Abstract
The antidiuretic hormone vasopressin (VP) is produced by the hypothalamus and is stored and secreted from the posterior pituitary. VP acts via VP type 2 receptors (V2Rs) on the basolateral membrane of principal cells of the collecting duct (CD) to regulate fluid permeability. The VP-evoked endocrine pathway is essential in determining urine concentrating capability. For example, a defect in any component of the VP signaling pathway can result in polyuria, polydipsia, and hypotonic urine, collectively termed diabetes insipidus (DI). A lack of VP production precipitates central diabetes insipidus (CDI), which can be managed effectively by VP supplementation. A majority of cases of nephrogenic diabetes insipidus (NDI) result from V2R mutations that impair receptor sensitivity. No specific therapy is currently available for management of NDI. Evidence is evolving that (pro)renin receptor (PRR), a newly identified member of the renin-angiotensin system, is capable of regulating VP production and action. As such, PRR should be considered strongly as a therapeutic target for treating CDI and NDI. The current review will summarize recent advances in understanding the physiology of renal and central PRR as it relates to the two types of DI.
Keywords: aquaporin-2, collecting duct, renin-angiotensin system, site-1 protease, soluble (pro)renin receptor
INTRODUCTION
A major function of the kidney is to concentrate urine in an effort to maintain fluid homeostasis during situations that require water conservation (e.g., dehydration). This important responsibility of the kidney is controlled, in large part, by the antidiuretic hormone vasopressin (VP). VP is produced in the paraventricular nucleus (PVN) and supraoptic nucleus (SON) of the hypothalamus, is stored in the posterior pituitary, and is secreted from the posterior pituitary into the circulation in response to increased plasma osmolality. VP binds to VP type 2 receptors (V2Rs) in the principle cells of the collecting duct (CD) to increase membrane water permeability via cAMP-dependent regulation of aquaporin-2 (AQP2). Defective hypothalamic VP production and / or V2R sensitivity underlies central (CDI) and nephrogenic (NDI) diabetes insipidus, respectively, which are characterized by polyuria, polydipsia, and hypotonic urine. While patients with CDI can be managed in a straightforward manner by replacing VP, no effective therapy currently is available for NDI.
The (pro)renin receptor (PRR), first cloned by Nguyen et al. (45), is a transmembrane protein that binds to both renin and its precursor (pro)renin. PRR has a unique structure comprising a large NH4-terminal extracellular domain, a single transmembrane protein, and a short cytoplasmic domain (6). The extracellular domain contains the ligand binding sites related to the renin-angiotensin system, whereas the intracellular domain (M8.9) represents a subunit of vacuolar H+-ATPase, an ATP-dependent multisubunit proton pump. Because of the unique structure and function of PRR, it also is referred to as ATP6AP2.
A 28-kDa soluble PRR (sPRR) that resides in the extracellular domain is generated following protease-mediated cleavage (14, 74). Upon cleavage three different forms of PRR are generated, i.e., PRR, sPRR, and M8.9. PRR has pleiotropic functions ranging from physiological (e.g., embryogenesis; Refs. 25, 38, 44, 60, 68) to pathophysiological [(e.g., cardiovascular and renal disease; Refs. 32, 36, 44, 53, 62, 68, 70). Specifically, circulating sPRR is elevated in early pregnancy (65, 66), preeclampsia (42, 58), gestational diabetes mellitus (3, 66), heart failure patients with renal dysfunction (16), obstructive sleep apnea syndrome (47, 48, 57), and chronic kidney disease (CKD) due to hypertension and type 2 diabetes (21). Like PRR, sPRR can bind to renin or (pro)renin to activate the tissue renin-angiotensin system (20).
Within the kidney, PRR is predominantly localized to the intercalated cells of the CD, an important site for hormonal regulation of fluid reabsorption and final urine output. A large body of experimental evidence from our group and others has established that CD PRR is essential for determining urine concentrating capability (37, 53). Furthermore, the antidiuretic action of CD PRR is mediated by sPRR, which directly stimulates AQP2 transcription and improves the urine concentrating defect in a mouse model of V2R antagonism (37). In addition to its renal actions, activation of PRR in the central nervous system stimulates VP release (31, 56). The major objective of this review is to summarize recent findings regarding the function of sPRR in regulation of fluid homeostasis and the potential for its manipulation in the treatment/management of DI.
CHALLENGES IN NDI THERAPY
Congenital NDI is a rare disease with a prevalence estimated at 1–2/1,000,000 and is caused, in large part, by inherited X-linked mutations of V2R, whereas a smaller number of cases is precipitated by mutations of AQP2 (10). Acquired NDI can be secondary to conditions/treatments including hypokalemia, hypercalcemia, postobstructive acidosis, or therapeutic lithium. Existing therapies for NDI patients include thiazide diuretics (4), nonsteroid anti-inflammatory drugs (40), and/or amiloride (28). Unfortunately, these management strategies are only partially effective and patients still display significant polyuria and polydipsia. The mechanism of action of these therapies is poorly defined and even counterintuitive, e.g., diuretic treatment of polyuria. Furthermore, substantial side effects are reported including hypokalemia associated with thiazides and abdominal pain and gastric bleeding that are often concurrent with nonsteroid anti-inflammatory drug treatment.
While multiple investigative drugs have been examined in preclinical models to improve the treatment of patients with NDI, no clinical trials have been initiated. One example involves the selective EP4 PGE2 receptor agonist ONO-AE1-329 that acts via cAMP to increase water reabsorption in the CD. In 2009, a seminal study reported that ONO-AE1-329 remarkably attenuates polyuria and polydipsia in mice with inducible deletion of V2R and restores renal AQP2 expression (29). In support of these findings, conditional deletion of EP4 in the CD induces NDI (18). Despite these promising data, there has been no progression of EP4-based technology into clinical trials. Recently, a strategy to disrupt the association between A-kinase anchoring proteins and PKA reportedly increased renal AQP2 expression and ameliorated NDI induced by V2R antagonism (2). A lead compound of the disrupter of A-kinase anchoring proteins and PKA, 3,3′-diamino-4,4′-dihydroxydiphenylmethane (FMP-API-1), and its derivatives increased AQP2 expression to the same extent as VP (2).
A number of repurposed medications (e.g., metformin, simvastatin) have demonstrated potential for treatment of NDI. Metformin, used typically to treat overweight patients with type 2 diabetes, has recently been identified as a novel therapy for NDI based on evidence from animal experiments (12). Metformin increased AQP2 and the urea transporter UT-A1 expression in the inner medulla of V2R knockout mice to an extent that increased urine osmolality. While the mechanism of action is unclear, metformin is thought to indirectly activate AMPK or directly target the mitochondrial respiratory-chain complex 1 (1). Because metformin is used widely at present and toxicity issues have been addressed, the chances of this compound being repurposed to treat patients with NDI is enhanced.
Simvastatin is used clinically to lower cholesterol levels. With regard to its use for treating NDI, Brattleboro rats display an increase in apical membrane AQP2 through Rho GTPase activity downregulation and inhibition of endocytosis, which consequentially increases urine osmolality (33). Although simvastatin has not been tested formally in the context of NDI, urinary AQP2 is reportedly increased in hypercholesterolemic patients treated with this statin, providing strong proof of concept that this compound could be efficacious in this regard (33). Another statin with documented cholesterol-lowering capability is fluvastatin. When fluvustatin is administered concurrently with secretin, urine-concentrating ability increases in V2R knockout mice (51). Fluvastatin is not without side effects, however, as it may result in flushing of the face, vomiting, diarrhea, and fainting.
ANTIDIURETIC ACTION OF CD PRR
VP determines water permeability of the CD primarily via V2R-dependent activation of AQP2. An overwhelming body of experimental evidence provides robust information about the detailed signaling mechanism by which VP acutely induces AQP2 trafficking to the apical membrane (15, 46). Briefly, VP binds V2R on the basolateral membrane and via Gsα activates adenylcyclase to elevate intracellular cAMP and stimulate the cAMP-dependent protein kinase PKA. Activated PKA induces phosphorylation of multiple serine residues including S256, S261, and S269. Of these, S256 appears to be solely responsible for directing apical translocation of AQP2 (17, 43). Although the importance of AQP2 trafficking to the apical membrane is well recognized, AQP2 gene transcription also is critical (11, 23, 39). For example, cAMP-responsive element binding protein (24, 71), AP-1 (71), NF-κB (22), GATA family (52), ETS family (73), NFAT5 and NFATc (30) proteins, C/EBPβ (27), and β-catenin (26) all have been demonstrated to play a role in AQP2 gene transcription in vitro. Of these, the role of β-catenin should be underscored because it is the only transcription factor wherein both in vitro and in vivo data are available. In this regard, β-catenin has been documented to regulate AQP2 expression and influence urine concentrating capability (26, 37). The relative importance of the other transcription factors is undefined at present and requires further study.
PRR represents a new regulator of VP signaling in CD cells (69, 70, 74). Although not involved in regulating AQP2 trafficking per se, PRR activation appears indispensable for upregulating AQP2 expression in response to chronic VP treatment. In primary rat inner medullary collecting duct (IMCD) cells on Transwells, VP elevated AQP2 protein expression, which was sensitive to 1) pharmacological inhibition of PRR using PRO20, 2) a small and interfering RNA, and 3) the neutralizing PRR antibody (64). Furthermore, activation of PRR with (pro)renin, but not renin, induced a marked increase in AQP2 expression. The release of sPRR along with (pro)renin but not renin is elevated by exposure to VP, an observation that is recapitulated in an M1 CD cell line (19).
Evidence from studies using alternative approaches support that PRR mediates VP regulation of AQP2 expression in CD cells. For example, downregulation of renal AQP2 expression and impairment of urine concentrating capability are consistently observed in mice with deletion of PRR in the CD (63) or the entire renal tubule (53, 60), and also in rats infused with the PRR inhibitor PRO20 under basal conditions or following water deprivation (63). During water deprivation, VP release from the hypothalamus is elevated and renal medullary osmolality is increased as part of the urine concentrating mechanism. Our recent study reports that hyperosmolality directly stimulates PRR expression and sPRR release in cultured CD cells via NF-κB (55). It is likely that VP and hyperosmolality may act in concert to stimulate renal medullary PRR expression. Taken together, these studies provide solid evidence that PRR is an important mediator of VP regulation of AQP2 expression in CD cells.
PRR is a single transmembrane protein with a large extracellular domain, which is cleaved by a protease to generate the 28-kDa sPRR. We reported first that sPRR has biological relevance by upregulating AQP2 expression in cultured CD cells through its interaction with frezzled-8 and subsequent stimulation of β-catenin signaling (37). Based on these findings, the following paracrine model is proposed to explain the mechanism of action of sPRR in the CD, i.e., sPRR is generated from the intercalated cells and is secreted to the urine where it acts from the luminal side to stimulate AQP2 expression in the principal cells. sPRR is detected in the urine, and therefore, the apical action of sPRR in upregulation of AQP2 suggests that urinary sPRR may exert a physiological function in the control of fluid reabsorption in the CD. While furin (9) and ADAMP19 (72) were thought to mediate sPRR production, recent work from our group (14) and others (41) has consistently identified site-1 protease (S1P) as the predominant PRR cleavage protease. This discovery allows investigation of the functional role of S1P-derived endogenous sPRR in regulating VP signaling and urine concentrating capability.
Li et al. reported that global deletion of angiotensin II type 1a receptors (AT1aR) induced polyuria and decreased urine osmolality secondary to impaired urine concentrating ability and downregulation of AQP2 expression and AVP signaling (34, 35). The phenotype in AT1aR KO mice is similar to that in PRR KO mice, suggesting a possible interaction between PRR and AT1aR. Whether AT1aR signaling regulates PRR or vice versa is an interesting topic for future investigators.
THERAPEUTIC POTENTIAL OF SPRR IN NDI
V2R mutations account for almost 80% of the cases of congenital NDI and effective interventions are not available. Therapeutic strategies designed to target signaling mediators downstream of the V2R have been investigated [e.g., 1) elevate the production of intracellular cAMP (49), 2) inhibit phosphodiesterase (8), and 3) activate PKA (2)], but none have progressed to the point of examination in clinical trials.
Our previous study identified the antidiuretic action of sPRR as a downstream mediator of V2R in the CD cells. This novel pathway has important implications concerning a new therapeutic strategy to treat NDI. In a mouse model of NDI induced by administration of a V2R antagonist, urinary sPRR excretion was significantly reduced but a short-term (3 day) treatment with histidine-tagged recombinant sPRR (sPRR-His) via osmotic minipump reduced polyuria by ~40% (37). Patients with congenital NDI develop severe polyuria and nocturia (awakening during sleep periods for urination), producing large urine volumes (e.g., 10–20 liters per day), which significantly impairs quality of life, social activity, and mental well-being (10). Therefore, a 40% reduction of polyuria would provide significant amelioration of these deleterious direct and indirect consequences of NDI. A longer term of sPRR-His treatment may be even more effective and should be evaluated in future studies. Nevertheless, our findings provide strong proof of concept for further exploration of sPRR-His in the treatment of NDI secondary to V2R mutations.
A sPRR-His-based approach may prove advantageous when compared with existing experimental therapies for NDI. For example, while the cAMP/PKA pathway is clearly important in mediating trafficking and expression of AQP2 downstream of the V2R, inhibiting this ubiquitous pathway has the potential for multiple off-target side effects. Because the action of sPRR is observed to the greatest extent in the kidney as compared with other tissues, the potential for unwanted, off-target side effects is decreased. In this regard, we observed no toxicity of sPRR-His in the context of our experimental conditions (37). Furthermore, sPRR-His may have an additional benefit in stimulating VP release, an advantage not shared with other therapies. The latter possibility currently is being investigated in our laboratory.
We sought to determine whether sPRR-His is similarly effective in other types of NDI such as that induced by lithium treatment. This is relevant clinically because lithium is commonly used for treatment of bipolar disease but is limited by its renal complications characterized by acquired NDI. To address this, NDI was induced in mice via 2-wk lithium treatment (40 mmol/kg diets) and sPRR-His was administered at 30 µg·kg−1·day−1 during the last 7 days. The 14-d LiCl treatment produced the expected elevation of water intake and urine volume and decreased urine osmolality, but none of these NDI indexes, to our surprise, were affected by concurrent sPRR-His treatment (67). While these data were contrary to our initial hypothesis, the distinct effects of sPRR-His treatment in the two experimental models of NDI (i.e., V2R antagonism and LiCl treatment) likely will provide important insight into designing a precision therapy pertinent to subpopulations of NDI patients with V2R mutations. In this regard, although downregulation of AQP2 is a phenomenon common to NDI evoked by V2R mutations and LiCl treatment, the underlying mechanisms directly responsible for NDI are etiology specific. While signaling consequences due to V2R mutations are well defined, the mechanism of lithium-induced NDI remains elusive despite intensive study. For example, investigations have implicated a role for glycogen synthesis kinase-3, the inositol and protein kinase C, cAMP, and renal interstitial fibrosis (40, 50). At present, however, the precise mechanism responsible for LiCl-induced NDI is unclear. With regard to our study, it appears that not only is exogenous sPRR-His ineffective in a mouse model of lithium-induced NDI but the production of endogenous sPRR is also unaltered (67), suggesting strongly that PRR/sPRR does not contribute to LiCl-induced NDI in mice.
We observed no toxicity associated with sPRR-His treatment in the current experimental model. However, sPRR-His administration has been shown to enhance inflammation and local renin activity, resulting in renal injury induced by protein overloading (13). Further research is warranted concerning this potential side effect.
CENTRAL EFFECT OF PRR ON VP SECRETION
In addition to its peripheral effect on VP signaling, PRR is shown to regulate central VP production. Within the central nervous system, PRR is predominantly expressed in neurons relevant to the cardiovascular system including the PVN, SON (54, 56), and the subfornical organ (7). In particular, PRR is colocalized with VP and oxytocin in the SON and PVN in humans (56). PRR knockdown in the SON of spontaneously hypertensive rats decreases blood pressure, heart rate, and plasma VP (54). In the same study, targeted overexpression of human PRR in the SON of Wistar-Kyoto rats induced a twofold increase in plasma VP and a threefold increase in urinary VP, in parallel with enhancing urine concentrating capability. These results demonstrated an important role of central PRR in regulating VP secretion. Furthermore, the increased VP secretion has been shown to contribute to the pathogenesis of hypertension in spontaneously hypertensive rats (54).
Abundant evidence suggests that sPRR mediates the peripheral action of PRR in transcriptional upregulation of AQP2 expression in the CD in response to VP treatment. It is conceivable that sPRR may similarly contribute to central VP release. If so, sPRR may have dual benefits for the management of both CDI and NDI (Fig. 1). To date, studies addressing this issue have not been performed. Furthermore, it is important to test the capability of VP-generating neurons to produce sPRR under basal conditions and/or during water deprivation. Can sPRR stimulate central VP secretion? Does S1P exist in the VP-producing neurons or will its activity or expression be altered by dehydration? Although our previous study found that sPRR-His infusion did not influence VP production (37), it is unclear whether exogenous sPPR-His can readily cross the blood-brain barrier. This issue could potentially be resolved by targeted delivery of the agent to the brain, e.g., intraventricular infusion or via genetic manipulation of S1P in neurons.
Fig. 1.
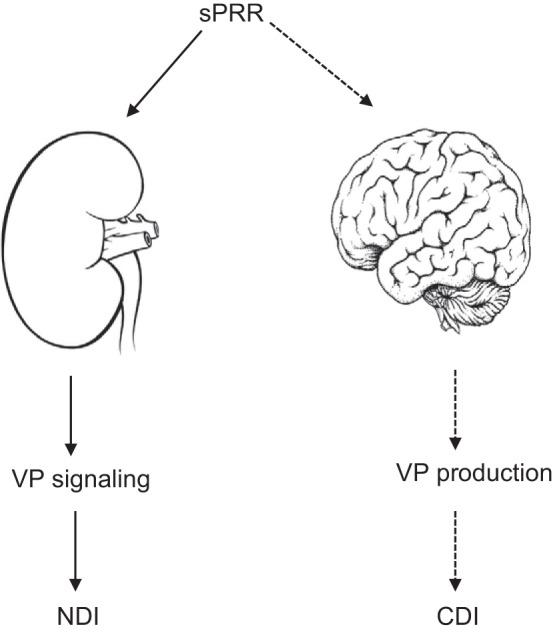
Schematic illustration of the antidiuretic action of soluble (pro)renin receptor (sPRR). Experimental evidence is available to demonstrate that sPRR stimulates renal aquaporin-2 expression and promotes urine concentrating capability by mediating the antidiuretic action of vasopressin (VP). Since sPRR acts downstream of VP type 2 receptor (V2R) in the kidney, it is effective in management of nephrogenic diabetes insipidus (NDI) induced by V2R antagonism. On the other hand, PRR is shown to regulate VP release from the central nervous system and such action is speculated to be mediated by sPRR. Solid line, experimentally confirmed; dashed line, not confirmed. CDI, central diabetes insipidus.
The mechanism of how PRR regulates VP production remains elusive. Our previous study employing renal mIMCD-K2 cells defines PRR as an osmosensitive gene (55). In response to hypertonicity, the protein abundance of both PRR and sPRR is upregulated dependent of NF-κB (55). A possibility exists that PRR may be involved in the osmosensing pathway for regulation of VP secretion. Plasma osmolality is maintained within a narrow range because hypertonicity can cause irreversible tissue damage and lethal neurological trauma (61). As small as 1% increase in plasma osmolality (~3 mosmol/kgH2O) will induce VP secretion to the circulation (5). The organum vasculosum laminae terminalis harbors osmosensitive neurons that sense changes in osmolality and sends axonal projections into the SON to regulate the firing activity of VP neurons. It will be interesting to test a possible role of PRR in the osmosensing capability of the organum vasculosum laminae terminalis.
CONCLUSIONS
Evidence is emerging concerning the influence of the PRR in regulating VP signaling in the CD. Administration of sPRR-His attenuates indexes of NDI induced by V2R antagonism, whereas a similar sPRR-His treatment was without effect on lithium-induced NDI. Hence, a sPRR-based approach may offer a novel intervention for subgroups of NDI patients with V2R mutations. The central action of PRR in regulating VP production is well demonstrated and may imply involvement of sPRR as seen in the kidney. The central production and action of sPRR should be explored in future studies.
GRANTS
This work was supported by NIH Grants DK-104072, HL-135851, and HL-139689; Veterans Affairs Merit Review from the Department of Veterans Affairs; and National Natural Science Foundation of China Grants 31330037, 81570377, 91439205, and 81630013. T. Yang is Research Career Scientist in Department of Veterans Affairs. J. D. Symons was supported by the American Heart Association Grant 16GRNT31050004) and National Institutes of Health Grants R03-AG-O52848 and R01-HL-141540).
DISCLOSURES
No conflicts of interest, financial or otherwise, are declared by the authors.
AUTHOR CONTRIBUTIONS
K.T.Y. and T.Y. prepared figures; K.T.Y. drafted manuscript; T.Y. and J.D.S. edited and revised manuscript; T.Y. and J.D.S. approved final version of manuscript.
REFERENCES
- 1.An H, He L. Current understanding of metformin effect on the control of hyperglycemia in diabetes. J Endocrinol 228: R97–R106, 2016. doi: 10.1530/JOE-15-0447. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Ando F, Mori S, Yui N, Morimoto T, Nomura N, Sohara E, Rai T, Sasaki S, Kondo Y, Kagechika H, Uchida S. AKAPs-PKA disruptors increase AQP2 activity independently of vasopressin in a model of nephrogenic diabetes insipidus. Nat Commun 9: 1411, 2018. doi: 10.1038/s41467-018-03771-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Bonakdaran S, Azami G, Tara F, Poorali L. Soluble (Pro) renin receptor is a predictor of gestational diabetes mellitus. Curr Diabetes Rev 13: 555–559, 2017. doi: 10.2174/1573399812666160919100253. [DOI] [PubMed] [Google Scholar]
- 4.Bouley R, Hasler U, Lu HA, Nunes P, Brown D. Bypassing vasopressin receptor signaling pathways in nephrogenic diabetes insipidus. Semin Nephrol 28: 266–278, 2008. doi: 10.1016/j.semnephrol.2008.03.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Bourque CW. Central mechanisms of osmosensation and systemic osmoregulation. Nat Rev Neurosci 9: 519–531, 2008. doi: 10.1038/nrn2400. [DOI] [PubMed] [Google Scholar]
- 6.Burcklé C, Bader M. Prorenin and its ancient receptor. Hypertension 48: 549–551, 2006. doi: 10.1161/01.HYP.0000241132.48495.df. [DOI] [PubMed] [Google Scholar]
- 7.Cao T, Feng Y. The (pro)renin receptor and body fluid homeostasis. Am J Physiol Regul Integr Comp Physiol 305: R104–R106, 2013. doi: 10.1152/ajpregu.00209.2013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Coffey AK, O’Sullivan DJ, Homma S, Dousa TP, Valtin H. Induction of intramembranous particle clusters in mice with nephrogenic diabetes insipidus. Am J Physiol Renal Physiol 261: F640–F646, 1991. doi: 10.1152/ajprenal.1991.261.4.F640. [DOI] [PubMed] [Google Scholar]
- 9.Cousin C, Bracquart D, Contrepas A, Corvol P, Muller L, Nguyen G. Soluble form of the (pro)renin receptor generated by intracellular cleavage by furin is secreted in plasma. Hypertension 53: 1077–1082, 2009. doi: 10.1161/HYPERTENSIONAHA.108.127258. [DOI] [PubMed] [Google Scholar]
- 10.Dabrowski E, Kadakia R, Zimmerman D. Diabetes insipidus in infants and children. Best Pract Res Clin Endocrinol Metab 30: 317–328, 2016. doi: 10.1016/j.beem.2016.02.006. [DOI] [PubMed] [Google Scholar]
- 11.Ecelbarger CA, Nielsen S, Olson BR, Murase T, Baker EA, Knepper MA, Verbalis JG. Role of renal aquaporins in escape from vasopressin-induced antidiuresis in rat. J Clin Invest 99: 1852–1863, 1997. doi: 10.1172/JCI119352. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Efe O, Klein JD, LaRocque LM, Ren H, Sands JM. Metformin improves urine concentration in rodents with nephrogenic diabetes insipidus. JCI Insight 1: e88409, 2016. doi: 10.1172/jci.insight.88409. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Fang H, Deng M, Zhang L, Lu A, Su J, Xu C, Zhou L, Wang L, Ou JS, Wang W, Yang T. Role of (Pro)Renin Receptor in Albumin Overload-Induced Nephropathy in Rats. Am J Physiol Renal Physiol ajprenal.00071.2018, 2018. doi: 10.1152/ajprenal.00071.2018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Fang H, Xu C, Lu A, Zou CJ, Xie S, Chen Y, Zhou L, Liu M, Wang L, Wang W, Yang T. (Pro)renin receptor mediates albumin-induced cellular responses: role of site-1 protease-derived soluble (pro)renin receptor in renal epithelial cells. Am J Physiol Cell Physiol 313: C632–C643, 2017. doi: 10.1152/ajpcell.00006.2017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Fenton RA, Knepper MA. Mouse models and the urinary concentrating mechanism in the new millennium. Physiol Rev 87: 1083–1112, 2007. doi: 10.1152/physrev.00053.2006. [DOI] [PubMed] [Google Scholar]
- 16.Fukushima A, Kinugawa S, Homma T, Masaki Y, Furihata T, Abe T, Suga T, Takada S, Kadoguchi T, Okita K, Matsushima S, Tsutsui H. Increased plasma soluble (pro)renin receptor levels are correlated with renal dysfunction in patients with heart failure. Int J Cardiol 168: 4313–4314, 2013. doi: 10.1016/j.ijcard.2013.04.176. [DOI] [PubMed] [Google Scholar]
- 17.Fushimi K, Sasaki S, Marumo F. Phosphorylation of serine 256 is required for cAMP-dependent regulatory exocytosis of the aquaporin-2 water channel. J Biol Chem 272: 14800–14804, 1997. doi: 10.1074/jbc.272.23.14800. [DOI] [PubMed] [Google Scholar]
- 18.Gao M, Cao R, Du S, Jia X, Zheng S, Huang S, Han Q, Liu J, Zhang X, Miao Y, Kang J, Gustafsson JA, Guan Y. Disruption of prostaglandin E2 receptor EP4 impairs urinary concentration via decreasing aquaporin 2 in renal collecting ducts. Proc Natl Acad Sci USA 112: 8397–8402, 2015. doi: 10.1073/pnas.1509565112. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Gonzalez AA, Cifuentes-Araneda F, Ibaceta-Gonzalez C, Gonzalez-Vergara A, Zamora L, Henriquez R, Rosales CB, Navar LG, Prieto MC. Vasopressin/V2 receptor stimulates renin synthesis in the collecting duct. Am J Physiol Renal Physiol 310: F284–F293, 2016. doi: 10.1152/ajprenal.00360.2015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Gonzalez AA, Lara LS, Luffman C, Seth DM, Prieto MC. Soluble form of the (pro)renin receptor is augmented in the collecting duct and urine of chronic angiotensin II-dependent hypertensive rats. Hypertension 57: 859–864, 2011. doi: 10.1161/HYPERTENSIONAHA.110.167957. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Hamada K, Taniguchi Y, Shimamura Y, Inoue K, Ogata K, Ishihara M, Horino T, Fujimoto S, Ohguro T, Yoshimoto Y, Ikebe M, Yuasa K, Hoshino E, Iiyama T, Ichihara A, Terada Y. Serum level of soluble (pro)renin receptor is modulated in chronic kidney disease. Clin Exp Nephrol 17: 848–856, 2013. doi: 10.1007/s10157-013-0803-y. [DOI] [PubMed] [Google Scholar]
- 22.Hasler U, Leroy V, Jeon US, Bouley R, Dimitrov M, Kim JA, Brown D, Kwon HM, Martin PY, Féraille E. NF-kappaB modulates aquaporin-2 transcription in renal collecting duct principal cells. J Biol Chem 283: 28095–28105, 2008. doi: 10.1074/jbc.M708350200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Hasler U, Vinciguerra M, Vandewalle A, Martin PY, Féraille E. Dual effects of hypertonicity on aquaporin-2 expression in cultured renal collecting duct principal cells. J Am Soc Nephrol 16: 1571–1582, 2005. doi: 10.1681/ASN.2004110930. [DOI] [PubMed] [Google Scholar]
- 24.Hozawa S, Holtzman EJ, Ausiello DA. cAMP motifs regulating transcription in the aquaporin 2 gene. Am J Physiol Cell Physiol 270: C1695–C1702, 1996. doi: 10.1152/ajpcell.1996.270.6.C1695. [DOI] [PubMed] [Google Scholar]
- 25.Huang J, Siragy HM. Sodium depletion enhances renal expression of (pro)renin receptor via cyclic GMP-protein kinase G signaling pathway. Hypertension 59: 317–323, 2012. doi: 10.1161/HYPERTENSIONAHA.111.186056. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Jung HJ, Kim SY, Choi HJ, Park EJ, Lim JS, Frøkiaer J, Nielsen S, Kwon TH. Tankyrase-mediated β-catenin activity regulates vasopressin-induced AQP2 expression in kidney collecting duct mpkCCDc14 cells. Am J Physiol Renal Physiol 308: F473–F486, 2015. doi: 10.1152/ajprenal.00052.2014. [DOI] [PubMed] [Google Scholar]
- 27.Jung HJ, Raghuram V, Lee JW, Knepper MA. Genome-wide mapping of DNA accessibility and binding sites for CREB and C/EBPβ in vasopressin-sensitive collecting duct cells. J Am Soc Nephrol 29: 1490–1500, 2018. doi: 10.1681/ASN.2017050545. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Knoers N, Monnens LA. Nephrogenic diabetes insipidus: clinical symptoms, pathogenesis, genetics and treatment. Pediatr Nephrol 6: 476–482, 1992. doi: 10.1007/BF00874020. [DOI] [PubMed] [Google Scholar]
- 29.Li JH, Chou CL, Li B, Gavrilova O, Eisner C, Schnermann J, Anderson SA, Deng CX, Knepper MA, Wess J. A selective EP4 PGE2 receptor agonist alleviates disease in a new mouse model of X-linked nephrogenic diabetes insipidus. J Clin Invest 119: 3115–3126, 2009. doi: 10.1172/JCI39680. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Li SZ, McDill BW, Kovach PA, Ding L, Go WY, Ho SN, Chen F. Calcineurin-NFATc signaling pathway regulates AQP2 expression in response to calcium signals and osmotic stress. Am J Physiol Cell Physiol 292: C1606–C1616, 2007. doi: 10.1152/ajpcell.00588.2005. [DOI] [PubMed] [Google Scholar]
- 31.Li W, Peng H, Cao T, Sato R, McDaniels SJ, Kobori H, Navar LG, Feng Y. Brain-targeted (pro)renin receptor knockdown attenuates angiotensin II-dependent hypertension. Hypertension 59: 1188–1194, 2012. doi: 10.1161/HYPERTENSIONAHA.111.190108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Li W, Peng H, Mehaffey EP, Kimball CD, Grobe JL, van Gool JM, Sullivan MN, Earley S, Danser AH, Ichihara A, Feng Y. Neuron-specific (pro)renin receptor knockout prevents the development of salt-sensitive hypertension. Hypertension 63: 316–323, 2014. doi: 10.1161/HYPERTENSIONAHA.113.02041. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Li W, Zhang Y, Bouley R, Chen Y, Matsuzaki T, Nunes P, Hasler U, Brown D, Lu HA. Simvastatin enhances aquaporin-2 surface expression and urinary concentration in vasopressin-deficient Brattleboro rats through modulation of Rho GTPase. Am J Physiol Renal Physiol 301: F309–F318, 2011. doi: 10.1152/ajprenal.00001.2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Li XC, Shao Y, Zhuo JL. AT1a receptor knockout in mice impairs urine concentration by reducing basal vasopressin levels and its receptor signaling proteins in the inner medulla. Kidney Int 76: 169–177, 2009. doi: 10.1038/ki.2009.134. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Li XC, Shao Y, Zhuo JL. AT1a receptor signaling is required for basal and water deprivation-induced urine concentration in AT1a receptor-deficient mice. Am J Physiol Renal Physiol 303: F746–F756, 2012. doi: 10.1152/ajprenal.00644.2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Li Z, Zhou L, Wang Y, Miao J, Hong X, Hou FF, Liu Y. (Pro)renin receptor is an amplifier of Wnt/β-catenin signaling in kidney injury and fibrosis. J Am Soc Nephrol 28: 2393–2408, 2017. doi: 10.1681/ASN.2016070811. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Lu X, Wang F, Xu C, Soodvilai S, Peng K, Su J, Zhao L, Yang KT, Feng Y, Zhou SF, Gustafsson JA, Yang T. Soluble (pro)renin receptor via β-catenin enhances urine concentration capability as a target of liver X receptor. Proc Natl Acad Sci USA 113: E1898–E1906, 2016. doi: 10.1073/pnas.1602397113. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Matavelli LC, Huang J, Siragy HM. In vivo regulation of renal expression of (pro)renin receptor by a low-sodium diet. Am J Physiol Renal Physiol 303: F1652–F1657, 2012. doi: 10.1152/ajprenal.00204.2012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Matsumura Y, Uchida S, Rai T, Sasaki S, Marumo F. Transcriptional regulation of aquaporin-2 water channel gene by cAMP. J Am Soc Nephrol 8: 861–867, 1997. [DOI] [PubMed] [Google Scholar]
- 40.Moeller HB, Rittig S, Fenton RA. Nephrogenic diabetes insipidus: essential insights into the molecular background and potential therapies for treatment. Endocr Rev 34: 278–301, 2013. doi: 10.1210/er.2012-1044. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Nakagawa T, Suzuki-Nakagawa C, Watanabe A, Asami E, Matsumoto M, Nakano M, Ebihara A, Uddin MN, Suzuki F. Site-1 protease is required for the generation of soluble (pro)renin receptor. J Biochem 161: 369–379, 2017. doi: 10.1093/jb/mvw080. [DOI] [PubMed] [Google Scholar]
- 42.Nartita T, Ichihara A, Matsuoka K, Takai Y, Bokuda K, Morimoto S, Itoh H, Seki H. Placental (pro)renin receptor expression and plasma soluble (pro)renin receptor levels in preeclampsia. Placenta 37: 72–78, 2016. [Erratum in Placenta 47: 130, 2016]. doi: 10.1016/j.placenta.2015.11.007. [DOI] [PubMed] [Google Scholar]
- 43.Nejsum LN, Zelenina M, Aperia A, Frøkiaer J, Nielsen S. Bidirectional regulation of AQP2 trafficking and recycling: involvement of AQP2-S256 phosphorylation. Am J Physiol Renal Physiol 288: F930–F938, 2005. doi: 10.1152/ajprenal.00291.2004. [DOI] [PubMed] [Google Scholar]
- 44.Nguyen G. Renin and prorenin receptor in hypertension: what’s new? Curr Hypertens Rep 13: 79–85, 2011. doi: 10.1007/s11906-010-0172-9. [DOI] [PubMed] [Google Scholar]
- 45.Nguyen G, Delarue F, Burcklé C, Bouzhir L, Giller T, Sraer JD. Pivotal role of the renin/prorenin receptor in angiotensin II production and cellular responses to renin. J Clin Invest 109: 1417–1427, 2002. doi: 10.1172/JCI0214276. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Nielsen S, Frøkiaer J, Marples D, Kwon TH, Agre P, Knepper MA. Aquaporins in the kidney: from molecules to medicine. Physiol Rev 82: 205–244, 2002. doi: 10.1152/physrev.00024.2001. [DOI] [PubMed] [Google Scholar]
- 47.Nishijima T, Tajima K, Takahashi K, Sakurai S. Elevated plasma levels of soluble (pro)renin receptor in patients with obstructive sleep apnea syndrome: association with polysomnographic parameters. Peptides 56: 14–21, 2014. doi: 10.1016/j.peptides.2014.03.008. [DOI] [PubMed] [Google Scholar]
- 48.Nishijima T, Tajima K, Yamashiro Y, Hosokawa K, Suwabe A, Takahashi K, Sakurai S. Elevated plasma levels of soluble (Pro)renin receptor in patients with obstructive sleep apnea syndrome in parallel with the disease severity. Tohoku J Exp Med 238: 325–338, 2016. doi: 10.1620/tjem.238.325. [DOI] [PubMed] [Google Scholar]
- 49.Olesen ET, Rützler MR, Moeller HB, Praetorius HA, Fenton RA. Vasopressin-independent targeting of aquaporin-2 by selective E-prostanoid receptor agonists alleviates nephrogenic diabetes insipidus. Proc Natl Acad Sci USA 108: 12949–12954, 2011. doi: 10.1073/pnas.1104691108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Poulsen SB, Kristensen TB, Brooks HL, Kohan DE, Rieg T, Fenton RA. Role of adenylyl cyclase 6 in the development of lithium-induced nephrogenic diabetes insipidus. JCI Insight 2: e91042, 2017. doi: 10.1172/jci.insight.91042. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Procino G, Milano S, Carmosino M, Barbieri C, Nicoletti MC, Li JH, Wess J, Svelto M. Combination of secretin and fluvastatin ameliorates the polyuria associated with X-linked nephrogenic diabetes insipidus in mice. Kidney Int 86: 127–138, 2014. doi: 10.1038/ki.2014.10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Rai T, Uchida S, Marumo F, Sasaki S. Cloning of rat and mouse aquaporin-2 gene promoters and identification of a negative cis-regulatory element. Am J Physiol Renal Physiol 273: F264–F273, 1997. doi: 10.1152/ajprenal.1997.273.2.F264. [DOI] [PubMed] [Google Scholar]
- 53.Ramkumar N, Stuart D, Calquin M, Quadri S, Wang S, Van Hoek AN, Siragy HM, Ichihara A, Kohan DE. Nephron-specific deletion of the prorenin receptor causes a urine concentration defect. Am J Physiol Renal Physiol 309: F48–F56, 2015. doi: 10.1152/ajprenal.00126.2015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Shan Z, Shi P, Cuadra AE, Dong Y, Lamont GJ, Li Q, Seth DM, Navar LG, Katovich MJ, Sumners C, Raizada MK. Involvement of the brain (pro)renin receptor in cardiovascular homeostasis. Circ Res 107: 934–938, 2010. doi: 10.1161/CIRCRESAHA.110.226977. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Su J, Liu X, Xu C, Lu X, Wang F, Fang H, Lu A, Qiu Q, Li C, Yang T. NF-κB-dependent upregulation of (pro)renin receptor mediates high-NaCl-induced apoptosis in mouse inner medullary collecting duct cells. Am J Physiol Cell Physiol 313: C612–C620, 2017. doi: 10.1152/ajpcell.00068.2017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Takahashi K, Hiraishi K, Hirose T, Kato I, Yamamoto H, Shoji I, Shibasaki A, Kaneko K, Satoh F, Totsune K. Expression of (pro)renin receptor in the human brain and pituitary, and co-localisation with arginine vasopressin and oxytocin in the hypothalamus. J Neuroendocrinol 22: 453–459, 2010. doi: 10.1111/j.1365-2826.2010.01980.x. [DOI] [PubMed] [Google Scholar]
- 57.Takahashi K, Ohba K, Tajima K, Nishijima T, Sakurai S. Soluble (Pro)renin receptor and obstructive sleep apnea syndrome: oxidative stress in brain? Int J Mol Sci 18: 1313, 2017. doi: 10.3390/ijms18061313. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Thomason J, Reyes M, Allen SR, Jones RO, Beeram MR, Kuehl TJ, Suzuki F, Uddin MN. Elevation of (Pro)renin and (Pro)renin receptor in preeclampsia. Am J Hypertens 28: 1277–1284, 2015. doi: 10.1093/ajh/hpv019. [DOI] [PubMed] [Google Scholar]
- 60.Trepiccione F, Gerber SD, Grahammer F, López-Cayuqueo KI, Baudrie V, Păunescu TG, Capen DE, Picard N, Alexander RT, Huber TB, Chambrey R, Brown D, Houillier P, Eladari D, Simons M. Renal Atp6ap2/(Pro)renin receptor is required for normal vacuolar H+-ATPase function but not for the renin-angiotensin system. J Am Soc Nephrol 27: 3320–3330, 2016. doi: 10.1681/ASN.2015080915. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Verbalis JG. Brain volume regulation in response to changes in osmolality. Neuroscience 168: 862–870, 2010. doi: 10.1016/j.neuroscience.2010.03.042. [DOI] [PubMed] [Google Scholar]
- 62.Wang F, Lu X, Liu M, Feng Y, Zhou SF, Yang T. Renal medullary (pro)renin receptor contributes to angiotensin II-induced hypertension in rats via activation of the local renin-angiotensin system. BMC Med 13: 278, 2015. doi: 10.1186/s12916-015-0514-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Wang F, Lu X, Peng K, Fang H, Zhou L, Su J, Nau A, Yang KT, Ichihara A, Lu A, Zhou SF, Yang T. Antidiuretic action of collecting duct (pro)renin receptor downstream of vasopressin and PGE2 receptor EP4. J Am Soc Nephrol 27: 3022–3034, 2016. doi: 10.1681/ASN.2015050592. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Wang F, Lu X, Peng K, Fang H, Zhou L, Su J, Nau A, Yang KT, Ichihara A, Lu A, Zhou SF, Yang T. Antidiuretic action of collecting duct (Pro)renin receptor downstream of vasopressin and PGE2 receptor EP4. J Am Soc Nephrol 27: 3022–3034, 2016. doi: 10.1681/ASN.2015050592. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Watanabe N, Bokuda K, Fujiwara T, Suzuki T, Mito A, Morimoto S, Jwa SC, Egawa M, Arai Y, Suzuki F, Sago H, Ichihara A. Soluble (pro)renin receptor and blood pressure during pregnancy: a prospective cohort study. Hypertension 60: 1250–1256, 2012. doi: 10.1161/HYPERTENSIONAHA.112.197418. [DOI] [PubMed] [Google Scholar]
- 66.Watanabe N, Morimoto S, Fujiwara T, Suzuki T, Taniguchi K, Mori F, Ando T, Watanabe D, Kimura T, Sago H, Ichihara A. Prediction of gestational diabetes mellitus by soluble (pro)renin receptor during the first trimester. J Clin Endocrinol Metab 98: 2528–2535, 2013. doi: 10.1210/jc.2012-4139. [DOI] [PubMed] [Google Scholar]
- 67.Yang KT, Wang F, Lu X, Peng K, Yang T, David Symons J. The soluble (Pro) renin receptor does not influence lithium-induced diabetes insipidus but does provoke beiging of white adipose tissue in mice. Physiol Rep 5: e13410, 2017. doi: 10.14814/phy2.13410. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Yang T. Crosstalk between (Pro)renin receptor and COX-2 in the renal medulla during angiotensin II-induced hypertension. Curr Opin Pharmacol 21: 89–94, 2015. doi: 10.1016/j.coph.2014.12.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Yang T. Unraveling the physiology of (Pro)renin receptor in the distal nephron. Hypertension 69: 564–574, 2017. doi: 10.1161/HYPERTENSIONAHA.116.08318. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Yang T, Xu C. Physiology and pathophysiology of the intrarenal renin-angiotensin system: an update. J Am Soc Nephrol 28: 1040–1049, 2017. doi: 10.1681/ASN.2016070734. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Yasui M, Zelenin SM, Celsi G, Aperia A. Adenylate cyclase-coupled vasopressin receptor activates AQP2 promoter via a dual effect on CRE and AP1 elements. Am J Physiol Renal Physiol 272: F443–F450, 1997. doi: 10.1152/ajprenal.1997.272.4.F443. [DOI] [PubMed] [Google Scholar]
- 72.Yoshikawa A, Aizaki Y, Kusano K, Kishi F, Susumu T, Iida S, Ishiura S, Nishimura S, Shichiri M, Senbonmatsu T. The (pro)renin receptor is cleaved by ADAM19 in the Golgi leading to its secretion into extracellular space. Hypertens Res 34: 599–605, 2011. doi: 10.1038/hr.2010.284. [DOI] [PubMed] [Google Scholar]
- 73.Yu MJ, Miller RL, Uawithya P, Rinschen MM, Khositseth S, Braucht DW, Chou CL, Pisitkun T, Nelson RD, Knepper MA. Systems-level analysis of cell-specific AQP2 gene expression in renal collecting duct. Proc Natl Acad Sci USA 106: 2441–2446, 2009. doi: 10.1073/pnas.0813002106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Zhu Q, Yang T. Enzymatic sources and physio-pathological functions of soluble (pro)renin receptor. Curr Opin Nephrol Hypertens 27: 77–82, 2018. doi: 10.1097/MNH.0000000000000396. [DOI] [PMC free article] [PubMed] [Google Scholar]


