Abstract
Recent magnetic resonance spectroscopy (MRS) studies suggest that abnormalities of the glutamatergic system in schizophrenia may be dependent on illness stage, medication status, and symptomatology. Glutamatergic metabolites appear to be elevated in the prodromal and early stages of schizophrenia but unchanged or reduced below normal in chronic, medicated patients. However, few of these studies have measured metabolites with high-field 7T MR scanners, which offer higher signal-to-noise ratio and better spectral resolution than 3T scanners and facilitate separation of glutamate and glutamine into distinct signals. In this study, we examined glutamate and other metabolites in the dorsal anterior cingulate cortex (ACC) of first-episode schizophrenia patients. Glutamate and N-acetylaspartate (NAA) were significantly lower in schizophrenia patients vs controls. No differences were observed in levels of glutamine, GABA, or other metabolites. In schizophrenia patients but not controls, GABA was negatively correlated with the total score on the Repeatable Battery for the Assessment of Neuropsychological Status (RBANS) as well as the immediate memory and language subscales. Our findings suggest that glutamate and NAA reductions in the ACC may be present early in the illness, but additional large-scale studies are needed to confirm these results as well as longitudinal studies to determine the effect of illness progression and treatment. The correlation between GABA and cognitive function suggests that MRS may be an important technique for investigating the neurobiology underlying cognitive deficits in schizophrenia.
Keywords: first-episode schizophrenia, 7 Tesla, magnetic resonance spectroscopy, glutamate, anterior cingulate cortex
Introduction
Understanding the molecular and structural mechanisms underlying clinical features of schizophrenia is an important step in identifying biomarkers and potential targets for novel medications, treatment strategies, and interventions. Converging evidence suggests that the pathophysiology of schizophrenia may include elevated glutamatergic activity in the early phases of the disorder1 as well as progressive loss of brain volume.2 It is possible that elevated glutamate levels reflect an excitotoxic process that contributes to the observed structural deficits in schizophrenia.3–5 Early detection of altered glutamatergic activity could provide an opportunity to slow the potentially damaging effects of excitotoxicity.
In vivo studies using proton magnetic resonance spectroscopy (MRS) have reported glutamatergic abnormalities in schizophrenia that may depend on illness stage, medication status, and symptomatology. Multiple studies found that glutamate or glutamine are elevated in individuals at high-risk for developing schizophrenia,6–8 in patients in the early stages of schizophrenia,5,6,9–13 in unmedicated patients,3,14 and in treatment-resistant patients.15,16 In contrast, glutamate appears to be at normal or below normal levels in chronic and medicated patients.17–22 In the largest study to date, glutamate in the anterior cingulate cortex (ACC) was lower in adults with schizophrenia compared to controls, and ACC glutamate decreased linearly with age, whereas ACC glutamine increased with age.23 Consistent with the latter finding, in another recent study with a large sample,24 ACC glutamine was elevated in chronic and medicated patients. Glutamatergic alterations may be regionally-specific as has been observed in the prodromal phase7 and the medicated and unmedicated states.14,25 Therefore, additional studies are needed to clarify the effects of illness stage, medication, clinical status, and brain region on MRS measures of glutamate and glutamine.
Most MRS studies of schizophrenia have been performed at field strengths of 1.5–4T. Higher field strengths provide better signal-to-noise ratio (SNR) and spectral resolution, which in turn allows for better detection and quantification of metabolite peaks. Lower field strengths, particularly fields ≤3T, are limited in their ability to separately quantify the overlapping signals of glutamate and glutamine.26 As a result, many studies report a combined peak, Glx, that represents the sum of glutamate and glutamine, which makes interpretation difficult. High-field (7T) MRS offers improved detection of distinct glutamate and glutamine signals. To date, only 4 studies of schizophrenia using 7T MRS have been published.27–30 Rowland et al29 found elevated glutamine/glutamate ratio in the ACC, Thakkar et al30 observed reduced glutamate and GABA in the occipital cortex, and Brandt et al27 found that ACC glutamate decreased with age in patients but not controls. Marsman et al28 observed no significant differences in glutamate levels in medial prefrontal or parieto-occipital cortices. However, they observed lower GABA in patients, and GABA inversely correlated with cognitive functioning.28 Notably, these 7T studies used mixed or older samples with relatively long illness durations. Any observed alterations in glutamate levels could be attributed to illness chronicity or effects of antipsychotic medications.
To better understand biochemistry in the early stages of schizophrenia and identify potential targets for the development of early interventions, we examined the neurochemistry of the ACC using high-field 7T MRS in a sample of first-episode patients with schizophrenia. We focused on the ACC because it plays a key role in emotion and cognition and is known to be affected in schizophrenia.19,31 Based on previous studies of patients in the early phase of schizophrenia,5,6,12,13 we hypothesized that glutamate would be elevated in first-episode patients and that glutamate would correlate with positive symptoms. Given evidence of reduced levels of N-acetylaspartate (NAA) in first-episode schizophrenia,32 we also hypothesized that NAA levels would be reduced in patients.
METHODS
Participants
23 individuals with schizophrenia or schizoaffective disorder and 26 age- and sex-matched healthy controls were recruited from the University of Alabama at Birmingham psychiatric clinics and the general community and enrolled in this study. Exclusion criteria were major medical conditions, substance abuse within 6 months of imaging, neurologic disorders, previous serious head injury with a loss of consciousness for more than 2 minutes, and pregnancy. Two patients (one due to claustrophobia and one due to scanner size restrictions) and 5 controls (one failed drug screen, one MR contraindication, and 3 lost to follow-up) did not complete the study. Therefore, final analyses include 21 patients and 21 controls.
In this study, first-episode was defined as being within 2 years of starting treatment. Specifically, with only one exception, 15 patients were within 1 year and 5 patients were within 2 years of starting treatment. At the time of scanning, patients had been treated for an average of 55.3 weeks (table 1) and were on the following medications: 1 unmedicated, 16 risperidone, 2 aripiprazole, 1 clozapine, and 1 combination of clozapine and ziprasidone. Diagnoses were established by a psychiatrist (A.C.L.) and confirmed through review of patient medical records. Patients’ symptom severity was assessed using the 20-item Brief Psychiatric Rating Scale (BPRS) and its positive and negative subscales. The UCSD Performance-Based Skills Assessment (UPSA)33 was used to assess patients’ functional capacity. General cognitive function for patients and controls was assessed with the Repeatable Battery for the Assessment of Neuropsychological Status (RBANS).34 All participants gave written informed consent. Before signing consent, all patients were evaluated for their ability to provide consent by completing a questionnaire probing their understanding of the study. The Institutional Review Boards of the University of Alabama at Birmingham and Auburn University approved this study.
Table 1.
Demographicsa
| Measure | Control (n = 21) | Schizophrenia (n = 21) | Statistic | P value |
|---|---|---|---|---|
| Age, years | 23.5 (4.5) | 23.2 (4.4) | t(40) = 0.21 | .84 |
| Sex, F/M | 5/16 | 5/16 | χ2(1) = 0 | 1 |
| Smoker, yes/no | 0/21 | 6/15 | χ2(1) = 7.0 | .01 |
| Parental SESb | 3.4 (3.3) | 4.3 (4.4) | t(39) = 0.72 | .48 |
| RBANSc | 95.1 (8.6) | 74.0 (15.0) | t(35) = 5.32 | <.001 |
| Immediate memory | 99.8 (12.8) | 79.3 (14.5) | t(35) = 4.58 | <.001 |
| Visuospatial/constructional | 89.7 (13.8) | 78.8 (17.3) | t(35) = 2.13 | .04 |
| Language | 105.2 (10.5) | 83.4 (10.8) | t(35) = 6.21 | <.001 |
| Attention | 97.2 (14.2) | 76.1 (19.8) | t(35) = 3.74 | .001 |
| Delayed memory | 92.3 (8.7) | 79.9 (19.0) | t(35) = 2.58 | .01 |
| Duration of untreated psychosis, weeks | — | 25.7 (44.9) | — | — |
| Treatment duration, weeks | — | 55.3 (65.0) | — | — |
| BPRSd | ||||
| Total | — | 32.3 (9.8) | — | — |
| Positive | — | 5.5 (3.3) | — | — |
| Negative | — | 5.9 (2.4) | — | — |
| UPSAe | — | 80.6 (10.8) | — | — |
Note: aMean (SD).
bSocioeconomic Status. Ranks determined from the Diagnostic Interview for Genetic Studies (1–18 scale); higher rank (lower numerical value) corresponds to higher socioeconomic status. Information not available for 1 patient.
cRepeatable Battery for the Assessment of Neuropsychological Status. Information not available for 3 patients and 2 controls.
dBrief Psychiatric Rating Scale. Scored on a 1–7 scale. Positive subscale: conceptual disorganization, hallucinatory behavior, and unusual thought content. Negative subscale: emotional withdrawal, motor retardation, and blunted affect. Information not available for 2 patients.
eUCSD Performance-Based Skills Assessment. Information not available for 4 patients.
MR Imaging
Imaging was performed at the Auburn University MRI Research Center on a MAGNETOM 7T MR scanner (Siemens Healthineers) equipped with a 32-channel head coil (Nova Medical). Three-dimensional structural images were acquired for anatomical reference (MPRAGE, TR/TE/TI = 2200/2.96/1050 ms, flip angle = 7°, GRAPPA acceleration factor = 2, field of view = 224 × 224 mm, matrix = 320 × 320, slice thickness = 0.7 mm, 0.7-mm isotropic resolution, sagittal acquisition). Following shimming with FASTESTMAP (fast, automatic shim technique using echo-planar signal readout for mapping along projections) and optimization of the radiofrequency power, spectra were acquired from the dorsal ACC (27 × 20 × 10 mm) using an ultra-short echo time (TE) stimulated echo acquisition mode (STEAM) sequence (TR/TE/TM = 10000/5/45 ms, 32 averages, 4 kHz spectral bandwidth, 2048 points) with outer volume suppression and VAPOR (variable power RF pulses and optimized relaxation delays) water suppression.35
MRS Processing
MRS analysis was performed in LCModel (version 6.3-1J)36 using a simulated basis set and LCModel’s default processing parameters. The basis set included alanine, aspartate, ascorbate, creatine (Cr), GABA, glucose, glutamine (Gln), glutamate (Glu), glycerophosphocholine (GPC), glutathione (GSH), myo-Inositol (mI), scyllo-Inositol, lactate, phosphocreatine (PCr), phosphocholine (PCh), phosphorylethanolamine, NAA, N-acetylaspartylglutamate (NAAG), taurine, and macromolecules. The macromolecule data were acquired from 4 healthy subjects using inversion recovery and STEAM (TR = 2000 ms, TI = 675 ms, 1632 averages total) and processed as previously described.37 Spectra were eddy current corrected and quantified using the unsuppressed water signal (2 averages). SNR and line widths (full-width at half maximum, FWHM) were used to assess spectral quality. Cramer-Rao lower bounds (CRLB) were used as a measure of fit, and metabolites with CRLB < 20% were included in further analysis, except for NAAG for which the cutoff was 25%.
Metabolite levels were corrected for partial volume using Gasparovic et al’s method.38 Given our long TR (10 s) and ultra-short TE (5 ms), we did not include the corrections for T1 and T2 effects. To calculate the fractions of tissue and cerebrospinal fluid (CSF) in the MRS voxel, the structural MPRAGE image was segmented into gray matter (GM), white matter (WM), and CSF using SPM8. Binary images of the MRS voxels were created in MATLAB using the MRS raw data headers, and these images were then used to mask the tissue types. Tissue proportions were calculated in MATLAB. Metabolite levels are presented in institutional units.
Statistical Analyses
Statistical analyses were performed in SPSS (version 22). Independent-samples t tests and chi-square tests, as appropriate, were used to compare demographics between patients and controls. Univariate ANCOVA covarying for age, sex, and smoking was used to compare metabolite levels between the groups. The quantified metabolites were glutamate, glutamine, GABA, total NAA (tNAA = NAA + NAAG), total creatine (tCr = Cr + PCr), total choline (tCho = GPC + PCh), glutathione, myo-Inositol, taurine, and aspartate. The planned comparisons based on our hypotheses were glutamate and NAA. The remaining ANCOVAs were exploratory. Pearson correlation coefficients were used to assess relationships among metabolites and symptom severity (BPRS), cognition (RBANS), and functional capacity (UPSA). With the exception of the correlation between BPRS positive subscale and glutamate, all correlations were exploratory. If a significant correlation was observed with the RBANS total score, then we conducted post hoc correlations with the subscales. Statistical significance was P < .05. To correct for multiple correlations, we used Benjamini-Hochberg false discovery rate set at P < .05.
RESULTS
Demographics are summarized in table 1. Patients and controls did not differ in age, sex, or parental socioeconomic status. The patient group had a significantly greater number of smokers. As expected, the controls scored significantly higher on the RBANS.
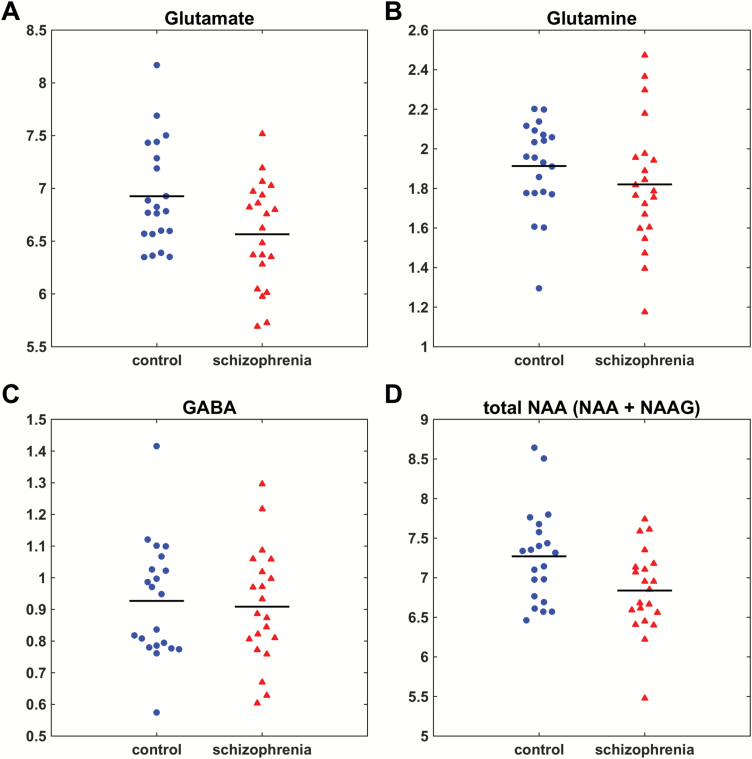
Representative ACC voxel and spectra are shown in figure 1. Metabolite levels (institutional units) for patients and controls are presented in table 2. Some metabolites were excluded from analysis based on the CRLB cutoffs as described in the methods (creatine: 1 patient; aspartate: 2 patients and 1 control; NAAG: 5 patients and 1 control). Patients had significantly lower levels of glutamate and tNAA compared to controls after controlling for age, gender, and smoking (figure 2). There were no significant differences in the other metabolites, including glutamine, GABA, and glutamine/glutamate ratio (table 2, figure 2, and supplementary figure S1). The ANCOVA revealed significant effects of age for glutamine and myo-Inositol as well as significant effects of gender for glutamate, GABA, glutathione, tNAA, taurine, and phosphocreatine (all P < .05). In post hoc analyses of glutamate and tNAA, the GM proportion [GM / (GM + WM)] was used as an additional covariate, which resulted in no significant group differences (glutamate: P = .14; tNAA: P = .19).
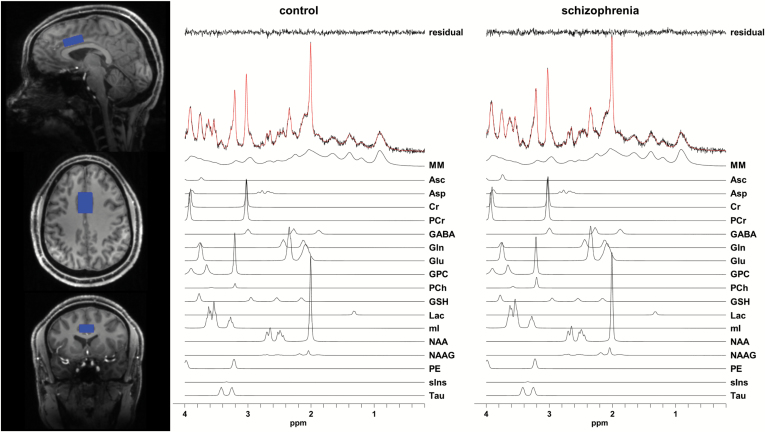
Fig. 1.
Representative voxel (blue) and metabolite spectra. The spectral fits are shown in red over the acquired spectra. The individual metabolite fits are shown below each spectrum. The difference between the spectrum and fit (residual) is shown at the top of the figure. Abbreviations: Asc: ascorbate, Asp: aspartate, Cr: creatine, GABA: gamma-aminobutyric acid, Gln: glutamine, Glu: glutamate, GPC: glycerophosphocholine, GSH: glutathione, Lac: lactate, mI: myo-Inositol, MM: macromolecules, NAA: N-acetylaspartate, NAAG: N-acetylaspartylglutamate, PCh: phosphocholine, PCr: phosphocreatine, PE: phosphorylethanolamine, sIns: scyllo-Inositol, Tau: taurine. For color, please see the figure online.
Table 2.
MRS Metabolite Levels and Quality Measuresa,b
| Measure | Control (n = 21) | Schizophrenia (n = 21) | Statistic | P value |
|---|---|---|---|---|
| Glutamate | 6.93 (0.50) | 6.57 (0.50) | F(1,37) = 4.70 | .04 |
| CRLB | 2.0% | 2.1% | ||
| Glutamine | 1.91 (0.23) | 1.82 (0.32) | F(1,37) = 1.15 | .29 |
| CRLB | 5.8% | 6.7% | ||
| Glutamine/Glutamate | 0.28 (0.04) | 0.28 (0.04) | F(1,37) = 0.01 | .92 |
| GABA | 0.93 (0.18) | 0.91 (0.18) | F(1,37) = 0.03 | .87 |
| CRLB | 11.0% | 11.9% | ||
| Total NAA (NAA + NAAG) | 7.27 (0.60) | 6.84 (0.53) | F(1,37) = 5.06 | .03 |
| CRLB | 1.4% | 1.6% | ||
| NAA | 6.82 (0.53) | 6.46 (0.50) | F(1,37) = 3.56 | .07 |
| CRLB | 1.7% | 2.0% | ||
| NAAG | 0.46 (0.10) | 0.45 (0.08) | F(1,31) = 1.21 | .28 |
| CRLB | 16.4% | 17.0% | ||
| Total Creatine (Cr + PCr) | 5.74 (0.38) | 5.73 (0.42) | F(1,37) = 0.45 | .51 |
| CRLB | 1.7% | 1.9% | ||
| Creatine (Cr) | 2.39 (0.22) | 2.33 (0.34) | F(1,36) = 0.64 | .43 |
| CRLB | 8.6% | 9.3% | ||
| Phosphocreatine (PCr) | 3.36 (0.35) | 3.44 (0.43) | F(1,37) = 0.07 | .79 |
| CRLB | 6.9% | 7.2% | ||
| Total Choline (Cho + PCh) | 1.30 (0.17) | 1.34 (0.16) | F(1,37) = 0.07 | .79 |
| CRLB | 3.3% | 3.4% | ||
| Glutathione | 0.75 (0.10) | 0.70 (0.11) | F(1,37) = 1.48 | .23 |
| CRLB | 8.9% | 10.4% | ||
| myo-Inositol | 5.03 (0.50) | 4.88 (0.47) | F(1,37) = 1.92 | .17 |
| CRLB | 2.3% | 2.5% | ||
| Taurine | 1.48 (0.12) | 1.59 (0.27) | F(1,37) = 1.42 | .24 |
| CRLB | 7.4% | 7.4% | ||
| Aspartate | 1.49 (0.21) | 1.45 (0.23) | F(1,34) = 1.18 | .29 |
| CRLB | 13.6% | 14.7% | ||
| SNR | 35.0 (3.6) | 31.7 (7.0) | t(40) = 1.89 | .07 |
| FWHM, ppm | 0.03 (0.003) | 0.03 (0.005) | t(40) = 1.73 | .09 |
| GM, % | 71.4 (7.8) | 73.6 (8.6) | t(40) = 0.88 | .39 |
| WM, % | 25.4 (7.7) | 23.7 (8.7) | t(40) = 0.67 | .51 |
| CSF, % | 2.5 (1.6) | 2.1 (1.7) | t(40) = 0.69 | .49 |
| GM/(GM+WM), % | 73.7 (8.0) | 75.6 (9.0) | t(40) = 0.72 | .47 |
Note: Cho, choline; Cr, creatine; CRLB, Cramer-Rao lower bounds; CSF, cerebrospinal fluid; FWHM, full-width at half maximum; GABA, gamma-aminobutyric acid; GPC, glycerophosphocholine; GM, gray matter; NAA, N-acetylaspartate; NAAG, N-acetylaspartylglutamate; PCh, phosphocholine; PCr, phosphocreatine; ppm, parts-per-million; SNR, signal-to-noise ratio; WM, white matter.
aMean (SD).
bAll metabolite levels are in institutional units.
Fig. 2.
Metabolite levels (corrected for partial volume effects) in the dorsal ACC in controls and patients. (A) Glutamate, P = .04. (B) Glutamine, n.s. (C) GABA, n.s. (D) Total NAA (NAA+NAAG), P = .03.
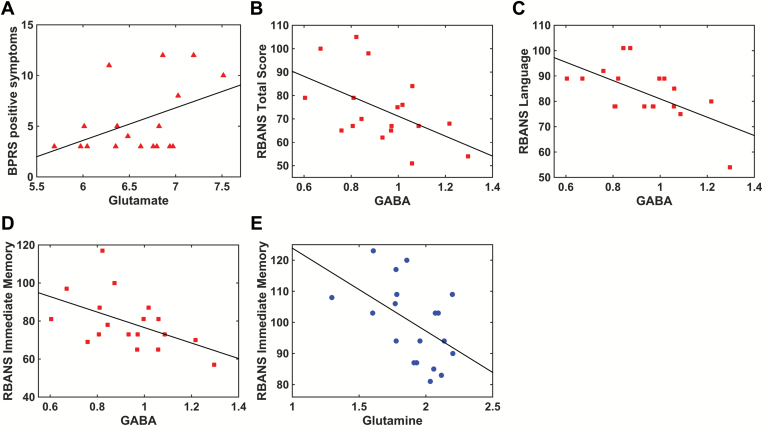
There was a trend-level correlation between the BPRS positive subscale and glutamate (r = .45, P = .053) (figure 3). Glutamate, glutamine, and GABA did not significantly correlate with the BPRS total score or the BPRS negative subscale. In patients but not controls, GABA negatively correlated with the RBANS total score (patients: r = −.51, P = .03; controls: r = −.03, P = .89), the RBANS immediate memory subscale (patients: r = −.50, P = .03; controls: r = .004, P = .99) and the RBANS language subscale (patients: r = −.60, P = .008; controls: r = −.21, P = .39) (figure 3). In controls but not patients, glutamine negatively correlated with the RBANS immediate memory subscale (controls: r = −.49, P = .03; patients: r = −.23, P = .37) (figure 3). Glutamate did not correlate with the duration of untreated psychosis (P = .98) or the length of treatment (P = .36). The correlation analyses did not survive correction for Benjamini-Hochberg false discovery rate (supplementary table S1).
Fig. 3.
Metabolite correlations with clinical measures (patients: red, controls: blue). (A) There was a trend-level relationship between glutamate and positive symptoms measured with the Brief Psychiatric Rating Scale (BPRS) (r = .45, P = .053). (B) In patients, GABA negatively correlated with the total score on the Repeatable Battery for the Assessment of Neuropsychological Status (RBANS) (r = −.51, P = .03). (C) In patients, GABA negatively correlated with the RBANS language subscale (r = −.60, P = .008). (D) In patients, GABA negatively correlated with the RBANS immediate memory subscale (r = −.50, P = .03). (E) In controls, glutamine negatively correlated with the RBANS immediate memory subscale (r = −.49, P = .03).
Discussion
This is the first 7T MRS study of first-episode schizophrenia. We used high-field 7T MRS to investigate the neurochemistry of the ACC. We observed reduced levels of glutamate and tNAA in the patient group. We found correlations between GABA and cognitive function in patients and between glutamine and cognitive function in controls. We also observed a trend for glutamate to correlate with positive symptoms in patients.
Based on recent MRS studies examining glutamate and glutamine in the early phases of schizophrenia and in unmedicated patients with schizophrenia, we hypothesized that glutamate would be elevated in this sample of first-episode patients. Hyperglutamatergic activity is consistent with evidence of hypofunction of the N-methyl-D-aspartate (NMDA) receptors in the pathophysiology of schizophrenia.4,39 Blocking NMDA receptors with ketamine or phencyclidine causes excessive glutamate release,40–43 so one possible mechanism for the hyperglutamatergic state might be hypofunction of NMDA receptors on GABAergic interneurons that leads to disinhibition of glutamatergic neurons.4 Supporting this hypothesis, recent MRS studies have found elevated glutamate in the striatum of medication-naïve patients6,13 and in the medial prefrontal cortex and hippocampus of unmedicated patients.3,14 Furthermore, treatment with antipsychotic medications is associated with reductions in glutamate levels in the frontal cortex,12 thalamus,11 temporal cortex,44 and striatum13 in patients as assessed with MRS.
Contrary to expectations, we observed lower glutamate in our sample of medicated first-episode patients. It is possible that reduced glutamate in our sample may be due to medication13,44 because patients were treated for approximately 1 year. Alternatively, it is possible that our findings as well as those of Gallinat et al,25 Wang et al,45 and Kegeles et al14 reflect regional glutamatergic abnormalities in schizophrenia. Gallinat et al25 found that glutamate levels were significantly lower in the ACC but significantly higher in the hippocampus of medicated patients. This is notable because we also observed lower glutamate in the ACC, but our sample was younger with shorter illness and treatment durations, suggesting that lower ACC glutamate may be present in the early phase of the illness. This interpretation is supported by the findings of Wang et al45 who observed lower glutamate + glutamine (Glx) in the medial prefrontal cortex of medication-naïve first-episode patients. Furthermore, Kegeles et al14 observed elevated glutamate in the medial prefrontal cortex but not the dorsolateral prefrontal cortex of unmedicated patients, many of whom were antipsychotic-naïve, suggesting differential effects even within the prefrontal cortex. Given that regional variations in the concentrations of glutamate and GABA in cingulate subregions have been identified in healthy subjects,46 which parallels findings of heterogeneous proportions of excitatory and inhibitory receptors in postmortem autoradiographic studies,47 it is not surprising that glutamatergic abnormalities in schizophrenia might be expressed in a regionally-specific way. Thus, we need additional studies measuring glutamate in multiple brain regions of the same patients.
Currently, there are only 4 published studies using 7T MRS to investigate neurometabolic abnormalities in schizophrenia. In these studies, the average age of the patients ranged from 27.628 to 37.527 years, and the average illness duration ranged from 6.528 to 14.030 years (although one study did not report illness duration27). In contrast, the average age of our sample was 23.2 years with an average treatment length of 55.3 weeks. Our finding of reduced glutamate is consistent with Thakkar et al30 who observed lower glutamate in the occipital cortex, but their patients were older with chronic schizophrenia. Brandt et al27 reported that ACC glutamate decreased with age in a group of older patients. Although we did not find differences in the glutamine/glutamate ratio, lower glutamate levels could potentially increase this ratio, which Rowland et al29 found to be elevated in the ACC of chronic patients. In summary, 7T MRS studies of schizophrenia have shown reduced glutamate in chronic and early-phase schizophrenia, in patients medicated for approximately 1 year and those medicated for numerous years, and in the ACC and the occipital cortex. Therefore, when considered as a whole, these studies suggest that reduced glutamate is not simply a consequence of illness progression, chronicity, or treatment and that the effect is not limited to a specific brain region. Additional studies are needed, particularly longitudinal studies with large samples of medication-naïve patients, to directly assess the effects of treatment, illness progression, and brain region. Ideally, these studies should aim to leverage the advantages offered by high-field MR systems.
In addition to reduced glutamate, we observed lower levels of tNAA in the ACC. NAA is thought to reflect neuronal health and integrity, and lower NAA might be expected when glutamate is elevated due to potential excitotoxic effects.3,5 However, we report that both glutamate and NAA are reduced in the ACC of first-episode patients. Our finding is consistent with Kegeles et al14 who reported lower NAA in the dorsolateral prefrontal cortex but not the medial prefrontal cortex of unmedicated patients, which is noteworthy because glutamate levels were normal in the dorsolateral prefrontal cortex. In a large study, Bustillo et al24 found reduced NAA in the dorsal ACC, but their finding was concurrent with elevated glutamine. Nevertheless, all these results are consistent with a meta-analysis showing significant NAA reductions in the frontal lobe of both first-episode and chronic patients.32 Lower NAA levels are consistent with postmortem evidence of reduced cortical thickness and decreased synaptic and mitochondrial densities in the ACC of people with schizophrenia.48,49 We acknowledge that it is possible that the NAA reduction in our sample is due to treatment with antipsychotic medications, so additional studies with larger samples of medication-naïve patients are needed to better understand the nature of NAA reductions.
We observed a trend for glutamate levels to correlate with positive symptoms as measured by the BPRS. Though not statistically significant, this finding may be clinically informative because it replicates prior studies. Kegeles et al14 also reported that glutamate in the medial prefrontal cortex correlated with the positive symptoms, and Bustillo et al24 found that glutamine in the dorsal ACC positively correlated with positive symptoms. This is important considering recent studies showing that glutamate is elevated in symptomatic nonresponders to treatment.15,16 Glutamate and glutamine have the potential to serve as targets for novel drug development as well as predictors of treatment response. We did not observe a correlation between glutamate and the UPSA, which contrasts with the recent report of Wijtenburg et al.23 This discrepancy may be due to differences in the patient samples: they studied a large group of young and old patients whereas we studied a group of young first-episode patients. The field would benefit from additional studies examining the association between glutamate and functional capacity as well as the effects of illness stage on this relationship.
Because of recent reports of correlations between metabolites and cognitive function, we conducted exploratory correlation analyses with the RBANS. We observed that GABA levels in the patient group were negatively correlated with the RBANS. Importantly, this correlation did not survive correction for multiple comparisons; however, it replicates a recent finding by Marsman et al28 who reported a negative correlation between intelligence and GABA in the medial prefrontal cortex of patients but not controls. The RBANS is designed to characterize general cognitive function across 5 domains: immediate memory, visuospatial/constructional, language, attention, and delayed memory.34 Our observation appeared to be driven by the immediate memory subscale and the language subscale. Postmortem studies of schizophrenia have shown evidence of GABAergic abnormalities, particularly in GABAergic interneurons, which may negatively impact cognitive function in people with schizophrenia.50 Supporting this idea, drugs that modulate GABA have been shown to affect working memory in people with schizophrenia and healthy controls.51 Our observation as well as the finding of Marsman et al28 contrasts with that of Rowland et al20 who reported that GABA in the ACC was positively correlated with a coding test of attention for a pooled group of patients and controls. These differences could be due to the assessment instrument, imaging methodology, or participant characteristics. Nevertheless, our observation suggests that MRS may be a valuable tool for investigating the neurobiology underlying cognitive deficits in schizophrenia.
We acknowledge several limitations of our study. First, the sample size in this study is small. This is the first 7T MRS study of first-episode patients, and several of our results are consistent with previous studies, including reduced NAA,32 an inverse correlation between GABA and cognition,28 and a trend correlation between glutamate and positive symptoms.14 Despite these consistencies, the metabolite differences that we report are small, and only large-scale studies with sufficient power will be able to provide convincing results. Second, patients were treated with antipsychotic medications and were not medication-naïve, which could influence metabolite levels. A recent study showed that glutamate levels in the striatum were lower following only 4 weeks of treatment.13 Therefore, additional longitudinal studies of medication-naïve, first-episode patients are needed to better determine the effect of treatment on MRS measurements of glutamate and other metabolites in cortical and sub-cortical regions. Third, only one brain region was assessed, so we could not directly test whether the observed effects were specific to the ACC. Fourth, none of the correlations between metabolites and clinical variables survived correction for multiple comparisons. Fifth, we did not use an MRS technique designed to optimize detection of GABA. However, Wijtenburg et al52 previously demonstrated good reproducibility of GABA in the ACC using the STEAM sequence at 7T. The purpose of our study was not to determine accurate concentrations of metabolites (ie, absolute quantification) but to examine relative group differences between patients and controls. Near et al53 show that even at 3T, short-TE GABA measurements are significantly correlated with edited GABA measurements. In addition, at high SNR and narrow linewidth available at 7T, although the GABA concentration may be underestimated, the reproducibility is good, indicating consistency in the measurements. For studies of group differences, Near et al53 conclude that measures of reproducibility are important for determining measurement efficacy. Nevertheless, the voxel size that we used was smaller than that of Wijtenburg et al52 and may be too small for reliable GABA measurements, so additional studies using editing techniques are needed to validate our findings and further our understanding of GABA abnormalities in schizophrenia. Finally, when the GM proportion was added as a covariate, there were no significant differences in glutamate or tNAA, suggesting that the observed effect may be due to variations in tissue composition. Notably, the patients had a numerically greater proportion of GM in the MRS voxel in the context of lower metabolite levels, so future studies should not only account for these variations in tissue composition but also further investigate the impact of differential tissue-specific effects on the MRS metabolite signals.
In summary, we investigated neurometabolite levels in the ACC of young patients with schizophrenia using high-field 7T MRS. Our findings suggest that glutamate and NAA reductions in the ACC may be present early in the illness, although large-scale studies are needed to confirm these results as well as longitudinal studies to determine the effect of illness progression and treatment. The correlations between GABA and cognitive function and between glutamate and positive symptoms suggest that MRS may be an important technique for investigating the neurobiology underlying cognitive deficits and symptomatology.
Funding
This work was supported by the UAB School of Medicine Imaging Steering Committee; the UAB Comprehensive Neuroscience Center; the Auburn University MRI Research Center; the National Institutes of Health (grant number R01MH102951 to A.C.L.); and the National Science Foundation (grant number IOS 0622318 to T.J.G.). The authors have declared that there are no conflicts of interest in relation to the subject of this study.
Supplementary Material
Acknowledgments
We thank Dr. Dinesh Deelchand and Dr. Gulin Oz (Center for Magnetic Resonance Research, University of Minnesota) for providing the basis set for MRS analysis. We thank Dr. Martha Forloines, Dr. Andie Thompkins, and Dr. Paul Anglin for assistance with scanning. The authors have declared that there are no conflicts of interest in relation to the subject of this study.
References
- 1. Poels EM, Kegeles LS, Kantrowitz JT, et al. . Glutamatergic abnormalities in schizophrenia: a review of proton MRS findings. Schizophr Res. 2014;152:325–332. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2. Hulshoff Pol HE, Kahn RS. What happens after the first episode? A review of progressive brain changes in chronically ill patients with schizophrenia. Schizophr Bull. 2008;34:354–366. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Kraguljac NV, White DM, Reid MA, Lahti AC. Increased hippocampal glutamate and volumetric deficits in unmedicated patients with schizophrenia. JAMA Psychiatry. 2013;70:1294–1302. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Olney JW, Newcomer JW, Farber NB. NMDA receptor hypofunction model of schizophrenia. J Psychiatr Res. 1999;33:523–533. [DOI] [PubMed] [Google Scholar]
- 5. Plitman E, Patel R, Chung JK, et al. . Glutamatergic metabolites, volume and cortical thickness in antipsychotic-naive patients with first-episode psychosis: implications for excitotoxicity. Neuropsychopharmacology. 2016;41:2606–2613. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. de la Fuente-Sandoval C, Leon-Ortiz P, Favila R, et al. . Higher levels of glutamate in the associative-striatum of subjects with prodromal symptoms of schizophrenia and patients with first-episode psychosis. Neuropsychopharmacology 2011;36:1781–1791. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Stone JM, Day F, Tsagaraki H, et al. . Glutamate dysfunction in people with prodromal symptoms of psychosis: relationship to gray matter volume. Biol Psychiatry. 2009;66:533–539. [DOI] [PubMed] [Google Scholar]
- 8. Tandon N, Bolo NR, Sanghavi K, et al. . Brain metabolite alterations in young adults at familial high risk for schizophrenia using proton magnetic resonance spectroscopy. Schizophr Res. 2013;148:59–66. [DOI] [PubMed] [Google Scholar]
- 9. Théberge J, Bartha R, Drost DJ, et al. . Glutamate and glutamine measured with 4.0 T proton MRS in never-treated patients with schizophrenia and healthy volunteers. Am J Psychiatry. 2002;159:1944–1946. [DOI] [PubMed] [Google Scholar]
- 10. Théberge J, Williamson KE, Aoyama N, et al. . Longitudinal grey-matter and glutamatergic losses in first-episode schizophrenia. Br J Psychiatry. 2007;191:325–334. [DOI] [PubMed] [Google Scholar]
- 11. Aoyama N, Théberge J, Drost DJ, et al. . Grey matter and social functioning correlates of glutamatergic metabolite loss in schizophrenia. Br J Psychiatry. 2011;198:448–456. [DOI] [PubMed] [Google Scholar]
- 12. Goto N, Yoshimura R, Kakeda S, et al. . Six-month treatment with atypical antipsychotic drugs decreased frontal-lobe levels of glutamate plus glutamine in early-stage first-episode schizophrenia. Neuropsychiatr Dis Treat. 2012;8:119–122. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. de la Fuente-Sandoval C, Leon-Ortiz P, Azcarraga M, et al. . Glutamate levels in the associative striatum before and after 4 weeks of antipsychotic treatment in first-episode psychosis: a longitudinal proton magnetic resonance spectroscopy study. JAMA Psychiatry 2013;70:1057–1066. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Kegeles LS, Mao X, Stanford AD, et al. . Elevated prefrontal cortex γ-aminobutyric acid and glutamate-glutamine levels in schizophrenia measured in vivo with proton magnetic resonance spectroscopy. Arch Gen Psychiatry. 2012;69:449–459. [DOI] [PubMed] [Google Scholar]
- 15. Egerton A, Brugger S, Raffin M, et al. . Anterior cingulate glutamate levels related to clinical status following treatment in first-episode schizophrenia. Neuropsychopharmacology. 2012;37:2515–2521. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Mouchlianitis E, Bloomfield MA, Law V, et al. . Treatment-resistant schizophrenia patients show elevated anterior cingulate cortex glutamate compared to treatment-responsive. Schizophr Bull. 2016;42:744–752. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Kraguljac NV, Reid MA, White DM, den Hollander J, Lahti AC. Regional decoupling of N-acetyl-aspartate and glutamate in schizophrenia. Neuropsychopharmacology. 2012;37:2635–2642. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Lutkenhoff ES, van Erp TG, Thomas MA, et al. . Proton MRS in twin pairs discordant for schizophrenia. Mol Psychiatry. 2010;15:308–318. [DOI] [PubMed] [Google Scholar]
- 19. Reid MA, Stoeckel LE, White DM, et al. . Assessments of function and biochemistry of the anterior cingulate cortex in schizophrenia. Biol Psychiatry. 2010;68:625–633. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Rowland LM, Kontson K, West J, et al. . In vivo measurements of glutamate, GABA, and NAAG in schizophrenia. Schizophr Bull. 2013;39:1096–1104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. Théberge J, Al-Semaan Y, Williamson PC, et al. . Glutamate and glutamine in the anterior cingulate and thalamus of medicated patients with chronic schizophrenia and healthy comparison subjects measured with 4.0-T proton MRS. Am J Psychiatry. 2003;160:2231–2233. [DOI] [PubMed] [Google Scholar]
- 22. Wood SJ, Yücel M, Wellard RM, et al. . Evidence for neuronal dysfunction in the anterior cingulate of patients with schizophrenia: a proton magnetic resonance spectroscopy study at 3 T. Schizophr Res. 2007;94:328–331. [DOI] [PubMed] [Google Scholar]
- 23. Wijtenburg SA, Wright SN, Korenic SA, et al. . Altered glutamate and regional cerebral blood flow levels in schizophrenia: a 1H-MRS and pCASL study. Neuropsychopharmacology. 2017;42:562–571. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24. Bustillo JR, Chen H, Jones T, et al. . Increased glutamine in patients undergoing long-term treatment for schizophrenia: a proton magnetic resonance spectroscopy study at 3 T. JAMA Psychiatry. 2014;71:265–272. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25. Gallinat J, McMahon K, Kühn S, Schubert F, Schaefer M. Cross-sectional study of glutamate in the anterior cingulate and hippocampus in schizophrenia. Schizophr Bull. 2016;42:425–433. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26. Harris AD, Saleh MG, Edden RA. Edited 1 H magnetic resonance spectroscopy in vivo: methods and metabolites. Magn Reson Med. 2017;77:1377–1389. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27. Brandt AS, Unschuld PG, Pradhan S, et al. . Age-related changes in anterior cingulate cortex glutamate in schizophrenia: A (1)H MRS Study at 7 Tesla. Schizophr Res. 2016;172:101–105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28. Marsman A, Mandl RC, Klomp DW, et al. . GABA and glutamate in schizophrenia: a 7 T ¹H-MRS study. Neuroimage Clin. 2014;6:398–407. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29. Rowland LM, Pradhan S, Korenic S, et al. . Elevated brain lactate in schizophrenia: a 7T magnetic resonance spectroscopy study. Transl Psychiatry. 2016;6:e967. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30. Thakkar KN, Rösler L, Wijnen JP, et al. . 7T proton magnetic resonance spectroscopy of gamma-aminobutyric acid, glutamate, and glutamine reveals altered concentrations in patients with schizophrenia and healthy siblings. Biol Psychiatry. 2017;81:525–535. [DOI] [PubMed] [Google Scholar]
- 31. Lahti AC, Weiler MA, Holcomb HH, Tamminga CA, Carpenter WT, McMahon R. Correlations between rCBF and symptoms in two independent cohorts of drug-free patients with schizophrenia. Neuropsychopharmacology. 2006;31:221–230. [DOI] [PubMed] [Google Scholar]
- 32. Brugger S, Davis JM, Leucht S, Stone JM. Proton magnetic resonance spectroscopy and illness stage in schizophrenia–a systematic review and meta-analysis. Biol Psychiatry. 2011;69:495–503. [DOI] [PubMed] [Google Scholar]
- 33. Patterson TL, Goldman S, McKibbin CL, Hughs T, Jeste DV. UCSD Performance-Based Skills Assessment: development of a new measure of everyday functioning for severely mentally ill adults. Schizophr Bull. 2001;27:235–245. [DOI] [PubMed] [Google Scholar]
- 34. Randolph C, Tierney MC, Mohr E, Chase TN. The Repeatable Battery for the Assessment of Neuropsychological Status (RBANS): preliminary clinical validity. J Clin Exp Neuropsychol. 1998;20:310–319. [DOI] [PubMed] [Google Scholar]
- 35. Tkác I, Andersen P, Adriany G, Merkle H, Ugurbil K, Gruetter R. In vivo 1H NMR spectroscopy of the human brain at 7 T. Magn Reson Med. 2001;46:451–456. [DOI] [PubMed] [Google Scholar]
- 36. Provencher SW. Estimation of metabolite concentrations from localized in vivo proton NMR spectra. Magn Reson Med. 1993;30:672–679. [DOI] [PubMed] [Google Scholar]
- 37. Deelchand DK, Adanyeguh IM, Emir UE, et al. . Two-site reproducibility of cerebellar and brainstem neurochemical profiles with short-echo, single-voxel MRS at 3T. Magn Reson Med. 2015;73:1718–1725. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38. Gasparovic C, Song T, Devier D, et al. . Use of tissue water as a concentration reference for proton spectroscopic imaging. Magn Reson Med. 2006;55:1219–1226. [DOI] [PubMed] [Google Scholar]
- 39. Moghaddam B, Javitt D. From revolution to evolution: the glutamate hypothesis of schizophrenia and its implication for treatment. Neuropsychopharmacology. 2012;37:4–15. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40. Adams B, Moghaddam B. Corticolimbic dopamine neurotransmission is temporally dissociated from the cognitive and locomotor effects of phencyclidine. J Neurosci. 1998;18:5545–5554. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41. Moghaddam B, Adams B, Verma A, Daly D. Activation of glutamatergic neurotransmission by ketamine: a novel step in the pathway from NMDA receptor blockade to dopaminergic and cognitive disruptions associated with the prefrontal cortex. J Neurosci. 1997;17:2921–2927. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42. Kraguljac NV, Frölich MA, Tran S, et al. . Ketamine modulates hippocampal neurochemistry and functional connectivity: a combined magnetic resonance spectroscopy and resting-state fMRI study in healthy volunteers. Mol Psychiatry. 2017;22:562–569. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43. Rowland LM, Bustillo JR, Mullins PG, et al. . Effects of ketamine on anterior cingulate glutamate metabolism in healthy humans: a 4-T proton MRS study. Am J Psychiatry. 2005;162:394–396. [DOI] [PubMed] [Google Scholar]
- 44. Szulc A, Galinska B, Tarasow E, et al. . Proton magnetic resonance spectroscopy study of brain metabolite changes after antipsychotic treatment. Pharmacopsychiatry. 2011;44:148–157. [DOI] [PubMed] [Google Scholar]
- 45. Wang J, Tang Y, Zhang T, et al. . Reduced γ-aminobutyric acid and glutamate+glutamine levels in drug-naïve patients with first-episode schizophrenia but not in those at ultrahigh risk. Neural Plast. 2016;2016:3915703. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46. Dou W, Palomero-Gallagher N, van Tol MJ, et al. . Systematic regional variations of GABA, glutamine, and glutamate concentrations follow receptor fingerprints of human cingulate cortex. J Neurosci. 2013;33:12698–12704. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47. Palomero-Gallagher N, Vogt BA, Schleicher A, Mayberg HS, Zilles K. Receptor architecture of human cingulate cortex: evaluation of the four-region neurobiological model. Hum Brain Mapp. 2009;30:2336–2355. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48. Todtenkopf MS, Vincent SL, Benes FM. A cross-study meta-analysis and three-dimensional comparison of cell counting in the anterior cingulate cortex of schizophrenic and bipolar brain. Schizophr Res. 2005;73:79–89. [DOI] [PubMed] [Google Scholar]
- 49. Roberts RC, Barksdale KA, Roche JK, Lahti AC. Decreased synaptic and mitochondrial density in the postmortem anterior cingulate cortex in schizophrenia. Schizophr Res. 2015;168:543–553. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50. Lewis DA, Curley AA, Glausier JR, Volk DW. Cortical parvalbumin interneurons and cognitive dysfunction in schizophrenia. Trends Neurosci. 2012;35:57–67. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51. Menzies L, Ooi C, Kamath S, et al. . Effects of gamma-aminobutyric acid-modulating drugs on working memory and brain function in patients with schizophrenia. Arch Gen Psychiatry. 2007;64:156–167. [DOI] [PubMed] [Google Scholar]
- 52. Wijtenburg SA, Rowland LM, Edden RA, Barker PB. Reproducibility of brain spectroscopy at 7T using conventional localization and spectral editing techniques. J Magn Reson Imaging. 2013;38:460–467. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53. Near J, Andersson J, Maron E, et al. . Unedited in vivo detection and quantification of γ-aminobutyric acid in the occipital cortex using short-TE MRS at 3 T. NMR Biomed. 2013;26:1353–1362. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.