Abstract
Imitation plays a key role in social learning and in facilitating social interactions and likely constitutes a basic building block of social cognition that supports higher-level social abilities. Recent findings suggest that patients with schizophrenia have imitation impairments that could contribute to the social impairments associated with the disorder. However, extant studies have specifically assessed voluntary imitation or automatic imitation of emotional stimuli without controlling for potential confounders. The imitation impairments seen might therefore be secondary to other cognitive, motoric, or emotional deficits associated with the disorder. To overcome this issue, we used an automatic imitation paradigm with nonemotional stimuli to assess automatic imitation and the top-down modulation of imitation where participants were required to lift one of 2 fingers according to a number shown on the screen while observing the same or the other finger movement. In addition, we used a control task with a visual cue in place of a moving finger, to isolate the effect of observing finger movement from other visual cueing effects. Data from 33 patients (31 medicated) and 40 matched healthy controls were analyzed. Patients displayed enhanced imitation and intact top-down modulation of imitation. The enhanced imitation seen in patients may have been medication induced as larger effects were seen in patients receiving higher antipsychotic doses. In sum, we did not find an imitation impairment in schizophrenia. The results suggest that previous findings of impaired imitation in schizophrenia might have been due to other cognitive, motoric, and/or emotional deficits.
Keywords: social influence, social cognition, mimicry, top-down control, antipsychotic medication
Introduction
Imitation refers to the translation of perceived actions into executed actions.1 Imitation is a likely foundation for important social behaviors, ranging from social learning (eg, skill or language acquisition) to the ability to understand the intentions and feelings of others.2 Imitation also contributes to smoothness, predictability, and feelings of affiliation in social interactions.3,4 As such, research on imitation is crucial for understanding disorders that are characterized by impairments in social behavior and may provide a better understanding of the underlying deficits involved in such conditions.
Patients with schizophrenia display impairments that may involve imitation-related systems, including understanding the intentions and feelings of others5 and they tend to have difficulties with social interactions.6 Behavioral studies on imitation in schizophrenia suggest that patients are impaired at imitating others7–12, and it has been suggested that schizophrenia constitutes a disorder of imitation.13 However, there is clear heterogeneity between studies when looking at the biological foundations of the impairment. Some imaging and transcranial magnetic stimulation (TMS) studies find intact activity14–17 in the mirror neuron system (MNS)—which is thought to form the neural basis of imitation18–21—during action observation and execution; while others find this activity to be reduced,22,23 altered,13 or enhanced.14,24 The core MNS circuitry includes the pars opercularis of the inferior frontal gyrus and adjacent ventral premotor cortex (Brodmann area 44 and 6) and the rostral inferior parietal lobule as well as the superior temporal sulcus, which processes biological motion.
Several issues also remain unresolved with respect to the behavioral studies. Experimental work within this area can be roughly categorized into 4 domains: imitation can either be voluntary or automatic, and it can be of either emotional (eg, facial expressions) or nonemotional stimuli (eg, manual movements). Studies have primarily focused on voluntary imitation where participants, eg, are required to imitate certain movements. This research has consistently shown that patients make more errors when asked to imitate facial and manual movements8–10,25–28 and facial expressions7,10,26,29–31 compared to healthy individuals.
Meanwhile, research on automatic imitation has been limited, in particular when it comes to imitation of nonemotional stimuli. Such research is important for 3 main reasons. First, voluntary tasks are (to varying degrees) taxing on a range of cognitive processes that are known to be impaired in schizophrenia,32 but which are not specifically tied to imitation. For instance, voluntary imitation requires working memory, attention to detail, planning, and self-monitoring of accuracy. It is therefore unclear whether the imitation impairments seen are due to specific imitation deficits or due to general cognitive deficits, which, among other things, should also be expected to impact voluntary imitation. Automatic processes, on the other hand, are generally much less taxing on such cognitive processes.33
Second, assessing someone’s ability to voluntarily imitate is not the same as assessing their tendency to imitate or potential difficulties in the ability to inhibit involuntary imitation. This is important because reduced imitation tendency may result in worse social interactions,3 while overimitation may result in catatonic symptoms like echolalia or echopraxia seen in schizophrenia.34,35 The top-down modulation of imitation and the MNS is subserved by structures related to perspective taking or mentalizing (including medial prefrontal cortex and temporoparietal junction) as well as structures related to general cognitive control processes. Imitation-inhibition and general inhibition processes are thus thought to be at least partially distinct.36–44 Interestingly, reduced top-down modulation of imitation has also been associated with reduced mentalizing and perspective-taking ability,36,40 abilities known to be impaired in schizophrenia.45
Third, because patients display a variety of problems in processing of emotions,46 it is unclear whether a deficit in voluntary or automatic imitation of emotional stimuli11,12,47,48 would reflect a specific impairment in imitation, or other aspects of emotion processing: eg, differences in visual processing of faces (avoiding salient regions like the eyes and the mouth),49 the experience of emotional states, or emotional reactions to the stimuli.50 For instance, a characteristic symptom of schizophrenia is blunted affect.29,51 This makes it difficult to distinguish between direct effects of action observation on action execution (imitation) and those mediated by emotional states.
Automatic imitation of nonemotional stimuli has not been studied experimentally in patients with schizophrenia and would overcome the limitations mentioned above. We therefore set out to investigate whether the basic mechanisms of imitation and the top-down modulation of these (imitation-inhibition) are altered in schizophrenia, compared to healthy individuals.
Participants were asked to perform an automatic imitation task,52 where they performed certain finger movements according to the number shown on a screen, while observing the same or another finger move on the screen. Although the performed finger movements are voluntary, any effect of imitation on these is accidental since participants are not instructed to imitate, and general imitation results in poorer task performance. By assessing automatic processes, we effectively reduce the cognitive load; however, lower level attentional processes are still recruited and may be affected by schizophrenia. We therefore included a control task52 to be able to delineate imitation and imitation-inhibition from any attentional deficits or deficits in distractor-inhibition (for detailed task description, see Methods). This also allowed us to control for any motor deficits.
We predicted that patients would have slower reaction times (RTs) and make more errors than healthy individuals since patients display cognitive32 and motor deficits53 (eg, psychomotor slowing/reduced processing speed54). Importantly, we had 4 core research questions (1–4), we wanted to answer: we were interested in determining whether patients would show deficits beyond those that could be attributed merely to other cognitive or motoric deficits, indicating a specific deficit in either (1) basic imitation or (2) the top-down control of it (ie, imitation-inhibition). In addition, since 2 recent studies13,22 indicate that antipsychotic medication might normalize putative mirror neuron activity during action observation and imitation, we assessed whether (3) imitation or (4) imitation-inhibition tendency in patients was associated with antipsychotic medication dose. Finally, in case of group differences in imitation or imitation-inhibition, we were interested in assessing whether these were associated with the patients’ level of functioning.
Methods
Participants
Thirty-nine patients with an ICD-10 DCR diagnosis of schizophrenia or schizoaffective disorder and 40 matched healthy controls were included in the study. Diagnosis was confirmed using the Schedules for Clinical Assessment in Neuropsychiatry (SCAN).55,56 Controls were pairwise matched to the patients according to age, gender, childhood residence, as well as commenced educational level (or parents’ educational attainment if higher than the patients’) and parental socioeconomic status when possible (see table 1). Two of the controls were matched to patients that had to be excluded as they did not fulfill inclusion criteria and one patient did not have a matched control as they did not complete the whole study.
Table 1.
Demographic and Clinical Characteristics of Patients and Controls
| Schizophrenia (n = 33) | Controls (n = 40) | |
|---|---|---|
| Age, mean (SD) | 36.7 (10.1) | 39.3 (10.5) |
| Number of males:females | 22:11 | 27:13 |
| Number of right:left handed | 30:3 | 36:4 |
| Educational level commenceda, mean (SD) | 2.1 (0.6) | 2.3 (0.7) |
| Years of education, mean (SD) | 12.1 (2.6) | 14.4 (3.2) |
| Number of high:middle parental SESb | 11:22 | 13:27 |
| Level of functioning (PSP), mean (SD) | 57.5 (15.7) | 86.1 (5.1) |
| Positive symptoms (SAPS)c, mean (SD) | 4.8 (4.2) | — |
| Negative symptoms (SANS)d, mean (SD) | 8.15 (4.9) | — |
| CPZ equivalent dose in mg, mean (SD) | 809 (687) | — |
Note: CPZ, chlorpromazine; PSP, Personal and Social Performance Scale; SAPS, Scale for the Assessment of Positive Symptoms; SANS, Scale for the Assessment of Negative Symptoms; SES, socioeconomic status.
aEducational level commenced divided into 4 levels: 1: primary school (up to 10 y of education), 2: secondary school/professional training, 3: bachelor program, 4: master program.
bParental SES was divided into 3 levels; however, none of the parents had a low SES.
cSAPS is the total score of the 4 global items. It was not possible to obtain a SAPS score for one of the patients.
dSANS is the total score of the 5 global items.
Patients were recruited through the Psychiatric Centre of the National Hospital of the Faroe Islands. Controls were contacted based on their age and gender and, if they fulfilled the inclusion criteria and matched a patient, were offered to participate in the study. Participants were between 18 and 55 years old. Exclusion criteria included current psychoactive substance use disorders (except nicotine), a neurological or medical disorder that could affect brain functioning, and an estimated IQ below 70 based on prior history or testing. In addition, the controls were excluded if they or a first-degree relative had a history of severe mental disorder. The participants were screened for recent use of psychoactive substances (tetrahydrocannabinol/cannabis, opiates, amphetamine, ecstasy, benzodiazepines, cocaine) using a urine stick (NanoSticka 200–32). Patients, without a prescription who had a positive test, were excluded. None of the controls had a positive test. At the time of testing, all but 2 patients were taking antipsychotic medication. We converted antipsychotic doses to chlorpromazine equivalents57,58 (see supplementary table 1S for details). Some patients also took other types of medication (see supplementary table 2S).
Six patients had hand tremor that made it difficult or impossible for them to keep their fingers stable enough to complete the task. Data from these were excluded from the analysis. Data from 33 patients and 40 controls were included in the analysis.
General Procedure
The imitation task was administered as part of a larger battery of cognitive tasks. In addition, symptom severity and level of functioning were assessed with the Scale for the Assessment of Positive/Negative Symptoms (SAPS/SANS)51,59 and the Personal and Social Performance Scale (PSP),60 respectively (see table 1). The study complied with the ethical standards of the relevant national and institutional committees and with the Helsinki Declaration. Written informed consent was obtained from all participants after the procedure had been explained.
Imitation and Effector Priming Control Task
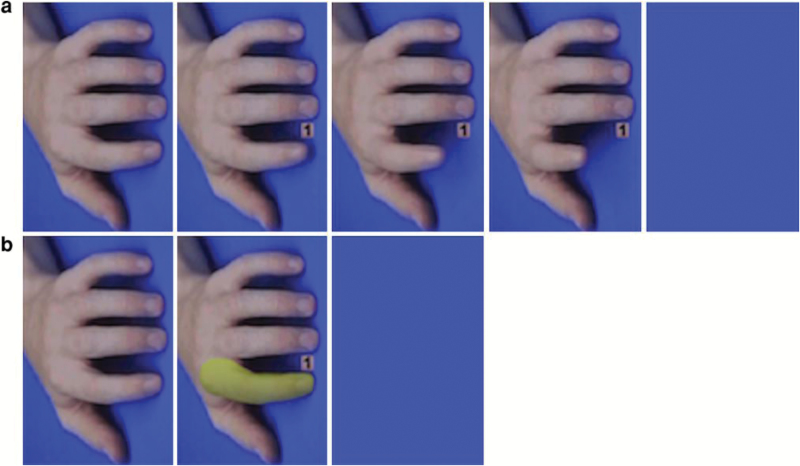
The task is a modified version of the automatic imitation and effector priming control tasks described in Cook and Bird (2011).52 Briefly, short video sequences of a human hand were presented on a computer screen (see figure 1) comprising 4 different conditions in a 2 × 2 factorial design with the factors task (imitation or control) and congruency between the targeted finger on the screen and the required finger movement (congruent or incongruent finger). There were 120 trials in total, 30 for each condition.
Fig. 1.
Example of the 5 frames shown in the imitation task (a) and the 3 frames shown in the control task (b). Both examples are from congruent trials. The first frame in both tasks displayed a resting hand that was shown for 800–2.400 ms. In the imitation task, the second (34 ms), third (34 ms), and fourth frame (500 ms) displayed the number 1 or 2 between the 2 fingers and the lifting movement of one of the fingers. In the control task, the second frame displayed the number 1 or 2 between the 2 fingers and one of the fingers was covered by a mask (display time: 568 ms). The last frame in both tasks was a blank screen, which remained blank until the participant had placed both fingers back on the keyboard. In both tasks, this screen appeared when the participant lifted a finger. Reprinted from Cook and Bird (2012).61
Participants were required to place the index and middle fingers of their right hand on the number 1 and 2 (letter N and M on the keyboard), respectively. On each trial, participants had to lift either their index or middle finger as fast as possible according to the number shown on the screen and then replace it on the same key: if “1” was shown, they had to lift their index finger and if “2” was shown, they had to lift their middle finger.
In the imitation task (see figure 1a), half of the trials depicted an action that was congruent with the required finger movement (eg, index finger lift required and index finger lift shown) and the other half were incongruent. Similarly, in the control task (see figure 1b), on half of the trials a semi-transparent mask appeared on the finger corresponding to the instructed finger movement (congruent trials), and on the other half, the mask appeared on the opposite finger (incongruent trials, eg, index finger lift required and mask appeared on the middle finger). During the control task, the fingers remained still for the whole trial. Trials were pseudo-randomized so that the same trial type never occurred more than twice in a row. In order to differentiate automatic imitation from spatial compatibility,52 response movements were orthogonal to stimulus postures (see figure 1). The task was programed in Presentation v. 16.3 (Neurobehavioral Systems).
Before the testing, participants were given standardized instructions and a practice session where they had to make 8 correct responses in a row to ensure their ability to perform the task. The whole process took approximately 15 minutes.
Data Analysis
As in Cook and Bird (2011),52 RTs shorter than 150 ms and longer than 2.000 ms were excluded from the analysis. In addition, error trials in which the participant lifted the incorrect finger were removed from the RT analyses. All analyses were run using mixed effects regression models. To assess whether there was a specific imitation (question 1) or imitation-inhibition (question 2) deficit in schizophrenia beyond nonspecific cognitive or motoric deficits, we ran 2 separate models with RTs as outcome and group (schizophrenia, control) by task (imitation, control) as predictors, including only congruent trials to answer question 1 and only incongruent trials to answer question 2 (see table 2). The analyses accounted for the pairwise matching of participants (when present), by assigning the matched individuals a common identifier and entering it as a random intercept. Random slopes were included for group, task, and finger (index vs middle finger).
Table 2.
Interaction Between Group and Task on Reaction Time During (1) Congruent Trials or (2) Incongruent Trials
| Factor | β | SE | t | P |
|---|---|---|---|---|
| Congruent trials | ||||
| Intercept | −0.22 | 0.05 | −4.24 | <.001 |
| Task | −0.09 | 0.03 | −2.48 | .014 |
| Group | 0.39 | 0.13 | 2.99 | .005 |
| Task × Group | −0.11 | 0.05 | −2.32 | .021 |
| Incongruent trials | ||||
| Intercept | −0.04 | 0.07 | −0.64 | .521 |
| Task | 0.02 | 0.04 | 0.63 | .531 |
| Group | 0.48 | 0.15 | 3.27 | .001 |
| Task × Group | −0.07 | 0.05 | −1.29 | .195 |
Note: RT = reaction time. The 2 models were defined as: RT = β0i + β1iTask + β2iGroup + β3TaskGroup + ε.
To assess whether there was an association between antipsychotic medication dose and imitation (question 3) or imitation-inhibition (question 4), we ran 2 separate models in the patients only with RTs as outcome and medication dose by task (imitation, control) as predictors, including only congruent trials to answer question 3 and only incongruent trials to answer question 4 (see table 3). Random slopes were included for task and finger.
Table 3.
Interaction Between Task and Antipsychotic Medication Dose (CPZ) on Reaction Time During (3) Congruent Trials or (4) Incongruent Trials
| Factor | β | SE | t | P |
|---|---|---|---|---|
| Congruent trials | ||||
| Intercept | 0.21 | 0.13 | 1.65 | .110 |
| Task | −0.20 | 0.05 | −4.17 | <.001 |
| CPZ | 0.17 | 0.14 | 1.18 | .245 |
| Task × CPZ | −0.13 | 0.05 | −2.47 | .015 |
| Incongruent trials | ||||
| Intercept | 0.34 | 0.13 | 2.68 | .012 |
| Task | −0.04 | 0.06 | −0.76 | .454 |
| CPZ | 0.05 | 0.15 | 0.38 | .708 |
| Task × CPZ | 0.09 | 0.07 | 1.27 | .215 |
Note: CPZ, chlorpromazine; RT = reaction time. The 2 models were defined as: RT = β0i + β1iTask + β2CPZ + β3TaskCPZ + ε.
Finally, follow-up control analyses were run to assess whether a potential group difference could be due to generally slower RTs in patients (supplementary table 6S patients, supplementary table 7S controls) and whether any medication effects would still hold when excluding patients with recent antipsychotic medication changes and when controlling for potential confounders such as other medications (supplementary table 8S), symptom severity (supplementary table 9S), or level of functioning (supplementary table 10S). Here we also assessed whether a group difference in imitation or imitation-inhibition was associated with level of functioning in the patients (supplementary table 10S). A full description of these models as well as the models assessing that the task worked as expected can be found in supplementary tables 4S–10S. Note, that effect sizes are reported in the form of standardized beta coefficients for all linear models. In case of null results on theoretically meaningful comparisons, we performed a follow-up Bayes factor (BF) analysis on the mixed effects models to assess the evidence in favor of the null hypothesis, with values below 0.3 indicating substantial evidence in favor of the null. For a more detailed explanation of mixed effects models, BF, and the computational implementation, cf. supplementary material.
Results
Errors and Reaction Times
As predicted, patients made more errors than controls (patients: 8.79%, M = 10.55, SD = 7.47; controls: 5.27%, M = 6.33, SD = 6.44). This was the case for all 3 types of errors: wrong finger lifted (β = 0.61, SE = 0.16, z = 3.74, P < .001), RTs shorter than 150 ms (β = 0.98, SE = 0.23, z = 4.34, P < .001), and RTs longer than 2000 ms (β = 0.29, SE = 0.13, z = 2.27, P = .023). Patients also displayed slower RTs across conditions compared to controls (patients: M = 592 ms/trial, SD = 114.97; controls: M = 534.91 ms, SD = 62.66; β = 0.37, SE = 0.14, t = 2.73, P = .01). For details on the error rate for each type of error by condition and group, see supplementary table 3S.
Imitation Effect: Movement Facilitation Due To Imitation vs a Control Cue
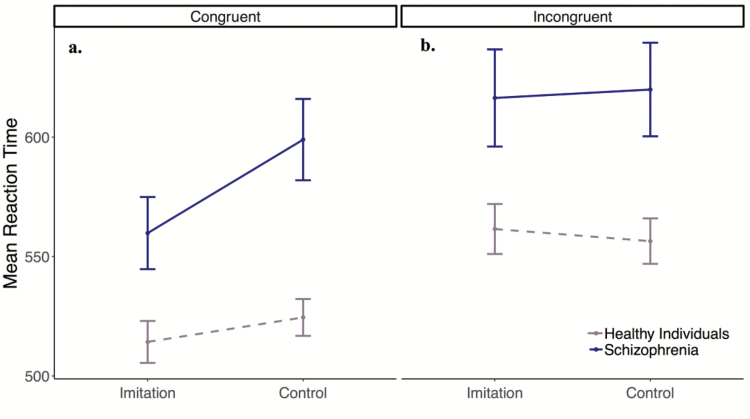
When assessing imitation in the 2 groups (question 1), we compared RTs (outcome) in the 2 tasks (imitation, control) by group (predictors), looking at congruent trials only. Controls responded significantly faster to imitation trials compared to control trials. In addition, there was a significant interaction between task and group on RT (see table 2: congruent trials). Specifically, patients showed an even larger difference in RTs between imitation and control trials than the healthy controls, see figure 2a.
Fig. 2.
Mean reaction times (RTs) during congruent (a) and incongruent trials (b) in the imitation and control tasks for each group. Error bars: ±1 SEM.
To assess whether this larger difference seen in the patients could be due to their generally slower RTs, we assessed the interaction between mean RT during congruent control trials and task (imitation vs control) in the 2 groups separately. For the patients, there was a significant interaction, where the slower the RT, the larger the difference between imitation trials and control trials (β = −0.25, SE = 0.08, t = −3.22, P = .002; supplementary table 6S). In contrast, there was no such relationship in the controls (β = 0.07, SE = 0.10, t = 0.65, P = .518, BF = 0.07; supplementary table 7S).
Imitation-Inhibition Effect: Inhibition of Imitation vs a Control Distractor Cue
Next, we looked at whether the groups responded differently to imitation-inhibition vs distractor-inhibition (question 2), by comparing incongruent trials in the 2 tasks. Controls had similar RTs during inhibition of imitation and distractor cue (BF < 0.001) and there was no significant interaction between task and group (BF = 0.07, table 2: incongruent trials), see figure 2b.
Imitation Tendency, Antipsychotic Medication Dose, and Level of Functioning
Finally, we assessed the influence of antipsychotic medication dose on imitation (question 3) and imitation- inhibition (question 4). We first looked at congruent trials in the 2 tasks (imitation, control) for the patients (question 3). We observed a significant interaction between task and medication dose on RTs. Specifically, patients receiving a higher dose showed faster RTs during imitation vs control trials (see table 3: congruent trials). This effect could not be easily explained by slower RTs in patients receiving higher doses, as there was no significant association between RT on congruent control trials and medication dose (β = 0.17, SE = 0.14, t = 1.2, P = .24, BF = 0.46). The interaction between task and medication dose remained significant when excluding the 4 patients, who had changes made in their antipsychotic medication within the last 3 weeks prior to testing and adjusting for other types of medication (supplementary table S8), for symptom severity (supplementary table S9), or for level of functioning (supplementary table S10). Note, that there was an interaction between level of functioning and task in this last model, ie, the higher the level of functioning, the faster RTs during imitation vs control trials (β = −0.14, SE = 0.06, t = −2.18, P = .032; supplementary table S10 and supplementary figure S1). When analyzing the incongruent trials (question 4), there was no significant interaction between medication dose and task (BF = 0.22, see table 3: incongruent trials).
Discussion
This study investigated automatic imitation and top-down modulation of imitation in schizophrenia. We found that patients with schizophrenia, although generally slower, displayed enhanced automatic imitation and intact imitation-inhibition compared to matched healthy individuals. The fact that we did not observe reduced imitation in schizophrenia stands in marked contrast to previous reports of imitation impairments in this patient group. However, previous studies assessed either voluntary imitation7–10,25–31 or automatic imitation of emotional stimuli12,13,32,33 while not controlling for the cognitive, motoric, and/or emotional deficits associated with the disorder. Thus, it is not possible to assess whether the imitation deficits seen in previous studies were primary or secondary to the aforementioned general deficits. By using an imitation task with nonemotional stimuli and a control task, we were able to delineate imitation-based effects from nonspecific cognitive or motoric effects. Indeed, when controlling for these confounders, patients with schizophrenia do not display the reduced imitation suggested by previous studies. This finding is in line with imaging and TMS studies showing intact14–17 or even enhanced14,24 MNS activity in patients during action observation or imitation and with work done on social motor coordination in schizophrenia, where spontaneous coordination is preserved.62
It could be argued that the enhanced imitation seen in patients actually reflects overimitation. Indeed, overimitation is sometimes seen in schizophrenia in the form of symptoms like echolalia or echopraxia.31,32 However, there are several factors that suggest that this was not the case. First, patients generally had slower RTs. This may have left more room for “improvement” compared to the controls, ie, the controls could not respond much faster than they already were (floor-effect). The association between longer RTs and larger imitation effect in the patient group supports this hypothesis. Second, there was an association between higher antipsychotic dose and a larger imitation effect even when controlling for potential confounders such as symptom severity. This suggests that the enhanced imitation seen may be medication induced rather than a consequence of the disorder. Third, the larger the patient’s tendency to imitate, the higher the level of functioning was seen. This is opposite to what would be expected if the increased imitation indeed reflected a deficit and rather suggests that increased susceptibility to social influence is an advantage for the patients. While no patients with symptoms like echolalia and echopraxia were present in our sample, we would expect them not to show enhanced imitation, but more likely impaired ability to inhibit imitation, consistent with studies on patients with frontal lesions,40 which may also display symptoms of overimitation.63 Future studies should test this hypothesis in patients displaying such catatonic symptoms.
The association between higher antipsychotic medication dose and increased imitation tendency is consistent with findings of increased MNS activity when receiving higher doses of antipsychotic medication13 or when being on antipsychotic medication (compared to off).22 In these studies, medication was associated with activity more similar to that of the healthy controls, suggesting a therapeutic effect. The underlying mechanism is not understood. We speculate that it could reflect the oxytocin-enhancing effect of antipsychotics.64,65 Indeed, oxytocin has been shown to enhance MNS activity in healthy individuals66,67 and in patients with schizophrenia,68 and to increase imitation.69,70 Future studies could explore this relationship further.
There are certain limitations of our interpretation. First, 31 out of 33 patients were medicated. It is therefore unclear whether unmedicated patients would display similar behaviors as controls. Second, as patients were not randomized to different types or doses of antipsychotic medication, we cannot exclude that unmeasured individual differences accompanying medication dose contributed to the observed effects.
In conclusion, we did not find reduced imitation in schizophrenia. Rather, patients displayed enhanced imitation and intact imitation-inhibition. The enhanced imitation may have been medication induced. The results suggest that previous findings of impaired imitation in schizophrenia may have been secondary to other cognitive, motoric, and/or emotional deficits and that schizophrenia should not be conceptualized as a disorder of imitation. These findings could have important implications for how the imitation system might be harnessed to facilitate social learning and interaction in patients with schizophrenia, as well as contribute to a growing mechanistic model of the social deficits accompanying the disorder.
Funding
Lundbeck Foundation, Denmark (R102-A9118 and R155-2014-1724); the Psychosis Research Unit, AUHR; the Interacting Minds Centre, Aarhus University.
Supplementary Material
Acknowledgments
We thank Marjun Biskopstø and Oddbjørg Johansen for their assistance in the data collection process and Jennifer Cook for providing generous support in the implementation of the paradigm.
References
- 1. Mazza M, Lucci G, Pacitti F, et al. . Could schizophrenic subjects improve their social cognition abilities only with observation and imitation of social situations?Neuropsychol Rehabil. 2010;20:675–703. [DOI] [PubMed] [Google Scholar]
- 2. Hurley SL. Perspectives on Imitation: Mechanisms of Imitation and Imitation in Animals. Vol 1 Cambridge, MA: MIT Press; 2005. [Google Scholar]
- 3. Chartrand TL, Bargh JA. The chameleon effect: the perception-behavior link and social interaction. J Pers Soc Psychol. 1999;76:893–910. [DOI] [PubMed] [Google Scholar]
- 4. Dale R, Fusaroli R, Duran N, Richardson DC. The self-organization of human interaction. Psychol Learn Motiv. 2013;59:43–95. [Google Scholar]
- 5. Green MF, Horan WP, Lee J. Social cognition in schizophrenia. Nat Rev Neurosci. 2015;16:620–631. [DOI] [PubMed] [Google Scholar]
- 6. Lavelle M, Healey PG, McCabe R. Nonverbal behavior during face-to-face social interaction in schizophrenia: a review. J Nerv Ment Dis. 2014;202:47–54. [DOI] [PubMed] [Google Scholar]
- 7. Schwartz BL, Mastropaolo J, Rosse RB, Mathis G, Deutsch SI. Imitation of facial expressions in schizophrenia. Psychiatry Res. 2006;145:87–94. [DOI] [PubMed] [Google Scholar]
- 8. Walther S, Vanbellingen T, Müri R, Strik W, Bohlhalter S. Impaired gesture performance in schizophrenia: particular vulnerability of meaningless pantomimes. Neuropsychologia. 2013;51:2674–2678. [DOI] [PubMed] [Google Scholar]
- 9. Matthews N, Gold BJ, Sekuler R, Park S. Gesture imitation in schizophrenia. Schizophr Bull. 2013;39:94–101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Park S, Matthews N, Gibson C. Imitation, simulation, and schizophrenia. Schizophr Bull. 2008;34:698–707. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Varcin KJ, Bailey PE, Henry JD. Empathic deficits in schizophrenia: the potential role of rapid facial mimicry. J Int Neuropsychol Soc. 2010;16:621–629. [DOI] [PubMed] [Google Scholar]
- 12. Haker H, Rössler W. Empathy in schizophrenia: impaired resonance. Eur Arch Psychiatry Clin Neurosci. 2009;259: 352–361. [DOI] [PubMed] [Google Scholar]
- 13. Thakkar KN, Peterman JS, Park S. Altered brain activation during action imitation and observation in schizophrenia: a translational approach to investigating social dysfunction in schizophrenia. Am J Psychiatry. 2014;171:539–548. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. McCormick LM, Brumm MC, Beadle JN, Paradiso S, Yamada T, Andreasen N. Mirror neuron function, psychosis, and empathy in schizophrenia. Psychiatry Res. 2012;201:233–239. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Horan WP, Pineda JA, Wynn JK, Iacoboni M, Green MF. Some markers of mirroring appear intact in schizophrenia: evidence from mu suppression. Cogn Affect Behav Neurosci. 2014;14:1049–1060. [DOI] [PubMed] [Google Scholar]
- 16. Horan WP, Iacoboni M, Cross KA, et al. . Self-reported empathy and neural activity during action imitation and observation in schizophrenia. Neuroimage Clin. 2014;5:100–108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Andrews SC, Enticott PG, Hoy KE, Thomson RH, Fitzgerald PB. No evidence for mirror system dysfunction in schizophrenia from a multimodal TMS/EEG study. Psychiatry Res. 2015;228:431–440. [DOI] [PubMed] [Google Scholar]
- 18. Iacoboni M. Neural mechanisms of imitation. Curr Opin Neurobiol. 2005;15:632–637. [DOI] [PubMed] [Google Scholar]
- 19. Rizzolatti G. The Mirror Neuron System and Imitation. Vol 1 Cambridge, MA: The MIT Press; 2005. [Google Scholar]
- 20. Molenberghs P, Cunnington R, Mattingley JB. Brain regions with mirror properties: a meta-analysis of 125 human fMRI studies. Neurosci Biobehav Rev. 2012;36:341–349. [DOI] [PubMed] [Google Scholar]
- 21. Keysers C, Gazzola V. Towards a unifying neural theory of social cognition. Prog Brain Res. 2006;156:379–401. [DOI] [PubMed] [Google Scholar]
- 22. Mehta UM, Thirthalli J, Basavaraju R, Gangadhar BN, Pascual-Leone A. Reduced mirror neuron activity in schizophrenia and its association with theory of mind deficits: evidence from a transcranial magnetic stimulation study. Schizophr Bull. 2014;40:1083–1094. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23. Enticott PG, Hoy KE, Herring SE, Johnston PJ, Daskalakis ZJ, Fitzgerald PB. Reduced motor facilitation during action observation in schizophrenia: a mirror neuron deficit?Schizophr Res. 2008;102:116–121. [DOI] [PubMed] [Google Scholar]
- 24. Lee JS, Chun JW, Yoon SY, Park HJ, Kim JJ. Involvement of the mirror neuron system in blunted affect in schizophrenia. Schizophr Res. 2014;152:268–274. [DOI] [PubMed] [Google Scholar]
- 25. Walther S, Vanbellingen T, Müri R, Strik W, Bohlhalter S. Impaired pantomime in schizophrenia: association with frontal lobe function. Cortex. 2013;49:520–527. [DOI] [PubMed] [Google Scholar]
- 26. Braun C, Bernier S, Proulx R, Cohen H. A deficit of primary affective facial expression independent of bucco-facial dyspraxia in chronic schizophrenics. Cogn Emot. 1991;5:147–159. [Google Scholar]
- 27. Walther S, Stegmayer K, Sulzbacher J, et al. . Nonverbal social communication and gesture control in schizophrenia. Schizophr Bull. 2015;41:338–345. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28. Stegmayer K, Moor J, Vanbellingen T, et al. . Gesture performance in first- and multiple-episode patients with schizophrenia spectrum disorders. Neuropsychobiology. 2016;73:201–208. [DOI] [PubMed] [Google Scholar]
- 29. Trémeau F, Malaspina D, Duval F, et al. . Facial expressiveness in patients with schizophrenia compared to depressed patients and nonpatient comparison subjects. Am J Psychiatry. 2005;162:92–101. [DOI] [PubMed] [Google Scholar]
- 30. Putnam KM, Kring AM. Accuracy and intensity of posed emotional expressions in unmedicated schizophrenia patients: vocal and facial channels. Psychiatry Res. 2007;151:67–76. [DOI] [PubMed] [Google Scholar]
- 31. Gaebel W, Wölwer W. Facial expression and emotional face recognition in schizophrenia and depression. Eur Arch Psychiatry Clin Neurosci. 1992;242:46–52. [DOI] [PubMed] [Google Scholar]
- 32. Schaefer J, Giangrande E, Weinberger DR, Dickinson D. The global cognitive impairment in schizophrenia: consistent over decades and around the world. Schizophr Res. 2013;150:42–50. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33. Gilbert SJ, Burgess PW. Executive function. Curr Biol. 2008;18:R110–R114. [DOI] [PubMed] [Google Scholar]
- 34. Strous RD, Stryjer R, Zerzion M, Weiss M, Bar F. Accent echoing: a newly described imitation phenomenon of psychosis?Isr Med Assoc J. 2003;5:61–62. [PubMed] [Google Scholar]
- 35. Francis A. Catatonia: diagnosis, classification, and treatment. Curr Psychiatry Rep. 2010;12:180–185. [DOI] [PubMed] [Google Scholar]
- 36. Spengler S, Bird G, Brass M. Hyperimitation of actions is related to reduced understanding of others’ minds in autism spectrum conditions. Biol Psychiatry. 2010;68:1148–1155. [DOI] [PubMed] [Google Scholar]
- 37. Brass M, Ruby P, Spengler S. Inhibition of imitative behaviour and social cognition. Philos Trans R Soc Lond B Biol Sci. 2009;364:2359–2367. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38. Brass M, Derrfuss J, von Cramon DY. The inhibition of imitative and overlearned responses: a functional double dissociation. Neuropsychologia. 2005;43:89–98. [DOI] [PubMed] [Google Scholar]
- 39. Spengler S, von Cramon DY, Brass M. Control of shared representations relies on key processes involved in mental state attribution. Hum Brain Mapp. 2009;30:3704–3718. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40. Spengler S, von Cramon DY, Brass M. Resisting motor mimicry: control of imitation involves processes central to social cognition in patients with frontal and temporo-parietal lesions. Soc Neurosci. 2010;5:401–416. [DOI] [PubMed] [Google Scholar]
- 41. Hogeveen J, Obhi SS, Banissy MJ, et al. . Task-dependent and distinct roles of the temporoparietal junction and inferior frontal cortex in the control of imitation. Soc Cogn Affect Neurosci. 2015;10:1003–1009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42. Brass M, Derrfuss J, Matthes-von Cramon G, von Cramon DY. Imitative response tendencies in patients with frontal brain lesions. Neuropsychology. 2003;17:265–271. [DOI] [PubMed] [Google Scholar]
- 43. Marsh LE, Bird G, Catmur C. The imitation game: effects of social cues on ‘imitation’ are domain-general in nature. Neuroimage. 2016;139:368–375. [DOI] [PubMed] [Google Scholar]
- 44. Santiesteban I, White S, Cook J, Gilbert SJ, Heyes C, Bird G. Training social cognition: from imitation to theory of mind. Cognition. 2012;122:228–235. [DOI] [PubMed] [Google Scholar]
- 45. Chung YS, Barch D, Strube M. A meta-analysis of mentalizing impairments in adults with schizophrenia and autism spectrum disorder. Schizophr Bull. 2014;40:602–616. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46. Tremeau F. A review of emotion deficits in schizophrenia. Dialogues Clin Neurosci. 2006;8:59–70. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47. Kring AM, Kerr SL, Earnst KS. Schizophrenic patients show facial reactions to emotional facial expressions. Psychophysiology. 1999;36:186–192. [PubMed] [Google Scholar]
- 48. Falkenberg I, Bartels M, Wild B. Keep smiling! Facial reactions to emotional stimuli and their relationship to emotional contagion in patients with schizophrenia. Eur Arch Psychiatry Clin Neurosci. 2008;258:245–253. [DOI] [PubMed] [Google Scholar]
- 49. Morris RW, Weickert CS, Loughland CM. Emotional face processing in schizophrenia. Curr Opin Psychiatry. 2009;22:140–146. [DOI] [PubMed] [Google Scholar]
- 50. Moody EJ, McIntosh DN, Mann LJ, Weisser KR. More than mere mimicry? The influence of emotion on rapid facial reactions to faces. Emotion. 2007;7:447–457. [DOI] [PubMed] [Google Scholar]
- 51. Andreasen NC. Scale for the Assessment of Negative Symptoms. Iowa City, IA: University of Iowa; 1983. [Google Scholar]
- 52. Cook J, Bird G. Social attitudes differentially modulate imitation in adolescents and adults. Exp Brain Res. 2011;211:601–612. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53. Bachmann S, Degen C, Geider FJ, Schröder J. Neurological soft signs in the clinical course of schizophrenia: results of a meta-analysis. Front Psychiatry. 2014;5:185. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54. Morrens M, Hulstijn W, Matton C, et al. . Delineating psychomotor slowing from reduced processing speed in schizophrenia. Cogn Neuropsychiatry. 2008;13:457–471. [DOI] [PubMed] [Google Scholar]
- 55. Wing JK, Sartorius N, Üstün TB.. Diagnosis and Clinical Measurement in Psychiatry: A Reference Manual for SCAN. Cambridge, England: Cambridge University Press; 1998. [Google Scholar]
- 56. Computer Assisted Personal Interviewing Application for the Schedules for Clinical Assessment in Neuropsychiatry Version 2.1 and Diagnostic Algorithms for WHO ICD 10 chapter V DCR and for Diagnostic and Statistical Manual of Mental Disorders, Version IV [computer program]. Version 1.0.4.6, Win9x, NT. Geneva, Switzerland: World Health Organization; 2005. [Google Scholar]
- 57. Leucht S, Samara M, Heres S, Patel MX, Woods SW, Davis JM. Dose equivalents for second-generation antipsychotics: the minimum effective dose method. Schizophr Bull. 2014;40:314–326. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58. Gardner DM, Murphy AL, O’Donnell H, Centorrino F, Baldessarini RJ. International consensus study of antipsychotic dosing. Am J Psychiatry. 2010;167:686–693. [DOI] [PubMed] [Google Scholar]
- 59. Andreasen NC. Scale for the Assessment of Positive Symptoms. Iowa City, IA: University of Iowa; 1984. [Google Scholar]
- 60. Morosini PL, Magliano L, Brambilla L, Ugolini S, Pioli R. Development, reliability and acceptability of a new version of the DSM-IV Social and Occupational Functioning Assessment Scale (SOFAS) to assess routine social functioning. Acta Psychiatr Scand. 2000;101:323–329. [PubMed] [Google Scholar]
- 61. Cook JL, Bird G. Atypical social modulation of imitation in autism spectrum conditions. J Autism Dev Disord. 2012;42:1045–1051. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62. Varlet M, Marin L, Raffard S, et al. . Impairments of social motor coordination in schizophrenia. PLoS One. 2012;7:e29772. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63. Archibald SJ, Mateer CA, Kerns KA. Utilization behavior: clinical manifestations and neurological mechanisms. Neuropsychol Rev. 2001;11:117–130. [DOI] [PubMed] [Google Scholar]
- 64. Beckmann H, Lang RE, Gattaz WF. Vasopressin–oxytocin in cerebrospinal fluid of schizophrenic patients and normal controls. Psychoneuroendocrinology. 1985;10:187–191. [DOI] [PubMed] [Google Scholar]
- 65. Uvnäs-Moberg K, Alster P, Svensson TH. Amperozide and clozapine but not haloperidol or raclopride increase the secretion of oxytocin in rats. Psychopharmacology (Berl). 1992;109:473–476. [DOI] [PubMed] [Google Scholar]
- 66. Perry A, Bentin S, Shalev I, et al. . Intranasal oxytocin modulates EEG mu/alpha and beta rhythms during perception of biological motion. Psychoneuroendocrinology. 2010;35:1446–1453. [DOI] [PubMed] [Google Scholar]
- 67. Levy J, Goldstein A, Zagoory-Sharon O, et al. . Oxytocin selectively modulates brain response to stimuli probing social synchrony. Neuroimage. 2016;124:923–930. [DOI] [PubMed] [Google Scholar]
- 68. Singh F, Nunag J, Muldoon G, Cadenhead KS, Pineda JA, Feifel D. Effects of intranasal oxytocin on neural processing within a socially relevant neural circuit. Eur Neuropsychopharmacol. 2016;26:626–630. [DOI] [PubMed] [Google Scholar]
- 69. De Coster L, Mueller SC, T’Sjoen G, De Saedeleer L, Brass M. The influence of Oxytocin on automatic motor simulation. Psychoneuroendocrinology. 2014;50:220–226. [DOI] [PubMed] [Google Scholar]
- 70. Korb S, Malsert J, Strathearn L, Vuilleumier P, Niedenthal P. Sniff and mimic – intranasal oxytocin increases facial mimicry in a sample of men. Horm Behav. 2016;84:64–74. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.