Abstract
Despite evidence for a role of the dopamine system in the pathophysiology of schizophrenia, there has not been substantial evidence that this disorder originates from a pathological change within the dopamine system itself. Current data from human imaging studies and preclinical investigations instead point to a disruption in afferent regulation of the dopamine system, with a focus on the hippocampus. We found that the hippocampus in the methylazoxymethanol acetate (MAM) rodent developmental disruption model of schizophrenia is hyperactive and dysrhythmic, possibly due to loss of parvalbumin interneurons, leading to a hyperresponsive dopamine system. Whereas current therapeutic approaches target dopamine receptor blockade, treatment at the site of pathology may be a more effective therapeutic avenue. This model also provided insights into potential means for prevention of schizophrenia. Specifically, given that stress is a risk factor in schizophrenia, and that stress can damage hippocampal parvalbumin interneurons, we tested whether alleviating stress early in life can effectively circumvent transition to schizophrenia-like states. Administering diazepam prepubertally at an antianxiety dose in MAM rats was effective at preventing the emergence of the hyperdopaminergic state in the adult. Moreover, multiple stressors applied to normal rats at the same time point resulted in pathology similar to the MAM rat. These data suggest that a genetic predisposition leading to stress hyper-responsivity, or exposure to substantial stressors, could be a primary factor leading to the emergence of schizophrenia later in life, and furthermore treating stress at a critical period may be effective in circumventing this transition.
Keywords: dopamine, psychosis, hippocampus, parvalbumin, antipsychotics, stress
Introduction
Schizophrenia is a devastating disorder that affects 1%–1.5% of the population worldwide. Current therapeutic approaches focus exclusively on blockade of dopamine (DA) D2 receptors.1 However, there is little evidence that the DA system itself is pathologically impacted in this disorder. Over the past decade a shift in thinking about schizophrenia has occurred, in which the DA system is considered normal, but is likely disrupted by pathology in afferent systems that control DA system dynamics.2 In order to provide insight into the pathophysiology, treatment, and ultimately prevention of schizophrenia, it is essential to understand how the DA system is regulated, how dysfunction in afferent systems that show altered activity in schizophrenia impact DA system activity, and the etiological factors that lead to systemwide disruptions that are observed in schizophrenia patients. Due to increasing evidence that the risk for schizophrenia is based on an interaction between genetic susceptibility and early life stressors,3 we examined the system-level interactions in a rodent developmental disruption model of schizophrenia based on the Research Domain Criteria (RDoC) system which addresses psychopathology from a behavioral construct representing a phenotype closely related to clinical manifestations on the one hand and to circuit-based interactions on the other.4–6 From this model, insights were gleaned into how susceptibility can interact with environmental factors to lead to pathophysiology, and how this transition could be circumvented with early intervention.
Dopamine Neuron Regulation and its Relevance for Psychopathology
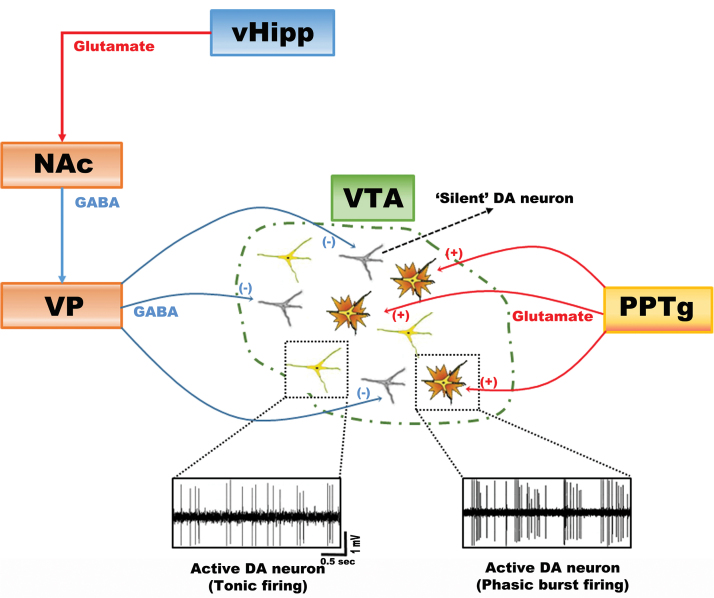
DA neurons display several activity states that are differentially regulated by afferent inputs and are proposed to impact behavior in unique manners. The dimensions of activity that influence DA neuron output are firing pattern, firing rate, and the proportion of neurons that are firing spontaneously. With respect to pattern, DA neurons fire in 1 of 2 configurations: Slow, irregular single spike firing and burst firing (figure 1). Burst firing is a brief phasic activation that occurs when an animal is exposed to a behaviorally salient event; hence it could be considered the rapid, behaviorally relevant phasic signal that is produced in response to discrete events. For the DA neurons in the ventral tegmental area (VTA), this is driven by glutamate originating primarily in the pedunculopontine tegmental afferents acting on NMDA receptors.7 However, for a DA neuron to burst fire it must be depolarized and spontaneously active.7,8 If the DA neuron is hyperpolarized and nonfiring, there is a magnesium block of the NMDA channel and stimulation fails to change its firing pattern.9 In the normal animal, approximately 50% of the DA neurons are firing spontaneously; the remainder appear to be held in a hyperpolarized, nonfiring state primarily by potent GABAergic inputs arising from the ventral pallidum (VP).7 Therefore, the VP controls the number of DA neurons that can be driven to burst fire. In essence, the pedunculopontine glutamatergic input drives the rapid, phasic signal, but the VP controls the gain of the system by determining how many DA neurons are in the responsive state (figure 1).
Fig. 1.
Dopamine (DA) neurons exist in distinct states of activity: baseline tonic population activity (ie, proportion firing spontaneously) and rapid, salience-driven phasic burst firing. In normal rats, approximately one half are firing spontaneously, with the other half in an inhibited, nonfiring state. This inhibition is maintained by a GABAergic inhibitory input from the ventral pallidum (VP). This spontaneous tonic firing state is regulated by the ventral hippocampus (vHipp) through excitatory projections to the nucleus accumbens (NAc), which, in turn, inhibits the ventral pallidum (VP) and releases silent DA neurons from inhibition. But only active DA neurons can be driven to phasic firing by the pedunculopontine tegmentum (PPTg). Therefore, the number of neurons that are active determines the amplitude of the phasic DA response.
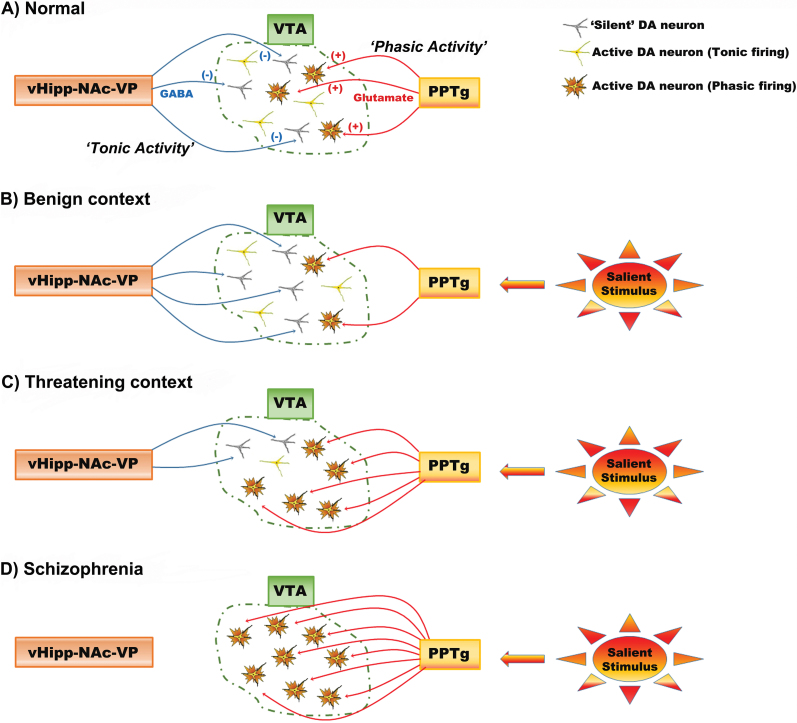
The interplay of the phasic burst signal with spontaneous activity gain plays an important role in regulating the responsivity of the DA system. In particular, the number of neurons firing, which we termed tonic population activity, is regulated by a pathway originating in the ventral hippocampus (vHipp). This glutamatergic projection activates the ventral striatal GABAergic neurons, which in turn inhibit the VP. This releases the DA neurons from the inhibitory influence of the VP and thereby increases the number of neurons firing (figure 1).2,9 The vHipp plays an important role in regulating the response of the organism to environmental conditions. Specifically, this region is proposed to determine context-dependent responses, or determining the appropriate response given the context in which the stimulus occurs.10 For example, a loud noise experienced from a safe context, such as one’s own house, would not engender as much of a response as the same noise occurring when walking in an unsafe area. By adjusting the number of DA neurons active, the vHipp can adjust the gain of the DA signal to appropriately fit the context. Therefore, in the dangerous environment, the vHipp causes a high level of DA population activity, such that a noise will activate a higher proportion of DA neurons, leading to greater phasic DA release that can drive a rapid orientation toward the stimulus to decide on an appropriate response (figure 2). However, a dysfunction in the ability to modulate the DA system in a context-dependent manner is proposed to underlie the inappropriate interpretation of events that occurs with psychosis.2
Fig. 2.
The hippocampus regulates DA neuron responsivity based on context. (A) The ventral hippocampus (vHipp) activates the nucleus accumbens (NAc) to inhibit the ventral pallidum (VP), driving VTA DA neuron tonic population activity. Pedunculopontine tegmentum (PPTg) input acts on tonically active DA neurons to generate phasic bursts of firing: this constitute the behaviorally salient rapid DA response. (B) If an organism is in a benign context where rapid reactions are not necessary, the number of DA neurons firing is kept low and the PPTg will only activate phasic bursting in a small population of neurons. As a result, a salient stimulus will trigger a calm orienting response. (C) In contrast, if the organism is in a threatening environment, the vHipp-NAc-VP pathway causes a large population of DA neurons to be active, increasing vigilance to the environment. Now the same salient stimulus will cause a larger phasic response, driving the organism to rapidly orient to the stimulus to prepare an appropriate response. (D) In schizophrenia, DA neuron population activity is in a constant high-activity state. Hence, most of the stimuli the organism encounters will lead to maximal dopamine output, resulting in the attribution of strong behavioral importance to stimuli, even to stimuli that should be safely ignored.
Developmental Disruption Model of Schizophrenia
Schizophrenia is a uniquely human disorder; as such, development of animal models has been problematic. Initial models were based on a hyperdopaminergic state, and used acute increases in DA transmission such as with amphetamine which, if given at high doses repeatedly, can produce a model of psychosis in humans.11 This approach was augmented by studies showing that functional antagonism of the NMDA receptor with phencyclidine (PCP) and ketamine produces a schizophrenia-like state in healthy individuals that included the entire symptom spectrum of schizophrenia.12,13 These data pointed to an interaction between NMDA and DA as a pathophysiological basis for schizophrenia. However, such acute pharmacological models fell short in mimicking the broad array of symptoms, their persistence, and the delayed onset of schizophrenia in humans.
A major breakthrough was the introduction of the neonatal vHipp lesion model.14 Drawing from imaging studies showing smaller hippocampal volume in the afflicted monozygotic twin,15 a lesion of the vHipp performed during the early postnatal period was found to engender a hyperdopaminergic state that was not present until the rat reached adulthood.14 This turned attention toward the concept of schizophrenia as a developmental disorder. We approached this model system using a different methodology. There is substantial evidence from epidemiological studies that insults that occur to the pregnant mother during the second trimester increase the incidence of schizophrenia births.16 Furthermore, we know that there is a strong genetic predisposition for schizophrenia, in that the concordance of 2 individuals for schizophrenia is a function of the proportion of shared genes.17 Using this information, we found that administering the DNA alkylating agent methylazoxymethanol acetate (MAM) during gestational day 17 (functionally equivalent to the human second trimester) produced offspring that displayed many of the characteristics associated with schizophrenia.5,6 This included thinning of the limbic cortical structures in regions homologous to those showing thinning in schizophrenia postmortem brains.5 Furthermore, as in schizophrenia,18 there was also a selective loss in parvalbumin-labeled GABAergic interneurons in the vHipp and frontal cortex.19,20 These rats also displayed deficits in executive or cognitive function typically associated with cortical dysfunction, deficits in sensory gating, disruption of emotional regulation, and enhanced behavioral response to PCP and to amphetamine.5,21 Interestingly, the hypersensitivity to amphetamine only developed in the adult, but was not present prepubertally,5 consistent with the late adolescence/early adulthood onset of schizophrenia in humans.
The increased response to amphetamine in MAM rats is consistent with a DA dysfunction in schizophrenia. Indeed, there is substantial evidence for an involvement of DA in schizophrenia. Thus, all antipsychotic drugs in use today act on DA D2 receptors.1 Furthermore, drugs that increase DA release will exacerbate schizophrenia symptoms and can mimic psychosis in normal.11,22 Finally, imaging studies examining raclopride displacement as a measure of endogenous DA release showed that schizophrenia patients exhibit significantly greater amphetamine-induced raclopride displacement in the associative striatum, with the amplitude of the increase proportional to the worsening of psychosis.23 Furthermore, there is an increase in fluorodopa uptake in DA terminals in the associative striatum, a measure believed to reflect number of active terminals,2 in schizophrenia patients as well as patients showing attenuated psychotic signs.24,25 Nonetheless, despite decades of research, there is little evidence for a dysfunction within the DA system itself; instead, current thinking revolves around the concept that the DA system is normal, but is being dysregulated by other structures.
The Role of the Hippocampus in Schizophrenia
One structure that has attracted attention is the hippocampus. This is the area that was found to be smaller in afflicted individuals15 and was targeted in the neonatal vHipp lesion model.14 Imaging studies have shown that the anterior hippocampus of schizophrenia patients is hyperactive, with the level of hyperactivity corresponding to the psychotic state.26 Preclinical studies have provided the link between hippocampal hyperactivity and a hyperresponsive DA system. Thus, activation of the vHipp of the rat (which is functionally equivalent to the human anterior limbic hippocampus)2,27 leads specifically to an increase in the number of DA neurons firing spontaneously, or population activity.8,28 An increase in DA population activity would make the DA system more responsive to stimuli driving phasic output. In the MAM rat, a similar phenomenon is observed—ie, a hyperactivity of the vHipp, along with an increase in VTA DA neuron population activity. Furthermore, this increased population activity and the behavioral hyper-responsivity to amphetamine is normalized by inactivation of the vHipp.28 The increased fluorodopa uptake observed in schizophrenia patients, which is believed to reflect an increase in the number of active terminals, is consistent with an increase in the number of active DA neurons driving these terminals.2
An important consideration relates to which DA neurons are affected. The DA system is comprised of a number of subcomponents that are topographically distinct with respect to their location, projection site, and function. In the rat, the most medial portion of the VTA projects to the reward-related nucleus accumbens/ventral striatum. At the transition between the lateral VTA and the substantia nigra (SN) are the DA neurons that project to associative striatum, with the lateral SN projecting primarily to the motor- and habit formation-related dorsolateral and dorsomedial striatum, respectively.29 In primates, the organization is somewhat different, in that primates do not have a large VTA; instead, the limbic and cortical/associative striatum projecting DA neurons are located in the SN with the motor-related neurons; the dorsal tier of the SN comprising the limbic and associative projecting neurons, and the ventral SN tier comprising the more motor-related systems.30,31 The DA neuron population that is activated in the MAM rat is primarily located in the lateral VTA,32 a region that projects to the associative striatum, which is the striatal area found to be hyperresponsive in schizophrenia patients.33,34
How could a hippocampally driven hyperresponsive DA system contribute to the psychotic symptoms of schizophrenia? As outlined above, a major function of the vHipp in rats is context-dependency10 that determines the state of responsivity of the DA system by adjusting the number of DA neurons that are responsive to stimuli. This is dependent on environmental contingencies in the normal individual. However, in schizophrenia patients, this fine-tuning of DA system responsivity appears subverted; with the vHipp being constantly overactive, all of the DA neurons would be in a maximally responsive state independent of context. Now any stimulus that arrives, salient or not, will cause a massive DA response. In these conditions, the schizophrenia patient would over-respond to all stimuli as if they were all maximally important, with an inability to segregate salient from nonsalient stimuli. Furthermore, the high response given to what should be neutral stimuli, such as strangers talking to each other across the room, could lead to confabulation in order to explain the intense emotional/threat response originating from the DA system. This has been termed “aberrant salience.”35,36 This would also be consistent with the reported inability of schizophrenia patients to respond in a context-dependent manner37 due to dysfunctions within the vHipp salience network.
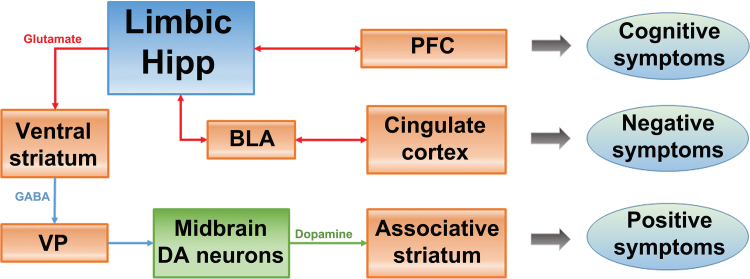
This raises the question of why the limbic hippocampal region is hyperactive in schizophrenia patients. One of the most robust findings in postmortem schizophrenia brains is the loss of the calcium-binding protein parvalbumin (PV) that is contained in a class of GABAergic inhibitory local-circuit neurons. This marker is decreased in both the prefrontal cortex (PFC) and hippocampus of schizophrenia patients.18 Because PV is a high-affinity calcium binding protein, PV allows neurons to fire at high frequencies by shortening the spike after hyperpolarization. For this reason, PV neurons are required for the generation of high-frequency evoked gamma rhythms.18 Stimulus-evoked gamma rhythms are found to be disrupted in schizophrenia18 as well as in the PFC and vHipp of MAM rats.20 Therefore, it is likely that the decrease in inhibitory PV neurons in the vHipp leads to a disruption of rhythmic activity and increased responsivity of the DA system. Moreover, the vHipp projects to regions beyond those that regulate DA neuron activity, such as the PFC and the amygdala. Given that the hippocampal inputs to the PFC and to the amygdala are important for cognitive performance and emotional regulation,38,39 respectively, a disruption of vHipp activity and rhythmicity could lead to dysregulation in these domains, which may underlie the cognitive and negative symptoms of this disorder (figure 3).2
Fig. 3.
In schizophrenia, the hippocampus is proposed to be hyperactive, leading to an overdrive in the responsivity of midbrain DA neurons that project to the associative striatum. This is proposed to underlie the positive symptoms of schizophrenia. Additionally, a hyperactive, dysrhythmic limbic hippocampus can also interfere with the function of other circuits. Thus, the hippocampus-prefrontal cortex (PFC) projection would lead to disruption of PFC activity and rhythmicity, leading to cognitive disruption. Moreover, the hippocampus-basolateral amygdala (BLA) projection would interfere with the BLA-limbic cortical control of emotional responses, possibly leading to negative symptoms. Therefore, a hyperactive, dysrhythmic limbic hippocampus potentially disrupts multiple interconnected circuits, and could contribute to all 3 symptom classes of schizophrenia.
From Pathophysiology to Treatment
Given evidence that psychosis may be due to an overactive limbic hippocampus driving a hyperresponsive DA system, what approach could be used to normalize this activity state? Current treatment strategies rely on blockade of D2 receptors.1 However, blockade of receptors may not represent the therapeutic mechanism of action—ie, with receptor blockade the system has a number of homeostatic mechanisms to compensate for this loss of input, including increased DA neuron population activity, increased tyrosine hydroxylase activity, increased D2 receptor number, etc.40,41 Furthermore, this would not impact the pathologically high DA neuron population activity. Another possibility is antipsychotic drug-induced depolarization block.41 In normal rats, administration of first- or second-generation antipsychotic drugs for 3 weeks or longer leads to a depolarization-induced inactivation of DA neuron activity,42,43 which would functionally decrease the heightened population activity. Moreover, the site of action of the depolarization block is consistent with the therapeutic effect, with first-generation drugs that have therapeutic efficacy but also extrapyramidal side-effects causing block in both the VTA and SN DA neurons, whereas second-generation drugs, which are unlikely to induce extrapyramidal effects at therapeutic doses, only cause block in the VTA DA neurons.42,43 One factor that is not consistent with this, however, is that antipsychotic drugs have a much more rapid onset of action in schizophrenia. However, in contrast to the normal rat, in MAM rats only a single dose of a first- or second-generation antipsychotic drug is necessary to induce depolarization block in the VTA,44 which is consistent with the clinical picture.45 We believe that this is due to the already-present hippocampal overdrive causing maximal DA population activity, which removes a compensatory mechanism for D2 receptor blockade. In this excited state, the feedback excitation caused by D2 blockade would combine with hippocampal overdrive leading to a rapid induction of depolarization block. Since a more active VTA DA system is more susceptible to induction of depolarization block, one would predict that a higher degree of psychosis would correspond to a faster/better antipsychotic action, which is a consistent finding in schizophrenia. Furthermore, if the DA system is not sufficiently overdriven at the basal state, induction of depolarization block would be more difficult to achieve—this is consistent with studies showing that schizophrenia patients with a low f-DOPA uptake (which we believe is a correlate of DA population activity) are more likely to be treatment non-responders.46
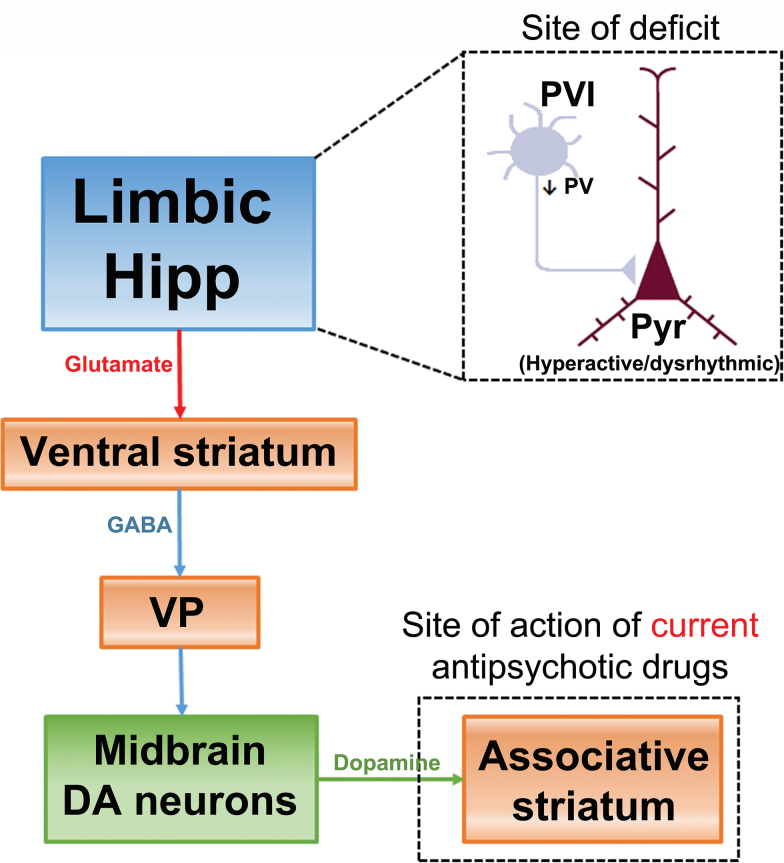
It should be noted that induction of depolarization block does not return the DA system to its “normal” state. This is likely due to the fact that the drugs are not working at what we believe is the site of deficit in the schizophrenia brain (ie, loss of hippocampal PV neurons), but instead is working 5 synapses downstream from this deficit, in blocking D2 receptors in the associative striatum (figure 4). A more effective approach would be to treat at the site of deficit, which would be the decreased PV-GABA inhibition of pyramidal neurons in the vHipp. One means to increase GABA transmission is with a positive allosteric modulator, such as a benzodiazepine. However, benzodiazepine receptors are ubiquitous in the brain, and strongly potentiating GABA would induce sleep. GABAA receptors are pentameric structures, with benzodiazepines binding to the alpha subunit. There are actually 7 different alpha subunits, with different pharmacological properties.47 However, only the alpha 5 is found in high concentrations in the hippocampus.48 Therefore, we tested a novel alpha 5 GABAA positive allosteric modulator in this system. We found that this compound is selective for the vHipp as opposed to other cortical areas, and moreover in the MAM rat reversed vHipp hyperactivity, amphetamine hyper-locomotion, and restored DA neuron population activity to baseline, without producing substantial effects in controls.49
Fig. 4.
Data from preclinical models and schizophrenia imaging/postmortem findings suggest that the site of deficit in the schizophrenia brain likely involves a decreased PV interneuron inhibition of pyramidal neurons in the limbic hippocampus. Current therapeutic approaches to schizophrenia rely on blocking DA receptors in the associative striatum to attenuate the impact of DA system overdrive. However, blocking D2 receptors is acting 5 synapses downstream from where we believe the deficit driving schizophrenia is located. As a result, it is not surprising that D2 antagonist antipsychotic drugs are not as efficacious as one that targets diminished inhibition in the limbic hippocampus.
These data suggest that this novel alpha 5 GABAA positive allosteric modulator could be an effective antipsychotic drug. However, similar drugs designed to work on this system, while showing promise preclinically, have failed in the clinic; this includes Roche’s glycine transport inhibitor, Lilly’s mGluR2,3 agonist, and Pfizer’s PDE-10 inhibitor.50–52 Why are these drugs that appear to be effective preclinically by selectively targeting the deficit in the schizophrenia patient’s brain found to be ineffective in the clinic? One likely problem relates to the patient population tested. Whereas the MAM rats treated with the GABAergic drug had no prior exposure to antipsychotics, in clinical trials the novel compounds are tested on patients that have been treated with D2 antagonist antipsychotic drugs for many years or even decades, and then are withdrawn from the drug for only 1–2 weeks. While such a washout period may be adequate to decrease blood levels of the antipsychotic drug, it is clear that there are many other actions of the drug that will not reverse in so short of a time; in particular, DA supersensitivity from increased D2 receptor number.41 Indeed, we found that if we pretreated MAM rats with haloperidol for just 3 weeks (sufficient to induce depolarization block) and then withdrew the drug for 1 week, the alpha 5 GABAA positive allosteric modulator was now completely ineffective at reversing amphetamine hyperresponsivity or DA population activity; in fact, even vHipp inactivation did not restore the basal state.53 This would be due to the trial design—with such a short washout and the presence of DA supersensitivity, the only approach that could effectively treat the supersensitive hyperdopaminergic state would be another D2 antagonist. Therefore, it is likely that many of these compounds that failed in the clinic may have done so due to issues with respect to prior drug exposure.
Early Intervention as a Means of Prevention
While novel therapeutic targets may be more effective in treating schizophrenia, a more effective approach would be to prevent the transition to psychosis earlier in life. It is known that the duration of untreated psychosis leads to a worse prognosis. One risk factor that has been associated with worsening of psychosis in schizophrenia is stress.54 Indeed, stress may play a role in the etiology of this disorder. Thus, in studies of children at risk for schizophrenia, the individuals that showed the greatest stress response tended to convert to schizophrenia later in life.55 Furthermore, epidemiological studies have shown that childhood stress or trauma is positively associated with schizophrenia onset in late adolescence/early adulthood.54 When examining MAM rats, studies have shown that prepubertally there is little in the way of the pathology observed in the adult rat with respect to DA dysfunction.5 However, during the late prepubertaly period MAM rats exhibit several indices of increased stress, including increased vocalization to footshock indicative of increased response to stressors, anxiety-like behavior, and increased baseline basolateral amygdala neuron firing.56–58 Furthermore, studies have shown that stress59 as well as induced seizures in the amygdala60 can lead to PV neuron loss in the hippocampus. If stress plays a role in the etiology of schizophrenia, then decreasing stress during the vulnerable prepubertal period could circumvent the damage that leads to the emergence of schizophrenia later in life. Indeed, we found that administering the anti-anxiety agent diazepam at a dose sufficient to attenuate anxiety and reverse amygdala hyperactivity58 decreased PV neuron loss and prevented the emergence of DA hyper-responsivity in the adult MAM rat.57,61
Stress and the Critical Period
The fact that stress-induced changes consistent with the transition to psychosis can be prevented in the MAM rat raises an interesting concept; ie, if MAM can be circumvented by relieving stress, then MAM does not “cause” schizophrenia, but instead MAM causes the animal to be hypersensitive to the deleterious effects of environmental stressors.62 Indeed, such a concept may be extended to the schizophrenia patient—ie, it is known that genetics alone does not determine schizophrenia, since identical twins have only a 50% concordance despite identical genetic makeup,17 it is know that environmental stressors can increase the probability of psychosis in susceptible individuals,3,63 and finally as much as 1/3 of schizophrenia is not familial.17 If this were the case, one might expect that stressing a normal rat to a sufficient extent could lead to a pathology similar to that observed in the MAM rat. To test this, normal rats were given stressors during the same prepubertal period used in the diazepam experiment in MAM rats. We found that only normal rats exposed to combined footshock and restraint stress during postnatal day (PD) 31–40 showed increased amphetamine-induced locomotion and increased VTA DA neuron population activity as adults that was similar to that observed in MAM rats.64 Interestingly, the primary DA neuron population that was affected by prepubertal combined stressors was the lateralmost VTA, which is the DA neuron population that projects to the associative striatal regions; the same regions showing hyper-DA states in schizophrenia patients.33,34 In contrast, administering the combined stressor in adulthood had a very different effect—there was a short-term decrease in VTA population activity,65 a condition that is more consistent with depression than with schizophrenia.66–68
What would make an individual hyperresponsive to environmental stressors? One brain region that has been shown to potently attenuate stress responses in the amygdala is the medial PFC.69,70 Thus, stimulation of the prelimbic PFC region will attenuate basolateral amygdala neuronal responses to stressors.69 This would suggest that loss of activity in this region may disrupt stress regulation. Indeed, we found that lesions of the prelimbic PFC conducted at PD25 caused normal rats to exhibit increased anxiety. Furthermore, exposure to footshock alone during this prepubertal period, which was insufficient to change VTA DA neuron population activity in intact rats, resulted in increased DA neuron population activity and increased amphetamine-induced locomotion in the adult rats that had received the early prelimbic prefrontal lesions.64 Therefore, a combination of predisposition in terms of effectively dealing with stress, combined with prepubertal stress exposure, may determine whether an individual transitions to psychosis later in life.
Why is the prepubertal period unique in its susceptibility to stress-induced PV interneuron damage? Studies have shown that the PV interneurons play a unique role in neuronal systems development. Early in life, PV neurons exhibit a substantial amount of plasticity, with glutamatergic synapses forming and being removed as the organism learns to deal with environmental contingencies. However, this plasticity comes at a price, in that the PV neurons are highly vulnerable to stressors at this time point. Thus, oxidative stress, glutamate drive, high-frequency firing, could all contribute to pathology and cell death. However, after puberty the PV neurons develop a perineuronal net that protects them from damage.71 For this reason, the timing of the stressor is critical in determining the pathology produced.
Summary
In this review, we provide an overview of schizophrenia pathophysiology and etiology, and how animal models can inform us about the circuitry that may underlie transition to psychosis. While this admittedly advances one of many possible circuits underlying schizophrenia etiology, validation is critically dependent on parallel experiments conducted in schizophrenia patients to both provide insight into circuits that are involved in schizophrenia. This also provides information regarding the impact of stress on psychiatric disorders, and particularly with respect to the timing of these stressors—stressors that impact an individual before puberty can lead to PV neuron loss and the emergence of schizophrenia in the adult; however, stressors experienced as an adult are more likely to lead to depression. Indeed, this common factor of stress vulnerability may be why both schizophrenia and depression run together in families. This points to a delicate balance between genetic predisposition to the impact of stressors, the balance between plasticity and stress-induced damage to circuits, and the resilience to stressors experienced by an individual. It may also point to a mechanism to prevent schizophrenia—ie, by identifying individuals at high risk for schizophrenia based on family history and early hyper-responsivity to stressors. One approach that can be used in humans would be psychosocial interventions, either via cognitive behavioral therapy or family intervention, to treat the vulnerable person how to best circumvent stress. In this way, translating animal models to the human condition and back again can provide us with the unique insights necessary to understand the etiology and pathophysiology of schizophrenia, develop novel treatments that move beyond serendipity to target the site of pathology, and interventions early in life that may decrease the emergence of psychosis.
Funding
Research activity of the authors is supported by grants from US National Institutes of Health (MH57440 to A.A.G.). A.A.G. also has received funds from Johnson & Johnson, Lundbeck, Pfizer, GlaxoSmithKline, Merck, Takeda, Dainippon Sumitomo, Otsuka, Lilly, Roche, Asubio, Abbott, Autofony, Janssen, and Alkermes.
Acknowledgment
The authors have declared that there are no conflicts of interest in relation to the subject of this study.
References
- 1. Kapur S, Remington G. Dopamine D(2) receptors and their role in atypical antipsychotic action: still necessary and may even be sufficient. Biol Psychiatry. 2001;50:873–883. [DOI] [PubMed] [Google Scholar]
- 2. Grace AA. Dysregulation of the dopamine system in the pathophysiology of schizophrenia and depression. Nat Rev Neurosci. 2016;17:524–532. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. van Os J, Kenis G, Rutten BP. The environment and schizophrenia. Nature. 2010;468:203–212. [DOI] [PubMed] [Google Scholar]
- 4. Modinos G, Allen P, Grace AA, McGuire P. Translating the MAM model of psychosis to humans. Trends Neurosci. 2015;38:129–138. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Moore H, Jentsch JD, Ghajarnia M, Geyer MA, Grace AA. A neurobehavioral systems analysis of adult rats exposed to methylazoxymethanol acetate on E17: implications for the neuropathology of schizophrenia. Biol Psychiatry. 2006;60:253–264. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Gomes FV, Rincón-Cortés M, Grace AA. Adolescence as a period of vulnerability and intervention in schizophrenia: Insights from the MAM model. Neurosci Biobehav Rev. 2016;70:260–270. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Floresco SB, West AR, Ash B, Moore H, Grace AA. Afferent modulation of dopamine neuron firing differentially regulates tonic and phasic dopamine transmission. Nat Neurosci. 2003;6:968–973. [DOI] [PubMed] [Google Scholar]
- 8. Lodge DJ, Grace AA. The hippocampus modulates dopamine neuron responsivity by regulating the intensity of phasic neuron activation. Neuropsychopharmacology. 2006;31:1356–1361. [DOI] [PubMed] [Google Scholar]
- 9. Grace AA, Floresco SB, Goto Y, Lodge DJ. Regulation of firing of dopaminergic neurons and control of goal-directed behaviors. Trends Neurosci. 2007;30:220–227. [DOI] [PubMed] [Google Scholar]
- 10. Maren S. Neurotoxic or electrolytic lesions of the ventral subiculum produce deficits in the acquisition and expression of Pavlovian fear conditioning in rats. Behav Neurosci. 1999;113:283–290. [DOI] [PubMed] [Google Scholar]
- 11. Angrist B, Sathananthan G, Wilk S, Gershon S. Amphetamine psychosis: behavioral and biochemical aspects. J Psychiatr Res. 1974;11:13–23. [DOI] [PubMed] [Google Scholar]
- 12. Lahti AC, Weiler MA, Tamara Michaelidis BA, Parwani A, Tamminga CA. Effects of ketamine in normal and schizophrenic volunteers. Neuropsychopharmacology. 2001;25:455–467. [DOI] [PubMed] [Google Scholar]
- 13. Murray JB. Phencyclidine (PCP): a dangerous drug, but useful in schizophrenia research. J Psychol. 2002;136:319–327. [DOI] [PubMed] [Google Scholar]
- 14. Lipska BK, Weinberger DR. To model a psychiatric disorder in animals: schizophrenia as a reality test. Neuropsychopharmacology. 2000;23:223–239. [DOI] [PubMed] [Google Scholar]
- 15. Weinberger DR, Berman KF, Daniel DG. Mesoprefrontal cortical dopaminergic activity and prefrontal hypofunction in schizophrenia. Clin Neuropharmacol. 1992;15:568A–569A. [DOI] [PubMed] [Google Scholar]
- 16. Canetta SE, Brown AS. Prenatal infection, maternal immune activation, and risk for schizophrenia. Transl Neurosci. 2012;3:320–327. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Kahn RS, Sommer IE, Murray RM, et al. Schizophrenia. Nat Rev Dis Primers. 2015;1:15067. [DOI] [PubMed] [Google Scholar]
- 18. Lewis DA, Hashimoto T, Volk DW. Cortical inhibitory neurons and schizophrenia. Nat Rev Neurosci. 2005;6:312–324. [DOI] [PubMed] [Google Scholar]
- 19. Gill KM, Grace AA. Corresponding decrease in neuronal markers signals progressive parvalbumin neuron loss in MAM schizophrenia model. Int J Neuropsychopharmacol. 2014;17:1609–1619. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Lodge DJ, Behrens MM, Grace AA. A loss of parvalbumin-containing interneurons is associated with diminished oscillatory activity in an animal model of schizophrenia. J Neurosci. 2009;29:2344–2354. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. Lodge DJ, Grace AA. Hippocampal dysfunction and disruption of dopamine system regulation in an animal model of schizophrenia. Neurotox Res. 2008;14:97–104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. Janowsky DS, el-Yousel MK, Davis JM, Sekerke HJ. Provocation of schizophrenic symptoms by intravenous administration of methylphenidate. Arch Gen Psychiatry. 1973;28:185–191. [DOI] [PubMed] [Google Scholar]
- 23. Laruelle M, Abi-Dargham A. Dopamine as the wind of the psychotic fire: new evidence from brain imaging studies. J Psychopharmacol. 1999;13:358–371. [DOI] [PubMed] [Google Scholar]
- 24. Howes OD, Bose SK, Turkheimer F, et al. Dopamine synthesis capacity before onset of psychosis: a prospective [18F]-DOPA PET imaging study. Am J Psychiatry. 2011;168:1311–1317. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25. Howes OD, Kambeitz J, Kim E, et al. The nature of dopamine dysfunction in schizophrenia and what this means for treatment. Arch Gen Psychiatry. 2012;69:776–786. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26. Heckers S, Konradi C. Hippocampal pathology in schizophrenia. Curr Top Behav Neurosci. 2010;4:529–553. [DOI] [PubMed] [Google Scholar]
- 27. Grace AA. Dopamine system dysregulation by the hippocampus: implications for the pathophysiology and treatment of schizophrenia. Neuropharmacology. 2012;62:1342–1348. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28. Lodge DJ, Grace AA. Aberrant hippocampal activity underlies the dopamine dysregulation in an animal model of schizophrenia. J Neurosci. 2007;27:11424–11430. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29. Ikemoto S. Dopamine reward circuitry: two projection systems from the ventral midbrain to the nucleus accumbens-olfactory tubercle complex. Brain Res Rev. 2007;56:27–78. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30. Szabo J. Organization of the ascending striatal afferents in monkeys. J Comp Neurol. 1980;189:307–321. [DOI] [PubMed] [Google Scholar]
- 31. Lynd-Balta E, Haber SN. The organization of midbrain projections to the striatum in the primate: sensorimotor-related striatum versus ventral striatum. Neuroscience. 1994;59:625–640. [DOI] [PubMed] [Google Scholar]
- 32. Lodge DJ, Grace AA. Divergent activation of ventromedial and ventrolateral dopamine systems in animal models of amphetamine sensitization and schizophrenia. Int J Neuropsychopharmacol. 2012;15:69–76. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33. Kegeles LS, Abi-Dargham A, Frankle WG, et al. Increased synaptic dopamine function in associative regions of the striatum in schizophrenia. Arch Gen Psychiatry. 2010;67:231–239. [DOI] [PubMed] [Google Scholar]
- 34. Howes OD, Montgomery AJ, Asselin MC, et al. Elevated striatal dopamine function linked to prodromal signs of schizophrenia. Arch Gen Psychiatry. 2009;66:13–20. [DOI] [PubMed] [Google Scholar]
- 35. Kapur S. Psychosis as a state of aberrant salience: a framework linking biology, phenomenology, and pharmacology in schizophrenia. Am J Psychiatry. 2003;160:13–23. [DOI] [PubMed] [Google Scholar]
- 36. Howes OD, Kapur S. The dopamine hypothesis of schizophrenia: version III–the final common pathway. Schizophr Bull. 2009;35:549–562. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37. Javitt DC, Shelley AM, Silipo G, Lieberman JA. Deficits in auditory and visual context-dependent processing in schizophrenia: defining the pattern. Arch Gen Psychiatry. 2000;57:1131–1137. [DOI] [PubMed] [Google Scholar]
- 38. Herman JP, Mueller NK. Role of the ventral subiculum in stress integration. Behav Brain Res. 2006;174:215–224. [DOI] [PubMed] [Google Scholar]
- 39. Sirota A, Montgomery S, Fujisawa S, Isomura Y, Zugaro M, Buzsáki G. Entrainment of neocortical neurons and gamma oscillations by the hippocampal theta rhythm. Neuron. 2008;60:683–697. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40. Zigmond MJ, Abercrombie ED, Berger TW, Grace AA, Stricker EM. Compensations after lesions of central dopaminergic neurons: some clinical and basic implications. Trends Neurosci. 1990;13:290–296. [DOI] [PubMed] [Google Scholar]
- 41. Grace AA, Bunney BS, Moore H, Todd CL. Dopamine-cell depolarization block as a model for the therapeutic actions of antipsychotic drugs. Trends Neurosci. 1997;20:31–37. [DOI] [PubMed] [Google Scholar]
- 42. Chiodo LA, Bunney BS. Typical and atypical neuroleptics: differential effects of chronic administration on the activity of A9 and A10 midbrain dopaminergic neurons. J Neurosci. 1983;3:1607–1619. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43. White FJ, Wang RY. Differential effects of classical and atypical antipsychotic drugs on A9 and A10 dopamine neurons. Science. 1983;221:1054–1057. [DOI] [PubMed] [Google Scholar]
- 44. Valenti O, Cifelli P, Gill KM, Grace AA. Antipsychotic drugs rapidly induce dopamine neuron depolarization block in a developmental rat model of schizophrenia. J Neurosci. 2011;31:12330–12338. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45. Agid O, Seeman P, Kapur S. The “delayed onset” of antipsychotic action–an idea whose time has come and gone. J Psychiatry Neurosci. 2006;31:93–100. [PMC free article] [PubMed] [Google Scholar]
- 46. Demjaha A, Murray RM, McGuire PK, Kapur S, Howes OD. Dopamine synthesis capacity in patients with treatment-resistant schizophrenia. Am J Psychiatry. 2012;169:1203–1210. [DOI] [PubMed] [Google Scholar]
- 47. McKernan RM, Whiting PJ. Which GABAA-receptor subtypes really occur in the brain?Trends Neurosci. 1996;19:139–143. [DOI] [PubMed] [Google Scholar]
- 48. Serwanski DR, Miralles CP, Christie SB, Mehta AK, Li X, De Blas AL. Synaptic and nonsynaptic localization of GABAA receptors containing the alpha5 subunit in the rat brain. J Comp Neurol. 2006;499:458–470. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49. Gill KM, Lodge DJ, Cook JM, Aras S, Grace AA. A novel α5GABA(A)R-positive allosteric modulator reverses hyperactivation of the dopamine system in the MAM model of schizophrenia. Neuropsychopharmacology. 2011;36:1903–1911. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50. Bugarski-Kirola D, Iwata N, Sameljak S, et al. Efficacy and safety of adjunctive bitopertin versus placebo in patients with suboptimally controlled symptoms of schizophrenia treated with antipsychotics: results from three phase 3, randomised, double-blind, parallel-group, placebo-controlled, multicentre studies in the SearchLyte clinical trial programme. Lancet Psychiatry. 2016;3:1115–1128. [DOI] [PubMed] [Google Scholar]
- 51. Geerts H, Spiros A, Roberts P. Phosphodiesterase 10 inhibitors in clinical development for CNS disorders. Expert Rev Neurother. 2017;17:553–560. [DOI] [PubMed] [Google Scholar]
- 52. Stauffer VL, Millen BA, Andersen S, et al. Pomaglumetad methionil: no significant difference as an adjunctive treatment for patients with prominent negative symptoms of schizophrenia compared to placebo. Schizophr Res. 2013;150:434–441. [DOI] [PubMed] [Google Scholar]
- 53. Gill KM, Cook JM, Poe MM, Grace AA. Prior antipsychotic drug treatment prevents response to novel antipsychotic agent in the methylazoxymethanol acetate model of schizophrenia. Schizophr Bull. 2014;40:341–350. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54. Corcoran C, Walker E, Huot R, et al. The stress cascade and schizophrenia: etiology and onset. Schizophr Bull. 2003;29:671–692. [DOI] [PubMed] [Google Scholar]
- 55. Johnstone EC, Lawrie SM, Cosway R. Genetic liability, illicit drug use, life stress and psychotic symptoms: preliminary findings from the Edinburgh study of people at high risk for schizophrenia. Soc Psychiatry Psychiatr Epidemiol. 2001;36:338–342. [DOI] [PubMed] [Google Scholar]
- 56. Zimmerman EC, Bellaire M, Ewing SG, Grace AA. Abnormal stress responsivity in a rodent developmental disruption model of schizophrenia. Neuropsychopharmacology. 2013;38:2131–2139. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57. Du Y, Grace AA. Peripubertal diazepam administration prevents the emergence of dopamine system hyperresponsivity in the MAM developmental disruption model of schizophrenia. Neuropsychopharmacology. 2013;38:1881–1888. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58. Du Y, Grace AA. Amygdala hyperactivity in MAM model of schizophrenia is normalized by peripubertal diazepam administration. Neuropsychopharmacology. 2016;41:2455–2462. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59. Steullet P, Cabungcal JH, Kulak A, et al. Redox dysregulation affects the ventral but not dorsal hippocampus: impairment of parvalbumin neurons, gamma oscillations, and related behaviors. J Neurosci. 2010;30:2547–2558. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60. Berretta S, Lange N, Bhattacharyya S, Sebro R, Garces J, Benes FM. Long-term effects of amygdala GABA receptor blockade on specific subpopulations of hippocampal interneurons. Hippocampus. 2004;14:876–894. [DOI] [PubMed] [Google Scholar]
- 61. Du Y, Grace AA. Loss of Parvalbumin in the hippocampus of MAM schizophrenia model rats is attenuated by peripubertal diazepam. Int J Neuropsychopharmacol. 2016;19:1–5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62. Grace AA. Dopamine system dysregulation and the pathophysiology of schizophrenia: insights from the methylazoxymethanol acetate model. Biol Psychiatry. 2017;81:5–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63. Tsuang M. Schizophrenia: genes and environment. Biol Psychiatry. 2000;47:210–220. [DOI] [PubMed] [Google Scholar]
- 64. Gomes FV, Grace AA. Prefrontal cortex dysfunction increases susceptibility to schizophrenia-like changes induced by adolescent stress exposure. Schizophr Bull. 2017;43:592–600. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65. Gomes FV, Zhu X, Grace AA. The ability of stress during adolescence or adulthood to produce schizophrenia-like pathophysiology is dependent on the state of the critical period. Biol Psychiatry. 2017; 81:S340–S341 [Google Scholar]
- 66. Chang CH, Grace AA. Amygdala-ventral pallidum pathway decreases dopamine activity after chronic mild stress in rats. Biol Psychiatry. 2014;76:223–230. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67. Belujon P, Grace AA. Restoring mood balance in depression: ketamine reverses deficit in dopamine-dependent synaptic plasticity. Biol Psychiatry. 2014;76:927–936. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68. Moreines JL, Owrutsky ZL, Grace AA. Involvement of infralimbic prefrontal cortex but not lateral habenula in dopamine attenuation after chronic mild stress. Neuropsychopharmacology. 2017;42:904–913. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69. Rosenkranz JA, Moore H, Grace AA. The prefrontal cortex regulates lateral amygdala neuronal plasticity and responses to previously conditioned stimuli. J Neurosci. 2003;23:11054–11064. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70. Hariri AR, Mattay VS, Tessitore A, Fera F, Weinberger DR. Neocortical modulation of the amygdala response to fearful stimuli. Biol Psychiatry. 2003;53:494–501. [DOI] [PubMed] [Google Scholar]
- 71. Cabungcal JH, Steullet P, Morishita H, et al. Perineuronal nets protect fast-spiking interneurons against oxidative stress. Proc Natl Acad Sci U S A. 2013;110:9130–9135. [DOI] [PMC free article] [PubMed] [Google Scholar]