Abstract
Accumulation of myeloid cells in the liver, notably dendritic cells (DCs) and monocytes/macrophages (MCs), is a major component of the metainflammation of obesity. However, the mechanism(s) stimulating hepatic DC/MC infiltration remain ill defined. Herein, we addressed the hypothesis that adipose tissue (AT) free fatty acids (FFAs) play a central role in the initiation of hepatic DC/MC accumulation, using a number of mouse models of altered FFA supply to the liver. In two models of acute FFA elevation (lipid infusion and fasting) hepatic DC/MC and triglycerides (TGs) but not AT DC/MC were increased without altering plasma cytokines (PCs; TNFα and monocyte chemoattractant protein 1) and with variable effects on oxidative stress (OxS) markers. However, fasting in mice with profoundly reduced AT lipolysis (AT-specific deletion of adipose TG lipase; AAKO) failed to elevate liver DC/MC, TG, or PC, but liver OxS increased. Livers of obese AAKO mice that are known to be resistant to steatosis were similarly protected from inflammation. In high-fat feeding studies of 1, 3, 6, or 20-wk duration, liver DC/MC accumulation dissociated from PC and OxS but tracked with liver TGs. Furthermore, decreasing OxS by ~80% in obese mice failed to decrease liver DC/MC. Therefore, FFA and more specifically AT-derived FFA stimulate hepatic DC/MC accumulation, thus recapitulating the pathology of the obese liver. In a number of cases the effects of FFA can be dissociated from OxS and PC but match well with liver TG, a marker of FFA oversupply.
Keywords: adipose tissue, dendritic cells, free fatty acids, inflammation, liver, monocytes
INTRODUCTION
Obesity is associated with a state of chronic, low-grade inflammation or so-called metainflammation (7, 12, 23, 31), which is proposed to be pathogenic for the initiation and/or exacerbation of insulin resistance and other metabolic abnormalities, primarily systemic dyslipidemia and tissue steatosis. A characteristic of the obese inflammatory response is the infiltration of immune cells into tissues of importance to metabolic function, such as adipose tissue (AT) (3, 8, 9, 28, 29, 34, 38, 43–46), liver (9, 29, 38, 46), and less clearly, skeletal muscle (13, 41). A number of studies (16, 21, 27, 29) have demonstrated the importance of monocyte recruitment and their subsequent maturation to M1 polarized macrophages in this process. Furthermore, we and others (8, 15, 38, 46) have demonstrated that the infiltration of dendritic cells (DCs)—both plasmacytoid (p)DCs and conventional (c)DCs—into AT and liver is an important component of obesity-associated inflammation, whereas the number of liver-resident macrophages (Kupffer cells) (22, 26, 38) remain unchanged.
Although there is some evidence that monocyte chemoattractant protein 1 (MCP-1) (16, 29, 42), free fatty acids (FFAs) (1, 21, 27, 36), and gut-derived lipopolysaccharide (5, 6) act as signals for monocyte/macrophage (MC) recruitment into AT, the signals/mechanisms responsible for initiating MC or DC recruitment into the liver have not been described. Two studies suggest that FFA (4) and lipopolysaccharide (6) activate the proinflammatory NF-κB pathway in the liver, but whether they stimulate hepatic immune cell infiltration was not addressed. One study (29) has described a role for C-C chemokine receptor type 2/MCP-1 in the uptake of intravenously injected exogenous MC into the liver, but the relevance of this observation to the infiltration of endogenous MC/DC is unclear. Of importance to the current study, in obesity the liver is exposed to excess FFAs, predominantly as long-chain saturates derived from AT, with a lesser quantity derived from hepatic lipase action on intermediate-density lipoproteins (IDL) and short-chain fatty acids derived from the gut microbiota through the portal circulation. The goal of the current study was to test the hypothesis that fatty acids, and more specifically, AT-derived FFAs, are a mechanism initiating the infiltration of DCs and/or MCs into liver. The data demonstrate that AT-derived FFAs are a necessary and sufficient signal in this regard, that the effects of FFAs are, in many cases, dissociated from hepatic oxidative stress and in all cases are dissociated from plasma cytokine levels but match well with liver triglyceride accumulation.
MATERIALS AND METHODS
Animal Care and Maintenance
Male C57BL/6J mice were purchased from the Jackson Laboratory (Bar Harbor, ME). Mice containing an adipocyte-specific deletion of adipose triglyceride lipase [Atgl (AAKO)] were derived from C57BL/6NTac mice and bred as previously described (35). All mice were housed in the University of Pittsburgh facility under standard conditions with ad libitum access to water and food unless otherwise noted. Experiments were conducted in compliance with National Institutes of Health guidelines, and all procedures were approved by the University of Pittsburgh Institutional Animal Care and Use Committee.
Lipid Infusion
Surgery and lipid infusion were performed as described previously (2, 32). Briefly, mice were catheterized in the femoral vein and allowed 3 days for recovery. During the recovery period, mice had ad libitum access to food and water and were monitored closely for normal activity and food intake. Three days after surgery, mice were infused with lipid emulsion (Intralipid, 10% final concentration) in heparinized saline (10 U/ml final concentration) for 16 h at a constant rate of 200 µl/h. Control animals underwent the same surgical procedure and were infused with heparinized saline (10 U/ml final concentration) for 16 h. Importantly, this model has been meticulously validated to have minimal impact on various makers of physiologic health, including blood pressure, heart rate, body weight, and organ (heart, lung, brain) weight (2, 32, and unpublished observations). Additionally, mice receiving lipid naturally adjust food intake so the total caloric intake (lipid infusion + chow consumed) matches that of control mice (chow alone).
Fasting and High-Fat Feeding Studies
Overnight fasting experiments.
One group of mice was fasted for 16 h before euthanasia. Control mice had ad libitum access to food during this time, and all groups of mice had ad libitum access to water.
High-fat feeding studies.
For 1-, 3-, and 6-wk studies mice were fed with a high-saturated fat, high-sucrose diet (41% calories from fat, 300 g/kg sucrose, 4.4 kcal/g; cat. no. 96001, Teklad) or a matched low-fat, low-sucrose control diet (13% calories from fat, 100g/kg sucrose, 3.6 kcal/g; cat. no. 110340, Teklad). For obesity studies, mice were fed a high-fat diet (60% calories from fat, 5.2 kcal/g; cat. no. D-12492, Research Diets) or a low-fat control diet (14% calories from fat, 3.5 kcal/g; cat. no. 5-P-76, LabDiet) for 20 wk. Length of feeding time in weeks is noted for each experiment.
Cytokine, FFA, and Triglyceride Measurements
MCP-1 and TNFα cytokines were quantified with commercial ELISA kits (R&D Systems) following the manufacturer’s instructions. Plasma FFAs were quantified using a colormetric kit (Wako Diagnostics) following the manufacturer’s instructions. Liver triglyceride levels were determined as previously described (37).
Flow Cytometry
Liver, AT, and spleen processing and cell staining were performed as described previously (38), and only viable CD45+ cells were used for analysis. Antibodies used include B220 (clone RA3–6B2, eBioscience), CD3 (clone 145–2C11, eBioscience), CD4 (clone GK1.5, eBioscience), CD8 (clone 53–5.8, BD Biosciences), CD11b (clone M1/70, eBioscience), CD11c (clone N418, eBioscience), FC-block CD16/32 (clone 93, eBioscience), CD45 (clone 30-F11, BD Biosciences), F4/80 (clone BM8, eBioscience), and NK1.1 (clone PK136, eBioscience). Aqua Live-Dead (Life Technologies) was used for viability detection. Flow cytometry was performed on an LSR II (BD Biosciences), and results were analyzed using FACSDiva software (BD Biosciences). Total cells (liver and spleen) or total cells normalized to tissue mass (AT) were determined for each population analyzed. Values for each population were then normalized to the appropriate control (either saline, fed, standard chow, or lean).
Febuxostat Experiments
Obese C57BL/6J mice (see above) received febuxostat (Axon Mechem) dissolved in their drinking water (50 mg/l). All mice were given ad libitum access to food during treatment, and control mice were given ad libitum access to regular drinking water. Mice were provided febuxostat or control water for the last 7 wk of the experiment (from weeks 13–20 of high-fat feeding).
Oxidative Stress Measurements
GSH.
Fresh liver samples were homogenized in 5% 5-sulfosalicylic acid and immediately flash-frozen. GSH levels were determined using the GSSG/GSH Quantification Kit (Dojindo Molecular Technologies, Inc.) following the manufacturer’s instructions.
Xanthine oxidase activity.
Xanthine oxidase activity was assessed electrochemically by reverse phase HPLC analysis of uric acid production (ESA Coul-Array System, Chelmsford, MA; 1 unit = 1 µmol of urate/min), as previously described (17).
Protein carbonyls.
Electrophoresis was performed on liver lysates, and proteins were transferred to PVDF membranes. Total carbonylated protein content was determined using the OxiSelect Protein Carbonyl Immunoblot Kit (Cell Biolabs) following the manufacturer’s instructions.
Immuno-spin trapping.
5,5-Dimethyl-1Pyrroline-N-Oxide (DMPO; Dojindo Molecular Technologies, Inc.) was injected intraperitoneally 24, 12, and 6 h before euthanasia. Each injection was dosed at 0.5 g/kg body wt for a total dose of 1.5 g/kg. Livers were excised, fixed, and prepared for immunofluorescence. DMPO-adducted biomolecular free radicals were visualized using a polyclonal anti-DMPO antibody (Enzo Life Sciences) as previously described (18, 19).
Statistics
Data are expressed as mean ± SE. Statistical significance was determined by t-test or one-way ANOVA, with Tukey’s multiple comparisons post hoc test utilized where appropriate. A P value < 0.05 was considered significant. Statistical analysis was performed using GraphPad Prism (GraphPad Software Inc.) and SPSS (IBM).
RESULTS
Liver Myeloid Cell Infiltration Is Induced by a Lipid Infusion and Associates with Increased Hepatic Triglycerides but Unaltered Oxidative Stress or Systemic Cytokine Levels
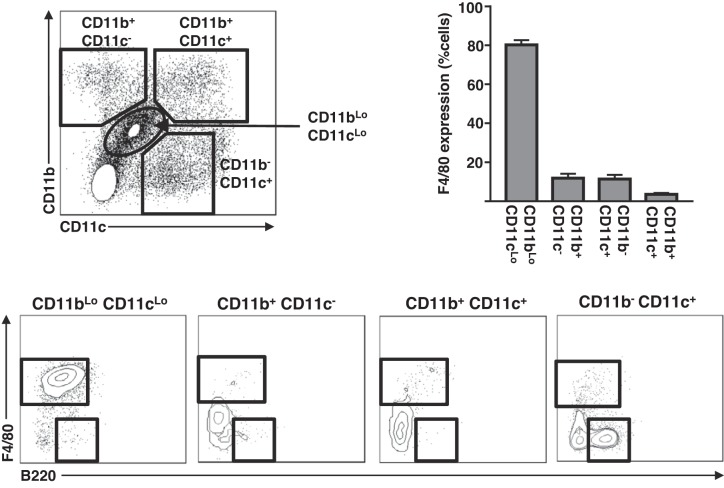
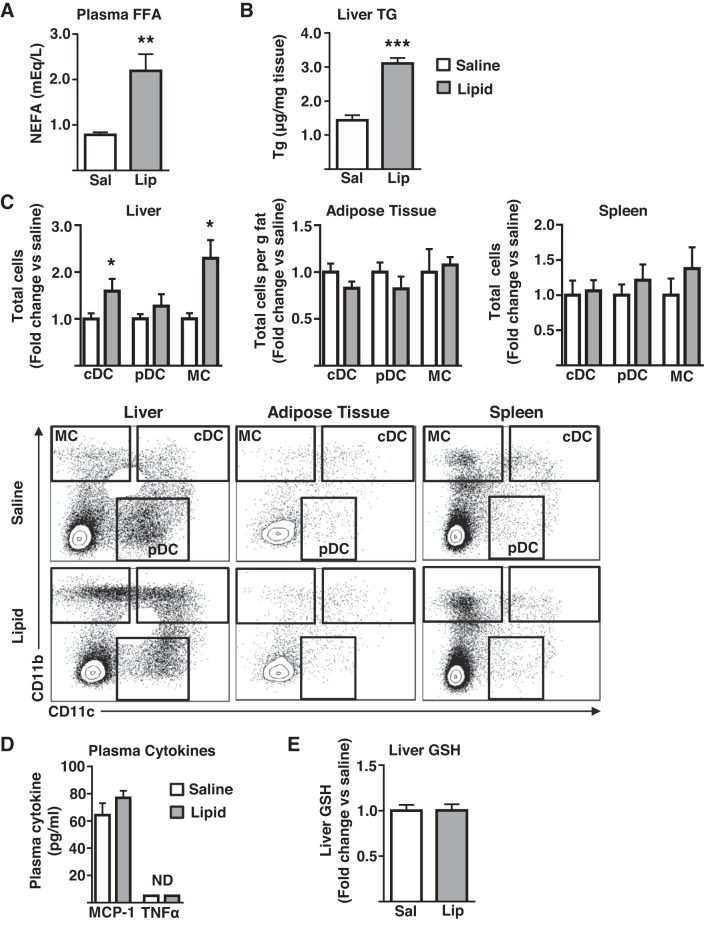
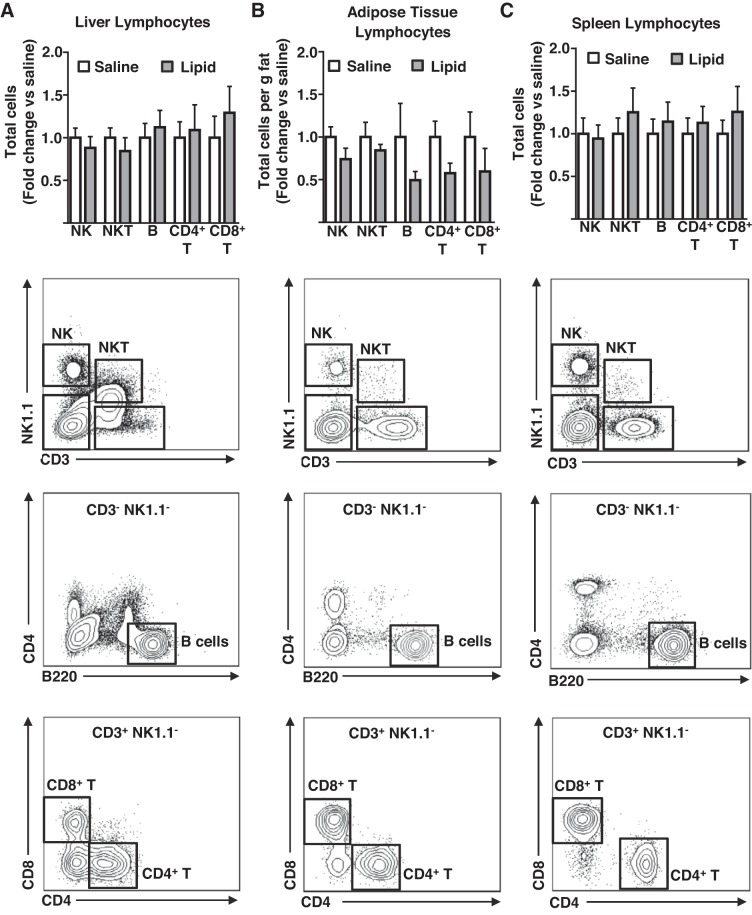
Previously, we have demonstrated that CD11b+ CD11c− (MC), CD11b+ CD11c+ (cDC), and CD11b− CD11c+ B220+ (pDC) (Fig. 1) are the primary myeloid cells that accumulate in the liver in obesity (38), whereas resident F4/80Hi CD11bLo CD11cLo (Kupffer cells) (Fig. 1) remain relatively unchanged (22, 26, 38). More recently, we have also confirmed functional differences between liver cDC and pDC using the mixed leukocyte reaction (unpublished data). As such, we focused on understanding FFA-mediated recruitment of hepatic MC, cDC, and pDC myeloid populations throughout this study (Fig. 1 for gating strategy). To determine the capacity of elevated FFA delivery to stimulate immune cell recruitment to the liver in the absence of the confounding variables associated with obesity, chronically catheterized mice were continuously infused with either saline or a lipid emulsion for 16 h. Subsequently, the liver, AT, and spleen were isolated and immune cell content determined by flow cytometry using the gating strategies shown in Figs. 2 and 3. As expected, lipid-infused mice displayed higher circulating FFA than saline-infused controls (Fig. 2A). The lipid infusion stimulated substantial increases in cDC and MC content of the liver, whereas the pDC content remained unchanged (Fig. 2C). Importantly, the effects of the lipid infusion were particular to the liver, because AT and spleen DC and MC content were unaltered by the lipid infusion (Fig. 2C). Unlike the increases in the myeloid cell populations, cells of the lymphoid lineage (NK, NKT, B, CD4+T, and CD8+T) were unaffected in all tissues examined (Fig. 3). Importantly, plasma levels of two prototypical chemokines, MCP-1 and TNFα, which are associated with inflammation and monocyte recruitment, were unaltered by a lipid infusion (Fig. 2D). Furthermore, liver oxidative stress, as measured by the levels of the small molecule antioxidant GSH, which decreases under the conditions of increased oxidative stress, was similar in saline and lipid-infused mice (Fig. 2E). Notably, liver triglyceride levels were elevated in the lipid-infused mice (Fig. 2B), likely because of a partitioning of a proportion of the increased fatty acid supply into the hepatic triglyceride pool. Together, these data demonstrate that a 16-h elevation in circulating FFA increases liver cDC and MC content, and that these effects are tissue specific and are restricted to immune cells of the myeloid lineage. Furthermore, the liver increases in DC/MC are independent of both alterations in liver oxidative stress and the circulating concentrations of chemokines associated with T helper type 1 inflammatory responses but are associated with elevated hepatic triglyceride content.
Fig. 1.
Myeloid populations in the liver. Livers from male mice were used to assess the shown myeloid cell populations by flow cytometry. Representative plots showing CD11b and CD11c expression of CD45+ mononuclear cells (top left), quantitated F4/80 expression of CD11bLo CD11cLo, CD11b+ CD11c−, CD11b+ CD11c+, and CD11b− CD11c+ populations (n = 7) (top right), and representative plots showing F4/80 and B220 expression of the indicated populations (bottom). All data are presented as means ± SE.
Fig. 2.
The effects of a lipid infusion on tissue DC/MC cell content. Chronically catheterized male mice received an infusion of saline/heparin or lipid emulsion/heparin for 16 h before analyses. A: plasma nonesterified fatty acids (FFA, n = 5); B: liver triglycerides (TG; n = 6–9); C: liver, adipose tissue, and splenic dendritic cells (cDC, pDC), and monocyte/macrophage (MC) populations as assessed by flow cytometry (n = 6–8); D: Plasma MCP-1 and TNFα (n = 3–4); E: liver glutathione (GSH; n = 6). All data are presented as mean ± SE and significant differences are indicated (*P < 0.05, **P < 0.01, ***P < 0.001 saline vs. lipid). cDC, conventional DC; DC, dendritic cell; FFA, free fatty acid; Lip, lipid; MCP, monocyte chemoattractant protein; ND, not detected; pDC, plasmacytoid DC; Sal, saline; NEFA, nonesterified fatty acid.
Fig. 3.
The effects of a lipid infusion on tissue lymphocyte content. Chronically catheterized male mice received an infusion of saline/heparin or lipid emulsion/heparin for 16 h before analyses. A: liver lymphocytes; B: adipose tissue lymphocytes; C: splenic lymphocytes as assessed by flow cytometry (n = 6–8). Representative flow plots for each tissue are shown below quantitation. All quantitative plots represent mean ± SE.
Fasting, a Physiological Stimulus of Increased AT FFA Delivery to the Liver, Is Sufficient to Initiate Hepatic Myeloid Cell Infiltration
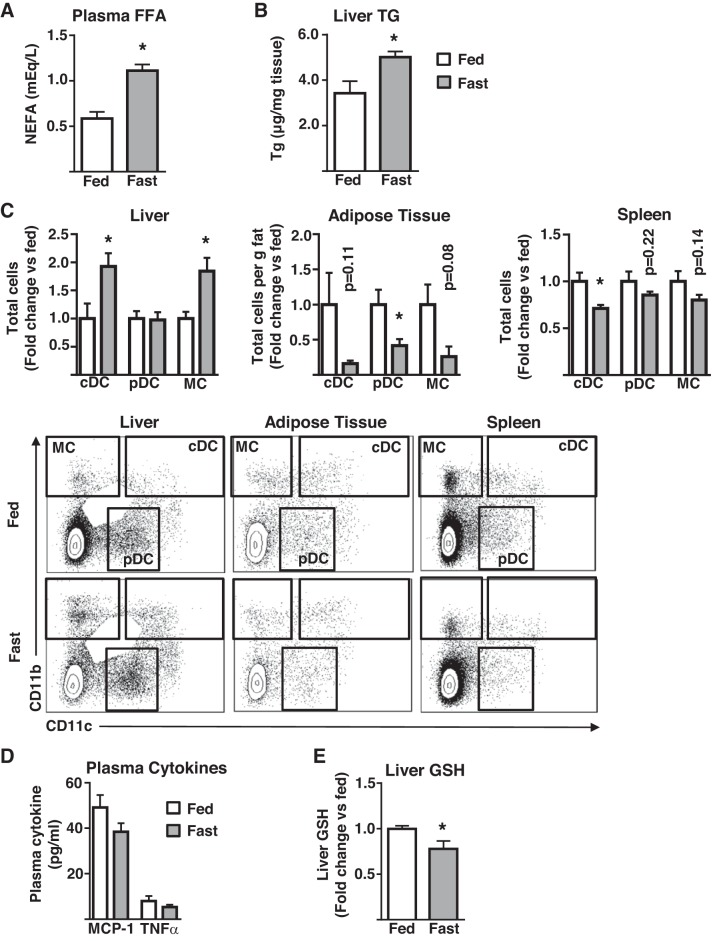
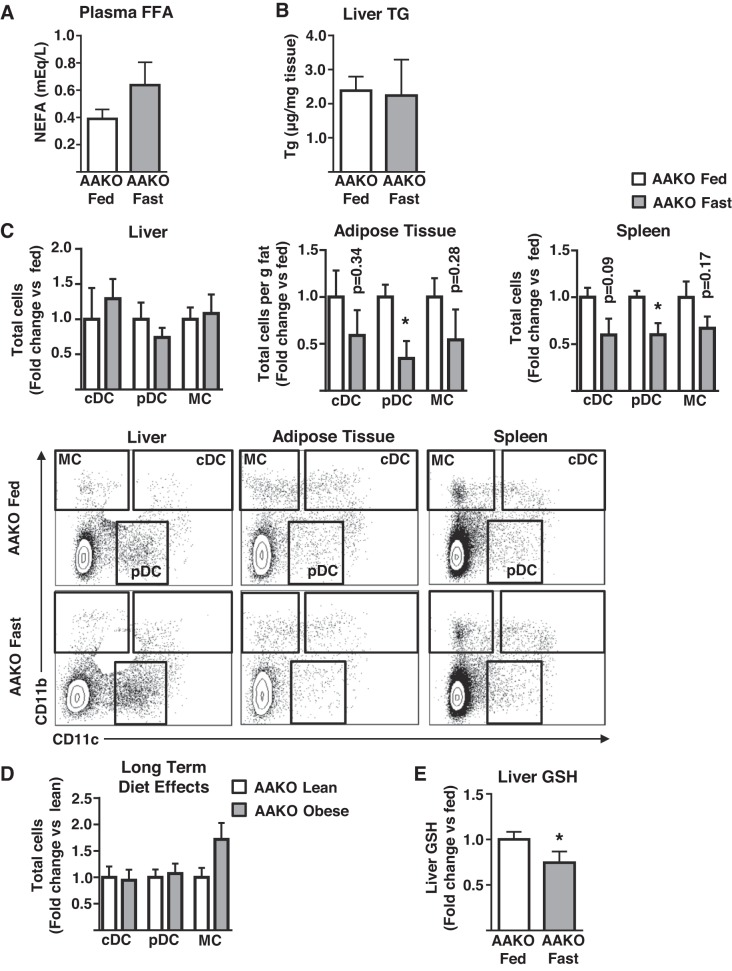
As AT is the primary source of FFAs for the liver, and FFA delivery to the liver from AT is elevated in obesity, we hypothesized that increasing AT FFA delivery to the liver in the absence of obesity would be sufficient to initiate hepatic myeloid cell infiltration. Fasting is a physiological situation of increased FFA release from adipocytes and delivery to the liver and has been described as having a role in monocyte recruitment to AT. Thus, mice were challenged with a 16-h fast, followed by liver immunophenotype assessment. As expected, fasting increased plasma FFA by ~twofold (Fig. 4A). Similar to lipid infusions, plasma TNFα and MCP-1 were unaltered by fasting (Fig. 4D), and liver triglycerides were elevated (Fig. 4B). However, unlike with lipid infusions, liver oxidative stress was increased as evidenced by lowered GSH levels (Fig. 4E). Consistent with lipid infusion results, fasting increased cDC and MC content of the liver, with no effects on pDC (Fig. 4C). Notably, AT and splenic myeloid cells decreased (Fig. 4C). To address the contribution more precisely of AT-derived FFA to this phenotype, similar fasting experiments were performed in AAKO mice that have substantially reduced lipolytic activity in response to fasting and β-adrenergic stimulation (35). As anticipated, neither circulating FFA nor liver triglyceride levels were altered in fasted AAKO mice (Fig. 5, A–B). In contrast to wild-type mice, fasting did not result in altered hepatic myeloid cell content in AAKO mice (Fig. 5C), data that are consistent with the protection of the AAKO liver from obesity-induced hepatic inflammation, as previously reported (35) (Fig. 5D). Similar to wild-type mice, AT and splenic myeloid cells decreased or trended toward a decrease in fasted AAKO mice (Fig. 5C). Notably, despite the reduction in FFA supply to the liver, fasting also induced hepatic oxidative stress in AAKO mice (Fig. 5E). Together, these data demonstrate that AT-derived FFA are necessary and sufficient to initiate hepatic DC and MC accumulation in the liver, independent of plasma cytokine alterations, and that oxidative stress, although occurring in the liver in response to fasting, does not result in myeloid cell infiltration when AT-derived FFA supply to the liver is inhibited.
Fig. 4.
The effects of fasting on tissue DC/MC content. Male mice were fasted for 16 h (fast) or allowed ad libitum access to food (fed) before analyses. A: plasma nonesterified fatty acids (FFA, n = 5); B: liver triglyceride (TG; n = 5); C: liver, adipose tissue, and splenic dendritic cells (cDC, pDC), and monocyte/macrophage (MC) populations as assessed by flow cytometry (n = 4–5); D: plasma MCP-1 and TNFα (n = 2–7); and E: liver glutathione (GSH; n = 4). All plots represent mean ± SE and significant differences are indicated (*P < 0.05 fed vs. fast). cDC, conventional DC; DC, dendritic cell; FFA, free fatty acid; MCP, monocyte chemoattractant protein; pDC, plasmacytoid DC; NEFA, nonesterified fatty acid.
Fig. 5.
The effects of impaired adipose tissue lipolysis on fasting-induced increases in hepatic DC/MC content. Male mice containing an adipocyte-specific Atgl deletion (AAKO) were fasted for 16 h (fast) or allowed ad libitum access to food (fed) before analyses. A: plasma nonesterified fatty acids (FFA, n = 4); B: liver triglyceride levels (TG, n = 4); C: liver, adipose tissue, and splenic dendritic cells (cDC, pDC), and monocyte/macrophage (MC) populations as assessed by flow cytometry (n = 4); D: similar populations as in C were assessed in the liver of lean and obese AAKO mice (n = 7); E: liver glutathione (GSH; n = 4). All plots represent mean ± SE and significant differences are indicated (*P < 0.05 AAKO fed vs. AAKO fast). cDC, conventional DC; DC, dendritic cell; FFA, free fatty acid; MCP, monocyte chemoattractant protein; pDC, plasmacytoid DC; NEFA, nonesterified fatty acid.
Myeloid Cell Content of the Liver in States of Short- and Long-Term Overnutrition Dissociates from Oxidative Stress Responses but Does Track with Liver Triglyceride Levels
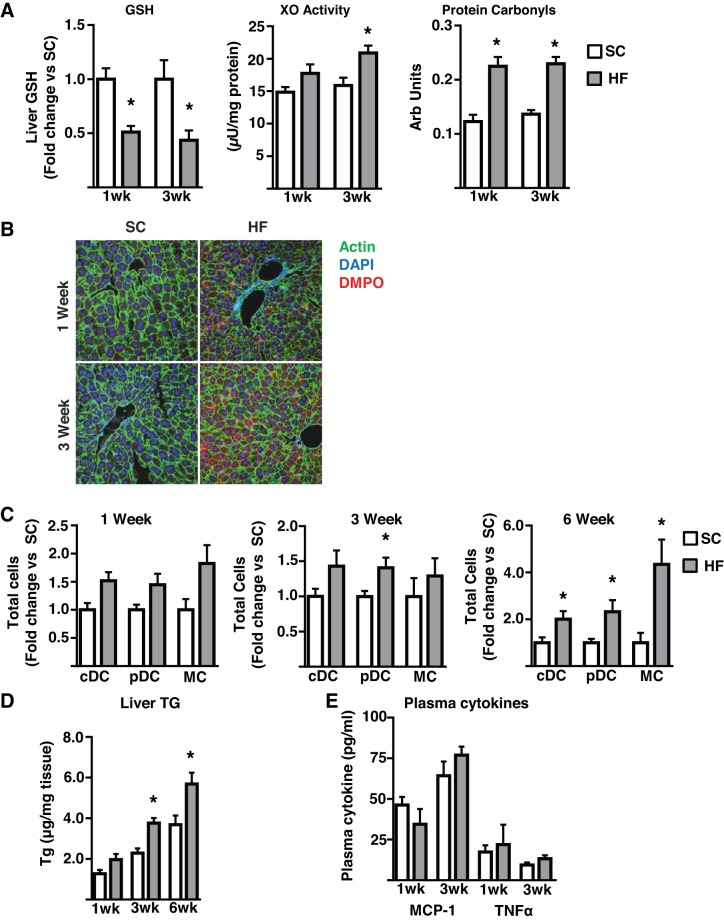
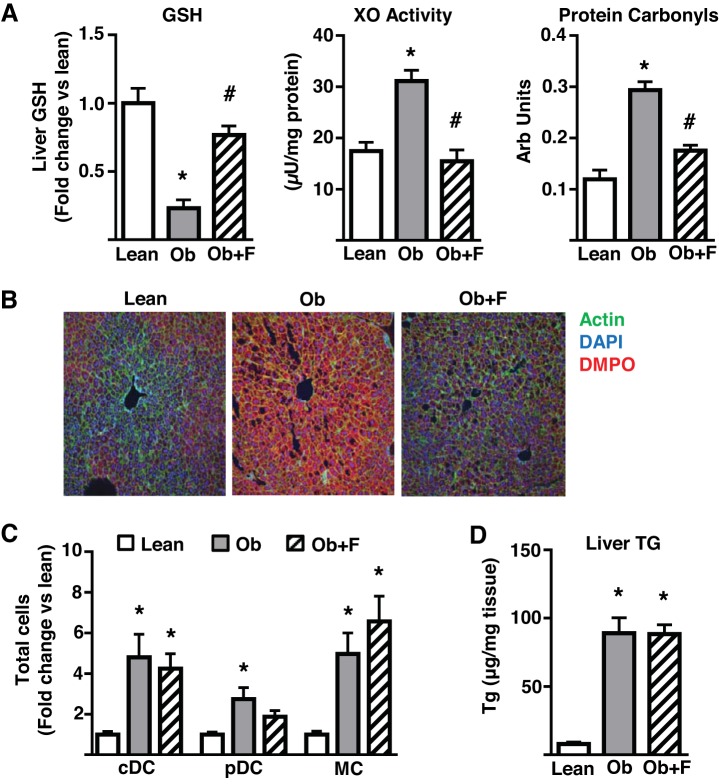
The data obtained from the lipid infusion, fasting, and AAKO mice are generally consistent with the concept that the increased DC/MC infiltration of liver in response to FFA oversupply is independent of measureable alterations in plasma cytokines but tracks closely to liver triglyceride levels, a surrogate marker of FFA oversupply to the liver. However, our data are inconsistent in respect of the hepatic oxidative stress response and MC/DC infiltration, suggesting that the oxidative stress response may be separable from myeloid cell accumulation in the liver, at least in the models utilized herein. To investigate this issue further, we determined the effects of a short-term, high-fat (41% of calories), high-sucrose diet (1, 3, and 6 wk) on liver oxidative stress and DC/MC content using a number of separate markers of oxidative stress. The selected diet is less obesogenic than the calorically denser 60% high-fat diet, therefore increasing the possibility of dissociating (temporally) oxidative stress and immunological alterations. Thus, a 1-wk diet was sufficient to alter a panel of liver oxidative stress markers (GSH levels, xanthine oxidase activity, protein carbonyls, and biomolecular free radicals as measured by immuno-spin trapping) (Fig. 6, A–B). However, despite a trend toward an increase, this length of diet had no significant effect on hepatic DC/MC content (Fig. 6C). Notably, also at this 1-wk time point, hepatic triglycerides trended upwards (Fig. 6D) whereas plasma MCP-1 and TNFα were unaltered (Fig. 6E). By 3 wk of diet, the oxidative stress response was similar to that observed with 1 wk of diet (Fig. 6A), liver triglycerides were significantly elevated (Fig. 6D), and plasma TNFα and MCP-1 remained unchanged (Fig. 6E). Here again there was no significant change in cDC or MC content, but there was a significant increase in liver pDC content (Fig. 6C). However, by 6 wk of diet, DC (pDC and cDC) and MC content were highly elevated (Fig. 6C), as were liver triglycerides (Fig. 6D); oxidative stress was not assessed at this time point because of lack of tissue. In further studies, we administered febuxostat, a xanthine oxidase inhibitor (30), in drinking water of diet-induced obese mice (20 wk of obesogenic diet), having established that this intervention decreases obesity-related liver oxidative stress by ~85% without altering body weight or caloric intake (unpublished observations). As expected, obese mice displayed pronounced hepatic oxidative stress (Fig. 7, A–B). Concurrent high-fat diet feeding and febuxostat treatment for 7 wk was sufficient to reduce all markers of obesity-induced oxidative stress to levels approaching those observed in lean mice (Fig. 7, A–B). Consistent with previous findings, livers from obese mice displayed highly elevated DC/MC (Fig. 7C) and triglyceride levels (Fig. 7D). However, the reduction in oxidative stress had no impact on hepatic DC/MC or triglyceride accumulations. These data demonstrate that diet-induced hepatic oxidative stress can be temporally and pharmacologically decoupled from hepatic DC/MC content. Furthermore, hepatic triglyceride levels track more closely to DC/MC content than oxidative stress. Together, these observations are consistent with the data obtained from the acute models of altered FFA delivery to the liver.
Fig. 6.
The effects of a short-term high-fat, high-sucrose diet on hepatic oxidative stress, triglyceride accumulation, and DC/MC accumulation. Male mice were fed either standard chow (SC) or a high-fat, high-sucrose (HF) diet for the indicated length of time before analyses. A: glutathione (GSH), xanthine oxidase (XO) activity, protein carbonyls. B: representative immunofluorescence images of liver biomolecular free radical adducts assessed by immuno-spin trapping following intraperitoneal injections of 5-Dimethyl-1Pyrroline-N-Oxide (DMPO) 24, 12, and 6 h before euthanasia (n = 3–4 all groups); C: liver dendritic cells (cDC, pDC) and monocyte/macrophage (MC) populations as assessed by flow cytometry (n = 4–6 all groups); D: liver triglyceride levels (n = 4–7); E: plasma MCP-1 and TNFα (n = 4–6). All plots represent mean ± SE and significant differences are indicated (*P < 0.05, HF vs. SC). cDC, conventional DC; DC, dendritic cell; MCP, monocyte chemoattractant protein; pDC, plasmacytoid DC; TG, triglyceride.
Fig. 7.
The effects of lowered oxidative stress on obesity-induced hepatic DC/MC accumulation. Male mice were fed either a control (Lean) or a high-fat (Ob) diet for 20 wk. Mice treated with febuxostat (Ob + F) were fed similarly to Ob mice but were provided febuxostat (50 mg/l) in their drinking water for the final 7 wk of the study. A: glutathione (GSH), xanthine oxidase (XO) activity, protein carbonyls. B: representative immunofluorescence images of liver biomolecular free radical adducts assessed by immuno-spin trapping following intraperitoneal injections of 5-Dimethyl-1Pyrroline-N-Oxide (DMPO) 24, 12, and 6 h before euthanasia (n = 7 all groups); C: liver dendritic cells (cDC, pDC), and monocyte/macrophage (MC) populations as assessed by flow cytometry (n = 6 all groups); D: liver triglyceride levels (TG; n = 5–7). All plots represent mean ± SE and significant differences are indicated (*P < 0.05 Ob vs. Lean; #P < 0.05 Ob + F vs. Ob). cDC, conventional DC; DC, dendritic cell; pDC, plasmacytoid DC; TG, triglyceride.
DISCUSSION
The goal of the current study was to understand the role of AT-derived FFAs in the initiation of liver myeloid cell (DC and MC) infiltration associated with obesity. The data support a series of conclusions. In a number of independent in vivo models, the data demonstrate that 1) hepatic DC/MC infiltration is rapidly induced in situations of increased FFA supply to the liver, and is absent when AT lipolysis is impaired, 2) that FFA, and more specifically AT-derived FFAs, are necessary and sufficient to induce DC/MC infiltration, 3) that the onset or maintenance of DC/MC infiltration is not strongly correlated to the presence of hepatic oxidative stress or plasma cytokine levels, and 4) that liver triglyceride levels represent the strongest correlate with hepatic DC/MC infiltration as an aggregate of all the models studied, likely reflecting elevated FFA delivery to the liver.
We utilized a number of in vivo models to evaluate the role for FFAs in initiating liver DC/MC infiltration. One, the lipid infusion model, results in increased circulating FFA concentrations to levels observed in obesity. Previous use of this model by a number of groups (20, 36), including ours (2, 11, 32), has demonstrated lipid accumulation in liver and skeletal muscle and the induction of insulin resistance. The current data in essence adds another important piece to the relationships between the triumvirate of dyslipidemia, insulin resistance, and inflammation. That these three pathologies track each other is hardly surprising given their lockstep occurrence in many rodent obesity models and in the metabolic syndrome in humans. The design of our lipid studies (i.e., a 16-h infusion) was devised to mimic the more physiological model of overnight fasting, the second model we used to manipulate FFA levels. The impetus to utilize a fasting model was based on previous work that demonstrated a role for AT lipolysis in the initiation of F4/80+ cell accumulation in AT (21). Similarly, our data showed that increased FFA mobilization results in elevated myeloid cell recruitment to the liver. Surprisingly, we did not observe the expected increase in AT myeloid cells following fasting. Indeed, in our analysis these cells decreased in both AT and spleen. The reason for this discrepancy is unclear. One possibility, that the difference in the data may have resulted from the previous study tracking cell accumulation using a different set of cell markers (F4/80 vs. CD11b/CD11c), was ruled out after we reanalyzed our data gating on F4/80+ cells and found similar decreases (data not shown). It should be noted though that Kosteli et al. (21) used immunohistochemistry and quantitative PCR for their analysis, and previous findings from our group using similar techniques also demonstrated that fasting increases AT crown-like structure formation and elevates AT Cd11c and F4/80 mRNA (35). However, regardless of this issue, our data identify the liver as another focal tissue for myeloid cell accumulation in response to elevated lipolysis.
The experiments discussed above relied on elevating circulating FFA, in one case in the physiological setting of fasting. The opposing experiment where the physiological fasting response remains intact whereas the elevated FFA supply to the liver is removed can be achieved by the inhibition of lipolysis. Herein, we utilized the AAKO mouse, a model of severely impaired AT lipolysis that contains an adipocyte-specific deletion of Atgl. Previous studies using this model involving our group demonstrated that AAKO mice are protected against obesity-induced steatosis and insulin resistance, despite becoming as obese as wild-type mice on a high-fat diet (35). Importantly, obese AAKO mice are also resistant to hepatic inflammation (35), which suggested that AT-derived FFAs play a prominent role in promoting hepatic inflammation in obesity. Given the effects of lipid infusions and fasting on the myeloid cell content of liver, our expectation was that this genetic intervention would prevent fasting-induced DC/MC cell accumulation. This indeed turned out to be the case, providing compelling evidence for a central role of adipocyte-derived FFAs in stimulating the recruitment of DC/MC to the liver.
A notable, if unexpected, observation arising from our studies was the lack of a strong positive relationship between markers of oxidative stress and DC/MC cell infiltration of the liver. Thus, although a lipid infusion induced immune cell infiltration, this event occurred in the absence of changes in oxidative stress as assessed by GSH levels. Conversely, fasting induced both oxidative stress and myeloid cell infiltration, suggesting a causal relationship. However, when the fasting experiment was performed in the AAKO mouse, the oxidative stress response was maintained, yet myeloid cell infiltration was prevented. The inconsistent relationship between FFA delivery, DC/MC infiltration, and oxidative stress in these studies led us to investigate further the temporal relationship between the initiation of inflammation (as measured by DC/MC infiltration) and oxidative stress under conditions of high-fat feeding. Importantly, little dietary FFA goes directly to the liver. Following absorption from the diet, FFAs (excluding short-chain and medium-chain FFAs, which are minimally present in the normal diet) are packaged into triglycerides and subsequently into chylomicrons. Chylomicron-associated FFAs (derived from hydrolyzed triglycerides, a reaction catalyzed by lipoprotein lipase) then enter peripheral tissues such as AT (lipoprotein lipase is not expressed in the adult liver) and are reesterified to triglycerides. Subsequently, the stored AT FFAs are mobilized as needed and delivered to other tissues, the liver being a predominant recipient. Interestingly, a similar lack of correlation between hepatic oxidative stress and myeloid cell infiltration was observed under short-term diet conditions. Thus, a 1-wk high-fat diet induced hepatic oxidative stress yet did not increase myeloid cell infiltration significantly. A further 2 wk of diet was required to demonstrate a positive correlation between liver myeloid cell increases and oxidative stress, but even here only one of the three populations studied (pDC) was elevated. Finally, in further studies in obese mice, an intervention (febuxostat) that reduced markers of liver oxidative stress by ~80% had minimal impact on the hepatic inflammatory response. Although these data do not rule out a role for oxidative stress in the initiation of liver inflammation in states of overnutrition, it does not appear to be necessary or sufficient for the DC/MC cell infiltration associated with acute/short-term oversupply of FFA to the liver. However, it is likely that oxidative stress impacts inflammatory function once recruited cells appear in the liver. Indeed, intracellular ROS activates the NLRP3 inflammasome (40)—a multiprotein complex that leads to aggressive IL-1β production in macrophages and DCs—and oxidized mitochondrial DNA elevates the immune activity of pDCs (33). Therefore, it is possible that oxidative stress has a greater capacity to activate immune cells within the liver than to facilitate their recruitment. Also, in reference to our studies using febuxostat, because this intervention inhibits only one pathway of oxidative stress, we cannot rule out that other sources of reactive oxygen species such as increased β-oxidation and/or endoplasmic reticulum stress may contribute to myeloid cell accumulation.
One particular aspect of our study that requires further comment is the functional role of the two different DC subsets assessed. The work of a number of groups based on marker profiling, molecular fingerprinting, and functional assays have defined at least two major DC subsets in the liver: plasmacytoid (marker profile CD11b−CD11c+B220+) and conventional (marker profile CD11b+CD11c+), with additional subsets reported within the cDC population (14, 25, 39). Indeed, the work of Miller et al. (25) and Gautier et al. (14) demonstrates that in liver, practically all CD11c+ cells are likely DCs, unlike other tissues, including AT, where CD11c can also be used as a macrophage marker. In general, cDCs more efficiently process and present antigen and produce higher levels of proinflammatory cytokines such as TNFα and IL-12 than pDCs, whereas pDCs are involved in maintaining antigen tolerance and responding to viral infection rapidly (24). Although functional effects of progressing obesity on subset-specific DCs are unknown, the two CD11c+ populations evaluated in this study responded differently to acute FFA treatment and expanded at different rates during obesity progression. Thus, cDC accumulated rapidly in response to FFA delivery, whereas pDC were the first to increase after exposure to a western diet. Notably, cDC eventually surpasses pDC content in liver in long-term obesity studies. Together, these data suggest that there are likely different mechanisms of recruitment to, or expansion within, the liver, and that although there is a clear case to be made for a direct role of FFA in the recruitment of cDC the same cannot be said of pDC. Interestingly, the monocyte/macrophage (CD11b+CD11c−) cell accumulation mirrored that of cDC in the liver. Although this population is not believed to contain DCs per se, it includes a large fraction of CCR2+ monocytes (26) that have the capacity to differentiate into inflammatory macrophages and DCs in other tissues (10). Although it has yet to be shown, it is possible that once recruited to the liver, these cells can differentiate into cDCs and that one source of the elevation in liver cDC is ultimately derived from infiltrating MC. This would help explain why cDCs accumulate more rapidly in response to acute FFA delivery and why their numbers increase at similar rates as MC cells under most of our experimental conditions. Although beyond the scope of the current study, it would certainly be important to delineate more closely the sources of and roles for the different myeloid populations that accumulate in the liver in response to elevated FFA supply and obesity.
In conclusion, mounting evidence indicates that obesity-associated chronic inflammation in liver contributes to metabolic dysfunction, but the mechanisms that initiate immune cell influx, and therefore the initiation of the altered immnunophenotype of the obese liver, remain incompletely defined. Our findings provide the first in vivo evidence, to our knowledge, that AT-derived FFAs can initiate an important aspect of the metainflammation of liver and suggest that lowering FFA supply from the AT may improve hepatic metabolic function by reducing a signal required for this process, and subsequent inflammatory responses, to occur.
GRANTS
This work was supported by Grant Nos. NIH-R01-DK102839 and NIH-T32-DK007052 (to R. O’Doherty), NIH Grant No. R01-DK-090166 (to E. Kershaw), NIH Grant No. P01-AG-043376-02S1 and National Institute of General Medical Sciences Grant No. P20 GM109098 (to E. Kelley). C. Wu was supported by the Xiangya Medical School of Central South University-University of Pittsburgh Research Training Program, and D. Harmon was supported by NIH Grant No. T32-DK-007052. Relevant studies were supported by the Unified Flow Cytometry Core and the Center for Biological Imaging at the University of Pittsburgh.
DISCLOSURES
No conflicts of interest, financial or otherwise, are declared by the authors.
AUTHOR CONTRIBUTIONS
D.B.H., C.W., M.S.-R., G.S., C.P.O., L.C.A., E.E. Kershaw, E.E. Kelley, and R.M.O. conceived and designed research; D.B.H., C.W., N.D., I.J.S., M.S.-R., and G.S. performed experiments; D.B.H., C.W., N.D., I.J.S., M.S.-R., G.S., and R.M.O. analyzed data; D.B.H., C.W., M.S.-R., G.S., and R.M.O. interpreted results of experiments; D.B.H. and R.M.O. prepared figures; D.B.H. and R.M.O. drafted manuscript; D.B.H., M.S.-R., and R.M.O. edited and revised manuscript; D.B.H., C.W., N.D., I.J.S., M.S.-R., G.S., C.P.O., L.C.A., E.E. Kershaw, E.E. Kelley, and R.M.O. approved final version of manuscript; R.M.O. is the guarantor of this study.
REFERENCES
- 1.Abe T, Hirasaka K, Kagawa S, Kohno S, Ochi A, Utsunomiya K, Sakai A, Ohno A, Teshima-Kondo S, Okumura Y, Oarada M, Maekawa Y, Terao J, Mills EM, Nikawa T. Cbl-b is a critical regulator of macrophage activation associated with obesity-induced insulin resistance in mice. Diabetes 62: 1957–1969, 2013. doi: 10.2337/db12-0677. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Alonso LC, Yokoe T, Zhang P, Scott DK, Kim SK, O’Donnell CP, Garcia-Ocaña A. Glucose infusion in mice: a new model to induce beta-cell replication. Diabetes 56: 1792–1801, 2007. doi: 10.2337/db06-1513. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Bertola A, Ciucci T, Rousseau D, Bourlier V, Duffaut C, Bonnafous S, Blin-Wakkach C, Anty R, Iannelli A, Gugenheim J, Tran A, Bouloumié A, Gual P, Wakkach A. Identification of adipose tissue dendritic cells correlated with obesity-associated insulin-resistance and inducing Th17 responses in mice and patients. Diabetes 61: 2238–2247, 2012. doi: 10.2337/db11-1274. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Boden G, She P, Mozzoli M, Cheung P, Gumireddy K, Reddy P, Xiang X, Luo Z, Ruderman N. Free fatty acids produce insulin resistance and activate the proinflammatory nuclear factor-kappaB pathway in rat liver. Diabetes 54: 3458–3465, 2005. doi: 10.2337/diabetes.54.12.3458. [DOI] [PubMed] [Google Scholar]
- 5.Caesar R, Reigstad CS, Bäckhed HK, Reinhardt C, Ketonen M, Lundén GO, Cani PD, Bäckhed F. Gut-derived lipopolysaccharide augments adipose macrophage accumulation but is not essential for impaired glucose or insulin tolerance in mice. Gut 61: 1701–1707, 2012. doi: 10.1136/gutjnl-2011-301689. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Cani PD, Amar J, Iglesias MA, Poggi M, Knauf C, Bastelica D, Neyrinck AM, Fava F, Tuohy KM, Chabo C, Waget A, Delmée E, Cousin B, Sulpice T, Chamontin B, Ferrières J, Tanti JF, Gibson GR, Casteilla L, Delzenne NM, Alessi MC, Burcelin R. Metabolic endotoxemia initiates obesity and insulin resistance. Diabetes 56: 1761–1772, 2007. doi: 10.2337/db06-1491. [DOI] [PubMed] [Google Scholar]
- 7.Chawla A, Nguyen KD, Goh YP. Macrophage-mediated inflammation in metabolic disease. Nat Rev Immunol 11: 738–749, 2011. doi: 10.1038/nri3071. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Cho KW, Zamarron BF, Muir LA, Singer K, Porsche CE, DelProposto JB, Geletka L, Meyer KA, O’Rourke RW, Lumeng CN. Adipose tissue dendritic cells are independent contributors to obesity-induced inflammation and insulin resistance. J Immunol 197: 3650–3661, 2016. doi: 10.4049/jimmunol.1600820. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.de Meijer VE, Sverdlov DY, Le HD, Popov Y, Puder M. Tissue-specific differences in inflammatory infiltrate and matrix metalloproteinase expression in adipose tissue and liver of mice with diet-induced obesity. Hepatol Res 42: 601–610, 2012. doi: 10.1111/j.1872-034X.2011.00960.x. [DOI] [PubMed] [Google Scholar]
- 10.Domínguez PM, Ardavín C. Differentiation and function of mouse monocyte-derived dendritic cells in steady state and inflammation. Immunol Rev 234: 90–104, 2010. doi: 10.1111/j.0105-2896.2009.00876.x. [DOI] [PubMed] [Google Scholar]
- 11.Dube JJ, Bhatt BA, Dedousis N, Bonen A, O’Doherty RM. Leptin, skeletal muscle lipids, and lipid-induced insulin resistance. Am J Physiol Regul Integr Comp Physiol 293: R642–R650, 2007. doi: 10.1152/ajpregu.00133.2007. [DOI] [PubMed] [Google Scholar]
- 12.Ferrante AW., JR The immune cells in adipose tissue. Diabetes Obes Metab 15, Suppl 3: 34–38, 2013. doi: 10.1111/dom.12154. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Fink LN, Costford SR, Lee YS, Jensen TE, Bilan PJ, Oberbach A, Blüher M, Olefsky JM, Sams A, Klip A. Pro-inflammatory macrophages increase in skeletal muscle of high fat-fed mice and correlate with metabolic risk markers in humans. Obesity (Silver Spring) 22: 747–757, 2014. doi: 10.1002/oby.20615. [DOI] [PubMed] [Google Scholar]
- 14.Gautier EL, Shay T, Miller J, Greter M, Jakubzick C, Ivanov S, Helft J, Chow A, Elpek KG, Gordonov S, Mazloom AR, Ma’ayan A, Chua WJ, Hansen TH, Turley SJ, Merad M, Randolph GJ; Immunological Genome Consortium . Gene-expression profiles and transcriptional regulatory pathways that underlie the identity and diversity of mouse tissue macrophages. Nat Immunol 13: 1118–1128, 2012. doi: 10.1038/ni.2419. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Ghosh AR, Bhattacharya R, Bhattacharya S, Nargis T, Rahaman O, Duttagupta P, Raychaudhuri D, Liu CS, Roy S, Ghosh P, Khanna S, Chaudhuri T, Tantia O, Haak S, Bandyopadhyay S, Mukhopadhyay S, Chakrabarti P, Ganguly D. Adipose recruitment and activation of plasmacytoid dendritic cells fuel metaflammation. Diabetes 65: 3440–3452, 2016. doi: 10.2337/db16-0331. [DOI] [PubMed] [Google Scholar]
- 16.Kanda H, Tateya S, Tamori Y, Kotani K, Hiasa K, Kitazawa R, Kitazawa S, Miyachi H, Maeda S, Egashira K, Kasuga M. MCP-1 contributes to macrophage infiltration into adipose tissue, insulin resistance, and hepatic steatosis in obesity. J Clin Invest 116: 1494–1505, 2006. doi: 10.1172/JCI26498. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Kelley EE, Trostchansky A, Rubbo H, Freeman BA, Radi R, Tarpey MM. Binding of xanthine oxidase to glycosaminoglycans limits inhibition by oxypurinol. J Biol Chem 279: 37231–37234, 2004. doi: 10.1074/jbc.M402077200. [DOI] [PubMed] [Google Scholar]
- 18.Khoo NK, Cantu-Medellin N, Devlin JE, St Croix CM, Watkins SC, Fleming AM, Champion HC, Mason RP, Freeman BA, Kelley EE. Obesity-induced tissue free radical generation: an in vivo immuno-spin trapping study. Free Radic Biol Med 52: 2312–2319, 2012. doi: 10.1016/j.freeradbiomed.2012.04.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Khoo NK, Cantu-Medellin N, St Croix C, Kelley EE. In vivo immuno-spin trapping: imaging the footprints of oxidative stress. Curr Protoc Cytom 74: 12.42.1–12.42.11, 2015. doi: 10.1002/0471142956.cy1242s74. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Kim JK, Fillmore JJ, Sunshine MJ, Albrecht B, Higashimori T, Kim DW, Liu ZX, Soos TJ, Cline GW, O’Brien WR, Littman DR, Shulman GI. PKC-theta knockout mice are protected from fat-induced insulin resistance. J Clin Invest 114: 823–827, 2004. doi: 10.1172/JCI200422230. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Kosteli A, Sugaru E, Haemmerle G, Martin JF, Lei J, Zechner R, Ferrante AW JR. Weight loss and lipolysis promote a dynamic immune response in murine adipose tissue. J Clin Invest 120: 3466–3479, 2010. doi: 10.1172/JCI42845. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Leroux A, Ferrere G, Godie V, Cailleux F, Renoud ML, Gaudin F, Naveau S, Prévot S, Makhzami S, Perlemuter G, Cassard-Doulcier AM. Toxic lipids stored by Kupffer cells correlates with their pro-inflammatory phenotype at an early stage of steatohepatitis. J Hepatol 57: 141–149, 2012. doi: 10.1016/j.jhep.2012.02.028. [DOI] [PubMed] [Google Scholar]
- 23.Lumeng CN, Saltiel AR. Inflammatory links between obesity and metabolic disease. J Clin Invest 121: 2111–2117, 2011. doi: 10.1172/JCI57132. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Merad M, Sathe P, Helft J, Miller J, Mortha A. The dendritic cell lineage: ontogeny and function of dendritic cells and their subsets in the steady state and the inflamed setting. Annu Rev Immunol 31: 563–604, 2013. doi: 10.1146/annurev-immunol-020711-074950. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Miller JC, Brown BD, Shay T, Gautier EL, Jojic V, Cohain A, Pandey G, Leboeuf M, Elpek KG, Helft J, Hashimoto D, Chow A, Price J, Greter M, Bogunovic M, Bellemare-Pelletier A, Frenette PS, Randolph GJ, Turley SJ, Merad M; Immunological Genome Consortium . Deciphering the transcriptional network of the dendritic cell lineage. Nat Immunol 13: 888–899, 2012. doi: 10.1038/ni.2370. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Morinaga H, Mayoral R, Heinrichsdorff J, Osborn O, Franck N, Hah N, Walenta E, Bandyopadhyay G, Pessentheiner AR, Chi TJ, Chung H, Bogner-Strauss JG, Evans RM, Olefsky JM, Oh DY. Characterization of distinct subpopulations of hepatic macrophages in HFD/obese mice. Diabetes 64: 1120–1130, 2015. doi: 10.2337/db14-1238. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Nguyen MT, Favelyukis S, Nguyen AK, Reichart D, Scott PA, Jenn A, Liu-Bryan R, Glass CK, Neels JG, Olefsky JM. A subpopulation of macrophages infiltrates hypertrophic adipose tissue and is activated by free fatty acids via Toll-like receptors 2 and 4 and JNK-dependent pathways. J Biol Chem 282: 35279–35292, 2007. doi: 10.1074/jbc.M706762200. [DOI] [PubMed] [Google Scholar]
- 28.Nishimura S, Manabe I, Nagasaki M, Eto K, Yamashita H, Ohsugi M, Otsu M, Hara K, Ueki K, Sugiura S, Yoshimura K, Kadowaki T, Nagai R. CD8+ effector T cells contribute to macrophage recruitment and adipose tissue inflammation in obesity. Nat Med 15: 914–920, 2009. doi: 10.1038/nm.1964. [DOI] [PubMed] [Google Scholar]
- 29.Oh DY, Morinaga H, Talukdar S, Bae EJ, Olefsky JM. Increased macrophage migration into adipose tissue in obese mice. Diabetes 61: 346–354, 2012. doi: 10.2337/db11-0860. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Okamoto K, Eger BT, Nishino T, Kondo S, Pai EF, Nishino T. An extremely potent inhibitor of xanthine oxidoreductase. Crystal structure of the enzyme-inhibitor complex and mechanism of inhibition. J Biol Chem 278: 1848–1855, 2003. doi: 10.1074/jbc.M208307200. [DOI] [PubMed] [Google Scholar]
- 31.Olefsky JM, Glass CK. Macrophages, inflammation, and insulin resistance. Annu Rev Physiol 72: 219–246, 2010. doi: 10.1146/annurev-physiol-021909-135846. [DOI] [PubMed] [Google Scholar]
- 32.Pascoe J, Hollern D, Stamateris R, Abbasi M, Romano LC, Zou B, O’Donnell CP, Garcia-Ocana A, Alonso LC. Free fatty acids block glucose-induced β-cell proliferation in mice by inducing cell cycle inhibitors p16 and p18. Diabetes 61: 632–641, 2012. doi: 10.2337/db11-0991. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Pazmandi K, Agod Z, Kumar BV, Szabo A, Fekete T, Sogor V, Veres A, Boldogh I, Rajnavolgyi E, Lanyi A, Bacsi A. Oxidative modification enhances the immunostimulatory effects of extracellular mitochondrial DNA on plasmacytoid dendritic cells. Free Radic Biol Med 77: 281–290, 2014. doi: 10.1016/j.freeradbiomed.2014.09.028. [DOI] [PubMed] [Google Scholar]
- 34.Reynolds CM, McGillicuddy FC, Harford KA, Finucane OM, Mills KH, Roche HM. Dietary saturated fatty acids prime the NLRP3 inflammasome via TLR4 in dendritic cells-implications for diet-induced insulin resistance. Mol Nutr Food Res 56: 1212–1222, 2012. doi: 10.1002/mnfr.201200058. [DOI] [PubMed] [Google Scholar]
- 35.Schoiswohl G, Stefanovic-Racic M, Menke MN, Wills RC, Surlow BA, Basantani MK, Sitnick MT, Cai L, Yazbeck CF, Stolz DB, Pulinilkunnil T, O’Doherty RM, Kershaw EE. Impact of reduced ATGL-mediated adipocyte lipolysis on obesity-associated insulin resistance and inflammation in male mice. Endocrinology 156: 3610–3624, 2015. doi: 10.1210/en.2015-1322. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Shi H, Kokoeva MV, Inouye K, Tzameli I, Yin H, Flier JS. TLR4 links innate immunity and fatty acid-induced insulin resistance. J Clin Invest 116: 3015–3025, 2006. doi: 10.1172/JCI28898. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Stefanovic-Racic M, Perdomo G, Mantell BS, Sipula IJ, Brown NF, O’Doherty RM. A moderate increase in carnitine palmitoyltransferase 1a activity is sufficient to substantially reduce hepatic triglyceride levels. Am J Physiol Endocrinol Metab 294: E969–E977, 2008. doi: 10.1152/ajpendo.00497.2007. [DOI] [PubMed] [Google Scholar]
- 38.Stefanovic-Racic M, Yang X, Turner MS, Mantell BS, Stolz DB, Sumpter TL, Sipula IJ, Dedousis N, Scott DK, Morel PA, Thomson AW, O’Doherty RM. Dendritic cells promote macrophage infiltration and comprise a substantial proportion of obesity-associated increases in CD11c+ cells in adipose tissue and liver. Diabetes 61: 2330–2339, 2012. doi: 10.2337/db11-1523. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Sumpter TL, Abe M, Tokita D, Thomson AW. Dendritic cells, the liver, and transplantation. Hepatology 46: 2021–2031, 2007. doi: 10.1002/hep.21974. [DOI] [PubMed] [Google Scholar]
- 40.Tschopp J, Schroder K. NLRP3 inflammasome activation: The convergence of multiple signalling pathways on ROS production? Nat Rev Immunol 10: 210–215, 2010. doi: 10.1038/nri2725. [DOI] [PubMed] [Google Scholar]
- 41.Varma V, Yao-Borengasser A, Rasouli N, Nolen GT, Phanavanh B, Starks T, Gurley C, Simpson P, McGehee RE JR, Kern PA, Peterson CA. Muscle inflammatory response and insulin resistance: synergistic interaction between macrophages and fatty acids leads to impaired insulin action. Am J Physiol Endocrinol Metab 296: E1300–E1310, 2009. doi: 10.1152/ajpendo.90885.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Weisberg SP, Hunter D, Huber R, Lemieux J, Slaymaker S, Vaddi K, Charo I, Leibel RL, Ferrante AW JR. CCR2 modulates inflammatory and metabolic effects of high-fat feeding. J Clin Invest 116: 115–124, 2006. doi: 10.1172/JCI24335. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Weisberg SP, McCann D, Desai M, Rosenbaum M, Leibel RL, Ferrante AW JR. Obesity is associated with macrophage accumulation in adipose tissue. J Clin Invest 112: 1796–1808, 2003. doi: 10.1172/JCI200319246. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Xu H, Barnes GT, Yang Q, Tan G, Yang D, Chou CJ, Sole J, Nichols A, Ross JS, Tartaglia LA, Chen H. Chronic inflammation in fat plays a crucial role in the development of obesity-related insulin resistance. J Clin Invest 112: 1821–1830, 2003. doi: 10.1172/JCI200319451. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Yang H, Youm YH, Vandanmagsar B, Ravussin A, Gimble JM, Greenway F, Stephens JM, Mynatt RL, Dixit VD. Obesity increases the production of proinflammatory mediators from adipose tissue T cells and compromises TCR repertoire diversity: implications for systemic inflammation and insulin resistance. J Immunol 185: 1836–1845, 2010. doi: 10.4049/jimmunol.1000021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Yekollu SK, Thomas R, O’Sullivan B. Targeting curcusomes to inflammatory dendritic cells inhibits NF-κB and improves insulin resistance in obese mice. Diabetes 60: 2928–2938, 2011. doi: 10.2337/db11-0275. [DOI] [PMC free article] [PubMed] [Google Scholar]