Abstract
Improved understanding of neuroimmune communication and the neural regulation of immunity and inflammation has recently led to proposing the concept of the “neuroimmune communicatome.” This advance is based on experimental evidence for an organized and brain-integrated reflex-like relationship and dialogue between the nervous and the immune systems. A key circuitry in this communicatome is provided by efferent vagus nerve fibers and cholinergic signaling. Inflammation and metabolic alterations coexist in many disorders affecting the liver and the gastrointestinal (GI) tract, including obesity, metabolic syndrome, fatty liver disease, liver injury, and liver failure, as well as inflammatory bowel disease. Here, we outline mechanistic insights regarding the role of the vagus nerve and cholinergic signaling in the regulation of inflammation linked to metabolic derangements and the pathogenesis of these disorders in preclinical settings. Recent clinical advances using this knowledge in novel therapeutic neuromodulatory approaches within the field of bioelectronic medicine are also briefly summarized.
Keywords: cholinergic, GI tract, inflammation, liver, vagus
INTRODUCTION
Ongoing research reveals that neuroimmune communication and the neural regulation of immunity and inflammation occur in an organized and integrated manner (43) (Fig. 1). This latest insight was recently outlined, and the term “neuroimmune communicatome” was conceptualized (41). Neural circuitries in the neuroimmune communicatome regulate immune function in a manner similar to how the nervous system regulates cardiovascular, gastrointestinal (GI), respiratory, and other physiological functions. Neural, central nervous system (CNS)-integrated reflex pathways play important roles in both. Moreover, experimental evidence indicates some overlap, but also differences between neural circuits and receptor signaling identified in immune regulation and those regulating metabolism and GI and other physiological functions (49). These allow for integrated and efficient control of homeostatic alterations. An important neuroimmunomodulatory circuitry within the neuroimmune communicatome is the vagus nerve-based “inflammatory reflex” (48, 62). Alterations in peripheral levels of cytokines and other inflammatory molecules are detected by afferent vagus nerve peripheral endings and communicated to the brain stem nucleus tractus solitarius (NTS) (14). NTS is anatomically and functionally linked with the dorsal motor nucleus of the vagus (DMN), which is a major source of efferent (motor) vagus nerve neurons that regulate peripheral immune responses and cytokine release (43, 48). Substantial mechanistic insight into the inflammatory reflex and its efferent arm, the cholinergic anti-inflammatory pathway, has been generated (46).
Fig. 1.
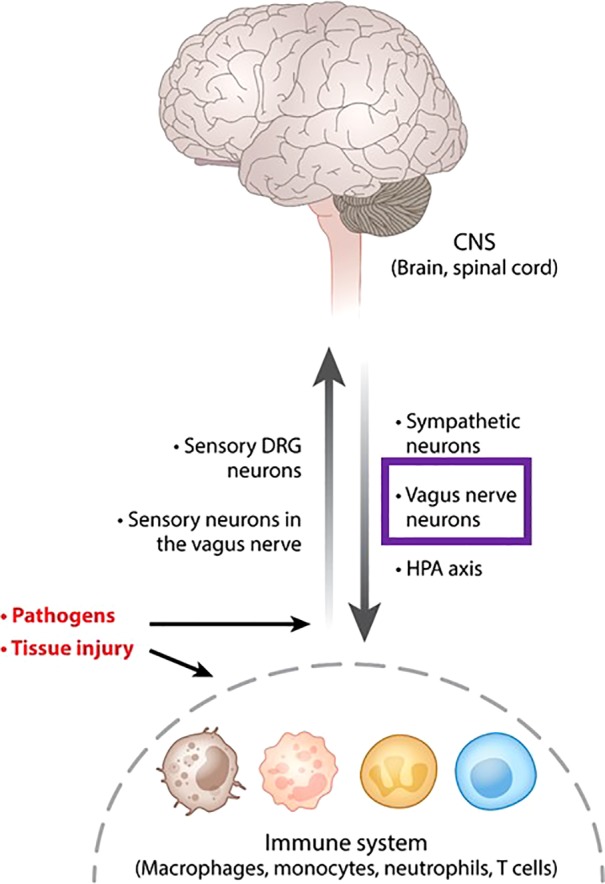
Communication between the central nervous system (CNS) and the immune system. The nervous system and the immune system communicate in response to pathogen invasion, tissue injury, and other homeostatic threats. Macrophages, neutrophils, monocytes, T lymphocytes, and other immune cells detect the presence of pathogen components and tissue injury molecules and release cytokines and other signaling molecules. These alterations in peripheral immune homeostasis are detected by sensory neurons residing in the dorsal root ganglia (DRG) and vagus nerve afferent neurons, which signal to the spinal cord and the brain. Pathogens also directly activate afferent neurons. These signals are integrated in the central nervous system with descending signaling via sympathetic and efferent vagus nerve fibers (purple box) with the release of catecholamines and ACh, respectively. These neurotransmitters interact with immune cells and control immune cell function and responses. Other neurotransmitters released from neurons also play a role in immune control. The hypothalamic-pituitary-adrenal (HPA) axis with the release of corticosteroids also provides a conduit of brain-to immune regulation (the use of this figure originally published in Ref. 43 has been granted by Annual Review of Immunology).
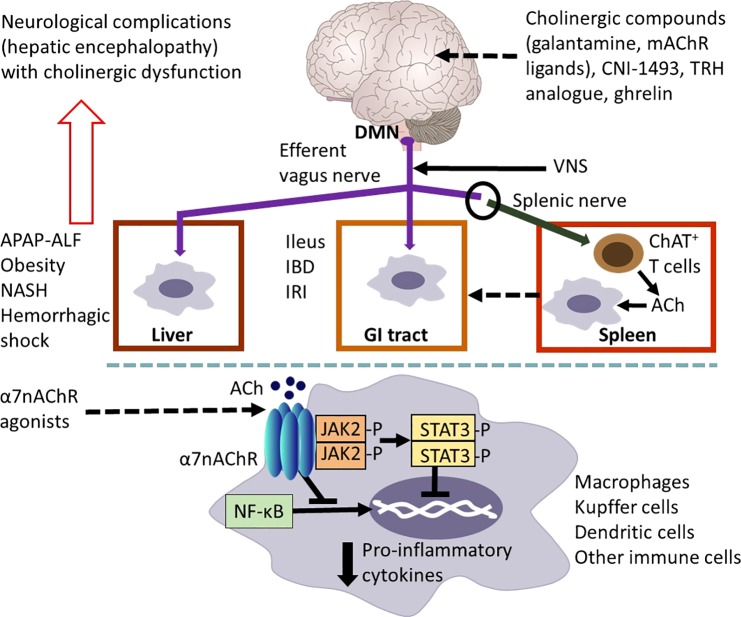
Efferent vagus nerve cholinergic signaling controls both the levels of proinflammatory cytokines and important mediators involved in the resolution of inflammation (14, 38). Experimental evidence indicates that the α7 nicotinic ACh receptor (α7nAChR) on immune cells importantly mediates efferent vagus nerve cholinergic suppression of proinflammatory cytokine production (67). While nicotinic receptors mediate fast ganglionic transmission (between preganglionic and postganglionic efferent vagus nerve fibers), “classical” regulatory functions of the efferent vagus nerve are mediated through different subtypes of muscarinic ACh receptors (mAChRs) on cardiac myocytes, smooth muscle cells, and glandular cells. These functions include modulation of heart rate, gastrointestinal motility and secretion, and respiratory function. This receptor-based difference in cholinergic control of physiological function is interesting and important because it may allow fine-tuned modulation and selectivity in therapeutic targeting. A functional cooperation between the efferent vagus nerve and the splenic nerve within the inflammatory reflex leads to the release of ACh in the spleen (43, 55). A subset of T lymphocytes contains the enzyme choline acetyltransferase (ChAT) required for the production of ACh; these ChAT+ T cells are the key source of splenic ACh in response to splenic nerve catecholaminergic signaling through β-adrenoreceptors (43, 55). Released ACh then interacts with α7nAChRs expressed on macrophages and other immune cells to suppress proinflammatory cytokine production (43, 55) (Fig. 2).
Fig. 2.
Vagus nerve cholinergic signaling regulates inflammation in the gastrointestinal (GI) tract and the liver. Efferent vagus nerve preganglionic cholinergic fibers (shown in purple) originating predominantly from the dorsal motor nucleus of the vagus (DMN) innervate the liver and the GI tract and regulate local immune responses, inflammation, and metabolism. The use of vagotomy, vagus nerve stimulation (VNS), knockout mice, and the administration of cholinergic compounds, including α7 nicotinic receptor (α7nAChR) agonists have been instrumental in identifying the functional anatomy and molecular components of this regulation. Cholinergic signaling has been implicated in suppressing hepatic inflammation and alleviating metabolic derangements in the context of acetaminophen (APAP)-induced liver injury and acute liver failure (ALF), obesity, nonalcoholic steatohepatitis (NASH) and hemorrhagic shock. Cholinergic signals also control GI inflammation and metabolic disease manifestations in experimental postoperative ileus, inflammatory bowel disease (IBD), and ischemia and reperfusion injury (IRI). A functional cooperation between the efferent vagus nerve and the splenic nerve with the release of acetylcholine (ACh) from a subset of T cells that contain the enzyme choline acetyltransferase (ChAT+ T cells) plays a major mediating role in the inflammatory reflex. Activation of this regulatory circuitry triggered by the central (in the brain) action of the acetylcholinesterase inhibitor, galantamine, and muscarinic ACh receptor (mAChR) ligands plays a role in suppressing colonic inflammation and alleviating the severity of the disease in murine IBD (colitis). Other compounds, including CNI-1493, a stable thyrotropin-releasing hormone (TRH) analog and ghrelin trigger activation of vagus nerve cholinergic signaling that controls immune responses and suppresses inflammation in experimental models of liver and GI disorders. The α7nAChR expressed by macrophages, Kupffer cells, and dendritic cells is a major cellular component of cholinergic regulation of hepatic and GI inflammation. Peripheral cholinergic α7nAChR-mediated activation inhibits NF-κB nuclear translocation, activates JAK2/STAT3 signaling, and suppresses proinflammatory cytokine production. The brain plays an important integrative and regulatory role in the neuroimmune communicatome in the context of liver and GI diseases with documented involvement of cholinergic signaling. However, the brain function can be severely affected (neurological complications known as hepatic encephalopathy) in severe liver diseases with considerable inflammatory involvement. These brain alterations, including cognitive deterioration, are associated with brain cholinergic dysfunction. Findings in preclinical settings have indicated the use of VNS, α7AChR agonists, and centrally acting AChE inhibitors, including galantamine, as therapeutic cholinergic modalities for liver and GI inflammatory disorders. Recent clinical trials with VNS in patients with IBD and with galantamine in patients with the metabolic syndrome have generated promising results [see text for details. The image of the brain has been adapted from Fig. 1, originally published in (43)].
Immune and metabolic dysregulations are characteristic features of many acute and chronic disorders, including obesity, metabolic syndrome, acute and chronic liver injury and failure, and inflammatory bowel disease (IBD) (48, 49). Moreover, excessive or nonresolved inflammation plays an important role in disease pathogenesis and is interrelated with metabolic dysregulation (e.g., insulin resistance) in many of these diseases (49). Here, we focus on the role of the efferent vagus nerve and cholinergic signaling in the regulation of immune function and inflammation in disorders associated with liver and GI pathologies. We briefly elaborate on the role of the brain in this context and highlight recent clinical developments based on insights generated in preclinical settings within the field of bioelectronic medicine.
EFFERENT VAGUS NERVE CIRCUITRY TO LIVER AND THE GI TRACT: ANATOMY AND FUNCTION
The efferent vagus nerve comprises preganglionic and postganglionic neurons; very long preganglionic neurons originating in brain stem nuclei communicate with relatively short postganglionic neurons in ganglia located either within or in close proximity to the innervated organs. The origin of efferent vagus nerve preganglionic fibers innervating the GI tract and more specifically the stomach and colon has been associated with the DMN (Fig. 2), and to a lesser extent in the nucleus ambiguus (NA) in the brain stem medulla oblongata (3, 59, 63). Integration of afferent and efferent signaling along the vagus nerve, which plays a role in vago-vagal reflexes controlling GI motility and secretion, takes place through anatomical and functional interactions between the NTS and DMN (59, 63). Interactions between efferent vagus neurons and neurons of the enteric nervous system are implicated in controlling GI function (9, 65). The left DMN has been identified as the main source of efferent vagus nerve preganglionic fibers innervating the liver (Fig. 2). In addition, some efferent vagus nerve neurons have been traced to the left anterior NA and the right DMN (54). The hepatic branch of the vagus nerve is supplied mainly through the anterior trunk, which is an extension of the left vagus nerve under the diaphragm. Efferent vagus nerve signaling to the liver regulates hepatic metabolic function, such as the control of hepatic glucose production (gluconeogenesis) (29, 49–51). Interactions between the DMN, NTS, thalamus, hypothalamus, amygdala, cortex areas, and other brain regions mediate forebrain regulation of the autonomic control of GI and hepatic function, and its coordination with behavioral, cognitive, and endocrine regulation (49).
VAGUS NERVE CHOLINERGIC REGULATION OF HEPATIC IMMUNE RESPONSES AND INFLAMMATION
Abundant experimental evidence indicates that in addition to controlling “classical” physiological processes, efferent vagus nerve cholinergic signaling regulates immunity and inflammation. Cholinergic modulation of liver inflammation by the vagus nerve was first reported by Tracey and colleagues (11) almost 20 years ago. They demonstrated that electrical stimulation of the cervical vagus nerve directly attenuated LPS-induced TNF production in the liver and circulation and prevented the development of endotoxemia-related hypotension in rodents (11). At the cellular level, ACh, the major neurotransmitter released by efferent vagus nerve endings, suppresses TNF production by macrophages, acting through α7nAChR-mediated mechanisms (11, 67). The term cholinergic anti-inflammatory pathway was introduced to define this neuroimmune circuit (11). The role of cholinergic signaling in reducing hepatic and circulating TNF levels and preventing shock was further demonstrated using electrical vagus nerve stimulation (VNS) in the setting of experimental ischemia reperfusion injury after acute aortic occlusion (6). In addition, nicotine, a nonspecific nAChR agonist, and PNU-282987, a selective α7nAChR agonist, were shown to suppress hepatic inflammation following ischemia-reperfusion injury (31, 42). Mechanistically, vagus nerve cholinergic suppression of hepatic TNF production is mediated through the inhibition of NF-κB nuclear translocation, as determined using a rat model of hypovolemic hemorrhagic shock (64) (Fig. 2). Moreover, hepatoprotective antiapoptotic effects of vagus nerve α7nAChR-mediated cholinergic signaling have been reported using vagotomy and a selective α7nAChR agonist in a mouse model of Fas-induced hepatitis (24).
Acute liver failure is a life-threatening condition that includes hepatic encephalopathy and requires expensive life-saving intensive care treatment (5). While the most common cause of acute liver injury and failure in the world is hepatitis A and E infections, the most common cause of acute liver injury and failure in the United States is acetaminophen (N-acetyl-p-aminophenol, APAP, Tylenol) overdose (5, 52). Critical care management of patients with acute liver failure continues to be challenging with limited treatment options. Sterile inflammation mediated by the release of cytokines and immune cell infiltration into the liver is a characteristic feature of acute liver failure pathobiology (15). In a mouse model of APAP-induced acute liver injury, enhanced cholinergic signaling has been related to hepatoprotective effects. Pretreatment with centrally acting acetylcholinesterase (AChE) inhibitors (that stimulate cholinergic signaling), including huperzine A, donepezil, and rivastigmine, suppresses TNF, IL-6, and HMGB1 levels and alleviates experimental hepatic injury [assessed by serum alanine aminotransferase (ALT) and aspartate aminotransferase levels, and histopathological findings] (75). In contrast, pretreatment with the peripherally acting AChE inhibitor, neostigmine, has no significant effect on liver injury in this model (75). This lack of efficacy of the peripherally acting neostigmine is in agreement with a previous report that neostigmine (in a nonlethal dose) does not alleviate interstitial inflammation and liver injury (1). Others have reported that neostigmine enhances survival, reduces liver damage (as determined by ALT levels, histopathologic signs of liver injury, and liver apoptosis), and decreases serum proinflammatory cytokine levels in an APAP murine model (60). We have recently observed (unpublished data) the superior hepatic protection exerted by administration of the centrally acting AChE inhibitor, galantamine, as compared with neostigmine in an APAP-induced liver injury model. Galantamine, which is clinically approved for Alzheimer’s disease, also has been shown to promote vagus nerve signaling (66) and exert anti-inflammatory activity via central activation of the vagus nerve-based cholinergic anti-inflammatory pathway (26, 45, 72). Therefore, it is interesting and important to study the therapeutic use of VNS in the context of acute liver injury and acute liver failure. It is also of therapeutic interest to examine the potential role of cholinergic signaling in a broader scope of liver diseases with autoimmune and autoinflammatory etiology, including primary biliary cirrhosis, chronic autoimmune hepatitis, and primary sclerosing cholangitis. The fact that galantamine and other AChE inhibitors are FDA-approved drugs will facilitate further development of these compounds in treatment approaches for liver diseases.
Chronic low-grade inflammation plays an important pathogenic role in obesity and obesity-driven disorders, including metabolic syndrome, Type 2 diabetes, and nonalcoholic steatohepatitis (NASH), by promoting insulin resistance and hepatic metabolic derangements (49). Experimental evidence supports a role for cholinergic signaling in controlling inflammation and metabolic derangements in obesity-driven conditions. α7nAChR knockout (KO) mice fed a high-fat diet have increased M1 macrophage infiltration and both TNF and CCL2 (MCP-1) expression when compared with wild-type controls (68). The expression of α7nAChR by human adipocytes is decreased during obesity, and the functional role of this receptor has been related to suppression of proinflammatory gene expression (12). Treatment of obese db/db mice with a selective α7nAChR agonist (TC-7020) results in significant suppression of circulating TNF, glucose, and triglyceride levels, accompanied by lower weight gain and decreased food consumption (34).
A specific hepatic vagus nerve α7nAChR-mediated anti-inflammatory function was indicated in the context of murine NASH (40). Hepatic vagotomy exacerbates liver inflammation indicated by increased levels of TNF, IL-12, and CCL2 (MCP-1) in mice with diet-induced NASH (40). Moreover, NASH development is accelerated in α7nAChR KO chimeric mice (produced by transplanting α7nAChR bone marrow cells into γ-irradiated and Kupffer cell-depleted wild-type recipients). This is indicated by Kupffer cell activation, a significant increase in proinflammatory cytokine expression and abnormal lipid metabolism (40). Treatment of mice with high-fat diet-induced obesity with galantamine significantly reduces inflammation, insulin resistance, and hepatic lipid accumulation (hepatic steatosis) (57). In addition, galantamine is efficient in alleviating disease severity in nonobese diabetic mice and mice with streptozotocin-induced Type 1 diabetes (2, 23). Treatment with galantamine improves glucose homeostasis and liver lipid profiles and reduces both liver inflammation (via suppression of NF-κB activation) and oxidative stress (via Nrf2 and MDA) (2). Consistent with these preclinical findings, a recent clinical trial revealed that galantamine in doses clinically approved for the treatment of Alzheimer’s disease, significantly reduces inflammation associated with metabolic syndrome in humans (16). In this randomized, double-blind, placebo-controlled trial, patients with metabolic syndrome who were treated with galantamine (8 mg daily for 4 wk followed by 16 mg daily for 8 wk) exhibited significantly reduced circulating TNF and leptin levels and higher IL-10 and adiponectin levels when compared with patients treated with placebo. Galantamine treatment also lowered plasma insulin levels, alleviated insulin resistance, and modulated sympathovagal autonomic regulation toward vagal predominance (16). Together, these findings support further studies of vagus nerve-targeted cholinergic treatments for obesity-associated immune dysfunction and inflammation.
VAGUS NERVE CHOLINERGIC CONTROL OF GASTROINTESTINAL IMMUNE RESPONSES AND INFLAMMATION
A large body of experimental evidence indicates that vagus nerve cholinergic signaling suppresses excessive inflammation in murine models of postoperative ileus and IBD (8, 58, 65). Postoperative ileus is a frequent complication of abdominal surgery associated with delayed gastric empting that requires long hospitalizations. Aberrant inflammation and alterations in neural reflex regulation are major components of this condition. The vagus nerve has been demonstrated to be an important regulator of intestinal inflammation in experimental postoperative ileus (17). VNS significantly suppresses intestinal inflammation in a macrophage-dependent manner and ameliorates surgically induced ileus in mice (17). In addition, nicotine decreases TNF, CXCL2 (also known as macrophage inflammatory protein 2α or MIP-2α), and IL-6 release from peritoneal macrophages in an α7nAChR-dependent manner, a finding that supports the role of cholinergic output from the efferent vagus nerve in this context (17). Subsequent activation of the JAK2/STAT3 pathway has been indicated as a critical downstream intracellular mechanism suppressing cytokine and chemokine production by macrophages (17) (Fig. 2).
Ulcerative colitis and Crohnʼs disease are the two forms of IBD; they are chronic, relapsing deleterious disorders, with intestinal inflammation mediated, in large part, by the release of TNF and other proinflammatory cytokines. Surgical interruption of the vagus nerve by subdiaphragmatic vagotomy has been shown to significantly increase colonic levels of TNF and other proinflammatory cytokines and to exacerbate the severity of acute colitis (manifested by increased disease activity and histological and macroscopic scores) in two different murine models (21). By contrast, vagotomy has no effect on disease severity in M-CSF-deficient mice, supporting macrophage-mediated regulation (21). In addition, administration of hexamethonium (an nAChR antagonist) worsens outcomes in sham-operated animals with colitis, while treatment with nicotine improves outcomes in vagotomized mice with colitis (21). An intriguing positive correlation between impaired vagus nerve cholinergic control of macrophage activation and proinflammatory function and enhanced susceptibility to intestinal inflammation and colitis has been demonstrated in mice with depressive-like behavior (20). Chronic VNS in freely moving animals with colitis (for 5 days, 3 times a day), substantially alleviates colonic inflammation and improves both histological scores of lesions and the multivariate disease index (35). Very recently, VNS was demonstrated to significantly alleviate inflammation and the severity of murine oxazolone-induced colitis, an aggressive form of this disease (36). Additionally, the translational potential of VNS for treating patients with Crohn’s disease was recently demonstrated (10). VNS, using an implanted bioelectronic device, led to clinical remission in five out of seven patients with Crohn’s disease (10). This clinical improvement was associated with a significant decrease in C-reactive protein and fecal calprotectin (indicators of active disease), and alleviation of patients’ perceived abdominal pain (10). This chronic VNS also improved vagus nerve tone indicated by analyzing heart rate variability in these patients (10). The efficacy of implanted device-generated VNS in patients with Crohn’s disease is currently being tested in multicenter clinical trials (NCT02951650 and NCT02311660). These studies and the recently demonstrated efficacy of VNS in patients with rheumatoid arthritis (28) clearly indicate the progress in developing neuromodulatory treatments for a broad range of diseases within the framework of the new field of bioelectronic medicine (43).
BRAIN CONTROL OF THE VAGUS NERVE REGULATION OF GASTROINTESTINAL AND HEPATIC INFLAMMATION
The brain plays a major role in controlling and integrating vagus nerve-regulated physiological processes, including cardiovascular, gastrointestinal, and respiratory function and metabolism (49). Accumulating evidence points to brain mechanisms in the regulation of peripheral immune homeostasis and inflammation through efferent vagus nerve cholinergic output (43, 48). Brain cholinergic mAChR-mediated signaling has been shown to play an important role in this regulation (30, 44, 56), which includes control of hepatic and intestinal inflammation in experimental conditions of APAP-induced liver injury, hemorrhagic shock, and colitis (22, 26, 37, 39, 75). Treatment of mice with colitis with the centrally acting AChE inhibitor, galantamine, and central, intracerebroventricular administration of mAChR ligands suppresses mucosal inflammation (26, 39) (Fig. 2). This is indicated by lower major histocompatibility complex II and proinflammatory cytokine levels and substantial amelioration of the disease, evident by significantly improved histological scores (26). These effects are mediated through brain-to-spleen neural circuitry in the inflammatory reflex, because they are abrogated in mice following either vagotomy, splenic nerve transection, or splenectomy, and are dependent on splenic CD11c+ cells and α7nAChR signaling (26). Activation of the brain-to-spleen neural axis in the inflammatory reflex also plays a major role in alleviating murine colitis severity by central, intracerebroventricular administration of the M1 mAChR agonist, McN-A-343 (39) (Fig. 2). In this condition, cholinergic activation suppresses cytokine release by splenic dendritic cells through α7nAChR and NF-κB-mediated signaling mechanisms, which, in turn, decreases CD4+CD25− T-cell priming (39). This is associated with suppressed Th1/Th17 colonic immune responses and proinflammatory cytokine release in mice with colitis (39).
The efferent vagus nerve-mediated cholinergic anti-inflammatory pathway can be centrally activated using CNI-1493, an experimental drug and mAChR ligand (7). Brain (intracerebroventricular) administration of this drug alleviates intestinal inflammation and improves gastric empting in an experimental model of postoperative ileus in mice (61) (Fig. 2). These protective effects of the centrally administered CNI-1493 are abolished in mice following vagotomy and are associated with neuronal activation in the NTS, DMN, and the paraventricular nucleus (PVN) (61). These observations point to a brain activation of the efferent vagus nerve-based cholinergic anti-inflammatory pathway as a major physiological mechanism. Recently, the efficacy of another approach of brain activation of the cholinergic anti-inflammatory pathway in alleviating experimental postoperative ileus was demonstrated (73). Central (intracisternal) administration of the stable thyrotropin-releasing hormone analog, RX-77368, causes neuronal stimulation in the DMN and gastric efferent vagus nerve activation (73). This central vagus nerve stimulation by RX-77368 suppresses the activation of M1 macrophages located in proximity to cholinergic neurons in the gastric myenteric plexus; this was accompanied by decreased proinflammatory gene expression and inflammation, and improved gastric emptying following abdominal surgery (73) (Fig. 2). Another study has reported a role for the vagus nerve and brain involvement in mediating the anti-inflammatory effects, the protection against organ injury and mortality following exogenous administration of the orexigenic hormone, ghrelin, to rats with intestinal ischemia-reperfusion injury (71). These effects are abolished in rats following vagotomy, and recapitulated by intracerebroventricular administration of ghrelin (71) (Fig. 2).
Brain cholinergic mAChR-mediated signaling also plays a role in the regulation of hepatic inflammatory responses. In rats with hemorrhagic shock, brain mAChR-mediated activation of efferent vagus nerve signaling to liver by melanocortin ACTH (1–24) causes significant suppression of hepatic TNF release, mediated in the periphery through nAChR-dependent inhibition of NF-κB activation (22). These effects are associated with suppression of the systemic inflammatory response and reversal of shock conditions (22). These findings together with the efficacy of VNS in ameliorating postoperative ileus (17) suggest the potential of using brain pharmacological or peripheral (bioelectronic) activation of vagus nerve cholinergic signaling to the liver and GI tract for treating aberrant GI and hepatic inflammation.
In addition to modulating vagus nerve regulation of GI and hepatic inflammation, brain cholinergic signaling plays a major role in regulating cognition (70). Interestingly, cognitive impairment and alterations in the brain cholinergic system have been reported in the context of disorders characterized by GI and hepatic inflammatory and metabolic derangements (47, 74) (Fig. 2). Cognitive impairment is an important manifestation of hepatic encephalopathy (18), and brain cholinergic deterioration has been documented in patients with cirrhosis and in rats with liver failure (19). An intriguing overlap of cholinergic pathways and neuroimaging abnormalities in the brain has been indicated in patients with hepatic encephalopathy and other patients with cognitive deterioration and delirium (25). Moreover, a correlation between brain cholinergic impairment and lower vagus nerve activity has been reported in patients with cirrhosis and hepatic encephalopathy (4, 33). Decreased vagus nerve activity and autonomic dysfunction have also been reported in obesity, metabolic syndrome, and IBD (13, 27, 32, 53).
These findings highlight an intriguing relationship between brain and peripheral vagus nerve cholinergic signaling in immune and metabolic regulation and suggest that their functional capacity may be impaired in conditions with inflammatory and metabolic etiology. Accordingly, interventions aimed at augmenting these regulatory circuitries hold the potential to treat immune, metabolic, and cognitive deterioration in related diseases.
CONCLUSIONS
Seeing the big picture and “not losing sight of the forest for the trees” is important in science. Therefore, we recently attempted to conceptualize the emerging “big picture” in the field of neuroimmune interactions and neural control of immune responses and inflammation under the umbrella of the neuroimmune communicatome (41). It is also important to implement a reductionist approach and focus on delineating the components of this communicatome in certain physiological and pathophysiological contexts. Herein, we have outlined mechanistic insights focusing on vagus nerve cholinergic signaling in controlling liver and GI inflammation. The use of VNS, cholinergic compounds, and methodologies based on advances in molecular genetics have been instrumental in identifying the mechanisms and evaluating therapeutic options in preclinical models of liver diseases, IBD, and postoperative ileus. Recent and ongoing clinical trials using implanted VNS devices and pharmacological cholinergic modalities have begun to validate the clinical relevance of these findings in patients with IBD, metabolic syndrome, and other diseases. Implementing contemporary research tools and approaches, including optogenetics, viral vector neuronal mapping, and single-cell genomics are critical for generating further relevant anatomical and functional insights (48). This will advance our understanding of efferent vagus nerve cholinergic regulation and other aspects of the neuroimmune communicatome, including immunomodulation through afferent (sensory) neurons, sympathetic catecholaminergic neurons, and the hypothalamic-pituitary-adrenal axis (14, 41, 65, 69) (Fig. 1). This knowledge will allow precise modulation of specific GI and hepatic neural circuitries using microdevice technology for therapeutic benefit within the scope of bioelectronic medicine (43).
GRANTS
This work was supported by National Institute of General Medical Sciences Grants R01GM089807 and R01GM128008.
DISCLOSURES
C.N.M. and V.A.P. are inventors on patents broadly related to the topic of this review and have assigned their rights to the Feinstein Institute for Medical Research.
AUTHOR CONTRIBUTIONS
C.N.M. and V.A.P. drafted the manuscript; C.N.M. and V.A.P edited and revised the manuscript; C.N.M. and V.A.P approved the final version of the manuscript.
ACKNOWLEDGMENTS
We thank Dr. Kevin J. Tracey for critically reading this review and his suggestions.
REFERENCES
- 1.Akinci SB, Ulu N, Yondem OZ, Firat P, Guc MO, Kanbak M, Aypar U. Effect of neostigmine on organ injury in murine endotoxemia: missing facts about the cholinergic antiinflammatory pathway. World J Surg 29: 1483–1489, 2005. doi: 10.1007/s00268-005-0073-2. [DOI] [PubMed] [Google Scholar]
- 2.Ali MA, El-Abhar HS, Kamel MA, Attia AS. Antidiabetic effect of galantamine: novel effect for a known centrally acting drug. PLoS One 10: e0134648, 2015. doi: 10.1371/journal.pone.0134648. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Altschuler SM, Escardo J, Lynn RB, Miselis RR. The central organization of the vagus nerve innervating the colon of the rat. Gastroenterology 104: 502–509, 1993. doi: 10.1016/0016-5085(93)90419-D. [DOI] [PubMed] [Google Scholar]
- 4.Barron HV, Alam I, Lesh MD, Strunk A, Bass NM. Autonomic nervous system tone measured by baroreflex sensitivity is depressed in patients with end-stage liver disease. Am J Gastroenterol 94: 986–989, 1999. doi: 10.1111/j.1572-0241.1999.01000.x. [DOI] [PubMed] [Google Scholar]
- 5.Bernal W, Wendon J. Acute liver failure. N Engl J Med 369: 2525–2534, 2013. doi: 10.1056/NEJMra1208937. [DOI] [PubMed] [Google Scholar]
- 6.Bernik TR, Friedman SG, Ochani M, DiRaimo R, Susarla S, Czura CJ, Tracey KJ. Cholinergic antiinflammatory pathway inhibition of tumor necrosis factor during ischemia reperfusion. J Vasc Surg 36: 1231–1236, 2002. doi: 10.1067/mva.2002.129643. [DOI] [PubMed] [Google Scholar]
- 7.Bernik TR, Friedman SG, Ochani M, DiRaimo R, Ulloa L, Yang H, Sudan S, Czura CJ, Ivanova SM, Tracey KJ. Pharmacological stimulation of the cholinergic antiinflammatory pathway. J Exp Med 195: 781–788, 2002. doi: 10.1084/jem.20011714. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Bonaz B. Is here a place for vagus nerve stimulation in inflammatory bowel diseases? Bioelectronic Med 4: 4, 2018. doi: 10.1186/s42234-018-0004-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Bonaz B, Bazin T, Pellissier S. The vagus nerve at the interface of the microbiota-gut-brain axis. Front Neurosci 12: 49, 2018. doi: 10.3389/fnins.2018.00049. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Bonaz B, Sinniger V, Hoffmann D, Clarencon D, Mathieu N, Dantzer C, Vercueil L, Picq C, Trocme C, Faure P, Cracowski JL, Pellissier S. Chronic vagus nerve stimulation in Crohn's disease: a 6-month follow-up pilot study. Neurogastroenterol Motil 28: 948–953, 2016. doi: 10.1111/nmo.12792. [DOI] [PubMed] [Google Scholar]
- 11.Borovikova LV, Ivanova S, Zhang M, Yang H, Botchkina GI, Watkins LR, Wang H, Abumrad N, Eaton JW, Tracey KJ. Vagus nerve stimulation attenuates the systemic inflammatory response to endotoxin. Nature 405: 458–462, 2000. doi: 10.1038/35013070. [DOI] [PubMed] [Google Scholar]
- 12.Cancello R, Zulian A, Maestrini S, Mencarelli M, Della Barba A, Invitti C, Liuzzi A, Di Blasio AM. The nicotinic acetylcholine receptor α7 in subcutaneous mature adipocytes: downregulation in human obesity and modulation by diet-induced weight loss. Int J Obes 36: 1552–1557, 2012. doi: 10.1038/ijo.2011.275. [DOI] [PubMed] [Google Scholar]
- 13.Carnethon MR, Jacobs DR Jr, Sidney S, Liu K; CARDIA study . Influence of autonomic nervous system dysfunction on the development of type 2 diabetes: the CARDIA study. Diabetes Care 26: 3035–3041, 2003. doi: 10.2337/diacare.26.11.3035. [DOI] [PubMed] [Google Scholar]
- 14.Chavan SS, Pavlov VA, Tracey KJ. Mechanisms and therapeutic relevance of neuro-immune communication. Immunity 46: 927–942, 2017. doi: 10.1016/j.immuni.2017.06.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Chung RT, Stravitz RT, Fontana RJ, Schiodt FV, Mehal WZ, Reddy KR, Lee WM. Pathogenesis of liver injury in acute liver failure. Gastroenterology 143: e1–e7, 2012. doi: 10.1053/j.gastro.2012.07.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Consolim-Colombo FM, Sangaleti CT, Costa FO, Morais TL, Lopes HF, Motta JM, Irigoyen MC, Bortoloto LA, Rochitte CE, Harris YT, Satapathy SK, Olofsson PS, Akerman M, Chavan SS, MacKay M, Barnaby DP, Lesser ML, Roth J, Tracey KJ, Pavlov VA. Galantamine alleviates inflammation and insulin resistance in patients with metabolic syndrome in a randomized trial. JCI Insight 2: 2017. doi: 10.1172/jci.insight.93340. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.de Jonge WJ, van der Zanden EP, The FO, Bijlsma MF, van Westerloo DJ, Bennink RJ, Berthoud HR, Uematsu S, Akira S, van den Wijngaard RM, Boeckxstaens GE. Stimulation of the vagus nerve attenuates macrophage activation by activating the Jak2-STAT3 signaling pathway. Nat Immunol 6: 844–851, 2005. [Erratum in Nat Immunol 6: 954, 2005]. doi: 10.1038/ni1229. [DOI] [PubMed] [Google Scholar]
- 18.Felipo V. Hepatic encephalopathy: effects of liver failure on brain function. Nat Rev Neurosci 14: 851–858, 2013. doi: 10.1038/nrn3587. [DOI] [PubMed] [Google Scholar]
- 19.García-Ayllón MS, Cauli O, Silveyra MX, Rodrigo R, Candela A, Compañ A, Jover R, Pérez-Mateo M, Martínez S, Felipo V, Sáez-Valero J. Brain cholinergic impairment in liver failure. Brain 131: 2946–2956, 2008. doi: 10.1093/brain/awn209. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Ghia JE, Blennerhassett P, Collins SM. Impaired parasympathetic function increases susceptibility to inflammatory bowel disease in a mouse model of depression. J Clin Invest 118: 2209–2218, 2008. doi: 10.1172/JCI32849. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Ghia JE, Blennerhassett P, Kumar-Ondiveeran H, Verdu EF, Collins SM. The vagus nerve: a tonic inhibitory influence associated with inflammatory bowel disease in a murine model. Gastroenterology 131: 1122–1130, 2006. doi: 10.1053/j.gastro.2006.08.016. [DOI] [PubMed] [Google Scholar]
- 22.Guarini S, Cainazzo MM, Giuliani D, Mioni C, Altavilla D, Marini H, Bigiani A, Ghiaroni V, Passaniti M, Leone S, Bazzani C, Caputi AP, Squadrito F, Bertolini A. Adrenocorticotropin reverses hemorrhagic shock in anesthetized rats through the rapid activation of a vagal anti-inflammatory pathway. Cardiovasc Res 63: 357–365, 2004. doi: 10.1016/j.cardiores.2004.03.029. [DOI] [PubMed] [Google Scholar]
- 23.Hanes WM, Olofsson PS, Kwan K, Hudson LK, Chavan SS, Pavlov VA, Tracey KJ. Galantamine attenuates Type 1 diabetes and inhibits anti-insulin antibodies in non-obese diabetic mice. Mol Med 21: 1, 2015. doi: 10.2119/molmed.2015.00142. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Hiramoto T, Chida Y, Sonoda J, Yoshihara K, Sudo N, Kubo C. The hepatic vagus nerve attenuates Fas-induced apoptosis in the mouse liver via α7 nicotinic acetylcholine receptor. Gastroenterology 134: 2122–2131, 2008. doi: 10.1053/j.gastro.2008.03.005. [DOI] [PubMed] [Google Scholar]
- 25.Hshieh TT, Fong TG, Marcantonio ER, Inouye SK. Cholinergic deficiency hypothesis in delirium: a synthesis of current evidence. J Gerontol A Biol Sci Med Sci 63: 764–772, 2008. doi: 10.1093/gerona/63.7.764. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Ji H, Rabbi MF, Labis B, Pavlov VA, Tracey KJ, Ghia JE. Central cholinergic activation of a vagus nerve-to-spleen circuit alleviates experimental colitis. Mucosal Immunol 7: 335–347, 2014. doi: 10.1038/mi.2013.52. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Karason K, Mølgaard H, Wikstrand J, Sjöström L. Heart rate variability in obesity and the effect of weight loss. Am J Cardiol 83: 1242–1247, 1999. doi: 10.1016/S0002-9149(99)00066-1. [DOI] [PubMed] [Google Scholar]
- 28.Koopman FA, Chavan SS, Miljko S, Grazio S, Sokolovic S, Schuurman PR, Mehta AD, Levine YA, Faltys M, Zitnik R, Tracey KJ, Tak PP. Vagus nerve stimulation inhibits cytokine production and attenuates disease severity in rheumatoid arthritis. Proc Natl Acad Sci USA 113: 8284–8289, 2016. doi: 10.1073/pnas.1605635113. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Lam TK, Pocai A, Gutierrez-Juarez R, Obici S, Bryan J, Aguilar-Bryan L, Schwartz GJ, Rossetti L. Hypothalamic sensing of circulating fatty acids is required for glucose homeostasis. Nat Med 11: 320–327, 2005. doi: 10.1038/nm1201. [DOI] [PubMed] [Google Scholar]
- 30.Lee ST, Chu K, Jung KH, Kang KM, Kim JH, Bahn JJ, Jeon D, Kim M, Lee SK, Roh JK. Cholinergic anti-inflammatory pathway in intracerebral hemorrhage. Brain Res 1309: 164–171, 2010. doi: 10.1016/j.brainres.2009.10.076. [DOI] [PubMed] [Google Scholar]
- 31.Li F, Chen Z, Pan Q, Fu S, Lin F, Ren H, Han H, Billiar TR, Sun F, Li Q. The protective effect of PNU-282987, a selective α7 nicotinic acetylcholine receptor agonist, on the hepatic ischemia-reperfusion injury is associated with the inhibition of high-mobility group box 1 protein expression and nuclear factor-κB activation in mice. Shock 39: 197–203, 2013. [DOI] [PubMed] [Google Scholar]
- 32.Lindgren S, Stewenius J, Sjölund K, Lilja B, Sundkvist G. Autonomic vagal nerve dysfunction in patients with ulcerative colitis. Scand J Gastroenterol 28: 638–642, 1993. doi: 10.3109/00365529309096103. [DOI] [PubMed] [Google Scholar]
- 33.Mani AR, Montagnese S, Jackson CD, Jenkins CW, Head IM, Stephens RC, Moore KP, Morgan MY. Decreased heart rate variability in patients with cirrhosis relates to the presence and degree of hepatic encephalopathy. Am J Physiol Gastrointest Liver Physiol 296: G330–G338, 2009. doi: 10.1152/ajpgi.90488.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Marrero MB, Lucas R, Salet C, Hauser TA, Mazurov A, Lippiello PM, Bencherif M. An α7 nicotinic acetylcholine receptor-selective agonist reduces weight gain and metabolic changes in a mouse model of diabetes. J Pharmacol Exp Ther 332: 173–180, 2010. doi: 10.1124/jpet.109.154633. [DOI] [PubMed] [Google Scholar]
- 35.Meregnani J, Clarencon D, Vivier M, Peinnequin A, Mouret C, Sinniger V, Picq C, Job A, Canini F, Jacquier-Sarlin M, Bonaz B. Anti-inflammatory effect of vagus nerve stimulation in a rat model of inflammatory bowel disease. Auton Neurosci Basic Clin 160: 82–89, 2011. [DOI] [PubMed] [Google Scholar]
- 36.Meroni E, Stakenborg N, Gomez-Pinilla PJ, De Hertogh G, Goverse G, Matteoli G, Verheijden S, Boeckxstaens GE. Functional characterization of oxazolone-induced colitis and survival improvement by vagus nerve stimulation. PLoS One 13: e0197487, 2018. doi: 10.1371/journal.pone.0197487. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Miceli PC, Jacobson K. Cholinergic pathways modulate experimental dinitrobenzene sulfonic acid colitis in rats. Auton Neurosci Basic Clin 105: 16–24, 2003. [DOI] [PubMed] [Google Scholar]
- 38.Mirakaj V, Dalli J, Granja T, Rosenberger P, Serhan CN. Vagus nerve controls resolution and pro-resolving mediators of inflammation. J Exp Med 211: 1037–1048, 2014. doi: 10.1084/jem.20132103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Munyaka P, Rabbi MF, Pavlov VA, Tracey KJ, Khafipour E, Ghia JE. Central muscarinic cholinergic activation alters interaction between splenic dendritic cell and CD4+CD25- T cells in experimental colitis. PLoS One 9: e109272, 2014. doi: 10.1371/journal.pone.0109272. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Nishio T, Taura K, Iwaisako K, Koyama Y, Tanabe K, Yamamoto G, Okuda Y, Ikeno Y, Yoshino K, Kasai Y, Okuno M, Seo S, Sakurai T, Asagiri M, Hatano E, Uemoto S. Hepatic vagus nerve regulates Kupffer cell activation via α7 nicotinic acetylcholine receptor in nonalcoholic steatohepatitis. J Gastroenterol 52: 965–976, 2017. doi: 10.1007/s00535-016-1304-z. [DOI] [PubMed] [Google Scholar]
- 41.Olofsson PS, Metz CN, Pavlov VA. The neuroimmune communicatome in inflammation. In: Inflammation: From Molecular and Cellular Mechanisms to the Clinic, edited by Cavaillon J and Singer M. Weinheim, Germany: Wiley‐VCH Verlag, 2017, p. 1485–1516. [Google Scholar]
- 42.Park J, Kang JW, Lee SM. Activation of the cholinergic anti-inflammatory pathway by nicotine attenuates hepatic ischemia/reperfusion injury via heme oxygenase-1 induction. Eur J Pharmacol 707: 61–70, 2013. doi: 10.1016/j.ejphar.2013.03.026. [DOI] [PubMed] [Google Scholar]
- 43.Pavlov VA, Chavan SS, Tracey KJ. Molecular and functional neuroscience in immunity. Annu Rev Immunol 36: 783–812, 2018. doi: 10.1146/annurev-immunol-042617-053158. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Pavlov VA, Ochani M, Gallowitsch-Puerta M, Ochani K, Huston JM, Czura CJ, Al-Abed Y, Tracey KJ. Central muscarinic cholinergic regulation of the systemic inflammatory response during endotoxemia. Proc Natl Acad Sci USA 103: 5219–5223, 2006. doi: 10.1073/pnas.0600506103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Pavlov VA, Parrish WR, Rosas-Ballina M, Ochani M, Puerta M, Ochani K, Chavan S, Al-Abed Y, Tracey KJ. Brain acetylcholinesterase activity controls systemic cytokine levels through the cholinergic anti-inflammatory pathway. Brain Behav Immun 23: 41–45, 2009. doi: 10.1016/j.bbi.2008.06.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Pavlov VA, Tracey KJ. The cholinergic anti-inflammatory pathway. Brain Behav Immun 19: 493–499, 2005. doi: 10.1016/j.bbi.2005.03.015. [DOI] [PubMed] [Google Scholar]
- 47.Pavlov VA, Tracey KJ. Neural circuitry and immunity. Immunol Res 63: 38–57, 2015. doi: 10.1007/s12026-015-8718-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Pavlov VA, Tracey KJ. Neural regulation of immunity: molecular mechanisms and clinical translation. Nat Neurosci 20: 156–166, 2017. doi: 10.1038/nn.4477. [DOI] [PubMed] [Google Scholar]
- 49.Pavlov VA, Tracey KJ. The vagus nerve and the inflammatory reflex—linking immunity and metabolism. Nat Rev Endocrinol 8: 743–754, 2012. doi: 10.1038/nrendo.2012.189. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Pocai A, Lam TK, Gutierrez-Juarez R, Obici S, Schwartz GJ, Bryan J, Aguilar-Bryan L, Rossetti L. Hypothalamic KATP channels control hepatic glucose production. Nature 434: 1026–1031, 2005. doi: 10.1038/nature03439. [DOI] [PubMed] [Google Scholar]
- 51.Pocai A, Obici S, Schwartz GJ, Rossetti L. A brain-liver circuit regulates glucose homeostasis. Cell Metab 1: 53–61, 2005. doi: 10.1016/j.cmet.2004.11.001. [DOI] [PubMed] [Google Scholar]
- 52.Reuben A, Koch DG, Lee WM; Acute Liver Failure Study Group . Drug-induced acute liver failure: results of a U.S. multicenter, prospective study. Hepatology 52: 2065–2076, 2010. doi: 10.1002/hep.23937. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Richter WO, Geiss HC, Aleksic S, Schwandt P. Cardiac autonomic nerve function and insulin sensitivity in obese subjects. Int J Obes Rel Metab Disord 20: 966–969, 1996. [PubMed] [Google Scholar]
- 54.Rogers RC, Hermann GE. Central connections of the hepatic branch of the vagus nerve: a horseradish peroxidase histochemical study. J Auton Nerv Syst 7: 165–174, 1983. doi: 10.1016/0165-1838(83)90044-9. [DOI] [PubMed] [Google Scholar]
- 55.Rosas-Ballina M, Olofsson PS, Ochani M, Valdés-Ferrer SI, Levine YA, Reardon C, Tusche MW, Pavlov VA, Andersson U, Chavan S, Mak TW, Tracey KJ. Acetylcholine-synthesizing T cells relay neural signals in a vagus nerve circuit. Science 334: 98–101, 2011. doi: 10.1126/science.1209985. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Rosas-Ballina M, Valdés-Ferrer SI, Dancho ME, Ochani M, Katz D, Cheng KF, Olofsson PS, Chavan SS, Al-Abed Y, Tracey KJ, Pavlov VA. Xanomeline suppresses excessive proinflammatory cytokine responses through neural signal-mediated pathways and improves survival in lethal inflammation. Brain Behav Immun 44: 19–27, 2015. doi: 10.1016/j.bbi.2014.07.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Satapathy SK, Ochani M, Dancho M, Hudson LK, Rosas-Ballina M, Valdes-Ferrer SI, Olofsson PS, Harris YT, Roth J, Chavan S, Tracey KJ, Pavlov VA. Galantamine alleviates inflammation and other obesity-associated complications in high-fat diet-fed mice. Mol Med 17: 599–606, 2011. doi: 10.2119/molmed.2011.00083. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Seyedabadi M, Rahimian R, Ghia JE. The role of α7 nicotinic acetylcholine receptors in inflammatory bowel disease: involvement of different cellular pathways. Expert Opin Ther Targets 22: 161–176, 2018. doi: 10.1080/14728222.2018.1420166. [DOI] [PubMed] [Google Scholar]
- 59.Shapiro RE, Miselis RR. The central organization of the vagus nerve innervating the stomach of the rat. J Comp Neurol 238: 473–488, 1985. doi: 10.1002/cne.902380411. [DOI] [PubMed] [Google Scholar]
- 60.Steinebrunner N, Mogler C, Vittas S, Hoyler B, Sandig C, Stremmel W, Eisenbach C. Pharmacologic cholinesterase inhibition improves survival in acetaminophen-induced acute liver failure in the mouse. BMC Gastroenterol 14: 148, 2014. doi: 10.1186/1471-230X-14-148. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.The F, Cailotto C, van der Vliet J, de Jonge WJ, Bennink RJ, Buijs RM, Boeckxstaens GE. Central activation of the cholinergic anti-inflammatory pathway reduces surgical inflammation in experimental post-operative ileus. Br J Pharmacol 163: 1007–1016, 2011. doi: 10.1111/j.1476-5381.2011.01296.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Tracey KJ. The inflammatory reflex. Nature 420: 853–859, 2002. doi: 10.1038/nature01321. [DOI] [PubMed] [Google Scholar]
- 63.Travagli RA, Hermann GE, Browning KN, Rogers RC. Brainstem circuits regulating gastric function. Annu Rev Physiol 68: 279–305, 2006. doi: 10.1146/annurev.physiol.68.040504.094635. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Vergoni AV, Marrama D, Guarini S, Tagliavini S, Bazzani C, Maugeri A, Bertolini A. Afferent vagal fibres and central cholinergic mechanisms are involved in the TRH-induced reversal of haemorrhagic shock. Pharmacol Res 23: 271–278, 1991. doi: 10.1016/S1043-6618(05)80086-6. [DOI] [PubMed] [Google Scholar]
- 65.Verheijden S, Boeckxstaens GE. Neuroimmune interaction and the regulation of intestinal immune homeostasis. Am J Physiol Gastrointest Liver Physiol 314: G75–G80, 2018. doi: 10.1152/ajpgi.00425.2016. [DOI] [PubMed] [Google Scholar]
- 66.Waldburger JM, Boyle DL, Edgar M, Sorkin LS, Levine YA, Pavlov VA, Tracey K, Firestein GS. Spinal p38 MAP kinase regulates peripheral cholinergic outflow. Arthritis Rheum 58: 2919–2921, 2008. doi: 10.1002/art.23807. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Wang H, Yu M, Ochani M, Amella CA, Tanovic M, Susarla S, Li JH, Wang H, Yang H, Ulloa L, Al-Abed Y, Czura CJ, Tracey KJ. Nicotinic acetylcholine receptor α7 subunit is an essential regulator of inflammation. Nature 421: 384–388, 2003. doi: 10.1038/nature01339. [DOI] [PubMed] [Google Scholar]
- 68.Wang X, Yang Z, Xue B, Shi H. Activation of the cholinergic antiinflammatory pathway ameliorates obesity-induced inflammation and insulin resistance. Endocrinology 152: 836–846, 2011. doi: 10.1210/en.2010-0855. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Willemze RA, Welting O, van Hamersveld HP, Meijer SL, Folgering JHA, Darwinkel H, Witherington J, Sridhar A, Vervoordeldonk MJ, Seppen J, de Jonge WJ. Neuronal control of experimental colitis occurs via sympathetic intestinal innervation. Neurogastroenterol Motil 30: 2018. doi: 10.1111/nmo.13163. [DOI] [PubMed] [Google Scholar]
- 70.Woolf NJ, Butcher LL. Cholinergic systems mediate action from movement to higher consciousness. Behav Brain Res 221: 488–498, 2011. doi: 10.1016/j.bbr.2009.12.046. [DOI] [PubMed] [Google Scholar]
- 71.Wu R, Dong W, Ji Y, Zhou M, Marini CP, Ravikumar TS, Wang P. Orexigenic hormone ghrelin attenuates local and remote organ injury after intestinal ischemia-reperfusion. PLoS One 3: e2026, 2008. doi: 10.1371/journal.pone.0002026. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Yang YP, Yang J. Galantamine protects against hydrochloric acid aspiration-induced acute respiratory distress syndrome in rabbits. Trop J Pharm Res 17: 17, 2018. [Google Scholar]
- 73.Yuan PQ, Taché Y. Abdominal surgery induced gastric ileus and activation of M1-like macrophages in the gastric myenteric plexus: prevention by central vagal activation in rats. Am J Physiol Gastrointest Liver Physiol 313: G320–G329, 2017. doi: 10.1152/ajpgi.00121.2017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Zaghloul N, Addorisio ME, Silverman HA, Patel HL, Valdés-Ferrer SI, Ayasolla KR, Lehner KR, Olofsson PS, Nasim M, Metz CN, Wang P, Ahmed M, Chavan SS, Diamond B, Tracey KJ, Pavlov VA. Forebrain cholinergic dysfunction and systemic and brain inflammation in murine sepsis survivors. Front Immunol 8: 1673, 2017. doi: 10.3389/fimmu.2017.01673. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.Zhang J, Zhang L, Sun X, Yang Y, Kong L, Lu C, Lv G, Wang T, Wang H, Fu F. Acetylcholinesterase inhibitors for Alzheimer’s disease treatment ameliorate acetaminophen-induced liver injury in mice via central cholinergic system regulation. J Pharmacol Exp Ther 359: 374–382, 2016. doi: 10.1124/jpet.116.233841. [DOI] [PubMed] [Google Scholar]