Abstract
Under certain circumstances, podocytes can be partially replaced following their loss in disease. The inability of podocytes to proliferate suggests that replacement derives from other cell types. Because neural/glial antigen 2 (NG2)-expressing cells can serve as progenitors in other organs and because herein we showed increased NG2 staining in podocytes following their loss in experimental focal segmental glomerulosclerosis, we used lineage tracing in NG2-CreER tdTomato mice to test the hypothesis that partial podocyte replacement might derive from this cell population. The percentage of glomeruli with red fluorescence protein (RFP)-labeled NG2 cells increased following podocyte depletion, which was augmented by enalapril. However, BrdU was not detected in RFP-labeled cells, consistent with the migration of these cells to the glomerulus. Within glomeruli, RFP-labeled cells did not coexpress podocyte proteins (p57, synaptopodin, nephrin, or podocin) but did coexpress markers for mesangial (α8 integrin, PDGFβ receptor) and parietal epithelial cells (PAX8, src-suppressed C-kinase substrate). These results suggest that following podocyte depletion, cells of NG2 lineage do not serve as adult podocyte progenitors but have the ability to transdifferentiate to mesangial and parietal epithelial cell fates.
Keywords: bromodeoxyuridine, focal segmental glomerulosclerosis, human podocyte number, neural/glial antigen 2, parietal epithelial cell
INTRODUCTION
In disease, the mechanisms for the replacement of adult kidney cells and an increase in their number following loss vary depending on the cell type. Within the kidney glomerulus, adult parietal epithelial, mesangial, and endothelial cells have the ability to proliferate, whereas adult podocytes cannot. The inability of podocytes to self-replicate renders them unable to replace themselves (1–5). However, studies have shown that human podocyte number does increase during adolescence (27), and under certain experimental conditions podocytes can be at least partially replaced (15, 21, 30, 35, 42), suggesting alternate cell sources explaining these changes. Recent studies have shown that certain resident kidney cell types can transdifferentiate toward the fate of others and are likely important in restoring cell number. For example, a subset of parietal epithelial cells (PECs) might derive from cells of renin lineage (CoRLs) during normal murine development (8, 33), and in disease, mesangial cells can be derived from CoRLs (12, 33, 38), adult podocytes from PECs (6, 18) and CoRL (14, 20, 24–26), and adolescent podocytes from PECs (1). It is therefore likely that PECs and CoRLs serve as adult glomerular cell progenitors (9, 19, 34–36).
These studies beg the question if other resident kidney cell types also serve as adult glomerular progenitors and affords an opportunity to learn from other organs. To this end, cells of neural/glial antigen 2 (NG2) lineage can serve as regenerative progenitor reservoirs in multiple organs, including the central nervous system (CNS) (2), eye, heart, and mesenchymal stem cells (5, 45a). Recently, Stefańska et al. (39) reported that kidney NG2-lineage cells have characteristics of osteocytes, adipocytes, and chondrocytes, raising the possibility that cells of NG2 lineage might serve as progenitors in the kidney. Indeed, NG2-expressing cells have been previously reported in normal rat glomeruli in a mesangial distribution and increase in PECs in experimental diabetic kidney disease (43). However, it is unclear if cells of NG2 lineage might serve as progenitors for adult glomerular cells in disease.
The purpose of the current studies was to determine if cells of NG2 lineage might in part contribute to the partial replacement of adult podocytes following their loss in experimental disease in mice and if this cell population is altered by the administration of an angiotensin-converting enzyme inhibitor, which is accompanied by partial podocyte replacement.
MATERIALS AND METHODS
NG2 labeling in reporter mice.
NG2-CreER tdTomato mice were generated by crossing the commercially available NG2-CreER mice (46) (cat. no. 008538, The Jackson Laboratory, Bar Harbor, ME) with tdTomato mice (22) (cat. no. 007914, The Jackson Laboratory). Upon administration of tamoxifen to double transgenic offspring, there was conditional and permanent expression of the tdTomato reporter in NG2-expressing cells. This allowed for the lineage tracing of a temporally discrete population of NG2 cells, which may have turned off NG2 expression in response to adopting a new cellular fate. Accordingly, 8-wk-old NG2-CreER tdTomato mice were administered 100 mg/kg tamoxifen by intraperitoneal injection on alternate days for 6 days and given at least 4 wk of clearance before experimental procedures (see below). To control for tamoxifen, NG2-CreER tdTomato animals were administered an equivalent volume of corn oil, the vehicle for tamoxifen, and also killed 4 wk later with no labeling detected. Mice were housed in the animal care facility of the University of Washington under specific pathogen-free conditions with ad libitum food and water. Animal protocols were approved by the University of Washington Institutional Animal Care and Use Committee (2968-04).
Inducing experimental focal segmental glomerulosclerosis and treatment groups.
Experimental focal segmental glomerulosclerosis (FSGS) was induced with a cytotoxic antiglomerular antibody as we have reported previously (6, 14–16, 20, 26, 28, 31). In brief, two doses of sheep antiglomerular antibody were administered at 12 mg/20 g body wt via intraperitoneal injection 24 h apart, which induces abrupt podocyte depletion accompanied by glomerulosclerosis.
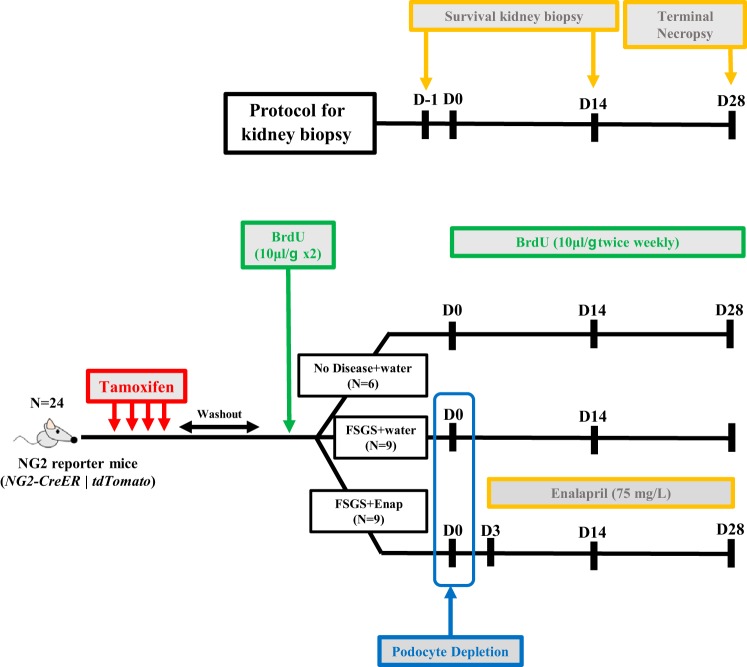
The study was designed to be able to study each individual mouse over the course of disease by performing two survival kidney biopsies in addition to the terminal harvesting of the kidney (Fig. 1). There was a total of 18 animals randomized into 3 groups: FSGS animals given water (n = 9), FSGS animals given enalapril (n = 9), and healthy mice that received a biopsy only (n = 6). The latter were included to ensure that the survival surgeries did not affect the reporting/labeling of study animals. Accordingly, following the tamoxifen washout period, all mice underwent a baseline left kidney biopsy. This kidney tissue served as the baseline for each mouse. Following a 2-wk recovery period, 18 mice were randomly selected for induction of experimental FSGS as described above. This group of mice was further randomized on FSGS day 3 to receive either drinking water or enalapril at 75 mg/l in drinking water. On day 14 of disease, all 24 mice, including those without disease, underwent a second survival biopsy of the right kidney, which served as the tissue for day 14 analysis. At day 28, a terminal necropsy was performed after mice were perfused with ice-cold PBS to remove excess red blood cells. Thus, kidney tissue was available on all mice at baseline, day 14, and day 28.
Fig. 1.
Schema of experimental design. Eight-week-old NG2-CreER tdTomato mice were given tamoxifen to label neural/glial antigen 2 (NG2) lineage cells with tdTomato, a red fluorescence protein (RFP) and then given a 4-wk tamoxifen washout period before initiation of experiments. Each mouse underwent two survival kidney biopsies in addition to the terminal necropsy of the kidney. There was a total of 24 animals randomized into 3 groups: focal segmental glomerulosclerosis (FSGS) animals given water (n = 9), FSGS animals given enalapril (Enap) (n = 9), and healthy mice that received a biopsy only (n = 6). Following the tamoxifen washout period, all mice underwent a survival baseline biopsy of the left kidney. This kidney tissue served as the baseline for each mouse. Following a 2-wk recovery period, 18 mice were randomly selected for induction of experimental FSGS (D0). This group of mice was further randomized on FSGS D3 to receive either drinking water or enalapril at 75 mg/l in drinking water. On D14 of disease, all 24 mice, including those without disease, underwent a second survival biopsy of the right kidney, which served as the tissue for D14 analysis. At D28, a terminal necropsy was performed. BrdU was administered throughout to identify cell proliferation. All animals received BrdU at 10 μl/g body wt before induction of FSGS and twice weekly after D0. D, day.
Immunostaining.
For all kidney tissues, one half was fixed overnight at 4°C in 10% neutral buffered formalin (Globe Scientific, Paramus, NJ), rinsed in 70% ethanol, processed, and embedded in paraffin. The other half was fixed for 45 min in 4% paraformaldehyde solution in PBS (Affymetrix, Santa Clara, CA), washed in 30% sucrose overnight at 4°C, patted dry, rinsed briefly in optimal cutting temperature medium (OCT), embedded in OCT, and frozen in a dry ice 100% ethanol bath. Sections were then cut from the paraffin or OCT blocks at a thickness of 4 μm.
Immunostaining was performed and assessed on all mice at baseline, day 14, and day 28. Indirect immunoperoxidase or immunofluorescence staining was performed on the paraffin sections as we have previously reported (14, 16). In brief, paraffin was removed using Histoclear (National Diagnostics, Atlanta, GA), and sections were rehydrated in a graded series of ethanol. Antigen retrieval was performed by boiling in 10 mM citric acid buffer at pH 6.0. Nonspecific protein binding was blocked using Background Buster (Accurate Chemical & Scientific, Westbury, NY) for 20 min at room temperature. Endogenous biotin activity was suppressed by an Avidin/Biotin Blocking kit (Vector Laboratories), and immunofluorescence staining was performed using the labeled streptavidin biotinylated antibody method. Antibodies were diluted in 1% IgG-free BSA in PBS. Initial primary antibodies were incubated overnight at 4°C. Secondary antibodies and streptavidin conjugates were incubated for 1 h at room temperature. All immunofluorescence-stained sections were mounted in Vectashield mounting medium with DAPI (Vector Laboratories).
BrdU labeling of mice to assess proliferation.
BrdU was administered to identify cell proliferation. All animals received BrdU at 10 μl/g body wt (BrdU-Amersham Cell Proliferation Labeling Reagent; GE Healthcare Life Sciences, Little Chalfont, United Kingdom) before induction of FSGS and twice weekly after the induction of FSGS. Double immunofluorescence for red fluorescence protein (RFP) and BrdU was performed on paraffin-embedded tissue as we have reported (11, 14, 20). Briefly, sections were incubated with a primary anti-BrdU antibody (1:200, GE Healthcare Life Sciences, Little Chalfont, UK) at 4°C overnight and then with biotinylated anti-mouse IgG secondary (1:500, Vector Laboratories), followed by streptavidin conjugated with Alexa Fluor 488 (1:200, Molecular Probes, Eugene, OR). BrdU staining was followed by RFP staining with the same protocol described above. To quantify the percentage of RFP+ cells with expression of BrdU, the total number of RFP+ cells and BrdU+ RFP+ cells was counted in a field magnified ×200. The cells along Bowman’s capsule, in the glomerular tuft, and outside the glomerulus were assessed, respectively. The percentage was calculated as number of BrdU+ RFP+ cells divided by RFP+ cells × 100 in each part of a field. Ten fields were assessed in each mouse.
Double staining for RFP and markers of mesangial cells, endothelial cells, podocytes, and PECs.
To investigate the phenotype of NG2-lineage cells at each time point, we performed double immunofluorescence staining for RFP and markers of mesangial, podocyte, and endothelial cells on paraffin-embedded tissues as follows: mesangial cell marker; goat anti-mouse α8 integrin antibody (1:100, Abcam, Cambridge, MA), podocyte marker; rabbit anti-mouse p57 (1:600, Santa Cruz Biotechnology, Santa Cruz, CA), endothelial cell marker; rat anti-mouse CD31 antibody (1:100, Dianova, Hamburg, Germany), PEC markers; biotinylated rabbit anti-mouse PAX8 antibody (1:500, Proteintech Group, Rosemont, IL) and rabbit anti-mouse src-suppressed C-kinase substrate (SSeCKS) antibody (1:200) (4). RFP was stained using the same protocol as described above. All immunofluorescence samples were mounted using mounting medium with DAPI (Vector Laboratories).
Identifying podocytes and PECs for quantification.
Immunostaining for p57 and PAX8 was performed with periodic acid-Schiff counterstaining to measure podocyte and PEC number, as previously reported (14, 15, 29, 31). In brief, paraffin sections were processed as described above, with antigen retrieval in 1 mM EDTA, pH 6.0. Endogenous peroxidase activity was quenched with 3% hydrogen peroxide, and sections were incubated overnight at 4°C with a primary rabbit anti-mouse p57 (1:800; Santa Cruz Biotechnology) or rabbit anti-mouse PAX8, followed by rabbit-on-rodent horseradish peroxidase polymer (Biocare Medical, Concord, CA). Visualization of immunostaining was by precipitation of diaminobenzidine (Sigma-Aldrich, St. Louis, MO). Counterstaining was performed with periodic acid-Schiff by incubating slides in fresh 0.5% periodic acid (Sigma-Aldrich) for 8 min, washing in double-distilled H2O for 5 min, incubating with Schiff reagent (Sigma-Aldrich) for 10 min at room temperature, fresh 0.5% sodium metabisulfate (Sigma-Aldrich) for 5 min, and washing for 5–10 min under running warm tap water. Slides were dehydrated in ethanol and mounted with Histomount.
Quantification of podocyte and PEC number.
An average of 47.21 ± 10.43 glomeruli for each animal at each time point were analyzed to determine podocyte density by use of the correction factor method as previously reported by Venkatareddy et al. (41). PECs were identified as cells lining Bowman’s capsule that showed strong nuclear staining for PAX8, with an average of 49.12 ± 8.23 glomeruli assessed for each animal at each time point.
Identifying reporter (RFP)-positive NG2 cells.
Double immunofluorescence for RFP and collagen type IV (SouthernBiotech, Birmingham, LA) was performed to identify glomeruli easily on kidney cross sections. For each animal at each time point, 43.52 ± 10.11 4-µm thick glomerular cross sections were analyzed for the absolute number of RFP+ cells found on each tuft and/or Bowman’s capsule. A positive glomerulus was defined as containing an RFP+ cell located on the glomerular tuft or Bowman’s capsule, and this was used to determine the percentage of RFP+ glomerular cross sections.
Statistical analysis.
Data are shown as the mean ± SE. Student’s t-test was applied for comparisons between groups. Multiple groups were compared using one-way ANOVA with post hoc Tukey’s honestly significant difference test. P values < 0.05 represented statistically significant differences. All statistical analysis was performed with GraphPad Prism (version 7.0, GraphPad Software, La Jolla, CA).
RESULTS
Increased glomerular NG2 staining following podocyte depletion.
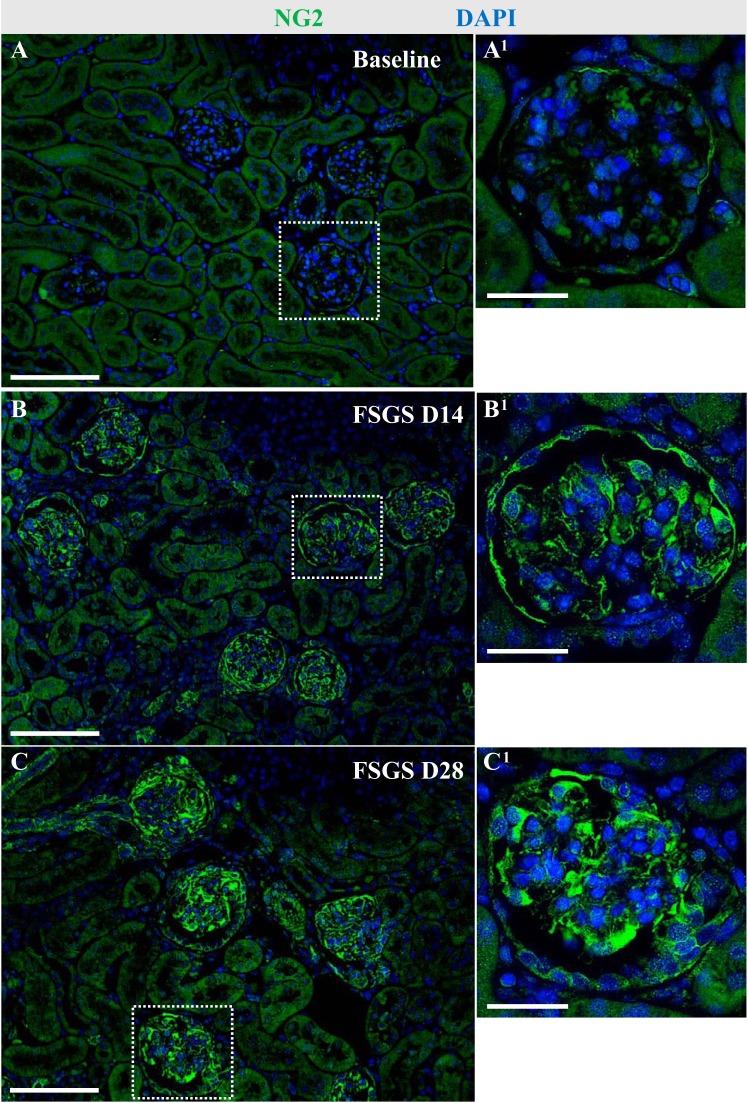
In glomeruli of healthy adult NG2-CreER tdTomato mice, faint staining for NG2 was detected in cells lining Bowman’s capsule and in the glomerular tuft in both a podocyte and mesangial cell distribution (Fig. 2). NG2 staining was also detected in a perivascular distribution in the interstitium (Fig. 2A). Because we have previously shown that perivascular CoRLs can serve as adult podocyte progenitors following loss (14, 20, 26), we used a model of experimental podocyte depletion to assess NG2 expression. Following abrupt podocyte depletion, the remaining podocytes had higher intensity of NG2 staining at day 14 (Fig. 2B) and day 28 (Fig. 2C) compared with baseline. This was accompanied by increased NG2 staining in PECs and mesangial cells (Fig. 2). These results show that the NG2 protein expression increases in glomerular epithelial and mesangial cells following podocyte depletion.
Fig. 2.
Glomerular distribution of neural/glial antigen 2 (NG2) staining following podocyte depletion. Immunofluorescence for NG2 (green) was performed to investigate the distribution of NG2-expressing cells in healthy mice and following podocyte depletion in experimental focal segmental glomerulosclerosis (FSGS). Nuclei were counterstained with DAPI (blue). Representative glomeruli indicated by the white dashed line are shown at higher magnification (×400) at each time point (A1–C1). In healthy adult NG2-CreER tdTomato mice, NG2+ cells are detected along Bowman’s capsule, in the glomerular tuft, and in perivascular cells outside the glomerulus (A). Following abrupt podocyte depletion at FSGS D14, the intensity of NG2 staining increased in cells in the glomerular tuft and along Bowman’s capsule (B). At D28, NG2 staining was similar to D14 (C). Scale bar: 100 μm (A–C) and 20 μm (A1–C1). D, day.
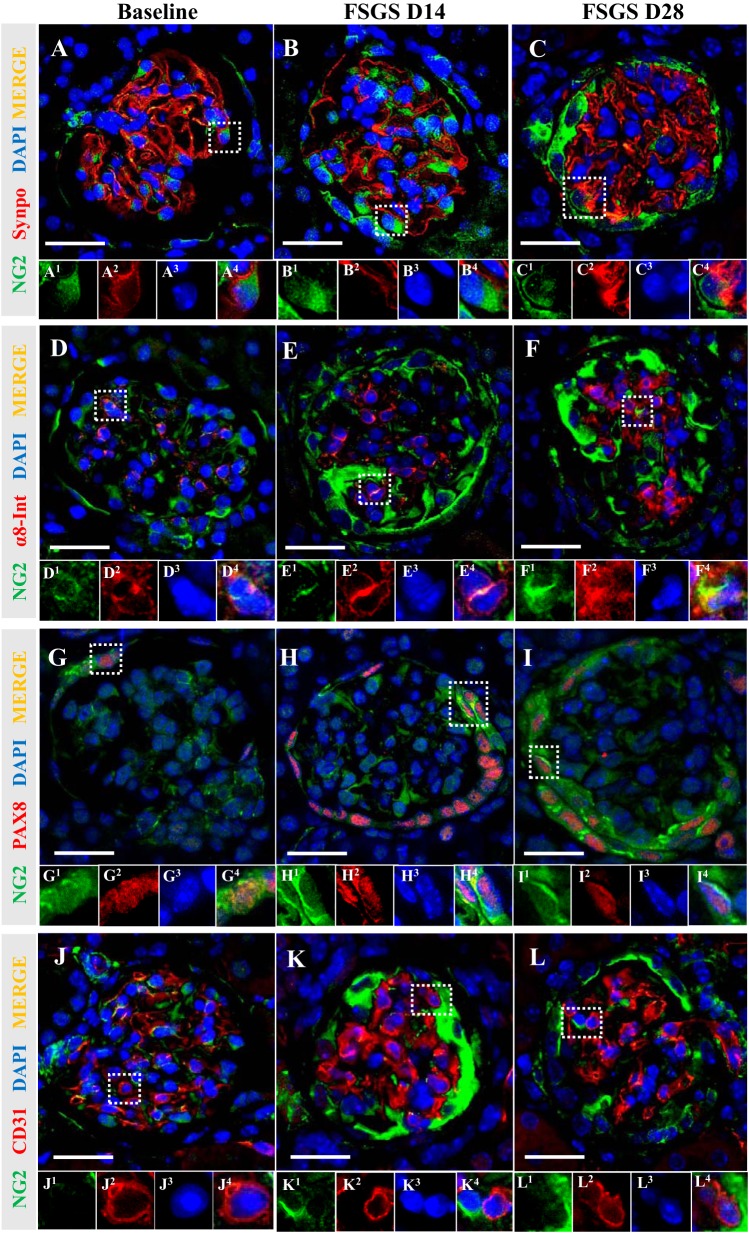
To discern exactly which glomerular cells expressed NG2 at baseline as well as FSGS day 14 and day 28, double immunofluorescence was performed for NG2 and synaptopodin (podocytes), α8 integrin (mesangial cells), PAX8 (PECs), and CD31 (endothelial cells). A subset of podocytes coexpressed NG2 (synpo+NG2+) at baseline, and double-positive cells increased at FSGS day 14 and 28 (Fig. 3, A–C). Likewise, there was costaining for NG2 and α8 integrin (α8 integrin+NG2+) in a subset of mesangial cells at baseline, which increased at FSGS day 14 and 28 (Fig. 3, D–F). Furthermore, double immunofluorescence for NG2 and PAX8 showed NG2 in PECs at baseline (PAX8+NG2+), which increased at FSGS day 14 and day 28 (Fig. 3, G–I). Glomerular endothelial cells did not express NG2 (CD31−NG2+) at any time point (Fig. 3, J–L).
Fig. 3.
Double immunofluorescence for neural/glial antigen 2 (NG2) and markers for podocytes, mesangial cells, and parietal epithelial cells (PECs) in health and disease. To determine which glomerular cell type expressed NG2, double immunofluorescence was performed for glomerular cell markers synaptopodin (Synpo), α8 integrin (α8-Int), PAX8, and CD31 (red), followed by staining for NG2 (green). Nuclei were counterstained by DAPI (blue). Representative glomeruli indicated by the white dashed line are shown at higher magnification at each time point (A1–4 to L1–4). Costaining for NG2/synpo and NG2/α8-Int showed that podocytes and mesangial cells expressed NG2 at baseline, and focal segmental glomerulosclerosis (FSGS) days 14 and 28 (A–F). Double immunofluorescence for NG2/PAX8 also showed that PECs expressed NG2 over the course of FSGS (G–I). CD31+ endothelial cells did not express NG2 at baseline or in experimental FSGS (J–L). These results show that podocytes, mesangial cells, and PECs stained for NG2 at baseline and disease. Original magnification, ×400. Scale bar: 20 μm.
Differential expression of glomerular cell markers in cells of NG2 lineage in permanently reporter-labeled healthy mice.
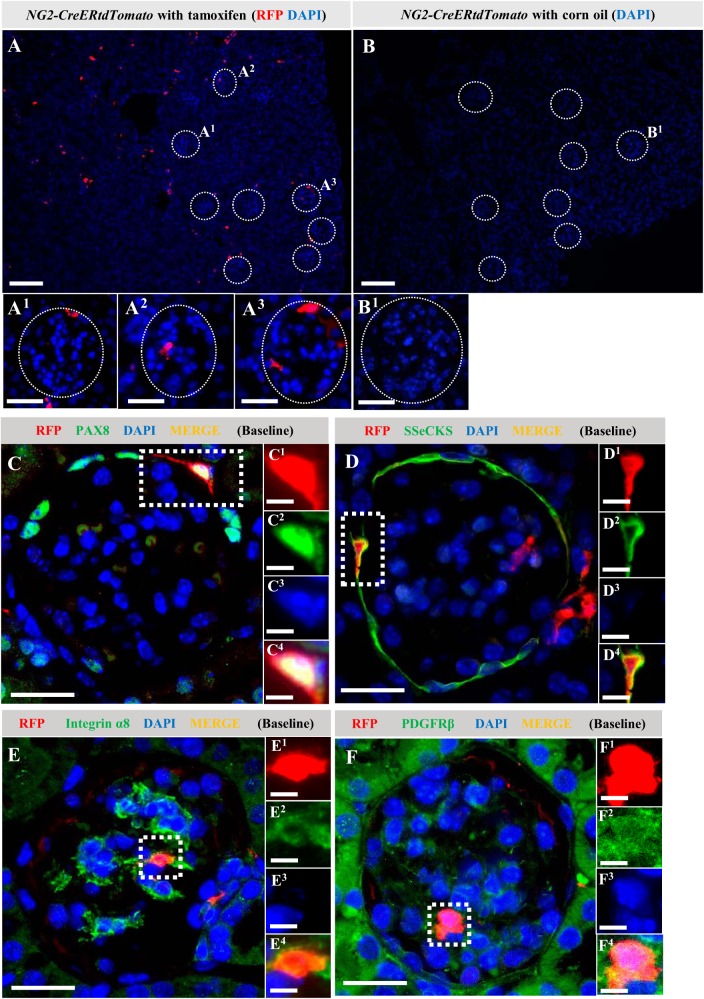
NG2-CreER tdTomato reporter mice were used to determine genetically if the higher staining for NG2 in podocytes in disease was due to de novo expression and/or if a subset of NG2-expressing podocytes derived from an NG2 lineage. Administering tamoxifen to NG2-CreER tdTomato mice induced temporally specific and permanent tdTomato reporter labeling in cells expressing NG2 (Fig. 4A). This enabled lineage tracing of a discrete population of cells of NG2 lineage following a 4-wk washout period where tamoxifen was no longer present for the labeling of de novo NG2-expressing cells. In healthy adult reporter mice, one or more RFP+ cells were detected in ≅20% of glomeruli (Fig. 4A). Within these glomeruli, three patterns emerged: RFP+ cells along Bowman’s capsule (Fig. 4A1), within the glomerular tuft (Fig. 4A2), or both (Fig. 4A3). To ensure labeling consistency across all mice before inducing podocyte depletion, mice underwent a survival biopsy following tamoxifen washout. RFP immunofluorescence showed that the labeling efficacy of RFP was not significantly different among the mice (data not shown). As expected, RFP was not detected in control NG2-CreER tdTomato mice given corn oil, the vehicle for tamoxifen (Fig. 4, B and B1).
Fig. 4.
Red fluorescence protein (RFP)+ neural/glial antigen 2 (NG2)-lineage cells along Bowman’s capsule express parietal epithelial cells (PEC) and mesangial cell markers at baseline. Administering tamoxifen to NG2-CreER tdTomato reporter mice induced temporally specific and permanent tdTomato reporter labeling in cells expressing NG2. To confirm tdTomato reporter labeling in NG2-lineage cells, immunofluorescence for RFP (red) was performed in NG2-CreER tdTomato mice administered tamoxifen and mice given corn oil, the vehicle for tamoxifen. Nuclei were counterstained with DAPI (blue). Glomeruli are indicated with white dotted lines (A). In mice treated with tamoxifen, RFP+ cells were detected in ≅20% of glomerular cross sections. Within these glomeruli, three patterns of RFP+ cells emerged: along Bowman’s capsule (A1), within the glomerular tuft (A2), or both (A3). RFP was not detected in control NG2-CreER tdTomato mice given corn oil (B). To clarify if labeled NG2-lineage cells in glomerular locations coexpressed resident glomerular cell markers in healthy glomeruli, double immunofluorescence was performed for RFP and cell-specific antibodies (green). Nuclei were counterstained with DAPI (blue). Ninety-eight percent of RFP+ cells along Bowman’s capsule coexpressed the PEC markers, PAX8 (C) and mouse src-suppressed C-kinase substrate (SSeCKS) (D). Within healthy glomerular tufts, 98%–100% of RFP+ cells coexpressed the mesangial cell markers α-ntegrin (E) and PDGFRβ (F). Representative RFP+ areas are indicated with square dotted lines (C–F) and magnified (C1–4 to F1–4). These results show that in the survival kidney biopsy in healthy mice, RFP-labeled NG2 cells coexpressed mesangial and PEC markers. Original magnification, ×100 (A and B), ×400 (A1–3, B1, and C1–4 to F1–4). Scale bar, 100 μm (A and B), 20 μm (A1–3, C–F), and 10 μm (C1–4 to F1–4).
To clarify which labeled cells of NG2 lineage within glomeruli coexpressed resident glomerular cell markers in healthy mice, double immunofluorescence was performed for RFP and glomerular cell-specific antibodies. Of the RFP+ cells along Bowman’s capsule, 98% coexpressed the PEC markers, PAX8 (Fig. 4C) or SSeCKS (Fig. 4D). Within healthy glomerular tufts, 98%–100% of RFP+ cells coexpressed the mesangial cell markers α8 integrin (Fig. 4E) or PDGFβ receptor (Fig. 4F).
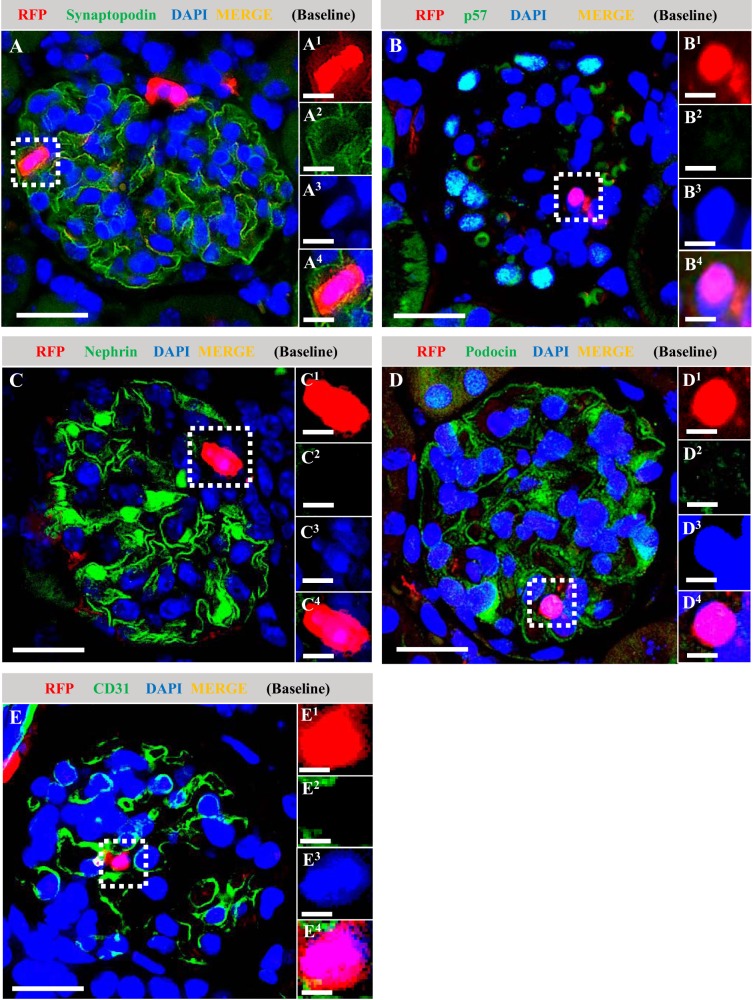
In contrast, in the healthy glomeruli RFP+ cells did not coexpress the podocyte markers synaptopodin (Fig. 5A), p57 (Fig. 5B), nephrin (Fig. 5C), or podocin (Fig. 5D), nor did RFP+ coexpress the endothelial cell marker CD31 (Fig. 5E).
Fig. 5.
Red fluorescence protein (RFP)+ neural/glial antigen 2 (NG2)-lineage cells do not coexpress podocyte or endothelial cell markers in healthy mice at baseline. Double immunofluorescence was performed on survival kidney biopsies for RFP (red), the podocyte markers synaptopodin, p57, nephrin, and podocin (all green), and the endothelial cell marker CD31 (green). Nuclei were counterstained with DAPI (blue). Representative RFP+ areas are indicated with square dotted lines and magnified (A1–4 to E1–4). At baseline, RFP+-labeled cells did not coexpress the podocyte markers synaptopodin (A), p57 (B), nephrin (C), podocin (D), or CD31 (E). Original magnification, ×400 (A–E). Scale bar, 20 μm (A–E) and 10 μm (A1–4 to E1–4).
Taken together, these results show that during the 6-day labeling window in young adult mice with tamoxifen, followed by 4 wk of washout, a subset of PECs and mesangial cells of NG2 lineage reside in healthy mouse glomeruli and were labeled with reporter. In contrast, neither podocytes nor glomerular endothelial cells were of NG2 lineage in healthy adult mouse glomeruli.
Podocyte depletion in NG2 reporter mice with experimental FSGS.
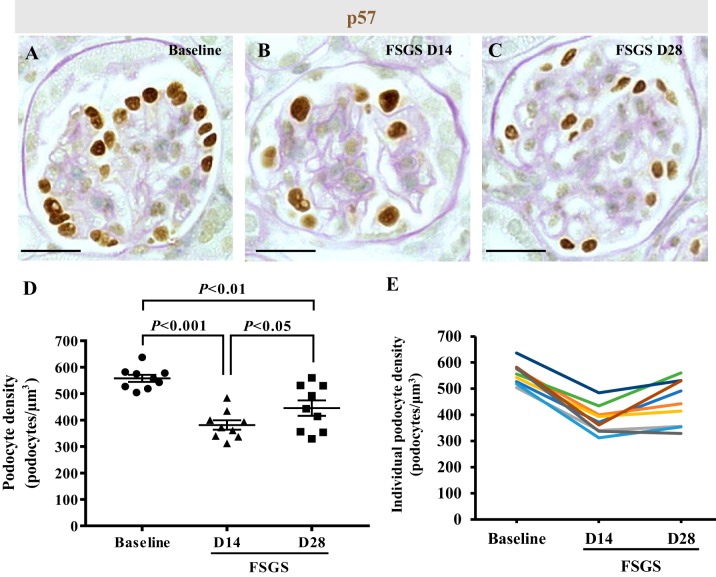
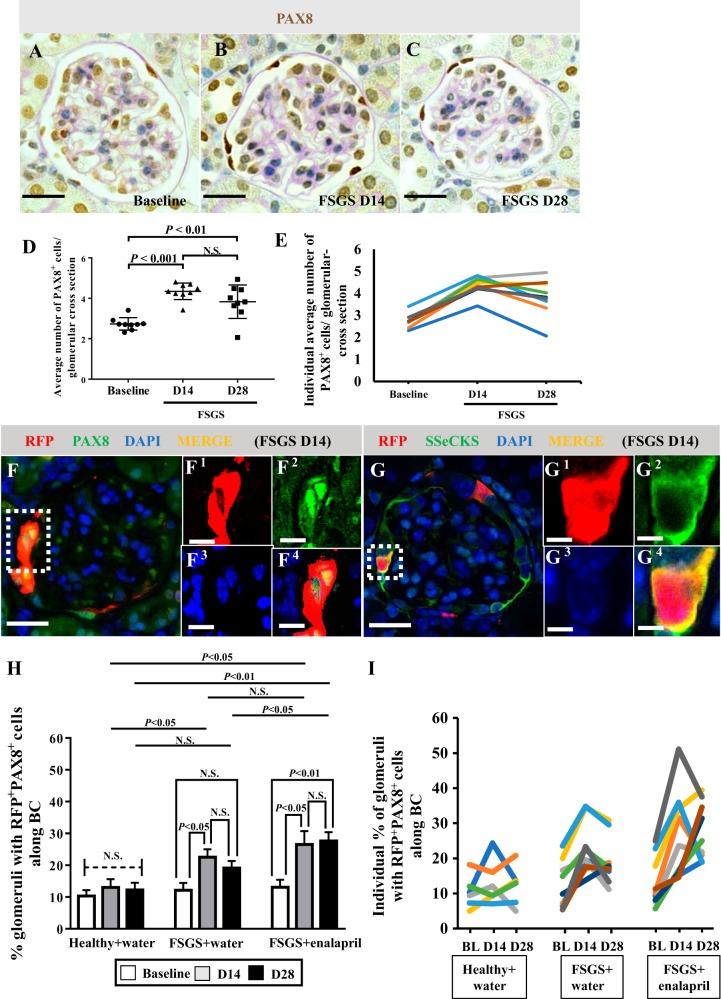
Because NG2 cells serve as progenitors for many nonkidney cell types (2, 5, 45a) and because our results showed increased NG2 staining in podocytes following depletion we asked if cells of NG2 lineage might serve an adult podocyte progenitor role following podocyte depletion in disease. Accordingly, podocyte depletion was induced in NG2-CreER tdTomato reporter mice. Because we performed two survival biopsies (one at baseline and one at day 14 of disease) in addition to obtaining kidney tissue at euthanasia on day 28, a time course for podocyte number measured by immunostaining for p57 was quantitated in each individual mouse separately at all 3 time points (Fig. 6). Results are reported at each time point as the mean ± SE for the group as a whole (Fig. 6D) and for individual mice (Fig. 6E). Podocyte density decreased from baseline by 31.6% in FSGS mice on day 14 (557.91 ± 13.51 vs. 381.4 ± 17.79 number of podocytes/glomerular volume in µm3, P < 0.001 vs. baseline) (Fig. 6D). However, on day 28, podocyte density was 16.78% higher than day 14 (445.46 number of podocytes/glomerular volume in µm3, P < 0.05 vs. day 14) (Fig. 6D). These results are consistent with abrupt podocyte depletion by day 14, followed by partial podocyte replacement at day 28 in this mouse strain, similar to what we have reported in this model in other mouse strains (15).
Fig. 6.
Podocyte depletion in neural/glial antigen 2 (NG2) reporter mice with experimental focal segmental glomerulosclerosis (FSGS). A–C: p57 immunohistochemistry was performed to measure podocyte density in NG2-CreER tdTomato mice and counterstained with periodic acid-Schiff to visualize glomeruli better. D: graph shows that podocyte density (number of podocytes/µm3) significantly decreased at FSGS D14 compared with baseline (P < 0.001). Although podocyte density was still lower at FSGS D28 compared with baseline (P < 0.01), there was partial recovery at FSGS D28 compared with FSGS D14 (P < 0.05). E: line graph showing podocyte density for individual mice, each represented by a different color. Original magnification, x400 (A–C). Scale bar, 20 μm. D, day.
BrdU was administered throughout the study to detect proliferating cells over an extended time course. BrdU staining was very rarely detected in diseased glomeruli, similar to our previous reports, with the occasional rare cell being a circulating cell type rather than a resident glomerular cell type (15). This was not a false negative because BrdU was readily detected in neighboring tubular epithelial cells, serving as an internal control. These results suggest that because podocytes did not proliferate, the origin for the partial replacement of podocytes is likely from another cell source.
Labeled cells of NG2 lineage increase in glomeruli following podocyte depletion.
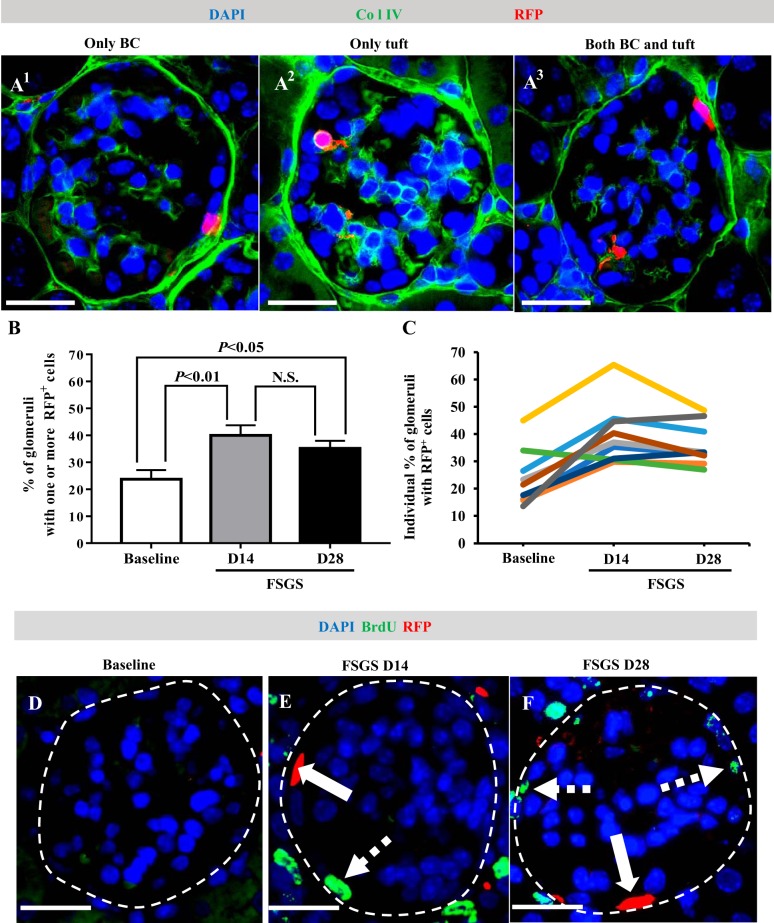
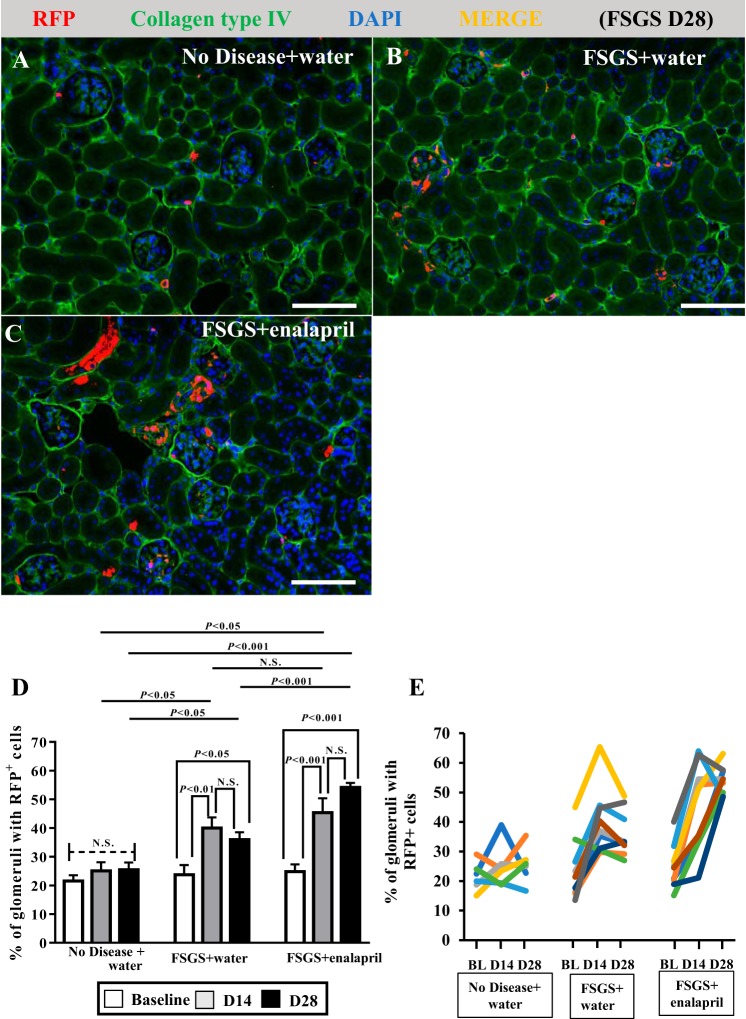
Following podocyte depletion, RFP+ cells were detected along Bowman’s capsule, in the glomerular tuft, and in both locations (Fig. 7A1–3). When quantitated, the percentage of glomerular cross sections with RFP+ cells increased from 23.71 ± 3.43% at baseline to 39.97 ± 3.73% at day 14 (P < 0.01 vs. baseline) (Fig. 7B). There was no further increase at day 28 (35.16 ± 2.81% of all glomeruli, P < 0.05, vs. baseline, P > 0.05 vs. day 14) (Fig. 7B). However, the mean number of RFP+ cells within individual glomeruli did not change over the course of disease (baseline: 1.35 ± 0.067, day 14: 1.516 ± 0.073, day 28: 1.42 ± 0.06/glomerular cross section, P > 0.05 comparison among each time point). The percentage of glomeruli with RFP+ cells did not change in age-matched animals that did not receive FSGS (data not shown). The results for individual mice are shown in Fig. 7C.
Fig. 7.
Red fluorescence protein (RFP)+ neural/glial antigen 2 (NG2)-lineage cells increase in glomeruli following podocyte depletion. Double immunofluorescence for RFP and collagen type IV (Col IV, green, used to demarcate glomerular outlines) was performed to assess the RFP+ NG2-lineage cells in glomeruli following podocyte depletion. Nuclei were counterstained with DAPI (blue). Three patterns emerged, and examples are shown (A). RFP+ cells distributed only along Bowman’s capsule (BC) (A1), only in the glomerular tuft (A2), or both (A3). Bar graph shows that the percentage of glomeruli with RFP+ cells increased at FSGS D14 (P < 0.01 vs. baseline) with no further increase at FSGS D28 (P < 0.05 vs. baseline, P > 0.05 vs. D14) (B). Line graph shows the percentage of glomeruli with RFP+ cells for individual mice, each represented by a different color (C). To measure proliferation in RFP+ NG2-lineage cells, double immunofluorescence was performed for RFP (red) and BrdU (green) (D–F). Glomeruli are outlined by white dashed line. Solid arrows indicate RFP+ cells along BC; dashed arrows indicate BrdU+ cells along BC. RFP+ cells in the glomerular tuft and along Bowman’s capsule did not coexpress BrdU in FSGS mice. These result support migration but not proliferation underlying the increase in labeled cells of NG2 lineage in the glomerulus following podocyte depletion. Original magnification, ×400 (A and D–F). Scale bar, 20 μm (A and D–F). D, day; N.S., not significant.
RFP+ cells in the glomerular tuft or along Bowman’s capsule did not coexpress BrdU (Fig. 7, D–F). The increase in the percentage of glomeruli with RFP+ cells in the absence of RFP+ cell proliferation is consistent with migration of RFP+ cells into the glomerulus.
Labeled NG2-lineage cells do not coexpress podocyte markers in diseased glomeruli.
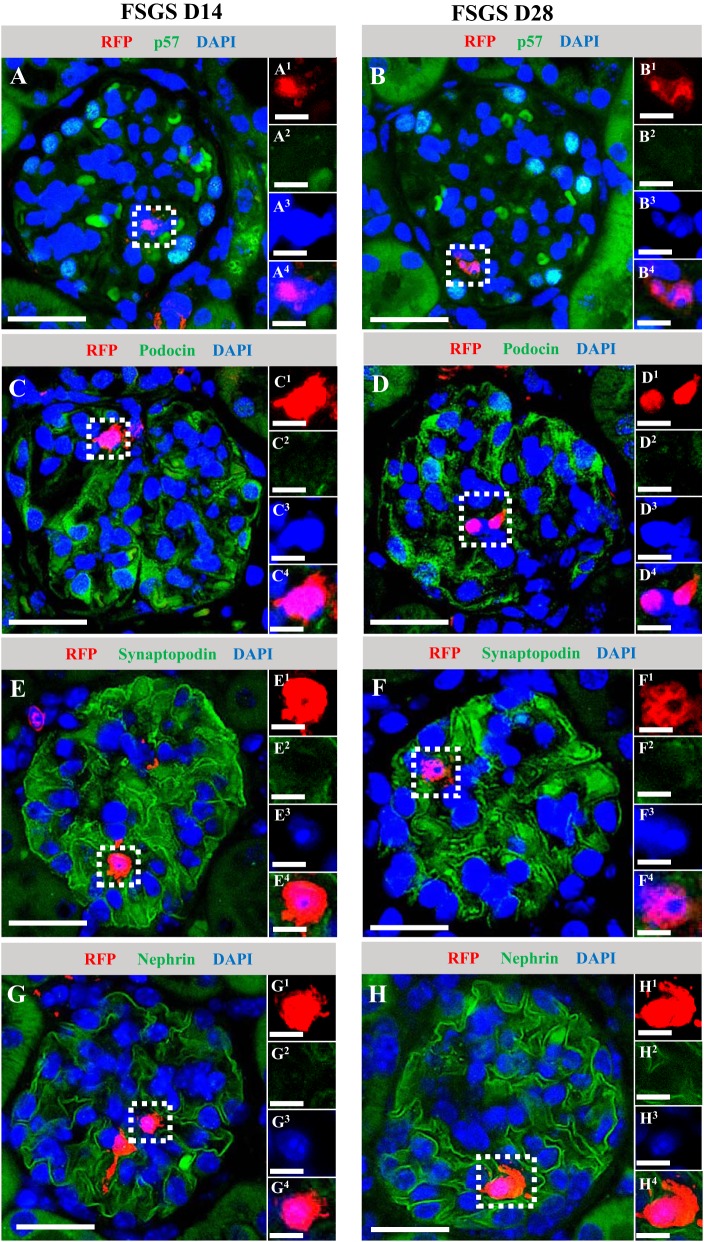
Because NG2-labeled cells increased in glomeruli of diseased mice typified by abrupt podocyte depletion followed by partial replacement, we hypothesized that cells of NG2 lineage might account for some of the increase in podocyte number. However, the results showed that at both day 14 and day 28 of disease, RFP+ cells did not costain for the podocyte markers p57 (Fig. 8, A–B), podocin (Fig. 8, C–D), synaptopodin (Fig. 8, E–F), or nephrin (Fig. 8, G–H).
Fig. 8.
Labeled neural/glial antigen 2 (NG2)-lineage cells that migrated to the glomerulus following podocyte depletion do not coexpress podocyte markers. To test the hypothesis that cells of NG2 lineage might account for some of the increase in podocyte number following podocyte depletion, double immunofluorescence was performed for red fluorescence protein (RFP) (red) and 4 podocyte markers (green) in mice with FSGS days 14 and 28. Nuclei were counterstained with DAPI (blue). Both at D14 and D28 of disease, RFP+ cells did not costain for the podocyte markers p57 (A and B), podocin (C and D), synaptopodin (E and F), or nephrin (G and H). Representative RFP+ areas are indicated by square dotted lines (A–H) and magnified (A1–4 to H1–4). Original magnification, ×400 (A–H). Scale bars, 20 μm (A–H) and 10 μm (A1–4 and H1–4). D, day.
Enalapril treatment stimulates increase of RFP+ cells in NG2 reporter mice with experimental FSGS.
We have reported that podocyte replacement can be augmented in this model when enalapril treatment is started at day 3, which coincides with marked podocyte depletion (20). Accordingly, we next asked if enalapril might enhance RFP-labeled cells of NG2 lineage to coexpress podocyte proteins. Assessment of RFP+ cells was performed with double immunofluorescence for RFP and collagen type IV as described earlier (Fig. 9, A–C). In FSGS mice given enalapril, the percentage of glomeruli with RFP+ cells significantly increased at day 14 of disease (42.34 ± 2.93%, P < 0.05 vs. 27.54 ± 4.36% at baseline). There was no further increase by day 28 of enalapril treatment (54.15 ± 1.59%, P > 0.05 vs. 42.34 ± 2.93%, P < 0.001 at day 14) (Fig. 9D). At day 28, the percentage of glomeruli with RFP+ cells was significantly higher in mice with FSGS given enalapril than in mice with FSGS given water (P < 0.001) (Fig. 9D). Results for individual mice are shown in Fig. 9E. Moreover, the number of RFP+ cells in these glomeruli also increased in mice with FSGS given enalapril at day 28 (1.97 ± 0.17/glomerular cross section, P < 0.05 vs. baseline 1.45 ± 0.10/glomerular cross section). However, despite the intraglomerular increase in RFP+ cells following enalapril treatment, no RFP+ cells coexpressed either p57, podocin, nephrin, or synpatopodin (data not shown). The results show that the partial replacement of podocytes, with or without enalapril, is unlikely to be from cells of NG2 lineage.
Fig. 9.
Enalapril treatment increases red fluorescence protein (RFP)+ cells in glomeruli in mice with experimental focal segmental glomerulosclerosis (FSGS). Double immunofluorescence was performed for RFP and collagen IV (green, to demarcate glomerular outlines). Nuclei were counterstained with DAPI (blue). Representative images at FSGS D28 show that RFP+ cells increased in glomeruli following podocyte depletion (B) and in mice given enalapril following podocyte depletion (C). In FSGS mice given enalapril, the percentage of glomeruli with RFP+ cells significantly increased at D14 of disease (P < 0.05 vs. BL), and there was no further increase by D28 of treatment (P > 0.05 vs. D14, P < 0.001 vs. BL) (D). In addition, at D28, this percentage was significantly higher in mice with FSGS given enalapril than that in mice with FSGS given water (P < 0.001). The results for individual mice are shown each represented by a different color (E). In healthy mice given water, there was no significant difference over the course of experiment, even with undergoing survival biopsies (D and E). Original magnification, ×200 (A–C). Scale bar, 100 μm (A–C). BL, baseline; D, day; N.S., not significant.
Percentage of glomeruli with NG2-labeled cells coexpressing PEC markers increases in FSGS, and enalapril treatment augments the increase.
Because PECs are activated following podocyte injury and increase in number, we next focused on asking if a subset of cells of NG2 lineage that migrated to Bowman’s capsule coexpressed PEC markers. Similar to previous studies in this model (23, 31, 44), PEC number, quantitated by PAX8 staining, increased at FSGS day 14 (4.35 ± 0.41/glomerular cross section vs. 2.73 ± 0.31, P < 0.001 vs. baseline) and FSGS day 28 (3.83 ± 0.83/glomerular cross section, P < 0.01 vs. baseline) (Fig. 10, A–D). Results for individual mice are shown in Fig. 10E.
Fig. 10.
The percentage of glomeruli with neural/glial antigen 2 (NG2)-labeled cells coexpressing parietal epithelial cell (PEC) markers increases following podocyte depletion, which is further augmented by enalapril. Examples of PAX8/periodic acid-Schiff (PAS) staining, used to quantitate PEC number (A–C). Graph shows that the average number of PAX8+ PECs increased at focal segmental glomerulosclerosis (FSGS) D14 (P < 0.001 vs. baseline) and FSGS D28 (P < 0.01 vs. baseline) (D). Line graph for PEC number for individual mice (E). PEC number increases along Bowman’s capsule (BC) following podocyte depletion. At FSGS D14, almost all (98%) RFP+ cells located along BC coexpressed either PAX8 (F), or src-suppressed C-kinase substrate (SSeCKS) (G). D28 results were similar (not shown). Representative red fluorescence protein (RFP)+ areas are indicated by square dotted lines (F and G) and magnified (F1–4 and G1–4). Original magnification, ×400 (A–C and F–G). Scale bars, 20 μm (A–C and F–G) and 10 μm (F1–4 and G1–4). The percentage of glomeruli with RFP+PAX8+ cells increased in mice with FSGS at D14 (P < 0.05 vs. baseline) but did not increase further at D28 (H). When enalapril was started at D3 following podocyte depletion, the percentage of glomeruli with RFP+PAX8+ cells along BC increased at D14 (P < 0.05 vs. baseline) and at D28 (P < 0.01 vs. baseline, P > 0.05 vs. D14). Furthermore, at D28 the percentage in mice with FSGS given enalapril was significantly higher compared with that in mice with FSGS given water (P < 0.05). The results for the number of RFP+PAX8+ cells along BC in individual mice are shown (I). In healthy mice given water, there was no significant difference over the course of experiment, even with undergoing survival biopsy (H and I). These results show that RFP+ cells that migrated to BC transdifferentiated to a PEC fate, which was augmented by enalapril. D, day.
Of the RFP+ cells located along Bowman’s capsule at day 14 FSGS, almost all (98%) coexpressed either PAX8 (Fig. 10F) or SSeCKS (Fig. 10G). Day 28 results were similar (not shown). The percentage of glomeruli with RFP+PAX8+ cells along Bowman’s capsule significantly increased in mice with FSGS (baseline; 12.13 ± 2.33%, vs. day 14; 22.53 ± 7.47%, P < 0.05 vs. baseline, and day 28; 19.11 ± 2.23%, P < 0.05 vs. baseline, P > 0.05 vs. day 14) (Fig. 10H). However, the average number of RFP+PAX8+ cells in individual glomeruli did not change in disease (baseline; 1.37 ± 0.079, day 14; 1.36 ± 0.075, day 28; 1.39 ± 0.074/glomerular cross section, P > 0.05 comparison between each time point), only the number of glomeruli that contained RFP+PAX8+ cells increased. When enalapril was started at day 3 following podocyte depletion, the percentage of glomeruli with RFP+PAX8+ cells along Bowman’s capsule further increased (baseline; 13.08 ± 2.33%, day 14; 26.52 ± 4.21%, P < 0.05 vs. baseline, day 28; 27.63 ± 2.73%, P < 0.01 vs. baseline, P > 0.05 vs. day 14) and was significantly higher compared with that in mice with FSGS given water (P < 0.05) at day 28 (Fig. 10H). The results for individual mice are shown in Fig. 10I. The number of RFP+PAX8+ cells along Bowman’s capsule also increased (baseline; 1.30 ± 0.078, day 14; 1.74 ± 0.14, P > 0.05 vs. baseline, day 28; 1.99 ± 0.18, P < 0.01 vs. baseline, P > 0.05 vs. day 14/glomerular cross section). Almost all RFP+ cells along Bowman’s capsule coexpressed PEC markers following enalapril treatment.
To determine if the increase in NG2+ cells along Bowman’s capsule was due to the proliferation of RFP+ cells with or without enalapril, double staining was performed for RFP and BrdU. As shown earlier, RFP+BrdU+ cells along Bowman’s capsule were barely detected in FSGS mice (at day 14: 2.91 ± 1.25% of all glomeruli had one or more RFP+BrdU+ cell; at day 28: 2.07 ± 1.47% of all glomeruli. P > 0.05 vs. 0% at baseline).
These results show that the increase in RFP+ cells along Bowman’s capsule was due to migration of cells of NG2 lineage to Bowman’s capsule. Coexpression of two PEC markers suggests transdifferentiation and that their migration but not proliferation was augmented by enalapril.
NG2-lineage cells coexpressing mesangial cell markers increases in FSGS.
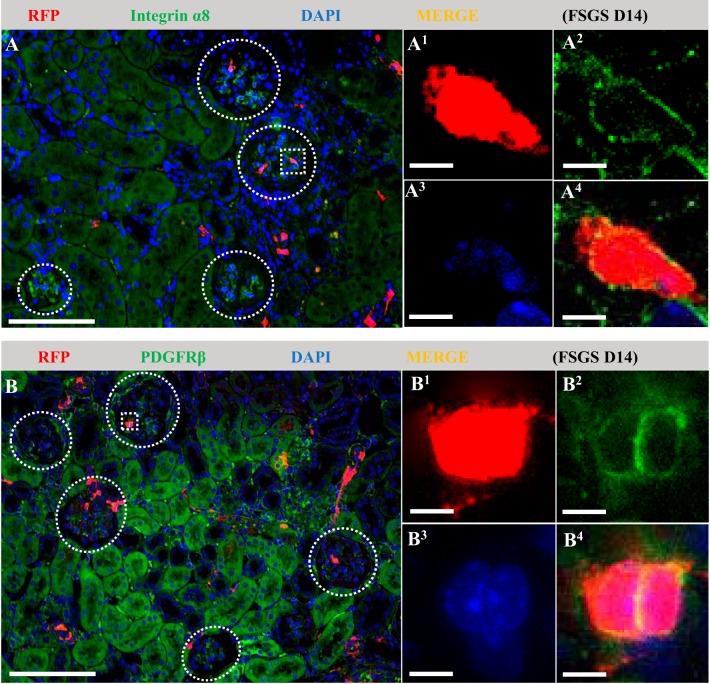
Finally, because RFP+ cells did not coexpress podocyte markers, we asked if the increased RFP+ cells in the glomerular tuft in disease coexpressed mesangial and/or endothelial cell markers. At day 14 and at day 28 of FSGS, almost all (97%) of RFP+ cells in the glomerular tuft coexpressed either α8 integrin (Fig. 11A) or PDGFβ receptor (Fig. 11B). The increase in glomeruli with RFP+ cells in the glomerular tuft following enalapril treatment was also accompanied by an increase in RFP+ α8 integrin+ cells and RFP+ PDGFβ receptor+ cells. BrdU did not increase in RFP+ cells in the glomerular tufts (day 14; 2.82 ± 1.21% and day 28; 1.91 ± 0.97% vs. 0% at baseline, P > 0.05). These results were not false negatives because BrdU was detected in occasional non-RFP+ cells along Bowman’s capsule, as well as tubular epithelial cells, which served as positive controls. In contrast, RFP+ cells in the glomerular tuft did not colocalize with the endothelial marker CD31 (data not shown).
Fig. 11.
A subpopulation of neural/glial antigen 2 (NG2)-lineage cells that migrate to the glomerulus following podocyte depletion coexpress mesangial cell markers. Double immunofluorescence was performed for red fluorescence protein (RFP) (red) and two mesangial markers (green). Nuclei were counterstained with DAPI (blue). Glomerular outlines are indicated by white circle dotted lines, and representative RFP+ areas indicated with square dotted lines are magnified. Representative images of focal segmental glomerulosclerosis (FSGS) D14 show that almost all (97%) of RFP+ cells in the glomerular tuft coexpressed either α8 integrin (A) or PDGFβ receptor (B). Original magnification, ×200 (A and B) and ×400 (A1–4 and B1–4). Scale bar, 100 μm (A and B) and 10 μm (A1–4 and B1–4). D, day.
These results suggest that cells of NG2 lineage migrated to the glomerular tuft in disease and transdifferentiated to mesangial cells but not endothelial cells, events that were augmented by enalapril.
DISCUSSION
Because adult podocytes are unable to self-replicate and are therefore unable to replace themselves following their loss in disease, any increase in their number has to derive from another cell source. To date, likely cell sources include PECs and CoRLs (17, 35, 36). The overall purpose of these studies was to explore if cells of NG2 lineage also serve an adult podocyte progenitor role, as they do in other organs and cell types such as the CNS (7). By using inducible NG2 reporter mice, the results showed that although RFP-labeled cells of NG2 lineage (referred to as RFP+) migrated to glomeruli following podocyte depletion, they did not transdifferentiate to a podocyte phenotype as we originally hypothesized. Rather, cells of NG2 lineage transdifferentiated toward either a PEC or mesangial cell phenotype.
Our results using double-staining with NG2 and glomerular cell markers showed that in healthy adult mice, NG2 staining was in podocyte and mesangial cells in the glomerular tuft and in PECs along Bowman’s capsule. However, following podocyte depletion NG2 staining increased in podocytes and PECs and decreased in mesangial cells. Increased NG2 staining in PECs has been previously reported in diabetic mice (43). The increased NG2 expression in podocytes formed the rationale for the hypothesis that cells of NG2 lineage might explain in part some of the partial replacement of podocytes following their loss in this model from day 14 to day 28.
To test this hypothesis, we used lineage tracing in an inducible NG2 reporter mouse. Following tamoxifen induction of inducible NG2 labeling with tdTomato in healthy mice, RFP+ cells were detected in approximately a quarter of glomeruli, distributed in both the glomerular tuft and along Bowman’s capsule. RFP+ cells coexpressed PEC and mesangial cell markers but not podocyte or endothelial cell markers. Next, we depleted podocytes by ~30% with a cytopathic antipodocyte antibody. This resulted in an increase in the percentage of glomeruli with RFP+ cells from baseline, which was further increased when diseased mice were given enalapril. Because the experimental design (Fig. 1) enabled lineage tracing at 3 separate time points (baseline, day 14, and day 28) in each individual mouse, the data showed that the increase in RFP in glomeruli occurred within the first 14 days and remained unchanged thereafter. BrdU labeling was given throughout the course of disease to capture proliferating cells. However, BrdU staining was not detected in RFP+ cells in glomeruli. These results show that the increase in RFP+ cells in glomeruli following podocyte depletion are consistent with the migration of NG2-lineage cells to the glomerular compartment.
The second finding in podocyte depleted reporter mice was that no RFP+ cells in the glomerulus coexpressed any of the four markers used to identify podocytes. Following podocyte depletion, enalapril treatment partially increased podocyte number in this mouse strain, similar to our previous reports (15, 20, 45). However, although enalapril increased the migration of RFP+ cells to the glomerulus, RFP+ cells did not coexpress podocyte proteins. Thus, our working hypothesis that NG2-lineage cells might serve as adult podocyte progenitors in this model under the given conditions was null. This is unlikely to be a methodological false negative because we have reported extensively that other kidney cells labeled with RFP can coexpress podocyte proteins (14, 20, 25, 26). It’s also unlikely to be a labeling issue, because we had baseline biopsies in each mouse validating consistent labeling across all mice at baseline and also showed that more glomeruli had RFP+ cells in disease compared with their own baseline.
The third finding was that glomerular RFP+ cells in disease either coexpressed two PEC proteins (PAX8 and SSeCKS) or two mesangial cell markers (PDGFβ receptor and α8 integrin), depending on their glomerular location. Almost all glomerular RFP+ cells coexpressed these proteins, and enalapril enhanced their number too. We interpret these results to mean that following podocyte depletion, cells of NG2 lineage migrate to the glomerulus, and a subset transdifferentiate to either PEC or mesangial cell fates but not a podocyte fate, nor a glomerular endothelial cell fate. It is noteworthy that we and others have shown increased PEC number in FSGS (3, 4, 23, 31, 37, 45) as occurs in this mouse strain, and others have shown an increase in mesangial number is associated with a poorer prognosis in FSGS (13, 32, 40). NG2-lineage cells and mesangial cells share a common precursor, where Foxd1-positive cells give rise to both NG2 lineages, i.e., fibroblasts, smooth muscle cells and pericytes, and mesangial cells in the course of kidney development. Xiong et al. (43) showed that overexpressing NG2 in mesangial cells leads to proliferation.
The current study has limitations. We do not offer a mechanistic explanation for either the migration of cells of NG2 lineage to the glomerulus, nor transdifferentiation to mesangial and PEC fates. We are unable to determine the origin from where migrated RFP+ cells that populated the glomerulus arise. A portion of the stroma throughout the kidney was labeled with RFP and included pericytes surrounding the vasculature of the tubular interstitium, as well as portions of the vasa recta. Therefore, the source could be the perivascular cells from these regions of the kidney. We did not check for RFP+ cells within the bone marrow in these studies, so it is entirely possible RFP+ cells could be coming from extrarenal sources. We do not know if NG2-lineage cells are detrimental to the glomerulus or reparative. Finally, we cannot explain the consequences, if any, for the increase in NG2 expression in resident podocytes in disease.
In summary, lineage tracing in an inducible reporter mouse demonstrates that NG2-lineage cells migrate to the glomerulus following podocyte depletion in experimental FSGS, which is augmented by enalapril. NG2-lineage cells can transdifferentiate to mesangial and PEC fates but not podocyte or glomerular endothelial cell fates. These results suggest that NG2-lineage cells are unlikely to serve as adult podocyte progenitors in disease.
GRANTS
This study was supported by NIH Grants 1R01AG046231-01A1, R01AG046231 (to S. J. Shankland); 5UH2DK107343-02, UH2DK107343 (to S. J. Shankland); and 5R01DK097598-05, R01DK097598 (to K. W. Gross and S. J. Shankland).
DISCLOSURES
No conflicts of interest, financial or otherwise, are declared by the authors.
AUTHOR CONTRIBUTIONS
T.S., D.G.E., A.D.M., J.W.P., and S.J.S. conceived and designed research; T.S., D.G.E., A.D.M., J.W.P., and S.J.S. performed experiments; T.S., D.G.E., A.D.M., J.W.P., and S.J.S. analyzed data; T.S., D.G.E., A.D.M., J.W.P., and S.J.S. interpreted results of experiments; T.S., D.G.E., A.D.M., J.W.P., and S.J.S. prepared figures; T.S., D.G.E., A.D.M., J.W.P., and S.J.S. drafted manuscript; T.S., D.G.E., A.D.M., J.W.P., and S.J.S. edited and revised manuscript; T.S., D.G.E., A.D.M., J.W.P., and S.J.S. approved final version of manuscript.
REFERENCES
- 1.Appel D, Kershaw DB, Smeets B, Yuan G, Fuss A, Frye B, Elger M, Kriz W, Floege J, Moeller MJ. Recruitment of podocytes from glomerular parietal epithelial cells. J Am Soc Nephrol 20: 333–343, 2009. doi: 10.1681/ASN.2008070795. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Belachew S, Chittajallu R, Aguirre AA, Yuan X, Kirby M, Anderson S, Gallo V. Postnatal NG2 proteoglycan-expressing progenitor cells are intrinsically multipotent and generate functional neurons. J Cell Biol 161: 169–186, 2003. doi: 10.1083/jcb.200210110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Benigni A, Morigi M, Rizzo P, Gagliardini E, Rota C, Abbate M, Ghezzi S, Remuzzi A, Remuzzi G. Inhibiting angiotensin-converting enzyme promotes renal repair by limiting progenitor cell proliferation and restoring the glomerular architecture. Am J Pathol 179: 628–638, 2011. doi: 10.1016/j.ajpath.2011.04.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Burnworth B, Pippin J, Karna P, Akakura S, Krofft R, Zhang G, Hudkins K, Alpers CE, Smith K, Shankland SJ, Gelman IH, Nelson PJ. SSeCKS sequesters cyclin D1 in glomerular parietal epithelial cells and influences proliferative injury in the glomerulus. Lab Invest 92: 499–510, 2012. doi: 10.1038/labinvest.2011.199. [DOI] [PubMed] [Google Scholar]
- 5.Crisan M, Yap S, Casteilla L, Chen C-W, Corselli M, Park TS, Andriolo G, Sun B, Zheng B, Zhang L, Norotte C, Teng P-N, Traas J, Schugar R, Deasy BM, Badylak S, Buhring H-J, Giacobino J-P, Lazzari L, Huard J, Péault B. A perivascular origin for mesenchymal stem cells in multiple human organs. Cell Stem Cell 3: 301–313, 2008. doi: 10.1016/j.stem.2008.07.003. [DOI] [PubMed] [Google Scholar]
- 6.Eng DG, Sunseri MW, Kaverina NV, Roeder SS, Pippin JW, Shankland SJ. Glomerular parietal epithelial cells contribute to adult podocyte regeneration in experimental focal segmental glomerulosclerosis. Kidney Int 88: 999–1012, 2015. doi: 10.1038/ki.2015.152. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Gensert JM, Goldman JE. Endogenous progenitors remyelinate demyelinated axons in the adult CNS. Neuron 19: 197–203, 1997. doi: 10.1016/S0896-6273(00)80359-1. [DOI] [PubMed] [Google Scholar]
- 8.Gomez RA, Belyea B, Medrano S, Pentz ES, Sequeira-Lopez ML. Fate and plasticity of renin precursors in development and disease. Pediatr Nephrol 29: 721–726, 2014. doi: 10.1007/s00467-013-2688-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Grahammer F, Wanner N, Huber TB. Podocyte regeneration: who can become a podocyte? Am J Pathol 183: 333–335, 2013. doi: 10.1016/j.ajpath.2013.04.009. [DOI] [PubMed] [Google Scholar]
- 11.Hamatani H, Eng DG, Kaverina NV, Gross KW, Freedman BS, Pippin JW, Shankland SJ. Lineage tracing aged mouse kidneys shows lower number of cells of renin lineage and reduced responsiveness to RAAS inhibition. Am J Physiol Renal Physiol 315: F97–F109, 2018. doi: 10.1152/ajprenal.00570.2017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Hugo C, Shankland SJ, Bowen-Pope DF, Couser WG, Johnson RJ. Extraglomerular origin of the mesangial cell after injury. A new role of the juxtaglomerular apparatus. J Clin Invest 100: 786–794, 1997. doi: 10.1172/JCI119592. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.El Karoui K, Hill GS, Karras A, Moulonguet L, Caudwell V, Loupy A, Bruneval P, Jacquot C, Nochy D. Focal segmental glomerulosclerosis plays a major role in the progression of IgA nephropathy. II. Light microscopic and clinical studies. Kidney Int 79: 643–654, 2011. doi: 10.1038/ki.2010.460. [DOI] [PubMed] [Google Scholar]
- 14.Kaverina NV, Eng DG, Largent AD, Daehn I, Chang A, Gross KW, Pippin JW, Hohenstein P, Shankland SJ. WT1 is necessary for the proliferation and migration of cells of renin lineage following kidney podocyte depletion. Stem Cell Reports 9: 1152–1166, 2017. doi: 10.1016/j.stemcr.2017.08.020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Kaverina NV, Eng DG, Schneider RRS, Pippin JW, Shankland SJ. Partial podocyte replenishment in experimental FSGS derives from nonpodocyte sources. Am J Physiol Renal Physiol 310: F1397–F1413, 2016. doi: 10.1152/ajprenal.00369.2015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Kaverina NV, Kadoya H, Eng DG, Rusiniak ME, Sequeira-López MLS, Gomez RA, Pippin JW, Gross KW, Peti-Peterdi J, Shankland SJ. Tracking the stochastic fate of cells of the renin lineage after podocyte depletion using multicolor reporters and intravital imaging. PLoS One 12: e0173891, 2017. doi: 10.1371/journal.pone.0173891. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Kopp JB. Replenishment of the podocyte compartment by parietal epithelial cells. Kidney Int 88: 934–935, 2015. doi: 10.1038/ki.2015.256. [DOI] [PubMed] [Google Scholar]
- 18.Lasagni L, Angelotti ML, Ronconi E, Lombardi D, Nardi S, Peired A, Becherucci F, Mazzinghi B, Sisti A, Romoli S, Burger A, Schaefer B, Buccoliero A, Lazzeri E, Romagnani P. Podocyte regeneration driven by renal progenitors determines glomerular disease remission and can be pharmacologically enhanced. Stem Cell Reports 5: 248–263, 2015. doi: 10.1016/j.stemcr.2015.07.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Lasagni L, Romagnani P. Basic research: podocyte progenitors and ectopic podocytes. Nat Rev Nephrol 9: 715–716, 2013. doi: 10.1038/nrneph.2013.247. [DOI] [PubMed] [Google Scholar]
- 20.Lichtnekert J, Kaverina NV, Eng DG, Gross KW, Kutz JN, Pippin JW, Shankland SJ. Renin-angiotensin-aldosterone system inhibition increases podocyte derivation from cells of renin lineage. J Am Soc Nephrol 27: 3611–3627, 2016. doi: 10.1681/ASN.2015080877. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Macconi D, Sangalli F, Bonomelli M, Conti S, Condorelli L, Gagliardini E, Remuzzi G, Remuzzi A. Podocyte repopulation contributes to regression of glomerular injury induced by ACE inhibition. Am J Pathol 174: 797–807, 2009. doi: 10.2353/ajpath.2009.080227. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Madisen L, Zwingman TA, Sunkin SM, Oh SW, Zariwala HA, Gu H, Ng LL, Palmiter RD, Hawrylycz MJ, Jones AR, Lein ES, Zeng H. A robust and high-throughput Cre reporting and characterization system for the whole mouse brain. Nat Neurosci 13: 133–140, 2010. doi: 10.1038/nn.2467. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Naito S, Pippin JW, Shankland SJ. The glomerular parietal epithelial cell’s responses are influenced by SM22 alpha levels. BMC Nephrol 15: 174, 2014. doi: 10.1186/1471-2369-15-174. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Pippin JW, Glenn ST, Krofft RD, Rusiniak ME, Alpers CE, Hudkins K, Duffield JS, Gross KW, Shankland SJ. Cells of renin lineage take on a podocyte phenotype in aging nephropathy. Am J Physiol Renal Physiol 306: F1198–F1209, 2014. doi: 10.1152/ajprenal.00699.2013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Pippin JW, Kaverina NV, Eng DG, Krofft RD, Glenn ST, Duffield JS, Gross KW, Shankland SJ. Cells of renin lineage are adult pluri-potent progenitors in experimental glomerular disease. Am J Physiol Renal Physiol 309: F341-F358, 2015. doi: 10.1152/ajprenal.00438.2014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Pippin JW, Sparks MA, Glenn ST, Buitrago S, Coffman TM, Duffield JS, Gross KW, Shankland SJ. Cells of renin lineage are progenitors of podocytes and parietal epithelial cells in experimental glomerular disease. Am J Pathol 183: 542–557, 2013. doi: 10.1016/j.ajpath.2013.04.024. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Puelles VG, Douglas-Denton RN, Cullen-McEwen LA, Li J, Hughson MD, Hoy WE, Kerr PG, Bertram JF. Podocyte number in children and adults: associations with glomerular size and numbers of other glomerular resident cells. J Am Soc Nephrol 26: 2277–2288, 2015. doi: 10.1681/ASN.2014070641. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Roeder SS, Barnes TJ, Lee JS, Kato I, Eng DG, Kaverina NV, Sunseri MW, Daniel C, Amann K, Pippin JW, Shankland SJ. Activated ERK1/2 increases CD44 in glomerular parietal epithelial cells leading to matrix expansion. Kidney Int 91: 896–913, 2017. doi: 10.1016/j.kint.2016.10.015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Roeder SS, Stefańska A, Eng DG, Kaverina N, Sunseri MW, McNicholas BA, Rabinovitch P, Engel FB, Daniel C, Amann K, Lichtnekert J, Pippin JW, Shankland SJ. Changes in glomerular parietal epithelial cells in mouse kidneys with advanced age. Am J Physiol Renal Physiol 309: F164–F178, 2015. doi: 10.1152/ajprenal.00144.2015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Ronconi E, Sagrinati C, Angelotti ML, Lazzeri E, Mazzinghi B, Ballerini L, Parente E, Becherucci F, Gacci M, Carini M, Maggi E, Serio M, Vannelli GB, Lasagni L, Romagnani S, Romagnani P. Regeneration of glomerular podocytes by human renal progenitors. J Am Soc Nephrol 20: 322–332, 2009. doi: 10.1681/ASN.2008070709. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Schneider RRS, Eng DG, Kutz JN, Sweetwyne MT, Pippin JW, Shankland SJ. Compound effects of aging and experimental FSGS on glomerular epithelial cells. Aging (Albany NY) 9: 524–546, 2017. doi: 10.18632/aging.101176. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Schoeneman MJ, Bennett B, Greifer I. The natural history of focal segmental glomerulosclerosis with and without mesangial hypercellularity in children. Clin Nephrol 9: 45–54, 1978. [PubMed] [Google Scholar]
- 33.Sequeira López MLS, Pentz ES, Nomasa T, Smithies O, Gomez RA. Renin cells are precursors for multiple cell types that switch to the renin phenotype when homeostasis is threatened. Dev Cell 6: 719–728, 2004. doi: 10.1016/S1534-5807(04)00134-0. [DOI] [PubMed] [Google Scholar]
- 34.Shankland SJ, Anders H-J, Romagnani P. Glomerular parietal epithelial cells in kidney physiology, pathology, and repair. Curr Opin Nephrol Hypertens 22: 302–309, 2013. doi: 10.1097/MNH.0b013e32835fefd4. [DOI] [PubMed] [Google Scholar]
- 35.Shankland SJ, Freedman BS, Pippin JW. Can podocytes be regenerated in adults? Curr Opin Nephrol Hypertens 26: 154–164, 2017. doi: 10.1097/MNH.0000000000000311. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Shankland SJ, Pippin JW, Duffield JS. Progenitor cells and podocyte regeneration. Semin Nephrol 34: 418–428, 2014. doi: 10.1016/j.semnephrol.2014.06.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Smeets B, Te Loeke NA, Dijkman HB, Steenbergen ML, Lensen JF, Begieneman MP, van Kuppevelt TH, Wetzels JF, Steenbergen EJ. The parietal epithelial cell: a key player in the pathogenesis of focal segmental glomerulosclerosis in Thy-1.1 transgenic mice. J Am Soc Nephrol 15: 928–939, 2004. doi: 10.1097/01.ASN.0000120559.09189.82. [DOI] [PubMed] [Google Scholar]
- 38.Starke C, Betz H, Hickmann L, Lachmann P, Neubauer B, Kopp JB, Sequeira-López ML, Gomez RA, Hohenstein B, Todorov VT, Hugo CP. Renin lineage cells repopulate the glomerular mesangium after injury. J Am Soc Nephrol 26: 48–54, 2015. doi: 10.1681/ASN.2014030265. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Stefańska A, Péault B, Mullins JJ. Renal pericytes: multifunctional cells of the kidneys. Pflugers Arch 465: 767–773, 2013. doi: 10.1007/s00424-013-1294-0. [DOI] [PubMed] [Google Scholar]
- 40.Taheri D, Chehrei A, Samanianpour P, Sadrarhami S, Keshteli AH, Shahidi S. The predictive role of histopathological findings in renal insufficiency and complete remission in a sample of Iranian adults with primary focal segmental glomerulosclerosis. J Res Med Sci 15: 14–19, 2010. [PMC free article] [PubMed] [Google Scholar]
- 41.Venkatareddy M, Wang S, Yang Y, Patel S, Wickman L, Nishizono R, Chowdhury M, Hodgin J, Wiggins PA, Wiggins RC. Estimating podocyte number and density using a single histologic section. J Am Soc Nephrol 25: 1118–1129, 2014. doi: 10.1681/ASN.2013080859. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Wanner N, Hartleben B, Herbach N, Goedel M, Stickel N, Zeiser R, Walz G, Moeller MJ, Grahammer F, Huber TB. Unraveling the role of podocyte turnover in glomerular aging and injury. J Am Soc Nephrol 25: 707–716, 2014. doi: 10.1681/ASN.2013050452. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Xiong J, Wang Y, Zhu Z, Liu J, Wang Y, Zhang C, Hammes H-P, Lang F, Feng Y. NG2 proteoglycan increases mesangial cell proliferation and extracellular matrix production. Biochem Biophys Res Commun 361: 960–967, 2007. doi: 10.1016/j.bbrc.2007.07.113. [DOI] [PubMed] [Google Scholar]
- 44.Zhang J, Pippin JW, Krofft RD, Naito S, Liu Z-H, Shankland SJ. Podocyte repopulation by renal progenitor cells following glucocorticoids treatment in experimental FSGS. Am J Physiol Renal Physiol 304: F1375–F1389, 2013. doi: 10.1152/ajprenal.00020.2013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Zhang J, Yanez D, Floege A, Lichtnekert J, Krofft RD, Liu Z-H, Pippin JW, Shankland SJ. ACE-inhibition increases podocyte number in experimental glomerular disease independent of proliferation. J Renin Angiotensin Aldosterone Syst 16: 234–248, 2015. doi: 10.1177/1470320314543910. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45a.Zhang H, Zhang X, Bie P, Miller RH, Bai L. Adult NG2-expressing cells in multiple organs: a novel progenitor in regenerative medicine. J Genet Syndr Gene Ther s3: 008, 2013. 10.4172/2157-7412.S3-008. [DOI] [Google Scholar]
- 46.Zhu X, Hill RA, Dietrich D, Komitova M, Suzuki R, Nishiyama A. Age-dependent fate and lineage restriction of single NG2 cells. Development 138: 745–753, 2011. doi: 10.1242/dev.047951. [DOI] [PMC free article] [PubMed] [Google Scholar]