Abstract
Randall’s plaque, an attachment site over which calcium oxalate stones form, begins in the basement membranes of thin limbs of the loop of Henle. The mechanism of its formation is unknown. Possibly, enhanced delivery of calcium out of the proximal tubule, found in many stone formers, increases reabsorption of calcium from the thick ascending limb into the interstitium around descending vasa recta, which convey that calcium into the deep medulla, and raises supersaturations near thin limbs (“vas washdown”). According to this hypothesis, plaque should form preferentially on ascending thin limbs, which do not reabsorb water. We stained serial sections of papillary biopsies from stone-forming patients for aquaporin 1 (which is found in the descending thin limb) and the kidney-specific chloride channel ClC-Ka (which is found in the ascending thin limb). Plaque (which is detected using Yasue stain) colocalized with ClC-Ka, but not with aquaporin 1 (χ2 = 464, P < 0.001). We conclude that plaque forms preferentially in the basement membranes of ascending thin limbs, fulfilling a critical prediction of the vas washdown theory of plaque pathogenesis. The clinical implication is that treatments such as a low-sodium diet or thiazide diuretics that raise proximal tubule calcium reabsorption may reduce formation of plaque as well as calcium kidney stones.
Keywords: ascending thin limb, calcium oxalate, nephrolithiasis, Randall’s plaque
INTRODUCTION
Randall’s plaque, a mixture of apatite and matrix, forms in the inner medulla and papillae of normal human kidneys but is more prominent in kidneys of idiopathic calcium stone formers (ICSF) (2). Calcium oxalate (CaOx) stones can form on Randall’s plaque (5). Plaque appears to begin in the basement membranes of thin limbs of the loop of Henle (tHL) (6).
Mechanisms that produce Randall’s plaque are not known. Perhaps the most straightforward theory is that plaque arises because of calcium phosphate supersaturations in the vicinity of the basement membranes of the tHL that are present in normal people and higher than normal in ICSF.
Idiopathic hypercalciuria (IH) is a very common trait in ICSF, and plaque abundance varies directly with the level of urine calcium excretion (12). We found increased delivery of sodium, calcium, and water out of the proximal tubule in ICSF with IH compared with normal subjects consuming the same diet (20). So IH raises the throughput of calcium along much of the nephron, including the tHL and collecting ducts.
In the inner stripe of the outer medulla, descending vas recta are surrounded by thick ascending limbs (TAL), which reabsorb calcium electrogenically without water (18). Increased delivery of calcium to the TAL will therefore increase reabsorption of calcium into the interstitium around descending vas recta, which can convey the “extra” calcium into the deep medulla and raise supersaturations.
We have proposed the “vas washdown” theory of calcium as a basis for plaque genesis via direct physical chemical forces (3). Movement of calcium out of inner medullary collecting ducts (IMCD) or the tHL is not a likely source of interstitial calcium loading, since neither segment exhibits significant transepithelial calcium transport.
A critical prediction of the vas washdown theory is that plaque forms preferentially in the basement membrane of ascending vs. descending tHL. Water extracted from descending tHL because of unbalanced osmotic forces must pass through the basement membrane into the interstitium and, in the process, lowers supersaturation in descending than ascending tHL, which are water-impermeable.
A failure to find preferential plaque formation on ascending vs. descending tHL would falsify this hypothesis. Accordingly, we test the vas washdown theory by determining whether plaque forms on one or both sides of the tHL and whether it forms preferentially on and around ascending limbs.
To perform this critical test, we exploit a well-known difference between the two thin limbs with respect to one transporter and one pore. Aquaporin 1 (AQP1) is limited to the descending thin limb, where it is the route for water permeability, while the kidney-specific chloride channel A (ClC-Ka) is limited to the ascending thin limb. Through use of these specificities, we imply nothing more than that they enable us to critically test the hypothesis at issue here.
MATERIALS AND METHODS
Patients and Biopsy Procedure
We studied papillary biopsies, obtained during surgical stone removal by percutaneous nephrolithotomy (PCNL), from 5 of our original 15 hypercalciuric ICSF patients (Table 1) (6). Briefly, during PCNL, biopsies were taken from the tip of one selected papilla in the upper, lower, and interpolar regions and from the outer cortex as the working sheath was pulled out of the access site. All biopsy samples were taken at sites of Randall’s plaque. Control tissue was obtained from two non-stone-forming subjects without plaque (one sample from the National Resource Center and another from Ohio State Medical Center, Midwestern Division of the Cooperative Human Tissue Network). Details of our surgical approach are presented elsewhere (6). The study was approved by the Institutional Review Board Committee for Indiana University Health (no. 98-073). Written informed consent was obtained from the patients before inclusion in the study.
Table 1.
Clinical data for idiopathic calcium oxalate stone-forming patients
| Stone Analysis |
||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Patient No. | Sex | Age at 1st stone, yr | No. of Stones | Age at study, yr | ESWL | URS | PNL | Prior treatment | PMH | n | CaOx, % | Apatite, % |
| 1 | M | 63 | 1 | 63 | 0 | 1 | 1 | None | Cholestectomy | 2 | 100 | 0 |
| 2 | M | 41 | 2 | 41 | 0 | 1 | 1 | None | Inguinal hernia, DM | 1 | 100 | 0 |
| 3 | F | 27 | 1 | 27 | 1 | 0 | 1 | None | VSD, UTI | 2 | 100 | 0 |
| 4 | M | 34 | 4 | 61 | 1 | 1 | 1 | None | UC, CAD | 1 | 100 | 0 |
| 5 | M | 24 | 4 | 75 | 0 | 0 | 1 | None | HTN, BPH, spinal disk surgery | 1 | 99 | 1 |
ESWL, extracorporeal shock wave lithotripsy; URS, ureteroscopy; PNL, percutaneous nephrolithotomy; Prior treatment refers to treatment for stone prevention; PMH, past medical history; DM, diabetes mellitus; VSD, ventricular septal defect; UTI, urinary tract infection; UC, ulcerative colitis without surgery; CAD, coronary artery disease; HTN, hypertension; BPH, benign prostatic hypertrophy; CaOx, calcium oxalate. In addition to the noted procedures, patient 4 also had nephrolithotomy for an earlier stone, and patient 5 had cystoscopy twice for an earlier stone.
Complete clinical histories were obtained, and old records were reviewed to obtain stone analyses. All five patients fulfilled the criteria for idiopathic CaOx stone formation (6), in that at least one stone was analyzed and shown to be composed of CaOx, and no stones contained uric acid, struvite, cystine, or >50% calcium phosphate. None of the five patients had systemic disorders such as primary hyperparathyroidism, sarcoidosis, vitamin D excess, hyperthyroidism, or renal tubular acidosis. Given that these patients required PCNL, they had a stone burden much above that readily treated via other modalities, but most did form an unusual number of stones (Table 1).
Clinical Evaluation
While patients were on a random diet, two 24-h urine samples were collected for measurements of volume, pH, calcium, oxalate, citrate, phosphate, uric acid, sodium, potassium, magnesium, sulfate, and ammonia (Table 2). From these measurements, we used the EQUIL2 computer program (19) to calculate supersaturations with respect to CaOx, calcium phosphate (as brushite calcium monohydrogen phosphate), and uric acid. Blood was drawn from patients in the fasting state and analyzed for calcium, phosphate, magnesium, sodium, potassium, chloride, creatinine, and carbon dioxide. Only the two most relevant blood measurements are presented here; they served to exclude systemic disorders noted above. Table 2 shows only the most relevant urine measurements. As is common among calcium formers, we found low urine volume, variably high urine calcium and oxalate, generally high supersaturation for CaOx, and a scattering of low urine citrate excretion rates.
Table 2.
Urine and serum data for idiopathic calcium oxalate stone-forming patients
| Supersaturation |
||||||||||
|---|---|---|---|---|---|---|---|---|---|---|
| Patient No. | Urine Volume, l/day (>1.5 liters) | Urine pH (5.8–6.2) | Urine Calcium, mg/day (100–200 mg/day) | Urine Oxalate, mg/day (20–45 mg/day) | Urine Citrate, mg/day (500–750 mg/day) | Urine Creat, mg/day | CaOx | CaP | Serum Creat, mg/dl (0.8–1.2 mg/dl) | Serum Ca, mg/dl (9–10.2 mg/dl) |
| 1 | 1.07 | 5.61 | 212 | 50 | 1,114 | 2,086 | 15.50 | 1.11 | 1.4 | 8.2 |
| 2 | 1.68 | 5.89 | 163 | 32 | 529 | 1,695 | 6.18 | 0.68 | 1.2 | 9.9 |
| 3 | 0.93 | 5.35 | 326 | 37 | 979 | 1,329 | 16.39 | 1.18 | 0.9 | 9.1 |
| 4 | 1.99 | 6.34 | 367 | 37 | 336 | 1,365 | 8.41 | 2.35 | 1.0 | 8.9 |
| 5 | 1.41 | 5.65 | 293 | 39 | 118 | 1,450 | 11.99 | 0.81 | 0.9 | 10.1 |
Normal range is shown in parentheses. SS, supersaturation; CaOx, calcium oxalate; CaP, calcium phosphate; Creat, creatinine.
Rationale
Our research strategy is based on well-established localization of specific channels in the medullary ascending and descending tHL. AQP1 localizes to descending tHL (14), whereas ClC-Ka localizes to ascending tHL and the bend of the loop (17). Plaque was identified using Yasue stain, as described in our prior publications (6, 7). IMCD were easily identified by their size and epithelial morphology.
Light Microscopy
General.
Biopsy samples taken during surgery were fixed in 5% fresh paraformaldehyde, as previously reported (6, 7), dehydrated through graded ethanol concentrations to 100% ethanol, and cleared in xylene and then embedded in Shandon precision-cut paraffin (Thermo Scientific). Serial sections were cut at 4 μm, placed on charged glass slides (Fisher Scientific), and stored at 4°C. The Yasue method uses 5% aqueous silver nitrate and rubeanic acid to generate dark brown-to-black histochemical staining of calcium deposits (22). There is a large amount of literature examining the effects of storage of formalin-fixed cut sections on loss of antigen expression. Cut sections must be stored in a refrigerator at low humidity (21). Grillo et al. reported a better way to ensure antigen preservation: storage of the formalin-fixed paraffin-embedded tissue blocks in a refrigerator (9). They found that tissue can be stored this way for decades with minimal loss of antigen expression. We used this method to store our samples, and all sections were retrieved from deep cuts into the paraffin block, another step that results in superior antigen expression.
AQP1 and ClC-Ka immunolocalization.
Slides were deparaffinized in xylene and rehydrated in graded ethanol from 100% to phosphate-buffered saline. For antigen retrieval, slides were steamed for 30 min in a rice cooker, allowed to cool, and then exposed to endogenous blocker for 1 h. Slides were rinsed with three changes of phosphate-buffered saline for 5 min each and transferred to Sequenza racks with 100-μl well coverslips. Protein blocker [0.5% Blotto + 10% cold-water fish gelatin (a very effective “blocking” agent) in Tris-buffered saline (TBS)] was applied for 1 h. Immediately thereafter (without rinses), goat AQP1 antibody (Santa Cruz Biotechnology), diluted 1:50 in Van Gogh Yellow (BioCare Medical), was applied. Slides were left overnight at room temperature in primary antibody, rinsed three times in TBS for 5 min each, incubated using an avidin-biotin complex (ABC) alkaline phosphatase kit (Vector Laboratories), and stained with alkaline phosphatase Vector Red.
For secondary staining, slides were rinsed three times in TBS and then stained with rabbit ClC-Ka (Sigma-Aldrich; 1:25 dilution). Slides were again left overnight at room temperature, rinsed three times in TBS, incubated using a rabbit ABC alkaline phosphatase kit, and stained with Vector Blue. Controls, in which the primary antibody was eliminated, showed no staining. Colocalization studies resulted in a change of color from red to magenta in AQP1-stained tubules. The stained sections were examined with a Leica DME microscope (Leica Microsystems, Deerfield, IL) equipped with a Slider digital camera (Spot Imaging Solutions, Sterling Heights, MI) and a ×20 objective.
Analysis of Tissue
We prepared three consecutive 4-μm serial sections of each biopsy: S1, stained for AQP1 only (not shown); S2, stained for AQP1 and Yasue, to enable colocalization of plaque with AQP1 (Fig. 1A); and S3, stained for AQP1 and ClC-Ka and Yasue (Fig. 1B). This allowed us to identify AQP1- and ClC-Ka-containing segments and differentiate one from the other in the presence of Yasue.
Fig. 1.
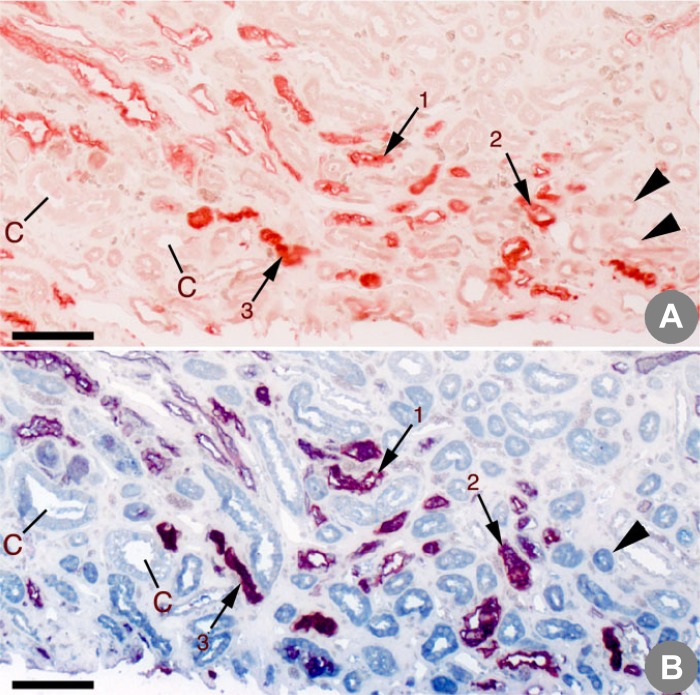
Serial sections from the same region of the inner medulla of a control patient. A: section stained with aquaporin 1 (AQP1) and Yasue. B: section stained with AQP1, kidney-specific chloride channel ClC-Ka, and Yasue. In the section stained only with APQ1 (A), positively stained loops are red; in the section stained with both APQ1 and ClC-Ka (B), positively stained descending thin loops are magenta and ClC-Ka-positive ascending thin loops are blue. Serial sections in A and B allow one to trace a single tubule between them. Small arrows mark the same AQP1-positive descending loops (1, 2, and 3) in A and B. Large arrowheads mark unstained ascending thin loops in A that are seen as ClC-Ka-positive ascending loop in B. No Yasue-positive deposits are noted in this control patient. C, medullary collecting ducts. Scale bars = 150 μm.
In Fig. 1, arrows 1–3 show tHL in serial sections stained for AQP1 in descending tHL; arrowheads denote ascending tHL, which are unstained in Fig. 1A and stained for ClC-Ka in Fig. 1B. In Fig. 1B, addition of ClC-Ka staining changes AQP1 stain color from red to magenta, a color change for the AQP1 stain that does not imply colocalization but merely a chemical change in the color created. Therefore, magenta staining is expected in AQP1-containing tubules double-stained for AQP1 and ClC-Ka, and red staining is expected when AQP1 stain is used alone with or without Yasue.
In Fig. 1, A and B, collecting ducts stain with neither antibody. Yasue stain is negative here, because this biopsy is from a non-stone-forming patient who lacked plaque. In other words, Yasue stain, when combined with our pore-staining antibodies, does not create artifacts.
Because we did not employ a tissue counterstain, we could not reliably identify tHL that did not stain with either antibody, even if they contained plaque. Plaque in the interstitium or in a tHL segment can look the same. Therefore, we only counted AQP1- or ClC-Ka-stained tHL. In principle, we could identify double-immunostained tHL (AQP1 + ClC-Ka-stained), but we found none, as expected, given the site specificity of the transporter and pore.
Using the S2 segment (AQP1 + Yasue), we classified all visible tHL as AQP1-positive and plaque-positive or plaque-negative, giving us two possible categories: APQ1 yes, plaque yes or no. The S1 segment was used to confirm AQP1 staining, because Yasue stain weakened the AQP1 staining intensity. Because we used 4-μm consecutive serial sections, this confirmation procedure was practical, as we could identify a given tubule on both sections.
Using the S3 segment, we classified each thin limb as ClC-Ka-positive or not and as plaque-positive or not. Likewise, we determined if any segments were AQP1- and ClC-Ka-positive.
In combination, the S2 and S3 segments gave us six theoretical categories: AQP1 yes or no, ClC-Ka yes or no, and plaque yes or no. In the absence of a counterstain, tHL-negative for both AQP1 and ClC-Ka could not be recognized. We did not find double-positive AQP1- and ClC-Ka-stained segments. This leaves only APQ1-positive or ClC-Ka-positive tubule segments, each with or without plaque.
We classified every identifiable tubule on photomicrographs of the S1–S3 segments for each of five patients and hand-numbered each segment on photographic plates, so that no stained segment could be double-counted. Finally, we reinspected each plate to ensure that no stained segment was left without a corresponding number written next to it. The 15 hand-numbered plates are the original data for this report.
Statistics
Results were tabulated by patient and tubule number as positive or negative for immunostains, as noted, and for plaque. Table analysis with χ2 calculations was performed routinely (SYSTAT, San Jose, CA).
RESULTS
Serial Sections from Stone-Forming Patients
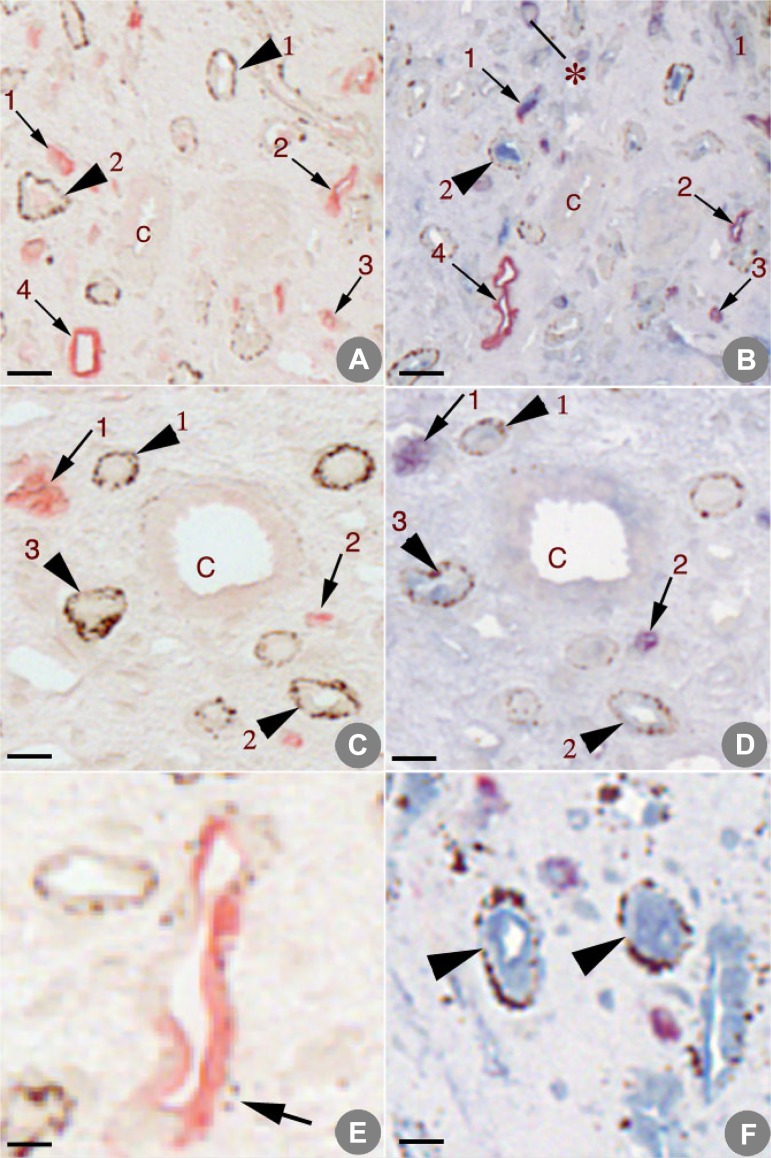
S2 sections from patients 1 and 2 (Table 1) illustrate AQP1-positive, plaque-negative tHL (Fig. 2, A and C, arrows 1–4 and arrows 1 and 2, respectively) and AQP1-negative, plaque-positive tHL (Fig. 2, A and C, arrowheads 1 and 2 and arrowheads 1–3, respectively). The AQP1-negative, plaque-positive tHL in Fig. 2, A and C, are confirmed as ClC-Ka-positive in the corresponding S3 sections (Fig. 2, B and D, arrowheads numbered to match those in A and C). Several ClC-Ka-positive tubules (Fig. 2F, arrowheads) are seen at high magnification to reveal numerous Randall’s plaque particles in the basement membrane. These serial sections allow visualization of exactly matching tubule segments. A ClC-Ka-positive tubule without plaque staining is shown in Fig. 2B.
Fig. 2.
A–D: 2 sets of serial sections from papillary biopsies from idiopathic calcium stone-forming patients. A and C: sections stained with aquaporin 1 (AQP1) and Yasue. B and D: sections stained with AQP1, kidney-specific chloride channel ClC-Ka, and Yasue. In A and C, arrows mark the same AQP1-positive tubules in A–D and arrowheads mark Yasue-positive tubules that are positive for ClC-Ka in B and D. Note ClC-Ka-positive tubule in B without Yasue stain (*). C, medullary collecting ducts. E: an AQP1-positive tubule with a few Yasue-positive particles. F: 2 ClC-Ka-positive tubules (arrowheads) at high magnification to reveal numerous Randall’s plaque particles in the basement membrane. Scale bars = 50 μm (A–D) and 20 μm (E and F).
As noted in Table 3, we found a few AQP1-positive tubules with small amounts of plaque (Fig. 2E, arrow). Two particles of plaque are adjacent to a tHL that stained positive for AQP1. Very sparse plaque around this AQP1-positive tHL was the rule, in contrast to the dense plaque that was commonly observed around ClC-Ka-positive tubules (Fig. 2C, arrowheads 1–3). Nevertheless, in our quantitative analysis, we did nothing to weight whether plaque was dense or sparse, a highly conservative approach, because this work is the critical test of a hypothesis.
Table 3.
Counts of stained tubules by presence of plaque
| AQP1-Positive Tubules |
ClC-Ka-Positive Tubules |
||||||
|---|---|---|---|---|---|---|---|
| Patient No. | Plaque | No plaque | Total | Plaque | No plaque | Total | All Tubules |
| 1 | 0 | 43 | 43* | 88 | 0 | 88* | 131 |
| 2 | 1 | 50 | 51* | 54 | 25 | 79* | 130 |
| 3 | 2 | 48 | 50* | 75 | 8 | 83* | 133 |
| 4 | 1 | 22 | 23* | 60 | 8 | 68* | 91 |
| 5 | 3 | 65 | 68* | 111 | 8 | 119* | 187 |
| All | 7 | 228 | 235* | 388 | 49 | 437* | 672 |
AQP1, aquaporin 1; ClC-Ka, kidney-specific chloride channel A.
χ2 P < 0.001.
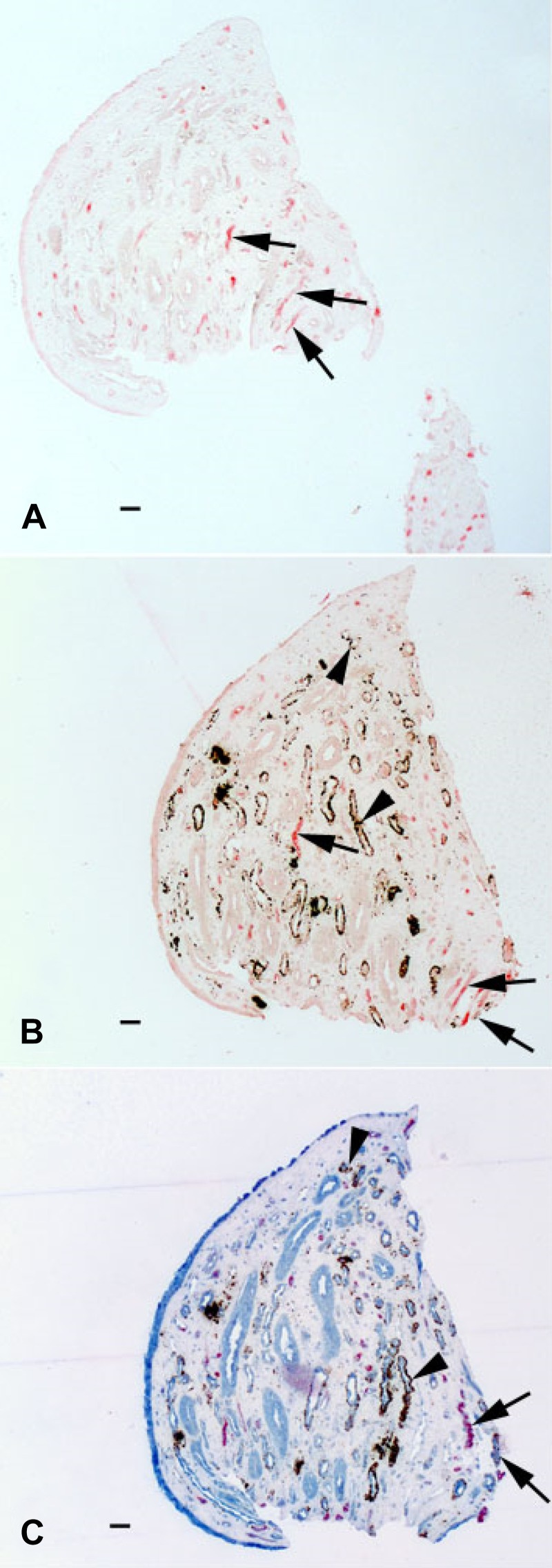
Figure 3 shows three serial sections from an ICSF stone-forming patient to demonstrate a set of S1, S2, and S3 sections: an S1 section stained only with AQP1, an S2 section stained with AQP1 and Yasue, and an S3 section stained with AQP1, Yasue, and ClC-Ka.
Fig. 3.
Three serial sections (S1, S2, and S3) from a papillary biopsy of an idiopathic calcium stone-forming patient. A: section stained with only aquaporin 1 (AQP1) (S1). B: section stained with AQP1 and Yasue (S2). C: section stained with AQP1, Yasue, and kidney-specific chloride channel ClC-Ka (S3). Arrows denote AQP1-positive tubules (A–C); arrowheads mark ClC-Ka-positive tubules (B and C). Scale bars = 50 μm.
Association of Plaque with Immunostaining
AQP1 staining was powerfully associated with a lack of plaque staining (Table 3), while ClC-Ka staining was the opposite: ClC-Ka-stained tubules were also plaque-positive in a majority of cases (χ2 = 464, P < 0.001). This pattern was clear (Table 3) and highly significant for all five patients taken separately.
DISCUSSION
Plaque Forms Preferentially in Ascending, But Not Descending, tHL
Our primary conclusion is that plaque forms in the basement membranes of ascending, but not descending, tHL. This finding fulfills a critical prediction of the vas washdown theory. Preloading of descending vas recta in the outer medulla with calcium could create topographically localized high calcium phosphate supersaturations more readily in the immediate vicinity of ascending than descending tHL. This occurs because water movement out of descending tHL must dilute calcium in the immediate interstitial vicinity, whereas little or no water leaves the ascending tHL. Therefore, the present work is a critical test of the theory.
The tHL asymmetry established here is one of a group of observations that support the vas washdown theory. The vascular bundles in the outer stripe of the outer medulla are surrounded by rings of the TAL, which reabsorb calcium without water (18). Being mainly electrogenic, the net movement of calcium out of the TAL will vary directly with calcium delivery. Patients with stones and abundant plaque often have IH, the physiology of which, especially in men, involves a higher-than-normal delivery of calcium out of the proximal tubule (11). Increased delivery to the TAL, because of IH, could result in greater-than-normal preloading of descending vas recta, and the preloaded blood would raise interstitial calcium concentration and, therefore, calcium phosphate supersaturation directly around the basement membrane of the tHL, fostering plaque.
The plaque asymmetry echoes the well-established anatomic asymmetry between ascending and descending tHL. The former, along with the vas recta, comprise the “vascular bundles.” In other words, descending vas recta, which, according to our theory, are preloaded with excess calcium in IH, lie preferentially near the ascending tHL, where plaque forms (18).
Possible Sources of Error
Our stains appear reliable, inasmuch as we readily demonstrated AQP1-positive and ClC-Ka-positive segments but no instances of both stains in a single segment. The transporter and pore are known to not overlap in their distributions.
Because plaque deposition could possibly interfere with AQP1 staining, we could not identify descending tHL with plaque. Because plaque develops in the basement membrane, but not on the apical membrane, where AQP1 resides, this is less plausible. The fact that we readily identified ClC-Ka staining in plaque-bearing tHL argues strongly that plaque does not interfere with immunostaining of membrane channels. ClC-Ka is located on the apical and basolateral membranes (17). The possibility that plaque deposits interfere with immunostaining for AQP1, but not ClC-Ka, seems remote.
Human tissue AQP1 staining of long loops becomes attenuated as they progress downward through the inner medulla, because AQP1 abundance falls (14). However, we readily identified AQP1-positive and plaque-negative thin segments at the same medullary depth as ClC-Ka-positive and plaque-positive tHL, yet we found almost no AQP1-positive and plaque-positive tHL.
In the absence of counterstain, we could not reliably identify tHL without immunostaining. Simple Yasue deposits could always be interstitial, even if the shape was suggestive of a tubule structure. However, this lack of tHL identification without AQP1 or ClC-Ka staining could not alter our main conclusion, unless a large fraction of unstained tubules was, indeed, AQP1-positive and plaque-positive. We have already put forth our argument against a selective deletion of AQP1 staining in plaque-bearing tubules.
Because selection bias is a concern, we counted every identifiable tHL segment in the S2 and S3 sections from our five patients, and we confirmed AQP1 staining for each such segment using the corresponding S1 section: 15 serial sections (3 from each of 5 patients) in all. Each counted segment was numbered directly on a photographic replica of the section, making a permanent laboratory record. Upon careful review, we could find no unnumbered segments. This process leaves little room for selection bias.
Alternative Mechanisms for Plaque Genesis
Although the aim of the present work was to test the vas washdown hypothesis, other mechanisms may well contribute to plaque genesis.
tHL fluid.
The sources of calcium for supersaturations to drive basement membrane crystallization are not likely to be primarily the tHL themselves. Even though estimates from animal micropuncture experiments indicate a high calcium phosphate supersaturation at the bend of the loops (4), epithelial calcium permeability is so low that delivery of calcium from the lumen into the basement membrane could create high supersaturations only if interstitial calcium concentrations were high enough to prevent diffusive losses. Our present data support no further discussion of this important matter.
IMCD.
IH itself could increase medullary calcium concentration via increased IMCD calcium reabsorption: more calcium per unit of time delivered into the IMCD. We have found only two reports of IMCD calcium reabsorption, both in rodents (1, 13), so whether it occurs in humans is unclear. Notably, plaque does not originate on IMCD basement membranes, although it may occur there. Perhaps this is because water conservation in the IMCD dissipates local interstitial supersaturations. The IMCD specifically clusters with neither tHL segment.
Bone formation.
Osteogenesis has been proposed as a mechanism for plaque production (10). A cell culture from a human kidney with medullary sponge abnormalities produced calcifications typically seen with cultured osteoblasts and expressed osterix, a bone transcriptional factor (15). We found no bone gene expression in regions of human plaque (8). Although the role of bone formation in plaque remains uncertain, future research will have to account for localization of such a mechanism preferentially in ascending vs. descending tHL.
Vascular calcification.
Possibly, plaque starts as calcification in vas recta (16). Neither we nor any other investigators have demonstrated plaque origin in vasa recta; rather, plaque deposits enlarge and surround these vessels. It would be difficult to envision how this mechanism would produce plaque formation selective to ascending vs. descending tHL.
This Was a Critical Test of Vas Washdown
Had plaque distributed equally between ascending and descending limbs or predominated at the descending limb, the premise of vas washdown would be contradicted and the theory overturned. This is because the theory depends utterly on local microenvironmental supersaturations that must be dissipated, to some extent, by water movement from descending thin limbs. Our finding of what the theory predicts does not mean the theory is true, merely that it has survived a critical test and can be considered viable for planning additional tests.
Summary
We have demonstrated an asymmetry of plaque locale massively preferential to ascending vs. descending tHL. Whereas one can imagine many possible explanations for the asymmetry, a most obvious mechanism would be the corresponding asymmetry of water movement: high through the descending segment and absent through the ascending segment.
Our proposed mechanism, vas washdown, requires this asymmetry as a necessary prediction, because it envisions an increased delivery of calcium into the region around ascending limbs. Water movement out of descending tHL must reduce any local interstitial supersaturation such calcium delivery might foster, whereas the ascending limb, lacking channels for such water movement, would be a perfect environment for supersaturation to increase and create nucleation of crystals. In other words, we have mounted a critical attempt to falsify the vas washdown hypothesis and have failed to falsify it, meaning that it remains a tested and, therefore, usable hypothesis.
GRANTS
This work was supported by National Institute of Diabetes and Digestive and Kidney Diseases Grant P01 DK-056788.
DISCLOSURES
No conflicts of interest, financial or otherwise, are declared by the authors.
AUTHOR CONTRIBUTIONS
A.P.E. conceived and designed research; A.P.E., J.E.L., and S.B.B. performed experiments; F.L.C. and E.M.W. analyzed data; A.P.E., F.L.C., and E.M.W. interpreted results of experiments; A.P.E. and E.M.W. prepared figures; A.P.E., F.L.C., and E.M.W. drafted manuscript; A.P.E., F.L.C., and E.M.W. edited and revised manuscript; A.P.E., F.L.C., and E.M.W. approved final version of manuscript.
REFERENCES
- 1.Bengele HH, Alexander EA, Lechene CP. Calcium and magnesium transport along the inner medullary collecting duct of the rat. Am J Physiol Renal Physiol 239: F24–F29, 1980. doi: 10.1152/ajprenal.1980.239.1.F24. [DOI] [PubMed] [Google Scholar]
- 2.Coe FL, Evan AP, Lingeman JE, Worcester EM. Plaque and deposits in nine human stone diseases. Urol Res 38: 239–247, 2010. doi: 10.1007/s00240-010-0296-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Coe FL, Worcester EM, Evan AP. Idiopathic hypercalciuria and formation of calcium renal stones. Nat Rev Nephrol 12: 519–533, 2016. doi: 10.1038/nrneph.2016.101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Deganello S, Asplin JR, Coe FL. Evidence that tubule fluid in the thin segment of the loop of Henle normally is supersaturated and forms a poorly crystallized hydroxyapatite that can initiate renal stones (Abstract). Kidney Int 37: 472, 1990. [Google Scholar]
- 5.Evan AP, Coe FL, Lingeman JE, Shao Y, Sommer AJ, Bledsoe SB, Anderson JC, Worcester EM. Mechanism of formation of human calcium oxalate renal stones on Randall’s plaque. Anat Rec (Hoboken) 290: 1315–1323, 2007. doi: 10.1002/ar.20580. [DOI] [PubMed] [Google Scholar]
- 6.Evan AP, Lingeman JE, Coe FL, Parks JH, Bledsoe SB, Shao Y, Sommer AJ, Paterson RF, Kuo RL, Grynpas M. Randall’s plaque of patients with nephrolithiasis begins in basement membranes of thin loops of Henle. J Clin Invest 111: 607–616, 2003. doi: 10.1172/JCI17038. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Evan AP, Lingeman JE, Worcester EM, Sommer AJ, Phillips CL, Williams JC, Coe FL. Contrasting histopathology and crystal deposits in kidneys of idiopathic stone formers who produce hydroxy apatite, brushite, or calcium oxalate stones. Anat Rec (Hoboken) 297: 731–748, 2014. doi: 10.1002/ar.22881. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Evan AP, Worcester EM, Williams JC Jr, Sommer AJ, Lingeman JE, Phillips CL, Coe FL. Biopsy proven medullary sponge kidney: clinical findings, histopathology, and role of osteogenesis in stone and plaque formation. Anat Rec (Hoboken) 298: 865–877, 2015. doi: 10.1002/ar.23105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Grillo F, Bruzzone M, Pigozzi S, Prosapio S, Migliora P, Fiocca R, Mastracci L. Immunohistochemistry on old archival paraffin blocks: is there an expiry date? J Clin Pathol 70: 988–993, 2017. doi: 10.1136/jclinpath-2017-204387. [DOI] [PubMed] [Google Scholar]
- 10.Khan SR, Canales BK. Unified theory on the pathogenesis of Randall’s plaques and plugs. Urolithiasis 43, Suppl 1: 109–123, 2015. doi: 10.1007/s00240-014-0705-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Ko B, Bergsland K, Gillen DL, Evan AP, Clark DL, Baylock J, Coe FL, Worcester EM. Sex differences in proximal and distal nephron function contribute to the mechanism of idiopathic hypercalcuria in calcium stone formers. Am J Physiol Regul Integr Comp Physiol 309: R85–R92, 2015. doi: 10.1152/ajpregu.00071.2015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Kuo RL, Lingeman JE, Evan AP, Paterson RF, Parks JH, Bledsoe SB, Munch LC, Coe FL. Urine calcium and volume predict coverage of renal papilla by Randall’s plaque. Kidney Int 64: 2150–2154, 2003. doi: 10.1046/j.1523-1755.2003.00316.x. [DOI] [PubMed] [Google Scholar]
- 13.Magaldi AJ, van Baak AA, Rocha AS. Calcium transport across rat inner medullary collecting duct perfused in vitro. Am J Physiol Renal Physiol 257: F738–F745, 1989. doi: 10.1152/ajprenal.1989.257.5.F738. [DOI] [PubMed] [Google Scholar]
- 14.Maunsbach AB, Marples D, Chin E, Ning G, Bondy C, Agre P, Nielsen S. Aquaporin-1 water channel expression in human kidney. J Am Soc Nephrol 8: 1–14, 1997. [DOI] [PubMed] [Google Scholar]
- 15.Mezzabotta F, Cristofaro R, Ceol M, Del Prete D, Priante G, Familiari A, Fabris A, D’Angelo A, Gambaro G, Anglani F. Spontaneous calcification process in primary renal cells from a medullary sponge kidney patient harbouring a GDNF mutation. J Cell Mol Med 19: 889–902, 2015. doi: 10.1111/jcmm.12514. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Taylor ER, Stoller ML. Vascular theory of the formation of Randall plaques. Urolithiasis 43, Suppl 1: 41–45, 2015. doi: 10.1007/s00240-014-0718-4. [DOI] [PubMed] [Google Scholar]
- 17.Uchida S, Sasaki S, Nitta K, Uchida K, Horita S, Nihei H, Marumo F. Localization and functional characterization of rat kidney-specific chloride channel, ClC-K1. J Clin Invest 95: 104–113, 1995. doi: 10.1172/JCI117626. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Wei G, Rosen S, Dantzler WH, Pannabecker TL. Architecture of the human renal inner medulla and functional implications. Am J Physiol Renal Physiol 309: F627–F637, 2015. doi: 10.1152/ajprenal.00236.2015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Werness PG, Brown CM, Smith LH, Finlayson B. EQUIL2: a BASIC computer program for the calculation of urinary saturation. J Urol 134: 1242–1244, 1985. doi: 10.1016/S0022-5347(17)47703-2. [DOI] [PubMed] [Google Scholar]
- 20.Worcester EM, Coe FL, Evan AP, Bergsland KJ, Parks JH, Willis LR, Clark DL, Gillen DL. Evidence for increased postprandial distal nephron calcium delivery in hypercalciuric stone-forming patients. Am J Physiol Renal Physiol 295: F1286–F1294, 2008. doi: 10.1152/ajprenal.90404.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Xie R, Chung JY, Ylaya K, Williams RL, Guerrero N, Nakatsuka N, Badie C, Hewitt SM. Factors influencing the degradation of archival formalin-fixed paraffin-embedded tissue sections. J Histochem Cytochem 59: 356–365, 2011. doi: 10.1369/0022155411398488. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Yasue T. Histochemical identification of calcium oxalate. Acta Histochem Cytochem 2: 83–95, 1969. doi: 10.1267/ahc.2.83. [DOI] [Google Scholar]