Abstract
p21 is upregulated in renal tubules in response to acute kidney injury (AKI). and localizes in the nucleus, where it induces cell cycle arrest (CCA). These events can mitigate early injury but can also facilitate the onset of the degenerative cell senescence/“aging” process. Hence, we asked the following: 1) can AKI-induced p21 upregulation be gauged by plasma and/or urinary p21 assay; 2) might p21 serve as an AKI/CCA biomarker; and 3) does p21 accumulate during normal renal aging, and might plasma p21 reflect this process? Mice were subjected to either ischemia-reperfusion (I/R) or nephotoxic (maleate) AKI. Renal cortical p21 expression (protein, mRNA) was assessed 2–18 h later and contrasted with plasma/urine p21 concentrations (ELISA). p21 mRNA/protein levels were also measured in aging mice (2, 12, 24 mo). AKI induced marked, progressive, increases in renal cortical p21 mRNA and protein levels. These changes were marked by acute (within 2–4 h) and profound increases (up to 200×) in both plasma and urine p21 concentrations. Renal I/R also activated p21 gene expression in extrarenal organs (heart, brain), consistent with so-called “organ cross talk”. p21 efflux from damaged cells was confirmed with studies of hypoxia-injured, isolated proximal tubules. Aging was associated with progressive renal cortical p21 expression, which correlated (r, 0.83) with rising plasma p21 concentrations. We concluded that 1) during AKI, renal p21 increases can be gauged by either plasma or urine p21 assay, serving as potentially useful AKI/CCA biomarkers; 2) AKI can activate p21 in extrarenal organs; and 3) plasma p21 levels may provide an index of the renal/systemic aging process.
Keywords: aging, cell cycle arrest, ischemia, maleate, nephotoxicity, senescence
INTRODUCTION
p21 is a critical cell cycle regulatory protein that promotes cell cycle arrest (CCA) by inhibiting multiple cyclin-dependent kinases, most notably, CDK2 (6, 8, 17). In addition, p21 can behave as a renal “stress” protein, being upregulated by diverse forms of acute kidney injury (AKI) (7, 10, 13, 14, 17). The latter process is denoted by prompt and dramatic p21 mRNA and protein increases after AKI induction.
Following synthesis, p21 rapidly traffics to, and accumulates in, the cell nucleus, where it exerts its cell cycle inhibitory effects (7, 13, 14, 17). However, it remains conceivable that nuclear membrane damage during AKI could release p21 from the nucleus with subsequent cell efflux. In kidney, this might lead to increased urinary p21 excretion. Alternatively, if p21 were to gain access to the peritubular space and the microvasculature, increased p21 plasma concentrations might result.
These considerations led us to hypothesize that plasma and/or urine p21 might serve as novel biomarkers of AKI, of intracellular p21 levels, and, hence, the potential for CCA. It is also possible that p21 could serve as a marker, or correlate, of cellular senescence, a p21-dependent process that can promulgate progressive renal damage (3, 12, 20). The present study was undertaken to seek support for each of these hypotheses.
METHODS
All AKI experiments were conducted using male CD-1 mice (35–40 g; Charles River Laboratories, Wilmington, DE) maintained under routine vivarium conditions with free food and water access thoughout. The animal protocols were approved by our Institutional Animal Care and Use Committee. Surgeries were performed under deep pentobarbital sodium anesthesia (40–50 mg/kg ip).
Renal ischemia protocol.
Ten mice were subjected to bilateral ischemic injury as follows. The abdominal cavities were opened, both renal pedicles were isolated, and then they were occluded for 22 min with atraumatic vascular clamps. Body temperature was maintained at 37°C with an external heating source. After vascular clamp removal, uniform reperfusion was confirmed by return of normal kidney color. The abdominal cavities were then sutured, and the mice were allowed to recover from anesthesia. At either 4 or 18 h post-renal ischemia (n = 5 mice at each time point), the abdominal cavities were reopened, a vena cava blood sample and a urine sample were obtained, and the kidneys were resected. Blood and urine were assayed for creatinine (Cr) using a commercially available assay (BioChain, no. 5030020; Newark NJ). Blood urea nitrogen (BUN) was also determined (BioChain, no. 5030020). Plasma and urine p21 levels were determined by ELISA using two distinct monoclonal antibodies (capture and detection antibodies; directed against 2 different p21 epitopes, thereby conferring p21 specificity; Abcam, Cambridge, MA; no. ab212072). The renal cortices were dissected on ice and extracted for RNA (RNeasy Mini+; Qiagen, Germantown, MD) and protein, the latter in the presence of protease inhibitors. Renal cortical p21 mRNA levels were determined by RT-PCR, factored by GAPDH, as previously described (10). Renal cortical p21 levels were measured by ELISA, as above. Five normal mice and five mice subjected to sham surgery for the above procedures, served as controls.
Maleate model of AKI.
Maleate is specifically transported into proximal tubules via the organic anion transporter and induces both mitochondrial inhibition/ATP depletion and oxidative stress (19). To ascertain whether this nephotoxic AKI model might impact plasma, renal cortical, and/or urinary p21 expression, 10 mice were injected with Na maleate (800 mg/kg ip in 1 ml of saline). Saline intraperitoneally injected mice served as controls. Either 4 or 18 h later (n = 5 each), plasma, renal cortical, and urine samples were obtained and assayed for BUN, Cr, and p21, as noted above.
Time-response relationship for maleate AKI.
To be useful as a biomarker, early detection of evolving injury is required. Hence, eight mice were injected with maleate, as above. At either 1 h (n = 4) or 2 h (n = 4) postinjection, renal cortical, plasma, and urine samples were obtained. Renal cortical p21 mRNA/protein levels, and plasma/urine p21 protein concentrations were determined as above. The results were compared with those derived from six normal controls.
p21 release from isolated proximal tubule segments.
Four normal mice had their kidneys removed, and cortical sections were cut and used to isolate proximal tubule segments (PTS) by collagenase digestion, sieving, and differential centrifugation though Percoll, as previously described (18). Each of the four tubule preparations was divided into four aliquots and added to a physiological incubation buffer (18). The aliquots were treated as follows: 1) control oxygenated incubation (95% O2/5% CO2); 2) hypoxic incubation (95% N2/5% CO2); 3) control incubation in the presence of 5 mM glycine; and 4) hypoxic incubation with 5 mM glycine. Glycine was added to prevent hypoxic cell death (5). After 30 min, the aliquots were pelleted via centrifugation, and the pellets underwent protein extraction in the presence of protease inhibitors. p21 was measured in both the tubule protein extract and incubation buffer. Lethal cell injury was gauged by percent lactate dehydrogenase (LDH) release.
AKI effects on extrarenal (cardiac and brain) p21 gene expression.
To assess whether AKI might affect p21 gene activity in vital extrarenal organs, five mice were subjected to the above-described ischemia-reperfusion (I/R) protocol. Eighteen hours later, heart, brain, and kidney tissue samples were obtained, RNA was extracted, and p21 mRNA levels were assessed. The results were compared with those obtained from five sham-operated controls.
p21 expression in aging animals.
Because cellular senescence develops with aging (e.g., as demonstrated in Ref. 7), we assessed whether plasma p21 levels would correlate with this process and also reflect intrarenal p21 levels. To these ends, male CD-1 mice of varying age (~2 mo, ~1 yr; ~2 yr; n = 4 each) were euthanized, and plasma, renal cortical, and urinary p21 protein levels were assessed.
Calculations and statistics.
All values are presented as means ± 1 SE. Urinary p21 concentrations were factored by urine Cr concentrations. Renal cortical p21 was expressed per milligram of tissue protein. Statistical comparisons were performed by unpaired Student’s t-test. If multiple comparisons were made, the Bonferroni correction was applied.
RESULTS
I/R model.
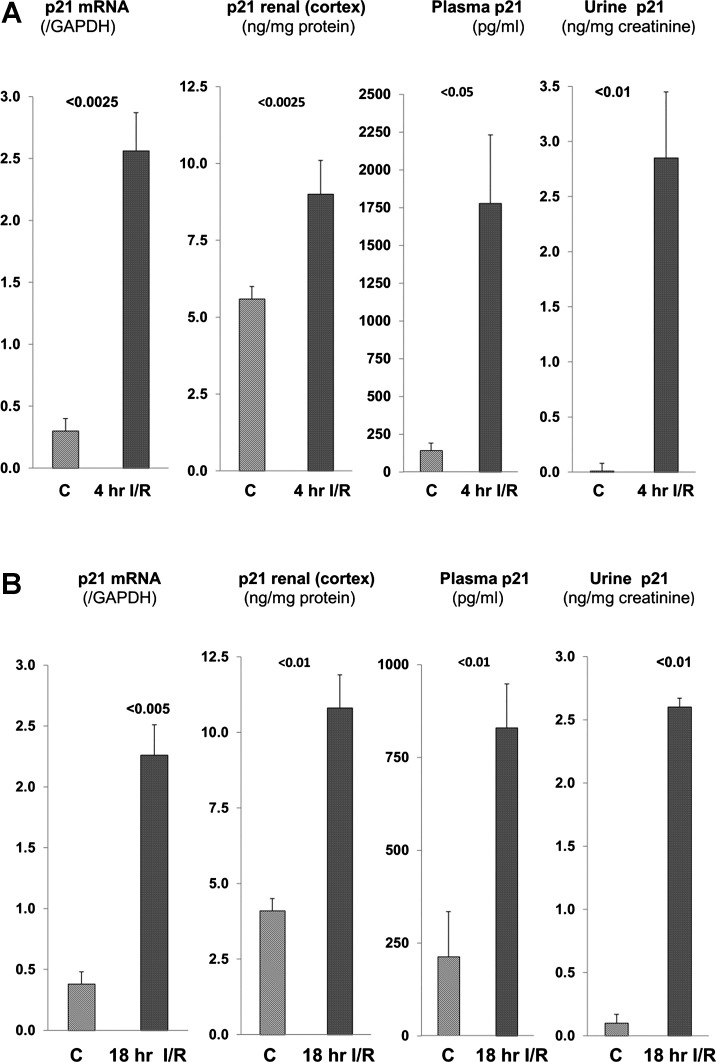
Within just 4 h of inducing renal injury, marked increases in renal cortical p21 mRNA and p21 protein concentrations were observed (Fig. 1A). These changes corresponded to a dramatic and unexpected 15-fold increase in plasma p21 concentrations. Normal urine had virtually undetectable p21 levels. However, remarkable urinary p21 increases were observed in the 4-h post-I/R samples (Fig. 1A, right). These renal cortical, plasma, and urinary p21 changes were durable with time, given that comparable results were found at the 18-h post-I/R time point (Fig. 1B). Azotemia was noted at 4 h post-I/R (BUN 50 ± 2 mg/dl, Cr 0.64 ± 0.03 mg/dl). By 18 h post-I/R, severe renal failure (BUN 110 ± 10, Cr 1.58 ± 0.35) had resulted. Control BUN and Cr values for these experiments were 23 ± 1 and 0.30 ± 0.01 mg/dl, respectively).
Fig. 1.
Renal p21 assessments at 4 h (A) and 18 h (B) after induction of ischemic acute kidney injury (AKI). Marked and sustained increases in renal cortical p21 mRNA and p21 protein concentrations were observed. These were associated with marked increases in both plasma and urinary p21 levels. C, control values; I/R, ischemia-reperfusion injury. Because of the large variation of urinary p21 levels in the AKI group, statistics were run after log base 10 conversion.
Maleate model.
Maleate recapitulated the findings of the I/R protocol, except that far greater urinary p21 increases were observed (Fig. 2, A and B). As with I/R, maleate caused progressive renal failure (BUN, 54 ± 6 and 124 ± 15 mg/dl at 4 and 18 h; plasma Cr, 1.06 ± 0.14 and 2.21 ± 0.2 mg/dl at 4 and 18 h; all P <0.05 vs. control values given above).
Fig. 2.
Renal p21 assessments at 4 h (A) and 18 h (B) post-Na maleate injection. Comparable findings were observed to those depicted in Fig. 1, with the exception that urinary p21 levels were markedly higher post-maleate vs. post-ischemia. C, controls; Mal, maleate.
Time-response relationship.
A progressive, statistically significant increase in renal cortical p21 mRNA was seen at 1 and 2 h post-maleate injection (0.5 ± 0.05, 0.90 ± 0.04; 1.44 ± 0.27 at baseline, 1 and 2 h post-maleate injection). By 2 h, statistically significant increases in plasma, urinary, and renal cortical p21 protein levels were first observed (plasma 381 ± 47 vs. 1,362 ± 173 pg/ml, P < 0.005; urine 0.2 ± 0 vs. 2.6 ± 0.9 ng/mg Cr, P < 0.05); cortex, 5.8 ± 0.4 vs. 7.3 ± 0.2 ng/mg tissue protein, P < 0.01), controls vs. 2-h post-maleate injection, respectively. These values increased further with time, as demonstrated at the 4- and 18-h time points, as depicted in Figs. 1 and 2.
Isolated tubule experiments.
Hypoxia induced lethal cell injury, as assessed by %LDH release (control oxygenation, 7 ± 1%, hypoxia, 35 ± 4%, P < 0.01). It also caused a threefold increase in p21 release (rising from 229 ± 58 to 656 ± 45 pg/ml in suspension medium, P < 0.005). Glycine addition prevented lethal cell injury [8 ± 2%, nonsignificant (NS) vs. control oxygenation] and mitigated p21 release into the suspension media (262 ± 50 pg/ml, NS) vs. oxygenated controls). Thus, p21 release appears to be a concomitant of early cell death, given that glycine blocked this process.
AKI effects on extrarenal (cardiac and brain) p21 gene expression.
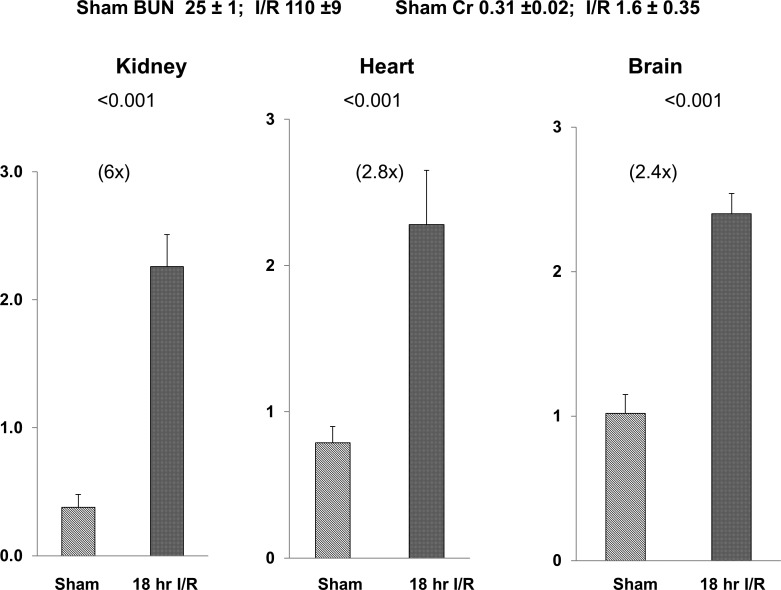
Ischemic AKI caused approximately two- to theefold increases in cardiac and brain p21 mRNA levels by 18 h post-AKI induction despite the fact that no direct cardiac or brain injury was induced. In comparison, the simultaneously obtained renal cortical p21 mRNA levels increased sixfold. Thus, the cardiac and cerebral increases were ~35–50% of those observed in damaged renal cortex. The associated BUN and Cr concentrations are presented in Fig. 3.
Fig. 3.
Cardiac and brain vs. renal p21 mRNA expression at 18 h post-induction of ischemic acute kidney injury (AKI). Renal ischemia caused a 6-fold increase in renal cortical p21 mRNA. It also induced a 2- to 3-fold p21 mRNA increase in heart and liver in the absence of any direct cardiac or hepatic tissue injury (P values vs. values observed in sham-operated mice). blood urea nitrogen (BUN) and creatinine (Cr) values observed in sham-operated and post-ischemic mice at 18 h are depicted. I/R, ischemia-reperfusion; sham, sham-operated mice.
Effect of aging on renal p21 expression.
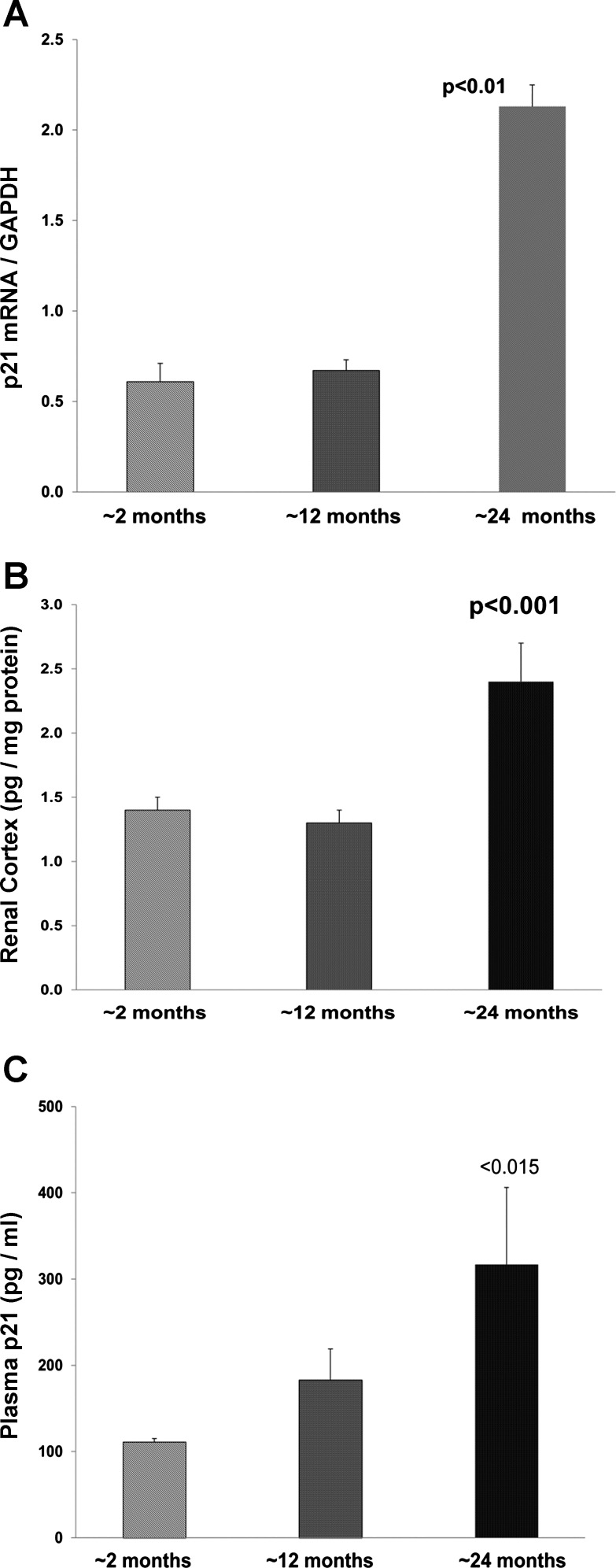
Aging was associated with increasing renal cortical p21 mRNA and protein concentrations. Furthermore, stepwise increases in plasma p21 concentrations were observed, which strongly correlated with renal p21 concentrations (r = 0.83, P < 0.01). Aging was not associated with increased p21 excretion (essentially undetectable; Fig. 4).
Fig. 4.
Renal cortical p21 mRNA (A), p21 protein levels (B), and p21 plasma levels (C) with aging. By 24 mo, marked increases in each were observed compared with values seen in 2-mo-old mice. These renal cortical changes were associated with progressive increases in plasma p21 concentrations (r = 0.83 plasma vs. same animal renal cortical levels).
DISCUSSION
There has been a multigenerational search for urinary biomarkers of AKI with the hope that early detection, with subsequent early therapeutic intervention, might abort the disease process. Thee broad categories of AKI biomarkers have emerged (reviewed in Ref. 9). These include the following: 1) proximal tubule stress proteins that are upregulated during early AKI and which are released into lumina for urinary excretion [e.g., neutrophil gelatinase-associated lipocalin (NGAL)], 2) freely filtered low-molecular-weight plasma proteins that escape the normal tubule reabsorption process due to tubular injury (e.g., β2-microglobulin), and 3) injury-induced release of constitutively expressed proximal tubule proteins from their normal intracellular compartments, thereby gaining urinary access [e.g., N-acetyl-glucosaminidase from lysosomes, cytochome c from mitochondria, LDH from cytoplasm, or kidney injury molecule-1 (KIM-1) from plasma membrane ectodomains]. The present report adds a surprising addition to these AKI biomarkers: the nuclear protein p21, which undergoes prompt (within ~2–4 h) urinary excretion in response to tubule damage. The available data indicate that multiple interactive mechanisms, encompassing those involved with each of the above three AKI biomarker families, are likely involved.
Pioneering work by Price and Saferstein et al. (13, 17) demonstrated that there is rapid p21 gene upregulation and increased p21 nuclear expression in response to AKI. The present findings of increased renal cortical p21 mRNA/protein levels within 2–4 h post-AKI are entirely consistent with these prior findings. Thus, p21, like NGAL and KIM 1, can be considered to be a renal tubular stress protein. However, unlike all of the above-noted biomarkers, p21 excretion is likely dependent, at least in part, on its release from the nuclear compartment. This could result from nuclear membrane damage and/or increased nuclear pore size, leading to nuclear, and ultimately cellular, p21 efflux. That tubular injury does, in fact, release p21 is supported by the current finding that hypoxic injury, imposed on isolated proximal tubules, induced theefold p21 elevations within the tubule suspension medium. Of note, AKI also caused marked plasma p21 increases, paralleling the urinary p21 elevations. Thus, plasma, in addition to urine, p21 assay would appear to have AKI biomarker potential. To our knowledge, these are the first sets of data to support the concept that a nuclear protein, p21, has potential utility as an AKI biomarker.
In addition to cellular efflux, it seems clear that decreased tubular reabsorption of filtered p21 also occurs. The current maleate results speak to this point. Following proximal tubule uptake via the organic anion transporter, maleate blocks mitochondrial ATP production and induces oxidative stress (19), culminating in AKI. During these events, preferential, and severe, damage to megalin (1) results. Given that megalin is a critical determinant of proximal tubule protein reabsorption, such injury would be expected to cause a dramatic increase in p21 excretion following its filtration by the glomerulus. Indeed, it is notable that maleate caused 10- to 100-fold greater urinary p21 excretion than did ischemia despite comparable degrees of renal injury (denoted by the observed BUN/Cr increases). Thus, much greater urinary p21 excretion with maleate vs. ischemic AKI speaks to the importance of megalin-mediated proximal tubule protein reabsorption as a key determinant of urinary p21 excretion. Of note, we (9, 16) have previously reported that maleate, as well as ischemia, also causes dramatic increases in urinary albumin levels. This underscores that decreased proximal tubule p21 reabsorption during AKI is not a p21-specific phenomenon.
A third mechanism that helps explain increased urinary p21 excretion during early AKI is a marked increase in plasma p21 concentrations. Within just 2–4 h of injury, ischemia and maleate raised plasma p21 levels 5- and 10-fold, respectively. These plasma p21 elevations clearly increase the glomerular p21 filtered load, particularly in early AKI when glomerular filtration rate is relatively well maintained. There are at least two plausible explanations for these dramatic plasma p21 increases. First, it is possible that, with marked renal p21 overproduction during AKI, p21 efflux into the renal interstitial compartment might occur. This would then permit p21 access to the renal microcirculation, thereby raising plasma p21 concentrations. In the presence of tubular injury, decreased tubular reabsorption of these resulting increases in the p21 filtered load would occur. Second, and perhaps more intriguing, were the findings that ischemic AKI, a renal-specific insult, upregulated extrarenal p21 gene expression, as evidenced by increases in myocardial and cerebral p21 mRNA. These observations imply that renal ischemia, presumably via the release of injury-associated molecules (e.g., DAMPs or damage-associated molecular patterns), evoke extrarenal p21 overproduction and systemic p21 release. Thus, it appears that p21 upregulation is a previously unrecognized consequence of the so-called AKI-induced “organ cross-talk” phenomenon (4).
While it is clear that p21 can mitigate early AKI, CCA can also occur and help initiate the process of tubular cell senescence (3, 10, 12, 20). Because senescent cells assume an “inflammatory phenotype”, they damage neighboring cells and, thus, may contribute to irreversible AKI or progressive renal disease (3, 10, 20). Given these considerations, there has been keen interest in developing CCA biomarkers. The “NephoCheck” assay, a recent FDA-approved product (2, 11, 15), measures the urinary concentrations of tissue inhibitor of metalloproteinase-2 (TIMP2) and insulin-like growth factor-binding protein-7 (IGFBP7), two cytoplasmic CCA proteins that undergo increased urinary excretion during AKI. Thus, the NephoCheck test is considered to be both an AKI and a CCA biomarker (2, 11, 15). However, p21 and the NephoCheck proteins behave very differently in response to AKI. Most notably, whereas p21 mRNA and cortical protein levels acutely rise in response to AKI, tubular TIMP2 and IGFB7 gene upregulation does not occur (9). Furthermore, proximal tubule cell TIMP2 and IGFBP7 concentrations fall in response to AKI, presumably due to tubule cell cytoplasmic release (9). Given that p21 levels rise, rather than fall, during AKI, it seems possible that p21 might be a more accurate CCA biomarker. However, this remains purely speculative at this time.
Finally, we considered whether plasma or urinary p21 levels might also be markers of CCA and associated cellular senescence in situations other than AKI. In this regard, as part of the normal aging process, increasing numbers of cells enter into a p21-enhanced senescent state (reviewed in Ref. 10). Hence, we measured p21 expression in kidneys from ~2-, ~12-, and ~24-mo-old mice. As shown in Fig. 4, with increasing age, increasing renal cortical concentrations of p21 mRNA and protein levels were observed. Consistent with the AKI results, plasma p21 levels strongly correlated (r = 0.83) with the renal cortical p21 levels. Of note, urinary p21 was undetectable thoughout the aging process. This implies relatively intact renal tubular function during aging, thereby allowing for complete reabsorption of the filtered p21 load. Despite the fact that plasma and renal cortical p21 levels were strongly correlated, it seems quite likely that both the kidney and extrarenal organs also contribute to the aging-associated increases in plasma p21 elevations. This raises the intriguing possibility that plasma p21 could serve as a potential biomarker of the renal and/or the systemic aging process. Whether p21 might better reflect so called “biological” vs. “chonological” age remains an unanswered, but intriguing, question.
In summary, urinary p21 can serve as an early AKI biomarker due to a number of interactive mechanisms, which include the following: 1) increased tubular p21 production, as evidenced by rapid AKI-induced increases in its mRNA and tubular protein levels; 2) increased p21 release from injured cells, as demonstrated by the current isolated proximal tubule segment experiments; 3) an increase in the filtered p21 load, as reflected by acute, and marked, plasma p21 elevations, the last maybe resulting from AKI-induced increases in both extrarenal (e.g., heart, brain) and intrarenal p21 production with subsequent cell release; and 4) AKI-induced tubular injury, which causes decreased tubular reabsorption of the filtered p21 load. Finally, plasma p21 levels progressively rise with aging and correlate with rising renal cortical p21 mRNA and protein levels. These latter findings suggest the potential value of plasma p21 assay as a biomarker of the renal and/or the systemic aging process. Thus, it is appears that a plasma p21 assay may have utility that extends beyond that of an AKI/CCA biomarker.
The ultimate utility of the plasma and/or urinary p21 assay will only be known after carefully performed clinical trials. Several questions will emerge. For example, what is the potential impact of concomitant extrarenal, as well as renal, tissue injury on plasma or urinary p21 levels? Do plasma and urinary p21 levels reflect degrees of renal dysfunction in multifactorial forms of AKI (e.g., endotoxemia, the cardiorenal syndrome)? Is prerenal azotemia associated with normal p21 levels (as would be important to know whenever any AKI biomarker is evaluated)? Thus, the present study represents only a starting point in the evaluation of p21 utility as an AKI/CCA biomarker.
GRANTS
This work was supported by discretionary research funds from Fred Hutchinson Cancer Research Center.
DISCLOSURES
R. A. Zager is a consultant for Renibus Therapeutics, which may submit a provisional patent based on the work described in this paper. A. C. Johnson has no conflict of interest, financial or otherwise, to disclose.
AUTHOR CONTRIBUTIONS
A.C.J. performed experiments; A.C.J. and R.A.Z. analyzed data; A.C.J. and R.A.Z. interpreted results of experiments; A.C.J. and R.A.Z. edited and revised manuscript; A.C.J. and R.A.Z. approved final version of manuscript; R.A.Z. prepared figures; R.A.Z. drafted manuscript.
REFERENCES
- 1.Bergeron M, Mayers P, Brown D. Specific effect of maleate on an apical membrane glycoprotein (gp330) in proximal tubule of rat kidneys. Am J Physiol Renal Physiol 271: F908–F916, 1996. doi: 10.1152/ajprenal.1996.271.4.F908. [DOI] [PubMed] [Google Scholar]
- 2.Bihorac A, Chawla LS, Shaw AD, Al-Khafaji A, Davison DL, Demuth GE, Fitzgerald R, Gong MN, Graham DD, Gunnerson K, Heung M, Jortani S, Kleerup E, Koyner JL, Krell K, Letourneau J, Lissauer M, Miner J, Nguyen HB, Ortega LM, Self WH, Sellman R, Shi J, Straseski J, Szalados JE, Wilber ST, Walker MG, Wilson J, Wunderink R, Zimmerman J, Kellum JA. Validation of cell-cycle arrest biomarkers for acute kidney injury using clinical adjudication. Am J Respir Crit Care Med 189: 932–939, 2014. doi: 10.1164/rccm.201401-0077OC. [DOI] [PubMed] [Google Scholar]
- 3.Clements ME, Chaber CJ, Ledbetter SR, Zuk A. Increased cellular senescence and vascular rarefaction exacerbate the progression of kidney fibrosis in aged mice following transient ischemic injury. PLoS One 8: e70464, 2013. doi: 10.1371/journal.pone.0070464. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Doi K, Rabb H. Impact of acute kidney injury on distant organ function: recent findings and potential therapeutic targets. Kidney Int 89: 555–564, 2016. doi: 10.1016/j.kint.2015.11.019. [DOI] [PubMed] [Google Scholar]
- 5.Dong Z, Venkatachalam MA, Weinberg JM, Saikumar P, Patel Y. Protection of ATP-depleted cells by impermeant strychnine derivatives: implications for glycine cytoprotection. Am J Pathol 158: 1021–1028, 2001. doi: 10.1016/S0002-9440(10)64049-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Dutto I, Tillhon M, Cazzalini O, Stivala LA, Prosperi E. Biology of the cell cycle inhibitor p21(CDKN1A): molecular mechanisms and relevance in chemical toxicology. Arch Toxicol 89: 155–178, 2015. doi: 10.1007/s00204-014-1430-4. [DOI] [PubMed] [Google Scholar]
- 7.Inguaggiato P, Gonzalez-Michaca L, Croatt AJ, Haggard JJ, Alam J, Nath KA. Cellular overexpression of heme oxygenase-1 up-regulates p21 and confers resistance to apoptosis. Kidney Int 60: 2181–2191, 2001. doi: 10.1046/j.1523-1755.2001.00046.x. [DOI] [PubMed] [Google Scholar]
- 8.Jackman M, Kubota Y, den Elzen N, Hagting A, Pines J. Cyclin A- and cyclin E-Cdk complexes shuttle between the nucleus and the cytoplasm. Mol Biol Cell 13: 1030–1045, 2002. doi: 10.1091/mbc.01-07-0361. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Johnson AC, Zager RA. Mechanisms underlying increased TIMP2 and IGFBP7 urinary excretion in experimental AKI. J Am Soc Nephol 29: 2157–2167, 2018. doi: 10.1681/ASN.2018030265. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Johnson ACM, Zager RA. Mechanisms and consequences of oxidant-induced renal preconditioning: an Nrf2-dependent, P21-independent, anti-senescence pathway. Nephol Dial Transplant. In press. doi: 10.1093/ndt/gfy029. [DOI] [PubMed] [Google Scholar]
- 11.Kashani K, Al-Khafaji A, Ardiles T, Artigas A, Bagshaw SM, Bell M, Bihorac A, Birkhahn R, Cely CM, Chawla LS, Davison DL, Feldkamp T, Forni LG, Gong MN, Gunnerson KJ, Haase M, Hackett J, Honore PM, Hoste EA, Joannes-Boyau O, Joannidis M, Kim P, Koyner JL, Laskowitz DT, Lissauer ME, Marx G, McCullough PA, Mullaney S, Ostermann M, Rimmelé T, Shapiro NI, Shaw AD, Shi J, Sprague AM, Vincent JL, Vinsonneau C, Wagner L, Walker MG, Wilkerson RG, Zacharowski K, Kellum JA. Discovery and validation of cell cycle arrest biomarkers in human acute kidney injury. Crit Care 17: R25, 2013. doi: 10.1186/cc12503. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Kim YY, Jee HJ, Um JH, Kim YM, Bae SS, Yun J. Cooperation between p21 and Akt is required for p53-dependent cellular senescence. Aging Cell 16: 1094–1103, 2017. doi: 10.1111/acel.12639. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Megyesi J, Andrade L, Vieira JM Jr, Safirstein RL, Price PM. Coordination of the cell cycle is an important determinant of the syndrome of acute renal failure. Am J Physiol Renal Physiol 283: F810–F816, 2002. doi: 10.1152/ajprenal.00078.2002. [DOI] [PubMed] [Google Scholar]
- 14.Nath KA. Provenance of the protective property of p21. Am J Physiol Renal Physiol 289: F512–F513, 2005. doi: 10.1152/ajprenal.00224.2005. [DOI] [PubMed] [Google Scholar]
- 15.Vijayan A, Faubel S, Askenazi DJ, Cerda J, Fissell WH, Heung M, Humpheys BD, Koyner JL, Liu KD, Mour G, Nolin TD, Bihorac A, Faubel S, Askenazi DJ, Basu RK, Bhatt UY, Bihorac A, Cerda J, Connor MJ Jr, Davidson AJ, de Caestecker MP, Doi K, Fissell WH, Golestaneh L, Heung M, Humpheys BD, Koyner JL, Liu KD, Mour GK, Singh P, Thakar CV, Vijayan A; American Society of Nephology Acute Kidney Injury Advisory Group . Clinical use of the urine biomarker [TIMP2] × [IGFBP7] for acute kidney injury risk assessment. Am J Kidney Dis 68: 19–28, 2016. doi: 10.1053/j.ajkd.2015.12.033. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Ware LB, Johnson AC, Zager RA. Renal cortical albumin gene induction and urinary albumin excretion in response to acute kidney injury. Am J Physiol Renal Physiol 300: F628–F638, 2011. doi: 10.1152/ajprenal.00654.2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Yu F, Megyesi J, Safirstein RL, Price PM. Identification of the functional domain of p21(WAF1/CIP1) that protects cells from cisplatin cytotoxicity. Am J Physiol Renal Physiol 289: F514–F520, 2005. doi: 10.1152/ajprenal.00101.2005. [DOI] [PubMed] [Google Scholar]
- 18.Zager RA, Burkhart KM, Conrad DS, Gmur DJ, Iwata M. Phospholipase A2-induced cytoprotection of proximal tubules: potential determinants and specificity for ATP depletion-mediated injury. J Am Soc Nephol 7: 64–72, 1996. [DOI] [PubMed] [Google Scholar]
- 19.Zager RA, Johnson AC, Naito M, Bomsztyk K. Maleate nephotoxicity: mechanisms of injury and correlates with ischemic/hypoxic tubular cell death. Am J Physiol Renal Physiol 294: F187–F197, 2008. doi: 10.1152/ajprenal.00434.2007. [DOI] [PubMed] [Google Scholar]
- 20.Zhu Y, Armstrong JL, Tchkonia T, Kirkland JL. Cellular senescence and the senescent secretory phenotype in age-related chonic diseases. Curr Opin Clin Nutr Metab Care 17: 324–328, 2014. doi: 10.1097/MCO.0000000000000065. [DOI] [PubMed] [Google Scholar]