Our data suggest that T3 repletion following weight loss exerts effects on EE similar to those observed following leptin repletion: in the aggregate, reversal of hypometabolism that characterizes the weight-reduced state. The leptin repletion data represent approximately twice as many subjects as previously reported and demonstrate a leptin-sensitive significant decline in REE that was not noted in the smaller population (33). While T3 repletion did result in a small, but significant, increase in resting pulse, there was no significant effect on blood pressure, implying that T3 repletion within the range reported here can be done safely.
The effects of leptin repletion on skeletal muscle are most likely mediated directly by the effects of leptin-stimulated increases in circulating concentrations of T3 on skeletal muscle gene expression (decreased MCH I and increased SERCA2) in the periphery following weight loss. However, it is also possible that leptin- and T3-mediated increases in SNS tone, as indicated by increased epinephrine and norepinephrine excretion following leptin or T3 repletion (Fig. 2), are also important mediators of skeletal muscle efficiency. The effects of T3 and leptin repletion on the ANS are likely to involve central effects of leptin on the hypothalamus via the ARC, PVN, dorsomedial hypothalamus, and ventromedial hypothalamus to stimulate an increase in SNS outflow, as discussed above (26). Thyroid hormone receptors affecting SNS outflow are more localized in the PVN (19). SNS signaling is impaired in hypothyroid states, suggesting that T3 repletion following weight loss may increase not only SNS tone but also signal transduction, especially in the periphery (1). The combination of direct effects of SNS inputs to decrease skeletal muscle work efficiency and augmentation of these effects via a T3-mediated increase in skeletal muscle sensitivity to adrenergic stimulation may be a part of the effects of leptin and T3 repletion following weight loss.
Both T3 and leptin repletion increased MHC II expression above baseline. Further study is needed to determine if the increased MHC II expression by T3 or leptin repletion is actually enhanced following weight loss. The effects of leptin administration in athyrotic individuals receiving fixed, as well as variable, doses of thyroid hormone would be informative as to the amount of hypometabolism following weight loss that is directly due to declines in thyroid hormones. The interplay between the HPT axis and SNS tone could be similarly explored in weight-reduced subjects receiving adrenergic antagonists.
The strengths of this study are the highly controlled environment and restriction of circulating concentrations of the molecules of interest to within a normal physiological range as measured by their nadir levels. The weaknesses of this study mainly relate to the dosing of T3 and leptin. Suppression of the T4-to-T3 ratio and further suppression of TSH by exogenous T3 have not been studied previously in subjects whose T4 and TSH levels were already low (in this case, as a result of weight loss). T3 was used to address the primary aim of this study. Dosing with T4 might be equally effective; however, high doses of T4 might be required, given that the “sick euthyroid hormone” increased conversion of T4 to reverse T3, which occurs in weight-reduced subjects. Also, circulating T3 concentrations do not reflect tissue (in this case, muscle) levels of T3, and changes in body weight have been shown to alter changes in muscle deiodinase activity that may further reduce local T3 levels following weight loss (9). In the case of leptin and T3 repletion, administration of these hormones as a single dose or twice a day does not mimic the normal circadian rhythms of these hormones and undoubtedly results in higher peak levels than would be seen under normal physiological conditions. This is a study of human physiology, not a “clinical trial.” These data do not constitute a recommendation that T3 should be routinely administered following weight loss, only that clinical trials using T3, T4, or some combination of T3 and T4 might be considered and studied as a means to ease some of the metabolic opposition to sustained weight loss.
In summary, this study indicates that repletion of T3 in weight-reduced subjects has effects on EE, skeletal muscle, and SNS similar to those observed following leptin repletion. Whether leptin-mediated increases in T3 following administration to weight-reduced subjects are themselves the sole cause of leptin-mediated increases in EE following weight loss or additive to other effects of leptin, such as increased SNS tone, environmental pollutants (10, 42), or dietary macronutrients (15, 40), requires further investigation, as does the potential role of T3 repletion as an adjunct to weight loss maintenance.
GRANTS
These studies were supported by National Institutes of Health Grants DK-64773, RR-00645, UL1 TR-000040, and P30-DK-26687.
DISCLOSURES
No conflicts of interest, financial or otherwise, are declared by the authors.
AUTHOR CONTRIBUTIONS
M.R., R.L.G., and R.L.L. conceived and designed research; M.R., R.L.G., F.H., K.M.B., R.S., and D.G. performed experiments; M.R. supervised the clinical care of all subjects; M.R., R.L.G., F.H., K.M.B., R.S., and D.G. analyzed data; M.R., R.L.G., R.S., and R.L.L. interpreted results of experiments; M.R. prepared figures; M.R. drafted manuscript; M.R., F.H., R.S., D.G., and R.L.L. edited and revised manuscript; M.R., R.L.G., F.H., K.M.B., R.S., D.G., and R.L.L. approved final version of manuscript.
ACKNOWLEDGMENTS
We gratefully acknowledge the invaluable assistance of our volunteers and the nursing and nutrition staffs of the Irving Center for Clinical Research at The New York Presbyterian Hospital, Columbia University College of Physicians and Surgeons. In particular, we acknowledge the invaluable contributions of our research coordinators, Ellen Murphy, Dr. Sanobar Parkar, Yomery Espinal, Elinor Naor, and Kalle Liimatta, as well as the contributions of Dr. Wahida Karmally (Director of the Bionutrition Core of Columbia University Clinical Research Resource) and Dr. Serge Cremers (Director of the Biomarkers Core Laboratory).
REFERENCES
- 1.Alkemade A. Central and peripheral effects of thyroid hormone signalling in the control of energy metabolism. J Neuroendocrinol 22: 56–63, 2010. doi: 10.1111/j.1365-2826.2009.01932.x. [DOI] [PubMed] [Google Scholar]
- 2.World Medical Association: American Physiological Society Guiding principles for research involving animals and human beings. Am J Physiol Regul Integr Comp Physiol 283: R281–R283, 2002. doi: 10.1152/ajpregu.00279.2002. [DOI] [PubMed] [Google Scholar]
- 3.Arruda AP, Oliveira GM, Carvalho DP, De Meis L. Thyroid hormones differentially regulate the distribution of rabbit skeletal muscle Ca2+-ATPase (SERCA) isoforms in light and heavy sarcoplasmic reticulum. Mol Membr Biol 22: 529–537, 2005. doi: 10.1080/09687860500412257. [DOI] [PubMed] [Google Scholar]
- 4.Baldwin KM, Joanisse DR, Haddad F, Goldsmith RL, Gallagher D, Pavlovich KH, Shamoon EL, Leibel RL, Rosenbaum M. Effects of weight loss and leptin on skeletal muscle in human subjects. Am J Physiol Regul Integr Comp Physiol 301: R1259–R1266, 2011. doi: 10.1152/ajpregu.00397.2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Caiozzo VJ, Baker MJ, Baldwin KM. Novel transitions in MHC isoforms: separate and combined effects of thyroid hormone and mechanical unloading. J Appl Physiol (1985) 85: 2237–2248, 1998. doi: 10.1152/jappl.1998.85.6.2237. [DOI] [PubMed] [Google Scholar]
- 6.Canepari M, Cappelli V, Pellegrino MA, Zanardi MC, Reggiani C. Thyroid hormone regulation of MHC isoform composition and myofibrillar ATPase activity in rat skeletal muscles. Arch Physiol Biochem 106: 308–315, 1998. doi: 10.1076/apab.106.4.308.4373. [DOI] [PubMed] [Google Scholar]
- 7.Causon RC, Carruthers ME. Measurement of catecholamines in biological fluids by high-performance liquid chromatography: a comparison of fluorimetric with electrochemical detection. J Chromatogr A 229: 301–309, 1982. doi: 10.1016/S0378-4347(00)84272-3. [DOI] [PubMed] [Google Scholar]
- 8.Celi FS, Zemskova M, Linderman JD, Babar NI, Skarulis MC, Csako G, Wesley R, Costello R, Penzak SR, Pucino F. The pharmacodynamic equivalence of levothyroxine and liothyronine: a randomized, double blind, cross-over study in thyroidectomized patients. Clin Endocrinol (Oxf) 72: 709–715, 2010. doi: 10.1111/j.1365-2265.2009.03700.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.De Andrade PB, Neff LA, Strosova MK, Arsenijevic D, Patthey-Vuadens O, Scapozza L, Montani JP, Ruegg UT, Dulloo AG, Dorchies OM. Caloric restriction induces energy-sparing alterations in skeletal muscle contraction, fiber composition and local thyroid hormone metabolism that persist during catch-up fat upon refeeding. Front Physiol 6: 254, 2015. doi: 10.3389/fphys.2015.00254. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Elobeid MA, Allison DB. Putative environmental-endocrine disruptors and obesity: a review. Curr Opin Endocrinol Diabetes Obes 15: 403–408, 2008. doi: 10.1097/MED.0b013e32830ce95c. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Faber J, Selmer C. Cardiovascular disease and thyroid function. Front Horm Res 43: 45–56, 2014. doi: 10.1159/000360558. [DOI] [PubMed] [Google Scholar]
- 12.Fitzgerald S, Bean N. The relationship between population T4/TSH set point data and T4/TSH physiology. J Thyroid Res 2016: 6351473, 2016. doi: 10.1155/2016/6351473. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Ghamari-Langroudi M, Vella KR, Srisai D, Sugrue ML, Hollenberg AN, Cone RD. Regulation of thyrotropin-releasing hormone-expressing neurons in paraventricular nucleus of the hypothalamus by signals of adiposity. Mol Endocrinol 24: 2366–2381, 2010. doi: 10.1210/me.2010-0203. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Goldsmith R, Joanisse DR, Gallagher D, Pavlovich K, Shamoon E, Leibel RL, Rosenbaum M. Effects of experimental weight perturbation on skeletal muscle work efficiency, fuel utilization, and biochemistry in human subjects. Am J Physiol Regul Integr Comp Physiol 298: R79–R88, 2010. doi: 10.1152/ajpregu.00053.2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Hall KD, Guo J. Obesity energetics: body weight regulation and effects of diet composition. Gastroenterology 152: 1718–1727.e3, 2017. doi: 10.1053/j.gastro.2017.01.052. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Kaptein EM, Beale E, Chan LS. Thyroid hormone therapy for obesity and nonthyroidal illnesses: a systematic review. J Clin Endocrinol Metab 94: 3663–3675, 2009. doi: 10.1210/jc.2009-0899. [DOI] [PubMed] [Google Scholar]
- 18.Kissileff H, Thornton M, Torres M, Pavlovich K, Leibel R, Rosenbaum M. Leptin reverses declines in satiation in weight-reduced obese humans. Am J Clin Nutr 95: 309–317, 2012. doi: 10.3945/ajcn.111.012385. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Lechan RM, Fekete C. The TRH neuron: a hypothalamic integrator of energy metabolism. Prog Brain Res 153: 209–235, 2006. doi: 10.1016/S0079-6123(06)53012-2. [DOI] [PubMed] [Google Scholar]
- 20.Légrádi G, Emerson CH, Ahima RS, Flier JS, Lechan RM. Leptin prevents fasting-induced suppression of prothyrotropin-releasing hormone messenger ribonucleic acid in neurons of the hypothalamic paraventricular nucleus. Endocrinology 138: 2569–2576, 1997. doi: 10.1210/endo.138.6.5209. [DOI] [PubMed] [Google Scholar]
- 21.Leibel R, Rosenbaum M. Metabolic response to weight perturbation. In: Novel Insights into Adipose Cell Functions. Research and Perspectives in Endocrine Interactions, edited by Clément K. Heidelberg: Springer-Verlag, 2010, p. 121–133. doi: 10.1007/978-3-642-13517-0_12. [DOI] [Google Scholar]
- 22.Leibel RL, Rosenbaum M, Hirsch J. Changes in energy expenditure resulting from altered body weight. N Engl J Med 332: 621–628, 1995. doi: 10.1056/NEJM199503093321001. [DOI] [PubMed] [Google Scholar]
- 23.Lovejoy JC, Smith SR, Bray GA, DeLany JP, Rood JC, Gouvier D, Windhauser M, Ryan DH, Macchiavelli R, Tulley R. A paradigm of experimentally induced mild hyperthyroidism: effects on nitrogen balance, body composition, and energy expenditure in healthy young men. J Clin Endocrinol Metab 82: 765–770, 1997. [DOI] [PubMed] [Google Scholar]
- 24.Müller MJ, Enderle J, Pourhassan M, Braun W, Eggeling B, Lagerpusch M, Glüer CC, Kehayias JJ, Kiosz D, Bosy-Westphal A. Metabolic adaptation to caloric restriction and subsequent refeeding: the Minnesota Starvation Experiment revisited. Am J Clin Nutr 102: 807–819, 2015. doi: 10.3945/ajcn.115.109173. [DOI] [PubMed] [Google Scholar]
- 25.Najjar M, Rowland M. Anthropometric reference data and prevalence of overweight. Vital Health Stat 11. Oct: 1–73, 1987. [PubMed] [Google Scholar]
- 26.Pandit R, Beerens S, Adan R. The role of leptin in energy expenditure. The hypothalamic perspective. Am J Regul Integr Comp Physiol 312: R938–R947, 2017. doi: 10.1152/ajpregu.00045.2016. [DOI] [PubMed] [Google Scholar]
- 27.Perello M, Cakir I, Cyr NE, Romero A, Stuart RC, Chiappini F, Hollenberg AN, Nillni EA. Maintenance of the thyroid axis during diet-induced obesity in rodents is controlled at the central level. Am J Physiol Endocrinol Metab 299: E976–E989, 2010. doi: 10.1152/ajpendo.00448.2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Phelan S, Wing RR. Prevalence of successful weight loss. Arch Intern Med 165: 2430, 2005. doi: 10.1001/archinte.165.20.2430-a. [DOI] [PubMed] [Google Scholar]
- 29.Pietrobelli A, Allison DB, Heshka S, Heo M, Wang ZM, Bertkau A, Laferrère B, Rosenbaum M, Aloia JF, Pi-Sunyer FX, Heymsfield SB. Sexual dimorphism in the energy content of weight change. Int J Obes Relat Metab Disord 26: 1339–1348, 2002. doi: 10.1038/sj.ijo.0802065. [DOI] [PubMed] [Google Scholar]
- 30.Pietrobelli A, Formica C, Wang Z, Heymsfield SB. Dual-energy X-ray absorptiometry body composition model: review of physical concepts. Am J Physiol Endocrinol Metab 271: E941–E951, 1996. [DOI] [PubMed] [Google Scholar]
- 31.Ravussin E, Lillioja S, Anderson TE, Christin L, Bogardus C. Determinants of 24-hour energy expenditure in man. Methods and results using a respiratory chamber. J Clin Invest 78: 1568–1578, 1986. doi: 10.1172/JCI112749. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Rodríguez-Rodríguez E, Aparicio A, Bermejo LM, López-Sobaler AM, Ortega RM. Changes in the sensation of hunger and well-being before and after meals in overweight/obese women following two types of hypoenergetic diet. Public Health Nutr 12: 44–50, 2009. doi: 10.1017/S1368980008001912. [DOI] [PubMed] [Google Scholar]
- 33.Rosenbaum M, Goldsmith R, Bloomfield D, Magnano A, Weimer L, Heymsfield S, Gallagher D, Mayer L, Murphy E, Leibel RL. Low-dose leptin reverses skeletal muscle, autonomic, and neuroendocrine adaptations to maintenance of reduced weight. J Clin Invest 115: 3579–3586, 2005. doi: 10.1172/JCI25977. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Rosenbaum M, Hirsch J, Murphy E, Leibel RL. Effects of changes in body weight on carbohydrate metabolism, catecholamine excretion, and thyroid function. Am J Clin Nutr 71: 1421–1432, 2000. doi: 10.1093/ajcn/71.6.1421. [DOI] [PubMed] [Google Scholar]
- 35.Rosenbaum M, Leibel RL. 20 years of leptin: role of leptin in energy homeostasis in humans. J Endocrinol 223: T83–T96, 2014. doi: 10.1530/JOE-14-0358. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Rosenbaum M, Sy M, Pavlovich K, Leibel RL, Hirsch J. Leptin reverses weight loss-induced changes in regional neural activity responses to visual food stimuli. J Clin Invest 118: 2583–2591, 2008. doi: 10.1172/JCI35055. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Rosenbaum M, Vandenborne K, Goldsmith R, Simoneau JA, Heymsfield S, Joanisse DR, Hirsch J, Murphy E, Matthews D, Segal KR, Leibel RL. Effects of experimental weight perturbation on skeletal muscle work efficiency in human subjects. Am J Physiol Regul Integr Comp Physiol 285: R183–R192, 2003. doi: 10.1152/ajpregu.00474.2002. [DOI] [PubMed] [Google Scholar]
- 38.Simonides WS, Thelen MH, van der Linden CG, Muller A, van Hardeveld C. Mechanism of thyroid-hormone regulated expression of the SERCA genes in skeletal muscle: implications for thermogenesis. Biosci Rep 21: 139–154, 2001. doi: 10.1023/A:1013692023449. [DOI] [PubMed] [Google Scholar]
- 39.Statsoft, Inc Statistica Release 5 for the Windows Operating System. Tulsa, OK: Statsoft, 1997. [Google Scholar]
- 40.Stiegler P, Cunliffe A. The role of diet and exercise for the maintenance of fat-free mass and resting metabolic rate during weight loss. Sports Med 36: 239–262, 2006. doi: 10.2165/00007256-200636030-00005. [DOI] [PubMed] [Google Scholar]
- 41.Sumithran P, Prendergast LA, Delbridge E, Purcell K, Shulkes A, Kriketos A, Proietto J. Long-term persistence of hormonal adaptations to weight loss. N Engl J Med 365: 1597–1604, 2011. doi: 10.1056/NEJMoa1105816. [DOI] [PubMed] [Google Scholar]
- 42.Tremblay A, Pelletier C, Doucet E, Imbeault P. Thermogenesis and weight loss in obese individuals: a primary association with organochlorine pollution. Int J Obes Relat Metab Disord 28: 936–939, 2004. doi: 10.1038/sj.ijo.0802527. [DOI] [PubMed] [Google Scholar]
- 43.Weir JB. New methods for calculating metabolic rate with special reference to protein metabolism. 1949. Nutrition 6: 213–221, 1990. [PubMed] [Google Scholar]
- 44.Yu F, Göthe S, Wikström L, Forrest D, Vennström B, Larsson L. Effects of thyroid hormone receptor gene disruption on myosin isoform expression in mouse skeletal muscles. Am J Physiol Regul Integr Comp Physiol 278: R1545–R1554, 2000. doi: 10.1152/ajpregu.2000.278.6.R1545. [DOI] [PubMed] [Google Scholar]