Abstract
Lower urinary tract dysfunction (LUTD) is a common problem in children and constitutes up to 40% of pediatric urology clinic visits. Improved diagnosis and interventions have been leading to better outcomes in many patients, whereas some children are left untreated or do not respond to the treatment successfully. In addition, many of these patients are lost by the pediatric urologists during their teenage years, and the outcome in later life largely remains unidentified. Studies suggest childhood LUTD is associated with subsequent adult urinary tract symptoms. However, whether and how early life LUTD attributes to urinary symptoms in those patients later in life remains to be elucidated. In the current study, we investigated the effects of early life voiding perturbation on bladder function using a neonatal maternal separation (NMS) protocol in mice. The NMS group displayed a delayed development of voluntary voiding behavior, a significant reduction of functional bladder capacity, and bladder overactivity compared with control mice later in life. In vitro evaluation of detrusor smooth muscle and molecular study showed a decrease in muscarinic contribution alongside an increase in purinergic contribution in detrusor contractility in NMS mice compared with control group. These results suggest that early life bladder dysfunction interfered with the normal maturation of the voluntary micturition control and facilitated LUTD in a later stage, which is at least partly attributed to an alteration of muscarinic and purinergic signaling in the urinary bladder.
Keywords: bladder dysfunction, cholinergic, overactive bladder, purinergic
INTRODUCTION
During the first few years of life, the urinary bladder undergoes a gradual development from an initially involuntary voiding pattern to a more socially conscious adult type of voluntary micturition control. The maturation of micturition control mechanisms relies on an intact neural circuitry, awareness of sensation of bladder fullness, and social norms (13). It requires continuous evolution, including the increase of functional bladder capacity, maturation of the detrusor-sphincter coordination, and progressive development of the voluntary control over the infantile voiding reflex (13, 23, 24). Therefore, various derangements of lower urinary tract function may occur during early development of normal voiding control mechanisms.
Lower urinary tract dysfunction (LUTD) is a common problem in children and constitutes up to 40% of pediatric urology clinic visits (12). The dynamics and functional disturbances of the lower urinary tract in children are different from those in adults and are often not associated with any identifiable anatomic abnormalities. Prior clinical studies suggested that considerable numbers of children who suffered from bladder dysfunction developed long-term lower urinary tract symptoms (LUTS) and subsequent renal problems (9, 15, 28). Despite accumulating clinical and animal research evidence, the pathophysiologic pathways involved in diverse types of bladder dysfunction in children and the etiology and the underlying mechanisms of long-term LUTS associated with bladder dysfunction in early life remain to be clarified.
The physiological properties and the development of the mechanism controlling micturition are believed to be very similar among laboratory animal species and humans. Voiding in neonatal rodents depends on perigenital stimulation through maternal licking to activate the somatovesical reflex processed in the spinal cord until voluntary bladder control emerges during 2 to 3 wk of age (34, 38). Although not a part of the normal bladder maturation process in humans, a similar reflex has been detected in infants indicating a common reflex arc (36). As the adult form of voiding controlled voluntarily by neural circuitry in the higher brain centers matures, the neonatal spinal-bladder reflex gradually subsides and eventually dissipates (17, 33). It has been shown that fetal and neonatal rodent urinary bladders undergo a series of rapid developmental changes in neural coordination as well as their structures during the first 3 wk of life (2, 38), similar to progressive development in young children (13, 23, 24). This means that adverse events in urinary function during this period could affect the anatomical and functional development of the lower urinary tract as well as neural circuits of voiding control.
In this study, we investigated the effects of early life voiding dysfunction on lower urinary tract function in adolescence by utilizing a neonatal maternal separation (NMS) protocol in mice, which induces a disruption of normal voiding cycle accompanying intermittent urinary retention and bladder distention in infancy and early childhood (38). We hypothesized that early voiding dysfunction would cause long-term abnormalities in detrusor function.
MATERIALS AND METHODS
Animals.
All procedures using animals were reviewed and approved by the Institutional Animal Care and Use Committee of the University of Colorado Anschutz Medical Campus.
Pregnant female C57BL/6J mice were received at gestational day 16 from The Jackson Laboratories (Bar Harbor, ME). Each dam was individually housed in a 12-h light/dark cycle with ad libitum access to food and water. Each litter of 5 to 10 pups was divided into a half and assigned to 2 groups: control or NMS. Control litters were culled on postnatal day (PND) 2 and immediately returned to their dam and remained with her continuously thereafter. The NMS group was removed from their dam and individually placed into a ventilated clean plastic container with nestling material from the home cage for 6 h (10 AM to 4 PM) daily from PND 2 to PND 14. Both male and female mice were utilized in this study. After weaning on PND 25, mice were group-housed with same-sex littermates.
Spontaneous void spot assay.
To evaluate voiding habits, void spot assays were conducted at 15 days, 3 wk, 4 wk, and 5 wk of age as previously described (N = 50 per group) (21). Briefly, mice were individually placed onto a steel wire mesh floor (0.64-cm2 opening) elevated from the cage bottom by 1 cm in a clean cage. A precut Whatman-grade filter paper (Thermo Fisher Scientific, Hampton, NH) was placed to cover the entire surface of the bottom of the cage. Each mouse had free access to water but no food during the 3-h test period, which started at 10 AM. The urine spots were imaged using ultraviolet light on a transilluminator, and the number of all urine spots greater than 8 mm2 (corresponding to 2.5 μl) on each filter was counted as voids. Areas of urine spots were converted to volumes by constructing a standard curve that relates to known volumes of mouse urine spotted on the filter paper using Adobe Photoshop (21). The numbers of large (≥25 μl) and small (<25 μl) micturition spots on the filter papers were counted, and the volume of each spot was summed to determine total volume of void. Mean volume of large voids was determined by averaging the volume of large voids on each filter paper.
Surgical procedure to catheterize the urinary bladder.
A subset of mice from each group underwent surgical catheter implantation in the bladder for cystometry studies at 5 wk old as previously described (21). Briefly, a flared-end polyethylene catheter (PE-10) was inserted through a puncture on the bladder dome and secured with a 7-0 Prolene suture (Ethicon, Somerville, NJ). The catheter was tunneled subcutaneously and exteriorized at the scapula region, where it was sutured to the skin. The catheter was filled with sterile saline after confirming no leakage at the bladder and then plugged to prevent leakage. The abdominal incision was closed, and animals were transferred to individual cages after the recovery from anesthesia. A single dose of carprofen (5 mg/kg) and penicillin (40,000 IU/kg) was given subcutaneously daily from 0 to 3 days after surgery.
Urodynamic evaluation of bladder function.
Urodynamic evaluation was performed in unanesthetized, unrestrained mice 1 wk after the surgery (N = 5–6 per sex in each group, 6 wk old) as described previously (21). The tip of the exteriorized bladder catheter located at the base of the mouse neck was connected to a pressure transducer and an infusion pump of the cystometry station (Small Animal Laboratory Cystometry Laboratory Station, Catamount Research and Development, St. Albans, VT). Room-temperature saline solution was infused into the bladder at a rate of 10 μl/min. Voided urine was monitored with the analytical balance connected to a force-displacement transducer integrated into the data acquisition system. Each animal was observed for up to eight voiding cycles of reproducible micturition patterns. Urodynamic values recorded continuously during testing, and the following urodynamic parameters were analyzed using Cystometry Analysis Software (SOF-552, Catamount Research and Development): maximum intravesical pressure at micturition, functional bladder capacity, voided volume, number of nonvoid contractions (NVCs) per voiding cycle, and intermicturition interval. The NVCs were defined as rhythmic intravesical pressure rises greater than one-third of average maximal voiding pressure in each animal without triggering micturition (21).
In vitro detrusor contractility measurements.
In vitro detrusor smooth muscle (DSM) contractility measurements were performed as previously described (22). Briefly, freshly isolated urinary bladders from mice in each group at 6 wk old (N = 10–12 per group) were cut into two halves longitudinally. Each strip (~3 mm × 6 mm, n = 20 per group) was placed in organ baths (Radnoti, Monrovia, CA) filled with oxygenated Tyrode’s buffer (in mM; 125 NaCl, 2.5 KCl, 23.8 NaHCO3, 0.5 MgCl2, 0.4 NaH2PO4, 1.8 CaCl2, and 5.5 glucose) at 37°C. Tissues were equilibrated for 45 min and then stretched to their optimum length for muscle contraction (Lo) in which the maximum force for muscle contraction was produced by electrical field stimulation (EFS; 70 V, 32 Hz). Once Lo was determined, each muscle strip was allowed to equilibrate a further 30 min in fresh Tyrode’s buffer. The tissues were subjected to several tests, including the contractile responses to EFS (70 V, 0.5–32 Hz), the acetylcholine receptor (AChR) agonist carbachol (CCh, 0.1 to 100 µM), high KCl (125 mM replaced NaCl in Tyrode’s buffer), 5 mM ATP, and α,β-methylene ATP [α,β-meATP, purinoceptor P2X1/3 agonist, 3 µM]. Contractile responses to EFS were also recorded after 15 min of incubation of the following substances: 1) 2,2,6,6-tetramethylpiperidin-4-yl heptanoate [TMPH; antagonist of neuronal nicotinic AChR (nAChR) formed by the combination of the α and β subunits (α3, α4, β2, and β4), 0.3 µM], the combination of 2) TMPH and atropine [muscarinic (mAChR) antagonist, 1 µM], 3) atropine and α,β-meATP (desensitizes P2X1/3), 4) atropine, α,β-meATP, and methyllycaconitine citrate [MLA; α7 nAChR blocker, 10 µM], and 5) tetrodotoxin [TTX; Na+ channel blocker, 1 µM]. Contractile parameters were measured using PowerLab Laboratory-Chart version 8.1.9 (AD Instruments, Colorado Springs, CO). Force measurements were performed and analyzed as previously described (22).
Histological analysis.
Paraformaldehyde-fixed paraffin sections (5-µm thickness) of the urinary bladders from each group at 6 wk old were stained with hematoxylin-eosin (H&E) and subjected for the morphological evaluation of urothelia, lamina propria, and DSM. The areas of 1) the total tissue section, 2) the muscle layer, and 3) the urothelial layer were measured using Adobe Photoshop software, and then we calculated the ratio of muscle and urothelial layer. In addition, the thicknesses of 1) total bladder wall, 2) the muscle layer, and 3) the urothelial layer were measured at two random locations on each section, and we compared the average of each parameter between control and NMS mice. Collagen fiber was visualized and imaged with second harmonic generation (SHG) microscopy (Carl Zeiss Microscopy, LLC, Thornwood, NY) (10). All SHG images were taken with the same parameter settings. The areas of collagen fibers (pseudocolored in red) in total tissue section and in muscle layer were measured by using Adobe Photoshop software. Then the ratio/amount of collagen fibers in total tissue section or in muscle layer was calculated and compared between two groups. Three sections that were at least 50 µm apart between each other from 4 mice per group were analyzed for reproducibility and the accuracy of measurements.
Gene expression analysis.
Total RNA was isolated from the urinary bladders (N = 4 per group) from 6-wk-old mice in each group using QIAzol lysis reagent (QIAGEN, Germantown, MD) and Direct-zol RNA kit (Zymo Research, Irvine, CA) and transcribed into cDNA using iScript Reverse Transcription kit (Bio-Rad, Hercules, CA). Real-time quantitative PCR was performed using a Light Cycler 480 (Roche Diagnostics, Indianapolis, IN). Expression levels of each gene were calculated as fold changes based on ΔΔCt values. Data were normalized to the housekeeping gene, β-actin (Actb). The protein extract from the bladders was separated by 4–15% Mini-PROTEAN TGX gels (Bio-Rad) and transferred to nitrocellulose membrane followed by blocking with Tris-buffered saline-Tween 20 (in mM: 25 Tris-HCl, pH 7.5, 150 NaCl, 50 KCl, 0.1% Tween 20) containing 5% nonfat dried milk. The filters were incubated with primary antibodies against P2X1 (1:500, cat. no. APR-022; Alomone Laboratories, Jerusalem, Israel), cholinergic receptor muscarinic 2 (Chrm2) (1:500, NBP2–26152, Novus Biologicals, Littleton, CO), or glyceraldehyde-3-phosphate dehydrogenase (Gapdh) (1:1,000, cat. no. sc-25778, Santa Cruz Biotechnology, Dallas, TX) diluted in Tris-buffered saline-Tween 20, followed by treatment with horseradish peroxidase-conjugated anti-rabbit IgG (Novus Biologicals) or anti-goat IgG (Jackson ImmunoResearch, West Grove, PA). Signals were detected by development using Pierce ECL Western Blotting Substrate (Thermo Fisher Scientific). The signals specific to each antibody were quantified using ImageJ software (National Institutes of Health, Bethesda, MD) and expressed as the ratio to a housekeeping gene, Gapdh.
Statistical analysis.
Differences were analyzed using the two-tailed Mann-Whitney test (GraphPad Instat, GraphPad Software, Inc., La Jolla, CA) between control and NMS groups. A probability value of P < 0.05 was regarded as significant. Results are expressed as means ± standard error (SE).
RESULTS
Delayed development of voluntary voiding control by early life urinary disturbance.
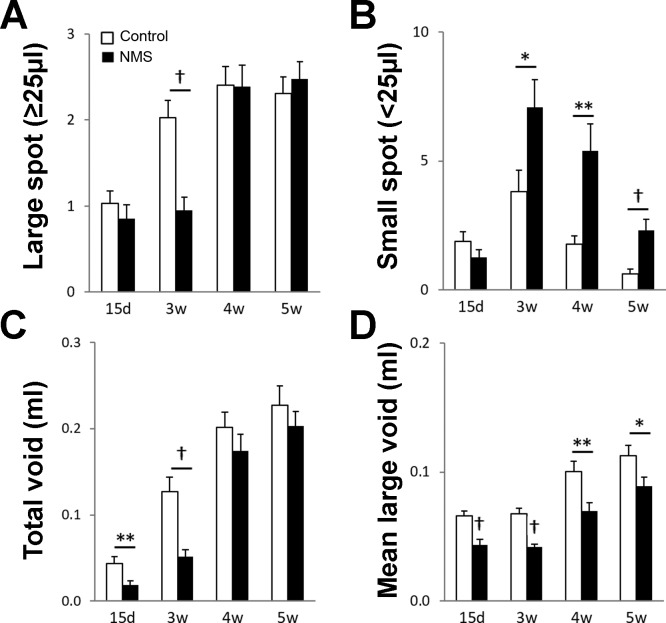
At 15 days of age spontaneous void was observed in two-thirds of control mice, which depicts the early onset of voluntary voiding around that age. However, only one-third of the animals in the NMS group had spontaneous voids at 15 days old, one-half in control group (P = 0.012 vs. control mice). At 3 wk and after, spontaneous voids were routinely seen in both groups (over 84%), suggesting that NMS delayed the development of voluntary micturition control. In the control group, the number of large voids was doubled at 3 wk from 15 days old (2.0 ± 0.2 vs. 1.0 ± 0.1, P < 0.001), reaching a plateau (2.3 to 2.4 ± 0.2, P > 0.20, 3 to 5 wk old). NMS mice showed no change in the number of large voids between 15 days and 3 wk of age. The difference between control and NMS mice at 3 wk old was statistically significant (P < 0.001). At 4 wk, the number of large voids was comparable between the two groups (Fig. 1A). The number of small voids showed a similar trend in both groups—it reached a peak at 3 wk and progressively declined afterwards. However, the frequent small voids persisted even at 5 wk in NMS mice when they were rarely observed in control mice (1.8- to 3.8-fold, P < 0.001 to 0.018, 3 to 5 wk old) (Fig. 1B). These data demonstrate that the adult form of voluntary control of voiding developed between the second and third weeks of life, which progressively took over the infantile form of voiding control characterized by small and frequent voids during the following weeks in mice. The total voided volume was significantly lower in NMS mice up to 3 wk old (41% to 43%, the volume in control mice was taken as 100% for comparison, P < 0.01), and then the difference became smaller and statistically insignificant (Fig. 1C). A significant decrease in mean volume of large voids was observed in NMS mice compared with the control group at all time points tested (62% to 79%, the volume in control mice taken as 100%, P < 0.0001 to 0.028) (Fig. 1D). This phenotype is consistent with the observation reported previously (34), which described that voided volume correlated with body size as NMS mice were smaller than control mice during tested period, 15 days to 5 wk of age (Table 1).
Fig. 1.
Micturition patterns in control and NMS groups at 15 days, 3 wk, 4 wk, and 5 wk of age. Number of the urine spots: large void (≥25 μl) (A) and small void (<25 μl) (B). Total voided volume (C). The volume per void in the large void spots (D). Open and closed bars represent control and NMS mice, respectively. Means ± SE, n = 50 per group. *P < 0.05, **P < 0.01, and †P < 0.001. NMS, neonatal maternal separation.
Table 1.
Comparison of the body weight and bladder weight at 6 wk of age in the control and NMS groups
| Body, g |
||||||
|---|---|---|---|---|---|---|
| 2 days | 15 days | 3 wk | 5 wk | 6 wk | Bladder, mg | |
| Male | 1.6 ± 0.0 | 7.7 ± 0.3 | 9.6 ± 0.2 | 19.5 ± 0.3 | 22.6 ± 0.5 | 19.8 ± 0.6 |
| Control | ||||||
| NMS | 1.6 ± 0.0 | 5.4 ± 0.1† | 6.5 ± 0.2† | 16.8 ± 0.4† | 21.4 ± 0.6 | 19.1 ± 0.9 |
| Female | 1.5 ± 0.0 | 7.6 ± 0.2 | 9.1 ± 0.3 | 16.3 ± 0.3 | 17.8 ± 0.6 | 15.9 ± 0.9 |
| Control | ||||||
| NMS | 1.5 ± 0.0 | 5.2 ± 0.2† | 6.3 ± 0.3† | 14.5 ± 0.3† | 17.1 ± 0.6 | 16.1 ± 1.3 |
Means ± SE; n = 21–25 and n = 12–13 per group each sex for body weight and bladder weight, respectively. NMS, neonatal maternal separation.
P < 0.0001 vs. control mice.
NMS induced the development of overactive bladder.
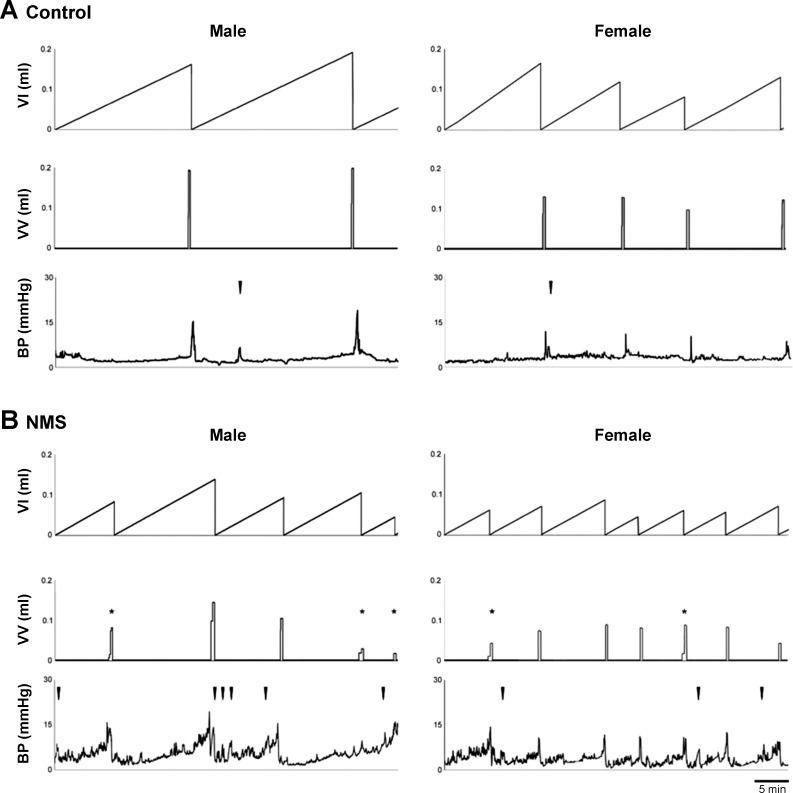
Urodynamic parameters in NMS mice were different from those in the control group for mice of both sexes (Fig. 2, Table 2). The maximal intravesical pressure at micturition was comparable between the two groups. However, NMS mice had a significantly smaller functional bladder capacity (shown as volume infused into the bladder) and voided volume than control mice (151 ± 10 vs. 106 ± 10, P = 0.0019 and 142 ± 8 vs. 98 ± 9, P = 0.0003). A few small and interrupted voids were occasionally noticed in NMS mice. There was no difference in emptying efficiency calculated as the ratio of voided volume to bladder capacity (92 ± 5 vs. 89 ± 4%, P > 0.5). Nonvoiding bladder contractions were consistently recorded in NMS mice whereas they were minimal in control mice (2.9 ± 1.0 vs. 0.7 ± 0.1 per cycle, P < 0.0001), indicating the development of bladder overactivity in NMS mice.
Fig. 2.
Functional bladder analyses in cystometry. Representative cystometrogram traces from unanesthetized, unrestrained control (A) and NMS (B) mice during a continuous intravesical infusion (10 μl/min) of room-temperature saline. Data from male and female mice are shown on the left and right, respectively. Volume infused (VI, top), voided volume (VV, middle), and bladder pressure (BP, bottom) are shown. *In voided volume traces (middle) show intermittent voids. Arrowheads in the bladder pressure traces (bottom) indicate examples of nonvoiding bladder contractions. NMS, neonatal maternal separation.
Table 2.
Comparison of urodynamic parameters in each group
| Infused, µl | Void, µl | Pves max, mmHg | Nonvoid contractions | |
|---|---|---|---|---|
| Control | 151 ± 10 | 142 ± 8 | 16.5 ± 0.9 | 0.7 ± 0.1 |
| NMS | 106 ± 10** | 98 ± 9† | 15.1 ± 0.5 | 2.9 ± 0.4† |
| Male | Infused, µl | Void, µl | Pves max, mmHg | Nonvoid contractions |
|---|---|---|---|---|
| Control | 164 ± 16 | 162 ± 10 | 18.3 ± 0.8 | 0.4 ± 0.1 |
| NMS | 121 ± 16 | 112 ± 15** | 16.9 ± 0.6 | 2.5 ± 0.5† |
| Female | Infused, µl | Void, µl | Pves max, mmHg | Nonvoid contractions |
|---|---|---|---|---|
| Control | 136 ± 11 | 118 ± 9 | 14.3 ± 1.5 | 1.1 ± 0.3 |
| NMS | 90 ± 8** | 81 ± 9** | 13.1 ± 0.7 | 3.3 ± 0.5† |
Means ± SE. NMS mice showed a significant decrease in bladder capacity, voided volume, and a significant increase in the number of nonvoid contractions compared with those in the control group (N = 10–11 per each group). Both male and female mice showed the same patterns of urodynamic changes in NMS mice (N = 5–6 per each group). NMS, neonatal maternal separation; Pves max, maximum intravesical pressure at micturition.
P < 0.01;
P < 0.001 vs. control mice.
Decreased mAChR-mediated detrusor contractility paralleled increased purinergic responses in NMS mice.
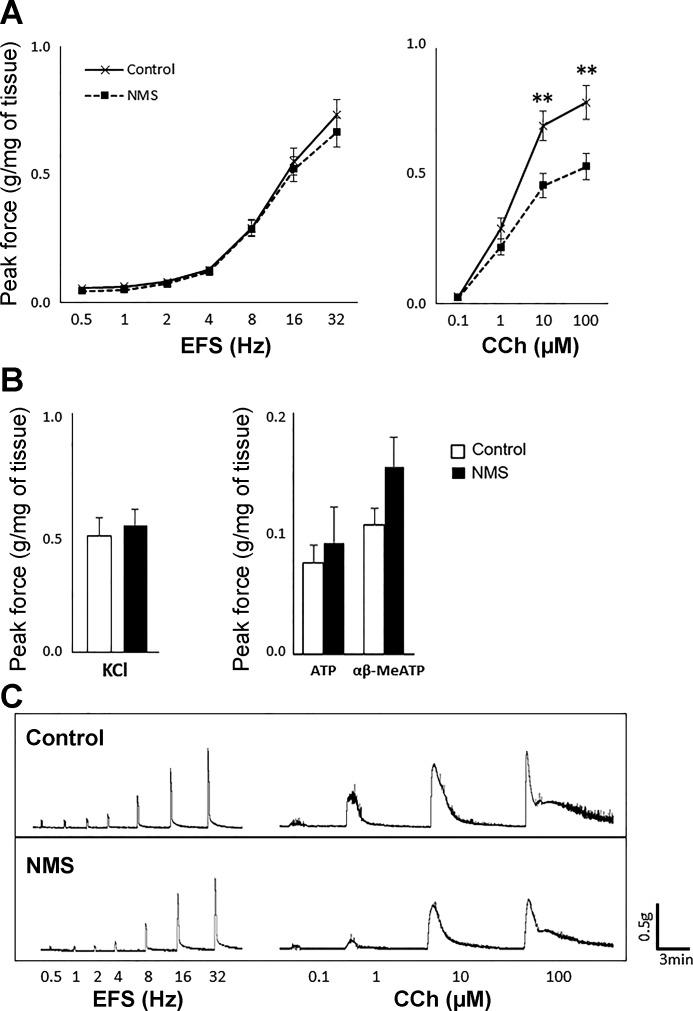
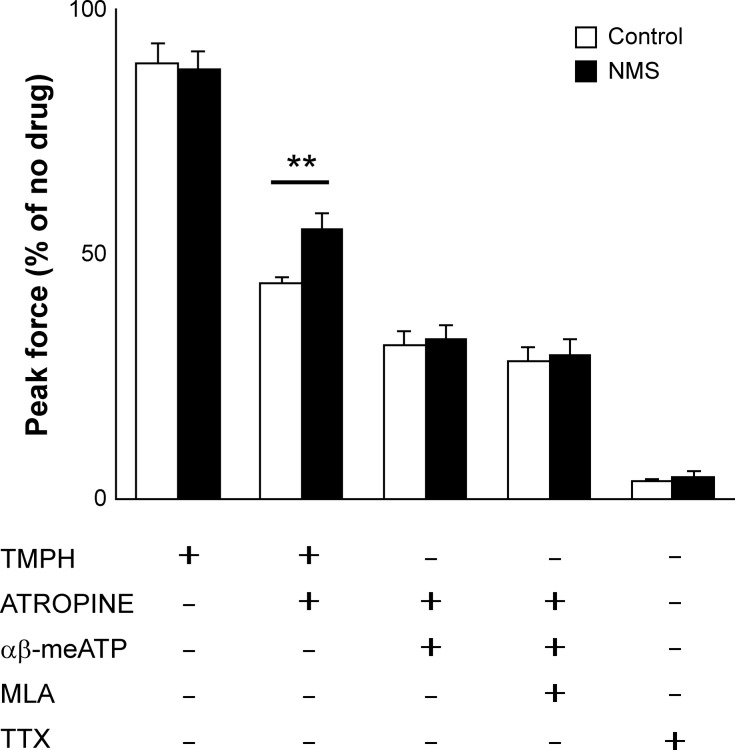
At 6 weeks of age, DSM contractility in response to EFS or KCl was comparable between the two groups. However, the response to CCh was significantly decreased in NMS mice compared with control mice (66% to 68% for 10 and 100 μM, control taken as 100%, P < 0.005) (Fig. 3). This result implies that NMS caused an impairment of AChR-mediated DSM contractions without affecting overall nerve-mediated contractility and detrusor contractile machinery. Because ATP is known to mediate DSM contractions besides mAChRs (17), we examined the response to ATP and α,β-meATP. NMS mice showed a trend of increase in contractility in response to both chemicals compared with control mice, although the change was statistically insignificant. Preincubation with TMPH or a combination of atropine and α,β-meATP with or without MLA reduced the contractile force ~10%, 68%, or 71% in both groups, respectively, indicating an equal level of involvement of neuronal nAChRs in DSM contractility. A combination of TMPH and atropine caused a significantly smaller impact in NMS mice compared with control mice (45% vs. 66% decrease, P = 0.009), implicating a reduced muscarinic input coupled with an increased contribution of cholinergic-independent pathway in NMS mice (Fig. 4). Considering that ATP and α,β-meATP induced larger contraction in the bladders in NMS mice compared with control group, purinergic mechanisms appeared to be mainly accountable for maintaining DSM contractility. TTX reduced 96% of EFS-evoked DSM contraction in both groups, indicating that TTX-insensitive neural or myogenic contributions were minimal in response to EFS.
Fig. 3.
Contractility of bladder strips at 6 wk of age. Peak contractile force in response to EFS (left) and CCh (right) (A), KCl (left) and ATP and α,β-meATP (right) (B); N = 10–12 per group. Open and closed bars represent control and NMS groups, respectively. Representative raw traces of contractions in response to EFS and CCh from each group (C). The force was normalized with tissue weight. Means ± SE, **P < 0.005. α,β-meATP, α,β-methylene ATP; CCh, carbachol; EFS, electric field stimulation; NMS, neonatal maternal separation.
Fig. 4.
Contractility of bladder strips in presence of inhibitors. Peak contractile force in response to electric field stimulation (EFS) with TMPH (nAChR inhibitor), atropine (mAChR inhibitor), α,β-meATP (P2X1/3 desensitizer), MLA (α7 nAChR inhibitor), and TTX (Na+ channel blocker). The force was normalized with tissue weight and expressed as relative to the response to EFS in absence of inhibitors (N = 5–7 per group). Open and closed bars represent control and NMS groups, respectively. Means ± SE, **P < 0.01. α,β-meATP, α,β-methylene ATP; AChR, acetylcholine receptor; mAChR, muscarinic AChR; MLA, methyllycaconitine citrate; nAChR, nicotinic AChR; NMS, neonatal maternal separation; TMPH, 2,2,6,6-tetramethylpiperidin-4-yl heptanoate; TTX, tetrodotoxin.
NMS did not affect bladder morphology.
NMS mice were significantly smaller when compared with the control group from 15 days to 5 wk old [N = 21–27 per group]; however, at 6 wk old both groups became comparable in terms of body weight and urinary bladder weight in each sex [N = 12 per group (Table 1)]. Bladder histology obtained by H&E and SHG was comparable in terms of the bladder wall thickness, the distribution and the amount of the extracellular matrix, and urothelial structure between the two groups (Fig. 5), indicating that NMS did not affect gross bladder morphology. Also, no gross morphological change was detected in kidneys by H&E staining (data not shown).
Fig. 5.
Histological analysis of bladders. Representative images of H&E staining (top) and SHG (bottom) of bladders from control (left) or NMS (right) mice at 6 wk old. Collagen fibers and cytoplasm were shown in pseudo-red and pseudo-green in SHG images, respectively. Scale bars = 500 μm. H&E, hematoxylin-eosin; NMS, neonatal maternal separation; SHG, second harmonic generation.
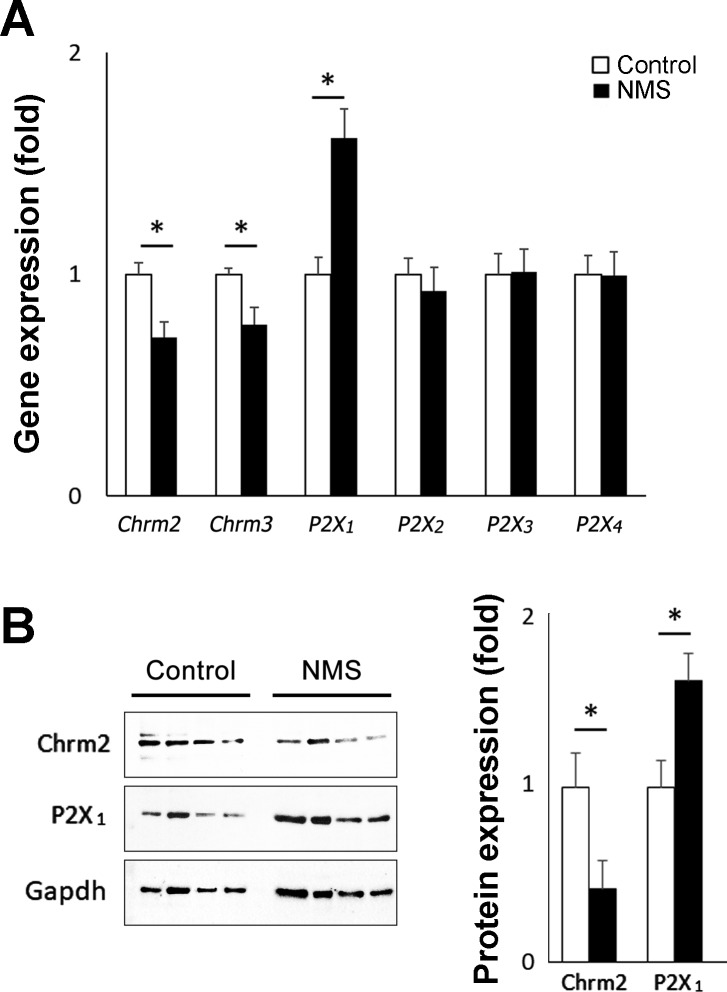
NMS induced an alteration of gene expression of mAChRs and P2X1 receptors in the bladder.
The quantitative PCR revealed a significant upregulation of purinoceptor P2X1 besides a significant downregulation of mAChR 2 and 3 (Chrm2 and Chrm3) in the urinary bladders in NMS mice compared with the control group (167 ± 13, 70 ± 7, and 77 ± 8%, respectively, control taken as 100%) (Fig. 6A). This result was consistent with the observations from in vitro DSM physiological studies. By Western blotting, the upregulation of P2X1 and the downregulation of Chrm2 were confirmed at the protein level (168 ± 21 and 42 ± 16%, respectively, control taken as 100%) (Fig. 6B). We performed Western blotting to examine the Chrm3 protein expression with two different commercially available antibodies in the bladders from control and NMS mice. However, we could not demonstrate the level of Chrm3 protein expression as neither antibody detected any specific signal with molecular weight close to the predicted (66 kDa) even for the protein samples from mouse brain used as positive control. No difference was detected in the expression level of other genes tested; nAChRs (α3–5 and β2), other purinoceptors (P2X2–4 and P2Y1–14), adrenergic receptors (α2, β2, β3), choline acetyl transferase (ChAT), and vesicular acetylcholine transporter (VAChT) (87% to 110%, data not shown).
Fig. 6.
Expression analyses of neurotransmitter receptors in bladders. A: a significant decrease in mRNA expression levels of muscarinic receptors (Chrm2 and Chrm3) and a significant increase of P2X1 were detected in bladders from NMS mice compared with control mice. Normalized with Actb (n = 4 per group). B: a decrease in Chrm2 and an increase in P2X1 protein expression were detected in bladders from NMS mice compared with the control group. Representative Western blotting results (left) and the summary of changes in Chrm2 and P2X1 at protein level normalized with Gapdh (right) (n = 4 per group). Means ± SE fold difference (control group taken as 1). White and black bars represent control and NMS groups, respectively. *P < 0.05. NMS, neonatal maternal separation.
DISCUSSION
The development of the adult form of voluntary micturition control is a continuous and gradual process that requires maturation of the nervous system, an increase in functional bladder capacity, maturation of the urethral sphincter function, and attainment of voluntary control over the bladder-sphincter unit (13, 23). Our data from void spot assay demonstrated that the earliest spontaneous voiding occurred in most animals in control and NMS groups at 15 days and 3 wk of age, respectively, suggesting that early life bladder functional disturbance delayed the development of voluntary void control. In addition, unlike the control group, NMS mice routinely had small voids with 1–2 large voids even at 5 wk old. This phenotype could be because of sustained involuntary spinal-bladder reflex as previous reports showed that perigenital-bladder reflex was induced ~40% in NMS but 20% in control rats at 6 wk old (38). In cystometry, NMS mice showed a decreased functional bladder capacity and an increased number of NVCs compared with control group, as well as occasional small and intermittent voids without notable changes in bladder pressures at micturition. Studies have shown that infants and small children often have interrupted voiding because of detrusor-sphincter dyscoordination during voiding before achieving bladder control (23, 33). Accordingly, we speculate that NMS mice had immature detrusor-sphincter coordination, which was manifested as small and intermittent voids in cystometry and frequent small voids in void spot assays. A previous study showed that about one-third of rats exposed to NMS but none in the control group retained perigenital reflex at 7 wk old suggesting an incomplete maturation of voiding control in the NMS animals (38), which agrees with the findings in this study. More recent studies demonstrated that 1) NMS mice had a significantly higher sensitivity upon urinary bladder distension in adulthood compared with the control group, 2) the hypersensitivity to urinary bladder distension mice was further increased after exposure to water avoidance stress in adult NMS mice but not in control mice, and 3) NMS mice had an increase in the number of voids compared with the control mice at 8 wk old (19, 29, 30). These results suggest the effects from NMS on bladder sustained in young adulthood, which also support our findings.
In vitro physiological examination revealed a decreased DSM contractility in response to CCh but not to EFS in NMS mice, indicating hypofunction of AChRs in DSM. Besides M3 mAChR (Chrm3) as the main contributor to DSM contraction, it has been reported that α3-containing (α3*) and α7 neuronal nAChRs expressed in the urothelium affect DSM contraction and bladder activity in a counteracting manner (4, 11). We found an equal level of DSM contractility attenuation in response to EFS in both groups by inhibiting either α3* or α7 nAChRs with TMPH and MLA, respectively, suggesting that the final effects of nAChRs on DSM contractility were unchanged. As expected, blocking mAChRs by atropine induced a substantial attenuation of contraction in control mice, whereas the observed effects were diminished in NMS mice. This result further suggested an impairment of the mAChR pathway coupled with an induction of alternative noncholinergic excitatory components and/or a suppression of inhibitory pathway in bladders of NMS mice. EFS excites bladder nerves causing a release of both excitatory and inhibitory neurotransmitters, including noradrenaline, ATP, nitric oxide, and neurokinins besides ACh from their terminals (5, 17). Of those, ATP has been recognized to play a prominent role in both motor and sensory functions in the urinary bladder through diverse types of P2X and P2Y purinoceptors (16, 37). ATP released from parasympathetic nerves participates in DSM excitability and contraction via P2X1 receptor, whereas ATP released from urothelial cells activates P2X3, P2X2/3, and P2Y receptors on afferent nerves and conveys bladder sensory information (5, 8, 14, 17, 37). α,β-meATP is a nonselective agonist that activates P2X1 and P2X3 receptor subtypes. Pretreatment of bladder strips with α,β-meATP abolished the elevated noncholinergic-mediated contraction in NMS mice, suggesting that purinergic pathways mediated via P2X1 and/or P2X3 are augmented as a compensatory mechanism for reduced contribution of the mAChR pathway to DSM contraction. Gene expression analysis revealed a downregulation in mAChRs (Chrm2 and Chrm3) and an upregulation of P2X1, whereas only minimal change was observed in other genes related to neurotransmitters involved in DSM contractility. We consider that an augmentation of inhibitory pathway unlikely play a role in producing equal DSM contractility in response to EFS as the expression of the β3 adrenergic receptor was unchanged in the bladders from both groups. Comparable expression of ChAT and VAChT combined with the DSM physiological data suggests that the bladder receives equal level of input from efferent nerves in terms of ACh release in both groups. Bladder histology and DSM contractility data suggest that NMS did not affect overall growth and development of the bladder, including its architecture and the functionality regarding the excitability of DSM contractile machinery and parasympathetic nerves. However, NMS facilitated the decline of mAChRs and the alternative increase of P2X1 receptor in the bladders, resulting in a shift in proportional contribution to DSM contraction between muscarinic and purinergic pathways in mice. In other words, an attenuation of mAChR-mediated DSM contractility, which is compensated by an elevated purinergic contribution. Accumulating evidence indicates that alteration of purinergic signaling and changes in profiles of purinoceptors are associated with pathophysiology of diverse urological problems in human and various animal species (3, 5, 20, 35). Of particular interest is that an elevated expression of P2X1 per DSM was reported in the bladders of patients with symptomatic bladder outlet obstruction (27). The NMS procedure in mice induces LUTS as a result of bladder distension and urinary retention at very young age, although the condition is intermittent not continuous as in bladder outlet obstruction cases. Another line of evidence showed a correlation between enhanced P2X1 receptor expression and increased voiding frequency and decreased bladder capacity induced by ketamine in rats, which recreated the symptoms in human ketamine abusers (25). It is well established that urothelium releases ATP upon distension, which primarily acts on afferent nerves to convey sensory and/or nociceptive signals from the bladder to the central nervous system (14, 17). Urothelial-derived ATP also acts as an autocrine signal between urothelial cells and acts as a paracrine signal affecting suburothelial interstitial cells of Cajal (ICC) or myofibroblasts (5, 26). Several lines of evidence demonstrated that the bladder mucosa and ICC/myofibroblasts release ATP at similar level as urothelium and small ATP release from DSM cells in rat and porcine bladders (7, 31, 32). Considering restricted expression of P2X1 in DSM membrane and unchanged level of expression of P2X3 in bladders (27, 37), we reason that the LUTD presented in the NMS mice in cystometry was at least partly mediated via neuron-independent DSM excitation. One plausible explanation is an elevated DSM sensitivity to ATP because of increased expression of P2X1 receptors in NMS mice. Moderate magnitude of ATP released from the urothelium, ICC/myofibroblasts, and DSM during filling phase could be captured by more P2X1 receptors on DSM located subjacent to the urothelium and lamina propria. Activation of P2X1 receptors causes excitation of small and locally limited DSM they reside on even without efferent input. The excitation of individual DSM cells then propagates to contiguous cells coupled with gap junction forming a functional unit (18), leading to larger contraction in the bladder. With the hypersensitivity, fraction of ATP could cause weak and/or local contractions, which exhibited as NVCs and bladder overactivity observed in NMS mice in cystometry. Antimuscarinics and beta agonists are typically used as first-line treatments of overactive bladder-related symptoms. Despite that those drugs offer a major improvement in symptoms and health-related quality of life in many patients, some patients do not respond to the treatment (6). Studies showed changes in mAChR functions in the bladders from patients with different types of LUTD (1). The results of this study suggest a demand for an alternative treatment to target the purinergic receptors selectively.
In conclusion, this study provides evidence that early life voiding disturbance interfered with the normal maturation of the voluntary micturition control and promoted LUTD in later stage. Further studies are required to discover the effects of early life disturbance of bladder function on the LUT as well as on neural pathways involved in the control of micturition at different stages of life, and the underlying pathophysiological mechanisms. Future studies using compounds to selectively inhibit P2X1 receptor would be useful to evaluate potential clinical application.
GRANTS
This study was supported by Ponzio Family Endowment Fund (D. T. Wilcox). Histological study was supported by University of Colorado Denver Research Histology Shared Resource funded by Cancer Center Support Grant No. P30CA046934.
DISCLOSURES
No conflicts of interest, financial or otherwise, are declared by the authors.
AUTHOR CONTRIBUTIONS
N.I. and A.P.M conceived and designed research; N.I. performed experiments; N.I. analyzed data; N.I. and D.T.W. interpreted results of experiments; N.I. prepared figures; N.I. drafted manuscript; D.T.W. edited and revised manuscript; D.T.W. approved final version of manuscript.
ACKNOWLEDGMENTS
The authors thank Dr. Ricardo Pineda and Dr. M. İrfan Dönmez from University of Colorado Denver for providing insights and expertise that greatly assisted the research and Akane Ito from Nara Women’s University, Nara, Japan, for assistance with data analyses.
REFERENCES
- 1.Andersson KE. Antimuscarinic mechanisms and the overactive detrusor: an update. Eur Urol 59: 377–386, 2011. doi: 10.1016/j.eururo.2010.11.040. [DOI] [PubMed] [Google Scholar]
- 2.Ayres PH, Shinohara Y, Frith CH. Morphological observations on the epithelium of the developing urinary bladder of the mouse and rat. J Urol 133: 506–512, 1985. doi: 10.1016/S0022-5347(17)49042-2. [DOI] [PubMed] [Google Scholar]
- 3.Bayliss M, Wu C, Newgreen D, Mundy AR, Fry CH. A quantitative study of atropine-resistant contractile responses in human detrusor smooth muscle, from stable, unstable and obstructed bladders. J Urol 162: 1833–1839, 1999. doi: 10.1016/S0022-5347(05)68247-X. [DOI] [PubMed] [Google Scholar]
- 4.Beckel JM, Birder LA. Differential expression and function of nicotinic acetylcholine receptors in the urinary bladder epithelium of the rat. J Physiol 590: 1465–1480, 2012. doi: 10.1113/jphysiol.2011.226860. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Burnstock G. Purinergic signalling in the urinary tract in health and disease. Purinergic Signal 10: 103–155, 2014. doi: 10.1007/s11302-013-9395-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Buser N, Ivic S, Kessler TM, Kessels AG, Bachmann LM. Efficacy and adverse events of antimuscarinics for treating overactive bladder: network meta-analyses. Eur Urol 62: 1040–1060, 2012. doi: 10.1016/j.eururo.2012.08.060. [DOI] [PubMed] [Google Scholar]
- 7.Cheng Y, Mansfield KJ, Sandow SL, Sadananda P, Burcher E, Moore KH. Porcine bladder urothelial, myofibroblast, and detrusor muscle cells: characterization and ATP release. Front Pharmacol 2: 27, 2011. doi: 10.3389/fphar.2011.00027. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Cockayne DA, Hamilton SG, Zhu QM, Dunn PM, Zhong Y, Novakovic S, Malmberg AB, Cain G, Berson A, Kassotakis L, Hedley L, Lachnit WG, Burnstock G, McMahon SB, Ford AP. Urinary bladder hyporeflexia and reduced pain-related behaviour in P2X3-deficient mice. Nature 407: 1011–1015, 2000. doi: 10.1038/35039519. [DOI] [PubMed] [Google Scholar]
- 9.Costantini E, Illiano E, Giannitsas K, Prestipino M, Pastore AL, Carbone A, Palleschi G, Balsamo R, Natale F, Villari D, Bini V, Maruccia S, Filocamo MT, Zucchi A. Urological dysfunction in young women: an inheritance of childhood? BJU Int 121: 453–457, 2018. doi: 10.1111/bju.14081. [DOI] [PubMed] [Google Scholar]
- 10.Cox G, Kable E, Jones A, Fraser I, Manconi F, Gorrell MD. 3-dimensional imaging of collagen using second harmonic generation. J Struct Biol 141: 53–62, 2003. doi: 10.1016/S1047-8477(02)00576-2. [DOI] [PubMed] [Google Scholar]
- 11.De Biasi M, Nigro F, Xu W. Nicotinic acetylcholine receptors in the autonomic control of bladder function. Eur J Pharmacol 393: 137–140, 2000. doi: 10.1016/S0014-2999(00)00008-X. [DOI] [PubMed] [Google Scholar]
- 12.Farhat W, Bägli DJ, Capolicchio G, O’Reilly S, Merguerian PA, Khoury A, McLorie GA. The dysfunctional voiding scoring system: quantitative standardization of dysfunctional voiding symptoms in children. J Urol 164: 1011–1015, 2000. doi: 10.1016/S0022-5347(05)67239-4. [DOI] [PubMed] [Google Scholar]
- 13.Feldman AS, Bauer SB. Diagnosis and management of dysfunctional voiding. Curr Opin Pediatr 18: 139–147, 2006. doi: 10.1097/01.mop.0000193289.64151.49. [DOI] [PubMed] [Google Scholar]
- 14.Ferguson DR, Kennedy I, Burton TJ. ATP is released from rabbit urinary bladder epithelial cells by hydrostatic pressure changes–a possible sensory mechanism? J Physiol 505: 503–511, 1997. doi: 10.1111/j.1469-7793.1997.503bb.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Fitzgerald MP, Thom DH, Wassel-Fyr C, Subak L, Brubaker L, Van Den Eeden SK, Brown JS; Reproductive Risks for Incontinence Study at Kaiser Research Group . Childhood urinary symptoms predict adult overactive bladder symptoms. J Urol 175: 989–993, 2006. doi: 10.1016/S0022-5347(05)00416-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Ford AP, Gever JR, Nunn PA, Zhong Y, Cefalu JS, Dillon MP, Cockayne DA. Purinoceptors as therapeutic targets for lower urinary tract dysfunction. Br J Pharmacol 147, Suppl 2: S132–S143, 2006. doi: 10.1038/sj.bjp.0706637. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Fowler CJ, Griffiths D, de Groat WC. The neural control of micturition. Nat Rev Neurosci 9: 453–466, 2008. doi: 10.1038/nrn2401. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Fry CH, Sui GP, Severs NJ, Wu C. Spontaneous activity and electrical coupling in human detrusor smooth muscle: implications for detrusor overactivity? Urology 63, Suppl 1: 3–10, 2004. doi: 10.1016/j.urology.2003.11.005. [DOI] [PubMed] [Google Scholar]
- 19.Fuentes IM, Pierce AN, Di Silvestro ER, Maloney MO, Christianson JA. Differential influence of early life and adult stress on urogenital sensitivity and function in male mice. Front Syst Neurosci 11: 97, 2018. doi: 10.3389/fnsys.2017.00097. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Harvey RA, Skennerton DE, Newgreen D, Fry CH. The contractile potency of adenosine triphosphate and ecto-adenosine triphosphatase activity in guinea pig detrusor and detrusor from patients with a stable, unstable or obstructed bladder. J Urol 168: 1235–1239, 2002. doi: 10.1016/S0022-5347(05)64632-0. [DOI] [PubMed] [Google Scholar]
- 21.Iguchi N, Dönmez MI, Malykhina AP, Carrasco A JR, Wilcox DT. Preventative effects of a HIF inhibitor, 17-DMAG, on partial bladder outlet obstruction-induced bladder dysfunction. Am J Physiol Renal Physiol 313: F1149–F1160, 2017. doi: 10.1152/ajprenal.00240.2017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Iguchi N, Malykhina AP, Wilcox DT. Inhibition of HIF reduces bladder hypertrophy and improves bladder function in murine model of partial bladder outlet obstruction. J Urol 195: 1250–1256, 2016. doi: 10.1016/j.juro.2015.08.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Kokoua A, Homsy Y, Lavigne JF, Williot P, Corcos J, Laberge I, Michaud J. Maturation of the external urinary sphincter: a comparative histotopographic study in humans. J Urol 150: 617–622, 1993. doi: 10.1016/S0022-5347(17)35563-5. [DOI] [PubMed] [Google Scholar]
- 24.Leung VY, Chu WC, Yeung CK, Sreedhar B, Liu JX, Wong EM, Metreweli C. Nomograms of total renal volume, urinary bladder volume and bladder wall thickness index in 3,376 children with a normal urinary tract. Pediatr Radiol 37: 181–188, 2007. doi: 10.1007/s00247-006-0376-y. [DOI] [PubMed] [Google Scholar]
- 25.Meng E, Chang HY, Chang SY, Sun GH, Yu DS, Cha TL. Involvement of purinergic neurotransmission in ketamine induced bladder dysfunction. J Urol 186: 1134–1141, 2011. doi: 10.1016/j.juro.2011.04.102. [DOI] [PubMed] [Google Scholar]
- 26.Merrill L, Gonzalez EJ, Girard BM, Vizzard MA. Receptors, channels, and signalling in the urothelial sensory system in the bladder. Nat Rev Urol 13: 193–204, 2016. doi: 10.1038/nrurol.2016.13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.O’Reilly BA, Kosaka AH, Chang TK, Ford AP, Popert R, McMahon SB. A quantitative analysis of purinoceptor expression in the bladders of patients with symptomatic outlet obstruction. BJU Int 87: 617–622, 2001. doi: 10.1046/j.1464-410x.2001.02179.x. [DOI] [PubMed] [Google Scholar]
- 28.Petrangeli F, Capitanucci ML, Marciano A, Mosiello G, Alvaro R, Zaccara A, Finazzi-Agro E, De Gennaro M. A 20-year study of persistence of lower urinary tract symptoms and urinary incontinence in young women treated in childhood. J Pediatr Urol 10: 441–445, 2014. doi: 10.1016/j.jpurol.2014.01.003. [DOI] [PubMed] [Google Scholar]
- 29.Pierce AN, Di Silvestro ER, Eller OC, Wang R, Ryals JM, Christianson JA. Urinary bladder hypersensitivity and dysfunction in female mice following early life and adult stress. Brain Res 1639: 58–73, 2016. doi: 10.1016/j.brainres.2016.02.039. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Pierce AN, Eller-Smith OC, Christianson JA. Voluntary wheel running attenuates urinary bladder hypersensitivity and dysfunction following neonatal maternal separation in female mice. Neurourol Urodyn 37: 1623–1632, 2018. doi: 10.1002/nau.23530. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Sadananda P, Kao FC, Liu L, Mansfield KJ, Burcher E. Acid and stretch, but not capsaicin, are effective stimuli for ATP release in the porcine bladder mucosa: are ASIC and TRPV1 receptors involved? Eur J Pharmacol 683: 252–259, 2012. doi: 10.1016/j.ejphar.2012.02.041. [DOI] [PubMed] [Google Scholar]
- 32.Sadananda P, Shang F, Liu L, Mansfield KJ, Burcher E. Release of ATP from rat urinary bladder mucosa: role of acid, vanilloids and stretch. Br J Pharmacol 158: 1655–1662, 2009. doi: 10.1111/j.1476-5381.2009.00431.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Sillén U. Bladder function in healthy neonates and its development during infancy. J Urol 166: 2376–2381, 2001. doi: 10.1016/S0022-5347(05)65594-2. [DOI] [PubMed] [Google Scholar]
- 34.Sugino Y, Kanematsu A, Hayashi Y, Haga H, Yoshimura N, Yoshimura K, Ogawa O. Voided stain on paper method for analysis of mouse urination. Neurourol Urodyn 27: 548–552, 2008. doi: 10.1002/nau.20552. [DOI] [PubMed] [Google Scholar]
- 35.Sun Y, Keay S, De Deyne PG, Chai TC. Augmented stretch activated adenosine triphosphate release from bladder uroepithelial cells in patients with interstitial cystitis. J Urol 166: 1951–1956, 2001. doi: 10.1016/S0022-5347(05)65726-6. [DOI] [PubMed] [Google Scholar]
- 36.Tran A, Fortier C, Giovannini-Chami L, Demonchy D, Caci H, Desmontils J, Montaudie-Dumas I, Bensaïd R, Haas H, Berard E. Evaluation of the bladder stimulation technique to collect midstream urine in infants in a pediatric emergency department. PLoS One 11: e0152598, 2016. doi: 10.1371/journal.pone.0152598. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Vial C, Evans RJ. P2X receptor expression in mouse urinary bladder and the requirement of P2X(1) receptors for functional P2X receptor responses in the mouse urinary bladder smooth muscle. Br J Pharmacol 131: 1489–1495, 2000. doi: 10.1038/sj.bjp.0703720. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Wu HY, de Groat WC. Maternal separation uncouples reflex from spontaneous voiding in rat pups. J Urol 175: 1148–1151, 2006. doi: 10.1016/S0022-5347(05)00321-6. [DOI] [PMC free article] [PubMed] [Google Scholar]