Abstract
Background
Psychotic experiences (PEs) are considered part of an extended psychosis phenotype and are associated with an elevated risk of developing a psychotic disorder. Risk of transition increases with persistence of PEs, and this is thought to be modulated by genetic and environmental factors. However, it is unclear if persistence is associated with progressive schizophrenia-like changes in neuroanatomy.
Methods
We examined cortical morphometry using MRI in 247 young adults, from a population-based cohort, assessed for the presence of PEs at ages 18 and 20. We then incorporated a polygenic risk score for schizophrenia (PRS) to elucidate the effects of high genetic risk. Finally, we used atlas-based tractography data to examine the underlying white matter.
Results
Individuals with persisting PEs showed reductions in gyrification (local gyrification index: lGI) in the left temporal gyrus as well as atypical associations with brain volume (TBV) in the left occipital and right prefrontal gyri. No main effect was found for the PRS, but interaction effects with PEs were identified in the orbitofrontal, parietal, and temporal regions. Examination of underlying white matter did not provide strong evidence of further disturbances.
Conclusions
Disturbances in lGI were similar to schizophrenia but findings were mostly limited to those with persistent PEs. These could reflect subtle changes that worsen with impending psychosis or reflect an early vulnerability associated with the persistence of PEs. The lack of clear differences in underlying white matter suggests our findings reflect early disturbances in cortical expansion rather than progressive changes in brain structure.
Keywords: psychotic experiences, psychosis, polygenic risk, magnetic resonance imaging, gyrification
Introduction
Delusions and hallucinations are key psychopathological features of psychotic disorders, but these phenomena are also prevalent in the general population at levels that do not reach clinical thresholds1,2 and are commonly referred to as psychotic experiences (PEs). Expression of PEs is most common during childhood and adolescence, a critical period in neurodevelopment, but higher prevalence of PEs during adolescence than of psychotic disorders in adults suggests that PEs dissipate over time.3 Despite their frequently transitory nature, the mere presence of PEs in an individual is associated with an elevated risk of transitioning to clinical psychosis.3 The manifestation of PEs can be considered part of an atypical developmental trajectory that presents a vulnerability to psychosis but one that is further modulated by environmental and genetic risk factors.4 PEs can then become abnormally persistent and this increases the already present risk of transitioning to a clinical psychotic outcome.5 In line with the notion of a continuum, PEs similarly show familial clustering1 and share many risk factors, including low intelligence quotient (IQ) and substance use, with psychotic disorders.6,7 However, PEs are not associated with the typical cognitive impairments found in psychotic disorders, but instead to minor impairments in verbal knowledge and working memory.8 PEs have also been linked to nonpsychotic disorders.9,10
Magnetic resonance imaging (MRI) techniques have been used extensively to study the prodromal phase of psychosis in an effort to map the neural correlates of vulnerability to psychosis and have consistently found reduced gray matter volume (GMV) in individuals at high risk, in prefrontal, temporal, and cingulate regions.11,12 More importantly, studies have found progressive changes in brain anatomy associated with transitioning to psychosis,13–19 supporting the idea there may be an early vulnerability to psychosis but that additional processes underlie transition. Further support for this idea comes from studies of cortical thickness that have reported cortical thinning before the onset of a psychotic disorder.17–19 There is an emerging literature describing alterations in cortical surface morphometry in relation to schizophrenia, with widespread progressive disturbances in gyrification.20,21 Gyrification represents a measure of early cortical expansion, constrained by axonal tension pulling densely interconnected areas together to grow outward bulging gyri, and is considered a relatively stable neurodevelopmental marker of cortical complexity.22,23 The degree of gyrification during early development scales linearly with brain volume but maintains a relatively constant ratio from birth to adulthood despite the overall increase in the size of the brain.23–25 Both hypo- and hypergyria have been reported in schizophrenia.20,21 Studies of high-risk cohorts report reduced prefrontal gyrification, but these effects did not remain when taking into account differences in GMV between groups.26–28
There are few structural imaging studies that have focused solely on PEs but these implicate frontal, temporal, and cingulate brain regions. A small study on children with PEs found increased GMV in the right orbitofrontal cortex, left superior temporal gyrus and angular gyrus, and reduced volume in the left inferior temporal gyrus.29 Studies on adults with PEs have reported a decrease in GMV bilaterally in the orbitofrontal cortex,30 the left supramarginal gyrus, and right prefrontal cortex,31 and an increase in the posterior cingulate cortex and precuneus.32 Two studies have examined PEs across childhood to adulthood and report global reductions in GMV and converging evidence of temporal lobe disturbances.33,34 To date, only one study has looked at cortical thickness in association with PEs and reported no effects.35 PEs have further been associated with abnormalities in white matter structures, connecting frontal regions with parietal and occipital regions,29,36–38 and functional connectivity of frontal and temporal regions.39,40 Unsurprisingly, alterations in brain structure and connectivity seem to congregate on the frontal lobes.
As mentioned, studies have highlighted a genetic component to the brain abnormalities found in schizophrenia. Advances in genetics granted the opportunity to assess the cumulative effect of multiple alleles that show small effects on risk for schizophrenia as a polygenic risk score (PRS) that can be used to examine how genetic risk is expressed in the general population. Neuroimaging studies have used this approach and have found influences on brain volume41 and prefrontal brain function42–44 across schizophrenia and healthy controls, hippocampal volume45 across high risk and first-episode patients, and mean cortical thickness46 and parietal gyrification47 in healthy controls.
This study aimed to examine cortical morphometry and underlying white matter in relation to the manifestation of PEs as well as genetic risk for schizophrenia. Previous studies of PEs have highlighted volumetric reductions and we expect that, much like in psychosis, assessment of the cortical surface will identify focal abnormalities in morphometry. In this article, we set out to test 3 hypotheses. We hypothesized that PEs are associated with disturbances in gyrification, with greater disparities in those with persisting PEs (H1). We were interested in white matter connectivity in relation to cortical gray matter and hypothesized that observed abnormalities in gyrification would coincide with disturbances in the underlying white matter (H2). There is little research on the relation between cortical morphometry and the PRS so far, and we will pursue an exploratory approach to associations with gyrification. However, transition to psychosis has been associated with greater reductions in cortical thickness so we did test the hypothesis that individuals with persistence of PEs and high genetic risk for schizophrenia will exhibit reductions in cortical thickness (H3).
Methods
Participants
A total of 4724 young adults who were part of the Avon Longitudinal Study of Parent and Children (ALSPAC; see http://www.bristol.ac.uk/alspac/researchers for more information about the resource and the ALSPAC data access policy.) were assessed for the presence of PEs at the age of 18. Based on their assessment, 126 out of 433 individuals rated as having had PEs underwent structural MRI. From a random sample of those with no report of PEs, 126 age-matched individuals were scanned as a control group.
The recruited sample differed slightly from the larger ALSPAC cohort in terms of childhood IQ and general mental health (see appendix 1, supplementary table 1). We also examined self-reported alcohol, tobacco, and cannabis consumption at age 18 and found no indication of substance abuse (see appendix 1, supplementary table 2).
At the time of the scan, all participants were 19–20 years old and were reassessed for the presence of PEs on the day. Ethical approval for this study was granted by the Cardiff University School of Psychology Ethics Committee and the ALSPAC Ethics and Law Committee. All participants gave their informed consent before taking part in this study.
Psychotic Experiences
The presence of PEs was assessed using the semi-structured Psychosis-Like Symptom Interview (PLiKSi).3,48 This instrument assesses hallucinations, delusions, and thought interference in the past 6 months. Interviewees were asked a series of questions about experiencing PEs and further cross-questioning was carried out if a positive answer was given. PEs were rated as absent, suspected, or definitely present and a credible example was required for PEs to be rated as definitely present. We combined the rating at each assessment to label participants based on the duration of PEs (table 1). Individuals with PEs at both time-points were labelled as persistent PEs. Those who no longer had PEs (resolving PEs) as well as individuals who only reported PEs at the second assessment (emergent PEs) were labelled as transient PEs. Individuals with no PEs at either time-point were labelled as healthy controls.
Table 1.
Classification of Participants Based on PEs Assessment at Ages 18 and 20 and Allocation of Participants in Specific Subsamples for Analyses
| Age 18 | Age 20 | Label | Sample | PRSa Subsample |
|---|---|---|---|---|
| n = 252 | n = 247 | n = 246 | n = 180 | |
| 126 HC | 111 HC | HC | 111 | 79 |
| 11 emergent PEsb | Transient PEs | 67 | 52 | |
| 126 PEs | 56 resolving PEsc | |||
| 69 PEs | Persistent PEsd | 68 | 49 |
HC, healthy controls; PEs, psychotic experiences; PRS, polygenic risk score.
aResolving PEs were those who were rated as having had PEs at age 18 but with no indication of any PEs at age 20.
bOut of the 69 individuals with persistent PEs, one participant was found to have an enlarged ventricle and was excluded from further analysis.
cOnly 180 out of the 247 individuals with PEs at both time-points had a PRS for schizophrenia available.
dEmergent PEs were individuals that were rated as not having had any PEs at age 18, but were rated as having had PEs at age 20.
Genetic Risk
PRS has been calculated for each individual in the ALSPAC cohort with genetic data available. Construction of this PRS was done using the results of the second Psychiatric Genomics Consortium schizophrenia GWAS49 as a training set. Candidate single nucleotide polymorphisms (SNP) were pruned for linkage disequilibrium (P ≤ .05 and r2 ≤ .25 within 500 kb windows) and polygenic scores were calculated by summing the number of risk alleles present for each SNP weighted by the logarithm of its odds ratio for schizophrenia.50 PRS scores were available for 183 individuals who were scanned and this led to a total of 180 individuals with PEs ratings at both time-points and PRS computed. Group means for the PRS are reported in table 2.
Table 2.
Descriptive and Inferential Statistics for the 3 Psychotic Experiences Groups With Psychosis Risk Score and Brain Volume
| Variable | Healthy Controls | Transient PEs | Persistent PEs | Test Statistic |
|---|---|---|---|---|
| n = 246 | 111 | 67 | 68 | |
| Agea | 20.2 (0.7) | 20.1 (0.6) | 20.2 (0.5) | |
| Female | 68 (61.26%) | 47 (70.15%) | 45 (66.18%) | χ2(2, 246) = 1.50, P = .47 |
| Male | 43 (38.74%) | 20 (29.85%) | 23 (33.82%) | |
| IQ | 112.3 (14.8) | 104.9 (14.0) | 106.0 (14.1) | F 2,243 = 7.01, P = .001b |
| TBVc | 1469.9 (130.9) | 1430.7 (155.6) | 1457.5 (153.0) | F 2,243 = 1.55, P = .214 |
| CIS-R | 4 (10) | 7 (12) | 11 (18) | χ2 (2) = 19.07, P < .001d |
| n = 180 | 79 | 51 | 50 | |
| Age | 20.1 (0.7) | 20.1 (0.7) | 20.0 (0.6) | |
| Female | 47 (40.50%) | 34 (66.67%) | 31 (62.00%) | χ2(2, 180) = 0.49, P = .78 |
| Male | 32 (59.49%) | 17 (33.33%) | 19 (38.00%) | |
| IQ | 112.2 (15.1) | 104.6 (15.8) | 108.1 (13.3) | F 2,177 = 4.22, P = .016e |
| TBVb | 1471.6 (125.3) | 1443.4 (161.3) | 1469.1 (149.6) | F 2,177 = 0.67, P = .515 |
| PRS | 0.06 (0.90) | −0.15 (1.25) | −0.07 (1.09) | F 2,177 = 0.62, P = .538 |
CSI-R, revised Clinical Interview Schedule; HC, healthy controls; IQ, intelligence quotient; PEs, psychotic experiences; PRS, polygenic risk score.
aAge is reported for 110 healthy controls, 66 transient PEs, and 68 persistent PEs.
bPost hoc comparisons using Bonferroni correction revealed that only HC differed from the other groups with PEs.
cTotal brain volume (TBV) is reported in cm3.
dPost hoc comparisons using Bonferroni correction showed that persistent PEs scored significantly higher than HC.
ePost hoc comparison revealed only a difference between HC and transient PEs.
Other Variables
Gender and age were recorded for each participant. The revised Clinical Interview Schedule (CIS-R) was carried out at age 18 and we included participant scores to get an estimate on general mental health. A childhood IQ, estimated at age 8 using the Wechsler Intelligence Scale for Children, was obtained from the ALSPAC database. Missing data points for childhood IQ were estimated using regression imputation51 across the entire ALSPAC dataset (n = 13971). Total brain volume was calculated and included in analyses.
Image Acquisition
Data were acquired at Cardiff University Brain Research Imaging Centre (CUBRIC) on a 3 Tesla General Electric HDx (GE Medical Systems) using an 8 channel head coil. T1-weighted structural images with a 1 mm isotropic resolution were acquired using a fast spoiled gradient echo (FSPGR) sequence (TR = 7.8 ms, TE = 3.0 ms, TI = 450 ms, flip angle = 20°, acquisition matrix = 256 × 192, zero-padded matrix = 256 × 256). High angular resolution diffusion weighted images (HARDI) were acquired with 60 gradient orientations (b = 1200 s/mm2) and 6 unweighted scans (b = 0 s/mm2) with a 2.4 mm isotropic resolution using a spin-echo echo-planar imaging sequence (TR = cardiac-gated, TE = 87 ms, acquisition matrix = 96 × 96, zero-padded matrix = 128 × 128). Following zero-padding, the reconstructed image resolution for the HARDI scans was 1.8 × 1.8 × 2.4 mm3.
Cortical surface reconstruction was carried out using Freesurfer version 5.3 (surfer.nmr.mgh.harvard.edu), and ExploreDTI v4.8.352 (http://exploredti.com) was used to analyse the diffusion imaging data. Processing steps and details are reported in the supplementary materials (see appendix 2 for details on Freesurfer and ExploreDTI processing steps).
Statistical Analysis
Parameter estimates for CT and lGI were estimated by fitting a general linear model at each vertex. Group membership was modelled as a categorical factor with 3 levels and gender was included as a categorical factor with 2 levels in each group. The input for each group is separately modelled but males and females were pooled together in statistical comparisons.
Our initial analyses focused on the main sample (n = 247) and we hypothesized that persistence of PEs will be associated with greater aberrations in morphometry. This is modelled by fitting a linear trend to the regressors for group that tests the contrast HC (“No Pes”) > persistent PEs and assumes transient PEs lies intermediate. In the presence of an effect, a quadratic trend was also tested to check if transient PEs did not differ from the other groups (results reported in supplementary materials). The amount of cortical expansion, and thus volume, is an important regulator of the degree of cortical folding in the brain23,53 and as prior studies have highlighted a confounding effect of brain volume on gyrification,27,28,54 we included TBV in our model. A main effect of TBV was tested and an interaction with group was examined by fitting the same linear trend to the regressors that model the slope for TBV in each group. Total brain volume was mean centered before inclusion.
We repeated the analysis with the inclusion of childhood IQ as a covariate. We initially omitted childhood IQ as a covariate due to differences between groups (table 2). As with TBV, we tested for a main effect of childhood IQ as well as an interaction with group on cortical morphometry measures.
In our following analyses, we incorporated the PRS and examined the smaller sample (n = 180). We tested for a main effect of the PRS while covarying for gender, TBV, and childhood IQ. Next, we included group membership (PEs assessment) and reran the linear trend (HC > persistent PEs) to assess if including the PRS as a covariate would change prior findings. We also tested a main effect of the PRS, covarying for group membership, and tested our hypothesis that a combination of a high PRS and persistent PEs would be associated with reductions in cortical thickness by fitting a linear contrast to the regressors for PRS in each group.
Analysis of diffusion metrics was carried out in R55. The mean value for tract length was extracted and Hartigans’ dip test56,57 was employed to assess the presence of a nonunimodal distribution that could indicate the presence of different tract classes, such as short-range association tracts and long-range projection tracts. We further tested for group differences in volume of interest (VOI) size that may confound findings. Due to the nature of the extracted tractography data we examined the role of PEs and potential impact of the PRS by computing 95% confidence intervals (CI) around bootstrapped estimates,58,59 resampled 10000 times, of the difference between selected groups and around correlation coefficients between the white matter indices and either the PRS or the cluster lGI. A Bonferroni correction was carried out in the presence of an effect. Visualisation of statistically identified effects was carried out using the ggplot2 package.60
Results
Demographics
There was no indication of a difference in gender distribution between groups in the whole sample or in the smaller subset. There was a group difference in CIS-R scores; post hoc testing revealed a significant difference between persistent PEs and HC. There was no indication of a difference between our groups in terms of TBV. Childhood IQ was higher in HC compared to transient and persistent PEs, and in the subsample IQ only differed between HC and transient PEs. Groups did not differ on the PRS. A summary of the demographics and statistical tests is given in table 2.
Cortical Morphometry
Our initial model examined the main effects of group and TBV as well as an interaction effect between group and TBV. There was no evidence of a main effect of group on either cortical thickness (CT) or gyrification (lGI). There were widespread positive associations between TBV and the lGI (see appendix 3,supplementary table 4 and supplementary figure 1, row A). Considering an interaction between group and TBV highlighted 2 clusters that followed the proposed linear trend (HC > transient PEs > persistent PEs); one cluster in the left occipital lobe, encompassing the lateral occipital and lingual gyrus and extending into the precuneus and fusiform gyrus, and one cluster covering the right middle and superior frontal gyri.
Including childhood IQ in the statistical model did not eliminate our findings. Testing for a main effect of childhood IQ highlighted positive associations with lGI bilaterally spanning the insular cortex and superior temporal gyrus as well as the pre- and postcentral gyri in the right hemisphere (see appendix 3, supplementary table 4 and supplementary figure 1, row B). A negative effect of IQ on CT was also identified in the right orbitofrontal cortex. More importantly, this model identified a main effect of PEs as well as an interaction between group and IQ on lGI in the left hemisphere across the middle and superior temporal gyri.
There was no evidence of a main effect of the PRS on either CT or lGI in our subsample. Incorporating both the PRS and group membership (HC, transient and persistent PEs) in the model did not eliminate our initial effects in frontal and occipital clusters. Additional interaction effects between group and TBV were identified on gyrification in the left precentral gyrus and on cortical thickness in the right middle frontal gyrus (see appendix 3, supplementary table 5 and supplementary figure 1, row C). Considering interaction effects between group and PRS on morphometry revealed further effects on gyrification. In the left medial orbitofrontal gyrus, there was an interaction effect that highlighted a difference in regressors that model the PRS between groups. This interaction showed a difference in the slope between HC and persistent PEs. Additional interaction effects were found when testing for a quadratic contrast (HC < transient PEs > persistent PEs) and are reported in supplementary materials (appendix 3, supplementary table 5 and supplementary figure 1, row D).
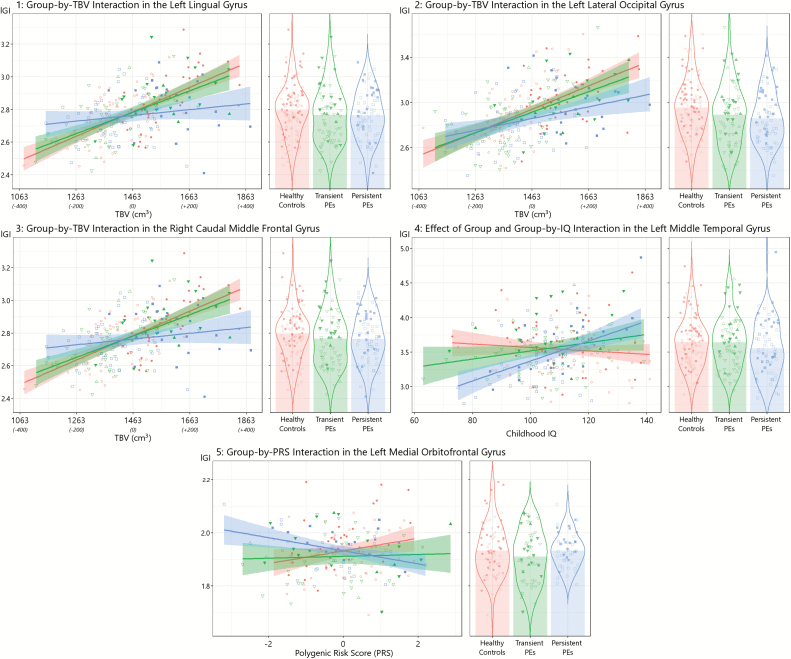
The clusters highlighting alterations associated with persistent PEs are displayed in figure 1 and identified clusters are summarized in table 3. The average lGI of each cluster was extracted and the linear relationship with TBV or IQ in each group is visualized in figure 2.
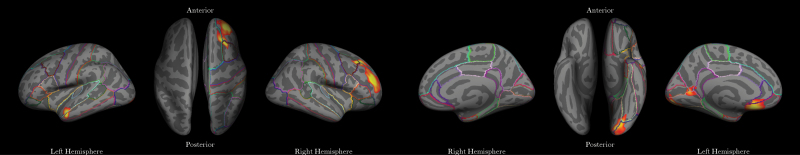
Fig. 1.
Reductions in local gyrification index (lGI) that highlight a main effect of group (healthy control > transient psychotic experiences [PEs] > persistent PEs) or interaction effects with as a difference between groups in the regressors that model the slope for total brain volume or PRS on lGI. Clusters are depicted on the MNI305 inflated brain template and show the outline of regions based on the Desikan–Killiany atlas.60 From left to right: Main effect of group in the left temporal lobe, group-by-TBV interaction effect in the right middle frontal lobe, group-by-TBV interaction effects in the occipital lobe, group-by-PRS interaction effect in the left orbitofrontal lobe.
Table 3.
Group Differences and Interaction Effects With Total Brain Volume, Childhood IQ, and PRS.
| Effect | Region | Cluster Size (mm2) | MNI Coordinates | P valuea | z Scoreb | ||
|---|---|---|---|---|---|---|---|
| X | Y | Z | |||||
| Group × TBV | R caudal middle frontal gyrus | 2233.6 | 29.0 | 24.1 | 41.8 | 0.0002 | 2.63 |
| Group × TBV | L lateral occipital gyrusc | 1476.4 | −12.1 | −90.9 | −8.4 | 0.0002 | 2.63 |
| Group × TBV | L lingual gyrus | 467.9 | −26.6 | −65.2 | 3.4 | 0.0100 | 1.82 |
| Group | L middle temporal gyrus | 505.1 | −54.7 | 0.4 | −28.5 | 0.0066 | 1.92 |
| Group × IQ | L middle temporal gyrus | 437.7 | −54.7 | 0.4 | −28.5 | 0.0149 | 1.72 |
| Group × PRS | L medial orbito-frontal gyrus | 791.1 | −5.2 | 24.4 | −19.4 | 0.0002 | 2.63 |
IQ, intelligence quotient; TBV, total brain volume; PRS, polygenic risk score.
Region and coordinates are based on the location of the cluster peak.
aCluster-wise corrected P-value based on a precomputed Monte Carlo simulation with a z-distribution with a vertex-wise threshold of P <.001 and a cluster threshold of P <.05.
bComputed by taking the inverse complementary error function of each cluster-wise P-value.
cThe cluster peak was present in the lingual gyrus, but the majority of this cluster was in the lateral occipital gyrus and using that name avoids confusion about the 2 clusters.
Fig. 2.
Differences in gyrification (lGI) between persistent psychotic experiences (PEs) (squares), transient PEs (triangles), and (circles). Females are shown as open symbols and males are filled symbols. Emergent and resolving PEs are denoted as upward-facing and downward-facing triangles, respectively. Panels 1–3 visualize differences in the scaling of the cluster average lGI with total brain volume (group-by-TBV interaction) and the x-axis in the scatterplot denotes the actual TBV with mean centred scores in parentheses. Panel 4 depicts the differences in slope of lGI with increasing intelligence quotient (group-by-IQ interaction) in the scatterplot and gives the main effect of group in the violin plot. Panel 5 highlights the different slope for lGI in persistent PEs with increasing polygenic risk score (group-by-PRS interaction).
Atlas-Based Tractography
All clusters that showed an association with group membership as well as TBV or the PRS were used as VOIs to examine underlying white matter connectivity. The size of the lingual gyrus VOI was found to differ between groups (appendix 3, supplementary table 6). All VOIs showed a unimodal distribution for tract length (appendix 3, supplementary table 7). We examined the difference between PE groups and HC and a summary of the white matter indices and estimated CIs are reported in supplementary tables 8 and 9 in appendix 3. At the 95% confidence level, we observed mixed effects of group membership on diffusion indices, but no effect survived correcting for multiple comparisons.
Additionally, we sought to examine the effect of the PRS on VOIs that demonstrated a PEs-by-PRS interaction effect by estimating CIs for the bootstrapped correlation coefficient. There was an indication of a positive correlation between the PRS and tract volume in the left inferior parietal gyrus. Confidence intervals for the correlation coefficient between each diffusion scalar and the PRS are reported in supplementary table 11. Interestingly, the correlations between the cluster lGI and white matter appeared mostly limited to measures of volume and number of streamlines (see appendix 3, supplementary tables 10 and 11).
Discussion
The principal aim of this study was to examine cortical morphometry in individuals with PEs and those at higher genetic risk of developing schizophrenia. Furthermore, we sought to link alterations in cortical gray matter to underlying white matter connectivity for a better understanding of the impact of PEs and genetic risk on cortical anatomy. We had hypothesized that the manifestation of PEs is associated with an atypical neurodevelopmental trajectory and this would be reflected in abnormalities in gyrification with greater disparities in those with persisting PEs (H1). We expected to find coinciding disturbances in the underlying white matter in the presence of aberrant gyrification (H2). Finally, individuals with abnormal persistence of PEs and a high genetic risk for schizophrenia (PRS) would exhibit further signs of cortical abnormality akin to that seen in psychosis (H3).
PEs, Persistence, and Gyrification
We initially did not find an effect of PE group on gyrification (H1) or cortical thickness. Taking IQ into consideration, we did identify a reduction in lGI across the middle and superior temporal gyrus associated with persistence of PEs. Focal aberrations in the temporal lobe are frequently reported in association with psychosis,61,62 and a few studies have reported on reduced gyral complexity.63–65 Testing for an interaction effect with total brain volume did reveal lower lGI in those with persistent PEs with greater brain volume in the middle frontal gyrus, extending into the superior frontal gyrus. Reduced cortical folding in the middle frontal gyrus has been reported in schizophrenia66,67 and seems to be present at onset of psychosis.63,68,69 Several high risk studies have described an opposite effect,27,28,54 but they also reported on local gray matter volume differences and crucially, their findings on increased gyrification did not remain after taking brain volume into account. In the occipital lobe we found similar effects; those with persistent PEs did not show a positive association between lGI and brain volume. Reduced occipital gyral complexity has previously been found in psychosis,67,69 but increased curvature has also been reported70,71 hence more research is needed to clarify these complex findings. Interestingly, there was no indication of deviations in lGI associated with transient PEs and this does suggests that these findings are associated with the persistence rather than the manifestation of PEs. It is important to note that our recruited HC sample had a higher childhood IQ than those with PEs but also compared to those in the ALSPAC cohort that were not recruited for this study. This puts some limits to the generalizability of our findings.
Though we did not test for gender-specific effects, we do believe this may have been a confounder in some of our findings. Most notably, visualizations of the interaction effects between group and TBV highlights a gender divide in TBV and suggests that lower lGI despite greater brain volume is predominantly seen in the males with persistent PEs. Gender differences in PEs have been reported, with mixed results,4,7,72 but it is not known if persistence is more prevalent in males or how this may relate to the differences in brain structure.
Polygenic Risk for Schizophrenia and Gyrification
There was no overall association between the PRS for schizophrenia and cortical morphometry (H3). There were interaction effects between PRS and PEs, though these did not follow a similar pattern. In the medial orbitofrontal cortex, those with persistent PEs displayed a decrease in lGI with a higher PRS score. Gyrification was lower in the temporal and parietal areas for those with transient PEs and a higher PRS score. The association between gyrification of the parietal lobules and a PRS score has previously been reported on,47 though this did not include an assessment of PEs. Our findings were unexpected as we identified more abnormalities associated with transient, rather than persistent, PEs. It is important to note that this sample was not optimally selected for a genetic imaging study and it would be important to examine the relation between PEs and polygenic risk using a larger and more representative sample. Though we did not find evidence of a cumulative effect of PRS and duration of PEs, we do believe these findings point toward potential synergistic effects of genetic risk and expression of PEs and highlight the need for further research into these effects.
Underlying White Matter Connectivity
In terms of white matter, there was no strong indication of differences between groups. Additionally, the measures of diffusion were not found to show associations with gyrification (H2), though gyrification did seem to correlate with metrics of volume, length, and number of streamlines. In tractography, the best strategy for ROI or VOI placement is often dependent on the shape, size, and location of the region and we cannot exclude small error placements during transformation from playing a role in our results. As such, it is vital to isolate the implicated pathways for examination with regards to psychosis and neurodevelopment.
Gyrification, Brain Volume, and White Matter Connectivity
Due to the cross-sectional nature of the imaging in this study, it is difficult to gain direct insight into the underlying neurodevelopmental processes. However, the combination of cortical thickness, gyrification, and diffusion metrics does allow for some conjecture. Typically, cortical folding is considered a process that begins during gestation and remains relatively stable despite the overall growth of the brain.73,74 From childhood to adolescence there is an increase in cortical complexity,75 particularly of the prefrontal lobe,76 but toward adulthood and onward there are age-related decreases in gyrification.77 As such, it is uncertain if focal disturbances in gyrification in those with persistent PEs are markers of an early neurodevelopmental insult that could be considered a vulnerability factor for psychosis, or if these occur at a later stage of development that coincides with the manifestation of PEs. Risk of developing a psychotic disorder increases with persistence of PEs following a dose–response relation5 and it is possible that these changes are associated with the transition to psychotic disorder.
Changes in cortical folding from adolescence to adulthood are not independent from other developmental changes in gray and white matter occurring at this time.23,78 During adolescence there is a general reduction in cortical gray matter volume and this is considered to be reflective of both synaptic pruning and increased myelination.78,79 Synaptic pruning reduces tension along neuronal fibres and this is thought to play a role in the widening of sulci with age.80,81 This process is particularly pronounced during adolescence and continues on into adulthood at a slower rate.77 If our findings on gyrification were part of a recent disturbance in development during adolescence, we would expect to observe coinciding changes in white matter. Previous research from our group on macro- and microstructure in relation to PEs instead linked severity of PEs to disturbances in cortical myelination in the inferior parietal lobe and orbitofrontal cortex31 and to reduced fractional anisotropy in frontomedial white matter pathways.82 Our findings highlight many subtle differences in cortical complexity but with little evidence of any disturbances in the underlying white matter tracts. Therefore, we propose that these variations in cortical folding are the result of early neurodevelopmental deviations rather than changes that take place during adolescence when the brain goes through complex reorganization.
Conclusion
In this study, we found differences in cortical folding patterns in those with persistent PEs with no evidence of differences in underlying connectivity, brain volume, and cortical thickness. Alterations in gyrification were comparable to those present in schizophrenia but were only evident when brain volume was taken into account. We propose that the atypical relationship between brain volume and cortical complexity is indicative of an early neurodevelopmental insult that results in cortical expansion plateauing sooner in those that manifest PEs that persist. This would explain why the degree of gyrification does not increase as expected with brain volume, as the former remains relatively stable throughout life despite a vast increase in overall brain size during development.73 Though far from conclusive, our findings emphasize the role of early developmental deviations without evidence of progressive changes with time though these were further bound to the persistence of PEs. Further longitudinal studies are essential to clarify the role of persistence in the alterations present in gyrification as either an indicator of developing psychosis or as a lingering vulnerability.
Limitations and Future Directions
Our findings allude to some effects of polygenic risk for schizophrenia on both cortical gray matter and white matter pathways but the exact mechanism and how it relates to PEs is still unclear. As noted above, our sample was not designed for rigorous examination of genetic effects on the brain; replication using much larger sample sizes would be needed before speculating more confidently on the implications of our findings. However, our work does show that covarying for polygenic risk does not alter findings we attribute to PEs and complements previous studies that have reported weak evidence of an association between PEs and the PRS.50,83 This is an important point moving forward in studying both effects. There have been genetic imaging studies that have highlighted an association between the PRS and MRI parameters,41–47 but the data are not conclusive. Cortical patterning has been found to be mediated by genetic factors84 but more research is needed to establish the contribution of common polymorphisms associated with schizophrenia.
In our work, we focused on the impact of persisting PEs during early adulthood in relation to brain structure. However, it is equally of importance to understand what drives the persistence of PEs and to take into consideration additional confounders on brain structure. Our study contained more female than male participants and we were unable to exclude the possibility that gender had a confounding effect. Future studies may want to consider a design that allows examination of gender-specific effects. We chose not to pursue substance use as a confounder; those who did respond on the questionnaires did not show signs of substance abuse. Additionally, previous research from our group did not find a link between brain structure and substance use.31,82 There was a clear difference in general psychopathology, driven by those with persisting PEs, but no group scored above clinically relevant thresholds and it is important to note this measure was obtained at age 18. Previous work from our group did not link CIS-R scores to gray matter volume abnormalities in PEs,31 but a mediation analysis did suggest general psychopathology could confound the abnormalities found in white matter and cortical myelination that were associated with severity of PEs.31,82
In this study, we did not consider the differences between emergent and resolved PEs. Our data does not hint at a clear difference between emergent and resolved PEs, but it is possible that emergent PEs will go on to be considered persistent after a follow-up assessment at a later stage. As such, longitudinal studies into PEs to untangle the different possible trajectories of symptoms are vital; what factors drive the emergence, persistence, and resolution of PEs?
Funding
This work was funded by a grant from the UK Medical Research Council (G0901885). A.S.D. was also supported by the National Institutes of Health Research Biomedical Research Centre at the South London & Maudsley Hospital Foundation NHS Trust and the IoPPN, King’s College London.
Supplementary Material
Acknowledgments
We would like to thank all the families who took part in this study, the midwives who helped recruit them, and the whole ALSPAC team. We also acknowledge the support of Dr Greg Parker at CUBRIC. All authors report no biomedical financial interests or potential conflicts of interest.
References
- 1. van Os J, Hanssen M, Bijl RV, Ravelli A. Strauss (1969) revisited: a psychosis continuum in the general population?Schizophr Res. 2000;45:11–20. [DOI] [PubMed] [Google Scholar]
- 2. McGrath JJ, Saha S, Al-Hamzawi A, et al. . Psychotic experiences in the general population: a cross-national analysis based on 31,261 respondents from 18 countries. JAMA Psychiatry. 2015;72:697–705. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Zammit S, Kounali D, Cannon M, et al. . Psychotic experiences and psychotic disorders at age 18 in relation to psychotic experiences at age 12 in a longitudinal population-based cohort study. Am J Psychiatry. 2013;170:742–750. [DOI] [PubMed] [Google Scholar]
- 4. van Os J, Linscott RJ, Myin-Germeys I, Delespaul P, Krabbendam L. A systematic review and meta-analysis of the psychosis continuum: evidence for a psychosis proneness-persistence-impairment model of psychotic disorder. Psychol Med. 2009;39:179–195. [DOI] [PubMed] [Google Scholar]
- 5. Kaymaz N, Drukker M, Lieb R, et al. . Do subthreshold psychotic experiences predict clinical outcomes in unselected non-help-seeking population-based samples? A systematic review and meta-analysis, enriched with new results. Psychol Med. 2012;42:2239–2253. [DOI] [PubMed] [Google Scholar]
- 6. Polanczyk G, Moffitt TE, Arseneault L, et al. . Etiological and clinical features of childhood psychotic symptoms: results from a birth cohort. Arch Gen Psychiatry. 2010;67:328–338. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Johns LC, Cannon M, Singleton N, et al. . Prevalence and correlates of self-reported psychotic symptoms in the british population. Br J Psychiatry. 2004;185:298–305. [DOI] [PubMed] [Google Scholar]
- 8. Mollon J, David AS, Morgan C, et al. . Psychotic experiences and neuropsychological functioning in a population-based Sample. JAMA Psychiatry. 2016;73:129–138. [DOI] [PubMed] [Google Scholar]
- 9. Yung AR, Buckby JA, Cosgrave EM, et al. . Association between psychotic experiences and depression in a clinical sample over 6 months. Schizophr Res. 2007;91:246–253. [DOI] [PubMed] [Google Scholar]
- 10. Varghese D, Scott J, Welham J, et al. . Psychotic-like experiences in major depression and anxiety disorders: a population-based survey in young adults. Schizophr Bull. 2011;37:389–393. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Fusar-Poli P, Borgwardt S, Crescini A, et al. . Neuroanatomy of vulnerability to psychosis: a voxel-based meta-analysis. Neurosci Biobehav Rev. 2011;35:1175–1185. [DOI] [PubMed] [Google Scholar]
- 12. Fusar-Poli P, Radua J, McGuire P, Borgwardt S. Neuroanatomical maps of psychosis onset: voxel-wise meta-analysis of antipsychotic-naive VBM studies. Schizophr Bull. 2012;38:1297–1307. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Pantelis C, Yücel M, Wood SJ, et al. . Structural brain imaging evidence for multiple pathological processes at different stages of brain development in schizophrenia. Schizophr Bull. 2005;31:672–696. [DOI] [PubMed] [Google Scholar]
- 14. Pantelis C, Velakoulis D, Wood SJ, et al. . Neuroimaging and emerging psychotic disorders: the Melbourne ultra-high risk studies. Int Rev Psychiatry. 2007;19:371–381. [DOI] [PubMed] [Google Scholar]
- 15. Wood SJ, Pantelis C, Velakoulis D, Yücel M, Fornito A, McGorry PD. Progressive changes in the development toward schizophrenia: studies in subjects at increased symptomatic risk. Schizophr Bull. 2008;34:322–329. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Smieskova R, Fusar-Poli P, Allen P, et al. . Neuroimaging predictors of transition to psychosis—a systematic review and meta-analysis. Neurosci Biobehav Rev. 2010;34:1207–1222. [DOI] [PubMed] [Google Scholar]
- 17. Fornito A, Yung AR, Wood SJ, et al. . Anatomic abnormalities of the anterior cingulate cortex before psychosis onset: an MRI study of ultra-high-risk individuals. Biol Psychiatry. 2008;64:758–765. [DOI] [PubMed] [Google Scholar]
- 18. Cannon TD, Chung Y, He G, et al. . Progressive reduction in cortical thickness as psychosis develops: a multisite longitudinal neuroimaging study of youth at elevated clinical risk. Biol Psychiatry. 2015;77:147–157. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Chung Y, Jacobson A, He G, et al. . Prodromal symptom severity predicts accelerated gray matter reduction and third ventricle expansion among clinically high risk youth developing psychotic disorders. Mol Neuropsychiatry. 2015;1:13–22. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. White T, Hilgetag CC. Gyrification and neural connectivity in schizophrenia. Dev Psychopathol. 2011;23:339–352. [DOI] [PubMed] [Google Scholar]
- 21. White T, Gottesman I. Brain connectivity and gyrification as endophenotypes for schizophrenia: weight of the evidence. Curr Top Med Chem. 2012;12:2393–2403. [DOI] [PubMed] [Google Scholar]
- 22. Zilles K, Armstrong E, Schleicher A, Kretschmann HJ. The human pattern of gyrification in the cerebral cortex. Anat Embryol (Berl). 1988;179:173–179. [DOI] [PubMed] [Google Scholar]
- 23. White T, Su S, Schmidt M, Kao CY, Sapiro G. The development of gyrification in childhood and adolescence. Brain Cogn. 2010;72:36–45. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24. Im K, Lee JM, Lyttelton O, Kim SH, Evans AC, Kim SI. Brain size and cortical structure in the adult human brain. Cereb Cortex. 2008;18:2181–2191. [DOI] [PubMed] [Google Scholar]
- 25. Ventura-Antunes L, Mota B, Herculano-Houzel S. Different scaling of white matter volume, cortical connectivity, and gyrification across rodent and primate brains. Front Neuroanat. 2013;7:3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26. Harris JM, Yates S, Miller P, Best JJ, Johnstone EC, Lawrie SM. Gyrification in first-episode schizophrenia: a morphometric study. Biol Psychiatry. 2004;55:141–147. [DOI] [PubMed] [Google Scholar]
- 27. Harris JM, Moorhead TW, Miller P, et al. . Increased prefrontal gyrification in a large high-risk cohort characterizes those who develop schizophrenia and reflects abnormal prefrontal development. Biol Psychiatry. 2007;62:722–729. [DOI] [PubMed] [Google Scholar]
- 28. Stanfield AC, Moorhead TW, Harris JM, Owens DG, Lawrie SM, Johnstone EC. Increased right prefrontal cortical folding in adolescents at risk of schizophrenia for cognitive reasons. Biol Psychiatry. 2008;63:80–85. [DOI] [PubMed] [Google Scholar]
- 29. Jacobson S, Kelleher I, Harley M, et al. . Structural and functional brain correlates of subclinical psychotic symptoms in 11–13 year old schoolchildren. Neuroimage. 2010;49:1875–1885. [DOI] [PubMed] [Google Scholar]
- 30. Pelletier-Baldelli A, Dean DJ, Lunsford-Avery JR, et al. . Orbitofrontal cortex volume and intrinsic religiosity in non-clinical psychosis. Psychiatry Res Neuroimaging. 2014;222:124–130. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31. Drakesmith M, Dutt A, Fonville L, et al. . Volumetric, relaxometric and diffusometric correlates of psychotic experiences in a non-clinical sample of young adults. NeuroImage Clin. 2016;12:550–558. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32. Modinos G, Mechelli A, Ormel J, Groenewold NA, Aleman A, McGuire PK. Schizotypy and brain structure: a voxel-based morphometry study. Psychol Med. 2010;40:1423–1431. [DOI] [PubMed] [Google Scholar]
- 33. Satterthwaite TD, Wolf DH, Calkins ME, et al. . Structural brain abnormalities in youth with psychosis spectrum symptoms. JAMA Psychiatry. 2016;73:515–524. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34. Roalf DR, Quarmley M, Calkins ME, et al. . Temporal lobe volume decrements in psychosis spectrum youths. Schizophr Bull. 2016;43:13–15. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35. Córdova-Palomera A, Alemany S, Falcón C, et al. . Cortical thickness correlates of psychotic experiences: examining the effect of season of birth using a genetically informative design. J Psychiatr Res. 2014;56:144–149. [DOI] [PubMed] [Google Scholar]
- 36. DeRosse P, Ikuta T, Peters BD, Karlsgodt KH, Szeszko PR, Malhotra AK. Adding insult to injury: childhood and adolescent risk factors for psychosis predict lower fractional anisotropy in the superior longitudinal fasciculus in healthy adults. Psychiatry Res. 2014;224:296–302. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37. Smallman RP, Barkus E, Azadbakht H, et al. . MRI diffusion tractography study in individuals with schizotypal features: a pilot study. Psychiatry Res. 2014;221:49–57. [DOI] [PubMed] [Google Scholar]
- 38. O’Hanlon E, Leemans A, Kelleher I, et al. . White matter differences among adolescents reporting psychotic experiences: a population-based diffusion magnetic resonance imaging study. JAMA Psychiatry. 2015;72:668–677. [DOI] [PubMed] [Google Scholar]
- 39. Diederen KMJ, Neggers SFW, de Weijer AD, et al. . Aberrant resting-state connectivity in non-psychotic individuals with auditory hallucinations. Psychol Med. 2013;43:1685–1696. [DOI] [PubMed] [Google Scholar]
- 40. Orr JM, Turner JA, Mittal VA. Widespread brain dysconnectivity associated with psychotic-like experiences in the general population. Neuroimage Clin. 2014;4:343–351. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41. Terwisscha van Scheltinga AF, Bakker SC, van Haren NEM, et al. . Genetic schizophrenia risk variants jointly modulate total brain and white matter volume. Biol Psychiatry. 2013;73:525–531. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42. Walton E, Geisler D, Lee PH, et al. . Prefrontal inefficiency is associated with polygenic risk for schizophrenia. Schizophr Bull. 2014;40:1263–1271. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43. Kauppi K, Westlye LT, Tesli M, et al. . Polygenic risk for schizophrenia associated with working memory-related prefrontal brain activation in patients with schizophrenia and healthy controls. Schizophr Bull. 2015;41:736–743. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44. Lancaster TM, Ihssen N, Brindley LM, et al. . Associations between polygenic risk for schizophrenia and brain function during probabilistic learning in healthy individuals. Hum Brain Mapp. 2016;37:491–500. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45. Harrisberger F, Smieskova R, Vogler C, et al. . Impact of polygenic schizophrenia-related risk and hippocampal volumes on the onset of psychosis. Transl Psychiatry. 2016;6:e868. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46. French L, Gray C, Leonard G, et al. . Early cannabis use, polygenic risk score for schizophrenia and brain maturation in adolescence. JAMA Psychiatry. 2015;72:1002–1011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47. Liu B, Zhang X, Cui Y, et al. . Polygenic risk for schizophrenia influences cortical gyrification in 2 independent general populations. Schizophr Bull. 2016;43:673–680. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48. Horwood J, Salvi G, Thomas K, et al. . IQ and non-clinical psychotic symptoms in 12-year-olds: results from the ALSPAC birth cohort. Br J Psychiatry. 2008;193:185–191. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49. Schizophrenia Working Group of the Psychiatric Genomics Consortium. Biological insights from 108 schizophrenia-associated genetic loci. Nature. 2014;511:421–427. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50. Jones HJ, Stergiakouli E, Tansey KE, et al. . Phenotypic manifestation of genetic risk for schizophrenia during adolescence in the general population. JAMA Psychiatry. 2016;73:221–228. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51. Buck SF. A method of estimation of missing values in multivariate data suitable for use with an electronic computer. J R Stat Soc Ser B Stat Methodol. 1960;22:302–306. [Google Scholar]
- 52. Leemans A, Jeurissen B, Sijbers J, Jones D. ExploreDTI: a graphical toolbox for processing, analyzing, and visualizing diffusion MR data. In: Proceedings 17th Scientific Meeting, International Society for Magnetic Resonance in Medicine. Vol. 17; 2009:3537. [Google Scholar]
- 53. Budday S, Steinmann P, Kuhl E. Physical biology of human brain development. Front Cell Neurosci. 2015;9:1–17. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54. Harris JM, Whalley H, Yates S, Miller P, Johnstone EC, Lawrie SM. Abnormal cortical folding in high-risk individuals: a predictor of the development of schizophrenia?Biol Psychiatry. 2004;56:182–189. [DOI] [PubMed] [Google Scholar]
- 55. R Core Team. R: A Language and Environment for Statistical Computing. Vienna, Austria: R Foundation for Statistical Computing; 2016. ISBN 3-900051-07-0. [Google Scholar]
- 56. Hartigan JA, Hartigan PM. The dip test of unimodality. Ann Stat. 1985;13:70–84. [Google Scholar]
- 57. Hartigan PM. Algorithm as 217: computation of the dip statistic to test for unimodality. Appl Stat. 1985;34:320–325. [Google Scholar]
- 58.Davison AC, Hinkley DV. Bootstrap Methods and Their Application. Vol 1. Cambridge, Cambridge University Press; 1997. [Google Scholar]
- 59. Canty A, Ripley D.. boot: Bootstrap R (S-Plus) Functions. R Package Version. 2017;1:3–20. [Google Scholar]
- 60. Wickham H. ggplot2: Elegant Graphics for Data Analysis. New York: Springer-Verlag, 2009. [Google Scholar]
- 61. Desikan RS, Ségonne F, Fischl B, et al. . An automated labeling system for subdividing the human cerebral cortex on MRI scans into gyral based regions of interest. Neuroimage. 2006;31:968–980. [DOI] [PubMed] [Google Scholar]
- 62. Honea R, Crow TJ, Passingham D, Mackay CE. Regional deficits in brain volume in schizophrenia: a meta-analysis of voxel-based morphometry studies. Am J Psychiatry. 2005;162:2233–2245. [DOI] [PubMed] [Google Scholar]
- 63. Vita A, De Peri L, Deste G, Sacchetti E. Progressive loss of cortical gray matter in schizophrenia: a meta-analysis and meta-regression of longitudinal MRI studies. Transl Psychiatry. 2012;2:e190. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64. Cachia A, Paillère-Martinot ML, Galinowski A, et al. . Cortical folding abnormalities in schizophrenia patients with resistant auditory hallucinations. Neuroimage. 2008;39:927–935. [DOI] [PubMed] [Google Scholar]
- 65. Nesvåg R, Schaer M, Haukvik UK, et al. . Reduced brain cortical folding in schizophrenia revealed in two independent samples. Schizophr Res. 2014;152:333–338. [DOI] [PubMed] [Google Scholar]
- 66. Palaniyappan L, Liddle PF. Differential effects of surface area, gyrification and cortical thickness on voxel based morphometric deficits in schizophrenia. Neuroimage. 2012;60:693–699. [DOI] [PubMed] [Google Scholar]
- 67. Palaniyappan L, Mallikarjun P, Joseph V, White TP, Liddle PF. Folding of the prefrontal cortex in schizophrenia: regional differences in gyrification. Biol Psychiatry. 2011;69:974–979. [DOI] [PubMed] [Google Scholar]
- 68. Palaniyappan L, Liddle PF. Diagnostic discontinuity in psychosis: a combined study of cortical gyrification and functional connectivity. Schizophr Bull. 2014;40:675–684. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69. Palaniyappan L, Marques TR, Taylor H, et al. . Cortical folding defects as markers of poor treatment response in first-episode psychosis. JAMA Psychiatry. 2013;70:1031–1040. [DOI] [PubMed] [Google Scholar]
- 70. Gay O, Plaze M, Oppenheim C, et al. . Cortex morphology in first-episode psychosis patients with neurological soft signs. Schizophr Bull. 2013;39:820–829. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71. Schultz CC, Wagner G, Koch K, et al. . The visual cortex in schizophrenia: alterations of gyrification rather than cortical thickness—a combined cortical shape analysis. Brain Struct Funct. 2013;218:51–58. [DOI] [PubMed] [Google Scholar]
- 72. Schultz CC, Koch K, Wagner G, et al. . Increased parahippocampal and lingual gyrification in first-episode schizophrenia. Schizophr Res. 2010;123:137–144. [DOI] [PubMed] [Google Scholar]
- 73. Maric N, Krabbendam L, Vollebergh W, de Graaf R, van Os J. Sex differences in symptoms of psychosis in a non-selected, general population sample. Schizophr Res. 2003;63:89–95. [DOI] [PubMed] [Google Scholar]
- 74. Armstrong E, Schleicher A, Omran H, Curtis M, Zilles K. The ontogeny of human gyrification. Cereb Cortex. 1995;5:56–63. [DOI] [PubMed] [Google Scholar]
- 75. Gautam P, Anstey KJ, Wen W, Sachdev PS, Cherbuin N. Cortical gyrification and its relationships with cortical volume, cortical thickness, and cognitive performance in healthy mid-life adults. Behav Brain Res. 2015;287:331–339. [DOI] [PubMed] [Google Scholar]
- 76. Dombroski B, Nitzken M, Elnakib A, et al. . Cortical surface complexity in a population-based normative sample. Transl Neurosci. 2014;5:17–24. [Google Scholar]
- 77. Blanton RE, Levitt JG, Thompson PM, et al. . Mapping cortical asymmetry and complexity patterns in normal children. Psychiatry Res. 2001;107:29–43. [DOI] [PubMed] [Google Scholar]
- 78. Sandu AL, Izard E, Specht K, Beneventi H, Lundervold A, Ystad M. Post-adolescent developmental changes in cortical complexity. Behav Brain Funct. 2014;10:44. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79. Mills KL, Goddings AL, Herting MM, et al. . Structural brain development between childhood and adulthood: convergence across four longitudinal samples. Neuroimage. 2016;141:273–281. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80. Giedd JN, Blumenthal J, Jeffries NO, et al. . Brain development during childhood and adolescence: a longitudinal MRI study. Nat Neurosci. 1999;2:861–863. [DOI] [PubMed] [Google Scholar]
- 81. Kochunov P, Mangin JF, Coyle T, et al. . Age-related morphology trends of cortical sulci. Hum Brain Mapp. 2005;26:210–220. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82. Klein D, Rotarska-Jagiela A, Genc E, et al. . Adolescent brain maturation and cortical folding: evidence for reductions in gyrification. PLoS One. 2014;9:e84914. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 83. Drakesmith M, Dutt A, Fonville L, et al. . Mediation of developmental risk factors for psychosis by white matter microstructure in young adults with psychotic experiences. JAMA Psychiatry. 2016;73:396–406. [DOI] [PubMed] [Google Scholar]
- 84. Zammit S, Hamshere M, Dwyer S, et al. . A population-based study of genetic variation and psychotic experiences in adolescents. Schizophr Bull. 2014;40:1254–1262. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 85. Peng Q, Schork A, Bartsch H, et al. ; Pediatric Imaging, Neurocognition and Genetics Study; Alzheimer’s Disease Neuroimaging Initiative Conservation of distinct genetically-mediated human cortical pattern. PLoS Genet. 2016;12:e1006143. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.