Abstract
The pyruvate dehydrogenase complex (PDC) converts pyruvate to acetyl-CoA and is an important control point for carbohydrate (CHO) oxidation. However, the importance of the PDC and CHO oxidation to muscle metabolism and exercise performance, particularly during prolonged or high-intensity exercise, has not been fully defined especially in mature skeletal muscle. To this end, we determined whether skeletal muscle-specific loss of pyruvate dehydrogenase alpha 1 (Pdha1), which is a critical subunit of the PDC, impacts resting energy metabolism, exercise performance, or metabolic adaptation to high-fat diet (HFD) feeding. For this, we generated a tamoxifen (TMX)-inducible Pdha1 knockout (PDHmKO) mouse, in which PDC activity is temporally and specifically ablated in adult skeletal muscle. We assessed energy expenditure, ex vivo muscle contractile performance, and endurance exercise capacity in PDHmKO mice and wild-type (WT) littermates. Additionally, we studied glucose homeostasis and insulin sensitivity in muscle after 12 wk of HFD feeding. TMX administration largely ablated PDHα in skeletal muscle of adult PDHmKO mice but did not impact energy expenditure, muscle contractile function, or low-intensity exercise performance. Additionally, there were no differences in muscle insulin sensitivity or body composition in PDHmKO mice fed a control or HFD, as compared with WT mice. However, exercise capacity during high-intensity exercise was severely impaired in PDHmKO mice, in parallel with a large increase in plasma lactate concentration. In conclusion, although skeletal muscle PDC is not a major contributor to resting energy expenditure or long-duration, low-intensity exercise performance, it is necessary for optimal performance during high-intensity exercise.
Keywords: contraction, exercise training, glucose metabolism, high-fat diet, mitochondria
INTRODUCTION
Glucose catabolism is a fundamental contributor to ATP production in predominantly glycolytic organs such as the brain and erythrocytes, as well as tissues with high substrate flexibility, such as heart and skeletal muscle (1, 17, 20, 26, 35). Broadly, ATP production via oxidative glucose metabolism comprises glycolytic conversion of glucose to pyruvate in the cytosol, followed by ATP generation via the mitochondrial tricarboxylic acid cycle and electron transport chain (12). In fact, the ability of skeletal muscle to maintain force production during exercise (i.e., performance), particularly with increasing intensity, is closely tied to its ability to metabolize glucose (22, 27, 55). However, although the necessity of glucose for exercise performance has long been appreciated, the precise contribution of carbohydrate (CHO) oxidation to skeletal muscle metabolism and exercise performance has not been fully defined.
The pyruvate dehydrogenase complex (PDC) is a central control point for CHO oxidation and the tricarboxylic acid cycle, as it mediates the oxidative decarboxylation of cytosolic pyruvate to mitochondrial acetyl-CoA (46). The PDC is a multiprotein complex consisting of several copies of the three main catalytic subunits, pyruvate dehydrogenase (PDH), dihydrolipoamide acetyltransferase (DLAT), and dihydrolipoamide dehydrogenase (DLD) (3, 18). PDC is primarily localized to the inner mitochondrial membrane, with the PDH enzyme being a heterotetramer consisting of two α- and two β-subunits encoded by Pdha1 and Pdhb1, respectively (30). The activity of the PDC, and rate of glucose oxidation, is regulated via a balance of inhibitory phosphorylations on the PDHα subunit by PDH kinases (PDK; mainly PDK2 and PDK4 in skeletal muscle) and dephosphorylation by pyruvate dehydrogenase phosphatases (PDP1 and PDP2) (9, 46). Obesity and diabetes increase protein abundance and activity of PDK2 and PDK4 in skeletal muscle, thereby inhibiting PDC activity (23, 47, 72), which has been linked to impaired glucose tolerance and skeletal muscle insulin resistance (9, 74). Contrariwise, exercise increases activity of PDP1 and reduces PDK4 transcription (9, 74). This leads to a rapid increase in PDC activity in muscle during an acute exercise bout as well as long-term adaptation in response to exercise training, resulting in enhanced PDC expression and activity in skeletal muscle (21, 34, 51, 61, 68, 69). Moreover, although it is clear that CHO oxidation is important for exercise performance, as evidenced by the increased reliance on glucose oxidation with increasing exercise intensity (37, 54, 55), the contribution of the PDC in skeletal muscle to maximal exercise capacity and exercise adaptation has not been fully elucidated.
A major limitation in the field has been the lack of an appropriate mouse model to study PDC biology in skeletal muscle. Mice with whole-body deletion of Pdha1 (i.e., systemic loss of PDC activity) die in utero, which emphasizes the developmental importance of the PDC (30). Additionally, mice with a skeletal muscle- and heart-specific knockout (KO) of Pdha1 die within 7 days of weaning onto a normal rodent chow diet (59). The premature lethality in this model was attributed to pathological cardiac remodeling (59), and as a result, further studies of the contribution of PDC activity to resting metabolism and exercise capacity, in vivo, was prevented. To this end, we generated a mouse model with inducible, skeletal muscle-specific KO of Pdha1 (PDHmKO). The advantage of this model is that it affords temporal deletion of PDHα specifically in adult skeletal muscle. We hypothesized that loss of PDC activity would impair exercise capacity and that this impairment would be more pronounced during high-intensity exercise. Additionally, because reduced PDC activity has been associated with skeletal muscle insulin resistance, we hypothesized that loss of PDC activity in PDHmKO mice would exacerbate skeletal muscle insulin resistance in response to high-fat diet (HFD) feeding.
MATERIALS AND METHODS
Animals and diets.
PDHmKO mice were generated by breeding mice harboring LoxP sites flanking exon 8 of the Pdha1 gene (30) together with mice carrying a tamoxifen (TMX)-inducible Cre recombinase expressed under the inducible human α-skeletal actin promoter (iHSA-Cre) (39). Mice were kept in a conventional facility with a 12-h light/12-h dark cycle and had free access to food and water unless otherwise noted. Pdha1 is an X-linked gene, therefore, all male mice used in this study had one floxed (FL) Pdha1 allele (Pdha1Ex8FL/Y) whereas female mice had two floxed alleles (Pdha1Ex8FL/FL). Studies were conducted on male or female PDHmKO mice on a C57BL/6J background, with floxed but iHSA-Cre-negative littermates used as experimental controls [referred to as wild-type (WT)]. TMX (2 mg) was administered via oral gavage to all mice for 5 consecutive days, starting at 4–5 wk of age. Experimental interventions were conducted a minimum of 4 wk after TMX administration. For diet studies, 2 wk after TMX administration mice were switched to an HFD (20 kcal% protein, 20 kcal% CHOs, 60 kcal% fat) (cat. no. D-12492, Research Diets, Inc., New Brunswick, NJ) or remained on a normal chow diet (25 kcal% protein, 58 kcal% CHOs, 17 kcal% fat) (CHOW; cat. no. 7912 irradiated, Envigo Teklad, East Millstone, NJ) for 12 wk. Diet and exercise studies were done in separate cohorts of mice. Tissues were excised from fasted (4 h) and anesthetized animals, snap-frozen in liquid nitrogen, and stored at −80°C for subsequent analysis. All experiments were approved by and conducted in accordance with the Animal Care Program at the University of California, San Diego.
Energy expenditure and body composition.
Body composition was analyzed by magnetic resonance imaging using an EchoMRI-100 analyzer (EchoMRI Medical Systems, Houston, TX). Whole-body energy expenditure was assessed through indirect calorimetry, using the Comprehensive Lab Animals Monitoring System (Columbus Instruments, Columbus, OH). Whole-body oxygen consumption (V̇o2), carbon dioxide production (V̇co2), respiratory exchange ratio (RER; V̇co2/V̇o2), activity counts (number of beam breaks), and food intake were continuously monitored for 3 consecutive days in the Comprehensive Lab Animals Monitoring System. Day 1 was considered acclimatization, with dark and light phase values being the average of days 2 and 3. All tests were performed at room temperature (25°C, standard housing temperature).
Ex vivo 2-deoxy glucose uptake.
2-deoxy glucose uptake (2DOGU) assay was performed as previously described (71). An insulin concentration of 0.36 nM (60 μU/ml) (Humulin R; Eli Lilly, Indianapolis, IN) was used for all experiments. Frozen soleus (SOL) and extensor digitorum longus (EDL) muscles were weighed, homogenized, and 2DOGU was calculated as previously described (57).
Oral glucose tolerance test.
After a 4-h fast, mice were orally gavaged with 2 g of dextrose per kg body wt, and blood glucose was measured in tail vein blood at 0 (before gavage), 20, 30, 45, 60, 90, and 120 min using a standard handheld glucose meter. Area under the glucose curve was calculated using Prism 6 (GraphPad Software Incorporated, La Jolla, CA) and was calculated from baseline (i.e., time “0” min).
Run to exhaustion treadmill testing.
Animals were acclimatized to running on an open treadmill (Columbus Instruments, Columbus, OH) for 10 min, 10 m/min, 10° incline, on 2 consecutive days before the start of the run to exhaustion (RTE) tests. For the low intensity “endurance” RTE trial, mice started the run at 10 m/min for 10 min, and the speed was increased in 2-m/min increments every 10 min until exhaustion. For the high-intensity “sprint” RTE trial, mice started the run at 6 m/min for 3 min, and the speed was increased in 1-m/min increments every minute until exhaustion. Mice were motivated to run using bristled brushes at the back of the treadmill and were considered exhausted when they could no longer run in response to this stimulus. The experimenter was blinded to the genotype of the mice during the run tests. In a separate cohort of mice, blood lactate was measured in tail vein blood before the start of the run, at 20 m/min, 26 m/min, and at exhaustion, using a handheld lactate meter (Nova Biomedical, Waltham, MA).
Voluntary wheel running exercise training.
Exercise-trained (EXT) mice were individually housed and had free access to food, water, and a running wheel for 21 days, whereas sedentary (SED) control groups were kept in a cage without a running wheel. Average speed, distance run, and time spent running were digitally recorded with data collected daily at 12:00 PM. After 21 days, voluntary wheel running (VWR) mice were moved to a cage without a running wheel, and tissues were dissected 24 h later, as described above.
Ex vivo muscle mechanics.
Muscle function was assessed in the fifth toe muscle of the EDL, as previously described (33, 48). Briefly, muscle sarcomere length was normalized using laser diffraction (73). Individual supramaximal stimulation conditions were established by single 0.5-ms twitch pulses, gradually increasing current until force plateaued, and 50% more than this level was used for experimental testing. Muscles were electrically stimulated (model no. S-88; Astro-Med, West Warwick, RI) via parallel platinum electrodes (300-ms train duration and 0.5-ms pulse duration) to assess twitch characteristics (twitch tension and time-to-peak-tension). After a 10-min rest, muscles were stimulated at increasing frequencies (1–120 Hz) with 120-s intervals between contractions to determine the force-frequency relationship. From this, fusion frequency was calculated as the lowest frequency where oscillations in force are no longer evident. For all analyses, force was normalized to the muscle physiologic cross-sectional area (6).
Single myofiber force production.
Single myofiber force production was assessed in myofibers from the tibialis anterior (TA) muscle, as previously described (56).
PDH activity.
PDH activity was assessed in 25 mg of snap-frozen gastrocnemius (GA) using a commercially available PDH activity assay kit, according to the manufacturer’s instructions (cat. no. MAK-183, MilliporeSigma, Burlington, MA). PDH activity was normalized to protein content.
Immunoblotting.
Equal amounts of proteins (30 µg) were separated by SDS-PAGE on XT Criterion Precast gels (Bio-Rad Laboratories, Hercules, CA) under reducing conditions and transferred to Amersham Protran nitrocellulose membranes (MilliporeSigma). Proteins on nitrocellulose membranes were reversibly stained with a Ponceau S solution [0.1% (wt/vol) Ponceau S in 5% acetic acid]. The following antibodies were used: PDHα (cat. no. 3205, Cell Signaling), eukaryotic translation elongation factor 2 (cat. no. 2332, Cell signaling), ATP synthase subunit alpha, ubiquinol-cytochrome C reductase core protein 2, mitochondrially encoded cytochrome C oxidase I, succinate dehydrogenase subunit B, NADH/ubiquinone oxidoreductase subunit B8 (cat. no. MS-604, MitoSciences), acyl-Coenzyme A dehydrogenase, very long-chain; cat. no. ab-155138, Abcam), acyl-Coenzyme A dehydrogenase, long-chain (cat. no. ab-82853, Abcam), hexokinase 2 (HK2; cat. no. 2857, Cell Signaling), lactate dehydrogenase A (cat. no. ABN-896, MilliporeSigma), Akt (cat. no. 2920, Cell Signaling), pAkt S473 (cat. no. 4058, Cell Signaling), GSK3α/β (5676, Cell signaling), pGSK3α/β S21/S9 (9331, Cell signaling), PDK4 (cat. no. NBP1-07047SS, Novus Biologicals), phosphofructokinase 1 (cat. no. sc-377346, Santa Cruz Biotechnology). Densitometric analysis of immunoblots was performed on five to nine individual samples using Image Lab Software (Bio-Rad Laboratories, Hercules, CA, USA), and a representative selection is presented in each figure.
RNA extraction and RT-PCR.
RNA extraction and RT-PCR were conducted in the GA, as previously described (65). Relative expression levels for each gene of interest were calculated with the ΔΔCt method, using either 36b4 or Ppib as normalization control. Primer sequences used in this study can be found in Table 1.
Table 1.
Quantitative PCR primer sequences
| Primer | Forward | Reverse |
|---|---|---|
| Pdha1-Ex8 | TGGATATCTTGTGCGTCCGAG | CCATGGTAGCGGTAAGTCTGG |
| Pdhb | AGTTGCCCAGTATGAGGTG | TCTGAGATGGGGGTGTCGAT |
| Pdhx | TGCCAGAGGAGTACCAAGGA | GAATCTCCCAACGGCCAAGA |
| Dlat | AGTTCCCGAAGCAAACTCGT | AAGTCCTGCAGGGGTACTGA |
| Dld | TGGGGCAGGAGTAATTGGTG | TCCACCCACATGACCCAAAA |
| Pdk2 | CGGGCGCTGTTGAAGAATGC | GCATTGCTGGATCCGAAGTCT |
| Pdk4 | GAAGCTGATGACTGGTGTATCC | GACCCACTTTGATCCCGTAAA |
| Pdp1 | GTGTTGACCTCCATGTGGCTA | GAGAGTGTGACTGCTGACCAA |
| Cd36 | GCCCAATGGAGCCATCTTTG | GCTGCTACAGCCAGATTCAGA |
| Slc25a20 (Cact) | CATGTGCCTGGTGTTTGTGG | GGTGGCTGTCCAGACAAACT |
| Acadvl | CTTTGCAGGGACTCAAGGAA | CAAGCGAGCATACTGGGTATTA |
| 36b4 | CCACGAAAATCTCCAGAGGCA | GAGAAGGGGGAGATGTTCAGC |
| Ppib | AGCGCAATATGAAGGTGCTCT | CTTATCGTTGGCCACGGAGG |
Statistics.
Data were analyzed using an unpaired Student’s t-test, two-way or three-way ANOVA (using repeated measurement where appropriate), followed by a Tukey’s post hoc test, with significant differences at P < 0.05. Statistical analyses were performed using Prism 6 (GraphPad Software Incorporated). All data are expressed as mean ± SE.
RESULTS
Validation of Pdha1 muscle KO mouse.
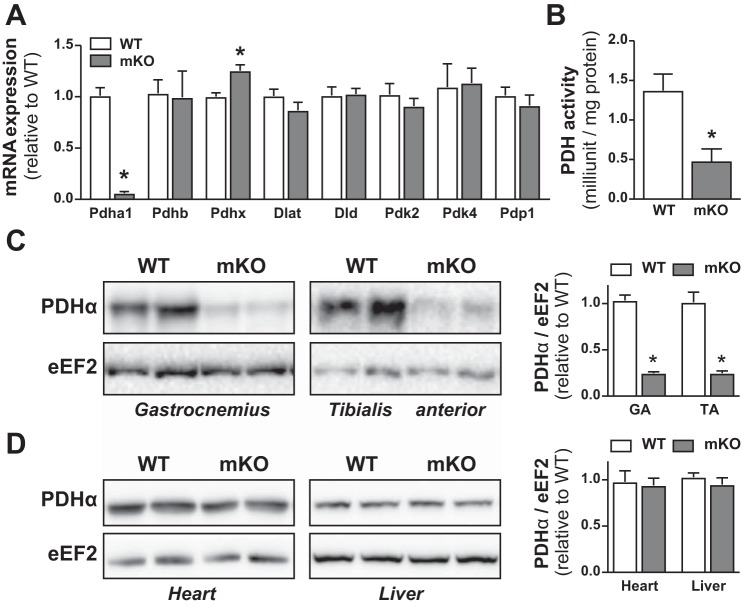
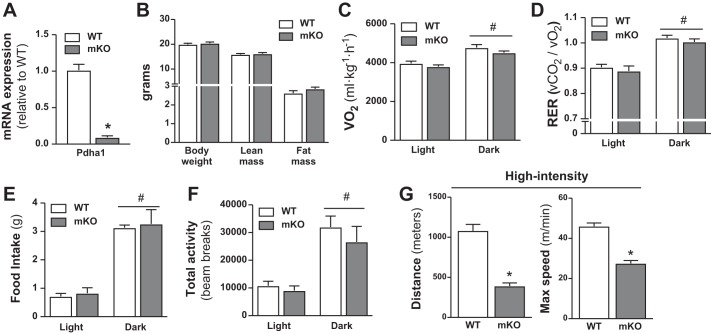
Pdha1 mRNA in gastrocnemius (GA) muscle was reduced ~94% in PDHmKO mice compared with WT littermates (Fig. 1A). PDHα protein abundance was reduced ~75% in GA and tibialis anterior (TA) of PDHmKO versus WT mice (Fig. 1C). Accompanying this, total PDH activity was reduced in GA from PDHmKO versus WT mice (Fig. 1B). There were no differences in PDHα protein abundance in heart or liver of PDHmKO versus WT mice (Fig. 1D). Apart from a modest increase in Pdhx mRNA abundance in GA of PDHmKO mice (Fig. 1A), there were no genotype differences in the expression of Pdhb, other PDC subunits (Dlat, Dld), or regulators of PDC activity (Pdk2, Pdk4, Pdp1) (Fig. 1A). PDHα was significantly reduced (~75%) but was not absent in whole-muscle lysates from PDHmKO mice (Fig. 1C). As we (70), and others (19, 25), have previously demonstrated using Cre-LoxP approaches in skeletal muscle, a lack of complete KO of the protein target in whole muscle is due to the presence of several different cell types (e.g., connective tissue fibroblasts, adipocytes, endothelial cells, immune cells) that also express PDHα (53, 75). Overall, these data demonstrate that we successfully generated a mouse model where Pdha1 is significantly reduced, specifically in skeletal muscle.
Fig. 1.
Knockout of Pdha1 in PDHmKO mice. Transcript levels in gastrocnemius (GA) muscle from PDHmKO and WT mice, normalized to 36b4 (n = 5/5) (A). PDH activity in GA muscle of PDHmKO and WT mice (B). Representative blots of PDHα protein abundance in GA and tibialis anterior (TA) muscle (C), heart and liver (D) of PDHmKO and WT mice. Bar graphs represent quantification of PDHα protein abundance (n = 6/7) in PDHmKO and WT mice, normalized to eEF2. Data reported as means ± SE. Student’s t-test, *P < 0.05 compared with WT. eEF2, eukaryotic translation elongation factor 2; PDHmKO, tamoxifen-inducible Pdha1 knockout mice; WT, wild-type.
Whole-body metabolism is not affected in PDHmKO mice.
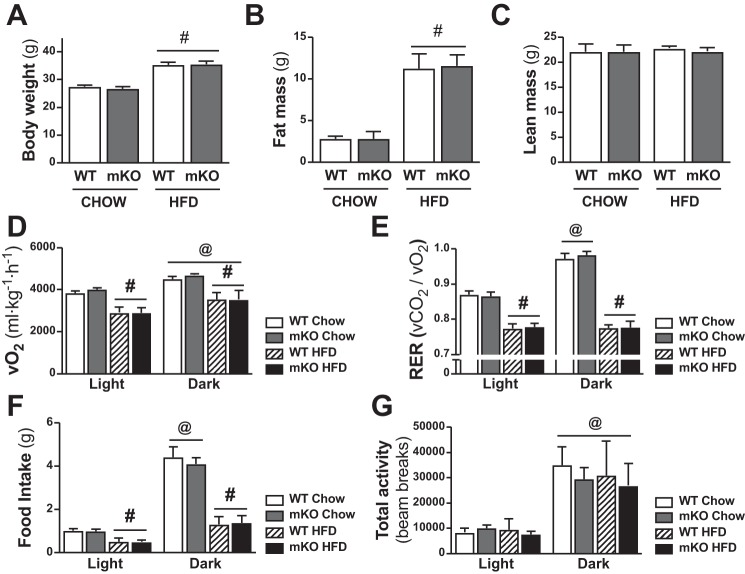
PDHmKO and WT mice were studied after 12 wk of ad libitum feeding of either CHOW or a HFD. Body weight (Fig. 2A), whole-body fat mass (Fig. 2B), and epididymal fat pad weight (Table 2) were higher in HFD versus chow-fed mice, but there was no diet effect on lean mass (Fig. 2C) or skeletal muscle, liver, or heart weight (Table 2). Moreover, there were no genotype differences in any of these parameters in chow- or HFD-fed cohorts (Fig. 2, A–C, Table 2). Whole-body V̇o2, RER, and food intake (Fig. 2, D–F) were reduced in HFD-fed PDHmKO and WT mice compared with chow-fed littermates. However, there were no genotype differences in diurnal rhythms of V̇o2 (Fig. 2D), RER (Fig. 2E), food intake (Fig. 2F), or total activity (Fig. 2G) in either chow- or HFD-fed groups.
Fig. 2.
Comparable whole-body energy expenditure in PDHmKO compared with WT mice. WT and PDHmKO mice were fed either a chow or a high-fat diet (HFD) for 12 wk. Body weight (A), fat mass (B), and lean mass (C) in chow- or HFD-fed WT and PDHmKO mice (Chow, n = 8/8; HFD, n = 6/8). Whole-body oxygen consumption (V̇o2) (D), respiratory exchange ratio (RER; V̇co2/V̇o2) (E), food intake (F), and total activity counts (beam breaks) (G) during light and dark phases recorded over 48 h in chow- or HFD-fed WT and PDHmKO mice (Chow, n = 6/6; HFD, n = 6/6). Data reported as means ± SE two-way ANOVA, #P < 0.05, main effect of diet (A–C); three-way ANOVA, #P < 0.05, main effect of diet (D–G); @P < 0.05 main effect of time. PDHmKO, tamoxifen-inducible Pdha1 knockout mice; V̇co2, carbon dioxide production; WT, wild-type.
Table 2.
Tissue weights of chow- and HFD-fed PDHmKO and WT mice
| CHOW WT | CHOW PDHmKO | HFD WT | HFD PDHmKO | |
|---|---|---|---|---|
| TA, mg | 44.5 ± 3.2 | 44.2 ± 4.2 | 44.8 ± 4.0 | 45.9 ± 5.5 |
| GA, mg | 128.8 ± 11.4 | 127.8 ± 9.8 | 133.6 ± 8.2 | 131.3 ± 11.4 |
| Liver, mg | 1,358.5 ± 100.9 | 1,320.6 ± 111.1 | 1,257.1 ± 206.3 | 1,237.9 ± 169.6 |
| Heart, mg | 121.0 ± 12.7 | 120.7 ± 8.0 | 128.9 ± 16.1 | 128.6 ± 9.7 |
| eWAT, mg | 443.4 ±79.5 | 433.8 ± 156.5 | 1,977.8 ± 293.8# | 2,026.6 ± 500.2# |
eWAT, epididymal fat; GA, gastrocnemius; HFD, high-fat diet; PDHmKO, tamoxifen-inducible Pdha1 knockout mice; TA, tibialis anterior; WT, wild-type. Two-way ANOVA:
P < 0.05 main effect of diet.
Unaltered skeletal muscle insulin sensitivity in PDHmKO mice.
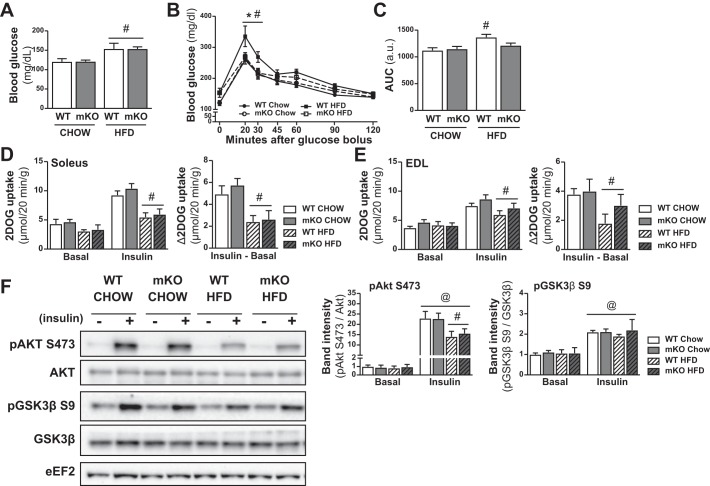
Fasting blood glucose was higher in HFD- versus chow-fed mice, but there was no genotype difference (Fig. 3A). WT mice on an HFD showed impaired glucose tolerance, as indicated by the reduced glucose excursion rate during an oral glucose tolerance test in HFD-fed WT mice (Fig. 3, B and C). PDHmKO mice showed unaltered glucose tolerance with HFD feeding, as compared with chow-fed WT and PDHmKO mice (Fig. 3, B and C). Skeletal muscle insulin sensitivity in soleus (SOL) and extensor digitorum longus (EDL) muscles was worsened with HFD feeding, as indicated by reduced insulin-stimulated (i.e., Insulin-Basal) glucose uptake in HFD-fed compare with chow-fed mice (Fig. 3, D and E). There was a decrease in insulin-stimulated Akt phosphorylation (S473) in HFD-fed WT and PDHmKO mice compared with chow-fed littermates (Fig. 3F), but no diet effects on the insulin-mediated phosphorylation of GSK3β (S9) (Fig. 3F). Importantly, despite a small improvement in glucose tolerance in HFD-fed PDHmKO mice compared with WT mice (Fig. 3, B and C), there were no differences in insulin signaling or insulin-stimulated glucose uptake between WT and PDHmKO mice in either the chow-fed or HFD-fed cohorts (Fig. 3, D–F).
Fig. 3.
Unchanged insulin sensitivity in skeletal muscle of PDHmKO compared with WT mice. WT and PDHmKO mice were fed either a chow or a high-fat diet (HFD) for 12 wk. Blood glucose concentration in tail-vein blood sample of WT and PDHmKO mice after a 3-h fast (Chow, n = 8/8; HFD, n = 6/8) (A). Blood glucose concentration during an oral glucose tolerance test (OGTT) (B). Mice were gavaged with 2 g/kg body wt dextrose. Bar graph shows area under the curve for OGTT experiment (Chow, n = 8/8; HFD, n = 6/8) (C). 2-deoxyglucose (2DOG) uptake in soleus (D) or extensor digitorum longus (E) (EDL) muscles, in the basal state or in response to insulin stimulation. Insulin-stimulated 2-deoxy glucose uptake (2DOGU) was calculated as (2DOGUBASAL – 2DOGUINSULIN) (Chow, n = 8/8; HFD, n = 6/8). Representative blot of Akt and Akt phosphorylated on S473, as well as GSK3β and GSK3β phosphorylated on S9 (F). Bar graphs show quantification of phosphorylated Akt and GSK3β relative to total protein abundance of Akt and GSK3β (n = 4 basal and 4 insulin-stimulated per genotype and diet). All samples were derived at the same time and processed in parallel. Data reported as means ± SE two-way ANOVA, #P < 0.05, main effect of diet (A); 2-way ANOVA with repeated measures, Tukey’s post hoc test, *P < 0.05, HFD-fed PDHmKO compared with HFD-fed WT (B); #P < 0.05, HFD WT compared with chow-fed WT; 2-way ANOVA, Tukey’s post hoc test, #P < 0.05, HFD WT compared with chow WT (C); 2-way ANOVA, #P < 0.05, main effect of diet (D–E); three-way ANOVA, #P < 0.05, main effect of diet, @P < 0.05 main effect of insulin (F). PDHmKO, tamoxifen-inducible Pdha1 knockout mice; WT, wild-type.
Metabolic proteins with HFD feeding.
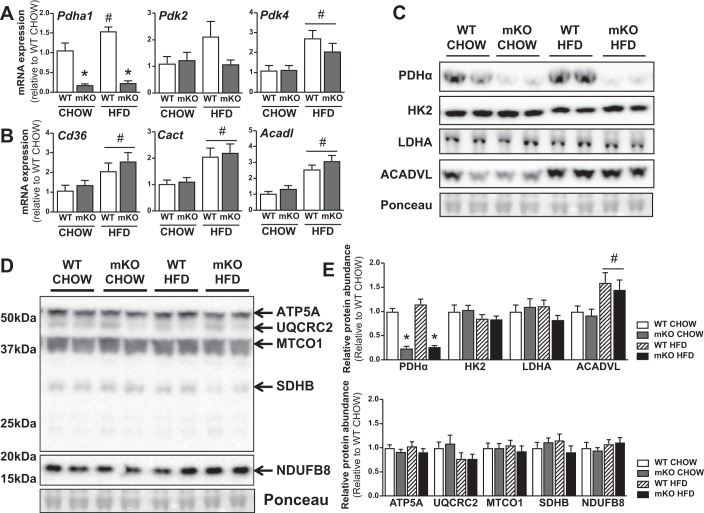
Pdha1 mRNA expression was increased in skeletal muscle of HFD fed versus chow-fed WT mice, although this expression was abrogated in chow- and HFD-fed PDHmKO mice (Fig. 4A). Additionally, Pdk4 but not Pdk2 mRNA expression was significantly elevated in skeletal muscle from HFD versus chow-fed WT and PDHmKO mice (Fig. 4A). Similarly, mRNA expression of proteins involved in fatty acid uptake (cluster of differentiation 36; Cd36), mitochondrial uptake of fatty acids (carnitine-acylcarnitine translocase; Cact), and oxidation (Acyl-CoA dehydrogenase very long chain; Acadvl) were elevated in skeletal muscle to the same extent in HFD-fed PDHmKO and WT mice compared with chow-fed littermates (Fig. 4B). Although ACADVL protein abundance was elevated in skeletal muscle from HFD- versus chow-fed mice (Fig. 4, C and E), there were no differences in protein abundance of other metabolic proteins (Fig. 4, C and E) or mitochondrial electron transport chain components (Fig. 4, D and E) between either chow- or HFD-fed WT and PDHmKO mice.
Fig. 4.
Metabolic and mitochondrial proteins in HFD-fed PDHmKO and WT mice. WT and PDHmKO mice were fed either a chow or a HFD for 12 wk. Transcript abundance of Pdha1, Pdk2, and Pdk4 (A) and Cd36, Cact (Slc25a20), and Acadl (B) in skeletal muscle from chow- or HFD-fed PDHmKO and WT mice, normalized to Ppib (Chow, n = 5/6; HFD, n = 6/7). Representative blot of PDHα, HK2, LDHA, ACADVL (C), and ATP5A, UQCRC2, MTCO1, SDHB, and NDUFB8 (D) protein abundance in skeletal muscle from chow- or HFD-fed PDHmKO and WT mice. Bar graphs show quantification of protein abundance in skeletal muscle relative to ponceau (Chow, n = 6/6; HFD, n = 6/6) (E). Data reported as means ± SE two-way ANOVA, #P < 0.05, main effect of diet, *P < 0.05, main effect of genotype (A–E). HFD, high-fat diet; PDHmKO, tamoxifen-inducible Pdha1 knockout mice; WT, wild-type.
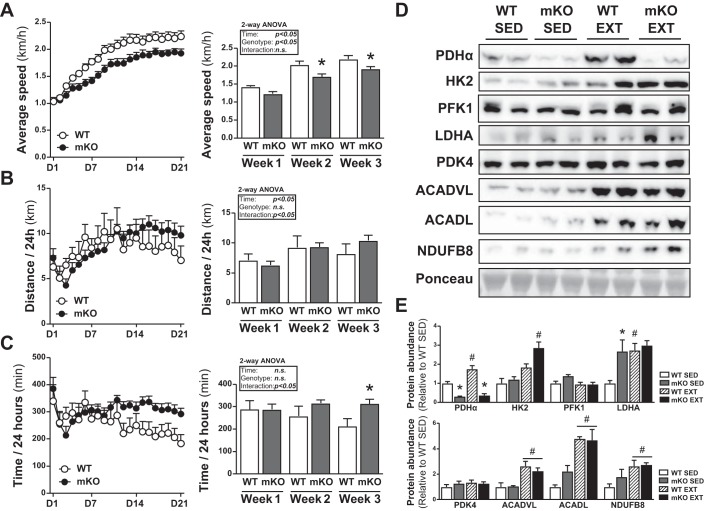
PDHmKO mice run at a lower average running speed during VWR.
We next studied the adaptation of PDHmKO mice to endurance exercise training. Voluntary wheel running (VWR) enhanced exercise capacity in both genotypes, as illustrated by an increased 24-h average run speed during weeks 2 and 3, as compared with week 1 (Fig. 5A). However, at weeks 2 and 3, PDHmKO mice ran at a lower self-selected speed compared with WT mice (Fig. 5A). Although there were no significant genotype differences in total distance run per 24 h (Fig. 5B), PDHmKO mice spent a longer time running per 24-h period compared with WT mice during the last week of the training period (Fig. 5C). Compared with sedentary (SED) littermates, exercise trained (EXT) PDHmKO and WT mice had reduced epididymal fat weight and increased heart weight when normalized to total body weight at the end of the 21-day exercise period (Table 3).
Fig. 5.
PDHmKO mice have reduced running speed during voluntary wheel running (VWR). Weekly average speed (km/h) (A), distance run per 24-h period (B), and time/24 h spent running (C) over 21 days VWR (n = 7/6). Representative blots of PDHα, HK2, PFK1, LDH, PDK4, ACADVL, ACADL, and NDUFB8 protein abundance in skeletal muscle from sedentary (SED) or exercise-trained (EXT) PDHmKO and WT mice (D). Bar graphs show quantification of protein abundance in skeletal muscle relative to ponceau (SED, n = 5/5; EXT, n = 7/6) (E). Data reported as means ± SE 2-way ANOVA, Sidak’s post hoc test, *P < 0.05, PDHmKO compared with WT (A–C); 2-way ANOVA, Tukey’s post hoc test, #P < 0.05, EXT compared with SED, *P < 0.05, PDHmKO compared with WT (D–E). PDHmKO, tamoxifen-inducible Pdha1 knockout mice; WT, wild-type.
Table 3.
Tissue weights of SED and EXT PDHmKO and WT mice
| SED WT | SED PDHmKO | EXT WT | EXT PDHmKO | |
|---|---|---|---|---|
| BW, g | 25.6 ± 1.6 | 26.1 ± 1.5 | 23.5 ± 0.8# | 21.7 ± 1.5# |
| TA, mg | 48.1 ± 5.6 | 49.3 ± 2.4 | 40.7 ± 3.3# | 39.4 ± 4.6# |
| mg/g body wt | 1.88 ± 0.19 | 1.89 ± 0.03 | 1.73 ± 0.11 | 1.81 ± 0.09 |
| GA, mg | 122.5 ± 5.9 | 129.4 ±8.9 | 110.4 ± 7.8# | 107.9 ± 4.9# |
| mg/g body wt | 4.79 ± 0.22 | 4.96 ± 0.21* | 4.69 ± 0.23 | 4.97 ± 0.17* |
| TRI, mg | 114.8 ± 4.5 | 115.9 ± 4.4 | 99.9 ± 4.2# | 92.3 ± 4.3# |
| mg/g body wt | 4.49 ± 0.18 | 4.45 ± 0.27 | 4.25 ± 0.27 | 4.25 ± 0.23 |
| Heart, mg | 108.7 ± 6.0 | 112.3 ± 9.1 | 117.0 ± 5.9 | 113.7 ± 6.1 |
| mg/g body wt | 4.25 ± 0.16 | 4.30 ± 0.27 | 5.00 ± 0.30# | 5.25 ± 0.19# |
| eWAT, mg | 474.9 ±102.8 | 415.2 ± 161.2 | 247.6 ± 67.9# | 187.3 ± 13.6# |
| mg/g body wt | 18.5 ± 3.7 | 15.7 ± 5.5 | 10.5 ± 2.7# | 8.7 ± 0.7# |
BW, body weight; GA, gastrocnemius; eWAT, epididymal fat; EXT, exercise-trained; PDHmKO, tamoxifen-inducible Pdha1 knockout mice; SED, sedentary; TA, tibialis anterior; TRI, triceps; WT, wild-type. Two-way ANOVA:
P < 0.05 main effect of diet;
P < 0.05 main effect of genotype.
Effect of VWR on abundance of metabolic proteins in skeletal muscle.
PDHα protein abundance in triceps muscle was elevated in EXT versus SED WT mice, and this induction was abrogated in PDHmKO mice (Fig. 5, D and E). Protein abundance of LDHA, ACADVL, ACADL, and NDUFB8 were increased to the same extent in both EXT WT and PDHmKO mice compared with SED littermates (Fig. 5, D and E). Interestingly, EXT PDHmKO mice showed an ~1.7-fold greater induction of hexokinase 2 (HK2) compared with EXT WT mice (82.4% vs. 136.3% induction) (Fig. 5, D and E). There were no genotype differences in protein abundance of PDK4 or phosphofructokinase 1 in either SED or EXT mice (Fig. 5, D and E).
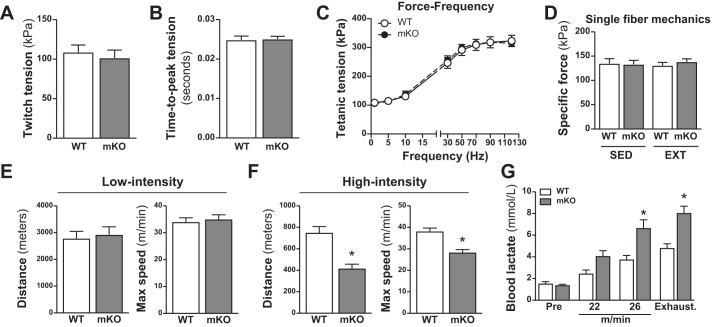
Muscle contractile function is unaffected by the loss of Pdha1.
Twitch (Fig. 6, A and B) and tetanic (Fig. 6C) contractile parameters of the fifth-toe EDL muscle were comparable between PDHmKO and WT mice. Furthermore, there were no differences in force production of isolated TA muscle fibers between genotypes in either SED or EXT cohorts (Fig. 6D).
Fig. 6.
Unaltered ex vivo contractile properties in skeletal muscle but reduced exercise performance in PDHmKO mice. Ex vivo twitch and force-frequency (F-F) characteristics in extensor digitorum longus (EDL) fifth-toe muscle from mixed male and female PDHmKO and WT mice (n = 7/7). Net peak twitch tension (A), time-to-peak tension (B), and F-F curve (C). Intrinsic force production in myofibers isolated from tibialis anterior (TA) of sedentary (SED) and exercise-trained (EXT) WT and PDHmKO mice (D). Total distance run and maximal speed reached during low-intensity exercise (+2 m/min every 10 min) in PDHmKO and WT mice (n = 6/6) (E). Total distance run and maximal speed reached during high-intensity exercise (+1 m/min every 1 min) in PDHmKO and WT mice (n = 6/6) (F). Blood lactate during high-intensity exercise in WT and PDHmKO mice, preexercise, at 22 m/min, 26 m/min, and at exhaustion (n = 7/7) (G). Data reported as means ± SE Student’s t-test (A–B); two-way ANOVA (C–D); Student’s t-test, *P < 0.05 compared with WT (E–F); two-way ANOVA, Tukey’s post hoc test, *P < 0.05, PDHmKO compared with WT (G). PDHmKO, tamoxifen-inducible Pdha1 knockout mice; WT, wild-type.
PDHmKO mice have impaired exercise performance during high-intensity exercise.
Because PDHmKO mice had a lower overall running speed during VWR (Fig. 5A), we next assessed maximal exercise performance in WT and PDHmKO mice. Mice ran on a treadmill until exhaustion using either a low-intensity endurance protocol or a high-intensity sprint protocol. With the low-intensity protocol, there were no differences between PDHmKO and WT mice in the total distance run or maximal run speed (Fig. 6E). However, for the high-intensity protocol, total distance and maximal run speed were reduced in PDHmKO mice compared with WT littermates (Fig. 6F). The impaired exercise performance during high-intensity exercise in PDHmKO mice was associated with elevated blood lactate levels during exercise and at exhaustion as compared with WT mice (Fig. 6G).
Impaired exercise performance in female PDHmKO mice.
To investigate if the minor effects of PDHα ablation in skeletal muscle were sex-specific, we repeated a subset of the primary end points in female WT and PDHmKO mice. Female PDHmKO mice had reduced mRNA expression of PDHα in skeletal muscle (Fig. 7A). Female PDHmKO mice had unaltered body composition (Fig. 7B) compared with WT littermates, and their whole-body V̇o2 (Fig. 7C), RER (Fig. 7D), food intake (Fig. 7E), and activity levels (Fig. 7F) were also similar. In line with our findings in male mice (Fig. 6F) for the high-intensity sprint protocol, total distance, and maximal run speed were lower in female PDHmKO mice compared with WT littermates (Fig. 7G).
Fig. 7.
Unchanged whole-body energy expenditure but reduced exercise performance in female PDHmKO mice. Transcript abundance in gastrocnemius (GA) muscle from female PDHmKO and WT mice (n = 5/5) (A). Body weight, lean mass, and fat mass in female WT and PDHmKO mice (n = 6/7) (B). Whole-body oxygen consumption (V̇o2) (C), respiratory exchange ratio (RER; V̇co2/V̇o2) (D), food intake (E), and total activity counts (beam breaks) (F) during light and dark phases recorded over 48 h in female WT and PDHmKO mice (n = 6/6). Total distance run and maximal speed reached during high-intensity exercise (+1 m/min every 1 min) in female PDHmKO and WT mice (n = 6/6) (G). Data reported as means ± SE Student’s t-test, *P < 0.05, PDHmKO compared with WT (A–B, G); 2-way ANOVA, #P < 0.05, main effect of time (C–F). PDHmKO, tamoxifen-inducible Pdha1 knockout mice; V̇co2, carbon dioxide production; WT, wild-type.
DISCUSSION
CHO oxidation is a central metabolic pathway in skeletal muscle and is the predominant contributor to ATP production in muscle during high-intensity exercise (22, 37, 41, 55). Although it is known that impairments in skeletal muscle glucose handling negatively affect exercise capacity (16, 32, 49), the contribution of PDH to basal muscle physiology at rest or in response to exercise has not been fully elucidated. Moreover, reduced PDC activity as a result of elevated PDK4 activity is a hallmark sign of HFD-induced alterations in skeletal muscle glucose oxidation and insulin sensitivity (9, 23, 47, 72). However, it is not fully elucidated whether loss of PDH in skeletal muscle would exacerbate insulin resistance upon HFD feeding. To address these points directly, we generated PDHmKO mice, which allow temporal KO of Pdha1 specifically in skeletal muscle of adult mice. Interestingly, loss of PDHα in skeletal muscle did not affect body composition, whole-body metabolism, or skeletal muscle insulin sensitivity. Neither did it affect muscle contractile properties, performance during low-intensity exercise, or adaptation of mitochondrial proteins in skeletal muscle in response to exercise training. However, exercise performance during high-intensity exercise was severely impaired in PDHmKO mice, which supports the importance of oxidative glucose metabolism for high-intensity exercise performance.
At rest, skeletal muscle is estimated to account for ~20%–30% of total energy expenditure in humans (76), with the majority of energy derived from fatty acid metabolism (1, 26). Therefore, given that glucose oxidation is a minor contributor to skeletal muscle energy expenditure at rest (1, 26), it not surprising that we find no impact of a loss of PDH on resting V̇o2 or RER. However, during muscle contractions the energetic demand on skeletal muscle drastically increases, with as much as a 20-fold increase in systemic V̇o2 during maximal exercise as compared with rest (12). Exercise intensity also changes fuel utilization in muscle, with a higher proportion of ATP derived from CHOs versus fats at higher exercise intensities (22, 37, 41, 55). This could explain why the exercise impairment in PDHmKO mice is only evident when treadmill speed is increased in shorter increments, forcing the mice to run at a higher exercise intensity, thus increasing the reliance on oxidative CHO metabolism. PDC-mediated CHO oxidation produces 18 times more ATP per glucose molecule than nonoxidative glycolysis, and deletion of Pdha1 thus generates an energetically unfavorable setting in PDHmKO muscles, which impinges exercise performance. This is analogous to a physiological setting where during intense exercise, oxygen delivery to the muscle cannot match the rates of CHO oxidation, thus lowering exercise efficiency (58).
During states of high energy demand, lactic acid accumulation lowers the cellular and interstitial pH in muscle (63). Blood lactate concentrations were ~70%–80% higher in PDHmKO mice compared with WT mice at submaximal and maximal exercise intensities. Given that pH plays an important role in regulating cross-bridge cycling (10, 14) it is possible that the reduced performance in PDHmKO is not only due to reduced capacity to generate ATP via nonoxidative glycolysis but also a greater drop in muscle pH because of excessive lactate generation. Interestingly, even though PDHmKO mice run at a lower overall speed compared with control mice during VWR, they show no impairments in exercise-mediated elevation of mitochondrial or metabolic proteins in skeletal muscle. Because HK2, a glycolytic enzyme, was elevated in muscle of PDHmKO mice with exercise training compared with WT mice, this could potentially support increased glucose uptake from the blood and a higher capacity to generate ATP via glycolysis.
PDC activity has also been linked to muscle force production. Herbst et al. (21) demonstrated that KO of PDH kinase 4 (PDK4) resulted in increased skeletal muscle PDC activity, correlating with increased ex vivo muscle force production (21). In contrast, we now demonstrate that ablation of PDH specifically in skeletal muscle does not impair ex vivo muscle force production. Thus, although increased PDH activity is associated with enhanced force production in mice (21), absence of PDH does not impact the ability of the muscle to generate force. It will be interesting to determine in future studies whether the increased force generation in PDK4 KO mice (21) is dependent on changes in PDH activity.
Obesity, HFD feeding, and diabetes increase protein abundance and activity of PDK2 and PDK4 in skeletal muscle in both rodents and humans (9, 23, 47, 72), which inhibits PDC activity and shifts the utilization from glucose to fatty acids as an energy source (9, 74). HFD-mediated inactivation of PDC activity has been linked to impaired glucose tolerance and worsened insulin sensitivity in skeletal muscle (9, 74). Glucose tolerance is improved in HFD-fed PDK4 KO mice (24, 28) and in chow-fed PDK2/PDK4 KO mice (29). Similarly, treatment with dichloroacetate, a competitive inhibitor of PDKs (5), reduces hyperglycemia in diabetic subjects (62). However, Rahimi et al. (52) showed that although PDK2/PDK4 KO mice have reduced hyperglycemia and increased glucose oxidation in skeletal muscle, insulin-stimulated glucose uptake into muscle was impaired, which was attributed to activation of PKC-θ and inhibition of insulin signaling. Although increased PDC activity is correlated with reduced hyperglycemia in chow and HFD-fed mice (24, 28, 29), we find that loss of PDHα and reduced PDC activity in skeletal muscle does not exacerbate hyperglycemia or skeletal muscle insulin resistance after 12 wk of HFD feeding in mice. Although a hyperinsulinemic-euglycemic clamp would provide more detailed insight into muscle insulin sensitivity in vivo, these findings highlight that inactivation of PDC activity in skeletal muscle is not the sole mechanism driving skeletal muscle insulin resistance during HFD-feeding.
Genetic background plays an important role in how a specific KO model responds to metabolic stressors (e.g., HFD feeding) (15, 43). PDHmKO mice and WT littermates are bred on a C57BL/6J genetic background, and thus carry a null-mutation in the nicotinamide nucleotide transhydrogenase (NNT) gene (15, 43). NNT is a mitochondrial protein participating in a mitochondrial redox cycle with PDC (13). NNT-mutant mice are more susceptible to the negative effect of HFD-feeding (e.g., hepatic steatosis), which coincides with an accumulation of peroxides in mitochondria (42). Interestingly, in liver, this specific phenotype could be ameliorated through activation of PDH (42). Therefore, considering this connection between PDC and NNT, it would be of interest for future studies to investigate the effects of PDH ablation in mice carrying a WT NNT gene. Regardless, in mice on a chow diet, our results clearly demonstrate that loss of Pdha1 does not impact skeletal muscle insulin sensitivity.
Whole-body KO of Pdha1 in mice is embryonically lethal (30), which emphasizes the importance of CHO oxidation to development. In line with this, when mitochondrial pyruvate import is ablated in either mitochondrial pyruvate carrier 1 (36, 66) or mitochondrial pyruvate carrier 2 (67) KO mice, a similar prenatal lethality phenotype is observed. The majority of tissue-specific KO models of Pdha1 generated to date display a milder phenotype, with only the neuron-specific Pdha1 deletion being embryonically lethal (50); this is likely due to the high reliance of the brain on glucose oxidation during development (40). On the other hand, mice with a KO of Pdha1 specifically in liver (7, 38), pancreatic β-cells (60), cardiac muscle (64), or cardiac and skeletal muscles (59) are not embryonically lethal. Interestingly, specific to striated muscle, Sidhu et al. (59) demonstrated that mice with Pdha1 deletion in striated muscles die within a week of being weaned on a regular rodent chow (i.e., 70% of the calories coming from CHOs), and a high mortality rate was later confirmed in a cardiac-specific KO model of Pdha1 (64). The cause of death in these mice was attributed to impaired glucose utilization in cardiac muscle and aberrant cardiac hypertrophy and ventricular dysfunction (59). Because of this heart-specific phenotype, no conclusions could be made on the role of PDH function in resting skeletal muscle function. We have now generated a mouse model in which deletion of Pdha1 occurs specifically in adult skeletal muscle, and not in the heart (39), allowing us to circumvent the lethality of previous muscle-specific Pdha1 KO models and to study the role of PDC in adult skeletal muscle. Because PDHmKO mice show no overt functional or metabolic phenotypes in the resting state, this might suggest a more prominent role for PDC activity in heart, as compared with skeletal muscle.
PDC deficiency is associated with congenital lactic acidosis and a range of neurological disorders (e.g., neuropathy, epilepsy, and ataxia) (2, 11, 45). In humans, mutations in the X-linked PDHA1 gene accounts for the majority of cases with clinical manifestation of PDC deficiency (11, 45), although mutations in several additional genes (e.g., PDHB1, DLD, DLAT, and more) also contribute (11, 44, 45). Importantly, skeletal muscle has been suggested as one of the primary organs affected in PDH-deficient individuals (8, 45), with reported occurrences of muscle weakness, associated with intramuscular lipid accumulation, type 1 fiber atrophy, and fiber type clustering (4, 8, 31). However, few studies have thoroughly investigated the muscle phenotype of patients with PDC deficiency. Our data show that in mice, skeletal muscle-specific PDC deficiency does not affect muscle contractile function, or spontaneous activity, apart from a lower self-selected average speed during VWR. Importantly, although blood lactate concentration was elevated during exercise in PDHmKO mice, there were no differences between genotypes at rest. Therefore, although it is important to be cautious when extrapolating data from rodents to humans, loss of skeletal muscle PDH activity in mice does not result in lactic acidosis, as is found in humans with PDC deficiencies (4, 8, 11, 45). However, loss of Pdha1 in mouse skeletal muscle is associated with exercise-induced hyperlactatemia.
In conclusion, we demonstrate that KO of Pdha1 in adult skeletal muscle does not affect resting energy metabolism, skeletal muscle insulin sensitivity, muscle contractile function, low-intensity exercise capacity, or skeletal muscle adaptation to exercise training. However, our data demonstrate that PDH, and therefore PDC, is necessary for maximal exercise capacity during high-intensity exercise, supporting the importance of oxidative glucose metabolism to high-intensity endurance exercise performance.
GRANTS
This work was supported by National Institutes of Health (NIH) Grants R01-AG043120 and P30-DK063491 (Pilot and Feasibility Award from the University of California San Diego/University of California, Los Angeles Diabetes Research Center) to S. Schenk, NIH Grants F30-DK115035 and T32-AR060712 and Graduate Student Research Support from the University of California San Diego Institute of Engineering in Medicine and the Office of Graduate Studies to V. F. Martins, a postdoctoral fellowship from the University of California San Diego Frontiers of Innovation Scholars Program to S. Schenk, and a postdoctoral fellowship from the Swiss National Science Foundation to K. Svensson.
DISCLOSURES
No conflicts of interest, financial or otherwise, are declared by the authors.
AUTHOR CONTRIBUTIONS
K.S., J.O., and S.S. conceived and designed research; K.S., J.R.D., S.T., V.F.M., A.S., and J.O. performed experiments; K.S., J.R.D., S.T., V.F.M., A.S., J.O., and S.S. analyzed data; K.S., J.O., and S.S. interpreted results of experiments; K.S. prepared figures; K.S. drafted manuscript; K.S., J.O., M.S.P., and S.S. edited and revised manuscript; K.S., J.R.D., S.T., V.F.M., A.S., J.O., M.S.P., and S.S. approved final version of manuscript.
REFERENCES
- 1.Andres R, Cader G, Zierler KL. The quantitatively minor role of carbohydrate in oxidative metabolism by skeletal muscle in intact man in the basal state; measurements of oxygen and glucose uptake and carbon dioxide and lactate production in the forearm. J Clin Invest 35: 671–682, 1956. doi: 10.1172/JCI103324. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Bhandary S, Aguan K. Pyruvate dehydrogenase complex deficiency and its relationship with epilepsy frequency–an overview. Epilepsy Res 116: 40–52, 2015. doi: 10.1016/j.eplepsyres.2015.07.002. [DOI] [PubMed] [Google Scholar]
- 3.Biensø RS, Knudsen JG, Brandt N, Pedersen PA, Pilegaard H. Effects of IL-6 on pyruvate dehydrogenase regulation in mouse skeletal muscle. Pflugers Arch 466: 1647–1657, 2014. doi: 10.1007/s00424-013-1399-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Blass JP, Kark AP, Engel WK. Clinical studies of a patient with pyruvate decarboxylase deficiency. Arch Neurol 25: 449–460, 1971. doi: 10.1001/archneur.1971.00490050083007. [DOI] [PubMed] [Google Scholar]
- 5.Bowker-Kinley MM, Davis WI, Wu P, Harris RA, Popov KM. Evidence for existence of tissue-specific regulation of the mammalian pyruvate dehydrogenase complex. Biochem J 329: 191–196, 1998. doi: 10.1042/bj3290191. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Chleboun GS, Patel TJ, Lieber RL. Skeletal muscle architecture and fiber-type distribution with the multiple bellies of the mouse extensor digitorum longus muscle. Acta Anat (Basel) 159: 147–154, 1997. doi: 10.1159/000147977. [DOI] [PubMed] [Google Scholar]
- 7.Choi CS, Ghoshal P, Srinivasan M, Kim S, Cline G, Patel MS. Liver-specific pyruvate dehydrogenase complex deficiency upregulates lipogenesis in adipose tissue and improves peripheral insulin sensitivity. Lipids 45: 987–995, 2010. doi: 10.1007/s11745-010-3470-8. [DOI] [PubMed] [Google Scholar]
- 8.Chung SJ, Asoh S, Yamanaka T, Okamura-Oho Y, Toshima K, Woo M, Nonaka I. Muscle involvement in pyruvate dehydrogenase complex (PDHC) deficiency. Brain Dev 9: 9–15, 1987. doi: 10.1016/S0387-7604(87)80003-7. [DOI] [PubMed] [Google Scholar]
- 9.Constantin-Teodosiu D. Regulation of muscle pyruvate dehydrogenase complex in insulin resistance: effects of exercise and dichloroacetate. Diabetes Metab J 37: 301–314, 2013. doi: 10.4093/dmj.2013.37.5.301. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Debold EP, Beck SE, Warshaw DM. Effect of low pH on single skeletal muscle myosin mechanics and kinetics. Am J Physiol Cell Physiol 295: C173–C179, 2008. doi: 10.1152/ajpcell.00172.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.DeBrosse SD, Okajima K, Zhang S, Nakouzi G, Schmotzer CL, Lusk-Kopp M, Frohnapfel MB, Grahame G, Kerr DS. Spectrum of neurological and survival outcomes in pyruvate dehydrogenase complex (PDC) deficiency: lack of correlation with genotype. Mol Genet Metab 107: 394–402, 2012. doi: 10.1016/j.ymgme.2012.09.001. [DOI] [PubMed] [Google Scholar]
- 12.Egan B, Zierath JR. Exercise metabolism and the molecular regulation of skeletal muscle adaptation. Cell Metab 17: 162–184, 2013. doi: 10.1016/j.cmet.2012.12.012. [DOI] [PubMed] [Google Scholar]
- 13.Fisher-Wellman KH, Lin C-T, Ryan TE, Reese LR, Gilliam LAA, Cathey BL, Lark DS, Smith CD, Muoio DM, Neufer PD. Pyruvate dehydrogenase complex and nicotinamide nucleotide transhydrogenase constitute an energy-consuming redox circuit. Biochem J 467: 271–280, 2015. doi: 10.1042/BJ20141447. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Fitts RH. The cross-bridge cycle and skeletal muscle fatigue. J Appl Physiol (1985) 104: 551–558, 2008. doi: 10.1152/japplphysiol.01200.2007. [DOI] [PubMed] [Google Scholar]
- 15.Fontaine DA, Davis DB. Attention to background strain is essential for metabolic research: C57BL/6 and the international knockout mouse consortium. Diabetes 65: 25–33, 2016. doi: 10.2337/db15-0982. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Fueger PT, Li CY, Ayala JE, Shearer J, Bracy DP, Charron MJ, Rottman JN, Wasserman DH. Glucose kinetics and exercise tolerance in mice lacking the GLUT4 glucose transporter. J Physiol 582: 801–812, 2007. doi: 10.1113/jphysiol.2007.132902. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Griffin TM, Humphries KM, Kinter M, Lim HY, Szweda LI. Nutrient sensing and utilization: Getting to the heart of metabolic flexibility. Biochimie 124: 74–83, 2016. doi: 10.1016/j.biochi.2015.10.013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Guevara EL, Yang L, Birkaya B, Zhou J, Nemeria NS, Patel MS, Jordan F. Global view of cognate kinase activation by the human pyruvate dehydrogenase complex. Sci Rep 7: 42760, 2017. doi: 10.1038/srep42760. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Hamilton DL, Philp A, MacKenzie MG, Baar K. A limited role for PI(3,4,5)P3 regulation in controlling skeletal muscle mass in response to resistance exercise. PLoS One 5: e11624, 2010. doi: 10.1371/journal.pone.0011624. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Henriksson J. Muscle fuel selection: effect of exercise and training. Proc Nutr Soc 54: 125–138, 1995. doi: 10.1079/PNS19950042. [DOI] [PubMed] [Google Scholar]
- 21.Herbst EAF, Dunford ECE, Harris RA, Vandenboom R, Leblanc PJ, Roy BD, Jeoung NH, Peters SJ. Role of pyruvate dehydrogenase kinase 4 in regulating PDH activation during acute muscle contraction. Appl Physiol Nutr Metab 37: 48–52, 2012. doi: 10.1139/h11-136. [DOI] [PubMed] [Google Scholar]
- 22.Holloszy JO, Kohrt WM, Hansen PA. The regulation of carbohydrate and fat metabolism during and after exercise. Front Biosci 3: D1011–D1027, 1998. doi: 10.2741/A342. [DOI] [PubMed] [Google Scholar]
- 23.Holness MJ, Kraus A, Harris RA, Sugden MC. Targeted upregulation of pyruvate dehydrogenase kinase (PDK)-4 in slow-twitch skeletal muscle underlies the stable modification of the regulatory characteristics of PDK induced by high-fat feeding. Diabetes 49: 775–781, 2000. doi: 10.2337/diabetes.49.5.775. [DOI] [PubMed] [Google Scholar]
- 24.Hwang B, Jeoung NH, Harris RA. Pyruvate dehydrogenase kinase isoenzyme 4 (PDHK4) deficiency attenuates the long-term negative effects of a high-saturated fat diet. Biochem J 423: 243–252, 2009. doi: 10.1042/BJ20090390. [DOI] [PubMed] [Google Scholar]
- 25.Jackson KC, Tarpey MD, Valencia AP, Iñigo MR, Pratt SJ, Patteson DJ, McClung JM, Lovering RM, Thomson DM, Spangenburg EE. Induced Cre-mediated knockdown of Brca1 in skeletal muscle reduces mitochondrial respiration and prevents glucose intolerance in adult mice on a high-fat diet. FASEB J 32: 3070–3084, 2018. doi: 10.1096/fj.201700464R. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Jackson RA, Hamling JB, Blix PM, Nabarro JDN. Relationship among peripheral glucose uptake, oxygen consumption, and glucose turnover in postabsorptive man. J Clin Endocrinol Metab 59: 857–860, 1984. doi: 10.1210/jcem-59-5-857. [DOI] [PubMed] [Google Scholar]
- 27.Jensen J, Rustad PI, Kolnes AJ, Lai YC. The role of skeletal muscle glycogen breakdown for regulation of insulin sensitivity by exercise. Front Physiol 2: 112, 2011. doi: 10.3389/fphys.2011.00112. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Jeoung NH, Harris RA. Pyruvate dehydrogenase kinase-4 deficiency lowers blood glucose and improves glucose tolerance in diet-induced obese mice. Am J Physiol Endocrinol Metab 295: E46–E54, 2008. doi: 10.1152/ajpendo.00536.2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Jeoung NH, Rahimi Y, Wu P, Lee WNP, Harris RA. Fasting induces ketoacidosis and hypothermia in PDHK2/PDHK4-double-knockout mice. Biochem J 443: 829–839, 2012. doi: 10.1042/BJ20112197. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Johnson MT, Mahmood S, Hyatt SL, Yang HS, Soloway PD, Hanson RW, Patel MS. Inactivation of the murine pyruvate dehydrogenase (Pdha1) gene and its effect on early embryonic development. Mol Genet Metab 74: 293–302, 2001. doi: 10.1006/mgme.2001.3249. [DOI] [PubMed] [Google Scholar]
- 31.Kinoshita H, Sakuragawa N, Tada H, Naito E, Kuroda Y, Nonaka I. Recurrent muscle weakness and ataxia in thiamine-responsive pyruvate dehydrogenase complex deficiency. J Child Neurol 12: 141–144, 1997. doi: 10.1177/088307389701200212. [DOI] [PubMed] [Google Scholar]
- 32.Kitaoka Y. McArdle disease and exercise physiology. Biology (Basel) 3: 157–166, 2014. doi: 10.3390/biology3010157.http:// [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.LaBarge SA, Migdal CW, Buckner EH, Okuno H, Gertsman I, Stocks B, Barshop BA, Nalbandian SR, Philp A, McCurdy CE, Schenk S. p300 is not required for metabolic adaptation to endurance exercise training. FASEB J 30: 1623–1633, 2016. doi: 10.1096/fj.15-281741. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.LeBlanc PJ, Peters SJ, Tunstall RJ, Cameron-Smith D, Heigenhauser GJF. Effects of aerobic training on pyruvate dehydrogenase and pyruvate dehydrogenase kinase in human skeletal muscle. J Physiol 557: 559–570, 2004. doi: 10.1113/jphysiol.2003.058263. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Levine R, Haft DE. Carbohydrate homeostasis. N Engl J Med 283: 175–183, 1970. doi: 10.1056/NEJM197007232830405. [DOI] [PubMed] [Google Scholar]
- 36.Li X, Li Y, Han G, Li X, Ji Y, Fan Z, Zhong Y, Cao J, Zhao J, Mariusz G, Zhang M, Wen J, Nesland JM, Suo Z. Establishment of mitochondrial pyruvate carrier 1 (MPC1) gene knockout mice with preliminary gene function analyses. Oncotarget 7: 79981–79994, 2016. doi: 10.18632/oncotarget.13210. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.van Loon LJC, Greenhaff PL, Constantin-Teodosiu D, Saris WHM, Wagenmakers AJM. The effects of increasing exercise intensity on muscle fuel utilisation in humans. J Physiol 536: 295–304, 2001. doi: 10.1111/j.1469-7793.2001.00295.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Mahmood S, Birkaya B, Rideout TC, Patel MS. Lack of mitochondria-generated acetyl-CoA by pyruvate dehydrogenase complex downregulates gene expression in the hepatic de novo lipogenic pathway. Am J Physiol Endocrinol Metab 311: E117–E127, 2016. doi: 10.1152/ajpendo.00064.2016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.McCarthy JJ, Srikuea R, Kirby TJ, Peterson CA, Esser KA. Inducible Cre transgenic mouse strain for skeletal muscle-specific gene targeting. Skelet Muscle 2: 8, 2012. doi: 10.1186/2044-5040-2-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Mergenthaler P, Lindauer U, Dienel GA, Meisel A. Sugar for the brain: the role of glucose in physiological and pathological brain function. Trends Neurosci 36: 587–597, 2013. doi: 10.1016/j.tins.2013.07.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Mul JD, Stanford KI, Hirshman MF, Goodyear LJ. Exercise and regulation of carbohydrate metabolism. Prog Mol Biol Transl Sci 135: 17–37, 2015. doi: 10.1016/bs.pmbts.2015.07.020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Navarro CDC, Figueira TR, Francisco A, Dal’Bó GA, Ronchi JA, Rovani JC, Escanhoela CAF, Oliveira HCF, Castilho RF, Vercesi AE. Redox imbalance due to the loss of mitochondrial NAD(P)-transhydrogenase markedly aggravates high fat diet-induced fatty liver disease in mice. Free Radic Biol Med 113: 190–202, 2017. doi: 10.1016/j.freeradbiomed.2017.09.026. [DOI] [PubMed] [Google Scholar]
- 43.Nicholson A, Reifsnyder PC, Malcolm RD, Lucas CA, MacGregor GR, Zhang W, Leiter EH. Diet-induced obesity in two C57BL/6 substrains with intact or mutant nicotinamide nucleotide transhydrogenase (Nnt) gene. Obesity (Silver Spring) 18: 1902–1905, 2010. doi: 10.1038/oby.2009.477. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Okajima K, Korotchkina LG, Prasad C, Rupar T, Phillips JA III, Ficicioglu C, Hertecant J, Patel MS, Kerr DS. Mutations of the E1beta subunit gene (PDHB) in four families with pyruvate dehydrogenase deficiency. Mol Genet Metab 93: 371–380, 2008. doi: 10.1016/j.ymgme.2007.10.135. [DOI] [PubMed] [Google Scholar]
- 45.Patel KP, O’Brien TW, Subramony SH, Shuster J, Stacpoole PW. The spectrum of pyruvate dehydrogenase complex deficiency: clinical, biochemical and genetic features in 371 patients. Mol Genet Metab 106: 385–394, 2012. doi: 10.1016/j.ymgme.2012.03.017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Patel MS, Nemeria NS, Furey W, Jordan F. The pyruvate dehydrogenase complexes: structure-based function and regulation. J Biol Chem 289: 16615–16623, 2014. doi: 10.1074/jbc.R114.563148. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Peters SJ, Harris RA, Wu P, Pehleman TL, Heigenhauser GJ, Spriet LL. Human skeletal muscle PDH kinase activity and isoform expression during a 3-day high-fat/low-carbohydrate diet. Am J Physiol Endocrinol Metab 281: E1151–E1158, 2001. doi: 10.1152/ajpendo.2001.281.6.E1151. [DOI] [PubMed] [Google Scholar]
- 48.Philp A, Chen A, Lan D, Meyer GA, Murphy AN, Knapp AE, Olfert IM, McCurdy CE, Marcotte GR, Hogan MC, Baar K, Schenk S. Sirtuin 1 (SIRT1) deacetylase activity is not required for mitochondrial biogenesis or peroxisome proliferator-activated receptor-gamma coactivator-1alpha (PGC-1alpha) deacetylation following endurance exercise. J Biol Chem 286: 30561–30570, 2011. doi: 10.1074/jbc.M111.261685. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Philp A, Hargreaves M, Baar K. More than a store: regulatory roles for glycogen in skeletal muscle adaptation to exercise. Am J Physiol Endocrinol Metab 302: E1343–E1351, 2012. doi: 10.1152/ajpendo.00004.2012. [DOI] [PubMed] [Google Scholar]
- 50.Pliss L, Pentney RJ, Johnson MT, Patel MS. Biochemical and structural brain alterations in female mice with cerebral pyruvate dehydrogenase deficiency. J Neurochem 91: 1082–1091, 2004. doi: 10.1111/j.1471-4159.2004.02790.x. [DOI] [PubMed] [Google Scholar]
- 51.Putman CT, Spriet LL, Hultman E, Lindinger MI, Lands LC, McKelvie RS, Cederblad G, Jones NL, Heigenhauser GJ. Pyruvate dehydrogenase activity and acetyl group accumulation during exercise after different diets. Am J Physiol Endocrinol Metab 265: E752–E760, 1993. doi: 10.1152/ajpendo.1993.265.5.E752. [DOI] [PubMed] [Google Scholar]
- 52.Rahimi Y, Camporez J-PG, Petersen MC, Pesta D, Perry RJ, Jurczak MJ, Cline GW, Shulman GI. Genetic activation of pyruvate dehydrogenase alters oxidative substrate selection to induce skeletal muscle insulin resistance. Proc Natl Acad Sci USA 111: 16508–16513, 2014. doi: 10.1073/pnas.1419104111. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Richard AJ, Hang H, Stephens JM. Pyruvate dehydrogenase complex (PDC) subunits moonlight as interaction partners of phosphorylated STAT5 in adipocytes and adipose tissue. J Biol Chem 292: 19733–19742, 2017. doi: 10.1074/jbc.M117.811794. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Romijn JA, Coyle EF, Sidossis LS, Zhang XJ, Wolfe RR. Relationship between fatty acid delivery and fatty acid oxidation during strenuous exercise. J Appl Physiol (1985) 79: 1939–1945, 1995. doi: 10.1152/jappl.1995.79.6.1939. [DOI] [PubMed] [Google Scholar]
- 55.Romijn JA, Coyle EF, Sidossis LS, Gastaldelli A, Horowitz JF, Endert E, Wolfe RR. Regulation of endogenous fat and carbohydrate metabolism in relation to exercise intensity and duration. Am J Physiol 265: E380–E391, 1993. doi: 10.1152/ajpendo.1993.265.3.E380. [DOI] [PubMed] [Google Scholar]
- 56.Ross JA, Levy Y, Svensson K, Philp A, Schenk S, Ochala J. SIRT1 regulates nuclear number and domain size in skeletal muscle fibers. J Cell Physiol 233: 7157–7163, 2018. doi: 10.1002/jcp.26542. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Schenk S, McCurdy CE, Philp A, Chen MZ, Holliday MJ, Bandyopadhyay GK, Osborn O, Baar K, Olefsky JM. Sirt1 enhances skeletal muscle insulin sensitivity in mice during caloric restriction. J Clin Invest 121: 4281–4288, 2011. doi: 10.1172/JCI58554. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Scott C. Misconceptions about aerobic and anaerobic energy expenditure. J Int Soc Sports Nutr 2: 32–37, 2005. doi: 10.1186/1550-2783-2-2-32. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Sidhu S, Gangasani A, Korotchkina LG, Suzuki G, Fallavollita JA, Canty JM Jr, Patel MS. Tissue-specific pyruvate dehydrogenase complex deficiency causes cardiac hypertrophy and sudden death of weaned male mice. Am J Physiol Heart Circ Physiol 295: H946–H952, 2008. doi: 10.1152/ajpheart.00363.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Srinivasan M, Choi CS, Ghoshal P, Pliss L, Pandya JD, Hill D, Cline G, Patel MS. ß-Cell-specific pyruvate dehydrogenase deficiency impairs glucose-stimulated insulin secretion. Am J Physiol Endocrinol Metab 299: E910–E917, 2010. doi: 10.1152/ajpendo.00339.2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.St. Amand TA, Spriet LL, Jones NL, Heigenhauser GJ. Pyruvate overrides inhibition of PDH during exercise after a low-carbohydrate diet. Am J Physiol Endocrinol Metab 279: E275–E283, 2000. doi: 10.1152/ajpendo.2000.279.2.E275. [DOI] [PubMed] [Google Scholar]
- 62.Stacpoole PW, Moore GW, Kornhauser DM. Metabolic effects of dichloroacetate in patients with diabetes mellitus and hyperlipoproteinemia. N Engl J Med 298: 526–530, 1978. doi: 10.1056/NEJM197803092981002. [DOI] [PubMed] [Google Scholar]
- 63.Street D, Bangsbo J, Juel C. Interstitial pH in human skeletal muscle during and after dynamic graded exercise. J Physiol 537: 993–998, 2001. doi: 10.1113/jphysiol.2001.012954. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Sun W, Quan N, Wang L, Yang H, Chu D, Liu Q, Zhao X, Leng J, Li J. Cardiac-specific deletion of the Pdha1 gene sensitizes heart to toxicological actions of ischemic stress. Toxicol Sci 151: 193–203, 2016. doi: 10.1093/toxsci/kfw035. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Svensson K, LaBarge SA, Martins VF, Schenk S. Temporal overexpression of SIRT1 in skeletal muscle of adult mice does not improve insulin sensitivity or markers of mitochondrial biogenesis. Acta Physiol (Oxf) 221: 193–203, 2017. doi: 10.1111/apha.12897. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Vanderperre B, Herzig S, Krznar P, Hörl M, Ammar Z, Montessuit S, Pierredon S, Zamboni N, Martinou J-C. Embryonic lethality of mitochondrial pyruvate carrier 1 deficient mouse can be rescued by a ketogenic diet. PLoS Genet 12: e1006056, 2016. doi: 10.1371/journal.pgen.1006056. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Vigueira PA, McCommis KS, Schweitzer GG, Remedi MS, Chambers KT, Fu X, McDonald WG, Cole SL, Colca JR, Kletzien RF, Burgess SC, Finck BN. Mitochondrial pyruvate carrier 2 hypomorphism in mice leads to defects in glucose-stimulated insulin secretion. Cell Reports 7: 2042–2053, 2014. doi: 10.1016/j.celrep.2014.05.017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Ward GR, Sutton JR, Jones NL, Toews CJ. Activation by exercise of human skeletal muscle pyruvate dehydrogenase in vivo. Clin Sci (Lond) 63: 87–92, 1982. doi: 10.1042/cs0630087. [DOI] [PubMed] [Google Scholar]
- 69.Watt MJ, Heigenhauser GJF, LeBlanc PJ, Inglis JG, Spriet LL, Peters SJ. Rapid upregulation of pyruvate dehydrogenase kinase activity in human skeletal muscle during prolonged exercise. J Appl Physiol (1985) 97: 1261–1267, 2004. doi: 10.1152/japplphysiol.00132.2004. [DOI] [PubMed] [Google Scholar]
- 70.White AT, LaBarge SA, McCurdy CE, Schenk S. Knockout of STAT3 in skeletal muscle does not prevent high-fat diet-induced insulin resistance. Mol Metab 4: 569–575, 2015. doi: 10.1016/j.molmet.2015.05.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.White AT, Philp A, Fridolfsson HN, Schilling JM, Murphy AN, Hamilton DL, McCurdy CE, Patel HH, Schenk S. High-fat diet-induced impairment of skeletal muscle insulin sensitivity is not prevented by SIRT1 overexpression. Am J Physiol Endocrinol Metab 307: E764–E772, 2014. doi: 10.1152/ajpendo.00001.2014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Wu P, Inskeep K, Bowker-Kinley MM, Popov KM, Harris RA. Mechanism responsible for inactivation of skeletal muscle pyruvate dehydrogenase complex in starvation and diabetes. Diabetes 48: 1593–1599, 1999. doi: 10.2337/diabetes.48.8.1593. [DOI] [PubMed] [Google Scholar]
- 73.Young KW, Dayanidhi S, Lieber RL. Polarization gating enables sarcomere length measurements by laser diffraction in fibrotic muscle. J Biomed Opt 19: 117009, 2014. doi: 10.1117/1.JBO.19.11.117009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Zhang S, Hulver MW, McMillan RP, Cline MA, Gilbert ER. The pivotal role of pyruvate dehydrogenase kinases in metabolic flexibility. Nutr Metab (Lond) 11: 10, 2014. doi: 10.1186/1743-7075-11-10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.Zhong Y, Huang R, Li X, Xu R, Zhou F, Wang J, Fan H, Goscinski M, Zhang M, Wen JG, Nesland JM, Suo Z. Decreased expression of PDHE1α predicts worse clinical outcome in esophageal squamous cell carcinoma. Anticancer Res 35: 5533–5538, 2015. [PubMed] [Google Scholar]
- 76.Zurlo F, Larson K, Bogardus C, Ravussin E. Skeletal muscle metabolism is a major determinant of resting energy expenditure. J Clin Invest 86: 1423–1427, 1990. doi: 10.1172/JCI114857. [DOI] [PMC free article] [PubMed] [Google Scholar]