Abstract
Response inhibition (RI) is a component of the cognitive control systems that support optimal cognition. Cognitive control deficits are well-described in schizophrenia, but are not well characterized in individuals at clinical high risk (CHR) for developing psychosis. Functional magnetic resonance imaging during Go/NoGo task performance was collected from 30 CHR youth, 23 early illness schizophrenia patients (ESZ), and 72 healthy adolescents and young adults (HC). Voxelwise main effects of group were examined (P < .005 height threshold, family-wise error-corrected cluster threshold, P < .05) for correct NoGo-Go contrast values and task-based functional connectivity. CHR and ESZ groups had slower and more variable reaction times (RT) on Go trials compared to HCs. Significant main effects of group in bilateral dorsal anterior cingulate (dACC) and right inferior frontal cortex stemmed from CHR and ESZ groups showing significantly less NoGo-Go activation, relative to HCs. Faster responding HCs had less functional coupling between dACC and medial prefrontal cortex, a default mode network (DMN) region during NoGo vs Go trials. This functional connectivity–performance relationship was not present in ESZ or CHR groups. The pattern of findings suggests CHR and ESZ groups were deficient in developing strong and consistent prepotent responding, based on their slow and variable motor responses and decreased engagement of dACC and right inferior frontal regions implicated in inhibitory control. Furthermore, only the control group showed a functional connectivity relationship consistent with greater response prepotency requiring more decoupling of inhibitory control regions from DMN regions during RI.
Keywords: schizophrenia prodrome, ultra-high risk for psychosis, first-episode schizophrenia, motor disinhibition, prefrontal cortex, adolescent mental health
Introduction
Response inhibition (RI), the voluntary “top-down” suppression of motor actions, is part of the cognitive control systems enabling efficient, flexible behavior.1 Cognitive control is thought to be a primary function of the prefrontal cortex (PFC).2,3 Human lesion,4 functional magnetic resonance imaging (fMRI),1,5 and non-human primate studies6,7 implicate PFC, particularly ventrolateral regions (VLPFC), in inhibitory control. Right VLPFC, presupplementary and supplementary motor areas, striatum and thalamic/subthalamic nuclei contribute to the functional neuroanatomy of RI.1,5,8–10 Also relevant are anatomically well-defined cortico-striatal (“indirect”) and cortico-sub-thalamic (“hyperdirect”) circuits that inhibit motor output via thalamic modulation of cortex.1,11 FMRI-based connectivity studies corroborate cortico-striatal-thalamic functional connectivity during RI12 (though see Erika-Florence13). Contributions from dorsolateral PFC (DLPFC), anterior cingulate cortex (ACC), and posterior parietal cortex likely stem from additional task demands on working memory, sustained attention, and/or response selection.9,10,14
Cognitive deficits, particularly in PFC-mediated executive functions such as inhibitory control, are common in schizophrenia15–17 and are associated with negative symptoms, disorganization, and poor functioning.18 Such deficits may be intermediary phenotypic markers of psychosis vulnerability, given their attenuated presence in first-degree relatives of patients with schizophrenia.19 An fMRI meta-analysis of executive functioning reported hypoactivation in schizophrenia in DLPFC, ACC, and thalamus, implicating a generalized cognitive control deficit.20
Behavioral,21–23 electrophysiological,24–27 and fMRI24,27–29 techniques reveal alterations in temporal and spatial features of inhibition-related brain responses and behavior in schizophrenia. However, not all studies report motor disinhibition in schizophrenia,24,28–31 suggesting that deficient use of context to establish task-appropriate response tendencies, rather than poor inhibition, may explain observed RI alterations.24,30 This fits with well-documented deficits in context-guided behavior and accompanying brain alterations in patients with schizophrenia,15,16,32–34 unaffected siblings,35,36 and individuals at elevated risk for psychosis.37,38 Deficits in context processing and developing prepotent responding have a countervailing influence on inhibitory control because deficient use of task context to establish strong response prepotency results in less need for RI.
Most prior studies of RI in schizophrenia focused on chronically ill patients. Little is known about neural substrates relevant for establishing and inhibiting prepotent responses in patients closer to illness onset or at clinical high risk (CHR) for psychosis. Accordingly, we used a Go/NoGo paradigm that engages fronto-parietal regions in healthy individuals12,24,39 to assess RI-related brain functioning in early illness schizophrenia patients (ESZ) and CHR youth. Because CHR and ESZ populations differ in age, and RI maturation is expected during neurodevelopment,40–42 we recruited a large (n = 72) adolescent and young adult healthy comparison (HC) group to account for normal aging effects. We hypothesized CHR and ESZ groups would show decreased PFC activation during RI compared to HCs, with ESZ patients exhibiting more severe abnormalities. Further, to identify inhibition-related alterations in functional network dynamics, we analyzed task-based connectivity.
Methods
Participants
CHR participants (n = 30), recruited from Yale University’s early psychosis clinic, met the Criteria of Prodromal Syndromes (COPS) based on the Structured Interview for Prodromal Syndromes (SIPS).43,44 COPS criteria comprise attenuated psychotic symptoms (APS), brief intermittent psychotic states (BIPS), and genetic risk with functional deterioration. All CHR participants met APS criteria. Approximately 20%–35% of individuals designated as CHR develop a psychotic disorder within 2–3 years45,46 with the APS subgroup conferring less conversion risk than BIPS.46 Symptom severity in CHR patients was assessed with the SIPS Scale of Prodromal Symptoms (SOPS). None of the CHR participants were on antipsychotic medication (n = 0/30).
ESZ patients (n = 23) were recruited from the community, and similar to our prior studies,47–49 were within 5 years of illness onset (mean onset 1.98 years ± 1.41; range 0.4–5 years). Schizophrenia or schizoaffective disorder was confirmed using the Structured Clinical Interview for DSM-IV (SCID).50 Symptom severity was assessed with the Positive and Negative Syndrome Scale (PANSS).51 Most ESZ patients were on antipsychotic medication (n = 20/23).
HCs (n = 72) were recruited from the community. The SCID (or the Schedule for Affective Disorders and Schizophrenia for School-Age Children,52 if <16 years old) ruled out Axis I disorders. Across groups, good physical health, English fluency, and 11–30 years of age were required. Past-year substance dependence (excepting nicotine), head injury, neurological illness, or, for HCs, a first-degree relative with a psychotic disorder were exclusionary criteria. The Hollingshead Index assessed parental socioeconomic status (pSES).53 Participants provided consent as approved by VA Connecticut Healthcare System, Hartford Hospital, and Yale University Institutional Review Boards.
Go/NoGo Task
Participants performed 2 runs of a well-validated event-related Go/NoGo task.12,24,39 Instructions emphasized responding with speed and accuracy by button pressing to Go stimuli (412 total “X” trials; probability = 0.84) and withholding response to NoGo stimuli (78 total “K” trials; probability = 0.16). Stimulus presentation was 250 ms, with a pseudorandomly jittered stimulus onset asynchrony (SOA) (1000, 2000, 3000 ms; mean SOA = 1675 ms). Behavioral variables were median Go reaction time (RT), accuracy, and the signal detection measure d′. Go accuracy was the percentage of correct Go trials (hits; lower scores reflect omissions). NoGo accuracy was the percentage of correctly inhibited NoGo trials (correct rejections; lower scores reflect commissions). Proportion of NoGo/total errors was also examined.24 Go stimuli were presented frequently to maintain a sustained hemodynamic response that would serve as a baseline against which the well-spaced, jittered NoGo stimuli would elicit a detectable hemodynamic response. For this reason, the contrast of NoGo–Go was the main unit of analysis, and individual condition betas were not subject to statistical analysis
Neuroimaging Acquisition, Processing, and Modeling
Axial echoplanar (EPI) images were acquired at the Olin Neuropsychiatry Research Center on a Siemens Allegra 3 Tesla magnet: TR = 1500 ms, TE = 27 ms, flip angle = 60°, FOV = 220 mm, 29 4 mm slices (1 mm gap), 3.44 mm × 3.44 mm × 5 mm voxels; 288 1.5 s volumes (7:12 min) per run. Each run’s first 4 TRs were discarded to mitigate nonequilibrium effects. Axial magnetization-prepared rapid gradient-echo (MPRAGE) T1-weighted high-resolution images were collected: TR = 2500 ms, TE = 2.74 ms, flip angle = 8°, FOV = 256 mm, 1.0 mm3 voxels.
Image processing was performed with Statistical Parametric Mapping (SPM8). Images were motion corrected via affine registration (INRIAlign) and slice-time corrected. Denoising was conducted with ACompCor, which performs a principal component analysis on time series data from white matter and ventricular cerebrospinal fluid regions, defined from segmented T1-weighted anatomical images co-registered to functional data.54
For individual (first-level) analyses, SPM’s canonical hemodynamic response function (HRF; a double gamma function with a temporal derivative term) was convolved with task-vectors (correct Go; incorrect Go; correct NoGo; incorrect NoGo). Only correct responses were included in contrast images subjected to second-level analyses. First-level models specified regressors for task vectors, ACompCor noise components, and run (weighted by the number of correct responses). After high-pass temporal filtering (128s), parameters (beta coefficients) representing each regressor’s fit to a voxel’s time series were estimated using the general linear model. Voxelwise beta estimates were adjusted by their temporal derivative beta images to reduce latency-induced amplitude bias.55 Mean functional images were normalized to Montreal Neurological Institute’s EPI template and smoothed (6 mm FWHM Gaussian filter).
Age-Adjustment Z-scoring
To account for brain maturation during the age range studied and age differences between the clinical groups, normal maturation effects were removed from the fMRI data.48,56,57 Aging effects for each voxel in the HC group image were modeled, and voxelwise age-adjusted z-scores for all subjects based on the HC age-regression were calculated:
Thus, a participant’s age-adjusted z-score voxelwise map reflects deviation in fMRI activation, expressed in standard deviations, from that expected for a HC of the same age. Behavioral data were similarly adjusted for normal aging. All regions showing a significant main effect of group were evaluated for curvilinear effects of age within the HC group, and in no region did the quadratic age term account for significant variance in NoGo vs Go response beyond the linear age effect (.79 < P < .87) supporting the application of linear regression to control effects of age. Behavioral metrics were evaluated for curvilinear effects of age within the HC group, and the quadratic age term did not account for significant variance in NoGo vs Go response beyond the linear age effect in 5 of the 6 behavioral variables examined (.18 < P < .82). For the remaining behavioral variable, RT variability, no significant differences were found in analyses based on age-adjustment with the linear vs polynomial age regression models, and so the more parsimonious linear adjustment was retained.
Functional Connectivity
To assess connectivity patterns relevant to group differences in NoGo–Go responses, whole-brain voxelwise functional connectivity, seeded from regions showing a main effect of group in the activation analysis, was calculated using generalized psychophysiological interaction (gPPI).58,59 PPI analysis requires 3 principal regressors: (a) the psychological regressor, reflecting the task design convolved with the canonical HRF, (b) the physiological regressor, derived from the first eigenvariate of the seed’s BOLD time series, and (c) the interaction of a × b. The “PPI” interaction term identifies brain regions differentially correlated with activity in the seed region during the task conditions being compared, reflecting functional connectivity modulated by task condition. Seeds were 10 mm spheres around significant NoGo–Go main effect of group activation maxima. First-level gPPI analyses were conducted in native space. Contrast and beta images were normalized, smoothed, and age-corrected, as described above, before second-level group analyses.
Data Analysis
Analysis of variance (ANOVA) compared continuous demographic and behavioral variables; chi-square tests compared categorical variables. Scan motion was calculated as median motion displacement,60 the root mean square (RMS) of the scan-to-scan change in x-y-z translation or roll-pitch-yaw rotation. Omnibus ANOVA follow-up tests were corrected with Tukey honest significant difference tests (HSD) for all pairwise comparisons except for RT and RT variability, for which planned contrasts compared (a) HC vs combined CHR and ESZ groups and (b) CHR vs ESZ groups, given the a priori expectation that RTs would be slower and more variable in the clinical groups relative to HCs.61
Voxelwise ANOVA of the age-adjusted main effect of group activation F-map compared NoGo to Go (ie, correct rejections vs hits). To correct for multiple comparisons, we applied a cluster-defining height threshold (P < .005; extent > 20 voxels) and a family-wise error (FWE) cluster-level threshold, P < .05. Mean contrast values from FWE-corrected clusters showing a significant main effect of group were extracted for all participants and corrected with Tukey HSD tests to determine pairwise group differences.
Voxelwise ANOVA was conducted on age-adjusted PPI contrast maps using the same thresholds for group comparisons as the activation analyses (P < .005 height threshold; P < .05 FWE cluster-corrected). If no significant group PPI effects were detected, main effects across groups were tested in nonage-corrected data, identifying regions showing significant differences in connectivity with the seed region during NoGo vs Go. Given the greater statistical power to detect task condition (relative to group) PPI effects, a more stringent voxelwise FDR-corrected threshold (P < .001; extent > 20 voxels) was applied.
Correlations with task performance were examined within HC, CHR, and ESZ groups by testing for group differences in regression slopes relating activation or connectivity to RT. Correlations with symptoms (positive and negative symptom totals) were examined within CHR and ESZ groups within regions that showed main effects of group or condition.
Results
Demographics and Task Performance
Groups did not differ on gender, handedness, or scan displacement (rotational or translational motion) (P > .16). A significant main effect of group (P < .001) on age (ESZ > HC > CHR) was explained by an older ESZ group vs HC (P = .005) and CHR (P < .001) groups; the HC group was marginally older than the CHR group (P = .06). A significant main effect of group (P = .001) on pSES was explained by lower pSES in the ESZ group compared to HC (P = .001) and CHR (P = .04) groups.
Analysis of age-adjusted performance indicated comparable accuracy among groups for NoGo, Go, and d′ (P > .37). When false alarm errors were expressed as a proportion of total errors, there was a trend toward a main effect of group (P = .08) explained by increased proportional false alarms in HCs vs the ESZ group (P = .08). There were significant main effects of group on Go median RT (P = .02) and RT variability (P < .001). ESZ and CHR RTs were significantly slower (P = .005) and more variable (P < .001) compared to HCs, but the 2 clinical groups did not differ from each other (P > .82). RT distribution analyses are presented in supplementary information.
There were no group differences in the relationship between NoGo accuracy and RT to Go [Group × RT term F(2, 124) = .914, P > .4] but there was a significant common slope collapsed across groups [RT term F(1, 124) = 22.58, P < .001], indicating the expected speed-accuracy tradeoff was present, regardless of group. See table 1 for demographic, behavioral, and symptom measures.
Table 1.
Demographics of Healthy Control (HC), Clinical High Risk for Psychosis (CHR), and Early Illness Schizophrenia (ESZ) Participants
| HC | CHR | ESZ | |
|---|---|---|---|
| N a | 72 | 30 | 23 |
| Gender (% male) | 51.39 | 60.00 | 73.91 |
| Mean ± SD | Mean ± SD | Mean ± SD | |
| *Age (years) [range] | 19.13 ± 4.82 [11.3–29.7] | 16.96 ± 3.58 [12.1–25.2] | 22.51 ± 3.85 [13.8–29.6] |
| *Parental SESb | 28.08 ± 12.61 | 31.73 ± 14.75 | 41.57 ± 16.78 |
| Handedness (% right-handed) | 88.06 | 79.31 | 91.30 |
| Chlorpromazinec equivalent | — | — | 268.64 ± 168.68 |
| Median translation motion displacement (mm) | .088 ± .044 | .093 ± .048 | .107 ± .079 |
| Median rotation motion displacement (degrees) | .048 ± .029 | .052 ± .028 | .048 ± .042 |
| Behavioral performance (age-adjusted z-scores) | |||
| Go accuracy (% correct) | 96.49 ± 6.67 [.00 ± .99] | 94.30 ± 9.02 [−.24 ± 1.34] | 96.23 ± 4.16 [−.19 ± .66] |
| NoGo accuracy (% correct) | 58.07 ± 14.36 [.00 ± .99] | 57.82 ± 18.51 [.05 ± 1.24] | 61.76 ± 13.41 [.16 ± .89] |
| d′d | 2.46 ± .78 [.00 ± .99] | 2.26 ± 1.05 [−.14 ± 1.27] | 2.34 ± .82 [−.36 ± 1.03] |
| NoGo errors relative to total errors | 79.87 ± .23 [.00 ± .99] | 72.06 ± .26 [−.26 ± 1.11] | 70.95 ± .21 [−.53 ± .97] |
| Total accuracy (% correct, across both trial types) | 90.37 ± 5.90 [.00 ± .99] | 88.49 ± 8.72 [−.21 ± 1.44] | 90.75 ± 4.62 [−.12 ± .79] |
| *Go median reaction time (ms) | 349.47 ± 34.71 [.00 ± .99] | 376.30 ± 48.40 [.66 ± 1.46] | 361.39 ± 40.90 [.57 ± 1.38] |
| *Reaction time variability (ms) | 109.36 ± 26.13 [.00 ± 0.99] | 130.43 ± 26.68 [0.73 ± 1.00] | 126.01 ± 28.47 [0.77 ± 1.15] |
| Mean symptom ratings for patient groupse | |||
| SOPS positive | — | 2.26 ± 0.97 | — |
| SOPS negative | — | 1.98 ± 1.16 | — |
| SOPS disorganized | — | 1.46 ± 0.97 | — |
| SOPS general | — | 2.31 ± 0.98 | — |
| PANSS positive | — | — | 2.28 ± 0.65 |
| PANSS negative | — | — | 2.54 ± 0.85 |
| PANSS general | — | — | 2.10 ± 0.43 |
aData from 1 HC was not included in this report due to excessive motion artifact (ie, 73 HC images were originally collected). Parental socioeconomic status (pSES) scores not available for 8 HC, 0 CHR, 2 ESZ; Handedness scores not available for 5 HC, 1 CHR, 0 ESZ; Clinical ratings not available for 2 CHR participants and 2 ESZ participants; 1 HC was removed from reaction time (RT) analyses due to a median RT >3 within-group standard deviations.
bpSES measured by the Hollingshead 2-factor index. Higher Hollingshead scores indicate lower SES. All other assessment measures are scaled such that higher scores reflect greater levels of the measured variable.
cChlorpromazine equivalent dosage based on 14/23 ESZ patients due to 3 un-medicated patients and 6 patients with incomplete dosing information.62 CHR participants were not taking antipsychotic medication at the time of scanning, and therefore no CPZ equivalents are provided. Among CHR participants 20 were psychotropic medication-free while 10 were taking one or more of the following: antidepressants, n = 8 (eg, selective serotonin reuptake inhibitors, norepinephrine-dopamine reuptake inhibitors); stimulant or nonstimulant ADHD medication, n = 3 (eg, atomoxetine, methylphenidate) and/or anticonvulsants, n = 2 (eg, gabapentin, topiramate).
dThe sensitivity index, d′, is calculated by taking the inverse cumulative distribution function of the hit rate minus the inverse cumulative distribution function of the false alarm rate, for each individual. A higher d′ value reflects better signal detection.
eClinical symptom mean ratings are derived from the Scale of Prodromal Symptoms (SOPS) for the CHR group and from the Positive and Negative Syndrome Scale (PANSS) for the ESZ group.
*Significant omnibus test, P ≤ .05:
*Age: F (2, 122) = 10.49, P < .001; Tukey–Kramer HSD post hoc tests HC > CHR, P = .06; HC < ESZ, P = .005; CHR < ESZ, P < .001.
*pSES: F (2, 112) = 7.35, P = .001; Tukey–Kramer HSD post hoc tests HC < ESZ, P = .001; CHR < ESZ, P = .04.
*Go trial reaction time, age-adjusted F (2, 121) = 4.19, P = .017; planned contrasts HC < CHR + ESZ, P = .005; CHR vs ESZ, P = .82.
*Go trial reaction time variability, age-adjusted F (2, 121) = 8.13, P < .001; planned contrasts HC < CHR + ESZ, P < .001; CHR vs ESZ, P = .87.
Nonsignificant (P ≥ .05) comparisons:
Gender: χ2(2, N = 125) = 3.71, P = .16.
Handedness: χ2(4, N = 119) = 4.07, P = .40.
Translational motion displacement, F (2, 122) = 1.14, P = .32.
Rotational motion displacement, F (2, 122) = 0.17, P = .85.
Go trial accuracy, age-adjusted F (2, 122) = 0.68, P = .50.
NoGo trial accuracy, age-adjusted F (2, 122) = 0.21, P = .81.
NoGo accuracy (adjusted for total accuracy), age-adjusted F (2, 122) = 2.56, P = .08; Tukey–Kramer HSD post hoc tests HC > ESZ, P = .08.
d′, age-adjusted F(2, 122) = 0.99, P = .37.
Activation Analyses
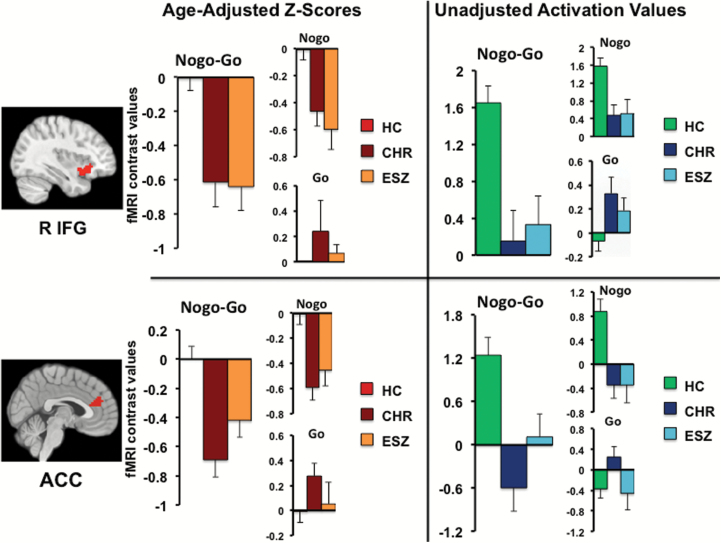
As expected from prior studies using this task12,24,39 significant main effects of condition for NoGo–Go activation were observed in largely bilateral fronto-parietal and subcortical regions including premotor cortex (BA 6), dorsal ACC (BA 32), inferior and superior parietal lobules (BA 7, 40), thalamus and striatum (FDR P < .001). The reverse contrast (Go–NoGo) yielded no significant activation at this threshold. Between-group analyses of NoGo–Go contrast values revealed significant main effects of group in right inferior frontal gyrus (RIFG; extending into right insula) and bilateral dorsal ACC. See table 2 for pairwise test statistics. Tukey HSD follow-up tests indicated that within RIFG, both ESZ (P = .001) and CHR (P < .001) groups showed less NoGo, relative to Go, response than HC. The same pattern was observed within the bilateral ACC region for ESZ (P = .04) and CHR (P < .001) compared to HC. This pattern of attenuated NoGo > Go response in the clinical groups arose primarily from reduced NoGo responses rather than increased Go responses, relative to HC (figure 1). Significant group effects persisted after controlling, at the between-subject level, for interindividual differences in RT, RT variability, and performance (d′) using ANCOVA models (P < .05). Significant group effects also persisted in these regions when using an age-matched grouping strategy in place of age z-scores (supplementary information).
Table 2.
Brain Regions showing a Significanta NoGo-Go Main Effect of Group Comparing Healthy Control (HC), Clinical High-Risk for Psychosis (CHR), and Early Illness for Schizophrenia (ESZ) Groups
| Cluster Neuroanatomy | Omnibus Group F-map FWE Cluster- Corrected P-value | Pairwise Follow-up Testsb | Pairwise Cohen’s d | Cluster Size (in 3 mm3 Voxels) | Peak MNI Coordinates (x, y, z) |
| Bilateral anterior cingulate cortex BA: 9, 10, 24, 32 | P = .047 | HC > CHR, P < .001 | HC vs CHR d = 0.98 | 105 | 6, 44, 16 |
| 3, 44, 25 | |||||
| HC > ESZ, P < .04 | HC vs ESZ d = 0.63 | −12, 44, 13 | |||
| Right inferior frontal cortex (extending into insular cortex) BA: 13, 47 | P = .035 | HC > CHR, P < .001HC > ESZ, P = .001 | HC vs CHR d = 0.84HC vs ESZ d = 0.96 | 95 | 36, 23, −1124, 23, −1136, 14, −14 |
aCluster-corrected P-values are family-wise error (FWE) corrected, at a height threshold of P < .005; extent = 20 voxels. Brodmann area = BA.
bTukey–Kramer HSD post hoc tests, 2-tailed, P < .05.
Fig. 1.
Bar graphs display age-adjusted z-score (left) and age un-adjusted (right) cluster mean (±standard error of the mean) by group for regions showing a significant main effect of group (P < .005 height threshold; P < .05 FWE cluster-corrected). Contrast values are displayed for NoGo–Go, whereas individual conditions (NoGo, Go) represent contrasts with the implicit baseline. Both CHR and ESZ groups showed significantly reduced NoGo–Go response relative to the HC group in right inferior frontal gyrus (RIFG) and anterior cingulate cortex (ACC) regions.
Functional Connectivity Analyses
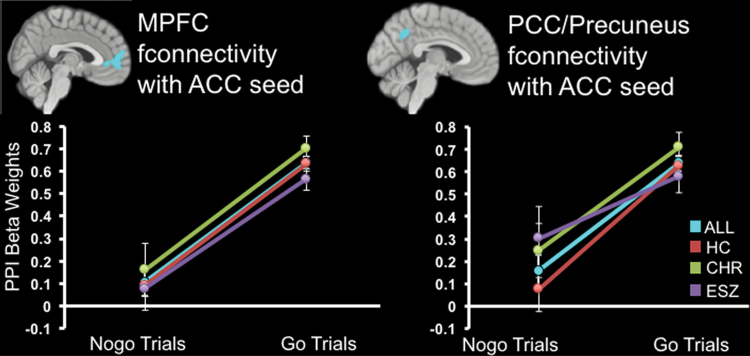
Functional connectivity analyses were seeded around main effect of group activation cluster maxima (RIFG: 36x 23y −11z; ACC: 6x 44y 16z). Age-adjusted PPI maps showed no significant group effects (P < .005 height threshold, P < .05 FWE cluster-corrected). We interrogated PPI main effects of condition (not age-corrected) to characterize differential modulation of functional connectivity by Go vs NoGo task conditions across groups. For the RIFG analysis, no significant effects survived. For the ACC analysis, there was significantly less coupling during NoGo relative to Go trials between the ACC and bilateral medial PFC (mPFC; Brodmann area 10), posterior cingulate cortex (PCC), and precuneus (whole-brain FDR corrected, P < .001; figure 2). These regions overlapped with anterior and posterior nodes of the default mode network (DMN); see supplementary figure 1. This pattern suggests that functional connectivity between dorsal ACC and midline DMN regions was modulated differently by Go vs NoGo trials. That is, across all groups, DMN and task-activated ACC functional connectivity was present during Go, but significantly decoupled during NoGo.
Fig. 2.
Plots show task condition psychophysiological interaction (PPI) effects, depicting less functional connectivity (fconnectivity) between the anterior cingulate seed region and medial prefrontal and posterior cingulate/precuneus cortices during NoGo, relative to Go trials, across all participant groups (main effect of condition FDR whole brain P < .001 corrected). Plot depicts PPI effect within each participant group (HC = red; CHR = green; ESZ = purple) and collapsed across groups (ALL = aqua).
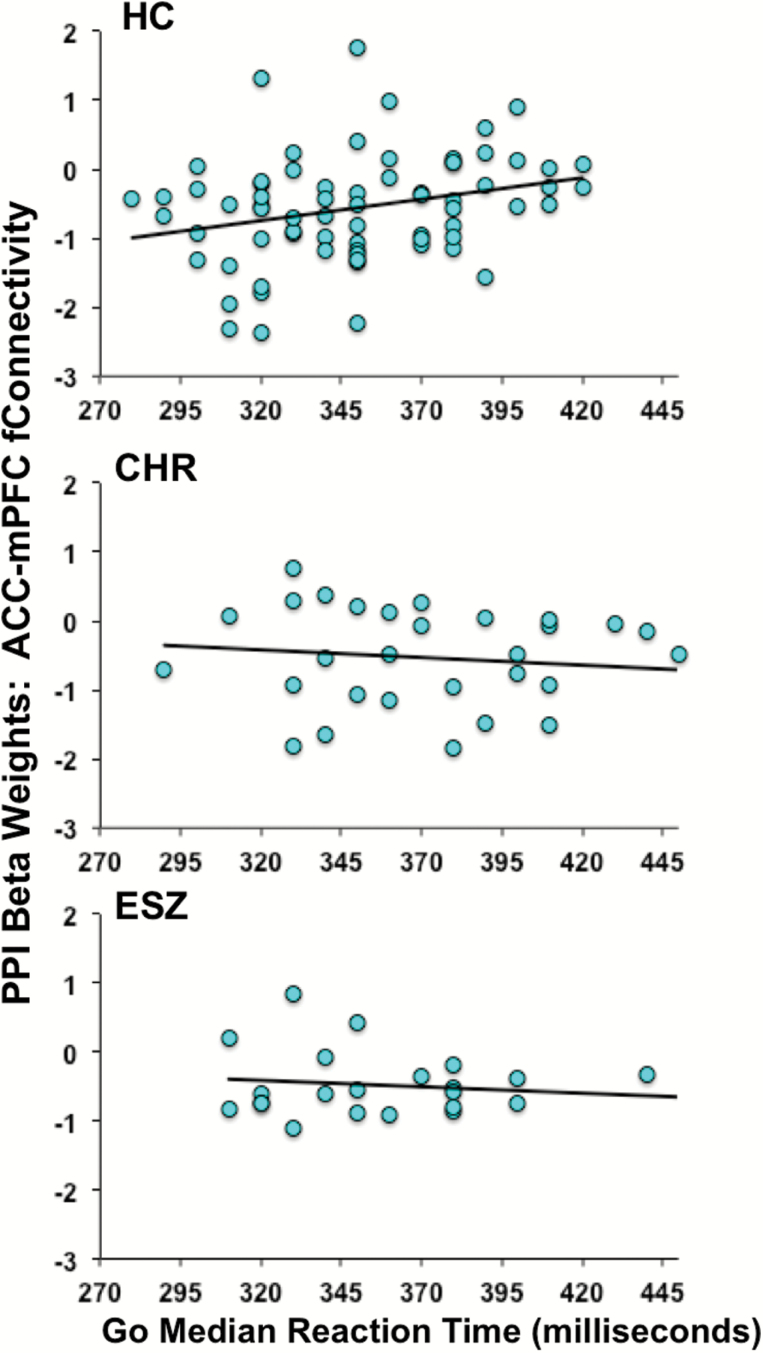
Lastly, we interrogated significant regions of ACC-seeded task condition-dependent functional connectivity (ie, regions showing significant PPI main effects of condition) for the presence of PPI–RT relationships, including testing group differences in regression slopes relating functional connectivity to RT. Within the mPFC region, slopes were significantly different between groups [group × RT interaction: F (2, 118) = 3.96, P = .02], even after removing an HC outlier whose median RT value was 3 SD slower than the HC group mean [group × RT interaction: F (2, 117) = 3.35, P = .04]; figure 3. Follow-up tests of correlations within each group were not significant in CHR (r = −.15, P = .43) or ESZ (r = −.16 P = .46) groups, whereas a significant positive relationship was present in HCs (r = .32, P = .006) indicating that the greater the HC attenuation of mPFC–ACC functional connectivity during RI, the faster the RT to Go trials. Within the PCC region, there were no significant relationships between PPI values and RT, for either between-group slope differences [group × RT interaction: F (2, 117) = 0.98, P = .38] or the common slope of the PPI-RT regression line [RT term F (1, 119) = 0.98, P = .32].
Fig. 3.
Significant (P < .05) group differences in the relationship between reaction time to Go trials vs functional connectivity with the anterior cingulate cortex (ACC) and medial prefrontal cortex (mPFC) in NoGo, relative to Go, trials.
Additional Correlates
Analyses examining NoGo–Go activation association with symptoms, medication dosage, and RT-activation slopes were not significant (P > .05) (supplementary information).
Discussion
Compared to HCs, CHR and ESZ groups had slower and more variable Go RTs, decreased RIFG and ACC NoGo activation, and an absence of the normal relationship in which ACC–mPFC decoupling during NoGo covaries with faster Go RTs. CHR and ESZ participants were inconsistent and slow, but not inaccurate. Further, analysis of RT distributions indicated both clinical groups had increased RT variability independent of long, slow responses characteristic of lapses (supplementary figure 3). RT slowing and increased variability are hallmark findings in schizophrenia61 and there tends to be a linear relationship between these 2 metrics.63 In the setting of the Go/NoGo task, slow and variable responding may best be understood as indicators of inefficient response preparation, which could explain why we observe performance differences in speed and consistency of responding, rather than in inhibitory control. The task fosters strong prepotent responding, with a higher probability of Go (84%) vs NoGo (16%) trials.12,39 This context implicitly elicits a prepotent tendency to “go,” intensifying the need for RI, recruitment of inhibition-related neural circuitry, and false alarm propensity. Our data suggest that HCs made use of this task context, responding more quickly and consistently than CHR and ESZ groups on Go trials, with a tendency to commit more false alarms than omission errors (based on examination of NoGo errors relative to total errors), similar to the pattern we observed in HCs relative to chronic schizophrenia patients.24 The strong prepotent responding in HCs may contribute to their significantly greater activation of inhibitory control circuitry during NoGo trials. However, controlling for behavioral indicators of prepotency did not eliminate group differences in NoGo–Go activation, demonstrating that prefrontal hypoactivation in clinical groups is not confounded with task performance.
Consistent with study hypotheses, CHR and ESZ groups, compared to HCs, showed significantly reduced NoGo–Go contrast values in bilateral dorsal ACC and RIFG/insula (figure 1), regions associated with response conflict64,65 and RI.1,4 These findings are consistent with the cognitive control literature in schizophrenia. A meta-analysis across executive functioning tasks found prefrontal hypoactivation, including ACC, in schizophrenia.20 More specifically, prior Go/NoGo fMRI studies of chronic schizophrenia report prefrontal hypoactivation during RI, in the absence of group performance differences, in ACC24,28,30 and right VLPFC.29 Although fewer studies have used the stop signal task to examine motor stopping in schizophrenia, reduced RIFG27 and ACC28 activation during successful stop trials, has been reported in patients with schizophrenia relative to controls using that paradigm as well, though intact activation to reactive stopping31 has also been reported. Our results replicate findings of prefrontal hypoactivation in chronic schizophrenia, and extend them to early illness and CHR samples. Our data also support 2 prior studies of the psychosis risk syndrome that observed prefrontal hypoactivation using different executive tasks.37,38 In particular, the ACC and RIFG regions that showed hypoactivation in the CHR group in our study anatomically overlap with ACC and RIFG hypoactivation in CHR individuals in the Colibazzi et al37 study (supplementary figure 2). There were no significant activation differences between CHR and ESZ groups in our study. Deficits in context-guided response acquisition and/or inhibition may, therefore, reflect vulnerability for psychosis with no progression if full psychosis develops.
In addition to signaling a need for RI, NoGo stimuli are infrequent and unexpected. NoGo–Go hypoactivation in the clinical groups could also reflect reduced response to infrequent stimuli. This alternative interpretation fits with (a) recent findings showing inferior frontal cortex (IFC) and IFC–ACC connectivity patterns respond equivalently across tasks that require infrequent response cue monitoring, regardless of inhibition demands13 and (b) the IFC’s well-established role in stimulus-driven attention.66 Observed activation differences may be explained by noninhibitory demands of the task, and could relate to long-recognized schizophrenia-associated alterations in processing expectancy.67 Consistent with this possibility, fMRI studies of schizophrenia using oddball tasks report reduced IFC activation to infrequent stimuli.68,69
Task-dependent functional connectivity analysis seeded from regions with group activation effects (RIFG; ACC) revealed no group differences. However, a significant PPI main effect of condition indicated that, across all groups, ACC activation positively correlated with mPFC and precuneus/PCC activation during Go trials, while these correlations were significantly absent during NoGo trials (figure 2). The mPFC and precuneus/PCC regions showing this task-modulated connectivity with the ACC overlapped with DMN nodes (supplementary figure 1), revealing that the ACC is positively coupled to DMN during prepotent Go responding and decoupled when responses are inhibited during NoGo. Further, within HCs, faster Go responders showed greater ACC–mPFC decoupling during NoGo. This HC pattern of task substrate-DMN decoupling during RI correlating with faster Go responses was not present in CHR or ESZ groups (figure 3). We speculate that the prepotent responding contextually encouraged by the task was strongest in the fastest HCs, who consequently required more ACC–DMN decoupling when inhibiting responses. Optimal responding may require greater recruitment of task-relevant inhibitory control circuitry to override the prepotent “Go” motor plan, as well as greater suppression of DMN activity to devote resources to overcoming prepotency. Consistent with this interpretation, task-induced DMN deactivation increases with task difficulty.70,71 That equivalent inverse coupling of these regions does not correspond with faster RTs in the clinical groups could indicate that this functional connectivity pattern is more effectively translated into adaptive behavior in HCs. Together, these activation and connectivity results extend our previous work demonstrating reduced task-induced DMN suppression in CHR and ESZ participants.48 Our results are consistent with broader findings indicating the expected reciprocity between DMN and external “task positive” networks may be attenuated in schizophrenia,72 and demonstrate that DMN-related deficits associated with schizophrenia precede illness onset.
These findings are limited by several factors. Foremost, we have insufficient clinical follow-up to examine psychosis conversion. Additionally, medication status differed among groups. Within ESZ patients, chlorpromazine dose equivalents were not associated with NoGo–Go contrast values or connectivity measures (supplementary information). The similar magnitude and direction of brain activation and behavioral effects within the antipsychotic-free CHR individuals argues against antipsychotic medication status accounting for ESZ group effects. Future research is needed to determine the extent to which observed alterations of in vivo brain functioning covary with adaptive functioning and clinical course. Despite these limitations, we combined analysis of activation and task-based connectivity to show similar behavioral and functional brain alterations in CHR and ESZ patients, demonstrating that inhibitory control alterations in schizophrenia predate psychosis onset. Both clinical groups showed less differentiation between NoGo and Go activity than HCs, a pattern that may best be explained by deficits in establishing appropriate response patterns based on contextual learning that are well-described in schizophrenia15–17,24,32 and detectable during the CHR phase,37,38 rather than by an inhibitory deficit per se.
Funding
National Institutes of Health (RO1 MH076989); Department of Veteran Affairs (CX001028).
Supplementary Material
Acknowledgments
Dr Mathalon reports that he is a consultant for Boehringer Ingelheim, Alkermes, and Upsher-Smith Laboratories. The authors have declared that there are no conflicts of interest in relation to the subject of this study.
References
- 1. Aron AR. The neural basis of inhibition in cognitive control. Neuroscientist. 2007;13:214–228. [DOI] [PubMed] [Google Scholar]
- 2. Fuster J. The Prefrontal Cortex, Vol. 10 Raven: Academic Press; 2008:S0896–S6273. [Google Scholar]
- 3. Miller EK, Cohen JD. An integrative theory of prefrontal cortex function. Annu Rev Neurosci. 2001;24:167–202. [DOI] [PubMed] [Google Scholar]
- 4. Aron AR, Fletcher PC, Bullmore ET, Sahakian BJ, Robbins TW. Stop-signal inhibition disrupted by damage to right inferior frontal gyrus in humans. Nat Neurosci. 2003;6:115–116. [DOI] [PubMed] [Google Scholar]
- 5. Chambers CD, Garavan H, Bellgrove MA. Insights into the neural basis of response inhibition from cognitive and clinical neuroscience. Neurosci Biobehav Rev. 2009;33:631–646. [DOI] [PubMed] [Google Scholar]
- 6. Iversen SD, Mishkin M. Perseverative interference in monkeys following selective lesions of the inferior prefrontal convexity. Exp Brain Res. 1970;11:376–386. [DOI] [PubMed] [Google Scholar]
- 7. Watanabe M. Prefrontal unit activity during delayed conditional Go/No-Go discrimination in the monkey. I. Relation to the stimulus. Brain Res. 1986;382:1–14. [DOI] [PubMed] [Google Scholar]
- 8. Buchsbaum BR, Greer S, Chang WL, Berman KF. Meta-analysis of neuroimaging studies of the Wisconsin card-sorting task and component processes. Hum Brain Mapp. 2005;25:35–45. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Simmonds DJ, Pekar JJ, Mostofsky SH. Meta-analysis of Go/No-go tasks demonstrating that fMRI activation associated with response inhibition is task-dependent. Neuropsychologia. 2008;46:224–232. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Swick D, Ashley V, Turken U. Are the neural correlates of stopping and not going identical? Quantitative meta-analysis of two response inhibition tasks. Neuroimage. 2011;56:1655–1665. [DOI] [PubMed] [Google Scholar]
- 11. Jahanshahi M, Obeso I, Rothwell JC, Obeso JA. A fronto-striato-subthalamic-pallidal network for goal-directed and habitual inhibition. Nat Rev Neurosci. 2015;16:719–732. [DOI] [PubMed] [Google Scholar]
- 12. Stevens MC, Kiehl KA, Pearlson GD, Calhoun VD. Functional neural networks underlying response inhibition in adolescents and adults. Behav Brain Res. 2007;181:12–22. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Erika-Florence M, Leech R, Hampshire A. A functional network perspective on response inhibition and attentional control. Nat Commun. 2014;5: 4073. doi:10.1038/ncomms5073. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. van Belle J, Vink M, Durston S, Zandbelt BB. Common and unique neural networks for proactive and reactive response inhibition revealed by independent component analysis of functional MRI data. Neuroimage. 2014;103:65–74. [DOI] [PubMed] [Google Scholar]
- 15. Barch DM, Carter CS, Braver TS, et al. Selective deficits in prefrontal cortex function in medication-naive patients with schizophrenia. Arch Gen Psychiatry. 2001;58:280–288. [DOI] [PubMed] [Google Scholar]
- 16. Cohen JD, Servan-Schreiber D. Context, cortex, and dopamine: a connectionist approach to behavior and biology in schizophrenia. Psychol Rev. 1992;99:45–77. [DOI] [PubMed] [Google Scholar]
- 17. MacDonald AW III, Carter CS. Event-related FMRI study of context processing in dorsolateral prefrontal cortex of patients with schizophrenia. J Abnorm Psychol. 2003;112:689–697. [DOI] [PubMed] [Google Scholar]
- 18. Lesh TA, Niendam TA, Minzenberg MJ, Carter CS. Cognitive control deficits in schizophrenia: mechanisms and meaning. Neuropsychopharmacology. 2011;36:316–338. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Snitz BE, Macdonald AW III, Carter CS. Cognitive deficits in unaffected first-degree relatives of schizophrenia patients: a meta-analytic review of putative endophenotypes. Schizophr Bull. 2006;32:179–194. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Minzenberg MJ, Laird AR, Thelen S, Carter CS, Glahn DC. Meta-analysis of 41 functional neuroimaging studies of executive function in schizophrenia. Arch Gen Psychiatry. 2009;66:811–822. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. Badcock JC, Michie PT, Johnson L, Combrinck J. Acts of control in schizophrenia: dissociating the components of inhibition. Psychol Med. 2002;32:287–297. [DOI] [PubMed] [Google Scholar]
- 22. Bellgrove MA, Chambers CD, Vance A, Hall N, Karamitsios M, Bradshaw JL. Lateralized deficit of response inhibition in early-onset schizophrenia. Psychol Med. 2006;36:495–505. [DOI] [PubMed] [Google Scholar]
- 23. Lipszyc J, Schachar R. Inhibitory control and psychopathology: a meta-analysis of studies using the stop signal task. J Int Neuropsychol Soc. 2010;16:1064–1076. [DOI] [PubMed] [Google Scholar]
- 24. Ford JM, Gray M, Whitfield SL, et al. Acquiring and inhibiting prepotent responses in schizophrenia: event-related brain potentials and functional magnetic resonance imaging. Arch Gen Psychiatry. 2004;61:119–129. [DOI] [PubMed] [Google Scholar]
- 25. Kiehl KA, Smith AM, Hare RD, Liddle PF. An event-related potential investigation of response inhibition in schizophrenia and psychopathy. Biol Psychiatry. 2000;48:210–221. [DOI] [PubMed] [Google Scholar]
- 26. Weisbrod M, Kiefer M, Marzinzik F, Spitzer M. Executive control is disturbed in schizophrenia: evidence from event-related potentials in a Go/NoGo task. Biol Psychiatry. 2000;47:51–60. [DOI] [PubMed] [Google Scholar]
- 27. Hughes ME, Fulham WR, Johnston PJ, Michie PT. Stop-signal response inhibition in schizophrenia: behavioural, event-related potential and functional neuroimaging data. Biol Psychol. 2012;89:220–231. [DOI] [PubMed] [Google Scholar]
- 28. Rubia K, Russell T, Bullmore ET, et al. An fMRI study of reduced left prefrontal activation in schizophrenia during normal inhibitory function. Schizophr Res. 2001;52:47–55. [DOI] [PubMed] [Google Scholar]
- 29. Kaladjian A, Jeanningros R, Azorin JM, Grimault S, Anton JL, Mazzola-Pomietto P. Blunted activation in right ventrolateral prefrontal cortex during motor response inhibition in schizophrenia. Schizophr Res. 2007;97:184–193. [DOI] [PubMed] [Google Scholar]
- 30. Arce E, Leland DS, Miller DA, Simmons AN, Winternheimer KC, Paulus MP. Individuals with schizophrenia present hypo- and hyperactivation during implicit cueing in an inhibitory task. Neuroimage. 2006;32:704–713. [DOI] [PubMed] [Google Scholar]
- 31. Zandbelt BB, van Buuren M, Kahn RS, Vink M. Reduced proactive inhibition in schizophrenia is related to corticostriatal dysfunction and poor working memory. Biol Psychiatry. 2011;70:1151–1158. [DOI] [PubMed] [Google Scholar]
- 32. Ford JM, Roach BJ, Miller RM, Duncan CC, Hoffman RE, Mathalon DH. When it’s time for a change: failures to track context in schizophrenia. Int J Psychophysiol. 2010;78:3–13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33. MacDonald AW III, Carter CS, Kerns JG, et al. Specificity of prefrontal dysfunction and context processing deficits to schizophrenia in never-medicated patients with first-episode psychosis. Am J Psychiatry. 2005;162:475–484. [DOI] [PubMed] [Google Scholar]
- 34. Servan-Schreiber D, Cohen JD, Steingard S. Schizophrenic deficits in the processing of context. A test of a theoretical model. Arch Gen Psychiatry. 1996;53:1105–1112. [DOI] [PubMed] [Google Scholar]
- 35. Delawalla Z, Csernansky JG, Barch DM. Prefrontal cortex function in nonpsychotic siblings of individuals with schizophrenia. Biol Psychiatry. 2008;63:490–497. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36. MacDonald AW III, Pogue-Geile MF, Johnson MK, Carter CS. A specific deficit in context processing in the unaffected siblings of patients with schizophrenia. Arch Gen Psychiatry. 2003;60:57–65. [DOI] [PubMed] [Google Scholar]
- 37. Colibazzi T, Horga G, Wang Z, et al. Neural dysfunction in cognitive control circuits in persons at clinical high-risk for psychosis. Neuropsychopharmacology. 2016;41:1241–1250. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38. Niendam TA, Lesh TA, Yoon J, et al. Impaired context processing as a potential marker of psychosis risk state. Psychiatry Res. 2014;221:13–20. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39. Steele VR, Aharoni E, Munro GE, et al. A large scale (N=102) functional neuroimaging study of response inhibition in a Go/NoGo task. Behav Brain Res. 2013;256:529–536. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40. Luna B, Sweeney JA. The emergence of collaborative brain function: FMRI studies of the development of response inhibition. Ann N Y Acad Sci. 2004;1021:296–309. [DOI] [PubMed] [Google Scholar]
- 41. Rubia K, Smith AB, Woolley J, et al. Progressive increase of frontostriatal brain activation from childhood to adulthood during event-related tasks of cognitive control. Hum Brain Mapp. 2006;27:973–993. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42. Tamm L, Menon V, Reiss AL. Maturation of brain function associated with response inhibition. J Am Acad Child Adolesc Psychiatry. 2002;41:1231–1238. [DOI] [PubMed] [Google Scholar]
- 43. Miller TJ, McGlashan TH, Rosen JL, et al. Prodromal assessment with the structured interview for prodromal syndromes and the scale of prodromal symptoms: predictive validity, interrater reliability, and training to reliability. Schizophr Bull. 2003;29:703–715. [DOI] [PubMed] [Google Scholar]
- 44. Miller TJ, McGlashan TH, Rosen JL, et al. Prospective diagnosis of the initial prodrome for schizophrenia based on the Structured Interview for Prodromal Syndromes: preliminary evidence of interrater reliability and predictive validity. Am J Psychiatry. 2002;159:863–865. [DOI] [PubMed] [Google Scholar]
- 45. Cannon TD, Cadenhead K, Cornblatt B, et al. Prediction of psychosis in youth at high clinical risk: a multisite longitudinal study in North America. Arch Gen Psychiatry. 2008;65:28–37. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46. Fusar-Poli P, Cappucciati M, Borgwardt S, et al. Heterogeneity of psychosis risk within individuals at clinical high risk: a meta-analytical stratification. JAMA Psychiatry. 2016;73:113–120. [DOI] [PubMed] [Google Scholar]
- 47. Fryer SL, Roach BJ, Wiley K, Loewy RL, Ford JM, Mathalon DH. Reduced amplitude of low-frequency brain oscillations in the psychosis risk syndrome and early illness schizophrenia. Neuropsychopharmacology. 2016;41:2388–2398. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48. Fryer SL, Woods SW, Kiehl KA, et al. Deficient suppression of default mode regions during working memory in individuals with early psychosis and at clinical high-risk for psychosis. Front Psychiatry. 2013;4:92. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49. Perez VB, Woods SW, Roach BJ, et al. Automatic auditory processing deficits in schizophrenia and clinical high-risk patients: forecasting psychosis risk with mismatch negativity. Biol Psychiatry. 2014;75:459–469. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50. First MB, Spitzer RL, Gibbon MG, Williams JBW. Structured clinical interview for DSM-IV-TR axis I disorders – patient edition (SCID-I/P, 11/2002 revision). New York, NY; 2002. [Google Scholar]
- 51. Kay SR, Fiszbein A, Opler LA. The positive and negative syndrome scale (PANSS) for schizophrenia. Schizophr Bull. 1987;13:261–276. [DOI] [PubMed] [Google Scholar]
- 52. Kaufman J, Birmaher B, Brent D, et al. Schedule for Affective Disorders and Schizophrenia for School-Age Children-Present and Lifetime Version (K-SADS-PL): initial reliability and validity data. J Am Acad Child Adolesc Psychiatry. 1997;36:980–988. [DOI] [PubMed] [Google Scholar]
- 53. Hollingshead A, Redlich F.. Social Class and Mental Illness. New York: John Wiley and Sons; 1958. [Google Scholar]
- 54. Behzadi Y, Restom K, Liau J, Liu TT. A component based noise correction method (CompCor) for BOLD and perfusion based fMRI. Neuroimage. 2007;37:90–101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55. Calhoun VD, Stevens MC, Pearlson GD, Kiehl KA. fMRI analysis with the general linear model: removal of latency-induced amplitude bias by incorporation of hemodynamic derivative terms. Neuroimage. 2004;22:252–257. [DOI] [PubMed] [Google Scholar]
- 56. Mathalon DH, Pfefferbaum A, Lim KO, Rosenbloom MJ, Sullivan EV. Compounded brain volume deficits in schizophrenia-alcoholism comorbidity. Arch Gen Psychiatry. 2003;60:245–252. [DOI] [PubMed] [Google Scholar]
- 57. Pfefferbaum A, Lim KO, Zipursky RB, et al. Brain gray and white matter volume loss accelerates with aging in chronic alcoholics: a quantitative MRI study. Alcohol Clin Exp Res. 1992;16:1078–1089. [DOI] [PubMed] [Google Scholar]
- 58. Friston KJ, Buechel C, Fink GR, Morris J, Rolls E, Dolan RJ. Psychophysiological and modulatory interactions in neuroimaging. Neuroimage. 1997;6:218–229. [DOI] [PubMed] [Google Scholar]
- 59. McLaren DG, Ries ML, Xu G, Johnson SC. A generalized form of context-dependent psychophysiological interactions (gPPI): a comparison to standard approaches. Neuroimage. 2012;61:1277–1286. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60. Van Dijk KR, Sabuncu MR, Buckner RL. The influence of head motion on intrinsic functional connectivity MRI. Neuroimage. 2012;59:431–438. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61. Nuechterlein KH. Reaction time and attention in schizophrenia: a critical evaluation of the data and theories. Schizophr Bull. 1977;3:373–428. [DOI] [PubMed] [Google Scholar]
- 62. Woods SW. Chlorpromazine equivalent doses for the newer atypical antipsychotics. J Clin Psychiatry. 2003;64:663–667. [DOI] [PubMed] [Google Scholar]
- 63. Wagenmakers EJ, Brown S. On the linear relation between the mean and the standard deviation of a response time distribution. Psychol Rev. 2007;114:830–841. [DOI] [PubMed] [Google Scholar]
- 64. Kerns JG, Cohen JD, MacDonald AW III, Cho RY, Stenger VA, Carter CS. Anterior cingulate conflict monitoring and adjustments in control. Science. 2004;303:1023–1026. [DOI] [PubMed] [Google Scholar]
- 65. MacDonald AW III, Cohen JD, Stenger VA, Carter CS. Dissociating the role of the dorsolateral prefrontal and anterior cingulate cortex in cognitive control. Science. 2000;288:1835–1838. [DOI] [PubMed] [Google Scholar]
- 66. Corbetta M, Patel G, Shulman GL. The reorienting system of the human brain: from environment to theory of mind. Neuron. 2008;58:306–324. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67. Jeon YW, Polich J. Meta-analysis of P300 and schizophrenia: patients, paradigms, and practical implications. Psychophysiology. 2003;40:684–701. [DOI] [PubMed] [Google Scholar]
- 68. Calhoun V, Wu L, Kiehl K, Eichele T, Pearlson G. Aberrant processing of deviant stimuli in schizophrenia revealed by fusion of FMRI and EEG data. Acta Neuropsychiatr. 2010;22:127–138. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69. Wynn JK, Jimenez AM, Roach BJ, et al. Impaired target detection in schizophrenia and the ventral attentional network: findings from a joint event-related potential-functional MRI analysis. Neuroimage Clin. 2015;9:95–102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70. McKiernan KA, Kaufman JN, Kucera-Thompson J, Binder JR. A parametric manipulation of factors affecting task-induced deactivation in functional neuroimaging. J Cogn Neurosci. 2003;15:394–408. [DOI] [PubMed] [Google Scholar]
- 71. Tomasi D, Ernst T, Caparelli EC, Chang L. Common deactivation patterns during working memory and visual attention tasks: an intra-subject fMRI study at 4 Tesla. Hum Brain Mapp. 2006;27:694–705. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72. Whitfield-Gabrieli S, Ford JM. Default mode network activity and connectivity in psychopathology. Annu Rev Clin Psychol. 2012;8:49–76. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.