Abstract
Cirrhosis is associated with a systemic proinflammatory milieu, endotoxemia, and gut dysbiosis. The oral cavity could be an additional source of inflammation. We aimed to determine the effect of periodontal therapy in cirrhosis through evaluating endotoxemia, inflammation, cognition, and quality of life (QOL). Age-matched cirrhotic and noncirrhotic subjects exhibiting chronic gingivitis and/or mild or moderate periodontitis underwent periodontal therapy with follow-up at 30 days. Saliva/stool for microbial composition and serum for Model for End-stage Liver Disease (MELD) score, endotoxin and lipopolysaccharide binding protein (LBP) and immune-inflammatory markers (IL-1β; IL-6; histatins 1, 3, 5; and lysozyme) were collected at baseline and day 30. The cognitive function and QOL were also evaluated similarly. A separate group of cirrhotic patients were followed for the same duration without periodontal therapy. Cirrhotics, especially those with hepatic encephalopathy (HE), demonstrated improved dysbiosis in stool and saliva, and improved endotoxin, LBP, and salivary and serum inflammatory mediators following periodontal therapy. These parameters, which were higher in HE at baseline, became statistically similar posttherapy. Pretherapy vs. posttherapy QOL and cognition also improved in HE patients following oral interventions. On the other hand, LBP and endotoxin increased over time in cirrhotic patients not receiving therapy, but the rest of the parameters, including microbiota remained similar over time in the no-therapy group. This proof-of-concept study demonstrates that periodontal therapy in cirrhosis, especially in those with HE, is associated with improved oral and gut dysbiosis, systemic inflammation, MELD score, and cognitive function, which was not observed in those who did not receive therapy over the same time period.
NEW & NOTEWORTHY Systematic periodontal therapy in cirrhotic outpatients improved endotoxemia, as well as systemic and local inflammation, and modulated salivary and stool microbial dysbiosis over 30 days. This was associated with improved quality of life and cognition in patients with prior hepatic encephalopathy. In a cirrhotic group that was not provided periodontal therapy, there was an increase in endotoxin and lipopolysaccharide binding protein in the same duration. The oral cavity could be an important underdefined source of inflammation in cirrhosis.
Keywords: cognition, hepatic encephalopathy, inflammation, microbiota, quality of life
INTRODUCTION
Cirrhosis and chronic liver diseases are one of the leading causes of morbidity and mortality (41). Most of these negative outcomes in cirrhosis are due to development of complications such as hepatic encephalopathy (HE) and infections. The development of these complications is linked with a systemic proinflammatory milieu and altered gut microbiota through endotoxemia (42). Therefore, treatments for cirrhosis complications are focused on resolving gut dysbiosis, but endotoxemia and systemic inflammation often persist despite these standard therapies (39). Complicating the picture is the use of nonabsorbable and systemic antibiotics in cirrhotic patients. Periodontitis is one of the most common inflammatory diseases worldwide, which leads to destruction of tooth-supporting structures (15). Although a dysbiotic biofilm structure initiates the disease, the periodontal tissue destruction occurs as a result of dysregulated immune response to the microbial insult (19). Chronic, persistent immune responses to this complex microbiome not only can lead to tooth loss, but are also associated with increased risk for several systemic complications (13, 20). The proposed mechanisms linking periodontal disease and systemic conditions include shared risk factors, direct effects of oral bacteria, and continuous exposure to inflammatory mediators. Recent evidence has also showed that cirrhotic patients have an altered salivary microbiota and inflammation, which has led to the hypothesis of an oral-gut-hepatic axis (4, 33). Patients with cirrhosis had a higher predicted functionality related to endotoxemia in their salivary microbiota compared with healthy controls (4). There are also reports showing that periodontal diseases are associated with negative outcomes in cirrhotic patients who use alcohol and tobacco (17, 34). Increased inflammation has also been linked to HE and cognitive dysfunction in cirrhosis (40). Therefore, the oral cavity could be a target to reduce the systemic inflammatory burden and potentially future negative outcomes.
We hypothesized that the oral tissue microenvironment, including oral microbiota and inflammation, will be associated with the changes in gut microbiota and endotoxemia. The impact of the modification of the oral-gut-hepatic axis on systemic inflammation, endotoxemia, cognitive function, quality of life, and hospitalizations was studied in patients with cirrhosis and healthy controls after periodontal therapy and compared with a group of cirrhotic patients not undergoing oral interventions as a proof of concept.
MATERIALS AND METHODS
Study subjects.
Consecutive outpatients with cirrhosis and age-balanced healthy controls were included. All eligible subjects were cirrhotic patients or healthy controls who were diagnosed with chronic generalized gingivitis and/or mild-to-moderate chronic periodontitis (36). We excluded subjects who were actively drinking or using tobacco products, those with unstable clinical courses, inability to provide consent, with dentures/partials or without 20 natural teeth, and those with severe cavitated lesions and severe periodontitis. All detailed criteria are in Table 1. They were recruited from the Virginia Commonwealth University (VCU) Medical Center and McGuire VA Medical Center Hepatology Clinics (cirrhotic patients) and through word of mouth (healthy controls). All subjects underwent serum, saliva, and stool collection at baseline following dental examination (36). Only HE patients on stable lactulose and rifaximin and those who could give informed consent (mini-mental status exam >25) were included. This was a nonrandomized, open-label trial of periodontal therapy with respect to the clinical providers (clinical hepatology, periodontics, and research coordinators).
Table 1.
Eligibility criteria
| Inclusions | Exclusions |
|---|---|
| • Age 21–75 yr able to give informed consent (mini-mental status exam score >25)• Cirrhosis diagnosed using biopsy, fibroscan or radiological features or endoscopic evidence of varices in a subject with chronic liver disease | By history or initial examination: • Unclear diagnosis of cirrhosis, MELD score ≥25 • Absorbable antibiotics or probiotics within 4 wk • Smoking, oral tobacco, and alcohol abuse within 3 mo |
| • Mild-to-moderate gingivitis or periodontitisFor patients with HE: | • Edentulous or less than 20 natural teeth, those requiring prophylactic antibiotics before periodontal therapy |
| • Controlled on lactulose and rifaximin for at least 3 mo | • Prisoners |
| • Pregnant women | |
| • Diagnosed bleeding disorders or on anti- coagulation | |
| • INR >1.5 or platelet count <50,000 within 1 mo | |
| • Uncontrolled diabetes (on insulin or HgbA1c>7 within 3 mo) | |
| • End-stage diseases (COPD on home oxygen, congestive heart failure, dialysis, disseminated cancer) | |
| • Unlikely to follow- up in the opinion of the PI | |
| • Last periodontal prophylactic therapy within 3 mo | |
| • HIV infection | |
| • Last hospitalization within 1 mo | |
| • Undergoing or cleared HCV in the prior month | |
| By screening dental examination for all subjects: | |
| • Severe periodontitis | |
| • ≥2 Severe cavitated caries lesions | |
| • Oral soft-tissue lesions such as ulcers or presence of abscesses |
COPD, chronic obstructive pulmonary disease; HCV, hepatitis C virus; HE, hepatic encephelopathy; HIV, human immunodeficiency virus; INR, international normalized ratio; MELD, Model for End-Stage Liver Disease.
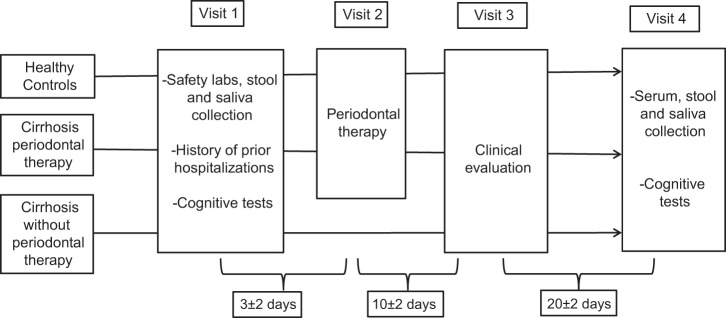
After informed consent, all subjects in the above two groups received periodontal therapy by a calibrated periodontist, which consisted of prophylaxis or scaling and root planing followed by oral hygiene instructions at the VCU Dental School (36). Patients with abscesses, severe periodontitis, and severe cavitated lesions were excluded. Subjects were followed at day 10 for safety, day 30 for safety and for serum, saliva, and stool collection, and at day 90 to evaluate for safety and hospitalizations (Fig. 1). The composition of oral and gut microbiome was assessed in saliva and stool. Cognitive testing consisting of PHES (psychometric hepatic encephalopathy score) (46), Stroop EncephalApp (3), and health-related quality of life (HRQOL) testing using Sickness Impact Profile (SIP) was also performed at days 0 and 30 (9, 32).
Fig. 1.
Schematic of the study design.
An additional cirrhotic outpatient group that fulfilled all of the criteria of those given periodontal therapy was also included and followed for the same duration without any periodontal therapy.
The Psychometric Hepatic Encephalopathy Score (46) (PHES) is a paper-and-pencil battery test, including Number Connection Test-A/B (NCT-A/B; in which subjects “join the dots” between numbers or numbers and letters in a timed fashion). The Digit Symbol Test (DST) is a test in which subjects need to pair numbers with special symbols correctly within 120 s. In the Line Tracing Test (LTT), subjects trace a line between two parallel lines, and the time required is noted (errors were not recorded at all sites). In the Serial Dotting Test (SDT), subjects need to dot the center of a group of blank circles. A high score on DST and low score on the rest indicates good performance. Different versions of these tests were given at baseline and retesting visits.
The EncephalApp Stroop test (3) was administered using standard iPod screens. The task has two components: “Off” and “On” state depending on the discordance or concordance of the stimuli. Both components were administered after two training runs. In the easier “Off” state, the subject views a neutral stimulus, and pound signs (###) are presented in red, green or blue, one at a time, and the subject has to respond as quickly as possible by touching the matching color of the stimulus to the colors displayed at the bottom of the screen. The colors at the bottom of the screen are also randomized and not fixed to their respective positions. This continues until a total of 10 presentations are completed correctly, which is one run. Outcome values are included and the total time taken for the run, as well as the individual responses. If the subject makes a mistake, i.e., presses a wrong color, the run stops and has to restart again. Therefore, the number of runs required to make five correct runs also indicates the number of mistakes. We continued the off state until the subject had achieved five correct runs. The “On” state is more challenging from a cognitive standpoint in that incongruent stimuli are presented in 9 of the 10 stimuli. In this portion, the subject has to accurately touch the color of the word presented, which is actually the name of the color in discordant coloring, i.e., the word “RED” is displayed in blue color and the correct response is blue, not red. Like the “off” state, we gave two training runs and then continued the task until five correct runs were achieved. The specific outcomes at the end of the EncephalApp app were 1) total time for five correct runs in the “off” state (OffTime), 2) number of runs needed to complete the five correct “Off” runs, 3) total time for five correct runs in the “on” state (OnTime), and 4) number of runs needed to complete the five correct “On” runs. Offtime+Ontime has been found in prior EncephalApp studies to be the best discriminator and was used to determine cognition.
The Sickness Impact Profile (SIP) (9) is a validated health-related quality of life (QOL) questionnaire. This consists of 136 items. There are two domains: psychosocial and physical, which are made of 12 subscales: sleep and rest, eating, work, home management, recreation and pastimes, ambulation, mobility, body care and movement, social interaction, alertness, emotional behavior, and communication. Subjects are asked to mark only those questions that are pertinent to their health over the past 24 h. The number of questions are weighted and divided from a total number to achieve a percentage that translates into the ultimate measure. A high score indicates poor health-related quality of life.
The trial is registered at https://clinicaltrials.gov/number NCT03030820.
Statistics.
Statistical analysis was performed using traditional parametric (paired t-tests) and nonparametric (Wilcoxon signed-rank paired tests) for pretherapy and posttherapy analyses. Unpaired t-tests, ANOVA, and Kruskal-Wallis tests were used to compare clinical variables between subject groups as needed. A P value of 0.05 was considered significant, and data are presented as means ± SD or median and interquartile range.
Inflammatory analysis.
Serum samples were analyzed for endotoxin using LAL assay and LBP, IL-1 β, and IL-6 using ELISA using published techniques (Assaygate, Ijamsville, MD) (6). Salivary inflammatory cytokines (IL-1β and IL-6), histatins 1, 3, and 5, and lysozyme were also determined using published techniques (Assaygate) (4). Serum analysis was also performed in the cohort that was studied twice without interventions.
Microbiota analysis.
Microbial DNA was isolated from saliva and stool samples, as previously described (26).
Bacterial ribosomal RNA gene sequencing.
The V1 and V2 variable regions of the bacterial 16S ribosomal RNA (rRNA) gene were sequenced using Multitag fusion primers and sequenced on an Ion Torrent Personal Genome Machine next-generation sequencer.
Bioinformatics analysis.
16S rRNA gene sequence data were used for bioinformatics analysis. Fasta files were demultiplexed using custom PERL scripts, and sequences were filtered for quality scores and length. The clean 16S sequences were clustered into operational taxonomic units (OTUs) using the USEARCH algorithm (14). A sequence identity of 97% was used to generate OTUs representing distinct bacterial species. The taxonomic identity of reference sequences was determined using the RDP11 Classifier (45). We used the George Mason University Metabiome Portal to organize raw data, track clinical metadata, and track analysis. The portal consists of a Drupal-based interface wrapped around a MYSQL database that uses PHP to manage the relational database. The system has built-in safeguards to curate the data, maintain security, and to ensure quality control. We then analyzed the data for this project through these pipelines and distributed the data through this interface.
Microbial biostatistical analysis.
Bacterial community composition was characterized using OTU counts generated as described above. OTU counts were converted to measures of relative abundance to account for variation in sequencing coverage between samples. Statistical analysis was carried out using the statistical software package R (www.r-project.org). Changes in abundance of individual taxa were analyzed using traditional univariate nonparametric statistical methods and UniFrac (28). We used LEfSe (linear discriminant analysis effect size) to determine the microbial taxa most likely to explain differences between and within groups after periodontal therapy (38). In addition, we focused on oral-origin taxa (Streptococcaceae, Veillonellaceae, and Porphyromonadaceae), autochthonous taxa (Lachnospiraceae and Ruminococcaceae) and potentially pathogenic taxa (Pasteurellaceae and Enterobacteriaceae) in pretesting and posttesting.
The microbiota in the stool and saliva were also compared before and after follow-up in the cohort that was followed without intervention. The protocol was approved by the Institutional Review Board at Virginia Commonwealth University and McGuire VA Medical Center, and all subjects gave informed consent before study procedures.
RESULTS
Subject characteristics.
For the periodontal therapy group, 72 subjects were considered between December 2016 to November 2017, of which 22 were excluded (14 had less than 20 natural teeth, 5 refused to participate, 2 had other medical problems that would impair adherence). The remaining 50 subjects (30 cirrhosis and 20 noncirrhotic controls) underwent dental examination.
Four cirrhotic patients had exclusions (three severe periodontitis and one severe cavities) noted during the dental examination. The demographics and details of cirrhosis are shown in Table 2. The cirrhotic patients included were statistically similar to controls in age and sex. Baseline cognitive performance in cirrhotic patients was like controls on PHES (psychometric hepatic encephalopathy score), but worse on EncephalApp Stroop (Table 2).
Table 2.
Baseline values of cohorts included in the study
| Cirrhosis Observed without Therapy | Cirrhosis with Periodontal Therapy | Controls with Periodontal Therapy | |
|---|---|---|---|
| N | 24 | 26 | 20 |
| Age | 61.2 ± 4.6 | 60.1 ± 12.4 | 56.6 ± 7.4 |
| Sex | 16/8 | 17/9 | 12/8 |
| Prior smoking | 7 | 7 | 0 |
| Prior alcohol abuse | 6 | 6 | 0 |
| Gingivitis/Peridontitis | 11/13 | 10/16 | 11/9 |
| Diabetes | 14 | 14 | 0 |
| Etiology (HCV, Alcohol, Alcohol+HCV, NASH, others) | 5/6/5/8/0 | 7/5/6/8/0 | |
| Prior HE | 10 | 10 | |
| On lactulose | 10 | 10 | |
| On rifaximin | 10 | 10 | |
| Prior ascites | 8 | 7 | |
| Prior variceal bleeding | 4 | 4 | |
| WBC count | 5.81 ± 1.9 | 6.12 ± 1.90 | 7.12 ± 1.7 |
| Platelet count | 138.7 ± 79.3# | 148.5 ± 51.9# | 235.1 ± 35.4 |
| INR | 1.32 ± 0.65# | 1.36 ± 0.29# | 1.08 ± 0.23 |
| MELD score | 9.8 ± 3.0 | 10.5 ± 4.2 | |
| PHES total score (median IQR) | −2.0 (−5.0, 1.0)# | −0.5 (−3.0, 2.0) | 1.0 (−1.0, 2.0) |
| EncephalApp Stroop Off+On Time, s | 185.9 ± 34.2# | 181.7 ± 45.5# | 146.6 ± 16.9 |
| SIP total | 10.4 ± 5.66# | 9.2 ± 8.1# | 0.13 ± 2.0 |
| SIP physical | 7.8 ± 9.92# | 7.2 ± 9.4# | 0.11 ± 0.24 |
| SIP psychosocial | 11.1 ± 12.42# | 10.6 ± 16.2# | 0.34 ± 0.9 |
| Serum values | |||
| Endotoxin, EU/ml, median IQR | 0.30 (0.32)# | 0.21 (0.11)# | 0.11 (0.05) |
| Lipopolysaccharide binding protein, ng/ml, median IQR | 316 (1428)# | 478 (829) | 112 (1921) |
| IL-6, pg/ml, median IQR | 5.7 (6.2)# | 4.9 (7.0)# | 1.1 (1.4) |
| IL-1β, pg/ml, median IQR | 0.49 (0.39)# | 0.54 (0.42) | 0.51 (0.42) |
No significant differences were found between cirrhotic patients who ultimately underwent periodontal therapy compared with those who did not. SIP denotes Sickness Impact Profile; a higher score indicates poor health-related quality of life. PHES denotes Psychometric Hepatic Encephalopathy Score; a low score indicates poor cognitive function. A high score on Stroop EncephalApp indicates poor cognition. HE, hepatic encephelopathy; HCV, hepatitis C virus; INR, international normalized ratio; IQR, interquartile range; NASH, nonalcoholic steatohepatitis; WBC, white blood cell.
P < 0.05 in controls vs. compared cirrhosis group using Mann-Whitney U-test or unpaired t-test based on data.
A separate group of cirrhotic subjects who fulfilled the criteria as the ones undergoing periodontal therapy was recruited between September 2017 and March 2018. This group of 24 cirrhotic patients had statistically similar clinical scores, demographics, cirrhosis severity, prior HE and decompensation, cognitive and HRQOL performance at baseline compared with the cirrhotic patients who underwent the periodontal therapy (Table 2). This cohort was similarly worse off compared with controls on HRQOL and EncephalApp Stroop.
Baseline dental examination.
Patients with cirrhosis with a higher mean plaque score (64.8 ± 20.2 vs. 48.6 ± 21.9, P = 0.05) and mean pocket depth (2.8 ± 0.5 vs. 2.4 ± 0.28, P = 0.05) and mean clinical attachment level (2.5 ± 1.2 vs. 1.3 ± 1.1, P = 0.007) and a trend toward higher bleeding on probing (25.9 ± 18.7 vs. 17.7 ± 13.1, P = 0.18) compared with controls. The relative presence of gingivitis and periodontitis was similar between groups and shown in Table 1. The procedures were performed safely and tolerated well in those in the periodontal therapy group.
In cirrhotic patients ultimately undergoing periodontal therapy, at baseline, the WBC count was similar between cirrhotic patients with and without HE (6.1 ± 1.2 vs. 6.2 ± 2.2, P = 0.98). As expected, the endotoxin levels (0.22 ± 0.05 vs. 0.15 ± 0.04, P = 0.04, Fig. 3A) and Model for End-stage Liver Disease (MELD) scores (13.2 ± 3.1 vs. 7.8 ± 1.7, P = 0.02) were higher in cirrhotic patients with HE compared with those without HE. Also, HE patients had a worse cognitive performance on PHES [median (interquartile range, IQR) −3.0 (−4.0, −1.0) HE vs. 1.0 (−2.0, 2.0) non-HE, P = 0.002] and EncephalApp Stroop Off+Ontime seconds (217.3 ± 45.0 HE vs. 156.9 ± 29.1 non-HE, P = 0.001, Fig. 2, A and B). As expected, HE patients had a significantly worse QOL compared with non-HE patients on total SIP (HE: 11.2 ± 5.3 vs. non-HE: 7.8 ± 9.0, P = 0.03), psychosocial domain (HE: 12.1 ± 20.7 vs. non-HE: 9.4 ± 3.5, P = 0.03), and physical domains (HE: 10.8 ± 12.5 vs. 4.7 ± 6.0, P = 0.01, Fig. 2C).
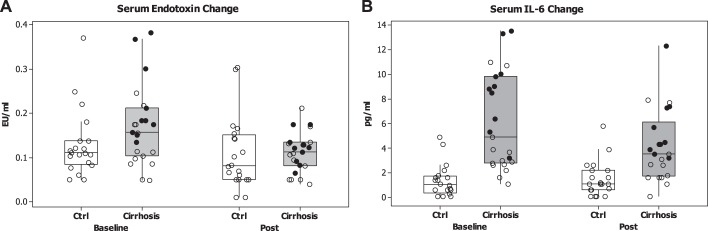
Fig. 3.
Changes in systemic inflammation after periodontal therapy. Data are presented as medians and 95% CI with individual data points. In the cirrhosis groups, the black dots are patients with HE (hepatic encephalopathy), while the others are without HE. A: change in serum endotoxin before or after periodontal therapy shows reduction in the cirrhosis group, and the HE group (P = 0.02, Wilcoxon matched-pairs test). Control values remained statistically similar. B: change in serum IL-6 before or after periodontal therapy shows reduction in the cirrhosis group, and the HE group (P = 0.42, Wilcoxon matched-pairs test). Control IL-6 levels were similar before or after therapy.
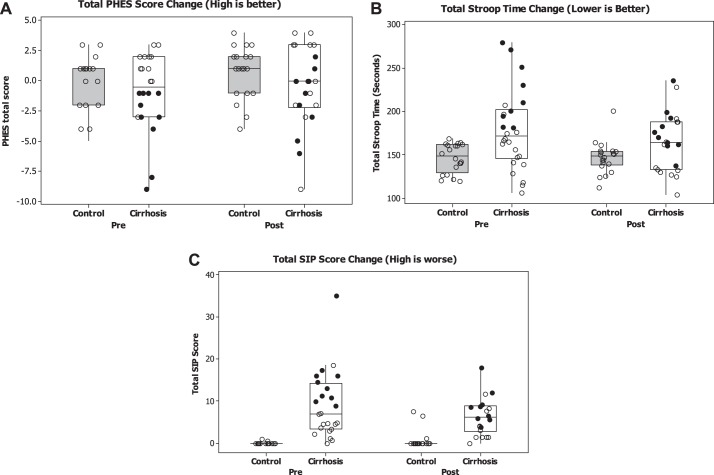
Fig. 2.
Changes in cognition and health-related quality of life after periodontal therapy. Data are presented as median and 95% CI with individual data points. In the cirrhosis groups, the black dots are patients with HE (hepatic encephalopathy), while the others are without HE. A: change in Psychometric Hepatic Encephalopathy Score (PHES) before/after periodontal therapy shows improvement in the cirrhosis, particularly the HE group (P < 0.05, Wilcoxon matched-pairs test). A higher PHES score indicates better performance. Control values were similar pretherapy/posttherapy. B: change in EncephalApp OffTime+OnTime before and after periodontal therapy shows improvement in the cirrhosis, particularly the HE group (P < 0.05, Wilcoxon matched-pairs test). A higher OffTime+OnTime value indicates worse performance. Control values were similar before and after therapy. C: change in Sickness Impact Profile (SIP) total values before or after periodontal therapy shows improvement in the cirrhosis, particularly the HE group (P < 0.05, Wilcoxon matched-pairs test). A higher SIP total value indicates worse health-related quality of life. Control values were similar before and after therapy.
Follow-up inflammatory changes.
All subjects who received periodontal therapy returned for follow-up at day 30 posttherapy. There was no change in the underlying cirrhosis status. In addition, none of the subjects was prescribed antibiotics, developed HE, used probiotics, or were hospitalized during that 30-day period. There was a significant improvement in the MELD score, IL-6, IL-1β, WBC count, and endotoxin levels between the visits for the cirrhotic subjects. After periodontal therapy, the endotoxin levels and MELD score became statistically similar between cirrhotic patients with and without HE. On salivary markers, there was no change in lysozyme or histatin with therapy, but a significant decrease in salivary IL-1β was noted in cirrhotic patients, especially in those with HE (Table 3 and Fig. 3). The coefficient of variation for endotoxin values was 8%, for LBP, it was 7.4%, for IL-6, it was 7.5%, and for IL-1β, it was 8.4%.
Table 3.
Changes in clinical and inflammatory markers pre/post periodontal therapy
| Cirrhosis |
Controls |
|||
|---|---|---|---|---|
| Pretherapy | 30-Day Post | Pretherapy | 30-Day Post | |
| WBC count | 6.11 ± 1.90 | 5.34 ± 1.85* | 7.12 ± 1.7 | 7.01 ± 1.5 |
| MELD score | 10.4 ± 4.3 | 8.7 ± 3.2* | ||
| PHES total score (median IQR) | −0.5 (−3.0, 2.0) | 0.0 (−2.0,3.0)* | 1.0 (−1.0, 2.0) | 1.0 (−2.0, 2.0) |
| SIP total | 9.1 ± 7.96# | 7.7 ± 8.1#* | 0.13 ± 2.0 | 0.8 ± 2.3 |
| SIP physical | 7.0 ± 9.3# | 4.8 ± 5.4#* | 0.11 ± 0.24 | 0.11 ± 0.45 |
| SIP psychosocial | 10.4 ± 16.3# | 5.5 ± 7.7#* | 0.34 ± 0.9 | 0.51 ± 1.65 |
| Serum values | ||||
| Endotoxin, EU/ml, median IQR | 0.21 (0.11)# | 0.11 (0.05)* | 0.11 (0.05) | 0.08 (0.10) |
| LBP, ng/ml, median IQR | 478 (829)# | 330 (1,114) | 112 (1,921) | 122 (1,955) |
| IL-6, pg/ml, median IQR | 4.9 (7.0)# | 3.5 (4.4)# | 1.1 (1.4) | 1.1 (1.6) |
| IL-1β, pg/ml, median IQR | 0.54 (0.42) | 0.35 (0.40)#* | 0.51 (0.42) | 0.54 (0.42) |
| Stroop Off+On Time, s | 178.7 ± 45.3# | 165.0 ± 33.7#* | 146.6 ± 16.9 | 147.1 ± 18.3 |
| Salivary values | ||||
| Lysozyme, ng/ml | 47,421 (71, 732) | 47,933 (64, 097) | 29,328 (69, 541) | 28,300 (75, 734) |
| Histatin 1, µg/ml | 5.0 (7.2) | 4.5 (9.5)# | 6.2 (19.6) | 10.9 (18.4) |
| Histatin 3, µg/ml | 429.5 (267.3) | 420.6 (178.2)# | 498.4 (360.5) | 523.1 (252.7) |
| Histatin 5, µg/ml | 6.2 (1.8)# | 5.9 (2.8)# | 4.7 (3.3) | 4.9 (3.1) |
| IL-6, pg/ml, median IQR | 6.4 (8.3) | 5.0 (10.2) | 7.2 (9.7) | 7.4 (10.8) |
| IL-1β, pg/ml, median IQR | 111.3 (328.3)# | 92.7 (248.1) | 68.0 (112.1) | 60.3 (109.8) |
All data are presented as means ± SD unless noted otherwise. These data pertain to the group that received periodontal therapy. IQR, interquartile range; LBP, LPS binding protein; MELD, Model for End-stage Liver Disease; PHES, psychometric hepatic encephalopathy score; SIP, Sickness Impact Profile; WBC, white blood cell. High score indicates a worse quality of life. Posttherapy indicates 30 days after periodontal therapy.
P < 0.05, pre vs. post Wilcoxon signed-rank sum tests for median and paired t-test for means.
P < 0.05, cirrhosis vs. controls using Mann-Whitney U-test for medians and unpaired t-test for means.
In patients who were followed without periodontal therapy, there was again no change in the underlying cirrhosis status or hospitalizations within those 30 days. However, there was a significant increase in endotoxin levels [pretreatment: 0.30 (0.32) vs. posttreatment: 0.62 (0.34), P = 0.001) and LBP levels [316 (143) vs. 414 (168), P = 0.03] over time in these patients. The WBC count (5.81 ± 1.9 vs. 6.02 ± 2.1), MELD score (9.8 ± 3.0 vs. 9.4 ± 3.6), International Normalized Ratio (INR; 1.32 ± 0.65 vs. 1.35 ± 0.48), or platelet count (138.7 ± 79.3 vs. 136.4 ± 64.2) remained similar between the visits. Serum IL-6 [5.7 (6.2) vs. 4.3 (5.9)] or IL-1β [0.49 (0.39) vs. 0.48 (0.32)] was similar between visits.
Effect on cognition and HRQOL.
There was a significant improvement in PHES and Stroop Off+OnTime scores in cirrhotic patients after periodontal therapy (Fig. 2, A and B). This was accompanied also by an improvement in total, physical, and psychosocial domains of the SIP (Fig. 2C). There was a significant improvement in cognition in HE patients after periodontal therapy on PHES [median (IQR) −3.0 (−4.0, − 1.0) pretreatment vs. posttreatment −2.0 (−3.0, 0.0), P = 0.05] and EncephalApp Stroop Off+Ontime seconds (pretreatment 217.3 ± 45.0 vs. posttreatment 194.1 ± 29.2. P = 0.03). The change in non-HE patients was not significant on PHES [median (IQR), pretreatment: 1.0 (−1.0, 2.0) vs. posttreatment: 1.0 (−1.0, 3.0), P = 0.09] or EncephalApp (pretreatment: 156.9 ± 29.1 vs. posttreatment: 152.2 ± 27.4, P = 0.35). There were significant improvements in HRQOL parameters after periodontal therapy in HE patients on total SIP (pretreatment: 11.2 ± 5.3 vs. posttreatment: 7.2 ± 2.9, P = 0.03), psychosocial domain (pretreatment: 12.1 ± 20.7 vs. posttreatment: 5.1 ± 2.8, P = 0.01), and physical domains (HE 10.8 ± 12.5 vs. 5.4 ± 2.8, P = 0.001). There was a nonsignificant trend toward QOL improvement in non-HE patients on total SIP (pretreatment: 7.80 ± 9.03 vs. posttreatment: 7.2 ± 9.6, P = 0.23), and physical domain (pretreatment: 4.7 ± 6.0 vs. posttreatment: 4.6 ± 7.0, P = 0.83), but there was an improvement in the psychosocial domain (pretreatment: 9.4 ± 13.8 vs. posttreatment: 5.7 ± 9.2, P = 0.04). No changes in healthy controls were seen on these parameters before or after therapy given the normal performance at baseline (Table 4).
Table 4.
Changes in serum and salivary inflammatory markers in cirrhotic patients with and without HE pre/post periodontal therapy
| Cirrhosis without HE |
Cirrhosis with HE |
|||
|---|---|---|---|---|
| Pretherapy | 30-day Post | Pretherapy | 30-day Post | |
| Serum | ||||
| Endotoxin, EU/ml | 0.17 (0.10) | 0.12 (0.08)* | 0.23 (0.06) | 0.10 (0.04)* |
| LBP, ng/ml | 491 (945) | 313 (1,100) | 347 (1,322) | 264 (1,187)* |
| IL-6, pg/ml | 3.6 (6.4) | 3.2 (3.9) | 8.8 (6.1)# | 4.3 (5.8)* |
| IL-1b, pg/ml | 0.37 (0.40) | 0.32 (0.40) | 0.72 (0.41) | 0.67 (0.42) |
| Saliva | ||||
| Lysozyme, ng/ml | 45,390 (56,966) | 46,009 (58,913) | 66,107 (93,190) | 50,918 (65,273) |
| Histatin 1, µg/ml | 4.3 (6.9) | 4.6 (9.5) | 7.6 (14.3) | 3.3 (17.1) |
| Histatin 3, µg/ml | 391.0 (360.6) | 404.5 (172.9) | 431.0 (247.0) | 475.4 (199.9) |
| Histatin 5, µg/ml | 6.7 (1.8) | 6.4 (2.6) | 5.7 (3.2) | 5.6 (2.4) |
| IL-6, pg/ml | 4.5 (7.4) | 6.7 (8.2) | 10.6 (10.5) | 12.6 (14.0) |
| IL-1β, pg/ml | 187.9 (272.3) | 173.6 (342.9) | 83.2 (99.1)# | 36.8 (98.9)#* |
Data are presented as medians (interquartile range, IQR); n = 16 and 10 patients without and with HE, respectively. These data are for the group that received periodontal therapy. Posttherapy is 30 days after periodontal therapy. LBP, LPS binding protein.
P < 0.05, pre vs. post-Wilcoxon signed-rank sum tests.
P < 0.05, HE vs. non-HE using the Mann-Whitney U-test.
In the group that did not receive interventions, cognitive dysfunction and HRQOL remained similarly affected between visits: PHES [−2.0 (−5.0, 1.0) vs. −2.0 (−4.5, 1.0)], or EncephalApp Stroop (185.9 ± 34.2 vs. 178.6 ± 45.5), pre vs. post total SIP (10.4 ± 5.66 vs. 9.9 ± 7.0), psychosocial SIP (11.1 ± 12.42 vs. 10.7 ± 10.68), and physical SIP (7.8 ± 9.92 vs. 7.4 ± 9.01).
Microbiota changes at baseline.
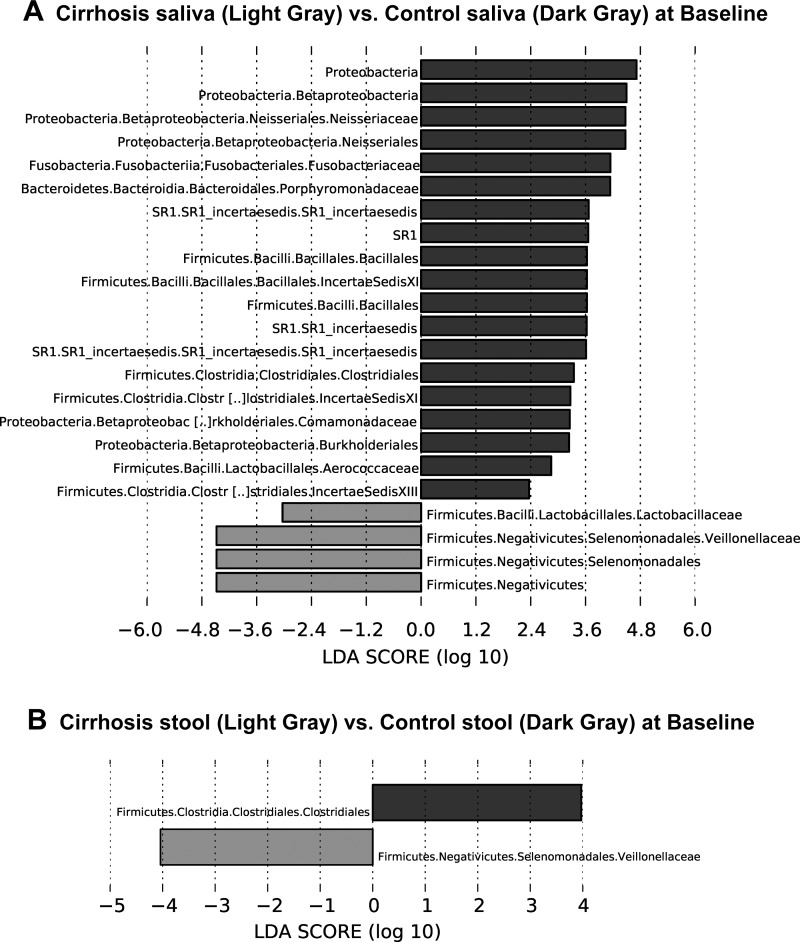
In cirrhotic patients who ultimately underwent periodontal therapy and in controls using UniFrac, there was a significant difference in β-diversity at baseline between controls vs. cirrhosis stool and salivary microbiota (all, P < = 1.0e-02). On LEFSe, the taxa that were higher in cirrhotic patients’ saliva at baseline compared with controls were related to Veillonellaceae and Lactobacillaceae, while a large swathe of microbiota, including Porphyromonadaceae, Neissareaceae, Clostridia, and Fusobacteriaceae were less abundant (Fig. 4A). Interestingly similar changes in Veillonellaceae and Clostridiales were observed in the stool, as found in the saliva at baseline between groups (Fig. 4B).
Fig. 4.
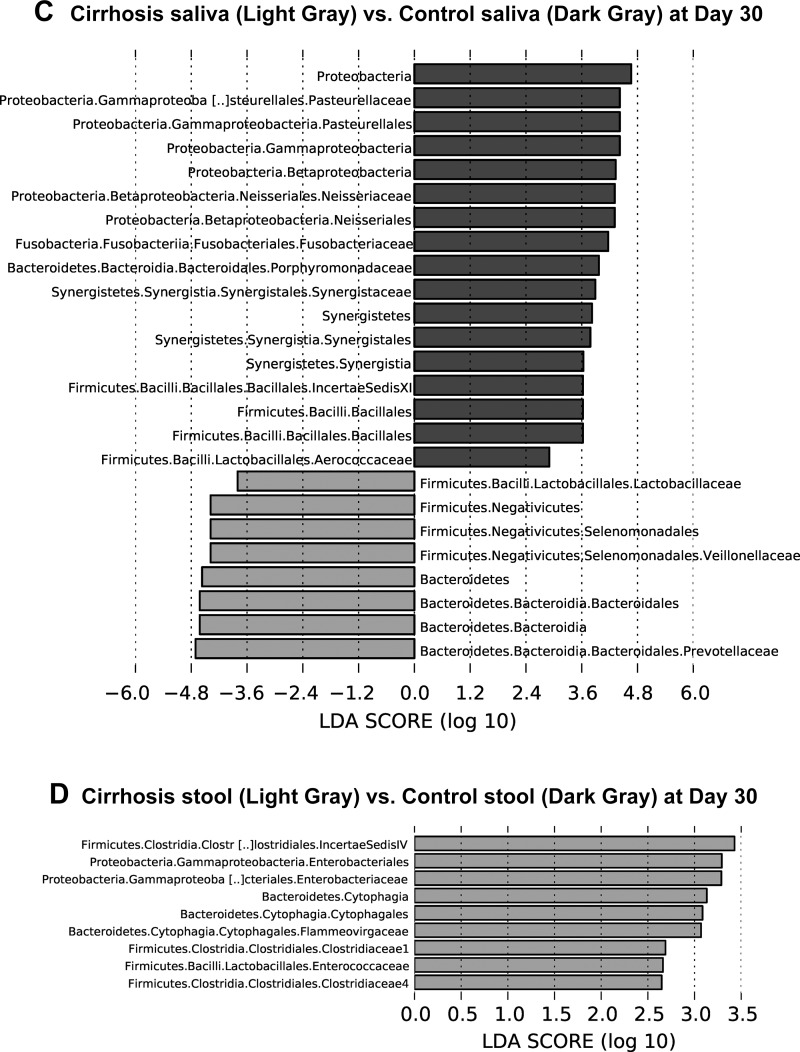
Linear discriminant function effect size (LEfSe) comparisons of microbiota family-level changes between groups and tissues. The x-axis is the log change in linear discriminant function between the groups displayed. A: changes between Cirrhosis saliva (light gray) and Control saliva (dark gray) at baseline. B: changes between Cirrhosis stool (light gray) and Control stool (dark gray) at baseline. C: changes between Cirrhosis saliva (light gray) vs. Control saliva (dark gray) at day 30. D: changes between Cirrhosis stool vs. Control stool (light gray) at day 30 only showed a decrease in taxa shown in cirrhosis and an increase in controls.
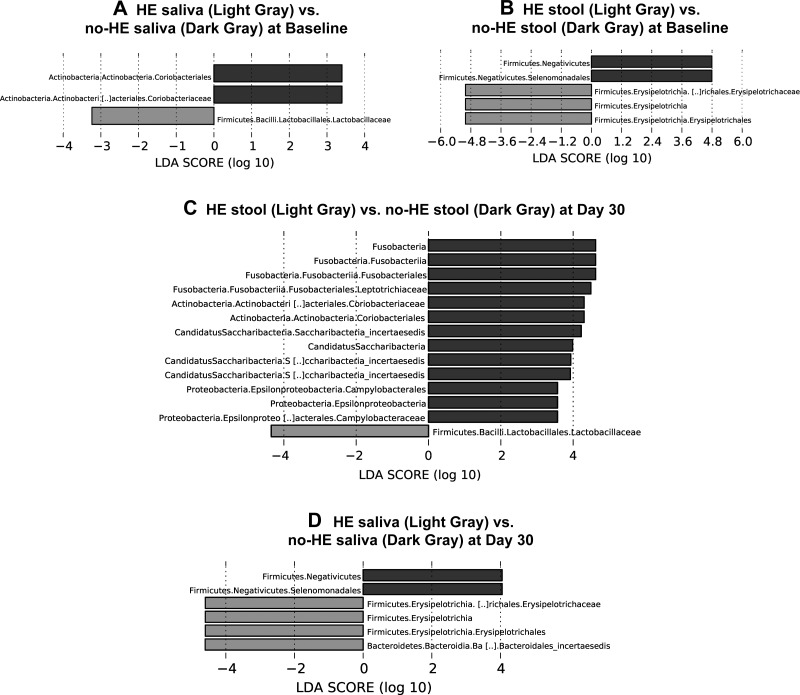
Using UniFrac there was a significant difference in β-diversity at baseline between cirrhotic patients with/without HE on stool, and salivary microbiota (all, P < = 1.0e-02). Specific taxa higher in HE patients’ saliva were Enterococcaceae, Lactobacillaceae, and lower Coriobacteriaceae compared with the cirrhotics with non-HE (Fig. 5A). Stool samples from HE patients also exhibited differences with higher abundance of Erysipelothriceaeae and lower Selomonadales and Veillonellaceae (Fig. 5B) compared with non-HE patients. We studied the cirrhosis dysbiosis ratio (CDR) and salivary dysbiosis ratio before and after therapy in HE and non-HE patients (4, 6). There was a significantly worse (lower) salivary CDR (HE: 1.1 ± 0.7 vs. non-HE 1.79 ± 1.1, P = 0.05) and CDR (HE: 0.59 ± 0.17 vs. non-HE 2.1 ± 1.2, P = 0.001) in HE patients pretherapy compared with those without HE. After therapy, there was a significant improvement (increase) in salivary CDR (pretreatment: 1.1 ± 0.7 vs. posttreatment: 1.78 ± 0.90, P = 0.05) and CDR (pretreatment: 0.59 ± 0.17 vs. posttreatment: 2.3 ± 2.1, P = 0.03) in HE patients but no significant change in non-HE patients.
Fig. 5.
Linear discriminant function effect size (LEfSe) comparisons of microbiota family-level changes between cirrhotic patients with and without hepatic encephalopathy (HE). The x-axis is the log change in linear discriminant function between the groups displayed. HE patients are always displayed in light gray, while cirrhotic patients without HE are displayed in dark gray. A: changes between HE saliva (light gray) vs. non-HE saliva (dark gray) at baseline. B: changes between HE stool (light gray) vs. non-HE stool (dark gray) at baseline. C: changes between HE stool (light gray) vs. non-HE stool (dark gray) at day 30. D: changes between HE saliva (light gray) vs. non-HE saliva (dark gray) at day 30.
Comparisons between groups changed 30 days after the periodontal therapy.
Using UniFrac, we found that there was a significant difference in β-diversity again at day 30 posttherapy between controls vs. cirrhosis stool and salivary microbiota (all, P < = 1.0e-02). On LEFSe, the taxa that were higher in cirrhotic patients’ saliva were similar to the baseline results displaying higher Veillonellaceae and Lactobacillaceae (Fig. 4C). In addition, Prevotellaceae also appeared in cirrhotic saliva at day 30. Saliva samples from noncirrhotic controls remained higher on Porphyromonadaceae, Neisseraceae, and Clostridia with a new increase in Pasteurellaceae and Synergesticaeae. Analyses of the cirrhotic samples at day 30 revealed significantly lower Enterobacteriaceae, Enterococcaceae, and Clostridiaceae compared with the controls (Fig. 4D). On UniFrac, there was a significant difference in β-diversity again at day 30 between cirrhotic patients with and without HE on stool and salivary microbiota (all, P < = 1.0e-02). Although higher Lactobacillaceae and lower Coriobacteriace levels observed at baseline in the saliva of HE patients remained similar at day 30, HE patients displayed significantly lower levels of Campylobacteriaceae and Fusobacteriaceae (Fig. 5C). At 30 days, the stool profile comparisons remained like baseline between patients with and without HE (Fig. 5D).
Changes in microbiota composition within groups after periodontal therapy.
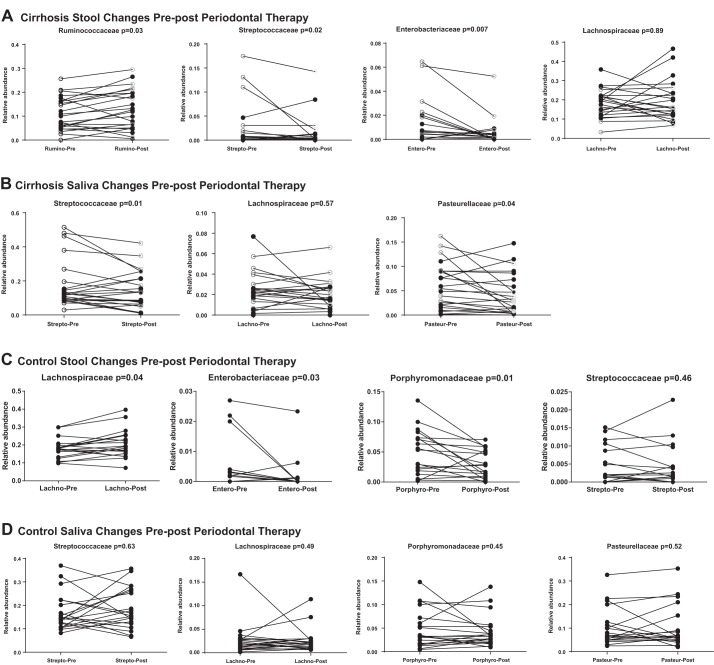
On Wilcoxon matched-pairs analysis, there were significant changes in cirrhosis stool with reduction in Enterobacteriaceae and Streptococcaceae and increase in Ruminococcaceae, which were more prominent in HE patients (Fig. 6A). This was accompanied by a reduction in cirrhosis salivary Pasteurellaceae and Streptococcaceae (Fig. 6B), which was again higher in HE patients. In controls, there were nonsignificant changes in saliva (Fig. 6D), but stool was again beneficially affected by decreased Enterobacteriaceae and Porphyromonadaceae and higher relative abundance of Lachnospiraceae (Fig. 6C).
Fig. 6.
Prominent microbiota family changes before and 30 days after periodontal therapy. Individual data points for subjects pre and post are displayed, and statistics employed are Wilcoxon-matched pair rank tests for family-level relative abundance. P values for this comparison are displayed in the title of each subpart of the figure. A: cirrhosis stool changes showed significant reduction in the relative abundance of Enterobacteriaceae and Streptococcaceae and increase in Ruminococcaceae in the entire group and in hepatic encephalopathy (HE) patients specifically. Lachnospiraceae were unaffected. HE patients are marked by ○, while the remaining are patients without HE. B: cirrhosis saliva changes showed significant reduction in the relative abundance of Streptococcaceae and Pasteurellaceae in the entire group and in hepatic encephalopathy (HE) patients specifically. Lachnospiraceae were unaffected. HE patients are marked by ○, while the remaining are patients without HE. C: control stool changes showed a significant reduction in the relative abundance of Porphyromonadaceae and Enterobacteriaceae and an increase in Lachnospiraceae. No changes in Streptococcaceae were seen. D: control saliva changes did not show any significant change in Lachnospiraceae, Pasteurellaceae, Streptococcaceae, or Porphyromonadaceae. E: cirrhosis stool changes in patients who did not receive periodontal therapy. There were no statistically significant changes in the relative abundance of specific taxa of interest in the entire group and in HE. HE patients are marked by ○, while the remaining are patients without HE. F: cirrhosis saliva changes in patients who did not receive periodontal therapy. There were no statistically significant changes in the relative abundance of specific taxa of interest in the entire group and in HE. HE patients are marked by ○, while the remaining are patients without HE.
Comparison within cirrhotic group followed without intervention.
On UniFrac there were no changes in β-diversity at day 30 compared with baseline on either stool or salivary microbiota. Similarly, there were no changes on LEFSe in saliva or stool between visits. In addition, on Wilcoxon matched-pairs analysis, none of the specific autochthonous taxa nor oral-origin taxa changes between visits (Fig. 6, E and F) regardless of HE status.
DISCUSSION
Cirrhosis and its complications are linked with gut microbial dysbiosis, endotoxemia, and systemic inflammation. However, despite treatments directed toward the gut milieu, patients with cirrhosis continue to exhibit systemic inflammation and endotoxemia, which can predict negative outcomes, such as hospitalizations, cognitive impairment, and psychological effects. While the oral cavity has been implicated in the liver health and inflammation in prior studies, the specific determinants and the effect of oral interventions on these changes are unclear (1, 2, 18). The current proof-of-concept study aimed to systemically characterize the effect of periodontal therapy on oral and systemic microbiota and immune and inflammatory markers and assess the cognitive and psychological effects in a well-defined group of patients with and without cirrhosis and comparing them to cirrhotic patients not subjected to the interventions.
Our results revealed a relationship between the oral cavity and the gut microbiota that can be modulated by changing the oral milieu. Specifically, we were able to demonstrate that periodontal therapy can reduce endotoxemia and systemic inflammation, as well as salivary inflammation in cirrhotic patients, especially those with HE. Indeed, the analysis showed favorable changes with higher relative abundance of autochthonous taxa (Ruminococcaceae and Lachnospiraceae) and reduction in potentially pathogenic (Enterobacteriaceae) and oral-origin taxa (Porphyromonadaceae and Streptococcaceae) in stools of controls and cirrhotic patients, especially those with HE. In addition, the salivary microbiota significantly changed beneficially over time within cirrhotic patients with lower relative abundance of endotoxin-producing Pasteurellaceae and also Streptococcaceae (31). There were persistent differences between groups at baseline and day 30 but largely salivary and stool microbial changes in cirrhotic patients represented improvement at day 30 compared with their baseline differences, especially in those with HE, who had worse dysbiosis at baseline. Importantly, in a similar outpatient cirrhotic group followed over 30 days, there was a significant increase in serum endotoxin and LBP without periodontal therapy, which was improved in the periodontal therapy group.
The potential mechanisms behind the change in the oral and gut microbial composition after periodontal therapy could be related to a reduction of oral inflammation leading to lower systemic inflammation, or due to lower inflammatory burden being swallowed and affecting the gut microbial milieu. Indeed, in prior studies, introduction of a periodontal bacteria, Porphyromonas gingivalis, led to hepatic inflammation in germ-free mice; however, this phenomenon is not specific because it can also be caused by Enterococcus fecalis (27, 30). Moreover, given the impaired local and systemic immune system and the lack of development of periodontitis, the germ-free mice may not be an ideal model to study these interactions (24).
The route of reduction of oral and systemic inflammation that enhances gut microbiota composition by improving the overall inflammatory milieu may be the likely mechanism.
In addition to the microbiota, there was a lowering of the MELD score, and a significant improvement in cognitive performance and HRQOL in HE patients. The results are in direct support of a prior study that linked oral dysbiosis and subsequent hospitalizations and reveal for the first time that periodontal interventions may potentially benefit adverse clinical outcomes in cirrhotic patients (4). Our findings underscore the importance of targeting all sources of systemic inflammation and endotoxemia in cirrhosis. Of note, the patients with prior HE, had higher endotoxemia despite being on modulators of the gut milieu (6). Following periodontal therapy, endotoxins, IL-6 and IL-1β levels in this group of patients returned to the levels of the patients without HE. This observation implies that in addition to liver disease severity, endotoxemia and systemic inflammation are likely mediated by other sources, such as the oral cavity, which persists despite treating the unfavorable gut milieu. Also as a counterbalance, the endotoxin and LBP levels increased in the group with cirrhosis that did not receive periodontal therapy, likely reflecting the underlying nature of the ongoing inflammatory process.
These findings are even more striking, given that we excluded patients with severe periodontitis and those who were smokers or actively abusing alcohol, which are all factors associated with unfavorable dental clinical outcomes regardless of cirrhosis (16, 34). Therefore, an improvement in oral and gut dysbiosis, improvement in liver disease severity and a decrease in systemic inflammation and endotoxemia that persist several days after periodontal therapy suggests a clinically relevant interrelationship between these physiological systems. It is intriguing that inflammatory improvement in controls was not expected. A recent study has found that the subgingival microbiota in cirrhosis is different in those with cirrhosis and periodontitis compared with noncirrhotic periodontitis patients (22). We could hypothesize that the changes because of oral interventions could have a greater impact on cirrhotic patients rather than controls with a relatively lower impact on systemic inflammation. This would also be explained by the fact that we excluded active alcohol use, smoking, severe periodontitis, yet allowed gingivitis patients.
As prior research has suggested, there is a multidimensional impairment in the immune-inflammatory system in cirrhosis, which results in a global mucosal-immune change (5, 37). This is reflected in dysbiosis in stool, large and small intestine, liver, serum, and saliva in cirrhotic patients (4, 7, 10, 37). In prior reports, the innate defenses of the saliva, including histatin and lysozyme, were impaired in cirrhotic patients along with local inflammatory activation (5). Following periodontal interventions, we observed a reduction in salivary IL-1β in cirrhosis patients with HE. Yet, there was no change in salivary histatin, lysozyme, or IL-6. Although the mechanism of these differential changes is unclear, it is likely that periodontal therapy may favor enhancement of the local humoral immune response. Therefore, further studies are warranted to understand disease pathophysiology at a molecular level and fully elucidate the mechanistic link between liver and oral diseases.
Although it is crucial to define the key players related to host and microbiome to develop better treatment strategies, translation to the clinics requires elicitation of patient-reported outcomes (23). Therefore, we also assessed the cognitive performance and HRQOL in our study population. Our results demonstrated significant improvement in two separate cognitive testing strategies, PHES and EncephalApp Stroop, in HE patients after periodontal therapy without any other changes in their underlying HE episodes or therapy (3, 46). This was not observed to a significant degree in cirrhotic patients without HE and healthy controls who were not cognitively impaired or had issues with HRQOL and remained similar in cirrhotic patients who were not treated. Given prior literature about impaired learning capacity in patients with prior HE (8, 35), this improvement is unlikely due to repeated exposures to the tests and potentially linked with improvement in the overall inflammatory milieu. The improvement in HRQOL and cognition in HE patients, who were already on lactulose and rifaximin, is relevant because there are no further U.S. Food and Drug Administration-approved therapies for alleviating cognition in this population in the United States (44). However, even though the tests and HRQOL values improved in prior HE patients, they only reached the non-HE patients and not the control values. This underlines the importance of the continued liver disease-related inflammation that persists despite reducing the oral contribution.
It is also important to note that the periodontal disease cohort included in the current study consisted of patients with chronic gingivitis, which is a disease characterized by gingival inflammation without alveolar bone loss and chronic mild-to-moderate periodontitis, which is the inflammation of gingival tissues accompanied by minimal bone loss. Both of these conditions can be managed by nonsurgical periodontal therapy, which was delivered in this study. On the contrary, the clinical management of severe forms of periodontal diseases usually requires more advanced procedures, including surgical interventions to control inflammation. Further, it is the severe and persistent forms of the disease that are associated with numerous other conditions through increased local and systemic inflammatory markers. Yet, despite the exclusion of patients with severe periodontal disease, alcohol, and tobacco use, we found significant changes in cirrhotic patients following oral interventions. However, while the MELD score and white blood cell (WBC) count changes were statistically significant, the clinical significance of this change magnitude is low, likely due to the fact that the baseline inflammation related to periodontal disease was low (12, 42). In fact, we also noted only very minor changes in oral and stool dysbiosis or local/systemic inflammation in the noncirrhotic controls despite periodontal therapy, which is consistent with the notion that more comprehensive oral interventions targeting severe forms of the periodontal diseases would have a more pronounced effect on systemic and subsequent cirrhosis outcomes. In noncirrhotic patients, gut microbial manipulation using probiotics can favorably change oral microbiota and local inflammation (25, 43). However, evidence supporting this in cirrhosis is needed. Therefore, further studies are warranted, including larger samples and severe cases to fully elucidate the impact of oral interventions on cirrhosis.
Our current results serve as proof of concept linking oral-gut-hepatic axis with statistically significant reductions in MELD and WBC count. Yet, clinical significance of our observations needs to be further tested in future clinical trials, and mechanistic studies, including severe periodontitis cases. The current study, if confirmed in larger numbers, will likely have significant implications for clinical practice. First, regular oral health care is not covered routinely in some public health care systems, such as the U.K. National Health Service or the U.S. Veterans Affairs, leading to a relative neglect of this important source of inflammation (11, 21). Second, all HE patients were already on the nonabsorbable rifaximin and lactulose, despite which, there was cognitive impairment and systemic inflammation compared with non-HE patients who showed significant cognitive improvement following oral interventions (44). Lastly, the need for dental evaluation and therapy in potential liver transplant candidates has already been established, which now potentially needs to be extended to all cirrhotic patients (29).
One of the limitations of our study was that the posttherapy dental clinical parameters were not assessed. Bleeding on probing could be explained partly by the low platelet count in cirrhosis, but ultimately, this platelet count and INR do not typically require additional correction in dental procedures. This was also not a randomized trial, but the cirrhotic patients who did not receive periodontal therapy were similar to the cirrhotic group that received this therapy. Improvements in HRQOL could be reflected by the greater visit frequency and knowledge of active dental interventions in affected subjects, but this did not improve in the group that was not given the periodontal therapy. There is an inherent variability in LAL assays for endotoxin, but the relatively narrow coefficient of variation and the similar trends found in LBP point toward endotoxin level alterations being reflective of underlying biological change.
In summary, we conclude that periodontal therapy in cirrhotic patients is associated with a reduction in endotoxemia, systemic and local inflammation, HRQOL, and cognitive performance against a background of oral and gut microbial modulation. The oral cavity could represent a treatment target to reduce inflammation and endotoxemia in patients with cirrhosis to improve clinical outcomes. Larger-scale, randomized, placebo-controlled trials are needed to define the impact of systematic oral interventions on clinical outcomes.
GRANTS
This article is supported through VA Merit Review Grant I0CX001076 and National Center for Advancing Translational Sciences grant R21TR002024 to J. S. Bajaj and National Institute of Dental and Craniofacial Research Grant R01DE025037 to S. E. Sahingur.
DISCLOSURES
No conflicts of interest, financial or otherwise, are declared by the authors.
AUTHOR CONTRIBUTIONS
J.S.B. and S.E.S. conceived and designed research; J.S.B., A.F., J.G.D., C.A., S.s.D., M.S., P.M.G., and S.E.S. analyzed data; J.S.B., P.M., A.F., J.G.D., C.A., and S.E.S. interpreted results of experiments; J.S.B. and M.S. prepared figures; J.S.B. and P.M.G. drafted manuscript; J.S.B., P.M., J.G.D., C.A., M.S., P.M.G., and S.E.S. edited and revised manuscript; J.S.B., P.M., M.B.W., A.F., J.G.D., C.A., S.s.D., M.S., P.M.G., and S.E.S. approved final version of manuscript; P.M., M.B.W., A.F., J.G.D., C.A., S.s.D., M.S., P.M.G., and S.E.S. performed experiments.
REFERENCES
- 1.Åberg F, Helenius-Hietala J, Meurman J, Isoniemi H. Association between dental infections and the clinical course of chronic liver disease. Hepatol Res 44: 349–353, 2014. doi: 10.1111/hepr.12126. [DOI] [PubMed] [Google Scholar]
- 2.Acharya C, Sahingur SE, Bajaj JS. Microbiota, cirrhosis, and the emerging oral-gut-liver axis. JCI Insight 2: 94416, 2017. doi: 10.1172/jci.insight.94416. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Allampati S, Duarte-Rojo A, Thacker LR, Patidar KR, White MB, Klair JS, John B, Heuman DM, Wade JB, Flud C, O’Shea R, Gavis EA, Unser AB, Bajaj JS. Diagnosis of minimal hepatic encephalopathy using Stroop encephalApp: a multicenter US-based, norm-based study. Am J Gastroenterol 111: 78–86, 2016. doi: 10.1038/ajg.2015.377. [DOI] [PubMed] [Google Scholar]
- 4.Bajaj JS, Betrapally NS, Hylemon PB, Heuman DM, Daita K, White MB, Unser A, Thacker LR, Sanyal AJ, Kang DJ, Sikaroodi M, Gillevet PM. Salivary microbiota reflects changes in gut microbiota in cirrhosis with hepatic encephalopathy. Hepatology 62: 1260–1271, 2015. doi: 10.1002/hep.27819. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Bajaj JS, Heuman DM, Hylemon PB, Sanyal AJ, White MB, Monteith P, Noble NA, Unser AB, Daita K, Fisher AR, Sikaroodi M, Gillevet PM. Altered profile of human gut microbiome is associated with cirrhosis and its complications. J Hepatol 60: 940–947, 2014. doi: 10.1016/j.jhep.2013.12.019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Bajaj JS, Hylemon PB, Ridlon JM, Heuman DM, Daita K, White MB, Monteith P, Noble NA, Sikaroodi M, Gillevet PM. Colonic mucosal microbiome differs from stool microbiome in cirrhosis and hepatic encephalopathy and is linked to cognition and inflammation. Am J Physiol Gastrointest Liver Physiol 303: G675–G685, 2012. doi: 10.1152/ajpgi.00152.2012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Bajaj JS, Schubert CM, Heuman DM, Wade JB, Gibson DP, Topaz A, Saeian K, Hafeezullah M, Bell DE, Sterling RK, Stravitz RT, Luketic V, White MB, Sanyal AJ. Persistence of cognitive impairment after resolution of overt hepatic encephalopathy. Gastroenterology 138: 2332–2340, 2010. doi: 10.1053/j.gastro.2010.02.015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Bergner M, Bobbitt RA, Carter WB, Gilson BS. The Sickness Impact Profile: development and final revision of a health status measure. Med Care 19: 787–805, 1981. doi: 10.1097/00005650-198108000-00001. [DOI] [PubMed] [Google Scholar]
- 10.Chen Y, Yang F, Lu H, Wang B, Chen Y, Lei D, Wang Y, Zhu B, Li L. Characterization of fecal microbial communities in patients with liver cirrhosis. Hepatology 54: 562–572, 2011. doi: 10.1002/hep.24423. [DOI] [PubMed] [Google Scholar]
- 11.Chief Business Office HEC Dental Benefits for Veterans. https://www.va.gov/healthbenefits/resources/publications/IB10-442_dental_benefits_for_veterans_2_14.pdf. [August 1, 2018].
- 12.Coltart I, Tranah TH, Shawcross DL. Inflammation and hepatic encephalopathy. Arch Biochem Biophys 536: 189–196, 2013. doi: 10.1016/j.abb.2013.03.016. [DOI] [PubMed] [Google Scholar]
- 13.Crump KE, Sahingur SE. Microbial nucleic acid sensing in oral and systemic diseases. J Dent Res 95: 17–25, 2016. doi: 10.1177/0022034515609062. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Edgar RC. Search and clustering orders of magnitude faster than BLAST. Bioinformatics 26: 2460–2461, 2010. doi: 10.1093/bioinformatics/btq461. [DOI] [PubMed] [Google Scholar]
- 15.Eke PI, Thornton-Evans GO, Wei L, Borgnakke WS, Dye BA. Accuracy of NHANES periodontal examination protocols. J Dent Res 89: 1208–1213, 2010. doi: 10.1177/0022034510377793. [DOI] [PubMed] [Google Scholar]
- 16.Grønkjær LL. Periodontal disease and liver cirrhosis: A systematic review. SAGE Open Med 3: 2050312115601122, 2015. doi: 10.1177/2050312115601122. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Grønkjær LL, Holmstrup P, Schou S, Schwartz K, Kongstad J, Jepsen P, Vilstrup H. Presence and consequence of tooth periapical radiolucency in patients with cirrhosis. Hepat Med 8: 97–103, 2016. doi: 10.2147/HMER.S113485. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Guggenheimer J, Eghtesad B, Close JM, Shay C, Fung JJ. Dental health status of liver transplant candidates. Liver Transpl 13: 280–286, 2007. doi: 10.1002/lt.21038. [DOI] [PubMed] [Google Scholar]
- 19.Hajishengallis G. Immunomicrobial pathogenesis of periodontitis: keystones, pathobionts, and host response. Trends Immunol 35: 3–11, 2014. doi: 10.1016/j.it.2013.09.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Hajishengallis G. Periodontitis: from microbial immune subversion to systemic inflammation. Nat Rev Immunol 15: 30–44, 2015. doi: 10.1038/nri3785. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Heister D, Brewer JB, Magda S, Blennow K, McEvoy LK; Alzheimer’s Disease Neuroimaging Initiative . Predicting MCI outcome with clinically available MRI and CSF biomarkers. Neurology 77: 1619–1628, 2011. doi: 10.1212/WNL.0b013e3182343314. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Jensen A, Ladegaard Grønkjær L, Holmstrup P, Vilstrup H, Kilian M. Unique subgingival microbiota associated with periodontitis in cirrhosis patients. Sci Rep 8: 10718, 2018. doi: 10.1038/s41598-018-28905-w. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Kanwal F. Patient-reported outcomes of cirrhosis. Clin Gastroenterol Hepatol 11: 1043–1045, 2013. doi: 10.1016/j.cgh.2013.07.006. [DOI] [PubMed] [Google Scholar]
- 24.Kim SC, Tonkonogy SL, Albright CA, Tsang J, Balish EJ, Braun J, Huycke MM, Sartor RB. Variable phenotypes of enterocolitis in interleukin 10-deficient mice monoassociated with two different commensal bacteria. Gastroenterology 128: 891–906, 2005. doi: 10.1053/j.gastro.2005.02.009. [DOI] [PubMed] [Google Scholar]
- 25.Krasse P, Carlsson B, Dahl C, Paulsson A, Nilsson A, Sinkiewicz G. Decreased gum bleeding and reduced gingivitis by the probiotic Lactobacillus reuteri. Swed Dent J 30: 55–60, 2006. [PubMed] [Google Scholar]
- 26.Liu TC, Gurram B, Baldridge MT, Head R, Lam V, Luo C, Cao Y, Simpson P, Hayward M, Holtz ML, Bousounis P, Noe J, Lerner D, Cabrera J, Biank V, Stephens M, Huttenhower C, McGovern DP, Xavier RJ, Stappenbeck TS, Salzman NH. Paneth cell defects in Crohn’s disease patients promote dysbiosis. JCI Insight 1: e86907, 2016. doi: 10.1172/jci.insight.86907. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Llorente C, Jepsen P, Inamine T, Wang L, Bluemel S, Wang HJ, Loomba R, Bajaj JS, Schubert ML, Sikaroodi M, Gillevet PM, Xu J, Kisseleva T, Ho SB, DePew J, Du X, Sørensen HT, Vilstrup H, Nelson KE, Brenner DA, Fouts DE, Schnabl B. Gastric acid suppression promotes alcoholic liver disease by inducing overgrowth of intestinal Enterococcus. Nat Commun 8: 837, 2017. [Erratum in Nat Commun 8: 2137, 2017.] doi: 10.1038/s41467-017-00796-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Lozupone CA, Stombaugh JI, Gordon JI, Jansson JK, Knight R. Diversity, stability and resilience of the human gut microbiota. Nature 489: 220–230, 2012. doi: 10.1038/nature11550. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Martin P, DiMartini A, Feng S, Brown R Jr, Fallon M. Evaluation for liver transplantation in adults: 2013 practice guideline by the American Association for the Study of Liver Diseases and the American Society of Transplantation. Hepatology 59: 1144–1165, 2014. doi: 10.1002/hep.26972. [DOI] [PubMed] [Google Scholar]
- 30.Nakajima M, Arimatsu K, Kato T, Matsuda Y, Minagawa T, Takahashi N, Ohno H, Yamazaki K. Oral administration of P. gingivalis induces dysbiosis of gut microbiota and impaired barrier function leading to dissemination of enterobacteria to the liver. PLoS One 10: e0134234, 2015. doi: 10.1371/journal.pone.0134234. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Nava GM, Stappenbeck TS. Diversity of the autochthonous colonic microbiota. Gut Microbes 2: 99–104, 2011. doi: 10.4161/gmic.2.2.15416. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Prasad S, Dhiman RK, Duseja A, Chawla YK, Sharma A, Agarwal R. Lactulose improves cognitive functions and health-related quality of life in patients with cirrhosis who have minimal hepatic encephalopathy. Hepatology 45: 549–559, 2007. doi: 10.1002/hep.21533. [DOI] [PubMed] [Google Scholar]
- 33.Qin N, Yang F, Li A, Prifti E, Chen Y, Shao L, Guo J, Le Chatelier E, Yao J, Wu L, Zhou J, Ni S, Liu L, Pons N, Batto JM, Kennedy SP, Leonard P, Yuan C, Ding W, Chen Y, Hu X, Zheng B, Qian G, Xu W, Ehrlich SD, Zheng S, Li L. Alterations of the human gut microbiome in liver cirrhosis. Nature 513: 59–64, 2014. doi: 10.1038/nature13568. [DOI] [PubMed] [Google Scholar]
- 34.Raghava KV, Shivananda H, Mundinamane D, Boloor V, Thomas B. Evaluation of periodontal status in alcoholic liver cirrhosis patients: a comparative study. J Contemp Dent Pract 14: 179–182, 2013. doi: 10.5005/jp-journals-10024-1296. [DOI] [PubMed] [Google Scholar]
- 35.Riggio O, Ridola L, Pasquale C, Nardelli S, Pentassuglio I, Moscucci F, Merli M. Evidence of persistent cognitive impairment after resolution of overt hepatic encephalopathy. Clin Gastroenterol Hepatol 9: 181–183, 2011. doi: 10.1016/j.cgh.2010.10.002. [DOI] [PubMed] [Google Scholar]
- 36.Sahingur SE, Xia XJ, Voth SC, Yeudall WA, Gunsolley JC. Increased nucleic Acid receptor expression in chronic periodontitis. J Periodontol 84: e48–e57, 2013. doi: 10.1902/jop.2013.120739. [DOI] [PubMed] [Google Scholar]
- 37.Santiago A, Pozuelo M, Poca M, Gely C, Nieto JC, Torras X, Román E, Campos D, Sarrabayrouse G, Vidal S, Alvarado-Tapias E, Guarner F, Soriano G, Manichanh C, Guarner C. Alteration of the serum microbiome composition in cirrhotic patients with ascites. Sci Rep 6: 25001, 2016. doi: 10.1038/srep25001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Segata N, Izard J, Waldron L, Gevers D, Miropolsky L, Garrett WS, Huttenhower C. Metagenomic biomarker discovery and explanation. Genome Biol 12: R60, 2011. doi: 10.1186/gb-2011-12-6-r60. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Shawcross DL. Is it time to target gut dysbiosis and immune dysfunction in the therapy of hepatic encephalopathy? Expert Rev Gastroenterol Hepatol 9: 539–542, 2015. doi: 10.1586/17474124.2015.1035257. [DOI] [PubMed] [Google Scholar]
- 40.Shawcross DL, Davies NA, Williams R, Jalan R. Systemic inflammatory response exacerbates the neuropsychological effects of induced hyperammonemia in cirrhosis. J Hepatol 40: 247–254, 2004. doi: 10.1016/j.jhep.2003.10.016. [DOI] [PubMed] [Google Scholar]
- 41.Stepanova M, De Avila L, Afendy M, Younossi I, Pham H, Cable R, Younossi ZM. Direct and indirect economic burden of chronic liver disease in the United States. Clin Gastroenterol Hepatol, 15: 759–766.e5, 2017. doi: 10.1016/j.cgh.2016.07.020. [DOI] [PubMed] [Google Scholar]
- 42.Tandon P, Garcia-Tsao G. Bacterial infections, sepsis, and multiorgan failure in cirrhosis. Semin Liver Dis 28: 26–42, 2008. doi: 10.1055/s-2008-1040319. [DOI] [PubMed] [Google Scholar]
- 43.Twetman S, Derawi B, Keller M, Ekstrand K, Yucel-Lindberg T, Stecksen-Blicks C. Short-term effect of chewing gums containing probiotic Lactobacillus reuteri on the levels of inflammatory mediators in gingival crevicular fluid. Acta Odontol Scand 67: 19–24, 2009. doi: 10.1080/00016350802516170. [DOI] [PubMed] [Google Scholar]
- 44.Vilstrup H, Amodio P, Bajaj J, Cordoba J, Ferenci P, Mullen KD, Weissenborn K, Wong P. Hepatic encephalopathy in chronic liver disease: 2014 Practice Guideline by the American Association for the Study of Liver Diseases and the European Association for the Study of the Liver. Hepatology 60: 715–735, 2014. doi: 10.1002/hep.27210. [DOI] [PubMed] [Google Scholar]
- 45.Wang Q, Garrity GM, Tiedje JM, Cole JR. Naive Bayesian classifier for rapid assignment of rRNA sequences into the new bacterial taxonomy. Appl Environ Microbiol 73: 5261–5267, 2007. doi: 10.1128/AEM.00062-07. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Weissenborn K, Ennen JC, Schomerus H, Rückert N, Hecker H. Neuropsychological characterization of hepatic encephalopathy. J Hepatol 34: 768–773, 2001. doi: 10.1016/S0168-8278(01)00026-5. [DOI] [PubMed] [Google Scholar]