Abstract
Individualized metacognitive training (MCT+) is a novel psychotherapy that has been designed to specifically target delusional beliefs in people with psychosis. It works by developing an awareness of the implausible content of delusional beliefs, while also targeting the cognitive biases that contribute to their formation and maintenance. It was expected that MCT+ would lead to significantly greater reductions in delusional severity compared to a cognitive remediation (CR) active control condition. A total of 54 patients with a schizophrenia spectrum disorder and active delusions were randomized into four 2-hourly sessions of MCT+ (n = 27) or CR (n = 27). All participants completed posttreatment assessment, and only 2 participants did not complete 6-month follow-up assessment, resulting in MCT+ (n = 26) and CR (n = 26) for final analysis. The primary outcome measures of delusional and positive symptom severity were assessed rater-blind; secondary outcome assessment was non-blinded and included clinical and cognitive insight, the jumping to conclusions (JTC) bias, and cognitive functioning. Participants in the MCT+ condition showed significant reductions in delusional and overall positive symptom severity (large effect) and improved clinical insight (moderate effect) relative to CR controls. In contrast, CR controls showed moderate improvement in problem-solving ability relative to MCT+, but no other cognitive domain. Importantly, these findings were maintained at 6-month follow-up. The study adds further efficacy to the MCT program, and suggests that even brief psychotherapy can help to ameliorate the symptoms of psychosis.
Keywords: schizophrenia, metacognition, cognitive-behavioral therapy, jumping to conclusions
Introduction
There has been a growing interest over the last decade in non-pharmacological treatments for delusions and other positive symptoms in psychosis. Of these, cognitive-behavioral therapy for psychosis (CBTp) has emerged as the most extensively implemented and studied psychosocial intervention.1 Recent meta-analyses have reported that CBTp is effective in reducing positive symptoms,2,3 and may be more effective than other psychological interventions for psychosis.4 However, recent meta-analyses report that when methodological limitations are taken into consideration (eg, lack of blinding, no control intervention), the therapeutic effect of CBTp is reduced.5–7 Moreover, other meta-analyses have shown that CBTp may not offer any advantage over other psychosocial treatments in the treatment of delusions specifically.3,7
To maximize the efficacy of CBTp, it has been suggested that interventions for psychosis should target the theoretical cognitive and emotional constructs that are responsible for the formation and maintenance of specific symptoms, such as delusions.7 Metacognitive training for psychosis (MCT) may represent one such intervention. Rather than targeting the idiosyncratic delusions specific to the individual client, this manualized group-based program indirectly targets the cognitive biases that decades of theoretical research has linked to the formation and maintenance of delusional beliefs (eg, overconfidence, belief inflexibility and the jumping to conclusions [JTC] bias).8 MCT encourages participants to “think about their thinking,” raising metacognitive awareness for these biases across several entertaining and collaborative exercises, and thereby indirectly “plants the seeds of doubt”.9,10
Several randomized controlled trials have shown the efficacy for MCT in reducing delusional severity, even at 3-year follow-up,11 and 2 recent meta-analyses have concluded that MCT exerts a moderate effect on delusions and positive symptoms.11,12 However, not all trials have yielded significant improvements for MCT,13 suggesting that the group program may not be appropriate for all clients with psychosis, particularly those with acute delusions or high levels of paranoia.14
Accordingly, an individually administered program of metacognitive training (or MCT+) was developed,15 which combines the “cognitive bias” focus of group MCT with elements of individual CBTp (Note: MCT+ is no longer referred to as “metacognitive therapy” to avoid confusion with the program developed by Adrian Wells). This hybrid approach allows therapists to simultaneously target the underlying cognitive biases that may be driving delusional content, while tailoring the therapeutic content to specific delusional beliefs, and allowing for greater use of CBTp techniques (eg, thought records, Socratic questioning) compared to the original group program (refer to the MCT+ treatment manual16 for an in-depth overview). Therefore, MCT+ was developed to maximize the efficiency, yet minimize the potential limitations, of both MCT and CBTp, and maybe a more effective treatment than either of these treatments used in isolation.
Relative to group-based MCT, the evidence-base for MCT+ is still emerging. The only published randomized controlled trial showed that participants who had received MCT+ had significantly lower delusion severity and higher levels of self-reflectiveness (medium effect size), relative to participants receiving an active control intervention.17 However, these group differences were no longer significant at the 6-month follow-up, which is contrary to the long-term effects typically observed in group-MCT trials.11,12,18 The authors noted a lower baseline delusion severity in the MCT+ group (despite randomization), which may have led to possible floor effects in this group, or larger regression to the mean in the control group. Moreover, the beneficial effects of MCT+ were more pronounced in a subset of participants who attended a minimum of 4 sessions of either intervention, highlighting the importance of lowering the potential attrition across sessions. One way of doing this is to combine multiple MCT+ modules together into fewer “extended” sessions (eg, 2-h vs 1-h), effectively reducing the number of overall sessions, while ensuring the essential therapeutic content is retained. There is tentative evidence that such extended versions of MCT+ are feasible and may still offer therapeutic benefit to people with psychosis. For example, a recent case study based on 2 individuals with active delusions, each receiving four 2-hourly MCT+ sessions (without concurrent antipsychotic medication), showed a reduction in delusional severity post-intervention.19 Another small-scale study similarly combined 2 MCT+ modules into a single individually administered module, finding that MCT participants (relative to TAU controls) also exhibited significant decreases in delusional severity, and significant improvements in clinical insight.20
The present study is the first independent randomized controlled trial of MCT+ conducted without direct involvement from the co-creators of the program. The primary aim of the study is to determine the efficacy of an extended-session MCT+ protocol in patients with delusions, compared to an active “cognitive remediation (CR)” control condition targeting neurocognitive symptoms. The four 2-hourly MCT+ sessions, delivered over a month, were designed to be flexible, adapting to the client’s therapeutic needs while ensuring that “cognitive biases” remained the focus, as per the MCT+ protocol. The primary outcome measure assessing for delusional and positive symptom severity was rater-blinded. It was hypothesized that MCT+ would lead to significantly greater reductions in positive symptoms compared to the CR control condition, which itself would be associated with greater improvements in neurocognitive functioning relative to MCT+.
Methods
Participants
A total of 54 participants with a schizophrenia spectrum diagnosis and current delusions were recruited among mental health outpatient consumers of the Northern Adelaide Local Health Network (NALHN) and Southern Adelaide Local Health Network (SALHN) catchment areas of South Australia. Participants were recruited between August 2013 and August 2016 and were referred to the trial by their NALHN/SALHN care coordinator. To be eligible for inclusion in the trial, participants were required to be aged between 18 and 65 years, diagnosed with a schizophrenia spectrum disorder (confirmed by Mini Neuropsychiatric Interview21), and have a current delusional belief (ie, score ≥ 3 on P1 [delusions] item of the Positive and Negative Syndrome Scale or PANSS22). All but 2 participants were currently taking antipsychotic medication.
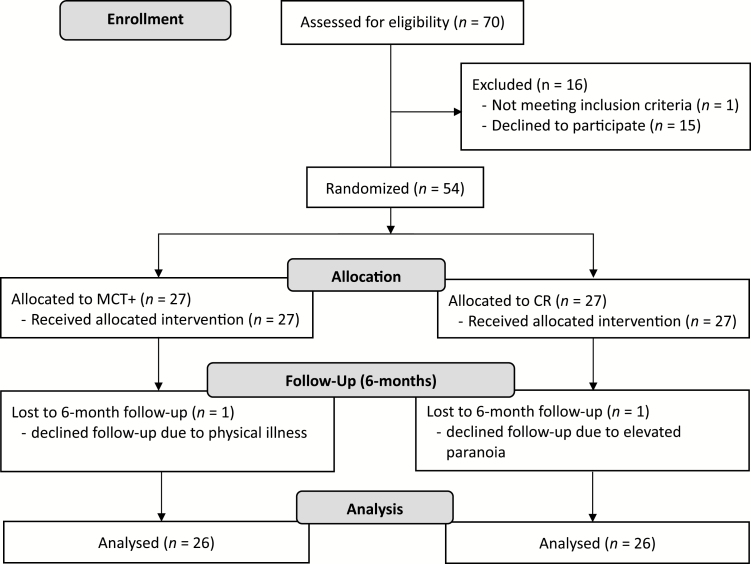
Exclusion criteria included a diagnosis of substance use disorder, alcohol dependence in the last 6 months, IQ < 70 (determined using the Wechsler Test of Adult Reading23), severe organic brain disorders, and previous experience with group MCT or any ongoing CBT-oriented psychotherapy. Participants continued to receive their medication throughout study participation and were offered financial remuneration (AU$50) on completion of posttreatment assessment. The study was approved by the Queen Elizabeth Hospital Human Research Ethics Committee (TQEH/LMH/MH) and all participants provided written informed consent before participating in the study (HREC number: 13/TQEHLMH/77). A CONSORT diagram is provided in figure 1.
Fig. 1.
CONSORT flow diagram.
Study Design
The study employed a randomized controlled experimental design. Participants were randomized to either the MCT+ condition or the CR control condition via a randomized fixed sequence. Group allocation was communicated to participants via a sealed opaque envelope at the first treatment session, ensuring allocation concealment from investigators conducting baseline assessment. Assessment was administered at baseline (T1), posttreatment (T2), and at posttreatment with 6-month follow-up (T3). Assessment of the primary delusional and positive symptom severity outcomes at T1 and T2 were conducted by raters blind to treatment allocation. Due to the relatively limited resources of the trial, assessment of the secondary outcome measures at T2 and T3 was conducted by the investigator who administered the interventions (or were self-report). Assessments for each participant were carried out by the same rater throughout the trial period (T1 to T3) to optimize reliability of the measures. Participants were blind to the study hypothesis at T1 and T2 and were fully debriefed about the central hypothesis after T3. The trial is registered on the Australian New Zealand Clinical Trials Registry (ACTRN 12616000976482).
Outcomes
The primary outcome measure was delusional and positive symptom severity as assessed by the P1 (delusions) item and overall Positive subscale on the PANSS at T2 and T3. The PANSS is widely used in both psychological and pharmacological clinical trials and has good psychometric properties.24 Secondary outcomes included PANSS Negative and General subscale scores. PANSS raters were trained clinicians who were blind to group allocation.
Assessment of all other secondary outcome measures was not rater-blinded at T2 and T3, and included:
- Psychotic Symptom Rating Scale (PSYRATS),25 which like the PANSS, was used to assess delusional severity across a number of domains (conviction, distress, preoccupation).
- Brief Assessment of Cognition in Schizophrenia (BACS)26 was used to assess the aspects of cognition that are most impaired in patients with schizophrenia. The BACS is composed of 6 brief assessments, each measuring a specific cognitive domain: List Learning (verbal memory); Digit Sequencing (working memory); Verbal Fluency (processing speed); Token Motor Task (motor/processing speed); Symbol Coding (attention); and Tower Task (problem-solving).
- Clinical insight (ie, awareness of and attitudes towards mental illness) was estimated using the Schedule for Assessment of Insight (SAI),27 and cognitive insight (ie, the ability to evaluate beliefs and misperceptions as distorted) with the Beck Cognitive Insight Scale (BCIS),28 which assesses levels of self-reflectiveness (or flexibility) and self-certainty (or overconfidence).
- The JTC bias, which has been linked to delusional severity,29 was assessed using an 80:20 computerized version of the beads task,8 where lower draws to decision (DTD) is the marker for the bias.
Interventions
- Experimental intervention: MCT+ is a manualized intervention comprising 9 audio-visual modules.16 The therapeutic goal of MCT+ is encourage patients to become more aware of their own thinking biases and more reflective on the tenacity of their own beliefs. Except for the introductory session, the modules do not follow a set order and have overlapping concepts. Most sessions include prescribed homework exercises, consistent with CBTp. Participants in the current trial completed four 2-hourly MCT+ treatment blocks, typically broken up into 2 consecutive 60-minute sessions. The audio-visual modules, while prescriptive in nature, were used as more of a guide to ensure therapy remained on topic, to illustrate therapeutic content, and to stimulate discussion and reinforce key concepts. At least 1 specific cognitive bias was discussed at length for each participant, but participants could cover up to 5 biases across sessions. The primary therapist was a psychologist trained in CBT and had received training in MCT/MCT+ from its co-developers.
- Control intervention: HAPPYNeuron Pro,30 a web-based and therapist-led CR program, was used as the active control intervention. The primary goal of the program is to improve neurocognitive deficits. This program was selected for the current study due to its highly customizable content (eg, difficulty-level can be tailored for the consumer) and suitability for psychosis populations.31 The cognitive domains targeted were selected to match the domains tested in the BACS. Controls participants completed 4 sessions of CR, which ranged between 90-minutes and 2-hours per session. Sessions were consumer-paced, and a minimal of 3 cognitive domains were completed by each participant.
Statistical Analyses
G*Power 332 was used to calculate the sample size required to detect medium-sized interactions (Cohen’s f = 0.25) in mixed model, 2 × 3 ANOVAs with α = .05, and conservatively assuming correlations between measurement occasions of r = .6 (based on test-retest correlations for the PANSS22). The estimated total sample size required for 95% power was 36.
Analyses were conducted using SPSS Version 23. On the PANSS, posttreatment and follow-up data were available for 27 MCT+ participants and 26 CR participants. For the remaining variables, we had complete data for 26 per group. Therefore, analyses of outcomes were performed on cases with complete data. Running analyses with the last observation carried forward did not alter the results.
As our main interest was in change over time, the normally distributed outcome variables were subjected to 2 (group: MCT+, CR) × 3 (time: baseline, posttreatment, follow-up) mixed model ANOVAs. Significant interactions were followed by pairwise comparisons of baseline (T1) with posttreatment (T2), and posttreatment with follow-up (T3) within each group. Mann-Whitney U tests or Friedman tests were used for skewed variables, and Chi-square tests were used for analyses involving categorical variables.
For ANOVAs, effect sizes are η2 (ie, the proportion of sample variance explained). To quantify the size of change over time, Cohen’s d is provided for differences between measurement occasions within each group. We calculated d = Mdiff/spre because it yields a readily interpretable effect size (and is less inflated than some repeated-measures versions of d33). We used the pooled baseline standard deviation from both groups because the combination of groups gives a superior estimate of variability,33 baseline is free of the possible influence of treatment, and comparisons for the 2 groups use the same metric. Differences for Mann-Whitney U tests were converted to r, and Kendall’s W is reported for Friedman tests.
Results
Table 1 presents baseline demographic, clinical, and cognitive features of the 2 groups, along with tests of intergroup differences. PANSS delusions scores reflected moderate severity for both groups. Preliminary analyses indicated the groups did not differ significantly on most demographic, clinical or cognitive variables, including the key PANSS subscales. However, mean baseline PSYRATS delusions was significantly higher in the MCT+ than CR group (d = 0.61). Correspondingly, the baseline difference in PANSS P1 (delusions) approached significance (d = 0.54). The MCT+ group also scored higher than CR on the self-reflection subscale of the BCIS (d = 0.56) and on the verbal fluency subscale of the BACS (d = 0.56).
Table 1.
Baseline Demographic and Clinical Characteristics (With SDs in Parentheses)
| MCT+ (n = 27) | CR (n = 27) | Test Statistic | P | |
|---|---|---|---|---|
| Age | 35.37 (9.84) | 39.04 (7.48) | t(52) = 1.54 | .13 |
| Gender: male | n = 15; 56% | n = 17; 63% | χ2(1) = 0.58 | .78 |
| Education (y) | 11.30 (1.96) | 11.52 (2.05) | t(52) = 0.41 | .69 |
| WTAR (FSIQ) | 99.70 (8.44) | 97.96 (9.76) | t(52) = 0.70 | .49 |
| Diagnosis | ||||
| Schizophrenia | 66.7% | 74.1% | χ2 (2) = 0.40 | .80 |
| Schizoaffective | 22.2% | 18.5% | ||
| Psychosis other | 11.1% | 7.4% | ||
| Illness duration (y) | 9.85 (8.47) | 12.37 (7.95) | t(52) = 1.13 | .27 |
| Antipsychotic (Chlorpromazine equivalent in mg) | 609.11 (420.70) | 425.00 (361.68) | t(50) = 1.67 | .10 |
| PANSS | ||||
| P1 (delusions) | 4.30 (1.10) | 3.74 (1.02) | t(52) = 1.91 | .06 |
| Positive | 17.89 (4.87) | 16.56 (4.35) | t(52) = 1.06 | .29 |
| Negative | 13.19 (4.11) | 14.70 (4.93) | t(52) = 1.23 | .23 |
| General | 31.81 (7.23) | 30.81 (7.44) | t(52) = 0.50 | .62 |
| PSYRATS delusions | 15.96 (3.89) | 13.59 (4.06) | t(52) = 2.19 | .033 |
| DTD (median) | 4 | 2 | U = 214.00 | .008 |
| Clinical insight: SAI | 11.25 (3.92) | 11.33 (3.40) | t(52) = 0.07 | .94 |
| Self-reflect: BCIS | 12.74 (4.78) | 10.37 (3.54) | t(47.9) = 2.07 | .044 |
| Self-certainty: BCIS | 6.89 (3.37) | 6.11 (3.04) | t(52) = 0.89 | .38 |
| BACS | ||||
| Digit sequencing | 17.63 (4.28) | 16.56 (4.01) | t(52) = 0.95 | .35 |
| Symbol coding | 47.11 (11.71) | 47.04 (10.71) | t(52) = 0.02 | .98 |
| Token motor task | 70.81 (16.54) | 65.07 (15.33) | t(52) = 1.32 | .19 |
| Tower task | 15.52 (3.78) | 15.07 (4.39) | t(52) = 0.40 | .69 |
| Verbal fluency | 47.56 (10.86) | 41.52 (10.90) | t(52) = 2.04 | .047 |
| List learning | 36.04 (11.57) | 31.19 (7.20) | t(43.5) = 1.85 | .07 |
Note: MCT+, Individualized Metacognitive Therapy for psychosis. CR, cognitive remediation control; WTAR (FSIQ), Wechsler Test of Adult Reading (full-scale IQ); PANSS, Positive and Negative Syndrome Scale; PSYRATS, Psychotic Symptom Rating Scales; DTD, draws to decision on the beads task; SAI, Schedule for Assessment of Insight; BCIS, Beck Cognitive Insight Scale; BACS, Brief Assessment of Cognition in Schizophrenia.
The distribution of DTD was markedly positively skewed, as observed elsewhere.34 A Mann-Whitney U test indicated significantly higher baseline DTD in the MCT+ group (r = .36). In other words, they gathered more evidence. Removal of 3 outliers (with DTD greater than 8; 2 in MCT+ and 1 in CR) did not resolve the group difference. Therefore, analyses of DTD were confined to simple tests of differences between time periods within each group.
Primary Outcomes
Table 2 presents descriptive statistics for the PANSS subscales by group and time, plus within-group effect sizes. Inferential statistics for the ANOVAs are in table 3, including the significance of pairwise differences between adjacent times. As PANSS P1 (delusions) was a key outcome, and there was a marginally significant baseline difference between groups, a preliminary 2 (group) × 2 (time: posttreatment, follow-up) ANCOVA was performed with baseline as the covariate. The covariate × time interaction was nonsignificant, F(1,50) = 0.10, P = .76, suggesting that the initial difference did not have the potential to bias assessments of change over time.
Table 2.
Means (SDs) and Effect Sizes (Cohen’s d) for Clinical and Cognitive Variables, by Group and Time
| MCT+ | CR | |||||||||
|---|---|---|---|---|---|---|---|---|---|---|
| T1 | T2 | T3 | T2-T1 (d) | T3-T2 (d) | T1 | T2 | T3 | T2-T1 (d) | T3-T2 (d) | |
| PANSS | ||||||||||
| P1 (delusions) | 4.30 (1.10) | 2.93 (1.30) | 2.63 (1.31) | −1.36 | −0.30 | 3.60 (0.91) | 3.56 (0.92) | 3.32 (1.07) | −0.04 | −0.24 |
| Positive | 17.89 (4.87) | 14.04 (4.27) | 12.70 (3.79) | −0.83 | −0.29 | 16.46 (4.41) | 15.77 (4.94) | 14.81 (4.72) | −0.15 | −0.21 |
| Negative | 13.19 (4.11) | 12.19 (3.81) | 12.15 (3.89) | −0.22 | −0.01 | 14.80 (5.07) | 14.20 (4.75) | 13.96 (4.41) | −0.13 | −0.05 |
| General | 31.81 (7.26) | 27.56 (7.67) | 26.19 (6.12) | −0.57 | −0.18 | 30.69 (7.56) | 28.96 (7.86) | 28.77 (8.03) | −0.23 | −0.03 |
| PSYRATS delusions | 16.08 (3.92) | 11.42 (4.16) | 10.60 (4.12) | −1.16 | −0.20 | 13.65 (4.13) | 13.58 (4.19) | 14.15 (4.86) | −0.02 | 0.14 |
| Clinical insight: SAI | 11.23 (3.99) | 13.85 (3.83) | 13.27 (3.32) | 0.70 | −0.15 | 11.35 (3.46) | 12.42 (3.31) | 11.12 (4.33) | 0.29 | −0.35 |
| Self-reflectiveness: BCIS | 12.81 (4.87) | 14.03 (4.33) | 12.96 (3.93) | 0.28 | −0.25 | 10.31 (3.60) | 11.12 (2.80) | 10.42 (3.94) | 0.19 | −0.16 |
| Self-certainty: BCIS | 6.85 (3.43) | 5.35 (2.50) | 6.08 (2.94) | −0.46 | 0.22 | 6.19 (3.07) | 6.50 (3.10) | 5.35 (3.05) | 0.10 | −0.35 |
| BACS | ||||||||||
| Digit sequencing (working memory) | 17.69 (4.35) | 18.19 (4.56) | 18.57 (4.79) | 0.12 | 0.09 | 16.77 (3.93) | 18.46 (4.20) | 18.04 (4.28) | 0.41 | −0.10 |
| Symbol coding (attention) | 47.08 (11.93) | 48.65 (8.93) | 49.42 (14.34) | 0.14 | −0.07 | 47.19 (10.89) | 47.58 (10.45) | 48.31 (8.70) | 0.03 | 0.06 |
| Token motor task (motor speed) | 70.62 (16.84) | 75.77 (15.39) | 77.31 (11.16) | 0.32 | 0.10 | 64.26 (15.04) | 70.08 (16.60) | 73.15 (12.97) | 0.36 | −0.19 |
| Tower task (problem-solving) | 15.42 (3.81) | 15.35 (4.63) | 15.81 (3.76) | −0.02 | 0.11 | 15.31 (4.31) | 17.92 (3.58) | 17.12 (3.23) | 0.64 | −0.20 |
| Verbal fluency (processing speed) | 47.12 (10.82) | 47.81 (13.20) | 48.38 (11.00) | 0.06 | 0.05 | 41.92 (10.91) | 47.42 (13.90) | 46.46 (12.70) | 0.51 | −0.10 |
| List learning (verbal memory) | 36.04 (11.80) | 36.81 (11.39) | 37.23 (11.97) | 0.08 | 0.04 | 30.73 (6.93) | 36.35 (7.67) | 34.81 (6.48) | 0.59 | −0.12 |
Note: MCT+, Individualized Metacognitive Therapy for psychosis; CR, cognitive remediation control; PANSS, Positive and Negative Syndrome Scale; PSYRATS, Psychotic Symptom Rating Scales; SAI, Schedule for Assessment of Insight; BCIS, Beck Cognitive Insight Scale; BACS, Brief Assessment of Cognition in Schizophrenia; T1, baseline; T2, posttreatment; T3, 6-mo follow-up. Significant interactions were followed up with tests of within-group differences: significant effects are indicated by bold values of d.
Table 3.
Results of 2 (Group) × 3 (Time) Mixed Model ANOVAs on Clinical Variables (Main Effects of Group are Omitted; all Were Small and Nonsignificant), and Outcomes of Pairwise Comparisons of Times for Significant Interactions
| Effect | Test Statistic | P | η2 | Within-Groups Differences (P) | |||
|---|---|---|---|---|---|---|---|
| MCT+ | CR | ||||||
| T2 vs T1 | T3 vs T2 | T2 vs T1 | T3 vs T2 | ||||
| PANSS P1 (delusions) | |||||||
| Time | F(2,102) = 55.47 | <.001 | .40 | ||||
| Group × Time | F(2,102) = 32.39 | <.001 | .23 | <.001 | .045 | .75 | .073 |
| PANSS Positive | |||||||
| Time | F(2,102) = 64.79 | <.001 | .48 | ||||
| Group × Time | F(2,102) = 20.14 | <.001 | .15 | <.001 | .004 | .057 | .038 |
| PANSS Negative | |||||||
| Time | F(2,102) = 3.08 | .05 | .06 | ||||
| Group × Time | F(2,102) = 0.17 | .85 | .003 | — | — | — | — |
| PANSS General | |||||||
| Time | F(2,102) = 19.46 | <.001 | .26 | ||||
| Group × Time | F(2,102) = 4.39 | .02 | .06 | <.001 | .15 | .02 | .84 |
| PSYRATS delusions | |||||||
| Time | F(2,100) = 16.43 | <.001 | .19 | ||||
| Group × Time | F(2,100) = 20.41 | <.001 | .24 | <.001 | .25 | .89 | .42 |
| Clinical Insight: SAI | |||||||
| Time | F(2,100) = 12.57 | <.001 | .19 | ||||
| Group × Time | F(2,100) = 4.94 | .009 | .07 | <.001 | .26 | .017 | .012 |
| Self-reflection: BCIS | |||||||
| Time | F(2,100) =2.24 | .11 | .04 | ||||
| Group × Time | F(2,100) = 0.10 | .91 | .002 | — | — | — | — |
| Self-certainty: BCIS | |||||||
| Time | F(2,100) = 2.19 | .12 | .04 | ||||
| Group × Time | F(2,100) = 3.54 | .03 | .06 | .011 | .21 | .59 | .050 |
| BACS | |||||||
| Digit Sequencing | |||||||
| Time | F(2,100) = 4.49 | .014 | .08 | ||||
| Group × Time | F(2,100) = 1.06 | .35 | .02 | ||||
| Symbol Coding | |||||||
| Time | F(2,100) = 1.40 | .25 | .03 | ||||
| Group × Time | F(2,100) = 0.23 | .80 | .004 | ||||
| Token Motor Task | |||||||
| Time | F(2,100) = 7.86 | .001 | .14 | ||||
| Group × Time | F(2,100) = 0.16 | .87 | .002 | ||||
| Tower Task | |||||||
| Time | F(2,100) = 4.86 | .010 | .08 | ||||
| Group × Time | F(2,100) = 4.65 | .012 | .08 | .91 | .48 | <.001 | .22 |
| Verbal Fluency | |||||||
| Time | F(2,100) = 5.24 | .007 | .09 | ||||
| Group × Time | F(2,100) = 2.63 | .077 | .05 | ||||
| List Learning | |||||||
| Time | F(2,100) = 5.32 | .006 | .09 | ||||
| Group × Time | F(2,100) = 2.72 | .071 | .05 | ||||
Note: MCT+, Individualized Metacognitive Therapy for psychosis; CR, cognitive remediation control; PANSS, Positive and Negative Syndrome Scale; PSYRATS, Psychotic Symptom Rating Scales; SAI, Schedule for Assessment of Insight; BCIS, Beck Cognitive Insight Scale; BACS, Brief Assessment of Cognition in Schizophrenia; T1, baseline; T2, posttreatment; T3, 6-mo follow-up.
For both PANSS P1 and PANSS Positive, the ANOVAs yielded strong, significant group × time interactions. There was a large reduction in PANSS P1 from baseline to posttreatment following MCT+, and a further small, significant improvement from posttreatment to follow-up. In contrast, no significant changes were observed in the CR group. For the broader spectrum of positive symptoms (PANSS Positive), significant improvements occurred from baseline to posttreatment (large effect) and posttreatment to follow-up (small effect) in the MCT+ group. For the CR group, while the improvement from baseline to posttreatment did not reach significance, follow-up was significantly lower than posttreatment (small effect).
Secondary Outcomes
Psychopathology.
Contrary to the positive symptoms, for PANSS Negative, the group × time interaction was nonsignificant. There was only a relatively small main effect of time (P = .05). Overall, negative symptoms diminished slightly but not meaningfully from baseline (M = 14.00, SD = 4.59) to posttreatment (M = 13.23, SD = 4.36), P = .042, d = 0.18, and there was no change from post to follow-up (M = 13.06, SD = 4.18), P = .64, d = .04.. For PANSS General, a main effect of time indicated significant improvements across both groups from baseline to posttreatment, d = 0.40, (MBaseline = 31.26, SD = 7.36; MPost = 28.25, SD = 7.72; MFollow-up = 27.45, SD = 7.18). A significant but modest interaction reflected greater improvement from baseline to post for MCT+ (medium effect) than for CR (small effect). There were no differences between post and follow-up within either group. Although administration of the PSYRATS was not blind, PSYRATS delusions was strongly correlated with PANSS P1 at every time point (rs ranged from .69 to .70). Results for PSYRATS delusions mirrored those for PANSS P1 in that there was a large interaction effect that arose from a substantial improvement from baseline to posttreatment in the MCT+ group, which was maintained at follow-up, but no change over time in the CR group.
Jumping to Conclusions.
For the beads task, Friedman tests (with 3 levels of time) were performed on each group separately. For MCT+, there was a small, significant effect of time, χ2(2) = 8.13, P = .017, Kendall’s W = .17. Pairwise posthoc comparisons showed that, relative to baseline (Mdn = 4, mean rank = 2.23), DTD was lower at follow-up (Mdn = 3, mean rank = 1.65), P = .04, but posttreatment (Mdn = 3, mean rank = 2.12) did not differ significantly from either of the other times. For the CR group, the effect of time was nonsignificant, χ2(2) = 0.47, P = .79, Kendall’s W = .01, and median DTD was 2 at all 3 time points. In addition, Spearman correlations showed that DTD was not significantly related to PANSS P1 or PANSS Positive at any of the measurement points (rs ranged from .04 to −.18).
Insight.
For both clinical insight (SAI) and self-certainty (BCIS), the ANOVAs yielded significant group × time interactions (for the means, see table 2). Pairwise comparisons showed that clinical insight increased significantly from baseline to post in both groups, with a larger effect size for MCT+. For MCT+, there was no further change to follow-up. In the CR group, insight diminished slightly but significantly from post to follow-up. For self-certainty, the only significant effects were a small decrease (improvement) at posttreatment following MCT+, and a small increase from post to follow-up in the CR group. There were no significant effects for BCIS self-reflectiveness.
Neurocognition.
Several effects were apparent on the BACS cognitive functioning measures (table 2). The most notable was a group × time interaction for the Tower Task (problem-solving), on which a medium-sized improvement occurred from baseline to post in the CR group, but no differences were seen in the MCT+ group.
Discussion
The present study was the first independent randomized controlled trial investigating the efficacy of MCT+, administered over 4 “intensive” sessions, relative to an active control group. Despite the brevity of the active treatment condition, the findings suggest that MCT+ led to strong and significant improvements in delusional severity and overall positive symptomology, which were maintained at 6-month follow-up. The fact that these improvements were observed against an active CR control condition is noteworthy given that a recent meta-analysis reported effect sizes in favor of CBTp diminish when the control intervention is not treatment-as-usual.7 The negligible attrition also highlights that both programs were feasible for people with active psychotic symptoms.
This set of findings is consistent with group-based MCT,11 which has also been shown to exert long-term improvement in delusional symptoms. The findings are also broadly consistent with the only other published trial on MCT+,18 although that study did not find significant improvements in favor of MCT+ at the 6-month follow-up. Of note, that study reported comparatively lower baseline delusional severity scores, particularly for the MCT+ group, suggesting that there was less opportunity for improvement on delusional symptoms compared to the current study. It is also possible that the current “extended-session” delivery of MCT+, where sessions lasted 2-hours, offered some unique therapeutic benefit to patients over the more typical 50-minute format. It has been suggested that individuals with psychosis may benefit from psychotherapy that is set at a slower pace, which could allow greater time for concepts to be understood and applied to daily life.35 MCT+ is well suited to an “extended” format, as the structured aspects of the therapy are presented within audio-visual modules, which helps maintain focus and retention of key concepts.
While both interventions were observed to improve general psychopathology and illness insight, MCT+ appeared to have a stronger and longer-term influence. Although previous trials have also shown that MCT can improve clinical insight,20 this was first trial to show that improvements could be maintained long-term. Of note, the MCT+ program includes content aimed at reducing self-stigma and improving self-esteem, which may have contributed to these findings.
Consistent with a growing body of work suggesting that CR programs can improve neurocognitive deficits in people with schizophrenia,36–38 the CR condition led to improvements in problem-solving ability, which were maintained at follow-up. Importantly, these improvements were not observed in the MCT+ group, again highlighting that the 2 interventions were targeting different symptom domains. However, improvements were not apparent within the other cognitive domains, suggesting that more sessions of CR might be required to be effective in these domains.
There were also inconsistent findings regarding cognitive insight. While improvements in self-certainty in favor of MCT+ were observed posttreatment, consistent with notion that MCT may exert its effect by reducing overconfidence,39 this was not maintained at follow-up. Moreover, self-reflectiveness (or cognitive flexibility) did not improve at all for MCT+, in contrast to other MCT trials.17,40 It is possible the significantly higher self-reflectiveness at baseline in the MCT+ condition (relative to CR) may have led to a potential ceiling effect, reducing room for improvement.
There was also no improvement in the JTC bias post-intervention for the MCT+ condition; in fact, DTD, the indicator for the bias, reduced for this group at posttreatment and follow-up (ie, higher tendency for a JTC bias). This may suggest that individualized MCT is not as effective as the original group-based program at reducing cognitive biases, since neither of the MCT+ trials to date have found improvement on the JTC bias. This could be because MCT+ provides less content on cognitive biases relative to group-based MCT. Further support for this comes from an earlier trial which reported a significant reduction in JTC in individuals receiving a hybrid of both MCT+ and group MCT.41
There is also recent evidence that the version of the beads task used in the current study, which involves a single bead sequence in the absence of a practice trial, is an unreliable measure of DTD, and thereby the JTC bias.42 Moreover, DTD did not correlate with delusional ideation, which is inconsistent with a recent meta-analysis tentatively linking JTC to delusional severity29 and, more generally, the hypothesis that JTC plays a casual role in the formation of delusions. This highlights the need for more longitudinal studies observing the potential association between the JTC bias and delusional severity, which would ideally use a more reliable version of the beads task.42 Nevertheless, the hypothesis that MCT+ works to reduce delusional severity by targeting the JTC bias is unsupported by the present findings.
Strengths of the present study include that it was randomized, the primary outcome measures were blinded, and assessors remained consistent across assessment. However, there were also several limitations, including that secondary outcome measures were not rater-blinded at posttreatment or follow-up. While this may have introduced some unintentional rater-bias, it is also worth noting that both measures of delusions (blinded PANSS and unblinded PSYRATS) were highly correlated. The current sample was also relatively high functioning given the severity of delusional symptoms, and it cannot be assumed the “extended therapy” format used would be appropriate for patients with lower functioning. Caution is also warranted given that the MCT+ group had more severe delusional symptoms at baseline relative to the control condition, despite randomization. This implies there were greater margins for change or larger regression to the mean in the MCT+ group, which may confound the between-group differences observed. Future MCT+ trials would benefit from using larger samples and ensure that all assessment of outcome measures remains blind to group allocation. Such trials, which are already underway,43 would also be more capable of identifying the “core modules” that offer the most therapeutic benefit. Ideally, future trials could also include a group-MCT condition which could be directly compared to MCT+.
In summary, the present study demonstrated that a relatively brief course of MCT+, a hybrid therapy program of group-based MCT and individualized CBTp, was effective at reducing delusional symptoms, relative to an active control condition receiving CR. These findings were maintained at 6-month follow-up. While larger multi-site trials investigating MCT+ are warranted, the present study adds to the growing literature that psychological interventions can be effective in people with psychosis, and challenges earlier conceptions that delusions are “un-understandable,”44 and by implication, resistant to rational counter-argument.
Funding
This research was supported by Flinders University and the Trevor Prescott Freemasons Memorial Scholarship from the SA Masonic Foundation to R.P.B.
Acknowledgments
The authors wish to acknowledge Steffen Moritz and Todd S. Woodward as the co-developers of metacognitive training, and who, in conjunction with Mahesh Menon, Christina Andreou and Tracey Wade, laid the conceptual groundwork for the present study. We would also like to thank mental health research nurses Bev Hisee and Imelda Cairney for conducting the PANSS assessments, and research assistants Ben McLean and Tomoko Nishizawa for helping to conduct MCT+ and CR sessions. The authors have declared that there are no conflicts of interest in relation to the subject of this study. R.P.B. conceived of and designed the study, secured ethics approval, recruited participants, conducted the majority of the MCT+ and CR sessions, assessed secondary outcomes, conducted preliminary data analysis and wrote the manuscript. J.M. analyzed the data, wrote the results section, and contributed to the other sections of the manuscript. P.D. assisted in the experimental design and contributed to the manuscript. D.L. helped to recruit participants contributed to the manuscript. C.G. helped to recruit participants, assisted with ethics approval and experimental design, and contributed to the manuscript.
References
- 1. Bucci S, Tarrier N. A cognitive behavioural case formulation approach to the treatment of psychosis. In: Tarrier N, Johnson J, eds. Case Formulation in Cognitive Behaviour Therapy: The Treatment of Challenging and Complex Cases. 2nd ed. East Sussex, UK: Routledge; 2016:166–187. [Google Scholar]
- 2. Wykes T, Steel C, Everitt B, Tarrier N. Cognitive behavior therapy for schizophrenia: effect sizes, clinical models, and methodological rigor. Schizophr Bull. 2008;34:523–537. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. van der Gaag M, Valmaggia LR, Smit F. The effects of individually tailored formulation-based cognitive behavioural therapy in auditory hallucinations and delusions: a meta-analysis. Schizophr Res. 2014;156:30–37. [DOI] [PubMed] [Google Scholar]
- 4. Turner DT, van der Gaag M, Karyotaki E, Cuijpers P. Psychological interventions for psychosis: a meta-analysis of comparative outcome studies. Am J Psychiatry. 2014;171:523–538. [DOI] [PubMed] [Google Scholar]
- 5. Jauhar S, McKenna PJ, Radua J, Fung E, Salvador R, Laws KR. Cognitive-behavioural therapy for the symptoms of schizophrenia: systematic review and meta-analysis with examination of potential bias. Br J Psychiatry. 2014;204:20–29. [DOI] [PubMed] [Google Scholar]
- 6. Lynch D, Laws KR, McKenna PJ. Cognitive behavioural therapy for major psychiatric disorder: does it really work? A meta-analytical review of well-controlled trials. Psychol Med. 2010;40:9–24. [DOI] [PubMed] [Google Scholar]
- 7. Mehl S, Werner D, Lincoln TM. Does cognitive behavior therapy for psychosis (CBTp) show a sustainable effect on delusions? A meta-analysis. Front Psychol. 2015;6:1450. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Garety PA, Freeman D. The past and future of delusions research: from the inexplicable to the treatable. Br J Psychiatry. 2013;203:327–333. [DOI] [PubMed] [Google Scholar]
- 9. Moritz S, Lysaker PH.. Metacognition – What did James H. Flavell really say and the implications for the conceptualization and design of metacognitive interventions. doi:10.1016/j.schres.2018.06.001 [DOI] [PubMed] [Google Scholar]
- 10. Moritz S, Andreou C, Schneider BC, et al. Sowing the seeds of doubt: a narrative review on metacognitive training in schizophrenia. Clin Psychol Rev. 2014;34:358–366. [DOI] [PubMed] [Google Scholar]
- 11. Eichner C, Berna F. Acceptance and efficacy of metacognitive training (MCT) on positive symptoms and delusions in patients with schizophrenia: a meta-analysis taking into account important moderators. Schizophr Bull. 2016;42:952–962. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Liu YC, Tang CC, Hung TT, Tsai PC, Lin MF. The efficacy of metacognitive training for delusions in patients with schizophrenia: a meta-analysis of randomized controlled trials informs evidence-based practice. Worldviews Evid Based Nurs. 2018;15:130–139. [DOI] [PubMed] [Google Scholar]
- 13. van Oosterhout B, Krabbendam L, de Boer K, et al. Metacognitive group training for schizophrenia spectrum patients with delusions: a randomized controlled trial. Psychol Med. 2014;44:3025–3035. [DOI] [PubMed] [Google Scholar]
- 14. Moritz S, Werner D, Menon M, Balzan RP, Woodward TS. Jumping to negative conclusions–a case of study-gathering bias?Psychol Med. 2016;46:59–61. [DOI] [PubMed] [Google Scholar]
- 15. Vitzthum FB, Veckenstedt R, Moritz S. Individualized metacognitive therapy program for patients with psychosis (MCT+): introduction of a novel approach for psychotic symptoms. Behav Cogn Psychother. 2014;42:105–110. [DOI] [PubMed] [Google Scholar]
- 16. Moritz S, Bohn F, Veckenstedt R, et al. Individualized metacognitive therapy program for psychosis (MCT+). 2nd ed. Hamburg, Germany: VanHam Campus Press; 2016. [Google Scholar]
- 17. Andreou C, Wittekind CE, Fieker M, et al. Individualized metacognitive therapy for delusions: a randomized controlled rater-blind study. J Behav Ther Exp Psychiatry. 2017;56:144–151. [DOI] [PubMed] [Google Scholar]
- 18. Moritz S, Veckenstedt R, Andreou C, et al. Sustained and “sleeper” effects of group metacognitive training for schizophrenia: a randomized clinical trial. JAMA Psychiatry. 2014;71:1103–1111. [DOI] [PubMed] [Google Scholar]
- 19. Balzan RP, Galletly C. Metacognitive therapy (MCT+) in patients with psychosis not receiving antipsychotic medication: a case study. Front Psychol. 2015;6:967. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Balzan RP, Delfabbro PH, Galletly CA, Woodward TS. Metacognitive training for patients with schizophrenia: preliminary evidence for a targeted, single-module programme. Aust N Z J Psychiatry. 2014;48:1126–1136. [DOI] [PubMed] [Google Scholar]
- 21. Sheehan DV, Lecrubier Y, Sheehan KH, et al. The mini-international neuropsychiatric interview (M.I.N.I.): the development and validation of a structured diagnostic psychiatric interview for DSM-IV and ICD-10. J Clin Psychiatry. 1998;59 (Suppl 20):22–33;quiz 34. [PubMed] [Google Scholar]
- 22. Kay SR, Fiszbein A, Opler LA. The positive and negative syndrome scale (PANSS) for schizophrenia. Schizophr Bull. 1987;13:261–276. [DOI] [PubMed] [Google Scholar]
- 23. Holdnack HA. Wechsler Test of Adult Reading: WTAR. San Antonio, TX: The Psychological Corporation; 2001. [Google Scholar]
- 24. Peralta V, Cuesta MJ. Psychometric properties of the positive and negative syndrome scale (PANSS) in schizophrenia. Psychiatry Res. 1994;53:31–40. [DOI] [PubMed] [Google Scholar]
- 25. Haddock G, McCarron J, Tarrier N, Faragher EB. Scales to measure dimensions of hallucinations and delusions: the Psychotic Symptom Rating Scales (PSYRATS). Psychol Med. 1999;29:879–889. [DOI] [PubMed] [Google Scholar]
- 26. Keefe RS, Harvey PD, Goldberg TE, et al. Norms and standardization of the Brief Assessment of Cognition in Schizophrenia (BACS). Schizophr Res. 2008;102:108–115. [DOI] [PubMed] [Google Scholar]
- 27. Amador XF, Strauss DH, Yale SA, Gorman JM. Awareness of illness in schizophrenia. Schizophr Bull. 1991;17:113–132. [DOI] [PubMed] [Google Scholar]
- 28. Beck AT, Baruch E, Balter JM, Steer RA, Warman DM. A new instrument for measuring insight: the Beck Cognitive Insight Scale. Schizophr Res. 2004;68:319–329. [DOI] [PubMed] [Google Scholar]
- 29. McLean BF, Mattiske JK, Balzan RP. Association of the jumping to conclusions and evidence integration biases with delusions in psychosis: a detailed meta-analysis. Schizophr Bull. 2017;43:344–354. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30. HAPPYneuron Pro. Cognitive Therapy Tools Rehabiltation Program 2018. https://www.happyneuronpro.com/en/. Accessed September 5, 2018.
- 31. Glenthøj LB, Fagerlund B, Randers L, et al. The FOCUS trial: cognitive remediation plus standard treatment versus standard treatment for patients at ultra-high risk for psychosis: study protocol for a randomised controlled trial. Trials. 2015;16:25. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32. Faul F, Erdfelder E, Lang AG, Buchner A. G*Power 3: a flexible statistical power analysis program for the social, behavioral, and biomedical sciences. Behav Res Methods. 2007;39:175–191. [DOI] [PubMed] [Google Scholar]
- 33. Cumming G. Understanding the New Statistics: Effect Sizes, Confidence Intervals, and Meta-analysis. New York: Routledge; 2013. [Google Scholar]
- 34. Ross RM, Pennycook G, McKay R, Gervais WM, Langdon R, Coltheart M. Analytic cognitive style, not delusional ideation, predicts data gathering in a large beads task study. Cogn Neuropsychiatry. 2016;21:300–314. [DOI] [PubMed] [Google Scholar]
- 35. Kingdon D, Turkington D.. Cognitive Therapy of Schizophrenia. New York, NY: The Guildford Press; 2008. [Google Scholar]
- 36. Wykes T, Huddy V, Cellard C, McGurk SR, Czobor P. A meta-analysis of cognitive remediation for schizophrenia: methodology and effect sizes. Am J Psychiatry. 2011;168:472–485. [DOI] [PubMed] [Google Scholar]
- 37. Galletly C, Rigby A. An overview of cognitive remediation therapy for people with severe mental illness. ISRN Rehabilitation. 2013;2013:1–6. [Google Scholar]
- 38. Medalia A, Saperstein AM. Does cognitive remediation for schizophrenia improve functional outcomes?Curr Opin Psychiatry. 2013;26:151–157. [DOI] [PubMed] [Google Scholar]
- 39. Köther U, Vettorazzi E, Veckenstedt R, et al. Bayesian analyses of the effect of metacognitive training on social cognition deficits and overconfidence in errors. J Exp Psychopathol. 2017;8:158–174. [Google Scholar]
- 40. Lam KC, Ho CP, Wa JC, et al. Metacognitive training (MCT) for schizophrenia improves cognitive insight: a randomized controlled trial in a Chinese sample with schizophrenia spectrum disorders. Behav Res Ther. 2015;64:38–42. [DOI] [PubMed] [Google Scholar]
- 41. Moritz S, Veckenstedt R, Randjbar S, Vitzthum F, Woodward TS. Antipsychotic treatment beyond antipsychotics: metacognitive intervention for schizophrenia patients improves delusional symptoms. Psychol Med. 2011;41:1823–1832. [DOI] [PubMed] [Google Scholar]
- 42. McLean BF, Mattiske JK, Balzan RP. Towards a reliable repeated-measures beads task. Psychiatry Res. 2018;265:200–207. [DOI] [PubMed] [Google Scholar]
- 43. Schneider BC, Brüne M, Bohn F, et al. Investigating the efficacy of an individualized metacognitive therapy program (MCT+) for psychosis: study protocol of a multi-center randomized controlled trial. BMC Psychiatry. 2016;16:51. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44. Jaspers K. Allgemeine Psychopathologie. Ein Leitfaden für Studierende, Ärzte und Psychologen. Berlin, Germany: J. Springer; 1913. [Google Scholar]